Digital health can be defined simply as the concept of health care meeting the Internet. In practical terms, this consists of (1) a wearable (or implanted) sensor with wireless communication, (2) a smartphone to receive the information, and (3) software (known as a mobile application or an app) to process the information. Overall, a common function of digital health technology is to provide some type of picture or interpretation of the data, or decision support, or in some cases, even an automatic action in the body triggered by the software.
The aim of digital health is to facilitate the four purposes of health care: diagnosis, monitoring, treatment, and prevention. Digital health can be useful to support the first two purposes and can lead to changes in behavior (such as adopting a healthy lifestyle or supporting healthy engagement with medications) to support the second two purposes. The benefits of digital health depend on the perspective of the stakeholder, including patients, health care professionals, and payers. Digital health tools need to demonstrate usability, clinical benefit, economic benefit, security, and safety to satisfy various stakeholders.1
Exciting Times
These are exciting times for digital health systems for diabetes. Many novel digital health tools, such as wearable/portable/implantable sensors and companion software to provide information, decision support, or even control of a drug delivery system are being developed.2,3 Data from the investment, academic, and regulatory communities indicate that there is a recent surge of interest in digital health as a paradigm for treating diabetes and other chronic diseases. Going forward, four trends could lead to significant advances in digital health moving to becoming mainstream in diabetes care. These trends include (1) increasing financial investment in digital health technology development, (2) accelerating execution of new ideas and technologies for digital health from academia and industry, (3) more streamlined regulation of the digital health industry by the Food and Drug Administration (FDA), and (4) increasing use of real-world data (RWD) collection by mobile apps to support clinical research (the latter to facilitate the input of data directly by participants, which can also be linked to electronic health data supporting traditional clinical trials, pragmatic trials, observational studies, and registries). Together these trends augur well for the use of digital health tools.
The Financial Community’s Recent Interest in Digital Health
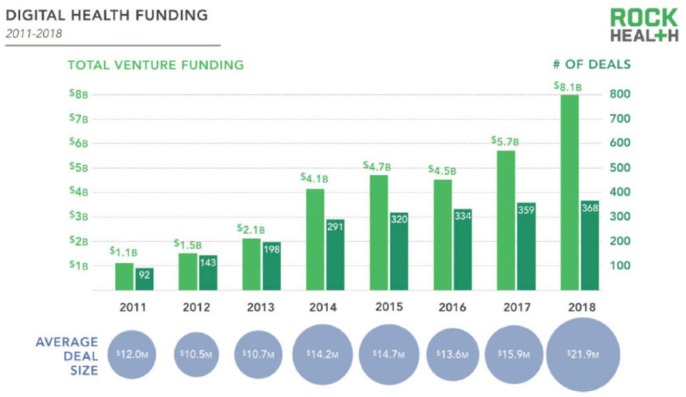
To assess the financial health of the digital health market, one can examine the annual amount of money directed at investments in digital health. Based on recent trends, according to Rock Health, 2018 was a record year for investments into digital health in terms of both total venture funding and number of completed deals. In 2018 investors put 8.1 billion into digital heath startup companies, which was an all-time high (see Figure 1).4 In general, companies in this space are hoping to make health care more accessible, maintain the quality, and cut costs by using these new technologies.5
Figure 1.
Digital health funding 2011—2018 according to the Rock Health Funding Database. Note: Includes U.S. deals >$2 million. Reproduced from Rock Health.4
It is clear that the business community sees great potential in health data 6 and considers digital health data to be a new type of natural resource that can be harnessed for profitability.7 Although a proportion of digital health data is generated automatically, physicians are required to enter data into the electronic health record that are intended more for population management than for their individual patients. In turn, increasingly the expectation for people with diabetes is that they will enter or allow access to their own data to create larger pools of big data to make it possible to analyze population trends. As such, people with diabetes must therefore accept the risk of a privacy breach that could expose their private information to third parties who may not be benevolent. At present, and based on most existing digital health company business plans, these contributions to the digital data resource are not often compensated, but to be fair, they will eventually need to be. 8
The Academic Community’s Recent Interest in Digital Health
Using the keywords “diabetes digital health,” a search of PubMed-indexed journals (accessed January 10, 2019) showed that in 2018, compared to any prior year, the most articles were published on this topic.9 However, looking at the number of articles from PubMed-indexed with the key words “diabetes mobile apps,” the trend has also been rising recently. This is further evidence that interest in publication of articles about digital health in PubMed-indexed medical journals is growing.
In contrast, there has been a shortage of clinically significant long-term outcomes data from randomized controlled clinical trials of digital tools to support their use. Pilot studies with small numbers of subjects for short durations are suggestive that this approach leads to better outcomes in diabetes, but the quality of data in this field is generally poor.10 At this time, better-designed clinical trials are needed to justify and maintain the attention and investment that digital health is now receiving. Many times users of digital health products lose interest over time, so it is useful to see long-term data of at least 6-12 months to understand whether new behavior facilitated by a digital health tool is likely to become established behavior.
The hyperbolic quality of research in digital health for diabetes and other chronic diseases has led to both hope and frustration. A hype cycle is a concept first developed by Gartner, which presents graphic portrayal of expectations over time divided into five stages of maturation and adoption of various technologies.11 The first stage (an innovation trigger) occurs whereby a potential technology breakthrough leads to early proof-of-concept stories and then media interest triggers significant publicity. The second stage (a peak of inflated expectations) occurs with the appearance of a collection of a few of highly publicized success stories, inflated expectations, and many stories of failures. The third stage (a trough of disillusionment) is characterized by waning interest as experiments and implementations fails to deliver and producers of the technology shake out or fail. The fourth stage (a slope of enlightenment) occurs with onset of a new understanding of how the technology can be useful along with the appearance of second- and third-generation products. The fifth stage (a plateau of productivity) occurs when mainstream adoption starts to take off (Figure 2).11
Figure 2.
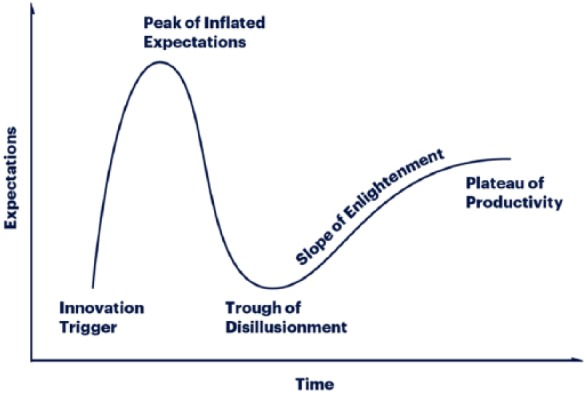
Gartner Methodologies, Gartner Hype Cycle,11 https://www.gartner.com/en/research/methodologies/gartner-hype-cycle.
From our research, one of the first digital health technologies that has seen expectations rise, fall, and rise again has been wearable/portable/implantable glucose sensors. In our view, the stages of hype for these diabetes technologies can be mapped onto the hype cycle. The “innovation trigger” of wearable glucose sensors12 was followed by the first real-time noninvasive glucose sensor, the Glucowatch. This highly publicized product was overly promoted, failed to live up to a “peak of inflated expectations,”13 and was taken off the market. Soon expectations plummeted to a “trough of disillusionment” for a succession of wearable noninvasive glucose sensors.14 A “slope of enlightenment” of rising expectations once again came with the appearance of continuous glucose monitors that each had an adjunctive indication and threshold alarms.15 Expectations for wearable sensors have continued to rise further as these products have now reached a “plateau of productivity”16 with the appearance of noncalibrated CGMs that have primary indications and intelligent mobile applications. Realistic expectations of the capabilities of wearable glucose sensors have finally emerged, following inappropriately high and inappropriately low expectations that previously reflected a hype cycle for this technology.
Digital Health and the FDA
The US FDA oversees most mobile apps that are intended to treat, diagnose, cure, mitigate, or prevent disease or other conditions as medical devices under federal statute.17 According to the FDA, such software-based technologies, including mobile medical apps, are now known as “software as a medical device” (SaMD). In recent years, FDA has been very active in revising its policies for regulating digital health products and currently is creating many new programs to respond to an expected tsunami of new digital health submissions.18 Table 1 presents eight recently enacted or announced regulatory policies toward digital health by the FDA.
Table 1.
Eight Recently Enacted or Announced Regulatory Policies Toward Digital Health by the FDA.
| 1. A new focus on oversight of mobile medical apps only for those that present higher risk to patients; and an intention to not enforce compliance for lower risk mobile apps.19
2. An intention to not focus oversight on technologies that receive, transmit, store, or display data from medical devices.19 3. An intention to not focus oversight on products that only promote general wellness.19 4. Development of new cybersecurity policies including encouragement of information exchange of cybersecurity information by the health care community.20-23 5. Publication of the Food and Drug Administration Safety and Innovation Act (FDASIA) Health IT report proposing a new framework for Health IT that includes collaboration and avoidance of regulatory duplication between FDA, the Federal Trade Commission (FTC), and the US Department of HHS Office of National Coordinator for Health Information Technology (ONC).24 6. An intention to create a Digital Health Software Precertification Program.25 7. An intention to create a center of excellence for digital health that will include oversight of cybersecurity issues.26 8. An intention to hire new staff for the Digital Health Center of Excellence.27,28 |
The early FDA publications on digital health contained three important novel initiatives: (1) clarity on the medical software provisions of the 21st Century Cures legislation, (2) launch of a pilot precertification program (called the FDA Pre-Certification for Software), and (3) expansion of the FDA’s digital health expertise by creating a Center of Excellence for Digital Health.29-31 At this time the FDA is also actively recruiting software engineers, artificial intelligence and machine learning engineers, security researchers, user interface/user experience designers, and product managers to be a part of the new Digital Health Center team.32 This team will focus on SaMD, software inside medical devices (SiMD), interoperable devices, and other novel digital health technologies.33
The FDA Digital Health Software Precertification Program will assess companies developing software products rather than the software products themselves. This program provides more streamlined and efficient regulatory oversight of software-based medical devices created by manufacturers who have (1) demonstrated a robust culture of quality and organizational excellence and (2) committed to monitor real-world performance of their software products after they come onto the US market. The intention is for precertified companies introducing SaMD products, to receive streamlined premarket review, and in exchange, they will be required to share risk-based postmarket performance metrics. FDA is hoping that this new voluntary regulatory pathway for clearance will both facilitate development as well as shorten time to market for new medical software because of the less onerous premarket requirements. At the same time, FDA is hoping that this program will also increase consumer and health care professional confidence in the safety and effectiveness of cleared SaMD because of the more rigorous postmarket review process for maintaining ongoing high quality.
In August 2018, Diabetes Technology Society convened a meeting of stakeholders in digital health for diabetes from (1) academia (including representatives from adult medicine, pediatric medicine, nursing, psychology, engineering, law, and the American Diabetes Association), (2) government (including representatives from FDA, NIH, and the US Department of Commerce), and (3) industry (including representatives from the software, hardware, and pharmaceutical industries). The aim was to discuss the impending FDA Digital Health Precertification Program. At the meeting, FDA provided information to the diabetes digital health community about their goals and plans. The digital health community in turn provided feedback and recommendations to the FDA. All parties agreed that this voluntary program has the potential to both streamline the premarket review process as well as also provide meaningful real-world postmarket performance data intended to assist patients and health care professionals to make informed choices for SaMD products.34
Increasing Use of Real-World Data Collection by Mobile Apps
Real-world data (RWD) are data obtained from outside of conventional clinical trials. Sources of RWD include electronic health records (EHRs), insurance claims, pharmacy records, patient registries, and mobile apps.35 The development of mobile apps, whose data can be linked to an electronic health record, has been a major contributing factor to the emergence of RWD as a viable source of clinical evidence. In diabetes care, collection of RWD data from mobile apps has the potential to understand better why diabetes interventions in the real world are often much less than those reported in randomized clinical trials.36 In addition, mobile apps have the potential to obtain consent, enroll, as well as gather self-reported RWD from participants in clinical trials at much lower costs than randomized controlled trials.37 For example in November 2018, FDA released an open source mobile app called “MyStudies,” which allows participants to input data that can be linked to their electronic health records for clinical trials and other types of health care research.38 FDA also expects that RWD collection with mobile apps will lead to more efficient product development, safety monitoring, and regulatory decisions.
Where We Go From Here?
We are now seeing increasing interest in digital health as a paradigm for delivering treatment for diabetes and other chronic diseases from the financial, academic, and regulatory communities. This interest comes at the same time we are seeing an explosion of interest in ubiquitous sensors as part of the Internet of Things, along with the use of artificial intelligence to interpret the avalanche of data that these sensors are delivering. With new financing, new ideas, new streamlined regulatory pathways for clearance, and new opportunities to collect RWD to inform regulatory decisions, digital health products are poised to thrive in the coming years.
Acknowledgments
The authors would like to thank Annamarie Sucher for her expert editorial assistance.
Footnotes
Abbreviations: EHR, electronic health record; FDA, Food and Drug Administration; FDASIA, Food and Drug Administration Safety and Innovation Act; FTC, Federal Trade Commission; ONC, Office of National Coordinator for Health Information Technology; RWD, real-world data; SaMD, software as a medical device; SiMD, software inside medical devices.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DCK is a consultant for Ascensia, EOFlow, Lifecare, Merck, Novo, Roche Diagnostics, and Voluntis. FK has nothing to disclose. DK is a medical advisor for Glooko and Vicentra and a consultant for NovoNordisk and Sanofi.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Klonoff DC, Kerr D. Overcoming barriers to adoption of digital health tools for diabetes. J Diabetes Sci Technol. 2018;12:3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dunn J, Runge R, Snyder M. Wearables and the medical revolution. Per Med. 2018;15:429-448. [DOI] [PubMed] [Google Scholar]
- 3. Belknap R, Weis S, Brookens A, et al. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLOS ONE. 2013;8:e53373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Day S, Zweig MTH. 2018. Year End Funding Report: Is digital health in a bubble? Available at: https://rockhealth.com/reports/2018-year-end-funding-report-is-digital-health-in-a-bubble/. Accessed January 10, 2019.
- 5. Dietsche E. Digital health investing: Looking back and forecasting the future. January 11, 2018. Available at: https://medcitynews.com/2018/01/digital-health-investing/. Accessed January 10, 2019.
- 6. Tanner A. The hidden trade in our medical data: Why we should worry. January 11, 2017. Available at: https://www.scientificamerican.com/article/the-hidden-trade-in-our-medical-data-why-we-should-worry/. Accessed January 10, 2019.
- 7. IBM. Big data: The new natural resource. 2012. Available at: https://www.ibmbigdatahub.com/infographic/big-data-new-natural-resource. Accessed January 10, 2019.
- 8. Blumenthal D. Realizing the value (and profitability) of digital health data. Ann Intern Med. 2017;166:842-843. [DOI] [PubMed] [Google Scholar]
- 9. Information NCfB. PubMed search results for “Diabetes Digital Health.” Available at https://www.ncbi.nlm.nih.gov/pubmed/?term=diabetes+digital+health. Accessed January 10, 2019.
- 10. Veazie S, Winchell K, Gilbert J, et al. Mobile Applications for Self-Management of Diabetes. Technical Brief No. 31. (Prepared by the Scientific Resource Center under Contract Nos. 290-2012-0004-C and 290-2017-00003-C.) AHRQ Publication No. 18-EHC010-EF. Rockville, MD: Agency for Healthcare Research and Quality; May 2018. Available at: https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/technical-brief-31-mobile-applications-for-self-management-of-diabetes.pdf. Accessed January 10, 2019. [PubMed] [Google Scholar]
- 11. Methodologies G. Gartner Hype Cycle. Available at: https://www.gartner.com/en/research/methodologies/gartner-hype-cycle. Accessed January 10, 2019.
- 12. Oberhardt BJ, Fogt EJ, Clemens AH. Glucose sensor characteristics for miniaturized portable closed-loop insulin delivery: a step toward implantation. Diabetes Care. 1982;5:213-217. [DOI] [PubMed] [Google Scholar]
- 13. Iafusco D, Errico MK, Gemma C, Prisco F. Usefulness or uselessness of GlucoWatch in monitoring hypoglycemia in children and adolescents. Pediatrics. 2004;113:175-176; author reply 6. [DOI] [PubMed] [Google Scholar]
- 14. Hoskins M. Non-Invasive diabetes technology: still dreaming. November 7, 2012. Available at: https://www.healthline.com/diabetesmine/non-invasive-diabetes-technology-still-dreaming#8. Accessed January 10, 2019.
- 15. McGarraugh G. Alarm characterization for continuous glucose monitors used as adjuncts to self-monitoring of blood glucose. J Diabetes Sci Technol. 2010;4:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolinsky H. Continuous glucose monitors: the long and winding road to acceptance, coverage. July 4, 2018. Available at: https://www.managedcaremag.com/archives/2018/7/continuous-glucose-monitors-long-and-winding-road-acceptance-coverage. Accessed January 10, 2019. [PubMed]
- 17. Shuren J, Patel B, Gottlieb S. FDA regulation of mobile medical apps. JAMA. 2018;320:337-338. [DOI] [PubMed] [Google Scholar]
- 18. Health P. Get ready for the mobile medical app Tsunami. July 2, 2018. Available at: https://www.premierhealth.com/Women-Wisdom-Wellness/Content/Get-Ready-for-the-Mobile-Medical-App-Tsunami/?HealthTopicTaxonomyID=21838. Accessed January 10, 2019.
- 19. US Food and Drug Administration. Mobile medical applications: guidance for industry and food and drug administration staff. February 9, 2015. Available at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM263366.pdf. Accessed January 10, 2019.
- 20. US Food and Drug Administration. Content of premarket submissions for management of cybersecurity in medical devices: guidance for industry and food and drug administration staff. October 2014. Available at: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm356190.pdf. Accessed January 10, 2019.
- 21. US Food and Drug Administration. Content of premarket submissions for management of cybersecurity in medical devices: draft guidance for industry and food and drug administration staff. October 18, 2018. Available at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM623529.pdf. Accessed January 10, 2019.
- 22. US Food and Drug Administration. Postmarket management of cybersecurity in medical devices guidance for industry and food and drug administration staff. December 28, 2016. Available at: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm482022.pdf. Accessed January 10, 2019.
- 23. US Food and Drug Administration. Statement from FDA Commissioner Scott Gottlieb, M.D. on FDA’s efforts to strengthen the agency’s medical device cybersecurity program as part of its mission to protect patients. October 1, 2018. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm622074.htm. Accessed January 10, 2019.
- 24. US Food and Drug Administration. FDASIA health information report: proposed strategy and recommendations for a risk-based framework. April 2014. Available at: https://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHReports/UCM391521.pdf. Accessed January 10, 2019.
- 25. US Food and Drug Administration. Digital health software precertification (Pre-Cert) program. September 27, 2018. Available at: https://www.fda.gov/MedicalDevices/DigitalHealth/DigitalHealthPreCertProgram/default.htm. Accessed January 10, 2019.
- 26. McGee MK. The FDA’s new digital health cyber unit: what would it do? September 27, 2018. Available at: https://www.govinfosecurity.com/fdas-new-digital-health-cyber-unit-what-would-do-a-11567. Accessed January 10, 2019.
- 27. Sweeney E. FDA begins filling positions for its new digital health unit. September 11, 2017. Available at: https://www.fiercehealthcare.com/regulatory/fda-begins-filling-positions-for-its-new-digital-health-unit. Accessed January 10, 2019.
- 28. US Food and Drug Administration. Medical devices: digital health. October 2, 2018. Available at: https://www.fda.gov/medicaldevices/digitalhealth/. Accessed January 10, 2019.
- 29. US Food and Drug Administration. Digital Health Action Plan. Available at: https://www.fda.gov/downloads/medicaldevices/digitalhealth/ucm568735.pdf. Accessed January 10, 2019.
- 30. Evans B. For entrepreneurs: what you need to know about FDA’s game-changing digital health innovation action plan. August 14, 2017. Available at: https://rockhealth.com/what-you-need-to-know-about-fdas-game-changing-digital-health-innovation-action-plan/. Accessed January 10, 2019.
- 31. US Food and Drug Administration. FDA budget matters: advancing innovation in digital health. September 26, 2018. Available at: https://www.fda.gov/NewsEvents/Newsroom/FDAVoices/ucm621675.htm. Accessed January 10, 2019.
- 32. US Food and Drug Administration. Digital health advisor job description. Available at: https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/UCM622320.pdf. Accessed January 10, 2019.
- 33. Orthogonal. The FDA gets serious about digital health: and that’s a good thing. August 7, 2017. Available at: https://medium.com/@orthogonal/the-fda-gets-serious-about-digital-health-14aab52de0c9. Accessed January 10, 2019.
- 34. King F, Klonoff DC, Ahn D, et al. Diabetes technology society report on the FDA digital health software precertification program meeting. J Diabetes Sci Technol. 2018:1932296818810436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khozin S, Blumenthal GM, Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109:djx187. [DOI] [PubMed] [Google Scholar]
- 36. Edelman SV, Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017;40:1425-1432. [DOI] [PubMed] [Google Scholar]
- 37. Crouthamel M, Quattrocchi E, Watts S, et al. Using a ResearchKit Smartphone App to collect rheumatoid arthritis symptoms from real-world participants: feasibility study. JMIR Mhealth Uhealth. 2018;6:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. US Food and Drug Administration. FDA’s MyStudies Application (App). November 6, 2018. Available at: https://www.fda.gov/Drugs/ScienceResearch/ucm624785.htm. Accessed January 10, 2019.