Abstract
Background
Smoking cessation is the most important treatment for smokers with chronic obstructive pulmonary disease (COPD), but little is known about the effectiveness of different smoking cessation interventions for this particular group of smokers.
Objectives
To evaluate the effectiveness of behavioural or pharmacological smoking cessation interventions, or both, in smokers with COPD.
Search methods
We searched all records in the Cochrane Airways Group Specialised Register of Trials. In addition to this electronic search, we searched clinical trial registries for planned, ongoing, and unpublished trials. We searched all databases from their inception. We checked the reference lists of all included studies and of other systematic reviews in relevant topic areas. We searched for errata or retractions from eligible trials on PubMed. We conducted our most recent search in March 2016.
Selection criteria
We included randomised controlled trials assessing the effectiveness of any behavioural or pharmacological treatment, or both, in smokers with COPD reporting at least six months of follow‐up abstinence rates.
Data collection and analysis
Two review authors independently extracted the data and performed the methodological quality assessment for each study. We resolved any disagreements by consensus.
Main results
We included 16 studies (involving 13,123 participants) in this systematic review, two of which were of high quality. These two studies showed that nicotine sublingual tablet and varenicline increased the quit rate over placebo (risk ratio (RR) 2.60 (95% confidence interval (CI) 1.29 to 5.24) and RR 3.34 (95% CI 1.88 to 5.92)). Pooled results of two studies also showed a positive effect of bupropion compared with placebo (RR 2.03 (95% CI 1.26 to 3.28)). When pooling these four studies, we found high‐quality evidence for the effectiveness of pharmacotherapy plus high‐intensity behavioural treatment compared with placebo plus high‐intensity behavioural treatment (RR 2.53 (95% CI 1.83 to 3.50)). Furthermore, we found some evidence that high‐intensity behavioural treatment increased abstinence rates when compared with usual care (RR 25.38 (95% CI 8.03 to 80.22)) or low‐intensity behavioural treatment (RR 2.18 (95% CI 1.05 to 4.49)). Finally, the results showed effectiveness of various combinations of psychosocial and pharmacological interventions.
Authors' conclusions
We found high‐quality evidence in a meta‐analysis including four (1,540 participants) of the 16 included studies that a combination of behavioural treatment and pharmacotherapy is effective in helping smokers with COPD to quit smoking. Furthermore, we conclude that there is no convincing evidence for preferring any particular form of behavioural or pharmacological treatment.
Plain language summary
What is the best way to help people with chronic obstructive pulmonary disease stop smoking?
Review question
We wanted to find out the best way to help people with chronic obstructive pulmonary disease (COPD) to stop smoking.
Background
Quitting smoking is the most important treatment for smokers with COPD. Treatments to help this group of people stop smoking can be categorised into behavioural support (such as motivational interviewing) and medication (such as nicotine replacement therapy). Although much research exists looking into what works for 'healthy' smokers, less is known about what is most effective for smokers with COPD.
Study characteristics
We looked for studies that included adult men and women who were current smokers and had a diagnosis of COPD. We included studies that assessed the effectiveness of any behavioural support or medication, or both as an aid to quit smoking. We included studies that compared different types of treatment or compared treatment to stop smoking with no treatment or 'usual care'. We only included studies that reported how many people had stopped smoking after at least six months of follow‐up. We carried out the most recent search for studies in March 2016.
Key results
We found high‐quality evidence from a collection of four (1,540 participants) of the total 16 included studies (13,123 participants) in this review. Overall, we found evidence that smokers with COPD who receive a combination of high‐intensity behavioural support and medication are more than twice as likely to quit as people who receive behavioural support alone. We found no clear evidence that one particular form of behavioural support or medication is better than another. It is still unclear whether smokers with COPD are different from smokers without COPD with regard to which treatments work best to help them stop smoking.
Quality of the evidence
We are quite confident in the finding that a combination of behavioural support and medication works better than behavioural support alone. However, we were not able to combine the results of many of the studies because the treatments or the outcomes of the studies were too different from each other.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD), a common and largely preventable disease, is characterised by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response to noxious particles in the airways and the lung (GOLD 2015). Exacerbations and comorbidities contribute to the overall severity in individual patients. The diagnosis must be confirmed by spirometry, the most widely available reproducible test of lung function (GOLD 2015).
COPD prevalence, morbidity, and mortality vary across countries and across different groups within countries (GOLD 2015). For example, a worldwide study showed that the prevalence of moderate COPD (that is Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage II) in women 40 years of age or older ranged from 3.1% to 12.6%, and in men from 6.7% to 13.1% (Buist 2007). This variation in prevalence is partly due to differences in pack‐years of smoking history. The odds ratio of COPD per 10‐year interval of pack‐years of smoking is 1.28 (95% confidence interval (CI) 1.15 to 1.42) among women and 1.16 (95% CI 1.12 to 1.21) among men (Buist 2007). Except for differences in exposure to risk factors, COPD prevalence variation is due to differences in diagnostic criteria, survey methods, and analytical approaches. Under‐recognition and underdiagnosis of COPD also affects the accuracy of prevalence and mortality data. In a large‐scale English study, the prevalence of COPD was highest among current smokers (19.3%), followed by ex‐smokers (15.2%) and never smokers (8.2%) (Shahab 2006). Furthermore, the prevalence of undetected COPD was high among asymptomatic smokers. The prevalence of previously undiagnosed COPD in male smokers 40 years of age or older ranged from 12% in a Greek research population, in Tzanakis 2004, to 30% in a Dutch research population (Geijer 2005). The Global Burden of Disease Study projected that COPD will become the third‐leading cause of death worldwide by 2020 (GOLD 2015). This increased mortality is driven mainly by the expanding epidemic of tobacco smoking, reduced mortality from other common causes of death, and aging of the world population (GOLD 2015).
The risk for developing COPD results from an interaction between genetic factors and many different environmental exposures (Mannino 2007). Cigarette smoking is the most commonly encountered risk factor for COPD in high‐income countries (Celli 2004; GOLD 2015; Kohansal 2009). Furthermore, a higher prevalence of respiratory symptoms and lung function abnormalities and a higher COPD mortality rate are seen among cigarette smokers with COPD than non‐smokers with COPD (GOLD 2015). For example, an overview of the literature showed the effect of smoking on forced expiratory volume in one second (FEV1) in males with mild to moderate COPD (Willemse 2004). The FEV1 of participants who continued to smoke declined within a range of 42 to 82 mL per year compared with 0 to 49 mL per year in participants who quit smoking at the start of the study (Willemse 2004). The latter decline is comparable with the physiological lung function decline that occurs naturally with increasing age (Kerstjens 1997).
Smoking cessation is the only evidence‐based intervention that reduces the risk of developing COPD and slows the accelerated decline in lung function in people with COPD (Anthonisen 2002). To date, none of the existing medications for COPD have been conclusively shown to modify the long‐term decline in lung function (GOLD 2015). Furthermore, smoking cessation is the single most cost‐effective way to reduce the risk of development of COPD and worsening of the disease (GOLD 2015).
Description of the intervention
For the current review, we included randomised controlled trials that assessed the effectiveness of any form of behavioural or pharmacological treatment, or combinations of both, as an aid to smoking cessation in people with COPD. Behavioural treatment refers to any psychotherapeutic approach aimed at identifying and modifying the behaviours associated with smoking (Tobacco Use and Dependence Guideline Panel 2008). Pharmacological treatments included nicotine replacement therapy (NRT), antidepressants for smoking cessation, nicotine receptor partial agonists, and nicotine vaccines. The following NRT delivery systems are currently available: nicotine chewing gum, nicotine inhaler, nicotine lozenge, nicotine patch, and nicotine sprays. Bupropion is the most commonly used antidepressant, as nortriptyline is not registered as a smoking cessation aid and is rarely used now. Varenicline is the most commonly used nicotine receptor partial agonist. Vaccines are not currently licensed for public use, although several vaccines are under development. A smoking cessation intervention is often a combination of a pharmacological and a behavioural treatment.
How the intervention might work
Smoking is an addictive behaviour. The chances of successful smoking cessation are therefore reduced without the use of behavioural or pharmacological treatments, or both. In the general smoking population, several behavioural and pharmacological treatments have demonstrated efficacy in helping to achieve abstinence. For example, the antidepressants nortriptyline and bupropion aid long‐term smoking cessation, and evidence suggests that the mode of action is independent of their antidepressant effects (Hughes 2012). Their efficacy is similar to that of NRT (Hughes 2014). A review of nicotine receptor partial agonists showed that varenicline increased the chances of successful long‐term smoking cessation compared with bupropion (Cahill 2013). Comparison of NRT and varenicline suggested a minor benefit of varenicline tending towards equivalency (Cahill 2013). Furthermore, a recent review showed the nicotine receptor partial agonist cytisine to be an effective treatment for smoking cessation (Hajek 2013). Interventions that combine behavioural and pharmacological treatments are even more successful in achieving abstinence (Stead 2015). Providing behavioural treatment in person or via telephone for people using pharmacotherapy to stop smoking showed a small but important effect (Stead 2015). However, these studies did not make a distinction between different kinds of smokers, even though there is some evidence that smokers with COPD are different from smokers without COPD. Jimenez‐Ruiz 2001 showed that smokers with COPD have more pack‐years of smoking history, stronger dependence on cigarettes, and use particular inhalation patterns while smoking. Furthermore, findings of a recent study endorsed the higher cigarette dependence in smokers with COPD and suggested that smokers with COPD have higher levels of depression and lower self efficacy to refrain from smoking (van Eerd 2015). This suggests that smokers are not a homogeneous group. It is therefore important to make the intervention fit best for each specific group, such as people with COPD (Borrelli 2010), for example by giving valuable and clear information on how smoking is related to COPD and to respiratory symptoms. It might also be useful to specifically address the increased comorbidities and cigarette dependence levels reported in people with COPD.
Why it is important to do this review
Smoking cessation is the most important intervention to reduce the risk of developing COPD and to improve the prognosis of people with the disease. People with COPD have a more urgent need to stop smoking than the average smoker; moreover, many often find it more difficult to do so (Tonnesen 2007). It is therefore important to provide an overview of the evidence base for different smoking cessation interventions directed at these patients.
Compared with smokers from the general population, smokers with COPD might have more difficulty quitting smoking because of their higher number of pack‐years of smoking history, stronger dependence on nicotine, higher levels of depression, and a lower self efficacy to refrain from smoking (Jimenez‐Ruiz 2001; Shahab 2006; van Eerd 2015). A Dutch study showed that smokers with mild to severe COPD were less likely to achieve abstinence than smokers without COPD; the prolonged abstinence rate after six months with nortriptyline therapy was 21% in smokers with COPD compared with 32% in smokers without COPD (Wagena 2005). However, that study did not control for baseline differences. Interventions aimed at smokers with COPD therefore might need to be tailored more specifically to the needs of people with COPD to increase their desire to stop and to address their increased levels of nicotine dependence and depression and their lower self efficacy (Shahab 2006; van Eerd 2015). However, evidence for the effectiveness of tailored versus general smoking cessation interventions in this patient group is scarce.
The scope of this updated review was the same as the original Cochrane review: to review the evidence 'whether and which treatments are effective in COPD smokers' (van der Meer 2003). However, results may have changed because new trials on this subject have been published since 2003.
Objectives
To evaluate the effectiveness of behavioural or pharmacological smoking cessation interventions, or both, in smokers with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We examined randomised controlled trials.
Types of participants
We included smokers with a diagnosis of COPD, according to criteria from the guidelines of the American Thoracic Society (ATS) (Qaseem 2011), the British Thoracic Society (BTS) (NICE 2010), or GOLD (GOLD 2015), or as confirmed by the treating physician.
Types of interventions
We included randomised controlled trials that assessed the effectiveness of any behavioural or pharmacological treatment, or both, as an aid to smoking cessation in participants with COPD. We categorised behavioural treatment as 'high' if more than one pre‐scheduled counselling session of greater than 10 minutes was offered with at least one face‐to‐face counselling session (Fiore 2008). Otherwise we categorised the behavioural treatment as 'low'.
We included the following comparisons.
Behavioural treatment versus no treatment or usual care.
One form of behavioural treatment versus a different form of behavioural treatment.
Pharmacological treatment versus placebo.
Pharmacological treatment versus a different pharmacological treatment.
Comparison of different combinations of behavioural and pharmacological treatments.
Types of outcome measures
As the primary outcome, we were interested in the percentage of participants who met the criteria for 'continuous or prolonged abstinence' over a period of six months or longer. 'Continuous abstinence' refers to abstinence periods that begin on the quit date. 'Prolonged abstinence' refers to continuous abstinence after an initial 'grace period' (Hughes 2003). The preferred outcome was biochemically validated continuous or prolonged abstinence at the longest‐reported time point. However, as biochemically validated abstinence might be lacking, we examined both self reported abstinence and biochemically validated abstinence. Unfortunately, lack of clarity is evident in the literature about the reliability of self reported abstinence (Hilberink 2011; Wilson 2011). When it was unclear whether the given quit rate was a point prevalence or a continuous or prolonged abstinence rate, we defined the quit rate as point prevalence. 'Point prevalence abstinence rate' refers to the proportion of participants who were non‐smokers at a specific point in time during follow‐up. It is a considerably less valid estimate of smoking abstinence than continuous/prolonged abstinence because participants could be classified as non‐smokers even if they had smoked a week before the reference date (Strassmann 2009; West 2005). We therefore used continuous or prolonged abstinence as the primary outcome measure, and point prevalence as the secondary outcome measure.
Primary outcomes
Percentage of participants with continuous or prolonged abstinence over a period of six months or longer.
Secondary outcomes
Percentage of participants with point prevalence abstinence over a period of six months or longer.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of Trials (CAGR), which is derived from systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy described in Appendix 2. In addition to the electronic search, we searched clinical trial registries for planned, ongoing, and unpublished trials (for example ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform). We searched all databases from their inception to March 2016, and applied no restriction on language of publication.
Searching other resources
We checked the reference lists of all included studies and other systematic reviews in relevant topic areas. We searched for errata or retractions from eligible trials in PubMed on 22 March 2016.
Data collection and analysis
Selection of studies
Two review authors (EVE and RVDM) independently selected studies for inclusion by applying the selection criteria. We resolved any disagreements through discussion, consulting a third review author (DK) if required. We identified and excluded duplicates and collated multiple reports on the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) flow diagram and a Characteristics of excluded studies table (Liberati 2009).
Data extraction and management
Two review authors (EVE and RVDM) independently extracted study characteristics from included studies. We adapted the standard data collection form for study characteristics and outcome data to the inclusion criteria. We noted in the Characteristics of included studies table whether outcome data were reported in an unuseable way. One review author (EVE) copied the data from the data collection form into the RevMan 2014 file. We double‐checked that the data had been entered correctly by comparing the study reports with the presentation of data in the systematic review. We extracted the following study characteristics.
Methods: study setting, study design, method of recruitment of participants, number of participants randomly assigned and followed up.
Participants: age, sex, cigarettes smoked per day, mean score on Fagerström Test for Nicotine Dependence (FTND) (Heatherton 1991), severity of COPD baseline lung function (FEV1 and forced vital capacity (FVC)).
Interventions: description of the experimental and control group(s), type and intensity of the behavioural treatment, therapist providing the treatment, dose of the pharmacological treatment.
Outcomes: primary and secondary outcomes, respectively, percentage of participants with prolonged or continuous abstinence, or both; percentage of participants with point prevalence abstinence; biochemical validation of abstinence.
Statistical analyses: complete‐case analyses or imputation of outcome data.
Notes: funding for trial, notable conflicts of interest of trial authors.
Assessment of risk of bias in included studies
Two review authors (EVE and RVDM) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author (DK). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of the outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. When information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section (van Eerd 2013).
Measures of treatment effect
We expressed results for dichotomous outcomes as risk ratios (RRs) and risk difference (RD). We calculated RRs as follows: (number of participants who quit smoking in the intervention group/number of participants randomly assigned to the intervention group)/(number of participants who quit smoking in the control group/number of participants randomly assigned to the control group). An RR greater than one favours the intervention group. RDs were the differences between the observed risks of abstinence in the intervention groups and the observed risks of abstinence in the control groups. We furthermore calculated 95% confidence interval for every study.
We undertook meta‐analyses only when this was meaningful, for example if interventions, participants, and the underlying clinical question were similar enough for pooling to make sense.
When multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (for example drug A versus placebo and drug B versus placebo) had to be entered into the same meta‐analysis, we combined the intervention groups into one comparison to avoid double counting.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
We contacted investigators to verify key study characteristics and to obtain missing numerical outcome data when possible (for example when a study is identified as abstract only).
Furthermore, regarding smoking cessation, we considered participants with missing outcome data as smokers (intention to treat).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. The I² statistic describes the percentage of variability in the summary estimate due to systematic heterogeneity rather than random chance stemming from sample error alone (Higgins 2011). Values greater than 50% suggest moderate heterogeneity, and values greater than 75% suggest substantial heterogeneity. When we identified substantial heterogeneity, we explored this through prespecified subgroup analysis.
Assessment of reporting biases
When we suspected reporting bias, we attempted to contact study authors to ask them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
When we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible publication biases.
Data synthesis
'Summary of findings' table
We created a 'Summary of findings' table by using the following outcomes: the primary outcome percentage of participants with prolonged or continuous abstinence, or both, and the secondary outcome percentage of participants with point prevalence abstinence. We used the five Grades of Recommendation, Assessment, Development and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Guyatt 2008). We used methods and recommendations as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and as used in GRADEpro GDT software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes and making comments to aid the reader's understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
To examine whether the intervention effect varies in different subgroups, we planned to carry out the following subgroup analyses, when applicable.
Severity of COPD: mild‐moderate versus severe‐very severe.
Level of behavioural treatment: low versus high.
Type of pharmacotherapy: nicotine replacement therapy, nicotine receptor partial agonists, antidepressants, nicotine vaccines.
Definition of abstinence: percentage of participants with continuous or prolonged abstinence over a period of 12 months or longer versus less than 12 months versus percentage of participants with point prevalence.
We used the following outcomes in the subgroup analysis.
Smoking status (% of group) at a minimum of six months from the quit date.
Sensitivity analysis
We undertook sensitivity analyses to assess the effect of removing studies with a high risk of bias.
Results
Description of studies
See: Characteristics of included studies tables.
Results of the search
We completed a PRISMA flow diagram (Liberati 2009; Figure 1). We identified 390 records by searching the Cochrane Airways Group Specialised Register of Trials, and additional sources added 20 records. Based on title and abstract, we excluded 287 records and assessed 119 full‐text articles for eligibility. We included a total of 16 studies in the review (Anthonisen 1994; Brandt 1997; Chen 2014; Christenhusz 2007; Crowley 1995; Gorecka 2003; Hilberink 2011; Kotz 2009; Lou 2013; Pederson 1991; Sundblad 2008; Tashkin 2001; Tashkin 2011; Tonnesen 2006; Wagena 2005; Wilson 2008). Five of these were also included in the previous version of this Cochrane review (van der Meer 2003). Of the 16 studies, four reported their data in two publications: Brandt 1997 and Kallan 1997, Christenhusz 2007 and Christenhusz 2011, Hilberink 2011 and Hilberink 2005, and Wagena 2005 and Van Schayck 2009. Two studies reported their data in three publications: Tashkin 2011, Tashkin 2011b, and Lock 2011; and Wilson 2008, Wilson 2010, and Wilson 2011. One study reported results in four publications: Kotz 2009, Kotz 2007, Kotz 2009b, and Kotz 2009c. One study reported the results in several different articles, of which we used five: Anthonisen 1994, Connett 1993, O'Hara 1993, Buist 1997, and Scanlon 2000.
1.
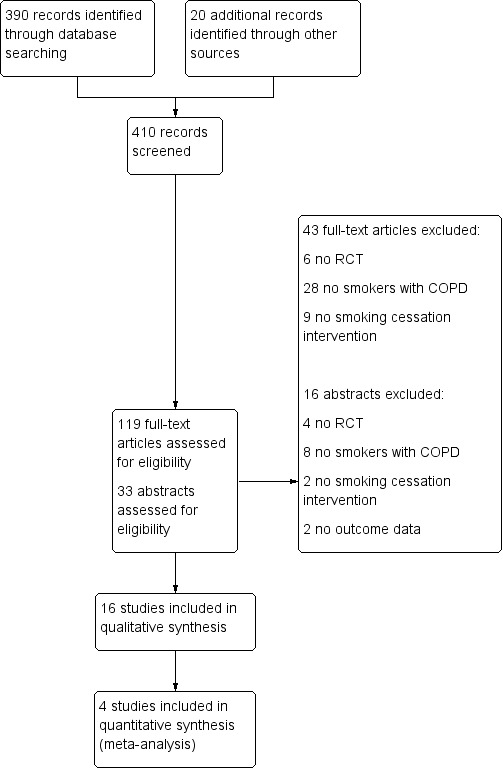
PRISMA study flow diagram
Included studies
We included 16 studies in this review update involving a total of 13,123 participants. An overview of the included studies is described in Table 6, and the key characteristics of the studies are described in detail in the Characteristics of included studies table.
1. Characteristics of included studies.
| Study | Country | Setting (in‐/outpatients) | N randomised | N completed | Intervention 1 | Intervention 2 | Control 1 | Control 2 |
| Anthonisen 1994 | CA & US | In | 5887 | Not reported | BH & PH‐NRT | NA | UC | NA |
| Brandt 1997 | DK | In | 56 | 45 | BH | NA | UC | NA |
| Chen 2014 | CN | Out | 85 | 80 | BH | NA | BH | NA |
| Christenhusz 2007 | NL | Out | 234 | 225 | BH & PH‐BUP | NA | BH | NA |
| Crowley 1995 | US | In & out | 53 | 36 | BH & PH‐NRT | NA | BH & PH‐NRT | BH & PH‐NRT |
| Gorecka 2003 | PL | Out | 70 | 69 | BH & PH‐BUP | NA | BH & PH‐NRT | NA |
| Hilberink 2011 | NL | Out | 697 | Not reported | BH | NA | UC | NA |
| Kotz 2009 | NL | Out | 296 | 248 | BH & PH‐NOR | NA | BH & PH‐NOR | UC |
| Lou 2013 | CN | Out | 3562 | 2607 | BH | NA | UC | NA |
| Pederson 1991 | CA | In | 74 | 64 | BH | NA | BH | NA |
| Sundblad 2008 | SE | Out | 478 | 391 | BH | NA | UC | NA |
| Tashkin 2001 | US | Out | 411 | 278 | BH & PH‐BUP | NA | BH | NA |
| Tashkin 2011 | US | Out | 504 | 499 | BH & PH‐VAR | NA | BH | NA |
| Tonnesen 2006 | DK | Out | 370 | 288 | BH & PH‐NRT | BH & PH‐NRT | BH | BH |
| Wagena 2005 | NL | Out | 255 | 220 | BH & PH‐BUP | BH & PH‐NOR | BH | NA |
| Wilson 2008 | IE | Out | 91 | 68 | BH & PH‐NRT | BH & PH‐NRT | UC | NA |
CA: Canada; CN: China; DK: Denmark; IE: Ireland; NL: Netherlands; PL: Poland; SE: Sweden; US: United States
BH: behavioural; BUP: bupropion; NA: not applicable; NOR: nortriptyline; NRT: nicotine replacement therapy; PH: pharmacological; UC: usual care; VAR: varenicline
Study participants
All participants were smokers with a diagnosis of COPD. All studies carried out spirometry to confirm the diagnosis of COPD, except for three studies that did not confirm the COPD diagnosis by spirometry (Brandt 1997; Hilberink 2011; Pederson 1991). Half of the studies included an equal distribution of men and women (Brandt 1997; Christenhusz 2007; Hilberink 2011; Lou 2013; Sundblad 2008; Tonnesen 2006; Wagena 2005; Wilson 2008); in the other half of the studies the percentage of men exceeded the percentage of women. The age of the participants in the different studies ranged from 48 to 66 years. Four studies included participants visiting inpatient clinics (Anthonisen 1994; Brandt 1997; Crowley 1995; Pederson 1991), whereas the remaining studies included participants visiting outpatient clinics or practices. Eight of the studies described severity of COPD according to Global Initiative for Chronic Obstructive Lung Disease/National Institute for Health and Care Excellence/European Respiratory Society/American Thoracic Society guidelines. One study, Crowley 1995, described severity of COPD according to their own definitions: early COPD (FEV1 70% to 90%) and late COPD (FEV1 less than 70%). Kotz 2009 and Tashkin 2001 included only participants classified with mild‐moderate COPD; the other six studies included a mix of mild‐moderate and severe‐very severe COPD participants. Four studies included mostly participants with mild‐moderate COPD, and two, Chen 2014 and Tonnesen 2006, included a large group of severe‐very severe COPD participants. In Chen 2014, 57% of COPD participants had mild‐moderate COPD, and 43% had severe‐very severe COPD. In Tonnesen 2006, 62% had mild‐moderate COPD, and 38% had severe‐very severe COPD.
Interventions
Eight studies evaluated behavioural treatments, in some of which pharmacotherapy was recommended or obligatory. Six studies evaluated pharmacotherapy, all of which included mandatory behavioural treatment. Five studies evaluated different combinations of behavioural and pharmacological treatment.
Behavioural treatments
The content of the behavioural treatments varied widely. All behavioural treatments contained some form of individual counselling, often combined with some form of group counselling, telephone counselling, and/or self help/written material. All of the behavioural treatments except for one, Brandt 1997, were high‐intensity treatments because they offered more than one pre‐scheduled counselling session of greater than 10 minutes with at least one face‐to‐face counselling session (Fiore 2008).
Pharmacological treatments
Placebo‐controlled trials included the investigation of nicotine replacement therapy (NRT) sublingual tablet (Tonnesen 2006), bupropion (Tashkin 2001; Wagena 2005), nortriptyline (Wagena 2005), and varenicline (Tashkin 2011). Three trials compared different pharmacotherapies: bupropion versus NRT patch (Gorecka 2003), NRT versus NRT with bupropion (Hilberink 2011), and bupropion versus nortriptyline (Wagena 2005). This last study was the only study using a double‐dummy design when investigating bupropion and nortriptyline against placebo (Wagena 2005). In contrast with the other studies, the use of pharmacotherapy in Hilberink 2011 was not mandatory and not provided for free, which resulted in only a few people using NRT or bupropion. All pharmacological treatments were accompanied by high‐intensity behavioural treatment. Tonnesen 2006 was the only study with a factorial design, as a result of which the behavioural component in the pharmacological comparison was a mix of high‐ and low‐intensity behavioural treatment.
Outcomes
Our primary outcome was continuous or prolonged abstinence over a period of six months or longer. Ten studies reported continuous, sustained, or prolonged abstinence. However, the definitions of abstinence used by the studies were diverse and sometimes vague, and did not always match our definition. Furthermore, most of these studies reported abstinence data after an initial grace period. We therefore clustered continuous, sustained, and prolonged abstinence data, which we categorised under the outcome 'prolonged abstinence'. Of the 10 studies reporting prolonged abstinence data, six studies reported six months' and seven studies reported 12 months' prolonged abstinence data. Few studies reported both six and 12 months' abstinence data. Furthermore, Wilson 2008 reported nine months' and Anthonisen 1994 reported two‐ to five‐year prolonged abstinence data of the Lung Health Study (LHS). In addition, Anthonisen 2002 reported prolonged abstinence 11 years after the start of the LHS.
Our secondary outcome was point prevalence abstinence over a period of six months or longer. Eleven of the 16 included studies reported this outcome. Nine studies reported six months' and eight studies reported 12 months' point prevalence abstinence data. A few studies reported both six and 12 months' abstinence data. Furthermore, Wilson 2008 reported nine months' and Sundblad 2008 reported three‐year point prevalence abstinence data. Lou 2013 described abstinence data as being continuous, however their definition of continuous abstinence was vague and corresponded with a mix of prolonged and point prevalence abstinence. In addition, all data except from the six months' follow‐up data were more consistent with point prevalence abstinence data, as the number of abstinent participants increased over time. We therefore categorised the six months' follow‐up data as prolonged abstinence data and the outcome data from six months onwards as point prevalence abstinence data.
All abstinence data were biochemically validated, however Brandt 1997 only measured exhaled carbon monoxide (CO) levels in self reported non‐smokers, and Sundblad 2008 only measured CO in a random sample of self reported non‐smokers. Brandt 1997 reported that the CO levels of the self reported non‐smokers confirmed their abstinence, and Sundblad 2008 reported that of the 35 tested, 33 had a CO level of less than 8 ppm, confirming that most of them were indeed abstinent. Eleven studies used exhaled CO measurement techniques, three studies measured cotinine levels in saliva, and three studies measured cotinine in urine. Pederson 1991 was an exception, as this study measured the CO level in blood samples. Cutoff levels varied: ≤ 8 to ≤ 10 ppm of CO in exhaled air, ≤ 10 to ≤ 20 ng/mL cotinine in saliva, and ≤ 50 to ≤ 60 ng/mL cotinine in urine.
Excluded studies
We excluded 59 of the potentially eligible studies: 10 studies were not randomised controlled trials, 36 studies did not include smokers with COPD, 11 studies were not studying smoking cessation interventions, and two did not report outcome data. These studies are listed with their reasons for exclusion in the Characteristics of excluded studies table.
Ongoing studies
We searched the clinical trial registers for ongoing studies and identified two potentially interesting studies that complied with the inclusion criteria for this review (www.ClinicalTrials.gov). One of the studies compares varenicline plus behavioural treatment with placebo plus behavioural treatment (NCT01694732). The estimated completion date of this study is November 2016. The other study compares behavioural treatment plus NRT with a different behavioural treatment plus NRT (NCT02148445). The estimated completion date of this study is September 2016. More information about these studies is summarised in the Characteristics of ongoing studies table.
Risk of bias in included studies
The risk of bias of each item for all included studies is summarised in Figure 2, and Figure 3 shows the percentages of the different items across all included studies.
2.
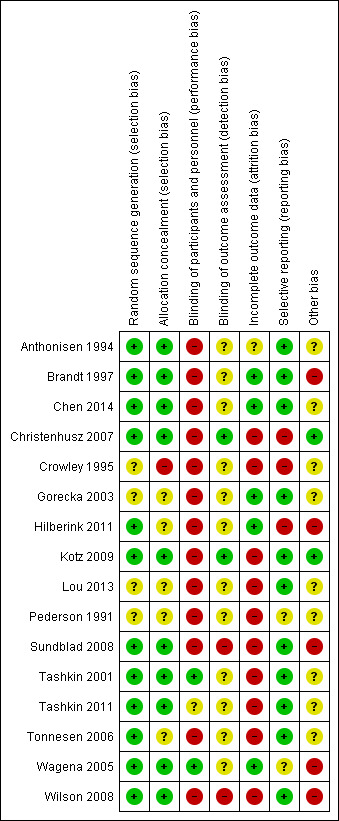
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
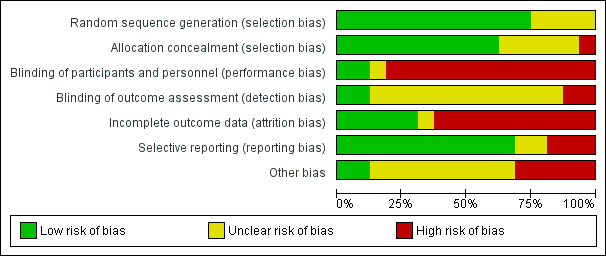
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
In conclusion, we recommend interpreting the results of six studies with caution, as we expect the risk of bias to have a significant influence on the results (Brandt 1997; Crowley 1995; Hilberink 2011; Sundblad 2008; Wagena 2005; Wilson 2008). In these studies, there was bias on several important components or bias on one crucial component; contamination bias; or poor adherence to the study protocol. In three studies, four or more components of risk of bias remained unclear and resulted in an overall unclear risk of bias (Gorecka 2003; Lou 2013; Pederson 1991).
Allocation
In four of the 16 studies, the random sequence generation method and allocation concealment were not described clearly enough for us to judge possible occurrence of selection bias (Crowley 1995; Gorecka 2003; Lou 2013; Pederson 1991).
Blinding
As almost all of the included studies evaluated different behavioural components, it was not possible to blind the participants and personnel for that part of the intervention, resulting in high 'Risk of bias' scores in almost all studies. Tashkin 2001 and Wagena 2005 were the only two pharmacological studies including identical behavioural treatment in all groups and describing the blinding of participants and personnel in detail. Blinding of outcome assessment referred to blinding of self reported or validated abstinence, or both. Only four studies reported any information about blinding. Christenhusz 2007 and Kotz 2009 were the only studies to report on how blinding was performed. Sundblad 2008 and Wilson 2008 explicitly reported that they did not blind participants and personnel for the outcome measurement.
Incomplete outcome data
Ten studies scored at high risk of bias for incomplete outcome data, due mostly to a high percentage of missing values or the presence of attrition bias, or both.
Selective reporting
We detected selective reporting of data in three studies: Christenhusz 2007 reported different outcome data in different papers and abstracts, Crowley 1995 did not report all prespecified outcomes, and Hilberink 2011 did not mention their randomised controlled trial (RCT) being a three‐armed RCT and only reported data from two arms in one of the publications.
Other potential sources of bias
Finally, we observed other potentially important forms of bias: adherence to study protocols for participants and personnel was not described in 11 of the studies; was found poor in three studies (Hilberink 2011; Wagena 2005; Wilson 2008); and was reported to be satisfactory in only two studies (Christenhusz 2007; Kotz 2009). Furthermore, contamination bias might have occurred in two studies (Brandt 1997; Hilberink 2011). In Figure 2 the final column shows the combined score for these sources of bias, per study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Behavioural treatment compared to no treatment or usual care in smokers with COPD.
| Behavioural treatment compared to no treatment or usual care in smokers with COPD | ||||||
| Patient or population: smokers with COPD Settings: in‐ and outpatients Intervention: behavioural treatment Comparison: no treatment or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment or usual care | Behavioural treatment | |||||
| Prolonged abstinence at longest follow‐up Follow‐up: 6 months | 2 per 1000 | 44 per 1000 (14 to 138) | RR 25.38 (95% CI 8.03, 80.22) | 3562 (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Point prevalence abstinence at longest follow‐up Follow‐up: 12 to 48 months | See comment | See comment | Not estimable | 3618 (2 studies) | See comment | Due to clinical and statistical heterogeneity, studies were not pooled. Individual RR were 1.98 (95% CI 0.74, 5.31) and RR 9.33 (95% CI 7.26, 11.99). 1 study had a high risk of bias due to a serious risk of contamination bias |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1This study had an unclear risk of bias as several important items were scored unclear, for example random sequence generation and allocation concealment. Downgrade once.
Summary of findings 2. One form of behavioural treatment compared to a different form of behavioural treatment in smokers with COPD.
| One form of behavioural treatment compared to a different form of behavioural treatment in smokers with COPD | ||||||
| Patient or population: smokers with COPD Settings: in‐ and outpatients Intervention: one form of behavioural treatment Comparison: a different form of behavioural treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| A different form of behavioural treatment | One form of behavioural treatment | |||||
| Prolonged abstinence at longest follow‐up Follow‐up: 6 to 12 months | See comment | See comment | Not estimable | 739 (4 studies) | See comment | No pooling due to clinical and statistical heterogeneity. Individual RR were 2.18 (1.05, 4.49), RR 0.97 (0.47, 1.99), RR 1.09 (0.59, 2.04), and RR not estimable. 3 of the 4 studies had a low risk of bias. 1 study had a high risk of bias due to poor adherence to the study protocol |
| Point prevalence abstinence at longest follow‐up Follow‐up: 6 to 12 months | See comment | See comment | Not estimable | 500 (3 studies) | See comment | No pooling due to clinical and statistical heterogeneity. Individual RR were 1.67 (0.68, 4.11), RR 1.35 (0.80, 2.28), and RR 0.15 (0.01, 2.83). 1 study had a low risk of bias, 1 study had a high risk of bias due to participants' poor adherence to the study protocol, and the remaining study had an unclear risk of bias |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. Pharmacological treatment compared to placebo in smokers with COPD.
| Pharmacological treatment compared to placebo in smokers with COPD | ||||||
| Patient or population: smokers with COPD Settings: outpatients Intervention: pharmacological treatment Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pharmacological treatment | |||||
| Prolonged abstinence at longest follow‐up Follow‐up: 6 to 12 months | 66 per 1000 | 168 per 1000 (136 to 207) | RR 2.53 (95% CI 1.83, 3.50) | 1429 (4 studies) | ⊕⊕⊕⊕ high1,2 | Point prevalence abstinence at longest follow‐up Follow‐up: 6 to 12 months RR 2.54 (95% CI 0.87, 7.44) |
| Prolonged abstinence at longest follow‐up; nicotine replacement therapy Follow‐up: 12 months | 54 per 1000 | 141 per 1000 (70 to 283) | RR 2.60 (95% CI 1.29, 5.24) | 370 (1 study) | ⊕⊕⊕⊕ high | Point prevalence abstinence at longest follow‐up; nicotine replacement therapy Follow‐up: 12 months RR 1.78 (95% CI 1.04, 3.05) |
| Prolonged abstinence at longest follow‐up; bupropion Follow‐up: 6 months | 87 per 1000 | 177 per 1000 (110 to 285) | RR 2.03 (95% CI 1.26, 3.28) | 503 (2 studies) | ⊕⊕⊕⊝ moderate3 | Point prevalence abstinence at longest follow‐up; bupropion Follow‐up: 6 months RR 1.46 (95% CI 0.97, 2.19) |
| Prolonged abstinence at longest follow‐up; varenicline Follow‐up: 12 months | 55 per 1000 | 184 per 1000 (104 to 326) | RR 3.34 (95% CI 1.88, 5.92) | 504 (1 study) | ⊕⊕⊕⊕ high | Point prevalence abstinence at longest follow‐up; varenicline Follow‐up: 12 months RR 1.83 (95% CI 1.27, 2.65) |
| Prolonged abstinence at longest follow‐up; nortriptyline Follow‐up: 6 months | 83 per 1000 | 212 per 1000 (72 to 620) | RR 2.54 (95% CI 0.87, 7.44) | 100 (1 study) | ⊕⊕⊝⊝ low4, 5 | No point prevalence data available |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Three studies had a low risk of bias. One study had a high risk of bias due to poor adherence to the study protocol. No downgrade. 2Sensitivity analyses including only those studies judged to be at low risk of bias did not impact the pooled results. No downgrade. 3One study had a low risk of bias. One study had a high risk of bias due to poor adherence to the study protocol. Downgrade once. 4This study had a high risk of bias due to poor adherence to the study protocol. Downgrade once.
5Point estimate for prolonged abstinence includes no difference. Downgrade once for imprecision.
Summary of findings 4. Pharmacological treatment compared to a different pharmacological treatment for smokers with COPD.
| Pharmacological treatment compared to a different pharmacological treatment for smokers with COPD | ||||||
| Patient or population: smokers with COPD Settings: outpatients Intervention: pharmacological treatment Comparison: a different pharmacological treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| A different pharmacological treatment | Pharmacological treatment | |||||
| Prolonged abstinence at longest follow‐up Follow‐up: 6 to 12 months | See comment | See comment | Not estimable | 166 (2 studies) | See comment | No pooling due to clinical and statistical heterogeneity. NRT vs bupropion RR 0.74 (95% CI 0.27, 2.05), bupropion vs nortriptyline RR 1.29 (95% CI 0.63, 2.63). 1 study had a high risk of bias due to poor adherence to the study protocol. 1 study had an unclear risk of bias |
| Point prevalence abstinence at longest follow‐up Follow‐up: 12 months | 62 per 1000 | 155 per 1000 (91 to 264) | RR 2.5 (1.47 to 4.26) | 543 (1 study) | ⊕⊕⊕⊝ moderate1 | NRT vs NRT plus bupropion |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; NRT: nicotine replacement therapy RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1This study had a high risk of bias due to contamination bias. Downgrade once.
Summary of findings 5. Combination interventions compared to different (combination) interventions or usual care for smokers with COPD.
| Combination interventions compared to different (combination) interventions or usual care for smokers with COPD | ||||||
| Patient or population: smokers with COPD Settings: in‐ and outpatients Intervention: combination interventions Comparison: different (combination) interventions or usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Different (combination) interventions or usual care | Combination interventions | |||||
| Prolonged abstinence at longest follow‐up Follow‐up: 6 to 60 months | See comment | See comment | Not estimable | 6431 (4 studies) | See comment | No pooling due to clinical and statistical heterogeneity. Individual RR 4.10 (3.36, 5.00), RR 2.22 (1.06, 4.68), RR 1.91 (0.65, 5.61), and RR not estimable. 3 studies had a low risk of bias, 1 study had a high risk of bias due to poor adherence to the study protocol |
| Point prevalence abstinence at longest follow‐up Follow‐up: 6 to 12 months | See comment | See comment | Not estimable | 1535 (4 studies) | See comment | No pooling. Individual RR were 1.60 (0.89, 2.89), RR 2.21 (0.89, 5.52), RR 3.41 (2.15, 5.41), RR 0.43 (0.02, 10.12), and RR 3.62 (0.40, 32.97). 3 studies had a high risk of bias due to contamination bias, poor adherence to the study protocol, or poor validation of outcome. 1 study had a low risk of bias |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; COPD: chronic obstructive pulmonary disease; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Outcome data from the included studies was based on complete‐case, intention‐to‐treat (ITT), and modified ITT principles. To increase consistency across study outcome data, we performed an ITT analysis on outcome data from all studies. In the following comparisons we only described subgroup analyses when a meta‐analysis was performed.
Comparison 1: Behavioural treatment versus no treatment or usual care
Two studies compared a behavioural treatment with usual care (Brandt 1997; Lou 2013). The content of the behavioural treatments varied widely, and the studies showed too much statistical heterogeneity for pooling to make sense. Brandt 1997 compared an intervention group in which the doctors used the term "smoker's lung" with a group receiving usual care by using the concept "chronic bronchitis". Only 12 months' point prevalence data were available for this study. In the "smoker's lung" group, 32% (8/25) quit smoking, and in the "chronic bronchitis" group, 16% (5/31) quit smoking (risk ratio (RR) 1.98, 95% confidence interval (CI) 0.74 to 5.31; risk difference (RD) 0.16, 95% CI ‐0.07 to 0.38) (Analysis 1.2). In Lou 2013, a cluster design was used, and the behavioural‐treatment group received a high‐intensity treatment: the participants were provided written material and individual counselling in the practice and during home visits, and they participated in group meetings. The content and number of usual‐care services were not standardised. Outcome data of this study were difficult to classify according to our definitions. As previously described, we included follow‐up data at six months' follow‐up as prolonged abstinence data and data from six months onwards as point prevalence abstinence data. The prolonged abstinence rate at six months was 4.4% (79/1814) in the behavioural‐treatment group, versus 0.2% (3/1748) in the usual‐care group. The RR was 25.38 (95% CI 8.03 to 80.22) and the RD was 0.04 (95% CI 0.03 to 0.05) (Analysis 1.1). All point prevalence abstinence rates at follow‐up from 12 to 48 months showed a positive effect of the behavioural‐treatment group on abstinence rates. At 48 months, the point prevalence abstinence rate was 33.6% (610/1814) in the behavioural‐treatment group compared with 3.6% (63/1748) in the usual‐care group, with a RR of 9.33 (95% CI 7.26 to 11.99) and a RD of 0.30 (95% CI 0.28 to 0.32) (Analysis 1.2). However, these effects may be overestimated, as the authors did not control for the cluster design of the RCT in their analysis.
1.2. Analysis.
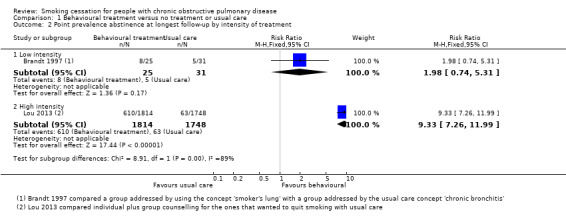
Comparison 1 Behavioural treatment versus no treatment or usual care, Outcome 2 Point prevalence abstinence at longest follow‐up by intensity of treatment.
1.1. Analysis.
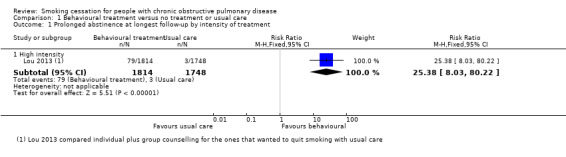
Comparison 1 Behavioural treatment versus no treatment or usual care, Outcome 1 Prolonged abstinence at longest follow‐up by intensity of treatment.
Comparison 2: One form of behavioural treatment versus a different form of behavioural treatment
Six studies compared two different behavioural treatments (Chen 2014; Crowley 1995; Kotz 2009; Pederson 1991; Tonnesen 2006; Wilson 2008). The content of the behavioural treatments varied widely, and the studies showed too much statistical heterogeneity for pooling to make sense.
Chen 2014 and Pederson 1991 compared a high‐intensity behavioural treatment with a low‐intensity behavioural treatment. In Chen 2014, participants received individual counselling, self help material, and telephone follow‐up calls. This behavioural‐treatment group was compared with a low‐intensity behavioural treatment that only provided simple advice to the participants. The six months' prolonged abstinence in the group with high‐intensity counselling was 40% (17/42) compared with 19% (8/43) in the group receiving simple advice. The RR was 2.18 (95% CI 1.05 to 4.49) and the RD was 0.22 (95% CI 0.03 to 0.41) (Analysis 2.1). Point prevalence data of this study were not reported. Pederson 1991 only examined point prevalence abstinence at six months' follow‐up. Rates for abstinence were 27% (10/37) in the group receiving high‐intensity counselling compared with 16.2% (6/37) in the group receiving simple advice (RR 1.67, 95% CI 0.68 to 4.11; RD 0.11, 95% CI ‐0.08 to 0.29) (Analysis 2.2).
2.1. Analysis.
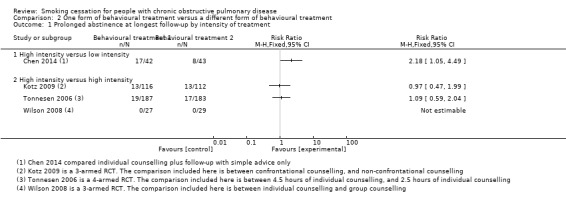
Comparison 2 One form of behavioural treatment versus a different form of behavioural treatment, Outcome 1 Prolonged abstinence at longest follow‐up by intensity of treatment.
2.2. Analysis.
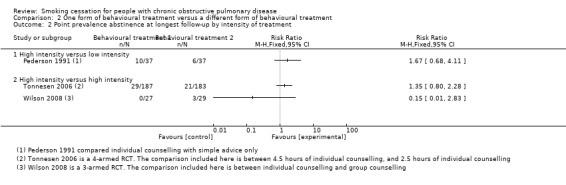
Comparison 2 One form of behavioural treatment versus a different form of behavioural treatment, Outcome 2 Point prevalence abstinence at longest follow‐up by intensity of treatment.
Crowley 1995, Tonnesen 2006, Kotz 2009, and Wilson 2008 compared a high‐intensity behavioural treatment with a different high‐intensity behavioural treatment. Crowley 1995 compared three different behavioural‐treatment groups. All groups received individual counselling and written material. They differed in the way the participants received lottery tickets for abstinence. Payment of lottery tickets according to abstinence occurred in two groups: one group received payment for self reported abstinence, the other group for CO‐validated abstinence. The third group received lottery tickets when their "buddy" in the other group also received lottery tickets; this was not linked with their smoking status. The authors stated that the three groups did not differ in six months' point prevalence abstinence rates, but there were no data available per group. Kotz 2009 compared individual and telephone counselling with confrontation with spirometry, with individual and telephone counselling without confrontation with spirometry. The prolonged abstinence at 12 months' follow‐up in the group with confrontation with spirometry was 11% (13/116) compared with 12% (13/112) in the group without confrontation with spirometry. The RR was 0.97 (95% CI 0.47 to 1.99) and the RD was ‐0.00 (95% CI ‐0.09 to 0.08) (Analysis 2.1). This study reported no point prevalence data. In Tonnesen 2006, the participants in one behavioural‐treatment group received high‐intensity individual counselling and proactive telephone counselling. This group was compared with less intensive ‐ but still high‐intensity according to our definition ‐ individual counselling and proactive telephone counselling. As Tonnesen 2006 was a study with factorial design, the same number of participants in both groups also received either NRT (sublingual tablet) or placebo. The prolonged abstinence rate at 12 months' follow‐up in the group with high‐intensity counselling was 10% (19/187) compared with 9% (17/183) in the group receiving less high‐intensive counselling. The RR was 1.09 (95% CI 0.59 to 2.04) and the RD was 0.01 (95% CI ‐0.05 to 0.07) (Analysis 2.1). The 12 months' point prevalence abstinence rate was 16% (29/187) in the high‐intensity counselling group and 11% (21/183) in the less high‐intensive counselling group (RR 1.35, 95% CI 0.80 to 2.28; and RD 0.04, 95% CI ‐0.86 to 0.94) (Analysis 2.2). Wilson 2008 compared individual counselling and group counselling. Both groups were also offered the opportunity to use NRT patches. In both groups none of the participants became prolonged abstinent at 12 months (Analysis 2.1). Point prevalence abstinences rates were 0% (0/27) in the individual‐counselling group and 10% (3/29) in the group‐counselling group (RR 0.15, 95% CI 0.01 to 2.83; and RD ‐0.10, 95% CI ‐0.23 to 0.02) (Analysis 2.2).
Comparison 3: Pharmacological treatment versus placebo
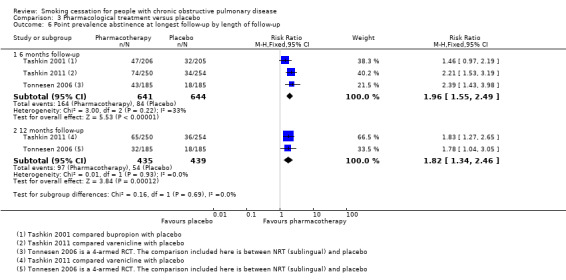
Four studies compared the following pharmacotherapy with placebo: NRT sublingual tablet (Tonnesen 2006), bupropion (Tashkin 2001; Wagena 2005), nortriptyline (Wagena 2005), and varenicline (Tashkin 2011). All studies except Wagena 2005 reported both prolonged and point prevalence abstinence data. Primary and secondary outcome data of the individual studies are presented in data and analyses table 3 (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6). All pharmacotherapy groups, except for the nortriptyline group from Wagena 2005, showed an increase in the chance of quitting compared with placebo. The prolonged abstinence rates in the pharmacotherapy groups ranged from 14% to 27%. The placebo group quit rates ranged from 5% to 9%. The pooled RR was 2.53 (95% CI 1.83 to 3.50) and the RD 0.10 (95% CI 0.07 to 0.14), with no evidence of heterogeneity (I² = 0%) (Analysis 3.1). The point prevalence abstinence rates in the pharmacotherapy groups ranged from 17% to 26%. The placebo group quit rates ranged from 10% to 16%. The pooled RR was 1.68 (95% CI 1.32 to 2.15) and the RD 0.09 (95% CI 0.05 to 0.13), with no evidence of heterogeneity (I² = 0%) (Analysis 3.4). In all studies, pharmacotherapy was combined with high‐intensity counselling.
3.1. Analysis.
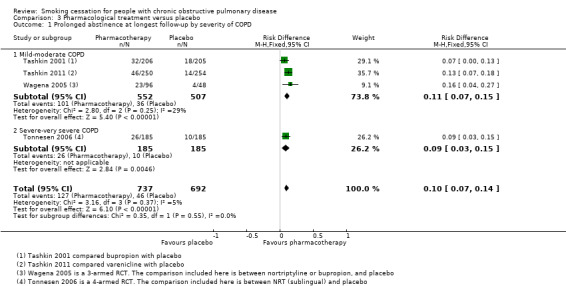
Comparison 3 Pharmacological treatment versus placebo, Outcome 1 Prolonged abstinence at longest follow‐up by severity of COPD.
3.2. Analysis.
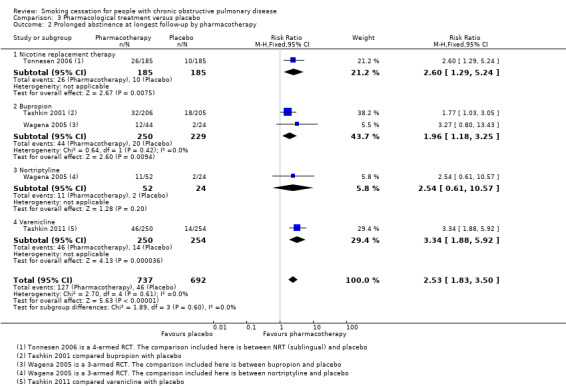
Comparison 3 Pharmacological treatment versus placebo, Outcome 2 Prolonged abstinence at longest follow‐up by pharmacotherapy.
3.3. Analysis.
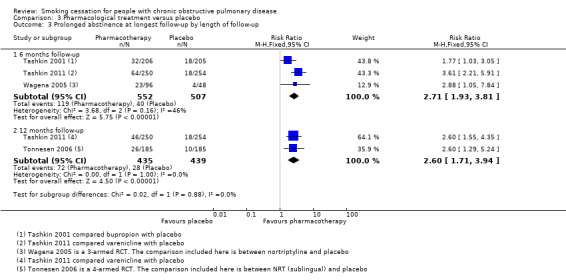
Comparison 3 Pharmacological treatment versus placebo, Outcome 3 Prolonged abstinence at longest follow‐up by length of follow‐up.
3.4. Analysis.
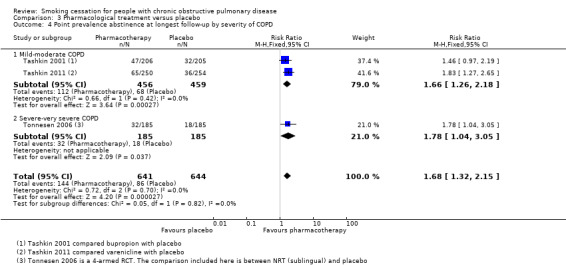
Comparison 3 Pharmacological treatment versus placebo, Outcome 4 Point prevalence abstinence at longest follow‐up by severity of COPD.
3.5. Analysis.
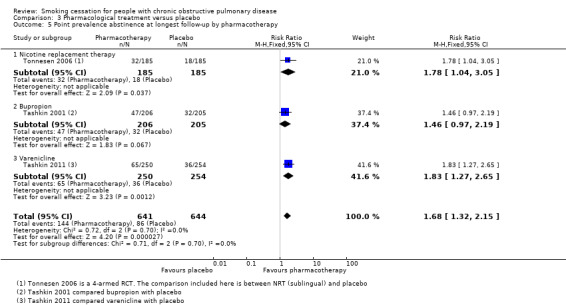
Comparison 3 Pharmacological treatment versus placebo, Outcome 5 Point prevalence abstinence at longest follow‐up by pharmacotherapy.
3.6. Analysis.
Comparison 3 Pharmacological treatment versus placebo, Outcome 6 Point prevalence abstinence at longest follow‐up by length of follow‐up.
All studies except Tonnesen 2006 included participants with mostly mild to moderate COPD. Tashkin 2001 included only participants with mild to moderate COPD. In Tashkin 2011, 89% had mild to moderate and 11% had severe to very severe COPD, and Wagena 2005 included 94% of mild to moderate severity and 6% of severe to very severe COPD patients. In a subgroup analysis, we distinguished between trials with mostly mild‐moderate COPD, Tashkin 2001, Tashkin 2011, and Wagena 2005, and a trial with a substantial number of people with severe‐very severe COPD (Tonnesen 2006). Both subgroups showed almost similar prolonged abstinence rates, with a RR of 2.51 (95% CI 1.74 to 3.62) and a RD of 0.11 (95% CI 0.07 to 0.15) in the group with mild‐moderate COPD, and a RR of 2.60 (95% CI 1.29 to 5.24) and a RD of 0.09 (95% CI 0.03 to 0.15) in the study with a substantial number of people with severe‐very severe COPD (Analysis 3.1). Point prevalence abstinence rates were also comparable between the group with mild‐moderate COPD patients (RR 1.66, 95% CI 1.26 to 2.18; RD 0.10, 95% CI 0.05 to 0.15) and the study with a substantial amount of people with severe‐very severe COPD (RR 1.78, 95% CI 1.04 to 3.05; RD 0.08, 95% CI 0.01 to 0.14) (Analysis 3.4).
The four studies in this comparison used different kinds of pharmacotherapy. Tonnesen 2006 used NRT (sublingual), Tashkin 2001 and Wagena 2005 used bupropion, Wagena 2005 also used nortriptyline, and Tashkin 2011 used varenicline. As previously mentioned, all pharmacotherapy, except for nortriptyline in the Wagena 2005 study, had a positive effect on prolonged abstinence compared with placebo (Analysis 3.2); the study using NRT had a RR of 2.60 (95% CI 1.29 to 5.24) and a RD of 0.09 (95% CI 0.03 to 0.15), the two studies using bupropion had a pooled RR of 2.03 (95% CI 1.26 to 3.28) and a RD of 0.09 (95% CI 0.03 to 0.15), the study of nortriptyline had a RR of 2.54 (95% CI 0.87 to 7.44) and a RD of 0.13 (95% CI ‐0.01 to 0.26), and varenicline had a RR of 3.34 (95% CI 1.88 to 5.92) and a RD of 0.13 (95% CI 0.07 to 0.18). The chances of point prevalence abstinence were also higher in the groups receiving pharmacotherapy compared with placebo (Analysis 3.5); the study using NRT had a RR of 1.78 (95% CI 1.04 to 3.05) and a RD of 0.08 (95% CI 0.01 to 0.14), the study using bupropion had a RR of 1.46 (95% CI 0.97 to 2.19) and a RD of 0.07 (95% CI ‐0.00 to 0.15), and the study using varenicline had a RR of 1.83 (95% CI 1.27 to 2.65) and a RD of 0.12 (95% CI 0.05 to 0.19).
We carried out a sensitivity analysis excluding the study by Wagena 2005, as this study showed a high risk of bias due to poor adherence to the treatment protocols. This did not result in a significant change to the prolonged abstinence rate (RR 2.49, 95% CI 1.77 to 3.50; and RD 0.10, 95% CI 0.06 to 0.13).
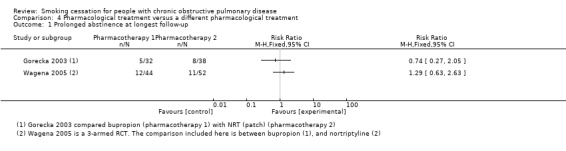
Comparison 4: Pharmacological treatment versus a different pharmacological treatment
Three studies compared pharmacotherapy with a different kind of pharmacotherapy (Gorecka 2003; Hilberink 2011; Wagena 2005). The comparisons the studies made between pharmacotherapies were too diverse for pooling to make sense. In Gorecka 2003 and Wagena 2005, the pharmacotherapy was combined with high‐intensity behavioural treatment. In Hilberink 2011, the intensity of the behavioural treatment was dependent on the level of motivation for quitting and varied between low and high.
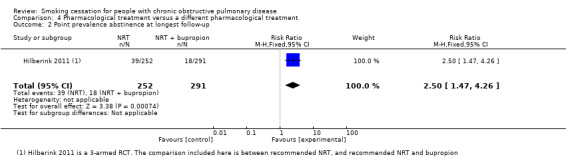
Gorecka 2003 compared bupropion with NRT patch. Prolonged abstinence rates at 12 months were 16% (5/32) in the bupropion group and 21% (8/38) in the NRT patch group with a RR of 0.74 (95% CI 0.27 to 2.05) and a RD of ‐0.05 (95% CI ‐0.23 to 0.13) (Analysis 4.1). No point prevalence data were available for this study. Hilberink 2011 compared a group in which they recommended NRT with a group in which they recommended NRT and bupropion. Prolonged abstinence data were not available, and 12 months' point prevalence rates were 15% (39/252) in the NRT group and 6% (18/291) in the NRT plus bupropion group (RR 2.50, 95% CI 1.47 to 4.26; RD 0.09, 95% CI 0.04 to 0.15) (Analysis 4.2). However, this is likely to be a misrepresentation, as contamination bias occurred in both groups. Wagena 2005 compared bupropion with nortriptyline in a study with double‐dummy design. In the group using bupropion, 27% (12/44) were prolonged abstinent at six months versus 21% (11/52) in the group using nortriptyline. The RR was 1.29 (95% CI 0.63 to 2.63) and the RD was 0.06 (95% CI ‐0.11 to 0.23) (Analysis 4.1). This study reported no other abstinence data.
4.1. Analysis.
Comparison 4 Pharmacological treatment versus a different pharmacological treatment, Outcome 1 Prolonged abstinence at longest follow‐up.
4.2. Analysis.
Comparison 4 Pharmacological treatment versus a different pharmacological treatment, Outcome 2 Point prevalence abstinence at longest follow‐up.
Comparison 5: Comparison of different combinations of behavioural and pharmacological treatments
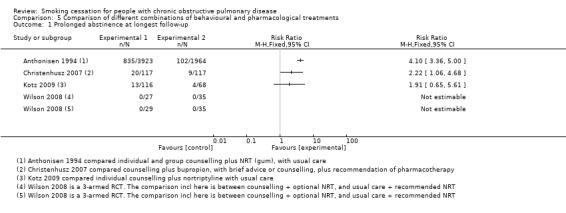
Six studies made comparisons between different kinds of pharmacological and behavioural treatments (Anthonisen 1994; Christenhusz 2007; Hilberink 2011; Kotz 2009; Sundblad 2008; Wilson 2008). The content of the treatments in the different studies was too diverse for pooling to make sense. The studies had in common that they all used high‐intensity behavioural treatments in their experimental group, except for the behavioural treatment in Hilberink 2011, which could be low or high intensive, depending on the motivational stage of the participant.
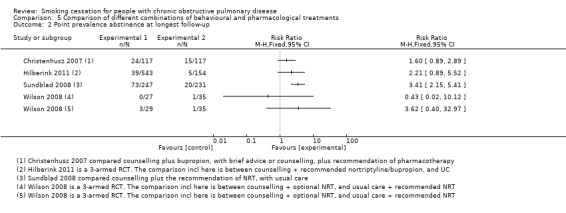
Five studies compared an experimental group with usual care. Anthonisen 1994 compared two different experimental groups with usual care. As receiving a bronchodilator or a placebo was the only difference between the two experimental groups, we combined them into one experimental group. The participants in this group all received counselling in combination with NRT gum. The participants in the control group received usual care. The prolonged abstinence rate at 12 months' follow‐up in the joined experimental group was 34% (1345/3923) and in the usual‐care group 9% (177/1964) (RR 3.80, 95% CI 3.28 to 4.41; and RD 0.25, 95% CI 0.23 to 0.27). The prolonged abstinence at five years' follow‐up in the joined experimental group was 21% (835/3923) and in the usual‐care group 5% (102/1964) (RR 4.10, 95% CI 3.36 to 5.00; and RD 0.16, 95% CI 0.14 to 0.18) (Analysis 5.1). At 11 years' follow‐up 16% (637/3923) in the joined experimental group and 4% (85/1964) in the usual‐care group had been prolonged abstinent (RR 3.75, 95% CI 3.01 to 4.67; and RD 0.12, 95% CI 0.10 to 0.13). We also combined the two experimental groups in Hilberink 2011 into one experimental group, as in both intervention groups contamination bias occurred, and the advice to use NRT and/or bupropion was given to all participants in both groups. This joined experimental group with a combination of participants using no pharmacotherapy, NRT, and/or bupropion in addition to a behavioural treatment was compared with a usual‐care group. The basis of the behavioural treatment was the minimal intervention strategy and in addition, dependent on the motivational stage, face‐to‐face and/or telephone counselling and written information. Only point prevalence abstinence rates were reported, and at 12 months' follow‐up 7% (39/543) in the joined experimental group and 3% (5/154) in the usual‐care group were abstinent (RR 2.21, 95% CI 0.89 to 5.52; RD 0.04, 95% CI 0.00 to 0.07) (Analysis 5.2). Kotz 2009 compared counselling and nortriptyline with usual care. Prolonged abstinence rates at 12 months were 11% (13/116) in the experimental group and 6% (4/68) in the usual‐care group (RR 1.91, 95% CI 0.65 to 5.61; RD 0.05, 95% CI ‐0.03 to 0.13) (Analysis 5.1). This study did not report point prevalence abstinence rates. In Sundblad 2008, the experimental group consisted of a high‐intensity behavioural treatment with hospitalisation in addition to the recommendation of NRT, which was compared with usual care. No prolonged abstinence rates were reported. The one‐year point prevalence rates were 43% (106/247) in the experimental group and 6% (15/231) in the usual‐care group (RR 6.61, 95% CI 3.97 to 11.01; RD 0.36, 95% CI 0.29 to 0.43). The three‐year point prevalence rates were 30% (73/247) and 7% (20/231), respectively (RR 3.41, 95% CI 2.15 to 5.41; RD 0.21, 95% CI 0.14 to 0.28) (Analysis 5.2). Finally, Wilson 2008 had two experimental groups, one with group and one with individual counselling, both plus optional NRT patches, in comparison to brief advice from the doctor. No participants in any of the groups became prolonged abstinent (Analysis 5.1). Point prevalence abstinence rates at 12 months were 0% (0/27) in the individual‐counselling group, 10% (3/29) in the group‐counselling group, and 3% (3/35) in the brief‐advice group (individual counselling plus optional NRT compared with brief advice (RR 0.43, 95% CI 0.02 to 10.12; RD ‐0.03, 95% CI ‐0.11 to 0.05), group counselling plus optional NRT compared with brief advice (RR 3.62, 95% CI 0.40 to 32.97; RD 0.07, 95% CI ‐0.05 to 0.20)) (Analysis 5.2).
5.1. Analysis.
Comparison 5 Comparison of different combinations of behavioural and pharmacological treatments, Outcome 1 Prolonged abstinence at longest follow‐up.
5.2. Analysis.
Comparison 5 Comparison of different combinations of behavioural and pharmacological treatments, Outcome 2 Point prevalence abstinence at longest follow‐up.
One study compared high‐intensity counselling plus bupropion with low‐ or high‐intensity counselling, depending on motivational stage, without pharmacotherapy (Christenhusz 2007). At 12 months 17% (20/117) of the participants in the counselling plus bupropion group were prolonged abstinent versus 8% (9/117) in the control group (RR 2.22, 95% CI 1.06 to 4.68; RD 0.09, 95% CI 0.01 to 0.18) (Analysis 5.1). Point prevalence abstinence rates at 12 months were 21% (24/117) in the counselling plus bupropion group and 13% (15/117) in the control group (RR 1.60, 95% CI 0.89 to 2.89; RD 0.08, 95% CI ‐0.05 to 0.20) (Analysis 5.2).
Discussion
Summary of main results
We reviewed the results of 16 RCTs (including a total of 13123 participants) evaluating the effectiveness of behavioural or pharmacological smoking cessation interventions, or both, in smokers with COPD. We found 11 additional studies in this update of the review (van der Meer 2003). The 'Summary of findings' tables 1 to 5 show the results of the main outcomes.
There was evidence from one study that high‐intensity behavioural treatment increased abstinence rates when compared with usual care (Lou 2013). However, we rated the quality of this study as moderate as we were unclear of the risk of bias due to lack of information (Table 1).
One study at low risk of bias showed higher abstinence rates for high‐intensity behavioural treatment over low‐intensity behavioural treatment (Chen 2014). Other studies that compared high‐intensity behavioural treatment with another high‐intensity behavioural treatment were too diverse to formulate univocal results (Table 2).
We pooled studies comparing pharmacotherapy with placebo and found high‐quality evidence that, in smokers with COPD, pharmacotherapy plus high‐intensity behavioural treatment was effective in increasing quit rates (RR 2.53, 95% CI 1.83 to 3.50) compared with placebo plus high‐intensity behavioural treatment (Tashkin 2001; Tashkin 2011; Tonnesen 2006; Wagena 2005). Among individual studies of pharmacotherapy, nortriptyline did not significantly increase the quit rate (Wagena 2005), whereas nicotine sublingual tablet, in Tonnesen 2006, and varenicline, in Tashkin 2011, did. Pooled results of two studies on bupropion, Tashkin 2001 and Wagena 2005, also showed a positive effect of bupropion compared with placebo (Table 3).
When comparing different kinds of pharmacological treatments, bupropion did not seem to be more effective than nortriptyline, in Wagena 2005, or nicotine patch, in Gorecka 2003. However, these studies were not pooled and therefore not rated for quality, but had an overall high and unclear risk of bias, respectively (Table 4).
Finally, we compared different combinations of behavioural and pharmacological treatments. These studies were not pooled and therefore not rated for quality. In a study at low risk of bias, high‐intensity behavioural treatment plus nicotine gum increased abstinence rates when compared with usual care (Anthonisen 1994). Furthermore, in a study at low risk of bias, high‐intensity behavioural treatment plus bupropion was superior to low‐ or high‐intensity behavioural treatment, depending on motivational stage, without pharmacotherapy (Christenhusz 2007). Also, high‐intensity behavioural treatment with hospitalisation plus recommendation of any NRT increased the chance of quitting compared with usual care (Sundblad 2008). However, this last study had a high risk of bias (Table 5).
The included studies reported a wide variety of outcome measures, and different studies used different definitions for the same outcome measure. Some studies used point prevalence as the outcome measure, while other studies used continuous abstinence, and some used both. According to Velicer 1992, the use of a combination of outcome measurements is often most appropriate in studies assessing the effects of a smoking cessation intervention.
Moreover, in different studies time points to assess the main outcome varied between six months and five years. The 'gold standard' for long‐term follow‐up is six or 12 months (Hatsukami 1999). Most relapses occur early in a quit attempt, and then persist. A measure taken at six months would certainly capture the majority of those relapse events, and 12 months' follow‐up would be even better (West 2005).
Also, six out of 16 studies had an overall high risk of bias, and three studies had an unclear risk of bias. This interfered with a clear and concise interpretation of the results. The most striking form of bias was the high proportion of missing data in 10 of the studies. Moreover, blinding was not possible in the behavioural interventions, resulting in a high risk of bias score in almost all of the included studies.
Finally, neither the exact content of the behavioural interventions nor that of usual care was always clearly described. This hampered the interpretation and implication of the results.
Overall completeness and applicability of evidence
We are confident that our rigorous search strategy ensured that we have identified the key research to inform this review. The studies in this review were conducted in different countries, which resulted in an international perspective of the results by comparing countries with cultural and economic differences. However, the trials on the same pharmacological intervention in smokers with COPD are limited to single studies only, except for two studies on bupropion. Also, the trials on behavioural interventions are very heterogeneous, and even though we categorised them into high‐ and low‐intensity interventions according to the literature, this still hampers drawing univocal conclusions. Furthermore, the completeness of the evidence was restricted to smoking cessation outcomes and did not include clinical outcomes, such as FEV1.
Quality of the evidence
In the 'Summary of findings' tables the quality of the evidence in the different comparisons is illustrated using the GRADE approach. One study was of low quality, two studies and one pool of studies were of moderate quality, and two studies and one pool of studies were of high quality. In the meta‐analysis, four studies, with a total of 1,429 participants, measured the effect of a pharmacological treatment plus behavioural treatment versus a placebo treatment plus behavioural treatment and showed strong and high‐quality evidence in favour of pharmacological treatment plus behavioural treatment for smoking cessation in smokers with COPD.
Potential biases in the review process
We undertook actions in order to decrease the chance of bias; to ensure that we included all relevant studies, we searched all relevant databases without major restrictions; in addition to the electronic search, we searched for planned, ongoing, and unpublished trials; we checked the reference lists of all included studies and of other systematic reviews in relevant topic areas and searched for errata or retractions from eligible trials; to decrease inter‐rater variability, two review authors independently selected studies for inclusion; these review authors also independently extracted study characteristics from included studies and independently assessed the risk of bias for each study; and any disagreement was resolved by involving a third review author.
We used an intensity classification system for the different behavioural interventions. We distinguished high‐ from low‐intensity behavioural treatment. This classification was based on existing criteria from the literature (Fiore 2008). However, not all included studies used these criteria, and we had to reclassify some behavioural treatments, which might have introduced bias. Two review authors independently reclassified the behavioural interventions, resolving any disagreements by involving a third review author.
Agreements and disagreements with other studies or reviews
There is evidence that in the general smoking population NRT, bupropion, nortriptyline, varenicline, and cytisine are effective pharmacotherapies for smoking cessation. Varenicline has been shown to be superior to single forms of NRT and bupropion, but not to combination NRT (Cahill 2013). Also, adding behavioural treatment to the use of pharmacological treatment shows a small but important effect in increasing the chances of quitting (Stead 2015).
In smokers with COPD, international guidelines recommend smoking cessation interventions that are similar to interventions for smokers without COPD (NICE 2008; West 2000). However, the literature suggests that smokers with COPD have specific characteristics that reduce their chance of successful quitting compared with other smokers (Jimenez‐Ruiz 2001; Jimenez‐Ruiz 2015; Shahab 2006; Tashkin 2009; van Eerd 2015). Currently, systematic reviews on smoking cessation interventions for smokers with COPD do not show differences compared to the general smoking population. Most of the existing reviews, including the previous version of this review (van der Meer 2003), included studies which showed that pharmacological treatment plus behavioural treatment is more effective than each strategy separately (Pires‐Yfantouda 2013; Strassmann 2009; Thabane 2012; Warnier 2013). This conclusion is endorsed by the results of this review. There is little evidence on the intensity of behavioural treatments and the type of pharmacotherapy used (Coronini‐Cronberg 2011; Strassmann 2009; Thabane 2012; Warnier 2013). This review adds that high‐intensity behavioural treatment, according to our classification, seemed to result in higher abstinence rates when compared with low‐intensity behavioural treatment. Furthermore, this review did not add conclusive information on the efficacy of the different pharmacological treatments, as the evidence from these studies had a high and unclear risk of bias.
Authors' conclusions
Implications for practice.
Based on four studies involving 1,540 participants, we found high‐quality evidence that a combination of behavioural treatment and pharmacotherapy is effective in helping smokers with COPD quit. Furthermore, we conclude that there is no convincing evidence for preferring any particular form of behavioural or pharmacological treatment.
Implications for research.
There is no evidence to change the present international smoking cessation guidelines for smokers with COPD. The question still remains of whether smokers with COPD require a different smoking cessation treatment than smokers without COPD. Although some evidence suggests that smokers with COPD have specific features that make it harder for them to quit (Jimenez‐Ruiz 2015; van Eerd 2015), more research in this field is needed. Future research on smoking cessation interventions for smokers with COPD could then focus on investigating which behavioural‐change techniques specifically motivate people with COPD to quit, and which could be added to existing interventions, such as symptom diaries or exercise capacity testing.
What's new
| Date | Event | Description |
|---|---|---|
| 5 March 2019 | Amended | 13 potentially eligible study records added to Studies awaiting classification. |
Acknowledgements
We would very much like to thank the editorial team of the Cochrane Airways Group (CAG), in particular Dr Emma Welsh and Emma Jackson, for their help in conducting and writing this systematic review. Furthermore, we would like to thank the CAG Information Specialist, Liz Stovold, for her advice.
We thank Ayalu Reda for his constructive comments on the review text.
We thank Carl Brandt, Juan Chen, Lieke Christenhusz, Sander Hilberink, Daniel Kotz, Britt‐Marie Sundblad, and Julie Wilson for providing additional information regarding their original studies.
We thank the following contributors for their respective translations in Farsi, Chinese, French, German, Polish, and Danish: Farhad Shokraneh, Ali Khamesipour, Zhi Hui Chang, Ben Shaw, Frederica Cassis, Samantha Lowry, Uwe Wollina, Filip Mejza, Marcin Stankiewicz, Jane Dennis, and Mia Schmidt‐Hansen.
Julia Walters was the Editor for this review and commented critically on the review.
The background and methods sections of this review are based on a standard template used by the CAG.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Search strategy for the Cochrane Airways Group Register
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Smoking
#8 MeSH DESCRIPTOR Smoking Cessation
#9 MeSH DESCRIPTOR Tobacco
#10 MeSH DESCRIPTOR Tobacco Use Disorder
#11 MeSH DESCRIPTOR Tobacco Use Cessation
#12 MeSH DESCRIPTOR Nicotine
#13 ((nicotin* or tobacco or smok* or cigarette*) NEAR5 (replac* or cessat* or ceas* or control* or quit* or stop* or abstin* or abstain* or self‐help* or "self help*" or behaviour* or behavior* or educat* or counsel* or support* or advice or treatment* or intervention*)):ti,ab,kw
#14 "nicotine replacement therapy" or NRT
#15 (nicotin*) NEAR3 (gum or patch or inhal* or nasal* or spray or lozenge* or polacrilex or agonist* or vaccin*)
#16 Varenicline
#17 Champix
#18 Chantix
#19 Bupropion
#20 Zyban
#21 Nortriptyline
#22 Nortrilen
#23 (nicotin* or tobacco or smok* or cigarette*) NEAR5 (antidepressant*)
#24 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 #6 and #24
NOTE: The Airways Register is maintained in specialist software developed for The Cochrane Collaboration; the CRS (Cochrane Register of Studies). Line #4 in the strategy denotes the field in the CRS reference record in which the record has been coded for condition, in this case, COPD.
Data and analyses
Comparison 1. Behavioural treatment versus no treatment or usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged abstinence at longest follow‐up by intensity of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 High intensity | 1 | 3562 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.38 [8.03, 80.22] |
| 2 Point prevalence abstinence at longest follow‐up by intensity of treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low intensity | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.74, 5.31] |
| 2.2 High intensity | 1 | 3562 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.33 [7.26, 11.99] |
Comparison 2. One form of behavioural treatment versus a different form of behavioural treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged abstinence at longest follow‐up by intensity of treatment | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 High intensity versus low intensity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 High intensity versus high intensity | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Point prevalence abstinence at longest follow‐up by intensity of treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 High intensity versus low intensity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 High intensity versus high intensity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Pharmacological treatment versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged abstinence at longest follow‐up by severity of COPD | 4 | 1429 | Risk Difference (M‐H, Fixed, 95% CI) | 0.10 [0.07, 0.14] |
| 1.1 Mild‐moderate COPD | 3 | 1059 | Risk Difference (M‐H, Fixed, 95% CI) | 0.11 [0.07, 0.15] |
| 1.2 Severe‐very severe COPD | 1 | 370 | Risk Difference (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.15] |
| 2 Prolonged abstinence at longest follow‐up by pharmacotherapy | 4 | 1429 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.83, 3.50] |
| 2.1 Nicotine replacement therapy | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.6 [1.29, 5.24] |
| 2.2 Bupropion | 2 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.18, 3.25] |
| 2.3 Nortriptyline | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.61, 10.57] |
| 2.4 Varenicline | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [1.88, 5.92] |
| 3 Prolonged abstinence at longest follow‐up by length of follow‐up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months follow‐up | 3 | 1059 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.93, 3.81] |
| 3.2 12 months follow‐up | 2 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.60 [1.71, 3.94] |
| 4 Point prevalence abstinence at longest follow‐up by severity of COPD | 3 | 1285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.32, 2.15] |
| 4.1 Mild‐moderate COPD | 2 | 915 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.26, 2.18] |
| 4.2 Severe‐very severe COPD | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.04, 3.05] |
| 5 Point prevalence abstinence at longest follow‐up by pharmacotherapy | 3 | 1285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.32, 2.15] |
| 5.1 Nicotine replacement therapy | 1 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.04, 3.05] |
| 5.2 Bupropion | 1 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.97, 2.19] |
| 5.3 Varenicline | 1 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.27, 2.65] |
| 6 Point prevalence abstinence at longest follow‐up by length of follow‐up | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months follow‐up | 3 | 1285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.55, 2.49] |
| 6.2 12 months follow‐up | 2 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.34, 2.46] |
Comparison 4. Pharmacological treatment versus a different pharmacological treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged abstinence at longest follow‐up | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Point prevalence abstinence at longest follow‐up | 1 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [1.47, 4.26] |
Comparison 5. Comparison of different combinations of behavioural and pharmacological treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prolonged abstinence at longest follow‐up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Point prevalence abstinence at longest follow‐up | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anthonisen 1994.
| Methods |
Design Randomised controlled trial Setting Inpatients |
|
| Participants |
Inclusion criteria Smokers, who smoked at least 10 cigarettes at 1 day in the prior 30 days; were aged 35 to 59 years; and had present mild airways obstruction (a ratio of FEV1 to FVC of 70% or less and an FEV1 between 55% and 90% of predicted normal). Exclusion criteria Screenees were excluded if they had serious illnesses such as cancer, heart attack, or stroke within the past 2 years, or other important medical conditions (e.g. high blood pressure) or previous chest or thoracic surgery and injuries that would confound results of spirometric studies. Also excluded were individuals taking certain physician‐prescribed medications including bronchodilators, B‐blockers, nitrates, and insulin. Screenees were excluded if they were likely to change their permanent residence to more than 75 miles from a clinical centre, were unwilling to enter the behavioural intervention program if randomised to SI, or if they consumed more than 25 alcoholic drinks per week or were binge drinkers (defined as 8 or more drinks per occasion, once or more often per month). Number randomised Total 5887, usual care (UC) 1962, smoking intervention plus placebo inhaler (SI‐P) 1962, smoking intervention plus active bronchodilator inhaler (SI‐A) 1963 Number followed up Not reported Age mean (SD): UC 48.4 (6.9), SI‐P 48.6 (6.8), SI‐A 48.4 (6.8) Sex % male: UC 63.8, SI‐P 64.0, SI‐A 60.8 Cigarettes/day mean (SD): UC 31.1 (12.8), SI‐P 31.5 (12.6), SI‐A 31.2 (13.2) FTND mean (SD): not reported Severity COPD FEV1% predicted, mean (SD): total 75 UC 75.1 (8.8), SI‐P 75.2 (8.8), SI‐A 75.1 (8.8) FEV1/FVC% mean (SD): total 63 UC 62.9 (5.5), SI‐P 63.0 (5.5), SI‐A 62.9 (5.6) |
|
| Interventions |
Behavioural treatment A. Smoking intervention plus placebo inhaler & B. Smoking intervention plus active bronchodilator inhaler Individual counselling: 1 structured session with a physician who strongly recommended to quit smoking related to the participant's lung impairment. Group counselling: 12 groups sessions over 10 weeks with health educator: behaviour modification techniques, with a quit date set early in the program. Participants who quit entered a maintenance program with monthly contacts through 8 months of abstinence and at least 3 meetings annually. Participants who relapsed were individually treated. C. Control Usual care Pharmacological treatment A. Smoking intervention plus placebo inhaler NRT gum 2 mg (free for 6 months, supplied to participants who believed that it might help with their nicotine dependence). Placebo inhaler 3 times daily (2 puffs per time). B. Smoking intervention plus active bronchodilator inhaler NRT gum 2 mg (free for 6 months, supplied to participants who believed that it might help with their nicotine dependence). Bronchodilator: ipratropium bromide 3 times daily (2 puffs per time). C. Control Usual care |
|
| Outcomes |
Abstinence Prolonged abstinence was defined as abstinence at each of annual visits 1 through 5 Validation Exhaled CO ≤ 10 ppm, measured at each visit, saliva cotinine ≤ 20 ng/mL, measured at each annual visit |
|
| Notes |
Funding Division of Lung Disease of the National Heart, Lung, and Blood Institute. Public Health Research grant from the National Center for Research Resources. Conflicts of interest Pharmaceutical companies supplied the drugs, Boehringer Ingelheim Pharmaceuticals Inc and Marion Merrell Dow Inc. Correspondence Additional information received from author for previous version of the review (van der Meer 2003). No additional information received after correspondence for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules were computer‐generated schedules, separately for each clinic. "The algorithm, based on blocks of random permutations of varying lengths, ensured approximate equality of numbers of participants in each of the three treatment groups." |
| Allocation concealment (selection bias) | Low risk | "Randomisation schedules were computer‐generated separately for each of the 10 clinics." "Backup system of randomisation envelopes was maintained. […] The envelope randomisation schedules were identical to those used by the randomisation program." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Results of salivary cotinine assessment were not revealed to participants." No further information about this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At year 1, questionnaires were completed for more than 94% of participants, and spirometry was completed for more than 89% of participants. At year 5 both questionnaires and spirometry were completed for more than 94% of participants. No further information about missing values on abstinence. They assumed in this analysis that participants who did not attend annual visits had continued smoking or had relapsed |
| Selective reporting (reporting bias) | Low risk | After correspondence for the previous review, we received reports of all outcome measures |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Brandt 1997.
| Methods |
Design Randomised controlled trial Setting Inpatients |
|
| Participants |
Inclusion criteria Smokers with intermittent or chronic dyspnoea, cough, varying degrees of bronchial obstruction and/or secretion problems. Exclusion criteria People whose lung disease was complicated by tuberculosis, lung cancer, silicosis, or asbestosis; people who had had previous thoracic surgery; people who had dementia, psychiatric illness, or needed an interpreter; people with terminal illness or who had a life expectancy of less than the year of follow‐up. Number randomised Total 56, smoker's lung (SL) 25, chronic bronchitis (CB) 31 Number followed up Total 45 Age mean (SD): SL 67.9 (7.3), CB 64.2 (10.5) Sex n (%) male: SL 11 (52), CB 14 (52) Cigarettes/day mean (SD): not reported FTND mean (SD): not reported Severity COPD (FEV1 and FEV1/FVC not reported) Peak flow, mean (SD): SL 225 (38), CB 236 (62) |
|
| Interventions |
Behavioural treatment All participants were given the same medical treatment and the same encouragement to stop smoking A. Smoker's lung Lung disease was designated 'smoker's lung' in information material and when the medical staff talked to the participants about their illness B. Chronic bronchitis Usual care; lung disease was designated 'chronic bronchitis' in information material and when the medical staff talked to the participants about their illness Pharmacological treatment None |
|
| Outcomes |
Abstinence Point prevalence abstinence at 12 months after discharge Validation Exhaled CO ≤ 8 ppm after 1 year in participants who said they were non‐smokers |
|
| Notes |
Funding Tobaksskaderaadet (the Tobacco‐Damage ‘Committee’) in the county of Fyen and Fru Carla Cornelia Storch Moeller’s grant (private fund) Conflicts of interest Not reported Correspondence Additional information received after correspondence. Additional article Kallan 1997 was translated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated consecutively. A secretary had 100 envelopes with 50 intervention group numbers and 50 control group numbers. The participants were put in groups of 10 with 5 in each intervention/control group. (Additional information from author) |
| Allocation concealment (selection bias) | Low risk | The secretary who managed the envelopes was not part of the research group, therefore no one in the research group could foresee assignment. (Additional information from author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | A. Participants' and personnel's adherence to the intervention protocol was not described. B. Contamination bias may have occurred as participants might have already been given the diagnosis smoker's lung before the start of the intervention or during the intervention in the control group when the term 'smoker's lung' received so much attention. C. Opportunities for staff to use the term 'smoker's lung' would have been determined by the frequency and level of care sought by the participants over the year; primary admission length, number of outpatient visits, and number of readmissions were similar in both groups. (Additional information from author) |
Chen 2014.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Smokers with COPD according to the GOLD criteria, who were > 18 years old. Exclusion criteria People with experience of smoking cessation medication and those with a history of asthma, asbestosis, silicosis, bronchiectasis, or lung cancer. Number randomised Total 85, COPD intervention group (COPD) 42, control group (C) 43 Number followed up Total 80 Age mean (SD): COPD 61.4 (8.6), C 61.6 (7.7) Sex n (%) male: COPD 41 (98), C 41 (95) Cigarettes/day mean (SD): COPD 17.5 (8.4), C 17.2 (9.6) FTND mean (SD): COPD 4.0 (2.0), C 4.1 (2.5) Severity COPD (FEV1/FVC not reported) FEV1% predicted: 11% mild ≥ 80% predicted, 46% moderate ≥ 50% to 80% predicted, 33% severe 30% to 50% predicted, 10% very severe < 30% predicted |
|
| Interventions |
Behavioural treatment A. COPD intervention Individual cognitive counselling (face‐to‐face) at baseline (20 minutes) by doctors with experience in professional smoking cessation treatment, self help materials, and 9 telephone follow‐ups (weeks 1, 2, 3, 4, 6, 8 and month 3, 4, and 5; > 10 minutes per call). Individual counselling was based on the five A’s principle. The content focused on the correlation between smoking and COPD. B. Control group Simple smoking cessation advice Pharmacological treatment None |
|
| Outcomes |
Abstinence Prolonged abstinence from week 4 to month 6 of the intervention Validation Exhaled CO < 10 ppm at week 4 and month 6 |
|
| Notes |
Funding Grants from the Chinese National Natural Science Foundation and the Chronic Respiratory Diseases Research Fund of the Chinese Medical Association Conflicts of interest Not reported Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Smokers in each category were assigned to the intervention or control group according to the randomised digital table..." |
| Allocation concealment (selection bias) | Low risk | A randomised digital table; central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The carbon monoxide levels were accessed by researchers, they were blinded for the participants', no information on blinding for the doctors who provided the counselling." (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of missing values and no differential attrition. (Additional information from author) |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Christenhusz 2007.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Clinically treated people with COPD with moderate COPD or severe COPD as defined by the ATS criteria. Who were current smoker, willing to participate in a smoking cessation program, between 40 and 75 years old, and had good knowledge and understanding of the Dutch language. Exclusion criteria A counter indication for the use of bupropion Number randomised Total 234, smoke‐stop‐therapy (SST) 114, minimal intervention strategy for lung patients (L‐MIS) 111 Number followed up Total 225 Age mean (SD): L‐MIS 59.6 (8.51), SST 57.0 (8.41) Sex n (%) male: L‐MIS 63 (57), SST 55 (48) Cigarettes/day mean (SD): L‐MIS 20.5 (13.5), SST 24.1 (13.8) FTND range 0 to 10, mean (SD): L‐MIS 4.98 (2.05), SST 5.84 (2.14) Severity COPD (FEV1/FVC not reported) FEV1% predicted, mean (SD): L‐MIS 65.8 (27.0), SST 64.6 (27.1) |
|
| Interventions |
Behavioural treatment A. Smoke‐stop‐therapy Individual counselling (1 session x 60 min; 3 sessions x 45 min), group counselling (4 sessions x 90 min), and telephone counselling (4 x 10 min). Participants could repeat part of the treatment in case of lapse within the first 3 months (recycling principle) B. Minimal intervention strategy for lung patients Brief advice to quit smoking by chest physician. When participants were motivated to quit smoking: individual counselling (1 session x 60 min; 2 sessions x 45 min) by the respiratory nurse, and telephone counselling (3 calls x 10 min) by the respiratory nurse Pharmacological treatment A. Smoke‐stop‐therapy Pharmacological support was strongly advised (mandatory), and bupropion was provided for free. B. Minimal intervention strategy for lung patients Pharmacological support was recommended during the treatment, but the use was voluntary and at participants’ costs |
|
| Outcomes |
Abstinence Prolonged (both 6 and 12 months) abstinence rates 1 year after the TQD Validation Salivary cotinine level < 20 ng/mL at both 6 and 12 months after the start of the intervention |
|
| Notes |
Funding The Asthma Foundation Netherlands (grant code: 3.4.01.67), the Comprehensive Cancer Centre Stedendriehoek Twente, and GlaxoSmithKline (provided bupropion (Zyban) free of charge) Conflicts of interest Not reported Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The data manager allocated the included participants based on a computerised randomisation list." |
| Allocation concealment (selection bias) | Low risk | The computerised randomisation list was managed by a research assistant. (Additional information from author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were not informed of their salivary cotinine level. A researcher did, afterwards, receive cotinine level information. (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Different numbers of participants in analysis and different missing values. (Additional information from author) |
| Selective reporting (reporting bias) | High risk | Different outcomes were reported in different articles/abstracts. Not all outcomes were reported. (Additional information from author) |
| Other bias | Low risk | Participants and personnel adhered to the intervention protocol. (Additional information from author) |
Crowley 1995.
| Methods |
Design Randomised controlled trial Setting In‐ and outpatients |
|
| Participants |
Inclusion criteria Participants with a diagnosis of COPD (advanced FEV1 ≤ 70% of predicted normal, less advanced FEV˜ 71% to 90% of predicted normal), at least 35 years of age, who were residence in their metro area, and had breath CO > 14 ppm at a regular visit to their physicians. Exclusion criteria Participants with plans for extended absence from Denver during the study, with job exposure to high CO, current pregnancy, temporomandibular joint disease or other dental conditions preventing use of nicotine gum, or psychosis, dementia, recent myocardial infarction, angina pectoris, serious arrhythmias, or diseases threatening death within 12 months. Candidates with bilirubin > 2 or blood urea nitrogen > 40 were also excluded. Number randomised Total 53, experimental group (exp) 18, control group 15, cigarette self report (CSR) group 16 Number followed up Total 36 Age mean (SD): exp 62.3, control 63, CSR 58.8 Sex n (%) male: exp 13 (72), control 12 (80), CSR 12 (75) Cigarettes/day mean (SD): not reported FTND mean: exp 5.4, control 5.8, CSR 5.1 Severity COPD FEV1% normal value, mean: exp 50.6, control 50.4, CSR 47.5 FEV1 = 70% to 90%; FEV1/FVC ratio < 70%, early COPD %: exp 5.6, control 6.7, CSR 6.3 FEV1 < 70%, late COPD %: exp 94.4, control 93.3, CSR 93.7 |
|
| Interventions |
Behavioural treatment All groups received individual counselling by a physician (1 x 30 minutes) and brochure encouraging complete tobacco abstinence. A. Experimental group Individual contact by technician (1 or 2 daily home visits on days 1 to 85, 10 min per visit). Contingent reinforcement (lottery tickets) for reduced CO values. Participants were informed about their CO values. B. Control group Individual contact by technician (1 or 2 daily home visits on days 1 to 85, 10 min per visit). Non‐contingent payment (lottery tickets) equal to pair‐mates' earnings from experimental group. These participants were not informed about their CO values. C. CSR group Individual contact by technician (1 or 2 daily home visits on days 1 to 85, 10 min per visit). Contingent reinforcement (lottery tickets) of self report of no smoking since previous home visit. These participants were not informed about their CO values. Pharmacological treatment All groups received NRT gum, 2 mg/piece up to 30 pieces/day, days 11 to 75 |
|
| Outcomes |
Abstinence Point prevalence abstinence at 6 months' follow‐up (last 24 hours) Validation Exhaled CCO < 10 (CCO = CO corrected for ambient‐air CO content) |
|
| Notes |
Funding The work was supported by Grant DA 03961 from the National Institute on Drug Abuse Conflicts of interest Not reported Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The first recruited participant in a category randomly was assigned to Experimental or CSR group (because of the pairing, a participant could not be assigned to the Control Group until a pair‐mate had been assigned to the Experimental Group)." |
| Allocation concealment (selection bias) | High risk | They used an open random allocation schedule. "The first recruited participant in a category randomly was assigned to Experimental or CSR group (because of the pairing, a participant could not be assigned to the Control Group until a pair‐mate had been assigned to the Experimental Group). If the first participant in the category randomly was assigned to Experimental, the second was assigned randomly to Control or CSR, and the third entered the remaining group. If the category's first participant randomly was assigned to CSR, the second was assigned sequentially to Experimental and the third to Control. Then the process began anew for the next participant in the category." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Only experimental participants were informed during monitoring visits of their CCO values, and they were informed only during the intervention period, but all participants knew that the measurements assessed recent smoking. Others were told that CCO calculations would be completed at our laboratory (they were), and that participants would be informed of their values after the study ended." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High percentage missing values. "36 participants who completed the 6‐month follow‐up; others dropped out (9 participants), moved away, or died (4 participants)." |
| Selective reporting (reporting bias) | High risk | 6 months' follow‐up outcome data were not specified per group |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Gorecka 2003.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Smokers with spirometry confirmed COPD (airflow limitation (FEV1/FVC < 0.7)) who responded to the invitation for the second spirometry testing (COPD confirmed on 2 spirometry tests done 1 year apart) and who did not stop smoking despite counselling done 1 year prior to the start of the study after the first spirometry. Exclusion criteria None Number randomised Total 70, nicotine patch (NP) 38, bupropion 32 Number followed up Total 69 Age mean (SD): total 55.8 (9.5) Sex n (%) male: total 40 (57) Cigarettes/day mean (SD): total 23.4 (9.1) FTND mean (SD): total 6.5 (2.1) Severity COPD (FEV1/FVC not reported) FEV1% predicted, mean (SD): total 73 (17) |
|
| Interventions |
Behavioural treatment For all groups counselling by respiratory physician to stop smoking during all 4 visits Pharmacological treatment A. Nicotine patch Transdermal nicotine patch (free of charge) 15 mg/day for 16 hrs every day. Nicotine patch was used for 8 weeks. B. Bupropion Buproprion (free of charge) 300 mg/day (1 x 150 mg for 3 days then 2 x 150 mg daily). Bupropion was used for 7 weeks |
|
| Outcomes |
Abstinence Prolonged abstinence (did not smoke a single cigarette for a year since the enrolment) at 12 months from start of the intervention. Validation Exhaled CO < 10 ppm |
|
| Notes |
Funding The study was part of the national program for early detection and prevention of COPD, and medications were provided by pharmaceutical companies; Pharmacia provided the nicotine patch and GlaxoSmithKline provided bupropion Conflicts of interest Not reported CorrespondenceGorecka 2003 was translated. All information was obtained from the translators |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence method not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Presumably no blinding as participants would have known if they were assigned to use a patch or a pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One person only was not to be contacted at the 1 year final follow‐up phone call.' |
| Selective reporting (reporting bias) | Low risk | Results for all outcome measures were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described. 13 participants did not come to the first follow‐up visit (at 2 weeks) when they were due to receive the remainder of the medications. Thus, 18.6% did not take medication according to the protocol. Unclear how this number was spread between the 2 treatment groups |
Hilberink 2011.
| Methods |
Design Cluster randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Practices Using 1 out of 4 suitable general practice electronic information systems Participants Age > 35 years; diagnosed as having COPD; recorded medication with ICPC code R95/96; prescription of at least 3 times of bronchodilators in the past year (ATC code R03a/bc); prescription of at least 2 times of inhaled anti‐inflammatory medication in the past year (ATC code R03). GPs were asked to confirm the diagnosis. Exclusion criteria None Number randomised: Practices Total 74, counselling and NRT (CN) 23, counselling, NRT, and bupropion (CNB) 26, usual care (UC) 25 Participants Total 697, CN 252, CNB 291, UC 154 Number followed up Not reported Age mean (SD): CN 58.0 (12.2), CNB 60.7 (11.2), UC 60.1 (11.5) Sex n (%) male: CN 11 (46.5), CNB 12 (47.8), UC 14 (55.4) Cigarettes/day mean (SD): CN 16.9 (10.3), CNB 16.9 (9.1), UC 16.8 (9.7) FTND mean (SD): CN 4.4 (2.4), CNB 4.6 (2.3), UC 4.3 (2.6) Severity COPD Not reported |
|
| Interventions |
Behavioural treatment A. Counselling plus NRT General practice level General practices received support for implementing a smoking cessation program consisting of a counselling strategy plus the recommendation of NRT Participant level Extended version of the minimal intervention strategy (MIS), specifically aimed at education and support of patients by GP. Participants were invited for control visit in accordance with COPD treatment guidelines. The first control visit focused on symptoms, health status and treatment, smoking behaviour, and motivational stage to quit smoking. Participants were divided into 3 categories: preparers (willing to quit within 1 month): next consultation was scheduled to set a quit date and to plan the follow‐up visits to GP (maximum of 2 follow‐up visits and proactive telephone calls by the practice nurse (maximum of 3 calls)); contemplators (willing to quit within 6 months) received self efficacy enhancing information by discussing how to cope with the various barriers to quit. Depending on their severity of nicotine dependence, they received additional info about NRT. Contemplators were invited again 2 weeks later; pre‐contemplators (not willing to quit) received information about the advantages of quitting. The participant education tools consisted of a booklet especially developed for the COPD population and a videotape. B. Counselling plus NRT plus bupropion General practice level General practices received support for implementing a smoking cessation program consisting of a counselling strategy plus the recommendation of NRT and bupropion Participant level Extended version of the MIS, specifically aimed at education and support of patients by GP. Participants were invited for control visit in accordance with COPD treatment guidelines. The first control visit focused on symptoms, health status and treatment, smoking behaviour, and motivational stage to quit smoking. Participants were divided into 3 categories: preparers (willing to quit within 1 month): next consultation was scheduled to set a quit date and to plan the follow‐up visits to GP (maximum of 2 follow‐up visits and proactive telephone calls by the practice nurse (maximum of 3 calls)); contemplators (willing to quit within 6 months) received self efficacy enhancing information by discussing how to cope with the various barriers to quit. Depending on their severity of nicotine dependence, they received additional info about NRT. Contemplators were invited again 2 weeks later; pre‐contemplators (not willing to quit) received information about the advantages of quitting. The participant education tools consisted of a booklet especially developed for the COPD population and a videotape. C. Usual care Consisted of regular check‐ups and COPD information. Pharmacological treatment A. Counselling plus NRT Information about and advice on using NRT (preparers and contemplators) B. Counselling plus NRT plus bupropion Information about and advice on using NRT, plus information/advice on using bupropion (preparers and contemplators) C. Usual care None |
|
| Outcomes |
Abstinence Point prevalence (did not smoke in the last 7 days) at 6 and 12 months Validation Urine cotinine ≤ 50 ng/mL (measured 12 months after baseline) |
|
| Notes |
Funding The present study was financed by the Asthma Foundation Netherlands, The Netherlands Organisation for Health Research and Development (ZonMw), Pharmacia, and GlaxoSmithKline. Conflicts of interest None Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "75 practices were allocated to one of the three arms by way of a stratified randomisation procedure, taking into account experience with the Minimal Intervention Strategy, delegation of tasks to the practice assistant and practice size." It was a computer‐generated randomisation. (Additional information from author) |
| Allocation concealment (selection bias) | Unclear risk | Allocation was not concealed for the GPs because they had to be trained. Participants were not told to which group they were randomised, but it is presumable that participants saw through if they got the intervention or not. (Additional information from author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Self report of abstinence was blinded for the GPs, not for the researchers. Cotinine validation was blinded for the participants and GPs, not for the researchers. (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of missing values is low, and there was no differential attrition |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes reported, however in one of the articles the authors did not mention that it was a 3‐armed RCT: "the study is a two armed randomised controlled trial". One arm was entirely ignored |
| Other bias | High risk | A. GPs' adherence to the protocol was poor: "The majority of the GPs (70–83%) complied with six out of the nine aspects of the protocol. Less than a third advised participants to use pharmacotherapy to help them quit smoking. More than 50% of the GPs restarted the protocol after relapse following initial smoking cessation." B. Use of bupropion or NRT, or both was not only reserved for the participants for whom it was mandatory. (Additional information from author) |
Kotz 2009.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Smoking history of 10 pack‐years; competent in reading and speaking Dutch; and reporting at least 1 of the respiratory symptoms (cough, sputum production, or shortness of breath), being motivated to stop smoking. Exclusion criteria Evidence of a prior respiratory diagnosis; people were also excluded if they had undergone spirometry during the preceding 12 months. One or more contraindications for using the smoking cessation medication (nortriptyline) was also criteria for exclusion, as was the current use of antidepressants. Number randomised Total 296, experimental group (exp) 116, control group 1 (co1) 112, control group 2 (co2) 68 Number followed up Total 248 Age mean (SD): exp 53.8 (7.0), co1 54.9 (8.0), co2 53.0 (7.6) Sex n (%) male: exp 71 (61.2), co1 74 (66.1), co2 40 (58.8) Cigarettes/day mean (SD): exp 23.9 (8.4), co1 23.2 (9.9), co2 22.7 (9.6) FTND mean (SD): exp 4.6 (1.5), co1 4.5 (1.5), co2 4.4 (1.5) Severity COPD FEV1 post‐bronchodilator % predicted, mean (SD): exp 80.5 (14.7), co1 83.7 (16.8), co2 79.7 (14.0) FVC post‐bronchodilator % predicted, mean (SD): exp 103.9 (14.9), co1 107.6 (17.8), co2 105.4 (14.4) FEV1/FVC post‐bronchodilator, mean (SD): exp 103.9 (14.9), co1 107.6 (17.8), co2 105.4 (14.4) |
|
| Interventions |
Behavioural treatment A. Experimental Individual counselling, lung minimal intervention strategy (L‐MIS), by respiratory nurse, CONFRONTATION with spirometry. FC1: day 1 (40 minutes); FC2: day 8 (40 minutes); TQD: day 14; FC3: day 15 (40 minutes); FC4: day 22 (40 minutes). Telephone counselling day 15 (5 to 15 minutes). B. Control 1 Individual counselling, L‐MIS, by respiratory nurse, NO CONFRONTATION with spirometry. FC1: day 1 (40 minutes); FC2: day 8 (40 minutes); TQD: day 14; FC3: day 15 (40 minutes); FC4: day 22 (40 minutes). Telephone counselling day 15 (5 to 15 minutes). C. Control 2 Referral to GP for usual care, NO CONFRONTATION with spirometry. Pharmacological treatment A. Experimental Nortriptyline, 25 mg day 1 to 3; 50 mg day 4 to 7, 75 mg day 8 to end of treatment period B. Control 1 Nortriptyline, 25 mg day 1 to 3; 50 mg day 4 to 7, 75 mg day 8 to end of treatment period C. Control 2 Nortriptyline, 25 mg day 1 to 3; 50 mg day 4 to 7, 75 mg day 8 to end of treatment period |
|
| Outcomes |
Abstinence Prolonged abstinence at 5 to 26 weeks from TQD and 5 to 52 weeks from TQD Validation Urine cotinine level < 50 ng/mL |
|
| Notes |
Funding This study is funded by grants from the Asthma Foundation Netherlands, PICASSO for COPD, and Maastricht University Hospital Conflicts of interest None Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The database of the trial incorporates a randomisation system of seven participants per block, allowing an unequal group allocation of 3 : 3 : 1; experimental group : control group 1 : control group 2. When eligible subjects are contacted by telephone, the RA randomises the subject by pressing a button on the computer screen. The database then randomly allocates the subject." |
| Allocation concealment (selection bias) | Low risk | "Neither the primary researcher nor any other person involved in the study can predict or influence which treatment group the next participant will be allocated to." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Blinding of neither participants nor RNs is possible, because we want to assess behavioural interventions. All participants know what kind of counselling they receive, as do the respiratory nurses who provide the counselling. However, analysts assessing the cotinine levels and assistants entering data from questionnaires are blinded for the group allocation of participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cotinine analysis in urine was blinded for all participants and research staff. (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition occurred |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Low risk | Participants' and personnel's adherence to the intervention protocol was not described, but the author explains that the number of counselling sessions was similar in both groups. Furthermore, all respiratory nurses filled in a checklist with subjects that had to be discussed in that particular session, and this was done concisely and there was no difference between the groups. (Additional information from author) |
Lou 2013.
| Methods |
Design Cluster randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria COPD diagnosed according to GOLD criteria. All participants were aged 35 years or older, had smoked 1 cigarette or more per day for the previous year, and had not stopped smoking for more than 3 months during that year. Exclusion criteria People who had any serious or unstable medical disorders (e.g. psychiatric disorder) that might affect lung function or they had a current diagnosis of major depression. Number randomised: Practices Total 14, intervention 7, control 7 Participants Total 3562, intervention 1814, control 1748 Number followed up Total 2607 Age mean (SD): intervention 61.6 (10.2), control 61.5 (10.1) Sex n (%) male: intervention 868 (48), control 840 (48) Cigarettes/day mean (SD): not reported FTND mean (SD): intervention 5.1 (2.1), control 5.0 (1.9) Severity COPD Not reported |
|
| Interventions |
Behavioural treatment A. Experimental At baseline participants were strongly encouraged to stop smoking by the general practitioner, and they received brief smoking cessation advice. The participants who would like to quit smoking were given a booklet that included information on smoking and quitting. Furthermore, there was individual counselling during home visits. If a participant had quit smoking, the general practitioners were to follow up the participant once a week at the first month and afterwards once a month until the end of the study. There were group meetings once a month by the participants. Every 2 months the professional group (including respiratory, rehabilitation, nutrition, sports, and psychology specialists) visited the participants in the healthcare centres. B. Control Participants in the control group were treated by healthcare providers or general practitioners in usual manner. Pharmacological treatment None |
|
| Outcomes |
Abstinence Prolonged abstinence from smoking for 2 years from the start of month 24 to the end of month 30, prolonged abstinence during months 24 to 36 and 24 to 48. Validation Exhaled CO ≤ 10 ppm (baseline and every 6 months follow‐up) |
|
| Notes |
Funding This research was funded by the Science and Technology Projects of Xuzhou City in 2007 (XM07C037) Conflicts of interest None Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The healthcare centres were classified in two classes: with high or low task delegation from general practitioners to nurses. The healthcare centres in the classes were then randomly allocated to the groups." No further information reported |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of missing values and differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Pederson 1991.
| Methods |
Design Randomised controlled trial Setting Inpatients |
|
| Participants |
Inclusion criteria Cigarette smoking patients, admitted to the Chest Unit of a hospital, previously diagnosed COPD as defined by the ACCP‐ATS. Exclusion criteria Suggestions of congestive heart failure and other acute disease Number randomised Total 74, treatment group 37, control group 37 Number followed up Total 64 Age mean (SD): total 53 (14) Sex n (%) male: total 51 (69) Cigarettes/day: 93% > 10 FTND mean (SD): not reported Severity COPD Not reported |
|
| Interventions |
Behavioural treatment A. Experimental Individual counselling: advice to quit smoking by their physician prior to admission, follow‐up intervention by trained assistant (2 to 8 sessions) x (15 to 20 minutes), and self help (cessation manual) B. Control Individual counselling: advice to quit smoking by their physician prior to admission Pharmacological treatment None |
|
| Outcomes |
Abstinence Point prevalence abstinence at 6 months after admission to the study Validation COHb analysis < 2.0% at 6 months from a random sample of 20 participants |
|
| Notes |
Funding Not reported Conflicts of interest Not reported Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough information. "...if they agreed, they would be randomised..." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Physicians were blinded as to group membership." However, it is not possible to blind participants and intervention staff to a behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of missing values |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not clearly prespecified |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Sundblad 2008.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria COPD (according to ERS guidelines), smoked more than 8 cigarettes/day, aged 40 to 60 years, lived in or in the vicinity of 9 Swedish towns and cities. Exclusion criteria Linguistic difficulties, severe comorbidities, or inability to stay away from home or work (either through reluctance or practical considerations). Number randomised Total 478, smoking cessation group (SC) 247, control group 231 Number followed up Total 391 Age mean (range): SC 53 (41 to 62), control 52 (41 to 61) Sex n (%) male: SC 111 (45), control 109 (47) Cigarettes/day mean (SD): not reported FTND mean (SD): SC 4.7 (2.0), control 4.8 (2.0) Severity COPD (FEV1/FVC not reported) FEV1% predicted, mean (SD): SC 74 (16), control 77 (14) |
|
| Interventions |
Behavioural treatment A. Smoking cessation Preparation time at home (2 weeks); registration of craving of cigarettes and number of smoked cigarettes; try reducing smoking by 25% Hospitalisation: 11 days in groups of 4 to 10 members (third day, cessation day); use of NRT was recommended; physical exercise; 1‐hour daily meeting with trained cessation nurse (individual counselling); structured educational program (topics: nicotine, health effects, dietary, training, lung function testing by doctor, physiotherapist, dietitian, technician, psychologist, occupational therapist, nurse) (group counselling); registration of craving, coping strategies, number of cigarettes At home: 2 to 3 months; registration with feedback: craving, coping strategies, number of cigarettes (telephone counselling, 1 weekly 5 to 30 min per call) Hospitalisation: 2 to 4 days; spouses invited; group discussions with the spouses: how to sustain smoking abstinence and avoid relapse At home: 10 months; registration with feedback of craving, coping strategies, number of cigarettes (telephone/email counselling) B. Control Participants in the usual‐care group were referred to primary healthcare centres and were informed about the COPD diagnosis and that smoking cessation had been recommended. Pharmacological treatment A. Smoking cessation Use of NRT was recommended B. Control Not specifically recommended |
|
| Outcomes |
Abstinence Point prevalence abstinence at 1 and 3 years from start of the intervention Validation Exhaled CO < 8 ppm, tested at 3 years' follow‐up, in a random sample of quitters |
|
| Notes |
Funding The study was supported by the research department of AFA Insurance Company, Stockholm, Sweden Conflicts of interest Not reported Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to a prearranged schedule, subjects were assigned into two groups by a person outside the study either to participate in the smoking cessation program or to be in a control group receiving usual care." A computer‐generated randomisation list was used. (Additional information from author) |
| Allocation concealment (selection bias) | Low risk | The allocation of participants to active treatment or control was totally concealed; when a participant was to be randomised, a phone call was made to an external person in a different location who picked the next number from his pre‐generated randomisation table. (Additional information from author) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The assessment of smoking abstinence was open and based on self reporting. However, in a random sample of approximately 50% of those who stated they were abstinent, an open (both study participant and personnel) carbon monoxide measurement was performed. (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | High risk | A. Participants' and personnel's adherence to the intervention protocol was not clearly described. It is only reported that "the one year long intervention followed a detailed protocol and the care providers did not change during the study." (Additional information from author) B. Exhaled CO was tested at 3 years' follow‐up in a random sample of quitters |
Tashkin 2001.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Current smokers with stage I or stage II COPD (ATS), aged 35 years or older, had smoked 15 cigarettes or more per day for the previous year, and had not stopped smoking for more than 3 months during that year. Motivated to quit smoking. Exclusion criteria People with any serious or unstable medical disorders that might affect lung function or for which bupropion SR was contraindicated, or if they had a current diagnosis of major depression. Number randomised Total 411, bupropion group 206, placebo group 205 Number followed up Total 278 Age mean (SD): bupropion 53.2 (9.0), placebo 54.5 (9.5) Sex n (%) male: bupropion 113 (55), placebo 113 (55) Cigarettes/day mean (SD): bupropion 28.7 (11.1), placebo 27.6 (10.2) FTND mean (SD): bupropion 7.1 (1.7), placebo 7.0 (1.7) Severity COPD (FEV1/FVC not reported) FEV1% predicted, mean (SD): bupropion 73.2 (19.4), placebo 69.4 (17.3) |
|
| Interventions |
Behavioural treatment Both groups received proactive telephone counselling by a trained health professional (1 session 3 days after the target quit date) and brief face‐to‐face individual counselling (9 sessions at week 1 to 7, 10, and 12) to encourage smoking cessation and prevent relapse. Pharmacological treatment A. Bupropion Bupropion SR 150 mg once daily for days 1 to 3, 150 mg twice daily on days 4 to 84. Bupropion was taken 1 week before quit date. B. Placebo Placebo SR 150 mg once daily for days 1 to 3, 150 mg twice daily on days 4 to 84. Placebo was taken 1 week before quit date |
|
| Outcomes |
Abstinence Prolonged abstinence weeks 4 to 26 from baseline on and point prevalence at week 26 (abstinence during the previous 7 days) Validation Exhaled CO ≤10 ppm |
|
| Notes |
Funding This study was funded by Glaxo Wellcome Inc Conflicts of interest Not reported Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done according to a randomisation code provided by Glaxo Wellcome, using block sizes of four stratified by centre. Within each block of four, two participants were assigned placebo and two bupropion SR." |
| Allocation concealment (selection bias) | Low risk | "The randomisation codes were kept at the study sites during the trial and we instructed investigators to break the code only for a medical emergency." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was a double‐blind, randomised, placebo‐controlled trial. "The randomisation codes were kept at the study sites during the trial and we instructed investigators to break the code only for a medical emergency." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low follow‐up rates and differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Tashkin 2011.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria Men and women aged ≥ 35 years, with clinical diagnosis of mild to moderate COPD (GOLD stages I and II), motivated to quit smoking, smoked on average ≥ 10 cigarettes/day over the past year with no period of abstinence > 3 months over that time. Exclusion criteria People were excluded if they had been treated with systemic corticosteroids or hospitalised for a COPD exacerbation during the 4‐week period prior to screening. Other exclusion criteria included unstable or uncontrolled medical conditions; a diagnosis of depression or treatment with antidepressants in the past year; drug or alcohol abuse in the past year; history of psychosis, panic disorder, or bipolar disorder; and use of a smoking cessation medication in the past month. Number randomised Total 504, varenicline 250, placebo 254 Number followed up Total 499 Age mean (SD): varenicline 57.2 (9.1), placebo 57.1 (9.0) Sex n (%) male: varenicline 155 (62.5), placebo 156 (62.2) Cigarettes/day mean (range): varenicline 25.3 (10 to 99), placebo 23.6 (10 to 60) FTND mean (SD): varenicline 6.2 (2.2), placebo 5.9 (2.1) Severity COPD FEV1/FVC ratio, mean: 61.1% to 62.9% FEV1 post‐bronchodilator % predicted, mean (SD): varenicline 70.8 (17.0), placebo 69.1 (16.9) |
|
| Interventions |
Behavioural treatment At the baseline visits, eligible participants received a smoking cessation self help booklet and 10 min of individual counselling. The target quit date (TQD) coincided with the week 1 visit. Counselling (10 min) was provided at clinic visits (weekly from weeks 1 to 13, then at weeks 16, 24, 32, 40, 48, and 52) and by telephone 3 days after the TQD and then in weeks 14, 20, 28, 36, and 44. Pharmacological treatment A. Varenicline Varenicline 0.5 mg once daily for 3 days, 0.5 mg bid for 4 days, then 1.0 mg bid, for a total of 12 weeks B. Placebo Placebo 0.5 mg once daily for 3 days, 0.5 mg twice daily for 4 days, then 1.0 mg twice daily, for a total of 12 weeks |
|
| Outcomes |
Abstinence Prolonged abstinence from week 9 to 24 and week 9 to 52 from start of the intervention and 7‐day point prevalence abstinence at weeks 24 and 52 Validation Exhaled CO ≤ 10 ppm |
|
| Notes |
Funding This study was funded by Pfizer Inc Conflicts of interest Several authors received grants from different pharmaceutical companies and attended advisory board meetings from different companies Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible participants were randomised at the baseline visit. Investigators obtained subject randomisation numbers and treatment group assignments through a central web‐based, or telephone call‐in drug management system or through instruction from the sponsor." |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised to varenicline or placebo in a 1:1 ratio using a block randomisation procedure with investigative site as the stratification variable. Investigators obtained subject randomisation numbers and treatment group assignments through a central web‐based, or telephone call‐in drug management system or through instruction from the sponsor." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study is described as a double‐blind trial, but there is no information on how this was realised |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low follow‐up percentages and differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Tonnesen 2006.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria People enrolled were aged ≥ 18 years; were smoking 1 or more cigarettes a day; had persistent airway obstruction with FEV1/FVC < 70% and FEV1 < 90% (ERS/ATS guidelines) of predicted normal value; and were willing to follow the study protocol. Exclusion criteria People who had used either nicotine sublingual tablets or bupropion during the previous 7 days, or who had any disease with predicted survival < 1 year, or were unable to adhere to the protocol. Number randomised Total 370, nicotine low support (NLS) 95, nicotine high support (NHS) 90, placebo low support (PLS) 88, placebo high support (PHS) 97 Number followed up Total 288 Age mean (SD): NLS 59.2 (10.3), NHS 61.3 (9.6), PLS 62.5 (9.3), PHS 61.2 (9.4) Sex n (%) male: NLS 45 (47), NHS 46 (51), PLS 40 (45), PHS 46 (47) Cigarettes/day mean (SD): NLS 20.1 (10.7), NHS 18.3 (10.4), PLS 20.2 (9.6), PHS 19.9 (8.8) FTND mean (SD): NLS 6.0 (2.1), NHS 5.9 (2.2), PLS 6.4 (1.9), PHS 6.4 (1.8) Severity COPD FEV1% predicted, mean (SD): total 55.8, PLS 56.0 (19.1), PHS 58.2 (17.8), NLS 55.1 (15.4), NHS 53.4 (19.4) FEV1/FVC%, mean (SD): PLS 56.2 (9.5), PHS 59.1 (10.0), NLS 57.0 (9.4), NHS 55.4 (11.3) |
|
| Interventions |
Behavioural treatment A. High support Individual counselling (7 sessions: baseline, 2, 4, 8, 12 weeks, 6 and 12 months) and proactive telephone counselling (5 calls: 1, 6, 10 weeks and 4.5 and 9 months) by nurses. Self help. Total amount of contact time: 7 x 20 to 30 min + 5 x 10 min = 4.5 hours. B. Low support Individual counselling (4 sessions: baseline, 2 weeks, 6 and 12 months) and proactive telephone counselling (6 calls: 1, 4, 6, 9, and 12 weeks and 9 months) by nurses. Self help. Total amount of contact time: 4 x 20 to 30 min + 6 x 10 min = 2.5 hours. Pharmacological treatment A. High support Recommendation of NRT sublingual tablet 2 mg (free of charge). Doses depending on baseline cigarettes use: ≥ 16 cigarettes per day: 1 or 2 tablets per hour (min 10 – max 40 per day), 10 to 15 cigarettes a day: 1 tablet per hour (6 to 30 tablets per day), 6 to 9 cigarettes a day: 1 tablet per hour (3 to 10 tablets per day). Duration: 12 weeks to up to 12 months. B. Low support Recommendation of placebo sublingual tablet 3 microgram capsaicin (free of charge). Doses depending on baseline cigarettes use; ≥ 16 cigarettes per day: 1 or 2 tablets per hour (min 10 to max 40 per day), 10 to 15 cigarettes a day: 1 tablet per hour (6 to 30 tablets per day), 6 to 9 cigarettes a day: 1 tablet per hour (3 to 10 tablets per day). Duration: 12 weeks to up to 12 months. |
|
| Outcomes |
Abstinence Prolonged abstinence from week 2 to month 12 after start of the intervention and point prevalence abstinence at 6 and 12 months after start of the intervention Validation Exhaled CO < 10 ppm |
|
| Notes |
Funding The Danish Medical Research Council provided the major grant for this study ($375,000; No. 9900732). Pfizer Consumer Healthcare, Sweden, supplied the study drugs used in the trial and provided grant support ($25,000). Conflicts of interest Dr. Tønnesen has served on advisory boards regarding smoking cessation agents, has participated in clinical trials with smoking cessation drugs, has received honorary for scientific talks and travel grants from Pfizer, GlaxoSmithKline, and Sanofi Aventis. Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were allocated to one of the four treatment groups using a block randomisation list at each centre." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind for behavioural treatment. It is stated this is a double‐blind trial, but it is not described how this blinding was realised |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High percentage (> 20%) of missing values |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported |
| Other bias | Unclear risk | Participants' and personnel's adherence to the intervention protocol was not described |
Wagena 2005.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria The study population consisted of current daily smokers at risk for COPD or with COPD (GOLD criteria). All participants were aged between 30 and 70 years, had to have a smoking history of at least 5 years, smoked on average at least 10 cigarettes per day during the last year, and were motivated to stop smoking. Exclusion criteria We excluded people who reported having used or who were still using bupropion SR or nortriptyline, people who were using nicotine therapy or psychoactive medication at the time of assessment, and people who had any serious or unstable medical disorders that might affect lung function or for which bupropion SR or nortriptyline was contraindicated. Number randomised Total 255, bupropion 86, nortriptyline 80, placebo 89, people with COPD 155 (following data accounts for all randomised participants and is not stratified for participants with COPD) Number followed up Total 220 Age mean (SD): bupropion 51.1 (8.3), nortriptyline 51.2 (9.1), placebo 51.3 (8.4) Sex n (%) male: bupropion 34 (40), nortriptyline 44 (55), placebo 46 (52) Cigarettes/day mean (SD): bupropion 24.2 (9.4), nortriptyline 22.2 (7.6), placebo 23.6 (8.8) FTND mean (SD): bupropion 6.2 (2.1), nortriptyline 6.0 (2.2), placebo 5.9 (2.1) Severity COPD FEV1 post‐bronchodilator % predicted, mean (SD): bupropion 86.3 (21.0), nortriptyline 83.1 (21.7), placebo 87.4 (23.0) FEV1/FVC ratio, x 100, mean (SD): bupropion 66.7 (13.4), nortriptyline 65.5 (13.6), placebo 65.1 (15.3) |
|
| Interventions |
Behavioural treatment For all groups at the baseline visit, the target quit date (TQD) was set for the second week, usually day 11 from the start of the medication. Individual face‐to‐face counselling by trained counsellor (baseline and 1 and 3 weeks after the TQD; 10 to 20 minutes per session). Supportive telephone calls by trained counsellor (TQD and at 2, 4, 6, 8, and 11 weeks after the TQD). Pharmacological treatment A. Bupropion group Bupropion 1 x 150 mg (day 1 to 6); 2 x 150 mg (day 7 to 84) AND placebo nortriptyline 1 x 25 mg (day 1 to 3); 1 x 50 mg (day 3 to 7); 1 x 75 mg (day 8 to 84) B. Nortriptyline group Nortriptyline 1 x 25 mg (day 1 to 3); 1 x 50 mg (day 3 to 7); 1 x 75 mg (day 8 to 84) AND placebo bupropion 1 x 150 mg (day 1 to 6); 2 x 150 mg (day 7 to 84) C. Placebo group Placebo bupropion 1 x 150 mg (day 1 to 6); 2 x 150 mg (day 7 to 84) AND placebo nortriptyline 1 x 25 mg (day 1 to 3); 1 x 50 mg (day 3 to 7); 1 x 75 mg (day 8 to 84) |
|
| Outcomes |
Abstinence Prolonged abstinence from week 4 to 26 after the TQD and point prevalence at 6 months Validation Urinary cotinine values ≤ 60 ng/mL at weeks 4, 12, and 26 after TQD |
|
| Notes |
Funding This study was funded by grant 3.2.00.21 from the Asthma Foundation Netherlands, Leusden; and by grant 2200.0111 from the The Netherlands Organisation for Health Research and Development, The Hague. Lundbeck BV, Amsterdam, the Netherlands, provided the nortriptyline for free. Conflicts of interest Not reported Correspondence No additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done according to a computer‐generated randomisation list provided by the pharmacist of Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands, stratified for COPD severity, using blocks of 33." |
| Allocation concealment (selection bias) | Low risk | "Randomization was done according to a computer‐generated randomisation list provided by the pharmacist." This is central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "No participant, research nurse, counsellor, investigator, or any other staff member was aware of the treatment assignments for the duration of the study. Blinding of participants was checked 1 week after the TQD and at the end of treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | High follow‐up rates (> 80%) in all groups, no differential attrition |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported, however for smokers with COPD only prolonged abstinence at week 26 was reported, while this was not a prespecified outcome |
| Other bias | High risk | Participants' and personnel's adherence to the intervention protocol was poor. "Medication compliance was assessed by counting number of pills allotted minus those returned." "55% of participants using 80% or fewer of the pills that they were asked to take. Poor compliance may have resulted in lower overall abstinence rates in our study population." "Forty participants (16%) discontinued medication because of adverse events: 8 (9%) in the placebo group, 12 (15%) in the bupropion SR‐treated group, and 19 (24%) in the nortriptyline treated group. The rate of discontinuation of treatment was statistically significantly higher among the participants receiving nortriptyline (P 0.01) compared with the participants receiving placebo." |
Wilson 2008.
| Methods |
Design Randomised controlled trial Setting Outpatients |
|
| Participants |
Inclusion criteria People were included in the study if they were self reported cigarette smokers attending the RRC with a diagnosis of COPD (NICE guidelines). Exclusion criteria People were excluded if they had any alcohol/drug‐related problems, contraindications to nicotine replacement therapy, or they stated they had no intention of stopping smoking. Number randomised Total 91, individual support (IS) 27, group support (GS) 29, usual care (UC) 35 Number followed up Total 68 Age mean (SD): IS 61.0 (8), GS 60.4 (9), UC 61.4 (8) Sex n (%) male: IS 14 (52), GS 9 (41), UC 18 (51) Cigarettes/day mean (SD): IS 20.8 (11), GS 20.1 (11), UC 17.5 (7) FTND mean (SD): not reported Severity COPD (FEV1/FVC not reported) FEV1, mean (SD): IS 52.1 (20), GS 54.6 (23), UC 54.3 (20) |
|
| Interventions |
Behavioural treatment A. Individual support Individual counselling by chest clinic sister or respiratory nurses, or both (5 weekly session x 60 min). Stage‐matched interventions. Discussing attitudes, subjective norms, self efficacy, former quit attempts and relapse plus CO measurement. B. Group support Group counselling by chest clinic sister or respiratory nurses, or both (5 weekly session x 60 min). Stage‐matched interventions. Discussing attitudes, subjective norms, self efficacy, former quit attempts and relapse plus CO measurement. C. Usual care Brief advice by doctor (1 x 5 to 10 min) included: assessing participant's interest in stopping smoking based on stages of change; advising on personal benefits of cessation and NRT; encouragement to set a stop date and enlist the support of family and friends. Pharmacological treatment A. Individual support 12‐week course of NRT (patches) for participants wanting to stop smoking (for free). Each NRT patch administered 16 h of nicotine (8 weeks 15 mg, 2 weeks 10 mg, and 2 weeks 5 mg). B. Group support 12‐week course of NRT (patches) for participants wanting to stop smoking (for free). Each NRT patch administered 16 h of nicotine (8 weeks 15 mg, 2 weeks 10 mg, and 2 weeks 5 mg). C. Usual care Advice to use NRT |
|
| Outcomes |
Abstinence Prolonged and point prevalence abstinence at 6, 9, and 12 months' follow‐up Validation Exhaled CO ≤ 10, salivary cotinine ≤ 10 ng/mL |
|
| Notes |
Funding Smith & Nephew Foundation and Pharmacia Conflicts of interest Not reported Correspondence Additional information received after correspondence |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects included in the study were allocated to one of three treatment groups by a computer‐generated list of random numbers." (Additional information from author) |
| Allocation concealment (selection bias) | Low risk | The randomisation numbers were sealed in an opaque, consecutively numbered envelope, therefore all individuals involved in the study were blind to the randomisation sequence. The sealed envelopes were kept in a secure place by the researcher |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is not possible to blind for behavioural treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher and participants were not blinded for self reported smoking status and exhaled CO measurement. The participant, researcher, and laboratory staff were blinded until after completion of the study for salivary cotinine measurement. (Additional information from author) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low follow‐up rates (< 80%) and differential attrition |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported. (Additional information from author) |
| Other bias | High risk | Participants' adherence to the intervention protocol was poor. "Attendance to the nursing interventions was poor in both groups (Fig. 2). Only 10 participants (37%) in the IS group and 7 participants (24%) in the GS group attended at least 3 weeks of the 5‐week intervention." "Only 16 (59%) participants in the IS group and 12 (41%) participants in the GS group used the free NRT patches offered." Personnel's adherence is not reported. (Additional information from author) |
ACCP‐ATS: American College of Chest Physicians ‐ American Thoracic Society; ATC: Anatomical Therapeutic Chemical classification; CB: chronic bronchitis; CCO: corrected carbon monoxide; CN: counselling and NRT; CNB: counselling, NRT, and bupropion; CO: carbon monoxide; co1: control group 1; co2: control group 2; COHb: carboxyhaemoglobin; COPD: chronic obstructive pulmonary disease; CSR: cigarette self report; ERS: European Respiratory Society; exp: experimental group; FC: face‐to‐face counselling; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; FTND: Fagerström Test for Nicotine Dependence; GOLD: Global Initiative for Chronic Obstructive Lung Disease; GP: general practitioner; GS: group support; ICPC: International Classification of Primary Care; IS: individual support; L‐MIS: minimal intervention strategy for lung patients; MIS: minimal intervention strategy; NHS: nicotine high support; NICE: National Institute for Health and Care Excellence; NLS: nicotine low support; NP: nicotine patch; NRT: nicotine replacement therapy; PHS: placebo high support; PLS: placebo low support; ppm: parts per million; RRC: Regional Respiratory Centre;
SC: smoking cessation group; SD: standard deviation; SI‐A: smoking intervention plus active bronchodilator inhaler; SI‐B: smoking intervention plus placebo inhaler; SL: smoker's lung; SR: sustained release; SST: smoke‐stop‐therapy; TQD: target quit date; UC: usual care.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andreeva 2007 | No smokers with COPD |
| Bastian 2015 | No smokers with COPD |
| Bendstrup 1997 | No smokers with COPD |
| Berlin 2011 | No smokers with COPD |
| Borglykke 2008 | Not an RCT |
| Bunker 2012 | No smokers with COPD |
| Cai 2006 | Not an RCT |
| Carone 2002 | No smokers with COPD |
| Cheng 2001 | No smokers with COPD |
| Damcevska 2007 | No smokers with COPD |
| Dheda 2004 | No smokers with COPD |
| Efraimsson 2008 | No smokers with COPD |
| Evans 1974 | No smokers with COPD |
| Fish 2012 | No smokers with COPD |
| Florou 2011 | Not an RCT |
| Folz 2015 | No smokers with COPD |
| George 2006 | No smokers with COPD |
| Gunthner 1970 | Not an RCT |
| Harter 2015 | No smokers with COPD |
| Heslop 2013 | No smokers with COPD |
| Hesselink 2004 | No smokers with COPD |
| Hoogendoorn 2010 | No smokers with COPD |
| Irizar‐Aramburu 2013 | No smokers with COPD |
| James 2012 | No smokers with COPD |
| Jennings 2015 | No smokers with COPD |
| Jimenez 2007 | No smokers with COPD |
| Jonsdottir 2015 | No smokers with COPD |
| Kato 2005 | No smokers with COPD |
| Kiser 2012 | No smokers with COPD |
| Kruis 2015 | No smokers with COPD |
| Lewis 1998 | No smokers with COPD |
| Lofdahl 1998 | Not an RCT |
| Lou 2015 | No smokers with COPD |
| Meyer 2015 | No outcome data |
| Møller 2004 | Not an RCT |
| Nohlert 2009 | No smokers with COPD |
| Ozol 2008 | Not an RCT |
| Parker 2013 | No smokers with COPD |
| Parkes 2008 | No smokers with COPD |
| Pauwels 1992 | No smoking cessation intervention |
| Rose 1992 | No smokers with COPD |
| Sachs 2009 | Not an RCT |
| Sajol 2015 | No smokers with COPD |
| Sakharova 2006 | No smokers with COPD |
| Shaban 2005 | No smokers with COPD |
| Shaker 2011 | No smoking cessation intervention |
| Signes‐Costa 2004 | Not an RCT |
| Smith 2013 | No smokers with COPD |
| Steurer‐Stey 2014 | No smokers with COPD |
| Stratelis 2006 | No smokers with COPD |
| Suhaj 2016 | No smokers with COPD |
| Tonnesen 1988 | No smokers with COPD |
| Voncken‐Brewster 2013 | No smokers with COPD |
| Voncken‐Brewster 2015 | No smokers with COPD |
| Walters 2012 | No smokers with COPD |
| Weling‐Scheepers 2005 | Not an RCT |
| Yuan 2015 | No smokers with COPD |
| Zhou 2010 | No smokers with COPD |
| Zhou 2011 | No outcome data |
| Zwar 2012 | No smokers with COPD |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01694732.
| Trial name or title | Efficacy of varenicline on smoking cessation at the acute phase of an exacerbation of chronic obstructive pulmonary disease (SAVE) |
| Methods |
Design Randomised controlled trial Setting Inpatients |
| Participants |
Inclusion criteria People were included in the study if they were aged ≥ 18 years, smoked ≥ 10 cigarettes per day in the last year, were affected by COPD (ATS/ERS criteria), were admitted to a pulmonology or intensive care unit for at least 24 hours due to an exacerbation, were included during this hospitalisation, were motivated to quit smoking, were able to understand the information and give written consent, and were available for 1‐year follow‐up. Exclusion criteria People were excluded if they were not entitled to the National Health Insurance Coverage, presented contraindications to the pharmacotherapy, were participating in other smoking cessation trials, were pregnant or breastfeeding, had a (family) history of or had a current psychiatric disorder (list of disorders is provided), had hepatic or renal impairment, had any alcohol/drug‐related problems, or had a life expectancy of less than 12 months. |
| Interventions |
Behavioural treatment A. Experimental Intensive smoking cessation counselling B. Placebo Intensive smoking cessation counselling Pharmacological treatment A. Experimental Varenicline B. Placebo Placebo |
| Outcomes | Abstinence At 6, 9, and 12 months' follow‐up |
| Starting date | August 2012 |
| Contact information | francis.couturaud@chu‐brest.fr |
| Notes | Funding University Hospital, Brest |
NCT02148445.
| Trial name or title | Smoking cessation versus long‐term nicotine replacement among high‐risk smokers |
| Methods | Design Randomised controlled trial |
| Participants |
Inclusion criteria People were included in the study if they were aged ≥ 18 years, had physician‐diagnosed COPD, smoked ≥ 5 cigarettes per day and had smoked on 25 or more of the last 30 days, spoke English or Spanish, and were willing to take nicotine replacement therapy for up to 1 year and participate in study procedures. Exclusion criteria People were excluded if they resided in a facility that did not allow smoking, did not have an address and telephone, had a unstable cardiac condition, were pregnant or breastfeeding, or had a terminal illness with a life expectancy of less than 12 months. |
| Interventions |
Behavioural treatment A. Smoking cessation Standard smoking cessation program B. Guided maintenance therapy Counselling focused on medication adherence and smoking reduction Pharmacological treatment A. Smoking cessation NRT (patch plus choice of gum/lozenge) B. Guided maintenance therapy NRT (patch plus choice of gum/lozenge) |
| Outcomes |
Abstinence Prolonged and point prevalence abstinence at 6 and 12 months' follow‐up Validation CO measurement |
| Starting date | May 2015 |
| Contact information | thutcheson@kumc.edu |
| Notes | Funding University of Kansas |
ATS: American Thoracic Society; CO: carbon monoxide; COPD: chronic obstructive pulmonary disease; ERS: European Respiratory Society; NRT: nicotine replacement therapy.
Differences between protocol and review
In the protocol we wrote that we would only include studies that focused exclusively on participants with COPD. However, in this review we also included studies involving smokers without COPD when a stratified group of smokers with COPD was randomised (Chen 2014; Wagena 2005).
In the protocol we did not explicitly state that we would not include studies that reported abstinence rates after less than six months of follow‐up. However, we only included studies that reported how many people had stopped smoking after at least six months of follow‐up.
Ten studies reported, according to their definition, continuous, sustained, or prolonged abstinence. However, these definitions were diverse and sometimes vague, and did not always match our definitions. Furthermore, most of these studies reported abstinence data after an initial grace period. We therefore clustered prolonged, sustained, and continuous abstinence data, and categorised all of these under one outcome measure, prolonged abstinence.
It was not possible to distinguish subgroups by severity of COPD in the way we described (mild‐moderate versus severe‐very severe). Most studies contained a mix of these severities, and none of the studies contained solely severe‐very severe cases. We therefore did a subgroup analysis distinguishing studies with mostly participants with mild‐moderate COPD and studies with a substantial amount of participants with severe‐very severe COPD.
The subgroup analysis on low‐ and high‐intensity behavioural treatment was not possible because there was too much heterogeneity of the different treatment and control groups between the different studies.
In addition to the description of RRs in the analysis, we also reported a measure of the absolute effects in the different trial arms, the RDs.
Contributions of authors
EVE is the guarantor of the review. She extracted, managed, analysed and interpreted the data and wrote the review.
RVDM performed previous work that was part of the foundation of the current review, extracted the data, provided detailed advice on data management, analysis and interpretation and on writing the review.
CVS provided general advice and comments on the review draft.
DK provided a third opinion when the first two review authors disagreed and provided detailed comments on data interpretation and writing the review.
Sources of support
Internal sources
The authors declare that no such funding was received for this systematic review, Netherlands.
External sources
-
SBOH, Netherlands.
The employer for family doctor trainees in the Netherlands provided the salary for the first review author.
-
Ministry for Innovation, Science and Research, Germany.
DK is supported by the Ministry for Innovation, Science and Research of the German Federal State of North Rhine‐Westphalia ("NRW‐Rückkehrprogramm").
Declarations of interest
EVE and RVDM: None known.
CVS received unrestricted grants for nicotine addiction studies in both primary care and public health. CVS is author on two study reports included in this review (Kotz 2009; Wagena 2005). CVS's institution received money for consultancy from Asthma Foundation, Achmea (health insurance), Pfizer, and Boehringer Ingelheim.
DK received an unrestricted grant from Pfizer for an investigator‐initiated trial on the effectiveness of practice nurse counselling and varenicline for smoking cessation in primary care (Dutch Trial Register NTR3067). DK is author on one study report included in this review (Kotz 2009).
Edited (no change to conclusions)
References
References to studies included in this review
Anthonisen 1994 {published data only}
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 1994;272(19):1497‐505. [PUBMED: 7966841] [PubMed] [Google Scholar]
- Buist AS. The US Lung Health Study. Respirology (Carlton, Vic.) 1997;2(4):303‐7. [PUBMED: 9525302] [DOI] [PubMed] [Google Scholar]
- Connett JE, Bjornson‐Benson WM, Daniels K. Recruitment of participants in the Lung Health Study, II: Assessment of recruiting strategies. Controlled Clinical Trials 1993;14(2 Suppl):38S‐51S. [PUBMED: 8500312] [DOI] [PubMed] [Google Scholar]
- O'Hara P, Grill J, Rigdon MA, Connett JE, Lauger GA, Johnston JJ. Design and results of the initial intervention program for the Lung Health Study. The Lung Health Study Research Group. Preventive Medicine 1993;22(3):304‐15. [PUBMED: 8327414] [DOI] [PubMed] [Google Scholar]
- Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, et al for the Lung Health Study Research Group. Smoking cessation and lung function in mild‐to‐moderate chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161:381–90. [DOI] [PubMed] [Google Scholar]
Brandt 1997 {published data only}
- Brandt CJ, Ellegaard H, Joensen M, Kallan FV, Sorknaes AD, Tougaard L. Effect of diagnosis of "smoker's lung". RYLUNG Group. The Lancet (London, England) 1997;349(9047):253. [PUBMED: 9014918] [DOI] [PubMed] [Google Scholar]
- Kallan FV, Brandt CJ, Ellegaard H, Joensen MB, Sorknaes AD, Tougaard L. The diagnosis of "smoker's lung" encourages smoking cessation [Diagnosen "rygerlunger" fremmer rygeophor. RYLUNG‐gruppen]. Ugeskrift for Laeger 1997;159(44):6528‐30. [PUBMED: 9411973] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen J, Chen Y, Chen P, Liu Z, Luo H, Cai S. Effectiveness of individual counseling for smoking cessation in smokers with chronic obstructive pulmonary disease and asymptomatic smokers. Experimental and Therapeutic Medicine 2014;7(3):716‐20. [PUBMED: 24520273] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christenhusz 2007 {published data only}
- Christenhusz L, Pieterse M, Seydel E, Palen J. Prospective determinants of smoking cessation in COPD patients within a high intensity or a brief counseling intervention. Patient Education and Counseling 2007;66(2):162‐6. [PUBMED: 17196359] [DOI] [PubMed] [Google Scholar]
- Christenhusz LC, Prenger R, Pieterse ME, Seydel ER, Palen J. Cost‐effectiveness of an intensive smoking cessation intervention for COPD outpatients. Nicotine and Tobacco Research 2012;14(6):657‐63. [PUBMED: 22180589] [DOI] [PubMed] [Google Scholar]
Crowley 1995 {published data only}
- Crowley TJ, Macdonald MJ, Walter MI. Behavioral anti‐smoking trial in chronic obstructive pulmonary disease patients. Psychopharmacology 1995;119(2):193‐204. [PUBMED: 7659767] [DOI] [PubMed] [Google Scholar]
Gorecka 2003 {published data only}
- Gorecka D, Bednarek M, Nowinski A, Puscinska E, Goljan‐Geremek A, Zielinski J. Effect of treatment for nicotine dependence in patients with COPD [Wyniki leczenia uzaleznienia od nikotyny chorych na przewlekla obturacyjna chorobe pluc]. Pneumonologia I Alergologia Polska 2003;71(9‐10):411‐7. [PUBMED: 15052977] [PubMed] [Google Scholar]
Hilberink 2011 {published data only}
- Hilberink SR, Jacobs JE, Bottema BJ, Vries H, Grol RP. Smoking cessation in patients with COPD in daily general practice (SMOCC): six months' results. Preventive Medicine 2005;41(5‐6):822‐7. [PUBMED: 16203030] [DOI] [PubMed] [Google Scholar]
- Hilberink SR, Jacobs JE, Breteler MH, Vries H, Grol RP. General practice counseling for patients with chronic obstructive pulmonary disease to quit smoking: impact after 1 year of two complex interventions. Patient Education and Counseling 2011;83(1):120‐4. [PUBMED: 20430565] [DOI] [PubMed] [Google Scholar]
Kotz 2009 {published data only}
- Kotz D, Huibers MJ, West RJ, Wesseling G, Schayck OC. What mediates the effect of confrontational counselling on smoking cessation in smokers with COPD?. Patient Education and Counseling 2009;76(1):16‐24. [PUBMED: 19150590] [DOI] [PubMed] [Google Scholar]
- Kotz D, Vos R, Huibers MJ. Ethical analysis of the justifiability of labelling with COPD for smoking cessation. Journal of Medical Ethics 2009;35(9):534‐40. [PUBMED: 19717691] [DOI] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confrontational counselling for smoking cessation in smokers with previously undiagnosed mild to moderate airflow limitation: study protocol of a randomized controlled trial. BMC Public Health 2007;7:332. [PUBMED: 18005415] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz D, Wesseling G, Huibers MJ, Schayck OC. Efficacy of confronting smokers with airflow limitation for smoking cessation. European Respiratory Journal 2009;33(4):754‐62. [PUBMED: 19129277] [DOI] [PubMed] [Google Scholar]
Lou 2013 {published data only}
- Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Supporting smoking cessation in chronic obstructive pulmonary disease with behavioral intervention: a randomized controlled trial. BMC Family Practice 2013;14:91. [PUBMED: 23802809] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pederson 1991 {published data only}
- Pederson LL, Wanklin JM, Lefcoe NM. The effects of counseling on smoking cessation among patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. The International Journal of the Addictions 1991;26(1):107‐19. [PUBMED: 2066170] [DOI] [PubMed] [Google Scholar]
Sundblad 2008 {published data only}
- Sundblad BM, Larsson K, Nathell L. High rate of smoking abstinence in COPD patients: Smoking cessation by hospitalization. Nicotine and Tobacco Research 2008;10(5):883‐90. [PUBMED: 18569763] [DOI] [PubMed] [Google Scholar]
Tashkin 2001 {published data only}
- Tashkin D, Kanner R, Bailey W, Buist S, Anderson P, Nides M, et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double‐blind, placebo‐controlled, randomised trial. The Lancet (London, England) 2001;357(9268):1571‐5. [PUBMED: 11377644] [DOI] [PubMed] [Google Scholar]
Tashkin 2011 {published data only}
- Lock K, Wilson K, Murphy D, Riesco JA. A cost‐effectiveness model of smoking cessation based on a randomised controlled trial of varenicline versus placebo in patients with chronic obstructive pulmonary disease. Expert Opinion on Pharmacotherapy 2011;12(17):2613‐26. [PUBMED: 22017336] [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Hays JT, Ma W, Lawrence D, Lee TC. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest 2011;139(3):591‐9. [PUBMED: 20864613] [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Rennard S, Taylor Hays J, Lawrence D, Marton JP, Lee TC. Lung function and respiratory symptoms in a 1‐year randomized smoking cessation trial of varenicline in COPD patients. Respiratory Medicine 2011;105(11):1682‐90. [PUBMED: 21621992] [DOI] [PubMed] [Google Scholar]
Tonnesen 2006 {published data only}
- Tonnesen P, Mikkelsen K, Bremann L. Nurse‐conducted smoking cessation in patients with COPD using nicotine sublingual tablets and behavioral support. Chest 2006;130(2):334‐42. [PUBMED: 16899830] [DOI] [PubMed] [Google Scholar]
Wagena 2005 {published data only}
- Schayck CP, Kaper J, Wagena EJ, Wouters EF, Severens JL. The cost‐effectiveness of antidepressants for smoking cessation in chronic obstructive pulmonary disease (COPD) patients. Addiction 2009;104(12):2110‐7. [PUBMED: 19922576] [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Archives of Internal Medicine 2005;165(19):2286‐92. [PUBMED: 16246996] [DOI] [PubMed] [Google Scholar]
Wilson 2008 {published data only}
- Wilson JS, Elborn JS, Fitzsimons D. 'It's not worth stopping now': why do smokers with chronic obstructive pulmonary disease continue to smoke? A qualitative study. Journal of Clinical Nursing 2011;20(5‐6):819‐27. [PUBMED: 20738455] [DOI] [PubMed] [Google Scholar]
- Wilson JS, Elborn JS, Fitzsimons D, McCrum‐Gardner E. Do smokers with chronic obstructive pulmonary disease report their smoking status reliably? A comparison of self‐report and bio‐chemical validation. International Journal of Nursing Studies 2011;48(7):856‐62. [PUBMED: 21288520] [DOI] [PubMed] [Google Scholar]
- Wilson JS, Fitzsimons D, Bradbury I, Stuart Elborn J. Does additional support by nurses enhance the effect of a brief smoking cessation intervention in people with moderate to severe chronic obstructive pulmonary disease? A randomised controlled trial. International Journal of Nursing Studies 2008;45(4):508‐17. [PUBMED: 17184783] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andreeva 2007 {published data only}
- Andreeva S, Grechenko V, Antonova T, Sakharova G, Antonov N, Chuchalin A. Airway hyperresponsiveness and its correction during the smoking cessation program. European Respiratory Society 17th Annual Congress; 2007 Sep 16‐18; Stockholm. 2007.
Bastian 2015 {published data only}
- Bastian LA, Fish LJ, Gierisch JM, Stechuchak KM, Grambow SC, Keefe FJ. Impact of smoking cessation on subsequent pain intensity among chronically ill veterans enrolled in a smoking cessation trial. Journal of Pain and Symptom Management 2015;50(6):822‐9. [PUBMED: 26210348] [DOI] [PubMed] [Google Scholar]
Bendstrup 1997 {published data only}
- Bendstrup KE, Ingemann Jensen J, Holm S, Bengtsson B. Out‐patient rehabilitation improves activities of daily living, quality of life and exercise tolerance in chronic obstructive pulmonary disease. European Respiratory Journal 1997;10(12):2801‐6. [PUBMED: 9493664] [DOI] [PubMed] [Google Scholar]
Berlin 2011 {published data only}
- Berlin I, Jacob N, Coudert M, Perriot J, Schultz L, Rodon N. Adjustment of nicotine replacement therapies according to saliva cotinine concentration: the ADONIS* trial ‐ a randomized study in smokers with medical comorbidities. Addiction 2011;106(4):833‐43. [PUBMED: 21205047] [DOI] [PubMed] [Google Scholar]
Borglykke 2008 {published data only}
- Borglykke A, Pisinger C, Jorgensen T, Ibsen H. The effectiveness of smoking cessation groups offered to hospitalised patients with symptoms of exacerbations of chronic obstructive pulmonary disease (COPD). Clinical Respiratory Journal 2008;2(3):158‐65. [PUBMED: 20298324] [DOI] [PubMed] [Google Scholar]
Bunker 2012 {published data only}
- Bunker JM, Reddel HK, Dennis SM, Middleton S, Schayck C, Crockett AJ, et al. A pragmatic cluster randomized controlled trial of early intervention for chronic obstructive pulmonary disease by practice nurse‐general practitioner teams: study protocol. Implementation Science 2012;7:83. [PUBMED: 22958678] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cai 2006 {published data only}
- Cai S, Chen P, Chen Y, Liu ZJ. Effect of health education on the lung function and life quality in patients with stable chronic obstructive pulmonary diseases. Zhong nan da xue xue bao. Yi xue ban (Journal of Central South University. Medical Sciences) 2006;31(2):189‐93. [PUBMED: 16706112] [PubMed] [Google Scholar]
Carone 2002 {published data only}
- Carone M, Bertolotti G, Cerveri I, Benedetto F, Fogliani V, Nardini S, et al. EDU‐CARE, a randomised, multicentre, parallel group study on education and quality of life in COPD. Monaldi Archives for Chest Disease 2002;57(1):25‐9. [PUBMED: 12174697] [PubMed] [Google Scholar]
Cheng 2001 {published data only}
- Cheng X, Xu X, Zhang Z. Results of community intervention trial for chronic obstructive pulmonary diseases and chronic cor‐pulmonale from 1992 to 1999. Chinese Journal of Tuberculosis and Respiratory Diseases 2001;24:579‐83. [PubMed] [Google Scholar]
Damcevska 2007 {published data only}
- Damcevska J, Babalj E, Stojanovski Z, Minov J. Different methods of education and success in smoking cessation. European Respiratory Society 17th Annual Congress; 2007 Sep 16‐18; Stockholm. 2007.
Dheda 2004 {published data only}
- Dheda K, Crawford A, Hagan G, Roberts CM. Implementation of British Thoracic Society guidelines for acute exacerbation of chronic obstructive pulmonary disease: impact on quality of life. Postgraduate Medical Journal 2004;80(941):169‐71. [PUBMED: 15016940] [DOI] [PMC free article] [PubMed] [Google Scholar]
Efraimsson 2008 {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self‐care management education at a nurse‐led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178‐85. [PUBMED: 18489687] [DOI] [PubMed] [Google Scholar]
Evans 1974 {published data only}
- Evans JA, Morrison IM, Saunders KB. A controlled trial of prednisone, in low dosage, in patients with chronic airways obstruction. Thorax 1974;29(4):401‐6. [PUBMED: 4603999] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fish 2012 {published data only}
- Fish LJ, Gierisch JM, Stechuchak KM, Grambow SC, Rohrer LD, Bastian LA. Correlates of expected positive and negative support for smoking cessation among a sample of chronically ill veterans. Addictive Behaviors 2012;37(1):135‐8. [PUBMED: 21978930] [DOI] [PubMed] [Google Scholar]
Florou 2011 {published data only}
- Florou A, Eleftheriou K, Patentalakis G, Sachlas A, Michailidis D, Gratziou C. Smoking cessation effectiveness in smokers with obstructive respiratory disease. European Respiratory Society 21stAnnual Congress; 2011 Sep 24‐28; Amsterdam. 2011.
Folz 2015 {published data only}
- Folz H, Murphy B, Byrd J, Day M. Assessing the impact of pharmacist interventions versus standard care in the outpatient management of chronic obstructive pulmonary disease. American Pharmacists Association Conference; 2015 March 27‐30; San Diego. 2015.
George 2006 {published data only}
- George J, Kong DCM, Santamaria NM, Ioannides‐Demos LL, Stewart K. Smoking cessation: COPD patients' perspective. Journal of Pharmacy Practice and Research 2006;36(2):107‐10. [Google Scholar]
Gunthner 1970 {published data only}
- Gunthner W. Long‐term therapy of the chronic bronchitis syndrome using Tanderil [Langzeitbehandlung des chronischen bronchitischen syndroms mit Tanderil]. Munchener medizinische wochenschrift 1970;112(49):2244‐6. [PubMed] [Google Scholar]
Harter 2015 {published data only}
- Harter M, Bartsch AL, Egger N, Konig HH, Kriston L, Schulz H, et al. Evaluating a collaborative smoking cessation intervention in primary care (ENTER): study protocol for a cluster‐randomized controlled trial. Trials 2015;16:447. [PUBMED: 26452466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heslop 2013 {published data only}
- Heslop K, Newton J, Baker C, Burns G, Carrick‐Sen D, Soyza A. Effectiveness of cognitive behavioural therapy (CBT) interventions for anxiety in patients with chronic obstructive pulmonary disease (COPD) undertaken by respiratory nurses: the COPD CBT CARE study: (ISRCTN55206395). BMC Pulmonary Medicine 2013;13:62. [PUBMED: 24498939] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesselink 2004 {published data only}
- Hesselink AE, Penninx BW, Windt DA, Duin BJ, Vries P, Twisk JW, et al. Effectiveness of an education programme by a general practice assistant for asthma and COPD patients: results from a randomised controlled trial. Patient Education and Counseling 2004;55(1):121‐8. [PUBMED: 15476999] [DOI] [PubMed] [Google Scholar]
Hoogendoorn 2010 {published data only}
- Hoogendoorn M, Wetering CR, Schols AM, Rutten‐van Molken MP. Is INTERdisciplinary COMmunity‐based COPD management (INTERCOM) cost‐effective?. European Respiratory Journal 2010;35(1):79‐87. [PUBMED: 19574331] [DOI] [PubMed] [Google Scholar]
Irizar‐Aramburu 2013 {published data only}
- Irizar‐Aramburu MI, Martinez‐Eizaguirre JM, Pacheco‐Bravo P, Diaz‐Atienza M, Aguirre‐Arratibel I, Pena‐Pena MI, et al. Effectiveness of spirometry as a motivational tool for smoking cessation: a clinical trial, the ESPIMOAT study. BMC Family Practice 2013;14:185. [PUBMED: 24308728] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2012 {published data only}
- James S, Patry R. Pulmonary rehabilitation provided by a pharmacist and its impact on patient care. American Pharmacists Association Conference; 2012 March 9‐12; New Orleans. 2012.
Jennings 2015 {published data only}
- Jennings JH, Thavarajah K, Mendez MP, Eichenhorn M, Kvale P, Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest 2015;147(5):1227‐34. [PUBMED: 25940250] [DOI] [PubMed] [Google Scholar]
Jimenez 2007 {published data only}
- Jimenez G, Lopez J, Martinez M, Romero A, Gil B, Dios Luna J, et al. Effectiveness of a specialised intervention for smoking cessation in hospitalised patients. European Respiratory Society 17th Annual Congress; 2007 Sep 16‐18; Stockholm. 2007.
Jonsdottir 2015 {published data only}
- Jonsdottir H, Amundadottir OR, Gudmundsson G, Halldorsdottir BS, Hrafnkelsson B, Ingadottir TS, et al. Effectiveness of a partnership‐based self‐management programme for patients with mild and moderate chronic obstructive pulmonary disease: a pragmatic randomized controlled trial. Journal of Advanced Nursing 2015;71(11):2634‐49. [PUBMED: 26193907] [DOI] [PubMed] [Google Scholar]
Kato 2005 {published data only}
- Kato S, Oda K, Hasumi H. Clinical significance of the combination therapy of smoking cessation and seihaito for chronic obstructive pulmonary disease. Kampo to Saishin‐chiryo 2005;14:260‐5. [Google Scholar]
Kiser 2012 {published data only}
- Kiser K, Jonas D, Warner Z, Scanlon K, Shilliday BB, DeWalt DA. A randomized controlled trial of a literacy‐sensitive self‐management intervention for chronic obstructive pulmonary disease patients. Journal of General Internal Medicine 2012;27(2):190‐5. [PUBMED: 21935752] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruis 2015 {published data only}
- Kruis AL, Boland MRS, Assendelft WJJ, Gussekloo J, Tsiachristas A, Stijnen T, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: results of cluster randomised trial. BMJ 2014;349:g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1998 {published data only}
- Lewis SF, Piasecki TM, Fiore MC, Anderson JE, Baker TB. Transdermal nicotine replacement for hospitalized patients: a randomized clinical trial. Preventive Medicine 1998;27(2):296‐303. [PUBMED: 9579010] [DOI] [PubMed] [Google Scholar]
Lofdahl 1998 {published data only}
- Löfdahl CG. The Lung Health Study (LHS): importance of the interruption of smoking [La Lung Health Study (LHS): importance de l'interruption du tabagisme]. Revue française des maladies 1998;15:S42‐4. [Google Scholar]
Lou 2015 {published data only}
- Lou P, Chen P, Zhang P, Yu J, Wang Y, Chen N, et al. A COPD health management program in a community‐based primary care setting: a randomized controlled trial. Respiratory Care 2015;60(1):102‐12. [PUBMED: 25371402] [DOI] [PubMed] [Google Scholar]
Meyer 2015 {published data only}
- Meyer AC, Streck JM, Ochalek TA, Hruska B, Teneback CC, Dixon AE, et al. Financial incentives promote smoking abstinence among patients with pulmonary disease. Annual Meeting of the College on Problems of Drug Dependence; 2015 June 13‐18; Phoenix. 2015.
Møller 2004 {published data only}
- Møller AM. Smoking cessation and chronic obstructive pulmonary disease [Rygestop og kronisk obstruktiv lungesygdom]. Ungeskrift for læger 2004;166(42):3695‐7. [PubMed] [Google Scholar]
Nohlert 2009 {published data only}
- Nohlert E, Tegelberg A, Tillgren P, Johansson P, Rosenblad A, Helgason AR. Comparison of a high and a low intensity smoking cessation intervention in a dentistry setting in Sweden: a randomized trial. BMC Public Health 2009;9:121. [PUBMED: 19405969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozol 2008 {published data only}
- Özol D, Kocak OM, Özel H. Effectiveness of out‐patient based smoking cessation. Turkish Respiratory Journal 2008;9(1):10‐4. [Google Scholar]
Parker 2013 {published data only}
- Parker DR, Eaton CB, Ahern DK, Roberts MB, Rafferty C, Goldman RE, et al. The study design and rationale of the randomized controlled trial: translating COPD guidelines into primary care practice. BMC Family Practice 2013;14:56. [PUBMED: 23641803] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parkes 2008 {published data only}
- Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ 2008;336(7644):598‐600. [PUBMED: 18326503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauwels 1992 {published data only}
- Pauwels RA, Lofdahl CG, Pride NB, Postma DS, Laitinen LA, Ohlsson SV. European Respiratory Society study on chronic obstructive pulmonary disease (EUROSCOP): hypothesis and design. European Respiratory Journal 1992;5(10):1254‐61. [PUBMED: 1486974] [PubMed] [Google Scholar]
Rose 1992 {published data only}
- Rose G, Colwell L. Randomised controlled trial of anti‐smoking advice: final (20 year) results. Journal of Epidemiology and Community Health 1992;46(1):75‐7. [PUBMED: 1573365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sachs 2009 {published data only}
- Sachs DPL, Hodgkin JE, Bostrom AG. COPD & increasing nicotine−dependence severity (1989−2006): need to change the paradigm for tobacco−dependence treatment in COPD. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009.
Sajol 2015 {published data only}
- Sajol G, Glass M, Burnham CN, Castro M. Smoking cessation in patients with established airway disease: Initial characterization of cellular and biomarker sputum. American Thoracic Society International Conference; 2015 May 15‐20; Denver. 2015.
Sakharova 2006 {published data only}
- Sakharova GM, Antonov NS. The combination of NRT + COPD therapy is more effective for smoking cessation. Abstract. 2006.
Shaban 2005 {published data only}
- Shaban M, Nejati S, Mehran A, Saidi J. The effects of counseling on smoking cessation in patients suffering from COPD. Hayat 2005;11:73‐81. [Google Scholar]
Shaker 2011 {published data only}
- Shaker SB, Stavngaard T, Laursen LC, Stoel BC, Dirksen A. Rapid fall in lung density following smoking cessation in COPD. COPD 2011;8(1):2‐7. [PUBMED: 21299472] [DOI] [PubMed] [Google Scholar]
Signes‐Costa 2004 {published data only}
- Signes‐Costa J, Llombart M, Chiner E, Romero C, Arriero JM, Gomez‐Merino E, et al. Efficacy of different smoking cessation treatments in COPD patients. American Thoracic Society 100th International conference; 2004 May 21‐26; Orlando. 2004.
Smith 2013 {published data only}
- Smith BJ, Carson KV, Brinn MP, Labiszewski NA, Peters MJ, Fitridge R, et al. Smoking termination opportunity for inpatients (STOP): superiority of a course of varenicline tartrate plus counselling over counselling alone for smoking cessation: a 12‐month randomised controlled trial for inpatients. Thorax 2013;68(5):485‐6. [PUBMED: 22993168] [DOI] [PubMed] [Google Scholar]
Steurer‐Stey 2014 {published data only}
- Steurer‐Stey C, Markun S, Lana KD, Frei A, Held U, Wensing M, et al. The improving care in chronic obstructive lung disease study: CAROL improving processes of care and quality of life of COPD patients in primary care: study protocol for a randomized controlled trial. Trials 2014;15:96. [PUBMED: 24670200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratelis 2006 {published data only}
- Stratelis G, Molstad S, Jakobsson P, Zetterstrom O. The impact of repeated spirometry and smoking cessation advice on smokers with mild COPD. Scandinavian Journal of Primary Health Care 2006;24(3):133‐9. [PUBMED: 16923621] [DOI] [PubMed] [Google Scholar]
Suhaj 2016 {published data only}
- Suhaj A, Manu MK, Unnikrishnan MK, Vijayanarayana K, Mallikarjuna Rao C. Effectiveness of clinical pharmacist intervention on health‐related quality of life in chronic obstructive pulmonary disorder patients ‐ a randomized controlled study. Journal of Clinical Pharmacy and Therapeutics 2016;41(1):78‐83. [PUBMED: 26775599] [DOI] [PubMed] [Google Scholar]
Tonnesen 1988 {published data only}
- Tonnesen P, Fryd V, Hansen M, Helsted J, Gunnersen AB, Forchammer H, et al. Two and four mg nicotine chewing gum and group counselling in smoking cessation: an open, randomized, controlled trial with a 22 month follow‐up. Addictive Behaviors 1988;13(1):17‐27. [PUBMED: 3284283] [DOI] [PubMed] [Google Scholar]
Voncken‐Brewster 2013 {published data only}
- Voncken‐Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomised controlled trial testing a web‐based, computer‐tailored self‐management intervention for people with or at risk for chronic obstructive pulmonary disease: a study protocol. BMC Public Health 2013;13:557. [PUBMED: 23742208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voncken‐Brewster 2015 {published data only}
- Voncken‐Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomized controlled trial evaluating the effectiveness of a web‐based, computer‐tailored self‐management intervention for people with or at risk for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1061‐73. [PUBMED: 26089656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walters 2012 {published data only}
- Walters JA, Cameron‐Tucker H, Courtney‐Pratt H, Nelson M, Robinson A, Scott J, et al. Supporting health behaviour change in chronic obstructive pulmonary disease with telephone health‐mentoring: insights from a qualitative study. BMC Family Practice 2012;13:55. [PUBMED: 22694996] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weling‐Scheepers 2005 {published data only}
- Weling‐Scheepers CA, Creutzberg EC, Heber EJ, Wouters EF. Smoking cessation: a 'heavy' decision during pulmonary rehabilitation. European Respiratory Society 15th Annual Congress; 2005 Sep 17‐20; Copenhagen. 2005.
Yuan 2015 {published data only}
- Yuan X, Tao Y, Zhao JP, Liu XS, Xiong WN, Xie JG, et al. Long‐term efficacy of a rural community‐based integrated intervention for prevention and management of chronic obstructive pulmonary disease: a cluster randomized controlled trial in China's rural areas. Brazilian Journal of Medical and Biological Research (Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica) 2015;48(11):1023‐31. [PUBMED: 26352697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2010 {published data only}
- Zhou Y, Hu G, Wang D, Wang S, Wang Y, Liu Z, et al. Community based integrated intervention for prevention and management of chronic obstructive pulmonary disease (COPD) in Guangdong, China: cluster randomised controlled trial. BMJ 2010;341:c6387. [PUBMED: 21123342] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Zhou W, Wei X, Ke H. Psychiatric adverse reactions in a prospective, randomized clinical trial of varenicline for smoking cessation in patients with COPD. 16th Congress of the Asian Pacific Society of Respirology; 2011 Nov 3‐6; Shanghai. 2011.
Zwar 2012 {published data only}
- Zwar NA, Hermiz O, Comino E, Middleton S, Vagholkar S, Xuan W, et al. Care of patients with a diagnosis of chronic obstructive pulmonary disease: a cluster randomised controlled trial. Medical Journal of Australia 2012;197(7):394‐8. [PUBMED: 23025736] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Efraimsson 2008b {published data only}
- Efraimsson EO, Hillervik C, Ehrenberg A. Effects of COPD self‐care management education at a nurse‐led primary health care clinic. Scandinavian Journal of Caring Sciences 2008;22(2):178‐85. [DOI] [PubMed] [Google Scholar]
Hagens 2017 {published data only}
- Hagens P, Pieterse M, Valk P, Palen J. Effectiveness of intensive smoking reduction counselling plus combination nicotine replacement therapy in promoting long‐term abstinence in patients with chronic obstructive pulmonary disease not ready to quit smoking: protocol of the REDUQ trial. Contemporary Clinical Trials Communications 2017;8:248‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanhan 2015 {published data only}
- Hanhan H, Wei W, Litiao C. Study on effect of 5A intervention method combined with varenicline for smoking cessation of COPD patients in stable stage. Chinese Nursing Research 2015;29(2):667‐70. [Google Scholar]
Harter 2015b {published data only}
- Harter M, Bartsch AL, Egger N, Konig HH, Kriston L, Schulz H, et al. Evaluating a collaborative smoking cessation intervention in primary care (ENTER): study protocol for a cluster‐randomized controlled trial. Trials 2015;16(1):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irizar‐Aramburu 2013b {published data only}
- Irizar‐Aramburu MI, Martinez‐Eizaguirre JM, Pacheco‐Bravo P, Diaz‐Atienza M, Aguirre‐Arratibel I, Pena‐Pena MI, et al. Effectiveness of spirometry as a motivational tool for smoking cessation: a clinical trial, the ESPIMOAT study. BMC Family Practice 2013;14(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2017 {published data only}
- Liang J, Abramson MJ, Zwar N, Russell G, Holland AE, Bonevski B, et al. Interdisciplinary model of care (RADICALS) for early detection and management of chronic obstructive pulmonary disease (COPD) in Australian primary care: study protocol for a cluster randomised controlled trial. BMJ Open 2017;7(9):e016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2018 {published data only}
- Liang J, Abramson M, Zwar N, Russell G, Holland A, Bonevski B, et al. Pharmacist‐led smoking cessation intervention in Australian primary care targeting smokers at risk of developing COPD. Respirology 2018;23:40. [Google Scholar]
Loth 2017 {published data only}
- Loth FG, Bickhardt J, Heindl T, Muhlig S. Model project for tobacco cessation for patients with COPD in Saxony and Thuringia: methodology and initial results. Atemwegs‐ und lungenkrankheiten 2017;43(6):276‐87. [Google Scholar]
Melzer 2017 {published data only}
- Melzer AC, Clothier BA, Japuntich SJ, Noorbaloochi S, Hammett P, Burgess DL, et al. Comparative Effectiveness of Proactive Tobacco Treatment among Smokers with and without Chronic Lower Respiratory Disease. Annals of the American Thoracic Society 2017;15(3):341‐7. [DOI] [PubMed] [Google Scholar]
Streck 2018 {published data only}
- Streck JM, Ochalek TA, Miller ME, Meyer AC, Badger G, Teneback C, et al. Promoting smoking abstinence among patients with chronic obstructive pulmonary disease: initial feasibility. Preventive Medicine Reports 2018;11:176‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tromeur 2018 {published data only}
- Tromeur C, Le MR, Couturand F. Effect of varenicline on smoking cessation in COPD patients recovering from exacerbation: a randomized trial. Fundamental and Clinical Pharmacology. Conference: Annual Meeting of French Society of Pharmacology and Therapeutics, and INSERM Clinical Research Centers, CIC Meeting 2018. France 2018;32 (Supplement 1):32. [Google Scholar]
van Eerd 2017 {published data only}
- Eerd EAM, Schayck OCP, Wesseling G, Kotz D. Predictors of long‐term smoking cessation in patients with COPD: results from a randomised controlled trial. European Respiratory Journal 2017;49(6):1700561. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang DX, Gu CJ, Ni L, Li N, Li QY, Zhou JP. Assessment of efficacy of medication combined with WeChat platform for quitting smoking in patients with chronic obstructive pulmonary disease. Journal of Shanghai Jiaotong University (Medical Science) 2016;36(3):385‐9. [Google Scholar]
References to ongoing studies
NCT01694732 {unpublished data only}
- NCT01694732. Efficacy of varenicline on smoking cessation at the acute phase of an exacerbation of chronic obstructive pulmonary disease (SAVE). clinicaltrials.gov/ct2/show/NCT01694732 (Accessed 22 July 2016).
NCT02148445 {unpublished data only}
- NCT02148445. Smoking Cessation Versus Long‐term Nicotine Replacement Among High‐risk Smokers. clinicaltrials.gov/ct2/show/NCT02148445 (Accessed 22 July 2016).
Additional references
Anthonisen 2002
- Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of lung health study participants after 11 years. American Journal of Respiratory and Critical Care Medicine 2002;166(5):675‐9. [DOI] [PubMed] [Google Scholar]
Borrelli 2010
- Borrelli B, Hayes RB, Dunsiger S, Fava JL. Risk perception and smoking behavior in medically ill smokers: a prospective study. Addiction 2010;105(6):1100‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buist 2007
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD009329.pub2; PUBMED: 23728690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Celli 2004
- Celli BR, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23(6):932‐46. [DOI] [PubMed] [Google Scholar]
Coronini‐Cronberg 2011
- Coronini‐Cronberg S, Heffernan C, Robinson M. Effective smoking cessation interventions for COPD patients: a review of the evidence. JRSM short reports 2011;2(10):78. [PUBMED: 22046497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2008
- US Department of Health and Human Services. Public Health Service. Clinical practice guideline. Treating tobacco use and dependence: 2008 update. www.ncbi.nlm.nih.gov/books/NBK63952/ (accessed 17 November 2015).
Geijer 2005
- Geijer RM, Sachs AP, Hoes AW, Salome PL, Lammers JW, Verheij TJ. Prevalence of undetected persistent airflow obstruction in male smokers 40‐65 years old. Family Practice 2005;22(5):485‐9. [DOI] [PubMed] [Google Scholar]
GOLD 2015
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2015. www.goldcopd.org/guidelines‐global‐strategy‐for‐diagnosis‐management.html (accessed 17 November 2015).
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 30 June 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2013
Hatsukami 1999
- Hatsukami DK, Mooney ME. Pharmacological and behavioral strategies for smoking cessation. Journal of Clinical Psychology in Medical Settings 1999;6:11‐38. [Google Scholar]
Heatherton 1991
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction 1991;86(9):1119‐27. [PUBMED: 1932883] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Hughes 2003
- Hughes JR, Keely JP, Niaura RS, Ossip‐Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine and Tobacco Research 2003;5(1):13‐25. [PubMed] [Google Scholar]
Hughes 2012
- Hughes JR. Use of nicotine replacement after a smoking lapse. Nicotine and Tobacco Research 2012;14(6):751‐4. [DOI] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4; PUBMED: 24402784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jimenez‐Ruiz 2001
- Jimenez‐Ruiz CA, Masa F, Miravitlles M, Gabriel R, Viejo JL, Villasante C, et al. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest 2001;119(5):1365‐70. [DOI] [PubMed] [Google Scholar]
Jimenez‐Ruiz 2015
- Jimenez‐Ruiz CA, Andreas S, Lewis KE, Tonnesen P, Schayck CP, Hajek P, et al. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. European Respiratory Journal 2015;46(1):61‐79. [PUBMED: 25882805] [DOI] [PubMed] [Google Scholar]
Kerstjens 1997
- Kerstjens HA, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status: facts, figures, and fallacies. Thorax 1997;52(9):820‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohansal 2009
- Kohansal R, Martinez‐Camblor P, Agusti A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. American Journal of Respiratory and Critical Care Medicine 2009;180(1):3‐10. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [DOI] [PubMed] [Google Scholar]
Mannino 2007
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. The Lancet 2007;370(9589):765‐73. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. NICE guidelines: Stop smoking service. NICE guidelines [PH10]. www.nice.org.uk/guidance/ph10/chapter/introduction (accessed 17 November 2015).
NICE 2010
- NICE. Chronic obstructive pulmonary disease in over 16s: diagnosis and management (CG101). www.nice.org.uk/CG101 (accessed 30 June 2016).
Pires‐Yfantouda 2013
- Pires‐Yfantouda R, Absalom G, Clemens F. Smoking cessation interventions for COPD: a review of the literature. Respiratory Care 2013;58(11):1955‐62. [PUBMED: 23431304] [DOI] [PubMed] [Google Scholar]
Qaseem 2011
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine 2011;155(3):179‐91. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen. Review Manager (RevMan). Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014.
Shahab 2006
- Shahab L, Jarvis MJ, Britton J, West R. Prevalence, diagnosis and relation to tobacco dependence of chronic obstructive pulmonary disease in a nationally representative population sample. Thorax 2006;61(12):1043‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2015
- Stead LF, Koilpillai P, Lancaster T. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009670.pub3; PUBMED: 26457723] [DOI] [PubMed] [Google Scholar]
Strassmann 2009
- Strassmann R, Bausch B, Spaar A, Kleijnen J, Braendli O, Puhan MA. Smoking cessation interventions in COPD: a network meta‐analysis of randomised trials. European Respiratory Journal 2009;34(3):634‐40. [DOI] [PubMed] [Google Scholar]
Tashkin 2009
- Tashkin DP, Murray RP. Smoking cessation in chronic obstructive pulmonary disease. Respiratory Medicine 2009;103(7):963‐74. [PUBMED: 19285850] [DOI] [PubMed] [Google Scholar]
Thabane 2012
- Thabane M. Smoking cessation for patients with chronic obstructive pulmonary disease (COPD): an evidence‐based analysis. Ontario Health Technology Assessment Series 2012;12(4):1‐50. [PUBMED: 23074432] [PMC free article] [PubMed] [Google Scholar]
Tobacco Use and Dependence Guideline Panel 2008
- Tobacco Use, Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update. www.ncbi.nlm.nih.gov/books/NBK63950/ (accessed 5 December 2012).
Tonnesen 2007
- Tonnesen P, Carrozzi L, Fagerstrom KO, Gratziou C, Jimenez‐Ruiz C, Nardini S, et al. Smoking cessation in patients with respiratory diseases: a high priority, integral component of therapy. European Respiratory Journal 2007;29(2):390‐417. [DOI] [PubMed] [Google Scholar]
Tzanakis 2004
- Tzanakis N, Anagnostopoulou U, Filaditaki V, Christaki P, Siafakas N. Prevalence of COPD in Greece. Chest 2004;125(3):892‐900. [DOI] [PubMed] [Google Scholar]
van Eerd 2013
- Eerd EAM, Meer RM, Reda AA, Schayck CP, Kotz D. Smoking cessation in smokers with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD010744] [DOI] [Google Scholar]
van Eerd 2015
- Eerd EA, Rossem CR, Spigt MG, Wesseling G, Schayck OC, Kotz D. Do we need tailored smoking cessation interventions for smokers with COPD? A comparative study of smokers with and without COPD regarding factors associated with tobacco‐smoking. Respiration 2015 (published online); Vol. 90, issue 3:211‐9. [DOI] [PubMed]
Velicer 1992
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin 1992;111(1):23‐41. [DOI] [PubMed] [Google Scholar]
Warnier 2013
- Warnier MJ, Riet EE, Rutten FH, Bruin ML, Sachs AP. Smoking cessation strategies in patients with COPD. European Respiratory Journal 2013;41(3):727‐34. [PUBMED: 22936706] [DOI] [PubMed] [Google Scholar]
West 2000
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax 2000;55(12):987‐99. [PUBMED: 11083883] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2005
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299‐303. [PUBMED: 15733243] [DOI] [PubMed] [Google Scholar]
Willemse 2004
- Willemse BW, Postma DS, Timens W, Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. European Respiratory Journal 2004;23(3):464‐76. [DOI] [PubMed] [Google Scholar]
Wilson 2011
- Wilson JS, Elborn JS, Fitzsimons D, McCrum‐Gardner E. Do smokers with chronic obstructive pulmonary disease report their smoking status reliably? A comparison of self‐report and bio‐chemical validation. International Journal of Nursing Studies 2011;48(7):856‐62. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van der Meer 2003
- Meer RM, Wagena EJ, Ostelo RW, Jacobs JE, Schayck CP. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2003, Issue 8. [DOI: 10.1002/14651858.CD002999; PUBMED: 12804448] [DOI] [PMC free article] [PubMed] [Google Scholar]


