Abstract
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the skin, manifesting in chronic, recurrent painful pustules, nodules, boils and purulent draining abscesses. Our current understanding of the pathogenesis of the disease is incomplete. This review aims to identify available treatment options in HS and discuss the pharmacological mechanisms through which such agents function. Identifying common pathways may inform our understanding of the pathogenesis of HS as well as identify future therapeutic targets. The pharmacological mechanisms implicated in topical therapies, antibiotic, hormonal, systemic immunomodulatory and biologic therapies for HS are discussed. Significant differences exist between agents and implicated pathways in therapy for mild and severe disease. This is an expression of the possible dichotomy in inflammatory pathways (and treatment responses) in HS. Studies involving monoclonal antibodies provide the greatest insight into what these specific mechanisms may be. Their variable levels of clinical efficacy compared with placebo bolsters the suggestion that differential inflammatory pathways may be involved in different presentations and severity of disease. Nuclear factor kappa B (NF-κB), tumor necrosis factor (TNF)-α and other innate immune mechanisms are strongly represented in treatments which are effective in mild to moderate disease in the absence of scarring or draining fistulae, however complex feed-forward mechanisms in severe disease respond to interleukin (IL)-1 inhibition but are less likely to respond to innate immune inhibition (through NF-κB or TNF-α) alone. It is unclear whether IL-17 inhibition will parallel TNF-α or IL-1 inhibition in effect, however it is plausible that small molecule targets (Janus kinase1 and phosphodiesterase 4) may provide effective new strategies for treatment of HS.
Keywords: biologics, cytokines, hidradenitis suppurativa, interleukin-17, inflammation, tetracycline, tumor necrosis factor-α
Background
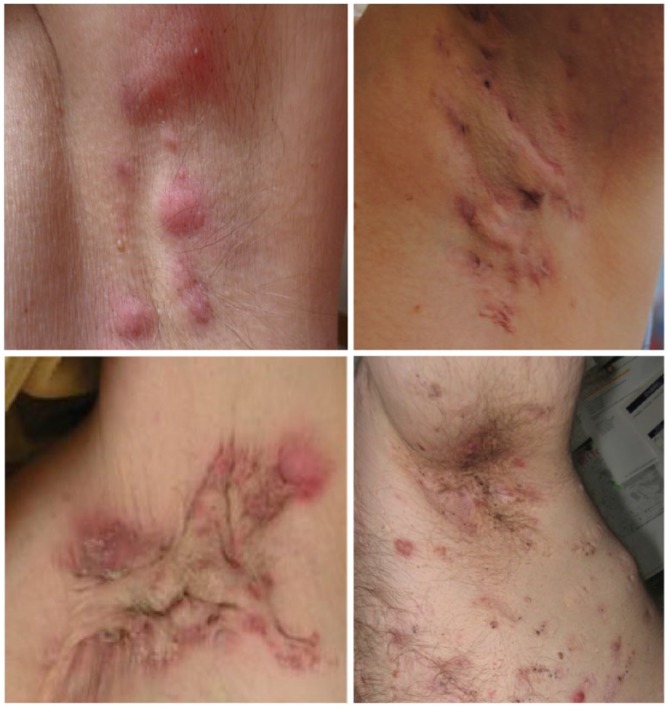
Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the skin, manifesting in chronic, recurrent, painful pustules, nodules, boils and purulent draining abscesses.1 It commonly affects the flexural areas of the axillae, sub-mammary folds, inguinal and gluteal regions. It has an estimated prevalence of 1–4%2 similar to other common dermatoses such as atopic dermatitis and psoriasis. Representative clinical manifestations of HS are presented in Figure 1.
Figure 1.
Clinical manifestations of hidradenitis suppurativa (HS) demonstrating inflammatory nodules in Hurley Stage 1 disease (top left), sinus tracts and inflammatory nodules separated by largely normal skin (Hurley Stage 2) (top right); widespread scarring inflammation and interconnected sinus tracts (Stage 3), (Bottom left) and follicular scarring and double ended comedones in PASH (Pyoderma Gangrenosum, Acne Congolobata and Suppurative Hidradenitis) Syndrome (Bottom right).
HS affects women three times more than men, with those of lower socioeconomic status disproportionately affected.1,2 Up to one-third of cases of HS are associated with pathogenic sequence variants identified in gamma secretase subunits as well as the inflammatory pathways associated with autoinflammatory diseases.3 Cigarette smoking, obesity, diabetes, inflammatory bowel disease and arthritis are all associated with HS.1,2 It has a significant impact upon quality of life, psychosexual function, and represents a notable financial and clinical burden on healthcare systems.4 Potential complications include secondary infection, hypertrophic scarring, development of squamous cell carcinoma, chronic pain, psychological and psychosexual complications.1 It is well documented that a lack of awareness and effective treatments contribute to diagnostic delay in HS, with an average of 7 years between onset and diagnosis.5 For many years, HS has been considered an orphan disease with very few effective treatments available. Diagnostic criteria for the disease are presented in Table 1.
Table 1.
Diagnostic criteria for HS as defined by the European S1 guideline for the treatment of HS/acne inversa.6 The presence of primary positive diagnostic criteria (either history or signs) are required and the presence of secondary positive diagnostic criteria are supportive of the diagnosis of HS.
| Primary positive diagnostic criteria: |
|---|
| • History: More than two recurrent, painful or suppurating lesions over a period of 6 months • Signs: Involvement of axilla, genitofemoral area, perineum, gluteal area and inframammary area of women. Presence of nodules, sinus tracts, abscesses, scarring. |
| Secondary positive diagnostic criteria: |
| • History: A family history of HS • Microbiology: A negative swab or presence of normal skin microbiota may be indicative of HS. |
HS, hidradenitis suppurativa.
Over the past decade, a flourish of basic and clinical research has led to an increase of awareness of HS, and the treatment options for patients. Recent United States Food and Drug Administration (US FDA) approval of adalimumab for HS7 has led to an increase in treatment options for disease control. However, our understanding of the disease pathogenesis remains incomplete.8 Multiple ongoing trials are testing existing monoclonal antibodies with the hope of identifying effective therapies and providing insight into the pathogenic mechanisms underlying HS.9,10 Whilst ‘trial by therapy’ does not systematically analyze the cellular mechanism underlying HS, alterations in the disease state can give inferential data regarding the role of specific inflammatory pathways in HS. Such data have challenged the existing pathogenic paradigm in HS. Currently, HS is considered a disease of follicular occlusion, which leads to the rupture and dermal seeding of bacteria and cellular debris1,12 leading to the influx of inflammatory cells and mediators. The response of HS to anti-inflammatory therapies has suggested that follicular occlusion may be a secondary phenomenon due to an aberrant inflammatory response to the cutaneous microbiome.8 A schematic illustration of inflammatory pathways in HS is presented in Figure 2.
Figure 2.
Pathogenesis of hidradenitis suppurativa and site of action of reported treatment modalities.
The purpose of this review is to provide a comprehensive overview of available treatment options in HS and to discuss the pharmacological mechanisms through which these agents function. This review aims to include a variety of therapies used in HS, not only those used by dermatologists, or those supported by clinical evidence, due to the fact that a paucity of high-level clinical evidence exists to guide treatment decisions in HS. Evaluations of the level of evidence for HS therapies are available from other high-quality publications.6,13 Through this broad review of therapies, we aim to identify common mechanistic pathways among these agents, allowing us to present an updated, testable model of HS and its immunopathogenesis and identify potential future therapeutic targets.
Topical therapies in HS
Topical therapy is seen as the mainstay of treatment in mild to moderate HS,6 although the level of evidence for its use is low13 (Table 2). A variety of topical medicaments with biocidal as well as anti-inflammatory mechanisms are used, with varying degrees of benefit dependent upon disease severity. It is likely that individual patient factors (including the relative contribution of the microbiome to inflammation in HS) may alter the response to topical therapy. Observed benefits include reducing the frequency of secondary infection14 but little evidence exists as to their efficacy in reducing flares of nodules, pustules, abscesses or preventing progression to more severe disease.
Table 2.
Topical therapies reported in HS and descriptions of antibacterial, keratolytic and anti-inflammatory effects. The associated quality of evidence supporting the use of topical therapies is reported in the far-right hand side column.
| Topical therapy | Mechanism(s) of action |
Quality of evidence15 | ||
|---|---|---|---|---|
| Antibacterial effect | Keratolytic effect | Anti-inflammatory effect | ||
| Chlorhexidine | Cell wall binding K+ efflux Poor effect on biofilm |
Nil | Nil | C |
| Povidone iodine | Free radical oxidation of DNA/RNA/membrane proteins Some biofilm activity |
Nil | Inhibition of MMP production, TNF-α | C |
| Pyrithione zinc | Bacteriostatic and antifungal: disruption of ATP and protein synthesis. Modulates cellular copper influx. Effect against yeast biofilms |
Nil evidence | Only in the presence of intracellular zinc ions. Can increase TNF-α and HSP-70 in keratinocytes at high concentrations | C |
| Hydrogen peroxide | Free Radical Oxidation of DNA/RNA/Membrane proteins Some biofilm activity |
Possible direct oxidation: nil evidence | Decrease ubiquination in NFκB pathway but higher concentrations display deleterious effects | C |
| Sodium hypochlorite | Free radical oxidation of DNA/RNA/membrane proteins High biofilm activity |
Possible direct oxidation: nil evidence | Decreases NF-κB signaling | C |
| Triclosan | Disruption of bacterial wall synthesis | Nil | Downregulates TLR signaling, IL-6, IL-1ß expression | C |
| Clindamycin | Bacteriostatic effect 50S ribosomal binding | Nil | NF-κB and AP-1 gene expression, regulation of macrophage function., reduction in expression of virulence factors | C |
| Azelaic Acid | Bacteriostatic and antifungal | Nil | Modulation of PPAR-γ function, reduction in IL-6, IL-1ß TNF activity | C |
| Retinoids | Nil | Indirect effect through AP-1 and NF-κB modulation | NF-κB and AP-1 modulation, TLR2, MMP1 and MMP9 downregulation |
C |
| Resorcinol | Membrane damage and K+ efflux | Minor at reported concentrations | Reported but mechanisms not described | C |
AP-1, activator protein 1; ATP, adenosine triphosphate; HS, hidradenitis suppurativa; IL, interleukin; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa B; PPAR, peroxisome proliferator-activated receptor; TNF, tumor necrosis factor; TLR, Toll-like receptor.
Chlorhexidine
Chlorhexidine is a biguanide broad spectrum biocide, the efficacy of which is highly pH dependent, with a maximum effect occurring within 20 s.16 Even in high concentrations, decreased biocidal activity was seen in the presence of biofilms [including Pseudomonas sp., methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli].17 Given the established association of biofilms18 and Gram-negative bacteria (Porphyromonas and Peptidophillus sp.)19 with disease activity in HS, chlorhexidine may reduce the stimulation of the immune system by resident bacteria, but not in the presence of biofilms. Clinical evidence for the use of chlorhexidine is low, and benefit is derived only from reducing the incidence of bacterial resistance compared with oral antibacterial therapy.14
Topical povidone iodine
Povidone iodine is reported in the treatment of HS.20 It demonstrates rapid bactericidal, tuberculocidal and viricidal effects through the release of free iodine radicals which attack free amino acids (methionine and cysteine).16 This results in destabilization of membrane fatty acids through reactions with unsaturated carbon bonds. Free oxidation of other vital pathogen structures (phospholipid, DNA/RNA/membrane-bound proteins) also occurs.21 Iodine also has multiple anti-inflammatory properties which function through the inhibition of matrix metalloproteinase (MMP) production, reduction in plasmin activity, and inhibition of tumor necrosis factor alpha (TNF-α).21 The role of MMP and TNF-α in HS8 may partially explain the effect. Surprisingly little published evidence surrounding the use of oral Saturated Solution of Potassium Iodide (SSKI) for HS and this would be an area to explore further in controlled clinical trials.
Topical pyrithione zinc
Pyrithione zinc is a coordination complex of zinc present in a number of anti-dandruff products. It has fungistatic and bacteriostatic properties which function via the disruption of adenosine triphosphate (ATP) levels and protein synthesis.22 Pyrithione zinc may also have some anti-inflammatory properties. Intracellular zinc can modulate the lipopolysaccharide (LPS)-stimulated maturation of dendritic cells via Toll-like receptors (TLRs);23 however, the action of pyrithione zinc is dependent upon adequate intracellular zinc and excessive concentrations can exert a proinflammatory effect.24 The clinical significance of the anti-inflammatory mechanisms of zinc is unclear as there is no evidence correlating the intake of dietary zinc to serum inflammatory markers in epidemiological studies.25 Other concerns include the pro-estrogenic action of zinc pyrithione (ER bioactivity = 0.237) which is comparable to the clinically relevant exposure to butyl parabens (ER bioactivity = 0.251).26
Hydrogen peroxide
Hydrogen peroxide is a widely available biocide with nonspecific activity against viruses, bacteria, yeasts and spores.16 It has greater activity against Gram-positive organisms; however, catalase positive organisms are more resistant at lower concentrations.16 The risk of air emboli has been reported when hydrogen peroxide is used in highly vascular enclosed cavities in hypovolemic patients. However, this complication has not been reported in HS patients. Hydrogen peroxide is 266-times less effective against biofilms than free bacteria,27 however efficacy can be increased with short contact times and novel irrigation methods in HS.28 Its use is reported in HS28 but no formal clinical studies have been undertaken. Alcohol-based formulations require longer exposure times to achieve the same bactericidal activity.16 Anti-inflammatory effects have been described in vitro through decreased ubiquitination in the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway leading to a reduction in TLR4 signaling after LPS stimulation at low concentrations.29,30 However, increased apoptosis and oxidative stress were observed at higher concentrations.31
Bleach baths (sodium hypochlorite solution)
Dilute bleach baths (sodium hypochlorite) are a well-established antimicrobial and anti-inflammatory treatment for atopic dermatitis,31 and its use has been extended to include HS. Dilute sodium hypochlorite is bactericidal via direct oxidative reactions with bacterial proteins as well as inhibition of bacterial growth with as little as 5 min of exposure.16 DNA synthesis is much more sensitive to sodium hypochlorite than protein synthesis at low concentrations32 and this is the likely mechanism in HS. It demonstrates some activity against biofilms, but has incomplete bactericidal activity even at high concentrations.33,34 No clinical studies have estimated the efficacy of bleach baths in HS; however, it may have anti-inflammatory activity mediated via modulation of NF-κB signaling32,35
Triclosan
Triclosan is a halogenated bisphenol derivative with broad spectrum bactericidal activity (excluding Pseudomonas sp.).36,37 It achieves this through disruption of bacterial cell wall synthesis.16,37 The efficacy of triclosan is highly dependent upon the formulation used, and increased potency is known to occur with ethylenediaminetetraacetic acid (EDTA).16 Triclosan is recommended in the HS literature for Hurley stage 1 and 2 disease.38 Triclosan exerts an anti-inflammatory effect independent of bactericidal activity via downregulation of LPS-stimulated TLR signaling with subsequent reduction in interleukin (IL)-6 and IL-1ß gene expression.36 Although triclosan monotherapy has poor activity against Pseudomonas sp., it has an adjuvant role in Pseudomonas biofilms when combined with oral aminoglycosides.39 The activity of triclosan as an adjuvant therapy in those individuals with known biofilm disease is an area of potential future research.
Topical clindamycin
Clindamycin is one of the pillars of treatments for Hurley stage 1 and 2 HS as outlined in European guidelines.6,40 It is also one of the few treatments to be examined in randomized controlled trials in HS.13 Overall, two randomized controlled trials have indicated the benefit of topical clindamycin over placebo41,42 (Relative Risk [RR] = 0.72, 0.14–3.64) with a benefit in pain reduction, pustules, and inflammatory nodules, but no significant difference compared with tetracyclines.42 Expert opinion considers it most beneficial for superficial pustules and solitary nodules, with recommendations to be used in the absence of deep-seated abscesses.14,43 Clindamycin is a semisynthetic lincomycin derivative with a bacteriostatic effect through the inhibition of protein synthesis by 50S ribosomal binding.44 It has broad Gram-positive cocci coverage and Gram-negative anaerobe coverage.44 The direct antimicrobial effects of clindamycin may be relevant to HS, as Porphyromonas sp. and Peptidophillus sp. have high sensitivity to clindamycin.36 Despite increasing reports of MRSA clindamycin resistance,44 clindamycin has been shown to reduce the expression of virulence factors45 including leucocidin, TSST01 and α-hemolysin. They also have direct effects on NF-κB and AP1 gene expression.45 There are also in vitro reports of regulation of macrophage function, and response of neutrophils to chemokines.
Topical azelaic acid
Azelaic acid is a topical organic acid, traditionally used in acne vulgaris and acne rosacea14,46 but has recently been recommended for use in HS as an adjuvant with topical clindamycin, particularly in pediatric cases.14 Azelaic acid functions through antifungal and bacteriostatic properties47 but also has well-documented anti-inflammatory properties through the modulation of peroxisome proliferator-activated receptor (PPAR)-γ activity and reduction in IL-6, TNF-α and IL-1ß.48 HS is associated with altered PPAR-γ signaling8 and the use of azelaic acid in early-stage HS lends credence to the thought of inflammation precedes follicular occlusion in HS.8
Topical retinoids
Retinoids are a class of compounds, chemically related to vitamin A. Topical and systemic retinoids are used in HS based upon their known anti-inflammatory properties49,50 and the underlying presumption that HS as a disorder of follicular occlusion and hyperkeratinization.51 No formal evaluation of the efficacy of topical retinoids in HS exists.52 Despite the follicular occlusion paradigm being brought into question,8 third-generation topical retinoids (adapalene, tazarotene) have significant anti-inflammatory activity through suppression of macrophage response to LPS stimulation, TNF-α, Toll Like Receptor 2 (TLR2) on monocytes, MMP1, MMP9 and VCAM1.53 All of these pathways are documented in the pathogenesis of HS8 The degree of cytokine reduction from topical retinoids is modest (IL-12 reduction from 840 pg/ml to 420 pg/ml) when compared with lesional HS cytokine levels.8 Further clinical studies are needed to assess the clinical efficacy of topical retinoids in early-stage HS.
Resorcinol
Resorcinol is a benzenediol or phenol used as a chemical peeling agent as well as having antiseptic and antipruritic activity. The 15% resorcinol has been reported in one retrospective and one prospective study in HS.54,55 Benefit was seen in patients with Hurley stage 1 and 2 disease, with twice daily application for 30 days with reduction in size of visible lesions, pain and erythema. The mechanism of action of resorcinol in HS is proposed to be keratolytic at the follicular infundibulum.54 However, given the pharmacokinetics of the drug,16 antiseptic properties dominate more than keratolytic activities at the concentrations used in HS. Resorcinol has potent antiseptic activity at a wide range of concentrations due to membrane damage resulting in K+ leakage, and direct uncoupling of oxidative phosphorylation16 Some anti-inflammatory activities of resorcinol and related compounds have also been proposed, although are not described in detail.56
Intralesional therapies
Intralesional triamcinolone
Intralesional triamcinolone is an effective rescue therapy to reduce the inflammation, pain and swelling from active nodules and abscesses in HS.57,58 Uncontrolled case series have identified a reduction in physician-assessed erythema, suppuration and size57 as well as the rates of systemic antibiotic therapy.58 However, a recent placebo-controlled trial suggested that intralesional steroids were not superior to placebo (saline) injections.59 This brings into question our understanding of the role of injections in symptom relief in HS as the benefit may be due to encourage spontaneous lesion rupture following injection rather than the pharmacological action of the steroid. Despite the lack of clarity regarding the mechanism of intralesional therapies in HS, oral corticosteroids are known to provide rapid relief in acute flares of HS and are beneficial as a low dose adjuvant with biologics and other immunomodulating agents. Therefore, the mechanisms of intralesional therapy in HS require further study.
Oral therapeutic agents
Anti-hyperglycemic agents
HS is positively associated with insulin resistance, even in multivariate analyses once adjusted for age sex and body mass index [2.51 (0.18) versus 1.92 (0.21); p = 0.04].60,61 Hypoglycemic agents have been used in the management of HS and they have an important role as an adjuvant anti-inflammatory therapy and help to address underlying proinflammatory comorbidities in HS.62 There is no evidence for their efficacy as a monotherapy62 (Table 3). They may also be vital in addressing microbiome-mediated inflammatory stimuli, given the role of short chain fatty acid and bile salt mediators, such as those produced in HS by Porphyromonas sp. and Peptinophillus sp.,63 as stimuli in other chronic inflammatory dermatoses.64,65 The proposed mechanisms of action include the inhibition of mTORC1 activity in metformin,66 leading to a decreased expression of IL-6, TNF-α67 as well as downregulation of Th17 activity and the NLRP3 inflammasome.68 Liraglutide, a glucagon-like 1 peptide analogue, increases the expression of transforming growth factor (TGF)-ß1 and decreases IL-17 expression.69 Dipeptidyl peptidase IV (DPP4) inhibitors reduce the metabolism of glucagon-like peptide. Members of this class include sitagliptin, saxagliptin and linagliptin.70 They have the additional benefit of slowing gastric emptying and promoting weight loss. Other anti-hyperglycemic agents including PPAR-γ agonists71 (pioglitazone, rosiglitazone) are known to decrease insulin resistance and decrease levels of IL-6, as well as having antiproliferative activity.71 Given the known role of PPAR-γ in sebaceous gland atrophy in HS,8 modulation of PPAR activity may be a future therapeutic avenue.
Table 3.
Mechanism of action of oral agents (including oral antimicrobials) in HS. Canonical effects are compared with documented anti-inflammatory effects proposed in HS. The quality of evidence regarding their use in HS is also listed in the far-right column.
| Oral therapy | Mechanism(s) of action |
Quality of evidence15 | |
|---|---|---|---|
| Canonical effect(s) | Documented anti-inflammatory effect(s) | ||
| Metformin | Decreased mTORC1 activity | Decreased IL-6, TNF-α, TH17, NLRP3 | C |
| DPP4 Inhibitors | Reduction of metabolism of GLP, slow gastric emptying, promote weight loss | Reduce adipose tissue associated TNF-α, IL-6 via NF-κB signaling | C |
| GP1 Analogues | Stimulate GP1 receptor | C | |
| PPAR-γ Agonists | Stimulate PPAR-γ activity | Decrease IL-6 anti-proliferative activity | C |
| Oral Contraception | Estrogen-response elements on gene transcription | Dendritic cell, T-cell, possible role of 16-α estrogens NF-κB, AP-1, PI3/Akt pathway modulation |
C |
| Finasteride | 5-α reductase inhibitor | IGF-1 dependent: possibly increased activity in insulin resistance/diabetic patients | C |
| Spironolactone | Aldosterone antagonist | Reduction in TNF-α, IL-6, NOS | C |
| Oral antimicrobials | |||
| Doxycycline | 30S ribosomal inhibition | Decreased IL-1, IL-6, IL-8, TNF-α, chemotaxis, lipo-oxygenase inhibition, MMP inhibition, NF-κB signaling inhibition | B |
| Minocycline | Downregulation of LPS-stimulated TLR2 activity; upregulated TIMP-1 | C | |
| Erythromycin | 50S ribosomal inhibition | Decreased IL-6, IL-8, TNF-α, GM-CSF activity. Downregulated AP-1 and NF-κB activity | C |
| Clindamycin | C | ||
| Rifampicin | Controversial: glucocorticoid receptor, | Reduced iNOS transcription, reduced NF-κB activity. Reduces TH17 differentiation | C |
| Ciprofloxacin | Inhibition of DNA gyrase and topoisomerase IV | Effects upon cAMP, NF-κB, AP-1, IL-8, IL-6 and gastrointestinal microbiome | C |
| Moxifloxacin | Reduction of IL-1ß, IL-8, TNF-α. Stabilization of IXb protein. Suppresses NF-κB signaling. Reduction in IL-17A |
C | |
| Metronidazole | Inhibition of nucleic acid synthesis | Impacts on gastrointestinal microbiome | C |
| Ertapenem | Binding to Penicillin-binding proteins and disruption of cell wall synthesis | Reduction in IL-6, IL-12, TNF-α | C |
| Linezolid | Inhibition of protein synthesis initiation | Reduction in IL-1β, IL-6, IL-8, TNF-α. Possible ERK1/2 signaling modulation |
C |
AP-1, activator protein 1; cAMP, cyclic adenosine monophosphate; GLP, glucagon like peptide; GM-CSF, granulocyte-macrophage colony-stimulating factor; HS, hidradenitis suppurativa; IGF-1, Insulin Like Growth Factor- 1; IL, interleukin; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MMP, matrix metalloproteinase; NF-κB, nuclear factor kappa B; NOS, nitric oxide synthase; PPAR, peroxisome proliferator-activated receptor; TLR2, Toll-like receptor; TNF, tumor necrosis factor.
Hormonal therapies
The evidence for the association between HS and estrogens, progestins, and androgen activity is contradictory. Whilst an epidemiological association with polycystic ovarian syndrome is known72 a recent systematic review did not identify a consistent hormonal abnormality in HS patients,73 although alterations in hormone levels are associated with disease flares.73 This has been well documented in perimenstrual, perimenopausal and postpartum women experiencing painful disease flares. It has been proposed that end-organ (follicular) activity of sex hormones may play a role in disease pathogenesis,74 however immunohistochemical findings show no evidence of dysregulated sex hormone receptors in the lesional skin of HS patients compared with healthy controls.8 Despite this lack of evidence, a clinical benefit is seen in HS patients with anti-androgen therapy, including finasteride, spironolactone, and antiandrogenic progestogens (cyproterone acetate, chlormadinone acetate, drospirenone;74,75 Table 3). It is acknowledged that variations in dosages and patient characteristics may confound the results of individual case reports; however, analysis of individual case reports in hormonal therapies is beyond the scope of this review. Sex hormones have immunomodulatory activity through their effects upon dendritic cells, T-cells maturation, differentiation, and suppression of the Th1 immune response.76 Bimodal immune activity is dependent upon sex, the specific inflammatory site, the cytokine milieu and the endogenous sex hormone levels.76,77 There is also evidence to suggest immune-modulating mechanisms of estrogens which are independent of canonical estrogen-response elements.76,77 These include important immune pathways such as NF-κB, SP1 and AP1,76,77 and signal transduction pathways including PI3K/AKT pathways77 implicated in HS.78 In line with other inflammatory rheumatological disorders, this may be mediated through estrogen metabolites (16-α estrogens), which are known to modulate local immune responses in arthritis and encourage the development of juxta-inflammatory adipose tissue and insulin resistance77 both seen in HS. Hence, identification of 16-α estrogens is a potential biomarker candidate for individuals that may benefit from hormonal therapies in HS.
A number of case reports have documented clinical improvements in HS with finasteride, a 5-α reductase antagonist.79 Anecdotally, Clark78reports that finasteride is more effective in obese patients with HS. Given that 5-α reductase is insulin-like growth factor (IGF) 1-dependent, it is conceivable that finasteride may have increased benefits in individuals with insulin resistance or diabetes, although this has not been systematically investigated. Spironolactone is an aldosterone antagonist and has been associated with reduced inflammatory cytokine levels in various tissues,80 as well as suppression of TNF-α, IL-6 and inhibition of NF-κB phosphorylation and nitric oxide synthesis,80 although the precise mechanism in HS is not well described.
Antimicrobial therapies
Oral antibiotics
There is a steady shift away from considering HS as a disease of infectious etiology to a chronic inflammatory condition.1,19 However the role of bacteria as either a driver of disease or a secondary bystander is unclear.19,81 Alterations to the cutaneous microbiome do influence disease activity, but through an aberrant immune response rather than a traditional infectious response82 Despite this, the role of antibiotics in HS is still seen by some patients and physicians as eliminating infection.83 Examining the types of antibiotics used in HS (Table 3) reveals that the most effective options include therapies with significant anti-inflammatory effect (carbapenems, aminoglycosides).
Tetracyclines in HS, which have shown clinical efficacy also have no effect on HS bacteriological cultures, suggesting an effect independent of bactericidal activity.84
Tetracyclines
Tetracyclines are recommended for Hurley stage 1 or early-stage 2 disease6,40 with one randomized controlled trial demonstrating no benefit compared with topical clindamycin.42 Tetracyclines have well described anti-inflammatory activities aside from their antibacterial role through inhibition of the 30S ribosomal unit. Tetracyclines reduce IL-1, IL-6, TNF-α, and IL-8.85 IL-8 is vital for inhibition of neutrophil chemotaxis, the inhibition of reactive oxygen species, MMPs and lipooxygenases.85 This MMP-blocking ability is functional at submicrobial doses and in tetracyclines that have been altered to remove their antimicrobial activity.85 Minocycline has the additional benefit of LPS-stimulated TLR2 inhibition and has similar reactive oxygen species scavenger activity to tocopherol (vitamin E). It also inhibits macrophage function through a reduction in oxidized lipids, upregulated tissue inhibitor of metalloproteinase (TIMP-1) and inhibits NF-κB signaling.86
Rifampicin
Rifampicin, in combination with clindamycin has been established as a beneficial treatment for HS.6 The anti-inflammatory mechanism of action is controversial and may act via the corticosteroid receptor.87 It also reduces the transcription of inducible nitric oxide synthase (iNOS) and competes with NF-κb for coactivator proteins and hence reduces NF-κB activity.87 Rifampicin is also known to reduce TH17 differentiation and modulates T-cell responses.88 From a proinflammatory standpoint it can also activate NF-κB and suppress PPAR-γ expression and activity.87 This suppression of PPAR-γ only occurs in the presence of the proinflammatory cytokine milieu88 although the clinical relevance of such data is unclear.
Clindamycin and erythromycin
Macrolide antibiotics function anti-microbially via the inhibition of the 50S ribosomal unit impairing bacterial protein synthesis.89 Macrolides also alter the bacterial biofilm structure by altering polysaccharide synthesis. Other anti-inflammatory effects include the decreasing activity of TNF-α, IL-8, IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) in neutrophils and epithelial cells via the augmentation of activator protein 1 (AP-1) and NF-κB activity in the nucleus.89 There is also some preliminary data suggesting augmentation of dendritic cell function with macrolides, with the effect varying between different forms of macrolide.89 A combination of rifampicin-clindamycin has been a widely recommended treatment.6 However, it has been recently proposed that rifampicin monotherapy may be sufficient for disease suppression and can be safely extended beyond the traditional 10-week mark, whilst the risks of Clostridium difficile infection remained elevated (coinciding with the use of clindamycin) during the 10 weeks of combined therapy.90
Moxifloxacin
Moxifloxacin is a fluoroquinolone antibiotic frequently used for the treatment of pneumonia91 and is part of a recommended combination including rifampicin and metronidazole for disease control.42 It has been demonstrated in vitro that moxifloxacin decreases activity of TNF-α, IL-8, IL-1ß via stabilization of the IXb protein and prevents translocation of NF-κB to the nucleus.91,92 However, there is conflicting evidence regarding the clinical relevance of this anti-inflammatory activity. Animal models indicate that IL-1ß and IL-17A are reduced by moxifloxacin in the presence of viable bacteria, but not bacteria inactivated by heat.93 Investigation in human models is needed to clarify the relevance of these results.
Metronidazole
As metronidazole is used as an adjuvant in therapy with moxifloxacin and rifampicin, the individual contribution of this drug to inflammation in HS is unclear. One unique aspect of metronidazole in comparison with other antibiotics used for the management of HS, is that is has well-documented impacts upon the gastrointestinal microbiome, resulting in metabolic dysregulation predisposing to obesity and insulin resistance.64,65,94 Therefore in addition to the anti-inflammatory mechanisms of antibiotics in HS, microbiome alterations may also have indirect anti-inflammatory effects in HS.
Intravenous antibiotics
The intravenous antibiotics ertapenem and linezolid have been reported as highly effective in chronic recalcitrant HS95,96 as a temporary bridge to definitive surgical management. Both drugs are known to have significant effects on the gut microbiota.95,96 Despite its efficacy during the initial 6-week course of therapy, relapses and flares are commonly reported after treatment cessation, requiring ongoing intermittent dosing, which raises concerns regarding emerging resistance of Pseudomonas sp. and Enterobacteriaceae sp.97 The shared anti-inflammatory activities of both agents include a reduction in IL-6, IL-12, TNF-α, as well as reduced activity of macrophages.95,97,98 The risk of antibiotic resistance needs to be weighed against the efficacy of alternative anti-inflammatory therapies in the use of these therapies in HS.
Systemic immunomodulators and biologics
Given the recognized role of inflammation in the pathogenesis of HS,8 systemic immunosuppression has been tested in HS with variable success (Table 4). The overall level of evidence for systemic immunosuppression in HS is low,13 with high-level evidence such as randomized controlled trials (RCTs) only available for etanercept99 adalimumab,100 infliximab,101 anakinra,102 apremilast103 MABp1104 and bermekimab (IFX-1).105
Table 4.
Effects of systemic immunomodulators in HS including assessment of the quality of evidence in each therapy.
| Systemic immunomodulator | Mechanism of action |
Quality of evidence15 | |
|---|---|---|---|
| Canonical effect | Proposed mechanism in HS | ||
| Methotrexate | Dihydrofolate reductase inhibitor | Correction of FoxP3/Treg function leading to correction of TH17/Treg ratio | C |
| Cyclosporine | Calcineurin inhibitor | JNK p53 NFAT inhibitor, specific inhibitor of T-cells | C |
| Acitretin | ATRA prodrug: RXR/RAR nuclear transcription factors. |
Alteration in AP-1, NF-κB transcription, Promotes conversion of naïve T-cells to Foxp3 regulatory T-cells |
C |
| Isotretinoin | C | ||
| Dapsone | Reduction in superoxide production and neutrophil function | Reduction of neutrophil chemotaxis and oxidative damage | C |
| Apremilast | PDE4 inhibitor, reducing intracellular cAMP | Direct effect on T-cells dendritic cells, macrophages and monocytes, reducing IFN-γ and IL-2. Increases IL-10 | B |
| INCB 57407 | JAK1 inhibitor | Multiple sites of action including suppression of inflammatory activity through alteration in gene regulation and expression in keratinocytes and leukocytes | Trials ongoing NCT 03607487 |
cAMP, cyclic adenosine monophosphate; HS, hidradenitis suppurativa; IFN, interferon; IL, interleukin; NCT, ClinicalTrials.gov identifier; PDE4, phosphodiesterase 4; Treg, T regulator cell.
Colchicine
Colchicine is an anti-inflammatory agent used in the treatment of gout as well as autoinflammatory conditions including familial Mediterranean fever.106 It functions via inhibition of tubulin polymerization, neutrophil function, suppression of NALP3 inflammasome, dendritic cell maturation as well as VEGF, S100A8, S100A9, NF-κB and Caspase 1.106,107 It has demonstrated some benefit in prospective trials in HS,107 however as in gout, it is limited by gastrointestinal side effects.
Methotrexate
Methotrexate has been reported in open-label studies but has not demonstrated any significant improvement in the degree of inflammation or frequency of flares in HS.108 Methotrexate is useful in the prevention of autoantibodies in the setting of adalimumab and infliximab therapy;109 however there is no indication in the literature that adjuvant methotrexate has a significant impact upon disease control in HS. Despite the fact that methotrexate has been shown to restore expression of FOXp3 and Treg function,110 methotrexate is reported as of ‘limited value’ for the treatment of HS108 with no patients in one prospective study demonstrating any improvement with the drug. This is suggestive that whilst Th17/Treg homeostasis is involved in the pathogenesis of HS,67 either it is not the sole inflammatory mechanism, or only a specific patient population may benefit from methotrexate therapy.
Cyclosporine
Cyclosporine therapy has been reported to result in substantial improvement in recalcitrant HS111 however the true efficacy is difficult to discern given the co-administration of prednisolone and oral antibiotics in many cases.112 The largest case series of 18 patients included only 2 patients who reported clinically significant improvement.112 Putative mechanisms include the suppression of IL-2 and interferon (IFN)-γ via the known mechanisms of calcineurin inhibition in cyclosporine.113 Where cyclosporine was co-administered with antibiotics it was unable to be elucidated whether the therapeutic effect was due to cyclosporine potentiating the effect of antibiotics (via CYP3A4) or the additive effects of both agents.
Acitretin and isotretinoin
Acitretin has been used in HS in various case series, with contradictory reports of success. A review by Blok and colleagues114 reported an improvement rate of 73%; however, a recent series by Tan and colleagues reported no improvement with acitretin monotherapy.115 A prospective study by Matusiak116 showed up to half of patients treated showed some improvement in symptoms, with a large proportion of stage 1 and 2 patients demonstrating improvement.116 Regarding the mechanisms of acitretin in HS, acitretin has been demonstrated to reduce the level of TH17 cells and serum IL-17 levels in psoriasis114 and also contributes to re-stabilizing the Th17/Treg imbalance proposed to be central to inflammation in the disease.67 The tolerability of treatment with retinoids has been hampered by the high dosages needed to sustain ongoing improvements.
Isotretinoin has been reported to have a moderate to significant improvement in younger, female patients with facial acne114,117 however no clinical benefit has been seen in Hurley stage 3 patients. Its mechanism of action in acne has been reported to be mediated by multiple metabolites, including via the RXR-γ receptor leading to reduction in the size of the sebaceous glands. Given the known atrophy of sebaceous glands in established HS8 it is more likely that it exerts an effect through immunomodulatory mechanisms. It is known that all-trans retinoic acid (of which isotretinoin is a prodrug) modulates the function of T-cells and monocytes,118 particularly the induction of TH17 cells via IL-6.67
Dapsone
Dapsone, similarly to retinoids, provides improvement in mild HS and has no documented response in severe Hurley stage 3 disease.119 Dapsone functions via anti-inflammatory and bacteriostatic properties.120 Given the sulfone-sensitive nature of microbial flora in HS,63 it is possible that the mechanism of action of dapsone may be contributed to by some antimicrobial effect. The anti-inflammatory effect of dapsone is mediated by suppression of superoxide production and it also downregulates the LPS-stimulated production of TNF-α and IL-8.120 As both of these cytokines are prevalent in HS,8 this could provide a mechanism for the partial response to dapsone. Dapsone is also known to reduce neutrophil chemotaxis120 which may explain the additional effect in early-stage disease.
Apremilast
Apremilast is a phosphodiesterase 4 (PDE4) inhibitor currently used in psoriasis and psoriatic arthritis.121 It has been explored as a potential treatment in other inflammatory conditions including HS, with one RCT104 of 20 patients, with 53% achieving the hidradenitis suppurativa clinical response (HiSCR) at week 16. The mechanism of apremilast is the accumulation of intracellular cyclic adenosine monophosphate (cAMP) resulting in protein kinase A activation and the regulation of multiple transcription factors including activating transcription factor 1 (ATF-1), CREB-binding protein and NF-κB, which results in the decreased production of IFN-γ and IL-2. IL-10 is also increased with reduced stimulation of T-cells, monocytes and macrophages.121,122 Given the diversity of the effects of apremilast this may be a promising avenue of investigation for the treatment of the inflammatory dysregulation in HS and shows a promising effect in well-established disease.
Vitamins, supplements and alternative treatments
Vitamins and supplements including zinc,123 myo-inositol,124 folic acid124 and magnesium124 have been reported in individual case reports in HS; however, there is no evidence to suggest increased efficacy over antibiotic therapy.124 There are also significant risks of toxicity and adverse effects with high dosages.125 In instances where anti-inflammatory activity has been identified, no link to clinically significant response has been seen that could not be accounted for by placebo.123,124 Overall, further investigation is needed into the mechanisms and role of alternative therapies in HS.
Monoclonal antibodies (biologics)
Monoclonal antibody therapy has revolutionized the treatment of chronic inflammatory disorders, such as psoriasis, rheumatoid arthritis and inflammatory bowel disease.126 Adalimumab is currently the only US FDA-approved monoclonal biologic therapy for HS,100 however a wide variety of monoclonal antibodies have been trialed as a therapy in HS (Table 5), including in current phase II clinical trials. Response rates vary between therapies, and given the specific targets of these drugs, this variation offers insights into important pathogenic pathways in HS. It may also confirm or refute the concept of pathogenic heterogeneity in HS.127
Table 5.
Monoclonal antibodies reported in HS including drugs under investigation.
| Monoclonal antibody | Mechanism(s) of action |
Quality of evidence15 | |
|---|---|---|---|
| Therapeutic target | Proposed mechanism in HS | ||
| Adalimumab | TNF-α | Reduction in TNF-α associated inflammation as well as keratinocyte-mediated feed-forward mechanisms. |
A |
| Etanercept | TNF-α | B | |
| Infliximab | TNF-α | B | |
| Anakinra | IL-1R | Interruption of keratinocyte-mediated feed-forward mechanisms as well as microbiome-associated inflammatory drive. | B NCT 01516749 NCT 01558375 |
| Bermekimab (MABp1) | IL-1α | B NCT 02643654 |
|
| Secukinumab | IL-17A | Preferential suppression of keratinocyte-induced feed-forward mechanisms and correction of Th17/Treg dysfunction | Trials ongoing NCT 03099980 |
| Bimikizumab | IL-17A / IL-17F | Trials ongoing NCT 03248531 |
|
| Ixekizumab | IL-17A | C (case reports) |
|
| Ustekinumab | IL-12 /IL-23 p40 subunit |
Trials ongoing NCT 01704534 |
|
| Guselkumab | IL-23 | Trials ongoing NCT 03628924 |
|
| IFX-1 | C5a | Indirect suppression of TNF-α via upstream pathways | Trials ongoing (NCT 03001622) |
| Efalizumab | LFA-1 | Interruption of ICAM-1-mediated inflammation No improvement in prospective trial (n = 5) |
NCT 00134134 |
| Drugs under investigation | |||
| MEDI8968 | IL-1 receptor I inhibitor | Interruption of keratinocyte-mediated feed-forward mechanisms as well as microbiome-associated inflammatory drive. | Trials ongoing NCT 01838499 |
| CJM112 | IL-17A inhibitor | Preferential suppression of keratinocyte-induced feed-forward mechanisms and correction of Th17/Treg dysfunction | Trials ongoing NCT 02421172 |
HS, hidradenitis suppurativa; IL, interleukin; NCT, ClinicalTrials.gov identifier; PDE4, phosphodiesterase 4; TNF, tumor necrosis factor; Treg, T regulator cell.
Elevated levels of TNF-α, IL-17, IL-1 and C5a have been identified in lesional tissue of HS patients8 and this has been the justification for selective targeting of these inflammatory pathways. TNF-α, as a nonspecific inflammatory cytokine, has inferior clearance and response rates to other therapies such as IL-17 and IL-23 inhibitors in psoriasis128 and the hope is that, similarly to psoriasis, novel HS-specific therapeutic targets will be identified for which monoclonal antibodies can be developed. TNF-α has broad anti-inflammatory effect including modulating the activity of dendritic cells, monocytes and neutrophils.128
The role of IL-17 inhibition in HS is currently under investigation in multiple phase II clinical trials (Table 5). The underlying premise behind IL-17 inhibition includes the presence of Th17 cells in HS lesional tissue,8 the role of IL-22, IL-17 and IL-23 in promoting the epidermal hyperplasia seen in HS8 and evidence from case reports of efficacy of IL-17 blockade in HS.129,130,131 Strong IL-17 signals are seen more in moderate and severe disease,8 so IL-17 may be beneficial in patients where TNF-α therapy has failed. A concern with the use of IL-17 antagonism in HS, however, is the association of HS with inflammatory bowel disease (IBD)131 and the inefficacy (and paradoxical worsening of disease) with IL-17 blockade in RCTs of IBD.132 Interestingly, genetic studies identified the absence of a minor allele (TNF-like ligand 1A) which was associated with a lack of response in these patients.132 Therefore, if IL-17 blockade does demonstrate an effect in HS, there may be pharmacogenomic indicators which may predict benefit in patients with HS and IBD. This would be an interesting area of further research.
Novel agents including IFX-1,104 targeting C5a anaphylatoxin, are purported to indirectly suppress TNF-α activity in HS. An RCT including 20 patients demonstrated response rates of 60% compared with 10% for placebo.104 C5a signaling is also involved in IL-17 and IL-23 signaling through the modulation of dendritic cell activity.133,134 However, the unaffected section of the complement cascade involving C5b-9 and membrane attack complex activation leads to multiple signal transduction activity including PI3K/Akt activation, STAT3 phosphorylation and cDC2 activation.132 The cDC2 activity mediates TNF-α activity as well as the modulation of transforming growth factor (TGF)-ß activity but does not block IL-22 production, which mediates Th17 activity.133,134 This mechanism may explain persistent inflammation in patients not responsive to IFX-1 and C5a blockade,104 but also gives hope that IFX-1 may be more effective in patients with more advanced (Hurley stage 3) disease with fistulae, hypertrophic scarring and high TGF-ß activity.8
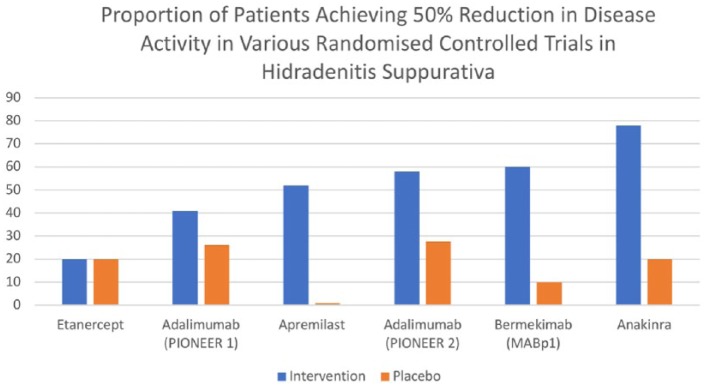
IL-1 blockade has been trialed in HS with the use of anakinra,102 an IL-1R antagonist and more recently with bermekimab105 a fully human anti-IL-1α antagonist. Both drugs have been investigated in RCTs (Figure 3) with response rates of 78%102 and 60%105 respectively. Cytokines of the IL-1 family (IL-1α, IL-1ß, IL-1R and IL-33) are important mediators of T-cell recruitment in inflammatory skin disease.8,135 It also has significant potential for activation of fibroblasts which may be involved in the development of scarring and fistulae in HS.8,135 The disparate rates in clinical response between anakinra and bermekimab also suggests that other members of the IL-1 family (such as IL-18 or IL-33) may be involved in HS which are targeted by anakinra but not by bermekimab.135
Figure 3.
Reported proportion of response in different RCTs in HS.
HS, hidradenitis suppurativa; RCT, randomized controlled trial.
Ustekinumab has been reported in a case reports136 and an open-label study137 as a therapy for HS, and elevation of IL12p40 has been identified in HS.8 Response rates (HiSCR) are reported as 47% with a positive response associated with low levels of LTA4H (leukotriene A4 hydrolaze)137 suggesting the activity of ustekinumab is more beneficial in the presence of active leukocyte activity,138 seen in early rather than advanced disease. Efalizumab (Raptiva®) has been investigated in a small cohort study139 of five women with moderate to severe, treatment-refractory HS. None of the individuals reported improvement of their symptoms.139 Efalizumab functions through the blocking the actions of LFA-1 (leukocyte function associated antigen 1), including ICAM-1 binding, ICAM-1 has been identified as involved in the inflammatory mechanisms of HS8 and is suggested to be involved in Porphyromonas and Peptinophillus-mediated inflammatory stimulation, including in the development of fistulae. Blockage of ICAM-1 binding to leukocytes may potentiate the epithelial activity of ICAM-1 leading to increases in epithelial-associated inflammation, accounting for the worsening of disease seen in these patients.139
Other medications currently under investigation in clinical trials include JAK1 inhibitors, which, through IL-6 uncoupling, may ameliorate IL-22-mediated inflammation as well as potentially the progression of scarring as is seen in other disorders including chronic GvHD.140 However, given the contribution of Th1 and Th17 to inflammation in HS,8 it may be that combination of JAK1 and JAK2 inhibition may be required in HS.141
Translating therapy into disease mechanisms
The common pathways implicated in topical, systemic and immunomodulatory therapies are illustrated in Figure 2. Significant differences exist between the agents and implicated pathways in therapy for mild as opposed to severe disease. This is an expression of the possible dichotomy in inflammatory pathways (and hence varying responses to treatment) that exists in HS.
A common theme in multiple agents recommended for use in mild to moderate disease is the augmentation of the NF-κB pathway as well as other inflammatory cytokines including TNF-α and IL-6, whether this be through topical or oral antibiotic therapy. This nonspecific inflammatory pathway, as well as the favorable rates of remission in mild HS, suggest that the disease in this stage is comparable to the chronic, relapsing inflammatory disorders including psoriasis and atopic dermatitis, which we are able to treat effectively. Continuous therapy can control the disease, but therapy must be balanced against adverse effects, patient preference and potential long-term sequelae. The role of 16-α estrogens as a potential biomarker for the utility of hormonal therapy in HS is also an area requiring further study.
The role of bacteria in HS is still controversial; however, what is agreed upon is that the inflammatory component of HS is likely to be an aberrant response to a dysbiotic cutaneous microbiome.19,63 In early disease, antibacterial actions may have an effect upon the disease process, but the evidence to date suggests that the majority of effects are due to anti-inflammatory alterations. The bacteriostatic effect of antibacterial treatments may also indicate that bacterial virulence factors (which are not eliminated by bacteriostatic effects) may also play a role in the pathogenesis of disease. There is a moderate impact on biofilm formation and prevalence, and the incomplete elimination of biofilms may contribute to disease recurrence along with the presence of a chronic feed-forward inflammatory drive. Despite this, the only proven major benefit of antibacterial therapies are the prevention of secondary colonization of impaired skin. These results are in direct conflict with the follicular occlusion paradigm in HS and implies that inflammation precedes occlusion, as in acne vulgaris. This is also supported by immunohistochemical studies of HS.8
When HS presents (or progresses) to moderate and severe disease (presence of scarring, fistulae and draining sinuses), therapeutic escalation to systemic immunomodulatory therapies and intravenous antibiotic therapies commonly ensues. It is difficult to untangle the role of the microbiome in moderate to severe HS, however, one hypothesis that high dose antibiotics, through profound changes in the gastrointestinal microbiome, may influence systemic inflammation, is captivating. The (albeit limited) benefit of systemic agents such as retinoids suggest that NF-κB and AP-1 transcription factors142,143 still play a central role in the inflammatory cascade of more advanced HS. However, the lack of efficacy of other therapies including hormonal therapies, methotrexate and cyclosporine suggest that other self-perpetuating inflammatory mechanisms are involved.
Studies involving monoclonal antibodies provide the greatest insight into what these specific mechanisms may be. The varying levels of clinical efficacy compared with placebo (Figure 3) bolsters the suggestion that differential inflammatory pathways may be involved in different presentations of disease. Within studies, particularly with adalimumab,100 the rates of efficacy were reduced for Hurley stage 3 patients than Hurley stage 2 patients. This is in contrast to bermekimab105 and anakinra,102 where response rates are highest; and the proportion of Hurley stage 3 patients are significantly higher. It is unclear whether IL-17 blockade will have similar results to TNF-α inhibition in HS (with greater benefit in mild disease as opposed to severe disease), or whether due to the augmentation in epithelial hyperplasia, keratinocyte-mediated feed-forward mechanisms may be interrupted, leading to similar response rates to IL-1 blockade (with greater utility in severe disease). Small molecule targets such as apremilast and JAK inhibition have the potential to be useful monotherapies and adjuvants and further clinical trials are sorely needed.
The significant placebo rates (up to 30%) in HS RCTs serve as a caveat to the interpretation of small degrees of clinical improvement in HS. Due to the lack of controlled, untreated natural history studies in HS, the rates of spontaneous resolution of lesions are difficult to discern in comparison with other chronic inflammatory skin diseases, such as atopic dermatitis and psoriasis. An additional complicating factor is the proinflammatory contribution of metabolic comorbidities (such as diabetes and obesity).8 Subgroup analysis in existing studies is unreliable due to small patient numbers, however it is feasible that specific therapies may be more beneficial in HS patients with specific comorbidities due to the specific cytokine stimulation caused by other comorbid conditions present in these patients.
Conclusion
Examining the therapeutic mechanisms in treatments for HS has identified a potential dichotomy in inflammatory processes between mild to moderate and moderate to severe disease. NF-κB, TNF-α and other innate immune mechanisms are strongly represented in treatments which are effective in mild to moderate disease in the absence of scarring or draining fistulae (Hurley stage 1 and Hurley stage 2 disease); however, complex feed-forward mechanisms in severe disease (Hurley stage 2 to Hurley stage 3) respond to IL-1 inhibition but are less likely to respond to innate immune inhibition (through NF-κB or TNF-α) alone. It is unclear whether IL-17 inhibition will parallel TNF-α or IL-1 inhibition in effect; however, it is plausible that small molecule targets may provide an effective new strategy. It is also plausible that personalized treatment regimens based upon comorbidities and genetic variants will be essential to identify the most effective therapy for HS patients.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JWF, JEH and JGK were supported in part by a grant # UL1 TR001866 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. JEH was supported in part by a grant # KL2TR001865 from the NCATS, NIH CTSA program.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JEH serves as an investigator/consultant for Pfizer Inc. and Novartis. JGK has been a consultant to, and has received research support from, the following companies that have developed or are developing therapeutics for psoriasis: AbbVie, Amgen, Boehringer, Bristol–Myers Squibb, Celgene, Dermira, Idera, Janssen, Leo, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, Serono, Sun, Valeant, and Vitae. The other authors have no financial conflicts of interest
ORCID iD: John W. Frew  https://orcid.org/0000-0001-5042-3632
https://orcid.org/0000-0001-5042-3632
Contributor Information
John W. Frew, Laboratory of Investigative Dermatology, The Rockefeller University, 1230 York Avenue, New York, NY 10065, USA.
Jason E. Hawkes, Laboratory of Investigative Dermatology, The Rockefeller University, New York, NY, USA
James G. Krueger, Laboratory of Investigative Dermatology, The Rockefeller University, New York, NY, USA
References
- 1. Hoffman LK, Ghias MH, Lowes MA. Pathophysiology of Hidradenitis Suppurativa. Semin Cutan Med Surg 2017; 36: 47–54. [DOI] [PubMed] [Google Scholar]
- 2. Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based clinical practice research datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol 2018; 178: 917–924. [DOI] [PubMed] [Google Scholar]
- 3. Frew JW, Vekic DA, Woods JA, et al. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol 2017; 177: 987–998. [DOI] [PubMed] [Google Scholar]
- 4. Kirby JS, Miller JJ, Adams DR, et al. Healthcare utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol 2014; 150: 937–944. [DOI] [PubMed] [Google Scholar]
- 5. Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173: 1546–1549. [DOI] [PubMed] [Google Scholar]
- 6. Zouboulis CC, Desai N, Emtestam L, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29: 619–644. [DOI] [PubMed] [Google Scholar]
- 7. Fotiadou C, Vakirlis E, Ionnides D. Spotlight on adalimumab in the treatment of active moderate-to-severe hidradenitis suppurativa. Clin Cosmet Invest Dermatol 2016; 9: 367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of inflammatory cytokine associations in hidradenitis suppurativa. F1000Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riis PT, Thorlacius LR, Jemec GB. Investigational drugs in clinical trials for hidradenitis suppurativa. Expert Opin Investig Drugs 2018; 27: 43–53. [DOI] [PubMed] [Google Scholar]
- 10. Maarouf M, Clark AK, Lee DE, et al. Targeted treatments for hidradenitis suppurativa: a review of the current literature and ongoing clinical trials. J Dermatolog Treat 2018; 29: 441–449. [DOI] [PubMed] [Google Scholar]
- 11. Frew JW, Hawkes JE, Krueger JG. A systematic review and critical evaluation of immunohistochemical associations in hidradenitis suppurativa [version 1; referees: awaiting peer review]. F1000Research 2018; 7: 1923, 10.12688/f1000research.17268.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pesce A, Capuzzo G, Cammisuli B, et al. Pilonidal disease, hidradenitis suppurativa and follicular occlusion syndrome: a diagnostic challenge. Eur Rev Med Phamacol Sci 2018; 22: 4755–4756. [DOI] [PubMed] [Google Scholar]
- 13. Ingram JR, Woo PN, Chua SL, et al. Interventions for hidradenitis suppurativa. Cochrane Database Syst Rev 2015; 10: CD010081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deckers IE, Prens EP. An update on medical treatment options for hidradenitis suppurativa. Drugs 2016; 76: 215–229. [DOI] [PubMed] [Google Scholar]
- 15. Robinson JK. Systematic reviews: grading recommendations and evidence quality. Arch Dermatol 2008; 144: 97–99. [DOI] [PubMed] [Google Scholar]
- 16. McDonnell G, Russell D. Antiseptics and disinfectants: activity, action and resistance. Clin Microbiol Reviews 1999; 12: 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonez PC, Filippidos C, Alves S, et al. Chlorhexidine activity against bacterial biofilms. Am J Infect Control 2013; 41: e119–e122. [DOI] [PubMed] [Google Scholar]
- 18. Fitzgerald KA, Davies A, Russell AD. Bacterial uptake of 14C-chlorhexidine diacetate and 14C-benzyl alcohol and the influence of phenoxyethanol and azolectin: studies with gram-negative bacteria. Microbios 1992; 70: 77–91. [PubMed] [Google Scholar]
- 19. Guet-Revillet H, Jais JP, Ungeheuer MN, et al. The microbiological landscape of anaerobic infections in hidradenitis suppurativa: a prospective metagenomic study. Clin Infec Dis 2017; 65: 282–291. [DOI] [PubMed] [Google Scholar]
- 20. Ingram JR, McPhee M. Management of hidradenitis suppurativa: a UK survey of current practice. Br J Dermatol 2015; 173: 1070–1072. [DOI] [PubMed] [Google Scholar]
- 21. Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, et al. Povidone iodine in wound healing: a review of current concepts and practices. Int J Surgery 2017; 44: 260–268. [DOI] [PubMed] [Google Scholar]
- 22. Chandler CJ, Segel IH. Mechanism of the antimicrobial action of pyrithione: effects on membrane transport, ATP levels, and protein synthesis. Antimicrob Agents Chemother 1978; 14: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitamura H, Morikawa H, Kamon H, et al. Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 2006; 7: 971–977. [DOI] [PubMed] [Google Scholar]
- 24. Lamore SD, Wondrak GT. Zinc pyrithione impairs zinc homeostasis and upregulates stress response gene expression in reconstructed human epidermis. BioMetals 2011; 24: 875–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yoon KS, Youn N, Gu H, et al. Estrogenic activity of zinc pyrithione: an in vivo and in vitro study. Environ Health Toxicol 2017; 32: e2017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perumal PK, Wand ME, Sutton JM, et al. Evaluation of the effectiveness of hydrogen peroxide-based disinfectants on biofilms formed by Gram-negative pathogens. J Hosp Infect 2014; 87: 227–233. [DOI] [PubMed] [Google Scholar]
- 27. Lajevardi SS, Abeysinghe J. Novel technique for management of axillary hidradenitis suppurativa using setons. Case Rep Surg 2015; 2015: 369657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Banerjee S, Zmijewski JW, Lorne E, et al. Modulation of SCFBetaTrCP dependent IkBa ubiquination by hydrogen peroxide. J Biol Chem 2010; 285: 2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wittmann C, Chockley P, Singh SK, et al. Hydrogen peroxide in inflammation: messenger, guide, and assassin. Adv Hematol 2012; 2012: 541471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang JT, Abrams M, Tlougan B, et al. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009; 123: e808–e814. [DOI] [PubMed] [Google Scholar]
- 31. Leung TH, Zhang LF, Wang J, et al. Topical hypochlorite ameliorates NF-κB-mediated skin diseases in mice. J Clin Invest 2013; 123: 5361–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eriksson S, van der Plas MJA, Morgelin M, et al. Antibacterial and antibiofilm effects of sodium hypochlorite against Staphylococcus aureus isolates derived from patients with atopic dermatitis. Br J Dermatol 2017; 117: 513–521. [DOI] [PubMed] [Google Scholar]
- 33. Epstein AK, Pokroy B, Seminara A, et al. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc Natl Acad Sci U S A 2011; 108: 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knowlden SA, Yoshida T, Perez-Nazario N, et al. Bleach baths promote early induction of inflammatory pathway genes with no effect on skin bacterial dysbiosis in AD subjects [abstract]. J Invest Dermatol 2017; 137: S50. [Google Scholar]
- 35. Barros SP, Wirojchanasak S, Barrow DA, et al. Triclosan inhibition of acute and chronic inflammatory gene pathways. J Clin Periodontol 2010; 37: 412–418. [DOI] [PubMed] [Google Scholar]
- 36. Lubarsky HV, Gerbersdorf SU, Hubas C, et al. Impairment of the bacterial biofilm stability by triclosan. PLoS One 2012; 7: e31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hessam S, Sand M, Meier NM, et al. Combination of oral zinc gluconate and topical triclosan: an anti-inflammatory treatment modality for initial hidradenitis suppurativa. J Dermatol Sci 2016; 84: 197–202. [DOI] [PubMed] [Google Scholar]
- 38. Maiden MM, Agostinho Hunt AM, Zachos MP, et al. Triclosan is an aminoglycoside adjuvant for the eradication of Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother 2018; 62: pii: e00146–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gulliiver W, Zouboulis CC, Prens E, et al. Evidence-based approach to treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord 2016; 17: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol 1983; 22: 325–328. [DOI] [PubMed] [Google Scholar]
- 41. Jemec GB, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998; 39: 971–974. [DOI] [PubMed] [Google Scholar]
- 42. Zouboulis CC, Bechara FG, Dickinson-Block JL, et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol. Epub ahead of print 23 October 2018. DOI: 10.1111/jdv.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hodille E, Badiou C, Bouveyron C, et al. Clindamycin suppresses virulence expression in inducible clindamycin-resistant Staphylococcus Aureus strains. Ann Clin Microbiol Antimicrob 2018; 17: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altenberg J, de Graaf CS, van de Werth TS, et al. Immunomodulatory effects of macrolide antibiotics - Part 1: biological mechanisms. Respiration 2011; 81: 67–74. [DOI] [PubMed] [Google Scholar]
- 45. Sieber MA, Hegel JK. Azelaic acid: properties and mode of action. Skin Pharmacol Physiol 2014; 27(Suppl. 1): 9–17. [DOI] [PubMed] [Google Scholar]
- 46. Charnock C, Brudeli B, Klaveness J. Evaluation of the antibacterial efficacy of diesters of azelaic acid. Eur J Pharm Sci 2004; 21: 589–596. [DOI] [PubMed] [Google Scholar]
- 47. Mastrofrancesco A, Ottaviani M, Aspite N, et al. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARγ activation. Exp Dermatol 2010; 19: 813–820. [DOI] [PubMed] [Google Scholar]
- 48. Dreno B, Gollnick HPM, Kang S, et al. Understanding innate immunity and inflammation in acne: implications for management. J Eur Acad Dermatol Venereol 2015; 29(Suppl. 4): 3–11. [DOI] [PubMed] [Google Scholar]
- 49. Bikowski JB. Mechanisms of the comedolytic and anti-inflammatory properties of topical retinoids. J Drugs Dermatol 2005; 4: 41–47. [PubMed] [Google Scholar]
- 50. Canavan TN, Chen E, Elewski BE. Optimizing non-antibiotic treatment for patients with acne: a review. Dermatol Ther (Heidelb) 2016; 6: 555–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gill L, Williams M, Hamzavi I. Update on hidradenitis suppurativa: connecting the tracts. F1000Prime Rep 2014; 6: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmidt N. Tretinoin: a review of its anti-inflammatory properties in the treatment of acne. J Clin Aesthet Dermatol 2011; 4: 22–29. [PMC free article] [PubMed] [Google Scholar]
- 53. Boer J, Jemec GB. Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa. Clin Exp Dermatol 2010; 35: 36–40. [DOI] [PubMed] [Google Scholar]
- 54. Pascual JC, Encabo B, Ruiz de Apodaca RF, et al. Topical 15% resorcinol for hidradenitis suppurativa: AN uncontrolled prospective trial with clinical and ultrasonographic follow-up. J Am Acad Dermatol 2017; 77: 1175–1178. [DOI] [PubMed] [Google Scholar]
- 55. Lightowler JE, Rylance HJ. On the anti-inflammatory activity of some substituted phenolic compounds. Br J Pharmacol Chemother 1964; 22: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J AM Acad Dermatol 2016; 75: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 57. Kraft JN, Searles GE. Hidradenitis suppurativa in 64 female patients: retrospective study comparing oral antibiotics and antiandrogen therapy. J Cutan Med Surg 2007; 11: 125–131. [DOI] [PubMed] [Google Scholar]
- 58. Wong D, Walsh S, Alhusayen R. Low-dose systemic corticosteroid treatment for recalcitrant hidradenitis suppurativa. J AM Acad Dermatol 2016; 75: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 59. Fajenbaum K. Intralesional triamcinolone, a standard of care treatment for acute hidradenitis suppurativa, does not prove to be superior to placebo. In: 2nd annual symposium on hidradenitis suppurativa, Hotel St Regis, Detroit, MI, USA, 4–5 November 2017. [Google Scholar]
- 60. Vilanova I, Hernandez JL, Mata C, et al. Insulin resistance in hidradenitis suppurativa: a case control study. J Eur Acad Dermatol Venereol 2018; 32: 820–824. [DOI] [PubMed] [Google Scholar]
- 61. Verdolini R, Clayton N, Smith A, et al. Metformin for the treatment of hidradenitis suppurativa: a little help along the way. J Eur Acad Dermatol Venereol 2013; 27: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 62. Ring HC, Thorsen J, Saunte DM, et al. The follicular skin microbiome in patients with hidradenitis suppurativa and healthy controls. JAMA Dermatol 2017; 153: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim CH. Immune regulation by microbiome metabolites. Immunology 2018; 154: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan JK, McKenzie C, Marino E, et al. Metabolite-sensing G protein coupled receptors – facilitators of diet-related immune regulation. Ann Rev Immunol 2017; 35: 371–402. [DOI] [PubMed] [Google Scholar]
- 65. Valeria de Vita Melnik BC. mTORC1 at the crossroad of metabolism and immunity in hidradenitis suppurativa. J Eur Acad Dermatol Venereol. Epub ahead of print 5 October 2018. DOI: 10.1111/jdv.15270. [DOI] [PubMed] [Google Scholar]
- 66. Melnik BC, John SM, Chen W, et al. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune conditions. Br J Dermatol 2018; 179: 260–272. [DOI] [PubMed] [Google Scholar]
- 67. Akdogan N, Alli N, Uysal PI. Visfatin and insulin levels and cigarette smoking are independent risk factors for hidradenitis suppurativa: a case control study. Arch Dermatol Res. Epub ahead of print 6 October 2018. DOI: 10.1007/s00403-018-1867. [DOI] [PubMed] [Google Scholar]
- 68. Insuela DRB, Carvalho VF. Glucagon and glucagon like peptide-1 as novel anti-inflammatory and immunomodulatory compounds. European J Pharmacol 2017; 812: 64–72. [DOI] [PubMed] [Google Scholar]
- 69. Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metabol 2016; 18: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dozsa A, Dezso B, Toth BI, et al. PPAR gamma-mediated and arachidonic acid dependent signaling is involved in differentiation and lipid production of human sebocytes. J Invest Dermatol 2014; 134: 910–920. [DOI] [PubMed] [Google Scholar]
- 71. Garg A, Neuren E, Strunk A. Hidradenitis suppurativa is associated with polycystic ovary syndrome: a population-based analysis in the United States. J Invest Dermatol 2018; 138: 1288–1292. [DOI] [PubMed] [Google Scholar]
- 72. Riis PT, Ring HC, Themstrup L, et al. The role of androgens and estrogens in hidradenitis suppurativa: a systematic review. Acta Derm Croat 2016; 24: 239–249. [PubMed] [Google Scholar]
- 73. Karagiannidis I, Nikolakis G, Sabat R, et al. Hidradenitis suppurativa/acne inversa: an endocrine skin disorder? Rev Endocr Metab Disord 2016; 17: 335–341. [DOI] [PubMed] [Google Scholar]
- 74. Karagiannidis I, Nikolakis G, Zouboulis CC. Endocrinological aspects of hidradenitis suppurativa. Derm Clin 2016; 34: 45–49. [DOI] [PubMed] [Google Scholar]
- 75. Bereshchenko O, Bruscoli S, Riccardi C. Glucocorticoids, sex hormones, and immunity. Front Immunol 2018; 9: 1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Straub RH. Interaction of the endocrine system with inflammation: a function of energy and volume regulation. Arthritis Res Ther 2014; 16: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xiao X, He Y, Li C, et al. Nicastrin mutations in familial acne inversa impact keratinocyte proliferation and differentiation through the Notch and phosphoinositide 3-kinase/AKT signaling pathways. Br J Dermatol 2016; 174: 522–532. [DOI] [PubMed] [Google Scholar]
- 78. Clark AK, Quinonez RL, Saric S. Sivamani hormonal therapy for hidradenitis suppurativa: review. Dermatol Online J 2017; 23. [PubMed] [Google Scholar]
- 79. Kato Y, Kamiya H, Koide N, et al. Spironolactone inhibits production of proinflammatory mediators in response to lipopolysaccharide via inactivation of nuclear factor-κB. Immunopharmacol Immunotoxicol 2014; 36: 237–241. [DOI] [PubMed] [Google Scholar]
- 80. Zhang L, Hao JB, Ren LS, et al. The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis. Lab Invest 2014; 94: 839–850. [DOI] [PubMed] [Google Scholar]
- 81. Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA 2017; 318: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 82. Naik HB, Nassif A, Ramesh MS, et al. Are bacteria infectious pathogens in hidradenitis suppurativa (HS)? Debate at the symposium for hidradenitis suppurativa advances meeting, November 2017. J Invest Dermatol Epub ahead of print 8 November 2018. DOI: 10.1016/j.jid.2018.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zaaroura H, Geffen Y, Bergman R, et al. Clinical and microbiological properties of Staphylococcus lugdunensis skin infections. J Dermatol 2018; 45: 994–999. [DOI] [PubMed] [Google Scholar]
- 84. Tilakaratne A, Soory M. Anti-inflammatory actions of adjunctive tetracyclines and other agents in periodontitis and associated comorbidities. Open Dent J 2014; 8: 109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sun J, Shigemi H, Tanaka Y, et al. Tetracyclines downregulate the production of LPS-included cytokines and chemokines in THP-1 cells via ERK, p38 and nuclear factor kB signaling pathways. Biochem Biophys Res 2015; 4: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schmidt KE, Kuepper JM, Schumak B, et al. Doxycycline inhibits experimental cerebral malaria by reducing inflammatory immune reactions and tissue-degrading mediators. PLos One 2018; 13: e0192717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yuhas Y, Berent E, Ovadiah H, et al. Rifampin augments cytokine-induced nitric oxide production in human alveolar epithelial cells. Antimicrob Agents Chemother 2006; 50: 396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ma K, Chen X, Chen JC, et al. Rifampicin attenuates experimental autoimmune encephalomyelitis by inhibiting pathogenic Th17 cells responses. J Neurochem 2016; 139: 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Altenberg J, de Graaf CS, van der Werf TS, et al. Immunomodulatory effects of macrolide antibiotics – part 1: biological mechanisms. Respiration 2011; 81: 67–74. [DOI] [PubMed] [Google Scholar]
- 90. Albrecht J, Barbaric J, Nast A. Rifampin alone may be enough. Is it time to abandon the oral clindamycin-rifampicin combination for hidradenitis suppurativa? Br J Derm. Epub ahead of print 15 November 2018. DOI: 10.1111/bjd.17422. [DOI] [PubMed] [Google Scholar]
- 91. Weiss T, Shalit I, Blau H, et al. Anti-Inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kB and mitogen activated protein kinase activation and of synthesis of pro-inflammatory cytokines. Antimicrob Agents Chemother 2004; 48: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Choi JH, Song MJ, Kim SH, et al. Effect of moxifloxacin on production of proinflammatory cytokines from human peripheral blood mononuclear cells. Antimicrob Agents Chemother 2003; 47: 3704–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Beisswenger C, Honecker A, Kamyschnikow A, et al. Moxifloxacin modulates inflammation during murine pneumonia. Respir Res 2014; 15: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rodrigues RR, Greer RL, Dong X, et al. Antibiotic induced alterations in gut microbiota are associated with changes in glucose metabolism in healthy mice. Front Microbiol 2017; 8: 2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chahine AA, Nahhas AF, Braunberger TL, et al. Ertapenem rescue therapy in hidradenitis suppurativa. JAAD Case Rep 2018; 4: 482–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yoshizawa S, Tateda K, Saga T, et al. Virulence suppressing effects of linezolid on methicillin-resistant Staphylococcus aureus: possible contribution to early defervescence. Anti Microb Agents Chemother 2012; 56: 1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mendes-Bastos P, Martorell A, Magina S. Ertapenem for the treatment of hidradenitis suppurativa: how much do we need it? Actas Dermosifiliogr 2018; 109: 582–583. [DOI] [PubMed] [Google Scholar]
- 98. Bode C, Muenster S, Diedrich B, et al. Linezolid, vancomycin and daptomycin modulate cytokine production, Toll-like receptors and phagocytosis in a human in vitro model of sepsis. J Antibiotics 2015; 68: 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Adams D, Yankura JA, Fogelberg AC, et al. Treatment of hidradenitis suppurativa with etanercept injection. Arch Dermatol 2010; 146: 501–504. [DOI] [PubMed] [Google Scholar]
- 100. Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375: 422–434. [DOI] [PubMed] [Google Scholar]
- 101. Grant A, Gonzales T, Montgomery MO, et al. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double-blind placebo-controlled crossover trial. J Am Acad Dermatol 2010; 62: 205–217. [DOI] [PubMed] [Google Scholar]
- 102. Tzanetakou V, Kanni T, Giatrakou S, et al. Safety and efficacy of anakinra in severe hidradenitis suppurativa a randomized clinical trial. JAMA Dermatol 2016; 152: 52–59. [DOI] [PubMed] [Google Scholar]
- 103. Vossen ARJV, van Doorn MBA, van der Zee HH, et al. Apremilast for moderate hidradenitis suppurativa: results of a randomized controlled trial. J Am Acad Dermatol 2019; 80(1): 80–88. [DOI] [PubMed] [Google Scholar]
- 104. Kanni T, Zenker O, Habel M, et al. Complement activation in hidradenitis suppurativa: a new pathway of pathogenesis? Br J Dermatol 2018; 179: 413–419. [DOI] [PubMed] [Google Scholar]
- 105. Kanni T, Argyropoulou M, Spyridopoulos T, et al. MABp1 Targeting IL-1alpha for moderate to severe hidradenitis suppurativa not eligible for adalimumab: a randomized study. J Invest Dermatol 2018; 138: 795–801. [DOI] [PubMed] [Google Scholar]
- 106. Leung YY, Hui LLY, Kraus VB. Colchicine: update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015; 45: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Armyra K, Kouris A, Markantoni V, et al. Hidradenitis suppurativa treated with tetracycline in combination with colchicine: a prospective series of 20 patients. Int J Dermatol 2017; 56: 346–350. [DOI] [PubMed] [Google Scholar]
- 108. Jemec GB. Methotrexate is of limited value in the treatment of hidradenitis suppurativa. Clin Exp Derm 2002; 27: 528–529. [DOI] [PubMed] [Google Scholar]
- 109. Heiberg MS, Rodevand E, Mikkelsen K, et al. Adalimumab and methotrexate is more effective than adalimumab alone in patients with established rheumatoid arthritis: results from a 6-month longitudinal observational multicenter study. Ann Rheum Dis 2006; 65: 1379–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cribbs AP, Kennedy A, Penn H, et al. Methotrexate restores regulatory T-cells function through demethylation of FOXP3 upstream enhancer in patients with rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 111. Bianchi L, Hansel K, Stingeni L. Recalcitrant severe hidradenitits suppurativa successfully treated with cyclosporine A. J Am Acad Dermtol 2012; 67: 278–279. [DOI] [PubMed] [Google Scholar]
- 112. Andreson MD, Zauli S, Bettoli V, et al. Cyclosporine treatment of severe hidradenitis suppurativa: a case series. J Dermatol Treat 2016; 27: 247–250. [DOI] [PubMed] [Google Scholar]
- 113. Fonseca ACRG, Carvalho E, Eriksson JW, et al. Calcineurin is an important factor involved in glucose uptake in human adipocytes. Mol Cell Biochem 2018; 445: 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Blok JL, van Hattem S, Jonkman MF, et al. Systemic therapy with immunosuppressive agents and retinoids in hidradenitis suppurativa: a systematic review. Br J Dermatol 2013; 168: 243–252. [DOI] [PubMed] [Google Scholar]
- 115. Tan MG, Shear NH, Walsh S, et al. Acitretin: monotherapy or combined therapy for hidradenitis suppurativa? J Cut Med Surg 2017; 21: 48–53. [DOI] [PubMed] [Google Scholar]
- 116. Matusiak L, Biniek A, Szepietowski JC. Acitretin treatment for hidradenitis suppurativa: a prospective series of 17 patients. Be J Dermtol 2014; 171: 170–174. [DOI] [PubMed] [Google Scholar]
- 117. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology 2017; 233: 120–125. [DOI] [PubMed] [Google Scholar]
- 118. Raverdeau M, Mills KH. Modulation of T-cell and innate immune responses by retinoic acid. J Immunol 2014; 192: 2953–2958. [DOI] [PubMed] [Google Scholar]
- 119. Yazdanyar S, Boer J, Ingvarsson G, et al. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology 2011; 222: 342–346. [DOI] [PubMed] [Google Scholar]
- 120. Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res 2014; 306: 103–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol 2012; 83: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 122. Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory bowel diseases. Front Pharmacol 2018; 9: 1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Poveda I, Vilarrasa E, Martorell A, et al. Serum zinc levels in hidradenitis suppurativa: a case control study. Am J Clin Dermatol 2018; 19: 771–777. [DOI] [PubMed] [Google Scholar]
- 124. Donnarumma M, Marasca C, Palma M, et al. An oral supplementation based on myo-inositol, folic acid and liposomal magnesium may act synergistically with antibiotic therapy and can improve metabolic profile in patients affected by hidradenitis suppurativa: our experience. G Ital Dermatol Venereol. Epub ahead of print 20 September 2018. DOI: 10.23736/S0392-0488.18.06012-1. [DOI] [PubMed] [Google Scholar]
- 125. Igic PG, Lee E, Harper W, et al. Toxic effects associated with consumption of zinc. Mayo Clin Proc 2002; 77: 713–716. [DOI] [PubMed] [Google Scholar]
- 126. Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis 2018; 77: 175–187. [DOI] [PubMed] [Google Scholar]
- 127. Frew JW, Vekic DA, Woods J, et al. Phenotypic heterogeneity implies heterogeneous pathogenic pathways in hidradenitis suppurativa. Exp Derm 2015; 24: 338–339. [DOI] [PubMed] [Google Scholar]
- 128. Hawkes JE, Yan BY, Chan TC, et al. Discovery of the IL-23/IL-17 signaling pathway and the treatment of psoriasis. J Immunol 2018; 201: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cotter C, Tobin AM, O’Connor R, et al. Severe refractory hidradenitis suppurativa: treatment with ixekizumab, two case reports. Br J Dermatol 2018; 179: 70. [Google Scholar]
- 130. Pandey K, Sekhri R, Sekhri V. Interleukin-17 inhibitor secukinumab in refractory hidradenitis suppurativa: a case report. J Am Acad Dermatol 2018; 79: AB156. [Google Scholar]
- 131. Principi M, Cassano N, Contaldo A, et al. Hidradentiis suppurativa and inflammatory bowel disease: an unusual but existing association. World J Gastroenterol 2016; 22: 4802–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012; 61: 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res 2011; 51: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schmudde I, Ströver HA, Vollbrandt T, et al. C5a receptor signaling in dendritic cells controls the development of maladaptive Th2 and Th17 immunity in experimental allergic asthma. Mucosal Immunol 2013; 6: 807–825. [DOI] [PubMed] [Google Scholar]
- 135. Jensen L. Targeting the IL-1 family in skin inflammation. Curr Opin Invest Drugs 2010; 11: 1211–1220. [PMC free article] [PubMed] [Google Scholar]
- 136. Collantes-Rodriguez C, Ossorio-Garcia L, Villegas-Romero I, et al. A single case of hidradenitis suppurativa, Crohn’s disease, and psoriasis treated with ustekinumab. J Am Acad Dermatol 2018; 79: AB24. [Google Scholar]
- 137. Blok JL, Li K, Brodmerkel C, et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol 2016; 174: 839–846. [DOI] [PubMed] [Google Scholar]
- 138. Low CM, Akthar S, Patel DF, et al. The development of novel LTA14 modulators to selectively target LTB4 generation. Sci Rep 2017; 7: 44449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Strober BE, Kim C, Siu K. Efalizumab for the treatment of refractory hidradenitis suppurativa. J Am Acad Dermatol 2007; 57: 1090–1091. [DOI] [PubMed] [Google Scholar]
- 140. Cavet J, Dickinson AM, Norden J, et al. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood 2001; 98: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 141. Welsch K, Holstein J, Laurence A, et al. Targeting JAK/STAT signaling in inflammatory skin diseases with small molecule inhibitors. Eur J Immunol 2017; 47: 1096–1107. [DOI] [PubMed] [Google Scholar]
- 142. Torma H. Regulation of keratin expression by retinoids. Dermatoendocrinol 2011; 3: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. van de Kerkhof PC. Update on retinoid therapy of psoriasis in: an update on the use of retinoids in dermatology. Dermatol Ther 2006; 19: 252–263. [DOI] [PubMed] [Google Scholar]