Abstract
Accumulating evidence suggests that autism spectrum disorder (ASD) may be conceptualized within a framework of reward processing impairments. The “Social Motivation Theory of Autism” posits that reduced motivation to interact with people and decreased pleasure derived from social interactions may derail typical social development and contribute to the emergence of core social communication deficits in ASD. Neuroinflammation may disrupt the development of mesolimbic dopaminergic systems that are critical for optimal functioning of social reward processing systems. This neuroinflammation-induced disturbance of mesolimbic dopaminergic functioning has been substantiated using maternal immune activation rodent models whose offspring show aberrant dopaminergic corticostriatal function as well as behavioral characteristics of ASD model systems. Preclinical findings are in turn supported by clinical evidence of increased mesolimbic neuroinflammatory responses in individuals with ASD. This review summarizes evidence for reward processing deficits and neuroinflammatory impairments in ASD and examines how immune inflammatory dysregulation may impair the development of dopaminergic mesolimbic circuitry in ASD. Finally, future research directions examining neuroinflammatory effects on reward processing in ASD are proposed.
Keywords: autism spectrum disorder, neuroinflammation, reward, dopamine
1). Introduction
Autism spectrum disorder (ASD) is a highly heterogeneous neurodevelopmental disorder estimated to affect 1 in 59 children in the United States (Baio et al., 2018). Individuals with ASD present with extensive differences in symptom presentations, cognitive abilities, and adaptive skills. Given the heterogeneity inherent to the disorder, it is likely that multiple distinct etiological and pathophysiological factors contribute to the development of ASD. Despite the breadth of research investigating the developmental pathophysiology of ASD, no unifying etiological model has yet been established for ASD. This is in large part due to the intrinsic complexity of the genetics of ASD (Sahin & Sur, 2015), multiple environmental influences that have been shown to confer risk for ASD (Rossignol, Genuis, & Frye, 2014), and differential expression and identification of symptoms in males and females (Mandy & Lai, 2017) and children and adults (St Pourcain et al., 2017; Woodman, Smith, Greenberg, & Mailick, 2016) with ASD. These challenges associated with identifying a common etiologic factor for all individuals with ASD have impaired the development of novel psychosocial and pharmacological treatment options.
2). The Social Motivation Hypothesis of Autism
One approach to improved ASD treatment development is to consider mechanistic accounts that may apply to at least a significant subgroup of those with ASD who may share a common pathophysiology, thereby providing a mechanistic and functional framework for targeted treatment approaches suitable for that subgroup. Social communication impairments and their associated neurobiological underpinnings have received the majority of research attention as a path forward to understand ASD pathophysiology (Pelphrey, Shultz, Hudac, & Vander Wyk, 2011). Another candidate mechanism that has emerged recently are neurobiological systems associated with reward processing impairments in ASD. The framework that certain individuals with ASD are less motivated to interact with others and derive less pleasure from social interactions was introduced over a decade ago (Dawson, Webb, & McPartland, 2005), and this model has been useful for describing behavioral profiles and neural mechanisms of those individuals with ASD with decreased social motivational and pleasure responses (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Dichter, Damiano, & Allen, 2012) as well as potential biomarkers for early emerging reduced social attention in ASD (Dawson, Bernier, & Ring, 2012).
The framework of the Social Motivation Hypothesis of Autism is that functional disruptions in brain circuits that support social motivation may constitute a primary deficit in ASD that may have downstream effects on the development of social cognition and communication (Chevallier, Kohls, et al., 2012; Kohls, Chevallier, Troiani, & Schultz, 2012). Decreased social motivation may not be the only mechanistic account of the full range of social deficits associated with ASD (e.g., some individuals with ASD have social interests and actively seek social interactions but fail to form friendships due to impaired social cognition and pragmatic language). However, even during the first year of life, infants with ASD demonstrate infrequent orienting to their own name and diminished eye contact (Ozonoff et al., 2010), suggesting that decreased social orienting and social interest are evident in infancy in ASD and may interfere with the development of social cognition in at least a significant proportion of individuals with ASD (Dichter & Adolphs, 2012).
Social motivation is critical for optimal social development and learning (Fareri, Martin, & Delgado, 2008). During typical development, orienting to social stimuli begins in early infancy (Farroni, Massaccesi, Menon, & Johnson, 2007), including a neonatal preference for faces over non-face stimuli (Johnson, Dziurawiec, Ellis, & Morton, 1991). This prioritization is critical for social-communicative development (Brooks & Meltzoff, 2005) and social adaptation through the lifespan (Emery, 2000). Attention to social stimuli, even in infancy, is hypothesized to be accompanied by feelings of pleasure (Dawson et al., 2004), resulting in increased social motivation (Schultz, 2005) that promotes the development of neural systems underlying social information processing. In turn, such reward processing mechanisms may serve to encode and consolidate positive memories of social experiences that influence future responses to social stimuli and influence the development of brain systems that mediate social functioning more broadly (Chevallier, Kohls, et al., 2012).
Accordingly, the social impairments that characterize ASD may reflect decreased motivation to engage in reciprocal social behaviors in infancy and early childhood that would ultimately result in fewer experiences with social sources of information and decreased social learning (Dawson et al., 2005). When young children with ASD lack the motivation to participate in activities where social skills are typically forged, the resulting impoverished social environment further compounds social communication impairments and, thereby, negatively impacts the development of social cognition and social communication (Kuhl, Coffey-Corina, Padden, & Dawson, 2005; Schultz et al., 2000). Consistent with this model, very young children with ASD demonstrate decreased orienting to social stimuli (Dawson, Meltzoff, Osterling, & Rinaldi, 1998; Klin, Lin, Gorrindo, Ramsay, & Jones, 2009), and atypical social orienting has been shown to predict decreased social competence in adolescents with ASD (Klin, Jones, Schultz, Volkmar, & Cohen, 2002). There is also evidence that social motivation remains impaired in individuals with ASD despite growth in other areas of cognitive development. For instance, older children with ASD report experiencing less pleasure from social rewards (Chevallier, Grezes, Molesworth, Berthoz, & Happe, 2012) and social stimuli are less salient for individuals with ASD (Chawarska et al., 2014; Sasson et al., 2007). More generally, ASD is characterized by lower levels of reward responsivity (for review see Clements et al., 2018) and impaired reward-based learning (Johnson, Yechiam, Murphy, Queller, & Stout, 2006). Finally, intriguing preliminary findings that social communication abilities of individuals with ASD appear to improve under certain motivational contexts suggests that core social communication impairments in ASD may be influenced by motivational factors (Peterson, Slaughter, Peterson, & Premack, 2013).
3). Brain reward circuitry and motivated social behavior
The mesocorticolimbic dopamine system is the primary neural network responsible for reward processing behaviors including reward approach, reward receipt, and reward-based learning (Schultz, 2015). Reward processing is mediated by dense dopaminergic projections originating from the ventral tegmental area (VTA) that project to the striatum, orbitofrontal cortex (OFC), ventromedial prefrontal cortex (vmPFC), and the anterior cingulate cortex, forming a mesolimbic dopamine pathway sensitive to the magnitude and probability of reward (Berridge, Robinson, & Aldridge, 2009; Schultz, 2000; Treadway & Zald, 2013). Reward-predictive dopamine bursts originating in VTA send signals to the striatum, including the nucleus accumbens (NAc), that mediate approach behaviors towards salient goals (Bjork & Hommer, 2007; Knutson & Cooper, 2005). Functioning of the mesocorticolimbic dopamine system can be assessed using a multitude of method, including dopamine levels, dopamine metabolite and precursor concentrations, and dopamine receptor binding activity.
The neural circuits that mediate reward processing may have evolved, at least in part, to facilitate social affiliation and attachment (Insel, 2003; Trezza, Damsteegt, Achterberg, & Vanderschuren, 2011). The ascending mesolimbic dopamine system mediates responses to social and nonsocial incentives (Gunaydin & Deisseroth, 2014) and dopamine transmission in the nucleus accumbens modulates social play behaviors (Manduca et al., 2016). Social interaction mobilizes the same mesolimbic network that is active during the processing of nonsocial rewards such as food, money, and drugs of addiction (Schultz, 1997; Zink, Pagnoni, Martin-Skurski, Chappelow, & Berns, 2004). Further, functioning of the mesolimbic circuit in the context of rewarding stimuli is associated with high valuation, incentive salience, and motivation (Smith, Berridge, & Aldridge, 2011). Therefore, reward mechanisms may serve to encode and consolidate positive memories of social experiences, facilitating social abilities hypothesized to be impaired in ASD (Chevallier, Kohls, et al., 2012).
4). Dopaminergic Impairments in ASD
A. Preclinical Evidence
Preclinical and clinical findings suggest that impaired mesolimbic dopamine functioning may contribute to cardinal features of ASD (Dichter & Adolphs, 2012; Hara et al., 2015; Staal, 2015). For example, the valproic acid model of ASD is one of the most widely used ASD animal models (Mabunga, Gonzales, Kim, Kim, & Shin, 2015); prenatal valproic acid exposure causes ASD-like phenotypes and a cascade of neurobiological changes, including excitatory/inhibitory neural imbalances linked to increased basal dopamine in the frontal cortex (Narita et al., 2002), hyperactive mesocortical dopamine in response to stress (Nakasato et al., 2008), and changes in locomotor behavior akin to that observed in striatal dopamine-depleted animals (Shaywitz, Yager, & Klopper, 1976). Additionally, SHANK3 has been proposed as candidate gene for ASD (Durand et al., 2007; Pinto et al., 2010). SHANK3 is expressed in the VTA, and manipulations that reduce social interaction, such as social defeat stress, lead to significant reductions in VTA SHANK3 expression (Warren et al., 2013). In mice, disruption of SHANK3 leads to impaired social reward phenotypes (Wang et al., 2011). Additionally, SHANK3 impairs the maturation of excitatory synapses onto VTA dopamine neurons and results in reduced in vivo burst activity of dopamine neurons. Further, optogenetic activation of VTA dopamine neurons increases social preference in SHANK3-deficient mice, linking sufficiency of dopamine neuron activity to social interaction functions (Bariselli et al., 2016).
B. Clinical Evidence
Clinically, there is evidence of impaired mesolimbic functioning in ASD. Individuals with ASD demonstrate altered effort-based decision making for rewards (Damiano, Aloi, Treadway, Bodfish, & Dichter, 2012; Mosner et al., 2017; Watson et al., 2015). Recent findings also show that individuals with ASD experience difficulty in social reward-based learning (Li et al., 2017). Additionally, social communicative abilities may improve in ASD under optimal motivational conditions (Chevallier, Kohls, et al., 2012; Lahaie et al., 2006; Wang, Dapretto, Hariri, Sigman, & Bookheimer, 2004). For instance, Peterson and colleagues (2013) demonstrated that adequate incentives boosted motivation and, as a result, improved performance on a theory-of-mind task in children with ASD.
Common polymorphisms of the dopamine D4 receptor gene and the dopamine transporter gene are related to challenging behaviors (Gadow, Devincent, Olvet, Pisarevskaya, & Hatchwell, 2010) and repetitive behaviors (Gadow, DeVincent, Pisarevskaya, et al., 2010) in ASD, and linkages have been reported between polymorphisms of the dopamine-3-receptor gene and striatal volumes (Staal, Langen, van Dijk, Mensen, & Durston, 2015) as well as symptoms of repetitive behaviors (Staal, 2015) in ASD. Oxytocinergic abnormalities in ASD (Bell, Nicholson, Mulder, Luty, & Joyce, 2006; Depue & Morrone-Strupinsky, 2005; Dolen, 2015; Ross, Cole, et al., 2009) and initial reports of the therapeutic effects of intranasal oxytocin administration for treating core ASD symptoms (Andari et al., 2010; Guastella et al., 2010; Guastella, Mitchell, & Dadds, 2008) suggest an etiologically-relevant role for mesolimbic dopamine functioning in ASD. Although oxytocin impacts multiple systems, there are dense oxytocin projections within the mesolimbic dopamine system, including oxytocin neurons that project to both the ventral tegmental area and the nucleus accumbens (Ferguson, Young, & Insel, 2002; Insel & Young, 2001; Ross, Freeman, et al., 2009), and oxytocin receptor activation plays an important role in the activation of reward pathways during pro-social behaviors, suggesting that oxytocin may improve social symptoms in ASD via effects on the mesolimbic dopamine system (Choe et al., 2015; Dolen, Darvishzadeh, Huang, & Malenka, 2013; Olazabal & Young, 2006).
Perhaps the strongest evidence for reward-sensitive mesolimbic impairment in ASD stems from functional neuroimaging studies (for review see Clements et al., 2018). These studies have generally, but not always, found striatal hypoactivation in individuals with ASD during the processing of monetary rewards in the context of incentive delay tasks (Delmonte et al., 2012; Dichter, Felder, et al., 2012; Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012) as well as a range of other non-reward cognitive tasks (Carlisi et al., 2017; Choi et al., 2015; Kohls et al., 2013; Schmitz et al., 2008; Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010; Solomon et al., 2015). Further, there is evidence for functional mesolimbic impairments in response to social rewards (Delmonte et al., 2012; Dichter, Felder, et al., 2012; Scott-Van Zeeland et al., 2010) and negative social reinforcements (Damiano et al., 2015) in ASD. It is noteworthy that functional mesolimbic impairments are evident in response to a range of rewards, including responses to food cues (Cascio et al., 2012) and special interests (Cascio et al., 2014; Dichter, Felder, et al., 2012), highlighting that functional mesolimbic impairments in ASD may not be specific to social stimuli but may extend more broadly to range of rewarding stimuli (Dichter & Adolphs, 2012).
5). Neuroinflammation as a Mechanistic Account of Reward Processing Impairments in ASD
The remainder of this review presents evidence that ASD is characterized by aberrant neuroinflammatory processes and that these processes represent a mechanistic factor contributing to the development and maintenance of reward processing impairments in ASD via effects on mesocorticolimbic dopamine systems. There is evidence to suggest a negative relationship between neuroinflammatory response and dopamine signaling via different pathways and receptors (i.e., D1, D2, D3). Specifically, decreases in dopaminergic activity have been linked with increased levels of inflammatory markers (Yan et al., 2015), while heightened dopamine responses are associated with attenuated cell-death as a result of immunological insult (e.g., lipopolysaccharide (LPS); Iravani et al., 2008). While dopaminergic signaling clearly impacts immunological responses, this review will largely provide evidence of the immunological effects on dopamine and its metabolites. These distinct perspectives may, in fact, reflect a bidirectional association between dopamine reactivity and inflammation. Linkages between neuroinflammation and mesocorticolimbic dopamine function have been previously described in the context of other psychiatric conditions (e.g., depression and anhedonia; Felger & Treadway, 2017) and neurodegenerative disorders (e.g., Parkinson’s disease; Kaur, Gill, Bansal, & Deshmukh, 2017), and there is emerging preclinical and clinical data that comparable linkages may be characteristic of ASD as well.
6). Overview of the Immune System
The immune system is a complex defense mechanism that serves to protect an organism from potentially harmful pathogens via innate and adaptive systems (see Parkin & Cohen, 2001 for a review). The innate immune system is a nonspecific first line of defense against antigens and includes physical barriers such as the skin, chemical barriers within the blood (e.g., antimicrobial proteins), and cells that attack potentially harmful foreign bodies. Cells involved within the innate system include neutrophils, dendritic cells, natural killer cells, mast cells, monocytes, and macrophages. Resident brain macrophages, known as microglia, serve a maintenance function by aiding in synaptic maturation and phagocytosis, a process by which pathogens or cell debris are degraded and destroyed (Takano, 2015). Microglia and other macrophages are also responsible for releasing cell-signaling molecules (i.e., cytokines) when activated by immunological insults. Cytokines, including interferons (e.g., IFN-α) and interleukins (e.g., IL-6, IL-1β), regulate pro- and anti-inflammatory responses and facilitate communication between the innate and adaptive arms of the immune system (Masi, Glozier, Dale, & Guastella, 2017). Adaptive immunity is then triggered when innate immunity is unable to ward off a pathogen, and this branch of the immune system is comprised of white blood cells, called B cells and T cells. In addition, the adaptive system serves a memory function for subsequent pathogen activations. Together, the innate and adaptive immune systems engage in complex communications with one another to maintain a defense against foreign and native threats (Filiano, Gadani, & Kipnis, 2015).
7). Effects of Neuroinflammation on Reward Processing
A. Preclinical Studies
The link between neuroinflammation and dopamine dysregulation has been explored in numerous studies using a variety of stimuli (e.g., interferon-alpha, IFN-α, endotoxin) known to induce systemic inflammatory responses. Shuto, Kataoka, Horikawa, Fujihara, and Oishi (1997) first observed significantly depleted dopamine levels in cytokine-treated mice compared to mice treated with saline. More recently, studies examining the offspring of immune-challenged rodents have reported significant dopamine disruptions in canonical reward processing brain regions (Kirsten, Taricano, Flório, Palermo-Neto, & Bernardi, 2010; Vuillermot, Weber, Feldon, & Meyer, 2010). Further, animals exposed to inflammatory stimuli have also shown behavioral impairments, such as decreased motivation to work for food (Felger et al., 2013; Nunes et al., 2014), inhibited environmental exploring (Felger et al., 2007), and decreased sexual motivation (Bernardi et al., 2010). Using positron emission tomography (PET) imaging, which allows for the examination of metabolic and neurochemical changes in the brain, Felger and colleagues (2013) observed reductions in dorsal striatal dopamine release following chronic IFN-α administration in rhesus monkeys. Similarly, single administrations of IFN-α in rats resulted in reduced striatal dihydroxphenylacetic acid (DOPAC), a metabolite of dopamine (Kamata, Higuchi, Yoshimoto, Yoshida, & Shimizu, 2000; Sato et al., 2006). IFN-α administration in rats has also been associated with decreased dopamine within the amygdala and raphe nucleus (Kitagami et al., 2003). Together, these findings demonstrate the potential for inflammatory responses to downregulate neural dopamine in preclinical samples. However, some rodent studies have also reported increases in neural dopamine following IFN-α administration (Kumai et al., 2000; Sato et al., 2006), and Felger and Miller (2012) suggest that this discrepancy within the preclinical literature may reflect differences in cytokine dosing and length of exposure. Overall, these preclinical findings contribute to a model of inflammation-induced disruption of corticostriatal dopamine pathways.
B. Clinical Studies
Consistent with preclinical evidence, human fMRI studies investigating the impact of inflammation on the brain have also shown distinct effects on dopaminergic neural circuitry (Capuron et al., 2007; Capuron et al., 2012; Dowell et al., 2016; Eisenberger et al., 2010; Felger et al., 2013). For example, experimentally-induced inflammation has been shown to attenuate ventral striatal activation in response to reward tasks (Capuron et al., 2012; Eisenberger et al., 2010). Healthy individuals exposed to a single administration of low-dose endotoxin showed significantly attenuated neural activation within the ventral striatum to monetary reward cues relative to placebo-treated controls (Eisenberger et al., 2010). Typhoid vaccination, which evokes a brief inflammatory response, has also been associated with decreased ventral striatal response to reward prediction errors, as well as increased insula encoding of punishment prediction error, relative to placebo-treated controls (Harrison et al., 2016). Similarly, patients with chronic hepatitis C virus (HCV) receiving IFN-α treatment showed diminished ventral striatal activation during a hedonic reward task compared to untreated patients (Capuron et al., 2012). Within this same study, attenuated ventral striatal activity was significantly correlated with patient reports of decreased motivation. However, in contrast to these findings of decreased striatal response to reward stimuli following an inflammatory challenge, there are reports of increased ventral striatal sensitivity to social rewards and social threats following endotoxin exposure (Inagaki et al., 2015; Muscatell et al., 2016). Consequently, Inagaki (2015) and Muscatell (2016) hypothesized that inflammation-induced neural responses may be highly sensitive to the context in which they are processed. These discrepancies highlight the need for future work to evaluate the effects of experimentally-induced inflammation on neural and behavioral responses to different classes of rewards.
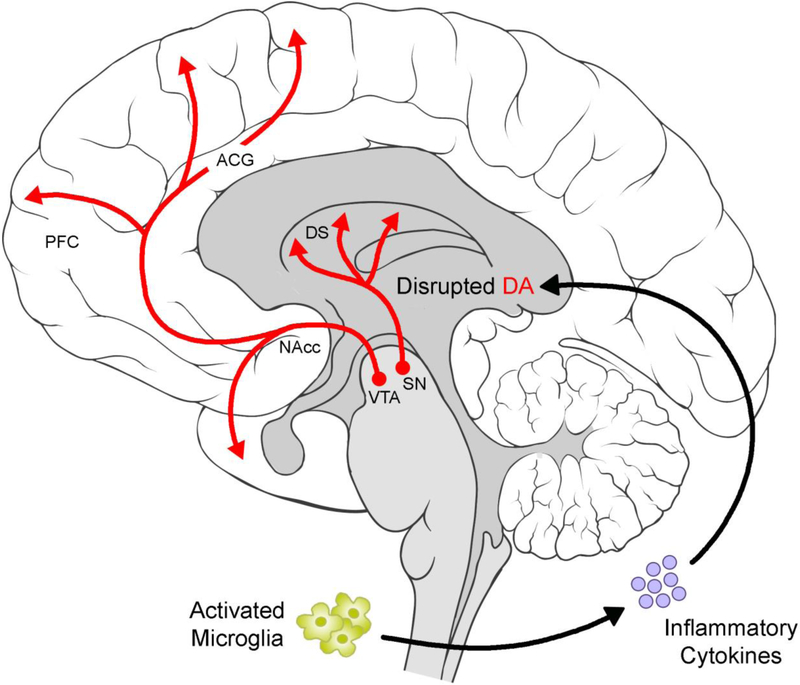
Molecular neuroimaging has further clarified linkages between neuroinflammation and impaired mesolimbic dopamine circuitry functioning. For example, examinations of neurometabolic functioning in HCV patients following a three-month-long IFN-α treatment course revealed increased resting state glucose metabolism in dorsal striatal regions (Juengling et al., 2000). Based on findings from the Parkinson’s disease literature, this hypermetabolic state is thought to reflect the loss of inhibitory dopamine neurons (Eidelberg et al., 1994; Mentis et al., 2002; Wichmann & DeLong, 1999). These findings have since been replicated and expanded upon by Capuron and colleagues (2007) using a sample of patients with malignant melanoma receiving IFN-α therapy over the course of four weeks: not only was dorsal striatal glucose metabolism enhanced following IFN-α treatment, but this effect was also significant within ventral striatal regions. Relatedly, Capuron and colleagues (2012) found that IFN-α therapy for patients with HCV resulted in increased uptake of dopamine neurotracer 18F-Dopa and decreased 18F-Dopa turnover within ventral and dorsal striatal regions, reflecting significant changes in presynaptic striatal dopamine function as a result of treatment. Specifically, these changes are indicative of decreased dopamine synthesis and release. Together these preclinical and clinical findings demonstrate the potential for inflammatory responses to alter dopaminergic activity within neural reward circuitry (see Figure 1), and thereby impact animal and human reward and motivational behavior.
Figure 1.
Activated microglia produce inflammatory cytokines, which contribute to impaired dopaminergic functioning within midbrain and corticostriatal regions (for a review see Felger & Treadway, 2017). ACG = anterior cingulate gyrus, DA = dopamine, DS = dorsal striatum, NAcc = nucleus accumbens, PFC = prefrontal cortex, SN = substantia nigra, VTA = ventral tegmental area. Brain art adapted from illustrations created by Patrick Lynch, medical illustrator, and C. Carl Jaffe, MD, cardiologist (available at https://commons.wikimedia.org/wiki/File:Brain_human_lateral_view.svg and https://commons.wikimedia.org/wiki/File:Brain_human_sagittal_section.svg) and licensed under a Creative Commons Attribution 2.5 Generic License, 2006 (CC BY 2.5).
Finally, some of the strongest documented links between immune dysfunction and reward-oriented behaviors have been illustrated in studies of individuals exposed to inflammatory stimuli (Eisenberger et al., 2010) or those undergoing immunotherapy to treat medical illness (Capuron et al., 2002; Capuron et al., 2012). Following an endogenous or experimentally-induced immune response, individuals in these studies have repeatedly exhibited symptoms collectively referred to as “sickness behavior”. Sickness behavior is a set of vegetative, somatic, and psychological responses that are considered to be adaptive in recovery to illness (Dantzer, O'Connor, Freund, Johnson, & Kelley, 2008). Behavioral presentation is characterized by low appetite, fatigue, social withdrawal, and generally low motivation (Dantzer & Kelley, 2007). Following exposure to a single administration of low-dose endotoxin, Eisenberger et al. (2010) reported that healthy adult participants displayed significantly greater levels of sickness behavior compared to placebo-treated participants. These symptoms of sickness behaviors were also accompanied by increased endorsement of symptoms of depression and anhedonia, conditions that are characterized by and diagnosed based on impaired responses to pleasure and decreased motivation (Nestler & Carlezon, 2006). These findings have been corroborated in patients receiving IFN-α treatments, who, compared to patients administered placebo medications, reported increased sickness behavior and depressed mood (Capuron et al., 2002; Capuron et al., 2007; Capuron et al., 2012).
8). Evidence of Neuroinflammation in ASD
A. Maternal Immune Activation and ASD Risk
Some of the strongest etiologic evidence of neuroinflammatory processes in ASD derive from studies of maternal immune activation (MIA). These studies highlight that immune dysregulation in ASD may be triggered as early as during fetal development by innate maternal antibodies, as well as maternal infection during pregnancy. Because the blood-brain barrier is increasingly permissive during gestation (Adinolfi, Beck, Haddad, & Seller, 1976), maternal immune cells are capable of accessing the developing fetal brain, making these developing neural networks more susceptible to harm (Karpiak & Rapport, 1975). Maternal infection during pregnancy has shown deleterious effects and hospital admissions to maternal viral infection in the first trimester or bacterial infection within the second trimester significantly increased the risk of offspring developing ASD (Atladóttir et al., 2010; Estes & McAllister, 2016). During maternal infection, children who go on to develop ASD may be exposed in utero to maternal anti-brain antibodies (Immunoglobulin G (IgG); Edmiston, Ashwood, & Van de Water, 2017). These antibodies appear to respond in a heightened manner to fetal brain tissue (i.e., fetal brain tissue is treated as a foreign pathogen), resulting in neurotoxic effects that increase the risk for developing ASD (Braunschweig & Van de Water, 2012; Zimmerman et al., 2007). Consistent with this framework, there are elevated rates of autoimmune disorders (e.g., Lupus, rheumatoid arthritis, celiac disease, hypothyroidism) in mothers of children with ASD (Comi, Zimmerman, Frye, Law, & Peeden, 1999; Keil et al., 2010), and children born to mothers with autoimmune disorders are 34% more likely to be diagnosed with ASD (Chen et al., 2016).
B. Autoimmune Anti-Brain Antibodies in ASD
Anti-brain antibodies are not only present in mothers of children with ASD but are also present in children and adults with ASD (Hoffmann et al., 2016). These anti-brain autoantibodies target a variety of neural components (Connolly et al., 1999; Singh, Singh, & Warren, 1997; Singh, Warren, Odell, Warren, & Cole, 1993) and are significantly more prominent in individuals with ASD (Goines et al., 2011; Wills et al., 2009). In addition, these antibodies appear to be most significantly elevated in ASD within brain regions associated with reward processing, including the dorsal striatum, prefrontal cortex (PFC) and anterior cingulate gyrus (ACG), as well as the cerebellum (Singer et al., 2006). In a study investigating the effects of human-derived serum antibodies on rodent brain proteins using immunoblotting with specific antigens for myelin basic protein and glial acidic fibrillary protein, Zimmerman and colleagues (2007) showed that sera from ASD children were significantly more reactive to rodent brain proteins than sera antibodies of unaffected siblings and their parents. This finding highlights the potentially neurotoxic effects of human-derived serum antibodies. In some instances, these anti-brain antibodies have also been associated with more severe cognitive, motor, adaptive, language and developmental impairments in individuals with ASD (Piras et al., 2014).
C. Aberrant Cytokine Concentrations in ASD
Glial cells (i.e., astrocytes and microglia) provide structural support and nutrients for neurons and play a critical role in phagocytosis, and there is evidence of increased glial activation in ASD. Upregulated glial activation has been shown to be directly related to alterations in peripheral and central cytokine expression in ASD (for review see Masi et al., 2017; Masi et al., 2015). This aberrant cytokine profile in ASD is evidenced by both increased pro-inflammatory responses (Ashwood et al., 2011b; Chez, Dowling, Patel, Khanna, & Kominsky, 2007) and decreased anti-inflammatory responses (Ashwood et al., 2008; El Gohary, El Aziz, Darweesh, & Sadaa, 2015; Okada et al., 2007), contributing to a largely pro-inflammatory profile in ASD. Altered cytokine concentrations have been associated with more severe behavioral features of the disorder, including: increased stereotypies, greater impairments in communication and daily living skills, increased hyperactivity, and increased overall ASD symptom severity (Ashwood et al., 2012; Ashwood et al., 2011b; El Gohary et al., 2015; Piras et al., 2014), as well as greater nonverbal and verbal developmental disruption (Careaga et al., 2017). Ashwood and colleagues (2011b) reported significant differences in cytokine concentrations between individuals with ASD who did and who did not experience regression, such that children with regressive ASD exhibited increased cytokine levels relative to children with no history of regression. These findings have provided support for the use of immunological markers in exploring endophenotypic subtypes of ASD and suggest a heightened inflammatory environment in some individuals with the disorder (Bryn et al., 2017).
Finally, increased lethargy in individuals with ASD has been associated with increases in eotaxin, IL-8, and IL-12p40, whereas greater stereotypy has been associated with increases in eotaxin, IL-1β, IL-6, IL-8, and IL-12p40 (Ashwood et al., 2011a, 2011b). More severe nonverbal communication impairments in individuals with ASD have been linked with enhanced concentrations of IL-4 (Ashwood et al., 2011b). Finally, increased hyperactivity and worsened cognitive and adaptive ability in ASD have been shown to accompany increased IL-8 levels (Ashwood et al., 2011b). Individuals with ASD with heightened proinflammatory profiles displayed greater impairment in social affect, as well as cognitive, language, and motor development, compared to individuals with ASD with relatively attenuated proinflammatory profiles (Careaga et al., 2017).
9). Effect of Neuroinflammation on Reward Behaviors and Dopaminergic Circuitry in ASD
A. Preclinical Findings
There is a growing literature examining the effects of experimentally-induced neuroinflammation on reward-related behaviors and associated mesocorticolimbic brain dopamine function in preclinical models. However, there is inconsistency with respect to the directionality of this disruption, perhaps due at least in part to the specific immunological stimuli administered. For example, both polyinosinic:polycytidylic acid (poly I:C) and lippopolysaccaride (LPS) have been administered to pregnant rodents to mimic viral and bacterial infections, respectively. Offspring of these immune-challenged mothers have repeatedly shown significant dysregulation in striatal and prefrontal brain regions, and they serve as ASD preclinical models of inflammation. Adult and peripubertal offspring of LPS-exposed rodents demonstrate consistently attenuated dopamine activity (measured by dopamine directly as well as its metabolites, DOPAC and homovanillic acid (HVA)) within striatal and thalamic brain regions (see Table 1; Kirsten & Bernardi, 2017; Kirsten, Taricano, Flório, et al., 2010; Soto et al., 2013). An exception to this trend includes a report of increased dopamine concentrations within the nucleus accumbens of adult rats born to LPS-exposed mothers (Romero, Guaza, Castellano, & Borrell, 2010). This same study reported a developmentally-mediated interaction between MIA and striatal dopamine levels such that peripubertal rats showed decreased striatal dopamine whereas adult rats showed increased striatal dopamine relative to saline-exposed offspring.
Table 1.
Evidence of neuroinflammation-induced dopamine alterations within corticostriatal brain regions in rodent MIA models of ASD
| Dopamine Marker | Developmental Stage |
Sex | Region | Finding | Study |
|---|---|---|---|---|---|
| Prenatal LPS Exposure | |||||
| DA | Peripubertal | Male/Female | NAcc | ↓ | (Romero et al., 2010) |
| DA | Adult | Male/Female | NAcc | ↑ | (Romero et al., 2010) |
| DA | Adult | Male | Striatum | ↓ | (Kirsten, Taricano, Flório, et al., 2010) |
| DA | Adult | Female | Striatum | ↓ | (Soto et al., 2013) |
| DOPAC | Adult | Male | Striatum | ↓ | (Kirsten, Taricano, Flório, et al., 2010) |
| DOPAC | Adult | Female | Striatum | ↓ | (Soto et al., 2013) |
| HVA | Adult | Male | Striatum | ↓ | (Kirsten, Taricano, Flório, et al., 2010) |
| HVA | Adult | Male/Female | Hypothalamus | ↓ | (Kirsten & Bernardi, 2017) |
| HVA | Adult | Female | Striatum | ↓ | (Soto et al., 2013) |
| Prenatal Poly I:C Exposure | |||||
| D1 Receptor Binding | Adult | Male/Female | DS, NAcc Shell | ↑ | (Vuillermot et al., 2010) |
| D1 Receptor Binding | Adult | Male | PFC | ↓ | (Meyer, Nyffeler, et al., 2008) |
| D2 Receptor Binding | Adult | Male | PFC | ↓ | (Meyer, Nyffeler, et al., 2008) |
| D2 Receptor Binding | Adult | Male/Female | Striatum | ↓ | (Ozawa et al., 2006) |
| DA | Adult | Male/Female | Striatum | ↑ | (Zuckerman, Rehavi, Nachman, & Weiner, 2003) |
| DA | Fetal and Adult | Male/Female | SN | ↑ | (Vuillermot et al., 2010) |
| DA | Adult | Male/Female | VTA | ↑ | (Vuillermot et al., 2010) |
| DAT | Peripubertal | Male/Female | DS, NAcc Core, NAcc Shell | ↓ | (Vuillermot et al., 2010) |
| DAT | Peripubertal | Male/Female | NAcc | ↓ | (Vuillermot et al., 2010) |
| DAT | Fetal | Male/Female | VTA, SN | ↑ | (Meyer, Engler, Weber, Schedlowski, & Feldon, 2008) |
| DOPAC | Adult | Male/Female | Striatum | ↑ | (Ozawa et al., 2006) |
| TH | Adult | Male | DS | ↑ | (Meyer, Nyffeler, et al., 2008) |
| TH | Fetal | Male/Female | DS | ↑ | (Vuillermot et al., 2010) |
| TH | Peripubertal | Male/Female | DS, NAcc Core, NAcc Shell | ↓ | (Vuillermot et al., 2010) |
| TH | Adult | Male/Female | NAcc Core, NAcc Shell | ↑ | (Vuillermot et al., 2010) |
| TH | Fetal | Male/Female | VTA, SN | ↑ | (Meyer, Engler, et al., 2008) |
Note. DA = dopamine; MIA = maternal immune activation; ASD = autism spectrum disorder; DAT = dopamine transporter; DOPAC = dihydroxyphenylacetic acid; HVA = homovanillic acid; TH = tyrosine hydroxylase; cerebral cortex; DS = dorsal striatum; NAcc = nucleus accumbens; PFC = prefrontal cortex; SN = substantia nigra; VTA = ventral tegmental area
Alternatively, offspring of poly I:C-exposed rodents primarily show a more variable dopamine profile that appears to be developmentally mediated (see Table 1; Meyer, Engler, et al., 2008; Meyer, Nyffeler, et al., 2008; Ozawa et al., 2006; Vuillermot et al., 2010; Zuckerman et al., 2003). Much like peripubertal rodents exposed to bacterial LPS in utero, peripubertal rodents born to viral poly I:C-exposed mothers show decreased levels of dopamine markers, including diminished tyrosine hydroxylase (TH) and dopamine transporter (DAT) expression within the NAcc and dorsal striatum (Vuillermot et al., 2010). Respectively, these proteins are tasked with dopamine biosynthesis and reuptake (Daubner, Le, & Wang, 2011; Vaughan & Foster, 2013). Poly I:C-induced alterations in dopamine binding have also been observed, although findings are mixed. D2 receptors in adult rodents downregulate binding in the striatum and PFC in response to prenatal poly I:C administration (Meyer, Nyffeler, et al., 2008; Ozawa et al., 2006) whereas the pattern for D1 receptors is less uniform. Vuillermot and colleagues (2010) reported increased D1 receptor binding within the dorsal striatum and the NAcc shell as a result of prenatal exposure to poly I:C, yet this pattern differed with previous findings that showed no difference between striatal D1 binding in rodents exposed to poly I:C in utero (Ozawa et al., 2006). This discrepancy may be attributed to differences in dopamine receptor function, as each receptor projects its output to unique neural regions via distinct pathways. Broadly, it has been suggested that these receptors interact in both synergistic and opposing fashions (Keeler, Pretsell, & Robbins, 2014). In addition, these receptor-specific responses may reflect D1 receptor tendencies to respond to phasic (i.e., more acute) dopamine activity, whereas D2 is more sensitive to tonic (i.e., extended) dopamine release (Dreyer, Herrik, Berg, & Hounsgaard, 2010). The precise timing of the prenatal immunological insult may also account for the observed difference in dopamine receptor response. Specifically, immunological insults presented earlier in gestation may result in hyperactive striatal DA, whereas insults encountered later in pregnancy may be associated with hypoactive striatal dopamine (Meyer, Yee, & Feldon, 2007).
Additionally, prenatal endotoxin exposures lead to increased maternal inflammatory response and enhanced cytokine concentrations in the offspring of affected mothers. For example, adult offspring of immune-challenged rodents showed significantly greater serum cytokines levels including: IL-1β, IL-2, IL-6, and TNF-α (Borrell, Vela, Arévalo-Martin, Molina-Holgado, & Guaza, 2002; Kirsten, Lippi, Bevilacqua, & Bernardi, 2013; Romero et al., 2010). This finding corroborates results describing increased inflammatory profiles in individuals with ASD and indicates that prenatal exposure to immunological insults may have lifelong impacts on neuroinflammatory profiles. Additionally, such inflammation-induced disruptions are associated with impairments in rodent social behaviors (Kirsten, Taricano, Maiorka, Palermo-Neto, & Bernardi, 2010) and restricted and repetitive behaviors, such as self-grooming (Kirsten & Bernardi, 2017). Discrepancies within the preclinical literature between broader neuroinflammation-induced dopamine dysregulation and ASD-specific immune activated models may reflect differences in the effect of MIA and post-natal immunological disruptions on dopamine function. It appears that the developmental stage in which the immunological disturbance occurs significantly influences the effect of distinct inflammatory stimuli on dopamine functioning. Although previous studies have noted significant impacts of direct LPS administration in adult rodents and adult offspring of LPS-treated mothers, it is unclear how these inflammation-induced dopamine disruptions differ from one another as a function of the timing of immunological insult (Dunn, 2006), highlighting the importance of a developmental framework to understand the impact of neuroinflammation on dopamine neural pathways.
B. Clinical Findings
Evidence of linkages between mesolimbic dopamine dysfunction and neuroinflammation in ASD is supported by the presence of anti-brain antibodies, heightened microglial activation, and increases in cytokine concentrations in mesocorticostriatal neural networks in ASD (see Table 2; Morgan et al., 2010; Singer et al., 2006; Vargas, Nascimbene, Krishnan, Zimmerman, & Pardo, 2005).
Table 2.
Studies demonstrating Corticostriatal Neuroinflammation in ASD
| Neuroinflammatory Marker | Sample | Technique | Region | Study |
|---|---|---|---|---|
| Microglial Activation | ASD, TDC Adults | PET Imaging | ACG, MFG, OFC | (Suzuki et al., 2013) |
| Anti-brain Antibody Reactivity | ASD, TDC Children | Western Immunoblotting | CN, CX | (Singh & Rivas, 2004) |
| Anti-brain Antibody Reactivity | ASD, TDC Children | Western Immunoblotting | CN, PT, PFC, CG | (Singer et al., 2006) |
| Anti-brain Antibody Reactivity | ASD, TDC Children | ELISA | PT | (Singer et al., 2006) |
| Astroglial Activation | ASD, TDC Post-mortem | Western Immunoblotting | ACG, MFG | (Vargas et al., 2005) |
| Cytokine: TGF-β1 | ASD, TDC Post-mortem | Protein Tissue Arrays | ACG, MFG | (Vargas et al., 2005) |
| Cytokine: TNF-α | ASD, TDC Post-mortem | Multiplexed Bead Analysis | CX | (Li et al., 2009) |
| Cytokine: IFN-γ | ASD, TDC Post-mortem | Multiplexed Bead Analysis | CX | (Li et al., 2009) |
| Cytokine: IL-6 | ASD, TDC Post-mortem | Protein Tissue Arrays | ACG | (Vargas et al., 2005) |
| Cytokine: IL-6 | ASD, TDC Post-mortem | Multiplexed Bead Analysis | CX | (Li et al., 2009) |
| Cytokine: IL-10 | ASD, TDC Post-mortem | Protein Tissue Arrays | ACG | (Vargas et al., 2005) |
| Microglial Activation | ASD, TDC Post-mortem | Iba-1 | PFC | (Morgan et al., 2010) |
| Microglial Density | ASD, TDC Post-mortem | Iba-1 | PFC | (Morgan et al., 2010) |
| Microglial Density | ASD, TDC Post-mortem | Immunocytochemistry | FI | (Tetreault et al., 2012) |
| Microglia-Neuron Clustering | ASD, TDC Post-mortem | K-function Spatial Clustering | PFC | (Morgan et al., 2012) |
Note. ASD = autism spectrum disorder; TDC = typically developing control; ELISA = enzyme-linked immunosorbent assay; Iba-1 = Iba-1 Immunohistochemistry Protocol; ACG = anterior cingulate gyrus; CG = cingulate gyrus; CN = caudate nucleus; CX = cerebral cortex; FI = frontoinsular cortex; MFG = medial frontal gyrus; OFC = orbitofrontal cortex; PFC = prefrontal cortex; PT = putamen
A study of 68 children with ASD and 30 typically developing children revealed that the serum from 49% of children with ASD contained anti-brain antibodies in the caudate nucleus, and another 18% of the ASD group had antibody-positive sera within the cerebral cortex, whereas no one within the TDC group presented with anti-brain antibodies within the caudate nucleus (Singh & Rivas, 2004). A study using western immunoblotting found increased serum antibodies in the caudate nucleus, putamen, and PFC in individuals with ASD (Singer et al., 2006), and subsequent enzyme-linked immunosorbent assay (ELISA) optical density readings replicated these findings within the putamen. Post-mortem studies have revealed evidence of hyperactive microglia and astroglia in ASD, a pattern that may lead to neuronal alternations mediated by the secretion of inflammatory cytokines. Vargas and colleagues (2005) described significant increases in anti-inflammatory cytokine tumor growth factor-β1 (TGF-β1) within the MFG, ACG, and white matter of the post-mortem brains of 11 individuals with ASD. Since this seminal finding, other studies have also reported increased microglial activation, as well as enhanced microglial volume, in the brain tissue of individuals with ASD (Morgan et al., 2012; Morgan et al., 2010; Suzuki et al., 2013). Other cytokines such as interleukin-10 (IL-10) and interleukin-6 (IL-6) are also increased in the ACG in individuals with ASD. Investigations of the broader cerebral cortices of individuals with ASD corroborated this finding of significantly enhanced IL-6 levels and also reported increased concentrations of tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ; Li et al., 2009). In some cases, immunocytochemical staining analyses have revealed the cellular sources of these cytokine increases. For example, IL-6 was shown to be prominently expressed within activated astrocytes in cortical, cerebellar and subcortical white matter regions, whereas TGF-β1 showed expressions within both activated microglia and astrocytes in cerebellar regions only (Vargas et al., 2005).
There is a single molecular imaging study assessing binding potential of the 18-kdopamine translocator protein (formerly peripheral benzodiazepine receptor), a putative measure of microglial activation during neuroinflammation, in ASD. Using ([11C](R)-PK11195 positron emission tomography (PET) imaging, Suzuki and colleagues (2013) reported hyperactive microglia in the ACG, MFG, and OFC in adults with ASD. Finally, though not a direct measure of neuroinflammation, it is noteworthy that two large-sample studies have reported elevated extra-axial cerebrospinal fluid in 6-24 month old infants who developed ASD in a manner that predicted the severity of ASD symptoms (Shen et al., 2017; Shen et al., 2013). Given the central role that extra-axial cerebrospinal fluid plays in beta-amyloid clearance (Xie et al., 2013), paravenous drainage of inflammatory cytokines (Iliff et al., 2012), and meningeal immune surveillance (Louveau et al., 2015), extra-axial cerebrospinal fluid elevations in infants who develop ASD is consistent with a neuroinflammatory model of ASD.
Taken together, we propose that neuroimmune dysregulations may contribute to the etiology and maintenance of ASD by preferentially disrupting mesocorticolimbic dopamine, and thereby impacting reward behaviors in individuals with the disorder. In addition, the preclinical work in this area suggests that this effect may be developmentally mediated.
10). Future Directions
Although research to date broadly supports that neuroinflammation impacts dopamine circuitry functioning in ASD, there are still important considerations that require further examination. First, few studies have used molecular imaging (i.e., PET and single-photon emission computed tomography (SPECT)) in ASD to directly measure dopamine functioning. FMRI studies in ASD have largely established a profile of underactive mesolimbic responses within corticostriatal areas to reward stimuli; however, little is known about dopamine and dopamine metabolite activity and binding in ASD (Zürcher, Bhanot, McDougle, & Hooker, 2015). Although the field has a breadth of preclinical work examining dopamine and its metabolites in ASD-models, this lack of clinical findings makes it difficult to compare results across mammalian samples. The few human studies that have examined dopamine and dopamine metabolite binding in ASD have reported results of both increased (Ernst, Zametkin, Matochik, Pascualvaca, & Cohen, 1997) and decreased (Nieminen-von Wendt et al., 2004) presynaptic dopamine activity in ASD within prefrontal cortical regions. This discrepancy may be attributed to methodological differences, including the use of anesthesia for sedation which decreases striatal dopamine release in preclinical models (Schulte et al., 2000). Similarly, mixed results have been reported regarding DAT binding in ASD. Results from PET and SPECT imaging studies have provided varied findings including increased DAT binding in OFC in ASD (Nakamura et al., 2010), as well as no significant differences (Makkonen, Riikonen, Kokki, Airaksinen, & Kuikka, 2008). Future ASD molecular imaging studies will be needed to clarify the impact of neuroinflammation on dopamine functioning and behavior in individuals with ASD with emphasis on contextual and environmental factors that influence neuroinflammation.
In addition, PET and SPECT imaging may help to address some of the current limitations of post-mortem neuroinflammation studies. Although post-mortem analyses of inflammatory responses yield important findings across varied developmental and functional levels, these analyses are limited in their ability to connect these inflammatory profiles with behavioral functioning as they rely exclusively on retroactive reports of diagnoses and symptom expression. Metabolic imaging, on the other hand, allows researchers to link in vivo dopamine activity to current behavior. To date, many ASD post-mortem studies examining neuroinflammation have been limited in scope based on a priori regional brain hypotheses. Although there is clear evidence of enhanced neuroinflammatory profiles in cortical regions in ASD, only a small number of studies have examined neuroinflammation in the striatum and broader midbrain in ASD. In addition, it is difficult to disentangle whether the observed neuroimmunological state of post-mortem tissue represents a consequence of the disorder or an etiological factor contributing to the development of the condition.
Future research should also address the correspondence in ASD between peripheral measures concentrations of cytokine levels in serum or cerebrospinal fluid (CSF) and central measures of cytokine profiles. Findings from several studies have reported discrepancies between peripheral and central inflammatory markers, calling into question the potential validity of peripheral measures (Hannestad et al., 2013; Sandiego et al., 2015). Molecular imaging and post-mortem analyses provide direct measures of central cytokine levels and microglial activation, and this may be used to provide more accurate measures of neuroinflammatory signatures, as well as potentially validate findings from studies of peripheral measures concentrations of cytokine levels in ASD. In addition, a more nuanced understanding of the mediating (pro-) or suppressing (anti-) inflammatory responses of specific cytokines should be addressed. Although ASD is typically characterized as a pro-inflammatory condition (Haroon, Raison, & Miller, 2012), this categorization is not wholly consistent within the literature (Krakowiak et al., 2015; Molloy et al., 2006), and may, therefore, be too simplistic given the multiple functions of specific cytokines (Cavaillon, 2001).
Neuroinflammatory profiles in ASD provide a promising opportunity to better understand the heterogeneous presentations and biological mechanisms of heterogeneous reward-oriented behaviors in individuals with ASD. Researchers have begun to evaluate the potential of inflammatory markers, particularly cytokines, to serve as viable biomarkers of treatment response to ASD pharmacological interventions (for review see Masi et al., 2017), but more research is still needed to understand the how these immune signatures might characterize individual behavioral presentations, and, in turn, guide diagnostic decisions and treatment planning in ASD (Colburn et al., 2001). Although there are strong theoretical linkages between neuroinflammation and impaired reward processing in ASD, to date no research has empirically examined this association. Future research will be needed to directly assess the influence of neuroinflammation on reward-oriented behaviors and neural processes in individuals with ASD. More broadly, it will be vital to understand what differentiates neuroimmunological disruptions that result in ASD from those that predispose to other psychiatric disorders, which have also been linked to immune dysregulation (e.g., schizophrenia, major depression). Differentiating the role immunology plays in the etiology of different psychiatric disorders may provide insights into the behavioral impact of specific neuroinflammatory aberrations. To date, the literature suggests a complex interaction between maternal and fetal genotype and heightened immune responsivity determines risk for the development of ASD (Traglia et al., 2018). As previously mentioned, it is also possible that the timing, duration, and severity of the immunological insult may result in unique behavioral presentations. Because both timing and development appear crucial in accurately understanding the mechanisms by which these immunological disruptions take effect, future studies should continue to employ a developmental framework and examine the long-term impacts of acute and prolonged immune dysregulation.
An additional future line of research should address the effects of neuroinflammation and subsequent nigrostriatal dopamine dysregulation on motor systems in ASD, given the strong influence of neuroinflammation on motor systems (Felger & Treadway, 2017). Motor disturbances are present in many individuals with ASD, and these impairments represent some of the earliest indicators of dysfunction in the disorder (Fatemi et al., 2012; Gowen & Hamilton, 2013). Notably, much of the neuroinflammation literature in ASD points to significant inflammation-induced impairment in the cerebellum (Vargas et al., 2005), a brain region critically-involved in sensorimotor control and perception (Manto et al., 2012).
Clearly, neural systems mediated primarily by non-dopaminergic mechanisms influence neuroinflammation and ASD symptom expression. For example, one such candidate system is serotonergic (5-HT) pathways: 5-Hydroxytryptamine produces inflammation via the 5-HT2A receptor (Pierce, Xie, Peroutka, Green, & Levine, 1995), serotonin antagonists reduce the severity of acute inflammatory episodes (Harbuz et al., 1996), and serotonin depletion reduces inflammation (Harbuz, Marti, Lightman, & Jessop, 1998). It is noteworthy in this regard that modulation of the release of 5-HT from dorsal raphe neurons in the nucleus accumbens bidirectionally modifies sociability in a mouse model (16p11.2 deletion mice) of ASD (Walsh et al., 2018). Additionally, chronic administration of 5-HT agents increase dopamine D3 receptor gene expression in the shell of the nucleus accumbens (Lammers, Diaz, Schwartz, & Sokoloff, 2000a, 2000b) and nonclinical studies in humans have demonstrated that 5-HT acts as a modulator of positive affect (Zald & Depue, 2001). Although no studies to date have assessed the effects of 5-HT agents on neuroinflammatory and reward processes in ASD, molecular imaging studies have documented 5-HT abnormalities in ASD (Chandana et al., 2005; Chugani, 2002) and 5-HT agents have been shown to improve ASD symptoms in some (Williams, Wheeler, Silove, & Hazell, 2011) but not all (King et al., 2009) studies, suggesting that agents that impact 5-HT systems remain a promising avenue for future drug development in ASD (Veenstra-VanderWeele et al., 2012).
In summary, emerging evidence suggests dysregulation of the neuroimmune system in ASD may provide insight into the etiology, maintenance, and heterogeneity of the disorder across development. These heightened neuroinflammatory responses in ASD appear to preferentially impact corticostriatal, and particularly mesolimbic, dopamine functioning, and may, therefore, represent a mechanistic account of reward processing deficits in ASD. Future research is needed to better understand the complexities of immune dysfunction in ASD and its relation to dopamine dysregulation, particularly over the course of development. Continued empirical investigations of these linkages will help researchers and clinicians better understand the heterogeneous presentation of reward processing impairments in ASD and may serve to improve diagnosis, treatment selection, and treatment response monitoring for individuals with the disorder.
Highlights.
Brain inflammation has been observed in individuals with autism
Brain inflammation impacts reward-related behaviors and dopamine functioning.
This review suggests that brain inflammation impairs reward processing in autism.
This linkage, suggests anti-inflammatory treatments should be investigated to treat autism.
Acknowledgements
Support for this review was provided by MH110027, MH110933, and an Independent Investigator Award from the Brain & Behavior Research Foundation to G. Dichter and the National Center for Advancing Translational Sciences KL2TR001109 and K23 MH113733 to E. Walsh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing interests.
References
- Adinolfi M, Beck SE, Haddad SA, & Seller MJ (1976). Permeability of the blood-cerebrospinal fluid barrier to plasma proteins during foetal and perinatal life. Nature, 259(5539), 140–141. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel J-R, Zalla T, Herbrecht E, Leboyer M, & Sirigu A (2010).Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America, 107(9), 4389–4394. doi: 10.1073/pnas.0910249107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen RL, Croen LA, … Van de Water J (2008). Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. Journal of neuroimmunology, 204(1), 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-picciotto I, Hansen R, Isaac N, & Water JVD (2012). Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology, 232, 196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011a). Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of neuroimmunology, 232, 196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, & Van de Water J (2011b). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain, Behavior, and Immunity, 25(1), 40–45. doi: 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atladóttir HÓ, Thorsen P, Østergaard L, Schendel DE, Lemcke S, Abdallah M, & Parner ET (2010). Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders, 40(12), 1423–1430. [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … White T (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariselli S, Tzanoulinou S, Glangetas C, Prevost-Solie C, Pucci L, Viguie J, … Bellone C (2016). SHANK3 controls maturation of social reward circuits in the VTA. Nat Neurosci, 19(7), 926–934. doi: 10.1038/nn.4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CJ, Nicholson H, Mulder RT, Luty SE, & Joyce PR (2006). Plasma oxytocin levels in depression and their correlation with the temperament dimension of reward dependence. Journal of Psychopharmacology, 20(5), 656–660. [DOI] [PubMed] [Google Scholar]
- Bernardi MM, Kirsten TB, Matsuoka SM, Teodorov E, Habr SF, Penteado SHWN, & Palermo-Neto J (2010). Prenatal lipopolysaccharide exposure affects maternal behavior and male offspring sexual behavior in adulthood. Neuroimmunomodulation, 17(1), 47–55. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: 'liking', 'wanting', and learning. Curr Opin Pharmacol, 9(1), 65–73. doi: 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, & Hommer DW (2007). Anticipating instrumentally obtained and passively-received rewards: a factorial fMRI investigation. Behav Brain Res, 177(1), 165–170. doi: 10.1016/j.bbr.2006.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arévalo-Martin A, Molina-Holgado E, & Guaza C (2002). Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. [DOI] [PubMed] [Google Scholar]
- Braunschweig D, & Van de Water J (2012). Maternal autoantibodies in autism. Archives of Neurology, 69(6), 693–699. doi: 10.1001/archneurol.2011.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R, & Meltzoff AN (2005). The development of gaze following and its relation to language. Dev Sci, 8(6), 535–543. doi: 10.1111/j.1467-7687.2005.00445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryn V, Aass HCD, Skjeldal OH, Isaksen J, Saugstad OD, & Ormstad H (2017). Cytokine Profile in Autism Spectrum Disorders in Children. Journal of Molecular Neuroscience, 61(1), 1–7. doi: 10.1007/s12031-016-0847-z [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, & Miller AH (2002). Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, … Miller AH (2007). Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology, 32(11), 2384–2392. doi: 10.1038/sj.npp.1301362 [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, … Miller AH (2012). Dopaminergic Mechanisms of Reduced Basal Ganglia Responses to Hedonic Reward During Interferon Alfa Administration. Archives of general psychiatry, 69(10), 1044–1044. doi: 10.1001/archgenpsychiatry.2011.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M, Rogers S, Hansen RL, Amaral DG, Van de Water J, & Ashwood P (2017). Immune Endophenotypes in Children With Autism Spectrum Disorder. Biological psychiatry, 81(5), 434–441. doi: 10.1016/j.biopsych.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisi CO, Norman LJ, Lukito SS, Radua J, Mataix-Cols D, & Rubia K (2017). Comparative Multimodal Meta-analysis of Structural and Functional Brain Abnormalities in Autism Spectrum Disorder and Obsessive-Compulsive Disorder. Biol Psychiatry, 82(2), 83–102. doi: 10.1016/j.biopsych.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock J, Schauder KB, Loring WA, Rogers BP, … Bolton S (2014). Affective neural response to restricted interests in autism spectrum disorders. J Child Psychol Psychiatry, 55(2), 162–171. doi: 10.1111/jcpp.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Foss-Feig JH, Heacock JL, Newsom CR, Cowan RL, Benningfield MM, … Cao A (2012). Response of neural reward regions to food cues in autism spectrum disorders. J Neurodev Disord, 4(1), 9. doi: 10.1186/1866-1955-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon J-M (2001). Pro-versus anti-inflammatory cytokines: myth or reality. CELLULAR AND MOLECULAR BIOLOGY-PARIS-WEGMANN-, 47(4), 695–702. [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, … Chugani DC (2005). Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. International Journal of Developmental Neuroscience, 23(2–3), 171–182. [DOI] [PubMed] [Google Scholar]
- Chawarska K, Shic F, Macari S, Campbell DJ, Brian J, Landa R, … Bryson S (2014). 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: a baby siblings research consortium study. J Am Acad Child Adolesc Psychiatry, 53(12), 1317–1327 e1311. doi: 10.1016/j.jaac.2014.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-w., Zhong X.-s., Jiang L.-n., Zheng X.-y., Xiong Y.-q., Ma S.-j., … Chen Q (2016). Maternal autoimmune diseases and the risk of autism spectrum disorders in offspring: A systematic review and meta-analysis. Behavioural Brain Research, 296(Supplement C), 61–69. doi: 10.1016/j.bbr.2015.08.035 [DOI] [PubMed] [Google Scholar]
- Chevallier C, Grezes J, Molesworth C, Berthoz S, & Happe F (2012). Brief report: Selective social anhedonia in high functioning autism. J Autism Dev Disord, 42(7), 1504–1509. doi: 10.1007/s10803-011-1364-0 [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, & Schultz RT (2012). The Social Motivation Theory of Autism. Trends in Cognitive Sciences, 16(4), 231–239. doi: 10.1016/j.tics.2012.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chez MG, Dowling T, Patel PB, Khanna P, & Kominsky M (2007). Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatric neurology, 36(6), 361–365. [DOI] [PubMed] [Google Scholar]
- Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, & Choi GB (2015). Oxytocin Mediates Entrainment of Sensory Stimuli to Social Cues of Opposing Valence. Neuron, 87(1), 152–163. doi: 10.1016/j.neuron.2015.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi US, Kim SY, Sim HJ, Lee SY, Park SY, Jeong JS, … Cheon KA (2015). Abnormal brain activity in social reward learning in children with autism spectrum disorder: an fMRI study. Yonsei Med J, 56(3), 705–711. doi: 10.3349/ymj.2015.56.3.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani DC (2002). Role of altered brain serotonin mechanisms in autism. Molecular psychiatry, 7(S2), S16. [DOI] [PubMed] [Google Scholar]
- Clements CC, Zoltowski AR, Yankowitz LD, Yerys BE, Schultz RT, & Herrington JD (2018). Evaluation of the Social Motivation Hypothesis of Autism: A Systematic Review and Meta-analysis. JAMA psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn W, DeGruttola VG, DeMets DL, Downing GJ, Hoth DF, Oates JA, … Woodcock J (2001). Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Biomarkers Definitions Working Group. Clinical Pharmacol & Therapeutics, 69, 89–95. [DOI] [PubMed] [Google Scholar]
- Comi AM, Zimmerman AW, Frye VH, Law PA, & Peeden JN (1999). Familial Clustering of Autoimmune Disorders and Evaluation of Medical Risk Factors in Autism. Journal of Child Neurology, 14(6), 388–394. doi: 10.1177/088307389901400608 [DOI] [PubMed] [Google Scholar]
- Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, & Deuel RK (1999). Serum autoantibodies to brain in Landau-Kleffner variant, autism, and other neurologic disorders. The Journal of pediatrics, 134(5), 607–613. [DOI] [PubMed] [Google Scholar]
- Damiano CR, Aloi J, Treadway M, Bodfish JW, & Dichter GS (2012). Adults with autism spectrum disorders exhibit decreased sensitivity to reward parameters when making effort-based decisions. J Neurodev Disord, 4(1), 13. doi: 10.1186/1866-1955-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano CR, Cockrell DC, Dunlap K, Hanna EK, Miller S, Bizzell J, … Dichter GS (2015). Neural mechanisms of negative reinforcement in children and adolescents with autism spectrum disorders. J Neurodev Disord, 7(1), 12. doi: 10.1186/s11689-015-9107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, & Kelley KW (2007). Twenty Years of Research on Cytokine-Induced Sickness Behavior. Brain Behaviour Immun, 21(2), 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, & Kelley KW (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci, 9(1), 46–56. doi: 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubner SC, Le T, & Wang S (2011). Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Archives of biochemistry and biophysics, 508(1), 1–12. doi: 10.1016/j.abb.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Bernier R, & Ring RH (2012). Social attention: a possible early indicator of efficacy in autism clinical trials. J Neurodev Disord, 4(1), 11. doi: 10.1186/1866-1955-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, & Rinaldi J (1998). Neuropsychological correlates of early symptoms of autism. Child Dev, 69. doi: 10.2307/1132265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, & Liaw J (2004). Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol, 40(2), 271–283. doi: 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, & McPartland J (2005). Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol, 27(3), 403–424. doi: 10.1207/s15326942dn2703_6 [DOI] [PubMed] [Google Scholar]
- Delmonte S, Balsters JH, McGrath J, Fitzgerald J, Brennan S, Fagan AJ, & Gallagher L (2012). Social and monetary reward processing in autism spectrum disorders. Mol Autism, 3(1), 7. doi: 10.1186/2040-2392-3-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depue R, & Morrone-Strupinsky J (2005). A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. The Behavioral and brain sciences, 28(3), 313. [DOI] [PubMed] [Google Scholar]
- Dichter GS, & Adolphs R (2012). Reward processing in autism: a thematic series. Journal of neurodevelopmental disorders, 4(1), 20–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, & Allen JA (2012). Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. Journal of neurodevelopmental disorders, 4(1), 0–0. doi: 10.1186/1866-1955-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, & Bodfish JW (2012). Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci, 7(2), 160–172. doi: 10.1093/scan/nsq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, & Bodfish JW (2012). Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord, 42(2), 147–160. doi: 10.1007/s10803-011-1221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G (2015). Autism: Oxytocin, serotonin, and social reward. Soc Neurosci, 10(5), 450–465. doi: 10.1080/17470919.2015.1087875 [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, & Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature, 501(7466), 179–184. doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell NG, Cooper EA, Tibble J, Voon V, Critchley HD, Cercignani M, & Harrison NA (2016). Acute Changes in Striatal Microstructure Predict the Development of Interferon-Alpha Induced Fatigue. Biological psychiatry, 79(4), 320–328. doi: 10.1016/j.biopsych.2015.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, & Hounsgaard JD (2010). Influence of Phasic and Tonic Dopamine Release on Receptor Activation. The Journal of Neuroscience, 30(42), 14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AJ (2006). Effects of cytokines and infections on brain neurochemistry. Clinical neuroscience research, 6(1), 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, … Bourgeron T (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet, 39(1), 25–27. doi: 10.1038/ng1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston E, Ashwood P, & Van de Water J (2017). Autoimmunity, autoantibodies, and autism spectrum disorder. Biological psychiatry, 81(5), 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelberg D, Moeller J, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, … Przedborski S (1994). The metabolic topography of parkinsonism. Journal of Cerebral Blood Flow & Metabolism, 14(5), 783–801. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, & Irwin MR (2010). Inflammation-Induced Anhedonia: Endotoxin Reduces Ventral Striatum Responses to Reward. Biological psychiatry, 68(8), 748–754. doi: 10.1016/j.biopsych.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Gohary TM, El Aziz NA, Darweesh M, & Sadaa ES (2015). Plasma level of transforming growth factor β 1 in children with autism spectrum disorder. Egyptian Journal of Ear, Nose, Throat and Allied Sciences, 16(1), 69–73. [Google Scholar]
- Emery NJ (2000). The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev, 24(6), 581–604. [DOI] [PubMed] [Google Scholar]
- Ernst M, Zametkin A, Matochik J, Pascualvaca D, & Cohen R (1997). Low medial prefrontal dopaminergic activity in autistic children. The Lancet, 350(9078), 638. [DOI] [PubMed] [Google Scholar]
- Estes ML, & McAllister AK (2016). Maternal immune activation: Implications for neuropsychiatric disorders. Science, 353(6301), 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, & Delgado MR (2008). Reward-related processing in the human brain: developmental considerations. Dev Psychopathol, 20(4), 1191–1211. doi: 10.1017/S0954579408000576 [DOI] [PubMed] [Google Scholar]
- Farroni T, Massaccesi S, Menon E, & Johnson MH (2007). Direct gaze modulates face recognition in young infants. Cognition, 102(3), 396–404. doi: 10.1016/j.cognition.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, … Dickson PE (2012). Consensus paper: pathological role of the cerebellum in autism. The Cerebellum, 11(3), 777–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, … Miller AH (2007). Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biological psychiatry, 62(11), 1324–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Miller AH (2012). Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology, 33(3), 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Mun J, Kimmel HL, Nye JA, Drake DF, Hernandez CR, … Howell LL (2013). Chronic interferon-α decreases dopamine 2 receptor binding and striatal dopamine release in association with anhedonia-like behavior in nonhuman primates. Neuropsychopharmacology, 38(11), 2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, & Treadway MT (2017). Inflammation Effects on Motivation and Motor Activity: Role of Dopamine. Neuropsychopharmacology, 42(1), 216–241. doi: 10.1038/npp.2016.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, & Insel TR (2002). The neuroendocrine basis of social recognition. Frontiers in neuroendocrinology, 23(2), 200–224. [DOI] [PubMed] [Google Scholar]
- Filiano AJ, Gadani SP, & Kipnis J (2015). Interactions of innate and adaptive immunity in brain development and function. Brain research, 1617, 18–27. doi: 10.1016/j.brainres.2014.07.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Devincent CJ, Olvet DM, Pisarevskaya V, & Hatchwell E (2010). Association of DRD4 polymorphism with severity of oppositional defiant disorder, separation anxiety disorder and repetitive behaviors in children with autism spectrum disorder. Eur J Neurosci, 32(6), 1058–1065. doi: 10.1111/j.1460-9568.2010.07382.x [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pisarevskaya V, Olvet DM, Xu W, Mendell NR, … Hatchwell E (2010). Parent-child DRD4 genotype as a potential biomarker for oppositional, anxiety, and repetitive behaviors in children with autism spectrum disorder. Prog Neuropsychopharmacol Biol Psychiatry, 34(7), 1208–1214. doi: 10.1016/j.pnpbp.2010.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, … Van de Water J (2011). Autoantibodies to cerebellum in children with autism associate with behavior. Brain, Behavior, and Immunity, 25(3), 514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen E, & Hamilton A (2013). Motor abilities in autism: a review using a computational context. Journal of autism and developmental disorders, 43(2), 323–344. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, & Hickie IB (2010). Intranasal Oxytocin Improves Emotion Recognition for Youth with Autism Spectrum Disorders. Biological Psychiatry, 67(7), 692–694. doi: 10.1016/j.biopsych.2009.09.020 [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, & Dadds MR (2008). Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry, 63(1), 3–5. doi: 10.1016/j.biopsych.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, & Deisseroth K (2014). Dopaminergic Dynamics Contributing to Social Behavior. Cold Spring Harb Symp Quant Biol, 79, 221–227. doi: 10.1101/sqb.2014.79.024711 [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot J-D, Lim K, Nabulsi N, Esterlis I, … Pelletier D (2013). The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: A [11 C] PBR28 PET study. Brain, Behavior, and Immunity, 33, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Takuma K, Takano E, Katashiba K, Taruta A, Higashino K, … Matsuda T (2015). Reduced prefrontal dopaminergic activity in valproic acid-treated mouse autism model. Behav Brain Res, 289, 39–47. doi: 10.1016/j.bbr.2015.04.022 [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Marti O, Lightman SL, & Jessop DS (1998). Alteration of central serotonin modifies onset and severity of adjuvant-induced arthritis in the rat. British journal of rheumatology, 37(10), 1077–1083. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Perveen-Gill Z, Lalies MD, Jessop DS, Lightman SL, & Chowdrey HS (1996). The role of endogenous serotonin in adjuvant-induced arthritis in the rat. Rheumatology, 35(2), 112–116. [DOI] [PubMed] [Google Scholar]
- Haroon E, Raison CL, & Miller AH (2012). Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology, 37(1), 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Voon V, Cercignani M, Cooper EA, Pessiglione M, & Critchley HD (2016). A Neurocomputational Account of How Inflammation Enhances Sensitivity to Punishments Versus Rewards. Biological psychiatry, 80(1), 73–81. doi: 10.1016/j.biopsych.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann C, Zong S, Mané-Damas M, Molenaar P, Losen M, & Martinez-Martinez P (2016). Autoantibodies in Neuropsychiatric Disorders. Antibodies, 5(2). doi: 10.3390/antib5020009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, … Nedergaard M (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med, 4(147), 147ra111. doi: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, … Eisenberger NI (2015). The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain, Behavior, and Immunity, 44, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR (2003). Is social attachment an addictive disorder? Physiol Behav, 79(3), 351–357. [DOI] [PubMed] [Google Scholar]
- Insel TR, & Young LJ (2001). The neurobiology of attachment. Nature Reviews Neuroscience, 2(2), 129–136. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Sadeghian M, Leung CCM, Tel BC, Rose S, Schapira AH, & Jenner P (2008). Continuous subcutaneous infusion of pramipexole protects against lipopolysaccharide-induced dopaminergic cell death without affecting the inflammatory response. Experimental Neurology, 212(2), 522–531. doi: 10.1016/j.expneurol.2008.04.037 [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, & Morton J (1991). Newborns' preferential tracking of face-like stimuli and its subsequent decline. Cognition, 40(1–2), 1–19. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Yechiam E, Murphy RR, Queller S, & Stout JC (2006). Motivational processes and autonomic responsivity in Asperger's disorder: evidence from the Iowa Gambling Task. J Int Neuropsychol Soc, 12(5), 668–676. doi: 10.1017/S1355617706060802 [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, … Lieb K (2000). Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology, 152(4), 383–389. doi: 10.1007/s002130000549 [DOI] [PubMed] [Google Scholar]
- Kamata M, Higuchi H, Yoshimoto M, Yoshida K, & Shimizu T (2000). Effect of single intracerebroventricular injection of α-interferon on monoamine concentrations in the rat brain. European Neuropsychopharmacology, 10(2), 129–132. [DOI] [PubMed] [Google Scholar]
- Karpiak SE, & Rapport MM (1975). Behavioral changes in 2-month-old rats following prenatal exposure to antibodies against synaptic membranes. Brain research, 92(3), 405–413. doi: 10.1016/0006-8993(75)90325-X [DOI] [PubMed] [Google Scholar]
- Kaur K, Gill JS, Bansal PK, & Deshmukh R (2017). Neuroinflammation-A major cause for striatal dopaminergic degeneration in Parkinson's disease. Journal of the Neurological Sciences, 381, 308–314. [DOI] [PubMed] [Google Scholar]
- Keeler JF, Pretsell DO, & Robbins TW (2014). Functional implications of dopamine D1 vs. D2 receptors: A ‘prepare and select’ model of the striatal direct vs. indirect pathways. Neuroscience, 282, 156–175. doi: 10.1016/j.neuroscience.2014.07.021 [DOI] [PubMed] [Google Scholar]
- Keil A, Daniels JL, Forssen U, Hultman C, Cnattingius S, Söderberg KC, … Sparen P (2010). Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology (Cambridge, Mass.), 21(6), 805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BH, Hollander E, Sikich L, McCracken JT, Scahill L, Bregman JD, … Sullivan L (2009). Lack of efficacy of citalopram in children with autism spectrum disorders and high levels of repetitive behavior: citalopram ineffective in children with autism. Archives of general psychiatry, 66(6), 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, & Bernardi MM (2017). Prenatal lipopolysaccharide induces hypothalamic dopaminergic hypoactivity and autistic-like behaviors: Repetitive self-grooming and stereotypies. Behavioural Brain Research, 331, 25–29. [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Lippi LL, Bevilacqua E, & Bernardi MM (2013). LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1β levels in adult rat offspring: relevance to autism. PLoS One, 8(12), e82244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Flório JC, Palermo-Neto J, & Bernardi MM (2010). Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behavioural Brain Research, 211(1), 77–82. doi: 10.1016/j.bbr.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Kirsten TB, Taricano M, Maiorka PC, Palermo-Neto J, & Bernardi MM (2010). Prenatal lipopolysaccharide reduces social behavior in male offspring. Neuroimmunomodulation, 17(4), 240–251. [DOI] [PubMed] [Google Scholar]
- Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, & Ohta T (2003). Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood–brain barrier. Brain research, 978(1), 104–114. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, & Cohen D (2002). Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry, 59(9), 809–816. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, & Jones W (2009). Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature, 459(7244), 257–261. doi: 10.1038/nature07868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, & Cooper JC (2005). Functional magnetic resonance imaging of reward prediction. Current opinion in neurology, 18(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Kohls G, Chevallier C, Troiani V, & Schultz RT (2012). Social 'wanting' dysfunction in autism: neurobiological underpinnings and treatment implications. J Neurodev Disord, 4(1), 10. doi: 10.1186/1866-1955-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, … Konrad K (2013). Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci, 8(5), 565–572. doi: 10.1093/scan/nss033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, & Van de Water J (2015). Neonatal cytokine profiles associated with autism spectrum disorder. Biological psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Coffey-Corina S, Padden D, & Dawson G (2005). Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci, 8(1), F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x [DOI] [PubMed] [Google Scholar]
- Kumai T, Tateishi T, Tanaka M, Watanabe M, Shimizu H, & Kobayashi S (2000). Effect of interferon-α on tyrosine hydroxylase and catecholamine levels in the brain of rats. Life sciences, 67(6), 663–669. [DOI] [PubMed] [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, & Saumier D (2006). Face perception in high-functioning autistic adults: evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology, 20(1), 30–41. doi: 10.1037/0894-4105.20.1.30 [DOI] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, & Sokoloff P (2000a). Dopamine D3 receptor gene expression in the shell of nucleus accumbens is increased by chronic antidepressant treatment. Molecular psychiatry, 5(3), 229. [DOI] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, & Sokoloff P (2000b). Selective increase of dopamine D 3 receptor gene expression as a common effect of chronic antidepressant treatments. Molecular psychiatry, 5(4), 378. [DOI] [PubMed] [Google Scholar]
- Li T, Wang X, Pan J, Feng S, Gong M, Wu Y, … Yi L (2017). Reward learning modulates the attentional processing of faces in children with and without autism spectrum disorder. Autism Res, 10(11), 1797–1807. doi: 10.1002/aur.1823 [DOI] [PubMed] [Google Scholar]
- Li X, Chauhn A, Shiekh AM, Patil S, Chauhn V, Li X-M, … Malik M (2009). Elevated Immune Response in the Brain of Autistic Patients. Journal of neuroimmunology, 207(1–2), 111–116. doi: 10.1016/j.jneuroim.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, … Kipnis J (2015). Structural and functional features of central nervous system lymphatic vessels. Nature, 523(7560), 337–341. doi: 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabunga DF, Gonzales EL, Kim JW, Kim KC, & Shin CY (2015). Exploring the Validity of Valproic Acid Animal Model of Autism. Exp Neurobiol, 24(4), 285–300. doi: 10.5607/en.2015.24.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, & Kuikka JT (2008). Serotonin and dopamine transporter binding in children with autism determined by SPECT. Developmental Medicine & Child Neurology, 50(8), 593–597. [DOI] [PubMed] [Google Scholar]
- Manduca A, Servadio M, Damsteegt R, Campolongo P, Vanderschuren LJ, & Trezza V (2016). Dopaminergic Neurotransmission in the Nucleus Accumbens Modulates Social Play Behavior in Rats. Neuropsychopharmacology. doi: 10.1038/npp.2016.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, & Lai MC (2017). Towards sex- and gender-informed autism research. Autism, 21(6), 643–645. doi: 10.1177/1362361317706904 [DOI] [PubMed] [Google Scholar]
- Manto M, Bower JM, Conforto AB, Delgado-García JM, da Guarda SNF, Gerwig M, … Timmann D (2012). Consensus Paper: Roles of the Cerebellum in Motor Control—The Diversity of Ideas on Cerebellar Involvement in Movement. Cerebellum (London, England), 11(2), 457–487. doi: 10.1007/s12311-011-0331-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Glozier N, Dale R, & Guastella AJ (2017). The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neuroscience Bulletin, 33(2), 194–204. doi: 10.1007/s12264-017-0103-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, Quintana DS, Glozier N, Lloyd AR, Hickie IB, & Guastella AJ (2015). Cytokine aberrations in autism spectrum disorder: a systematic review and meta-analysis. Molecular psychiatry, 20(4), 440–446. doi: 10.1038/mp.2014.59 [DOI] [PubMed] [Google Scholar]
- Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, … Eidelberg D (2002). Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. American Journal of Psychiatry, 159(5), 746–754. [DOI] [PubMed] [Google Scholar]
- Meyer U, Engler A, Weber L, Schedlowski M, & Feldon J (2008). Preliminary evidence for a modulation of fetal dopaminergic development by maternal immune activation during pregnancy. Neuroscience, 154(2), 701–709. doi: 10.1016/j.neuroscience.2008.04.031 [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, & Feldon J (2008). Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology, 33(2), 441–456. [DOI] [PubMed] [Google Scholar]
- Meyer U, Yee BK, & Feldon J (2007). The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? The Neuroscientist, 13(3), 241–256. [DOI] [PubMed] [Google Scholar]
- Molloy CA, Morrow AL, Meinzen-Derr J, Schleifer K, Dienger K, Manning-Courtney P, … Wills-Karp M (2006). Elevated cytokine levels in children with autism spectrum disorder. Journal of neuroimmunology, 172(1), 198–205. [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Abramson I, Semendeferi K, Courchesne E, & Everall IP (2012). Abnormal microglial-neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain research, 1456, 72–81. doi: 10.1016/j.brainres.2012.03.036 [DOI] [PubMed] [Google Scholar]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, … Everall IP (2010). Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biological psychiatry, 68(4), 368–376. doi: 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- Mosner MG, Kinard JL, McWeeny S, Shah JS, Markiewitz ND, Damiano-Goodwin CR, … Dichter GS (2017). Vicarious Effort-Based Decision-Making in Autism Spectrum Disorders. J Autism Dev Disord, 47(10), 2992–3006. doi: 10.1007/s10803-017-3220-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Moieni M, Inagaki TK, Dutcher JM, Jevtic I, Breen EC, … Eisenberger NI (2016). Exposure to an Inflammatory Challenge Enhances Neural Sensitivity to Negative and Positive Social Feedback. Brain, Behavior, and Immunity, 57, 21–29. doi: 10.1016/j.bbi.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, … Suzuki K (2010). Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Archives of general psychiatry, 67(1), 59–68. [DOI] [PubMed] [Google Scholar]
- Nakasato A, Nakatani Y, Seki Y, Tsujino N, Umino M, & Arita H (2008). Swim stress exaggerates the hyperactive mesocortical dopamine system in a rodent model of autism. Brain Res, 1193, 128–135. doi: 10.1016/j.brainres.2007.11.043 [DOI] [PubMed] [Google Scholar]
- Narita N, Kato M, Tazoe M, Miyazaki K, Narita M, & Okado N (2002). Increased monoamine concentration in the brain and blood of fetal thalidomide- and valproic acid-exposed rat: putative animal models for autism. Pediatr Res, 52(4), 576–579. doi: 10.1203/00006450-200210000-00018 [DOI] [PubMed] [Google Scholar]
- Nestler EJ, & Carlezon WA (2006). The mesolimbic dopamine reward circuit in depression. Biological psychiatry, 59(12), 1151–1159. [DOI] [PubMed] [Google Scholar]
- Nieminen-von Wendt TS, Metsähonkala L, Kulomäki TA, Aalto S, Autti TH, Vanhala R, … von Wendt LO (2004). Increased presynaptic dopamine function in Asperger syndrome. Neuroreport, 15(5), 757–760. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Estrada A, Epling B, Hart EE, Lee CA, … Salamone JD (2014). Effort-related motivational effects of the pro-inflammatory cytokine interleukin 1-beta: studies with the concurrent fixed ratio 5/chow feeding choice task. Psychopharmacology, 231(4), 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, … Sugihara G.-i. (2007). Decreased serum levels of transforming growth factor-β1 in patients with autism. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 31(1), 187–190. [DOI] [PubMed] [Google Scholar]
- Olazabal D, & Young L (2006). Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience, 141(2), 559–568. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, & Iyo M (2006). Immune Activation During Pregnancy in Mice Leads to Dopaminergic Hyperfunction and Cognitive Impairment in the Offspring: A Neurodevelopmental Animal Model of Schizophrenia. Biological psychiatry, 59(6), 546–554. doi: 10.1016/j.biopsych.2005.07.031 [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Young GS (2010). A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry, 49(3), 256–266 e251–252. [PMC free article] [PubMed] [Google Scholar]
- Parkin J, & Cohen B (2001). An overview of the immune system. The Lancet, 357(9270), 1777–1789. doi: 10.1016/S0140-6736(00)04904-7 [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, & Vander Wyk BC (2011). Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry, 52(6), 631–644. doi: 10.1111/j.1469-7610.2010.02349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CC, Slaughter V, Peterson J, & Premack D (2013). Children with autism can track others' beliefs in a competitive game. Developmental science, 16(3), 443–450. [DOI] [PubMed] [Google Scholar]
- Pierce PA, Xie G-X, Peroutka SJ, Green PG, & Levine JD (1995). 5-Hydroxytryptamine-induced synovial plasma extravasation is mediated via 5-hydroxytryptamine2A receptors on sympathetic efferent terminals. Journal of Pharmacology and Experimental Therapeutics, 275(1), 502–508. [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, … Betancur C (2010). Functional impact of global rare copy number variation in autism spectrum disorders. Nature, 466(7304), 368–372. doi: 10.1038/nature09146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras IS, Haapanen L, Napolioni V, Sacco R, Van de Water J, & Persico AM (2014). Anti-brain antibodies are associated with more severe cognitive and behavioral profiles in Italian children with Autism Spectrum Disorder. Brain, Behavior, and Immunity, 38, 91–99. doi: 10.1016/j.bbi.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, & Borrell J (2010). Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Molecular psychiatry, 15(4), 372–383. [DOI] [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, & Young LJ (2009). Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience, 162(4), 892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, & Young LJ (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. The Journal of Neuroscience, 29(5), 1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, & Frye RE (2014). Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry, 4, e360. doi: 10.1038/tp.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin M, & Sur M (2015). Genes, circuits, and precision therapies for autism and related neurodevelopmental disorders. Science, 350(6263). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiego CM, Gallezot J-D, Pittman B, Nabulsi N, Lim K, Lin S-F, … Huang Y (2015). Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proceedings of the National Academy of Sciences, 112(40), 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson N, Tsuchiya N, Hurley R, Couture SM, Penn DL, Adolphs R, & Piven J (2007). Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia, 45(11), 2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Suzuki E, Yokoyama M, SEMBA JI, Watanabe S, & Miyaoka H (2006). Chronic intraperitoneal injection of interferon-α reduces serotonin levels in various regions of rat brain, but does not change levels of serotonin transporter mRNA, nitrite or nitrate. Psychiatry and clinical neurosciences, 60(4), 499–506. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, & Murphy DG (2008). Neural correlates of reward in autism. Br J Psychiatry, 192(1), 19–24. doi: 10.1192/bjp.bp.107.036921 [DOI] [PubMed] [Google Scholar]
- Schulte D, Callado L, Davidson C, Phillips P, Roewer N, Schulte am Esch J, & Stamford J (2000). Propofol decreases stimulated dopamine release in the rat nucleus accumbens by a mechanism independent of dopamine D2, GABAA and NMDA receptors. British journal of anaesthesia, 84(2), 250–253. [DOI] [PubMed] [Google Scholar]
- Schultz RT (2005). Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci, 23. doi: 10.1016/j.ijdevneu.2004.12.012 [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, … Gore JC (2000). Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry, 57(4), 331–340. [DOI] [PubMed] [Google Scholar]
- Schultz W (1997). Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol, 7(2), 191–197. [DOI] [PubMed] [Google Scholar]
- Schultz W (2000). Multiple reward signals in the brain. Nat Rev Neurosci, 1(3), 199–207. doi: 10.1038/35044563 [DOI] [PubMed] [Google Scholar]
- Schultz W (2015). Neuronal Reward and Decision Signals: From Theories to Data. Physiological Reviews, 95(3), 853 LP-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, & Bookheimer SY (2010). Reward processing in autism. Autism Res, 3(2), 53–67. doi: 10.1002/aur.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Yager RD, & Klopper JH (1976). Selective brain dopamine depletion in developing rats: an experimental model of minimal brain dysfunction. Science, 191(4224), 305–308. [DOI] [PubMed] [Google Scholar]
- Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, Nordahl CW, … Gu H (2017). Increased Extra-axial Cerebrospinal Fluid in High-Risk Infants Who Later Develop Autism. Biol Psychiatry, 82(3), 186–193. doi: 10.1016/j.biopsych.2017.02.1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, … Amaral DG (2013). Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain, 136(Pt 9), 2825–2835. doi: 10.1093/brain/awt166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, & Oishi R (1997). Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain research, 747(2), 348–351. doi:Doi 10.1016/S0006-8993(96)01371-6 [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, & Zimmerman AW (2006). Antibrain antibodies in children with autism and their unaffected siblings. Journal of neuroimmunology, 178(1), 149–155. [DOI] [PubMed] [Google Scholar]
- Singh VK, & Rivas WH (2004). Prevalence of serum antibodies to caudate nucleus in autistic children. Neuroscience letters, 355(1–2), 53–56. doi: 10.1016/j.neulet.2003.10.026 [DOI] [PubMed] [Google Scholar]
- Singh VK, Singh EA, & Warren RP (1997). Hyperserotoninemia and serotonin receptor antibodies in children with autism but not mental retardation. Biological psychiatry, 41(6), 753–755. [DOI] [PubMed] [Google Scholar]
- Singh VK, Warren RP, Odell JD, Warren WL, & Cole P (1993). Antibodies to Myelin Basic Protein in Children with Autistic Behavior. Brain, Behavior, and Immunity, 7(1), 97–103. doi: 10.1006/brbi.1993.1010 [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, & Aldridge JW (2011). Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A, 108(27), E255–264. doi: 10.1073/pnas.1101920108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ragland JD, Niendam TA, Lesh TA, Beck JS, Matter JC, … Carter CS (2015). Atypical Learning in Autism Spectrum Disorders: A Functional Magnetic Resonance Imaging Study of Transitive Inference. J Am Acad Child Adolesc Psychiatry, 54(11), 947–955. doi: 10.1016/j.jaac.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto AMS, Kirsten TB, Reis-Silva TM, Martins MFM, Teodorov E, Flório JC, … Bondan EF (2013). Single early prenatal lipopolysaccharide exposure impairs striatal monoamines and maternal care in female rats. Life sciences, 92(14), 852–858. doi: 10.1016/j.lfs.2013.03.003 [DOI] [PubMed] [Google Scholar]
- St Pourcain B, Eaves LJ, Ring SM, Fisher SE, Medland S, Evans DM, & Davey Smith G (2017). Developmental Changes Within the Genetic Architecture of Social Communication Behavior: A Multivariate Study of Genetic Variance in Unrelated Individuals. Biol Psychiatry. doi: 10.1016/j.biopsych.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal WG (2015). Autism, DRD3 and repetitive and stereotyped behavior, an overview of the current knowledge. Eur Neuropsychopharmacol, 25(9), 1421–1426. doi: 10.1016/j.euroneuro.2014.08.011 [DOI] [PubMed] [Google Scholar]
- Staal WG, Langen M, van Dijk S, Mensen VT, & Durston S (2015). DRD3 gene and striatum in autism spectrum disorder. Br J Psychiatry, 206(5), 431–432. doi: 10.1192/bjp.bp.114.148973 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, … Tsuchiya KJ (2013). Microglial activation in young adults with autism spectrum disorder. JAMA psychiatry, 70(1), 49–58. [DOI] [PubMed] [Google Scholar]
- Takano T (2015). Role of Microglia in Autism: Recent Advances. Developmental Neuroscience, 37(3), 195–202. [DOI] [PubMed] [Google Scholar]
- Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, & Allman JM (2012). Microglia in the cerebral cortex in autism. Journal of autism and developmental disorders, 42(12), 2569–2584. doi: 10.1007/s10803-012-1513-0 [DOI] [PubMed] [Google Scholar]
- Traglia M, Croen LA, Jones KL, Heuer LS, Yolken R, Kharrazi M, … Weiss LA (2018). Cross-genetic determination of maternal and neonatal immune mediators during pregnancy. Genome medicine, 10(1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, & Zald DH (2013). Parsing Anhedonia: Translational Models of Reward Processing Deficits in Psychopathology. Curr Dir Psychol Sci, 22(3), 244–249. doi: 10.1177/0963721412474460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, & Vanderschuren LJ (2011). Nucleus accumbens mu-opioid receptors mediate social reward. J Neurosci, 31(17), 6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, & Pardo CA (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of Neurology, 57(1), 67–81. doi: 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Vaughan RA, & Foster JD (2013). Mechanisms of dopamine transporter regulation in normal and disease states. Trends in pharmacological sciences, 34(9), 10.1016/j.tips.2013.1007.1005. doi: 10.1016/j.tips.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, … Thompson BJ (2012). Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences, 201112345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuillermot S, Weber L, Feldon J, & Meyer U (2010). A longitudinal examination of the neurodevelopmental impact of prenatal immune activation in mice reveals primary defects in dopaminergic development relevant to schizophrenia. Journal of Neuroscience, 30(4), 1270–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, … Malenka RC (2018). 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, & Bookheimer SY (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry, 43(4), 481–490. doi: 10.1097/00004583-200404000-00015 [DOI] [PubMed] [Google Scholar]
- Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, … Jiang YH (2011). Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet, 20(15), 3093–3108. doi: 10.1093/hmg/ddr212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, … Bolanos-Guzman CA (2013). Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry, 73(1), 7–14. doi: 10.1016/j.biopsych.2012.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Miller S, Hannah E, Kovac M, Damiano CR, Sabatino-DiCrisco A, … Dichter GS (2015). Increased reward value of non-social stimuli in children and adolescents with autism. Front Psychol, 6, 1026. doi: 10.3389/fpsyg.2015.01026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann T, & DeLong MR (1999). Oscillations in the basal ganglia. Nature, 400(6745), 621–622. [DOI] [PubMed] [Google Scholar]
- Williams K, Wheeler DM, Silove N, & Hazell P (2011). Cochrane review: selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders (ASD). Evidence-Based Child Health: A Cochrane Review Journal, 6(4), 1044–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, & Van de Water J (2009). Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain, Behavior, and Immunity, 23(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman AC, Smith LE, Greenberg JS, & Mailick MR (2016). Contextual Factors Predict Patterns of Change in Functioning over 10 Years Among Adolescents and Adults with Autism Spectrum Disorders. J Autism Dev Disord, 46(1), 176–189. doi: 10.1007/s10803-015-2561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, … Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342(6156), 373–377. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, & Zhou R (2015). Dopamine Controls Systemic Inflammation through Inhibition of NLRP3 Inflammasome. Cell, 160(1), 62–73. doi: 10.1016/j.cell.2014.11.047 [DOI] [PubMed] [Google Scholar]
- Zald DH, & Depue RA (2001). Serotonergic functioning correlates with positive and negative affect in psychiatrically healthy males. Personality and Individual Differences, 30(1), 71–86. [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, & Pearce DA (2007). Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity, 21(3), 351–357. doi: 10.1016/j.bbi.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, & Berns GS (2004). Human striatal responses to monetary reward depend on saliency. Neuron, 42(3), 509–517. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, & Weiner I (2003). Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology, 28(10), 1778. [DOI] [PubMed] [Google Scholar]
- Zürcher NR, Bhanot A, McDougle CJ, & Hooker JM (2015). A systematic review of molecular imaging (PET and SPECT) in autism spectrum disorder: current state and future research opportunities. Neuroscience & Biobehavioral Reviews, 52, 56–73. [DOI] [PubMed] [Google Scholar]