Abstract
EPA and DHA protect against multiple metabolic and neurologic disorders. Although DHA appears more effective for neuroinflammatory conditions, EPA is more beneficial for depression. However, the brain contains negligible amounts of EPA, and dietary supplements fail to increase it appreciably. We tested the hypothesis that this failure is due to absorption of EPA as triacylglycerol, whereas the transporter at the blood-brain barrier requires EPA as lysophosphatidylcholine (LPC). We compared tissue uptake in normal mice gavaged with equal amounts (3.3 μmol/day) of either LPC-EPA or free EPA (surrogate for current supplements) for 15 days and also measured target gene expression. Compared with the no-EPA control, LPC-EPA increased brain EPA >100-fold (from 0.03 to 4 μmol/g); free EPA had little effect. Furthermore, LPC-EPA, but not free EPA, increased brain DHA 2-fold. Free EPA increased EPA in adipose tissue, and both supplements increased EPA and DHA in the liver and heart. Only LPC-EPA increased EPA and DHA in the retina, and expression of brain-derived neurotrophic factor, cyclic AMP response element binding protein, and 5-hydroxy tryptamine (serotonin) receptor 1A in the brain. These novel results show that brain EPA can be increased through diet. Because LPC-EPA increased both EPA and DHA in the brain, it may help in the treatment of depression as well as neuroinflammatory diseases, such as Alzheimer’s disease.
Keywords: Alzheimer’s disease, omega 3 fatty acids, brain lipids, fish oil, inflammation, lysophospholipid, nutrition/lipids, blood-brain barrier, retina, brain-derived neurotrophic factor, eicosapentaenoic acid, docosahexaenoic acid
The two major long-chain omega 3 FAs in animal tissues are EPA and DHA. Both of these FAs are known to have beneficial effects as anti-inflammatory agents and protect against various metabolic and neurologic diseases. Although the DHA concentration is higher than EPA in most tissues, the brain and retina are unique in having very high levels of DHA but virtually no EPA (1). The major dietary sources of EPA and DHA are fish, fish oil, and krill oil, all of which usually contain more EPA than DHA (2). However, the EPA levels in the brain are not increased significantly following the feeding of fish oil, krill oil, or even ethyl ester of EPA, although other tissues are enriched in both EPA and DHA (3–6). Interestingly, several clinical and preclinical studies showed that dietary EPA is superior to dietary DHA in the prevention and treatment of depression (7–9). It is therefore puzzling how EPA protects against depression without being incorporated appreciably into the brain. To explain this paradox, it has been proposed that the beneficial effects of EPA may result from the suppression of peripheral inflammation or from its hepatic conversion to DHA, rather than from a direct effect on the brain (10). However, the conversion of EPA to DHA cannot explain why dietary DHA does not have similar effects. The lack of enrichment of brain EPA by the dietary EPA has been explained by proposing that EPA is rapidly oxidized by the brain, in contrast to DHA (11, 12). This mechanism is supported by kinetic studies with labeled FAs showing the generation of more water-soluble degradation products from EPA, compared with DHA in the brain (10, 11). An alternative explanation that has not been explored is that the omega 3 FAs taken up into the brain do not accumulate in the brain if they are not taken up by the physiological transporter at the blood-brain barrier, namely major facilitator superfamily domain-containing protein 2a (Mfsd2a), which is specific for the lysophosphatidylcholine (LPC) form of the FA (13). This sodium-dependent symporter has been shown to be critical for the uptake and accumulation of DHA by the brain, and loss of its activity leads to a deficiency of brain DHA, microcephaly, and mental retardation (13, 14). Although it is expressed in several tissues (15), it is functionally more important in the endothelial cells of the blood-brain barrier (13), retina (16), and placenta (17). Mfsd2a has been shown to transport most long-chain FAs in the form of lysophospholipids, as well as acylcarnitines (18). However, the potential role of this transporter in the accrual of EPA by the brain has not been investigated. Interestingly, recent studies show that the concentration of LPC-EPA is significantly lower than that of LPC-DHA in the plasma (19), suggesting that the formation of LPC-EPA (presumably in the liver) may be a limiting factor in the accumulation of brain EPA. If so, increasing the plasma LPC-EPA concentration should result in its increased uptake and retention by the brain. We recently showed that feeding LPC-DHA (1 mg DHA per day or ∼40 mg/kg body weight) to normal mice for 30 days not only increased the plasma LPC-DHA concentration, but also increased the brain DHA content by 100% in normal adult mice and improved their memory function, as determined by the Morris water maze test (20). The same amount of unesterified (free) DHA, a surrogate for fish oil, krill oil, and other current supplements, neither increased brain DHA nor improved the memory. In the present study, we tested the hypothesis that the brain EPA levels can be similarly increased if it is provided in the form of LPC-EPA in the diet by comparing the uptake and accretion of dietary LPC-DHA and free EPA by the brain in normal adult mice. The results show, for the first time, that the brain EPA can indeed be increased several-fold using a physiological dose of dietary LPC-EPA, but not free EPA. Furthermore, the amount of DHA in the brain increased even more than that of EPA by dietary LPC-EPA, indicating that EPA taken up as LPC is efficiently converted to DHA. However, there was no change in brain DHA by dietary free EPA at the same concentration. The expression of the neurotrophic factor, brain-derived neurotrophic factor (BDNF), and its downstream targets, cyclic AMP response element binding protein (CREB) and 5-hydroxy tryptamine (serotonin) receptor 1A (5-HT1A), as well as the phosphorylation of CREB were significantly elevated in the brain following treatment with dietary LPC-EPA, but not free EPA, showing that the dietary LPC-EPA is functionally effective in the brain. Because of the known beneficial effects of EPA in patients with depression, the enrichment of brain EPA by this strategy might be potentially useful in the prevention and treatment of depression. Furthermore, because LPC-EPA increases both EPA and DHA in the brain, it may be more beneficial than dietary DHA for the treatment of depression as well as various neuroinflammatory diseases, including Alzheimer’s disease, which are more responsive to DHA.
MATERIALS AND METHODS
Chemicals
Free FAs (15:0, 17:0, 22:3, and 20:5) and tri-15:0 triacylglycerol (TAG) were purchased from Nu-Chek Prep Inc. (Elysian, MN). Di-17:0 phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were obtained from Avanti Polar Lipids (Alabaster, AL). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). All solvents (MS grade) were obtained from Thermo Fisher Scientific (Waltham, MA).
Preparation of LPC-EPA
The sn-1 EPA-LPC was prepared by the treatment of di-EPA PC with snake venom PLA2. di-EPA PC was synthesized by esterification of celite-bound glycerophosphorylcholine (265 mg) with EPA (1.48 g) in the presence of dimethylaminopyridine (0.31 g) and dicyclohexylcarbodiimide (1.01 g), essentially as described by Ichihara et al. (21). The PC was purified by silicic acid chromatography, dissolved in 20 ml of diethyl ether, and reacted with 10 mg Crotalus adamenteus venom dissolved in 0.5 ml Tris-HCl buffer (pH 7.4) containing 10 mM CaCl2. After 5 h of reaction on a metabolic shaker at room temperature, the precipitated LPC-EPA was washed with 5 ml of hexane to remove free EPA and extracted with chloroform:methanol (2:1 v/v) and used without further purification. The preparation gave a single spot on TLC plates corresponding to authentic LPC and contained only EPA by GC analysis.
Animals
All protocols were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee, and the procedures were performed in adherence to the institutional guidance and regulations. Male C57BL/6 mice (age, 2 months) were purchased from Jackson Laboratories (Bar Harbor, ME). The mice were fed a standard rodent chow (Teklad LM 485; Envigo, Indianapolis, IN) and water ad libitum throughout the experiment. All mice were housed in rooms with a 12 h light/dark cycle at controlled temperature (22°C ± 2°C). After 1 week of acclimatization, the mice were randomly divided into three groups (n = 6 each) and gavaged daily for 15 days with 80 μl of corn oil containing no EPA (control) or 1 mg EPA (3.3 μmol) either as free FA or as LPC.
The standard rodent chow, which contained 5.8% total fat, did not contain any EPA or DHA, but had 0.3% α-linolenic acid (18:3, n-3) and 2.6% linoleic acid (18:2, n-6). The food intake and the body weights were not different between the three groups of mice. After 15 days of treatment, the mice were fasted overnight and anesthetized with 2% isoflurane. Blood was drawn by cardiac puncture into a heparinized syringe, and plasma was separated by centrifugation at 1,500 g for 15 min at 4°C. The mice were then perfused transcardially with ice-cold 100 mM PBS (pH 7.4), and liver, heart, brain, retina, and adipose tissues were harvested. All samples were flash-frozen in liquid nitrogen and stored at −80°C until analysis.
Lipid extraction and FA analysis
The lipids were extracted from the fractions or tissues by the modified Bligh and Dyer procedure, as described by Ivanova et al. (22), after adding tri-15:0 TAG and di-17:0 PC as internal standards. Methylation of FAs of total lipids and GC/MS analysis of the methyl esters was performed as described previously (20, 23).
LC/MS analysis
LC/MS analysis of molecular species of lipids was performed on an ABSciex 6500 QTRAP mass spectrometer coupled with an Agilent 2600 UPLC system, as described previously (20). Quantification of EPA-, docosapentaenoic acid (DPA)-, and DHA-containing molecular species of PC, LPC, PE, and TAG in plasma and tissues was performed from the relative intensities of the various species and corresponding internal standards [17:0-17:0 PC, 17:0-LPC, 15:0-15:0 PE, 17:0-17:0 phosphatidylserine (PS), 14:1-17:0 phosphatidylinositol (PI), and 15:0-15:0-15:0 TAG]. The data processing was carried out using Analyst 1.6.2 (ABSciex, Redwood City, CA).
Western blotting
The brain tissue was homogenized in lysis buffer [50 mM Tris (pH 8.0), 25 mM KCl, 0.5 mM EDTA, Nonidet P-40, 0.1 mM EGTA, 10 μl/ml aprotinin, 1 mM MgCl2, 1 mM CaCl2, 1 mM Na4P2O7, 1 mM Na4MO4, 1% protease inhibitor cocktail, 1 mM phenyl sulfonyl fluoride, 10 mM sodium flouoride, and 1 mM NaV] and the suspension was left on ice for 30 min and centrifuged at 13,000 g at 4°C for 10 min. The supernatant (30 μg protein) was separated on 10% SDS-PAGE and the separated proteins were transferred on to methanol-rinsed polyvinylidene difluoride membranes (Millipore) The membranes were blocked with 5% (w/v) nonfat dried milk in TBS containing 0.1% Tween-20 (TBST) for 1 h at 4°C and probed with primary antibodies in TBST overnight at 4°C. The membranes were then washed with TBST three times and incubated with appropriate horseradish peroxide-conjugated secondary antibodies at room temperature for 1 h. Finally, the membranes were washed with TBST three times and developed with ECL detection kits (Bio-Rad, Hercules, CA) for 1 min, respective proteins were quantified in a Bio-Rad ChemiDoc MP imaging system, and the results were expressed as mean relative densitometric units.
ELISA
Brain tissue was homogenized in the lysis buffer. The homogenates were centrifuged at 10,000 g for 20 min, the supernatants were collected, and total protein concentration was determined by MicroBCA procedure (Pierce, Rockford, IL) using BSA as standard. Endogenous concentrations of BDNF were quantified using an ELISA kit (BDNF Emax ImmunoAssay System kit, (Promega Inc., Madison, WI) according to manufacturer’s protocol.
RNA isolation
Total RNA was isolated from the brain using Trizol reagent (Sigma) according to the manufacturer’s protocol. The resulting RNA was reverse transcribed to cDNA using dNTP, oligo(dT)12-18 as primer and Moloney murine leukemia virus reverse transcriptase (Invitrogen, Thermo Fisher) in a 20 μl reaction mixture.
Real-time PCR analysis
The mRNA quantification was performed using the ABI-Prism7700 sequence detection system (Applied Biosystems, Foster City, CA) using SYBR Green super mix (Quantabio, Beverly, MA) and the following primers for murine genes (Invitrogen): BDNF [forward (F): 5′-ATGGGACTCTGGAGAGCCTGAA-3′, reverse (R): 5′-CGCCAGCCAATTCTCTTTTTGC-3′]; CREB (F: 5′-TCAGCCGGGTACTACCATTC-3′, R: 5′-TTCAGCAGGCTGTGTAGGAA-3′); 5-HT1A (F: 5′-CTGTTTATCGCCCTGGATG-3′, R: 5′-ATGAGCCAAGTGAGCGAGAT-3′); TNFα (F: 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC3′, R: 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′); GAPDH (F: 5′-GGTGAAGGTCGGTGTGAACG3′, R: 5′-TTGGCTCCACCCTTCAAGTG-3′).
The mRNA expression of the targeted genes was normalized to the level of GAPDH mRNA. Data were processed by the ABI Sequence Detection system 1.6 software and analyzed by ANOVA.
Statistics
Unless otherwise indicated, all statistical analyses were performed using GraphPad Prism 7.0 software (La Jolla, CA). Analysis of significance was determined by one-way ANOVA with post hoc Tukey multiple comparison test.
RESULTS
Effect of feeding free EPA or LPC-EPA on plasma omega 3 FAs
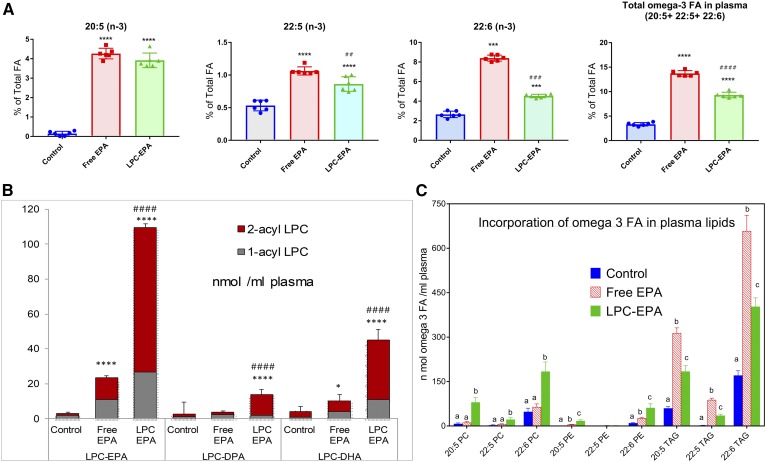
Figure 1A shows the composition of long-chain omega 3 FA levels in the plasma following the gavage of corn oil carrier alone (no-EPA control) or with free EPA or LPC-EPA at a dose of 1 mg EPA (3.3 μmol/day for 15 days). The total FA composition of plasma is shown in supplemental Table S1. The EPA concentration was very low in control plasma (0.15% of total FAs), but was increased significantly by free EPA (to 4.3% of total FAs) and by LPC-EPA (to 3.9% of total FAs) to a comparable extent. However, the increase in the elongation products, namely DPA (22:5, n-3) and DHA (22:6, n-3), was greater with free EPA than with LPC-EPA. The DHA content increased from 2.7% of total FAs (in control) to 8.4% of total by free EPA and to 4.5% of total by LPC-EPA. The DPA content rose from 0.53% of total (in control) to 1.06% of total in the free EPA group, and to 0.86% of total in the LPC-EPA group. The total omega 3 FA content (20:5 + 22:5 + 22:6) was thus significantly higher with free EPA (13.7% of total FAs) compared with LPC-EPA (9.3% of total FAs). The concentration of 20:4 decreased in both groups to a similar extent (supplemental Table S1).There were also significant decreases in 18:1 (n-9), 18:1 (n-7), and 16:1 (n-7) to a roughly equal extent by both treatments. These results show that both molecular forms of EPA are absorbed efficiently, and that EPA is converted to DPA and DHA in the liver and other tissues and secreted into plasma in the lipoproteins.
Fig. 1.
Effect of oral free EPA and LPC-EPA on plasma lipids. A: FA composition. The FA analysis was carried out by GC/MS, as described in the text. Only the omega 3 FAs are shown here. The total FA composition of plasma is shown in supplemental Table S1. The values shown are mean ± SD, n = 6 for each group. Statistical significance was determined by one-way ANOVA with post hoc Tukey multiple comparison test. *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001 compared with control (ANOVA). #P < 0.05; ##P < 0.01; ###P < 0.001; ####P < 0.0001, LPC-EPA compared with free EPA (ANOVA). B: LPC species in plasma (nanomoles per milliliter). The composition of LPC species containing omega 3 FA was analyzed by LC/MS/MS in the MRM mode, as described in the text. The values shown are mean ± SD, n = 6 per group. Statistical significance was determined by one-way ANOVA with post hoc Tukey multiple comparison test. P value symbols are as in A. C: Incorporation of omega 3 FA in plasma lipids. Molecular species of PC, PE, and TAG containing 20:5, 22:5, and 22:6 were analyzed by LC/MS/MS in the MRM mode, as described in the text. The values shown are nanomoles of each omega 3 FA associated with the indicated plasma lipid, and are mean ± SD (n = 6 per group). The individual values were calculated by adding all the molecular species of each lipid containing the indicated omega 3 FA. The values were corrected for the number of molecules of omega 3 FA expected in each molecular species (for example: for 16:0-20:5 PC, the nanomoles of 20:5 are the same as the nanomoles of PC, whereas for 20:5-20:5 PC, the nanomoles of 20:5 are double the nanomoles of PC, and for 20:5-20:5-20:5 TAG, there are three times the nanomoles of TAG). Bars with different letters are significantly different from each other (one-way ANOVA).
A significant increase in the net amount of plasma LPC-EPA (expressed as nanomoles per milliliter) occurred after feeding LPC-EPA compared with free EPA (Fig. 1B). There was also an increase in the net amounts of LPC-DHA and LPC-DPA (nanomoles per milliliter) in the mice fed LPC-EPA. These results show that part of EPA absorbed through the LPC-EPA pathway is converted to DPA and DHA in the liver and secreted into plasma in the form of LPC. It is possible that LPC-EPA in the plasma was derived from direct absorption from the gut as well as secretion from the liver. However, the isomer composition of LPC shows that over 75% of LPC-EPA and LPC-DHA were sn-2 acyl isomers. Because we fed only sn-1 EPA isomer, and the isomerization does not favor the formation of sn-2 acyl isomer (24), these results show that most of the omega 3 FA LPC in plasma was secreted by the liver, which is known to secrete predominantly sn-2 acyl (unsaturated) isomers (25–27). It may also be noted that the animals were fasted overnight before collection of the plasma, and therefore it is unlikely that the plasma LPC-EPA measured here is derived from the recently absorbed LPC.
The molecular species of PC, PE, and TAG containing the omega 3 FA were analyzed by LC/MS/MS to determine whether the metabolic fates of dietary free EPA and LPC-EPA differed after their absorption. Most of the lipid species containing EPA, DPA, and DHA were elevated in the plasma of mice fed LPC-EPA (Fig. 1C), supporting an efficient assimilation of the absorbed LPC-EPA by the liver, elongation to DHA, and subsequent secretion into plasma lipoproteins. In contrast to LPC-EPA, which increased the omega 3 FAs of both phospholipids and TAG, free EPA increased mostly the omega 3 FAs of TAG. The results also show that the majority of the omega 3 FA was present in plasma TAG in both the free EPA and LPC-EPA groups. However, a significantly higher fraction of plasma omega 3 FAs was incorporated into the phospholipids in the LPC-EPA group compared with the free EPA group.
Incorporation of dietary free EPA and LPC-EPA into brain lipids
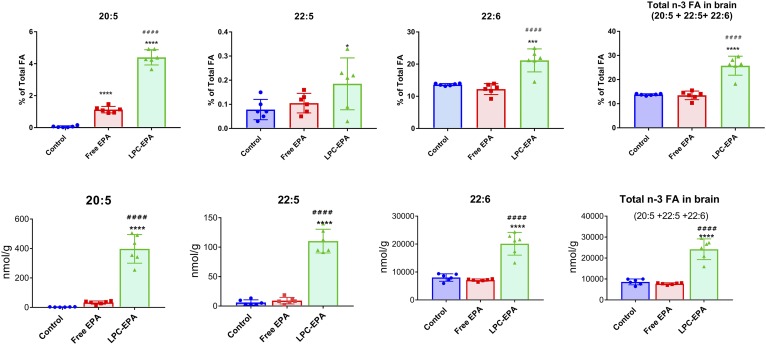
Figure 2 shows the percentage of omega 3 FAs in the brain lipids, as well as the concentration in nanomoles per gram of tissue in the three groups of mice. The total FA composition is shown in supplemental Table S2. The EPA concentration in the control group is very low (0.04% of total FAs), as reported by several previous studies (3, 4). Although this percentage was increased by both free EPA and LPC-EPA, the brain EPA content was 7-fold greater in the LPC-EPA group (4.14% of total FAs) compared with the free EPA group (0.56% of the total). Furthermore, there was a marked increase in DPA (22:5, n-3) and DHA (22:6) in the LPC-EPA group, but not in the free EPA group, whether expressed as percent of total FAs or as nanomoles per gram of tissue. Thus feeding LPC-EPA increased the net amount (nanomoles per gram) of DPA by 18-fold over the control and increased DHA by 2.5-fold over the control group. The total omega 3 FA content (nanomoles per gram) of the brain was increased by 2.8-fold over the no-EPA control by LPC-EPA, whereas it was not affected by the same dose of free EPA. These studies therefore show that LPC-EPA is efficiently transported into the brain and converted to DPA and DHA in addition to increasing the EPA content by >100-fold. It is likely that part of the increase in brain DHA is due to the uptake of LPC-DHA from the plasma, because the plasma LPC-DHA is also increased by oral LPC-EPA (Fig. 1B). We calculated that about 12% of the administered EPA (5.9 μmol out of 49.5 μmol) was incorporated into brain lipids over the period of 15 days, as indicated by the increase in omega 3 FAs of the brain. This is a much higher incorporation compared with the previous studies, which reported that less than 1% of the oral dose is deposited in the brain after ingestion of either TAG-DHA or PC-DHA (28, 29).
Fig. 2.
Omega 3 FA incorporation into brain lipids. A: Percent of total FAs. Total FA analysis of brain lipids was carried out by GC/MS. Only the values for the three long-chain omega 3 FAs are shown. The composition of all the FAs are shown in supplemental Table S2. The values shown are mean ± SD (n = 6 per group). Statistical significance was determined by one-way ANOVA with post hoc Tukey test. B: Nanomoles per gram of tissue. The concentration of the three long-chain omega 3 FAs in total brain lipids is expressed as nanomoles per gram. The statistical symbols are the same as in Fig. 1.
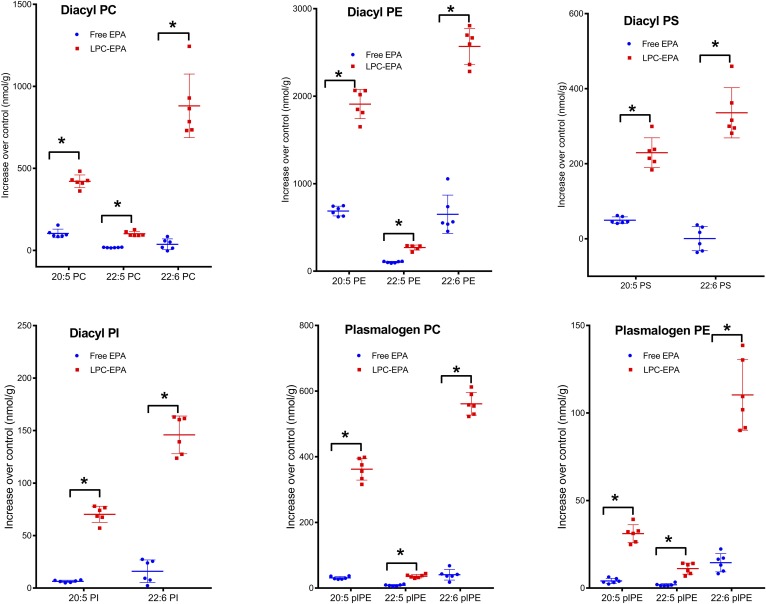
The increase in the net amount of each omega 3 FA (nanomoles per gram) in various brain phospholipid classes was analyzed by LC/MS/MS and is shown in Fig. 3. In all cases, the increase in omega 3 FA was greater in the LPC-EPA group compared with the free EPA group. The net increase in DHA was greater than that of EPA in all phospholipids. However, a relatively large fraction of the EPA was incorporated into PE and PS compared with PC. DPA was a minor component in all phospholipids. It is of interest to note that the increase in EPA (nanomoles per gram) was almost equal in diacyl PC and plasmalogen PC, although the concentration of diacyl PC is much greater, suggesting a preferential enrichment of plasmalogen PC. However, in the case of PE, the diacyl PE was more predominantly enriched compared with plasmalogen PE.
Fig. 3.
Increase in omega 3 FAs of brain phospholipids (nanomoles per gram). The molecular species of phospholipids containing the three omega 3 FAs were analyzed by LC/MS/MS, as described in the text. The increase in each species above the average of the control value was then calculated for the free EPA- and LPC-EPA-treated mice. The values shown are the sum of the increases in all molecular species containing the indicated FA (mean ± SD, n = 6 per group). *P < 0.0001, all differences between free EPA and LPC-EPA were significantly different by the unpaired t-test with Welch’s correction (GraphPad Prism). The increases in individual molecular species are shown in supplemental Figs. S1–S4.
The increases in individual molecular species of diacyl PC, diacyl PE, diacyl PS, and diacyl PI are shown in supplemental Figs. S1–S4. Most of the molecular species showed an increase above the no-EPA control, especially in the LPC-EPA group. An exception was the 20:4-22:6 species in all phospholipid classes, which showed a significant decrease in the case of the LPC-EPA group compared with the no-EPA group as well as the free EPA group. This is apparently because of the decrease in 20:4 in all phospholipids. There was a concomitant increase in the 22:6-22:6 species of all phospholipids, suggesting the replacement of 20:4 with 22:6.
Incorporation of free EPA and LPC-EPA into tissue lipids
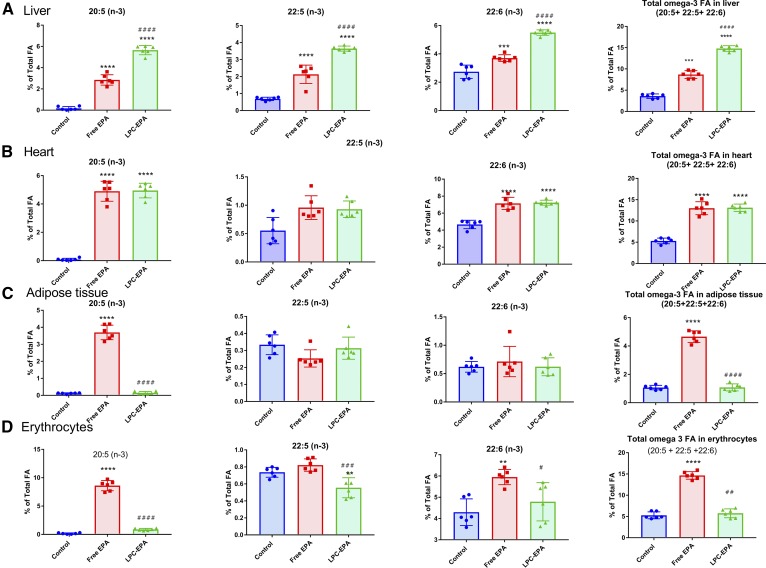
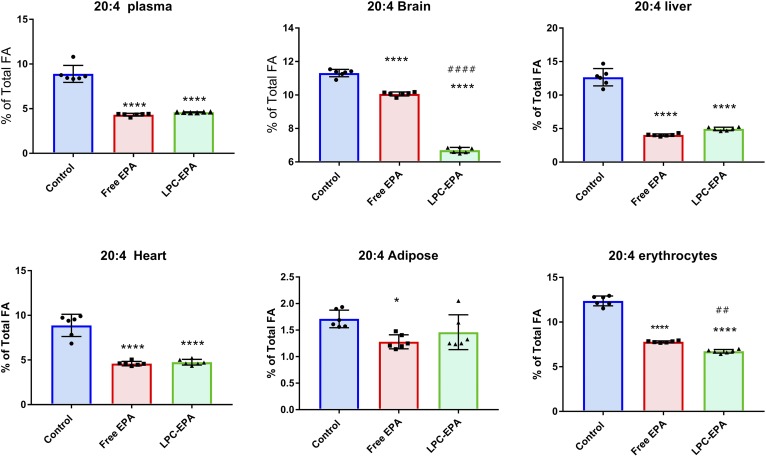
Feeding free EPA increased the liver EPA content significantly (from 0.17% to 2.85% of total FAs), but the increase was greater with LPC-EPA (5.65% of total FAs) (Fig. 4A). There was also a robust conversion of EPA to DPA and DHA, as evident from the increases in their percentages, by both free EPA and LPC-EPA. However, the conversion was greater in the LPC-EPA group compared with the free EPA group. In contrast to the brain, the liver accumulated significant amounts of DPA with both of the EPA supplements, suggesting a slower conversion of DPA to DHA. The total percentage of omega 3 FA in the liver was increased 2.4-fold by free EPA and 4.1-fold by LPC-EPA, showing that the latter was more efficient in enriching liver omega 3 FA, similar to the brain. With regard to the other FAs in the liver, the greatest percentage decrease occurred in 20:4 (supplemental Table S3, Fig. 5), and this decrease was equal with both supplements. Free EPA increased the percentage of 18:1 (n-9), 18:1 (n-7), 16:0, and 18:0 in the liver, and decreased 18:2 and 20:4; whereas, LPC-EPA decreased 18:2 and increased 18:0, in addition to the decrease in 20:4.
Fig. 4.
Omega 3 FA incorporation into tissue lipids: liver (A); heart (B); adipose tissue (C); and erythrocytes (D). The total FA composition was analyzed by GC/MS, as described in the text. Only the values for the omega 3 FAs are shown here. The total FA composition of the tissues is shown in supplemental Tables S3–S6. The values shown are mean ± SD (n = 6 per group). Statistical significance was determined by one-way ANOVA with post hoc Tukey test. The symbols for the P values are the same as in Fig. 1.
Fig. 5.
Effect of free EPA and LPC-EPA on ARA levels in tissues. The ARA values for each tissue are shown for comparison. The symbols are as described in Fig. 1.
Dietary free EPA and LPC-EPA were incorporated equally efficiently into heart lipids (Fig. 4B). Although the increase in DPA percentage did not reach statistical significance, the increase in percentage of DHA was highly significant and was equal for the two supplements (4.6% in control, 7.14% in the free EPA group, and 7.23% in the LPC-EPA group). The percentage of total omega 3 FA was also increased equally in both cases (from 5.3% in the control to 13% with both free and LPC-EPA). The decrease in the percentage of arachidonic acid (ARA) was also similar with free EPA (−48%) and LPC-EPA (−46%) (supplemental Table S4, Fig. 5). In addition to a decrease in 20:4, there was a marked decrease in the percentage of saturated FAs 16:0 and 18:0 with both the supplements. A significant increase in the percentage of α-linolenic acid [18:3 (n-3)] was also observed with both the supplements, possibly due to retro conversion of EPA and/or a slow turnover of α-linolenic acid.
In contrast to the other tissues, the EPA concentration in the white adipose tissue (peri-gonadal) was increased significantly by dietary free EPA, but not by LPC-EPA (Fig. 4C). There was no significant increase in the EPA metabolites, DPA or DHA, with either supplement. A significant decrease in the percentage of 20:4 occurred only after free EPA supplementation (supplemental Table S5, Fig. 5), further indicating that only free EPA was incorporated into adipose tissue. Small but significant decreases occurred in 18:1 (n-9) and 18:1 (n-7), especially in the free EPA group.
In the erythrocytes, only free EPA increased the percentages of EPA and DHA (Fig. 4D). The percentage of total omega 3 FAs of erythrocytes increased by >170% by free EPA, but was unaffected by LPC-EPA. Although both DPA and DHA were increased in the plasma by LPC-EPA, they were unchanged in the erythrocytes. In fact, there was a significant decrease in DPA by LPC-EPA. These results suggest that there was a selective exchange of EPA between erythrocytes and plasma. However, both free EPA and LPC-EPA significantly decreased the ARA content of erythrocytes, similar to plasma (supplemental Table S6).
ARA in tissues
The effect of free EPA and LPC-EPA on the ARA levels of various tissues is summarized in Fig. 5. As mentioned above, the ARA levels were significantly decreased by both molecular forms of EPA in all the tissues except the adipose tissue, where only the free EPA decreased the ARA. In general, the decrease in the percentage of ARA correlated with the increase in EPA and DHA, the exception being the brain, where free EPA decreased the percentage of ARA without increasing EPA or DHA. This suggests that free EPA may have increased transiently in the brain at the expense of ARA, but was subsequently depleted possibly due to β oxidation, as shown by Chen and Bazinet (11).
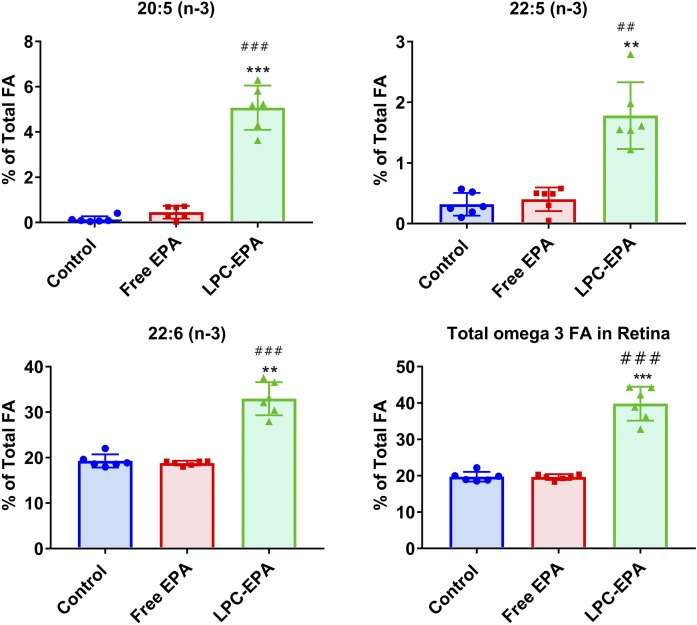
Effect on omega 3 FAs in retina
Because DHA is highly concentrated in the retina, and Mfsd2a is required for the accretion of DHA in this tissue (16), we have also determined the FA composition of the retina following the treatment with free and LPC-EPA. The total FA composition of the retina is shown in supplemental Table S7. As shown in Fig. 6, the percentages of all three omega 3 FAs were increased significantly by LPC-EPA, but not by free EPA. Retinal EPA increased from 0.14% to 5% of the total FAs, whereas the DPA increased from 0.3% to about 1.8% of the total, and DHA went up from 19% of the total in the control to 33% in LPC-EPA-treated animals. There was a >50% reduction in the percentage of arachidonate in the retina by LPC-EPA, but free EPA had no significant effect (supplemental Table S7). These results show that, similar to the brain, the retina is specifically enriched in all omega 3 FAs by dietary LPC-EPA presumably through the Mfsd2a pathway, whereas free EPA at the same dosage had no significant effect.
Fig. 6.
Effect of free EPA and LPC-EPA on the omega 3 FA composition of the retina. The total FA composition is shown in supplemental Table S7. Statistical significance was determined by one-way ANOVA with post hoc Tukey test. The symbols for the P values are the same as in Fig. 1.
Effect of EPA supplementation on the expression of selected genes in the brain
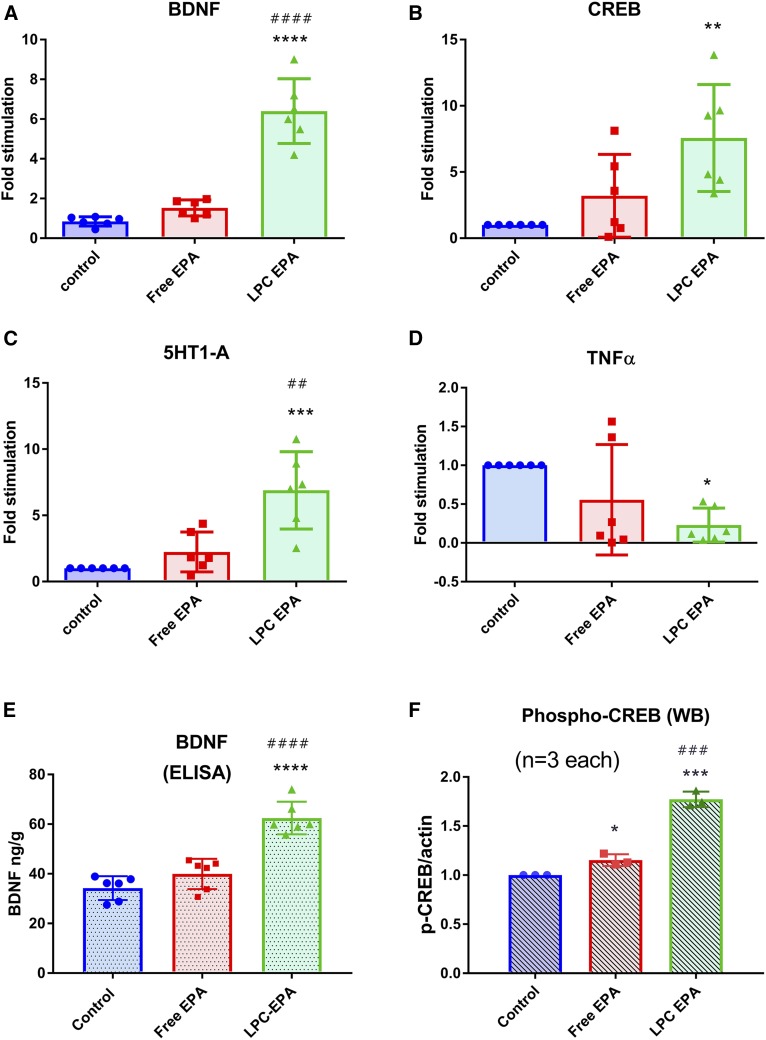
To examine the functional consequence of omega 3 FA enrichment of the brain by LPC-EPA, we determined the expression of several important genes known to be regulated by omega 3 FA by quantitative PCR. As shown in Fig. 7, the expression of BDNF, CREB, and 5-HT1A was increased significantly, whereas that of TNFα was decreased by LPC-EPA. Free EPA had no significant effect on any of the genes tested. In addition, the BDNF concentration was assayed by ELISA and was found be significantly increased by LPC-EPA, but not by free EPA. The phosphorylation of CREB, a downstream target of BDNF, was also increased by both free EPA and LPC-EPA, but the increase was more significant with the latter. All these results show that the increase in brain EPA and DHA by LPC-EPA resulted in the expected functional effects in the brain.
Fig. 7.
Effect of free EPA and LPC-EPA on gene expression and CREB phosphorylation in the brain. A–D: Gene expression: BDNF (A); CREB (B); 5-HT1A (C); TNFα (D). Quantitative PCR was carried out as described in the text. The values shown are the mean ± SD of six animals per group. E: BDNF protein levels were measured by ELISA. The values shown are the mean ± SD of six samples for each group. The free EPA value was not significantly different from the control. However, the BDNF level in in LPC-EPA-treated animals was significantly higher than the control or free EPA-treated animals, both at P < 0.0001 (ANOVA with post hoc Tukey multiple comparison test). F: Phosphorylated CREB was measured by Western blot as described in the text. Values shown are expressed as relative to control mean (n = 3 per group). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with control. ##P < 0.01, ###P < 0.001, and #### P < 0.0001 compared with free EPA (one-way ANOVA with post hoc Tukey multiple comparison test).
DISCUSSION
The two long-chain omega 3 FAs, EPA and DHA, have overlapping functions in the brain, but are not interchangeable. Thus, while DHA has been shown to be effective in inhibiting amyloid and Tau pathologies (30, 31) and improving cognition and memory (32, 33), it is not effective in improving depression (8, 9). On the other hand, several clinical and experimental studies have shown that EPA is more effective in the prevention and treatment of depression (7–9, 34), but it is less effective than DHA in improving memory and cognition (35). The EPA concentration in the brain is very low compared with DHA, ranging from 0 to <1% of the DHA concentration (36). Although theoretically the two FAs are interconvertible, the conversion efficiency is tissue dependent and is not bidirectional in most tissues. In the brain, EPA is converted to DHA, but the retro conversion of DHA to EPA is negligible (4, 37). Therefore, if EPA has certain unique benefits, such as its mood-improving effects, it is unlikely that DHA could substitute for it, as is evident from the preclinical and clinical trials (8, 9). On the other hand, because EPA is easily converted to DHA in the brain (37), it is possible to get the benefits of both EPA and DHA by increasing brain EPA levels. It appears that both EPA and DHA are required for the optimal effect because EPA increases the release of serotonin from the presynaptic neurons and DHA increases the activity of serotonin receptor in the postsynaptic neurons (38). However, it has been difficult to test this hypothesis clearly until now because the EPA levels in the brain are not increased after feeding fish oil or other EPA-rich supplements (3–6). The possible reasons for the low accumulation of EPA in the brain have been proposed to be not only due to its rapid oxidation through the β-oxidation pathway but also due to its rapid loss from the membrane lipids (36). However, a drawback of the previous studies is that they were conducted with labeled free EPA, which apparently enters the brain through diffusion (11), whereas the recent evidence suggests that the physiological transport of long-chain FAs, especially DHA, into the brain is through a sodium-dependent membrane transporter (Mfsd2a) in the molecular form of LPC (13). This mechanism supports the previous in vivo studies by Lagarde et al. (39), who showed that intravenously injected LPC-DHA is taken up more efficiently by the brain compared with free DHA. Although this mechanism has been challenged by the kinetic studies of Chen et al. (40), our recent studies on long-term feeding of free DHA and LPC-DHA showed that the net accumulation of brain DHA occurs only through dietary LPC-DHA, and not through free DHA (20). We showed that feeding LPC-DHA [1 mg DHA (3.04 μmol)/day for 30 days] resulted in a 2-fold increase in the net amount (nanomoles per gram) of brain DHA, whereas the same dose of free DHA had no effect. Therefore, we proposed that a similar enrichment of brain EPA can be achieved by providing dietary EPA in the form of LPC. The results presented here show for the first time that brain EPA can indeed be increased several fold by providing a clinically feasible dose of LPC-EPA. There was one report that showed a similar enrichment of brain phospholipid species with EPA after providing free EPA, but the dose used in that study (3% w/w of diet) is about 100-fold higher than the dose of LPC-EPA used in the present study, assuming an average daily consumption of 3 g of diet by the adult mouse. It is important to point out that such high doses of omega 3 FAs could cause systemic complications, such as increased bleeding times, and therefore the present studies employing a low dose of LPC-EPA are more translationally relevant.
It is of interest to note that dietary LPC-EPA markedly increased the omega 3 FAs not only in the brain but also in the retina, where Mfsd2a-mediated uptake is known to play a critical role (16). The protective role of EPA and DHA against retinopathies has been shown by several studies (41–43). The retina is unique in having very long-chain omega 3 FAs that give rise to pro-homeostatic molecules called elavonoids, which are essential for the integrity of the retinal pigmental epithelial cells (44). Because EPA is preferred over DHA for the synthesis of very long-chain omega 3 FAs in the retina (45, 46), LPC-EPA may be more beneficial than LPC-DHA for the prevention of retinopathies and macular degeneration, in addition to the neuroinflammatory diseases.
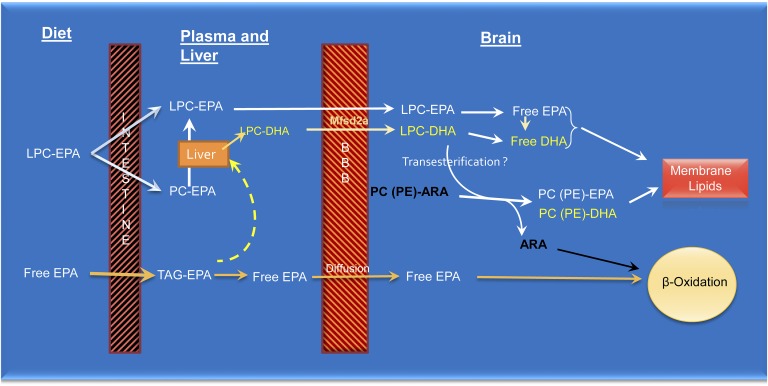
The exact pathways by which dietary LPC-EPA (but not dietary free EPA) specifically enriches brain EPA and DHA are not clear. LPC is known to be absorbed intact and converted to PC in the mucosal cells, following which it is transported in chylomicrons to various tissues (47). Part of the LPC may also be hydrolyzed to free EPA, which is then incorporated into chylomicron TAG. Our previous studies on LPC-DHA showed that some of the PC formed from the absorbed LPC is transported in HDL particles secreted by the intestine (23). It is also possible that a part of dietary LPC is absorbed intact and transported in portal blood as LPC because of its hydrophilic property, although experimental evidence for this pathway is lacking. The results presented here show that the majority of plasma LPC-EPA (in fasting plasma) was, in fact, sn-2 acyl isomer, suggesting the secretion of sn-2 EPA LPC by the liver following the absorption and assimilation of sn-1 EPA LPC. Whatever the isomeric form of LPC-EPA in the plasma, it is clear that it behaves differently from the dietary free EPA or TAG-EPA with regard to its uptake and metabolism by the brain (Figs. 2, 3). Because of the efficient uptake and retention of EPA derived from dietary LPC-EPA compared with dietary free EPA, we propose that the EPA taken up by the Mfsd2a-facilitated pathway enters a metabolic pool distinct from that of free DHA, which is apparently taken up by diffusion (11) (Fig. 8). Because there is a rapid and efficient conversion to DHA after the uptake of LPC-EPA, it is presumably hydrolyzed first to free EPA and activated to acyl-CoA, followed by elongation and desaturation in the endoplasmic reticulum and peroxisomes. LPC-EPA could also take part in various transacylation reactions with endogenous lipids (48), resulting in an exchange of EPA with endogenous ARA. Alternatively, LPC-DHA may be formed and secreted by the liver into the circulation, followed by its uptake by the brain. However, the following observations suggest that a significant portion of brain DHA was formed in the brain from elongation of EPA: 1) The amount of LPC-EPA was greater than LPC-DHA in the plasma and, therefore, more EPA than DHA would be taken up by the brain. Because the increase in brain DHA was greater than that of EPA, this suggests that the EPA taken up was converted to DHA in the brain. 2) There was very little LPC-22:5 in the plasma, but the brain 22:5 levels were significantly increased. This increase could have occurred only from the elongation of newly acquired EPA in the brain. 3) The conversion of DPA to DHA (as measured by the increase in DHA/increase in DPA) was much higher in the brain than in the liver, which indicates that the conversion of 22:5 to 22:6 may be more efficient in the brain than in the liver.
Fig. 8.
Proposed mechanisms for the differential effects of free EPA and LPC-EPA. Whereas free EPA (as well as EPA from fish oil and krill oil) is absorbed as TAG, LPC-EPA is absorbed as PC or LPC. The liver secretes more LPC-EPA and LPC-DHA after the uptake of phospholipid-EPA than after the uptake of TAG-EPA. The sodium-dependent symporter at the blood-brain barrier (Mfsd2a) facilitates the uptake of LPC-EPA and LPC-DHA by the brain. Part of the EPA taken up by the brain through this pathway is converted to DHA, and both EPA and DHA are incorporated into membrane lipids. LPC-EPA (and LPC-DHA) may also take part in transesterification reactions with endogenous ARA-containing phospholipids, resulting in the displacement of ARA and its subsequent oxidation of the latter by the β-oxidation pathway. This accounts for the loss of ARA after feeding EPA. In contrast to LPC-EPA, the free EPA generated in the plasma is taken up by diffusion and enters a different metabolic pool where it is preferentially oxidized by the β-oxidation pathway (10, 11) and, therefore, does not accumulate in the brain membranes.
The results presented here show that although normally the brain EPA levels are very low (0.03 μmol/g), they can be increased by over 100-fold (to 4.0 μmol/g) by feeding LPC-EPA. Furthermore, the brain DHA could be increased to the same extent as obtained with feeding LPC-DHA (20). Because both EPA and DHA of the brain are increased by LPC-EPA, it may be more beneficial than LPC-DHA (which increases only DHA in the brain) for the prevention and treatment of various neurological disorders, including Alzheimer’s disease. Moreover, this provides an opportunity to test whether the beneficial effects of EPA on mood disorders can be enhanced if its concentration in the brain is increased. The enhanced expression of BDNF, CREB, and 5-HT1A, and the increased phosphorylation of CREB as well as reduced expression of TNFα in the brain show that increasing the brain EPA levels does result in physiological responses commensurate with the known mechanisms for increased neuroplasticity and cognition. Our results also show a significant increase in the EPA and DHA content of the retina by LPC-EPA, but not by free EPA. Because the Mfsd2a-mediated transport of DHA has been shown to be essential for normal retinal function (16) and because the increase in retinal EPA may lead to increased production of elavonoids (44), dietary LPC-EPA could also benefit retinal health.
Supplementary Material
Footnotes
Abbreviations:
- ARA
- arachidonic acid
- BDNF
- brain-derived neurotrophic factor
- CREB
- cyclic AMP response element binding protein
- DPA
- docosapentaenoic acid (22:5, n-3)
- 5-HT1A
- 5-hydroxy tryptamine (serotonin) receptor 1A
- LPC
- lysophosphatidylcholine
- Mfsd2a
- major facilitator superfamily domain-containing protein 2a
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- TAG
- triacylglycerol
- TBST
- TBS containing 0.1% Tween-20
This research was supported by Center for Integrated Healthcare, U.S. Department of Veterans Affairs Merit Review Award I01 BX001090, National Institutes of Health Grant R21AT00847 from the National Center for Complementary and Integrative Medicine, and Office of the NIH Director Grant S10 OD010660 (LC/MS equipment) (P.V.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Veterans Administration.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Dyall S. C. 2015. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 7: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleiner A. C., Cladis D. P., and Santerre C. R.. 2015. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J. Sci. Food Agric. 95: 1260–1267. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues P. O., Lopes P. A., Ramos C., Miguueis S., Alfaia C. M., Pinto R. M. A., Rolo E. A., Bispo P., Batista I., Bandarra N. M., et al. 2014. Influence of feeding graded levels of canned sardines on the inflammatory markers and tissue fatty acid composition of Wistar rats. Br. J. Nutr. 112: 309–319. [DOI] [PubMed] [Google Scholar]
- 4.Kaur G., Begg D. P., Barr D., Garg M., Cameron-Smith D., and Sinclair A. J.. 2010. Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br. J. Nutr. 103: 32–37. [DOI] [PubMed] [Google Scholar]
- 5.Tou J. C., Altman S. N., Gigliotti J. C., Benedito V. A., and Cordonier E. L.. 2011. Different sources of omega-3 polyunsaturated fatty acids affects apparent digestibility, tissue deposition, and tissue oxidative stability in growing female rats. Lipids Health Dis. 10: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz-Hernandez C., Thakkar S. K., Moulin J., Oliveira M., Masserey-Elmelegy I., Dionisi F., and Destaillats F.. 2012. Benefits of structured and free monoacylglycerols to deliver eicosapentaenoic (EPA) in a model of lipid malabsorption. Nutrients. 4: 1781–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martins J. G. 2009. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 28: 525–542. [DOI] [PubMed] [Google Scholar]
- 8.Song C., Shieh C. H., Wu Y. S., Kalueff A., Gaikwad S., and Su K. P.. 2016. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: acting separately or synergistically? Prog. Lipid Res. 62: 41–54. [DOI] [PubMed] [Google Scholar]
- 9.Ross B. M. 2008. The emerging role of eicosapentaenoic acid as an important psychoactive natural product: some answers but a lot more questions. Lipid Insights. 2: 89–97. [Google Scholar]
- 10.Igarashi M., Chang L., Ma K., and Rapoport S. I.. 2013. Kinetics of eicosapentaenoic acid in brain, heart and liver of conscious rats fed a high n-3 PUFA containing diet. Prostaglandins Leukot. Essent. Fatty Acids. 89: 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C. T., and Bazinet R. P.. 2015. β-Oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot. Essent. Fatty Acids. 92: 33–40. [DOI] [PubMed] [Google Scholar]
- 12.Kaur G., Molero J. C., Weisinger H. S., and Sinclair A. J.. 2013. Orally administered [14C]DPA and [14C]DHA are metabolised differently to [14C]EPA in rats. Br. J. Nutr. 109: 441–448. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen L. N., Ma D., Shui G., Wong P., Cazenave-Gassiot A., Zhang X., Wenk M. R., Goh E. L. K., and Silver D. L.. 2014. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 509: 503–506. [DOI] [PubMed] [Google Scholar]
- 14.Guemez-Gamboa A., Nguyen L. N., Yang H., Zaki M. S., Kara M., Ben-Omran T., Akizu N., Rosti R. O., Rosti B., Scott E., et al. 2015. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat. Genet. 47: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Markhus M. W., Skotheim S., Graff I. E., Frøyland L., Braarud H. C., Stormark K. M., and Malde M. K.. 2013. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One. 8: e67617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong B. H., Chan J. P., Cazenave-Gassiot A., Poh R. W., Foo J. C., Galam D. L. A., Ghosh S., Nguyen L. N., Barathi V. A., Yeo S. W., et al. 2016. Mfsd2a is a transporter for the essential omega 3 fatty acid docosahexaenoic acid (DHA) in eye and is important for photoreceptor cell development. J. Biol. Chem. 291: 10501–10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naninck E. F. G., Hoeijmakers L., Kakava-Georgiadou N., Meesters A., Lazic S. E., Lucassen P. J., and Korosi A.. 2015. Chronic early life stress alters developmental and adult neurogenesis and impairs cognitive function in mice. Hippocampus. 25: 309–328. [DOI] [PubMed] [Google Scholar]
- 18.Quek D. Q. Y., Nguyen L. N., Fan H., and Silver D. L.. 2016. Structural insights into the transport mechanism of the human sodium-dependent lysophosphatidylcholine transporter MFSD2A. J. Biol. Chem. 291: 9383–9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okudaira M., Inoue A., Shuto A., Nakanaga K., Kano K., Makide K., Saigusa D., Tomioka Y., and Aoki J.. 2014. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res. 55: 2178–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugasini D., Thomas R., Yalagala P. C. R., Tai L. M., and Subbaiah P. V.. 2017. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 7: 11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichihara K., Iwasaki H., Ueda K., Takizawa R., Naito H., and Tomosugi M.. 2005. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem. Phys. Lipids. 137: 94–99. [DOI] [PubMed] [Google Scholar]
- 22.Ivanova P. T., Milne S. B., Byrne M. O., Xiang Y., and Brown H. A.. 2007. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 432: 21–57. [DOI] [PubMed] [Google Scholar]
- 23.Subbaiah P. V., Dammanahalli K. J., Yang P., Bi J., and O’Donnell J. M.. 2016. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim. Biophys. Acta. 1861: 723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiełbowicz G., Smuga D., Gładkowski W., Chojnacka A., and Wawrzeńczyk C.. 2012. An LC method for the analysis of phosphatidylcholine hydrolysis products and its application to the monitoring of the acyl migration process. Talanta. 94: 22–29. [DOI] [PubMed] [Google Scholar]
- 25.Croset M., Brossard N., Polette A., and Lagarde M.. 2000. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem. J. 345: 61–67. [PMC free article] [PubMed] [Google Scholar]
- 26.Graham A., Zammit V. A., Christie W. W., and Brindley D. N.. 1991. Sexual dimorphism in the preferential secretion of unsaturated lysophosphatidylcholine by rat hepatocytes but no secretion by sheep hepatocytes. Biochim. Biophys. Acta. 1081: 151–158. [DOI] [PubMed] [Google Scholar]
- 27.Scagnelli G. P., Cooper P. S., VandenBroek J. M., Berman W. F., and Schwartz C. C.. 1991. Plasma 1-palmitoyl-2-linoleoyl phosphatidylcholine. Evidence for extensive phospholipase A1 hydrolysis and hepatic metabolism of the products. J. Biol. Chem. 266: 18002–18011. [PubMed] [Google Scholar]
- 28.Graf B. A., Duchateau G. S. M. J., Patterson A. B., Mitchell E. S., van Bruggen P., Koek J. H., Melville S., and Verkade H. J.. 2010. Age dependent incorporation of 14C-DHA into rat brain and body tissues after dosing various 14C-DHA-esters. Prostaglandins Leukot. Essent. Fatty Acids. 83: 89–96. [DOI] [PubMed] [Google Scholar]
- 29.Kitson A. P., Metherel A. H., Chen C. T., Domenichiello A. F., Trepanier M. O., Berger A., and Bazinet R. P.. 2016. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 33: 91–102. [DOI] [PubMed] [Google Scholar]
- 30.Green K. N., Martinez-Coria H., Khashwji H., Hall E. B., Yurko-Mauro K. A., Ellis L., and LaFerla F. M.. 2007. Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 27: 4385–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm M. O. W., Kuchenbecker J., Grosgen S., Burg V. K., Hundsdorfer B., Rothhaar T. L., Friess P., de Wilde M. C., Broersen L. M., Penke B., et al. 2011. Docosahexaenoic acid reduces amyloid β production via multiple pleiotropic mechanisms. J. Biol. Chem. 286: 14028–14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chouinard-Watkins R., Vandal M., Leveille P., Pincon A., Calon F., and Plourde M.. 2017. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol. Aging. 57: 28–35. [DOI] [PubMed] [Google Scholar]
- 33.Arsenault D., Julien C., Tremblay C., and Calon F.. 2011. DHA improves cognition and prevents dysfunction of entorhinal cortex neurons in 3xTg-AD mice. PLoS One. 6: e17397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marangell L. B., Martinez J. M., Zboyan H. A., Kertz B., Kim H. F. S., and Puryear L. J.. 2003. A Double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am. J. Psychiatry. 160: 996–998. [DOI] [PubMed] [Google Scholar]
- 35.Che H., Zhou M., Zhang T., Zhang L., Ding L., Yanagita T., Xu J., Xue C., and Wang Y.. 2018. Comparative study of the effects of phosphatidylcholine rich in DHA and EPA on Alzheimer’s disease and the possible mechanisms in CHO-APP/PS1 cells and SAMP8 mice. Food Funct. 9: 643–654. [DOI] [PubMed] [Google Scholar]
- 36.Chen C. T., Domenichiello A. F., Trepanier M. O., Liu Z., Masoodi M., and Bazinet R. P.. 2013. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J. Lipid Res. 54: 2410–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson G. J., Connor W. E., and Corliss J. D.. 1990. Docosahexaenoic acid is the preferred dietary n-3 fatty acid for the development of the brain and retina. Pediatr. Res. 27: 89–97. [DOI] [PubMed] [Google Scholar]
- 38.Patrick R. P., and Ames B. N.. 2015. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 29: 2207–2222. [DOI] [PubMed] [Google Scholar]
- 39.Lagarde M., Bernoud N., Brossard N., Lemaitre-Delaunay D., Thies F., Croset M., and Lecerf J.. 2001. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16: 201–204. [DOI] [PubMed] [Google Scholar]
- 40.Chen C. T., Kitson A. P., Hopperton K. E., Domenichiello A. F., Trepanier M. O., Lin L. E., Ermini L., Post M., Thies F., and Bazinet R. P.. 2015. Plasma non-esterified docosahexaenoic acid is the major pool supplying the brain. Sci. Rep. 5: 15791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Querques G., Forte R., and Souied E. H.. 2011. Retina and omega-3. J. Nutr. Metab. 2011: 748361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez González-Herrero M. E., Ruiz M., López Román F. J., Marín Sánchez J. M., and Domingo J. C.. 2018. Supplementation with a highly concentrated docosahexaenoic acid plus xanthophyll carotenoid multivitamin in nonproliferative diabetic retinopathy: prospective controlled study of macular function by fundus microperimetry. Clin. Ophthalmol. 12: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connor K. M., SanGiovanni J. P., Lofqvist C., Aderman C. M., Chen J., Higuchi A., Hong S., Pravda E. A., Majchrzak S., Carper D., et al. 2007. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 13: 868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jun B., Mukherjee P. K., Asatryan A., Kautzmann M. A., Heap J., Gordon W. C., Bhattacharjee S., Yang R., Petasis N. A., and Bazan N. G.. 2017. Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity. Sci. Rep. 7: 5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh M., and Clandinin M. T.. 2005. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long-chain fatty acids (C24-C36) in retina. Curr. Eye Res. 30: 959–968. [DOI] [PubMed] [Google Scholar]
- 46.Yu M., Benham A., Logan S., Brush R. S., Mandal M. N., Anderson R. E., and Agbaga M. P.. 2012. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. J. Lipid Res. 53: 494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsson A. 1968. Intestinal absorption of lecithin and lysolecithin by lymph fistula rats. Biochim. Biophys. Acta. 152: 379–390. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita A., Hayashi Y., Nemoto-Sasaki Y., Ito M., Oka S., Tanikawa T., Waku K., and Sugiura T.. 2014. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 53: 18–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.