Abstract
Integrins, the major receptors for cell-extracellular matrix (ECM) interactions, regulate multiple cell biological processes including adhesion, migration, proliferation and growth factor-dependent signaling. The principal laminin (LM) binding integrins α3β1, α6β1 and α6β4 are usually co-expressed in cells and bind to multiple laminins with different affinities making it difficult to define their specific function. In this study, we generated kidney epithelial collecting duct (CD) cells that lack both the α3 and α6 integrin subunits. This deletion impaired cell adhesion and migration to LM-332 and LM-511 more than deleting α3 or α6 alone. Cell adhesion mediated by both α3β1 and α6 integrins was PI3K independent, but required K63-linked polyubiquitination of Akt by the ubiquitin-modifying enzyme TRAF6. Moreover, we provide evidence that glial-derived neurotrophic factor (GDNF) and fibroblast growth factor 10 (FGF10)- mediated cell signaling, spreading and proliferation were severely compromised in double integrin α3/α6- but not single α3- or α6-null CD cells. Interestingly, these growth factor-dependent cell functions required both PI3K- and TRAF6-dependent Akt activation. These data suggest that expression of the integrin α3 or α6 subunit is sufficient to mediate GDNF- and FGF10-dependent spreading, proliferation and signaling on LM-511. Thus, our study shows that α3 and α6 containing integrins promote distinct functions and signaling by CD cells on laminin substrata.
Keywords: kidney, basement membrane, receptor, cell migration
Introduction
Basement membranes (BMs) are specialized extracellular matrices that coat the basal aspect of epithelial and endothelial cells. The core structural components of BMs are laminins (LM), non-fibrillar collagens, nidogens, the heparan sulfate proteoglycans perlecan and agrin [1-3]. There are 16 different LMs that consist of an α, a β and a γ chain [1-5]. Epithelial cells adhere to LMs via integrins, which are heterodimeric transmembrane receptors that consist of non-covalently bound α and β subunits [1-3]. Integrins α3β1, α6β1 and α6β4 are the major receptors used by epithelial cells to bind LMs [6-8].
The kidney is composed of two major components, the glomerulus, which is the filtering unit, and the tubules comprised of terminally differentiated epithelial cells. The collecting duct (CD), which represents the distal kidney tubule, is the best studied tubular segment with respect to cell-ECM interactions. LM-511 and −332 are the major LMs expressed in CD BMs and epithelial cells bind to them via integrins α3β1, α6β1 and α6β4 [9-12]. Despite the high levels of expression of these LMs, deleting the α5, α3 or γ2 LM chain in the developing kidney collecting system causes relatively minor developmental phenotypes[11, 12]. Similarly, deleting the integrin α3 subunit in vivo causes a subtle developmental phenotype, while deleting the integrin α6 subunit does not cause any abnormal developmental phenotype [9, 10].
In contrast to the in vivo data, integrin α3-null CD cells exhibit major abnormalities with respect to function on LMs. Integrin α3β1 is required for CD cell adhesion to both LM-332 and −511 [9]. Furthermore, integrin α3β1-dependent CD cell adhesion to LMs requires Akt activation that is PI3K independent, but requires K63-linked polyubiquitination mediated by TRAF6 [9]. α6 containing integrins are not required for CD adhesion to LM-511 and they only have a minimal contribution to adhesion on LM-332. They also do not mediate integrin-dependent signaling on LMs [10]. These data indicate that α3 and α6 containing integrins play selective and diverse cell functions with α3β1 driving most of the cell adhesion to the two major LMs expressed in the CD BMs.
In this study, we further investigate the relative contribution of α3 versus α6 integrins on CD cell function by analyzing CD cells that lack both α3 and α6 integrin subunits. We provide evidence that the double integrin α3/α6 null CD cells have worse adhesion and migration than single integrin α3- or α6-null CD cells on both LM-332 and LM-511. In addition, α3/α6 -null CDs have severe defects in growth factor-mediated cell signaling, spreading and proliferation. Finally, we show that growth factor-induced cell functions on laminin substrata require both PI3K- and TRAF6-dependent Akt activation. Thus α3 and α6 containing integrins promote distinct functions and signaling by CD cells on laminin substrata.
Results
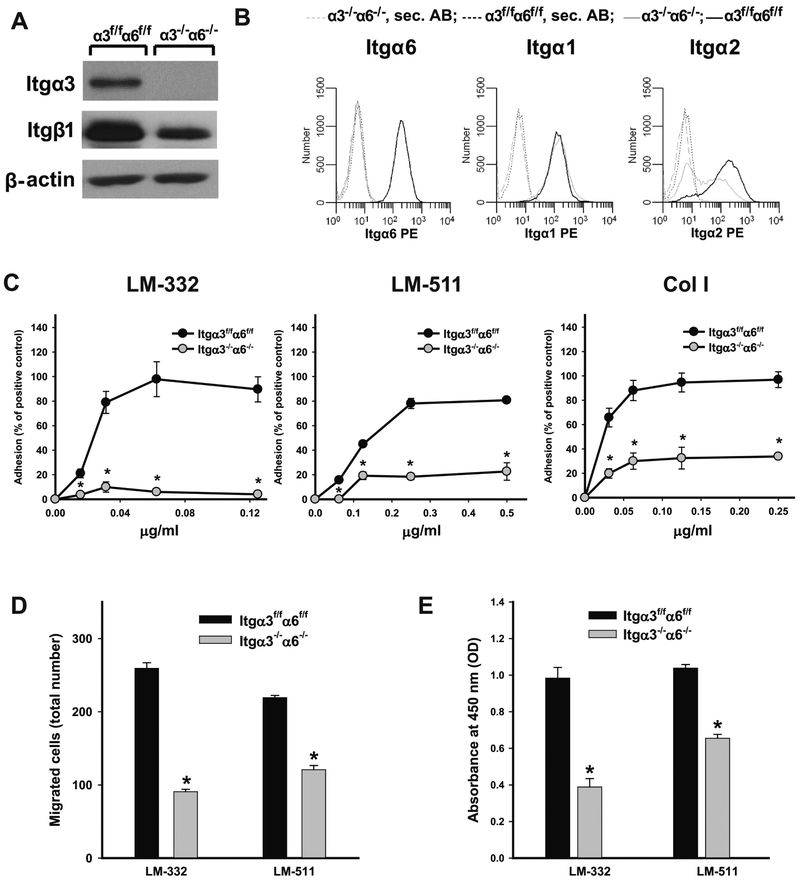
Integrin subunits α3 and α6 are critical for CD cell adhesion, proliferation and migration on LM-332 and LM-511.
We interbred integrin (Itg) α3flox/flox [13] and α6flox/flox [14] mice to obtain Itgα3flox/floxα6flox/flox (Itgα3f/fα6f/f) mice. CD cells were isolated from these mice and both α3 and α6 genes were deleted by adenovirus-Cre mediated recombination [9]. Successful generation of Itgα3−/−α6−/− CD cells was verified by immunoblotting for the integrin α3 subunit and flow cytometry of the α6 subunit (Fig. 1A-B). There was markedly decreased β1 integrin subunit expression (Fig. 1A-B). To determine whether deleting these two major LM receptors altered expression of other integrin α subunits, we evaluated the expression of the collagen receptors (integrins α1β1 and α2β1), the fibronectin binding integrin α5β1 and the αv containing integrins. While expression of α1 (Fig. 1B), α5 and αv (data not shown) integrin subunits were unchanged, surface expression of the α2 integrin subunit was decreased in Itgα3−/−α6−/− CD cells (Fig. 1B).
Figure 1. Integrin subunits α3 and α6 are critical for CD cell adhesion, proliferation and migration on LM-332 and LM-511.
Itgα3−/−α6−/− CD cells do not express integrin subunits α3 and α6 and have less β1 and α2 than Itgα3f/fα6f/f cells as assessed by immunoblot (A) and FACS analyses (B). Adhesion (C), migration (D) and proliferation (E) of Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells on LM-332, LM-511 and Col I were evaluated at 1 (C), 4 (D) and 24 (E) h after plating. Shown are mean measurements ±SEM of 4-6 experiments; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells.
We used the Itgα3−/−α6−/− CD cells to define the effects of complete deletion of the lamininbinding integrins on CD cell adhesion, migration and proliferation on LM-332, LM-511 and collagen I (Col I), the principal ligand for integrin α2β1. Itgα3−/−α6−/− CD cells were unable to adhere on LM-332 and LM-511 adhesion was severely compromised compared to the cells expressing both integrins (Fig. 1C). Itgα3−/−α6−/− CD cell adhesion to LM-511 was decreased further with the addition of a β1blocking antibody, but it was unaffected by antibodies directed against the α1, α2 or α5 subunits (data not shown). Consistent with the decreased expression of integrin α2β1, Itgα3−/−α6−/− CD cells had an adhesion defect on Col I (Fig. 1C). Itgα3−−α6−/−CD cells also showed significant migration and proliferation defects when plated on LM-332 and LM-511 (Fig. 1D-E). Thus, deleting both integrin α3 and α6 subunits causes severe adhesion, migration and proliferation defects when CD cells are plated on LMs.
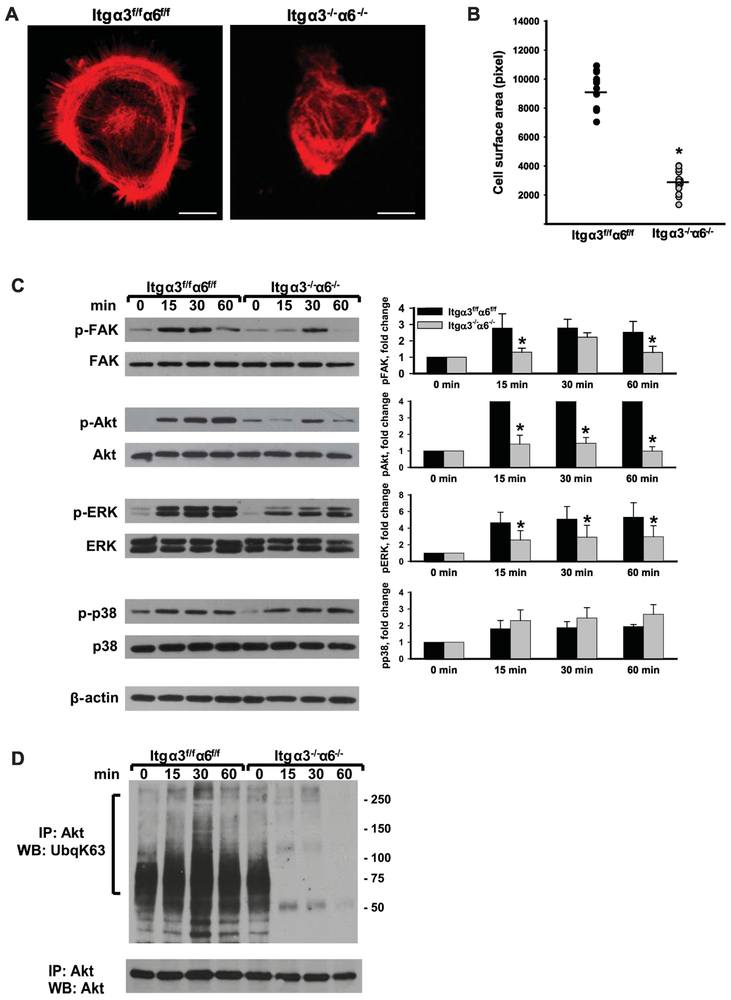
Integrin α3 and α6 subunits regulate CD cell spreading and signaling on LM-511.
We next defined the ability of Itgα3−/−α6−/− CD cells to spread and signal after adhesion to LM-511. We did not analyze their spreading and signaling on LM-332 due to their inability to adhere to this substrate (Fig. 1C). Itgα3−/−α6−/− CD cells spread significantly less than Itgα3f/fα6f/f CD cells (Fig. 2A-B). When replating assays of Itgα3−/−α6−/− and Itgα3f/fα6f/f CD cells on LM-511 were performed, we observed significantly decreased activation of focal adhesion kinase (FAK) and ERK, but not p-38 MAPK (Fig 2C). With respect to Akt phosphorylation, basal activity in Itgα3f/fα6f/f CD cells was low and it increased significantly following adhesion of these cells to LM-511. By contrast, Itgα3−/−α6−/− CD cells demonstrated higher basal activity of Akt with little increase after adhesion to LM-511. These results are similar to the ones observed with single Itgα3−/− CD cells and attributed to alterations in TRAF6-dependent K63-linked polyubiquitination of Akt [9]. Like in the single Itgα3−/− CD cells, there was K63-linked polyubiquitination of Akt in Itgα3−/−α6−/− CD cells at baseline, however, after plating on LM-511, this only increased in Itgα3f/fα6f/f and it decreased in Itgα3−/−α6−/− CD cells (Fig. 2D). Thus, deleting both α3 and α6 integrins in CD cells resulted in decreased FAK, ERK and Akt activation following adhesion to LM-511 and the decreased Akt activation was associated with diminished K63-linked polyubiquitination.
Figure 2. Integrin subunits α3 and α6 regulate CD cell spreading on LM-511 as well as Akt phosphorylation and K63-linked polyubiquitination.
(A-B) Spreading of Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells on LM-511 was evaluated 1 h after plating. (A) Representative confocal images of cells stained with rhodamine–phalloidin are shown; bar: 10 μM. (B) The individual values of cell surface area (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells. (C-D) Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells were plated on LM-511 (0.5 μg/ml) and lysed 15, 30 and 60 min later. Cell lysates were subjected to immunoblot analysis for phosphorylation of FAK, Akt, p38, ERK1/2 (C), or to immunoprecipitation with an anti-Akt antibody followed by immunoblot analyses for K63-linked polyubiquitination and Akt (D). Time point “0” represents non-adherent cells. Levels of phosphorylated proteins were measured by densitometry, normalized to total protein and β-actin levels, and expressed as fold-change relative to cells left in suspension, “0” time point. Values are the mean and ±SEM of 3 independent experiments; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells of the corresponding time point.
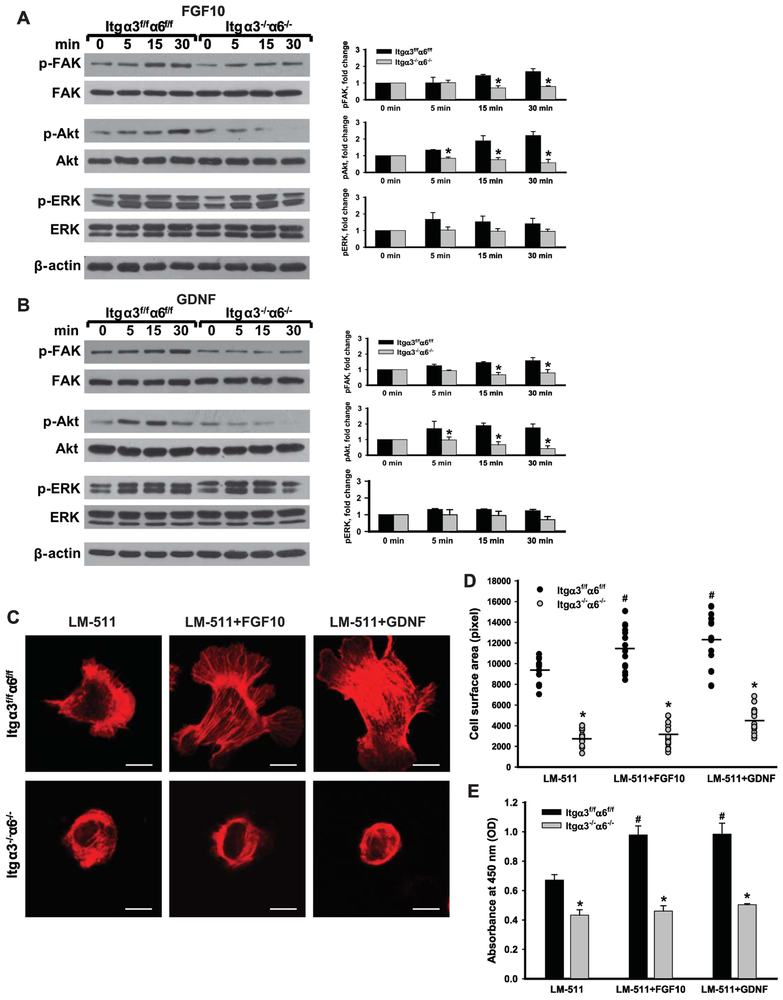
A single LM-binding integrin is sufficient for growth factor-dependent CD cell signaling, spreading and proliferation.
We previously showed that signaling mediated by growth factors such as GDNF, FGF2 and FGF10 in CD cells requires β1 containing integrins [15]. However, the relative and/or specific contribution of LM-binding integrins in mediating growth factor-induced signaling in CD cells is unknown. We therefore treated wild type or single Itgα3−/− and Itgα6−/− CD cells plated onto LM-511 with GDNF or FGF10 for various times. There were no differences in FGF10- or GDNF-mediated FAK, ERK or Akt signaling among the three cell populations (Supplementary Figure). In contrast to Itgα3f/f α6f/f, Itgα3−/− or Itgα6−/− CD cells, the Itgα3−/−Itgα6−/− CD cells failed to activate FAK or Akt in response to either FGF10 or GDNF, however no major changes in ERK phosphorylation were observed between any of the cell types (Fig. 3A-B). Consistent with the decrease in Akt signaling, relative to control CD cells, Itgα3−/−α6−/− CD cells were unable to spread on LM-511 in response to FGF10 or GDNF (Fig. 3C-D). Furthermore, while FGF10 or GDNF treatment induced a significant increase in proliferation of Itgα3f/f α6f/f cells plated on LM-511, they failed to induce this effect for Itgα3−/−α6−/− CD cells (Fig. 3E). These data suggest that expression of either integrin α3 or α6 subunit is sufficient to mediate growth factor signaling and biological effects such as cell spreading and proliferation on LM-511.
Figure 3. Integrin subunits α3 and α regulate FGF10- and GDNF-dependent Akt activation, cell spreading and proliferation on LM-511.
(A-B) Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells were plated on LM-511 for 1 h, treated with FGF10 (A) or GDNF (B) (10 ng/ml each) and lysed 5, 15 and 30 min after addition of growth factors. Cell lysates were subjected to immunoblot analysis for phospho- FAR, Akt and ERK1/2; β-actin was used as loading control. Levels of phosphorylated proteins were measured by densitometry, normalized to total protein and β-actin levels, and expressed as fold-change relative to the “0” time point. Values are the mean and ±SEM of 3-5 independent experiments; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells at the corresponding time point. (C-E) Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells were plated on LM-511 for 1 h and treated with FGF10 or GDNF (10 ng/ml each). Cell spreading (C-D) and proliferation (E) were evaluated at 1 and 24 h after addition of growth factors. (C) Representative confocal images of the cells stained with rhodamine-phalloidin; bar: 10 μM. (D) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells. # ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF (E) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown; *p≤0.05 between Itgα3f/fα6f/f and Itgα3−/−α6f/f CD cells; # p≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF.
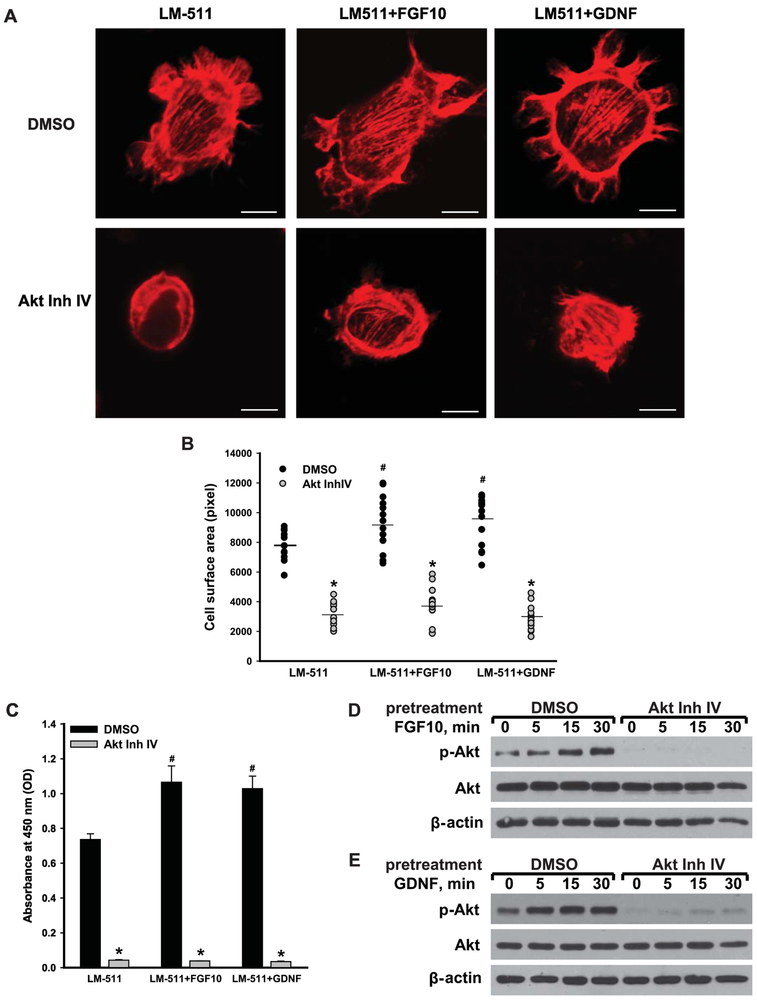
FGF10- and GDNF-induced CD cell spreading and proliferation is regulated by both PI3K dependent and independent Akt activation.
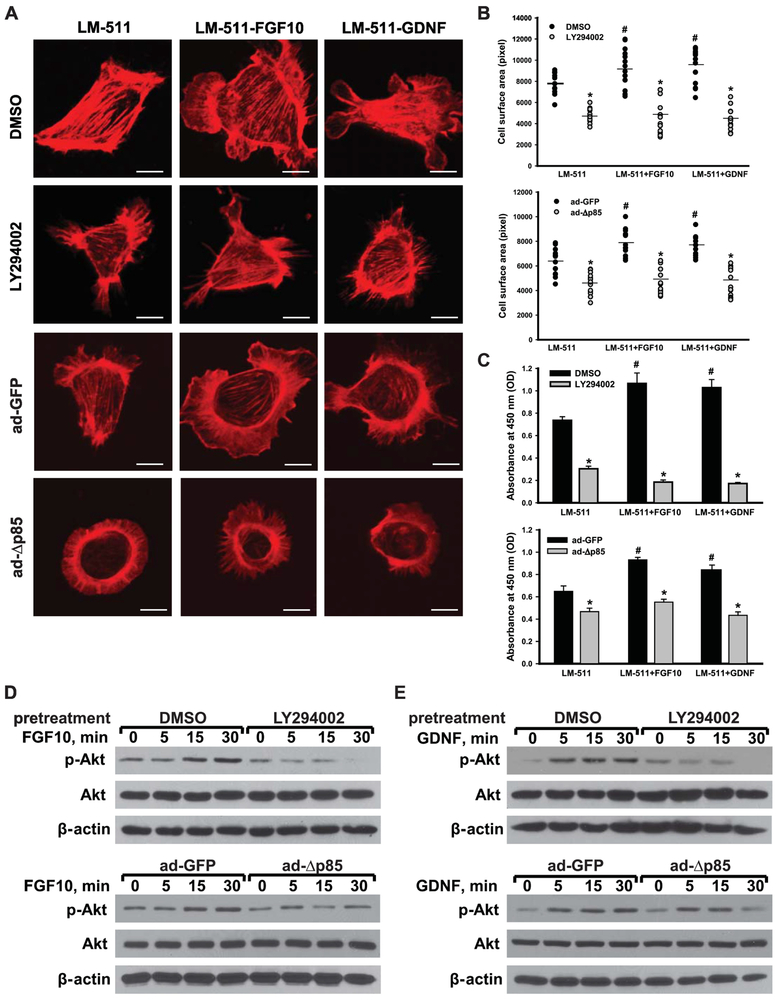
Since Itgα3−/−α6−/−, but not Itgα3−/− or Itgα6−/− CD cells are unable to respond to FGF10 and GDNF, we investigated the mechanisms underlying these abnormalities. We focused on Akt signaling, the principal pathway affected in the Itgα3−/−α6−/− CD cells. A role for Akt signaling in mediating FGF10- and GDNF-dependent spreading on LM-511 was confirmed by the observation that treatment of Itgα3f/fα6f/f CD cells with the Akt inhibitor IV prevented growth factor-induced spreading (Fig. 4A-B). In addition, Akt inhibitor IV decreased their proliferation both at baseline and in response to FGF10 or GDNF (Fig. 4C). The inhibitor efficacy in preventing Akt activation was verified by performing immunoblots on Itgα3f/fα6f/f CD cells plated on LM-511, pretreated with or without Akt inhibitor IV and exposed to FGF10 and GDNF (Fig. 4D-E).
Figure 4. FGF10- or GDNF-induced cellular spreading and proliferation mediated by LM-binding integrins is regulated by Akt.
Itgα3f/fα6f/f CD cells were treated with DMSO or Akt inhibitor IV (5 μM) for 1 h, plated on LM-511 for 1 h and then treated with or without FGF10 (A) or GDNF (B) (10 ng/ml each). Cell spreading (A-B) and proliferation (C) were evaluated at 1 and 24 h after addition of growth factors, respectively. (A) Representative confocal images of the cells stained with rhodamine-phalloidin are shown; bar: 10 μM. (B) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f CD cells pretreated with DMSO and Akt Inh. IV. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (C) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown; *p≤0.05 between cells treated with DMSO and Akt Inh.IV. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (D-E) Phosphorylation of Akt was evaluated 5, 15 and 30 min after addition of FGF10 (D) or GDNF (E). β-actin was used as loading control.
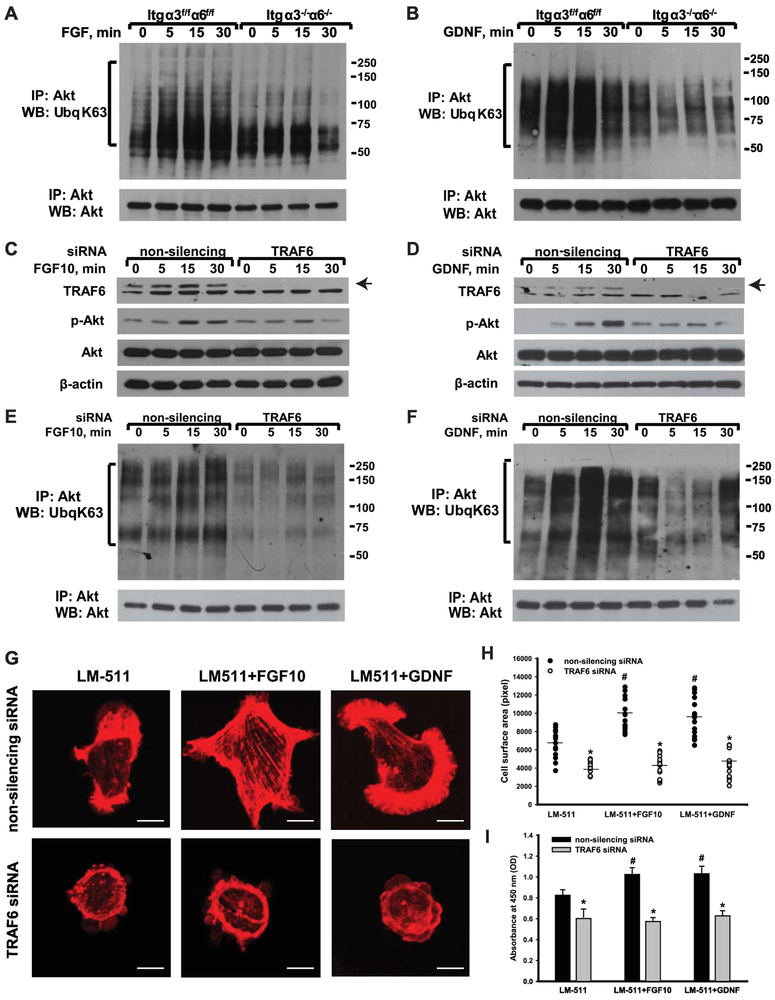
As TRAF6-mediated K63-linked polyubiquitination is a major mechanism of Akt activation when integrins bind to LMs, we assessed the levels of K63-linked polyubiquitination of Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells plated on LM-511 and stimulated with FGF10 or GDNF. K63-linked polyubiquitination of Akt increased over time when Itgα3f/fα6f/f CD cells were exposed to the growth factors, while no change was noted in the Itgα3−/−α6−/− CD cells (Fig. 5A-B). To investigate whether this K63-linked polyubiquitination of Akt is mediated by TRAF6, we downregulated TRAF6 expression in Itgα3f/fα6f/f CD cells using siRNA (Fig. 5C-J). Reduced TRAF6 expression resulted in a defect in FGF10- or GDNF-mediated Akt phosphorylation (Fig. 5C-D) and decreased K63-linked polyubiquitination of Akt (Fig. 5E-F). Consistent with the diminished Akt signaling, CD cell spreading (Fig. 5G-H) and proliferation (Fig. 5J) were significantly decreased in the TRAF6 siRNA treated Itgα3f/fα6f/f CD cells, however the effects were much less than those seen when Akt inhibitor IV was utilized (Fig. 4). It is interesting to note that downregulation of TRAF6 affected cell proliferation less than cell spreading. These data suggest that mechanism(s) other than TRAF6-mediated K63-linked polyubiquitination also regulate Akt-dependent cellular functions induced by growth factors.
Figure 5. FGF10- or GDNF-induced cellular spreading and proliferation mediated by LM-binding requires TRAF6-mediated K63-linked polyubiquitination of Akt.
(A-B) Itgα3f/fα6f/f and Itgα3−/−α6−/− CD cells were plated on LM-511 for 1 h, treated with FGF10 (A) or GDNF (B) (10 ng/ml each) and lysed at 5, 15 and 30 min later. Cell lysates were immunoprecipitated with an anti-Akt antibody and immunoblotted for K63-linked polyubiquitination or Akt. (C-J) Itgα3f/fα6f/f CD cells were transfected with non-silencing or TRAF6 siRNA (20 nM for 24 h), plated on LM-511 for 1 h and treated with FGF10 or GDNF (10 ng/ml each). (C-F) Cells were lysed 5, 15 and 30 min after addition of growth factors and immunoblotted for phosphorylation of Akt and TRAF6 protein levels (C-D) or immunoprecipitated with anti-Akt antibody and then immunoblotted for K63-linked polyubiquitination or Akt (E-F). β-actin was used as loading control (C-D). Cell spreading (G-H) and proliferation (J) were evaluated at 1 and 24 h after addition of growth factors, respectively. (G) Representative confocal images of the cells stained with rhodamine-phalloidin are shown; bar: 10 μM. (H) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f CD cells transfected with non-silencing and TRAF6 siRNA. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (J) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown. *p≤0.05 between cells transfected with nonsilencing and TRAF6 siRNA. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF.
Since PI3K is a canonical key regulator of Akt activity, we determined whether it plays a role in integrin-dependent growth factor-induced Akt activation. We inhibited PI3K activity either by using the PI3K inhibitor LY294002 or by infecting Itgα3f/fα6f/f CD cells with adenovirus carrying a PI3K catalytic subunit deletion mutant (ad-Δp85). Inhibiting PI3K activation by either of these mechanisms decreased CD cell spreading (Fig. 6A-B) and proliferation (Fig. 6C). In contrast to TRAF6 downregulation, which primarily affected cell-spreading, inhibition of PI3K-dependent Akt activation, resulted in a more significant decrease in growth factor-mediated cell proliferation (Fig. 5G-J compared to Fig. 6A-C). The efficacy of LY294002 and ad-Δp85 on inhibiting growth factor-dependent Akt activation was confirmed by immunoblotting lysates from CD cells treated with FGF10 or GDNF for phospho-Akt (Fig. 6D-E).
Figure 6. Integrin subunits α3 and α6 regulate FGF10- or GDNF-induced cellular spreading and proliferation on LM-511 via PI3K-dependent Akt activation.
Itgα3f/fα6f/f CD cells were treated with DMSO or the PI3K inhibitor LY294002 (25 μM) for 1 h and plated on LM-511 for 1 h . Itgα3f/fα6f/f CD cells were also infected with ad-GFP or ad-Δp85 for 48 h prior to plating on LM-511. The cells were treated with FGF10 or GDNF (10 ng/ml each). Cell spreading (A-B) and proliferation (C) were evaluated at 1 and 24 h after addition of growth factors, respectively. (A) Representative confocal images of the cells stained with rhodamine-phalloidin are shown; bar: 10 μM. (B) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between Itgα3f/fα6f/f CD cells pretreated with DMSO and LY; or between infected with ad-GFP and ad-Δp85. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (C) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown; *p≤0.05 between cells pretreated with DMSO and LY; or between infected with ad-GFP and ad-Δp85. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (D-E) Phosphorylation of Akt was evaluated at 5, 15 and 30 min after addition of FGF10 (D) or GDNF (E). β-actin was used as loading control.
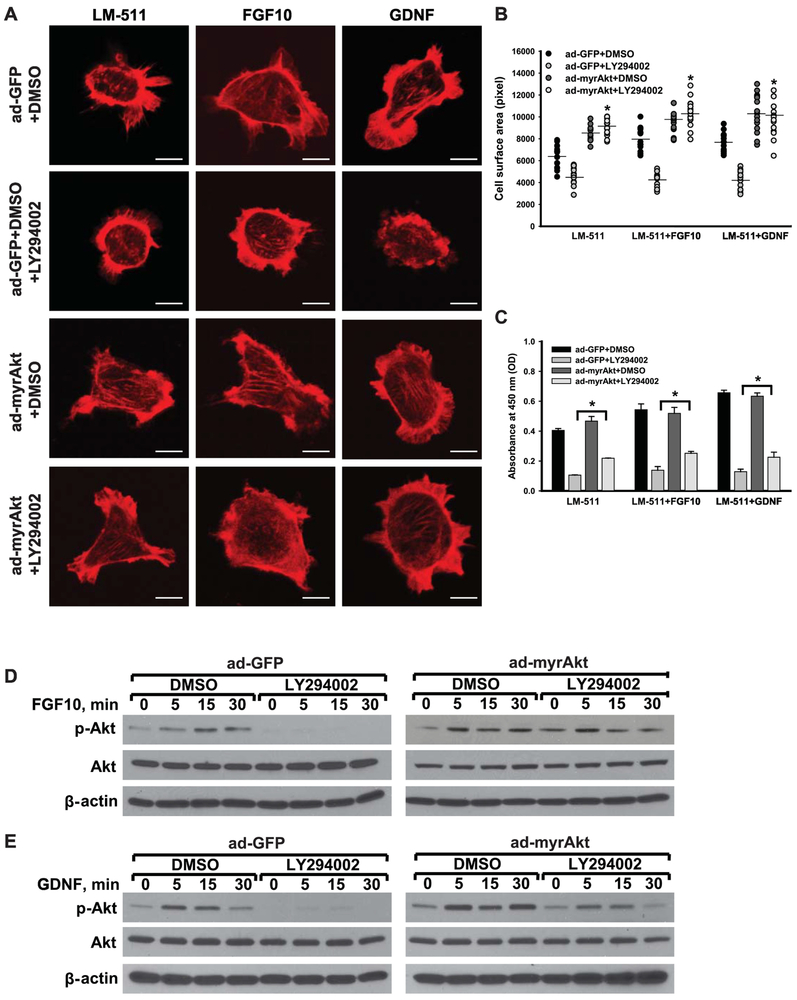
To verify that the effects of growth factor-induced CD cell spreading and proliferation were mediated via Akt activation, we infected Itgα3f/fα6f/f CD cells with either an adenovirus carrying GFP (ad-GFP) or myristoylated Akt (ad-myrAkt) that anchors Akt to the cellular membrane keeping it constitutively active. After plating on LM-511, the infected cells were treated with LY294002 before stimulation with FGF10 or GDNF (Fig. 7). Inhibiting PI3K with LY294002 did not affect growth factor-induced spreading (Fig. 7A-B) of cells expressing the constitutively active Akt despite decreasing this function in cells infected with the GFP vector. This constitutive form of Akt also significantly rescued proliferation of cells treated with LY294002, however it was only partial (Fig. 7C). We confirmed that introduction of a constitutively active Akt, reversed the effects of LY294002 with respect to Akt activation following exposure to FGF10 and GDNF (Fig. 7D-E).
Figure 7. Akt activation is sufficient to mediate LM-binding integrin-dependent FGF10- or GDNF-induced cellular spreading and proliferation.
Itgα3f/fα6f/f CD cells were infected with ad-GFP or ad-myrAkt for 48 h, treated with LY294002 (25 μM) for 1 h, plated on LM-511 for 1 h and stimulated with FGF10 or GDNF (10 ng/ml each). Cell spreading (A-B) and proliferation (C) were evaluated at 1 and 24 h after addition of growth factors, respectively. (A) Representative confocal images of the cells stained with rhodamine-phalloidin are shown; bar: 10 μM. (B) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean is shown; *p≤0.05 between cells treated with ad-GFP and LY294002 and ad-myrAkt and LY294002. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (C) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown; *p≤0.05 between cells treated with ad-GFP and LY294002 and ad-myrAkt andLY294002. ≤0.05 between Itgα3f/fα6f/f untreated or treated with FGF10 or GDNF. (D-E) Phosphorylation of Akt was evaluated at 5, 15 and 30 min after addition of FGF10 (D) or GDNF (E). β-actin was used as a loading control.
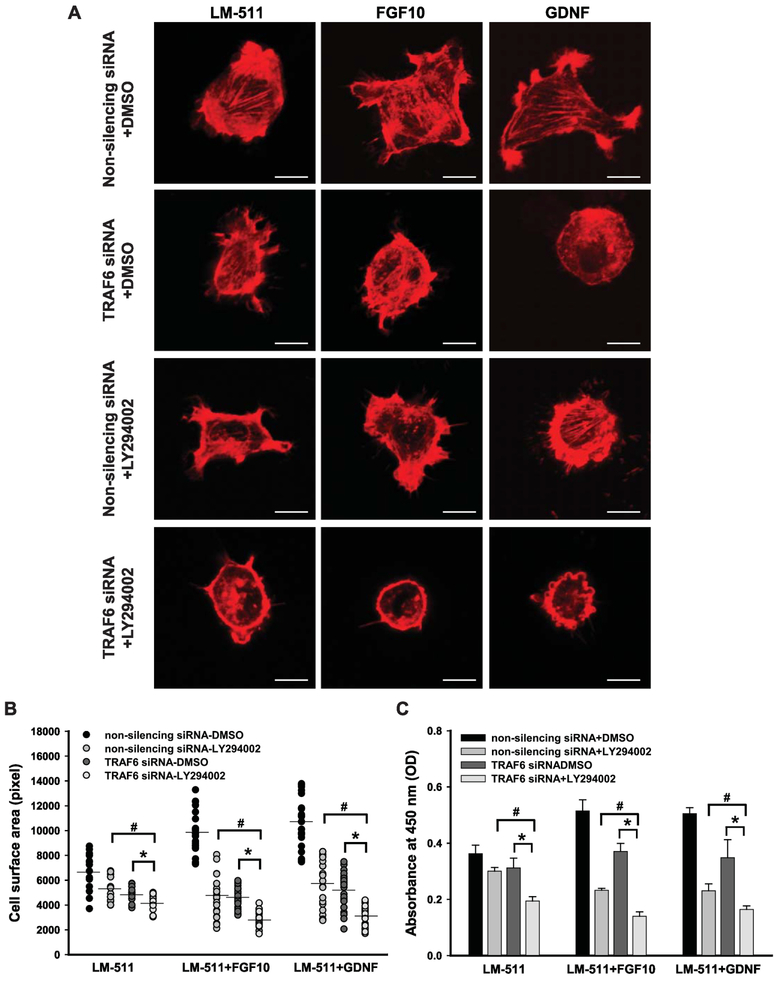
We next investigated whether both PI3K-dependent and TRAF6-mediated K63-linked polyubiquitination regulate growth factor-mediated Akt-dependent CD cell spreading and proliferation by treating Itgα3f/fα6f/f CD cells plated on LM- 511 with both TRAF6 siRNA and LY294002 prior to FGF10 or GDNF stimulation. While inhibition of either TRAF6 or PI3K partially decreased growth factor-induced cellular functions, their combined inhibition resulted in complete abrogation of cell spreading (Fig. 8A-B) and a severe decrease of proliferation (Fig. 8C) that were comparable to the results seen with Akt inhibitor IV (Fig. 4A-C). This result suggests that FGF10- and GDNF-induced CD cell spreading and proliferation are regulated by activation of Akt via both PI3K- and TRAF6-dependent mechanisms.
Figure 8. FGF10- or GDNF-induced cell spreading and proliferation is dually regulated by PI3K- and TRAF6--dependent activation of Akt.
Itgα3f/fα6f/f CD cells were transfected with non-silencing or TRAF6 siRNA (20 nM for 24 h), treated with DMSO or LY294002 (25 μM) for 1 h, plated on LM-511 for 1 h and stimulated with FGF10 or GDNF (10 ng/ml). Cell spreading (A-B) and proliferation (C) were evaluated at 1 and 24 h after addition of growth factors, respectively. (A) Representative confocal images of the cells stained with rhodamine-phalloidin are shown; bar: 10 μM. (B) The individual measurements of cell surface (in pixels) of 15-30 cells with the mean a 95% CI is shown. (C) Proliferation as measured by the OD of BrdU-positive cells ±SEM of 4-6 independent experiments is shown; #p≤0.05 between cells treated with a non-silencing siRNA and LY294002 and TRAF6 siRNA and LY294002. * #p≤0.05 between cells treated with TRAF6 siRNA and TRAF6 siRNA and LY294002 (B and C).
Discussion
The principal laminin integrins, α3β1, α6β1 and α6β4 are often co-expressed in the same cell, act synergistically in multiple cellular processes and functionally compensate for each other, making it difficult to define their specific signaling and functions. In this study we generated CD cells lacking both α3 and α6 integrin subunits, which enabled us to define the functional cooperation between these integrins. We show that both integrins α3 and α6 subunits contribute to CD cell adhesion, migration, spreading and signaling on LM-511 and LM-332. By contrast, expression of either the integrin α3 or α6 subunit is sufficient to mediate GDNF- and FGF10-dependent spreading, proliferation and signaling on LM-511. These growth factor-dependent functions are facilitated by both PI3K- and TRAF6-mediated K63-linked polyubiquitination-dependent activation of Akt. In contrast, activating integrins by LM only induces activation of Akt that is PI3K independent, but requires TRAF6-mediated K63-linked Akt polyubiquitination. Thus, laminin-binding integrins have specificity for some functions, yet they can compensate for each other in different circumstances.
By generating CD cells where either the integrin α3 or α6 subunits were deleted, we previously identified integrin α3β1 as the principal functional laminin-binding receptor that mediates CD cell adhesion, migration and proliferation on LM-332 or LM-511 [9]. We also demonstrated that deleting the α3 subunit caused decreased expression of the β1 integrin subunit [9]. We further showed that the α6 integrins do not play a role in CD cell interactions with LM-511 and only mildly affect CD cell adhesion, migration and proliferation on LM-332 [10]. In this study, we provide evidence that deleting both the integrin α3 and α6 subunits significantly worsens CD adhesion, migration and proliferation on LM-332 as well as LM-511 and no other β1 integrins played a significant role in these processes. There was also no additive decrease in β1 subunit expression compared to α3-null CD cells, suggesting that α3 is the major integrin subunit that interacts with the β1 subunit. Thus, the laminin binding integrins functionally synergize with each other to promote CD cell adhesion to LM substrata. We also show that integrin Itgα3−/−α6−/− CD cells adhere significantly less than Itgα3f/f α6f/f cells on collagen I. This is likely due to decreased integrin α2β1 (the major collagen I receptor) expression in the double null cells [16], however the mechanism whereby this occurs is yet to be identified.
We previously showed that deleting the integrin α3 subunit from CD cells diminished Akt signaling in response to adhesion to LM-511[9]. This effect is even more severe in integrin Itgα3−/−α6−/−CD cells where we also detected diminished FAK and ERK activation. This decreased integrin-dependent signaling is likely due to a major adhesion defect, which is the main mechanism controlling FAK activation and its downstream signaling pathways [17, 18]. The decreased Akt activation is at least in part due to diminished K63-linked polyubiquitination, which is consistent with the finding that integrin α3β1-dependent cell adhesion requires TRAF6-dependent K63-linked polyubiquitination of Akt [9]. Interestingly, TRAF6 overexpression in HEK293 cells activates PI3K and a program of actin polymerization resulting in filopodia growth [19], which supports our finding and suggests a potential role for TRAF6-dependent activation of Akt in modulating cytoskeletal function.
Integrin-dependent adhesion to ECM ligands is required for growth factor-dependent signaling of CD cells. Interestingly, single Itgα3−/− and Itgα6−/− CD cells respond normally to FGF10 and GDNF despite the severe adhesion defect present in the Itgα3−/−cells. By contrast, Itgα3−/−α6−/−CD cells show decreased spreading, proliferation and activation of FAK and Akt in response to these growth factors. Thus, expression of one of the major laminin binding integrins is sufficient to promote the action of growth factors on CD cells.
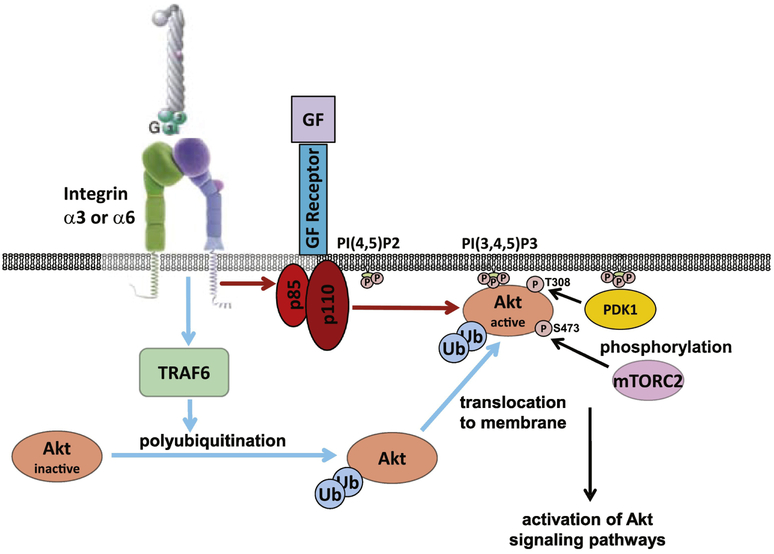
We previously demonstrated that integrin α3β1–mediated cell adhesion to laminin induces TRAF6-mediated K63-linked polyubiquitination-dependent and PI3K independent Akt activation. Integrin α3β1 did not alter the ability of phosphoinositide-dependent protein kinase 1 (PDK1) to phosphorylate Akt at T308, which is required for Akt activity, nor did it change the ability of rapamycin (mTOR) complex 2 (mTORC2) to phosphorylate S473 that is required for maximal Akt activation [9]. In the current study, we show that GDNF- and FGF10-dependent stimulation of Akt on laminin substrata requires both PI3K- and TRAF6-dependent activation mechanisms (Fig. 9). The predominant process whereby Akt regulates growth factor-induced proliferation is PI3K dependent, while the cytoskeletal effects (measured as cell spreading) are mediated by both TRAF6-dependent K63-linked polyubiquitination and PI3K-dependent mechanisms [9]. These data are consistent with the fact that receptor tyrosine kinases induce Akt activation by regulating the formation of the lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PI(3,4,5)P3) from phosphatidylinositol-4,5-diphosphate (PI(4,5)P2) by the PI3K family members; and that integrin α3β1-dependent Akt activation requires TRAF6-mediated K63-linked polyubiquitination [20-23].
Figure 9. Growth factors induce Akt activation in CD cells adherent on LM by TRAF6-mediated K63-linked polyubiquitination and PI3K-dependent phosphorylation.
Growth factors (GF: FGF10, GDNF) induce TRAF6-mediated K63-linked polyubiquination of Akt which facilitates Akt translocation to the cellular membrane (blue arrows). They also induce PI3K activation that changes phospholipid membrane composition resulting in Akt anchoring to the membrane where it is phosphorylated and fully activated (red arrows).
In conclusion, these studies show functional specificity by the LM-binding integrins and their ability to compensate for each other in different circumstances. They also verify that the complex integrin signaling induced by LMs is regulated at multiple levels, including the ligand, differential signaling mediated by the α3 and α6 subunits and the ability of the integrins to modulate GF-dependent signaling.
Materials and Methods
Reagents, adenovirus vectors, siRNAs and antibodies.
Human laminin 332 (LM-332) was produced, purified and evaluated as previously described [24]. Laminin 511 (LM-511) was produced and evaluated as previously described [9, 25]. Collagen I was purchased from BD Biosciences (San Jose, CA, USA,); fibronectin and vitronectin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Akt inhibitor IV and PI3K inhibitor LY294002 were purchased from Calbiochem (San Diego, CA, USA). Akt inhibitor IV is a cell permeable and reversible benzimidazole compound that inhibits Akt phosphorylation/activation by targeting the ATP binding site of a kinase upstream of Akt, but downstream of PI3K; it does not affect PI3K activity.
Adenoviruses with vectors encoding green fluorescent protein (GFP), ad-GFP, the dominant-negative catalytic subunit deletion mutant PI3K, ad-delta p85, and constitutively active myristoylated Akt (ad-myrAkt) were propagated in HEK293 cells, purified by column chromatography, quantitated, and used for cell infection based on particle yield as previously described [26].
Silencer Select pre-designed TRAF6 siRNAs (sequences (5’ −3’) CAUUAAGGAUGAUACAUUAtt and AGAAAAGAGUUGUAGUUUUtt) and Silencer Select Negative control #1 siRNA were bought from Ambion (Carlsbad, CA, USA).
The following antibodies were used in Western immunoblot analyses: integrin α3 (AB1920, Millipore, Temecula, CA, USA and AF2787, R&D Systems); phospho-AktThr308 (9275), phospho-AktSer473 (9271), total Akt (40D4), phospho-p38Thr180/Tyr182 (9211) and total p38 (9212), phospho-ERK1/2Thr202/Tyr204 (9101) and total ERK1/2 (9102), K63-linkage specific polyubiquitin (5621), (all from Cell Signaling Technologies); TRAF6 (ab94720, Abcam, Cambridge, MA, USA). Antibody for Akt (9272, Cell Signaling) were used for immunoprecipitation. Antibody to β-actin (A4700, Sigma-Aldrich) was used to evaluate protein loading. Anti-mouse α1 (555001), α2 (553819), and α6 (555734) integrin antibodies, which were used in flow cytometry analyses, were purchased from BD. R-phycoerythrin-conjugated secondary antibodies were bought from Invitrogen (Carlsbad, CA, USA).
Generation of Itgα3flox/floxItgα6flox/flox mice.
Integrin α3flox/flox (Itgα3flox/flox) mice [13] were crossed with integrin α6flox/flox (Itgα6flox/flox) mice [27]. The expression of floxed genes for integrin α3 and α6 subunits was confirmed by PCR.
Generation of integrin α3f/fα6f/f and α3−/−α6−/− CD cells.
CD cells were isolated from 5-6 weeks old Itgα3flox/floxItgα6flox/flox mice as described by Husted et al. [28] and immortalized with pSV40 plasmid. To knock out α3 and α6 integrin subunits, loci for these integrin subunits in CD cells were deleted with adenovirus expressing Cre recombinase. CD cells were grown in DMEM/F12 medium containing 10% FBS and 1% penicillin/streptomycin. Expression of α3 and α6 integrin subunits was confirmed by Western blot and flow cytometry analyses, respectively.
Flow cytometry analysis was performed as previously described [15]. CD cells were incubated with anti-mouse α1, α2 and α6 integrin antibodies followed by R-phycoerythrin-conjugated secondary antibodies.
Western blot analysis.
Cell lysates were prepared using M-PER reagent (Thermo Scientific, Waltham, MA, USA). Lysates were centrifuged at 17,000×g for 15 min at 4°C, and total protein concentration was determined using BCA reagent (Thermo Scientific). Protein extracts were subjected to Western immunoblot analysis and developed using the Western Lightning Chemiluminescence Plus detection system (PerkinElmer, Wellesley, MA, USA) according to the manufacturer’s protocol. Densitometry was performed using ImageJ program. To quantify levels of protein phosphorylation, OD of bands for phosphoprotein was normalized to total protein and β-actin.
Cell adhesion.
Cell adhesion assays were performed in 96-well plates as previously described [29]. Cells (1×105) were seeded in serum-free medium onto plates containing different concentrations of ECM for 1 h. Non-adherent cells were removed and the remaining cells were fixed, stained with crystal violet, and solubilized, and the optical densities of the cell lysates were read at 570 nm. Adhesion was calculated as percent of positive control (adhesion to serum).
Cell migration.
Cell migration was assayed as previously described [29]. Transwells with 8-μm pores were coated with different ECM components, and 1×105 cells were added to the upper well in serum-free medium. Cells that migrated through the filter after 4 h were counted.
Cell replating assays were performed on CD cells that were trypsinized, washed, suspended in serum-free DMEM, plated on LM-511 (0.5 μg/ml) and harvested at 0, 15, 30 and 60 min later.
Growth factor-dependent signaling.
CD cells grown on LM-511 for 1 h were stimulated with FGF10 or GDNF (10 ng/ml each) and harvested at 0, 5, 15 and 30 min later. Cells were washed in PBS and lysed using M-PER reagent with protease and phosphatases inhibitor cocktails (Sigma). Protein extracts (20-40 μg) were subjected to Western immunoblot analysis. When chemical inhibitors were used they were added 1 h prior to the assays. For silencing experiments, cells were transfected with non-silencing siRNA (20 nM, transfection control) or TRAF6 siRNA (20 nM) using Lipofectamine RNAiMAX according to manufacturer’s instructions. Transfected cells were utilized 24 h later. For experiments using adenoviruses, cells were infected with ad-GFP, ad-Δp85 or ad-myrAkt for 48 h prior to treatments.
Cell spreading assay.
CD cells were plated onto slides coated with LM-511 (0.5 μg/ml) for 1h and treated with growth factors for 30 minutes after which they were fixed, permeabilized, exposed to rhodamine–phalloidin (1:5000) and visualized under a fluorescence microscope. Fifteen to 30 HPFs were analyzed using ImageJ software.
Cell proliferation was assessed by measuring incorporation of 5-bromodeoxyuridine (BrdU) in an enzyme-linked immunosorbent assay–based 5-Bromo-2′-deoxy-uridine Labeling and Detection Kit III (Roche Applied Science, Indianapolis, IN) as previously described [30]. BrdU incorporation was quantified by a change of absorbance (optical density) at 405 nm. Various cellular treatments were as described in Cell replating assay.
Evaluation of K63-linked polyubiquitination of Akt.
For immunoprecipitation, anti-Akt antibody were covalently bound to protein A/G sepharose (Thermo Scientific, Waltham, MA, USA) as previously described [31]. Imunoprecipitations were performed with 0.1 mg of total cell lysates overnight at 4°C with rotating, after which the bound immune complexes were washed, resuspended in SDS-PAGE sample buffer, heated at 95 °C for 10 min, cleared by centrifugation, and subjected to Western immunoblot analysis for K63-linkage specific polyubiquitin or Akt.
Statistical analyses.
The mean and SEM of each treatment group were calculated for all experiments. At least 4 independent experiments (some in triplicates each) were performed. Student’s t test was used to compare two groups. All statistical tests were two-sided and statistical analysis was done with the use of SigmaStat software (Systat Software Inc., San Jose, CA). Statistical significance was defined as p less than or equal to 0.05.
Supplementary Material
Highlights.
Kidney epithelial collecting duct cells that lack both the α3 and α6 integrin subunits were generated.
Deleting both integrin subunits impaired cell adhesion and migration to laminins 332 and 511 more than deleting α3 or α6 alone.
Cell adhesion mediated by α3β1 and α6 integrins was PI3K independent, but required K63-linked polyubiquitination of Akt.
Growth factor-mediated cell signaling, spreading and proliferation were severely compromised in double integrin α3/α6- but not single α3- or α6-null CD cells.
Growth factor-dependent cell functions required both PI3K- and K63-linked polyubiquitination of Akt.
Acknowledgements:
This work was in part supported by Veterans Affairs Merit Reviews 1I01BX002025-01 (AP) and 1I01BX002196-01 (RZ); National Institutes of Health grants R01-CA162433 (AP), R01-DK095761 (AP), R01-DK069221 (RZ), R01-DK083187 (R.Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- [1].Miner JH, Yurchenco PD, Laminin functions in tissue morphogenesis, Annu Rev Cell Dev Biol 20 (2004) 255–84. [DOI] [PubMed] [Google Scholar]
- [2].Yurchenco PD, Extracellular matrix receptors in branched organs, Development 23(5) (2011) 547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yurchenco PD, Patton BL, Developmental and pathogenic mechanisms of basement membrane assembly, Curr Pharm Des 15(12) (2009) 1277–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pozzi A, Yurchenco PD, Iozzo RV, The nature and biology of basement membranes, Matrix Biol 57-58 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Miller RT, Mechanical properties of basement membrane in health and disease, Matrix Biol 57-58 (2017) 366–373. [DOI] [PubMed] [Google Scholar]
- [6].Pozzi A, Zent R, Extracellular matrix receptors in branched organs, Curr Opin Cell Biol 23(5) (2011) 547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mathew S, Chen X, Pozzi A, Zent R, Integrins in renal development, Pediatric nephrology 27(6) (2012) 891–900. [DOI] [PubMed] [Google Scholar]
- [8].Pozzi A, Zent R, Integrins in kidney disease, Journal of the American Society of Nephrology : JASN 24(7) (2013) 1034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yazlovitskaya EM, Tseng HY, Viquez O, Tu T, Mernaugh G, McKee KK, Riggins K, Quaranta V, Pathak A, Carter BD, Yurchenco P, Sonnenberg A, Bottcher RT, Pozzi A, Zent R, Integrin alpha3beta1 regulates kidney collecting duct development via TRAF6-dependent K63-linked polyubiquitination of Akt, Mol Biol Cell 26(10) (2015) 1857–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Viquez OM, Yazlovitskaya EM, Tu T, Mernaugh G, Secades P, McKee KK, Georges-Labouesse E, De Arcangelis A, Quaranta V, Yurchenco P, Gewin LC, Sonnenberg A, Pozzi A, Zent R, Integrin alpha6 maintains the structural integrity of the kidney collecting system, Matrix Biol 57-58 (2017) 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shannon MB, Patton BL, Harvey SJ, Miner JH, A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease, J Am Soc Nephrol 17(7) (2006) 1913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA, Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis, Development 136(5) (2009) 843–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A, Kidney failure in mice lacking the tetraspanin CD151, J Cell Biol 175(1) (2006) 33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Niculescu C, Ganguli-Indra G, Pfister V, Dupe V, Messaddeq N, De Arcangelis A, Georges-Labouesse E, Conditional ablation of integrin alpha-6 in mouse epidermis leads to skin fragility and inflammation, Eur J Cell Biol 90(2-3) (2011) 270–7. [DOI] [PubMed] [Google Scholar]
- [15].Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, Fassler R, Yurchenco P, Pozzi A, Zent R, beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity, Development 136(19) (2009) 3357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A, Inhibition of integrin alpha2beta1 ameliorates glomerular injury, Journal of the American Society of Nephrology : JASN 23(6) (2012) 1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitra SK, Hanson DA, Schlaepfer DD, Focal adhesion kinase: in command and control of cell motility, Nat Rev Mol Cell Biol 6(1) (2005) 56–68. [DOI] [PubMed] [Google Scholar]
- [18].Mitra SK, Schlaepfer DD, Integrin-regulated FAK-Src signaling in normal and cancer cells, Curr Opin Cell Biol 18(5) (2006) 516–23. [DOI] [PubMed] [Google Scholar]
- [19].Wang KZ, Wara-Aswapati N, Boch JA, Yoshida Y, Hu CD, Galson DL, Auron PE, TRAF6 activation of PI 3-kinase-dependent cytoskeletal changes is cooperative with Ras and is mediated by an interaction with cytoplasmic Src, J Cell Sci 119(Pt 8) (2006) 1579–91. [DOI] [PubMed] [Google Scholar]
- [20].Ornitz DM, Itoh N, The Fibroblast Growth Factor signaling pathway, Wiley Interdiscip Rev Dev Biol 4(3) (2015) 215–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ichihara M, Murakumo Y, Takahashi M, RET and neuroendocrine tumors, Cancer Lett 204(2) (2004) 197–211. [DOI] [PubMed] [Google Scholar]
- [22].Murakumo Y, Jijiwa M, Asai N, Ichihara M, Takahashi M, RET and neuroendocrine tumors, Pituitary 9(3) (2006) 179–92. [DOI] [PubMed] [Google Scholar]
- [23].Teven CM, Farina EM, Rivas J, Reid RR, Fibroblast growth factor (FGF) signaling in development and skeletal diseases, Genes Dis 1(2) (2014) 199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tripathi M, Nandana S, Yamashita H, Ganesan R, Kirchhofer D, Quaranta V, Laminin-332 is a substrate for hepsin, a protease associated with prostate cancer progression, J Biol Chem 283(45) (2008) 30576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McKee KK, Capizzi S, Yurchenco PD, Scaffold-forming and Adhesive Contributions of Synthetic Laminin-binding Proteins to Basement Membrane Assembly, The Journal of biological chemistry 284(13) (2009) 8984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tan J, Geng L, Yazlovitskaya EM, Hallahan DE, Protein kinase B/Akt-dependent phosphorylation of glycogen synthase kinase-3beta in irradiated vascular endothelium, Cancer research 66(4) (2006) 2320–7. [DOI] [PubMed] [Google Scholar]
- [27].Marchetti G, De Arcangelis A, Pfister V, Georges-Labouesse E, alpha6 integrin subunit regulates cerebellar development, Cell Adh Migr 7(3) (2013) 325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Husted RF, Hayashi M, Stokes JB, Characteristics of papillary collecting duct cells in primary culture, Am J Physiol 255(6 Pt 2) (1988) F1160–9. [DOI] [PubMed] [Google Scholar]
- [29].Chen D, Roberts R, Pohl M, Nigam S, Kreidberg J, Wang Z, Heino J, Ivaska J, Coffa S, Harris RC, Pozzi A, Zent R, Differential expression of collagen- and laminin-binding integrins mediates ureteric bud and inner medullary collecting duct cell tubulogenesis, American journal of physiology. Renal physiology 287(4) (2004) F602–11. [DOI] [PubMed] [Google Scholar]
- [30].Linkous AG, Yazlovitskaya EM, Hallahan DE, Cytosolic phospholipase A2 and lysophospholipids in tumor angiogenesis, J Natl Cancer Inst 102(18) (2010) 1398–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Persons DL, Yazlovitskaya EM, Cui W, Pelling JC, Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signalregulated kinase activity increases sensitivity to cisplatin, Clinical cancer research : an official journal of the American Association for Cancer Research 5(5) (1999) 1007–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.