Abstract
Rationale:
Mesenchymal stem cells (MSCs) are increased in the airways after allergen challenge. RhoA/ROCK signaling is critical in determining the lineage fate of MSCs in tissue repair/remodeling.
Objectives:
To investigate the role of RhoA/ROCK signaling in lineage commitment of MSCs during allergen-induced airway remodeling and delineate the underlying mechanisms.
Methods:
Active RhoA expression in asthmatic lung tissues and its role in cockroach allergen-induced airway inflammation and remodeling were investigated. The RhoA/ROCK signaling-mediated MSC lineage commitment was assessed in an asthma mouse model using MSC lineage tracing mice (nestin-Cre; ROSA26-EYFP). The role of RhoA/ROCK in MSC lineage commitment was also examined by MSCs expressing constitutively active RhoA (RhoA-L63) or dominant negative RhoA (RhoA-N19). Downstream RhoA-regulated genes were identified using the stem cell signaling array.
Results:
Lung tissues from asthmatic mice showed increased expression of active RhoA when compared with those from controls. Inhibition of RhoA/ROCK signaling with fasudil, a RhoA/ROCK inhibitor, reversed established cockroach allergen-induced airway inflammation and remodeling as assessed by more collagen deposition/fibrosis. Furthermore, fasudil inhibited MSC differentiation into fibroblasts/myofibroblasts, but promoted MSC differentiation into epithelial cells in asthmatic nestin-Cre; ROSA26-EYFP mice. Consistently, expression of RhoA-L63 facilitated the differentiation of MSCs to fibroblasts/myofibroblasts, whereas expression of RhoA-19 switched the differentiation toward epithelial cells. Gene Array identified the Wnt signaling effector Lef1 as the most up-regulated gene in RhoA-L63-transfected MSCs. Knockdown of Lef1 induced MSC differentiation away from fibroblasts/myofibroblasts but towards epithelial cells.
Conclusions:
These findings uncover a previously unrecognized role of RhoA/ROCK signaling in MSC-involved airway repair/remodeling in asthma.
Keywords: Asthma, Cockroach allergen, RhoA/ROCK, Mesenchymal stem cell, Lef1
Capsule summary
MSCs are critical in airway repair/remodeling in asthma. RhoA/ROCK signaling functions as a molecular switch for the lineage fate of MSCs during airway repair/remodeling. Targeting RhoA/ROCK signaling might be an effective therapeutic strategy for asthma.
INTRODUCTION
Alterations within the structural cells of the airway, together with abnormal immune responses, lead to the full manifestation of asthma1–3. Airway epithelial damage and repair occur almost immediately after epithelial injury4, 5, whereas airway remodeling, characterized by goblet cell hyperplasia, angiogenesis, smooth muscle hypertrophy, and sub-epithelial fibrosis6, occurs after repeated allergen exposure, inflammation and damage7–9. Therefore, therapies that can prevent/inhibit fibrotic remodeling but accelerate airway epithelium repair are needed.
Stem/progenitor cells are known to participate in tissue repair and remodeling10, 11. Mesenchymal stem cells (MSCs) are adult connective tissue progenitor cells with the ability of self-renewal and differentiation into multiple cell types12. MSCs from bone-marrow/circulating blood can migrate to the sites of tissue damage13–15. Especially, we and others have shown that MSCs were significantly increased in the lungs after allergen challenge16–20. Nestin, originally detected in neuronal stem cells, has recently been used as a marker for bone marrow-derived MSCs12, 21. Nestin positive MSCs were recruited to the injured/diseased arteries, contributing to pathological arterial remodeling22, 23. This population of cells was also recruited to other injured/diseased sites such as the reactive stroma resulting in overgrowth of prostate tissues24, subchondral bone of joints promoting osteoarthritis25, 26, and lung tissues leading to the suppression of airway inflammation and remodeling20, 27–29. It has been revealed that MSCs have the ability to differentiate into a number of mature cell types in the lungs such as epithelial cells4, 30–36 and fibroblasts/myofibroblasts16–20, 37–39. However, these studies were performed either in cultured MSCs or injected exogenous MSCs in mouse models of lung injury. Furthermore, the primary endogenous factors that control MSC differentiation at sites of airway damage as yet remain unclear. We have recently demonstrated that TGFβ1-activated RhoA/ROCK signaling in MSCs is a determinant for the lineage commitment of MSCs40. RhoA is an intracellular signal transducer of the Rho family of small GTPases41. The RhoA-ROCK pathway is a central coordinator of tissue injury response. RhoA and its downstream effectors, ROCK1 and ROCK2, regulate a number of cellular processes, including actin cytoskeleton organization, cell adhesion, migration, proliferation, survival, and permeability42–44. RhoA/ROCK signaling is also essential for multiple aspects of VEGF-mediated angiogenesis45. Importantly, RhoA/ROCK signaling has been linked with pathophysiological processes of asthma, including repeated allergen challenge-induced airway smooth muscle contraction, formation of asthmatic airway hyper-responsiveness, and chemotactic aggregation of eosinophils to the airway, and allergic inflammation and airway remodeling46–52. These findings raise the possibility that RhoA/ROCK signaling may play a role in modulating MSC differentiation and subsequently airway repair/remodeling in allergic asthma.
Here, we tested the hypothesis that RhoA/ROCK signaling plays a role in allergen-induced airway repair/remodeling by controlling lineage commitment of MSCs. We provide evidence that RhoA is activated in asthmatic lung tissues, and inhibition of RhoA/ROCK signaling protects against cockroach allergen-induced airway inflammation and remodeling. By using MSC lineage tracing mouse model, we demonstrate that inhibition of RhoA/ROCK signaling suppresses MSC fibroblast/myofibroblast differentiation, but promotes MSC differentiation into airway epithelial cells. Importantly, we identified Wnt signaling effector Lef1 as the most up-regulated gene in MSCs by RhoA/ROCK signaling and a key factor for MSC lineage commitment.
METHODS
Animals
All animal experimentations were carried out using six- to eight-week-old mice. C57BL/6 wild-type (WT), B6.Cg-Tg (Nes-cre) 1Kln/J mice (Stock No: 003771) and B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J mice (Stock No: 006148) were purchased from Jackson Lab. All animals were maintained in the animal facility of the Johns Hopkins University School of Medicine. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University, Baltimore, MD.
Allergen-induced asthma mouse model
A chronic cockroach allergen-induced mouse model of asthma was generated. Mice were sensitized once a day with 20 µg cockroach extract (CRE, Greer Laboratory) in 50µl of PBS under light anesthesia on day 0, 7, and 14. Following sensitization, mice were treated three times a week with CRE for 6 continuous weeks (Fig 2, A). Control mice received PBS during the sensitization and challenge phases. In some cases, mice were treated with fasudil (LC Laboratories; Woburn, Massachusetts, USA) at a dose of 30 mg/kg dissolved in buffered saline by intra-peritoneal administration 30 min before CRE challenge for prevention or once a day for two consecutive weeks after the last challenge for treatment of the established mouse model Vehicle-treated mice received water. Bronchoalveolar lavage (BAL) fluids were obtained with 2 flushes of 0.8 ml of PBS and BAL cells were collected for total and differential counts by flow cytometric analysis53, 54. Cell free BAL fluids were used for cytokine measurements. Lung tissues were dissected for histological analyses. Bloods were also taken to determine serum antibodies against CRE.
FIG 2.
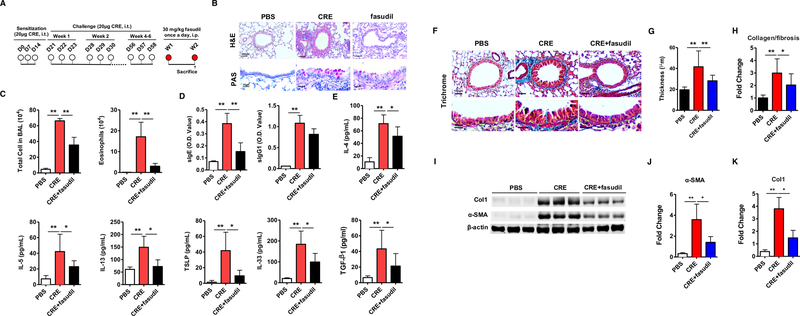
Inhibition of RhoA signaling reverses the established lung inflammation. A, Protocol for cockroach allergen-induced mouse model of asthma. CRE: cockroach extract, i.t., intra-tracheal, i.p., intra-peritoneal. B, Paraffin tissue sections of lung were stained with H&E (upper panel) and periodic acid-Schiff (PAS, lower panel). C, Bronchoalveolar lavage (BAL) total and eosinophil cell counts as assessed by flow cytometry. D-E, ELISA analyses for serum levels of cockroach allergen specific IgE and IgG1 (D) and BAL levels of cytokines (E). F, Representative images of the Masson’s trichrome staining of collagen on lung sections. G, Analyses of the epithelial layer thickness. H, airway collagen/fibrosis (blue) presented as fold change (blue/total area in each group normalized to PBS group). I, Representative immunoblots for α-SMA and collagen 1 (Col 1) in lung sections. β-actin was used as a control. n=3/group. J-K, Densitometric analysis for α-SMA (J) and Col 1 (K). C-H, n=6–8/group. Data represent mean ± SEM. *P<0.05, **P<0.01.
Analysis of lung inflammation
Lung inflammation was assessed as previously described53–55. Masson’s Trichrome Staining was carried out using the kit from Sigma Aldrich according to the manufacture and the procedure is provided in the Online Repository.
ELISA
Concentrations of IL-4, IL-5, IL-13, IL-17, TSLP, IL-33, and TGFβ1 in cell-free BAL fluids were measured by ELISA using the Ready-Set-Go! ELISA sets (ThermoFisher)53–55. Cockroach allergen-specific IgE and IgG1 serum levels were analyzed by ELISA as previously described 54.
Immunostaining
Immunostaining was performed as previously described53–56. The following primary antibodies were used: anti-active RhoA-GTPase (New East Biosciences), anti-E-cadherin (Cell Signaling), anti-αSMA (Sigma, clone SP171), anti-EYFP (Novus), anti-Vimentin (ThermoFisher, clone V9), and anti-Lef1 (Cell Signaling). The detailed information is provided in the Online Repository (Table E2). To determine the fluorescence signal in tissue sections, fluorescent positive cells in four different high-power fields from each lung section were quantified using ImageJ v1.50e (NIH) and presented as mean fluorescence intensity per square micrometer. Four to six lung sections from each sample were used for analysis.
TABLE E2.
Antibodies used for flow cytometry and immunohistochemical studies
| Antibody | Clone | Species | Application | Source |
|---|---|---|---|---|
| CD11b | M1/70 | Rat | FC (0.25 µg/105cells) | BioLegend |
| CD29 | HMβ1–1 | Hamster | FC (0.25 µg/105cells) | BioLegend |
| CD31 | 390 | Rat | FC (0.25 µg/105cells) | BioLegend |
| CD34 | HM34 | Hamster | FC (0.25 µg/105cells) | BioLegend |
| Collagen 1 | NB600–408 | Rabbit | WB (1:1000) | Novus Biologicals |
| E-cadherin | 24E10 | Rabbit | IHC (1:200) ICC (1:200) WB (1:1000) |
Cell Signaling Technology |
| Epi-CAM | G8.8 | Rat | IHC (1:100) | ThermoFisher |
| EYFP | GF28R | Mouse | IHC (1:100) | ThermoFisher |
| NB600–303 | Rabbit | IHC (1:100) | Novus Biologicals | |
| Lef1 | C12A5 | Rabbit | IHC (1:200) | Cell Signaling Technology |
| LepR | BAF497 | Goat | FC (1:50) | R&D Systems |
| Nestin | 1D11H2 | Mouse | FC (1:100) | Proteintech |
| pLef1 | SAB4504256 | Rabbit | WB (1:1000) | Sigma-Aldrich |
| RhoA- GTPase |
26904 | Mouse | IHC (1:100) ICC (1:50) WB (1:500) |
New East Biosciences |
| Sca1 | D7 | Rat | FC (0.25 µg/105cells) | BioLegend |
| TER-119 | BAM1125 | Rat | FC (1:50) | R&D Systems |
| Vimetin | V9 | Mouse | IHC (1:50) | ThermoFisher |
| α-SMA | SP171 | Rabbit | IHC (1:100) ICC (1:100) WB (1:1000) |
Sigma-Aldrich |
IHC, Immunohistochemistry; ICC, Immunocytochemistry; WB, Western blotting; FC, Flow cytrometry
Western blotting
Western blotting was assessed as previously described53–56. Primary antibodies include anti-active RhoA-GTPase, anti-αSMA, anti-COI, anti-E-cadherin, and anti-phospho-serine-42-Lef1 (Sigma, cat# SAB4504256), anti-Lef1, and β-actin (BioLegend, clone 2F1–1). Blots were washed and probed with IRDye 800CW or IRDye 680RD-conjugated secondary antibodies (LI-COR). Detection was performed using a LI-COR Odyssey CLx imaging system and fluorescent intensities were quantified with Image Studio Lite version 5.2.5 (LI-COR).
MSC lineage tracing mouse model
To trace the lineage of MSCs, nestin-Cre: ROSA26-EYFP mice, where nestin+ cells and their progenitors express EYFP, were used. Fasudil was given 30 min prior to every single CRE challenge in some mice at a dose of 30 mg/kg dissolved in water by intra-peritoneal administration. Mice were sacrificed 4 days after the last CRE-challenge.
MSC differentiation in vitro assay
Mouse MSCs were cultured from bone marrow of C57BL/6J mice as previously described40, 57 and characterized as described in the Online Repository. MSCs were transfected with a plasmid expressing a constitutively active RhoA (L63RhoA) or a dominant negative RhoA (N19RhoA) or empty vector (EV). Lef1 knockdown was accomplished by using MISSION® shRNA Plasmid (Sigma, TRCN0000225788). The methods for transfection and determination of transfection efficiency are provided in the Online Repository. For MSC differentiation into fibroblasts/myofibroblasts, these transfected MSCs were cultured in CRE-ECM or control medium for 7 days. Cells were fixed with 4% paraformaldehyde and immuno-stained for α-SMA. For MSC differentiation into epithelial cells, MSCs were cultured in Small Airway Epithelial Cell Growth Medium (Lonza, CC-3119 and CC-4124), or control medium for 14 days. Cells were then fixed with 4% paraformaldehyde and immune stained for E-cadherin.
Gene profiling of mesenchymal stem cells
For gene profiling, 5.0×105 MSCs were seeded on tissue culture plate 24 h prior to transfection with a plasmid expressing a constitutively active RhoA (RhoA-L63) or empty vector (EV) control using Lipofectamine 2000 (Thermo Fisher). Total RNA was isolated from the MSCs using Ribospin II RNA purification kit (GeneAll) and cDNA was synthesized using High Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher). The cDNA was used on the real-time RT2 Profiler PCR Array (QIAGEN) specifically designed for Mouse Stem Cell Signaling (PAMM-047Z) in combination with RT2 SYBR® Green qPCR Mastermix (QIAGEN). PCR Array data was analyzed using QIAGEN GeneGlobe Data Analysis Center and calculated fold change was done using the 2(-Delta Delta C(T)) method as described by Livak and Schmittgen58.
RT-PCR
Quantitative RT-PCR was performed as previously reported 53, 54, 56. Primer sequences are listed in Table E1 in the Online Repository.
TABLE E1:
Primers used for RT-PCR assays
| Gene | GenBank | Primer Sequence | Amplicon | PrimerBank |
|---|---|---|---|---|
| Accession | Size | ID* | ||
| Acta2 | NM_007392 | Fwd: 5’GTCCCAGACATCAGGGAGTAA Rev: 5’TCGGATACTTCAGCGTCAGGA |
102 | 6671507a1 |
|
Actb |
NM_007393 |
Fwd: 5’ GGCTGTATTCCCCTCCATCG Rev: 5’ CCAGTTGGTAACAATGCCATGT |
154 |
6671509a1 |
|
Cdh1 |
NM_009864 |
Fwd: 5’CAGGTCTCCTCATGGCTTTGC Rev: 5’CTTCAAGAAGGCTGTCC |
175 |
6753374a1 |
|
Lef1 |
NM_010703 |
Fwd: 5’TGTTTATCCCATCACGGGTGG Rev: 5’CATGGAAGTGTCGCCTGACAG |
67 |
27735019a1 |
|
Lifr |
NM_013584 |
Fwd: 5’TACGTCGGCAGACTCGATATT Rev: 5’TGGGCGTATCTCTCTCTCCTT |
113 |
4379217a1 |
|
Rhoa |
NM_016802 |
Fwd: 5’AGCTTGTGGTAAGACATGCTTG Rev: 5’GTGTCCCATAAAGCCAACTCTAC |
138 |
31542143a1 |
|
Smad7 |
NM_001042660 |
Fwd: 5’GGCCGGATCTCAGGCATTC Rev: 5’TTGGGTATCTGGAGTAAGGAGG |
125 |
6678778a1 |
|
Tgfβr3 |
NM_011578 |
Fwd: 5’GGTGTGAACTGTCACCGATCA Rev: 5’GTTTAGGATGTGAACCTCCCTTG |
125 |
33469109a1 |
|
Vimentin |
NM_011701 |
Fwd: 5’CGGCTGCGAGAGAAATTGC Rev: 5’CCACTTTCCGTTCAAGGTCAAG |
124 |
31982755a1 |
Primer identification number given by PrimerBank, a PCR primer database for quantitative gene expression analysis (https://pga.mgh.harvard.edu/primerbank/).
Statistical analysis
All data were analyzed with Graph Pad Prism statistical software program (GraphPad Inc., La Jolla, CA) and are expressed as mean ± SEM. The significance of differences among groups was determined by one-way ANOVA (nonparametric test), and between two groups was analyzed by an unpaired, 2-tailed Student’s t-test. A P<0.05 was considered statistical significance.
RESULTS
Expression of active RhoA is increased in asthmatic lung tissues
To test if RhoA is involved in the pathogenesis of asthma, we detected the expression of active RhoA in the lung tissues of asthmatic mouse model by immunofluorescence analysis. Increased expression of active RhoA (RhoA-GTP) was detected in the lung tissues of cockroach allergen-challenged mouse model relative to the PBS-treated mice as determined by immunostaining (Fig 1, A and B). The active RhoA was predominantly expressed in the airway epithelium as assessed by co-staining for both RhoA-GTP and Epi-CaM. The increased expression of RhoA was further supported by RT-PCR (Fig 1, C) and western blot analyses of lung tissues from these mice (Fig 1, D and E).
FIG 1.
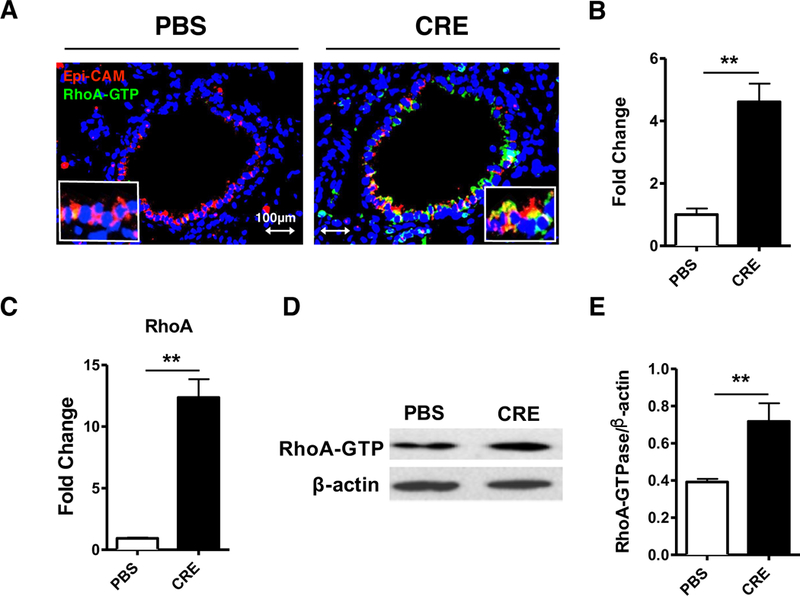
Expression of active RhoA (RhoA-GTP) in asthmatic lung tissues. A, Expression of active RhoA (green) in the airway epithelium (red) and lung tissue samples from CRE and PBS treated mice. B. Quantification of RhoA expression in lung sections using ImageJ v1.50e (NIH). C, Expression of RhoA was detected by RT-PCR. D, Representative Immunoblots for RhoA-GTP in lung tissues of CRE and PBS-treated mice. E, Densitometric analysis for RhoA expression. Data represent mean ± SEM, n=6/group. **P<0.01.
Inhibition of RhoA signaling reverses the established lung inflammation
We determined whether inhibiting RhoA signaling can reverse the established inflammatory phenotypes in a chronic mouse model of asthma (Fig 2, A)20, 53, 57. We found that cockroach allergen-challenged mice showed significantly increased lung inflammatory cell infiltration with goblet cell hyperplasia as assessed by H&E and PAS staining (Fig 2, B). In contrast, lungs from PBS control mice showed normal or undetectable airway inflammation. To determine whether inhibition of RhoA signaling reverses the established lung inflammation, fasudil, a selective RhoA/ROCK inhibitor59, was used after the asthmatic mouse model was established. We found that treatment with fasudil significantly reversed the established lung inflammation with goblet cell hyperplasia. Moreover, these fasudil treated allergen-challenged mice showed reduced numbers of total inflammatory cells and specifically eosinophils in the BAL fluids (Fig 2, C). Furthermore, fasudil-treated mice showed lower levels of serum cockroach specific IgE (sIgE) (Fig 2, D) and reduced levels of IL-4, IL-5, IL-13, TSLP, IL-33, and TGFβ1 (Fig 2, E) in the BAL fluids. Next, we investigated whether inhibiting RhoA signaling can prevent cockroach allergen-induced inflammatory phenotypes by using fasudil before cockroach allergen challenge (Fig E1, A). Similarly, we found that treatment with fasudil before the allergen challenge also suppressed lung inflammation, including the recruitment of inflammatory cells to the lungs with goblet cell hyperplasia on histological examination (Fig E1, B), numbers of total inflammatory cells and specifically eosinophils in the BAL fluids (Fig E1, C), levels of serum cockroach specific IgE and IgG1 (Fig E1, D), and levels of IL-4, IL-5, IL-13, and TGFβ1 in the BAL fluids (Fig E1, E). The results suggest that RhoA signaling participates in allergen-induced lung inflammation.
FIG E1.
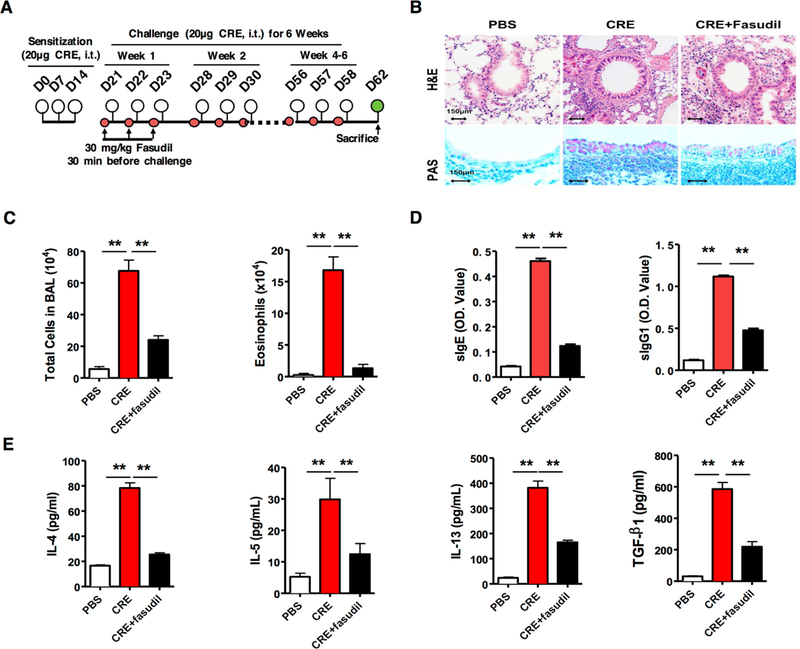
Inhibition of RhoA signaling reverses cockroach allergen-induced lung inflammation. A, Protocol for cockroach allergen-induced mouse model of asthma. CRE: cockroach extract, i.t., intra-tracheal. B, Paraffin tissue sections of lung were stained with H&E (upper panel) and periodic acid-Schiff (PAS, lower panel). Scale bars represent 150 µm. C, Bronchoalveolar lavage (BAL) total and eosinophil cell counts as assessed by flow cytometry. D-E, ELISA analyses for serum levels of cockroach allergen specific IgE and IgG1 (D) and BAL levels of cytokines (E). C-E, 10 to 12 mice were pooled from two independent experiments. Data represent mean ± SEM. *P<0.05, **P<0.01.
Inhibition of RhoA signaling reverses the established airway remodeling
Next, we asked whether RhoA signaling can reverse the established airway remodeling caused by chronic exposure to cockroach allergen. Compared to PBS-challenged mice, cockroach allergen-challenged mice showed increased airway thickening and more collagen deposition/fibrosis as assessed by Masson’s trichrome staining (Fig 2, F). Similarly, treatment with fasudil after the establishment of asthmatic mouse model reversed the established airway thickening (Fig 2, G) and collagen deposition/fibrosis (Fig 2, H). The reversed airway remodeling was further confirmed by western blot (Fig 2, I) with the reduced expression of α-SMA (Fig 2, J) and Collagen 1(CoI1) (Fig 2, K) in fasudil-treated vs. vehicle-treated asthmatic mice. The same pattern was also observed for the asthma mouse model treated with fasudil before allergen challenge (Fig E2). The results indicate that RhoA signaling may be critical in allergen-induced airway remodeling.
FIG E2.
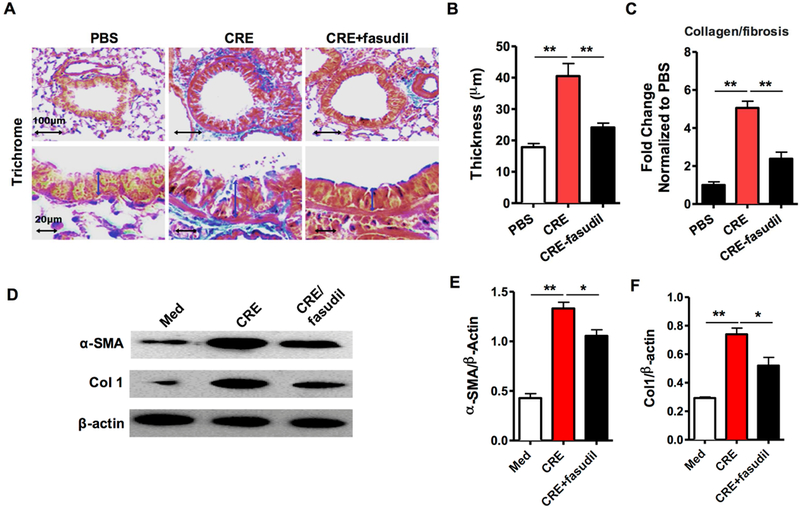
Inhibition of RhoA signaling reverses cockroach allergen-induced airway remodeling. A, Representative images of the Masson trichrome staining of collagen on lung sections. B, Analyses of the epithelial layer thickness. C, airway collagen/fibrosis (blue) presented as fold change (blue/total area in each group normalized to PBS group). D, Representative immunoblots for α-SMA and collagen 1 (Col 1) in lung sections. β-actin was used as a control. n=3. E-F, Densitometric analysis for α-SMA (E) and Col 1 (F). A-C, n=4–6/group. Quantification data represent mean ± SEM. *P<0.05, **P<0.01.
Cockroach allergen-challenged epithelium conditioned medium (CRE-ECM) activates RhoA/ROCK signaling through TGFβ1
To investigate the underlying mechanisms by which active RhoA signaling exacerbates airway remodeling, we first determined whether cockroach allergen-challenged epithelium conditioned medium (CRE-ECM) induces the activation of RhoA/ROCK signaling in MSCs. Mouse bone-marrow-derived MSCs were cultured and characterized by positive expression of CD29, Sca1, nestin, and LepR, and negative expression of CD11b, CD34, CD31, and TER-119 (Fig 3, A)40, 57. MSCs were treated with CRE-ECM, and expression of active RhoA in MSCs was analyzed after 24 h. Expression of active RhoA in MSCs was markedly augmented upon exposure to CRE-ECM as detected by immunostaining with antibody against active RhoA (Fig 3, B). We further determined whether the CRE-ECM-induced activation of RhoA is through TGFβ1, because we previously demonstrated that CRE-ECM contains active TGFβ1 and activates TGFβ1 signaling in MSCs 20. Interestingly, the augmented RhoA expression was significantly inhibited when TGFβ1 was removed from CRE-ECM by TGFβ1 neutralizing antibody (Fig 3, B). Similar results were also obtained by western blot (Fig 3, C).
FIG 3.
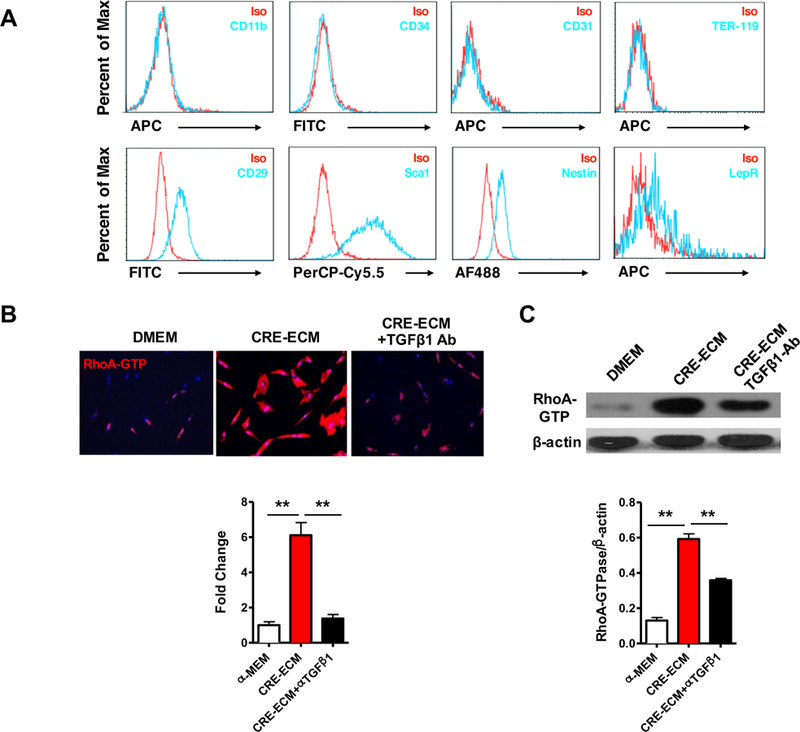
Characteristics of mesenchymal stem cells and CRE-ECM-induced RhoA activation. A, MSCs were characterized by positive expression of CD29, Sca1, nestin, and LepR, and negative expression of CD11b, CD34, CD31, and TER-119. B, Representative images of immunofluorescence staining for GTP-RhoA (red) in MSCs. C, Representative immunoblots for RhoA-GTP in MSCs. β-actin was used as a control. Data represent mean ± SEM of three independent experiments. **P<0.01.
Inactivation of RhoA/ROCK signaling promotes MSC differentiation into epithelial cells
To determine the role of RhoA/ROCK signaling in the lineage commitment of MSCs in asthma, we took advantage of the nestin-Cre; ROSA26-EYFP mice, where nestin+ cells and their progeny permanently express EYFP. Compared to PBS-challenged mice, CRE-challenged mice showed increased double YFP+/α-SMA+ (Fig 4, A, upper) and YFP+/vimentin+ cells (Fig 4, A, middle) in the sub-epithelial layer, but not double YFP+/E-cadherin+ in the airway epithelial layer, indicating that these migrated YFP-labelled cells differentiate into fibroblasts/myofibroblasts. Intriguingly, YFP+/α-SMA+ or YFP+/vimentin+ cells were nearly undetectable in the sub-epithelial layer, whereas double E-cad+YFP+ cells were remarkably increased in the airway epithelial layer of mice with fasudil treatment (Fig 4, A, lower), indicating that these YFP-labelled cells are differentiated away from fibroblasts/myofibroblasts but toward airway epithelial cells after treatment with fasudil. Expression of α-SMA, vimentin, and E-cadherin in lung tissues from these mice were also analyzed by RT-PCR. Consistently, compared to these PBS treated mice, CRE-treated mice showed increased expression of α-SMA and vimentin, but was significantly inhibited by fasudil (Fig 4, B). In contrast, E-cadherin showed no change between PBS and CRE-treated group, but increased in fasudil-treated CRE-challenged mice.
FIG 4.
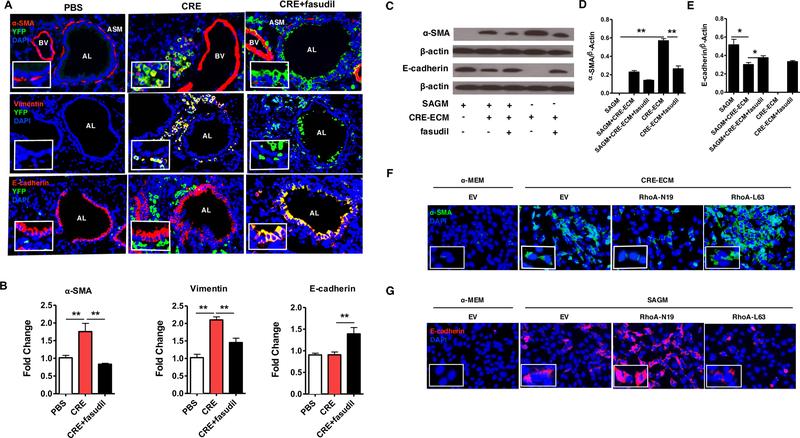
RhoA signaling regulates the lineage commitment of MSCs in asthma mouse model and in vitro. A, Representative image of double immunofluorescence staining with the primary antibodies against YFP, α-SMA (top), vimentin (meddle), and E-cadherin (bottom). n=6/group Nuclei were counterstained with DAPI. AL: airway lumen, BV: bronchial vein. ASM: airway smooth muscle. B, Expression of α-SMA, vimentin, and E-cadherin in lung tissues were analyzed by RT-PCR, n=6/group. C, Representative immunoblots for α-SMA and E-cadherin in MSCs. β-actin was used as a control. D-E, Densitometric analysis for α-SMA (D) and E-cadherin (E). F, Immunofluorescence staining of CRE-ECM-treated MSCs expressing empty vector (EV), RhoA-L63, and RhoA-N19 with the primary antibody against α-SMA. G, Immunofluorescence staining of SAGM-treated MSCs expressing EV, RhoA-L63, and RhoA-N19 with the primary antibody against E-cadherin. Data represent mean ± SEMs of three independent experiments. *P<0.05, **P<0.01.
The role of RhoA/ROCK signaling in controlling MSC lineage commitment was further confirmed with in vitro experiments. MSCs were cultured with CRE-ECM in the presence or absence of fasudil. We found that CRE-ECM promoted α-SMA expression in MSCs, but was significantly inhibited by fasudil (Fig 4, C and D). In contrast, E-cadherin was not expressed in the CRE-ECM-treated MSCs, but expressed in CRE-ECM-treated MSCs with fasudil (Fig 4, C and E). Similar pattern was observed for SAGM (small airway growth media). We found that SAGM induced E-cadherin expression, which was inhibited by CRE-ECM, but partially recovered by fasudil. As expected, α-SMA expression was not observed in SAGM-treated MSCs (Fig 4, C and D). To further validate these findings, we used MSCs expressing either a constitutively active RhoA (RhoA-L63) or dominant-negative (RhoA-N19). Compared to the cells transfected with control empty vector, MSCs expressing RhoA-L63 showed increased expression of α-SMA (Fig 4, F), but MSC expressing RhoA-N19 demonstrated decreased expression of α-SMA. Next, we tested whether inhibition of RhoA activation promotes MSCs differentiation toward epithelial cells in MSCs cultured with SAGM. MSCs expressing RhoA-N19 showed increased expression of E-cadherin when compared with those transfected with empty vector (Fig 4, G), whereas MSCs expressing RhoA-L63 had no such effect. Collectively inactivation of RhoA/ROCK signaling not only inhibits MSC fibroblasts/myofibroblast differentiation, but also triggers the switch of MSCs to differentiation into airway epithelial cells.
Identification of genes involved in RhoA-mediated MSC differentiation
To identify the molecules that mediate RhoA-induced MSC differentiation, we investigated the genes differentially expressed in mouse MSCs transfected with or without RhoA-L63 using the Stem Cell Signaling Array (PAMM-047Z, QIAGEN). This array profiles the expression of 84 key genes involved in signal transduction pathways important for embryonic stem cell (ESC) and induced pluripotent stem cell (iPSC) maintenance and differentiation (Fig 5, A). A total of 16 genes were differentially expressed in RhoA-L63 transfected MSCs compared to control cells with ranges from 2 to 56.61-fold change (Fig 5, B), including 8 genes up-regulated and 8 down-regulated (Fig 5, C). Of these, Lef-1 is the most up-regulated gene (56.61-fold), and the pluripotency maintenance pathway factor Lifr (leukemia inhibitory factor receptor) was the most down-regulated (13.55-fold). Especially, genes within TGF-β/BMP pathway and Wnt signaling factor showed the most up or down-regulation (Fig 5, D). Several selected up or down-regulated genes were further validated by qRT-PCR, including Lef1, Smad7, TGFβr3, and Lifr (Fig 5, E).
FIG 5.
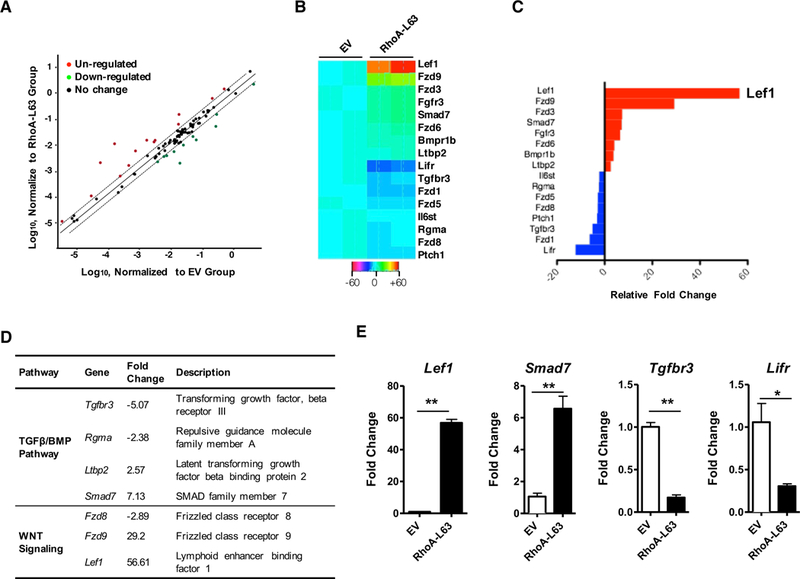
Identification of RhoA downstream genes by using Stem Cell Signaling Array. A, Differentially expressed genes in MSCs transfected with empty vector (EV) or RhoA-L63, n=4/group. B, Heatmap of gene expression analysis (Fold changes ≥2.0, P<0.01). C, Relative fold change of top up (red) or down (blue)-regulated genes. D, Most differentially expressed genes in TGFβ/BMP and WNT pathways. E, qRT-PCR analysis for Lef1, Smad7, Tgfbr3, and Lifr mRNA expression in MSCs expressing empty vector (EV) and RhoA-L63. Data represent mean ± SEMs of three independent experiments. *P<0.05, **P<0.01.
RhoA signaling regulates Lef1 activation
We then narrowed our focus down to Lef1 and investigated its regulation by RhoA signaling Compared to PBS-treated mice, cockroach allergen-challenged mice showed significantly up-regulated expression of Lef1 in lung tissues (Fig 6, A). The expression of Lef1 was attenuated if mice were treated with fasudil before challenge. These findings were further supported by RT-PCR analysis in lung tissues from these mice (Fig 6, B). Interestingly, although no difference was found for total Lef1, increased expression was noted for phospho-Lef1 (p-Lef1) in MSCs after exposure to CRE-ECM (Fig 6, C and D). The increased expression of p-Lef1 was blocked when fasudil was added to CRE-ECM.
FIG 6.
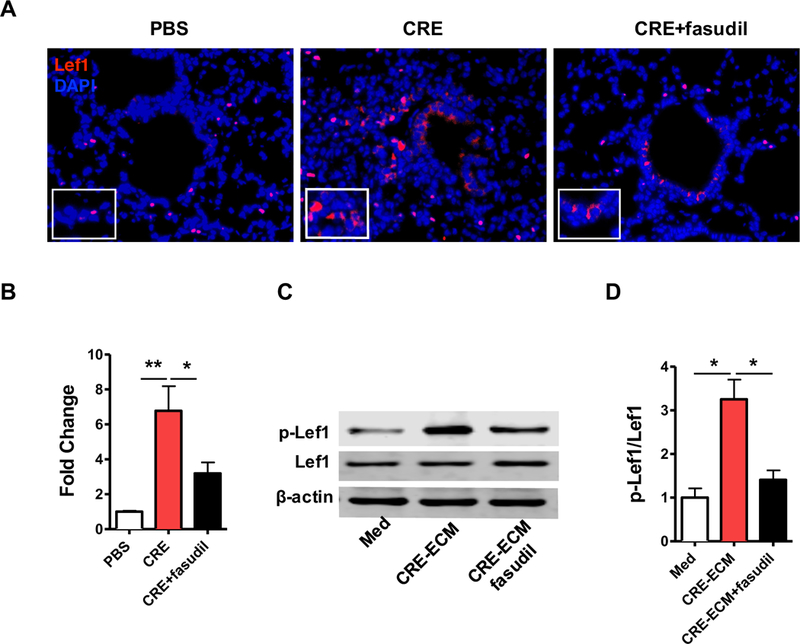
RhoA signaling induces Lef1 activation in MSCs. A, Representative image of immunofluorescence staining for lef1 in lung section (n=5/group). B, RT-PCR analysis of Lef1 expression in lung tissues (n=5/group). C, Representative immunoblots for total Lef1 and phosphote-Lef1 (p-Lef1) in MSCs. β-actin was used as a control. n=3. D, Densitometric analysis for Lef1. Data represent mean ± SEMs. *P<0.05, **P<0.01.
Lef1 regulates MSC differentiation
We tested whether the RhoA activated Lef1 plays a role in MSC differentiation by knocking down Lef1 with targeted small interfering RNA. We first confirmed the transfection efficacy of Lef1 shRNA using flow cytometry analysis (Fig 7, A). Moreover, a dose-dependent deletion of Lef1 was detected by RT-PCR (Fig 7, B) and western blot (Fig 7, C). A time-dependent deletion of Lef1 was also performed (Fig E3). While no difference was observed in these cells with or without Lef1 knockdown after treatment with control medium (Fig 7, D to G), significant changes on MSC differentiation were observed in cells cultured with either CRE-ECM or SAGM. Particularly, MSCs with Lef1 knockdown display an inhibition of MSC differentiation into fibroblasts compared to siRNA control-treated MSCs for α-SMA (Fig 7, D). The inhibition was further supported by western blotting (Fig 7, E). By contrast, these MSCs with Lef1 knockdown showed an increased differentiation into epithelial cells with higher expression of E-cadherin compared to shRNA control-treated MSCs (Fig 7, F) and western blot (Fig 7, G) Collectively, our findings suggest that knockdown of Lef1 can suppress MSC differentiation into fibroblasts, but promote differentiation of MSCs into epithelial cells.
Figure 7.
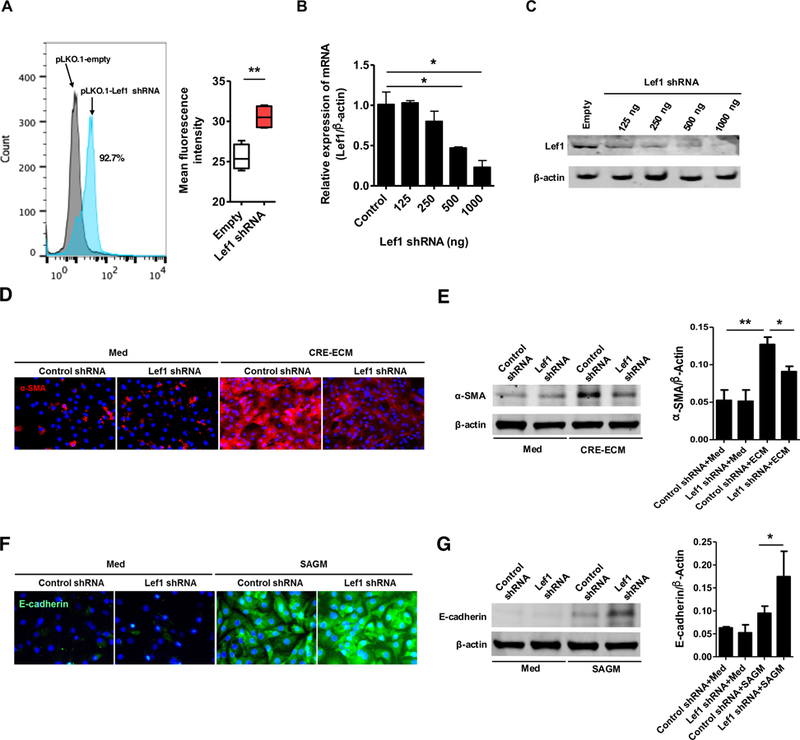
Lef1 regulates MSC differentiation. A, Transfection efficacy of Lef1 shRNA was confirmed using flow cytometry analysis. B-C, A dose-dependent deletion of Lef1 was detected by RT-PCR (B) and western blot (C). D, Immunofluorescence staining of MSCs transfected with control shRNA and Lef1 shRNA with the primary antibody against α-SMA. E, Representative immunoblots for α-SMA in MSCs transfected with control shRNA and Lef1 shRNA. F, Immunofluorescence staining of MSCs transfected with control shRNA and Lef1 shRNA with the primary antibody against E-cadherin. G, Representative immunoblots for E-Cadherin in MSCs transfected with control shRNA and Lef1 shRNA. Data represent mean ± SEMs of three independent experiments. *P<0.05, **P<0.01.
FIG E3.
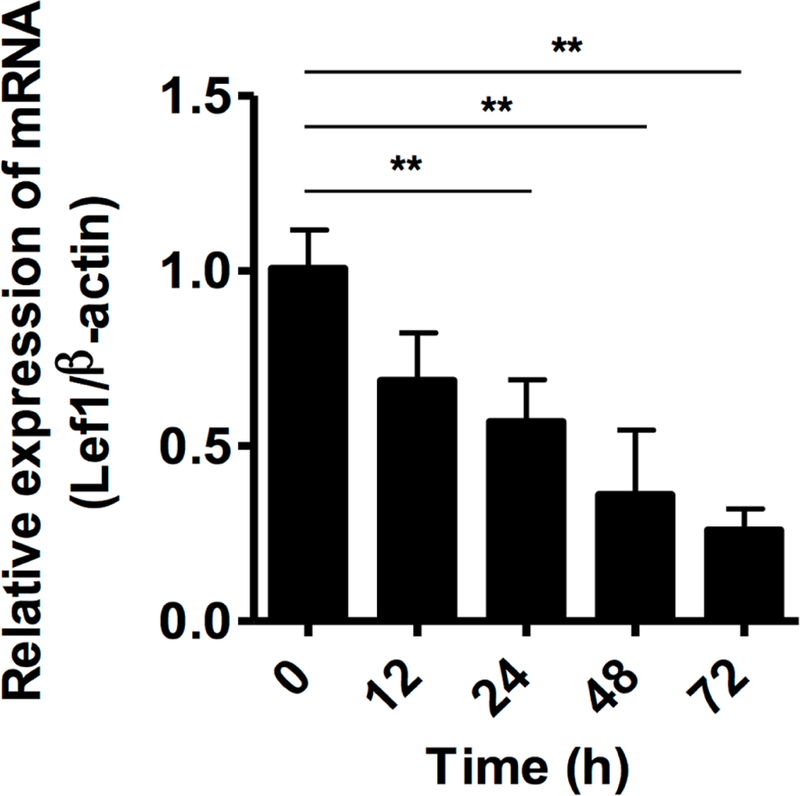
Lef1 knockdown in MSCs at different times was assessed by RT-PCR. **P<0.01.
DISCUSSION
In this study, we made a novel finding that TGFβ1 activated RhoA/ROCK signaling functions as a molecular switch for the lineage fate of the recruited MSCs in asthma: activation of the RhoA/ROCK signaling drives MSC differentiation toward fibroblasts/myofibroblasts and enhances airway remodeling, whereas inactivation of this signaling pathway diverts MSC differentiation away from fibroblasts/myofibroblasts to epithelial cells for airway repair. Our study also revealed that the RhoA/ROCK signaling-mediated MSC lineage commitment/differentiation is, at least partially, through Lef1. The results suggest that Lef1 is a novel key downstream target of RhoA signaling. Our findings provide evidence for the potential development of RhoA/ROCK inhibitor as a therapeutic target for asthma.
Studies have suggested that active TGFβ1 released from the injured tissues is essential for the recruitment of MSCs to the damaged tissues and tissue repair/remodeling20, 22, 40, 60. Our recent finding that TGFβ1-activated RhoA/ROCK signaling functions as a molecular switch regarding the fate of MSCs in arterial repair/remodeling after injury40, which led us to hypothesize that TGFβ1-activated RhoA/ROCK signaling may control the lineage commitment of MSCs in airway repair/remodeling in asthma. Indeed, numerous studies have suggested that RhoA is required for B cell61 and Th2 cell differentiation51, and is associated with pathophysiological processes of asthma46–52. Data presented in this study adds new understanding on the involvement of MSCs in the pathogenesis of asthma and reveals a novel role of RhoA in airway remodeling.
Our studies offered several lines of evidence to support the biological role of RhoA/ROCK signaling in asthma. First of all, we found an activated RhoA signaling in the lung tissues of cockroach allergen-induced mouse model of asthma. Next, we showed that RhoA/ROCK signaling is an important player in allergic inflammation, and specifically, inhibition of RhoA/ROCK signaling can not only reverse the established cockroach allergen-induced inflammation, but also prevent the development of allergen-induced inflammation However, we noted that inhibition of RhoA/ROCK signaling did not “completely” block the cockroach allergen-induced lung inflammation. Although the reason is unclear, we believe that RhoA signaling is not sole signaling activated by cockroach allergen that contributes to the cockroach allergen-induced lung inflammation. In particular, we have recently demonstrated that mannose receptor modulates macrophage polarization and allergic inflammation through miR-511–3p54, and miR-155 modulates cockroach allergen and oxidative stress-induced cyclooxygenase-2 in asthma55. PAR2 signaling has also been shown to play a key role in cockroach allergen-induced airway hyper-responsiveness and airway inflammation/remodeling in a chronic mouse model62. Furthermore, we showed that inhibition of RhoA/ROCK signaling reversed the established cockroach allergen-induced collagen deposition and airway wall thickening, an index of airway remodeling. These findings are consistent with several recent reports that inhibition of RhoA/ROCK signaling attenuates hyperoxia-induced pulmonary fibrosis in neonatal rats63, prevents and reverses intestinal fibrosis64, and contributes to the development of experimental pulmonary fibrosis65. Collectively, our studies provide further evidence that inhibition of RhoA/ROCK signaling could prevent or recover the cockroach allergen-induce chronic inflammation and remodeling in asthma.
One of the most important mechanisms elucidated from this study is that RhoA/ROCK signaling controls lineage commitment of the recruited MSCs during asthma. MSCs can differentiate into a number of mature cell types such as epithelial cells30–35 and fibroblasts/myofibroblasts38, 39. Particularly, we hypothesized that the migrated MSCs at early stages may suppress inflammation and repair the damaged airway through cell differentiation and paracrine effects (growth factors and cytokines at sites of tissue injury)15, 20, 35, 66–68, but when there are repetitive allergen exposures and sustained release of local growth factors, MSCs may contribute to progressive fibrosis and pathological remodeling due to their differentiation into myofibroblasts38. Indeed, this hypothesis was well-supported by our findings with the use of the nestin-Cre; ROSA26-EYFP mice, which directly trace MSC migration and differentiation. We found increased double YFP+/α-SMA+ or YFP+/vimentin+ cells in the sub-epithelial layer after being repeatedly exposed to cockroach allergen, whereas these cell populations were reduced when these mice were pre-treated with fasudil. In contrast, YFP+ cells were increased in the epithelial layer which were also positive for airway epithelial marker E-cadherin. These findings were further supported by in vitro experiments with MSCs expressing a constitutively active RhoA or dominant negative RhoA. Thus, this study provides a proof of concept that MSCs can differentiate into airway epithelial cells, and inactivation of RhoA/ROCK signaling not only inhibits MSC fibroblast/myofibroblast differentiation and airway remodeling but also triggers the switch of MSCs to participate in epithelium repair after airway damage.
The airway epithelium is the lung’s first line of defense against inhaled allergens and pollutants. Once injury occurs, airway epithelium must retain a robust capacity for self-repair. Airway basal progenitor cells are one of the most important factors involved with repair69, 70. A recent study has suggested that multipotent stem cell population in airway submucosal glands can proliferate, migrate, and transdifferentiate to repair surface epithelium following injury71. Furthermore, alveolar type 2 (AT2) cells also function as alveolar stem cells, and single-cell Wnt signaling niches maintain stemness of AT272. These studies mainly focused on the local resident progenitor cells, but migrated stem cells like bone marrow-derived MSCs are not considered. Thus, our studies filled the gap and illustrated that MSCs can migrate from bone marrow into airway and differentiate into airway epithelial cells after airway injury. However, future studies are needed to track the lineage commitment/differentiation of MSCs in asthma mouse model over time using an inducible MSC lineage tracing mouse model. Particularly, it would be of interest to determine the differentiation of MSCs into different subtypes of airway epithelial cells in these mouse models. While we focused on MSCs that may serve as progenitor cells for the epithelium, local epithelial cells (e.g., basal cells, club cells and AT-II)70, 73 are also critical as stem/progenitor cells for the epithelial repair. We assume that airway repair/remodeling in early stages may be due to local airway basal progenitor cells or local MSCs with ultimate loss of their proliferative and differentiation capacity (“progen itor exhaustion”) 70, but in late stages is due to migrated bone-marrow-derived MSCs following repeated challenges. Our studies here further recognized the downstream event of RhoA/ROCK signaling in MSC lineage commitment and demonstrated that genes within TGF-β/BMP pathway and Wnt signaling are the most up or down-regulation in RhoA over-expressed MSCs. Particularly, we identified Lef1 as a Wnt signaling factor to be the most up-regulated. Lef1 as a transcription factor belongs to a family of proteins that share homology with the high mobility group protein-1(HMG1)74. Interestingly, Lef1 can directly induce epithelial-mesenchymal cell transition (EMT)75 and is also required for the transition from mesenchymal to epithelial cells in the mouse embryonic mammary gland76 and lineage commitment of myoepithelial cells (stem cells for regenerating tracheal epithelium)77. Our studies provide novel evidence for the requirement of Lef1 in lineage commitment/MSCs to either myofibroblasts/fibroblasts or epithelial cells. While no difference was observed between untreated MSCs with or without Lef1 knockdown, it is likely that the trigger (s) or the activation of RhoA signaling is essential in Lef1-mediated MSC differentiation. These findings suggest that Lef1 is critical in regulating MSC differentiation, and blocking Lef1 can be an alternative approach to interfere with the TGFβ1-RhoA signaling pathway for airway repair or suppression of airway remodeling in asthma. However, the underlying mechanism regarding Lef1-mediated MSC differentiation remains unknown. β-catenin, a coactivator for transcription factors of the TCF/LEF family78, plays a role in proliferation, self-renewal, and differentiation of hematopoietic stem cells35, 75, 79, raising the possibility that β-catenin may mediate the RhoA-Lef1 interaction during MSC differentiation. In addition, TGFβ transcriptional coactivator Smad3, co-localized with Lef1 at canonical Wnt-responsive elements, directly interacts with Lef180. Furthermore, Lef1 has been demonstrated to bind the promoter regions of E-Cadherin81 or VEGF82 and regulate cell differentiation75, 83. Lastly, downstream genes are required for the Lef1-regulated MSC differentiation (e.g., vimentin84, Oct4 and nanog85) (Fig 8).
Figure 8.
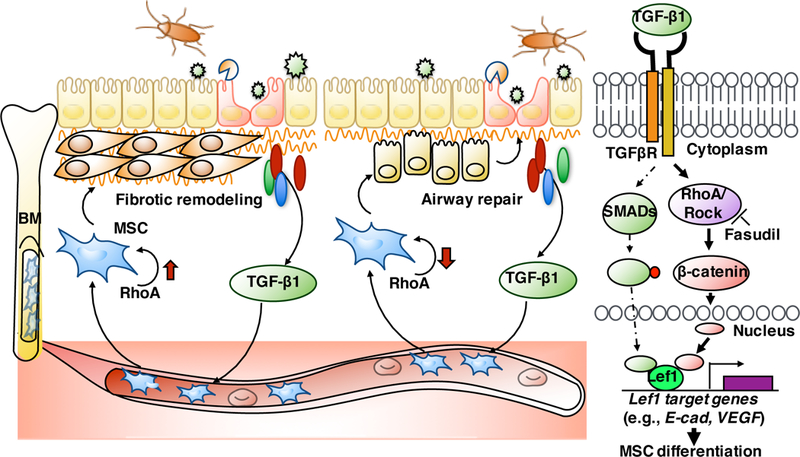
Schematic showing the role of TGFβ1-RhoA signaling in MSC recruitment, lineage commitment/differentiation, and its underlying molecular mechanism.
In summary, our observation in a chronic asthma mouse model yield important findings: RhoA/ROCK signaling plays a critical role in allergen-induced airway inflammation and remodeling, and activation of the RhoA/ROCK signaling drives MSC differentiation toward fibroblast/myofibroblasts and enhances airway remodeling. Inactivation of the RhoA/ROCK signaling promotes MSC differentiation into epithelial cells for airway repair through the Wnt signaling factor Lef1. These findings provide new understanding of the signaling pathways that control MSC lineage commitment in airway repair/remodeling in asthma and recognize a novel therapeutic target for patients with asthma.
METHODS
Masson’s Trichrome staining
The Masson Trichrome staining procedure stains the collagen-rich fibrotic regions in blue, it is suited to assess and visualize the extent of fibrosis in lung tissues. Masson’s Trichrome was conducted according to the guideline of the agent kit from Sigma (HT15, St. Louis, MO, USA). Briefly, frozen slides were fixed in Bouin’s solution overnight at room temperature. After incubation in Weigert’s Iron Hematoxylin Solution, the slides was stained with Biebrich Scarlet-Acid Fuchsin and Aniline Blue and dehydrated in ethanol and xylene. Extensive washes were done between each staining. Images where taken using a Nikon ETi microscope. The collagen fibers were stained blue, the nuclei were stained black and lung tissues were stained red. Quantification of collagen fiber from Masson Trichrome stained lung sections (n=4–6/group) were carried out using ImageJ v1.50e (NIH) with installed plugins, deconvolution of the color images, and image calculator. Airway thickness were also measured using ImageJ based on given length-to-pixel ratio.
Epithelial cell-derived conditioned medium
Cockroach allergen-induced epithelial conditioned medium (CRE-ECM) was prepared by treating TC-1, a mouse lung epithelial cell-line, with CRE (50 µg/ml) for 72 h in serum free DMEM. Cell free CRE-ECM was collected, filtered through a 0.22 µm filter (Millipore) and stored at −80°C until used.
Bone marrow-derived mesenchymal stem cell and characterization.
MSCs were cultured from bone marrow of C57BL/6J mice as previously described (E1, E2). MSCs were cultured in α-MEM containing 20% FBS (Gibco). MSCs (passage 6–8) were characterized by flow cytometry using the following markers: CD11b (Thermo Fisher, clone M1/70), CD34 (Thermo Fisher, clone RAM34), CD31 (Thermo Fisher, clone 390), TER-119 (Thermo Fisher), CD29 (Thermo Fisher, clone eBioHMb1–1), Sca-1 (Thermo Fisher, clone D7), Nestin (ThermoFisher, clone 1A4), and LepR (Thermo Fisher). These samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences) as previously described (E2).
MSC transfection
Approximately 1.0×105 MSCs were seeded onto a 6-well tissue culture plate 24 h prior transfection with a plasmid expressing a constitutively active RhoA (L63RhoA) or a dominant negative RhoA (N19RhoA) or empty vector (EV) control using FuGENE 6 (Promega) as instructed by the manufacturer. Transfection efficiency was assessed by GFP expression using flow cytometry.
Lef1 knockdown
Lef1 knockdown was accomplished by transfecting 1×105 MSCs with 0.5 µg of MISSION® shRNA Plasmid (Sigma, TRCN0000225788) expressing Lef1 specific shRNA using FuGENE 6 (Promega). pLKO.1-puro non-target shRNA (Sigma, SHC016) was used as a control. Lef1 knockdown was confirmed by RT-PCR and Western blot.
RT-PCR
Total RNA was extracted from CK14 ceramic beads (Cayman Chemical) homogenized tissues and reverse-transcribed to cDNA using High Capacity cDNA Reverse Transcriptase kit (Thermo Fisher). Quantification PCR assay was performed in triplicate using 7300 Real Time PCR System (Applied Biosciences) with PowerUp™ SYBR™ Green Master Mix (Thermo Fisher) with the appropriate primers (Table E1). Relative gene expression was calculated by using the 2−ΔΔCT methods as described by Livak and Schmittgen (E3). The mRNAs were normalized to the internal control gene β-actin. A ‘no template’ sample was used as a negative control.
Key Messages.
RhoA signaling is critical in airway inflammation and remodeling.
RhoA signaling controls lineage commitment of MSCs in asthma.
Wnt signaling effector Lef1 plays a role in RhoA signaling-mediated MSC differentiation.
ACKNOWLEDGEMENTS
This work was supported by grants from the US National Institutes of Health (NIH) (RO1ES021739, R21 AI109062, R21 AI121768, and R21 AI137547) and the National Science Foundation of China (NSFC) 81628001(to P Gao).
ABBREVIATIONS
- CRE
Cockroach extract
- BALF
Bronchoalveolar lavage fluid
- CRE-ECM
Cockroach allergen-epithelial conditioned medium
- MSC
Mesenchymal stem cell
- RhoA
Ras homolog family member A
- ROCK
Rho-associated protein kinase 1
- Lef1
Lymphoid enhancer-binding factor 1
- EMT
Epithelial-mesenchymal cell transition
- α-SMA
Alpha-smooth muscle actin
- WT
Wild-type
- EV
Empty vector
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc 2009; 6:655–9. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev 2011; 242:205–19. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012; 18:684–92. [DOI] [PubMed] [Google Scholar]
- 4.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest 2009; 119:336–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganesan S, Sajjan US. Repair and Remodeling of airway epithelium after injury in Chronic Obstructive Pulmonary Disease. Curr Respir Care Rep 2013; 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000; 161:1720–45. [DOI] [PubMed] [Google Scholar]
- 7.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol 2011; 128:451–62; quiz 63–4. [DOI] [PubMed] [Google Scholar]
- 8.Naveed SU, Clements D, Jackson DJ, Philp C, Billington CK, Soomro I, et al. Matrix Metalloproteinase-1 Activation Contributes to Airway Smooth Muscle Growth and Asthma Severity. Am J Respir Crit Care Med 2017; 195:1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res 2017; 367:551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 1998. 279:1528–30. [DOI] [PubMed] [Google Scholar]
- 11.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, et al. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J 2008; 22:1226–36. [DOI] [PubMed] [Google Scholar]
- 12.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013; 19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 2003; 48:3464–74. [DOI] [PubMed] [Google Scholar]
- 14.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium feasibility, cell migration, and body distribution. Circulation 2003; 108:863–8. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 2007; 104:11002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bentley JK, Popova AP, Bozyk PD, Linn MJ, Baek AE, Lei J, et al. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respir Res 2010; 11:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, LeClair L, Poynter ME, et al. Bone marrow-derived mesenchymal stromal cells inhibit Th2-mediated allergic airways inflammation in mice. Stem Cells 2011; 29:1137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther 2011. 2:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou-Yang HF, Huang Y, Hu XB, Wu CG. Suppression of allergic airway inflammation in a mouse model of asthma by exogenous mesenchymal stem cells. Exp Biol Med (Maywood) 2011; 236:1461–7. [DOI] [PubMed] [Google Scholar]
- 20.Gao P, Zhou Y, Xian L, Li C, Xu T, Plunkett B, et al. Functional effects of TGF-beta1 on mesenchymal stem cell mobilization in cockroach allergen-induced asthma. J Immunol 2014; 192:4560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L, Zeng X, Hu J, Chen Q. Characterization of Nestin, a Selective Marker for Bone Marrow Derived Mesenchymal Stem Cells. Stem Cells Int 2015; 2015:762098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan M, Li C, Zhen G, Jiao K, He W, Jia X, et al. Injury-activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells 2012; 30:2498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Li C, Pang L, Shi C, Guo F, Chen A, et al. Mesenchymal stem cells recruited by active TGFbeta contribute to osteogenic vascular calcification. Stem Cells Dev 2014. 23:1392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Xie L, Tintani F, Xie H, Li C, Cui Z, et al. Aberrant Transforming Growth Factor-beta Activation Recruits Mesenchymal Stem Cells During Prostatic Hyperplasia. Stem Cells Transl Med 2017; 6:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med 2013. 19:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L, Tintani F, Wang X, Li F, Zhen G, Qiu T, et al. Systemic neutralization of TGF-beta attenuates osteoarthritis. Ann N Y Acad Sci 2016; 1376:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogulur I, Gurhan G, Aksoy A, Duruksu G, Inci C, Filinte D, et al. Suppressive effect of compact bone-derived mesenchymal stem cells on chronic airway remodeling in murine model of asthma. Int Immunopharmacol 2014; 20:101–9. [DOI] [PubMed] [Google Scholar]
- 28.Takeda K, Webb TL, Ning F, Shiraishi Y, Regan DP, Chow L, et al. Mesenchymal Stem Cells Recruit CCR2(+) Monocytes To Suppress Allergic Airway Inflammation. J Immunol 2018; 200:1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinas-Pardo L, Mirones I, Amor-Carro O, Fraga-Iriso R, Lema-Costa B, Cubillo I, et al. Mesenchymal stem cells regulate airway contractile tissue remodeling in murine experimental asthma. Allergy 2014; 69:730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci U S A 2003; 100:2397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc 2008; 5:323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr., et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci U S A 2005; 102:186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Sun Z, Qiu X, Li Y, Qin J, Han X. Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 2009; 390:1309–14. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Wang Y, Gong X, Su H, Han X. Secretion of rat tracheal epithelial cells induces mesenchymal stem cells to differentiate into epithelial cells. Cell Biol Int 2012; 36:169–75. [DOI] [PubMed] [Google Scholar]
- 35.Cai SX, Liu AR, Chen S, He HL, Chen QH, Xu JY, et al. Activation of Wnt/beta-catenin signalling promotes mesenchymal stem cells to repair injured alveolar epithelium induced by lipopolysaccharide in mice. Stem Cell Res Ther 2015; 6:65. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Kokubun K, Pankajakshan D, Kim M-JJ, Agrawal DK. Differentiation of porcine mesenchymal stem cells into epithelial cells as a potential therapeutic application to facilitate epithelial regeneration. Journal of tissue engineering and regenerative medicine 2016; 10:83. [DOI] [PubMed] [Google Scholar]
- 37.Tang N, Zhao Y, Feng R, Liu Y, Wang S, Wei W, et al. Lysophosphatidic acid accelerates lung fibrosis by inducing differentiation of mesenchymal stem cells into myofibroblasts. J Cell Mol Med 2014; 18:156–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol 2007; 39:666–71. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 2016. 16:582–98. [DOI] [PubMed] [Google Scholar]
- 40.Li C, Zhen G, Chai Y, Xie L, Crane JL, Farber E, et al. RhoA determines lineage fate of mesenchymal stem cells by modulating CTGF-VEGF complex in extracellular matrix. Nat Commun 2016; 7:11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002; 420:629–35. [DOI] [PubMed] [Google Scholar]
- 42.Zhao ZS, Manser E. PAK and other Rho-associated kinases--effectors with surprisingly diverse mechanisms of regulation. Biochem J 2005; 386:201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247–69. [DOI] [PubMed] [Google Scholar]
- 44.Hall A Rho family GTPases. Biochem Soc Trans 2012; 40:1378–82. [DOI] [PubMed] [Google Scholar]
- 45.Bryan BA, Dennstedt E, Mitchell DC, Walshe TE, Noma K, Loureiro R, et al. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J 2010; 24:3186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaafsma D, Gosens R, Bos IS, Meurs H, Zaagsma J, Nelemans SA. Allergic sensitization enhances the contribution of Rho-kinase to airway smooth muscle contraction. Br J Pharmacol 2004; 143:477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. Am J Respir Cell Mol Biol 2006; 35:722–9. [DOI] [PubMed] [Google Scholar]
- 48.Kume H RhoA/Rho-kinase as a therapeutic target in asthma. Curr Med Chem 2008. 15:2876–85. [DOI] [PubMed] [Google Scholar]
- 49.Goto K, Chiba Y, Sakai H, Misawa M. Mechanism of inhibitory effect of prednisolone on RhoA upregulation in human bronchial smooth muscle cells. Biol Pharm Bull 2010. 33:710–3. [DOI] [PubMed] [Google Scholar]
- 50.Wei B, Shang YX, Li M, Jiang J, Zhang H. Cytoskeleton changes of airway smooth muscle cells in juvenile rats with airway remodeling in asthma and the RhoA/ROCK signaling pathway mechanism. Genet Mol Res 2014; 13:559–69. [DOI] [PubMed] [Google Scholar]
- 51.Yang JQ, Kalim KW, Li Y, Zhang S, Hinge A, Filippi MD, et al. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J Allergy Clin Immunol 2016; 137:231–45 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. American journal of physiology. Lung cellular and molecular physiology 2004; 287:44. [DOI] [PubMed] [Google Scholar]
- 53.Qu J, Do DC, Zhou Y, Luczak E, Mitzner W, Anderson ME, et al. Oxidized CaMKII promotes asthma through the activation of mast cells. JCI Insight 2017; 2:e90139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Do DC, Ishmael FT, Squadrito ML, Tang HM, Tang HL, et al. Mannose receptor modulates macrophage polarization and allergic inflammation through miR-511–3p. J Allergy Clin Immunol 2018; 141:350–64 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiu L, Zhang Y, Do DC, Ke X, Zhang S, Lambert K, et al. miR-155 Modulates Cockroach Allergen- and Oxidative Stress-Induced Cyclooxygenase-2 in Asthma. J Immunol 2018; 201:916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, Do DC, Liu J, Wang B, Qu J, Ke X, et al. Functional role of kynurenine and aryl hydrocarbon receptor axis in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol 2018; 141:586–600 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu T, Zhou Y, Qiu L, Do DC, Zhao Y, Cui Z, et al. Aryl Hydrocarbon Receptor Protects Lungs from Cockroach Allergen-Induced Inflammation by Modulating Mesenchymal Stem Cells. J Immunol 2015; 195:5539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]
- 59.Nagumo H, Sasaki Y, Ono Y, Okamoto H, Seto M, Takuwa Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am J Physiol Cell Physiol 2000; 278:C57–65. [DOI] [PubMed] [Google Scholar]
- 60.Zhao W, Wang C, Liu R, Wei C, Duan J, Liu K, et al. Effect of TGF-beta1 on the Migration and Recruitment of Mesenchymal Stem Cells after Vascular Balloon Injury Involvement of Matrix Metalloproteinase-14. Sci Rep 2016; 6:21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S, Zhou X, Lang RA, Guo F. RhoA of the Rho family small GTPases is essential for B lymphocyte development. PLoS One 2012; 7:e33773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asaduzzaman M, Davidson C, Nahirney D, Fiteih Y, Puttagunta L, Vliagoftis H. Proteinase-activated receptor-2 blockade inhibits changes seen in a chronic murine asthma model. Allergy 2018; 73:416–20. [DOI] [PubMed] [Google Scholar]
- 63.Qi XJ, Ning W, Xu F, Dang HX, Fang F, Li J. Fasudil, an inhibitor of Rho-associated coiled-coil kinase, attenuates hyperoxia-induced pulmonary fibrosis in neonatal rats. Int J Clin Exp Pathol 2015; 8:12140–50. [PMC free article] [PubMed] [Google Scholar]
- 64.Holvoet T, Devriese S, Castermans K, Boland S, Leysen D, Vandewynckel YP, et al. Treatment of Intestinal Fibrosis in Experimental Inflammatory Bowel Disease by the Pleiotropic Actions of a Local Rho Kinase Inhibitor. Gastroenterology 2017; 153:1054– 67. [DOI] [PubMed] [Google Scholar]
- 65.Knipe RS, Probst CK, Lagares D, Franklin A, Spinney JJ, Brazee PL, et al. The Rho Kinase Isoforms ROCK1 and ROCK2 Each Contribute to the Development of Experimental Pulmonary Fibrosis. Am J Respir Cell Mol Biol 2018; 58:471–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008; 8:726–36. [DOI] [PubMed] [Google Scholar]
- 67.Han X-PP, Zhang F-QQ, Tan X-SS, Liu L, Ma W-XX, Ou-Yang H-FF, et al. EPO modified MSCs can inhibit asthmatic airway remodeling in an animal model. Journal of cellular biochemistry 2018; 119: 1008–16. [DOI] [PubMed] [Google Scholar]
- 68.Shentu TP, Huang TS, Cernelc-Kohan M, Chan J, Wong SS, Espinoza CR, et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic differentiation. Sci Rep 2017; 7:18052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Volckaert T, De Langhe S. Lung epithelial stem cells and their niches: Fgf10 takes center stage. Fibrogenesis Tissue Repair 2014; 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghosh M, Miller YE, Vandivier RW. Reply to: Airway Basal Cell Reprogramming and EMT: Potential Key to Understanding Early COPD. Am J Respir Crit Care Med 2018. [DOI] [PMC free article] [PubMed]
- 71.Tata A, Kobayashi Y, Chow RD, Tran J, Desai A, Massri AJ, et al. Myoepithelial Cells of Submucosal Glands Can Function as Reserve Stem Cells to Regenerate Airways after Injury. Cell Stem Cell 2018; 22:668–83 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018; 359:1118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schilders KA, Eenjes E, van Riet S, Poot AA, Stamatialis D, Truckenmuller R, et al. Regeneration of the lung: Lung stem cells and the development of lung mimicking devices. Respir Res 2016; 17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Love JJ, Li X, Chung J, Dyson HJ, Wright PE. The LEF-1 high-mobility group domain undergoes a disorder-to-order transition upon formation of a complex with cognate DNA. Biochemistry 2004; 43:8725–34. [DOI] [PubMed] [Google Scholar]
- 75.Santiago L, Daniels G, Wang D, Deng F- MM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. American journal of cancer research 2017; 7:1389–406. [PMC free article] [PubMed] [Google Scholar]
- 76.Boras-Granic K, Chang H, Grosschedl R, Hamel PA. Lef1 is required for the transition of Wnt signaling from mesenchymal to epithelial cells in the mouse embryonic mammary gland. Developmental biology 2006; 295:219–31. [DOI] [PubMed] [Google Scholar]
- 77.Lynch TJ, Anderson PJ, Rotti PG, Tyler SR, Crooke AK, Choi SH, et al. Submucosal Gland Myoepithelial Cells Are Reserve Stem Cells That Can Regenerate Mouse Tracheal Epithelium. Cell Stem Cell 2018; 22:653–67 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clevers H Wnt/beta-catenin signaling in development and disease. Cell 2006;127:69–80. [DOI] [PubMed] [Google Scholar]
- 79.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423:409–14. [DOI] [PubMed] [Google Scholar]
- 80.Aloysius A, DasGupta R, Dhawan J. The transcription factor Lef1 switches partners from beta-catenin to Smad3 during muscle stem cell quiescence. Sci Signal 2018; 11. [DOI] [PubMed] [Google Scholar]
- 81.Nawshad A, Medici D, Liu CC, Hay ED. TGFbeta3 inhibits E-cadherin gene expression in palate medial-edge epithelial cells through a Smad2-Smad4-LEF1 transcription complex. J Cell Sci 2007; 120:1646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ke H, Masoumi KC, Ahlqvist K, Seckl MJ, Rydell-Tormanen K, Massoumi R. Nemo-like kinase regulates the expression of vascular endothelial growth factor (VEGF) in alveolar epithelial cells. Sci Rep 2016; 6:23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell 2012; 22:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 2003; 63:2658–64. [PubMed] [Google Scholar]
- 85.Huang C, Qin D. Role of Lef1 in sustaining self-renewal in mouse embryonic stem cells. J Genet Genomics 2010; 37:441–9. [DOI] [PubMed] [Google Scholar]
- E1:Li C, Zhen G, Chai Y, Xie L, Crane JL, Farber E, et al. RhoA determines lineage fate of mesenchymal stem cells by modulating CTGF-VEGF complex in extracellular matrix. Nat Commun 2016; 7:11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2:Xu T, Zhou Y, Qiu L, Do DC, Zhao Y, Cui Z, et al. Aryl Hydrocarbon Receptor Protects Lungs from Cockroach Allergen-Induced Inflammation by Modulating Mesenchymal Stem Cells. J Immunol 2015; 195:5539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E3:Livak KJ, Schmittgen TD Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–8. [DOI] [PubMed] [Google Scholar]


