Abstract
Evidence suggests that there is an association between polymorphisms in the α5 nicotinic acetylcholine receptor (nAChR) subunit and risk of developing alcohol dependence in humans. The α5 nAChR subunit has also recently been shown to modulate some of the acute response to ethanol in mice. The aim of the current study was to further characterize the role of α5-containing (α5*) nAChRs in acute ethanol responsive behaviors, ethanol consumption and ethanol preference in mice. We conducted a battery of tests in male α5 knockout (KO) mice for a range of ethanol-induced behaviors including hypothermia, hypnosis, and anxiolysis. We also investigated the effects of α5* nAChR on ethanol reward using the Conditioned Place Preference (CPP) assay. Further, we tested the effects of gene deletion on drinking behaviors using the voluntary ethanol consumption in a two-bottle choice assay and Drinking in the Dark (DID, with or without stress) paradigm. We found that deletion of the α5 nAChR subunit enhanced ethanol-induced hypothermia, hypnosis, and an anxiolytic-like response in comparison to wild-type controls. The α5 KO mice showed reduced CPP for ethanol, suggesting that the rewarding properties of ethanol are decreased in mutant mice. Interestingly, Chrna5 gene deletion had no effect on basal ethanol drinking behavior, or ethanol metabolism, but did decrease ethanol intake in the DID paradigm following restraint stress. Taken together, we provide new evidence that α5 nAChRs are involved in some but not all of the behavioral effects of ethanol. Our results highlight the importance of nAChRs as a possible target for the treatment of alcohol dependence.
Keywords: ethanol, alpha 5 nAChRs, nicotinic receptors, reward, mice
1. Introduction
Alcohol and nicotine are two of the most commonly abused drugs, contributing to over 500,000 deaths annually in the United States, with associated medical costs in excess of $500 billion per year (Centers for Disease Control and Prevention, 2014; 2015). Multiple studies have observed that up to 90% of individuals diagnosed with alcohol use disorder are cigarette smokers, with between 20–40% of them being heavy smokers, defined by 40+ cigarettes per day (Istvan and Matarazzo, 1984; DiFranza and Guerrera, 1990; Batel et al., 1995; Falk et al., 2006). Interestingly, increased alcohol abstinence rates have been reported in alcoholics who attempt to quit smoking and smoking cessation was shown not to disrupt alcohol abstinence (Gulliver et al., 2007; for review, see Hughes & Kalman, 2006). Evidence from both human and animal studies supports the notion that there are common genetic factors underlying these disorders (Schlaepfer et al., 2008; Cross et al., 2017). Here, we investigate the role of the alpha 5 subunit of nicotinic acetylcholine receptor, Chrna5, in mouse models of ethanol addiction-related behaviors (for review, see Bühler et al., 2015).
Investigations of the CHRNA5-CHRNA3-CHRNB4 gene cluster in humans have suggested a role for α5* nicotinic subunits in nicotine and alcohol dependence (Joslyn et al., 2008; Schlaepfer et al., 2008), as well as in the level of response to alcohol, which may impact liability to develop alcohol dependence. These studies have implicated single nucleotide polymorphisms (SNPs) in the CHRNA5 gene, encoding the α5 nAChR subunit, to be associated with an increased risk to develop alcohol dependence (Joslyn et al., 2008; Schlaepfer et al., 2008; Choquet et al., 2013). Furthermore, human genome-wide association studies have associated a promoter single nucleotide polymorphism (SNP) in CHRNA5, rs588765, with alcohol dependence in European Americans. Individuals homozygous for this SNP had significantly higher mRNA levels of CHRNA5 in the frontal cortex than heterozygotes or those without the SNP (Wang et al., 2009). Mouse genetic studies have reported that there is an allelic difference in Chrna5 between C57BL/6J and DBA/2J (alcohol-avoiding mice) that can be associated with alcohol preference in the strains (Symons et al., 2010). Furthermore, these researchers reported that whole brain Chrna5 mRNA levels were significantly increased in C57BL/6J mice compared to DBA/2J mice following ethanol treatment (Symons et al., 2010). Finally, using α5 knockout (KO) mice, Santos et al., (2013) study suggest that the α5 nAChR subunit is important for the sedative effects of ethanol but does not play a role in ethanol oral intake in mice. Overall, these human and mouse studies have suggested an important role for the α5 nicotinic subunit in alcohol responses and alcohol dependence.
Neuronal nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that form pentamers containing α (α2-α10) and/or β (β2-β4) subunits. Alpha 5 is an accessory subunit that forms pentamers with either α4β2, α3β4, or α3β2 subunits, and together, these constitute 10–37% of all nAChRs (Kuryatov et al., 2008; Baddick and Marks, 2011). Although high levels of expression are found only in few brain areas (Salas et al., 2003a), Chrna5 mRNA has been found in the majority of brain regions (Brown et al., 2007), suggesting that a5* nAChRs could have a substantial impact on brain function. For example, α5* nAChRs play a key role in nicotine intake, reward, and withdrawal in rodents (Salas et al., 2003a; Jackson et al., 2010; Jackson et al., 2008; Fowler et al., 2011: Morel et al., 2014). For example, α5 KO mice showed an enhancement of nicotine reward and intake (Jackson et al., 2010; Fowler et al., 2011) and a reduction in some nicotine wihdrawal signs (Salas et al., 2003; Jackson et al., 2008). Expression studies in Xenopus oocytes also found that ethanol potentiated currents produced by acetylcholine in certain nAChR subunit combinations (α4β2*, α4β4*, α2β4*, and α2β2*), had no effect on receptors containing α3β4 or α3β2 subunits, and inhibited the function of α7 receptors, demonstrating that the effects of ethanol depend on the nAChR subtype considered (Cardoso et al., 1999; Borghese et al., 2003). A variety of ethanol’s behavioral effects can be modulated by altered nAChR function (Kuzmin et al., 2008; Kamens et al., 2010, 2012; Santos et al., 2012; Sajja and Rahman, 2012). Importantly, Santos and colleagues (Santos et al., 2012), observed that mice lacking Chrna5 demonstrated prolonged sleep time following administration of a sedative dose of ethanol, showed greater impairment of locomotion by ethanol evidenced by a decreased latency to fall off of a rotarod, but consumed similar amounts of ethanol compared to wild-type mice in the Drinking in the Dark (DID) forced drinking model (Santos et al., 2012). These results indicate that more work is needed to fully understand the role α5* nAChRs plays in mediating phenotypes associated with alcohol dependence.
In this study, we seek to further characterize the role of the α5 nAChR subunit on ethanol responsive behaviors and rewarding effects in mice. We hypothesized that deletion of the Chrna5 gene would result in altered responses to ethanol in a battery of behavioral tests with emphasis on phenotypes associated with risk for alcoholism and across a range of alcohol doses. Using α5 knockout (KO) and wild-type (WT) mice, we explored the role of α5* nAChRs in three acute ethanol-responsive behaviors: hypothermia, loss of righting reflex, and anxiolysis. We also examined ethanol consumption and reward in multiple mouse paradigms of oral drinking and conditioned place preference (CPP).
2. Materials and methods
2.1. Animals
The α5 KO breeding pairs were procured from The Jackson Laboratory (Bar Harbor, ME) and bred in the animal facility at Virginia Commonwealth University (Richmond, VA). They were originally reported by Salas et al. (2003a). All mice used in each experiment were backcrossed for at least 12 generations on C57BL/6J background. Heterozygote KO/+ mice were crossed to generate homozygous mutant and WT control littermates. Male mice were group-housed in a temperature and humidity controlled animal care facility approved by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and had free access to food and water under a 12-h light/dark cycle (lights on at 6:00 am) schedule. All experiments were performed during the light cycle. Mice were 8–10 weeks old at the start of the experiments. The study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. Behavioral and pharmacological tests were performed on different cohorts of mice.
2.2. Drugs
200-proof ethanol (Pharmco-AAPER, USA) was dissolved in 0.9% saline to yield a 20% (v/v) solution and injected intraperitoneally (i.p) for acute experiments. For drinking studies, ethanol (3–30%) was administered orally (p.o.) in the drinking water. Ketamine HCl (100 mg/mL solution, KetaVed™), obtained from Vedco Inc (Saint Joseph, MO), was diluted to 10 mg/mL in saline. Sodium pentobarbital, USP (50 mg/mL), purchased from Virginia Commonwealth University Hospital Pharmacy, was diluted to 3 mg/mL in saline. Ketamine HCl and sodium pentobarbital were each administered i.p. at a volume of 0.1 mL per 10 g of mouse mass for doses of 100 mg/kg and 30 mg/kg, respectively. The doses of alcohol (2 – 4g/kg) used in acute behavioral studies and conditioned place preference test were based on previously published studies (Alkana et al., 1992, Browman et al., 2000; Tanchuck-Nipper et al., 2015; Putman et al., 2016; Slater et al., 2016; Guildford et al., 2016).
2.3. Body Temperature Measurement
Hypothermia was measured by rectally inserting a standard thermometer probe ~24mm (Fischer Scientific, Pittsburgh, PA). Baseline temperatures were recorded and five minutes later, mice were administered 2.5 g/kg ethanol or saline (i.p.). Body temperature was recorded 15, 30, 60, and 120 minutes following ethanol injection. Data were expressed as the mean temperature change (°C) ± SEM from baseline after ethanol treatment. The room temperature of the laboratory varied from 21–24°C.
2.4. Loss of Righting Reflex (LORR)
The LORR assay was used to assess the sedative-hypnotic effects of ethanol, pentobarbital, and ketamine. The α5 KO and WT mice were administered i.p. injections of ethanol (3.8 g/kg), pentobarbital (30 mg/kg) or ketamine (100 mg/kg), then placed into a supine position in a V-shaped trough. A mouse was confirmed to have achieved LORR only after it remained on its back for at least 30 sec. The time from ethanol, pentobarbital or ketamine injection until initial LORR (latency to LORR) was recorded. The time for a mouse to recover the ability to right itself three times within 30 sec was measured as the duration of LORR. Mice taking longer than five minutes for LORR onset were eliminated from the study due to the possibility of misplaced injection. Data were expressed as latency to LORR and LORR duration (in sec).
2.5. Elevated Plus Maze (EPM)
Anxiolytic-like behavior was assessed in the EPM, an elevated platform consisting of four black arms in a cross shape raised from the ground. Two arms have walls (closed arms) and the other two arms do not (open arms). Mice innately avoid open, elevated spaces, therefore, increased time spent in the open arms of the EPM is interpreted as anxiolytic-like behavior (Pellow et al. 1985). Prior to testing, mice were acclimated to the room for at least 1–2 hours. On test day, α5 WT and KO mice were administered 0 or 2.0 g/kg ethanol (i.p.) and returned to their home cage for 15 min to minimize interference from the hyperlocomotion produced by acute ethanol treatment. Each mouse was placed briefly into a plastic container, transferred to the center of the EPM, and allowed to roam freely for five minutes. (Data were expressed as the total time (sec) spent in the open arms. To control for locomotor effects, the total number of crossovers across the center of the maze was recorded.
2.6. Conditioned Place Preference (CPP)
An unbiased CPP paradigm was performed, as we previously described (Kota et al., 2007; Harenza et al., 2014). The experimenter handled each mouse for approximately 2 minutes for each of three days prior to the start of CPP. The apparatus (Med-Associates, St. Albans, VT, USA, ENV3013) consists of two conditioning chambers (20 × 20 × 20 cm each) that differ in color (black and white) and floor texture (white mesh and black rod) to provide two distinct conditioning environments. There is a smaller, gray holding chamber with a smooth PVC floor with partitions that allow closure from or access to the larger chambers. On day 1 (Pre-Test), animals were confined to the middle chamber for 5 min, then allowed to move freely explore the entire apparatus for 15 min. Time spent in each conditioning chamber was recorded and used to separate the animals into groups of approximately equal initial bias for the drug-paired chambers. For conditioning, days 2–4, the control group received saline (i.p.) in both chambers and the drug group received ethanol (2 g/kg, i.p., 10 min pretreatment) in one chamber and saline in the other chamber for 15 min. Treatments were counterbalanced equally in order to ensure that some mice received the unconditioned stimulus in the morning while others received it in the afternoon. The alcohol-paired chamber was randomized among all groups. Sessions were 4 hours apart and were conducted by the same investigator. On day 5 (Post-Test), mice were allowed to freely explore and time spent in each chamber was recorded. Preference scores (sec) were calculated as the difference in time spent in the drug-paired chamber, Post-Test time minus Pre-Test time.
2.7. Drinking in the Dark (DID)
DID is a limited-access drinking procedure used to model binge-drinking behavior in rodents (Rhodes et al., 2005; Thiele et al., 2014). The α5 KO and WT mice were single-housed in a reverse light-dark cycle (7:00 am – 7:00 pm) one week prior to testing with ad libitum access to food and water. At the end of the acclimation period, three hours into the dark cycle (10:00 am), the water bottle from each cage was replaced with one drinking tube containing 20% ethanol for four hours. Baseline alcohol intake was calculated using the average consumption across four days of ethanol presentation.
On day 5, we investigated the impact of restraint stress on ethanol intake. Alpha 5 KO and WT mice were assigned to either the experimental (Stress) or control (No Stress) groups. The stress procedure was performed in an adjacent room to avoid disrupting the control group. Mice were removed from their home cage and placed in a Plexiglas restraint tube (diameter: 25.4 mm; length: 83 mm) for 15 min. Restraint stress was performed on all mice at the same time each day (between 09:00 and 10:00 am) for 4 consecutive days. At the end of each restraint period, mice were returned to the DID procedure room for 4 hours drinking session. The No Stress group remained undisturbed in their home cages in the DID procedure room during this phase of the experiment except for routine handling.
2.8. Blood Ethanol Concentration (BEC) Analysis
To rule out the possibility of the α5 gene deletion directly altering ethanol metabolism, we assessed BEC in drug-naïve α5 KO and WT mice over a two-hour time course following administration of a single high dose of ethanol (4.0 g/kg, i.p.). Blood was collected into microtainers containing dipotassium EDTA (BD Biosciences, San Jose, CA, USA) via submandibular cheek punch at 15-, 30-, 60- or 120 min time-points after injection. Samples were prepared for analysis by aliquoting 20 µl of whole blood into 20 ml GC vials (Autosampler Guys, Alexandria, VA) containing 960 µl deionized (DI) water and 20 µl of 0.1 mg/ml 1-propanol standard. Vials were capped and stored at −20 ˚C until BECs were analyzed using headspace gas chromatography as described (Wolstenholme et al., 2011).
2.9. Statistical Analysis
Data were tested at an alpha level of 0.05 using either Student’s t-test, one-way or multi-way analysis of variance (ANOVA), using GraphPad Prism (GraphPad Inc., San Diego, CA). When multiple measurements were taken from individual mice, repeated measures ANOVAs were used. Significant ANOVAs were followed with Tukey post-hoc tests and results are reported with corrected p-values.
3. Results
3.1. Ethanol-induced hypothermia accentuated in α5 null mice
We first tested whether α5*nAChRs influence ethanol’s hypothermic effect. Treatment with 2.5 g/kg ethanol reduced body temperature up to 120 min in both WT and KO mice (Fig. 1A). Alpha 5 KO mice had a significantly larger decrease in body temperature as compared to WT mice. A three-way ANOVA with repeated measures (genotype x treatment x time) resulted in a significant genotype x treatment interaction, F(1,19) = 5.057; p= 0.031. A significant main effect of genotype [F(1,19) = 5.376; p= 0.0317] and treatment [F(1,19) = 66.028; p< 0.0001] was also found. Ethanol’s hypothermic effect was long lasting, as body temperature changes were relatively stable for the first hour of observation [Ftime(1, 19) = 3.300; p= 0.0851]. Body temperature did not differ between saline treated WT and α5 KO at any time point. These results suggest that the α5 subunit plays an important role for hypothermic response of ethanol. In addition, the increased hypothermic effect in α5 KO mice is not due to changes in alcohol metabolism, as BEC levels were the same in α5 KO and WT mice following a 4 mg/kg ethanol injection [Fig 1B, F treatment x time (3,18) = 1.16, p = 0.35].
Figure 1.
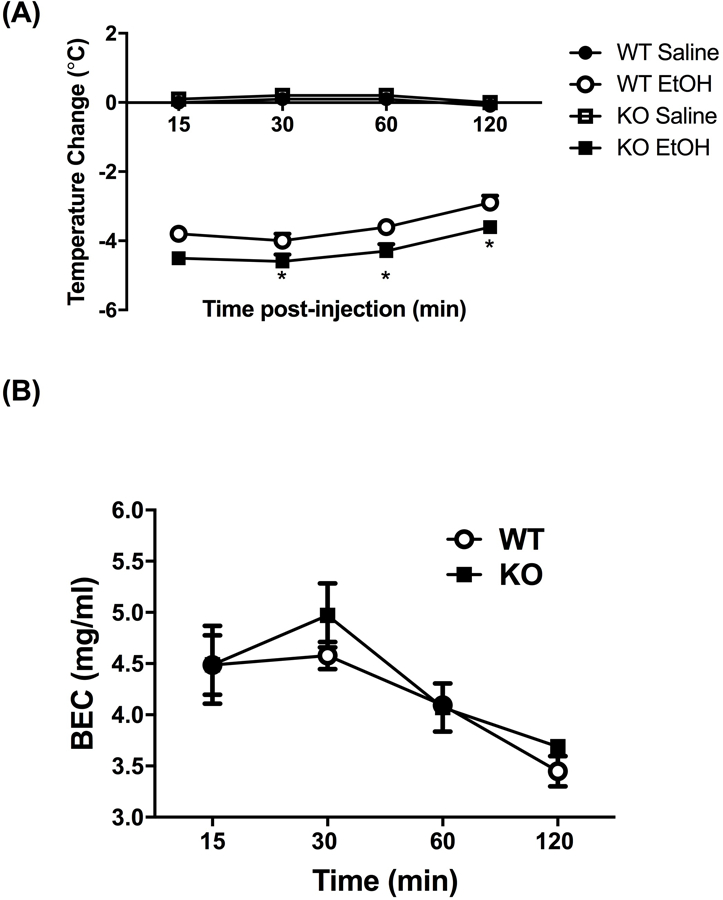
(A) Deletion of the Chrna5 gene enhances ethanol-induced hypothermia in mice. Data (mean ± SEM) represent the change in body temperature from baseline in degrees Celsius of α5 WT and KO mice at time points following an injection of 2.5 g/kg ethanol. Ethanol-induced hypothermia was significantly enhanced in α5 KO mice compared to WT mice at the 30-, 60-, and 120-min timepoints (*p<0.05, n=8 per group). (B) Deletion of the Chrna5 gene does not alter BEC levels in mice. Data (mean ± SEM) represent BEC (mg/mL) at 15, 30, 60, and 120 min after receiving an injection of 4.0 g/kg ethanol (n = 5 per group).
3.2. Deletion of Chrna5 enhances the duration of both ethanol- and pentobarbital-induced LORR
Our lab has previously highlighted the role of β2* nAChRs in the induction on LORR after ethanol treatment (Dawson et al., 2013). Given that ethanol has been shown to have distinct effects depending on the subunit composition of nAChRs (Cardoso et al., 1999; Borghese et al., 2003), we tested the latency and duration of ethanol-induced LORR after treatment with 3.8 g/kg ethanol in WT and α5 null mice. There was no significant difference between WT and α5 KO for the onset of LORR (Fig 2A), but there was a significant increase in the duration of LORR in the α5 KO (Fig 2B, [t=2.636, df=13; p= 0.02]. Our findings are similar to those reported by Santos and colleagues (Santos et al., 2012).
Figure 2.
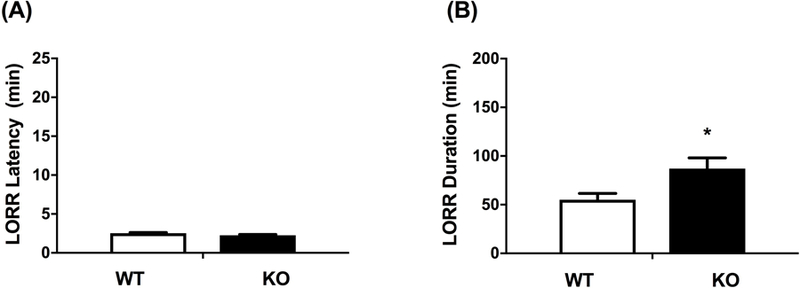
Deletion of the Chrna5 gene had no effect on latency to loss of righting reflex (LORR) (A) but enhanced ethanol-induced LORR duration (B). Data represent the mean ± SEM for α5 WT and KO mice after receiving 3.8 g/kg i.p. ethanol (*p<0.05, n= 7 per group).
We tested whether the interaction between α5*nAChRs and LORR effect was specific to ethanol or could be generalized to other sedative drugs, such as pentobarbital and ketamine. The α5 genotype did not alter the latency to LORR for either pentobarbital or ketamine (Fig. 3). In contrast, α5 KO mice exhibited a significantly longer duration of pentobarbital-induced LORR compared to WT mice (Fig 3, [tbarb=8.755 df=5; p < 0.001]), similar to what was observed in ethanol-treated mice. However, the α5 genotype had no effect on the duration of ketamine-induced LORR (Fig. 3). Given that both ethanol and pentobarbital modulate GABAA receptors, this suggests a possible potentiation of GABAA inhibitory tone in α5 KO mice.
Figure 3.
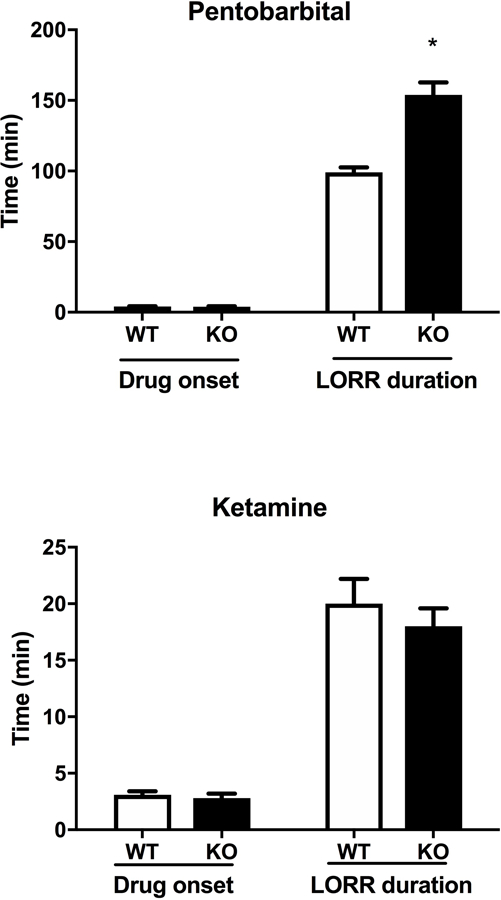
Deletion of Chrna5 enhances loss of righting reflex (LORR) duration following pentobarbital, but not ketamine. Data (mean ± SEM) represent Pentobarbital (30 mg/kg, i.p.) and Ketamine (100 mg/kg, i.p.) LORR latency and LORR duration in WT and α5 KO mice (*p<0.05 compared to correspondent WT mice, n= 8 per group).
3.3. Deletion of Chrna5 enhances ethanol-induced anxiolytic-like behavior
Ethanol is known to have anxiolytic-like effect in the EPM. As expected, ethanol increased the time spent in the open arms in both genotypes (anxiolytic-like effect) but had a significantly larger effect in the α5 KO mice relative to the WT mice (Fig. 4A). A two-way ANOVA (genotype x treatment) resulted in significant main effects of treatment [F(1,36) = 55.99; p< 0.0001] and genotype [F(1,36)= 9.88; p= 0.003], as well as a significant interaction [F(1,36)= 10.89; p= 0.002]. These differences were not due to changes in locomotor activity since the number of crossovers between the arms was the same for all groups (Figure 4B). Our EPM results suggest that α5 nAChR subunit plays an important role in the anxiolytic effect of acute ethanol.
Figure 4.
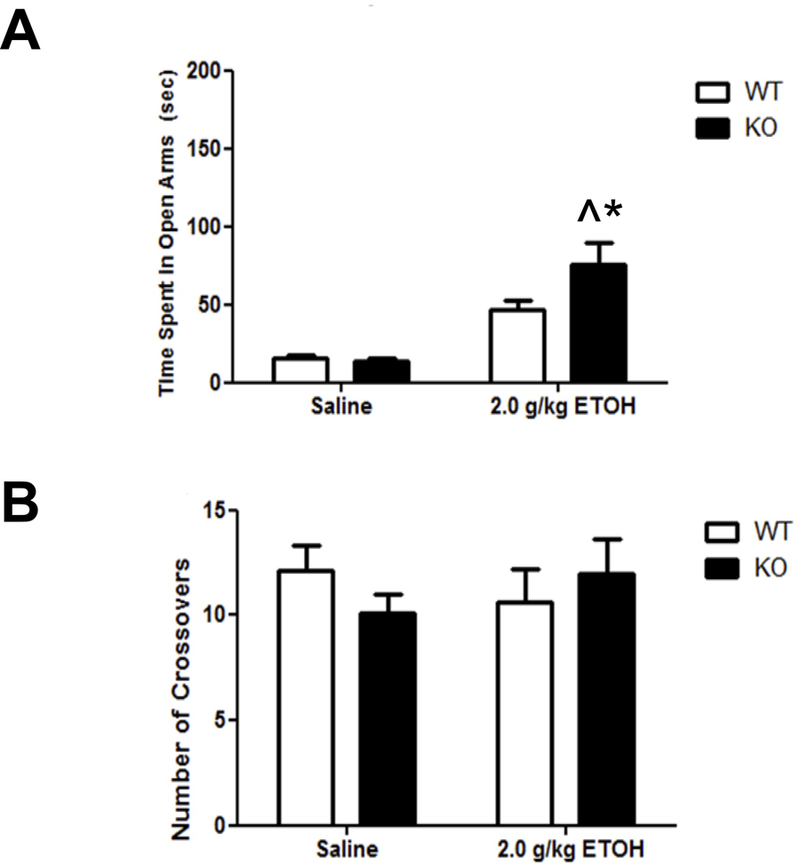
Deletion of the Chrna5 gene enhances ethanol-induced anxiolytic-like behavior. Data (mean ± SEM) represent (A) time spent in open arms and (B) total number of crossovers in WT and α5 KO mice following an i.p. injection of 2.0 g/kg ethanol (^p<0.05 compared to correspondent saline-treated group; *p<0.05 compared to correspondent WT mice, n= 8–13).
3.4. Deletion of Chrna5 reduced ethanol CPP
CPP was used to measure the influence of α5*nAChRs in the rewarding effects of ethanol. While WT animals displayed a robust development of ethanol CPP, as measured by a significant increase in the amount of time spent in the drug-paired chamber, there was no CPP produced in the α5 KO mice (Fig. 5, [Fgenotype x treatment (1,40) = 13.36, p < 0.001]). These data suggest that α5 nAChR subunit expression may be required for acquisition of ethanol CPP in mice.
Figure 5.
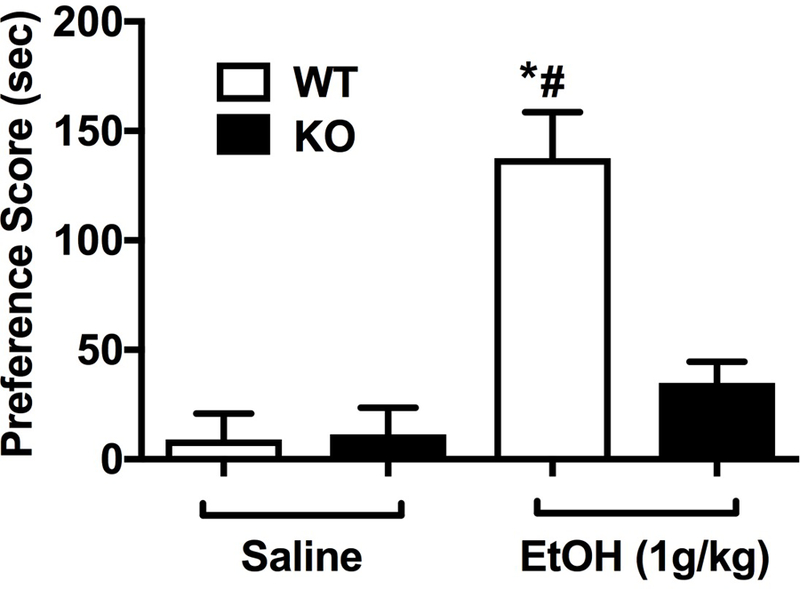
Deletion of the Chrna5 gene reduces ethanol conditional place preference in mice. Data (mean ± SEM) represent preference scores (sec) in WT and α5 KO mice conditioned with saline or ethanol (*p<0.05 compared to the correspondent saline-treated group; #p<0.05 compared to correspondent WT mice, n=11).
3.5. Deletion of Chrna5 reduces ethanol consumption following stress in mice
Given that α5 null mice display altered acute ethanol-induced responses and ethanol CPP, we hypothesized that α5* nAChRs could also influence alcohol consumption. We tested mice in two distinct drinking paradigms: 1) standard two-bottle choice, which measures ethanol preference; and 2) DID, which models binge-like ethanol consumption. Unlike the standard two-bottle choice test which allows for continuous access to ethanol, DID is thought to motivate compulsive-like drinking by limiting the amount of time mice have access to ethanol. Overall, the DID paradigm has been found to produce increased ethanol consumption and BEC than the standard two-bottle choice paradigm (Fritz and Boehm, 2016).
We assessed ethanol consumption (3–30% v/v) in the two-bottle choice paradigm over the course of 2 weeks (see supplemental methods for the procedure). We found that deletion of the α5 subunit did not affect ethanol consumption or preference at any concentration of ethanol (Fig. 1 Supplement). A two-way ANOVA with repeated measure revealed a significant main effect of ethanol concentration (F(4,117) = 86.390, p< 0.0001), but not to genotype (F (1,117) = 1.795, p= 0.194) or the interaction between genotype and ethanol concentrations (F(4,117) = 0.852, p=0.496).
Similar to what has been reported by Santos and colleagues (Santos et al., 2012), we found no differences in the average ethanol intake between WT and KO mice after 4 days of DID (p=0.529, t-test; Fig. 6, BL). Since ethanol reduces anxiety-like behavior in α5 KO mice, we explored the influence of stress on drinking behavior. α5 KO and WT mice were subjected to daily restraint stress once/day for 4 days before each DID session. We found that daily ethanol significantly decreased in α5 KO mice compared to WT counterparts in all stress days (F(7,48)=9.545, p<0.001) (Fig. 6).
Figure 6.
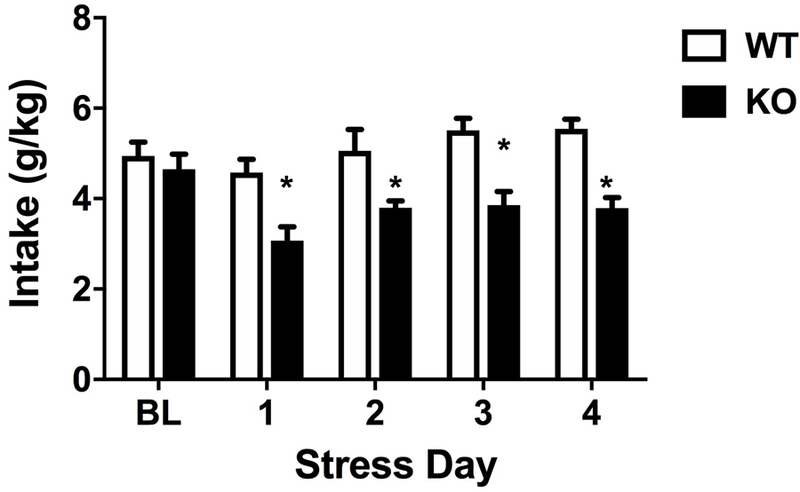
Comparison of ethanol consumption before and during repeated stress in α5 WT and KO mice using the DID paradigm. Deletion of the Chrna5 gene reduces DID drinking behavior after stress in mice. The baseline (BL) EtOH intake for α5 WT and KO mice is similar. Data (mean ± SEM) represent daily DID ethanol intake in g/kg for 4 hours in WT and α5 KO mice that underwent daily restraint stress for 15 min for 4 days (*p<0.05 compared to correspondent WT group, n = 8).
4. Discussion
The goal of this study was to characterize the role of the α5 nAChR subunit (Chrna5) in several ethanol-responsive behaviors using α5 KO mice and their WT littermates. We found that deletion of Chrna5 significantly enhanced ethanol’s effects on body temperature, increased LORR duration, and accentuated ethanol’s anxiolytic effects in the EPM in comparison to WT controls. Further, we investigated the involvement of α5 nAChRs in ethanol reward in the CPP, voluntary drinking in continuous access two-bottle choice and binge-like ethanol drinking in the DID paradigm. Deletion of Chrna5 attenuated ethanol CPP and reduced ethanol intake in the DID following restraint stress. However, drinking behavior was not changed in the continuous access two-bottle choice assay or DID without restraint stress. Taken together, our findings indicate that α5 nAChRs are involved in acute ethanol-responsive behaviors. Furthermore, the effects of α5 on ethanol drinking behaviors are more prominent under stressful conditions.
The LORR test is used to assess sensitivity to ethanol’s hypnotic effects. Our results are in agreement with a previous report where α5 KO mice showed increased ethanol-induced LORR duration without affecting LORR onset (Santos et al., 2012). One possibility for the effect of α5* nAChRs on ethanol-induced sedation could be due to changes in ethanol metabolism. This is unlikely, given that we found no difference in BEC between KO and WT mice during the exact time frame that we measured LORR behavior. The sedative effects of ethanol are known to be associated with hypoactivity of NMDA receptors and hyperactivity of GABA receptors (Beleslin et al., 1997). To understand the mechanism of α5 nAChRs effect on the hypnotic effects of ethanol, we used two different types of sedative/hypnotics. Pentobarbital is a GABAA receptor potentiator (Loscher and Rogawski, 2012), while sedative effects of ketamine are attributed to its inhibitory action on NMDA receptors and HC1 channels (MacDonald et al., 1987; Chen et al., 2009). Like ethanol, we found that pentobarbital significantly increased LORR duration without affecting LORR onset in α5 KO mice, suggesting that α5 plays a role in GABAA receptor function. In contrast, ketamine had no genotype-related changes in LORR duration or onset. Together, these results suggest that deletion of Chrna5 enhances ethanol-induced LORR, at least in part, by increasing the activity of GABAA receptor function without altering NMDA receptor or HC1 channel function.
The α5 subunit is co-expressed with α4β2* and α3β4* nAChRs subtypes to form functional receptor complexes (Ramirez-Latorre et al., 1996; Gotti et al., 2007). While the present study did not examine the exact composition of α5* nAChRs, work in the field using nAChR specific antagonist and nAChR knockout mice give insight into the possible receptor composition. Work in our lab has highlighted the role of α4β2* nAChRs in modulating acute-ethanol behavioral responses. Drugs that specifically block this receptor complex amplify LORR duration and changes in body temperature. Similar results were also found when testing the β2KO mice (Dawson et al., 2013). Human genetic studies have highlighted the influence of the CHRNA5, CHRNA3 and CHRNB4 gene cluster that encodes α5, α3, and β4 nAChR subunits respectively, in alcohol-related behaviors (Schlaepfer et al., 2008). Mice overexpressing the human α5, α3, β4 gene cluster, display normal ethanol-induced sedative-hypnotic effects, changes in body temperate and ataxia as measured via the balance beam and rotorod test (Gallego et al., 2012). Studies using β4 KO mice also found no significant difference between WT littermates in ethanol LORR and locomotor behavior (Kamens et al., 2017). Not surprisingly, neither overexpression of the human gene cluster or β4 null mutation affected ethanol metabolism. Overall, this suggest that the influence of α5* nAChRs in modulating the sedative, locomotor and temperature changes associated with acute ethanol are likely due to α4β2α5* and not α3β4α5* receptor subtypes.
One of the key findings is that α5 KO mice demonstrated an increased response to ethanol’s anxiolytic-like effects during the EPM test. There was no difference in the total number of crossovers in α5 KO compared to WT mice, indicating that the effect of α5 KO in the EPM is not due to changes in the locomotor activity. Our results are in line with a previous study where α5 KO mice demonstrated an enhanced anxiolytic phenotype in the EPM (Gangitano et al., 2009). It is possible that ethanol’s effect is mediated by α4β2α5 nAChRs, since α4β2* nAChR antagonist and β2 KO mice accentuate alcohol-induced anxiolytic behavior in the EPM (Dawson et al., 2013). At this point, the role of α5α3β4 nAChRs in modulating ethanol-induced anxiolysis cannot be excluded given that drugs targeting α3β4* nAChRs and β4 KO mice have not been tested. In addition, it is known that β4* nAChRs play role in regulating anxiety-like behavior in the EPM during basal conditions (Salas et al., 2003b).
Furthermore, we found that deficiency of α5 nAChRs did not increase ethanol intake in two-bottle choice or in the DID paradigm under basal conditions, suggesting that the effect of α5 is more complex than simple enhancement of ethanol-induced anxiolysis. Given that stress elevates ethanol drinking (Anderson et al., 2016), we further examined voluntary ethanol consumption in the DID assay following restraint-induced stress. Here, we found a profound effect of α5: While the WT mice showed a modest increase of ethanol consumption following stress, the α5 KO mice showed a significant reduction of ethanol intake following stress. These results suggest that deletion of Chrna5 may alter the sensitivity to stress and/or loss of α5 may decrease the effects of ethanol experienced by animals in stressful situations. Overall, the results from the EPM and drinking behaviors suggest that there could be additional aspects of anxiety or emotional behaviors regulated by α5 nAChRs. To further understand the nature of the role of α5 nAChR subunits in anxiolytic-like behaviors induced by acute ethanol, different tests for more aspects of anxiety-like behaviors may prove useful in future studies.
The results of our DID study confirms previously published data where deletion of Chrna5 did not change ethanol intake in this binge drinking assay (Santos et al., 2012). The fact that Chrna5 deletion did not change ethanol consumption in either the DID or two-bottle choice procedures during basal conditions suggests that α5* nAChRs do not have a strong role in ethanol consumption under non-stress conditions.
We used the CPP assay to examine the role of α5 in ethanol reward-like effect. We found for the first time that ethanol preference was absent in α5 KO mice in the CPP assay. This finding compliments our DID stress data where deletion of Chrna5 reduced ethanol consumption in response to restraint stress in a voluntary drinking assay. Interestingly, our observation of a decrease in the rewarding properties of ethanol in α5 KO mice is in contrast to our previous observation that they demonstrate increased preference for higher doses of nicotine in the CPP assay (Jackson et al., 2010). While unlikely, it is possible that emergent differences in ethanol metabolism between WT and KO mice occurring after 120 min post-alcohol injection could explain the difference in CPP scores since the interval between conditioning session was 4 hours.
In contrast to our observations with ethanol, in which we found that loss of the α5 subunit enhanced the hypnotic effects of ethanol, the α5 gene deletion confers decreased sensitivity to the locomotor depressant effect of nicotine (Jackson et al., 2010, Fowler et al., 2011). While the exact mechanisms underlying these results remain unknown, these results support the significance of the role of α5 nAChR in the early responses to both nicotine and alcohol exposure. Finally, it is possible that the effects observed in the α5 KO mice could be due to compensatory changes in the expression levels of other genes resulting from deletion of the Chrna5 gene. While the expression of major nAChR subunits is not affected by Chrna5 gene deletion (Salas et al., 2003a), compensatory mechanisms with other nicotinic or non-nicotinic pathways in the brain cannot be entirely ruled out. Despite this limitation, our data collectively confirm that Chrna5 gene plays a significant role in ethanol responsive behaviors and reward.
Supplementary Material
Deletion of the Chrna5 gene has no effect on two-bottle choice ethanol drinking intake and preference in mice. Data (mean ± SEM) represent (A) ethanol intake in g/kg and (B) ethanol preference ratio during 4 weeks of exposure to increasing concentrations of ethanol (n= 11).
Acknowledgments
The authors greatly appreciate the technical assistance of Tie Han and Cindy Evans. This research was supported by the National Institute on Drug Abuse DA- R01 DA032246 (MID and MFM).
This research was supported by the National Institute on Drug Abuse DA- (MID).
Footnotes
Conflict of Interest statement
All of the authors declare no conflict of interest.
References
- Alkana RL, Finn DA, Jones BL, Kobayashi LS, Babbini M, Bejanian M, Syapin PJ, 1992. Genetically determined differences in the antagonistic effect of pressure on ethanol-induced loss of righting reflex in mice. Alcohol Clin Exp Res 16,17–22. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC, 2016. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl) 233,2035–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddick CG, Marks MJ, 2011. An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol 82(8), 828–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B, 1995. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction (Abingdon, England) 90,977–980. [DOI] [PubMed] [Google Scholar]
- Beleslin DB, Djokanović N, Jovanović Mićić D, Samardzić R, 1997. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol 14,167–173. [DOI] [PubMed] [Google Scholar]
- Borghese C, Wang L, Bleck V, Harris RA, 2003. Mutation in neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes blocks ethanol action. Addiction Biology 8,313–318. [DOI] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P, 2007. Nicotinic alpha5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. Journal of Neurochemistry 103,204–215. [DOI] [PubMed] [Google Scholar]
- Browman KE, Rustay NR, Nikolaidis N, Crawshaw L, Crabbe JC, 2000. Sensitivity and tolerance to ethanol in mouse lines selected for ethanol-induced hypothermia. Pharmacol Biochem Behav 67,821–829. [DOI] [PubMed] [Google Scholar]
- Bühler KM, Giné E, Echeverry-Alzate V, Calleja-Conde J, Fonseca FR, López-Moreno JA, 2015. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict Biol 20,845–71. [DOI] [PubMed] [Google Scholar]
- Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA, 1999. Effects of ethanol on recombinant human neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. The Journal of Pharmacology and Experimental Therapeutics 289,774–780. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Excessive Drinking Costs U.S. $223.5 Billion Available at: http://www.cdc.gov/features/alcoholconsumption/.
- Centers for Disease Control and Prevention, 2015. Smoking and Tobacco Use: Fast Facts Available at: http://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/.
- Chen X, Shu S, Bayliss DA, 2009. HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 29,600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquet H, Joslyn G, Lee A, Kasberger J, Robertson M, Brush G, Schuckit MA, White R, Jorgenson E, 2013. Examination of Rare Missense Variants in the CHRNA5-A3-B4Gene Cluster to Level of Response to Alcohol in the San Diego Sibling Pair Study. Alcoholism: Clinical and Experimental Research 37,1311–1316. [DOI] [PubMed] [Google Scholar]
- Dawson A, Miles MF, Damaj MI, 2013. The β2 nicotinic acetylcholine receptor subunit differentially influences ethanol behavioral effects in the mouse. Alcohol 47, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP, 1990. Alcoholism and Smoking. J Studies Alcohol 51,130–135. [DOI] [PubMed] [Google Scholar]
- Falk DE, Yi H, Hiller-Sturmhöfel S, 2006. An epidemiologic analysis of co-occurring alcohol and tobacco use and disorders: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Res Health 29,162–171. [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular α5 nicotinic receptor subunit signaling controls nicotine intake. Nature 471,597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz B,M, Boehm SL 2nd., 2016. Rodent models and mechanisms of voluntary binge-like ethanol consumption: Examples, opportunities, and strategies for preclinical research. Prog Neuropsychopharmacol Biol Psychiatry 65, 297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego X, Ruiz-Medina J, Valverde O, Molas S, Robles N, Sabrià J, Crabbe JC, Dierssen M, 2012. Transgenic over expression of nicotinic receptor alpha 5, alpha 3, and beta 4 subunit genes reduces ethanol intake in mice. Alcohol 46, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano D, Salas R, Teng Y, Perez E, De Biasi M, 2009. Progesterone modulation of α5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav 8,398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SJ, Lotfipour S, Leslie FM, 2017. Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am J Drug Alcohol Abuse 43(2), 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M, 2007. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74, 1102–1111. [DOI] [PubMed] [Google Scholar]
- Guildford MJ, Sacino AV, Tapper AR, 2016. Modulation of ethanol reward sensitivity by nicotinic acetylcholine receptors containing the α6 subunit. Alcohol 57, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver SB, Kamholz PDBW, Helstrom AW, 2007. Smoking Cessation and Alcohol Abstinence: What Do the Data Tell Us? Alcohol Res Health 29,208–212. [PMC free article] [PubMed] [Google Scholar]
- Harenza JL, Muldoon PP, De Biasi M, Damaj MI, Miles MF, 2014. Genetic variation within the Chrna7 gene modulates nicotine reward-like phenotypes in mice. Genes Brain Behav 13,213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Kalman D, 2006. Do smokers with alcohol problems have more difficulty quitting? Drug Alcohol Depend 82,91–102. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazzo JD, 1984. Tobacco, alcohol, and caffeine use: a review of their interrelationships. Psychological Bull 95,301–326. [PubMed] [Google Scholar]
- Jackson KJ, Marks MJ, Vann RE, Chen X, Gamage TF, Warner JA, Damaj MI, 2010. Role of alpha5 Nicotinic Acetylcholine Receptors in Pharmacological and Behavioral Effects of Nicotine in Mice. J Pharmacol Exp Ther 334,137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential Role of Nicotinic Acetylcholine Receptor Subunits in Physical and Affective Nicotine Withdrawal Signs. Journal of Pharmacology and Experimental Therapeutics 325,302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn G, Brush G, Robertson M, Smith TL, Kalmijn J, Schuckit M, White RL, 2008. Chromosome 15q25.1 genetic markers associated with level of response to alcohol in humans. Proceedings of the National Academy of Sciences 105,20368–20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Hoft NR, Cox RJ, Miyamoto JH, Ehringer MA, 2012. The α6 nicotinic acetylcholine receptor subunit influences ethanol-induced sedation. Alcohol 46,463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Andersen J, Picciotto MR, 2010. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology (Berl) 208,613–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Silva C, McCarthy R, Cox RJ, Ehringer MA, 2017. No evidence of a role of the β4 subunit of the nicotinic acetylcholine receptor in alcohol-related behaviors. BMC Res Notes 10, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI, 2007. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther 322,399–407. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, Lindstrom J, 2008. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol 74(1), 132–43. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J, 2008. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology 203,99–108. [DOI] [PubMed] [Google Scholar]
- Löscher W, Rogawski MA, 2012. How theories evolved concerning the mechanism of action of barbiturates. Epilepsia 53, 12–25. [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Miljkovic Z, & Pennefather P, 1987. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol 58,251–266. [DOI] [PubMed] [Google Scholar]
- Morel C, Fattore L, Pons S, Hay YA, Marti F, Lambolez B, De Biasi M, Lathrop M, Fratta W, Maskos U, Faure P, 2014. Nicotine consumption is regulated by a human polymorphism in dopamine neurons. Mol Psychiatry 19,930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M, 1985. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14,149–167. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC, 2002. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther 302,257–263. [DOI] [PubMed] [Google Scholar]
- Putman AH, Wolen AR, Harenza JL, Yordanova RK, Webb BT, Chesler EJ, Miles MF, 2016, Identification of quantitative trait loci and candidate genes for an anxiolytic-like response to ethanol in BXD recombinant inbred strains. Genes Brain Behav 15(4), 367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L, 1996. Functional contributions of alpha5 subunit to neuronal acetylcholine receptor channels. Nature 38, 347–351. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC, 2005. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84,53–63. [DOI] [PubMed] [Google Scholar]
- Sajja RK, Rahman S, 2012. Neuronal nicotinic receptor ligands modulate chronic nicotine-induced ethanol consumption in C57BL/6J mice. Pharmacol Biochem Behav 102,36–43. [DOI] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M, 2003a. The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol 63,1059–1066. [DOI] [PubMed] [Google Scholar]
- Salas R, Pieri F, Fung B, Dani JA, De Biasi M, 2003b. Altered anxiety-related responses in mutant mice lacking the beta4 subunit of the nicotinic receptor. J Neurosci 23,6255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos N, Chatterjee S, Henry A, Holgate J, Bartlett SE, 2012. The α5 Neuronal Nicotinic Acetylcholine Receptor Subunit Plays an Important Role in the Sedative Effects of Ethanol But Does Not Modulate Consumption in Mice. Alcohol Clin Exp Res 37,655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ et al. , 2008. The CHRNA5/A3/B4 Gene Cluster Variability as an Important Determinant of Early Alcohol and Tobacco Initiation in Young Adults. Biological Psychiatry 63,1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, & Ehringer MA, 2008. The genetic components of alcohol and nicotine co-addiction: From genes to behavior. Current Drug Abuse Reviews, 1(2), 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater CA, Jackson A, Muldoon PP, Dawson A, O’Brien M, Soll LG, Abdullah R, Carroll FI, Tapper AR, Miles MF, Banks ML, Bettinger JC, Damaj MI, 2016. Nicotine Enhances the Hypnotic and Hypothermic Effects of Alcohol in the Mouse. Alcohol Clin Exp Res 40(1), 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons MN, Weng J, Diehl E, Heo E, Kleiber ML, Singh SM, 2010. Delineation of the Role of Nicotinic Acetylcholine Receptor Genes in Alcohol Preference in Mice. Behavior Genetics 40,660–671. [DOI] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA, 2015. Sex Differences in Ethanol’s Anxiolytic Effect and Chronic Ethanol Withdrawal Severity in Mice with a Null Mutation of the 5α-Reductase Type 1 Gene. Behav Genet 45(3), 354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL, 2014. “Drinking in the Dark”(DID): A Simple Mouse Model of Binge-Like Alcohol Intake. Curr Protoc Neurosci 68,9.49.1–9.49.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP et al. , 2009. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Molecular Psychiatry 14,501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Warner JA, Capparuccini MI, Archer KJ, Shelton KL, Miles MF, 2011. Genomic analysis of individual differences in ethanol drinking: evidence for non-genetic factors in C57BL/6 mice. PloS one 6,e21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Deletion of the Chrna5 gene has no effect on two-bottle choice ethanol drinking intake and preference in mice. Data (mean ± SEM) represent (A) ethanol intake in g/kg and (B) ethanol preference ratio during 4 weeks of exposure to increasing concentrations of ethanol (n= 11).


