Abstract
The incubation of cue-reinforced cocaine-seeking coincides with increased extracellular glutamate within the ventromedial prefrontal cortex (vmPFC). The vmPFC is comprised of two subregions that oppositely regulate drug-seeking, with infralimbic (IL) activity inhibiting, and prelimibic (PL) activity facilitating, drug-seeking. Thus, we hypothesized that increasing and decreasing endogenous glutamate within the IL would attenuate and potentiate, respectively, cue-reinforced drug-seeking behavior, with the converse effects observed upon manipulations of endogenous glutamate within the PL. Male Sprague-Dawley rats were trained to self-administer cocaine (0.25 mg/infusion; 6 h/day X 10 days), the delivery of which was signaled by a tone-light cue. Rats were then subdivided into 3 or 30 day withdrawal groups. For testing, rats were microinjected with vehicle, 20 mM of the mGlu2/3 agonist LY379268 (to lower endogenous glutamate), or 300 μM of the excitatory amino acid transporter inhibitor threo-β-benzyloxyaspartate (TBOA; to raise endogenous glutamate) into either the IL or PL (0.5 μl/side) and then given a 30-min test for cue-reinforced drug-seeking. Vehicle-infused rats exhibited incubated responding on the cocaine-associated lever. Neither LY379268 nor TBOA altered behavior at 3 days withdrawal, indicating that glutamate within neither subregion regulates cue-reinforced drug-seeking during early withdrawal. At 30 days withdrawal, intra-PL LY379268 microinjection significantly decreased drug-seeking behavior, while the effect was more modest when infused intra-IL. Interestingly, intra-IL TBOA attenuated incubated drug-seeking during protracted withdrawal, but did not affect behavior when infused intra-PL. These results argue that glutamate release within the PL in response to drug-seeking likely drives the manifestation of incubated cocaine-seeking during protracted withdrawal.
Keywords: Cocaine, Incubation, Prelimbic cortex, Infralimbic cortex, Glutamate, Drug-seeking, Craving
1. Introduction
Drug-associated cues elicit intense drug-craving (Childress et al., 1993; Goldstein and Volkow, 2011), and this ability strengthens during prolonged abstinence (Tran-Nguyen et al., 1998; Grimm et al., 2001; Lu et al., 2004; Pickens et al., 2011). In human cocaine addicts, craving in response to drug-associated cues presents itself in an inverse-U shape curve over time (Gawin and Kleber, 1986; Parvaz et al., 2017). This phenomenon, termed the incubation of craving (Grimm et al., 2001; Lu et al., 2004; Pickens et al., 2011), also manifests with other types of drugs of abuse (Nicotine: Bedi et al., 2011; Meth: Wang et al., 2013; Alcohol: Li et al., 2014) and may help elucidate a period of time where addicts are most vulnerable to cue-induced relapse. The functional neuroanatomy involved in the incubation of cocaine craving remains to be fully elucidated. However, it likely relates to neuro-adaptations within the prefrontal cortex (PFC), based on evidence that cocaine abusers consistently exhibit increased metabolic indices of hyperactivity within this region during cue-induced craving (Grant et al., 1996; Garavan et al., 2000; Bonson et al., 2002).
In laboratory rodents, the incubation of cue-induced craving is modeled by a withdrawal-dependent increase in the conditioned reinforcing properties of drug-paired discrete stimuli, typically assessed when the animal is in a drug-free state (Grimm et al., 2001; Pickens et al., 2011). Indeed, evidence from such preclinical studies also implicates the PFC, notably its more ventromedial aspects (vmPFC), as underpinning incubated craving. For one, neuropharmacological inactivation of the vmPFC, via infusion of a GABA agonist cocktail, decreases incubated drug-seeking (Koya et al., 2009). Cues signaling cocaine availability promote cocaine-seeking by elevating glutamatergic transmission in the nucleus accumbens (Suto et al., 2013), a major downstream projection of the vmPFC. Further, the expression of incubated cocaine-seeking is positively correlated with higher expression of neural activity markers within the vmPFC, such as p-ERK (Koya et al., 2009) and p-PKCε (Miller et al., 2016), as well as increases in extracellular glutamate during incubated drug-seeking (Shin et al., 2016). This cue-reinforced rise in extracellular glutamate in the vmPFC appears to be cocaine-selective as it is not apparent in sucrose- or neutral cue-reinforced rats (Shin et al., 2016). Put together, these data have led to the hypothesis that endogenous glutamate from the vmPFC may be a driving factor in incubated drug-seeking.
The vmPFC is divided into two subregions: the prelimbic and infralimbic cortices (respectively, PL and IL). Although some neuroanatomical overlap exists with respect to their innervation of nucleus accumbens (NAc) subregions, the PL mainly projects to the NAc core, while the IL mainly projects to the NAc shell (Vertes, 2004). Although adjacent to each other, these two regions oppositely control aspects of drug-seeking, particularly during protracted withdrawal. Specifically, neuropharmacological (Pelloux et al., 2013) and optogenetic (Stefanik et al., 2013, 2015; Ma et al., 2014) evidence argues that PL activity drives drug-seeking behavior. In contrast, IL activity inhibits aspects of cocaine-seeking in the extinction-reinstatement model of relapse (Peters et al., 2008; LaLumiere et al., 2012) and also during incubated cocaine-seeking (Ma et al., 2014). Likewise, 3,4-methylenedioxymethamphetamine (MDMA) reinstatement studies also point to dichotomous roles for the IL and PL in regulating drug-seeking behavior, as inactivation of the PL, but not the IL, completely blocked cue-induced reinstatement of MDMA-seeking behavior (Ball and Slane, 2012). Further, optogenetic and electrophysiological studies indicate that AMPA receptors in the NAc undergo differential remodeling during protracted withdrawal from cocaine self-administration to influence the incubation process (Conrad et al., 2008; Ma et al., 2014). More specifically, opto-genetically reversing the maturation of silent glutamatergic synapses e a phenomenon linked to incubated drug-seeking e in IL-NAc projections attenuates incubated cocaine-seeking, while reversing maturation in the PL-NAc projections potentiates incubated cocaine-seeking (Ma et al., 2014). As optogenetic approaches cannot inform as to the biochemical bases of incubation, we sought to delineate the relative role for glutamate within the PL versus IL in the incubation of cocaine-seeking using bidirectional neuropharmacological approaches. More specifically, endogenous glutamate within each vmPFC subregion was raised using the non-selective excitatory amino acid transporter (EAAT) reuptake inhibitor DL-threo-β-benzyloxyaspartate (TBOA) and lowered using the mGlu2/3 autoreceptor agonist LY379268. As the PL putatively drives drug-seeking behavior, we hypothesized that mimicking the cue-reinforced increase in glutamate (Shin et al., 2016) within this subregion upon TBOA infusion would augment incubated drug-seeking behavior, while inhibiting cue-reinforced glutamate release with LY379268 would produce the opposite effect. Conversely, as IL activity tends to inhibit drug-seeking, we hypothesized that increasing and lowering glutamate within this more ventral subregion would attenuate and promote incubated drug-seeking, respectively.
2. Material and methods
2.1. Subjects
Male Sprague-Dawley (N = 160) rats weighing 275–325 g were obtained from Charles River Laboratories (Hollister, CA, USA) and allowed 2 days to acclimate to the colony. Rats were pair-housed in a colony room that was temperature (25°C) and humidity (71%) controlled under a 12-hr reverse light cycle (lights off: 0700 h). Food and water was available ad libitum except during food training. All experimental protocols were consistent with the National Institute of Health Guide for Care and Use of Laboratory Animals and approved by the University of California, Santa Barbara, Institutional Animal Care and Use Committee.
2.2. Lever-response training
To be consistent with the procedures employed in our prior microdialysis study (Shin et al., 2016), rats were food-deprived to 16 g/day in order to promote lever-response training. Rats were placed into sound-attenuated operant-conditioning chambers (30 × 20 × 24 cm; Med Associates Inc., St. Albans, VT) for 16 h overnight. Each chamber contained two retractable levers, a stimulus light above each lever, a food trough between the levers, a house light on the wall opposite the levers, and a speaker connected to a tone generator (ANL-926, Med Associates). A lever press on the active lever resulted in the delivery of a 45 mg food pellet (Bio Serv, Frenchtown, NJ), along with a 1-sec presentation of light above the active lever. Once lever-response training was completed (acquisition criterion = 100 responses per active lever/session), rats were taken off food restriction, then slated to undergo surgery.
2.3. Jugular implantation and vmPFC cannula surgeries
Rats were anesthetized with a ketamine/xylazine cocktail (56.25 and 7.5 mg/kg, respectively, intramuscular) and then implanted with a chronic indwelling catheter into the right jugular vein, as previously described by our group (Ben-Shahar et al., 2013; Shin et al., 2016; Miller et al., 2016). Each catheter was comprised of Silastic tubing (13 cm long; 0.3 mm inner diameter, 0.64 mm outer diameter; Dow Corning, Midland, MI), attached to a threaded 22 gauge metal guide cannula (Plastics One, Roanoke, VA) that was cemented to a small square of polypropylene mesh (Bard Mesh, C.R. Bard, Cranston, RI), which ensured adherence to tissue around the animal’s back.
Immediately following intravenous catheter implantation, rats were transferred to a stereotaxic apparatus and implanted with a bilateral guide cannula (22 gauge, 1 mm c/c, 13 mm long; Plastics One) aimed 2 mm above either the PL (AP: +3 mm; ML: ±1.0; DV: −1.5 mm) or the IL (AP: +3 mm; ML: ±1.0; DV:−3 mm; Paxinos and Watson, 2006). Four small stainless steel screws and cranioplastic cement secured the guide cannulae to the skull. Stylets (Plastics One) were placed into each cannula in order to prevent occlusion.
Rats were given banamine (2 mg/kg; non-opiate analgesic; subcutaneous) to reduce postsurgical pain before surgeries and for 2 days post-surgery. On the subsequent days until the end of the experiment, rats were flushed intravenously with 0.1 ml of sterile gentamycin (2 mg/kg) and heparin + cefazolin (60 IU/ml and 1 mg/ml, respectively) in order to maintain catheter patency. All catheters were tested every week for patency using sodium brevitol (5 mg/kg, intravenous; JHP Pharmaceuticals, Parsippany, NJ, USA).
2.4. Self-administration
Allowing a minimum of 5 days for recovery after surgeries, rats were allowed to self-administer cocaine (0.25 mg/0.1 ml/infusion; Sigma-Aldrich, St. Louis, MO) on a fixed ratio 1 schedule of reinforcement, 6 h/day, for 10 consecutive days. This was done in the same operant chambers as lever-response training described above. Depression of the active lever activated a 20-sec light and tone (78 dB, 2 kHz) compound stimulus, which also served as a time-out period in which lever presses were recorded but had no consequences. The first two days of self-administration were capped at 100 and 102 infusions, respectively, to prevent overdose. The next 8 days of self-administration was unhindered, after which rats were left undisturbed in their home-cage for either 3 or 30 days, depending on withdrawal group. Only rats that exhibited stable levels of drug taking over the last 3 days of the self-administration phase of the study were tested for the effects of our neuropharmacological manipulations upon drug-seeking. Rats were randomly assigned to a microinjection treatment group (TBOA vs. vehicle; LY379268 vs. vehicle), with all groups exhibiting equivalent drug intake prior to testing.
2.5. Microinjection and test for cue-reinforced cocaine-seeking
DL-threo-β-Benzyloxyaspartic acid (TBOA; Tocris, Minneapolis, MN) was dissolved into 0.1 N NaOH and neutralized with 0.1 N HCl, then diluted with sterile water to a working concentration of 300 μM. This TBOA concentration was selected for study as it elevates extracellular glutamate within the PFC of rats (Melendez et al., 2005) and augments a cocaine-conditioned place-preference when infused intra-mPFC in mice (Lominac et al., 2016). LY379268 (Tocris) was mixed with sterile water to a concentration of 20 mM, a dose comparable to that demonstrated previously to lower cocaine-induced behavioral sensitization during protracted withdrawal when infused intra-mPFC (Lupinsky et al., 2010). Sterile water served for vehicle injections.
At either 3 or 30 days withdrawal, rats were microinjected bilaterally with 0.5 μl of their assigned drug (0.5 μl/min) for 1 min. Injectors were then left in place for an additional minute to allow drug diffusion. The microinjectors were removed and rats were tethered as per usual, then given a 30-min test for cue-reinforced responding (Extinction Test), during which each lever press resulted in the presentation of the cues associated with self-administration (i.e., light and tone), but no drug. TBOA infused rats were placed into the chambers immediately upon microinjection in a manner consistent with prior neuropharmacological studies using this drug (e.g., Kapasova and Szumlinski, 2008; Lominac et al., 2016). Rats slated for the LY379268 and LY379268’s corresponding vehicle group were left inside the operant chamber (levers retracted, doors open) for ~10 min in order for the drug to take effect, as conducted in prior neuropharmaco-logical studies (Bossert et al., 2004; Counotte et al., 2011; Myal et al., 2015), before starting the 30-min Extinction Test.
At the end of the Extinction Test, rats were anesthetized with 4% isoflurane and decapitated in order to extract the brain. Tissue was sliced on a vibratome (Leica, Nussloch, Germany) then stained with 0.1% cresyl violet acetate to visualize microinjector placement. Only data from rats exhibiting injector placement within the boundaries of the vmPFC (prelimbic and/or infralimbic areas) were employed in the statistical analyses.
2.6. Statistical analyses
As the four studies of the effects of intra-PL and intra-IL infusion of TBOA and LY379268 on incubated drug-seeking were each conducted independently, in series, the data from each experiment were analyzed separately. To confirm equivalent cocaine intake across the four experimental conditions within each study, the data for the average number of cocaine infusions earned during the last 3 days of self-administration training were analyzed using a Withdrawal (3 vs. 30 days withdrawal) X Treatment (TBOA/LY379268 vs. vehicle) ANOVA. The behavioral data for the Extinction Test was analyzed by a Withdrawal (3 vs. 30 days withdrawal) X Treatment (TBOA/LY379268 vs. vehicle) ANOVA. When appropriate, interactions were deconstructed using simple effects analyses of group differences between 3 and 30 days withdrawal rats. α = 0.05 for all analyses.
3. Results
3.1. Self-administration training
All rats were trained to self-administer cocaine, then randomly assigned into their respective withdrawal time-point and intracranial drug treatment groups. An analysis of the number of cocaine infusions earned over the last 3 days of self-administration training failed to indicate any group differences in cocaine intake in any of the studies (Withdrawal X Treatment ANOVAs, for PL-TBOA: all p’s > 0.08; for PL-LY379268: all p’s > 0.10; for IL-TBOA: all p’s > 0.30; for IL-LY379268: all p’s > 0.09; see Table 1).
Table 1.
Mean and SEMs of the number of cocaine infusions earned over the last 3 days of cocaine self-administration across treatment groups.
| Group | Cocaine Infusions | |
|---|---|---|
| 3WD | 30WD | |
| PL-TBOA | 90.19 ± 5.59 | 97.30 ± 8.12 |
| IL-TBOA | 93.14 ± 6.67 | 92.43 ± 5.86 |
| PL-LY38 | 96.24 ± 29.81 | 103.63 ± 29.65 |
| IL-LY38 | 91.73 ± 4.10 | 107.75 ± 7.90 |
3.2. TBOA infusion into the infralimbic, but not the prelimbic cortex, attenuates the incubation of cocaine-seeking
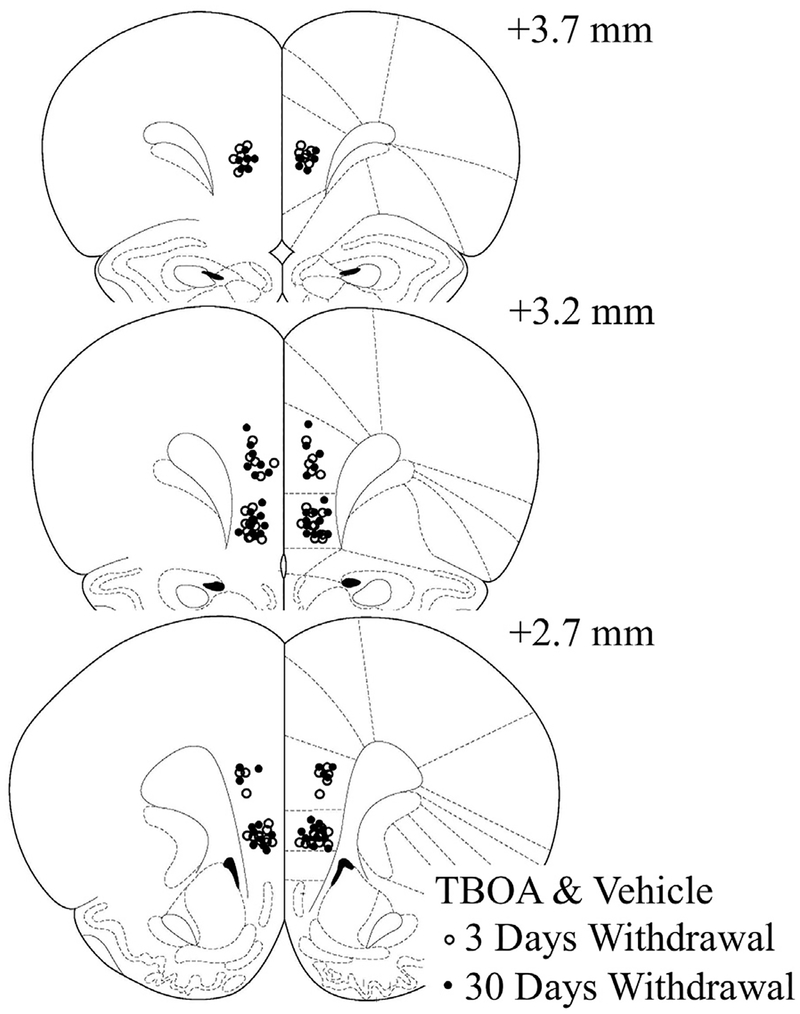
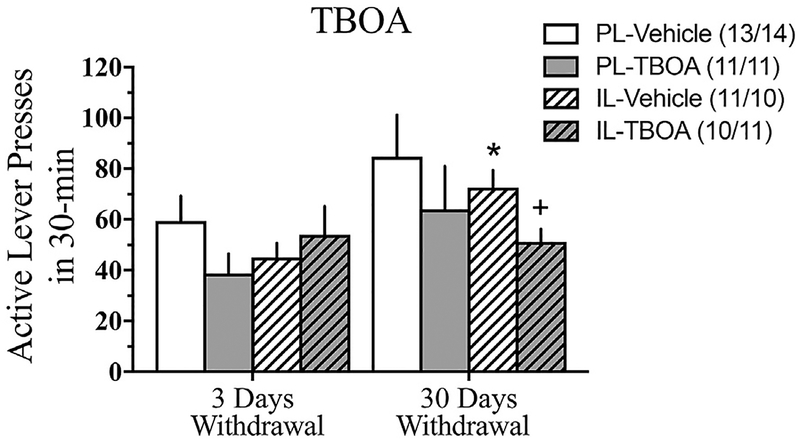
Localization of the microinjection sites for both vehicle- and TBOA-infused rats are presented in Fig. 1. In the PL experiment, infusion of TBOA did not affect cue-reinforced cocaine-seeking at either 3 or 30 days withdrawal, compared to vehicle [Withdrawal effect: p = 0.07; Withdrawal X Treatment: F (1,45) = 0.00, p = 0.99; see Fig. 2]. On the other hand, TBOA infusion into the IL significantly altered the time-dependent change in cue-reinforced cocaine-seeking behavior [Withdrawal X Treatment: F (1,38) = 4.18, p = 0.05]. Deconstruction of the interaction along the Withdrawal factor indicated that TBOA significantly decreased cocaine-seeking at 30 days withdrawal [F (1,19) = 6.23, p = 0.02] but not 3 days withdrawal [F (1,19) = 0.55, p = 0.47; Fig. 2]. No effect of TBOA infusion was observed for inactive lever presses, irrespective of subregion (data not shown) [Treatment X Withdrawal ANOVAs, for PL: all p’s > 0.30; for IL: all p’s > 0.40]. These latter results argue that TBOA infusion did not produce any adverse motor side-effects that might interfere with responding. Further, these data indicate that the intra-IL TBOA effect was selective for the formerly cocaine-reinforced lever.
Fig. 1.
Summary of bilateral placements of TBOA & vehicle microinjection cannulae within the PL and IL.
Fig. 2.
Comparison of the total number of active lever presses at 3 vs. 30 days withdrawal during the Extinction Test for TBOA- or vehicle-infusion into either the PL or IL. Data represents the means ± SEMs of the number of rats indicated in parentheses. *p < 0.05 vs. IL-3 days withdrawal vehicle, illustrating an incubation of drug-seeking. +p < 0.05 vs. IL-30 days withdrawal vehicle.
3.3. LY379268 infusion into the prelimbic, but not the infralimbic cortex, attenuates the incubation of cocaine-seeking
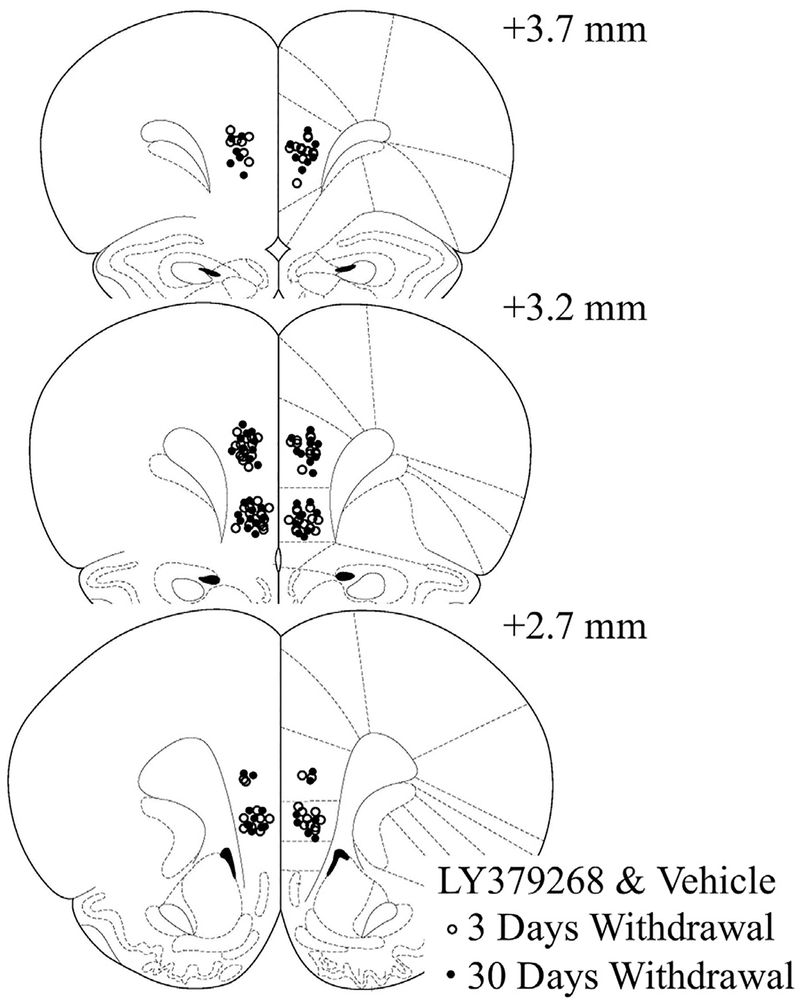
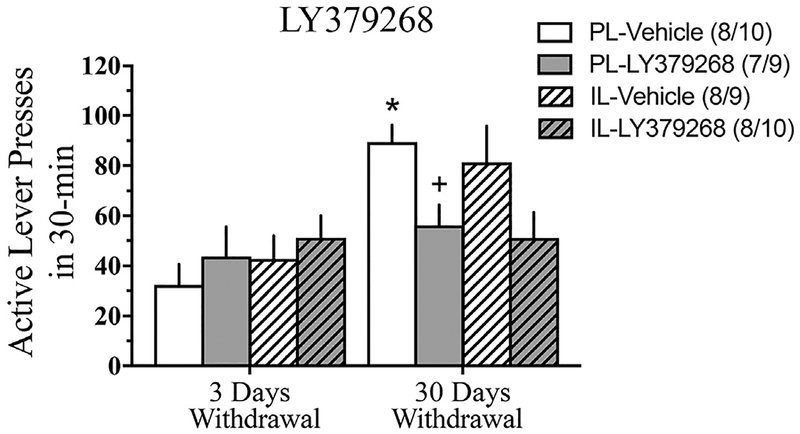
Localization of the microinjection sites for both vehicle- and LY379268-infused rats are presented in Fig. 3. In the PL experiment, LY379268 infusion significantly influenced the time-dependent change in cue-reinforced cocaine-seeking [Withdrawal X Treatment: F (1,30) = 6.66, p 0.02; main effect of withdrawal: F (1,30) = 16.10, p < 0.001; Fig. 4]. Deconstruction of the interaction along the Withdrawal factor demonstrated that LY379268 significantly decreased drug-seeking behavior at 30 days withdrawal [F (1,17) = 9.80, p = 0.006], but did not affect behavior at 3 days withdrawal [F (1,13) = 0.65, p = 0.44]. In contrast, intra-IL infusion of LY379268 had no statistically significant effect on drug-seeking at either time point [Treatment X Withdrawal ANOVA, all p’s > 0.08; Fig. 4]. No effect of LY379268 was apparent for inactive lever presses (data not shown) [Treatment X Withdrawal ANOVAs, for PL: all p’s > 0.06; for IL: all p’s > 0.20]. Thus, LY379268 infusion did not produce any overt motor side-effects that might interfere with responding. Further, these data indicate that the intra-PL LY379268 effect was selective for the formerly cocaine-reinforced lever.
Fig. 3.
Summary of bilateral placements of LY379268 & vehicle microinjection cannulae within the PL and IL.
Fig. 4.
Comparison of the total number of active lever presses at 3 vs. 30 days withdrawal during the Extinction Test for LY379268- or vehicle-infusion into either the PL or IL. Data represents the means ± SEMs of the number of rats indicated in parentheses. *p < 0.05 vs. PL-3 days withdrawal vehicle, illustrating an incubation of drug-seeking. +p < 0.05 vs. PL-30 days withdrawal vehicle.
4. Discussion
Extracellular glutamate levels are elevated in the vmPFC during incubated cocaine-seeking (Shin et al., 2016). However, at the outset of this study, the functional relevance that elevated glutamate plays in incubated drug-seeking was unknown. The vmPFC is subdivided into the PL and IL subregions, which are theorized to play opposing roles in regulating drug-seeking behavior, including the incubation of drug-seeking during protracted withdrawal (Ma et al., 2014; Ball and Slane, 2012). Through neuropharmacological manipulation of endogenous glutamate within the PL and IL, the present study demonstrated that endogenous glutamate in the PL is necessary, but not sufficient, for incubated drug-seeking as the local application of a mGlu2/3 autoreceptor agonist attenuated, while infusion of the non-selective neuronal EAAT inhibitor TBOA did not affect, incubated drug-seeking. Conversely, in the IL, local application of TBOA abolished incubated drug-seeking, while mGlu2/3 agonism produced no significant effect. Lastly, these neuropharmacological manipulations of endogenous GLU did not affect drug-seeking during short-term withdrawal, indicating a more critical role for excitatory glutamate neurotransmission within the vmPFC in regulating the increased cue-reactivity observed during protracted withdrawal.
4.1. Glutamate manipulations influence drug-seeking behavior during protracted, but not short-term, withdrawal
All drug effects observed in the present study were selective for protracted withdrawal. As none of our manipulations altered behavior during early withdrawal, it is unlikely that the reduction in incubated drug-seeking produced by our manipulations reflects gross impairments in general motivation, motor activity or cognitive function (i.e., the ability to recall the drug-cue/context association or the operant response). The temporal specificity of our drug effects for later withdrawal supports previous incubation studies in which neuropharmacological or peptide manipulations of either the cell body (Ben-Shahar et al., 2013; Miller et al., 2016; present study) or terminal (Conrad et al., 2008; Fischer et al., 2013; Li et al., 2013; Loweth et al., 2014) regions of corticoaccumbens projections, along with the general CEA (Lu et al., 2007), exerted effects on drug-seeking only in protracted withdrawal. These behavioral results, coupled with prior in vivo microdialysis (Shin et al., 2016), immunoblotting (Boudreau and Wolf, 2005; Lu et al., 2005; Ghasemzadeh et al., 2011; Ben-Shahar et al., 2013; Miller et al., 2016) and electrophysiological (Lee et al., 2013; Ma et al., 2014; Scheyer et al., 2016) evidence, further support the argument that the neural substrates of incubated cue-reinforced drug-seeking are distinct from those underpinning drug-seeking per se and that the passage of time in withdrawal is critical for the neuroadaptations within vmPFC that bring this structure “on-line” in protracted withdrawal to augment behavioral reactivity to drug-associated cues (Wolf, 2016; Shin et al., 2016). Indeed, others have also hypothesized glutamate-mediated mechanisms are involved in incubated versus non-incubated cocaine-seeking (Lu et al., 2005, 2007).
The neurophysiological properties of terminals within specific corticoaccumbens and amygdaloaccumbens projections are markedly different in early versus later withdrawal (Lee et al., 2013; Ma et al., 2014). Specifically, glutamate projections from the vmPFC to the NAc (Ma et al., 2014), as well as projections from the basolateral amygdala to NAc (Lee et al., 2013), undergo silencing or dematu-ration during early drug withdrawal. However, with the passage of time in withdrawal, these synapses re-mature and become “unsilenced”, owing to the insertion of specific AMPA receptor subunits, and possibly other yet unknown molecular adaptations that augment synapse excitability (Lee et al., 2013; Ma et al., 2014). Currently, it is not known whether or not synapses within the vmPFC proper also undergo similar forms of time-dependent metaplasticity. However, if synapse silencing does occur during early withdrawal in glutamate synapses within vmPFC, such a phenomenon might account for the relative ineffectiveness of our glutamate manipulations, as well as other neuropharmacological manipulations of the vmPFC (e.g., Ben-Shahar et al., 2013; Miller et al., 2016; present study), at earlier withdrawal time-points.
4.2. Endogenous glutamate in the prelimbic cortex is necessary, but not sufficient, for incubated drug-seeking
Stimulation of mGlu2/3 autoreceptors via LY379268 infusion into the PL significantly attenuated incubated drug-seeking, implicating endogenous glutamate within this subregion as a critical mediator of incubated responding. Our neuropharmacological result is consistent with evidence that the PL is implicated in cue-induced reinstatement (Capriles et al., 2003; Zavala et al., 2008; McGlinchey et al., 2016), with PL projections to the NAc core being recruited during, and in proportion to, cue-induced reinstatement of cocaine-seeking (McGlinchey et al., 2016). Further, inactivation of the PL is known to attenuate stress-induced, cue-induced, and cocaine-induced reinstatement of drug-seeking (Capriles et al., 2003; McLaughlin and See, 2003; Fuchs et al., 2004; Di Pietro et al., 2006), supporting a critical role for this vmPFC subregion in these animal models of relapse. More directly relevant to incubated cocaine-seeking, the PL exhibits enhanced coding of cocaine-associated stimuli (West et al., 2014), and the PL projection to the NAc core is strengthened (Suska et al., 2013; Ma et al., 2014; Luís et al., 2016) during protracted withdrawal from chronic cocaine self-administration. Further, the strengthening of this pathway coincides with increased electrophysiological indices of presynaptic glutamate release from PL-NAc projections (Suska et al., 2013; Luís et al., 2016). Taken together, we propose that a time-dependent increase in glutamate release within the PL is at least one neurochemical substrate driving the enhanced encoding of cocaine-associated cues by this subregion, as well as the resultant metaplasticity within the NAc (and other terminal regions) culminating in greater cue-reactivity and incubated responding. Important questions for future work relate to (1) the source of the glutamate within the PL that incubates during protracted withdrawal and is critical for the manifestation of incubated responding, (2) the neuronal or glial adaptations that occur within the PL resulting in increased cue-reactivity of glutmate and (3) the specific glutamate receptors activated by incubated drug-seeking glutamate release within the PL that promote the synaptic strengthening of corticoaccumbens projections to drive incubated responding.
At the outset of study, we hypothesized that if the cue-induced increase in glutamate within the PL drives incubated responding, then augmenting endogenous glutamate levels within this subregion via an infusion of the EAAT inhibitor TBOA might increase the magnitude of the incubated response. In contrast to our hypothesis, intra-PL TBOA infusion did not significantly affect drug-seeking in later withdrawal. This negative result does not likely reflect the dose of TBOA employed as 300 μM TBOA is within the range reported to produce a 2–3-fold increase in glutamate within PFC (Melendez et al., 2005) and, as discussed below, this dose was effective at altering incubated responding when infused into the IL. Given that responding for cocaine-associated cues during protracted withdrawal results in a near-doubling of glutamate within the vmPFC that is maintained throughout a 2-h cue-induced extinction-like test (Shin et al., 2016), it is likely that the failure to observe a potentiation of incubated drug-seeking by intra-PL TBOA infusion reflects a ceiling effect upon behavior. This being said, intra-PL TBOA infusion did not augment cocaine-seeking expressed by rats tested during early withdrawal ‒ a time when cocaine-associated cues do not influence glutamate within vmPFC (Shin et al., 2016) ‒ and thus a time point where an effect of TBOA should be apparent. Alternatively, given our findings for LY379268, the possibility exists that elevating endogenous glutamate prior to the test for incubated drug-seeking engaged autoregulatory and/or presynaptic axoaxonal inhibitory mechanisms that occluded our ability to detect behavioral potentiation. Indeed, incubated cocaine-seeking tended to be suppressed by intra-PL TBOA infusion in the present study, suggesting the possibility that inhibitory mechanisms may have been engaged by TBOA pretreatment.
The PFC expresses the glial transporters EAAT1 and EAAT2, as well as the neuronal transporter EAAT3 (Danbolt, 2001). The reported IC50 of TBOA for each of these transporters is 70 μM, 6 μM and 6 μM, respectively (Jabaudon et al., 1999) and thus, the 300 μM TBOA dose employed in the present study is well above that predicted to inhibit all three transporters. Indeed, intra-PFC infusion of TBOA doses between 250 and 500 μM is sufficient to produce a robust local increase in extracellular glutamate (while manipulations of sodium-independent transporters are without effect; Melendez et al., 2005), the relative contribution of glutamate release from glia versus neuronal sources and the role for sodium-dependent glial transporters in the maintenance and regulation of vmPFC glutamate remains to be determined, to the best of our knowledge. Thus, while it is presumed that the incubated glutamate release observed when rats respond for cocaine-associated cues (Shin et al., 2016) is derived from neuronal sources (a likely candidate being thalamocortical projections), there is no evidence negating the contribution of glia to this phenomenon. Thus, the intriguing, although speculative, possibility exists that the failure of TBOA to influence incubated drug-seeking reflects a greater involvement of glia-derived glutamate, which will be tested in future studies by manipulating EAAT1 expression.
4.3. Increased endogenous glutamate in the infralimbic cortex is sufficient to block incubated drug-seeking
In contrast to the PL, inhibition of glutamate re-uptake via TBOA infusion within the IL blocked incubated drug-seeking, arguing an inhibitory role for endogenous glutamate within this subregion. These neuropharmacological results complement the extant literature pertaining to the reinstatement of drug-seeking demonstrating that IL activation with AMPA agonists reduces cocaine-primed reinstatement of drug-seeking (Peters et al., 2008; LaLumiere et al., 2010). Further, our results are in line with opto-genetic evidence demonstrating that activation of IL projections to the NAc shell inhibits incubated cocaine-seeking (Ma et al., 2014) and extend these findings by implicating glutamate as an upstream mediator of this effect. It is noteworthy that in an earlier study by Koya et al. (2009), the local infusion of an inhibitory GABA agonist cocktail into the more ventral aspect of the vmPFC decreased incubated cocaine-seeking. While this result may seem contrary to the present findings, the microinjection sites in this prior study were not localized exclusively to the IL, with a fraction of the microinjections localized more dorsally within the PL (Koya et al., 2009). Given the present observations for LY379268’s effects within the PL and earlier optogenetics work (Ma et al., 2014), the inhibitory effect of the GABA agonist cocktail observed in Koya et al. (2009) could reflect the effects of inhibiting PL projections.
The above being said, an inspection of Fig. 4 indicates that the magnitude of the intra-IL LY379268 effect upon incubated behavior was comparable to that produced by an intra-PL infusion of this mGlu2/3 agonist. Further inspection of Fig. 4 suggests that the failure to detect a statistically significant effect of intra-IL LY379268 infusion likely reflected the variability in cue-elicited responding observed in the vehicle-infused controls in this particular experiment. The reduction in incubated drug-seeking upon intra-IL LY379268 infusion is peculiar in light of the discussion presented above, as well as prior work indicating little to no effect of IL inactivation upon stress- or cocaine-primed reinstatement of drug-seeking (Capriles et al., 2003), or upon cue-primed reinstatement of MDMA-seeking (Ball and Slane, 2012). Further, in contrast to the PL, IL neurons do not exhibit a withdrawal-dependent enhancement of the encoding of cocaine-associated stimuli (West et al., 2014). Although a body of literature argues an important role for IL activity in suppressing drug-seeking behavior, other data argue that IL activation may, in fact, drive such behavior (see Moorman et al., 2015 for detailed review). As one example, lesions of either the PL or the IL are reported to reduce cocaine-seeking after 7 days of withdrawal (Pelloux et al., 2013). Notably, however, the effect of the IL lesion was more modest than that observed for the PL, which is a finding consistent with the data in Fig. 4. Furthermore, immunohistochemical data indicate high levels of cellular activation within the IL when animals are exhibiting drug-seeking or drug-conditioned behaviors (e.g., Franklin and Druhan, 2000; Hamlin et al., 2008; Koya et al., 2009; Moorman and Aston-Jones, 2015; Nic Dhonnchadha et al., 2012; Zavala et al., 2007, 2008). Given the complexity of the vmPFC and the large overlap in projections from the PL and IL (Heidbreder and Groenewegen, 2003; Moorman et al., 2015; Vertes, 2004, 2006), it is entirely possible that the increased cue-elicited glutamate release observed in our prior study (Shin et al., 2016) emanated from either the PL or the IL (or both) to drive incubated drug-seeking. This hypothesis remains to be addressed empirically.
5. Conclusions
In conclusion, the present study demonstrates that endogenous glutamate in the vmPFC is necessary for the manifestation of incubated drug-seeking during protracted withdrawal, while elevating endogenous glutamate within the IL is sufficient to attenuate this behavioral phenomenon. In contrast, manipulations of endogenous glutamate within neither region altered, in any obvious manner, drug-seeking expressed during early withdrawal. These neuropharmacological data extend prior correlative evidence of a relationship between vmPFC glutamate and the incubation of cocaine-seeking and argue a critical role for the incubation of glutamate release within “pro-relapse circuits” involving the PL (and perhaps also the IL) in the incubation of cue-reactivity during extended drug abstinence. If relevant to the human condition, these data highlight the potential pharmacotherapeutic utility of glutamate autoreceptor agonists as a viable strategy for curbing excessive cue-elicited craving during protracted withdrawal and facilitating addiction recovery.
Funding
The authors declare no competing financial interests. This research was funded in part by an award from the W.M. Keck Foundation.
Abbreviations
- CEA
central nucleus of the amygdala
- Extinction Test
30-min cue-reinforced drug-seeking test
- EAAT
excitatory amino acid transporter
- GRM2
gene encoding metabotropic glutamate receptor 2
- IL
infralimbic cortex
- PFC
prefrontal cortex
- PL
prelimbic cortex
- mPFC
medial prefrontal cortex
- MDMA
3,4-methylenedioxymethamphetamine
- NAc
nucleus accumbens
- TBOA
DL-threo-β-Benzyloxyaspartic acid
- vmPFC
ventromedial prefrontal cortex
Footnotes
Conflicts of interest
None.
References
- Ball KT, Slane M, 2012. Differential involvement of prelimbic and infralimbic medial prefrontal cortex in discrete cue-induced reinstatement of 3,4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats. Psycho-pharmacology 224 (3), 377–385. 10.1007/s00213-012-2762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H, 2011. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry 69 (1), 708–711. 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK, 2013. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J. Neurosci 33 (2), 495–506a. 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED, 2002. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26 (3), 376–386. 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y, 2004. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J. Neurosci 24 (47), 10726–10730. 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME, 2005. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci 25 (40), 9144–9151. 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, 2003. A role for the prefrontal cortex in stress-and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168, 66–74. 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP, 1993. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res. Monogr 137, 73–95. [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME, 2008. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454 (7200), 118–121. 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Pattij T, Spijker S, 2011. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat. Neurosci 14 (4), 417–419. 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, 2001. Glutamate uptake. Prog. Neurobiol 65 (1), 1–105. 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM, 2006. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur. J. Neurosci 24 (11), 3285–3298. 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston ACW, Rebec GV, 2013. Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J. Neurosci 33 (22), 9319–9327. 10.1523/JNEUROSCI.3278-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP, 2000. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur. J. Neurosci 12 (6), 2097–2106. 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE, 2004. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J. Neurosci 24 (29), 6600–6610. 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA, 2000. Cue-induced cocaine craving: neuro-anatomical specificity for drug users and drug stimuli. Am. J. Psychiatry 157 (11), 1789–1798. 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD, 1986. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives General Psychiatry 43 (2), 107–113. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Giles C, Purgianto A, Seubert C, Mantsch JR, 2011. Glutamatergic plasticity in medial prefrontal cortex and ventral tegmental area following extended-access cocaine self-administration. Brain Res 1413, 60–71. 10.1016/j.brainres.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci 12, 652–669. 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A, 1996. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci 93 (21), 12040–12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y, 2001. Neuroadaptation: incubation of cocaine craving after withdrawal. Nature 412, 141–142. 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP, 2008. Renewal of extinguished cocaine-seeking. Neuroscience 151 (3), 659–670. 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ, 2003. The medial prefrontal cortex in the rat: evidence for a dorsoventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev 27 (6), 555–579. 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gähwiler BH, Gerber U, 1999. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc. Natl. Acad. Sci. U.S.A 96 (15), 8733–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK, 2008. Strain differences in alcohol-induced neuro-chemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol. Clin. Exp. Res 32 (4), 617–631. 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y, 2009. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 (Suppl. 1), 177–185. 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW, 2010. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn. Mem 17 (4), 168–175. 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW, 2012. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur. J. Neurosci 35 (3e4), 614–622. 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane N, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y, 2013. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat. Neurosci 16 (11), 1644–1651. 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, Ma MY, Xue MM, Luo YX, Yang FD, Bao YP, Shi J, Sun HQ, Lu L, 2014. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict. Biol 20 (3), 513–522. 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME, 2013. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J. Neurosci 33 (3), 1130–1142. 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK, 2016. Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur. J. Neurosci 43 (5), 689–702. 10.1111/ejn.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Xuan L, Ford KA, Tuan L, Olive MF, Szumlinski KK, Tseng KY, Wolf ME, 2014. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat. Neurosci 17 (1), 73–80. 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y, 2004. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47, 214–226. 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y, 2005. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat. Neurosci 8 (2), 212–219. 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y, 2007. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol. Psychiatry 61 (5), 591–598. 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lupinsky D, Moquin L, Gratton A, 2010. Interhemispheric regulation of the medial prefrontal cortical glutamate stress response in rats. J. Neurosci 30 (22), 7624–7633. 10.1523/JNEUROSCI.1187-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luís C, Cannella N, Spanagel R, Köhr G, 2016. Neurobiology of learning and memory persistent strengthening of the prefrontal cortex ‒ nucleus accumbens pathway during incubation of cocaine-seeking behavior. Neurobiol. Learn. Behav 138, 281–290. 10.1016/j.nlm.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Ma Y, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y, 2014. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83, 1453–1467. 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey EM, James MH, Mahler SV, Pantazis C, Aston-Jones G, 2016. Prelimbic to accumbens core pathway is recruited in a dopamine-dependent manner to drive cued reinstatement of cocaine seeking. J. Neurosci 36 (33), 8700–8711. 10.1523/JNEUROSCI.1291-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE, 2003. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol. Berl 168 (1–2), 57–65. 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW, 2005. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J. Pharmacol. Exp. Ther 314 (1), 139–147. 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Miller BW, Wroten MG, Sacramento AR, Silva HE, Shin CB, Vieira P, Ben-Shahar O, Kippin TE, Szumlinski KK, 2016. Cocaine craving during protracted withdrawal requires PKCε priming within vmPFC. Addict. Biol 22 (3), 629–639. 10.1111/adb.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G, 2015. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res 1628, 130–146. 10.1016/j.brainres.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G, 2015. Prefrontal neurons encode context-based response execution and inhibition in reward seeking and extinction. Proc. Natl. Acad. Sci 112 (30), 9472–9477. 10.1073/pnas.1507611112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myal S, O’Donnell P, Counotte DS, 2015. Nucleus accumbens injections of the mGluR2/3 agonist LY379268 increase cue-induced sucrose seeking following adult, but not adolescent sucrose self-administration. Neuroscience 305, 309–315. 10.1016/j.neuroscience.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nic Dhonnchadha BÁ, Lovascio BF, Shrestha N, Lin A, Leite-Morris KA, Man HY, Kaplan GB, Kantak KM, 2012. Changes in expression of c-Fos protein following cocaine-cue extinction learning. Behav. Brain Res 234 (1), 100–106. 10.1016/j.bbr.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ, 2017. Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73 (11), 1127–1134. 10.1001/jamapsychiatry.2016.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2006. The Rat Brain in Stereotaxic Coordinates, 6th ed. Academic Press, New York, NY. [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ, 2013. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur. J. Neurosci 38 (7), 3018–3026. 10.1111/ejn.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW, 2008. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci 28 (23), 6046–6053. 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y, 2011. Neurobiology of the incubation of drug craving. Trends Neurosci 34 (8), 411–420. 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Loweth JA, Christian DT, Uejima J, Rabei R, Le T, Stefanik MT, Murray CH, Sakas C, Wolf ME, 2016. AMPA receptor plasticity in accumbens core contributes to incubation of methamphetamine craving. Biol. Psychiatry 80 (9), 661–670. 10.1016/j.biopsych.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK, 2016. Incubation of cocaine-craving relates to glutamate over-flow within ventromedial prefrontal cortex. Neuropharmacology 102, 103–110. 10.1016/j.neuropharm.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Kalivas PW, 2015. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced transient synaptic potentiation and cocaine-seeking behavior. Brain Struct. Funct 221 (3), 1681–1689. 10.1007/s00429-015-0997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT, 2013. Optogenetic inhibition of cocaine seeking in rats. Addict. Biol 18 (1), 50–53. 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM, 2013. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc. Natl. Acad. Sci 110 (2), 713–718. 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Elmer GI, Wang B, You ZB, Wise RA, 2013. Bidirectional modulation of cocaine expectancy by phasic glutamate fluctuations in the nucleus accumbens. J. Neurosci 33 (21), 9050–9055. 10.1523/JNEUROSCI.0503-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen TL, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL, 1998. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology 19, 48–59. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2006. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience 142 (1), 1–20. 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Wang G, Shi J, Chen N, Xu L, Li J, Li P, Sun Y, Lu L, 2013. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS ONE 8 (7), 1–7. 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West EA, Saddoris MP, Kerfoot EC, Carelli RM, 2014. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur. J. Neurosci 39, 1891–1902. 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, 2016. Synaptic mechanisms underlying persistent cocaine craving. Nat. Publ. Group 17 (6), 351–365. 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL, 2007. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience 145, 438–452. 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL, 2008. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse 62 (6), 421–431. 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]