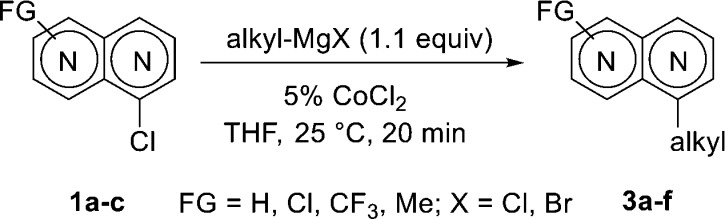Abstract
CoCl2 (5%) catalyzes cross-couplings of various halogenated naphthyridines with alkyl- and arylmagnesium halides. Also, arylzinc halides undergo smooth cross-couplings with various naphthyridines in the presence of CoCl2·2LiCl (5%) and sodium formate (50%), leading to polyfunctional arylated naphthyridines. Two of these arylated naphthyridines are highly fluorescent, with quantum efficiencies reaching 95% and long excited-state lifetimes of up to 12 ns.
N-Heterocyclic scaffolds are ubiquitous building blocks for pharmaceuticals, agrochemicals, and materials science.1 There is a need for new N-heterocyclic structures since novel ring systems may display original biological or physical properties. Recently, the naphthyridine scaffold has attracted increased attention.2 Its functionalization was found to be especially difficult, and only a few methods are available.3 Although Fe-catalyzed cross-couplings have been used to functionalize chloronaphthyridines, the scope of such cross-couplings is quite limited.4 Interestingly, Co-catalyzed cross-couplings generally display a broader reaction scope and have proved to be very useful for the functionalization of electron-deficient N-heterocycles.5 We recently showed that the addition of appropriate ligands (e.g., sodium formate or pivalate) considerably extends the scope of these cross-couplings.6 Herein we report that Co-catalyzed cross-couplings allow efficient functionalization of various halogenated naphthyridines.
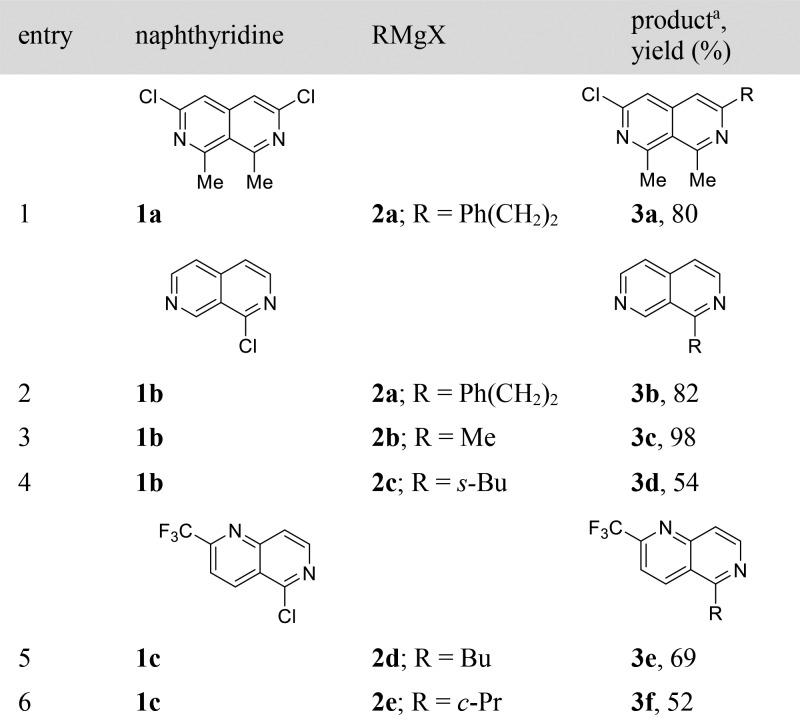
In preliminary experiments, we found that chloronaphthyridines 1a–c are easily alkylated using 5% CoCl2 in THF (Table 1). Thus, the reaction of 3,6-dichloro-1,8-dimethyl-2,7-naphthyridine (1a) with 2-phenylethylmagnesium bromide (2a) at 25 °C (30 min) provided monoalkylated naphthyridine 3a in 80% yield (Table 1, entry 1). Similarly, alkylmagnesium reagent 2a allowed the conversion of 1-chloro-2,7-naphthyridine (1b) to the expected 1-phenethyl-2,7-naphthyridine (3b) in 82% yield (entry 2). Treatment of 1b with MeMgCl (2b) gave the corresponding 1-methyl-2,7-naphthyridine (3c) in 98% yield (entry 3). Furthermore, sec-BuMgCl (2c) underwent cross-coupling with 1b to afford 2,7-naphthyridine 3d in 54% yield (entry 4). Co-catalyzed alkylation of 5-chloro-1,6-naphthyridine 1c with BuMgCl (2d) provided 1,6-naphthyridine 3e in 69% yield (entry 5). Additionally, the reaction of 5-chloro-1,6-naphthyridine 1c with cyclopropylmagnesium bromide (2e) gave 1,6-naphthyridine 3f in 52% yield (entry 6).
Table 1. Co-Catalyzed Alkylation of Chloronaphthyridines 1a–c with Alkylmagnesium Reagents 2a–e.
Isolated yields of analytically pure products.
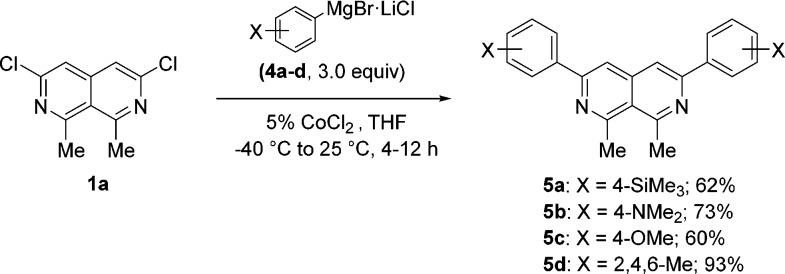
Furthermore, we have found that 3,6-dichloronaphthyridine 1a was easily bisarylated in the presence of 5% CoCl2 using arylmagnesium reagents7 such as 4a–d (Scheme 1). Thus, the reaction of 1a with 4-trimethylsilylphenylmagnesium bromide (4a) (3.0 equiv) at −40 °C provided bisarylated 2,7-naphthyridine 5a in 62% yield within 4 h. Similarly, 4-N,N-dimethylaminophenylmagnesium bromide (4b) and 4-anisylmagnesium bromide (4c) underwent Co-catalyzed cross-couplings (−40 °C, 4–12 h) with 1a, leading to 3,6-substituted naphthyridines 5b and 5c, respectively, in 60–73% yield. Sterically hindered mesitylmagnesium bromide (4d), however, reacted with 1a at 25 °C within 4 h, furnishing 2,7-naphthyridine 5d in 93% yield.
Scheme 1. Co-Catalyzed Bisarylation of Naphthyridine 1a with Various Arylmagnesium Bromides (4a–d).
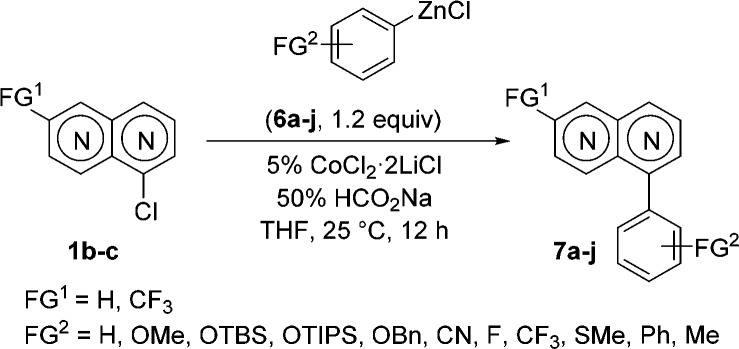
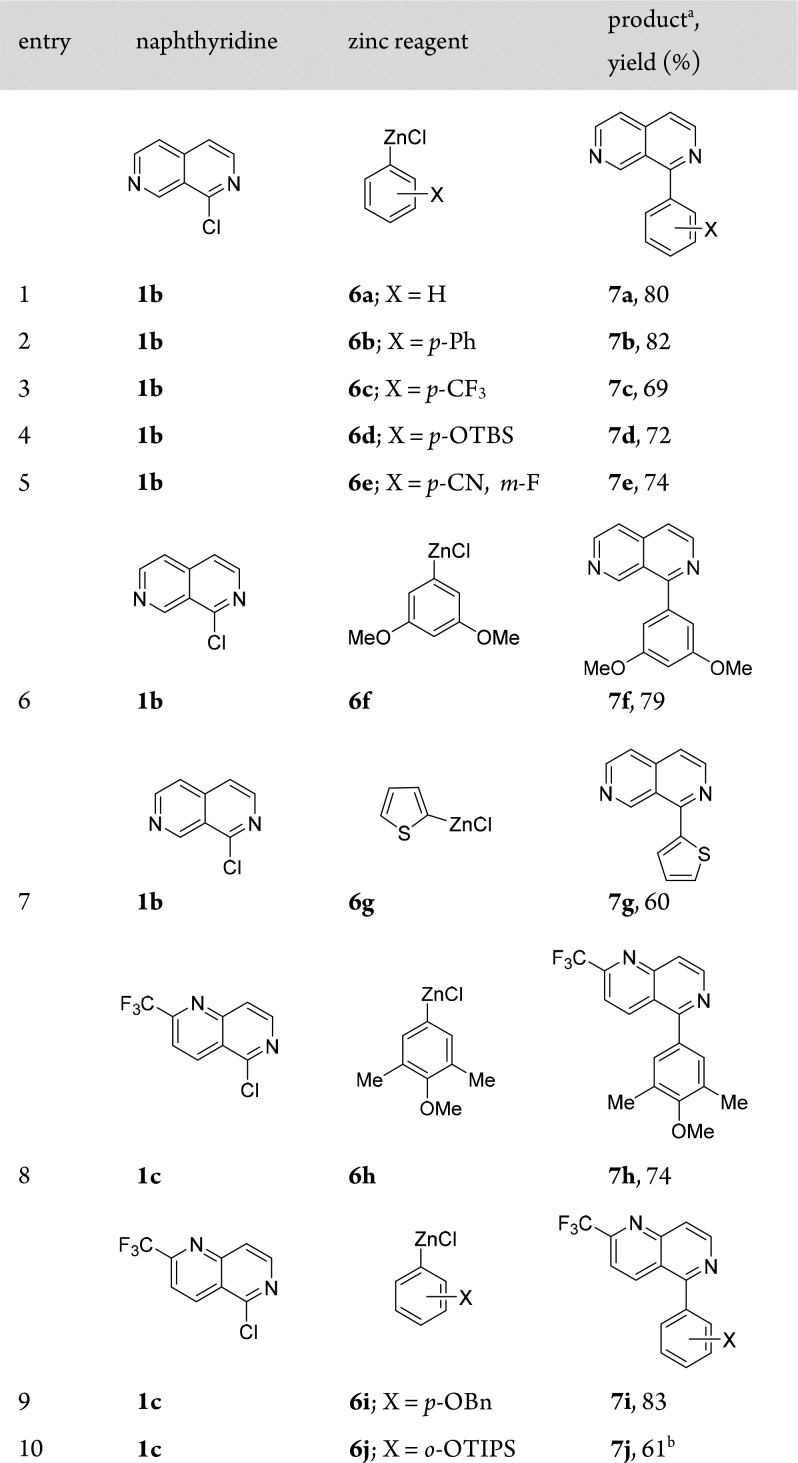
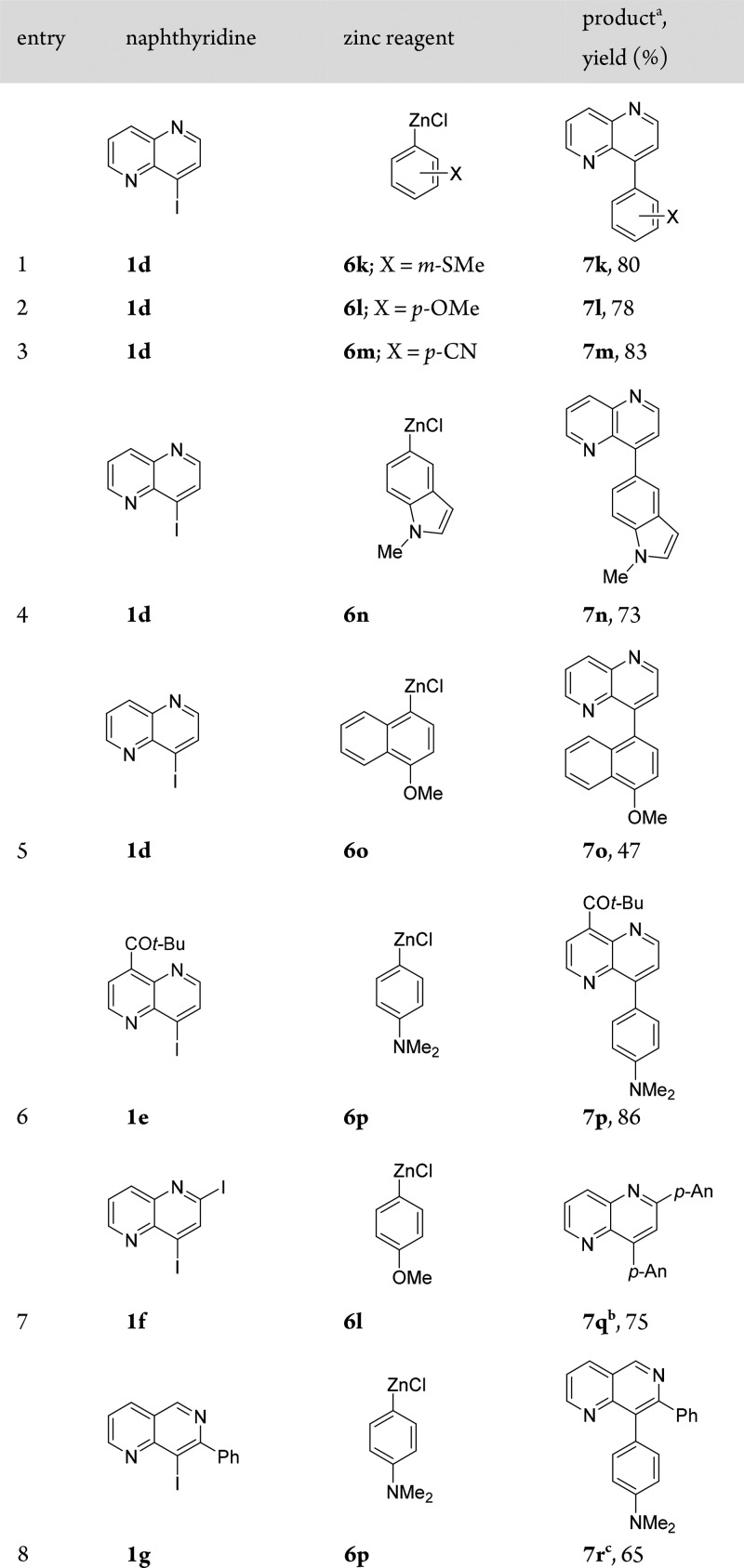
We noticed that C(sp2)–C(sp2) cross-couplings of naphthyridines 1b and 1c with PhMgCl using 5% CoCl2 led to low yields (<30%). This problem could be solved by replacing arylmagnesium halides with the corresponding arylzinc reagents and using HCO2Na as a ligand.6a Thus, 1b reacted smoothly with PhZnCl (6a) or [1,1′-biphenyl]-4-ylzinc chloride (6b) within 12 h at 25 °C, furnishing the corresponding arylated naphthyridines 7a and 7b in 80–82% yield (Table 2, entries 1 and 2). Furthermore, a range of arylzinc reagents 6c–f bearing various functional groups underwent such Co-catalyzed Negishi8 cross-couplings with 1b, providing the expected products 7c–f in 69–79% yield (entries 3–6). Heteroaryl–heteroaryl cross-couplings are utmost challenging because of catalyst deactivation when Pd or Ni catalysts are used.9 However, in the presence of THF-soluble CoCl2·2LiCl (5%) and HCO2Na (50%), the cross-coupling of 1-chloronaphthyridine 1b with 2-thienylzinc chloride (6g) afforded 2,7-naphthyridine 7g in 60% yield (entry 7). We further have shown that naphthyridine 1c was easily arylated under these conditions. Thus, the reaction of 1c with arylzinc chlorides 6h and 6i provided 1,6-naphthyridines 7h and 7i, respectively, in 74–83% yield (entries 8 and 9). Interestingly, the Co-catalyzed arylation of 1c with 2-(triisopropylsilyloxy)phenylzinc chloride (6j) succeeded at only elevated temperature (60 °C, 12 h) to give naphthyridyl alcohol derivative 7j in 61% yield (entry 10). Also, iodo-substituted naphthyridines were excellent substrates for such Co-catalyzed cross-couplings. Thus, the reaction of electron-rich arylzinc reagents 6k and 6l with 4-iodo-1,5-naphthyridine (1d) afforded 1,5-naphthyridines 7k and 7l, respectively, in 78–80% yield (Table 3, entries 1 and 2). Similarly, the reaction of electron-deficient p-NCC6H4ZnCl (6m) with 1d led to naphthyridine 7m in 83% yield (entry 3). Furthermore, heteroarylzinc reagent 6n reacted smoothly with 1d to afford naphthyridine 7n in 73% yield (entry 4). Remarkably, sterically demanding naphthylzinc reagent 6o was also converted with 1d to 1,5-naphthyridine 7o in 47% yield (entry 5).
Table 2. Co-Catalyzed Arylations of Arylzinc Reagents 6a–j with Chloronaphthyridines 1b and 1c.
Isolated yields of analytically pure products.
The cross-coupling reaction proceeded at 60 °C for 12 h.
Table 3. Co-Catalyzed Arylations of Arylzinc Reagents 6k and 6l with Iodonaphthyridines 1d–g.
Isolated yields of analytically pure products.
2.4 equiv of zinc reagent was used. p-An = p-MeOC6H4.
3.0 equiv of the arylzinc reagent was necessary for complete conversion.
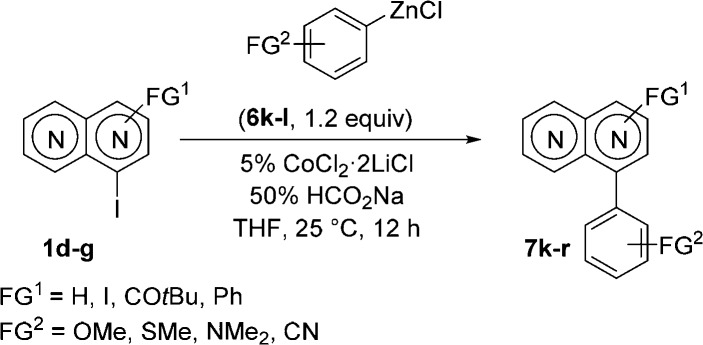
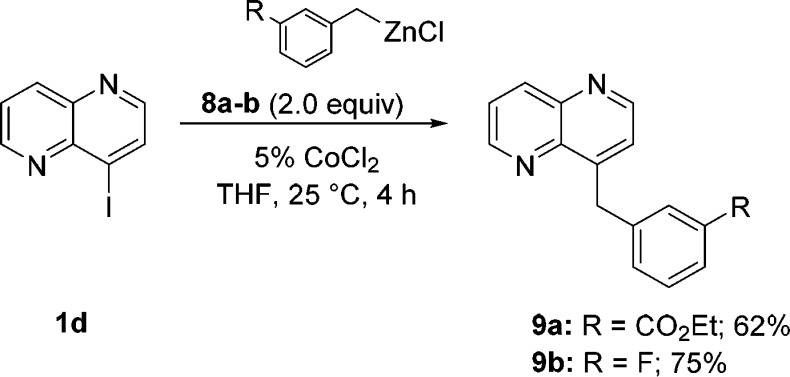
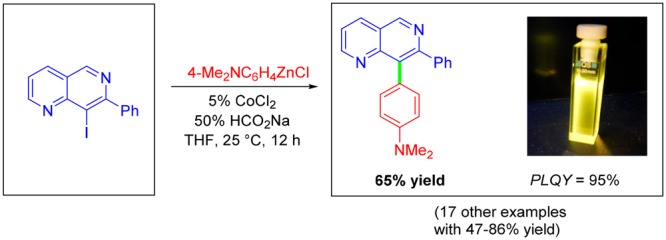
This mild method also allows the coupling of sensitive iodonaphthyridines. Thus, the coupling of zinc reagent 6p with 4-iodo-1,5-naphthyridine 1e provided the corresponding 4,8-functionalized 1,5-naphthyridine 7p in 86% yield (entry 6). Similarly to the bisarylation of dichloronaphthyridine 1a (Scheme 1), we also examined the Co-catalyzed reaction of 2,4-diiodo-1,5-naphthyridine (1f) with p-MeOC6H4ZnCl (6l) and obtained the corresponding bis(anisyl)naphthyridine 7q in 75% yield (entry 7). Finally, Co-catalyzed cross-coupling of sterically hindered 8-iodo-1,6-naphthyridine (1g)10 with p-Me2NC6H4ZnCl (6p) furnished naphthyridine 7r in 65% yield (entry 8). We also found that 1d smoothly reacts with benzylic zinc reagents.11 Thus, the couplings of 1d with benzylzinc chlorides 8a and 8b provided 1,5-naphthyridines 9a and 9b in 62–75% yield (Scheme 2).
Scheme 2. Co-Catalyzed Cross-Coupling of 4-Iodo-1,5-naphthyridine (1d) with Benzylic Zinc Reagents 8a and 8b.
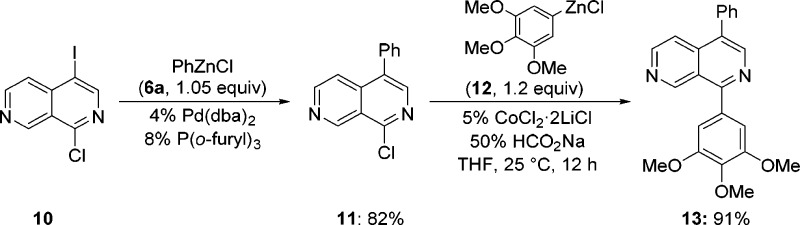
By using mixed halogenated naphthyridines, we observed that 1-chloro-4-iodo-2,7-naphthyridine (10) was regioselectively functionalized by stepwise cross-coupling utilizing successive Pd and Co catalyses (Scheme 3). Thus, Pd-catalyzed Negishi cross-coupling of 10 with PhZnCl (6a) selectively furnished 2,7-naphthyridine 11 in 82% yield. Subsequent Co-catalyzed cross-coupling with arylzinc chloride 12 gave the mixed bisarylated naphthyridine 13 in 91% yield.
Scheme 3. Regioselective Pd/Co-Catalyzed Cross-Coupling.
Naphthyridine derivatives have previously been applied as fluorescent probes12 or as ligands for fluorescent complexes.13 The newly prepared naphthyridines 5b and 7r are highly fluorescent in various organic solvents and display strong solvatochromism (Figure 1a,b and Supporting Information (SI) Figures 1–4). We suggest that these phenomena are based on strong interactions between the electron donor NMe2 and the electron-poor naphthyridine moiety as an electron acceptor. While the absolute photoluminescence quantum efficiencies (PLQEs) of 5b in various solvents are about 20%, the PLQE of 7r is almost quantitative in nonpolar solvents (toluene, 95 ± 5%; cyclohexane, 93 ± 5%) and drops only slightly in more polar solvents (CHCl3, 81 ± 5%; 1,4-dioxane, 80 ± 5%; THF, 71 ± 5%; see SI Figures 5 and 6 for details). These high emission efficiencies are accompanied by very long excited lifetimes of 3.8 and 12.0 ns for 5b and 7r, respectively (Figure 1c,d; see the SI for details).
Figure 1.
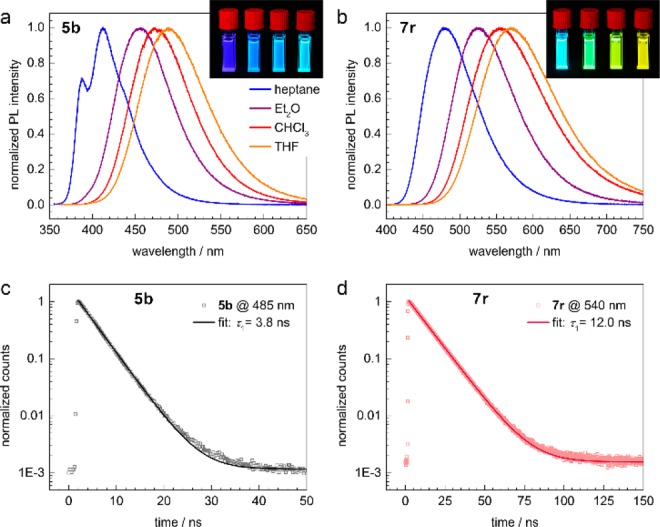
(a, b) PL spectra of compounds 5b and 7r, respectively, dissolved in heptane (blue), Et2O (purple), CHCl3 (red), and THF (orange). The excitation wavelengths were 360 and 390 nm, respectively. The insets show photographs of the solutions under UV illumination. (c, d) Time-correlated single-photon counting (TCSPC) traces of 5b and 7r in CHCl3, measured at the peaks of the PL spectra (open symbols). The solid lines are monoexponential fits.
In summary, we have prepared various novel polyfunctionalized naphthyridines by Co-catalyzed cross-couplings. Alkyl- and arylmagnesium reagents reacted smoothly with chloro-2,7-naphthyridines using CoCl2 (5%). The addition of sodium formate allowed an extension of the range of functionalized N-heterocycles by mild cross-coupling of chloro- and iodonaphthyridines with arylzinc reagents. Two of the new naphthyridines are highly fluorescent (PLQE = 20–95%) with tunable emission from blue to yellow and long excited-state lifetimes from 3.8 to 12.0 ns. Further extensions of organometallic naphthyridine functionalizations are currently underway in our laboratories.
Acknowledgments
We thank the DFG for financial support and Albemarle (Frankfurt) and BASF (Ludwigshafen) for gifts of chemicals.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.7b03242.
Experimental details, GC data, melting points, and IR, 1H and 13C NMR, and mass spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Snieckus V. Chem. Rev. 1990, 90, 879. 10.1021/cr00104a001. [DOI] [Google Scholar]; b Chinchilla R.; Nájera C.; Yus M. Chem. Rev. 2004, 104, 2667. 10.1021/cr020101a. [DOI] [PubMed] [Google Scholar]; c Modern Heterocyclic Chemistry; Alvarez-Builla J., Vaquero J. J., Barluenga J., Eds.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]; d Vo C.-V. T.; Bode J. W. J. Org. Chem. 2014, 79, 2809. 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]
- a Zhang J.; Chen F.; He Y.-M.; Fan Q.-H. Angew. Chem., Int. Ed. 2015, 54, 4622. 10.1002/anie.201411105. [DOI] [PubMed] [Google Scholar]; b Chen F.; Surkus A. E.; He L.; Pohl M.-M.; Radnik J.; Topf C.; Junge K.; Beller M. J. Am. Chem. Soc. 2015, 137, 11718. 10.1021/jacs.5b06496. [DOI] [PubMed] [Google Scholar]; c Shen Z.-L.; Dhayalan V.; Benischke A. D.; Greiner R.; Karaghiosoff K.; Mayer P.; Knochel P. Angew. Chem., Int. Ed. 2016, 55, 5332. 10.1002/anie.201600961. [DOI] [PubMed] [Google Scholar]; d Fernandez S.; Ganiek M. A.; Karpacheva M.; Hanusch F. C.; Reuter S.; Bein T.; Auras F.; Knochel P. Org. Lett. 2016, 18, 3158. 10.1021/acs.orglett.6b01373. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ma W.; Chen F.; Liu Y.; He M.-Y.; Fan Q.-H. Org. Lett. 2016, 18, 2730. 10.1021/acs.orglett.6b01186. [DOI] [PubMed] [Google Scholar]; f Jackl M. K.; Kreituss I.; Bode J. W. Org. Lett. 2016, 18, 1713. 10.1021/acs.orglett.6b00523. [DOI] [PubMed] [Google Scholar]; g Xiong B.; Zhang S.; Jiang H.; Zhang M. Org. Lett. 2016, 18, 724. 10.1021/acs.orglett.5b03699. [DOI] [PubMed] [Google Scholar]; h Zhang S.-L.; Deng Z.-Q. Org. Biomol. Chem. 2016, 14, 8966. 10.1039/C6OB01452F. [DOI] [PubMed] [Google Scholar]
- a Voight E. A.; Yin H.; Downing S. V.; Calad S. A.; Matsuhashi H.; Giordano I.; Goodman R. M.; Hennessy A. J.; Wood J. L. Org. Lett. 2010, 12, 3422. 10.1021/ol101235f. [DOI] [PubMed] [Google Scholar]; b Plodek A.; König M.; Bracher F. Eur. J. Org. Chem. 2015, 1302. 10.1002/ejoc.201403502. [DOI] [Google Scholar]; c Balkenhohl M.; Greiner R.; Makarov I. S.; Heinz B.; Karaghiosoff K.; Zipse H.; Knochel P. Chem. - Eur. J. 2017, 23, 13046. 10.1002/chem.201703638. [DOI] [PubMed] [Google Scholar]; d Ziegler D. S.; Greiner R.; Lumpe H.; Kqiku L.; Karaghiosoff K.; Knochel P. Org. Lett. 2017, 19, 5760. 10.1021/acs.orglett.7b02690. [DOI] [PubMed] [Google Scholar]
- Greiner R.; Blanc R.; Petermayer C.; Karaghiosoff K.; Knochel P. Synlett 2016, 27, 231. 10.1055/s-0035-1560703. [DOI] [Google Scholar]
- For recent Co-catalyzed cross-coupling reactions, see:; a Ohmiya H.; Yorimitsu H.; Oshima K. J. Am. Chem. Soc. 2006, 128, 1886. 10.1021/ja057560o. [DOI] [PubMed] [Google Scholar]; b Ohmiya H.; Yorimitsu H.; Oshima K. Org. Lett. 2006, 8, 3093. 10.1021/ol0611144. [DOI] [PubMed] [Google Scholar]; c Bégouin J.-M.; Gosmini C. J. Org. Chem. 2009, 74, 3221. 10.1021/jo900240d. [DOI] [PubMed] [Google Scholar]; d Bégouin J.-M.; Rivard M.; Gosmini C. Chem. Commun. 2010, 46, 5972. 10.1039/c0cc01055c. [DOI] [PubMed] [Google Scholar]; e Nicolas L.; Angibaud P.; Stansfield I.; Bonnet P.; Meerpoel L.; Reymond S.; Cossy J. Angew. Chem., Int. Ed. 2012, 51, 11101. 10.1002/anie.201204786. [DOI] [PubMed] [Google Scholar]; f Hammann J. M.; Haas D.; Knochel P. Angew. Chem., Int. Ed. 2015, 54, 4478. 10.1002/anie.201411960. [DOI] [PubMed] [Google Scholar]; g Kuzmina O. M.; Steib A. K.; Fernandez S.; Boudot W.; Markiewicz J. T.; Knochel P. Chem. - Eur. J. 2015, 21, 8242. 10.1002/chem.201500747. [DOI] [PubMed] [Google Scholar]
- a Haas D.; Hammann J. M.; Lutter F. H.; Knochel P. Angew. Chem., Int. Ed. 2016, 55, 3809. 10.1002/anie.201510665. [DOI] [PubMed] [Google Scholar]; b Hofmayer M. S.; Hammann J. M.; Lutter F. H.; Knochel P. Synthesis 2017, 49, 3925. 10.1055/s-0036-1588986. [DOI] [Google Scholar]; c Hammann J. M.; Lutter F. H.; Haas D.; Knochel P. Angew. Chem., Int. Ed. 2017, 56, 1082. 10.1002/anie.201610324. [DOI] [PubMed] [Google Scholar]
- Piller F. M.; Appukkuttan P.; Gavryushin A.; Helm M.; Knochel P. Angew. Chem., Int. Ed. 2008, 47, 6802. 10.1002/anie.200801968. [DOI] [PubMed] [Google Scholar]
- For recent transition-metal-catalyzed Negishi cross-coupling reactions, see:; a Avedissian H.; Bérillon L.; Cahiez G.; Knochel P. Tetrahedron Lett. 1998, 39, 6163. 10.1016/S0040-4039(98)01267-2. [DOI] [Google Scholar]; b Hossain K. M.; Takagi K. Chem. Lett. 1999, 28, 1241. 10.1246/cl.1999.1241. [DOI] [Google Scholar]; c Zhou J.; Fu G. C. J. Am. Chem. Soc. 2003, 125, 12527. 10.1021/ja0363258. [DOI] [PubMed] [Google Scholar]; d Nakamura M.; Ito S.; Matsuo K.; Nakamura E. Synlett 2005, 1794. 10.1055/s-2005-871541. [DOI] [Google Scholar]; e Organ M. G.; Avola S.; Dubovyk I.; Hadei N.; Kantchev E. A. B.; O’Brien C. J.; Valente C. Chem. - Eur. J. 2006, 12, 4749. 10.1002/chem.200600206. [DOI] [PubMed] [Google Scholar]; f Takahashi H.; Inagaki S.; Yoshii N.; Gao F.; Nishihara Y.; Takagi K. J. Org. Chem. 2009, 74, 2794. 10.1021/jo900142b. [DOI] [PubMed] [Google Scholar]; g Bedford R. B.; Huwe M.; Wilkinson M. C. Chem. Commun. 2009, 600. 10.1039/B818961G. [DOI] [PubMed] [Google Scholar]; h Ejiri S.; Odo S.; Takahashi H.; Nishimura Y.; Gotoh K.; Nishihara Y.; Takagi K. Org. Lett. 2010, 12, 1692. 10.1021/ol100210u. [DOI] [PubMed] [Google Scholar]; i Smith A. L.; Hardcastle K. I.; Soper J. D. J. Am. Chem. Soc. 2010, 132, 14358. 10.1021/ja106212w. [DOI] [PubMed] [Google Scholar]; j Kealey S.; Passchier J.; Huiban M. Chem. Commun. 2013, 49, 11326. 10.1039/c3cc47203e. [DOI] [PubMed] [Google Scholar]; k Haas D.; Hammann J.; Greiner R.; Knochel P. ACS Catal. 2016, 6, 1540. 10.1021/acscatal.5b02718. [DOI] [Google Scholar]
- a Kaes C.; Katz A.; Hosseini M. W. Chem. Rev. 2000, 100, 3553. 10.1021/cr990376z. [DOI] [PubMed] [Google Scholar]; b Bedel S.; Ulrich G.; Picard C.; Tisnès P. Synthesis 2002, 1564. 10.1055/s-2002-33347. [DOI] [Google Scholar]; c Comprehensive Coordination Chemistry II, Vol. 1; McCleverty J. A., Meyer T. J., Eds.; Elsevier: Oxford, U.K., 2004. [Google Scholar]; d Düfert M. A.; Billingsley K. L.; Buchwald S. L. J. Am. Chem. Soc. 2013, 135, 12877. 10.1021/ja4064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.; Hunter J. A.; Larock R. C. Org. Lett. 2001, 3, 2973. 10.1021/ol010136h. [DOI] [PubMed] [Google Scholar]
- a Metzger A.; Schade M. A.; Knochel P. Org. Lett. 2008, 10, 1107. 10.1021/ol7030697. [DOI] [PubMed] [Google Scholar]; b Bedford R. B.; Huwe M.; Wilkinson M. C. Chem. Commun. 2009, 600. 10.1039/B818961G. [DOI] [PubMed] [Google Scholar]; c Bégouin J.-M.; Claudel S.; Gosmini C. Synlett 2009, 3192. 10.1055/s-0029-1218307. [DOI] [Google Scholar]; d Cai Y.; Benischke A. D.; Knochel P.; Gosmini C. Chem. - Eur. J. 2017, 23, 250. 10.1002/chem.201603832. [DOI] [PubMed] [Google Scholar]
- a Sun Y.-Y.; Liao J.-H.; Fang J.-M.; Chou P.-T.; Shen C.-H.; Hsu C.-W.; Chen L.-C. Org. Lett. 2006, 8, 3713. 10.1021/ol061293p. [DOI] [PubMed] [Google Scholar]; b Andrew T. L.; VanVeller B.; Swager T. M. Synlett 2010, 3045. 10.1055/s-0030-1259060. [DOI] [Google Scholar]; c Wang K.-Y.; Chen C.; Liu J.-F.; Wang Q.; Chang J.; Zhu H.-J.; Li C. Org. Biomol. Chem. 2012, 10, 6693. 10.1039/c2ob25926e. [DOI] [PubMed] [Google Scholar]; d Xiao L.; Xing X.; Chen Z.; Qu B.; Lan H.; Gong Q.; Kido J. Adv. Funct. Mater. 2013, 23, 1323. 10.1002/adfm.201202194. [DOI] [Google Scholar]
- a Liao J.-H.; Chen C.-T.; Chou H.-C.; Cheng C.-C.; Chou P.-T.; Fang J.-M.; Slanina Z.; Chow T. J. Org. Lett. 2002, 4, 3107. 10.1021/ol0264096. [DOI] [PubMed] [Google Scholar]; b Li H.-J.; Fu W.-F.; Li L.; Gan X.; Mu W.-H.; Chen W.-Q.; Duan X.-M.; Song H.-B. Org. Lett. 2010, 12, 2924. 10.1021/ol1003725. [DOI] [PubMed] [Google Scholar]; c Liu S.-W.; Lee C.-C.; Lin C.-F.; Huang J.-C.; Chen C.-T.; Lee J.-H. J. Mater. Chem. 2010, 20, 7800. 10.1039/c0jm01049a. [DOI] [Google Scholar]; d Wu Y.-Y.; Chen Y.; Gou G.-Z.; Mu W.-H.; Lv X.-J.; Du M.-L.; Fu W.-F. Org. Lett. 2012, 14, 5226. 10.1021/ol302347m. [DOI] [PubMed] [Google Scholar]; e Krämer C.; Leingang S.; Hübner O.; Kaifer E.; Wadepohl H.; Himmel H.-J. Dalton Trans. 2016, 45, 16966. 10.1039/C6DT03166H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.