Abstract
There is some evidence of specific oro-detection of FFAs in rodents and humans. The aim of this study was to record gustatory evoked potentials (GEPs) in response to FFA solutions and to compare GEPs in response to linoleic acid solution with GEPs obtained after stimulation with sweet and salty tastants. Eighteen healthy men were randomly stimulated with fatty (linoleic acid), sweet (sucrose), and salty (NaCl) solutions at two concentrations in the first experiment. Control recordings (n = 14) were obtained during stimulation by a paraffin oil mixture without FFA or by water. In the second experiment, 28 men were randomly stimulated with five FFA solutions and a paraffin emulsion. GEPs were recorded with electroencephalographic electrodes at Cz, Fz, and Pz. GEPs were observed in response to FFA in all participants. GEP characteristics did not differ according to the quality and the concentration of the solutions in the first experiment and according to the FFA in the second experiment. This study describes for the first time GEPs in response to FFA and demonstrates that the presence of FFA in the mouth triggers an activation of the gustatory cortex. These data reinforce the concept that fat taste could be the sixth primary taste.
Keywords: long-chain fatty acids, medium-chain fatty acids, short-chain fatty acids, fatty taste
The gustatory processing system encodes the quality (sweet, salty, bitter, sour, or umami), intensity, and palatability of a sensory stimulus (1–3). Concerning fat stimulus, it is important to understand its representation in the brain, beyond its textural properties, which can increase the palatability of food (4). In fact, the fatty food consumption could have important implication in feeding behavior.
The cerebral representation of the fatty texture of food has already been studied using neurophysiological investigations in macaque monkey (5–7) and functional MRI (fMRI) in humans (8–10). The primary (anterior insular and frontal opercular cortices) and secondary (orbitofrontal cortex) cerebral taste cortices in nonhuman primates responded to the presentation of fat in the mouth by encoding the nontaste properties of food, such as its viscosity, texture, grittiness, or temperature (5, 11–15), because similar responses of the same neurons were observed using stimulation by mineral oils, which have a similar texture but different chemical composition compared with FAs (6, 7). In humans, the results about the representation of fat in the brain are not as clear as those in nonhuman primates. One previous study using fMRI reported lower activation of the gustatory and reward cerebral regions and higher activation in somatosensory cerebral regions following a fatty stimulus than that with sugar (10). However, other studies highlighted cerebral activation by fatty food in the orbito-frontal and cingulate cortices, suggesting that a fat stimulus play a role in the hedonic control of food intake (8, 9).
Other data demonstrated that long-chain fatty acids (LCFAs) could be detected through specific receptor located in the mouth of rodents (16–19) and humans (20, 21), as is the case for sweet, salty, bitter, sour, and umami tastes. Other studies found the same results for short-chain (22) and medium-chain (23, 24) FAs. However, there is not sufficient evidence to define fat taste as a primary taste (25). Because oro-sensory perception of FFAs seems to be uncertain in humans, it remains a matter of debate (25–28).
We recently reported a noninvasive method with high time resolution to study the gustatory pathway: gustatory evoked potentials (GEPs) (26, 27). GEPs have already been obtained in response to primary taste (salty, sweet, sour, bitter, and umami) solutions (29–31).
In the present study, we hypothesized that GEPs could be observed in response to FFA solutions, with the same characteristics as GEPs in response to other known primary taste stimuli. Recording GEPs in response to FFA solutions could be an objective argument to demonstrate the activation of the gustatory pathway by FFAs.
Therefore, the first aim was to record GEPs in response to unsaturated LCFA solutions, in two concentrations, applied in the oral cavity in healthy subjects, in comparison with control sessions (paraffin emulsion and water) (first experiment). We also aimed to compare the LCFA cortical responses with GEPs obtained in response to sweet and salty solutions in the same subjects (first experiment). The second aim was to compare GEPs in response to unsaturated LCFA solutions with other FFA solutions: saturated and unsaturated LCFAs and medium- and short-chain FAs (second experiment).
METHODS
Solutions and subjects
First experiment.
The taste stimuli consisted of LCFA emulsions, sodium chloride (NaCl), and sucrose solutions.
NaCl and sucrose (Cooper, Melun, France) were diluted in Evian water on the same day as GEP recordings. Evian water, which is almost deionized, was used as the control solution.
The preparation of the LCFA emulsion was described elsewhere (32). Linoleic acid (LA) were chosen because the Western diet is characterized by an overconsumption of LA and because they are known to bind and activate lipid receptors CD36 and G-protein-coupled receptor 120 (GPR120) in gustatory papillae (23, 33). Briefly, LA (Sigma-Aldrich, Saint Quentin Fallavier, France) oil-in-water emulsions (LA emulsions) were prepared in a solution of 5% acacia gum (Cooper) and 5% paraffin oil (Cooper) diluted in Evian mineral water. A mixture of paraffin oil and acacia gum without LA was used as a control solution (paraffin emulsion), to limit differences in viscosity and lubricity between control and taste solutions, which could be detected by the subjects. LA and paraffin emulsions were prepared for less than 24 h before tasting.
Healthy men were enrolled in this study. The mean age and BMI were 22 ± 2 years old (range: 19–34) and 23 ± 3 kg/m2 (range: 19–29), respectively. All of the subjects were nonsmokers or mild smokers who had not smoked during the day before GEP recordings. Heavy smokers (20 or more cigarettes per day) were not included. As shown in previous data (34), only heavy smoking alters gustatory pathways, and GEPs obtained in response to salty and sweet solutions were similar in mild and moderate smokers and nonsmokers (unpublished observations). None of the subjects had oral, dental, or neurological disorders or specific medical histories. Subjects who were currently undergoing medical treatment and obese subjects (BMI > 30 kg/m2) were excluded.
Eighteen subjects were investigated in six sessions separated by an interval of at least 1 day. Each session corresponded to a specific stimulus quality and concentration, which was randomly assigned. Each stimulus was applied in two different concentrations: 0.25% and 1% LA solutions, which were higher than the LA threshold (32), 5 and 20 g of sucrose per 100 ml of water, and 0.5 and 2 g of NaCl per 100 ml of water, also higher than the sweet and salty thresholds, respectively.
Eighteen subjects were investigated in two control sessions of GEP recordings: one with the paraffin emulsion and another with water. The GEPs of only 14 subjects were assessable because of some artifacts that hindered the absence of GEPs in these recordings.
Second experiment.
The taste stimuli consisted of five FFA solutions. The FFAs were as follow: polyunsaturated long-chain FFA (solution containing 0.25% LA), monounsaturated long-chain FFA (solution containing 0.25% oleic acid), saturated long-chain FFA (solution containing 0.25% stearic acid), medium-chain FFA (solution containing 0.25% lauric acid), and short-chain FFA (solution containing 0.1% caproic acid). These FFAs were chosen because of their overconsumption in the Western diet. The concentrations that were tested were higher than the FFA threshold and were tested to be similarly intense in preliminary studies. The similar intensity of the prepared FFA emulsions was confirmed in the present study (see below). The preparation of the FFA solutions was similar to that described above. Each subject had also a sixth GEP recording session in response to a control paraffin solution. Each session of GEP recordings, which was randomly assigned, was separated by an interval of at least 1 day.
Eighteen healthy men were enrolled in this second experiment. The mean age and BMI were 25 ± 6 years old (range: 19–37) and 22 ± 2 kg/m2 (range: 19–26), respectively. Age and BMI of the subjects were comparable in the two experiments (P > 0.05 for both). All of the subjects were nonsmokers or very mild smokers (<10 cigarettes per day) who had not smoked during the day before GEP recordings.
Ethical approval
The subjects were informed about the nature and aims of the experiments and provided informed consent. The study was approved by the regional Ethics Committee of Burgundy, France, in accordance with the latest revision of the Declaration of Helsinki and European Law (ISO EN 14155).
Experimental design
The taste-delivery system has been described in detail in previous studies (29, 30). Briefly, control and taste solutions were driven through the system by compressed air. Two parallel silicone tubes were used: one for the control solution and the other for the taste solution. Switching between the control and taste solutions was performed by two electromagnetic valves controlled by an electronic device. This electronic device (stimulator) sent a signal to the computer software (SystemPLUS EVOLUTION, 2007 Micromed S.p.A) when the taste solution was administered (with 1 ms precision), resulting in a precise time recording of the GEPs. Participants put the two parallel tubes (silicone tubing, P/N 10025-02S, Bio-Chem valve) in their mouth, placed 1.5 ± 0.5 cm from the dental arch on the midline of the tongue. Air was purged from the taste delivery system to avoid delaying stimulus presentation.
Solutions were delivered to the tongue through a hole at the end of each tube. A taste solution was intermittently delivered through the first tube. During the period without the taste solution, a control solution (water for salty and sweet solutions or paraffin emulsion for FFA emulsions) was continuously delivered through the second tube to minimize the likelihood that the subjects would feel different sensations from the injections from the two tubes.
During the control sessions, the experimental protocol was similar to the one previously described, and the paraffin emulsion (or water) was present in both the tubes and was therefore used as the stimulus.
Stimulation
All of the sessions were conducted at the same time of day, 2–4 h after lunch. The subjects were asked not to eat or drink anything except water during the time between lunch and the GEP recording. One session lasted approximately 40 min: 20 min to prepare for the GEP recording and 20 min for the GEP recording itself. In each session, the stimulus was presented for 1 s 20 times. Each stimulus was separated by a 1 min interval of water solution.
During the GEP recordings, the subjects listened to quiet music through their headphones to mask the switching clicks of the electromagnetic valves. No evoked potentials were recorded in our experiment in response to quiet music (checked with control GEP recordings). The subjects also had to close their eyes to avoid light stimulation and to wear a nose clip for each of the taste stimulations in order to avoid retronasal olfaction, because FFA solutions can activate olfactory receptors (35).
After GEP recordings were performed with the taste solutions, the subjects were asked to rate the hedonic value and the perceived intensity of each solution using a 10 cm visual analog scale (VAS) anchored by “not at all” and “extremely” at its extremities. They had to respond to the following questions: “How palatable was the taste solution?” and “How intense was the taste solution?”
GEP recording and data analysis
Electroencephalographic (EEG) measurements were recorded according to the international 10-20 system using a conventional EEG recording system. Five sites were recorded by surface electrodes defined by their scalp topography: centro-parietal electrode Pz, central electrode Cz, and frontal electrodes Fz, Fp1, Fp2. The electrodes were referenced against linked earlobes (ear clip electrodes enfolded by Ag, 10 mm diameter; SystemPLUS EVOLUTION). The ground electrode was placed on the forehead. Disposable cup electrodes enfolded by Ag-AgCl (6 mm diameter), with a long polyurethane cable (SystemPLUS EVOLUTION), were used. Electrodes were placed after using first a pumice paste and then a conductive and adhesive paste.
The EEG measurements were amplified, filtered, and digitized using Micromed software (SystemPLUS EVOLUTION, 2007 Micromed S.p.A), as follows: time constant, 1 s; sampling frequency, 2,048 Hz; 200 Hz low-pass filter; 0.4 Hz high-pass filter; 50 Hz filter. GEPs were averaged after each recording session (average of 20 stimuli). No baseline correction was applied during averaging.
GEP analysis was performed with the same software (SystemPLUS EVOLUTION). No contamination due to α waves was noted because baseline cortical activity in participants with closed eyes was mainly noted in occipital recordings. Moreover, averaging decreased α wave contamination. GEP was defined by three peaks, as described in previous studies (29, 30): P1 the first positive peak, N1 the higher negative peak, and P2 the second positive peak. P1 latency (in milliseconds), N1 latency (in milliseconds), and P1N1 amplitude (in microvolts) of the GEPs were registered for each recorded electrode. The P1 latency was defined as the time between stimulus delivery and the potential’s positive peak P1. The N1 latency was defined as the time between stimulus delivery and the potential’s negative peak. The amplitude of each response was calculated as the difference between the first positive and the negative peaks (P1N1 amplitude). The positive peak corresponded to the peak pointing down, whereas the negative peak corresponded to the peak pointing up. The software first averaged the GEPs (n = 20) and then detected the peaks. The GEP recordings were then analyzed by the same well-trained neurophysiologist and were processed with a standard and consistent method of EEG analysis, regardless of the quality and intensity of the taste solution and the hedonic value noted by the subject. The neurophysiologist was blinded to the taste solution applied. Because of constraints inherent to our software, prestimulus cerebral activity was not available. Many GEP recordings in Fp1 and Fp2 (in response to sweet, salty, and fatty stimuli), one GEP recording in Pz (in response to sweet stimulus), and 9 out of 108 GEP recordings in the second experiment were not analyzed because of artifacts.
Statistical analysis
At the end of the recordings for each stimulus and for each patient, an average of the responses of all subjects was made: It was called the “grand average” (see Figs. 1, 2, and 5). P1N1 amplitudes are minimized in the “grand average” compared with the statistical mean: in fact, there is a smoothing of the amplitude in the graph because GEP peaks of each subject do not have the same latency.
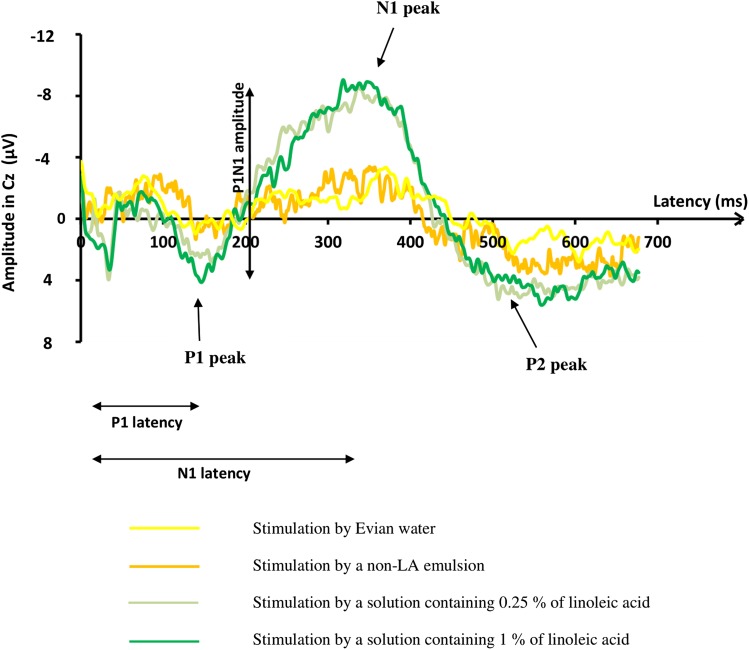
Fig. 1.
Grand averages (average of the responses of all subjects) of recordings of GEPs, on the Cz electrode. The start of the stimulation was at 0 ms. The yellow curve represent the grand average of GEPs of the 14 assessable subjects who were tested with water. The orange curve represents the grand average of GEPs of the 14 assessable subjects who were tested with the non-LA emulsion. The two others show the grand average of GEPs obtained in the 18 subjects in response to the LA 1% solution (dark green curve) and in response to the LA 0.25% solution (light green curve). No identifiable GEP was noted in response to water and non-LA emulsion, in contrast to taste stimulation by LA emulsions.
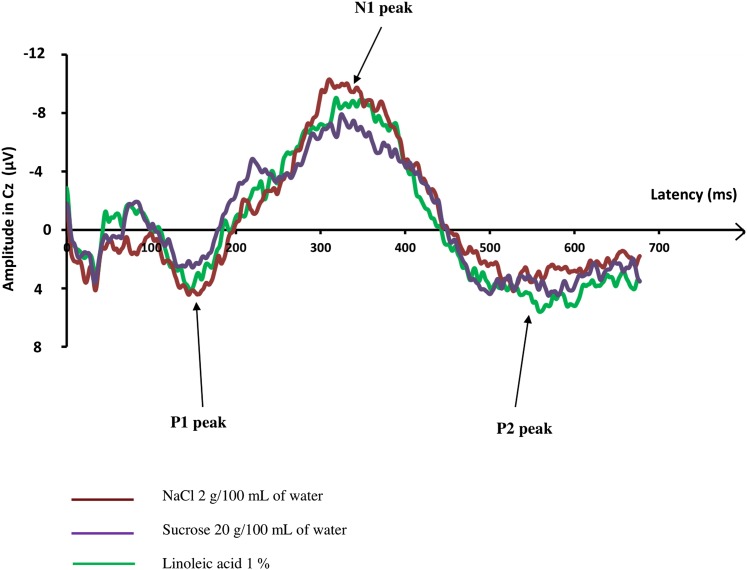
Fig. 2.
Grand averages (average of the responses of all subjects) of GEPs: recordings of GEPs in response to the three high concentrated taste solutions (LA, salty, and sweet solutions), on the Cz electrode, in all 18 participants. The start of the taste stimulation was at 0 ms. No difference of GEPs parameters was observed whatever the quality of the stimulus.
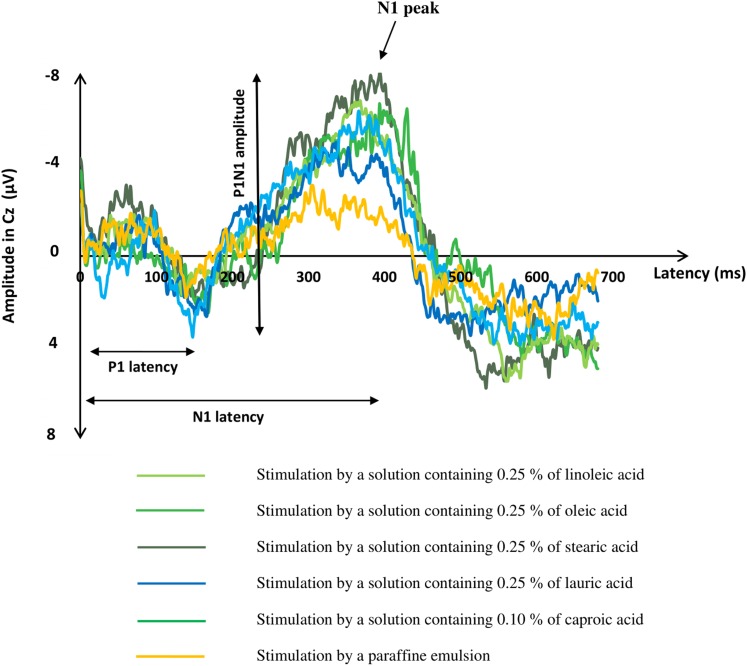
Fig. 5.
Grand averages (average of the responses of all subjects) of recordings of GEPs, on the Cz electrode. The start of the stimulation was at 0 ms. P1N1 amplitude was higher in response to the five FFA solutions than in response to paraffin emulsion (P < 0.001 for all).
P1 latency, N1 latency, and P1N1 amplitude of each GEP and the VAS results (for hedonic value and perceived intensity of the solutions) were expressed as means and standard deviations.
In the first experiment, P1 latency, N1 latency, and P1N1 amplitude of GEPs obtained in Pz, Cz, and Fz after stimulation by LA, paraffin emulsions, and water were compared using two-way ANOVA for repeated measures, to test the subject effect and the solution effect. P1 latency, N1 latency, and P1N1 amplitude of GEPs obtained in Pz, Cz, and Fz after stimulation by sweet, salty, and fatty solutions, were then compared using three-way ANOVA for repeated measures to test the subject effect, the effect of the taste quality, and the effect of the concentration of taste. Hedonic value and perceived intensity of taste were also analyzed by three-way ANOVA to test the subject effect, the effect of the taste quality, and the effect of the concentration of taste. Post hoc analyses (Tukey’s tests) were also performed when the result was found significant.
In the second experiment, hedonic value, perceived intensity of taste, P1 latency, N1 latency, and P1N1 amplitude of GEPs obtained in Pz, Cz, and Fz after stimulation by FFA and paraffin emulsions were compared using two-way ANOVA for repeated measures, to test the subject effect and the solution effect. Post hoc analyses (Tukey’s tests) were performed when the result was found to be significant.
Then, GEP parameters in response to paraffin solutions on the one hand, and in response to LA solutions on the other hand, were compared between both experiments using one-way ANOVA.
GEPs recorded in Fp1 and Fp2 electrodes were not analyzed because of the too large variability of the recording (artifacts due to eye movements, possible activation from an anticipation phenomenon, and projections from several cerebral sensory areas). A P value below 0.05 was considered statistically significant. SAS 9.2 software (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
Comparison of GEP cerebral recordings in response to LA emulsion, paraffin emulsion, and water (first experiment)
Tasting LCFA solutions triggered a typical gustative evoked potential: GEPs were observed in response to LA emulsions at the two concentrations (higher than the LA threshold) in all subjects and in all the analyzed recording electrodes. The grand average of GEPs recorded in the Cz electrode in response to LA is shown in Fig. 1. The mean values (±SD) of GEP parameters recorded by the Cz electrode were as follow: P1 latency was 160 ± 30 ms, N1 latency was 309 ± 50 ms, and P1N1 amplitude was 20 ± 11 µV in response to the 0.25% LA emulsion; and P1 latency was 148 ± 26 ms, N1 latency was 325 ± 53 ms, and P1N1 amplitude was 19 ± 7 µV in response to the 1% LA emulsion. The characteristics of the GEPs recorded by the Pz and Fz electrodes in response to LA emulsions were similar to those recorded by the Cz electrode (Table 1).
TABLE 1.
Mean ± SD of the GEP parameters (P1 latency, N1 latency, and P1N1 amplitude) recorded in response to the three taste stimuli (LA, salty, and sweet solutions) at two concentrations, on the Pz, Cz, and Fz electrodes, in the 18 participants
| LA | Sucrose | NaCl | ||||
| 0.25% | 1% | 5 g | 20 g | 0.5 g | 2 g | |
| P1 latency (ms) | ||||||
| Pz | 163 ± 28 | 150 ± 24 | 151 ± 20 | 154 ± 21 | 159 ± 29 | 152 ± 20 |
| Cz | 160 ± 30 | 148 ± 26 | 152 ± 20 | 154 ± 20 | 156 ± 30 | 152 ± 22 |
| Fz | 163 ± 32 | 152 ± 25 | 152 ± 21 | 150 ± 23 | 158 ± 28 | 151 ± 18 |
| N1 latency (ms) | ||||||
| Pz | 308 ± 48 | 333 ± 56 | 314 ± 45 | 314 ± 60 | 326 ± 42 | 321 ± 55 |
| Cz | 308 ± 50 | 325 ± 53 | 298 ± 59 | 308 ± 61 | 321 ± 40 | 315 ± 54 |
| Fz | 309 ± 57 | 337 ± 51 | 298 ± 65 | 305 ± 56 | 321 ± 41 | 314 ± 48 |
| P1N1 amplitude (µV) | ||||||
| Pz | 19.0 ± 9.5 | 18.6 ± 6.6 | 19.0 ± 9.0 | 16.0 ± 8.6 | 18.5 ± 9.6 | 20.0 ± 7.0 |
| Cz | 20.0 ± 10.0 | 19.0 ± 8.0 | 22.0 ± 11.6 | 19.0 ± 7.6 | 19.7 ± 8.6 | 20.7 ± 10.5 |
| Fz | 18.0 ± 10.0 | 16.6 ± 6.6 | 19.0 ± 8.5 | 16.6 ± 8.0 | 17.0 ± 8.6 | 20.0 ± 10.0 |
No significant difference in GEP parameters was observed between the six solutions.
No GEP was recorded in response to water solution in all subjects, whatever the electrodes.
In response to paraffin emulsion, a small evoked response was detected in only 3 out of 14 subjects. The mean P1N1 amplitude of this response was as follow: 4 ± 8 µV in Cz, 2 ± 4 µV in Fz, and 3 ± 7 µV in Pz, and differed significantly with the P1N1 amplitude of GEPs recorded in response to LA emulsions (P < 0.001).
Figure 1 shows the evoked potentials recorded by the Cz electrode in response to water and paraffin emulsion, in contrast to those recorded in response to LA emulsions.
Comparisons of GEP cerebral recordings, hedonic values, and perceived intensities in response to LA emulsions and sweet and salty solutions (first experiment)
The grand averages of GEPs recorded by the Cz electrode in response to the three different taste solutions at the higher concentration are presented in Fig. 2. The curves are similar for the solutions at the lower concentration (data not shown).
Table 1 summarizes the mean value (±SD) of GEP parameters recorded by the Cz, Pz, and Fz electrodes in response to the three taste stimuli at two concentrations for each taste. P1 latency, N1 latency, and P1N1 amplitude differed neither according to the quality of taste (sweet, salty, or fatty) nor the concentration of each solution, whatever the GEP electrode.
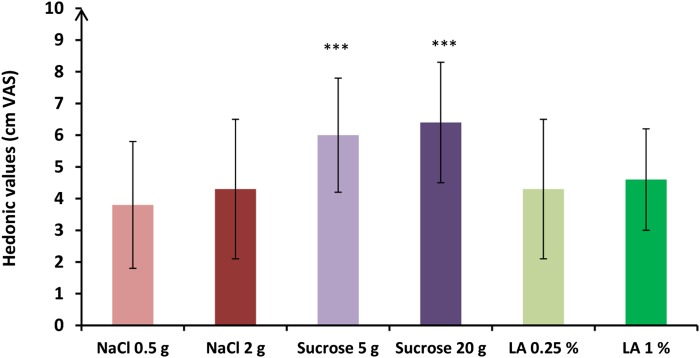
Hedonic values of the taste solutions are shown in Fig. 3. Hedonic values were different according to the quality of the solution [F(2,82) = 13.10; P < 0.001]. In posthoc analyses, subjects preferred the sweet solutions to the fatty or the salty solutions (P < 0.001). No difference in hedonic values was observed according to the concentration of the solution [F(1,82) = 1.04; P = 0.31].
Fig. 3.
Mean (± SD) hedonic values reported by the 18 participants for each of the taste solutions via VASs. VAS comparisons between the taste solutions obtained with post hoc analyses are expressed as follows. *** P < 0.001. The sweet solutions were perceived as more pleasant than the salty or the fatty one.
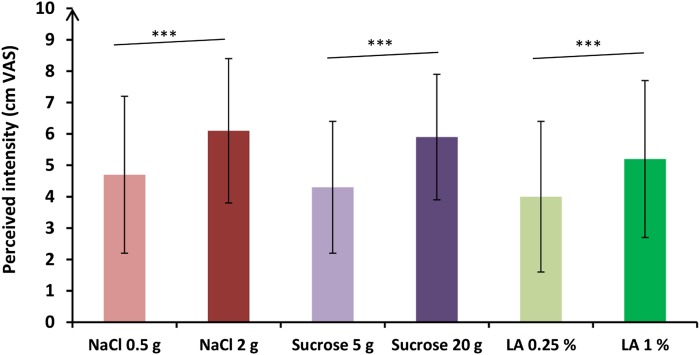
Perceived intensities of each taste solution are presented in Fig. 4. Intensity of the solutions that were perceived by the participants were different according to the concentration of the solutions [F(1,82) = 10.07; P < 0.01]. In posthoc analyses, high concentrated solutions were perceived as more intense than low concentrated solutions, regardless of the taste of the solution (P < 0.001). No difference in perceived intensity was observed according to the quality of taste (sweet, salty, or fatty) [F(2,82) = 1.26; P = 0.29].
Fig. 4.
Mean (± SD) perceived intensities reported by the 18 participants for each of the taste solutions via VASs. VAS comparisons between the taste solutions obtained with post hoc analyses are expressed as follows. *** P < 0.001. The highest concentrated solutions were perceived more intense than the lowest ones, regardless of the taste of the solution.
Comparisons of GEP cerebral recordings, perceived intensity, and hedonic values in response to the FFA and paraffin emulsions (second experiment)
Grand average of GEPs recorded by the Cz electrode in response to the five different FFA and paraffin emulsions are presented in Fig. 5. Table 2 summarizes the mean value (±SD) of GEP parameters recorded by the Cz, Pz, and Fz electrodes in response to the five different FFA and paraffin emulsions.
TABLE 2.
Mean ± SD of the GEP parameters (P1 latency, N1 latency, and P1N1 amplitude) recorded in response to the five FFA and paraffin emulsions, on the Pz, Cz, and Fz electrodes, in the 18 participants
| LA | Oleic Acid | Stearic Acid | Lauric Acid | Caproic Acid | Paraffin | |
| P1 latency (ms) | ||||||
| Pz | 165 ± 28 | 171 ± 27 | 172 ± 30 | 160 ± 26 | 177 ± 36 | 193 ± 46 |
| Cz | 161 ± 24 | 165 ± 20 | 173 ± 30 | 152 ± 20 | 176 ± 38 | 190 ± 46 |
| Fz | 161 ± 25 | 164 ± 18 | 169 ± 31 | 154 ± 19 | 178 ± 38 | 176 ± 79 |
| N1 latency (ms) | ||||||
| Pz | 340 ± 55 | 330 ± 67 | 337 ± 43 | 292 ± 56 | 342 ± 61 | 357 ± 82 |
| Cz | 335 ± 57 | 325 ± 68 | 330 ± 45 | 286 ± 55 | 336 ± 66 | 352 ± 84 |
| Fz | 332 ± 59 | 320 ± 69 | 330 ± 48 | 283 ± 56 | 329 ± 66 | 351 ± 84 |
| P1N1 amplitude (µV) | ||||||
| Pz | 15 ± 8 | 15 ± 8 | 16 ± 7 | 13 ± 7 | 17 ± 7 | 7 ± 8 |
| Cz | 15 ± 10 | 15 ± 9 | 18 ± 9 | 14 ± 8 | 18 ± 6 | 6 ± 8 |
| Fz | 14 ± 9 | 14 ± 6 | 15 ± 7 | 12 ± 6 | 15 ± 5 | 5 ± 7 |
P1N1 amplitudes of the GEPs in Pz, Cz, and Fz were different according to the taste solutions: P1N1 amplitude was higher in response to the five FFA solutions than in response to paraffin emulsion, whatever the electrodes (P < 0.001 for all).
Tasting FFA solutions triggered a typical gustative evoked potential: GEPs were observed in response to the five FFA emulsions (linoleic, oleic, stearic, lauric, and caproic acids) in all the analyzed recording electrodes. In response to paraffin emulsion, a small evoked response was observed in only 8 out of 18 subjects.
P1N1 amplitude of the GEPs in Pz, Cz, and Fz were different according to the taste solutions: F(5,76) = 8.21, P < 0.001 for Pz; F(5,76) = 9.28, P < 0.001 for Cz; and F(5,76) = 8.75, P < 0.001 for Fz. In posthoc analyses, P1N1 amplitude was higher in response to the five FFA solutions than in response to paraffin emulsion, whatever the electrodes (P < 0.001 for all). No difference in P1N1 amplitude was detected between the five FFA solutions.
P1 latency and N1 latency recorded in Pz, Cz, and Fz electrodes did not differ according to the six solutions.
Table 3 summarizes the mean value (±SD) of VAS results of hedonic values and perceived intensities of the five different FFA solutions and the paraffin emulsion. No difference was observed in hedonic value and perceived intensity of taste between the six solutions.
TABLE 3.
Mean ± SD of the 10 cm VAS results of the hedonic values and perceived intensities of the taste in response to the five FFA and paraffin emulsions, in the 18 participants
| LA | Oleic Acid | Stearic Acid | Lauric Acid | Caproic Acid | Paraffin | |
| Hedonic value | 2.0 ± 2.3 | 2.5 ± 2.2 | 2.3 ± 2.2 | 1.5 ± 2.0 | 2.5 ± 2.1 | 2.0 ± 2.6 |
| Perceived intensity | 2.6 ± 2.4 | 2.5 ± 1.9 | 2.6 ± 2.0 | 3.6 ± 3.3 | 3.0 ± 2.3 | 2.6 ± 2.5 |
The VAS was anchored by “not at all” (noted 0) and “extremely” (noted 10) at its extremities. No significant difference in hedonic values and perceived intensities of the taste was observed between the six solutions.
Comparisons of GEP cerebral recordings between the first and second experiments
Evoked response parameters in response to paraffin emulsions did not differ between the first and second experiment whatever the electrodes (P > 0.05). Similar results were obtained for parameters of GEPs recorded in response to LA solutions (P > 0.05).
DISCUSSION
In this study, we aimed to investigate GEPs in response to FFA solutions applied in the oral cavity. We observed that GEPs in response to FFA were recorded in the Pz, Cz, and Fz electrodes in all subjects. P1N1 amplitude of GEPs recorded in response to FFA was higher than the small evoked response observed after stimulation by paraffin emulsion, whatever the FFA and the electrodes. Moreover, no GEP was obtained in response to water. The characteristics of GEPs in response to LA (P1 and N1 latencies and P1N1 amplitudes) were similar to those obtained after taste stimulation by sucrose and sodium chloride, regardless of the concentrations of the sweet, fatty, and salty solutions.
This is the first study to record GEPs in response to FFA. To our knowledge, only GEPs in response to sweet, salty, bitter, sour, and umami stimuli have been recorded so far (29–31). The method used for taste stimulation and GEP recording was checked in pilot studies (29, 30), which is a strength for this study. The GEP parameters (P1 latency, N1 latency, and P1N1 amplitude) obtained after stimulation with the sucrose solutions in the present observation were similar to those recorded in our previous studies with the same experimental method and similar taste solutions (29, 30).
Besides the few limits inherent to our experimental design that have been presented elsewhere (29, 30), some specific limitations were taken into account in the methods or in the analysis of this study to minimize bias. First, the gustatory thresholds for salty, sweet, and FFA solutions were not determined for each subject before GEP recording, but the concentrations of the solutions used were higher than the usual reported gustatory thresholds (36, 37). Second, P1N1 amplitude of the GEPs was not different according to the concentration of the taste solutions, contrary to what was shown for sweet taste in a previous report (30). However, after complementary analyses of our previous results, it was observed that P1N1 amplitude was greater in response to high sweet concentration than low sweet concentration only for a small subgroup: the subjects who preferred the more concentrated sucrose solution (20 g/100 ml of water). In fact, in the previous study (contrary to the present one), the subjects were selected according to their hedonic sensation induced by sucrose solutions at different intensities, and they were divided into three groups according to their preferred sucrose solution (5, 10, or 20 g per 100 ml of water). This selection criterion could explain the difference between the results of the previous and the current studies. Third, the small evoked response obtained after stimulation by paraffin emulsions could correspond to a P300 event-related potential (38), which could also be a part of GEP recordings in response to FFA stimulation. However, the great significant difference in P1N1 amplitude between the response of FFA and paraffin solutions could not erase our results.
Several main results are underlined in this study. The presence of GEPs after FFA emulsion, whose P1N1 amplitude was higher than the small evoked response after stimulation by paraffin emulsion, brings new arguments to demonstrate that FFA can activate the gustatory cortex. Data from the previous literature, in which neurophysiological investigations were conducted in macaque monkeys and fMRI in humans, were not in agreement with this result, because they observed the same responses of orbito-frontal neurons to oral fatty stimulus (LA and lauric acid) and to stimulation with substances with a similar texture without FAs (silicone or paraffin oils) (5, 6). They also observed that some neurons of the anterior insula and frontal opercular cortex responded to fat by encoding its texture and viscosity and not its taste (7). As there is a topographical overlap between the somatosensory and gustatory cortices in the brain of primates, it may be difficult to distinguish between somesthesic and gustatory responses using fMRI, contrary to evoked potentials. In fact, latencies of somatosensory evoked potentials (39) are shorter than GEP latencies (29, 30). Nonetheless, two other studies, using fMRI, underlined a specific effect of FAs on orbito-frontal and cingulate cortices in the hedonic control of food intake in humans (8, 9). Hence, GEPs recorded in response to FA stimulation reinforce the concept that FA could be the sixth primary taste. In fact, seven basic conditions are required to consider an oro-sensation as a primary taste (40): the presence of an effective stimulus, a chemoreception system, a signaling cascade, a neural activation, a physiological impact, a regulation system, and a specific taste sensation. GEPs recorded in response to FAs in the present study bring some evidence of neural activation of FAs, which was lacking in humans (40).
The characteristics (P1 and N1 latencies and P1N1 amplitudes) of GEPs obtained in response to LA were similar to those obtained after taste stimulation with sucrose and sodium chloride, whatever the concentrations of the sweet, salty, and fatty solutions (first experiment). Several hypotheses could be put forward. First, sweet, salty, and fatty taste, which correspond to palatable food (15), are mediated by the same gustatory pathway globally explored by the GEPs. The same result was observed when comparing human GEPs in response to several sweet (glucose, fructose, and sucrose) solutions (unpublished observations). Second, it has been demonstrated that the same cerebral regions, defined as gustatory cortices and reward-related brain regions, such as the orbito-frontal and cingulate cortices, can be activated by fatty (8, 9) or salty (15, 41, 42) foods, as well as by sweet foods. Moreover, sodium chloride is also known to activate the insular taste cortex, such as sucrose (15, 42). Third, the similarities between GEPs in response to sucrose, LA, and sodium chloride can be explained by some similarities in taste receptor signaling in the oral cavity. It has been suggested that LCFAs are detected via specific receptors on taste buds (26, 27, 43), with the signals transmitted to the brain through taste nerves. In humans, two main channel proteins have been described in taste papillae for the detection of LCFA: CD36 (20) and GPR120 (21). LCFA recognition by the CD36 and GPR120 receptors is mediated by an increase in intracellular Ca2+ concentration via the endoplasmic reticulum (17, 44, 45), such as that observed for the sweet receptor T1R (46). Other channel proteins were discovered in mice with similar activities for detection of FAs (27): GPR40 or Transient Receptor Potential type 5 (TRPM5). Indeed, GPR120 has a seven-transmembrane structure, which is similar to that for sweet receptors (47), and mice knocked out for expression of CD36, GPR120, GPR40, or TRPM5 have a reduced spontaneous preference for fat (48). Likewise, subjects carrying genetic variants of taste receptors have decreased spontaneous fat perception. For example, common variants in the CD36 gene influence fat-ingestive behavior (49) and oral fat perception (50). All these data reinforce the fact that FAs have a specific effect on the gustatory pathway. Otherwise, the identity of the salt receptors remains controversial (51): beyond the well-known salt ionic amiloride-sensitive receptors, it has been demonstrated in mammalians, including humans, that the major mechanism mediating salt taste is amiloride insensitive (52, 53), which is composed of cation-nonselective Na+ receptors. Moreover, previous studies using GEPs did not observe differences in GEP latencies obtained in response to sucrose or sodium chloride [see Ohla et al. (31) for review].
The characteristics (P1 and N1 latencies and P1N1 amplitudes) of GEPs obtained in response to long-, medium-, and short-chain FAs were similar to each other. Hence, long-, medium-, and short-chain FFAs could similarly activate the gustatory pathway. This result is in accordance with previous studies that demonstrated human oral sensitivity to these FFAs using oral detection thresholds (54, 55). This orosensory detection of FFA seemed to be independent to degree of FA saturation (55). The same characteristics of GEPs recorded in the present study could be also explained by the fact that several channel proteins, with similar structures, have been described in taste buds receptors for the detection of short-chain FFAs (GPR41 and 43), medium-chain FFA (GPR40 and 120), and saturated and unsaturated long-chain FFA (GPR40 and 120) (22–24, 56, 57).
This study thus brings a new element of the existence of fat taste representation in the brain, and therefore it could have important implications in feeding behavior. The fatty texture as well as other textural properties can certainly increase the palatability of food, all the more so as fat is often associated with high-energy-density foods (4). But, beyond texture, it is important to understand the representation of fat in the brain, because overeating high-energy-density fatty foods is common in obese humans (58, 59). Moreover, oral hyposensitivity to FAs in animal leads to excess fatty food consumption and weight gain (60). This fatty taste sensation could also have an important role in anticipatory responses (lipase secretion or cholecystokinin release, for instance), leading to improved lipid digestion, absorption, and storage (32, 40). It could also slow gastric emptying and suppress appetite through the release of glucagon-like peptide-1 and peptide Y and the inhibition of ghrelin release (43).
In conclusion, the present study brings new arguments to demonstrate, using GEP recordings, that short-, medium-, and long-chain FFAs are able to activate the gustatory cortex. Like sweet and salty, fat taste appears to activate central feeding and reward-related brain regions, reinforcing the concept that fat taste could be the sixth primary taste. It is important to understand the representation of fat in the brain because of its implication in obesity.
Acknowledgments
The authors thank the electronic society “BEST Electronics” for designing the taste delivery stimulator, Michel Tavan (Centre des Sciences du Goût et de l’Alimentation) for his technical help regarding the taste delivery device, and Philip Bastable for reviewing the English.
Footnotes
Abbreviations:
- EEG
- electroencephalographic
- fMRI
- functional MRI
- GEP
- gustatory evoked potential
- GPR
- G-protein-coupled receptor
- LA
- linoleic acid
- LCFA
- long-chain fatty acid
This work was supported by Centre National de la Recherche Scientifique, Institut National de la Recherche Agronomique, the Dijon Association for Neurosciences, and Conseil Régional de Bourgogne-Franche-Comté (PARI 2 Sensorialité). The authors have no conflict of interests to declare.
REFERENCES
- 1.Cabanac M. 1971. Physiological role of pleasure. Science. 173: 1103–1107. [DOI] [PubMed] [Google Scholar]
- 2.Katz D. B., Nicolelis M. A. L., and Simon S. A.. 2002. Gustatory processing is dynamic and distributed. Curr. Opin. Neurobiol. 12: 448–454. [DOI] [PubMed] [Google Scholar]
- 3.Small D. M., Gregory M. D., Mak Y. E., Gitelman D., Mesulam M. M., and Parrish T.. 2003. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 39: 701–711. [DOI] [PubMed] [Google Scholar]
- 4.Montmayeur J. P., and Le Coutre J.. 2010. Fat Detection: Taste, Texture and Post-Ingestive Effects. Frontiers in Neuroscience. Vol. 609. S. A. Simon and M. L. Nicoletis, series editors. CRC Press, Boca Raton, FL. [PubMed] [Google Scholar]
- 5.Rolls E. T., Critchley H. D., Browning A. S., Hernadi I., and Lenard L.. 1999. Responses to the sensory properties of fat of neurons in the primate orbitofrontal cortex. J. Neurosci. 19: 1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhagen J. V., Rolls E. T., and Kadohisa M.. 2003. Neurons in the primate orbitofrontal cortex respond to fat texture independently of viscosity. J. Neurophysiol. 90: 1514–1525. [DOI] [PubMed] [Google Scholar]
- 7.Verhagen J. V., Kadohisa M., and Rolls E. T.. 2004. The primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature and taste of foods. J. Neurophysiol. 92: 1685–1699. [DOI] [PubMed] [Google Scholar]
- 8.De Araujo I. E., and Rolls E. T.. 2004. The representation in the human brain of food texture and oral fat. J. Neurosci. 24: 3086–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabenhorst F., Rolls E. T., Parris B. A., and D’Souza A. A.. 2010. How the brain represents the reward value of fat in the mouth. Cereb. Cortex. 20: 1082–1091. [DOI] [PubMed] [Google Scholar]
- 10.Stice E., Burger K. S., and Yokum S.. 2013. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 98: 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolls E. T. 2006. Brain mechanisms underlying flavor and appetite. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361: 1123–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rolls E. T. 2007. Sensory processing in the brain related to the control of food intake. Proc. Nutr. Soc. 66: 96–112. [DOI] [PubMed] [Google Scholar]
- 13.Rolls E. T. 2011. The neural representation of oral texture including fat texture. J. Texture Stud. 42: 137–156. [Google Scholar]
- 14.Rolls E. T. 2012. Taste, olfactory and food texture reward processing in the brain and the control of appetite. Proc. Nutr. Soc. 71: 488–501. [DOI] [PubMed] [Google Scholar]
- 15.Rolls E. T. 2015. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 127–128: 64–90. [DOI] [PubMed] [Google Scholar]
- 16.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., and Besnard P.. 2005. CD36 involvement in orosensory detection of dietary lipids: impact of spontaneous fat preference and digestive secretions. J. Clin. Invest. 115: 3177–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaillard D., Laugerette F., Darcel N., El Yassimi A., Passily-Degrace P., Hichami A., Khan N., Montmayeur P., and Besnard P.. 2008. The gustatory pathway is involved in CD36-mediated oro-sensory perception of long-chain fatty acids in the mouse. FASEB J. 22: 1458–1468. [DOI] [PubMed] [Google Scholar]
- 18.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., Le Coutre J., Ninomiya Y., and Damak S.. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30: 8376–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin C., Passilly-Degrace P., Gaillard D., Merlin J. F., Chevrot M., and Besnard P.. 2011. The lipid-sensors candidates CD36 and GPR120 are differentially regulated by dietary lipids in the mouse taste buds: impact on spontaneous fat preference. PLoS One. 6: e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons P. J., Kummer J. A., Luiken J. J., and Boon L.. 2011. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113: 839–843. [DOI] [PubMed] [Google Scholar]
- 21.Galindo M. M., Voigt N., Stein J., Van Lengerich J., Raguse J. D., Hofmann T., Meyerhof W., and Behrens M.. 2012. G protein-coupled receptors in human fat taste perception. Chem. Senses. 37: 123–139. [DOI] [PubMed] [Google Scholar]
- 22.Brown A. J., Goldsworthy S. M., Barnes A. A., Eilert M. M., Tcheang L., Daniels D., Muir A. I., Wigglesworth M. J., Kinghorn I., Fraser N. J., et al. . 2003. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278: 11312–11319. [DOI] [PubMed] [Google Scholar]
- 23.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., and Tsujimoto G.. 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11: 90–94. [DOI] [PubMed] [Google Scholar]
- 24.Itoh Y., Kawamata Y., Harada M., Kobayashi M., Fujii R., Fukusumi S., Ogl K., Hosoya M., Tanaka Y., Uejima H., et al. . 2003. Free fatty acids regulate insulin secretion from pancreatic cells through GPR40. Nature. 422: 173–176. [DOI] [PubMed] [Google Scholar]
- 25.Mattes R. D. 2011. Accumulating evidence supports a taste component for free fatty acids in humans. Physiol. Behav. 104: 624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart J. E., Feinle-Bisset C., and Keast R. S. J.. 2011. Fatty acid detection during food consumption and digestion: associations with ingestive behavior and obesity. Prog. Lipid Res. 50: 225–233. [DOI] [PubMed] [Google Scholar]
- 27.Fushiki T. 2014. Why fat is so preferable: from oral fat detection to inducing reward in the brain. Biosci. Biotechnol. Biochem. 78: 363–369. [DOI] [PubMed] [Google Scholar]
- 28.DiPatrizio N. V. 2014. Is fat taste ready for primetime? Physiol. Behav. 136: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacquin-Piques A., Gaudillat S., Mouillot T., Gigot V., Meillon S., Leloup C., Pénicaud L., and Brondel L.. 2016. Prandial states modify the reactivity of the gustatory cortex using gustatory evoked potentials in Humans. Front. Neurosci. 9: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacquin-Piques A., Mouillot T., Gigot V., Meillon S., Leloup C., Pénicaud L., and Brondel L.. 2016. Preference for sucrose solutions modulates taste cortical activity in humans. Chem. Senses. 41: 591–599. [DOI] [PubMed] [Google Scholar]
- 31.Ohla K., Busch N. A., and Lundström J. N.. 2012. Time for taste—a review of the early cerebral processing of gustatory perception. Chemosens. Percept. 5: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chevrot M., Passilly-Degrace P., Ancel D., Bernard A., Enderli G., Gomes M., Robin I., Issanchou S., Vergès B., Nicklaus S., et al. . 2014. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am. J. Clin. Nutr. 99: 975–983. [DOI] [PubMed] [Google Scholar]
- 33.Baillie A. G., Coburn C. T., and Abumrad N. A.. 1996. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J. Membr. Biol. 153: 75–81. [DOI] [PubMed] [Google Scholar]
- 34.Vennemann M. M., Hummel T., and Berger K.. 2008. The association between smoking and smell and taste impairment in the general population. J. Neurol. 255: 1121–1126. [DOI] [PubMed] [Google Scholar]
- 35.Ramirez I. 1993. Role of olfaction in starch and oil preference. Am. J. Physiol. 265: R1404–R1409. [DOI] [PubMed] [Google Scholar]
- 36.Yamauchi Y., Endo S., and Yoshimura I.. 2002. A new whole-mouth gustatory test procedure. II. Effects of aging, gender and smoking. Acta Otolaryngol. Suppl. 546: 49–59. [DOI] [PubMed] [Google Scholar]
- 37.Running C. A., and Mattes R. D.. 2015. Humans are more sensitive to the taste of linoleic and α-linolenic than oleic acid. Am. J. Physiol. Gastrointest. Liver Physiol. 308: G442–G449. [DOI] [PubMed] [Google Scholar]
- 38.Hansenne M. 2000. The P300 event-related potential. I. Theoretical and psychobiological perspectives. [Article in French]. Neurophysiol. Clin. 30: 191–210. [DOI] [PubMed] [Google Scholar]
- 39.Desmedt J. E., and Bourguet M.. 1985. Color imaging of parietal and frontal somatosensory potential fields evoked by stimulation of median or posterior tibial nerve in man. Electroencephalogr. Clin. Neurophysiol. 62: 1–17. [DOI] [PubMed] [Google Scholar]
- 40.Besnard P. 2016. Lipids and obesity: also a matter of taste? Rev. Endocr. Metab. Disord. 17: 159–170. [DOI] [PubMed] [Google Scholar]
- 41.O’Doherty J., Rolls E. T., Francis S., Bowtell R., and McGlone F.. 2001. Representation of pleasant and aversive taste in the human brain. J. Neurophysiol. 85: 1315–1321. [DOI] [PubMed] [Google Scholar]
- 42.Kadohisa M., Rolls E. T., and Verhagen J. V.. 2005. Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex and amygdala. Chem. Senses. 30: 401–419. [DOI] [PubMed] [Google Scholar]
- 43.Liu D., Archer N., Duesing K., Hannan G., and Keast R.. 2016. Mechanism of fat taste perception: association with diet and obesity. Prog. Lipid Res. 63: 41–49. [DOI] [PubMed] [Google Scholar]
- 44.El-Yassimi A., Hichami A., Besnard P., and Khan N. A.. 2008. Linoleic acid induces calcium signaling, Src-kinase phosphorylation B and neurotransmitters release in mouse CD-36-positive gustatory cells. J. Biol. Chem. 283: 12949–12959. [DOI] [PubMed] [Google Scholar]
- 45.Abdoul-Azize S., Selvakumar S., Sadou H., Besnard P., and Khan N. A.. 2014. Ca2+ signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie. 96: 8–13. [DOI] [PubMed] [Google Scholar]
- 46.Chandrashekar J., Hoon M. A., Ryba N. J., and Zuker C. S.. 2006. The receptors and cells for mammalian taste. Nature. 444: 288–294. [DOI] [PubMed] [Google Scholar]
- 47.Matsumura S., Mizushige T., Yoneda T., Iwanaga T., Tsuzuki S., Inoue K., and Fushiki T.. 2007. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed. Res. 28: 49–55. [DOI] [PubMed] [Google Scholar]
- 48.Gilbertson T. A., and Khan N. A.. 2014. Cell signaling mechanisms of oro-gustatory detection of dietary fat: advances and challenges. Prog. Lipid Res. 53: 82–92. [DOI] [PubMed] [Google Scholar]
- 49.Keller K. L., Liang L. C., Sakimura J., May D., van Belle C., Breen C., Driggin E., Tepper B. J., Lanzano P. C., Deng L., et al. . 2012. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring). 20: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ong H. H., Tan Y. N., and Say Y. H.. 2017. Fatty acid translocase gene CD36 rs1527483 variant influences oral fat perception in Malaysian subjects. Physiol. Behav. 168: 128–137. [DOI] [PubMed] [Google Scholar]
- 51.Lyall V., Leck G. L., Vinnikova A. K., Ghosh S., Phan T. H., Alam R. I., Russel O. F., Malik S. A., Bigbee J. W., and DeSimone J. A.. 2004. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 558: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feldman G. M., Mogyorosi A., Heck G. L., DeSimone J. A., Santos C. R., Clary R. A., and Lyall V.. 2003. Salt-evoked lingual surface potential in humans. J. Neurophysiol. 90: 2060–2064. [DOI] [PubMed] [Google Scholar]
- 53.Bachmanov A. A., Bosak N. P., Lin C., Matsumoto I., Ohmoto M., Reed D. R., and Nelson T. M.. 2014. Genetics of tastes receptors. Curr. Pharm. Des. 20: 2669–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattes R. D. 2009. Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem. Senses. 34: 145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalé-Rush A., Burgess J. R., and Mattes R. D.. 2007. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem. Senses. 32: 423–431. [DOI] [PubMed] [Google Scholar]
- 56.Le Poul E., Loison C., Struyf S., Springael J. Y., Lannoy V., Decobecq M. E., Brezillon S., Dupriez V., Vassart G., Van Damme J., et al. . 2003. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278: 25481–25489. [DOI] [PubMed] [Google Scholar]
- 57.Xiong Y., Miyamoto N., Shibata K., Valasek M. A., Motoike T., Kedzierski R. M., and Yanagisawa M.. 2004. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA. 101: 1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCrory M. A., Fuss P. J., McCallum J. E., Yao M., Vinken A. G., Hays N. P., and Robert S. B.. 1999. Dietary variety within food groups: association with energy intake and body fatness in men and women. Am. J. Clin. Nutr. 69: 440–447. [DOI] [PubMed] [Google Scholar]
- 59.Rolls E. T. 2000. The orbitofrontal cortex and reward. Cereb. Cortex. 10: 284–294. [DOI] [PubMed] [Google Scholar]
- 60.Gilbertson T. A., Liu L., York D. A., and Bray G. A.. 1998. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann. N. Y. Acad. Sci. 855: 165–168. [DOI] [PubMed] [Google Scholar]