Abstract
Behavioral and psychological symptoms of dementia (BPSD) include aggression, agitation, resistiveness to care, depression, anxiety, apathy, and hallucinations. BPSD are common in nursing home residents and can be ameliorated using person-centered approaches. Despite regulatory requirements, less than 2% of nursing homes consistently implement person-centered behavioral approaches. In a National Institute of Nursing Research-funded research protocol, we are implementing a pragmatic cluster randomized clinical trial designed to enable staff in nursing homes to reduce BPSD using behavioral approaches while optimizing function, preventing adverse events, and improving quality of life of residents. The implementation is based on use of the Evidence Integration Triangle (EIT), a parsimonious, community-engaged participatory framework that is well suited to the complexity and variability in the nursing home environment. A total of 50 nursing home communities will be randomized to EIT-4-BPSD or education only. Primary Aim 1 is to determine if communities exposed to EIT-4-BPSD demonstrate evidence of implementation evaluated by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) criteria. Primary Aim 2 is to evaluate the feasibility, utility, and cost of the EIT approach in EIT-4-BPSD communities.
Keywords: Alzheimer’s disease, behavioral and psychological symptoms, nursing home, pragmatic trial
1 |. INTRODUCTION
Behavioral and psychological symptoms of dementia (BPSD) include aggression, agitation, resistiveness to care, depression, anxiety, apathy, and hallucinations. BPSD are exhibited by up to 90% of nursing home residents living with dementia (Kales, Gitlin, Lyketsos, & Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia, 2014). BPSD result in negative health outcomes (Galynker, Roane, Miner, Feinberg, & Watts, 1995; Wunderlich & Kohler, 2000), decline in physical functioning (Galynker et al., 1995; Wunderlich & Kohler, 2000), and high cost of care (Herrmann et al., 2006). In addition, BPSD put residents at risk for inappropriate use of antipsychotic drugs and other restraining methods that reduce function (Kales et al., 2011), increase social isolation (Wunderlich & Kohler, 2000), and increase risk of physical abuse (Dyer, Pavlik, Murphy, & Hyman, 2000).
Behavioral approaches have been shown to have as much efficacy as antipsychotic medication and are endorsed as the first line of treatment for BPSD (Cooper, Mukadam, Katona, Blazer, & Livingston, 2013; Galik, Resnick, Hammersla, & Brightwater, 2014; Galik, Resnick, Lerner, Hammersla, & Gruber-Baldini, 2015; Grabowski et al., 2014; Kolanowski & Buettner, 2008; Kolanowski, Litaker, Buettner, Moeller, & Costa, 2011; Livingston et al., 2014; Richter, Meyer, Möhler, & Köpke, 2012; Van Haitsma et al., 2015). The Centers for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care and Reduce Antipsychotic Use in Nursing Homes requires that care for residents with dementia be delivered using person-centered behavioral approaches (Centers for Medicare and Medicaid Services, 2013). These approaches include, but are not limited to, the identification of resident preferences for everyday living and care, and inclusion of these preferences in the plan of care.
Despite regulatory requirements, less than 2% of nursing homes (also referred to here as communities) consistently implement person-centered behavioral approaches (Grabowski et al., 2014). Known barriers to use of behavioral approaches include limited staff knowledge, skills, and experience with non-pharmacological approaches, beliefs in the effectiveness of use of psychotropic medications over behavioral interventions to manage BPSD, lack of medical record systems that are able to integrate information seamlessly in useful and helpful ways, and lack of staff motivation to use non-pharmacologic strategies consistently (Kolanowski, Fick, Frazer, & Penrod, 2010; Kolanowski, Van Haitsma, & Penrod, 2015; Lemay et al., 2013; Marx et al., 2014). Developing and testing implementation strategies for addressing BPSD in nursing home residents has been identified as a research priority by international experts (Morley et al., 2014). An effective real-world implementation approach, however, is needed to engage communities that have been characterized as unstable and lacking in resources for managing BPSD (Buckwalter et al., 2009; Tabak, Khoong, Chambers, & Brownson, 2012).
In this paper we describe a National Institute of Nursing Research-funded research protocol for a Hybrid III pragmatic cluster randomized clinical trial (Curran, Bauer, Mittman, Pyne, & Stetler, 2012) designed to enable staff in nursing homes to reduce BPSD using behavioral approaches while optimizing function, preventing adverse events,and improving quality of life of residents. We selected this research design for two reasons: (a) successful implementation requires a community-wide and locally customized approach (thus the need for primary implementation outcomes at the community level and effectiveness outcomes at the resident level); and (b) to prevent treatment contamination that might occur if randomization were conducted within sites. The Pragmatic-Explanatory Continuum Indicator Summary (PRECIS) diagram in Figure 1 illustrates the degree of pragmatic versus controlled design in key features of the trial, as assessed by the research team.
FIGURE 1.
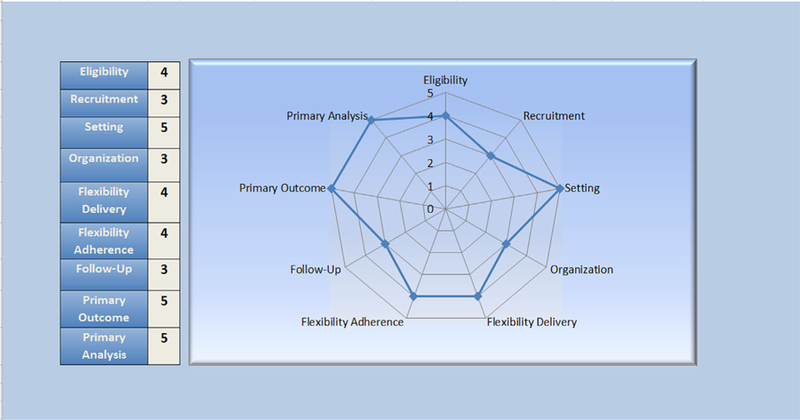
PRECIS wheel for pragmatic trials. Legend: 1. Very Explanatory; 2. Rather Explanatory; 3. Equally Pragmatic/Explanatory; 4. Rather Pragmatic; 5. Very Pragmatic; http://www.crispebooks.org/chapter-1-pragmatic-trials-18OF-1945R.html
2 |. BACKGROUND
To date, the major emphasis in research has been on the development of new knowledge. There has been limited focus on dissemination or implementation of findings into real world settings, which has resulted in a “voltage drop” (Glasgow, Kaplan, Ockene, Fisher, & Emmons, 2012; Glasgow et al., 2012c), or gap between the percentage of individuals who could benefit from evidence-based interventions and those who actually are exposed to these interventions (Glasgow, Eckstein, & Elzarrad, 2013; Helga et al., 2013; Lazenby, 2014). In particular, little has been done to optimally implement evidence-based interventions in nursing homes (Helga et al., 2013).
There are many organizational challenges and barriers to practice change, including staff members’ lack of belief in the utility and feasibility of the care approach; limited motivation and training of staff; insufficient support from administration; inadequate staffing levels; competing workload concerns; staff turnover; costs of the intervention; and lack of fit between the intervention and the philosophy of care (Beck et al., 2005; Finucane, Stevenson, Moyes, Oxenham, & Murray, 2013; Galik et al., 2008; Lekan-Rutledge, Palmer, & Belyea, 1998; Schnelle et al., 2002). Education of staff is not sufficient to change their behavior and improve clinical outcomes for residents (Beer et al., 2011; Cohen-Mansfield, 2001; Finucane et al., 2013; Kuske et al., 2007; McCabe, Davison, & George, 2007). Yet, staff education is the primary strategy used to decrease inappropriate use of psychotropics and increase use of behavioral approaches for BPSD (Rahman, Applebaum., Schnelle, & Simmons, 2012).
2.1 |. Implementation framework: The evidence integration triangle
The Evidence Integration Triangle (EIT) (Glasgow, Green, Taylor, & Stange, 2012) is a parsimonious, community-engaged participatory framework that is well suited to the complexity and variability of the nursing home environment. Our application of EIT brings together evidence-informed person-centered approaches for management of BPSD and community stakeholders. The three elements are a participatory implementation process with stakeholders, implementation of evidence-based approaches, and practical progress measures. Active engagement empowers stakeholders to identify their unique barriers to person-centered care and their goals for integrating evidence into practice (Kottke et al., 2008). Figure 2 illustrates the three-pronged Evidence Integration Triangle for management of behavioral and psychological symptoms of dementia (EIT-4-BPSD).
FIGURE 2.
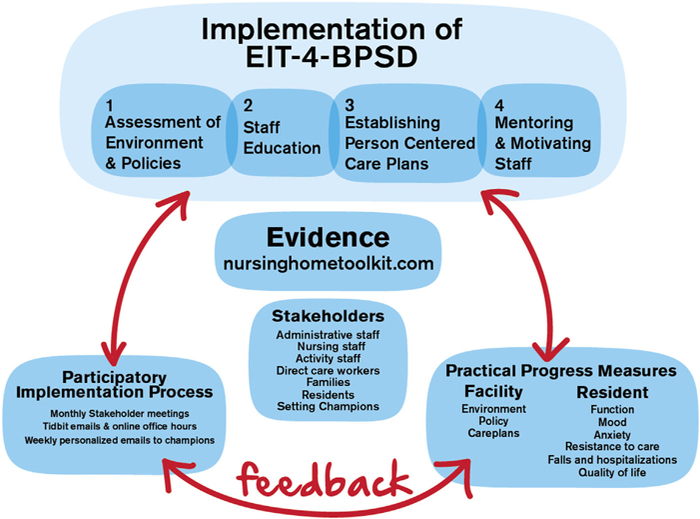
Evidence integration triangle applied to EIT-4-BPSD
Essential to the process are: 1. The Research Facilitator, an individual who has advanced healthcare education and experiential background in long-term care and behavioral interventions for BPSD; 2. An Internal Champion, a staff member selected by the community to work with the Research Facilitator and stakeholder group to bring about change in the way BPSD are managed; and 3. A stakeholder group, committed members of the community who assist with making needed system-wide changes for appropriately managing BPSD. Stakeholders usually include a nurse in a leadership position (e.g., director of nursing); a nurse practitioner or physician providing medication management of BPSD; a unit nurse; a nursing assistant; a family member; an activity staff; a social worker; and a resident.
Working together, these individuals enact the triad of components of EIT-4-BPSD, which include: (1) participatory implementation via a combination of in-person monthly meetings, weekly emails, and phone interactions between stakeholders and a Research Facilitator as they develop community goals and work towards achieving those goals; (2) implementation of the four steps shown in Figure 2 to assure that person-centered approaches to BPSD are sustainably integrated into routine care within the communities, with implementation led by the community-designated Internal Champion under the guidance of the Research Facilitator; and (3) practical progress measures, which are ongoing assessments of progress made toward implementation and goals, based on monitoring of community and resident data.
EIT allows for differences between communities and encourages tailoring of the implementation process, in contrast to an explanatory trial in which strict adherence to the intervention protocol is maintained. In pragmatic trials, a balance between treatment fidelity and implementation flexibility is critical because each community has different cultural, environmental and clinical challenges and must set its own specific goals for attaining the practice change. Participatory approaches such as EIT have been shown to increase the adoption of innovations by fostering a rapid learning environment and may speed translation of evidence into practice because of the relevance of community-identified goals (Kessler & Glasgow, 2011; Van De Ven & Johnson, 2006).
Although EIT has been shown to facilitate implementation of interventions in community-based primary care practices and in cancer outpatient communities (Glasgow et al., 2012c; Lazenby, 2014), to date it has not been extensively used or studied in nursing home settings (Resnick, Galik, Gruber-Baldini, & Zimmerman, 2013; Resnick, Galik, Vigne, & Payne, 2015),
2.2 |. Theoretical foundations of EIT-4-BPSD
In this protocol, approaches to behavior change among staff in nursing home settings via EIT-4-BPSD are based on concepts from the social ecological model (Gregson et al., 2003) and social cognitive theory (Bandura, 1986; Bandura, 1995; Bandura, 1997). In the social ecological model, behavior change is shaped by intrapersonal factors (e.g., age, years of experience, gender of staff); interpersonal factors (e.g., staff to staff, staff to family, and staff to resident interactions); environmental challenges (e.g., resources to help address BPSD); and policy issues (e.g., policies around inclusion of nursing assistants in resident care conferences). These concepts can be used to identify barriers and ways to overcome them and also can direct staff to change from a task-focused care approach to a person-centered approach to both prevent and manage BPSD.
In social cognitive theory, self-efficacy expectations, and outcome expectations shape behavior. Self-efficacy expectations are beliefs regarding whether one is able to initiate and sustain a course of action towards a desired goal. Outcome expectations are beliefs about what will occur if the desired behavior is performed. Social cognitive theory is used to strengthen staff self-efficacy and outcome expectations associated with implementing person-centered behavioral approaches for managing BPSD. The four sources of information to strengthen self-efficacy and outcome expectations include: 1) enactive mastery experience, such as engaging staff in successful performance of person-centered behavioral approaches; 2) verbal encouragement to provide person-centered behavioral approaches to BPSD; 3) vicarious experience, or sharing how others provide successful person-centered behavioral approaches; and 4) elimination of fear and frustration associated with implementing person-centered behavioral approaches. The Research Facilitator and Internal Champion play an active role in strengthening self-efficacy and outcome expectations of staff.
2.3 |. Specific aims
The purpose of this study is to test EIT-4-BPSD to determine if it is an effective implementation strategy to enable staff in nursing homes to reduce BPSD using behavioral approaches while optimizing function, preventing adverse events, reducing inappropriate use of psychotropic medications, and improving quality of life of residents. A total of 50 nursing communities will be randomized to EIT-4-BPSD or Education Only (EO).
Primary Aim 1 is to determine if communities exposed to EIT-4-BPSD demonstrate evidence of implementation at 12 months, when evaluated by the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) criteria (Virigina Tech, 2014). Specifically, we hypothesize that: (a) residents in EIT-4-BPSD communities will experience less BPSD, maintain or improve function, have reduced use of psychotropic medications, experience fewer adverse events, and have improved quality of life compared to residents in EO communities. Outcomes will be measured at baseline, 4 and 12 months post implementation of the intervention; and (b) EIT-4-BPSD communities will demonstrate improvements in Environment and Policy assessments that reflect support for person-centered behavioral approaches for BPSD, and will have a greater percentage of residents with person-centered behavioral approaches incorporated into their care plans at 12 months post-implementation when compared to EO communities. In addition, we will examine maintenance of EIT-4-BPSD community outcomes at 18 months and at 24 months post-implementation.
Primary Aim 2 is to evaluation the feasibility, utility, and cost of the EIT approach in EIT-4-BPSD communities. Throughout the study period, we will capture qualitative data on what occurs during the stakeholder meetings. We will hold a focus group with the stakeholder group and setting staff in each intervention site at the end of the 12 month intervention period. The focus group interview guide will address the experience of the stakeholders and staff regarding what helped them implement a philosophy of providing individualized behavioral interventions for BPSD while optimizing function and physical activity for all of their residents. We will determine the costs of implementation using an activity-based costing method.
3 |. METHODS
The study protocol received approval from the University of Maryland Institutional Review Board. A Data Safety and Monitoring Committee will convene yearly to monitor the scientific and ethical integrity of the study. This study was also registered in clinicaltrials.gov: identifier number NCT03014570.
3.1 |. Settings and sample
To reflect the real world of practice and increase external validity, community, and resident exclusion criteria are kept to a minimum. Nursing home communities in Pennsylvania and Maryland are eligible to participate in the testing of EIT-4-BPSD if they: (a) agree to actively partner with the research team to change practice; (b) have at least 50 (if all 50 are dedicated to memory care) to 100 beds; (c) identify an individual in the community who is recommended by administration and staff to serve as an Internal Champion for the practice change; and (d) are able to access email and websites via a phone, tablet, or computer.
Recruitment of communities is done by mailing invitations to eligible communities in Maryland and Pennsylvania, followed by telephone calls and site visits if requested by the community. We also post invitations on relevant websites, such as state-based long-term care organizations. When a nursing community expresses an interest in participating in the study, the community is randomized to EIT-4-BPSD or an EO control intervention based on a coin toss.
Within each study community, residents are eligible to participate if they: (a) are living in a participating nursing home; (b) are 55 years of age or older; (c) are English speaking; (c) within the past month have exhibited at least one BPSD as reported by nursing staff; (d) have cognitive impairment as determined by a score of 0–12 on the Brief Interview of Mental Status (BIMS; Brief Interview of Mental Status, 2011) (e) are not enrolled in hospice; and (f) are not in the nursing community for short-stay rehabilitation care.
As researchers and practitioners, we strive to uphold the ethical principle of autonomy in our work with individuals who live with dementia. We obtained University of Maryland Institutional Review Board approval for the following consent process. A list of all eligible residents is obtained from a designated staff member. We approach these residents initially for assent. If the resident does not assent, there is no further contact. If a resident does assent, we then conduct an evaluation of their decisional capacity, and proceed with obtaining their written/verbal consent if they demonstrate decisional capacity. If decisional capacity is impaired, we then approach the legally authorized representative for consent. Residents are approached by research assistants until 12–13 residents per community are recruited (N = 625 residents). This sampling approach respects the preferences of the resident, assures variability, and equal participation from each nursing community in the study, and accounts for the expected 20% attrition.
We are starting EIT-4-BPSD in 8–10 communities and the EO intervention in 8–10 communities annually over 3 years, for a total of three cohorts (50 communities in total). Sample size calculation was based on the hypotheses associated with the Effectiveness component of RE-AIM and our prior research (Resnick et al., 2016a; Resnick, Kolanowski, et al., 2013). For community-level outcomes, our prior work resulted in effect sizes of .9 for environmental and policy changes (Resnick, Galik, Vigne, & Carew, 2016; Resnick, Kolanowski, et al., 2013). With 50 communities, the statistical power for these two measures will be adequate (>.90 for both outcomes based on our analysis plan). For resident-level outcomes, person-centered approaches resulted in a small intervention effect (Cohen’s d=.19) to maintain or improve BPSD, function, well-being, or experience of adverse events (Galik et al., 2015; Kolanowski et al., 2011; Van Haitsma et al., 2015), Given a two-tailed alpha of .05, an estimated intra-class correlation coefficient (ICC) between clusters (communities) of .02, a correlation coefficient between repeated measures (baseline, 4 and 12 months) of .6, and assuming even dispersion of means, a sample of 500 residents will provide sufficient power (>.80) to detect a small effect size for group differences (Cohen & Cohen, 1983). Our prior research showed a 20% rate of attrition over 12 months (Galik et al., 2014). Therefore, we anticipate that a total of 625 residents from 50 nursing homes will be sufficient to achieve our specific aims. To achieve this sample, we will recruit 12–13 residents in each of 50 communities.
3.2 |. Intervention
3.2.1 |. The EIT-4-BPSD intervention
The intervention activities begin with a Stakeholder meeting. All EIT-4-BPSD related activities are done during scheduled working hours of the community-employed Stakeholders. Following the initial stakeholder meeting, EIT-4-BPSD activities are primarily implemented by the Research Facilitator working monthly with the Internal Champion and Stakeholders, using a four-step approach that combines face-to-face and internet-enhanced interventions. The four-step approach includes ongoing, iterative, and active participation of all Stakeholders.
Table 1 provides an overview of what is included in the initial Stakeholder team meeting. This meeting generally lasts between 2 and 4 hr, depending on the availability, interests and educational needs of the Stakeholder team members. For example, if the team has extensive knowledge about BPSD, less time is spent on this aspect of the overview. A critical component of the Stakeholder meeting is the Brainstorming done to establish community goals. These goals help to focus the work within the community and might include such aims as optimizing function among the residents as a way to decrease BPSD; increasing incorporation of person-centered approaches to manage BPSD in resident care plans; improving communication among staff and administration; or decreasing falls.
TABLE 1.
Content for first EIT-4-BPSD meeting with the stakeholder team
| Introduction and overview of EIT-4-BPSD |
| a. Welcome and overview of project; |
| b. Roles and responsibilities of research and stakeholder teams; |
| c. Background support for EIT-4-BPSD: 1. Review of current care practices/philosophies of care and evaluation of readiness to implement change in philosophy; 2. Task versus resident focused care; 3. BPSD and interventions to prevent or decrease BPSD; |
| d. Participatory implementation/brainstorming activity and identification of barriers/solutions to implementation; |
| e. Development of community-specific goals relevant to BPSD |
| Implementation of steps 1 and 2 in the 4-step process |
| a. Training to complete step 1: completing environment and policy assessments: use of assessments and examples of environment interventions and policies to facilitate use of behavioral approaches for BPSD; |
| b. Training to complete step 2: education of staff. Review educational materials developed by the research team and establish the community plan for staff training; |
| c. Prior to staff training, educational materials will be reviewed to make sure they are culturally relevant for staff within the community using seven factors from multiple sources (American Association of Colleges of Nursing (AACN), 2008; Giger & Davidhizer, 2002); |
| d. The research team will help assure that the education meets state and federal based requirements for education of nursing community staff (e.g., content and length of session) |
| Implementation of steps 3 and 4 in the 4-step process |
| a. Training to complete step 3: Establishing person-centered care plans for BPSD: |
| 1. Teach use of the Describe, Investigate, Create, and Evaluate (DICE) approach, the physical capability assessment, and the resident assessment of preferences |
| b. Training to complete step 4: Mentoring and motivating staff: |
| 1. Review self-efficacy based techniques (e.g., verbal encouragement); |
| 2. Training of Champion to complete use of Behavioral Interventions for BPSD measure with nursing assistants and provide motivational feedback to them (done at 0–2, 4–6, and 10–12 months); use of contests to motivate staff to adhere to person-centered care plans for BPSD |
| Summary of training day |
| a. Summary of training and findings from Brainstorming session, review of challenges/drivers and established community goals; |
| b. Discussion of ways the stakeholder team will achieve goals; |
| c. Opportunity for questions; |
| d. Review of next steps and timeline of project; |
| e. Homework given to Champions to complete the Environment and Policy Assessments which will be reviewed at the next monthly meeting |
EIT-4-BPSD, Evidence Integration Triangle for management of behavioral and psychological symptoms of dementia.
Table 1 also provides information about the four steps of the EIT-4-BPSD intervention implemented by the Internal Champion with the help of the Research Facilitator. These include: Step 1. Assessment of the environment and policies for evidence of resources/support for implementation of nonpharmacological interventions for BPSD; Step 2. Staff education, based on gaps in staff knowledge of BPSD and person-centered behavioral interventions; Step 3. Establishment of person-centered care plans, which are developed by staff with guidance from the Research Facilitator and Internal Champion; and Step 4. Mentoring and motivating of staff to integrate person-centered behavioral approaches routinely as they provide care to residents. Mentoring and motivating is done by the Internal Champion, supported by the Research Facilitator and Stakeholder team, using techniques from social cognitive theory such as verbal encouragement (Resnick et al., 2013).
Evidence-based resources for non-pharmacologic person-centered interventions are drawn from the previously developed Nursing Home Toolkit (nursinghometoolkit.com). The Nursing Home Toolkit is an online repository of resources that includes five components: introduction to the philosophy of person-centered care; system integration processes; education and leadership programs for responding to BPSD; tools for assessment of BPSD; and pragmatic behavioral approaches for BPSD. These resources are thus easily accessible to the staff and augment materials provided during education and ongoing interaction with the Research Facilitator.
Following the initial face-to-face training, each EIT-4-BPSD community is visited monthly for a total of 12 months by the Research Facilitator, who works with the Internal Champion and Stakeholder Team to implement EIT-4-BPSD. Table 2 delineates what is done during the monthly meetings, which last approximately 1–2 hr. In addition, each month the Research Facilitator explores challenges, celebrates successes, and gives feedback to the Stakeholder Team based on available pragmatic measures (evaluation of the environment, policies, and care planning) and works with the Internal Champion and staff to facilitate the implementation of the steps of EIT-4-BPSD (e.g., helps with education, resident specific care plan development, and observation of staff interaction with residents as an indicator of treatment fidelity). Monthly interactions with the Stakeholders provide them with an opportunity to make revisions to the implementation process, so that EIT-4-BPSD is standardized yet individualized for each community. This cyclic feedback pattern of review of progress and challenges will help guide staff intervention activities to best respond to contextual changes in the community over time.
TABLE 2.
Research facilitator monthly stakeholder team meetings to implement EIT-4-BPSD
| Activities for monthly meetings with stakeholder team | EIT-4-BPSD intervention month |
|
|
|
|
|
|
|
|
|
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Initial 2–4 hr training of internal champion/stakeholder team (see Table 1) | X | |||||||||||
| Review of environment and policy assessments (Galik et al., 2014; Resnick, Galik, & Vigne, 2014; Resnick et al., 2017) | X | |||||||||||
| Plan and implement education of nurses and families as relevant to the community goals; plan for new staff education | X | |||||||||||
| Review care plans of consented residents to assure that person-centered approaches to BPSD are established | X | X | X | X | X | X | X | X | X | X | ||
| Review of practical measures and data collected as part of the intervention (e.g., evaluation of care plans; observations of staff during care interactions); ongoing review of challenges/solutions identified by champion/stakeholders; review of motivational techniques to assure implementation of person-centered approaches | X | X | X | X | X | X | X | X | X | X | X | |
EIT-4-BPSD, Evidence Integration Triangle for management of behavioral and psychological symptoms of dementia.
In addition to the monthly visits, weekly emails will be sent to all Stakeholder Team members within the cohort to provide BPSD Tidbits. The Tidbits include updates about person-centered behavioral approaches for BPSD and will share individual community successes and strategies to deal with challenges (nursinghometoolkit.org). To motivate stakeholder teams across the cohort, contests will be held (e.g., winning example for overcoming a challenging bathing interaction with a resident with BPSD) and winners announced in the weekly emails.
3.2.2 |. Education-only (EO) control group
Communities randomized to EO will be provided with staff education about BPSD using a previously developed PowerPoint presentation in 30-min sessions, as is currently done in usual practice. The education is provided to communities in a preferred format (e.g., face-to-face; webinar, conference call), and the community is encouraged to record the necessary documentation to use the educational session to meet state and federal regulations for staff continuing education.
3.3 |. Measures/outcomes
A description of RE-AIM outcomes is provided in Table 3. Community-level and resident-level outcomes are obtained by trained research assistants (RAs) who are blind to community condition. Data are collected at baseline, 4 and 12 months from the following sources: designated administrative staff for community relevant data (e.g., staffing); participating residents’ medical charts; and observations by the RAs and input from the community staff working with the residents at the time of evaluation (approximately 10 min of staff time). The Research Facilitator will obtain Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) outcomes at baseline, 12, 18, and 24 months, in intervention sites only, as delineated.
TABLE 3.
Measures used to assess RE-AIM outcomes: Constructs, measurement level, source, and timing
| Construct | Measurement level | Source | Time | Measures |
|---|---|---|---|---|
| Descriptives/covariates | ||||
| Demographics | Community (All) | Nursing home compare website | Baseline | State; size; profit status; urban or rural; nursing staff ratio and mix; nursing home compare quality measure ratings (falls, pain, depressive symptoms, worsening of activities of daily living, antipsychotic medication use) |
| Demographics | Resident | RA from medical record | Baseline | Age, gender, race, ethnicity, marital status, education, length of stay |
| Cognitive status | Resident | RA from resident | Baseline , 4, 12 | Brief interview of mental status (BIMS) (Mansbacha) Macea,& Clarka,2014) |
| Health status and medications | Resident | RA from medical record | Baseline , 4, 12 | Cumulative illness rating scale (Linn et al., 1968; Parmelee, Thuras, Katz, & Lawton, 1995) total score; number, dosing, and frequency of medications prescribed |
| Reach | ||||
| Participation (defined as attending at least the first Stakeholder meeting in EIT-4-BPSD communities) | Community: All for volunteering; EIT-4-BPSD for stakeholder meeting participation | Research facilitator | Baseline; over 12 months | All communities: Percentage of communities that volunteer to participate (#volunteers/591 communities in both states). First 50 will be enrolled. Comparison of participating communities versus non-participating communities will be done. EIT communities: Percent of NHs that participate in the first Stakeholder meeting (#completed/25 EIT-4-BPSD) |
| Exposure of residents to person-centered interventions for BPSD | All residents in EIT-4-BPSD communities | RA from medical record | 12 months post implementation | Checklist for evidence of use of person-centered care Approaches to manage BPSD in care plans: Percentage of all residents living in the EIT-4-BPSD communities that have care plans reflecting at least 1 person-centered intervention to address BPSD (based on de-identified care plans for all residents in the EIT-4-BPSD communities) |
| Nurses exposed to educational session | Community (All) | Research facilitator | Following educational sessions | Percentage of nursing staff in all communities exposed to the educational sessions provided (#exposed/all nursing staff in the community). We will compare exposure between EIT-4-BPSD and EO communities |
| Effectiveness | ||||
| Environmental modifications | Community (All) | RA | Baseline, 12 | Environment assessment total score |
| Policy modifications | Community (All) | RA | Baseline, 12 | Policy assessment total score |
| BPSD: depression | Resident | RA from Staff | Baseline, 4,12 | Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos et al., 1988a, b) |
| BPSD: resistance to care | Resident | RA from Staff | Baseline, 4,12 | Resistiveness to Care Scale (Mahoney et al., 1999) |
| BPSD: agitation | Resident | RA from Staff | Baseline, 4, 12 | Cohen-Mansfield Agitation Inventory (CMAI) (Cohen-Mansfield, 2014; Finkel et al., 1992) |
| Function | Resident | RA from Staff | Baseline, 4, 12 | Barthel Index (BI) (Mahoney & Barthel, 1965) |
| Quality of life | Resident | RA from Staff | Baseline, 4, 12 | Quality of Life-AD (QoL-AD) (Logsdon et al., 2002) |
| Adverse events | Resident | RA from Medical Record | Baseline, 4, 12 | Number of falls; Number transferred to hospitals or emergency room, number of residents restrained |
| Medications: psychotropics | Resident | RA from Record | Baseline, 4, 12 | Medications (including PRN dosing) given in the 7 days prior to the Day of evaluation. |
| Adoption | ||||
| Participation in initial and monthly stakeholder team meetings | Community (EIT-4-BPSD only) | Research Facilitator | Over the 12 month study period | Number of communities completing the initial and the monthly meetings; number of champions/stakeholders attending monthly meetings; Differences in communities that participate versus don’t participate. |
| Environment and policy changes; care plan changes | Community(all | RA | Baseline, 12 | Environment/Policy Assessments; Checklist for Evidence of Use of Person Centered Care Approaches to Manage BPSD in Care plans: :Number of communities that complete Environment and Policy assessments, demonstrated change in environments, policies and care plans. Differences in communities that did or did not complete assessments or make changes (change in at least one item for each measure). |
| Rate of adoption | ||||
| • Early | Community (EIT-4-BPSD only) | RA | Baseline to 4 months | Environment, policy, and care plan Assessments: evidence of policy, environment and/or care plan changes in first four months |
| • Late | Community (EIT-4-BPSD only) | RA | Between 5–12 months | Environment, policy, and care plan assessments: evidence of policy, environment and/or care plan changes in months 5–12 |
| • No-adopters | Community (EIT-4-BPSD only) | RA | By 12 months | Environment, policy, and care plan assessments: no evidence of changes in policy, environment and/or care plan by 12 months |
| Implementation/fidelity | ||||
| Delivery of first EIT meeting with team | Community (EIT-4-BPSD only) | Research Facilitator | First month | Number of communities out of total 25 EIT-4-BPSD communities completing the initial Stakeholder training meeting |
| Delivery of four steps of EIT-4-BPSD Step 1: Environment/policy assessments; Step 2 Education of staff; Step 3: Development of care plans for BPSD; Step 4: Mentoring and motivating | Community (EIT-4-BPSD only) | Research facilitator | During 12 month period | Step 1: Completion of environment and policy assessments. Step 2: Percentage of staff participating in educational sessions; Step 3: Development of care plans with person centered interventions for BPSD based on checklist (Step 4: Evidence from the internal champion of use of information from weekly Tidbits; participation in contests; verbal encouragement of staff; completion of the observations using the Behavioral Interventions for BPSD measure at 0–2, 4–6, and 10–12 months post implementation of EIT-4-BPSD |
| Delivery: education for EO communities | EO community (all) | 0–2 months | Percentage of staff participating in educational sessions | |
| Receipt of education: step 2 in EIT-4-BPSD; EO | Community (all) | Internal champion; research facilitator | 0–2 months | Knowledge of behavioral interventions for BPSD: Evidence of receipt will be based on percentage of staff obtaining 80% or better total score on knowledge of behavioral interventions for BPSD |
| Enactment of behavioral approaches to BPSD (staff) | Community (EIT-4-BPSD only) | Internal champion; research facilitator | 0–2; 4–6; and 10–12 months | Observation tool: use of behavioral interventions for BPSD: increase in number of behavioral interventions provided during routine care interactions (based on Use of behavioral interventions for BPSD) |
| Maintenance | ||||
| Long term attrition | Community (All) | RA | 12 | Percentage of communities that withdraw by 12 months |
| Person centered care plans for BPSD | Community (All) | RA | 12, 18, 24 | Checklist for evidence of use of person centered care approaches to manage bpsd in care plans: evidence of person centered approaches for BPSD in care plans of recruited residents based on checklist for evidence of use of person centered care approaches to manage BPSD in care plan |
| Environment and policy | Community (EIT-4-BPSD) | RA | 12, 18, 24 | Environment/policy assessments: positive change in environment/policy assessments |
RE-AIM, Reach, Effectiveness, Adoption, Implementation, and Maintenance; EIT-4-BPSD, Evidence Integration Triangle for management of behavioral and psychological symptoms of dementia; EO, education only; RA, research assistant.
3.3.1 |. Descriptive measures
The following data will be obtained at baseline to describe communities and consider confounders: state; size; profit or non-profit ownership status; urban or rural; Nursing Home Compare descriptive measures (11 outcomes such as percentage of residents with pain); staffing ratio and mix (number of nursing assistants + nurses [registered; licensed practical nurses] + activity staff/number of residents). Resident descriptive information will include: age, race, gender, education, and marital status at baseline; and use of psychotropic medications (antipsychotics, antidepressants, sedative/hypnotics, anxiolytics). Also for descriptive purposes, cognitive status will be measured using the Brief Interview of Mental Status (BIMS) (Brief Interview of Mental Status, 2011), and health status will be measured using the Cumulative Illness Rating Scale (CIRS) (Linn, Linn, & Gurel, 1968; Parmalee, Lawton, & Katz, 1998).
3.3.2 |. Community and staff RE-AIM measures
The Environment and Policy Assessments and the Care Plan Checklist for Evidence of Person-Centered Approaches for BPSD are completed to evaluate RE-AIM components of Effectiveness, Adoption, and Maintenance. The Environment Assessment includes 24 items that affect care of residents with BPSD (e.g., “outdoor spaces are available”). Items are scored as present or not present and summed; higher scores are indicative of better environmental quality. There was prior evidence of inter-rater reliability and validity based on hypothesis testing (Galik et al., 2014; Resnick et al., 2013). The Policy Assessment includes 24 items that reflect policies that support behavioral approaches for BPSD (e.g., policies on use of restraints). Items are scored as present or not present and summed; higher scores indicate a greater number of policies supporting person-centered care. There was prior evidence of inter-rater reliability and validity (Galik et al., 2015; Resnick et al., 2013). The Care Plan Checklist for Evidence of Person Centered Approaches for BPSD (Resnick et al., 2017) is used to evaluate care plans for evidence of person-centered approaches that address common BPSD (apathy, agitation, inappropriate/disruptive vocalizations, aggression, wandering, repetitive behaviors, resistance to care, and sexually inappropriate behaviors).
The Knowledge of Behavioral Interventions for BPSD test is used to address RE-AIM components of Implementation and Receipt. The Knowledge test is a 10-item multiple-choice test of staff knowledge. Test-retest reliability was 0.92 and validity was supported by significant associations between test scores and the use of behavioral interventions for BPSD. Last, for community outcomes, the measure for Use of Behavioral Interventions for BPSD is used to address Implementation and Enactment components within RE-AIM. This is a reliable and valid eight-item observation of nursing assistants to assess implementation of appropriate interventions to prevent or manage BPSD when interacting with residents during routine care.
3.3.3 |. Residents’ RE-AIM measures of intervention effectiveness
To evaluate Effectiveness as indicated by the RE-AIM model, behavioral symptoms relevant to the residents will be evaluated. Depressive symptoms will be measured using the Cornell Scale for Depression in Dementia (CSDD) (Alexopoulos, Abrams, Young, & Shamoian, 1988a; Alexopoulos, Abrams, Young, & Shamoian, 1988b), a reliable and valid 19 item assessment of depressive symptoms in individuals with dementia (Alexopoulos et al., 1988a; Alexopoulos et al., 1988b; Barca, Engedal, Selbaek, 2010). Agitation will be measured using the Cohen-Mansfield Agitation Inventory (CMAI). The 14-item version of the CMAI uses a five-point Likert scale to rate the frequency of behavioral symptoms (Cohen-Mansfield, 2014; Finkel, Lyons, & Anderson, 1992). Prior use supported its reliability and validity (Cohen-Mansfield, 2014; Finkel et al., 1992). Resistiveness to care will be evaluated using the Resistiveness to Care Scale (Mahoney et al., 1999), a reliable and valid 13-item Likert scale that assesses residents’ behaviors during activities of daily living. Functional ability will be measured using the Barthel Index (BI) (Mahoney & Barthel, 1965), which is a 10-item measure of performance of activities of daily living with evidence of reliability and validity (Mahoney & Barthel, 1965; Resnick & Galik, 2007). Items are weighted to account for the amount of assistance required. Last, resident quality of life will be assessed using the Quality-of-Life-AD scale (QoL-AD) (Logsdon, Gibbons, McCurry, & Teri, 2002). The QoL-AD is a 13-item reliable and valid instrument designed to rate the resident’s quality of life from the staff perspective. Examples of items include physical condition, mood, relationships, and participation in meaningful activities. Ratings are obtained on a four-point scale (1 is poor and 4 is excellent), and total scores range from 13–52 (Logsdon et al., 2002).
Adverse events most relevant to BPSD will be obtained from medical records and designated individuals within the communities (e.g., quality assurance nurse). Adverse events will include falls and transfers to hospitals or emergency rooms, and physical and chemical restraint use. Baseline adverse events will include the 4 months prior to treatment implementation; 4-month follow-up will include adverse events that occur between baseline and 4 months; and 12 month follow-up will include those that occur between 4 and 12 months post implementation of the intervention. Restraint use will be based on the Minimum Data Base 3.0 definition of assessment for use of restraints (Centers for Medicare and Medicare Services, 2013; Rahman & Applebaum, 2009; Saliba & Buchanan, 2008).
3.4 |. Data analysis
Descriptive statistics including measures of central tendency, dispersion, and appropriate visualization approaches (e.g., box plots and spaghetti plots) will be performed on each outcome variable for residents and communities to ascertain distribution and ensure that the assumptions (e.g., normality) associated with the planned statistical procedures are met. When necessary, transformations will be performed.
All analyses will be done using an intent-to-treat philosophy. Baseline characteristics (both resident level and community level) will be compared between intervention and control groups and the relevant variables (e.g., age) that differ by group will be included as covariates in hypothesis testing. In particular, we will use constructs known to be correlated with BPSD, including gender, pain, health status, and cognitive and functional impairment.
Linear mixed models (LMMs) for longitudinal data (baseline, 4 and 12 months) will be used to assess the intervention effect on continuous outcomes (i.e., function, behavioral symptoms, and quality of life), accounting for clustering of residents within the same community and correlations between repeated measurements of each resident. Mixed-effect Poisson regression (i.e., Generalized Linear Mixed Model [GLMM]) will be conducted to assess the effect of the intervention on count outcomes (i.e., number of adverse events including number of falls, hospitalizations, emergency room visits, and restraint use). The fixed effects included in the models will be treatment group (EIT-4-BPSD vs. EO), time (baseline, 4 and 12 months), group-by-time interaction term, and the aforementioned relevant covariates (e.g., age). Random effects will include communities and residents. The hypotheses will be tested by evaluating the interaction term for each outcome variable. Similar LMMs will be used to compare changes in community-level measures (i.e., environments, policies) from baseline to 12 months with only community included as random effects.
For each hypothesis, exploratory analyses will be performed to assess model assumptions. Post-analysis diagnostic measures (e.g., residuals) will be explored to assess model fit. All tests will use a 5% significance level. The use of LMM will provide flexibility with regard to assumptions related to the covariance structure of the residuals and the presence of missing data for the repeated measures.
If there is significant dropout, beyond our anticipated 20% rate of attrition, we will identify baseline characteristics that differ between persons or communities that drop out. Maximum likelihood methods will be used for primary analyses, which address non-informative dropout (missing at random [MAR]). If “informative” dropout appears possible, we will consider sensitivity analyses that involve adding these relevant baseline covariates to make the MAR assumption more plausible. Qualitative data will also be collected to inform effectiveness outcomes.
3.4.1 |. Analysis of intervention adoption
Staff adoption will measured as the number of Internal Champions and Stakeholder team members participating in initial face-to-face training and monthly meetings among the 25 communities randomized to EIT-4-BPSD. We will also consider improvement in scores on the Use of Behavioral Interventions for BPSD measure at each testing time point as evidence of adoption among staff.
Community adoption will be based on changes in environments, policies, and care plans of residents at 4 and 12 months post implementation of EIT-4-BPSD, with a target of at least one item changed in each assessment area. Furthermore, we will describe differences among EIT-4-BPSD communities that demonstrate adoption early (in the first 4 months) versus late (between 5–12 months) versus non-adopters (no change by 12 months). Predictors such as community characteristics will be compared across the three potential times of adoption (early, late, nonadopters).
3.4.2 |. Analysis of intervention implementation
Delivery of the intervention will be evaluated by describing the number of treatment communities that are exposed to the first EIT-4-BPSD training session and the number of EO communities and staff exposed to education sessions. In addition, we will measure evidence of delivery for each step of the EIT-4-BPSD intervention: Step 1: Percent of environmental and policy assessments completed; Step 2: Percentage of staff exposed to education; Step 3: Percentage of residents with evidence of person-centered approaches to BPSD in care plans; Step 4: Description of mentoring and motivating activities (e.g., participation in contests); number of observations completed of staff-resident interactions using the Use of Behavioral Approaches for BPSD measure at 0–2, 4–6, and 10–12 months; and number of telephone conferences requested.
Receipt of intervention will be based on descriptive statistics of the Knowledge of Behavioral Interventions for BPSD Test, with mean scores of 80% or greater providing evidence of receipt. We will also compare differences in mean percentage correct on the Knowledge of Behavioral Interventions for BPSD Test between EIT-4-BPSD communities and EO communities.
Enactment of intervention will be based on percentage of care interactions in which person-centered approaches for BPSD in EIT-4-BPSD communities are provided, based on the Use of Behavioral Approaches for BPSD measure. This is done as part of Step 4 within EIT-4-BPSD at baseline (0–2 months), 4–6, and 10–12 months post-implementation.
3.4.3 |. Analysis of intervention maintenance
Finally, maintenance of the intervention will be evaluated by tracking communities that withdraw from the study within 12 months and reasons for attrition. We will compare EIT-4-BPSD communities with EO communities to determine if there is a differential rate of attrition. Maintenance of environment and policy changes and inclusion of person-centered approaches for BPSD in care plans will be examined at 18 and 24 months post-implementation, based on maintaining or improving Environment and Policy assessments and percentage of residents with person-centered approaches for management or prevention of BPSD in their care plans. LMMs will be used to compare changes in community-level measures from baseline to 24 months, with community included as random effects. Time will be included and recorded accordingly. Attrition and maintenance of changes at 12 and 24 months will be compared to determine maintenance over that period.
4 |. DISCUSSION
While there are resources available for provision of evidence-based non-pharmacologic interventions to residents with dementia, such as those within the web-based Nursing Home Toolkit, staff in nursing homes continue to need help with the implementation process (Beck et al., 2005; Bonner et al., 2015; Parrish, O’Malley, Adams, Adams, & Coleman, 2009; Schnelle et al., 2002). EIT-4-BPSD provides an implementation plan using a theoretically-based 4-step approach (Resnick, Galik, Gruber-Baldini, & Zimmerman, 2011; Resnick et al., 2013) guided by the Evidence Integration Triangle (EIT) framework. EIT-4-BPSD can facilitate a change in how BPSD is prevented and managed in long term-care settings (Glasgow, 2013; Glasgow et al., 2012a).
As with any study protocol, there are strengths and limitations to this work. We include volunteer communities from two states and focus on residents who have evidence of BPSD. Thus findings cannot be generalized to all residents and all nursing homes. Due to the limitation on the number of settings and residents recruited per setting, this study does not provide a true reflection of the reach of dissemination, one of the RE-AIM outcomes. It will, however, inform future dissemination research. The development of the EIT-4-BPSD took into account that all communities have unique challenges to implementation. We use the EIT to allow flexibility in implementation and have included in analyses known influences on study outcomes, such as size and staffing. There may be other factors we have not considered. To overcome this limitation, we will adjust for clustering and resident characteristics during data analysis.
Potential challenges to the implementation of EIT-4-BPSD that were anticipated include limited willingness of communities to participate in the study, lack of engagement of Internal Champions and Stakeholders, staff turnover, and loss of residents to follow-up. We are prepared to speak with and visit with interested settings to engage them in the study. We will work with settings to identify new Internal Champions if needed, and we will provide educational information about the study to new staff for their onboarding. We will also over-sample residents in recruitment to compensate for loss to follow-up.
In addition to anticipated challenges, we have experienced some unanticipated challenges in the implementation in our first cohort of communities. An example of an unanticipated challenge was the start of a major construction project at one of our intervention sites, which necessitated onboarding a new Internal Champion and re-visiting the timing of stakeholder meetings. The Research Facilitator met with the administrator and stakeholder group to re-affirm their commitment. Together they selected a replacement for the Internal Champion and reorganized meeting times to accommodate their disrupted schedules. In another site, the community was sold, and subsequent changes in leadership and policies required rescheduling and flexibility of intervention-related activities.
Despite these limitations, EIT-4-BPSD has the potential to make a significant impact on practice in our nation’s 15,633 nursing homes, by providing guidance on how to implement person-centered behavioral approaches for BPSD, the ultimate goal of the CMS National Partnership.
Acknowledgments
Funding information
National Institute of Nursing Research, Grant number: 1R01NR015982–01A1
Footnotes
CONFLICTS OF INTEREST
None.
REFERENCES
- Alexopoulos G, Abrams R, Young R, & Shamoian C (1988a). Cornell scale for depression in dementia. Biology and Psychiatry, 23, 271–284. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Abrams RC, Young RC, & Shamoian CA (1988b). Use of the Cornell Scale in non-demented patients. Journal of the American Geriatrics Society, 36, 230–236. [DOI] [PubMed] [Google Scholar]
- American Association of Colleges of Nursing (AACN) (2008). Cultural competency in baccalaureate nursing education, Retrieved from http://www.aacnnursing.org/Education-Resources/Tool-Kits/Cultural-Competency-in-Nursing-Education
- Bandura A (1986). Social Foundations of Thought and Action Englewood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Bandura A (1995). Self-efficacy in Changing Societies New York: Cambridge University Press. [Google Scholar]
- Bandura A (1997). Self-efficacy: The Exercise of Control New York: W.H.: Freeman and Company. [Google Scholar]
- Barca M, Engedal K, & Selbaek G (2010). A reliability and validity study of the Cornell scale among elderly inpatients, using various clinical criteria. Dementia and Geriatric Cognitive Disorders, 29, 438–447. [DOI] [PubMed] [Google Scholar]
- Beck C, Heacock P, Mercer S, Doan R, O’Sullivan P, Stevenson J, … Hoskins J (2005). Sustaining a best-care practice in a nursing home. Journal of Healthcare Quality,, 27(4), 5–16. [DOI] [PubMed] [Google Scholar]
- Beer C, Horner B, Flicker L, Scherer S, Lautenschlager N, Bretland N, … Almeida O (2011). A cluster-randomised trial of staff education to improve the quality of life of people with dementia living in residential care: The DIRECT study. PLoS ONE, 6(11), e28155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner A, Field T, Lemay C, Mazor K, Andersen D, Compher C, … Gurwitz J (2015). Rationales that providers and family members cited for the use of antipsychotic medications in nursing home residents with dementia. Journal of the American Geriatrics Society, 63, 302–308. [DOI] [PubMed] [Google Scholar]
- Brief Interview of Mental Status (2011). Retrieved from http://dhmh.dfmc.org/longtermcare/documents/bims_form_instructions.pdf
- Buckwalter K, Grey M, Bowers B, McCarthy A, Gross D, Funk M, & Beck C (2009). Intervention research in highly unstable environments. Research in Nursing & Health, 32, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2013). Dementia care in nursing homes: Clarification to Appendix P State Operations Manual (SOM) and Appendix in the SOM for F309-Quality of Care and F329-Unnecessary Drugs Retrieved from https://www.google.com/search?q=Dementia+care+in+nursing+homes%3A+Clarification+to+Appendix+P+State+Operations+Manual+%28SOM%29+and+Appendix+in+the+SOM+for+F309-+Quality+of+Care+and+F329-+Unnecessary+Drugs.&ie=utf-8&oe=utf-8
- Centers for Medicare and Medicare Services. (2013). Minimum data set Retrieved from http://www.cms.gov/apps/mds/
- Cohen J, & Cohen P (1983). Applied multiple regression/correlation analysis for the behavioral sciences Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Cohen-Mansfield J (2001). Nonpharmacologic interventions for inappropriate behaviors in dementia: A review, summary, and critique. American Journal of Geriatric Psychiatry, 9, 361–381. [PubMed] [Google Scholar]
- Cohen-Mansfield J (2014). Instructional manual for the Cohen-Mansfield Agitation Inventory Retrieved from http://www.dementiaassessment.com.au/symptoms/CMAI_Scale.pdf [Google Scholar]
- Cooper C, Mukadam N, Katona C, Lyketsos CG, Blazer D, Ames D, … Livingston G (2013). Systematic review of the effectiveness of pharmacologic interventions to improve quality of life and well-being in people with dementia. American Journal of Geriatric Psychiatry, 21, 173–183. [DOI] [PubMed] [Google Scholar]
- Curran G, Bauer M, Mittman B, Pyne J, & Stetler C (2012). Effectiveness-implementation hybrid designs. Medicare Care, 50, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer C, Pavlik V, Murphy K, & Hyman D (2000). The high prevalence of depression and dementia in elder abuse or neglect. Journal of the American Geriatrics Society, 48, 205–208. [DOI] [PubMed] [Google Scholar]
- Finkel S, Lyons J, & Anderson R (1992). Reliability and validity of the CMAI in institutionalized elderly. International Journal of Geriatric Psychiatry, 7, 487–490. [Google Scholar]
- Finucane A, Stevenson B, Moyes R, Oxenham D, & Murray S (2013). Improving end-of-life care in nursing homes: Implementation and evaluation of an intervention to sustain quality of care. Palliative Medicine, 27, 772–778. [DOI] [PubMed] [Google Scholar]
- Galik E, Resnick B, Lerner N, Hammersla M, & Gruber-Baldini A (2015). Function Focused Care for assisted living residents with dementia. The Gerontologist, 55(Suppl 1), S13–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galik E, Resnick B, Hammersla M, & Brightwater J (2014). Optimizing function and physical activity among nursing home residents with dementia: Testing the impact of Function Focused Care. The Gerontologist, 54(6), 11–20. [DOI] [PubMed] [Google Scholar]
- Galik E, Resnick B, Gruber-Baldini A, Nahm ES, Pearson K, & Pretzer-Aboff I (2008). Pilot testing of the Restorative Care Intervention for the cognitively impaired. Journal of the American Medical Directors Association, 9, 516–522. [DOI] [PubMed] [Google Scholar]
- Galynker II, Roane DM, Miner C, Feinberg T, & Watts B (1995). Negative symptoms in patients with Alzheimer’s disease. The American Journal of Geriatric Psychiatry, 3(1), 52–59. [DOI] [PubMed] [Google Scholar]
- Giger J, & Davidhizer R (2002). The giger and davidhizar transcultural assessment model. Journal of Transcultural Nursing, 13, 185. [DOI] [PubMed] [Google Scholar]
- Glasgow R (2013). What does it mean to be pragmatic? Pragmatic methods, measure, and models to facilitate research translation. Health Education and Behavior, 40, 257–264. [DOI] [PubMed] [Google Scholar]
- Glasgow R, Eckstein E, & Elzarrad M (2013). Implementation science perspectives and opportunities for HIV/AIDS research: Integrating science, practice, and policy. Journal of Acquired Immune Deficiency Syndrome, 63(Suppl 1), S26–S31. [DOI] [PubMed] [Google Scholar]
- Glasgow R, Green L, Taylor M, & Stange K (2012). An evidence integration triangle for aligning science with poliy and practice. American Journal of Preventive Medicine, 42, 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow R, Kaplan R, Ockene J, Fisher E, & Emmons K (2012). The need for practical patient report measures of health behaviors and psychosocial issues in electronic health records. Health Affairs, 31, 497–504. [DOI] [PubMed] [Google Scholar]
- Glasgow R, Vinson C, Chambers D, Khoury MJ, Kaplan RM, & Hunter C (2012). National Institutes of Health approaches to dissemination and implementation science: Current and future directions. American Journal of Public Health, 102, 1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski D, O’Malley A, Afendulis C, Caudry D, Elliot A, & Zimmerman S (2014). Culture change and nursing home quality of care. The Gerontologist, 54(Suppl 1), S35–S45. 10.1093/geront/gnt1143 [DOI] [PubMed] [Google Scholar]
- Gregson J, Foerster S, Orr R, Jones L, Benedict J, Clarek B, … Zotz K (2003). System, environment and policy changes: Using the Social Ecological Model as a framework for evaluating nutrition education and social marketing programs with low income audiences. Journal of Nutrition Education, 33, S4–S15. [DOI] [PubMed] [Google Scholar]
- Helga E, Breimaier H, Halfens R, Wilborn D, Meesterberends E, Nielsen G, & Lohrmann C (2013). Implementation interventions used in nursing homes and hospitals: A descriptive, comparative study between Austria, Germany, and the Netherlands. ISRN Nursing, 2013, article ID 706054 https://www.hindawi.com/journals/isrn/2013/706054/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann N, Lanctôt KL, Sambrook R, Lesnikova N, Hébert R, McCracken P, ... Nguyen E (2006). The contribution of neuropsychiatric symptoms to the cost of dementia care. International Journal of Geriatric Psychiatry, 21, 972–976. [DOI] [PubMed] [Google Scholar]
- Kales H, Gitlin L, Lyketsos C & Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. (2014). Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. Journal of the American Geriatrics Society, 62, 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales H, Zivin K, Kim HM, Valenstein M, Chiang C, Ignacio RV, … Blow FC(2011). Trends in antipsychotic use in dementia 1999–2007. Archives of General Psychiatry, 68, 190–197. [DOI] [PubMed] [Google Scholar]
- Kessler R, & Glasgow R (2011). A proposal to speed translation of healthcare research into practice. American Journal of Prevention Medicine, 40(6), 637–644. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, & Buettner L (2008). Prescribing activities that engage passive residents: An innovative method. Journal of Gerontological Nursing, 34(1), 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Fick D, Frazer C, & Penrod J (2010). It’s about time: Use of nonpharmacological interventions in the nursing home. Journal of Nursing Scholarship, 42, 214–222. [DOI] [PubMed] [Google Scholar]
- Kolanowski A, Litaker M, Buettner L, Moeller J, & Costa P (2011). A randomized clinical trial of theory-based activities for the behavioral symptoms of dementia in nursing home residents. Journal of the American Geriatrics Society, 59, 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolanowski A, Van Haitsma K, Penrod J, Hill N, & Yevchak A (2015). Wish we would have known that! Communication breakdown impedes person-centered care. The Gerontologist, 55(Supp 1), S50–S60. [DOI] [PubMed] [Google Scholar]
- Kottke T, Solberg L, Nelson A, Belcher DW, Caplan W, Green LW, … Woolf SH (2008). Optimizing practice through research: A new perspective to solve an old problem. Annals of Family Medicine, 6, 459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuske B, Hanns S, Luck T, Angermeyer C, Behrens J, & Riedel-Heller S (2007). Nursing home staff training in dementia care: A systematic review of evaluated programs. International Psychogeriatrics, 19, 818–841. [DOI] [PubMed] [Google Scholar]
- Lazenby M (2014). The international endorsement of US Distress Screening and Psychosocial Guidelines in Oncology: A model for dissemination. Journal of the National Comprehensive Cancer Network, 12, 221–227. [DOI] [PubMed] [Google Scholar]
- Lekan-Rutledge D, Palmer MH, & Belyea M (1998). In their own words: Nursing assistants’ perceptions of barriers to implementation of prompted voiding in long-term care. The Gerontologist, 38, 370–378. [DOI] [PubMed] [Google Scholar]
- Lemay C, Mazor K, Field T, Donovan J, Kanaan A, Briesacher BA, … Tjia J (2013). Knowledge of and perceived need for evidence-based education about antipsychotic medications among nursing home leadership and staff. Journal of the American Medical Directors Association, 14, 895–900. [DOI] [PubMed] [Google Scholar]
- Linn B, Linn M, & Gurel L (1968). Cumulative illness rating scale. Journal of the American Geriatrics Society, 16, 622–626. [DOI] [PubMed] [Google Scholar]
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, … Cooper C (2014). A systematic review of the clinical effectiveness and cost-effectiveness of sensory, psychological and behavioural interventions for managing agitation in older adults with dementia. Health Technology Assessment, 18(39), 1–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon R, Gibbons L, McCurry S, & Teri L (2002). Assessing quality of life in older adults with cognitive impairment. Psychosomatic Medicine, 64, 510–519. [DOI] [PubMed] [Google Scholar]
- Mahoney E, Hurley A, Volicer L, Bell M, Gianotis P, Hartshorn M, … Warden V (1999). Development and testing of the Resistiveness to Care Scale. Research in Nursing & Health, 22, 27–38. [DOI] [PubMed] [Google Scholar]
- Mahoney F, & Barthel D (1965). Functional evaluation: The Barthel Index. Maryland State Medical Journal, 14(2), 61–66. [PubMed] [Google Scholar]
- Mansbacha W, Macea R, & Clarka M (2014). Differentiating levels of cognitive functioning: A comparison of the brief interview for mental status (bims) and the brief cognitive assessment tool (BCAT) in a nursing home sample. Aging & Mental Health, 18, 921–928. [DOI] [PubMed] [Google Scholar]
- Marx K, Stanley I, Van Haitsma K, Moody J, Alonzi D, Hansen B, & Gitlin L (2014). Knowing versus doing: Education and training needs of staff in a chronic care hospital unit for individuals with dementia. Journal of Gerontological Nursing, 30, 1–9. 10.3928/00989134-20140905-00989101 [DOI] [PubMed] [Google Scholar]
- McCabe M, Davison T, & George K (2007). Effectiveness of staff training programs for behavioral problems among older people with dementia. Aging & Mental Health, 11, 505–519. [DOI] [PubMed] [Google Scholar]
- Morley J, Caplan G, Cesari M, Dong B, Flaherty J, Grossberg G, … Vellas B (2014). International survey of nursing home research priorities. Journal of the American Medical Directors Association, 15, 309–312. [DOI] [PubMed] [Google Scholar]
- Parmalee P, Lawton MP, & Katz I (1998). The structure of depression among elderly institiutionalized residents: Affective and somatic correlates of physical frailty. Journal of Gerontology A, Biological Sciences Medicine and Science, 53, M155–M162. [DOI] [PubMed] [Google Scholar]
- Parmelee P, Thuras P, Katz I, & Lawton M (1995). Validation of the cumulative illness rating scale in a geriatric residential population. Journal of the American Geriatrics Society, 43, 130–137. [DOI] [PubMed] [Google Scholar]
- Parrish M, O’Malley K, Adams R, Adams S, & Coleman E (2009). Implementation of the care transitions intervention: sustainability and lessons learned. Professional Case Management, 14, 282–293. [DOI] [PubMed] [Google Scholar]
- Rahman A, & Applebaum R (2009). The nursing home minimum data set assessment instrument: Manifest functions and unintended consequences—past, present, and future. The Gerontologist, 49, 727–735. [DOI] [PubMed] [Google Scholar]
- Rahman A, Applebaum R, Schnelle J, & Simmons S (2012). Translating research into practice in nursing homes: Can we close the gap? The Gerontologist, 52, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, Galik E, Gruber-Baldini A, & Zimmerman S (2011). Testing the impact of function focused care in assisted living. Journal of the American Geriatrics Society, 59, 2233–2240. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E, Kolanowski A, Van Haitsma K, Ellis J, Behrens L, … McDermott C (2017). Reliability and validity of the Care Plan Checklist for evidence of person-centered approaches for BPSD. Journal of the American Medical Directors Association, Advance online publication 10.1016/j.jamda.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, & Galik E (2007). Reliability and validity testing of the physical activity survey in long term care (PAS-LTC). Journal of Aging and Physical Activity, 15, 439–458. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E, Vigne E, & Payne A (2015). Testing the feasibility of implementing FFC-AL to 100 AL communities in Maryland. Health Education & Behavior, 43, 296–304.27178495 [Google Scholar]
- Resnick B, Galik E, & Vigne E (2014). Translation of function focused care to assisted living communities. Family and Community Health, 37, 101–165. [DOI] [PubMed] [Google Scholar]
- Resnick B, Galik E, Gruber-Baldini A, & Zimmerman S (2013). Understanding dissemination and implementation of a new intervention in assisted living settings: The case of Function Focused Care. Journal of Applied Gerontology, 32, 280–301. [DOI] [PubMed] [Google Scholar]
- Resnick B, Kolanowski A, Van Haitsma K, Boltz M, Galik E, Bonner A, … Mulhall PM (2016). Pilot testing of the EIT-4-BPSD intervention. American Journal of Alzheimer’s Disease and Other Dementias, 31, 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick B, Galik E, Vigne E, & Carew A (2016). Dissemination and implementation of function focused care-assisted living. Health Education & Behavior, 43(3), 12–16. [DOI] [PubMed] [Google Scholar]
- Richter T, Meyer G, Möhler R, & Köpke S (2012). Psychosocial interventions for reducing antipsychotic medication in care home residents. Cochrane Database Systematic Reviews, 12(CD008634), https://doi.org/0.1002/14651858.CD008634.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba D, & Buchanan J (2008). Development & validation of a revised nursing home assessment tool: MDS 3.0 Retrieved from http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/NursingHomeQualityInits/downloads/MDS30FinalReport.pdf [Google Scholar]
- Schnelle JF, Alessi CA, Simmons SF, Al-Samarrai NR, Beck JC, & Ouslander JG (2002). Translating clinical research into practice: A randomized controlled trial of exercise and incontinence care with nursing home residents. Journal of the American Geriatrics Society, 50, 1476–1483. [DOI] [PubMed] [Google Scholar]
- Tabak R, Khoong E, Chambers D, & Brownson R (2012). Models for dissemination and implementation research. American Journal of Preventive Medicine, 43, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Ven A, & Johnson P (2006). Knowledge of theory and practice. Academy Management Review, 31, 802–821. [Google Scholar]
- Van Haitsma K, Curyto K, Abbott K, Towsley G, Spector A, & Kleban M (2015). A randomized controlled trial for an individualized positive psychosocial intervention for the affective and behavioral symptoms of dementia in nursing home residents. The Journals of Gerontology Series B Psychological Sciences and Social Sciences, 70, 35–45. 10.1093/geronb/gbt10212 [DOI] [PubMed] [Google Scholar]
- Virigina Tech. (2014). What is RE-AIM Retrieved from http://www.re-aim.hnfe.vt.edu/about_re-aim/what_is_re-aim/index.html
- Wunderlich G, & Kohler P (2000). Improving the quality of long-term care Washington, DC: National Academy Press; Retrieved from http://www.nap.edu/openbook.php?record_id=9611 [PubMed] [Google Scholar]


