Summary
Lipodystrophies are rare, heterogeneous, genetic or acquired, disorders characterised by varying degrees of body fat loss and associated metabolic complications, including insulin resistance, dyslipidaemias, hepatic steatosis and predisposition to atherosclerotic vascular disease. The four main types of lipodystrophy, excluding antiretroviral therapy-induced lipodystrophy in HIV-infected patients, are congenital generalised lipodystrophy (CGL), familial partial lipodystrophy (FPLD), acquired generalised lipodystrophy (AGL) and acquired partial lipodystrophy (APL). This paper reviews the literature related to the prevalence of dyslipidaemias and atherosclerotic vascular disease in patients with lipodystrophies. Patients with CGL, AGL and FPLD have increased prevalence of dyslipidaemia but not those with APL. Patients with CGL as well as AGL present in childhood, and have severe dyslipidaemias (mainly hypertriglyceridaemia) and early onset diabetes mellitus as a consequence of extreme fat loss. However, only a few patients with CGL and AGL have been reported to develop coronary heart disease. In contrast, data from some small cohorts of FPLD patients reveal increased prevalence of atherosclerotic vascular disease especially among women. Patients with APL have a relatively low prevalence of hypertriglyceridaemia and diabetes mellitus. Overall, patients with lipodystrophies appear to be at high risk of atherosclerotic vascular disease due to increased prevalence of dyslipidaemia and diabetes and efforts should be made to manage these metabolic complications aggressively to prevent atherosclerotic vascular disease.
Keywords: Lipodystrophy, congenital generalised lipodystrophy, familial partial lipodystrophy, acquired generalised lipodystrophy, acquired partial lipodystrophy, metreleptin
INTRODUCTION
Lipodystrophies are a group of rare, heterogeneous, genetic or acquired, disorders characterised by varying degrees of body fat loss.1 Fat loss may be restricted to only small areas (localised), on the extremities (partial), or all over the body (generalised), as seen in Fig. 1. Depending upon the extent of fat loss, these patients suffer from metabolic complications, such as insulin resistance, impaired glucose tolerance, diabetes mellitus, dyslipidaemia, hepatic steatosis, polycystic ovarian syndrome, acanthosis nigricans, and their sequelae. A classification of various types of lipodystrophies is given in Table 1.
Fig. 1.
Clinical features of patients with various types of lipodystrophies. (A) Anterior view of a 33-year-old Hispanic female with congenital generalised lipodystrophy (also known as Berardinelli–Seip congenital lipodystrophy) type 1, due to homozygous c.589-2A>G mutation in AGPAT2 gene. The patient had generalised loss of subcutaneous (sc) fat with acanthosis nigricans in the axillae and neck. She has umbilical prominence and acromegaloid features (enlarged mandible, hands and feet). (B) Anterior view of a 27-year-old Native American Hispanic female with familial partial lipodystrophy of the Dunnigan variety (FPLD2) due to heterozygous p.Arg482Trp mutation in LMNA gene. She had marked loss of sc fat from the limbs and anterior truncal region. The breasts were atrophic. She had increased sc fat deposits in the face, anterior neck and vulvar regions. (C) Anterior view of an 8-year-old German boy with acquired generalised lipodystrophy. He had severe generalizsed loss of sc fat with marked acanthosis nigricans in the neck, axillae and groin. (D) Anterior view of a 39-year-old Caucasian female with acquired partial lipodystrophy (Barraquer–Simons syndrome). She had marked loss of sc fat from the face, neck, upper extremities, chest and on some areas of anterior thighs. She had increased sc fat deposition in the lower extremities. Modified and reproduced with permission from Fitzpatrick's Dermatology in General Medicine.126
Table 1.
Classification of lipodystrophies
| Lipodystrophy/type | Subtype | Gene |
|---|---|---|
| Genetic lipodystrophies | ||
| Autosomal recessive lipodystrophies | ||
| Congenital generalised lipodystrophy (CGL) | CGL1 | AGPAT2 |
| CGL2 | BSCL2 | |
| CGL3 | CAV1 | |
| CGL4 | CAVIN1 | |
| Familial partial lipodystrophy (FPLD) | FPLD5 | CIDEC |
| FPLD6 | LIPE | |
| Mandibuloacral dysplasia (MAD) | Type A | LMNA |
| Type B | ZMPSTE24 | |
| Autoinflammatory syndromes | JMP, CANDLE | PSMB8 |
| Wiedemann–Rautenstrauch syndrome (WRS) | Type C | POL3RA |
| Autosomal dominant lipodystrophies | ||
| Familial partial lipodystrophy (FPLD) | FPLD1 | Unknown |
| FPLD2 | LMNA | |
| FPLD3 | PPARG | |
| FPLD4 | PLIN1 | |
| FPLD7 | ADRA2A | |
| Other | AKT2 | |
| Atypical progeroid syndrome | LMNA | |
| SHORT syndrome | PIK3R1 | |
| MPD syndrome | POLD1 | |
| Wiedemann–Rautenstrauch syndrome (WRS), neonatal progeroid syndrome | Type A | FBN1 |
| Type B | CAV1 | |
| Acquired lipodystrophies | ||
| Acquired generalised lipodystrophies (AGL) | Panniculitis-associated | |
| Auto-immune Idiopathic | ||
| Acquired partial lipodystrophies (APL) | MPGN-associated | |
| Auto-immune Idiopathic | ||
| HAART-induced lipodystrophy in HIV-infected patients Localised lipodystrophies |
CANDLE, chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature; HAART, highly active anti-retroviral therapy; HIV, human immunodeficiency virus; JMP, joint contractures, muscle atrophy, microcytic anaemia, and panniculitis-induced; MDP, mandibular hypoplasia, deafness and progeroid features; MPGN, membranoproliferative glomerulonephritis; SHORT, short stature, hyperextensibility or inguinal hernia, ocular depression, Rieger anomaly, and teething delay.
The prevalence of lipodystrophies (excluding antiretroviral therapy-induced lipodystrophy in HIV-infected patients) in the general population is estimated to be only 1.3–4.7 cases per million, establishing these as rare disorders.2 The four most prevalent types of lipodystrophies are congenital generalised lipodystrophy (CGL), familial partial lipodystrophy (FPLD), acquired generalised lipodystrophy (AGL) and acquired partial lipodystrophy (APL) (Fig. 1). CGL and FPLD are autosomal recessive and autosomal dominant genetic disorders, respectively; each reported in approximately 500 patients worldwide; however, most of the other genetic lipodystrophies have been reported in about 30 patients or less. Thus, it is difficult to ascertain the prevalence of dyslipidaemias or atherosclerotic cardiovascular disease in these extremely rare subtypes of lipodystrophies.
The dyslipidaemia in patients with lipodystrophies is characterised by hypertriglyceridaemia, and low levels of high-density lipoprotein (HDL)-cholesterol. Some patients develop extreme hypertriglyceridaemia and chylomicronaemia resulting in recurrent attacks of acute pancreatitis, lipaemia retinalis, and tuberous and eruptive xanthomas. Even planar xanthomas in the palms and soles have been observed in some patients. Dyslipidaemia and diabetes mellitus can also predispose these patients to atherosclerotic vascular disease, including coronary heart disease, cerebrovascular accidents and peripheral vascular disease. However, because these disorders are rare, only anecdotal and limited data from some cohorts of patients are available about the prevalence of dyslipidaemia and atherosclerotic cardiovascular disease (ASCVD).
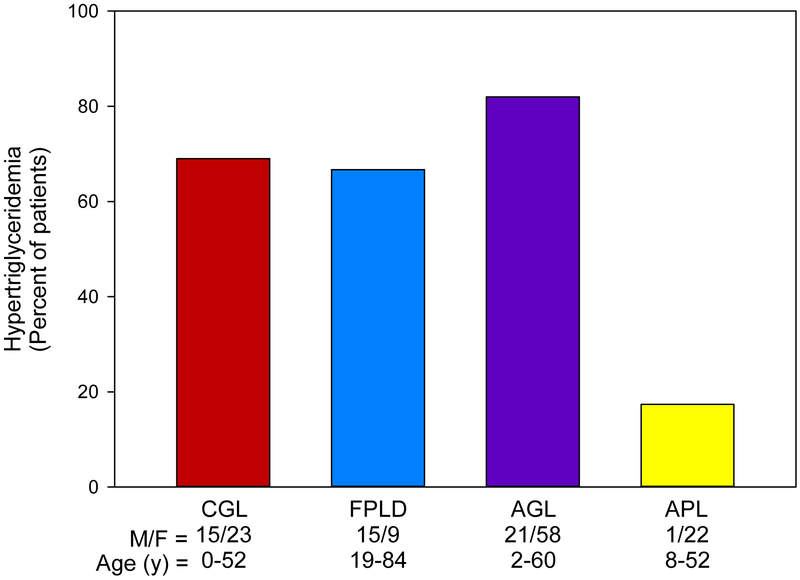
This paper reviews available literature on dyslipidaemias and ASCVD among various subtypes of genetic and acquired lipodystrophies. Localised lipodystrophies do not often result in metabolic complications because of the small amount of fat loss. The paper does not review dyslipidaemias in human immunodeficiency virus (HIV)-infected patients with highly active antiretroviral therapy-induced lipodystrophy, and readers are referred to other recent reviews.3–6 We reviewed the literature for anecdotal case reports of dyslipidaemia and ASCVD amongst the four major types of lipodystrophies. While there were only a few anecdotal reports of ASCVD among CGL, AGL and APL patients, there was more substantial literature on ASCVD among patients with FPLD. We also collected and analysed the data from case reports of FPLD patients to ascertain the prevalence of dyslipidaemia, diabetes mellitus, other risk factors and ASCVD. We have estimated the prevalence of hypertriglyceridaemia (fasting serum triglyceride level greater ≥150 mg/dL) in our cohort of patients at UT Southwestern Medical Center with lipodystrophies (Fig. 2). Patients with AGL had the highest prevalence of hypertriglyceridaemia, i.e., ~80% of patients, followed closely by patients with CGL and FPLD. Patients with APL had a relatively low prevalence of dyslipidaemia with less than 20% having hypertriglyceridaemia.
Fig. 2.
Prevalence of hypertriglyceridaemia in various types of genetic and acquired lipodystrophies from UT Southwestern Lipodystrophy Database. Hypertriglyceridaemia is defined as fasting serum triglyceride concentrations ≥150 mg/dL. The number of male (M) and female (F) patients in each group, along with their age ranges are provided under the x-axis. AGL, acquired generalised lipodystrophy; APL, acquired partial lipodystrophy; CGL, congenital generalised lipodystrophy; FPLD, familial partial lipodystrophy. Reproduced with permission from Dyslipidemias. Pathophysiology, Evaluation and Management.125
1. CONGENITAL GENERALISED LIPODYSTROPHY (CGL)
CGL is an autosomal recessive disorder with generalised lack of body fat and extreme muscularity present at birth or soon thereafter.7 The population prevalence of CGL has been estimated to be 1 in 10 million.7,8 CGL type 1 and type 2 are the most common subtypes of CGL, each reported in about 200–250 patients. CGL type 3 has been reported in a single patient.9 CGL type 4 has been reported in ~30 patients to date.10–18 Patients with CGL have acromegaloid features with enlarged mandible, hands and feet with muscular appearance, prominent veins and accelerated growth. They have a voracious appetite, hepatomegaly and/or splenomegaly and early onset of acanthosis nigricans.8,19–21 Some patients have also been reported to have hypertrophic cardiomyopathy and mild mental retardation.22,23
Patients with CGL develop metabolic abnormalities like hyperinsulinaemia, diabetes, hepatic steatosis and hypertriglyceridaemia at a young age, some even at birth or during infancy.7,19,23–25 The levels of HDL-cholesterol also tend to be low.26,27 However, atherosclerotic vascular complications such as coronary heart disease, strokes or peripheral vascular disease have only been reported in a few patients.
1.1. CGL type 1 (AGPAT2 mutations)
Following the linkage of CGL1 locus to chromosome 9q34,28 Agarwal et al.29 reported biallelic disease-causing mutations in AGPAT2, which encodes for the enzyme, 1-acylglycerol-3-phosphate acyltransferase 2, that plays a critical role in the biosynthesis of triglycerides and phospholipids. Affected patients with CGL1 have presence of mechanical adipose tissue in the palms, soles, orbits, under the scalp, perineum, vulva, peri-articular, and pericalyceal regions of the kidneys, but lack metabolically active adipose tissue in most subcutaneous areas, intra-abdominal and intra-thoracic regions and bone marrow.21,30
Agarwal et al.23 reported 38 patients [11 male, 27 females; age range 3–65 years; mean ± standard deviation (SD), 19.6 ± 15.2 years] belonging to 26 pedigrees with biallelic AGPAT2 mutations. Almost 64% of these patients had diabetes mellitus, 13% had cardiomyopathy, but there was no description of coronary artery disease. Van Maldergem et al.22 reported 21 CGL1 patients (6 males, 15 females) from 17 families, with mean ± standard error of the mean (SEM) age, 21.6 ± 2.8 years. Four (19%) patients had hypertrophic cardiomyopathy, and 35% had diabetes mellitus, but no mortality or coronary artery disease was described in any patient. Lupsa et al.31 reported 19 CGL1 patients (mean ± SD age, 23 ± 12 years), evaluated at the National Institutes of Health, USA, from 1999 to 2009. Ten patients with CGL1 had left ventricular (LV) hypertrophy (3 mild, 4 moderate, and 3 severe), and four patients had LV dysfunction. One patient with mild hypertrophic cardiomyopathy and normal LV function, developed severe coronary artery disease requiring coronary artery bypass grafting at age 45 years.31,32 Akinci et al.33 recently reported 16 CGL1 patients (10 females and 6 males; age 11–21 years) belonging to 10 families from Turkey. Over 56% had diabetes. Median (range) LDL-cholesterol level was 84 mg/dL (43–105 mg/dL), HDL-cholesterol was 29 mg/dL (25–36 mg/dL) and serum triglycerides were 532 mg/dL (164–1222 mg/dL). Three females with CGL1 had coronary artery disease between the ages of 30 and 62 years; and two of them died at 62 years due to myocardial infarction.
The pathology report from a 19-year-old female requiring heart transplantation for severe heart failure due to severe LV hypertrophy, dilated left ventricle, and severe LV dysfunction, showed biventricular dilatation with mild to moderate biventricular myocyte hypertrophy, subendocardial and epicardial fibrosis; but normal coronary arteries.31 Autopsy findings of a 32-year-old female with the biallelic AGPAT2 mutations showed mild LV hypertrophy in the posterolateral region, but there was no mention of coronary artery disease.31 We reported autopsy findings in a 24-year-old African American female with CGL1.34 Both left main and right coronary arteries showed approximately 20% stenosis with early atheromatous plaques. Scant atheromatous streaking of the distal aorta was noted.34 Thus, there was evidence for mild atherosclerosis at such young age in our patient.
1.2. CGL type 2 (BSCL2 mutations)
Magre et al.35 using linkage analysis and positional cloning were the first to report mutations in Berardinelli–Seip congenital lipodystrophy 2 (BSCL2) gene in patients with CGL2. BSCL2 encodes for the protein, Seipin, which has been recently reported to play a role in lipid droplet assembly and in adipocyte differentiation.36–38 Affected CGL2 patients have absence of both metabolically active and mechanical adipose tissue.21,30 Cardiomyopathy and mild mental retardation are commonly associated with CGL2.22,23,39,40
Agarwal et al.23 reported 17 patients with biallelic BSCL2 mutations (9 males, 8 females; age range 4–32 years; mean ± SD, 11.9 ± 7.7 years) from 11 pedigrees. Almost 53% of patients had diabetes mellitus, 42.9% had cardiomyopathy, but none was reported to have coronary artery disease. Van Maldergem et al.22 reported 45 CGL2 patients (27 males, 18 females) from 24 families. Eleven patients (4 females, and 7 males) had hypertrophic cardiomyopathy, and 35.5% had diabetes mellitus. Seven of these 45 (15.5%) patients died between 14 and 35 years of age; of these, three died due to cardiac failure, two due to renal failure, one with hepatic failure, and one from an unknown cause. Lupsa et al.31 reported 10 CGL2 patients, age 17 ± 7 years. Eight of these patients with CGL2 had increased LV mass (2 mild, 2 moderate, and 4 severe).31 Akinci et al.33 reported 11 CGL2 patients (5 females and 6 males; age 3–19 years) belonging to seven families from Turkey. Five (45.5%) patients had diabetes, and none had atherosclerosis or coronary artery disease. Median LDL-cholesterol level was 98 mg/dL (range 67–151 mg/dL), HDL-cholesterol was 27 mg/dL (16–33 mg/dL) and serum triglycerides were 405 mg/dL (254–676 mg/dL). Lima et al.41 reviewed causes of mortality in patients with CGL2, and showed that the mean ± SD age of death for 20 patients in Rio Grande do Norte, Brazil, with homozygous c.325dupA variant in BSCL2 gene was 27.1 ± 12.4 years. Nineteen patients had diabetes, and two of them had sudden deaths; one of which was caused by myocardial infarction. The majority of other patients died of infection (n = 7) and end stage liver disease (n = 7). Another study of 22 patients (14 females, 8 males; age 22 ± 9.7 years) with CGL2 from the same area revealed diabetes mellitus in 68.2% and hypertension in 50% patients.26 Mean HDL-cholesterol was 27.4 ± 7.7 mg/dL, and serum triglycerides were 332 ± 286 mg/dL. Calcification of the aortic valve was seen in 9.1%, mild aortic regurgitation in 4.5%, concentric left ventricular hypertrophy in ~50% and eccentric left ventricular hypertrophy in 4.5% of the patients.
Post-mortem pathology report from a 32-year-old male with CGL2, who died due to complications of pneumonia and respiratory failure, showed left ventricular patchy perivascular and interstitial fibrosis, and 50% occlusion of the left main coronary artery.31
Thus, overall despite high prevalence of marked dyslipidaemia and diabetes mellitus, there are only two reports of ASCVD among CGL2 patients. However, most of the reported patients have been young.
1.3. CGL type 4 (CAVIN1 mutations)
CGL type 4 is caused by mutations in Caveolae Associated Protein 1 (CAVIN1) gene, previously known as Polymerase I and Transcript Release Factor (PTRF).11 Cavin-1 is an essential factor in the biogenesis of caveolae and co-localises with caveolin 1 in the adipocytes. Patients with CGL4 develop generalised lipodystrophy during infancy, sparing the mechanical and bone marrow fat.12 These patients have predisposition to serious arrhythmias such as catecholaminergic polymorphic ventricular tachycardia (CPVT), prolonged QT interval, and there have been reports of sudden death, likely secondary to ventricular arrhythmias.10,13 Ten of 30 reported cases with CGL4 had arrhythmia, but there have been no reports of coronary artery disease or diabetes. Patients with CGL4 have hepatomegaly and metabolic abnormalities including hypertriglyceridaemia, hepatic steatosis, hyperinsulinaemia and insulin resistance but frank diabetes has not been reported.10,13,16 A recent autopsy report of a 15-year-old boy with CGL4 showed mild fibrous thickening of intima, but no plaques in coronary arteries.42
2. FAMILIAL PARTIAL LIPODYSTROPHY (FPLD)
FPLD is characterised by fat loss mostly involving the extremities (especially prominent on the lower extremities), with variable fat loss from the face, neck and trunk. Most FPLD patients have an autosomal dominant inheritance, but there are extremely rare patients with autosomal recessive inheritance.
2.1. FPLD2 (LMNA mutations)
The molecular basis of the most common subtype of FPLD, the Dunnigan variety (FPLD2) was discovered by Cao et al.43 following the linkage of the FPLD locus to chromosome 1q21-22.44 Subsequently, various heterozygous, missense mutations in the lamin A/C (LMNA) gene have been reported by several investigators.45–47 Lamins A and C are integral parts of the nuclear lamina and play a role in nuclear function and integrity. In our experience, ~75% of patients with FPLD2 have a missense mutation affecting the arginine residue at 482 position (p.R482Q, p.R482W, p.R482L), and these patients are considered to have ‘typical’ FPLD2, which usually presents with a severe phenotype, whereas FPLD2 patients harbouring mutations in other positions are considered to have ‘atypical’ variety and some of these patients may have milder phenotype. From our review of the literature of anecdotal cases, however, ~50% had ‘typical’ FPLD2 and the others had ‘atypical’ FPLD2. This discrepancy is likely due to inclusion of a large number of subjects with heterozygous mutation in LMNA from the Reunion Island.48
The loss of subcutaneous fat gives the appearance of increased muscularity. This is more easily recognised in females, as opposed to males, and may account for the approximately three times more preponderance of females with FPLD2 reported in the literature compared to males.
Women with FPLD also have more severe metabolic sequelae of insulin resistance compared to men.49
In a study that included eight well-characterised pedigrees, women with FPLD2 (n = 22) were shown to have a significantly higher prevalence of diabetes, with higher serum triglyceride concentrations and lower HDL-cholesterol concentrations, when compared to both normal females (n = 27) and males with FPLD2 (n = 17). These affected females had a significantly high rate of atherosclerotic vascular disease (including coronary heart disease, stroke and claudication) when compared to affected males. When affected males were compared with controls there was no difference, as both groups had 12% of subjects with atherosclerotic vascular disease. However, 45% of FPLD2 females had atherosclerotic vascular disease compared to 15% of the control female subjects.49
Another study included subjects from three extended Canadian FPLD2 kindreds, and compared clinical features of 35 FPLD2 patients with 51 controls. Affected patients were noted to have significantly higher prevalence of dyslipidaemia (68.6% compared to 7.8% in the controls). Patients with FPLD2, both males and females, had lower serum HDL cholesterol and higher serum triglyceride levels compared to unaffected subjects from the same kindreds. There was no significant difference in the prevalence of hypertension or glucose intolerance.50
The same three Canadian FPLD kindreds were reported in another paper and FPLD2 patients were compared with normal family members.52 FPLD2 patients had significantly more coronary heart disease (stable and unstable angina, myocardial infarction, coronary artery bypass grafting) compared to the control family members: 34.8% compared to 5.9% in all age groups, and 26.1% compared to 0% before age 55 years. As previously noted, FPLD2 patients also had significantly more dyslipidaemia, with hypertriglyceridaemia and low HDL-cholesterol. Interestingly, LDL cholesterol levels were higher in the controls compared to FPLD2 patients. FPLD2 patients had a mean age of onset of coronary heart disease of 46.5 ± 3.8 years. All of the patients with coronary heart disease also had diabetes, dyslipidaemia and hypertension, and only two of them were smokers. Hospitalisation rates for coronary artery bypass graft surgery among these female FPLD2 patients were noted to be higher than the general Canadian population at that time.52
Similar results were reported recently in FPLD2 patients from Turkey. As compared to controls (n = 30), FPLD2 patients (n = 52) had significantly higher serum triglyceride levels, lower HDL-cholesterol levels, and higher glucose levels. As compared to nine FPLD3 patients with PPARG mutations, FPLD2 patients with LMNA mutations had increased prevalence of hypertension and atherosclerosis.51
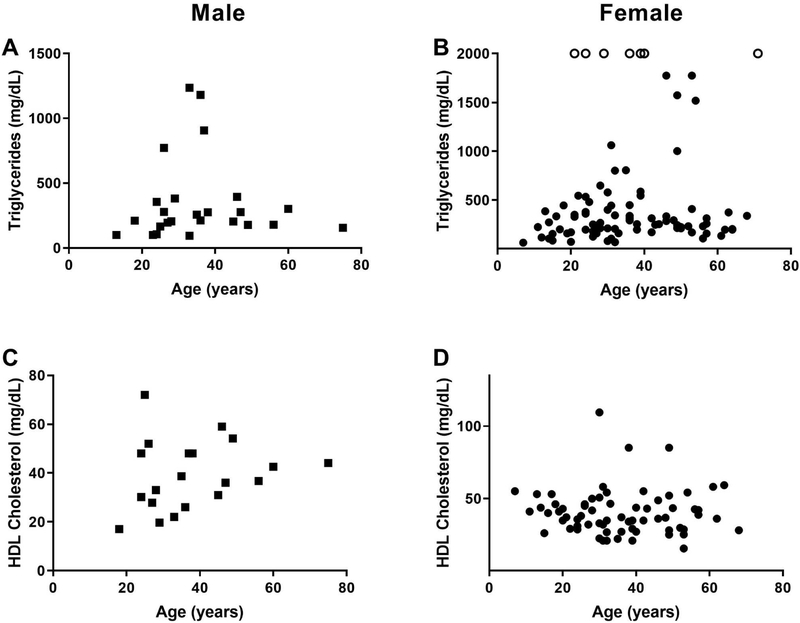
We analysed data from 258 previously reported FPLD2 patients (195 females and 63 males) to determine the type and extent of dyslipidaemia present in these patients (Fig. 3), as well as the presence of atherosclerotic disease, and other risk factors such as diabetes mellitus and hypertension.48,53–97 As compared to males, female patients with FPLD2 had significantly higher prevalence of diabetes mellitus. There was no significant difference between the prevalence of coronary artery disease between males and (mostly pre-menopausal) females (Table 2). It is interesting to note, that in the general population, males would be expected to have a higher prevalence of coronary artery disease compared to pre-menopausal women. Although serum triglycerides were not significantly different between males and females with FPLD2, more females had very severe hypertriglyceridaemia. Female FPLD2 patients were noted to have much higher triglyceride levels than their male counterparts. Most males had serum triglyceride levels of 400 mg/dL or less, with all of them having serum triglyceride levels of less than 1,400 mg/dL. On the other hand, female patients had serum triglyceride levels of up to 10,000 mg/dL. Many more females compared to males were noted to have severe and very severe hypertriglyceridaemia (triglycerides ≥1000–2000 mg/dL) predisposing them to complications such as acute pancreatitis.
Fig. 3.
Serum triglycerides and HDL-cholesterol levels in patients with familial partial lipodystrophy, Dunnigan variety (FPLD2) due to LMNA mutations previously reported in the literature according to age. Data in males are shown as squares and in females as circles. (A) Serum triglyceride levels in males (n = 25) showing that the majority have hypertriglyceridaemia, with some patients with triglyceride levels over 1,000 mg/dL. (B) Serum triglyceride levels in females (n = 93) showing that the majority have hypertriglyceridaemia. Females with serum triglyceride levels over 2,000 mg/dL (n = 8) are indicated with open circles. In these females, serum triglyceride values ranged from 2,500 mg/dL to 10,000 mg/dL. (C) Serum high-density lipoprotein (HDL)-cholesterol levels in males (n = 20) showing that the majority have levels below 40 mg/dL. (D) Serum high-density lipoprotein (HDL)-cholesterol levels in females (n = 69) showing that the majority have levels below 50 mg/dL.
Table 2.
Comparison of serum lipids and lipoproteins and clinical features of males and females with familial partial lipodystrophy, Dunnigan variety (FPLD2) due to LMNA mutations
| Males (n = 63) |
Females (n = 195) |
p value | |||
|---|---|---|---|---|---|
| n | Median (range) | n | Median (range) | ||
| Age (years) | 58 | 35.5 (7–75) | 185 | 39 (2–71) | 0.31 |
| T Chol | 20 | 220 (150–319) | 59 | 199 (127–603) | 0.11 |
| Triglycerides | 28 | 212 (58–1,235) | 95 | 266 (62–9,975) | 0.20 |
| HDL-C | 23 | 39 (17–72) | 71 | 30 (17–72) | 0.89 |
| LDL-C | 18 | 138 (75–227) | 44 | 113 (52–227) | 0.10 |
| DM (Y/N) | 41 | 16/25a (39%)b | 164 | 110/54a (67%)b | 0.001 |
| HTN (Y/N) | 23 | 11/12a (48%)b | 130 | 80/50a (62%)b | 0.25 |
| CAD (Y/N) | 14 | 7/7a (50%)b | 63 | 20/43a (32%)b | 0.26 |
| CHF (Y/N) | 20 | 4/16a (20%)b | 52 | 13/39a (25%)b | 0.76 |
All measurements for lipid profile are given in milligrams per deciliter (mg/dL). CAD, coronary artery disease; CHF, congestive heart failure; DM, diabetes mellitus; HDL-C, high-density lipoprotein (HDL)-cholesterol; HTN, hypertension; LDL-C, low density lipoprotein (LDL)-cholesterol; N, no or absent; n, number; range, minimum–maximum value; T Chol, total cholesterol; Y, yes or present.
Absolute number of patients with or without a clinical feature are given for the variable.
Percentage of individuals with each discrete trait is given in parentheses.
Total cholesterol levels in FPLD2 males and females were elevated; however, females tended to have higher cholesterol levels in general than males (although this did not reach statistical significance). The highest total cholesterol level in males was less than 350 mg/dL, whereas the highest total cholesterol reported in the female cohort was over 600 mg/dL. Serum cholesterol levels were reported to be high in younger patients also and did not seem to increase with age. HDL-cholesterol levels in the males and females with FPLD2 were not statistically different. This is an interesting observation because normally HDL-cholesterol levels in females are much higher than those in males.
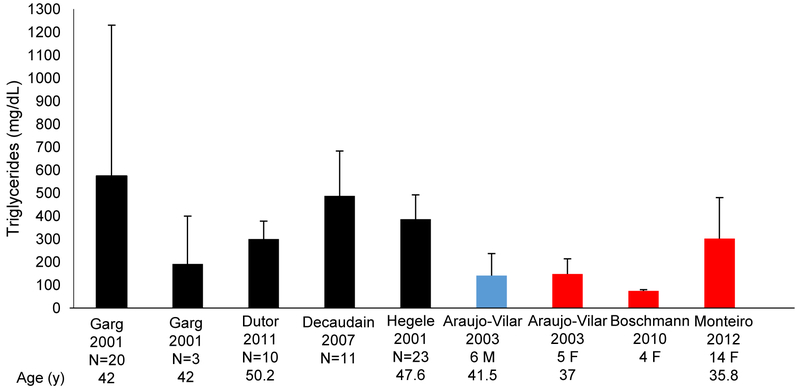
Several cohorts of FPLD2 patients have also been reported without description of individual data.52,54,98–105 These have been analysed together to evaluate severity of dyslipidaemia (Fig. 4). These cohorts do not separate out patients that have been treated with lipid-lowering medications, and do not indicate whether serum triglyceride levels are fasting values or not. Similarly, some patients that have individually been reported in the literature may have had baseline, fasting, untreated serum triglyceride levels reported, whereas others may be on lipid-lowering medications, or may have initiated dietary and lifestyle changes.
Fig. 4.
Analysis of cohorts of FPLD2 patients with various heterozygous missense LMNA mutations reported by several authors showing mean serum triglyceride levels with standard deviations in these groups. Black bars indicate that males and females were not reported separately in the cohort. Blue bars indicate only males were reported. And red bars indicate only females were reported. Three patients from the Garg 2001 cohort had heterozygous LMNA mutation p.R582H and had their lipid panels reported separately (second black bar), whereas the other 20 patients had various other mutations (1 patient had p.G465D; 4 patients had p.R482Q; and 15 patients had p.R482W) and lipid panels reported together for these patients (first black bar). M, male; F, female; N, number of patients reported; y, years.52,80,98,101,102,104,105
Hypertriglyceridaemia is present in most cohorts, although more elevated values are noted in cohorts reported by Garg in 200198 and Decaudain in 2007,80 closely followed by Hegele in 2001.52 The serum triglyceride levels are mildly elevated in the cohort reported by Araujo-Vilar in 2003104 (all with p.R482W mutations) and are not noted to be much different in males and females.104 However, this study also compared male and female FPLD2 patients with normal controls, and noted that the female FPLD2 patients had significantly higher serum triglyceride levels, higher LDL cholesterol levels, and lower HDL-cholesterol levels compared to normal controls.104 The serum lipid values of the male FPLD2 patients were not significantly different from normal male controls.104 This again suggests that females with FPLD2 are at a higher risk of dyslipidaemia and resultant atherosclerotic cardiovascular complications, compared to males with the same LMNA mutations.
2.2. FPLD3 (PPARG mutations)
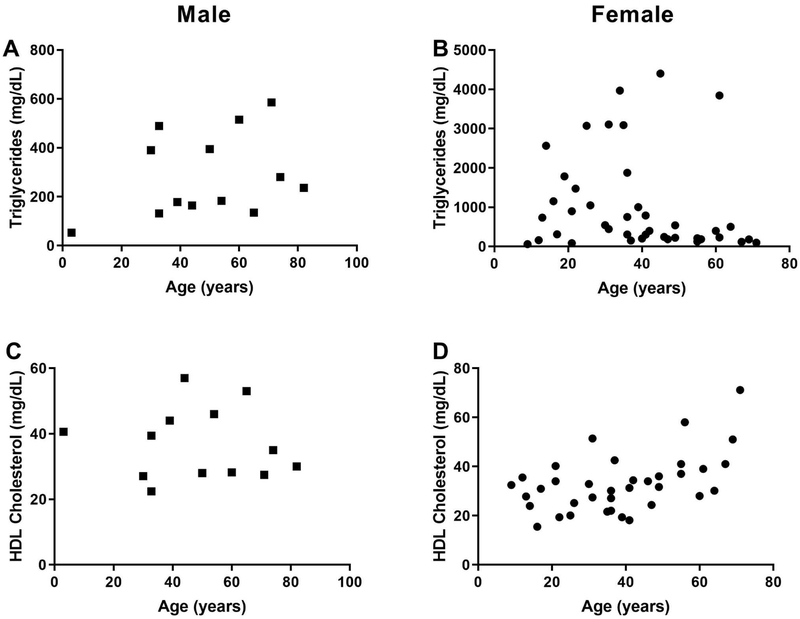
Agarwal and Garg106 were the first to report the association of FPLD phenotype with PPARG mutation. PPARG encodes for the well-known transcription factor, peroxisome proliferator-activated receptor γ, which is involved in adipocyte differentiation. A milder lipodystrophy phenotype is noted in patients with FPLD3 as compared to those with FPLD2.45 Consequently, these patients also have less severe metabolic complications. We have analysed data from 68 FLPD3 patients (49 females and 19 males) reported in the literature with regards to dyslipidaemia (Fig. 5) and ASCVD. Female patients with FPLD3 were noted to have significantly higher triglyceride levels, compared to males. The differences in total cholesterol and HDL-cholesterol were not significant. Similarly, there was no statistically significant difference in the prevalence of hypertension and diabetes mellitus in males and females with FPLD3 (Table 3). Only two female patients with FPLD3, age 41 and 44 years, have been reported with coronary artery disease.107 Because of milder phenotype, FPLD3 patients may not be as predisposed to atherosclerotic vascular disease as FPLD2 patients. This, along with the smaller number of reported cases compared to FPLD2, may explain paucity of cardiovascular data in this subset. Of note, the females (median age 37 years) were younger than the males (median age 50 years). This may be attributed to easier recognition of partial lipodystrophy in females leading to an earlier diagnosis.
Fig. 5.
Serum triglycerides and HDL-cholesterol levels in patients with familial partial lipodystrophy, type 3 (FPLD3) due to PPARG mutations previously reported in the literature according to age. Data in males are shown as squares and in females as circles. (A) Serum triglycerides levels in males (n = 12) showing that the majority have hypertriglyceridaemia. (B) Serum triglyceride levels in females (n = 41) showing that the majority have hypertriglyceridaemia. (C) Serum high-density lipoprotein (HDL)-cholesterol levels in males (n = 13) showing that the majority have levels below 40 mg/dL. (D) Serum high-density lipoprotein (HDL)-cholesterol levels in females (n = 36) showing that the majority have levels below 50 mg/dL.
Table 3.
Comparison of serum lipids and lipoproteins and clinical features of males and females with familial partial lipodystrophy, type 3 due to PPARG mutations
| Males (n = 14) |
Females (n = 48) |
p value | |||
|---|---|---|---|---|---|
| n | Median (range) | n | Median (range) | ||
| Age (years) | 13 | 50 (3–82) | 41 | 37 (9–71) | 0.045 |
| T Chol | 10 | 191 (149–268) | 26 | 209 (99–567) | 0.42 |
| Triglycerides | 13 | 236 (53–586) | 41 | 444 (62–4,403) | 0.038 |
| HDL-C | 13 | 35 (22–57) | 36 | 32 (16–71) | 0.26 |
| DM (Y/N) | 14 | 12/2a (86%)b | 44 | 32/12a (73%)b | 0.48 |
| HTN (Y/N) | 5 | 4/1a (80)b | 35 | 22/13a (63)b | 0.64 |
All measurements for lipid profile are given in milligrams per deciliter (mg/dL). DM, diabetes mellitus; HDL-C, high-density lipoprotein (HDL)-cholesterol; HTN, hypertension; N, no or absent; n, number; range, minimum–maximum value; T Chol, total cholesterol; Y, yes or present.
Absolute number of patients with or without a clinical feature are given for the variable.
Percentage of individuals with each discrete trait is given in parentheses.
3. ACQUIRED GENERALISED LIPODYSTROPHY (AGL)
Patients with AGL usually have generalised loss of subcutaneous fat loss associated with panniculitis (type 1) or autoimmunity (type 2). An idiopathic variety (type 3) where the cause of lipodystrophy is not identified is also recognised.108 A recent paper reports anti-perilipin 1 autoantibodies as a cause of generalised lipodystrophy in these patients.109 However, only limited number of patients were studied by the authors. Approximately 80 patients have been reported in the literature. Autoimmune diseases such as juvenile dermatomyositis, Sjogren’s syndrome autoimmune hepatitis, Hashimoto’s thyroiditis and juvenile rheumatoid arthritis have been associated with progressive fat loss. Since these conditions are more common in the females, this explains why females are about three times more likely to be affected with AGL compared to males. The fat loss associated with AGL typically occurs during childhood and adolescence, and is associated with insulin resistance and diabetes mellitus, severe hypertriglyceridaemia and hepatic steatosis.25
A review paper studied 79 patients (63 previously reported patients and 16 new patients),110 and noted severe hypertriglyceridaemia, that led to eruptive xanthomas, lipaemia retinalis, and acute pancreatitis in some cases, along with low levels of HDL-cholesterol that were reported in all age groups. Metabolic complications can predispose these patients to premature atherosclerosis and coronary heart disease. Four patients with AGL, all female (3 from the newly reported series,110 and one from the literature111), developed premature coronary heart disease, one patient developed carotid atherosclerosis, and two patients were diagnosed with peripheral vascular disease.110
They also tend to have low levels of serum complement 4,112 and have high risk of developing T-cell lymphomas,113 which has been reported more frequently in these patients than cardiovascular disease.
4. ACQUIRED PARTIAL LIPODYSTROPHY (BARRAQUER–SIMONS SYNDROME)
Acquired partial lipodystrophy is characterised by progressive loss of subcutaneous fat which initially starts in the face and then affects the arms and torso, but spares the lower extremities. This usually begins in childhood, and is associated with several autoimmune diseases, most commonly systemic lupus erythematosus and dermatomyositis. Patients have often been reported to have had various infections preceding the development of lipodystrophy. About 20–25% of these patients go on to develop membranoproliferative glomerulonephritis.108 Similar to AGL, the pathogenesis of fat loss in APL is attributed to autoimmunity108 and APL is about four times more prevalent in females compared to males. However, APL patients have a lower prevalence of insulin resistance and diabetes mellitus (6.7%) compared to other types of lipodystrophies. Hypertriglyceridaemia and low HDL-cholesterol are present in ~35% of cases, making metabolic complications less common when compared to FPLD. A paper including 35 cases, along with a review of 220 cases of APL in the literature, did not report any atherosclerotic vascular disease.108
5. MANAGEMENT OF DYSLIPIDAEMIA
Aggressive management of dyslipidaemias is important for lowering the risk of acute pancreatitis as well as ASCVD. For those with chylomicronaemia, an extremely low fat diet is recommended. In addition, fibrates as well as n-3 fatty acids from fish oils alone or in combination may lower serum triglycerides. Combination therapy with statins and fibrates should be used in rare patients with caution to avoid risk of myopathy. Patients with diabetes need to be managed intensively to achieve near normal HbA1c levels with high doses of insulin, if needed.114
Multiple prospective worldwide studies have shown metreleptin (recombinant analogue of human leptin) replacement therapy to be beneficial for improving metabolic complications, such as diabetes, hypertriglyceridaemia and hepatic steatosis in CGL and AGL patients.25,115–119 Metreleptin has been shown to improve fasting glucose and lower HbA1c by 2%;119,120 cause 60% reduction in triglycerides in 1 year;120 decrease LDL- and total cholesterol;117,121 and reduce hepatic steatosis within 6–12 months.119,122–124 The improvement in metabolic parameters in patients with FPLD has not been seen consistently. Metreleptin was approved for all lipodystrophy patients in Japan in 2013; for CGL and AGL in USA 2014; and for generalised and partial lipodystrophy in Europe in 2018. It is expected that marked improvement in dyslipidaemia and hyperglycaemia with metreleptin may also reduce the risk of ASCVD among CGL and AGL patients, and possibly in those with FPLD. However, the extremely high cost of therapy and restricted availability of metreleptin remain barriers to widespread use.
6. CONCLUSIONS AND FUTURE DIRECTIONS
Patients with lipodystrophies appear to be at high risk of atherosclerotic vascular disease due to increased prevalence of dyslipidaemia and diabetes. However, because these disorders are rare, increased prevalence of atherosclerotic vascular complications has not been documented and only anecdotal reports are available. Multicentre, collaborative effort is needed to characterise the severity of dyslipidaemia and diabetes among various different types and subtypes of lipodystrophies, the age of onset, and the prevalence of coronary heart disease, cerebrovascular accidents and peripheral vascular disease.
Metabolic complications, i.e., dyslipidaemia and diabetes mellitus, should be managed aggressively to prevent the risk of atherosclerotic vascular disease. Metreleptin therapy should be considered for patients with generalised lipodystrophy. Targeted novel therapies are needed to manage metabolic complications in patients with lipodystrophies to mitigate the risk of atherosclerotic vascular disease.
Acknowledgments:
We would like to thank Chandna Vasandani, PhD, for literature review and collecting data for FPLD3 patients, Carmel Towar, BS, for creating figures and graphs, Beverley Adams-Huet, MS, for statistical analysis, and Claudia Quittner, RN, for data collection.
Conflicts of interest and sources of funding: This work was supported by the National Institutes of Health grant RO1 DK105448, and CTSA Grant UL1RR024982, UL1TR001105 and Southwest Medical Foundation. The authors state that there are no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hussain I, Garg A. Lipodystrophy syndromes. Endocrinol Metab Clin North Am 2016; 45: 783–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiquette E, Oral EA, Garg A, Araujo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes 2017; 10: 375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman N, Whitten RA. HIV-Associated Lipodystrophy. Treasure Island, FL: StatPearls, 2018. [Google Scholar]
- 4.Non LR, Escota GV, Powderly WG. HIV and its relationship to insulin resistance and lipid abnormalities. Transl Res 2017; 183: 41–56. [DOI] [PubMed] [Google Scholar]
- 5.Alves MD, Brites C, Sprinz E. HIV-associated lipodystrophy: a review from a Brazilian perspective. Ther Clin Risk Manag 2014; 10: 559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freitas P, Carvalho D. Lipodystrophy: beyond generalization? Panminerva Med 2013; 55: 253–68. [PubMed] [Google Scholar]
- 7.Patni N, Garg A. Congenital generalized lipodystrophies–new insights into metabolic dysfunction. Nat Rev Endocrinol 2015; 11: 522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg A Acquired and inherited lipodystrophies. N Engl J Med 2004; 350: 1220–34. [DOI] [PubMed] [Google Scholar]
- 9.Kim CA, Delepine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab 2008; 93: 1129–34. [DOI] [PubMed] [Google Scholar]
- 10.Rajab A, Straub V, McCann LJ, et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet 2010; 6: e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi YK, Matsuda C, Ogawa M, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest 2009; 119: 2623–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simha V, Agarwal AK, Aronin PA, Iannaccone ST, Garg A. Novel subtype of congenital generalized lipodystrophy associated with muscular weakness and cervical spine instability. Am J Med Genet A 2008; 146A: 2318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shastry S, Delgado MR, Dirik E, Turkmen M, Agarwal AK, Garg A. Congenital generalized lipodystrophy, type 4 (CGL4) associated with myopathy due to novel PTRF mutations. Am J Med Genet A 2010; 152A: 2245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dwianingsih EK, Takeshima Y, Itoh K, et al. A Japanese child with asymptomatic elevation of serum creatine kinase shows PTRF-CAVIN mutation matching with congenital generalized lipodystrophy type 4. Mol Genet Metab 2010; 101: 233–7. [DOI] [PubMed] [Google Scholar]
- 15.Ardissone A, Bragato C, Caffi L, et al. Novel PTRF mutation in a child with mild myopathy and very mild congenital lipodystrophy. BMC Med Genet 2013; 14: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinci G, Topaloglu H, Akinci B, et al. Spectrum of clinical manifestations in two young Turkish patients with congenital generalized lipodystrophy type 4. Eur J Med Genet 2016; 59: 320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jelani M, Ahmed S, Almramhi MM, et al. Novel nonsense mutation in the PTRF gene underlies congenital generalized lipodystrophy in a consanguineous Saudi family. Eur J Med Genet 2015; 58: 216–21. [DOI] [PubMed] [Google Scholar]
- 18.Murakami N, Hayashi YK, Oto Y, et al. Congenital generalized lipodystrophy type 4 with muscular dystrophy: clinical and pathological manifestations in early childhood. Neuromuscul Disord 2013; 23: 441–4. [DOI] [PubMed] [Google Scholar]
- 19.Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy). Acta Paediatr Suppl 1996; 413: 2–28. [DOI] [PubMed] [Google Scholar]
- 20.Westvik J Radiological features in generalized lipodystrophy. Acta Paediatr Suppl 1996; 413: 44–51. [DOI] [PubMed] [Google Scholar]
- 21.Garg A, Fleckenstein JL, Peshock RM, Grundy SM. Peculiar distribution of adipose tissue in patients with congenital generalized lipodystrophy. J Clin Endocrinol Metab 1992; 75: 358–61. [DOI] [PubMed] [Google Scholar]
- 22.Van Maldergem L, Magre J, Khallouf TE, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet 2002; 39: 722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal AK, Simha V, Oral EA, et al. Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab 2003; 88: 4840–7. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab 2003; 14: 214–21. [DOI] [PubMed] [Google Scholar]
- 25.Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002; 346: 570–8. [DOI] [PubMed] [Google Scholar]
- 26.Rego AG, Mesquita ET, Faria CA, et al. Cardiometabolic abnormalities in patients with Berardinelli-Seip syndrome. (Portuguese.) Arq Bras Cardiol 2010; 94: 109–18. [DOI] [PubMed] [Google Scholar]
- 27.Gupta N, Asi N, Farah W, et al. Clinical features and management of non-HIV-related lipodystrophy in children: a systematic review. J Clin Endocrinol Metab 2017; 102: 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garg A, Wilson R, Barnes R, et al. A gene for congenital generalized lipodystrophy maps to human chromosome 9q34. J Clin Endocrinol Metab 1999; 84: 3390–4. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal AK, Arioglu E, De Almeida S, et al. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 2002; 31: 21–3. [DOI] [PubMed] [Google Scholar]
- 30.Simha V, Garg A. Phenotypic heterogeneity in body fat distribution in patients with congenital generalized lipodystrophy due to mutations in the AGPAT2 or Seipin genes. J Clin Endocrinol Metab 2003; 88: 5433–7. [DOI] [PubMed] [Google Scholar]
- 31.Lupsa BC, Sachdev V, Lungu AO, Rosing DR, Gorden P. Cardiomyopathy in congenital and acquired generalized lipodystrophy: a clinical assessment. Medicine (Baltimore) 2010; 89: 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rheuban KS, Blizzard RM, Parker MA, Carter T, Wilson T, Gutgesell HP. Hypertrophic cardiomyopathy in total lipodystrophy. J Pediatr 1986; 109: 301–2. [DOI] [PubMed] [Google Scholar]
- 33.Akinci B, Onay H, Demir T, et al. Natural history of congenital generalized lipodystrophy: a nationwide study from Turkey. J Clin Endocrinol Metab 2016; 101: 2759–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandalia M, Garg A, Vuitch F, Nizzi F. Postmortem findings in congenital generalized lipodystrophy. J Clin Endocrinol Metab 1995; 80: 3077–81. [DOI] [PubMed] [Google Scholar]
- 35.Magre J, Delepine M, Khallouf E, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 2001; 28: 365–70. [DOI] [PubMed] [Google Scholar]
- 36.Szymanski KM, Binns D, Bartz R, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci USA 2007; 104: 20890–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fei W, Shui G, Gaeta B, et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol 2008; 180: 473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang W, Thein S, Guo X, et al. Seipin differentially regulates lipogenesis and adipogenesis through a conserved core sequence and an evolutionarily acquired C-terminus. Biochem J 2013; 452: 37–44. [DOI] [PubMed] [Google Scholar]
- 39.Rahman OU, Khawar N, Khan MA, et al. Deletion mutation in BSCL2 gene underlies congenital generalized lipodystrophy in a Pakistani family. Diag Pathol 2013; 8: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhayana S, Siu VM, Joubert GI, Clarson CL, Cao H, Hegele RA. Cardiomyopathy in congenital complete lipodystrophy. Clin Genet 2002; 61: 283–7. [DOI] [PubMed] [Google Scholar]
- 41.Lima JG, Nobrega LHC, Lima NN, et al. Causes of death in patients with Berardinelli-Seip congenital generalized lipodystrophy. PLoS One 2018; 13: e0199052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patni N VF, Garg A. Post mortem findings in a young male with congenital generalized lipodystrophy, type 4 due to CAVIN1 mutations. J Clin Endocrinol Metabol 2018; November 21: (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet 2000; 9: 109–12. [DOI] [PubMed] [Google Scholar]
- 44.Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21–22. Nat Genet 1998; 18: 292–5. [DOI] [PubMed] [Google Scholar]
- 45.Simha V, Garg A. Inherited lipodystrophies and hypertriglyceridemia. Curr Opin Lipidol 2009; 20: 300–8. [DOI] [PubMed] [Google Scholar]
- 46.Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 2000; 24: 153–6. [DOI] [PubMed] [Google Scholar]
- 47.Speckman RA, Garg A, Du F, et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet 2000; 66: 1192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andre P, Schneebeli S, Vigouroux C, Lascols O, Schaaf M, Chevalier P. Metabolic and cardiac phenotype characterization in 37 atypical Dunnigan patients with nonfarnesylated mutated prelamin A. Am Heart J 2015; 169: 587–93. [DOI] [PubMed] [Google Scholar]
- 49.Garg A Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab 2000; 85: 1776–82. [DOI] [PubMed] [Google Scholar]
- 50.Hegele RA, Kraw ME, Ban MR, Miskie BA, Huff MW, Cao H. Elevated serum C-reactive protein and free fatty acids among nondiabetic carriers of missense mutations in the gene encoding lamin A/C (LMNA) with partial lipodystrophy. Arterioscler Thromb Vasc Biol 2003; 23: 111–6. [DOI] [PubMed] [Google Scholar]
- 51.Akinci B, Onay H, Demir T, et al. Clinical presentations, metabolic abnormalities and end-organ complications in patients with familial partial lipodystrophy. Metabolism 2017; 72: 109–19. [DOI] [PubMed] [Google Scholar]
- 52.Hegele RA. Premature atherosclerosis associated with monogenic insulin resistance. Circulation 2001; 103: 2225–9. [DOI] [PubMed] [Google Scholar]
- 53.Madej-Pilarczyk A, Niezgoda A, Janus M, et al. Limb-girdle muscular dystrophy with severe heart failure overlapping with lipodystrophy in a patient with LMNA mutation p.Ser334del. J Appl Genet 2017; 58: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegele RA, Cao H, Anderson CM, Hramiak IM. Heterogeneity of nuclear lamin A mutations in Dunnigan-type familial partial lipodystrophy. J Clin Endocrinol Metab 2000; 85: 3431–5. [DOI] [PubMed] [Google Scholar]
- 55.Haque WA, Oral EA, Dietz K, Bowcock AM, Agarwal AK, Garg A. Risk factors for diabetes in familial partial lipodystrophy, Dunnigan variety. Diabetes Care 2003; 26: 1350–5. [DOI] [PubMed] [Google Scholar]
- 56.Haque WA, Vuitch F, Garg A. Post-mortem findings in familial partial lipodystrophy, Dunnigan variety. Diabet Med 2002; 19: 1022–5. [DOI] [PubMed] [Google Scholar]
- 57.van der Kooi AJ, Bonne G, Eymard B, et al. Lamin A/C mutations with lipodystrophy, cardiac abnormalities, and muscular dystrophy. Neurology 2002; 59: 620–3. [DOI] [PubMed] [Google Scholar]
- 58.Owen KR, Donohoe M, Ellard S, Hattersley AT. Response to treatment with rosiglitazone in familial partial lipodystrophy due to a mutation in the LMNA gene. Diabet Med 2003; 20: 823–7. [DOI] [PubMed] [Google Scholar]
- 59.Savage DB, Soos MA, Powlson A, et al. Familial partial lipodystrophy associated with compound heterozygosity for novel mutations in the LMNA gene. Diabetologia 2004; 47: 753–6. [DOI] [PubMed] [Google Scholar]
- 60.Vantyghem MC, Pigny P, Maurage CA, et al. Patients with familial partial lipodystrophy of the Dunnigan type due to a LMNA R482W mutation show muscular and cardiac abnormalities. J Clin Endocrinol Metab 2004; 89: 5337–46. [DOI] [PubMed] [Google Scholar]
- 61.Subramanyam L, Simha V, Garg A. Overlapping syndrome with familial partial lipodystrophy, Dunnigan variety and cardiomyopathy due to amino-terminal heterozygous missense lamin A/C mutations. Clin Genet 2010; 78: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vantyghem MC, Vincent-Desplanques D, Defrance-Faivre F, et al. Fertility and obstetrical complications in women with LMNA-related familial partial lipodystrophy. J Clin Endocrinol Metab 2008; 93: 2223–9. [DOI] [PubMed] [Google Scholar]
- 63.Araujo-Vilar D, Lado-Abeal J, Palos-Paz F, et al. A novel phenotypic expression associated with a new mutation in LMNA gene, characterized by partial lipodystrophy, insulin resistance, aortic stenosis and hypertrophic cardiomyopathy. Clin Endocrinol (Oxf) 2008; 69: 61–8. [DOI] [PubMed] [Google Scholar]
- 64.Vouillarmet J, Laville M. A case of familial partial lipodystrophy: from clinical phenotype to genetics. Can J Diabetes 2016; 40: 376–8. [DOI] [PubMed] [Google Scholar]
- 65.Krawiec P, Melges B, Pac-Kozuchowska E, Mroczkowska-Juchkiewicz A, Czerska K. Fitting the pieces of the puzzle together: a case report of the Dunnigan-type of familial partial lipodystrophy in the adolescent girl. BMC Pediatr 2016; 16: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kutbay NO, Yurekli BS, Onay H, et al. A case of familial partial lipodystrophy caused by a novel lamin A/C (LMNA) mutation in exon 1 (D47N). Eur J Intern Med 2016; 29: 37–9. [DOI] [PubMed] [Google Scholar]
- 67.Demir T, Onay H, Savage DB, et al. Familial partial lipodystrophy linked to a novel peroxisome proliferator activator receptor -gamma (PPARG) mutation, H449L: a comparison of people with this mutation and those with classic codon 482 Lamin A/C (LMNA) mutations. Diabet Med 2016; 33: 1445–50. [DOI] [PubMed] [Google Scholar]
- 68.Lewandowski KC, Lewinski A, Dabrowska K, Jakubowski L, Gach A. Familial partial lipodystrophy as differential diagnosis of polycystic ovary syndrome. Endokrynol Pol 2015; 66: 550–4. [DOI] [PubMed] [Google Scholar]
- 69.Belo SP, Magalhaes AC, Freitas P, Carvalho DM. Familial partial lipodystrophy, Dunnigan variety – challenges for patient care during pregnancy: a case report. BMC Res Notes 2015; 8: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chirico V, Ferrau V, Loddo I, et al. LMNA gene mutation as a model of cardiometabolic dysfunction: from genetic analysis to treatment response. Diabetes Metab 2014; 40: 224–8. [DOI] [PubMed] [Google Scholar]
- 71.Thong KM, Xu Y, Cook J, et al. Cosegregation of focal segmental glomerulosclerosis in a family with familial partial lipodystrophy due to a mutation in LMNA. Nephron Clin Pract 2013; 124: 31–7. [DOI] [PubMed] [Google Scholar]
- 72.Nabrdalik K, Strozik A, Minkina-Pedras M, et al. Dunnigan-type familial partial lipodystrophy associated with the heterozygous R482W mutation in LMNA gene – case study of three women from one family. Endokrynol Pol 2013; 64: 306–11. [DOI] [PubMed] [Google Scholar]
- 73.Turk M, Wehnert M, Schroder R, Chevessier F. Multisystem disorder and limb girdle muscular dystrophy caused by LMNA p.R28W mutation. Neuromuscul Disord 2013; 23: 587–90. [DOI] [PubMed] [Google Scholar]
- 74.Weterings AA, van Rijsingen IA, Plomp AS, et al. A novel lamin A/C mutation in a Dutch family with premature atherosclerosis. Atherosclerosis 2013; 229: 169–73. [DOI] [PubMed] [Google Scholar]
- 75.Mory PB, Crispim F, Freire MB, et al. Phenotypic diversity in patients with lipodystrophy associated with LMNA mutations. Eur J Endocrinol 2012; 167: 423–31. [DOI] [PubMed] [Google Scholar]
- 76.Araujo-Vilar D, Victoria B, Gonzalez-Mendez B, et al. Histological and molecular features of lipomatous and nonlipomatous adipose tissue in familial partial lipodystrophy caused by LMNA mutations. Clin Endocrinol (Oxf) 2012; 76: 816–24. [DOI] [PubMed] [Google Scholar]
- 77.Laudes M, Oberhauser F, Walgenbach K, et al. Comparison of phenotypes in male and female individuals of a new family with Dunnigan type of familial partial lipodystrophy due to a lamin A/C R482W mutation. Horm Metab Res 2009; 41: 414–7. [DOI] [PubMed] [Google Scholar]
- 78.Moreau F, Boullu-Sanchis S, Vigouroux C, et al. Efficacy of pioglitazone in familial partial lipodystrophy of the Dunnigan type: a case report. Diabetes Metab 2007; 33: 385–9. [DOI] [PubMed] [Google Scholar]
- 79.Hegele RA, Al-Attar SA, Rutt BK. Obstructive sleep apnea in 2 women with familial partial lipodystrophy due to a heterozygous LMNA R482Q mutation. CMAJ 2007; 177: 743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decaudain A, Vantyghem MC, Guerci B, et al. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab 2007; 92: 4835–44. [DOI] [PubMed] [Google Scholar]
- 81.Vantyghem MC, Faivre-Defrance F, Marcelli-Tourvieille S, et al. Familial partial lipodystrophy due to the LMNA R482W mutation with multinodular goitre, extrapyramidal syndrome and primary hyperaldosteronism. Clin Endocrinol (Oxf) 2007; 67: 247–9. [DOI] [PubMed] [Google Scholar]
- 82.Lanktree M, Cao H, Rabkin SW, Hanna A, Hegele RA. Novel LMNA mutations seen in patients with familial partial lipodystrophy subtype 2 (FPLD2; MIM 151660). Clin Genet 2007; 71: 183–6. [DOI] [PubMed] [Google Scholar]
- 83.Ludtke A, Heck K, Genschel J, et al. Long-term treatment experience in a subject with Dunnigan-type familial partial lipodystrophy: efficacy of rosiglitazone. Diabet Med 2005; 22: 1611–3. [DOI] [PubMed] [Google Scholar]
- 84.Ludtke A, Genschel J, Brabant G, et al. Hepatic steatosis in Dunnigan-type familial partial lipodystrophy. Am J Gastroenterol 2005; 100: 2218–24. [DOI] [PubMed] [Google Scholar]
- 85.Young J, Morbois-Trabut L, Couzinet B, et al. Type A insulin resistance syndrome revealing a novel lamin A mutation. Diabetes 2005; 54: 1873–8. [DOI] [PubMed] [Google Scholar]
- 86.Drac H, Madej-Pilarczyk A, Gospodarczyk-Szot K, Gawel M, Kwiecinski H, Hausmanowa-Petrusewicz I. Familial partial lipodystrophy associated with the heterozygous LMNA mutation 1445G>A (Arg482Gln) in a Polish family. Neurol Neurochir Pol 2010; 44: 291–6. [DOI] [PubMed] [Google Scholar]
- 87.Klupa T, Szopa M, Skupien J, et al. LMNA gene mutation search in Polish patients: new features of the heterozygous Arg482Gln mutation phenotype. Endocrine 2009; 36: 518–23. [DOI] [PubMed] [Google Scholar]
- 88.Keller J, Subramanyam L, Simha V, Gustofson R, Minjarez D, Garg A. Lipodystrophy: an unusual diagnosis in a case of oligomenorrhea and hirsutism. Obstet Gynecol 2009; 114: 427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patel K, Roseman D, Burbank H, Attarian H. Obstructive sleep apnea in familial partial lipodystrophy type 2 with atypical skin findings and vascular disease. Sleep Breath 2009; 13: 425–7. [DOI] [PubMed] [Google Scholar]
- 90.Imachi H, Murao K, Ohtsuka S, et al. A case of Dunnigan-type familial partial lipodystrophy (FPLD) due to lamin A/C (LMNA) mutations complicated by end-stage renal disease. Endocrine 2009; 35: 18–21. [DOI] [PubMed] [Google Scholar]
- 91.Schmidt HH, Genschel J, Baier P, et al. Dyslipemia in familial partial lipodystrophy caused by an R482W mutation in the LMNA gene. J Clin Endocrinol Metab 2001; 86: 2289–95. [DOI] [PubMed] [Google Scholar]
- 92.Morel CF, Thomas MA, Cao H, et al. A LMNA splicing mutation in two sisters with severe Dunnigan-type familial partial lipodystrophy type 2. J Clin Endocrinol Metab 2006; 91: 2689–95. [DOI] [PubMed] [Google Scholar]
- 93.Rankin J, Auer-Grumbach M, Bagg W, et al. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A 2008; 146A: 1530–42. [DOI] [PubMed] [Google Scholar]
- 94.Bereziat V, Cervera P, Le Dour C, et al. LMNA mutations induce a non-inflammatory fibrosis and a brown fat-like dystrophy of enlarged cervical adipose tissue. Am J Pathol 2011; 179: 2443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gambineri A, Semple RK, Forlani G, et al. Monogenic polycystic ovary syndrome due to a mutation in the lamin A/C gene is sensitive to thiazolidinediones but not to metformin. Eur J Endocrinol 2008; 159: 347–53. [DOI] [PubMed] [Google Scholar]
- 96.Wiltshire KM, Hegele RA, Innes AM, Brownell AK. Homozygous lamin A/C familial lipodystrophy R482Q mutation in autosomal recessive Emery Dreifuss muscular dystrophy. Neuromuscul Disord 2013; 23: 265–8. [DOI] [PubMed] [Google Scholar]
- 97.Le Dour C, Schneebeli S, Bakiri F, et al. A homozygous mutation of prelamin-A preventing its farnesylation and maturation leads to a severe lipodystrophic phenotype: new insights into the pathogenicity of nonfarnesylated prelamin-A. J Clin Endocrinol Metab 2011; 96: E856–62. [DOI] [PubMed] [Google Scholar]
- 98.Garg A, Vinaitheerthan M, Weatherall PT, Bowcock AM. Phenotypic heterogeneity in patients with familial partial lipodystrophy (dunnigan variety) related to the site of missense mutations in lamin a/c gene. J Clin Endocrinol Metab 2001; 86: 59–65. [DOI] [PubMed] [Google Scholar]
- 99.Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 2000; 24: 153–6. [DOI] [PubMed] [Google Scholar]
- 100.Carboni N, Politano L, Floris M, et al. Overlapping syndromes in laminopathies: a meta-analysis of the reported literature. Acta Myol 2013; 32: 7–17. [PMC free article] [PubMed] [Google Scholar]
- 101.Monteiro LZ, Foss-Freitas MC, Junior Montenegro RM, Foss MC. Body fat distribution in women with familial partial lipodystrophy caused by mutation in the lamin A/C gene. Indian J Endocrinol Metab 2012; 16: 136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dutour A, Roll P, Gaborit B, et al. High prevalence of laminopathies among patients with metabolic syndrome. Hum Mol Genet 2011; 20: 3779–86. [DOI] [PubMed] [Google Scholar]
- 103.Spuler S, Kalbhenn T, Zabojszcza J, et al. Muscle and nerve pathology in Dunnigan familial partial lipodystrophy. Neurology 2007; 68: 677–83. [DOI] [PubMed] [Google Scholar]
- 104.Araujo-Vilar D, Loidi L, Dominguez F, Cabezas-Cerrato J. Phenotypic gender differences in subjects with familial partial lipodystrophy (Dunnigan variety) due to a nuclear lamin A/C R482W mutation. Horm Metab Res 2003; 35: 29–35. [DOI] [PubMed] [Google Scholar]
- 105.Boschmann M, Engeli S, Moro C, et al. LMNA mutations, skeletal muscle lipid metabolism, and insulin resistance. J Clin Endocrinol Metab 2010; 95: 1634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-g gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 2002; 87: 408–11. [DOI] [PubMed] [Google Scholar]
- 107.Agostini M, Schoenmakers E, Mitchell C, et al. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab 2006; 4: 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Misra A, Peethambaram A, Garg A. Clinical features and metabolic and autoimmune derangements in acquired partial lipodystrophy: report of 35 cases and review of the literature. Medicine (Baltimore) 2004; 83: 18–34. [DOI] [PubMed] [Google Scholar]
- 109.Corvillo F, Aparicio V, Lopez-Lera A, et al. Autoantibodies against perilipin 1 as a cause of acquired generalized lipodystrophy. Front Immunol 2018; 9: 2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Misra A, Garg A. Clinical features and metabolic derangements in acquired generalized lipodystrophy: case reports and review of the literature. Medicine (Baltimore) 2003; 82: 129–46. [DOI] [PubMed] [Google Scholar]
- 111.Wachslicht-Rodbard H, Muggeo M, Kahn CR, Saviolakis GA, Harrison LC, Flier JS. Heterogeneity of the insulin-receptor interaction in lipoatropic diabetes. J Clin Endocrinol Metab 1981; 52: 416–25. [DOI] [PubMed] [Google Scholar]
- 112.Savage DB, Semple RK, Clatworthy MR, et al. Complement abnormalities in acquired lipodystrophy revisited. J Clin Endocrinol Metab 2009; 94: 10–6. [DOI] [PubMed] [Google Scholar]
- 113.Brown RJ, Chan JL, Jaffe ES, et al. Lymphoma in acquired generalized lipodystrophy. Leuk Lymphoma 2016; 57: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab 2016; 101: 4500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Araujo-Vilar D, Sanchez-Iglesias S, Guillin-Amarelle C, et al. Recombinant human leptin treatment in genetic lipodystrophic syndromes: the long-term Spanish experience. Endocrine 2014; 49: 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beltrand J, Beregszaszi M, Chevenne D, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics 2007; 120: e291–6. [DOI] [PubMed] [Google Scholar]
- 117.Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract 2011; 17: 922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol 2013; 59: 131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007; 92: 532–41. [DOI] [PubMed] [Google Scholar]
- 120.Diker-Cohen T, Cochran E, Gorden P, Brown RJ. Partial and generalized lipodystrophy: comparison of baseline characteristics and response to metreleptin. J Clin Endocrinol Metab 2015; 100: 1802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chong AY, Lupsa BC, Cochran EK, Gorden P. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 2010; 53: 27–35. [DOI] [PubMed] [Google Scholar]
- 122.Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 2005; 41: 753–60. [DOI] [PubMed] [Google Scholar]
- 123.Simha V, Szczepaniak LS, Wagner AJ, DePaoli AM, Garg A. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 2003; 26: 30–5. [DOI] [PubMed] [Google Scholar]
- 124.Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 2002; 109: 1345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garg A Dyslipidemias. Pathophysiology, Evaluation and Management. Totowa, NJ: Humana Press, 2015. [Google Scholar]
- 126.Garg A Lipodystrophy In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York: McGraw Hill, 2012; 755–64. [Google Scholar]