Abstract
Incentives are primary determinants of if and how well an organism will perform a given behavior. Here, we examined how incentive valence and magnitude influence task switching, a critical cognitive control process, and test the predictions that the anterior cingulate cortex (ACC) and the ventral striatum (vStr) function as key nodes linking motivation and control systems in the brain. Our results indicate that reward and punishment incentives have both common and distinct effects on cognitive control at the behavioral and neurobiological levels. For example, reward incentives led to greater activity in the ACC during the engagement of control relative to punishments. Furthermore, the neural responses to reward and punishment differed as a function of individual sensitivity to each incentive valence. Functional connectivity analyses suggest a role for vStr in signaling motivational value during cognitive control and as a potential link between motivation and control networks. Overall, our findings suggest that similar changes in observed behavior (e.g. response accuracy) under reward and punishment incentives are mediated by, at least partially, distinct neurobiological substrates.
Keywords: anterior cingulate cortex, ventral striatum, cognitive control, punishment, reward
Introduction
Motivation and cognitive control are two critical aspects of behavior and brain function. Goal-directed behavior requires cognitive control in the forms of selective or sustained attention, inhibition of inappropriate reactions, task switching and learning about contextual changes. The effects of cognitive control and motivational influences on behavior may be closely interrelated: cognitive control processes are often necessary to resolve conflict between competing motivations however, motivation and incentives (e.g. rewards or punishments) can also facilitate specific cognitive functions (Della Libera and Chelazzi, 2006; Engelmann and Pessoa, 2007; Braem et al., 2013; Frober and Dreisbach, 2014; Umemoto and Holroyd, 2015). Consequently, incentives are ubiquitously used by principles (e.g. governments, teachers or parents) to shape agents’ (e.g. citizens, students and children) behaviors into socially acceptable patterns. Here, we used a rule switching task to examine how functional connectivity between motivational and control networks might modulate cognitive control processes. Reward contingencies can enhance both performance and local activation in task-relevant regions during different cognitive tasks (Pochon et al., 2002; Gilbert and Fiez, 2004; Taylor et al., 2004; Small et al., 2005; Taylor et al., 2006; Krawczyk et al.,2007; Locke and Braver, 2008; Engelmann et al., 2009; Jimura et al., 2010; Padmala and Pessoa, 2011; Krawczyk and D’Esposito, 2013; Xue et al., 2013; Paschke et al., 2015; Soutschek et al., 2015). Specifically, rewards improved information encoding and performance during various forms of task switching, and these effects have been associated with activity changes in prefrontal cortex (PFC) and parietal cortex (Kouneiher et al., 2009; Economides et al., 2014; Rudorf and Hare, 2014; Etzel et al., 2016). Furthermore, there is evidence for differential effects of reward and punishment on regions such as the orbitofrontal cortex (OFC), the anterior cingulate cortex (ACC), insula or amygdala (Small et al., 2005; Taylor et al., 2006; Wachter et al., 2009; Murty et al., 2012; Krawczyk and D’Esposito, 2013).
Although limited by the fact that separate reward and punishment incentives have only rarely been implemented in the same task and individuals, the available evidence suggests potentially different mechanisms of action for these two incentives. Reward incentives increased brain activity in task-relevant regions during highly demanding attention trials, while punishment did so across all trial types (Paschke et al., 2015). Furthermore, many studies have reported that humans have a stronger aversion losses compared to the preference for equivalent gains (Kahneman and Tversky, 1979) and others have demonstrated a negativity bias (Baumeister et al., 2001). Although such effects are not universal (Erev et al., 2008; Yechiam and Hochman, 2013; Yechiam and Hochman, 2014; Clay et al., 2017), they do suggest that rewarding gains may differ from punishing losses in motivating behavior. Overall, there is substantial evidence that different mechanisms may mediate the impact of reward and punishment on cognitive control networks, but it remains unknown to what extent, and in which precise contexts, their impact differs.
The impact of rewards and punishments may also differ due to individual differences in the sensitivity to either incentive in addition to valence-specific effects on cognitive and neural mechanisms. Individual differences in sensitivity to reward and punishment have been reported to modulate cognitive control (Gray and Braver, 2002; Engelmann et al., 2009; Avila et al., 2012; Fuentes-Claramonte et al., 2015; Bunford et al., 2017) or conflict adaptation (Braem et al., 2013). They have also been reported to mediate the impact of incentives on brain activation and task performance (Locke and Braver, 2008; Jimura et al., 2010). Therefore, we investigated how individual differences in sensitivity to reward and punishment as measured by the BIS/BAS scale (Carver and White, 1994) might modulate the impact of both incentives on cognitive control neural mechanisms.
We conducted whole-brain analyses of cognitive control-related activity, but especially sought to test the hypothesized roles of the ACC and ventral striatum (vStr) in linking motivation and control processes. The vStr has been shown to be crucial for signaling incentive salience and motivation (Knutson et al., 2001; Delgado, 2007; Izuma et al., 2008; Bartra et al., 2013). As part of the key cortico-basal ganglia reward circuitry (Haber and Knutson, 2010), it has a key role in integrating motivation and control. Therefore, we investigated the role of vStr and its functional connectivity in this integration during a response rule switching task, and how these contributions might be modified by incentive valence and magnitude.
The ACC is strongly interconnected both with limbic regions signaling incentive motivation as well as PFC and parietal regions crucial for cognitive control (Beckmann et al., 2009). Proposed as a key functional link between motivational and cognitive control brain networks (Pessoa and Engelmann, 2010; Shackman et al., 2011), recent theoretical accounts have attempted to explain its seemingly varied computations in relation to the evaluation of engaging control in order to alter current or default actions in favor of better or more appropriate alternatives (Rushworth et al., 2011; Holroyd and Yeung, 2012; Shenhav et al., 2013; Botvinick and Braver, 2015; Kolling et al., 2016). Therefore, improvements in cognitive control performance under increased incentive motivation could be associated with increased activity within the ACC and/or its functional connectivity with limbic regions and/or other prefrontal and parietal regions that support cognitive control abilities.
The goal of the current study was to further understand the neural mechanisms by which reward and punishment motivations improve cognitive control. We used a visually cued stimulus–response switching task together with low and high levels of incentives and functional magnetic resonance imaging (fMRI) to identify shared and differential effects of reward and punishment at the neural level. We found that high levels of incentive improved performance regardless of valence or trial type. However, activity within the fronto-parietal network and its functional connectivity with the vStr differed during the engagement of cognitive control under rewards versus punishments.
Materials and methods
Participants
Thirty right-handed healthy adults provided informed consent to participate in the study (mean age, 24 years; s.d., 2; 15 females). None of them reported any present or past psychiatric diagnosis. The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich). Participants received monetary compensation for their participation in the study (details below).
Incentivized switch task
This task uses a mixed blocked/event-related design to investigate the shared and unique neural substrates of reward and punishment incentive motivation during cognitive control processing (Fig. 1). On each trial, participants are presented with a picture of a house and a cue icon, located above the stimulus, which indicated the relevant aspect of the stimuli on that trial (see timing details in Fig. 1). Between stimuli, the color of a fixation cross (random duration: 500–1000 ms) indicates the incentive type for the current block. Each of the 3 separate runs consisted of 84 trials, presented in 6 pseudorandomized blocks [2 neutral blocks, 2 reward blocks (1 high, 1 low) and 2 punishment blocks (1 high, 1 low)] of 14 trials each, with 20–25 switch trials/run. A switch trial (change to a new cue that guides responses) is randomly presented after 2–5 repeat trials (in which the same cue guides responses). A 10 s yellow cross between blocks, followed by a 2-s green/red/white cross indicates that the block to come is a reward, punishment or neutral, respectively. In addition, all of the pictures in the block are shown within a colored frame, thereby providing constant indication of the current incentive condition. Reward and punishment magnitudes (low and high) are signaled by the size of the fixation cross and thickness of the picture frame.
Fig. 1.
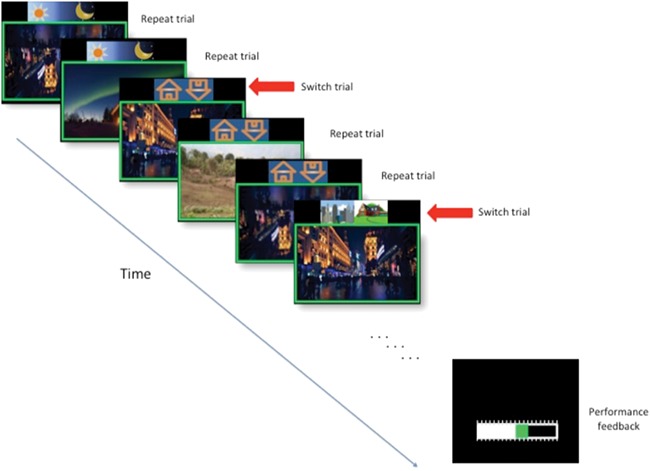
Schematic illustration of the incentive switch task. The figure shows trials during a low-reward incentive block as indicated by the thin green frame. Cues to respond according to day/night, upright/inverted or city/rural scene classifications were shown 250 ms before the pictures appeared and remained onscreen during the entire 1200 ms duration of stimulus presentation. Switches between response rules (i.e. switch trials) occurred after 2–5 repeat trials.
Before entering the scanner, participants were given instructions, shown examples of the cues and frames for each condition and performed a short practice version of the task. They were informed that they had been endowed with 10 points and that they could earn/lose points depending on their performance in the different blocks. The exact contingencies were not told to participants to avoid detailed value calculations (details in supplementary text). After each block, a horizontal bar showed the points they had before the block and how much they gained/lost in the latest block for 2 s. Following the scanning session, point totals were averaged across the three runs and exchanged for 0.5 Swiss Francs each.
Behavioral analyses
For one participant data from only two of the three runs were included, due to a technical failure during the process of saving the performance data.
Statistical analyses were conducted using the statistical software package SPSS v24. To investigate the effects of incentive (valence and magnitude, separately), trial type and any potential interaction effect we used the composite inverse efficiency scores [IES: mean reaction time (RT) in milliseconds/proportion of correct responses], which incorporates accuracy and RT in each trial category and allows us to investigate the potential presence of speed accuracy trade-offs. We conducted two 3 × 2 repeated measures analyses of variance, one with incentive valence (reward, neutral and punishment) and trial type (repeat and switch) as within-subject factors and a second with incentive magnitude (high, low and absent) and trial type as within-subject factors.
fMRI scanning parameters
Images were acquired using a Philips Achieva 3T whole-body scanner with an eight-channel sensitivity-encoding head coil at the Laboratory for Social and Neural Systems Research, University Hospital Zurich. Each of three functional runs comprised 125 volumes (37 slices per volume; field of view, 200 × 200 × 133 mm; slice thickness, 3 mm; gap, 0.6 mm; in-plane resolution, 2.5 * 2.5 mm; matrix, 80 * 78; repetition time, 2344 ms, echo time 30 ms; flip angle, 77 degrees) in ascending order in addition to five `dummy’ volumes at the start of each run. Magnetic field B0/B1 maps were collected (short echo time, 4.29; long echo time, 7.4 ms). A T1-weighted turbo field echo structural image was acquired for each participant (181 slices; field of view, 256 × 256 × 181 mm; slice thickness, 1 mm; no gap; in-plane resolution, 1 * 1 mm; matrix, 256 * 256; repetition time, 8.3 ms; echo time, 3.9 ms; flip angle, 8 degrees; see extended description in Supplementary data).
fMRI preprocessing
Image analysis was performed using SPM12 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK). Functional images were realigned and unwarped, segmented according to the corresponding T1-weighted structural images, normalized to the mean subject’s EPI template and smoothed using a 6 mm Full Width Half Maximum (FWHM) kernel. To account for physiological noise we used the PhysIO-toolbox implementation (http://www.translationalneuromodeling.org/tapas/) of RETROICOR.
fMRI general linear models
We computed general linear models (GLMs) at the single-subject level with SPM12, and results were examined at the group level using the randomize function from the FMRIB Software Library (http://fsl.fmrib.ox.ac.uk/fsl/) to implement one-sample t-test non-parametric permutations (n = 5000 permutations, with threshold-free cluster enhancement, TFCE). All the reported results are family-wise error (FWE) corrected at the voxel level and coordinates are given in Montreal Neurological Institute (MNI) space.
To examine the interaction between incentives and cognitive control both on transient and sustained BOLD activity, we used two different GLM models. We computed GLM-1, modeling switch and repeat events separately for each incentive condition [reward high (RH), reward low (RL), neutral (NEUT), punishment low (PL), punishment high (PH)]. The duration of all trial event regressors was set to be equal to the RT on that trial. We added two additional regressors to model the anticipatory cross before the start of each block, and the feedback screen presented at its end. In both cases, we included incentive valence and magnitudes as parametric modulators, (from +2 to −2) to identify RH, RL, NEUT, PL and PH blocks, respectively. These parametric regressors were included as both linear (to test for regions that showed a valence-dependent response to punishments and rewards) and quadratic (tests for regions that responded to incentive magnitude, but not valence) terms. The linear and quadratic parametric modulators on the anticipatory cross and feedback events were not orthogonalized and allowed to directly compete in explaining the variation in BOLD signals. Furthermore, incorrect and missed trials were also included as variables of no interest.
Following estimation of GLM-1 for each subject, we computed several contrasts of interest: (i) the parametric effects of the anticipatory cross (linear and quadratic), (ii) the main effect of cognitive control (switch > repeat across all trial types), (iii) the main effect of incentive magnitude [(switch >repeat trials during high incentives) > (switch > repeat trials during low incentives)], (iv) the main effect of incentive valence [(switch > repeat during all reward trials) > (switch > repeat during all punishment trials)] and (v) incentive-by-magnitude interactions (switch > repeat during RH vs switch > repeat during PH).
To try and account for individual variation in cognitive control and incentive sensitivity, all group-level contrasts included two covariates. One was the corresponding differences in individual IES scores as a covariate for each participant. Direct tests of the covariates did not reveal any regions in which BOLD activity differed as a function of these scores. The second was a dummy covariate that was equal to 1 for the first 5 (pilot) participants and 0 for the next 25 participants (further details in supplementary text).
In addition, we also examined how individual differences in reward or punishment sensitivity related to BOLD activity during cognitive control. We used individuals’ scores from the standardized BIS/BAS questionnaires (Carver and White, 1994) to perform the following group-level regressions: (i) BAS-drive scores (a measure of propensity to be motivated by rewards to pursue goals) against the increase in BOLD for switch > repeat trials in RH > RL blocks and (ii) BIS scores against the increase in BOLD for switch > repeat trials in PH > PL blocks. These regression analyses did not include covariates for performance.
In order to investigate the sustained effects of incentive condition on cognitive control, we created a second model (GLM-2) using a boxcar function to model the different blocks separately according to their incentive types (i.e. the combination of valence and magnitude: RH, RL, NEUT, PL, PH). As in GLM-1, we included the combination of incentive valence and magnitudes as a parametric modulator, (from +2 to −2) to identify RH, RL, NEUT, PL and PH blocks, respectively. These parametric regressors were included as both linear (to test for regions that showed a valence-dependent response to punishments and rewards) and quadratic (tests for regions that responded to incentive magnitude, but not valence) terms. The linear and quadratic parametric modulators on the anticipatory cross and feedback events were not orthogonalized and allowed to directly compete in explaining the variation in BOLD signals.
Following estimation of GLM-2 for each subject, we computed the following contrasts: (i) the main effect of incentive magnitude (high > low incentive blocks), (ii) the main effect of incentive valence (reward > punishment incentive blocks) and (iii) incentive-by-magnitude interactions (RH > PH).
For all BOLD GLMs, motion parameters and the physiological regressors from RETROICOR were included as regressors of no interest. Regressors were convolved with the canonical hemodynamic response function implemented in SPM12, high passed filtered (128 s) and modeled using AR(1) autoregression.
As before, all group-level contrasts included the corresponding differences in individual IES scores as a covariate for each participant.
Psychophysiological interaction modeling
In order to investigate the roles of the hypothesized key regions mediating the impact of incentives on cognitive control, we computed three separate psychophysiological interaction analyses. We computed the psychophysiological interaction between ACC activity and incentive type as follows. We created a functional ACC mask, defined by the significant voxels observed on the dorsal ACC during the second order parametric regressor of incentive magnitude at the time of the anticipatory cue for each upcoming block (independent of their magnitude and valence). Next, we extracted an ACC time series for each individual participant, averaged across all voxels within a 5 mm sphere centered on the participants’ peak response for the second-order parametric regressor of incentive magnitude at the time of the anticipatory block cue within the functionally defined ACC mask. The time series was then deconvolved (Gitelman et al., 2003) and used to create five psychophysiological interaction regressors. The five interaction terms were ACC * RH blocks, ACC * RL blocks, ACC * NEUT blocks, ACC * PL blocks and ACC * PH blocks. For the psychophysiological interaction (PPI) model, blocks were defined as beginning when the fixation cross cue that signified the upcoming block type disappeared until the start of the feedback for that block. Next, the regressors corresponding to the five interaction terms as well as the seed region time series and convolved psychological response functions for each block type (i.e. the main effects) were entered into a GLM. Motion parameters and the physiological regressors output from RETROICOR were also included in the PPI GLMs as regressors of no interest. The same procedure was followed to delineate the functional mask of the second area of interest, the vStr, as well as to define the psychophysiological terms and the GLM model. Finally, the procedure was repeated with a third cluster defined by the significant voxels observed on the dorsal ACC during the first-order parametric regressor of incentive magnitude at the time of the anticipatory cue for each upcoming block.
Following estimation of the PPI GLMs, we computed contrasts for (i) the main effect of incentive magnitude (high > low incentives PPIs), (ii) the main effect of incentive valence (reward > punishment incentives PPIs) and (iii) incentive-by-magnitude interactions (RH > PH PPIs). The outcomes of these contrasts were entered into one-sample t-tests conducted using the randomize permutation function with TFCE in FSL at the group level for statistical inference. All group-level PPI contrasts included the difference between each of the IES conditions in the relevant contrast as a covariate and a dummy covariate for the first five participants.
Results
Behavioral results
Both high levels of reward and punishment lead to better performance in the task-switching paradigm (Table 1, Fig. 2; see Supplementary data for further analyses of switch costs, accuracy and RTs). Consistent with the existing literature on switch costs, we found an effect of trial type [F(1,29) = 55.79, P < 0.001, 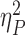 = 0.66], but no significant effect of incentive valence or interaction with trial type. Thus, on average, performance under reward, punishment and neutral conditions did not differ.
= 0.66], but no significant effect of incentive valence or interaction with trial type. Thus, on average, performance under reward, punishment and neutral conditions did not differ.
Table 1.
Main performance measures on the incentivized switch task
| Performance variable | Reward high | Reward low | Neutral | Punishment low | Punishment high | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Repeat | Switch | Repeat | Switch | Repeat | Switch | Repeat | Switch | Repeat | Switch | |
| Percent correct | ||||||||||
| Mean | 94 | 91 | 91 | 86 | 93 | 88 | 91 | 90 | 94 | 91 |
| (s.d.) | (4) | (11) | (6) | (13) | (6) | (8) | (6) | (14) | (6) | (11) |
| Response times | ||||||||||
| Mean | 649 | 703 | 661 | 730 | 673 | 736 | 668 | 723 | 667 | 731 |
| (s.d.) | (75) | (92) | (70) | (80) | (72) | (74) | (63) | (75) | (72) | (92) |
| Inverse efficiency | 690 | 788 | 728 | 872 | 730 | 846 | 736 | 845 | 712 | 821 |
| scores | (95) | (176) | (103) | (213) | (114) | (135) | (96) | (289) | (98) | (175) |
Response times were computed over correct trials only and are listed in milliseconds.
Inverse efficiency scores are calculated as response time in milliseconds divided by the proportion of correct responses.
Fig. 2.
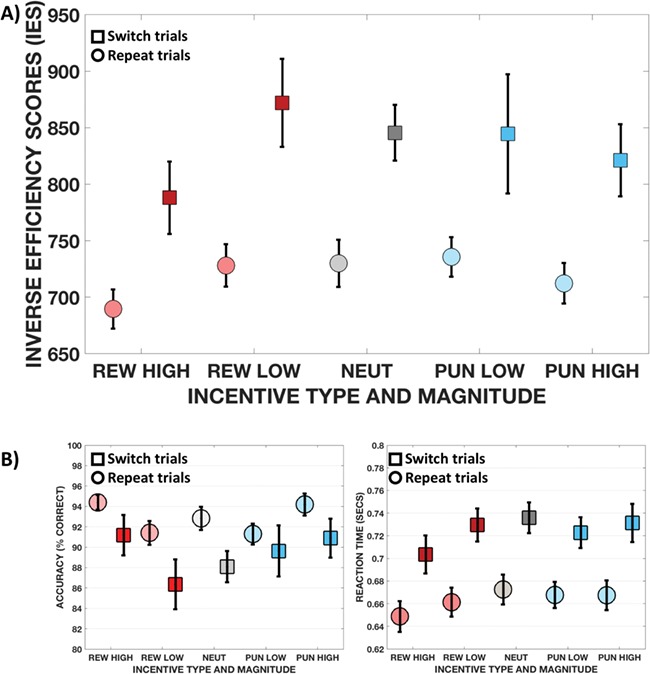
Bar charts showing performance. The upper panel shows the performance in the main outcome variable of the task, the IES for repeat and switch trials in the different incentive conditions. The lower panels show the performance in the two component measures of the IES: accuracy and RT. Circles depict repeat trials, squares indicate switch trials.
However, larger incentives resulted in better task performance regardless of valence, with a significant effect of both incentive magnitude [Fig. 2a; F(2, 28) = 12.817, P < 0.001, 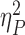 = 0.48] and trial type [F(1,29) = 60.133, P < 0.001,
= 0.48] and trial type [F(1,29) = 60.133, P < 0.001, 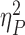 = 0.675], without significant interaction between them. Direct comparisons of the IES (collapsed across repeat and switch trials) in the RH and PH conditions revealed slightly better performance in RH than PH trials [t(29) = −2.27, P = 0.031]. When breaking this result down to see if differences in IES are the result of changes in accuracy or RT, we found that accuracy did not significantly differ [t(29) = 0.29, P = 0.77], but responses were faster in RH than PH trials [t(29) = −3.21, P = 0.003; Fig. 2b, Table 1].
= 0.675], without significant interaction between them. Direct comparisons of the IES (collapsed across repeat and switch trials) in the RH and PH conditions revealed slightly better performance in RH than PH trials [t(29) = −2.27, P = 0.031]. When breaking this result down to see if differences in IES are the result of changes in accuracy or RT, we found that accuracy did not significantly differ [t(29) = 0.29, P = 0.77], but responses were faster in RH than PH trials [t(29) = −3.21, P = 0.003; Fig. 2b, Table 1].
Lastly, we tested the association between changes in behavioral performance under the various incentives and individual differences in reward or punishment sensitivity, measured using the standardized BIS/BAS questionnaires (Carver and White, 1994). None of the correlations were significant.
These results show that the incentives used in this paradigm were effective in improving performance. Interestingly, under high levels of reward or punishment incentives, improvements were comparable for both the relatively simple repeat trials and the more cognitively demanding switch trials.
fMRI results
Cognitive control and incentive effects during task performance
We first contrasted switch and repeat trials to examine the effects of trial type across all incentives. Consistent with previous studies using task switching paradigms, the increased engagement of cognitive control was associated with greater activity in brain networks typically associated with cognitive control, including regions of medial and lateral prefrontal as well as parietal cortex (Fig. 3, red voxels; Table 2).
Fig. 3.
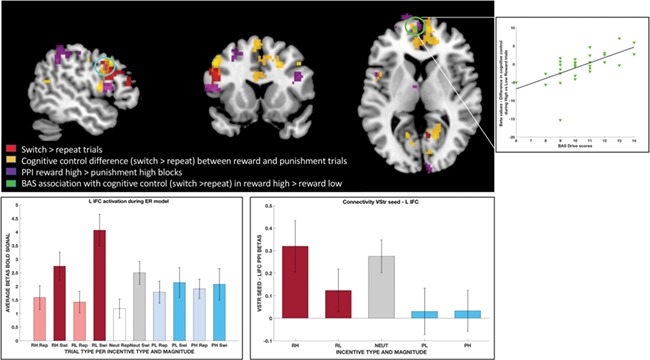
Overlap of results in the different analysis. Results from the different contrasts are shown in separate colors: (i) red voxels show increased activation for switch trials compared to repeat trials, (ii) yellow voxels show areas of increased activation during cognitive control under reward incentives relative to punishments, (iii) violet voxels show increased connectivity with the ventral striatum seed in the functional connectivity analyses and (iv) green voxels depict the increased activation during cognitive control in the context of increasing rewards that is associated with differences in reward sensitivity (BAS scores). The scatterplot on the upper right shows the beta coefficients for the contrast testing differences in cognitive control in the context of increasing rewards against differences in reward sensitivity (BAS scores). The bar graphs show beta coefficients in the left inferior prefrontal voxels circled in blue where contrasts (i), (ii) and (iii) overlap: (a) left: for each trial depending on the type, incentive valence and magnitude, in the GLM-1 model, allowing to see activation during the contrast of switch vs repeat trials and during the reward vs punishment trials during cognitive control; and (b) right: for each incentive block type, in the PPI connectivity analysis with the vStr.
Table 2.
Regions more active on switch versus repeat trials (i.e. effects of cognitive control)
| Region | Cluster size | x; y; z | TFCE T-stat |
|---|---|---|---|
| Precuneus cortex | 1267 | 17; −63; 32 | 5.36 |
| Cuneal cortex | |||
| Superior parietal lobule | |||
| Supramarginal gyrus, posterior division | |||
| Postcentral gyrus | |||
| Lateral occipital gortex, superior division | |||
| Inferior frontal gyrus, pars opercularis | 193 | −50; 11; 29 | 5.29 |
| Precentral gyrus | |||
| Middle frontal gyrus | |||
| Inferior frontal gyrus, pars triangularis | |||
| Frontal pole | |||
| Lingual gyrus | 5 | −17; −58; −14 | 5.04 |
| Temporal occipital fusiform cortex |
All reported regions are significant at P < 0.05 after whole-brain FWE correction at the voxel level and a cluster extent of 5 voxels. The FWE correction was based on 5000 permutations of the threshold free cluster enhancement (TFCE) values. The TFCE values and permutation-derived test statistics were calculated using the randomize function implemented in FSL. All coordinates are listed in MNI space and represent the peaks of all clusters formed by contiguous voxels as well as peaks >20 mm apart within the same cluster.
In order to investigate the influences of incentive valence during the deployment of cognitive control, we compared BOLD activity related to cognitive control (i.e. the switch > repeat contrast) under reward versus punishment incentives across magnitude levels using GLM-1 (see also Supplementary data and Supplementary Figure S1 for an additional specification of this model that included response times as a parametric modulator on all trials). This comparison revealed increased activation during reward compared to punishment in dorsal ACC, left inferior frontal cortex (IFC) and bilateral dorsolateral PFC (dlPFC), together with several other regions (Fig. 3, yellow voxels; Supplementary Table S2). No region was more active under punishment compared to reward after correcting for multiple comparisons. Also, there were no significant differences between reward and punishment in terms of sustained activity in our block-level model (GLM-2).
In order to investigate the influences of incentive magnitude during the deployment of cognitive control, we compared BOLD activity related to cognitive control (i.e. the switch > repeat contrast) under high > low incentives levels, regardless of the incentive valence (i.e. collapsing across reward and punishment). Consistent with the lack of magnitude-by-trial type interaction in the behavioral data, no brain region showed differential activity for this contrast.
Although our a priori hypothesis was that incentives would primarily influence performance on the more difficult switch trials, which required increased cognitive control, we found that high incentives improved performance independent of trial type. Therefore, we also tested for a main effect of incentive magnitude on brain activity. We found decreases in BOLD activity in the amygdalae, occipital and temporal cortex, as well as ventral prefrontal brain regions on high relative to low incentive trials [Supplementary Table S3 (results GLM-1) and Supplementary Table 4 (GLM-2)].
Lastly, although we found that performance was slightly better on trials with RH relative to PH incentives, there were no significant differences between them in the corresponding contrasts for BOLD activity during cognitive control [(high reward switch trials > high reward repeat trials) > (high punishment switch trials > high punishment repeat trials)] or when testing the mean effect across trial types.
Individual differences in the neural responses to reward and punishment incentives
We also investigated how individual differences in sensitivity to reward and punishment might modulate changes in local BOLD activity during cognitive control. We found that participants with higher BAS-drive showed increased activation in the rostromedial PFC during successful deployment of cognitive control as the level of reward incentive increased from low to high (Fig. 3, green voxels; Supplementary Table S5). On the other hand, increases in punishment sensitivity (BIS scores) led to decreased activity in left middle frontal cortex during successful deployment of cognitive control as punishment levels increased from low to high levels (Supplementary Table S5). Thus, we find that punishment and reward sensitivity are associated with activity in different prefrontal regions during the engagement of cognitive control in our task-switching paradigm.
Incentive anticipation effects at the time of cue presentation
In order to identify incentive-sensitive regions whose anticipatory activity might prepare the brain for better performance under reward or punishment incentives, we also investigated changes in BOLD activity during the presentation of the cross cue that identified the incentive type and level for the upcoming block.
We observed brain activity patterns reflecting both incentive valence and magnitude at the time of cue presentation. During the anticipatory cue presentation, we found significant linear effects of valence (i.e. regions that show greater activity for reward than punishment anticipation) in the right dlPFC and IFC, as well as the dorsal ACC (Table 3). The contrast for the quadratic regressor for incentive magnitude regardless of valence revealed increased activity for higher incentive levels in the vStr, as well as lateral OFC, ACC and several other brain regions (Fig. 4, Table 3). These findings indicate that incentive anticipation has a strong impact on neural activity and this activity may facilitate the engagement of task-related areas in order to perform the upcoming task.
Table 3.
Parametric effects of incentive at the time of incentive anticipation
| Region | Cluster size | x; y; z | TFCE T-stat |
|---|---|---|---|
| Anticipatory cross (linear effect) | |||
| Cingulate gyrus, anterior division; paracingulate gyrus | 132 | 7; 39; 14 | 5.87 |
| Juxtapositional lobule cortex (formerly supplementary motor cortex); precentral gyrus; superior frontal gyrus | 88 | 2; −8; 68 | 6.21 |
| Paracingulate gyrus; cingulate gyrus, anterior division; juxtapositional lobule cortex (formerly supplementary motor cortex); superior frontal gyrus | 64 | 2; 11; 47 | 4.95 |
| Cingulate gyrus, posterior division; cingulate gyrus, anterior division; precentral gyrus | 26 | 2; −21; 40 | 4.86 |
| Frontal pole, superior frontal gyrus, middle frontal gyrus | 25 | 20; 39; 29 | 5.64 |
| Frontal pole | 17 | −22; 46; 18 | 5.35 |
| Inferior frontal gyrus, pars opercularis; precentral gyrus; frontal operculum cortex; central opercular cortex | 9 | 37; 16; 18 | 4.22 |
| Frontal pole | 9 | 30; 56; 18 | 4.17 |
| Inferior frontal gyrus, pars triangularis; frontal orbital cortex; frontal operculum cortex; inferior frontal gyrus, pars opercularis | 8 | 55; 24; −4 | 4.3 |
| Inferior frontal gyrus, pars triangularis; frontal operculum cortex; inferior frontal gyrus, pars opercularis; frontal pole | 6 | 57; 29; 4 | 4.6 |
| Cingulate gyrus, anterior division | 6 | −2; 21; 18 | 4.6 |
| Anticipatory cross (2nd order effects) | |||
| Frontal orbital cortex; temporal pole; lateral occipital cortex, superior division; lateral occipital cortex, inferior division; intracalcarine cortex; cingulate gyrus, posterior division; precuneous cortex; cuneal cortex; lingual gyrus; temporal occipital fusiform cortex; occipital fusiform gyrus; occipital pole | 10750 | 20; 9; −11 | 6.77 |
| Superior frontal gyrus frontal pole; superior frontal gyrus; middle frontal gyrus; inferior frontal gyrus, pars opercularis; precentral gyrus; postcentral gyrus; superior parietal lobule; supramarginal gyrus, anterior division; juxtapositional lobule cortex (formerly supplementary motor cortex); paracingulate gyrus; cingulate gyrus, anterior division; cingulate gyrus, posterior division; left thalamus; left caudate; left putamen left pallidum; brain-stem; left accumbens; right thalamus; right caudate; right putamen; right hippocampus; right accumbens | 3307 | −10; 16; 68 | 6.64 |
| Postcentral gyrus supramarginal gyrus, anterior and posterior divisions; superior parietal lobule; precentral gyrus | 132 | 37; −33; 47 | 4.95 |
| Postcentral gyrus supramarginal gyrus, anterior division | 7 | 57; −16; 40 | 3.67 |
| Middle frontal gyrus precentral gyrus superior frontal gyrus | 6 | 35; −1; 65 | 4.23 |
| Insular cortex | 5 | 37; −1; −11 | 3.6 |
All reported regions are significant at P < 0.05 after whole-brain FWE correction at the voxel level and a cluster extent of 5 voxels. The FWE correction was based on 5000 permutations of the threshold-free cluster enhancement (TFCE) values. The TFCE values and permutation-derived test statistics were calculated using the randomize function implemented in FSL. All coordinates are listed in MNI space and represent the peaks of all clusters formed by contiguous voxels as well as peaks >20 mm apart within the same cluster.
Fig. 4.
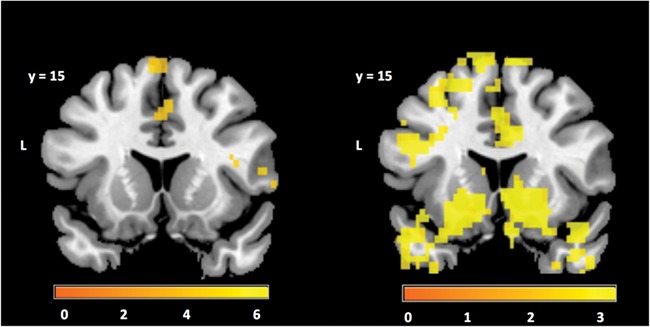
Regions showing incentive anticipation effects as revealed by the contrast for the first- and second-order parametric regressors (left and right, respectively) for incentive magnitude at the time when the upcoming block type was indicated (i.e. the fixation cross cue). The color bar scale indicates t-statistics derived from 5000 permutations of the data in voxels that are significant after correction for multiple comparisons (P < 0.05) at the whole brain level.
An anonymous reviewer pointed out that, according to Aston-Jones and Cohen (2005), the locus coeruleus (LC) might play a role in regulating arousal and optimizing behavioral performance during cognitive tasks. She or he suggested that we test for activity related to incentive magnitude in the LC during the anticipatory cue presentation. We did so by applying a small-volume correction for the incentive magnitude contrast using the LC mask from Keren et al., (2009). We did indeed observe a significant effect of incentive magnitude in the right LC consistent with the reviewer’s speculation (MNI coordinates: 5, −36, −18; TFCE t-stat = 2.73; 1 voxel). We note, however, that our scanning sequence was not optimized for detecting BOLD signals in the LC and these exploratory findings should be interpreted in light of that fact. Nevertheless, these results, together with our findings in the ACC, are consistent with theories that norepinephrine plays an important role in modulating attention and effort and suggest that further work in this direction is warranted.
Functional connectivity (PPI) analyses
Our analyses of local activity revealed that portions of the ACC as well as the vStr were sensitive to incentive magnitudes and/or valences beginning at the time of cue presentation. Given our hypotheses regarding the role of two regions in the integration of motivation and control systems, we examined ACC and vStr functional connectivity as a function of incentive (Supplementary Figure S2).
No significant differences in functional connectivity between incentive types were observed for the two dACC seed regions (Supplementary Figure S3).
Although there were also no main effects of incentive valence or magnitude on vStr connectivity, we did find a difference between the RH and PH conditions, consistent with the behavioral findings. Specifically, there was increased connectivity between the vStr and a range of regions including the ACC, left IFC/dlPFC, rostral medial and lateral PFC, as well as superior parietal regions (Fig. 3, violet voxels; Table 4). Interestingly, this left IFC/dlPFC cluster overlapped with portions of the left IFC showing local activation differences in the GLM-1 cognitive control contrast (i.e. switch > repeat) and greater activity for this cognitive control contrast under reward versus punishment incentives. Together, these findings suggest that functional connectivity between the vStr and left IFC may play a role in facilitating the enhancement of cognitive control seen under high levels of reward incentive.
Table 4.
Regions with increased functional connectivity with the vStr during high reward blocks compared to high punishment blocks
| Region | Cluster size | x; y; z | TFCE T-stat |
|---|---|---|---|
| Precentral gyrus, middle frontal gyrus, frontal pole, superior frontal gyrus, postcentral gyrus, paracingulate gyrus | 865 | −32; −11; 58 | 5.18 |
| Superior parietal lobule; lateral occipital cortex, superior division; supramarginal gyrus, posterior division; angular gyrus; supramarginal gyrus, anterior division | 346 | −25; −51; 43 | 5.95 |
| Occipital pole; occipital fusiform gyrus; lateral occipital cortex, inferior division; lingual gyrus | 157 | 22; −91; −18 | 5.57 |
| Frontal pole | 96 | −22; 59; 4 | 4.44 |
| Frontal pole | 69 | −2; 66; 11 | 5.31 |
| Inferior frontal gyrus, pars opercularis; precentral gyrus; frontal operculum cortex; central opercular cortex | 46 | −50; 11; 11 | 5.35 |
| Postcentral gyrus; precentral gyrus; supramarginal gyrus, anterior division | 36 | −57; −8; 29 | 4.68 |
| Occipital pole; lingual gyrus; occipital fusiform gyrus; lateral occipital cortex, inferior division | 27 | −7; −93; −22 | 5.36 |
| Middle frontal gyrus; inferior frontal gyrus, pars opercularis; precentral gyrus | 24 | −55; 6; 36 | 4.29 |
| Lateral occipital cortex, superior division; superior parietal lobule | 17 | 27; −63; 61 | 4.73 |
| Inferior frontal gyrus, pars opercularis; precentral gyrus; middle frontal gyrus | 14 | −42; 9; 25 | 3.99 |
| Left thalamus | 10 | −5; −3; 11 | 4.61 |
All reported regions are significant at P < 0.05 after whole-brain FWE correction at the voxel level and a cluster extent of 5 voxels. The FWE correction was based on 5000 permutations of the threshold-free cluster enhancement (TFCE) values. The TFCE values and permutation-derived test statistics were calculated using the randomize function implemented in FSL. All coordinates are listed in MNI space and represent the peaks of all clusters formed by contiguous voxels as well as peaks >20 mm apart within the same cluster.
Discussion
We investigated how incentive valence and magnitude influence task switching, a critical cognitive control process. We found that reward and punishment incentives have both common and distinct effects on cognitive control at the behavioral and neurobiological levels. High magnitudes of reward or punishment incentives led to similar performance improvements and elicited anticipatory brain activation in motivation and executive control regions. However, the comparison between reward and punishment incentives during cognitive control deployment showed stronger activation of lateral PFC, parietal cortex and ACC regions associated with control for reward than punishment. Thus, comparable changes in observed behavior (e.g. response accuracy) under reward and punishment incentives may be mediated by distinct neurobiological substrates.
Overall, our results indicate that the presence of explicit monetary reward or punishment incentives does not change the fundamental neural systems supporting cognitive control, but can enhance their functioning.
Greater BOLD activity for reward vs punishment incentives may be related to the simultaneous increase in performance and speed under reward incentives. Both reward and punishment have been linked to motor action selection and execution (Chen et al., 2018), which could translate into improved accuracy. However, high rewards might have energized/invigorated motor responses more than punishment incentives (Niv et al., 2007; Cools et al., 2009; Guitart-Masip et al., 2012; Beierholm et al., 2013; Rigoli et al., 2016). Greater invigoration could result in faster RTs and, as a consequence, increase the cognitive control level necessary to perform accurately during those trials. The increased levels of BOLD activity we observed for reward vs punishment incentives could potentially reflect the increased cost associated with simultaneously increasing both accuracy and speed during the task (Manohar et al., 2015). Indeed, Taylor et al. (2014) have suggested that when brain regions show greater activity in a condition with faster RTs it is indicative of high engagement of that region in the required cognitive processes.
Another possibility is that reward and punishment defer in the degree to which they promote proactive vs reactive control. Such a mechanism is not necessarily separate from the effects on invigoration postulated above and the two processes may interact. In either case, we propose that reward may improve performance mostly via the enhancement of proactive control. In contrast, punishment may have led primarily to better reactive control. This proactive vs reactive distinction is consistent with the enhanced functional connectivity between vStr during anticipatory cross and left IFC only under high reward incentives. Furthermore, it is in line with previous evidence from sustained attention studies (Locke and Braver, 2008). Enhanced proactive control would ensure task cues are kept active and prepare the system for fast and accurate responses, whereas reactive control strategies yield increased performance, but relatively slower responses than proactive strategies.
Both the invigoration and control mechanisms are consistent with the fact that there were effects of both incentive valence and magnitude on brain activity when the anticipatory cue revealed the upcoming incentive type. Anticipation of performing under increasing incentive magnitudes was associated with higher BOLD activity in brain regions linked to both motivation (e.g. vStr and OFC) and control (e.g. ACC, lateral prefrontal and parietal cortices, dorsal striatum) processes (Delgado, 2007; Jensen et al., 2007; Krebs et al., 2012; Bartra et al., 2013; Bolstad et al., 2013; Cho et al., 2013; Chib et al., 2014; Robertson et al., 2015). This suggests that information regarding the incentives for upcoming performance can initiate preparatory or readiness states that facilitate subsequent task performance because higher ACC and vStr activity preceded better performance in high reward and punishment blocks. Such a mechanism would also be in line with effects of motivation on cognitive functioning via increased proactive control and use of contextual information (Frober and Dreisbach, 2014; Chiew and Braver, 2016) or preparatory cognitive control processes (Botvinick and Braver, 2015).
Interestingly, although the local activity in vStr during performance anticipation/preparation showed a magnitude rather than salience effect, there was greater functional connectivity between the vStr and core cognitive control regions in prefrontal and parietal cortices under high reward compared to high punishment blocks. This suggests that vStr may differentially transfer motivation to cognitive control systems as a function of valence. Thus, although the initial anticipatory process may be similar for both reward and punishment, their mechanisms supporting actual performance might differ. Moreover, the left IFC cluster showing increased functional coupling with vStr overlapped with clusters showing increased activity for switch relative to repeat trials both on average and particularly during reward incentive blocks. The vStr-left IFC connectivity in our study may reflect signaling leading to increased recruitment of this IFC region in support of task performance when reward contingencies increase the value of engaging control. This would be consistent with recent evidence of better information encoding under reward incentives in fronto-parietal networks (Etzel et al., 2016) or enhanced activation in IFC during incongruent trials in a flanker task (Paschke et al., 2015). These results support our hypothesis that the vStr plays a key role in linking motivation and cognitive control.
In contrast to the vStr, local BOLD activity in the ACC differed as a function of both incentive magnitude and valence, but did not show incentive-dependent functional connectivity with other nodes in the brain’s cognitive control network. While ACC activity was correlated with activity patterns in brain regions showing increased activity on switch relative to repeat trials cognitive control regions, this correlation was not significantly different across incentive types. A constant level of integration would nevertheless still convey information about incentive magnitude or valence encoded in the varying levels of ACC activity to other cognitive control regions. Thus, our findings are consistent with the proposed role for ACC in signaling the value of engaging cognitive control.
This pattern of incentive-magnitude-dependent activity is also consistent with the idea that the ACC activity reflects salience. Portions of the ACC are thought to be part of a salience network (Qiao et al., 2018). In this view, the ACC, as well as vStr and other regions, signals the presence of motivationally salient stimuli and thereby promote the implementation of cognitive control either locally or by a network of other brain regions (Eisenreich et al., 2017; Alexander et al., 2018). Salience is often closely related to and may even contribute to the expected value of control. In the current task design we cannot distinguish the two measures. Moreover, the sets of brain regions reported to be associated with salience and control are highly overlapping, suggesting that they may influence the brain in similar ways. In general, the neural systems processing salient or arousing features of the environment most likely interact closely with attention, valuation and control mechanisms to guide behavior.
With regard to attention and arousal, we also found indications that anticipatory activity in the LC increased as a function of incentive magnitude. This would suggest a role for noradrenergic influences, potentially increasing arousal, attention or motivation to overcome effort costs (Aston-Jones and Cohen, 2005; Soutschek and Tobler, 2018; Walton and Bouret, 2018). However, the LC is a challenging area to image with fMRI (Liu et al., 2017) and our imaging sequence was not optimized to detect signals in this brain region. Therefore, we believe that future studies using optimized scanning procedures should be conducted to try and replicate this result.
Beyond differences related to valence and magnitude, we also found that the neural responses to incentives differed as a function of an individual’s sensitivity to reward and punishment. Rostral mPFC regions showed increased activation under reward relative to punishment during cognitive control deployment and greater increases in activity as a function of reward incentive magnitude in individuals with higher reward sensitivity. This is consistent with previous evidence for (i) a link between differences in personality and differences in cognitive control (Gray and Braver, 2002; Avila et al., 2012; Fuentes-Claramonte et al., 2015), (ii) the modulatory role of individual differences in sensitivity to reward on the impact of incentives on brain activation and/or behavior (Gray and Braver, 2002; Engelmann et al., 2009; Jimura et al., 2010; Braem et al., 2013; Bunford et al., 2017) and (iii) mPFC activity reflecting reward anticipation and receipt (Liu et al., 2011; Bartra et al., 2013; de la Vega et al., 2016). In addition, the importance of rostral mPFC in supporting cognitive control processes has been previously shown using both fMRI (Kim et al., 2011) and lesion mapping techniques (Glascher et al., 2012). As a whole, these data suggest that rostral mPFC plays a key role in promoting cognitive control in pursuit of earning more rewards.
On the other hand, individual differences in self-reported punishment sensitivity were associated with decreased activity in the left frontopolar cortex (FPC), in a region that was distinct from the portions of left lateral PFC whose activity increased on successful switch relative to repeat trials. Previous studies have shown that individual punishment sensitivity might modulate the impact of punishments on adaptations to conflict (Braem et al., 2013). Reduced activity in left FPC might potentially represent a shift toward more focal processing in high relative to low punishment blocks in individuals who are more sensitive to punishments. This mechanism would be in line with studies showing that shifts toward more focal neural processing are associated with improved performance in cognitive control tasks (Durston et al., 2006).
Conclusion
Our findings showing that monetary reward and punishment incentives can activate motivational neural circuitry and increase its functional coupling with the cognitive control networks that support task switching behavior raise interesting possibilities for future research and translational applications. We find that increasing levels of both incentive valences can improve performance in a healthy adult sample that is already performing with ~90% accuracy at baseline. Would individuals with lower levels of performance at baseline show even greater performance increases in behaviors requiring cognitive control in the context of incentives? Our results suggest that, in healthy young adults, reward and punishment do not fundamentally change the neural mechanisms mediating task switching, but rather enhance the engagement of these networks. This suggests that the existence of a sufficiently competent system is required before an incentive can be fully effective.
However, determining whether or not reward and punishment incentives have similar mechanisms of action in individuals with developing or dysfunctional neural networks will require further research. The use of reward and punishment to encourage or discourage specific behaviors is already commonplace in both clinical and educational settings. Given our results in healthy young adults, it is possible that incentives encourage the engagement of cognitive control abilities such as they are, and this engagement might have a feed-forward effect on competence (i.e. practice effects). If so, then rewards and punishments could be used to improve the function of cognitive control networks in cases of neuropathology or to facilitate their maturation in healthy development. The feasibility of using incentives to promote the engagement and refinement of more general cognitive control abilities that will transfer across domains remains an unknown, but exciting possibility.
Supplementary Material
Acknowledgments
Conflict of interest. None declared.
Funding
This work was supported in part by the Swiss National Science Foundation (SNSF grant number 100014_140277) and National Center of Competence in Research Affective sciences hosted by the University of Geneva (SNSF grant number 51NF40-104897).
References
- Alexander W.H., Brown J.W., Collins A.G.E., Hayden B.Y., Vassena E. (2018). Prefrontal cortex in control: broadening the scope to identify mechanisms. Journal of Cognitive Neuroscience, 30(8), 1061–1065. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. [DOI] [PubMed] [Google Scholar]
- Avila C., Garbin G., Sanjuán A., et al. (2012). Frontostriatal response to set switching is moderated by reward sensitivity. Social Cognitive and Affective Neuroscience, 7(4), 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister R.F., Bratslavsky E., Finkenauer C., Vohs K.D. (2001). Bad is stronger than good. Review of General Psychology, 5(4), 323. [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience, 29(4), 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierholm U., Guitart-Masip M., Economides M., et al. (2013). Dopamine modulates reward-related vigor. Neuropsychopharmacology, 38(8), 1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad I., Andreassen O.A., Reckless G.E., Sigvartsen N.P., Server A., Jensen J. (2013). Aversive event anticipation affects connectivity between the ventral striatum and the orbitofrontal cortex in an fMRI avoidance task. PLoS One, 8(6), e68494 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M., Braver T. (2015). Motivation and cognitive control: from behavior to neural mechanism. Annual Review of Psychology, 66, 83–113. [DOI] [PubMed] [Google Scholar]
- Braem S., Duthoo W., Notebaert W. (2013). Punishment sensitivity predicts the impact of punishment on cognitive control. PLoS One, 8(9), e74106 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunford N., Roberts J., Kennedy A.E., Klumpp H. (2017). Neurofunctional correlates of behavioral inhibition system sensitivity during attentional control are modulated by perceptual load. Biological Psychology, 127, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver C.S., White T.L. (1994). Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment—the bis bas scales. Journal of Personality and Social Psychology, 67(2), 319–333. [Google Scholar]
- Chen X.L., Holland P., Galea J.M. (2018). The effects of reward and punishment on motor skill learning. Current Opinion in Behavioral Sciences, 20, 83–88. [Google Scholar]
- Chib V.S., Shimojo S., O’Doherty J.P. (2014). The effects of incentive framing on performance decrements for large monetary outcomes: behavioral and neural mechanisms. Journal of Neuroscience, 34(45), 14833–14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew K.S., Braver T.S. (2016). Reward favors the prepared: Incentive and task-informative cues interact to enhance attentional control. Journal of Experimental Psychology. Human Perception and Performance, 42(1), 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y.T., Fromm S., Guyer A.E., et al. (2013). Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. Neuroimage, 66, 508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay S.N., Clithero J.A., Harris A.M., Reed C.L. (2017). Loss aversion reflects information accumulation, not bias: a drift–diffusion model study. Frontiers in Psychology, 8, 1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., Frank M.J., Gibbs S.E., Miyakawa A., Jagust W., D’Esposito M. (2009). Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. Journal of Neuroscience, 29(5), 1538–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega A., Chang L.J., Banich M.T., Wager T.D., Yarkoni T. (2016). Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. Journal of Neuroscience, 36(24), 6553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Della Libera C., Chelazzi L. (2006). Visual selective attention and the effects of monetary rewards. Psychological Science, 17(3), 222–227. [DOI] [PubMed] [Google Scholar]
- Durston S., Casey B.J. (2006). A shift from diffuse to focal cortical activity with development. Developmental Science, 9(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Economides M., Guitart-Masip M., Kurth-Nelson Z., Dolan R.J. (2014). Anterior cingulate cortex instigates adaptive switches in choice by integrating immediate and delayed components of value in ventromedial prefrontal cortex. Journal of Neuroscience, 34(9), 3340–3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich B.R., Akaishi R., Hayden B.Y. (2017). Control without controllers: toward a distributed neuroscience of executive control. Journal of Cognitive Neuroscience, 29(10), 1684–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.B., Damaraju E., Padmala S., Pessoa L. (2009). Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Frontiers in Human Neuroscience, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann J.B., Pessoa L. (2007). Motivation sharpens exogenous spatial attention. Emotion, 7(3), 668–674. [DOI] [PubMed] [Google Scholar]
- Erev I., Ert E., Yechiam E. (2008). Loss aversion, diminishing sensitivity, and the effect of experience on repeated decisions. Journal of Behavioral Decision Making, 21(5), 575–597. [Google Scholar]
- Etzel J.A., Cole M.W., Zacks J.M., Kay K.N., Braver T.S. (2016). Reward motivation enhances task coding in frontoparietal cortex. Cerebral Cortex, 26(4), 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frober K., Dreisbach G. (2014). The differential influences of positive affect, random reward, and performance-contingent reward on cognitive control. Cognitive, Affective, & Behavioral Neuroscience, 14(2), 530–547. [DOI] [PubMed] [Google Scholar]
- Fuentes-Claramonte P., Ávila C., Rodríguez-Pujadas A., et al. (2015). Reward sensitivity modulates brain activity in the prefrontal cortex, ACC and striatum during task switching. PLoS One, 10(4), e0123073 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert A.M., Fiez J.A. (2004). Integrating rewards and cognition in the frontal cortex. Cognitive, Affective, & Behavioral Neuroscience, 4(4), 540–552. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage, 19(1), 200–207. [DOI] [PubMed] [Google Scholar]
- Glascher J., Adolphs R., Damasio H., et al. (2012). Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 109(36), 14681–14686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.R., Braver T.S. (2002). Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive, Affective, & Behavioral Neuroscience, 2(1), 64–75. [DOI] [PubMed] [Google Scholar]
- Guitart-Masip M., Chowdhury R., Sharot T., Dayan P., Duzel E., Dolan R.J. (2012). Action controls dopaminergic enhancement of reward representations. Proceedings of the National Academy of Sciences of the United States of America, 109(19), 7511–7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd C.B., Yeung N. (2012). Motivation of extended behaviors by anterior cingulate cortex. Trends in Cognitive Sciences, 16(2), 122–128. [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D.N., Sadato N. (2008). Processing of social and monetary rewards in the human striatum. Neuron, 58(2), 284–294. [DOI] [PubMed] [Google Scholar]
- Jensen J., Smith A.J., Willeit M., et al. (2007). Separate brain regions code for salience vs. valence during reward prediction in humans. Human Brain Mapping, 28(4), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimura K., Locke H.S., Braver T.S. (2010). Prefrontal cortex mediation of cognitive enhancement in rewarding motivational contexts. Proceedings of the National Academy of Sciences of the United States of America, 107(19), 8871–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D., Tversky A. (1979). Prospect theory: an analysis of decision under risk. Econometrica, 47(2), 28. [Google Scholar]
- Keren N.I., Lozar C.T., Harris K.C., Morgan P.S., Eckert M.A. (2009). In vivo mapping of the human locus coeruleus. Neuroimage, 47(4), 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Johnson N.F., Cilles S.E., Gold B.T. (2011). Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. Journal of Neuroscience, 31(13), 4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, 21(16), RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N., Behrens T., Wittmann M.K., Rushworth M. (2016). Multiple signals in anterior cingulate cortex. Current Opinion in Neurobiology, 37, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouneiher F., Charron S., Koechlin E. (2009). Motivation and cognitive control in the human prefrontal cortex. Nature Neuroscience, 12(7), 939–945. [DOI] [PubMed] [Google Scholar]
- Krawczyk D.C., D’Esposito M. (2013). Modulation of working memory function by motivation through loss-aversion. Human Brain Mapping, 34(4), 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk D.C., Gazzaley A., D'Esposito M. (2007). Reward modulation of prefrontal and visual association cortex during an incentive working memory task. Brain Res, 1141, 168–177. [DOI] [PubMed] [Google Scholar]
- Krebs R.M., Boehler C.N., Roberts K.C., Song A.W., Woldorff M.G. (2012). The involvement of the dopaminergic midbrain and cortico-striatal-thalamic circuits in the integration of reward prospect and attentional task demands. Cerebral Cortex, 22(3), 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.Y., Marijatta F., Hammerer D., Acosta-Cabronero J., Duzel E., Howard R.J. (2017). Magnetic resonance imaging of the human locus coeruleus: a systematic review. Neuroscience & Biobehavioral Reviews, 83, 325–355. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. (2011). Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews, 35(5), 1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke H.S., Braver T.S. (2008). Motivational influences on cognitive control: behavior, brain activation, and individual differences. Cognitive, Affective, & Behavioral Neuroscience, 8(1), 99–112. [DOI] [PubMed] [Google Scholar]
- Manohar S.G., Chong T.T., Apps M.A., et al. (2015). Reward pays the cost of noise reduction in motor and cognitive control. Current Biology, 25(13), 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty V.P., Labar K.S., Adcock R.A. (2012). Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. Journal of Neuroscience, 32(26), 8969–8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y., Daw N.D., Joel D., Dayan P. (2007). Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology, 191(3), 507–520. [DOI] [PubMed] [Google Scholar]
- Padmala S., Pessoa L. (2011). Reward reduces conflict by enhancing attentional control and biasing visual cortical processing. Journal of Cognitive Neuroscience, 23(11), 3419–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschke L.M., Walter H., Steimke R., et al. (2015). Motivation by potential gains and losses affects control processes via different mechanisms in the attentional network. Neuroimage, 111, 549–561. [DOI] [PubMed] [Google Scholar]
- Pessoa L., Engelmann J.B. (2010). Embedding reward signals into perception and cognition. Frontiers in Neuroscience, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J.B., Levy R., Fossati P., et al. (2002). The neural system that bridges reward and cognition in humans: an fMRI study. Proceedings of the National Academy of Sciences of the United States of America, 99(8), 5669–5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Xu L., Che X., et al. (2018). The motivation-based promotion of proactive control: the role of salience network. Frontiers in Human Neuroscience, 12, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoli F., Chew B., Dayan P., Dolan R.J. (2016). The dopaminergic midbrain mediates an effect of average reward on pavlovian vigor. Journal of Cognitive Neuroscience, 28(9), 1303–1317. [DOI] [PubMed] [Google Scholar]
- Robertson B.D., Hiebert N.M., Seergobin K.N., Owen A.M., MacDonald P.A. (2015). Dorsal striatum mediates cognitive control, not cognitive effort per se, in decision-making: an event-related fMRI study. Neuroimage, 114, 170–184. [DOI] [PubMed] [Google Scholar]
- Rudorf S., Hare T.A. (2014). Interactions between dorsolateral and ventromedial prefrontal cortex underlie context-dependent stimulus valuation in goal-directed choice. Journal of Neuroscience, 34(48), 15988–15996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F., Noonan M.P., Boorman E.D., Walton M.E., Behrens T.E. (2011). Frontal cortex and reward-guided learning and decision-making. Neuron, 70(6), 1054–1069. [DOI] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 12(3), 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A., Botvinick M.M., Cohen J.D. (2013). The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron, 79(2), 217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D.M., Gitelman D., Simmons K., Bloise S.M., Parrish T., Mesulam M.M. (2005). Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cerebral Cortex, 15(12), 1855–1865. [DOI] [PubMed] [Google Scholar]
- Soutschek A., Stelzel C., Paschke L., Walter H., Schubert T. (2015). Dissociable effects of motivation and expectancy on conflict processing: an FMRI study. Journal of Cognitive Neuroscience, 27(2), 409–423. [DOI] [PubMed] [Google Scholar]
- Soutschek A., Tobler P.N. (2018). Motivation for the greater good: neural mechanisms of overcoming costs. Current Opinion in Behavioral Sciences, 22, 96–105. [Google Scholar]
- Taylor J.S., Rastle K., Davis M.H. (2014). Interpreting response time effects in functional imaging studies. Neuroimage, 99, 419–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., et al. (2006). Medial frontal cortex activity and loss-related responses to errors. Journal of Neuroscience, 26(15), 4063–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Welsh R.C., Wager T.D., Phan K.L., Fitzgerald K.D., Gehring W.J. (2004). A functional neuroimaging study of motivation and executive function. Neuroimage, 21(3), 1045–1054. [DOI] [PubMed] [Google Scholar]
- Umemoto A., Holroyd C.B. (2015). Task-specific effects of reward on task switching. Psychological Research, 79(4), 698–707. [DOI] [PubMed] [Google Scholar]
- Wachter T., Lungu O.V., Liu T., Willingham D.T., Ashe J. (2009). Differential effect of reward and punishment on procedural learning. Journal of Neuroscience, 29(2), 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M.E., Bouret S. (2018). What is the relationship between dopamine and effort? Trends in Neurosciences, S0166-2236(18)30272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G., Xue F., Droutman V., Lu Z.L., Bechara A., Read S. (2013). Common neural mechanisms underlying reversal learning by reward and punishment. PLoS One, 8(12), e82169 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E., Hochman G. (2013). Loss-aversion or loss-attention: the impact of losses on cognitive performance. Cognitive Psychology, 66(2), 212–231. [DOI] [PubMed] [Google Scholar]
- Yechiam E., Hochman G. (2014). Loss attention in a dual-task setting. Psychological Science, 25(2), 494–502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


