Abstract
Aims/hypothesis
This study evaluates whether the non-selective β-blocker, carvedilol, can be used to prevent counterregulatory failure and the development of impaired awareness of hypoglycaemia (IAH) in recurrently hypoglycaemic rats.
Methods
Sprague Dawley rats were implanted with vascular catheters and intracranial guide cannulas targeting the ventromedial hypothalamus (VMH). These animals underwent either three bouts of insulin-induced hypoglycaemia or received three saline injections (control group) over 3 days. A subgroup of recurrently hypoglycaemic animals was treated with carvedilol. The next day, the animals underwent a hypoglycaemic clamp with microdialysis without carvedilol treatment to evaluate changes in central lactate and hormone levels. To assess whether carvedilol prevented IAH, we treated rats that had received repeated 2-deoxyglucose (2DG) injections to impair their awareness of hypoglycaemia with carvedilol and measured food intake in response to insulin-induced hypoglycaemia as a surrogate marker for hypoglycaemia awareness.
Results
Compared with the control group, recurrently hypoglycaemic rats had a ~1.7-fold increase in VMH lactate and this was associated with a 75% reduction in the sympathoadrenal response to hypoglycaemia. Treatment with carvedilol restored VMH lactate levels and improved the adrenaline (epinephrine) responses. In 2DG-treated rats compared with control animals receiving saline, food intake was reduced in response to hypoglycaemia and increased with carvedilol treatment.
Conclusion/interpretation
We conclude that carvedilol may be a useful therapy to prevent counterregulatory failure and improve IAH.
Keywords: β-blocker, Carvedilol, Counterregulatory failure, Impaired hypoglycaemia awareness, Lactate, Recurrent hypoglycaemia, Ventromedial hypothalamus (VMH)
Introduction
Hypoglycaemia remains a major barrier to properly managing glucose levels in people with type 1 diabetes [1, 2]. However, loss of the ability to secrete glucagon in the early stages of diabetes, compounded by the fact that antecedent hypoglycaemia results in loss of the sympathoadrenal response to hypoglycaemia, not only reduces effective recovery from hypoglycaemia but also reduces symptomatic awareness of hypoglycaemia—a condition called hypoglycaemia-associated autonomic failure (HAAF) [3]. The mechanisms leading to HAAF are not known, but several mechanisms have been proposed, including, but not limited to, repeated activation of the adrenergic system and impairments in central glucose-sensing mechanisms [4-6]. The data from the current study suggest these two mechanisms may be linked.
We have reported that repeated activation of adrenergic receptors in the ventromedial hypothalamus (VMH) in the absence of peripheral hypoglycaemia raised local lactate concentrations and suppressed the counterregulatory responses (Sejling et al., unpublished data). Noradrenaline (norepinephrine) enhances lactate production from astrocytes through the activation of β2-adrenergic receptors (β2ARs) and can also increase lactate uptake into neurons by enhancing monocarboxylic acid transporter (MCT) expression [7-9]. Normally, in response to acute bouts of hypoglycaemia, VMH noradrenaline levels rise and act through β2ARs to enhance the sympathoadrenal response [10-12]. Less is known about the effects of recurrent hypoglycaemia on the VMH noradrenaline system or how repeated activation of β2ARs impacts the counterregulatory responses. It has been reported that VMH noradrenaline responses to hypoglycaemia remain intact in recurrently hypoglycaemic rats [13] and that the activity of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine synthesis, is not impaired by recurrent hypoglycaemia [14]. This suggests that recurrent hypoglycaemia does not impair the VMH noradrenaline response to subsequent bouts of hypoglycaemia, but, rather, defects leading to counterregulatory failure may lie downstream of noradrenaline release. In support of this finding, adrenergic blockade during antecedent hypoglycaemia prevents counterregulatory failure in healthy humans [15]. Therefore, while the acute activation of VMH adrenergic receptors may be beneficial in enhancing the counterregulatory response, repeated activation of this neurotransmitter system may contribute to counterregulatory failure. Hence, β-blockers may be a useful therapeutic strategy to help prevent the onset of HAAF. The objectives of the current study were to evaluate whether the non-specific β-blocker, carvedilol, can be used to prevent the development of counterregulatory failure and impaired awareness of hypoglycaemia (IAH) in rodents. We chose these models because the rat model of recurrent hypoglycaemia is an established model of HAAF that replicates many of the same features of HAAF found in humans, while the rodent model of IAH possesses the same counterregulatory defects as the recurrent hypoglycaemia model, but it also exhibits changes in feeding responses that allow us to objectively evaluate hypoglycaemia awareness.
Methods
Animals
Adult male Sprague Dawley rats (CD:SD, strain 001; Charles River, Wilmington, MA, USA) of 7–8 weeks of age and weighing ~300 g were individually housed in conventional rat cages in the University of Utah’s Comparative Medicine Center in temperature- (22±2°C) and humidity-controlled rooms. Cages were lined with wood chip bedding and the animals were provided with enrichment in the form of a red acrylic tube and a gnawing block. The animals had free access to rodent chow (Envigo Teklad; Madison, WI, USA) and water and were acclimatised to a 12 h light/dark cycle (lights on between 07:00 hours and 19:00 hours) for 1 week before experimental manipulation. The principles of laboratory animal care were followed, and experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Utah.
Carvedilol treatment
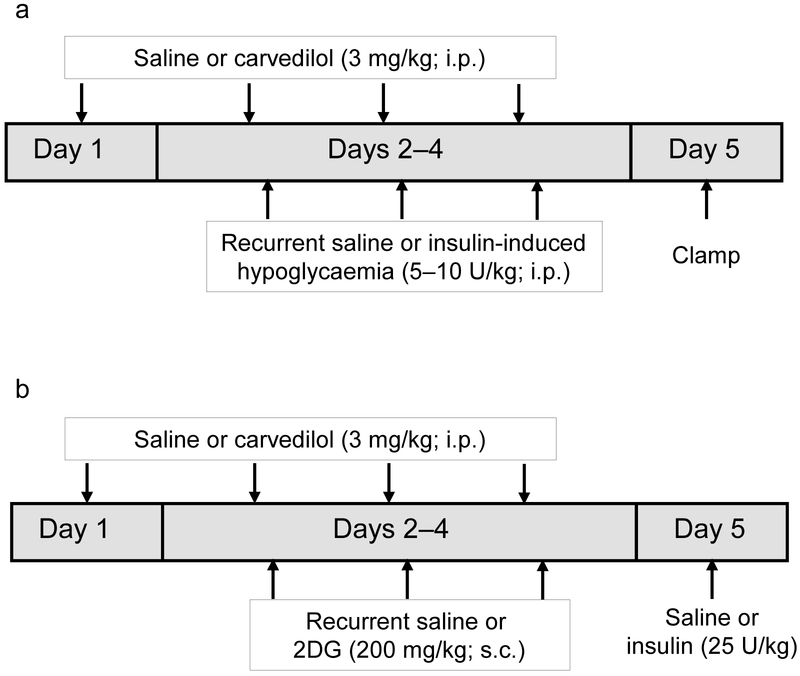
Carvedilol treatment (Fig. 1a,b) was initiated 1 day prior to the start of the recurrent hypoglycaemia or recurrent 2-deoxyglucose (2DG; Sigma-Aldrich, St Louis, MO, USA) treatment regimens described below. The animals were administered a single daily i.p. injection of carvedilol (3 mg/kg) dissolved in saline (154 mmol/l NaCl) at 08:00 hours in the laboratory. On each of the days that either insulin or 2DG was given, carvedilol was administered 1 h prior to the insulin or 2DG injection. Carvedilol treatment continued throughout the 3 days of recurrent hypoglycaemia or 2DG treatment, but the drug was not given on the day of the clamp or food-intake studies. Typical doses of carvedilol used in rodent studies range from 1 mg/kg to 30 mg/kg, with benefits evident even at the lower doses of 1 and 2 mg/kg [16-19]. As the goal of our study was to identify doses of carvedilol that would temper the β-adrenergic effects and not completely block β-adrenergic signalling, we evaluated two low doses, 1 and 3 mg/kg; we noted that the optimal dose for preventing counterregulatory failure in this rodent model of recurrent hypoglycaemia was 3 mg/kg (electronic supplementary material [ESM] Fig. 1).
Fig. 1.
Study design. (a) Schematic diagram showing carvedilol and recurrent saline or insulin treatment during days 1–4. All animals underwent a hypoglycaemic clamp on day 5. (b) Schematic diagram showing the 2DG treatment regimen. Carvedilol and 2DG were administered from day 1 to 4. On day 5, insulin was given to induce hypoglycaemia and total food consumption (g) in response to the hypoglycaemic challenge was measured over 4 h
Study 1: evaluating the counterregulatory hormone responses following carvedilol treatment
Vascular surgery
After the acclimatisation period, the animals underwent aseptic surgery under 2-2.5% isoflurane anaesthesia to have vascular catheters implanted into the left carotid artery and right jugular vein as described previously [20]. Animals were monitored every 15 min throughout the surgery. Compared with injectable anaesthetics, isoflurane allowed for more rapid recovery from surgical procedures.
Stereotaxic surgery
Subsequently, the animals were placed into a stereotaxic frame and bilateral stainless steel guide cannulas for microdialysis (Azuma, San Diego, CA, USA) were inserted to the level of the VMH (from bregma: 2.6 mm posterior, 3.8 mm lateral and 8.7 mm ventral at an angle of 16°) and secured with screws and dental acrylic as described previously [21]. The animals were then returned to their home cages for 4 days to recover before being randomly assigned to one of the following treatment groups: (1) saline control (n=6); (2) recurrent hypoglycaemia (n=7); and (3) recurrent hypoglycaemia + carvedilol (n=7). Treatment groups underwent the clamping procedure in random order.
Recurrent hypoglycaemia
Hypoglycaemia was induced using a single i.p. injection of insulin (5-10 IU/kg, Humulin R, Eli Lilly, Indianapolis, IN, USA). Insulin doses were tapered daily from 10 U/kg on day 1 to 8 U/kg on day 2 to 5 U/kg on day 3 to account for the development of counterregulatory failure and to avoid severe hypoglycaemia and seizures [22]. Plasma glucose concentrations were monitored through a tail nick every 30 min over 3 h using an AlphaTRAK 2 glucometer (Abbott Laboratories, Chicago, IL, USA). Target glucose levels were maintained between ~1.7 and 2.8 mmol/l for at least 2 h during this time. During recurrent hypoglycaemia, animals had access to water but not food. Following each episode of hypoglycaemia, plasma glucose levels were recovered to euglycaemic levels by providing free access to food and administering 0.5-1 ml 20% dextrose i.p., as needed. Once recovered, the animals were returned to their home cages. This procedure was repeated for 3 consecutive days (Fig. 1a). This treatment regimen was shown to effectively reduce the counterregulatory hormone responses to hypoglycaemia [22].
Hypoglycaemic clamp
Following an overnight fast, bilateral 1 mm microdialysis/microinjection probes (Azuma) were inserted through the guide cannulas of the animals and their vascular catheters were connected to the infusion pumps. After a 2.5 h recovery period, baseline blood samples were collected and the collection of baseline microdialysate samples was initiated for 45 min. Once baseline microdialysate samples were collected, a constant insulin (50 mU kg−1 min−1) and variable 20% glucose infusion was used to lower and maintain plasma glucose levels at 2.5±0.3 mmol/l for 90 min. Blood samples were collected every 5 min to assess plasma glucose levels (Analox GM9, Analox Instruments, Stourbridge, UK) and at 30, 60 and 90 min during the clamping period to assess plasma lactate and hormone responses. Once the plasma was collected and frozen, the erythrocytes were re-suspended in an equivalent volume of artificial plasma and re-infused back into the animal to prevent volume depletion and anaemia.
Probe placement
At the end of the experiment, the animals were euthanised with intravenous sodium pentobarbital. The brains were rapidly removed, frozen and stored at −80°C until they were analysed. Accuracy of probe position was histologically verified and only those animals with properly positioned probes were included in the analysis. The primary endpoint of the clamp procedure was to determine whether carvedilol prevented the development of counterregulatory failure in recurrently hypoglycaemic rats.
Study 2: determining the effects of recurrent hypoglycaemia and carvedilol treatment on VMH Mct expression
To evaluate the effects of recurrent hypoglycaemia and carvedilol treatment on expression of the monocarboxylic acid transporter in the VMH, groups of control rats (n=8), recurrently hypoglycaemic rats (n=9) and recurrently hypoglycaemic rats that received carvedilol (n=8) were killed on the day following their last treatment regimen. The VMH was micropunched and quantitative (q)RT-PCR was used to quantify mRNA levels of Mct1 (also known as Slc16a1; Taqman Assay ID: Rn00562332_m1) and Mct2 (also known as Slc16a7; Assay ID: Rn00568872_m1). Total RNA was extracted (Qiagen RNA extraction kit; Qiagen, Germantown, MD, USA) and reverse transcription was conducted using Taqman primers and RT-PCR assays. Briefly, reverse transcription was performed by adding 10 μl Taqman master mix to 125 ng RNA and running the following thermocycler settings: 25°C for 10 min, 37°C for 120 min, 85°C for 5 min and 4°C hold. PCR reactions were carried out by adding 18 μl reaction mix to 2 μl cDNA and running the following reactions: 50°C for 2 min, 95°C for 2 min, 95°C for 1 s, 60°C for 20 s for 40 cycles. Normalisation was carried out using β-actin (Assay ID: Rn01410374_m1) as the endogenous control. The 2−ΔΔC2 method was used to calculate relative fold change in gene expression between control and experimental groups [23].
Study 3: evaluating hypoglycaemia awareness
Impaired hypoglycaemia awareness model
In humans, IAH is determined using questionnaires querying the extent to which individuals feel various hypoglycaemic symptoms [24, 25]. This procedure is not feasible in animals and a model of IAH needed to be developed. In response to hypoglycaemia, animals typically seek and consume food [26]. Therefore, food consumption may be a useful and quantitatively objective surrogate marker of IAH in rodents. We anticipate that in such a model, those animals that are hypoglycaemia aware would consume more food when blood glucose levels decline, whereas those that are impaired aware would eat less. It has been reported that recurrent insulin-induced hypoglycaemia does not elicit a reduced feeding response to hypoglycaemia, whereas recurrent glucose deprivation using 2DG does [27, 28]. Of note, both recurrent insulin-induced hypoglycaemia and recurrent glucose deprivation lead to impairments of the counterregulatory response to hypoglycaemia [29]. The reason for this dichotomy in the feeding response is not fully understood, but it may be due to a more profound and/or prolonged reduction in utilisable glucose (at the cellular level) in the 2DG model. Therefore, we induced ‘IAH’ in our animals by administering a s.c. injection of 2DG (200 mg/kg) once daily for 3 consecutive days. Control animals received s.c. saline injections. During recurrent 2DG or saline administration, the animals had access to water but not food. Animals were randomly allocated to these treatment conditions.
Effects of carvedilol treatment on hypoglycaemia awareness
To evaluate whether carvedilol improved awareness of hypoglycaemia, we treated rats made ‘IAH’ with carvedilol and measured food intake in response to insulin-induced hypoglycaemia on day 4 as a surrogate marker of hypoglycaemia awareness (Fig. 1b). Again, carvedilol treatment was started prior to the start of the 2DG-treatment regimen and carvedilol was given 1 h prior to each injection of 2DG. On day 4, non-fasted rats were given either a single s.c. injection of saline or 25 U/kg NPH insulin (Lilly). This dose of insulin was necessary to induce and maintain the hypoglycaemic stimulus for the entire 4 h period that food consumption was evaluated. Lower insulin doses were insufficient to keep glucose levels at ~2.8 mmol/l once the animals started to eat. Moreover, this dose of insulin was comparable with the total amount of insulin used to maintain hypoglycaemia using insulin-clamp procedures while food consumption was evaluated (data not shown). Tail vein glucose and food consumption was evaluated every 2 h and total food intake over the course of 4 h was measured. The rats were randomly assigned to one of the following treatment groups (recurrent treatment + day 4 treatment): (1) saline + saline (n=6); (2) saline + insulin (n=8); (3) 2DG + insulin (n=8); and (4) 2DG and carvedilol + insulin (n=6). The primary endpoint of the awareness study was to determine whether carvedilol prevented the development of IAH.
Study 4: effects of carvedilol on body weight and feeding behaviour
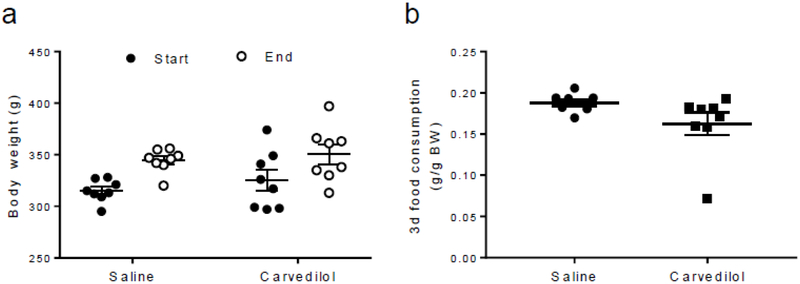
To control for the potential non-specific effects of carvedilol on feeding behaviour, we administered carvedilol (3 mg/kg; i.p.) once daily for 4 consecutive days to a group of animals (n=8) and monitored its effects on body weight and daily food consumption. This group was compared with a group administered a daily saline injection (n=8).
Hormone and microdialysate analysis
Plasma catecholamine concentrations were analysed by ELISA (Abnova, Taipei, Taiwan), while plasma glucagon concentrations were determined using commercial radioimmunoassays (MilliporeSigma, Burlington, MA, USA). Plasma lactate concentrations were determined using an Analox GL6 analyser (Analox Instruments). Microdialysate lactate concentrations were analysed using a fluorometric assay (Biovision, Milpitas, CA, USA).
Statistical analysis
Treatment effects were analysed using Student’s t-test, one- (for lactate, glucagon, adrenaline and food-consumption data) or two-way (for plasma glucose and glucose infusion rate [GIR] data) ANOVA for independent or repeated measures as appropriate, followed by Tukey’s pairwise comparisons using Prism GraphPad 7.0 statistical software. A value of p≤0.05 was set as the criterion for statistical significance. The sample sizes required for all studies were determined using statistical power calculations based on our experience with these experiments, with a p<0.10 for the β error and p<0.05 for the α error.
Results
Exogenous GIRs with recurrent hypoglycaemia
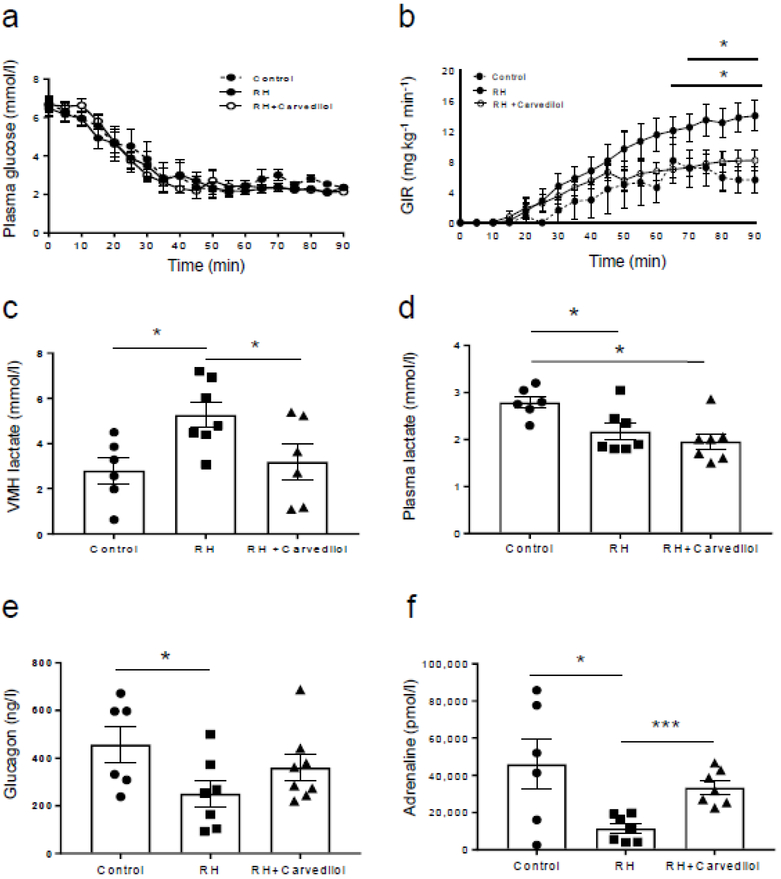
Plasma glucose levels were matched between treatment groups during the hypoglycaemic clamp (Fig. 2a). Despite similar plasma glucose concentrations, exogenous glucose requirements were significantly higher between 70 and 90 min in the recurrently hypoglycaemic group compared with the control group (Fig. 2b; p<0.05, control vs recurrent hypoglycaemia). Carvedilol treatment reduced GIRs to normal (p<0.05, recurrently hypoglycaemic vs recurrently hypoglycaemic + carvedilol).
Fig. 2.
Evaluation of VMH lactate and the counterregulatory hormone responses. Plasma glucose (a) and GIR (b) during the hypoglycaemic clamp; control group (n=6); recurrently hypoglycaemic (RH) rats (n=7); carvedilol-treated RH rats (n=7). GIRs at the end of the clamp were significantly higher in the RH group compared with the control group (*p<0.05, control vs RH, for time points shown below the bar). Carvedilol treatment reduced the GIR of RH animals to normal (†p<0.05, RH vs RH + carvedilol, for time points shown below the bar). (c) Basal extracellular (VMH) lactate levels were 66% elevated in the RH rats (n=7; *p<0.05 vs control) compared with the controls (n=6) and these levels were reduced to normal with carvedilol treatment (n=6; *p<0.05 vs RH). (d) Baseline plasma lactate concentrations were lower in RH animals (n=7) compared with controls (n=6; *p<0.05, control vs RH and RH + carvedilol). No differences were observed between carvedilol-treated RH animals (n=8) and the RH group. (e) In comparison with controls (n=6), peak plasma glucagon levels were reduced by 42% in the RH (n=7) group (*p<0.05 vs control). (f) Peak adrenaline concentrations during the hypoglycaemic clamp were significantly suppressed by 75% in the RH (n=7) group compared with controls (n=6) (*p<0.05 vs control). Treatment with carvedilol (n=7) restored these hormone responses to normal (***p<0.001 vs RH animals). Plasma glucose and GIR data were analysed using two-way repeated measures ANOVA and microdialysate and hormone data were analysed using one-way ANOVA with post hoc Tukey honest significant difference (HSD) test. Data are presented as mean ± SEM. RH, recurrently hypoglycaemic
Hormone response with recurrent hypoglycaemia
No significant differences in baseline hormone levels were noted between treatment groups (Table 1). As expected, recurrent hypoglycaemia raised baseline extracellular lactate concentrations in the VMH by 66% (p<0.05 vs control group; Fig. 2c). Treatment with carvedilol normalised VMH lactate concentrations. In contrast, plasma lactate concentrations were slightly, but significantly, lower in both recurrently hypoglycaemic and carvedilol-treated animals (p<0.05 vs control group; Fig. 2d). Peak glucagon responses, which generally occurred between 30 and 60 min after the start of insulin, were reduced by 42% in the recurrently hypoglycaemic group (p<0.05 vs control group; Fig. 2e). Although carvedilol improved the glucagon responses in recurrently hypoglycaemic animals, the improvement failed to reach statistical significance (p=0.09 vs recurrently hypoglycaemic). Peak adrenaline (epinephrine) concentrations, which generally occurred between 60 and 90 min after the start of insulin, were reduced by 75% compared with the control group (p<0.05 vs control group; Fig. 2f) and carvedilol fully restored the sympathoadrenal response in recurrently hypoglycaemic animals (p<0.001 vs recurrently hypoglycaemic).
Table 1.
Baseline hormone concentrations in groups of rats
| Variable | Control (n=6) |
Recurrently hypoglycaemic (n=7) |
Recurrently hypoglycaemic + carvedilol (n=7) |
|---|---|---|---|
| Body weight (g) | 315.8±9.4 | 294.1±19.1 | 323.4±5.0 |
| Plasma glucose (mmol/l) | 6.7±0.4 | 6.4±0.4 | 6.7±0.4 |
| Glucagon (ng/l) | 67.4±15.0 | 49.8±11.0 | 73.2±14.4 |
| Adrenaline (pmol/l) | 746.1±151.5 | 922.1±406.6 | 923.8±257.2 |
Data reported as mean ± SEM. No significant differences in baseline hormone levels were noted between treatment groups
Mct expression in the VMH with recurrent hypoglycaemia
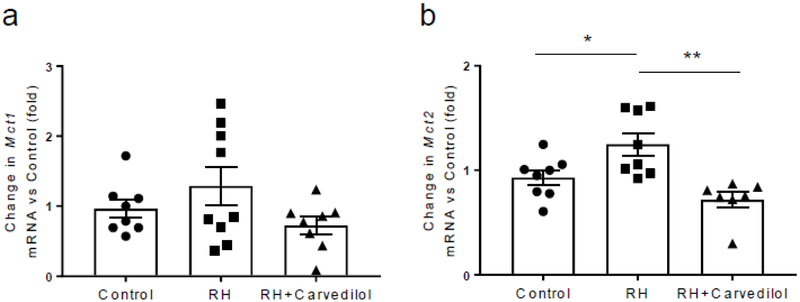
Recurrent hypoglycaemia did not significantly alter the level of Mct1 mRNA (Fig. 3a) in the VMH, but it increased the level of Mct2 mRNA (p<0.05 vs control group; Fig. 3b). Treatment with carvedilol reduced Mct2 mRNA levels to normal.
Fig. 3.
Mct1 and Mct2 mRNA levels in the VMH. (a) In comparison with the control group (n=8), Mct1 mRNA level in the VMH was not significantly altered by recurrent hypoglycaemia (n=9). Treatment of recurrently hypoglycaemic (RH) rats with carvedilol (n=8) reduced the Mct1 mRNA levels slightly, although the difference just failed to reach statistical significance (p=0.09 vs RH). (b) Mct2 mRNA expression in the VMH was significantly upregulated in RH animals (n=8) compared with controls (n=8) (*p<0.05 vs control); treatment with carvedilol (n=7) restored these levels to normal (**p<0.01 vs RH). Data were analysed using one-way ANOVA with post hoc Tukey honest significant difference (HSD) test and presented as mean ± SEM. RH, recurrently hypoglycaemic
Carvedilol treatment prevents the development of IAH
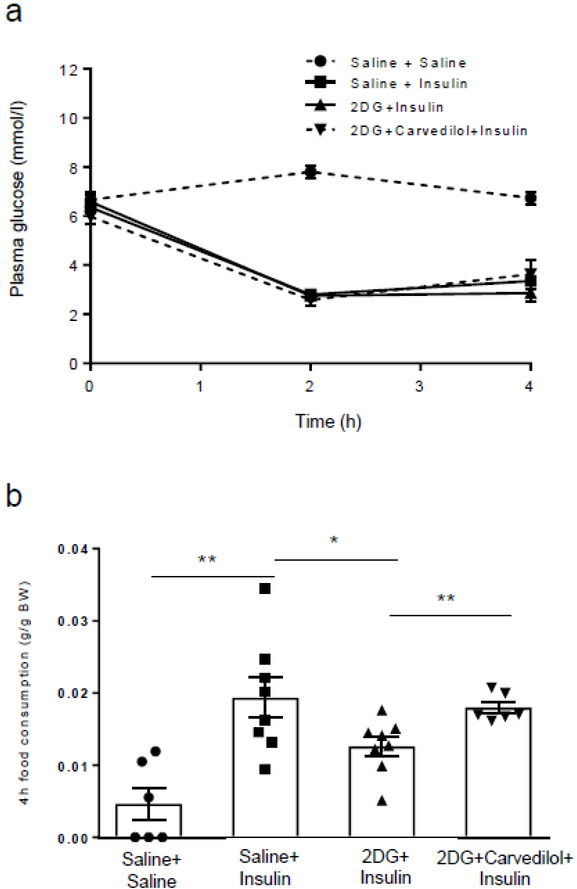
Plasma glucose levels were matched between all treatment groups during hypoglycaemia induction (Fig. 4a). Rats treated with saline for 3 days consumed significantly more food in response to insulin-induced hypoglycaemia than was consumed by saline-treated rats administered saline on day 4 (Fig. 4b). This demonstrated hypoglycaemia induces a significant feeding response. In contrast, 2DG-treated animals ate significantly less than the saline-treated animals during day 4 hypoglycaemia (p<0.05 vs saline + insulin), suggesting IAH. Treatment with carvedilol prevented the development of IAH in the 2DG-treated animals as they ate as much as the saline + insulin group, suggesting hypoglycaemia awareness was restored to normal.
Fig. 4.
Evaluation of hypoglycaemia awareness. (a) Plasma glucose levels during the induction of hypoglycaemia on day 5. (b) Hypoglycaemia-naive rats made hypoglycaemic (saline + insulin; n=8) on day 4 consumed more than four times more food than hypoglycaemia-naive rats given a saline injection (saline + saline; n=6) (**p<0.01, saline + insulin vs saline + saline). Rats treated with 2DG (2DG + insulin; n=8) ate 35% less food when hypoglycaemic compared with the saline + insulin group (*p<0.05, 2DG + insulin vs saline + insulin). In contrast, when the 2DG animals were treated with carvedilol (n=6), the animals consumed as much food as the insulin group (**p<0.01, 2DG + insulin vs 2DG + carvedilol + insulin). Data were analysed using one-way ANOVA with post hoc Tukey honest significant difference (HSD) test and presented as mean ± SEM. BW, body weight
Effects on feeding and body weight
Carvedilol treatment did not increase body weight (Fig. 5a) or overall food consumption (Fig. 5b) over the course of the 4 day regimen.
Fig. 5.
Carvedilol treatment did not affect body weight or food consumption. Treatment with low doses of carvedilol over the course of 4 days did not increase (a) body weight gain from the start of treatment (n=8) until the end of the treatment (n=8; p=NS, saline vs carvedilol) and nor did it affect (b) total food consumption between saline (n=8) and carvedilol-treated animals (n=8) over the course of the treatment (p=NS, saline vs carvedilol). Unpaired Student’s t tests were used for these comparisons. Data are presented as mean ± SEM. BW, body weight
Discussion
The treatment and prevention of hypoglycaemia is critical if we are to help individuals with diabetes reach and maintain their glycaemic targets. Previous research suggests that repeated activation of the adrenergic system during recurrent episodes of hypoglycaemia may potentially contribute to the development of HAAF [15]. The current study shows that carvedilol may be a useful therapy to prevent the development of HAAF.
Activation of VMH β2ARs is important for augmenting the counterregulatory responses to an acute bout of hypoglycaemia [11, 12]. This is consistent with observations by Beverly and colleagues who showed noradrenaline levels in the VMH rose during hypoglycaemia [10, 30]. However, it was noteworthy that recurrent hypoglycaemia did not alter this neurotransmitter response, despite the development of a defective sympathoadrenal response [13]. Consistent with these observations, we showed that repeated activation of VMH β2ARs in the absence of hypoglycaemia raised VMH lactate levels and induced counterregulatory failure (Sejling et al, unpublished data), suggesting the suppressive effects of noradrenaline are most likely mediated through β2ARs. In a subgroup of animals treated with noradrenaline in that prior study, we administered α-cyano-4-hydroxy-cinnamic acid (4CIN) into the VMH to block the uptake of lactate into neurons immediately prior to the hypoglycaemic clamp. In this group, we saw the suppressive effects of noradrenaline treatment on glucose counterregulation were completely abolished. Hence, these data suggest that repeated activation of VMH β2ARs plays an important role in the development of counterregulatory failure, in part by enhancing central lactate production. Therefore, the use of β-blockers may be a promising therapeutic strategy to help preserve the hormone responses to hypoglycaemia.
The current study shows that when carvedilol is administered in low doses, it can effectively prevent the development of counterregulatory failure in recurrently hypoglycaemic rats. Carvedilol-treated recurrently hypoglycaemic rats required less exogenous glucose during the hypoglycaemic clamp compared with recurrently hypoglycaemic animals treated with vehicle and, more importantly, we saw improvements in the sympathoadrenal response to hypoglycaemia in the carvedilol-treated recurrently hypoglycaemic animals that corresponded to the reduced GIRs, suggesting more effective recovery from hypoglycaemia. Of interest is the fact that carvedilol is a lipophilic drug that gains rapid access to the brain. It was reported that the level of 11C-labelled carvedilol reaches a peak in the brain within 10 min of systemic administration in mice and remains in the circulation with a half-life of 7-10 h [31]. In our studies, we administered carvedilol 1 h prior to inducing hypoglycaemia and this was sufficient to prevent the onset of counterregulatory failure in recurrently hypoglycaemic animals. The ability of carvedilol to penetrate and act within the brain was evident when examining lactate levels in the VMH—although recurrent hypoglycaemia raised lactate levels in the VMH, animals treated with carvedilol exhibited normal brain lactate levels. These changes occurred despite plasma lactate concentrations being slightly lower in recurrently hypoglycaemic animals, indicating that elevated brain lactate levels are generated locally and not taken up from the periphery, as lactate generally travels along its concentration gradient.
Carvedilol treatment did not affect plasma lactate levels in recurrently hypoglycaemic animals. This important observation underscores the potential for carvedilol to prevent some of the central adaptations that occur during recurrent hypoglycaemia. Elevated brain lactate levels have been implicated in the development of HAAF in both humans and rodents [32-37], but the mechanism(s) are still not known. De Feyter et al reported elevated brain lactate concentrations in people with type 1 diabetes that were not associated with increased lactate oxidation [32, 38]. In contrast, Wiegers et al reported enhanced brain lactate utilisation in individuals with type 1 diabetes with IAH, as evidenced by a decline in brain lactate levels during a hypoglycaemic clamp that was not observed in healthy individuals [35, 37]. Using exercise to raise peripheral lactate levels physiologically prior to a hypoglycaemic clamp, the same group also showed that lactate levels decreased to 30% below baseline levels during hypoglycaemia in people with type 1 diabetes with IAH compared with healthy individuals and people with type 1 diabetes with normal hypoglycaemia awareness, for whom brain lactate levels decreased to baseline levels [36]; this suggests increased lactate oxidation may contribute to IAH. This observation is consistent with our data showing enhanced VMH lactate uptake plays a major role in suppressing the counterregulatory response by enhancing GABAergic neurotransmission [34]. The increase in local lactate production is likely driven by repeated adrenergic activation as carvedilol prevented the rise and the development of IAH. Although the mechanism(s) through which noradrenaline enhances central lactate levels is not entirely clear, we speculate that if VMH noradrenergic neurotransmission remains intact following recurrent hypoglycaemia, then activation of VMH adrenergic receptors could potentially enhance glucose uptake [39] and stimulate the breakdown of glycogen into lactate [40-45] and the long-term re-synthesis of glycogen [46]. Importantly, as brain lactate levels remain chronically elevated and brain glycogen supplies are limited, an increase in glucose uptake may help replenish and sustain brain glycogen stores in recurrently hypoglycaemic animals. Whether these effects are mediated directly through β2ARs on astrocytes or indirectly through actions on other neurons [47] is less clear. These mechanisms need to be investigated in more detail.
Elevated brain lactate levels in recurrently hypoglycaemic animals were accompanied by increased Mct2 mRNA expression, suggesting recurrent hypoglycaemia leads to adaptations that enhance the capacity to transport lactate into neurons. The increase in MCT expression is consistent with those observed in rodent models of ischaemia, in which brain lactate levels are also elevated [48]. It is notable that Mct2 expression in recurrently hypoglycaemic animals was reduced with carvedilol treatment, coincident with the reduction in extracellular lactate levels.
Consistent with the animal data, studies in healthy humans showed that adrenergic blockade with propanolol during antecedent episodes of hypoglycaemia prevented suppression of the catecholamine responses [15]. Together, the rodent and human data support the idea that the development of counterregulatory failure and the potential loss of hypoglycaemia awareness stem from repeated activation of βARs. We therefore evaluated whether mild to moderate adrenergic blockade was a suitable therapeutic strategy to prevent the development of IAH in rodents. To address this question, we used repeated injections of 2DG to impair hypoglycaemia awareness in our animals. Hypoglycaemia triggers the onset of a number of different symptoms including tremulousness, increased heart rate and hunger. Hunger or food-seeking behaviour may be an objective and reliable indicator of hypoglycaemia awareness in rodents as it is both measurable and can be reliably triggered by hypoglycaemia. While hypoglycaemia awareness involves cognitive processes to recognise when one is low, that recognition should arguably translate into behavioural actions to correct hypoglycaemia (i.e. food seeking or eating). We therefore used food intake as a surrogate marker of hypoglycaemia awareness with the premise being that if the animal is aware that it is hypoglycaemic, it would consume more food compared with one that is less aware. Notably, Sanders et al demonstrated that recurrent insulin-induced hypoglycaemia was not able to suppress the feeding response to subsequent bouts of hypoglycaemia in rats [27], whereas recurrent glucose deprivation using 2DG did [28]. We therefore used recurrent 2DG treatment to induce IAH in our rats and quantified food consumption in response to insulin-induced hypoglycaemia. While the 2DG stimulus is not entirely the same as insulin-induced hypoglycaemia, glucose deprivation at the cellular level is likely to be similar between the two conditions, despite the hyperglycaemia generally found in the 2DG model. We showed that hypoglycaemia significantly increased food intake in saline-treated animals, whereas 2DG treatment significantly reduced food intake in response to hypoglycaemia, suggesting 2DG animals were less aware of hypoglycaemia. Treatment with carvedilol prevented the reduction in food intake in 2DG animals, suggesting the animals were more hypoglycaemia aware. Our data are consistent with studies in humans conducted by Hirsch and colleagues which showed that total symptom scores improved in the presence of propanolol, although the improvement did not reach statistical significance [49]. Importantly, neuroglycopenic symptoms were not reduced by propranolol.
It is important to note that treatment of diabetic individuals with beta-blockers is controversial because of the perceived potential to attenuate hypoglycaemic symptoms. However, there is a lack of convincing clinical evidence to support this notion. In the recent GEMINI (Glycemic Effect in Diabetes Mellitus: Carvedilol–Metoprolol Comparison in Hypertensives) study, which evaluated the use of two different beta-blockers in individuals with type 2 diabetes and hypertension, carvedilol was shown to effectively reduce overall hypoglycaemia burden scores [50]. Likewise, the majority of clinical studies show that beta-blocker treatment of non-diabetic individuals and those with type 1 diabetes improves counterregulatory hormone responses without increasing the frequency of hypoglycaemia or impairing hypoglycaemia awareness [51-56], although loss of tremulousness and reduced tachycardia were reported in some studies. In a prospective study of 150 people with diabetes, 50 of whom were on beta-blockers, no difference in the incidence of hypoglycaemic unconsciousness was noted between those taking beta-blockers and those not, suggesting the use of beta-blockers in type 1 diabetes does not increase the incidence of severe hypoglycaemia [52] and may be a safe therapeutic option that can be used in conjunction with insulin.
In conclusion, our data suggest that partial adrenergic blockade with carvedilol during antecedent hypoglycaemia may be useful for preventing IAH and the development of counterregulatory failure. To our knowledge, this is the first study to investigate the use of carvedilol as a treatment to prevent IAH. Future studies will be directed towards evaluating this therapy for the treatment of HAAF in the setting of diabetes.
Supplementary Material
Research in context.
What is already known about this subject?
Recurrent hypoglycaemia does not affect the release of noradrenaline in the ventromedial hypothalamus, despite impairments in the counterregulatory hormone responses
Increased lactate in the ventromedial hypothalamus may contribute to counterregulatory failure in recurrently hypoglycaemic animals
Repeated adrenergic activation induced by hypoglycaemia progressively impairs the sympathoadrenal response to hypoglycaemia in healthy humans
What is the key question?
Can low doses of the β-blocker carvedilol be used to improve hypoglycaemia awareness or prevent the development of counterregulatory failure?
What are the new findings?
Systemic treatment of Sprague Dawley rats with low doses of carvedilol reduces lactate levels in the ventromedial hypothalamus
Low-dose carvedilol treatment prevents the development of counterregulatory failure in recurrently hypoglycaemic rats
Low-dose carvedilol treatment improves hypoglycaemia awareness in rats
How might this impact on clinical practice in the foreseeable future?
Low doses of carvedilol may be a promising clinical therapy to improve hypoglycaemia awareness in diabetic individuals with impaired awareness of hypoglycaemia
Acknowledgments
Funding The authors are grateful for the generosity of the agencies that helped fund this study: the JDRF (3-SRA-2017-487-S-B), the National Institutes of Health (R01 DK099315) and the University of Utah’s Diabetes and Metabolism Research Center. NK was supported by the Undergraduate Research Opportunities Program at the University of Utah.
Abbreviations
- 2DG
2-Deoxyglucose
- β2AR
β2-Adrenergic receptor
- GIR
Glucose infusion rate
- HAAF
Hypoglycaemia-associated autonomic failure
- IAH
Impaired awareness of hypoglycaemia
- MCT
Monocarboxylic acid transporter
- qRT-PCR
Quantitative RT-PCR
- VMH
Ventromedial hypothalamus
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with the manuscript.
Contribution statement RF, GS, AS and NK researched the data. OC conceptualised and designed the studies. SJF developed the 2DG IAH rodent model. RF and OC drafted the manuscript. RF, GS, AS, NK, SJF and OC reviewed, revised and approved the final manuscript. OC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- [1].Cryer PE (2008) The barrier of hypoglycemia in diabetes. Diabetes 57: 3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cryer PE (2008) Hypoglycemia: still the limiting factor in the glycemic management of diabetes. EndocrPract 14: 750–756 [DOI] [PubMed] [Google Scholar]
- [3].Cryer PE (2001) Hypoglycemia-associated autonomic failure in diabetes. AmJPhysiol EndocrinolMetab 281: E1115–E1121 [DOI] [PubMed] [Google Scholar]
- [4].Cryer PE (2004) Diverse causes of hypoglycemia-associated autonomic failure in diabetes. NEnglJMed 350: 2272–2279 [DOI] [PubMed] [Google Scholar]
- [5].Cryer PE (2005) Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 54: 3592–3601 [DOI] [PubMed] [Google Scholar]
- [6].Chan O, Sherwin R (2013) Influence of VMH fuel sensing on hypoglycemic responses. Trends EndocrinolMetab 24: 616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chenal J, Pellerin L (2007) Noradrenaline enhances the expression of the neuronal monocarboxylate transporter MCT2 by translational activation via stimulation of PI3K/Akt and the mTOR/S6K pathway. Journal of neurochemistry 102: 389–397 [DOI] [PubMed] [Google Scholar]
- [8].Pralong E, Magistretti PJ (1994) Noradrenaline reduces synaptic responses in normal and tottering mouse entorhinal cortex via alpha 2 receptors. Neuroscience letters 179: 145–148 [DOI] [PubMed] [Google Scholar]
- [9].Pierre K, Debernardi R, Magistretti PJ, Pellerin L (2003) Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. Journal of neurochemistry 86: 1468–1476 [DOI] [PubMed] [Google Scholar]
- [10].Barnes MB, Lawson MA, Beverly JL (2011) Rate of fall in blood glucose and recurrent hypoglycemia affect glucose dynamics and noradrenergic activation in the ventromedial hypothalamus. AmJPhysiol RegulIntegrComp Physiol 301: R1815–R1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Szepietowska B, Zhu W, Chan O, Horblitt A, Dziura J, Sherwin RS (2011) Modulation of beta-adrenergic receptors in the ventromedial hypothalamus influences counterregulatory responses to hypoglycemia. Diabetes 60: 3154–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Szepietowska B, Zhu W, Sherwin RS (2013) beta2-Adrenergic receptor agonist administration promotes counter-regulatory responses and recovery from hypoglycaemia in rats. Diabetologia 56: 2517–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].de Vries MG, Lawson MA, Beverly JL (2004) Dissociation of hypothalamic noradrenergic activity and sympathoadrenal responses to recurrent hypoglycemia. AmJPhysiol RegulIntegrComp Physiol 286: R910–R915 [DOI] [PubMed] [Google Scholar]
- [14].Figlewicz DP, Van Dijk G, Wilkinson CW, Gronbeck P, Higgins M, Zavosh A (2002) Effects of repetitive hypoglycemia on neuroendocrine response and brain tyrosine hydroxylase activity in the rat. Stress 5: 217–226 [DOI] [PubMed] [Google Scholar]
- [15].Ramanathan R, Cryer PE (2011) Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes 60: 602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morsy MA, Ibrahim SA, Amin EF, Kamel MY, Abdelwahab SA, Hassan MK (2014) Carvedilol ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Biomed Res Int 2014: 105214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun YL, Hu SJ, Wang LH, Hu Y, Zhou JY (2005) Comparison of low and high doses of carvedilol on restoration of cardiac function and calcium-handling proteins in rat failing heart. Clin Exp Pharmacol Physiol 32: 553–560 [DOI] [PubMed] [Google Scholar]
- [18].Ohta Y, Watanabe K, Nakazawa M, et al. (2000) Carvedilol enhances atrial and brain natriuretic peptide mRNA expression and release in rat heart. J Cardiovasc Pharmacol 36 Suppl 2: S19–23 [DOI] [PubMed] [Google Scholar]
- [19].Watanabe K, Ohta Y, Nakazawa M, et al. (2000) Low dose carvedilol inhibits progression of heart failure in rats with dilated cardiomyopathy. Br J Pharmacol 130: 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chan O, Zhu W, Ding Y, McCrimmon RJ, Sherwin RS (2006) Blockade of GABAA Receptors in the Ventromedial Hypothalamus Further Stimulates Glucagon and Sympathoadrenal but Not the Hypothalamo-Pituitary-Adrenal Response to Hypoglycemia. Diabetes 55: 1080–1087 [DOI] [PubMed] [Google Scholar]
- [21].Chan O, Lawson M, Zhu W, Beverly JL, Sherwin RS (2007) ATP-sensitive K(+) channels regulate the release of GABA in the ventromedial hypothalamus during hypoglycemia. Diabetes 56: 1120–1126 [DOI] [PubMed] [Google Scholar]
- [22].Osundiji MA, Hurst P, Moore SP, et al. (2011) Recurrent hypoglycemia increases hypothalamic glucose phosphorylation activity in rats. Metabolism 60: 550–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- [24].Geddes J, Schopman JE, Zammitt NN, Frier BM (2008) Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabetic medicine : a journal of the British Diabetic Association 25: 501–504 [DOI] [PubMed] [Google Scholar]
- [25].Geddes J, Wright RJ, Zammitt NN, Deary IJ, Frier BM (2007) An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care 30: 1868–1870 [DOI] [PubMed] [Google Scholar]
- [26].Sprague JE, Arbelaez AM (2011) Glucose counterregulatory responses to hypoglycemia. Pediatr Endocrinol Rev 9: 463–473; quiz 474-465 [PMC free article] [PubMed] [Google Scholar]
- [27].Sanders NM, Figlewicz DP, Taborsky GJ Jr., Wilkinson CW, Daumen W, Levin BE (2006) Feeding and neuroendocrine responses after recurrent insulin-induced hypoglycemia. Physiol Behav 87: 700–706 [DOI] [PubMed] [Google Scholar]
- [28].Sanders NM, Ritter S (2000) Repeated 2-deoxy-D-glucose-induced glucoprivation attenuates Fos expression and glucoregulatory responses during subsequent glucoprivation. Diabetes 49: 1865–1874 [DOI] [PubMed] [Google Scholar]
- [29].Sanders NM, Taborsky GJ Jr., Wilkinson CW, Daumen W, Figlewicz DP (2007) Antecedent hindbrain glucoprivation does not impair the counterregulatory response to hypoglycemia. Diabetes 56: 217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].de Vries MG, Lawson MA, Beverly JL (2005) Hypoglycemia-induced noradrenergic activation in the VMH is a result of decreased ambient glucose. American journal of physiology Regulatory, integrative and comparative physiology 289: R977–981 [DOI] [PubMed] [Google Scholar]
- [31].Bart J, Dijkers EC, Wegman TD, et al. (2005) New positron emission tomography tracer [(11)C]carvedilol reveals P-glycoprotein modulation kinetics. Br J Pharmacol 145: 1045–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].De Feyter HM, Mason GF, Shulman GI, Rothman DL, Petersen KF (2013) Increased brain lactate concentrations without increased lactate oxidation during hypoglycemia in type 1 diabetic individuals. Diabetes 62: 3075–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herzog RI, Jiang L, Herman P, et al. (2013) Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. JClinInvest 123: 1988–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chan O, Paranjape SA, Horblitt A, Zhu W, Sherwin RS (2013) Lactate-induced release of GABA in the ventromedial hypothalamus contributes to counterregulatory failure in recurrent hypoglycemia and diabetes. Diabetes 62: 4239–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wiegers EC, Rooijackers HM, Tack CJ, Heerschap A, de Galan BE, van der Graaf M (2016) Brain Lactate Concentration Falls in Response to Hypoglycemia in Patients With Type 1 Diabetes and Impaired Awareness of Hypoglycemia. Diabetes 65: 1601–1605 [DOI] [PubMed] [Google Scholar]
- [36].Wiegers EC, Rooijackers HM, Tack CJ, et al. (2017) Effect of Exercise-Induced Lactate Elevation on Brain Lactate Levels During Hypoglycemia in Patients With Type 1 Diabetes and Impaired Awareness of Hypoglycemia. Diabetes 66: 3105–3110 [DOI] [PubMed] [Google Scholar]
- [37].Wiegers EC, Rooijackers HM, Tack CJ, et al. (2018) Effect of lactate administration on brain lactate levels during hypoglycemia in patients with type 1 diabetes. J Cereb Blood Flow Metab: 271678X18775884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mason GF, Petersen KF, Lebon V, Rothman DL, Shulman GI (2006) Increased brain monocarboxylic Acid transport and utilization in type 1 diabetes. Diabetes 55: 929–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Catus SL, Gibbs ME, Sato M, Summers RJ, Hutchinson DS (2011) Role of beta-adrenoceptors in glucose uptake in astrocytes using beta-adrenoceptor knockout mice. Br J Pharmacol 162: 1700–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Subbarao KV, Hertz L (1990) Noradrenaline induced stimulation of oxidative metabolism in astrocytes but not in neurons in primary cultures. Brain Res 527: 346–349 [DOI] [PubMed] [Google Scholar]
- [41].Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. ProcNatlAcadSciUSA 91: 10625–10629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fray AE, Forsyth RJ, Boutelle MG, Fillenz M (1996) The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol 496 (Pt 1): 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gibbs ME, Hutchinson DS, Summers RJ (2008) Role of beta-adrenoceptors in memory consolidation: beta3-adrenoceptors act on glucose uptake and beta2-adrenoceptors on glycogenolysis. Neuropsychopharmacology 33: 2384–2397 [DOI] [PubMed] [Google Scholar]
- [44].Walls AB, Heimburger CM, Bouman SD, Schousboe A, Waagepetersen HS (2009) Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience 158: 284–292 [DOI] [PubMed] [Google Scholar]
- [45].Obel LF, Andersen KM, Bak LK, Schousboe A, Waagepetersen HS (2012) Effects of adrenergic agents on intracellular Ca2+ homeostasis and metabolism of glucose in astrocytes with an emphasis on pyruvate carboxylation, oxidative decarboxylation and recycling: implications for glutamate neurotransmission and excitotoxicity. Neurotox Res 21: 405–417 [DOI] [PubMed] [Google Scholar]
- [46].Pellerin L, Stolz M, Sorg O, Martin JL, Deschepper CF, Magistretti PJ (1997) Regulation of energy metabolism by neurotransmitters in astrocytes in primary culture and in an immortalized cell line. Glia 21: 74–83 [DOI] [PubMed] [Google Scholar]
- [47].Lee JG, Choi IS, Park EJ, et al. (2007) beta(2)-Adrenoceptor-mediated facilitation of glutamatergic transmission in rat ventromedial hypothalamic neurons. Neuroscience 144: 1255–1265 [DOI] [PubMed] [Google Scholar]
- [48].Moreira TJ, Pierre K, Maekawa F, et al. (2009) Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J Cereb Blood Flow Metab 29: 1273–1283 [DOI] [PubMed] [Google Scholar]
- [49].Hirsch IB, Boyle PJ, Craft S, Cryer PE (1991) Higher glycemic thresholds for symptoms during beta-adrenergic blockade in IDDM. Diabetes 40: 1177–1186 [DOI] [PubMed] [Google Scholar]
- [50].McGill JB, Bakris GL, Fonseca V, et al. (2007) beta-blocker use and diabetes symptom score: results from the GEMINI study. Diabetes, obesity & metabolism 9: 408–417 [DOI] [PubMed] [Google Scholar]
- [51].Poterucha JT, Bos JM, Cannon BC, Ackerman MJ (2015) Frequency and severity of hypoglycemia in children with beta-blocker-treated long QT syndrome. Heart Rhythm 12: 1815–1819 [DOI] [PubMed] [Google Scholar]
- [52].Barnett AH, Leslie D, Watkins PJ (1980) Can insulin-treated diabetics be given beta-adrenergic blocking drugs? Br Med J 280: 976–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kerr D, MacDonald IA, Heller SR, Tattersall RB (1990) Beta-adrenoceptor blockade and hypoglycaemia. A randomised, double-blind, placebo controlled comparison of metoprolol CR, atenolol and propranolol LA in normal subjects. Br J Clin Pharmacol 29: 685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cameron OG (1989) Beta-adrenergic blockade does not prevent hypoglycemia awareness in non-diabetic humans. Psychosom Med 51: 165–172 [DOI] [PubMed] [Google Scholar]
- [55].Giugliano D, Acampora R, Marfella R, et al. (1997) Metabolic and cardiovascular effects of carvedilol and atenolol in non-insulin-dependent diabetes mellitus and hypertension. A randomized, controlled trial. Ann Intern Med 126: 955–959 [DOI] [PubMed] [Google Scholar]
- [56].Park MJ, Guest CB, Barnes MB, et al. (2008) Blocking of beta-2 adrenergic receptors hastens recovery from hypoglycemia-associated social withdrawal. Psychoneuroendocrinology 33: 1411–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.