Abstract
A new bacterium, strain AT3T, was isolated by microbial culturomics from a faecal sample from a Frenchman after bariatric surgery. The isolate exhibited 96.6% 16S ribosomal RNA gene nucleotide sequence similarity with Anaerotruncus colihominis strain WAL 14565T = CCUG 45055T = CIP 107754T. Phenotypic and genomic characteristics showed that the new strain represents a novel species, for which the name Anaerotruncus massiliensis sp. nov. is proposed. The type strain is strain AT3T = CSUR P2007T = DSM 100567T.
Keywords: Anaerotruncus, human gut microbiome, microbial culturomics, obesity, taxonogenomics
Introduction
Obesity is a major public health problem [1], [2]. It increases the risk of metabolic diseases such as type 2 diabetes and cardiovascular diseases such as high blood pressure [3]. The global obesity rate has been steadily increasing since 1980 [4]. The influence of the gut microbiota in human health and disease has been revealed in the recent years. Recently obesity has been associated with gut microbiota dysbiosis [5], [6], [7].
The various treatment regimens proposed to treat obesity are mainly dietary measures as well as medical and surgical treatments. Bariatric surgery has been documented as one of the most effective treatments for obesity and has been associated with increased microbial diversity [8], [9]. We studied stool samples from obese patients before and after bariatric surgery using the microbial culturomics approach [10]. During this study, a novel Gram-negative anaerobic bacterium was isolated from a stool sample collected after bariatric surgery from an obese man.
Here we characterize a novel bacterial species, strain AT3, belonging to Anaerotruncus genus [11], together with the genome sequencing assembly, annotation and comparison. At the time we described this new species, the genus Anaerotruncus only contained one species, the type species Anaerotruncus colihominis strain WAL 14565T = CCUG 45055T = CIP 107754T [11].
Methods
The stool sample was collected from a 47-year-old obese Frenchman with a body mass index of 35.3 kg/m2 in November 2011. Written informed consent was obtained from the patient at the nutrition, metabolic disease and endocrinology service at La Timone Hospital, Marseille, France. The study and the assent procedure were approved by the local ethics committee of IFR 48 (approval 09-022, 2010). Stool samples were stored at −80°C after collection until use.
Growth of strain AT3 was performed in May 2015. Sterile stool extract was preincubated in a blood culture bottle enriched with rumen fluid and sheep's blood, then seeded on 5% sheep's blood Columbia agar (bioMérieux, Marcy l’Etoile, France) as described elsewhere [12]. Strain purification and identification were performed as previously described [12], [13].
The 16S ribosomal RNA (rRNA) gene sequencing of the strain AT3 was performed as previously reported [14] using the fD1-rP2 primers, a GeneAmp PCR System 2720 thermal cycler (Applied Biosystems; Thermo Fisher Scientific, Waltham, MA, USA) and an ABI Prism 3130-XL capillary sequencer (Applied Biosciences, Saint Aubin, France). For taxonomic assignment, CodonCode Aligner (CodonCode, Centerville, MA, USA) software was used to correct sequences, and BLASTn searches were performed by the National Center for Biotechnology Information (NCBI) web server (http://blast.ncbi.nlm.nih.gov.gate1.inist.fr/Blast.cgi) for neighbour search. Sequences were aligned using CLUSTAL W [15] and phylogenetic inferences were obtained using the maximum likelihood method [16] by MEGA 7 software [17].
The colonies' morphology and pigmentation were observed after cultivation of the strain on a 5% sheep's blood Columbia agar plate (bioMérieux) at 37°C in anaerobic condition for 48 hours. Phenotypic characteristic analysis of the strain was performed as previously described [12], [18]. The growth of the strain for temperature ranged from 25 to 55°C when assessed on sheep's blood–enriched Columbia agar (bioMérieux) under different atmospheric conditions. The pH growth of strain AT3 was assessed at various pH range from 6 to 8.5 under anaerobic condition at 37°C. The saltiness of the strain using various concentrations (5, 10, 50, 75 and 100 g/L) of NaCl on Schaedler agar with 5% sheep's blood (bioMérieux) under an anaerobic atmosphere at 37°C was also tested.
Biochemical assays using API Gallery strips—API ZYM, API 20A, API 50 CH and rapid ID 32 A (bioMérieux)—were performed according the manufacturer's instruction.
Cellular fatty acid methyl ester and carbohydrate metabolism end product of strain AT3 were assessed as previously reported [12]. Antimicrobial susceptibility of the strain was also tested according the European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommendation [19], [20] using 12 antibiotics.
The antimicrobial agent susceptibility of the strain was tested according the EUCAST recommendations [19], [20] using 12 antibiotics, including Etest strips for amikacin (0.016–256 μg/mL), vancomycin (0.016–256 μg/mL), imipenem (0.002–32 μg/mL), ceftriaxone (0.016–256 μg/mL), rifampicin (0.002–32 μg/mL), benzyl penicillin (0.002–32 μg/mL), amoxicillin (0.016–256 μg/mL), cefotaxime (0.002–32 μg/mL), metronidazole (0.016–256 μg/mL), minocycline (0.016–256 μg/mL), teicoplanin (0.016–256 μg/mL), erythromycin (0.016–256 μg/mL) and daptomycin (0.016–256 μg/mL) (bioMérieux).
Genomic DNA of strain AT3 was sequenced on MiSeq Technology (Illumina, San Diego, CA, USA) with the mate-pair strategy and assembly deployed as previously described [18], [21]. The genome annotation of strain AT3 was performed as previously described [12] and compared to the genomes of his relatives species. For the comparison, genomes of closest species were automatically retrieved from the 16S rRNA tree and thus, Anaerotruncus colihominis strain WAL 14565T (= CCUG 45055T = CIP 107754T) (ABGD00000000) [11], Hydrogenoanaerobacterium saccharovorans strain SW512T (= AS 1.5070T = JCM 14861T) (FOCG00000000) [22], Ruthenibacterium lactatiformans strain 585–1T (= DSM 100348T = VKM B-2901T) (JXXK00000000) [23], Acutalibacter muris strain KB18T (= DSM 26090T = KCTC 15540T) (CP021422) [24], Acetanaerobacterium elongatum strain Z7T (= JCM 12359T = AS 1.5012T) (FNID00000000) [25], Ruminococcus flavefaciens strain C94T (= ATCC 19208T) (JAEF00000000) [26] and Ruminococcus champanellensis strain 18P13T (= DSM 18848T = JCM 17042T) (FP929052) [27] were selected. For each selected genome, complete genome sequence, proteome genome sequence and Orfeome genome sequence were retrieved from NCBI's FTP. All proteomes were analysed with proteinOrtho [28]. Then for a couple of genomes, a similarity score was computed. This score was the mean value of nucleotide similarity of average genomic identity of orthologous gene sequences between the two genomes studied [29]. An annotation of the entire proteome was performed to define the distribution of functional classes of predicted genes according to the clusters of orthologous groups of proteins (using the same method as for the genome annotation). The genome of strain AT3 was locally aligned 2 by 2 using the BLAT algorithm [30], [31] against each the selected genomes previously cited. Digital DNA-DNA hybridization values were estimated from a generalized model [32]. Average amino acid identity was also calculated on the basis of the overall similarity between two genomic data sets of the online proteins (http://enve-omics.ce.gatech.edu/aai/index) [33], [34].
Results
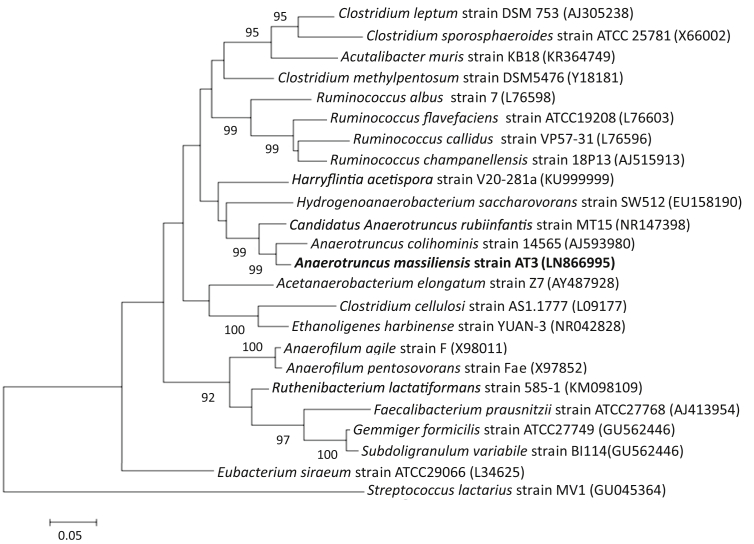
The spectrum generated from clean isolate spots was unable to match those in Bruker's and our own database, so the strain's 16S RNA gene was sequenced. BLASTn searches performed using the 16S rRNA gene sequences showed that strain AT3 exhibited 96.6% sequence similarity with A. colihominis strain WAL 14565T = CCUG 45055T = CIP 107754T [11], classified in the Ruminococcaceae family created by Rainey [35]. A maximum likelihood phylogenetic tree based on 16S rRNA gene sequences showed relationships between strain AT3 and some related taxa (Fig. 1). The numbers of nodes correspond to the percentages of bootstrap values obtained by repeating the analysis to 1000 times to generate a consensus tree.
Fig. 1.
16S rRNA-based maximum likelihood (ML) phylogenetic tree highlighting position of strain AT3 with closely related species. Corresponding GenBank accession numbers for 16S rRNA genes of each strain are indicated. Sequences were aligned using CLUSTAL W with default parameters and phylogenies were inferred by MEGA 7 software. Branches are scaled in terms of expected number of substitutions per site. Numbers above branches are support values when higher than 75% from ML bootstrapping. Scale bar represents a nucleotide sequence divergence of 2%.
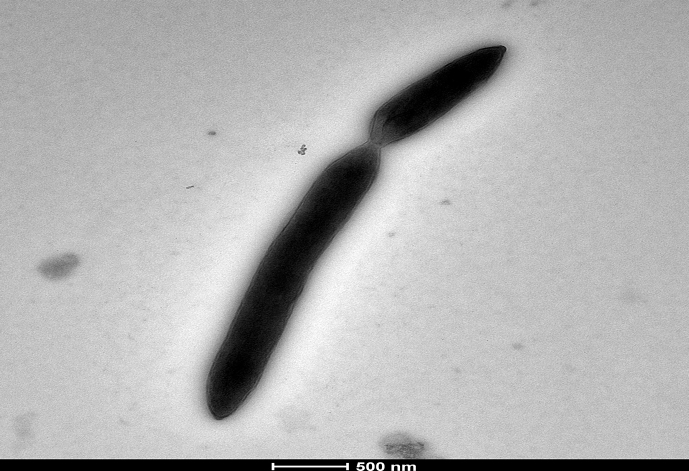
This new isolate was a Gram-negative–staining, nonmotile and rod-shaped bacterium. The strain was catalase positive but oxidase negative. The growth of the strain occurred between 28 to 45°C, but optimal growth was observed at 37°C after 72 hours' incubation in anaerobic atmosphere on Columbia agar with 5% sheep's blood (bioMérieux). No growth was observed in aerobic atmosphere. No growth of this bacterium was observed using 10 g/L of NaCl concentration on Schaedler agar (bioMérieux). The pH for growth ranged from 6 to 8.5, but the optimal pH was 7.5. The colonies were opalescent, with a mean diameter of 0.5 to 1.5 mm. Haemolysis was not observed on Columbia agar plate (bioMérieux). Cells had a diameter ranging from 0.3 to 2.9 μm by electron microscopy (Fig. 2).
Fig. 2.
Transmission electron microscopy of strain AT3 using a Tecnai G20 (FEI) at operating voltage of 60 kV. Scale bar, 500 μm.
Using the API 50 CH strip, positive reactions were observed with glycerol, erythritol, adonitol, methyl-βd-xylopyranoside, galactose, glucose, mannose, sorbitol, esculin, trehalose, inulin, glycogen, gentiobiose, tagatose and potassium 5–cetogluconate and starch, whereas arabinose, ribose, xylose, fructose, sorbose, rhamnose, dulcitol, inositol, mannitol, methyl-αd-mannopyranoside, methyl-αd-glucopyranoside, N-acetylglucosamine, amygdaline, arbutine, salicin, cellobiose, maltose, lactose, melibiose, saccharose, melezitose, raffinose, sylitol, turanose, lyxose, fucose, arabitol and potassium gluconate were negative. Using the API 20A strip, glucose, maltose, mannose, trehalose and rhamnose are fermented, while mannitol, lactose, saccharose, salicin, xylose, arabinose, glycerol, cellobiose, melezitose, raffinose, sorbitol and rhamnose are not. Aesculin and gelatine are hydrolyzed while urease and indole are not produced. Using the rapid ID 32 A strip, positive reactions were observed with alkaline phosphatase, arginine arylamidase, leucyl glycine arylamidase, phenylalanine arylamidase, leucine arylamidase, pyroglutamic acid arylamidase, tyrosine arylamidase, alanine arylamidase, glycine arylamidase, histidine arylamidase, glutamyl glutamic acid arylamidase, serine arylamidase and arginine dihydrolase. Mannose and raffinose are not fermented. Urease is not produced, while and indole are produced and nitrate is reduced. Using the API ZYM strip, positive reactions were observed with alkaline phosphatase, esterase (C-4), esterase lipase (C-8), acid phosphatase, naphthol-AS-BI-phosphohydrolase and α-glucosidase, while lipase, leucine arylamidase, valine arylamidase, cystine arylamidase, trypsin, α-chymotrypsin, α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, α-mannosidase and α-fructosidase were negative.
The major cellular fatty of the new strain were the anteiso-C15:0 (29%), C16:0 (20.6%) and C14:0 (19.5%). The different characteristics of strain AT3T compared to its closet species are listed in Table 1. The composition of cellular fatty acids for this isolate is shown in Table 2. The end products of carbohydrate metabolism of the strain AT3 after 72 hours' incubation in Wilkins Chalgren anaerobe broth (MilliporeSigma, St Louis, MO, USA) were acetic acid (12.3 ± 0.5 mM), butanoic acid (7.6 ± 2.0 mM), isobutanoic acid (1.4 ± 0.1 mM), isopentanoic acid (1.0 ± 0.2 mM) and isohexanoic acids (1.0 ± 0.1 mM).
Table 1.
Differential characteristics of strain AT3 compared to its closest species
| Properties | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Cells size (μm) | 0.3/2.9 | 0.5/2–5 | 0.3–0.4/2.0–14.5 | 1.6/0.4 | 0.2–0.4/4.0–8.0 | 0.8/0.9 | 0.9/1.3 | |
| Gram stain | — | + | _ | _ | — | + | + | + |
| Motility | — | — | _ | _ | — | + | — | — |
| Spore forming | — | — | — | _ | + | — | — | — |
| Aesculin hydrolysis | + | — | _ | + | + | + | — | + |
| Gelatine hydrolysis | + | — | _ | — | w | + | — | |
| Urease | — | — | — | — | — | — | NA | — |
| Indole | + | + | — | — | — | + | — | — |
| Catalase | + | — | — | — | — | — | — | — |
| Acid from: | ||||||||
| Arabinose | — | — | + | _ | — | + | + | — |
| Cellobiose | — | + | — | — | — | + | + | + |
| Glucose | + | + | + | + | — | + | — | — |
| Lactose | — | — | + | — | — | — | + | — |
| Maltose | v | + | NA | v | — | + | — | — |
| Mannose | v | + | + | — | — | — | — | — |
| Rafinose | — | — | + | _ | — | + | — | — |
| Sucrose | — | — | + | v | NA | + | — | — |
| Salicin | — | — | _ | v | — | + | — | — |
| Starch | + | — | — | + | — | — | NA | — |
| Trehalose | + | — | + | _ | — | — | — | — |
| Alkaline phosphatase | + | — | NA | — | + | w | NA | + |
| Major end product of carbohydrate metabolism | A, B, Ib, Ip, Ih | A, B | A, E | L, S, A | NA | A, E | A, F, E | A, S |
| G+C content (%) | 63.7 | 54 | 41.9 | 49.6 | 54.6 | 50.4 | 39–44 | 53.05 |
| Isolated from | Human faeces | Human faeces | UASB reactor | Human faeces | Human faeces | Human faeces | Human faeces | Human faeces |
+, positive result; −, negative result; v, variable result; w, weakly positive result; NA, data not available; UASB, upflow anaerobic sludge blanket; A, acetic acid; B, butyric acid; ib, isobutyric acid; Ip, isopentanoic acid; Ih, isohexanoic acid; L, lactic acid; E, ethanol; F, formic; S, succinic.
1, Strain AT3; 2, Anaerotruncus colihominis; 3, Hydrogenoanaerobacterium saccharovorans; 4, Ruthenibacterium lactatiformans; 5, Acutalibacter muris; 6, Acetanaerobacterium elongatum; 7, Ruminococcus flavefaciens, 8, Ruminococcus champanellensis. Data from [Lawson et al. 2004], [Song et al. 2004], [Shkoporov et al. 2016], [Lagkouvardos et al. 2016], [Chen et al. 2004] and [Chassard et al. 2012].
Table 2.
Cellular fatty acid composition of strain AT3
| Fatty acid | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Anteiso-C15:0 | 29.0 | NA | <1 | 9.8 | 6.04 | 19.56 |
| C16:0 | 20.6 | 29.1 | 6.6 | 22.1 | NA | NA |
| C14:0 | 19.5 | 15.6 | 2.2 | NR | 8.03 | NA |
| C18:1n9 | 5.6 | NA | 31.9 | 12.2 | NR | NA |
| Iso-C14:0 | 5.6 | NA | NA | NR | 32.11 | NA |
| Iso-C15:0 | 5.5 | 11.6 | NA | 16.8 | 42.83 | 26.57 |
NA, no data available. Values are percentages of total fatty acids.
1, Strain AT3; 2, Hydrogenoanaerobacterium saccharovorans; 3, Ruthenibacterium lactatiformans; 4, Acutalibacter muris; 5, Acetanaerobacterium elongatum; 6, Ruminococcus champanellensis. Data from [Song et al. 2004], [Shkoporov et al. 2016], [Lagkouvardos et al. 2016], [Chen et al. 2004] and [Chassard et al. 2012].
Antibiotic susceptibility tested according the EUCAST recommendation gave the following MIC results: >256, 0.125, 32, >256, 0.006, 0.016, 0.19, 0.016, 1.5, 0.125, 256 and 0.5 μg/mL, respectively, for amikacin, vancomycin, imipenem, ceftriaxone, rifampicin, benzyl penicillin, amoxicillin, metronidazole, minocycline, teicoplanin, erythromycin and daptomycin.
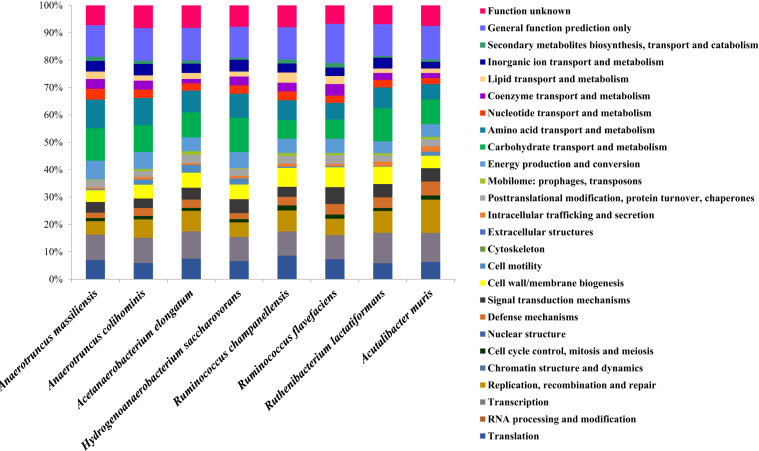
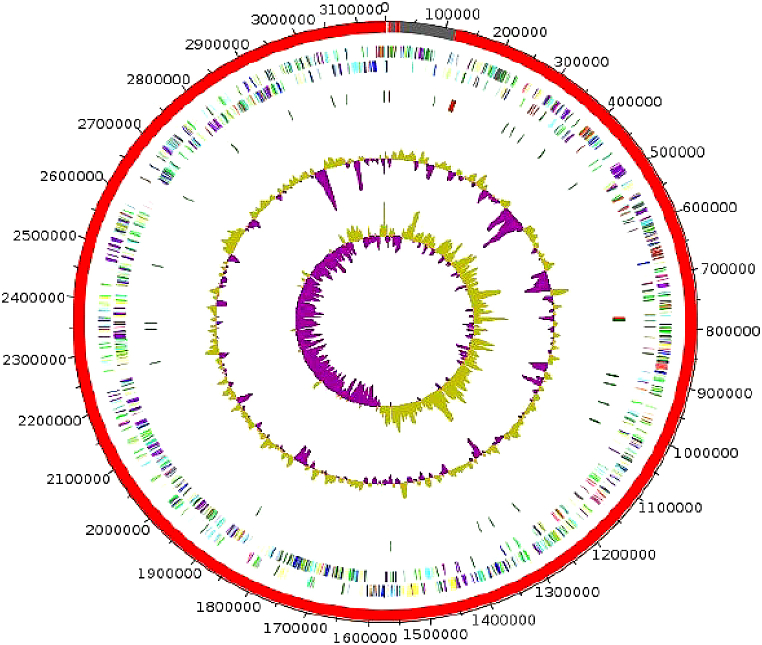
The draft genome of strain AT3 is 3 145 950 bp long with 63.6% G+C content (Fig. 3). It is composed of seven scaffolds (eight contigs). Within the 2927 predicted genes, 2868 were protein-coding genes and 59 were RNAs genes (four 5S rRNA genes, two 16S rRNA genes, two 23S rRNA genes and 51 tRNA genes). A total of 2106 genes (74.4%) were assigned to a putative function by COGs or by nonredundant blast. There were 398 genes (13.9%) associated with peptide signal proteins. ARG-ANNOT (Antibiotic Resistance Gene–Annotation) analysis found two genes (0.07%) associated with antimicrobial agent resistance [36] and four genes (0.14%) associated with polyketide synthase or nonribosomal peptide synthetases [37]. Using the online RAST Server version 2.0 tool, 20 genes were found to be associated with antibiotic resistance (four genes for fluoroquinolone resistance, four for tetracycline resistance and 12 for multidrug resistance efflux pumps), nine genes associated with invasion and intracellular resistance, five to copper homeostasis, nine to cobalt–zinc–cadmium resistance, two to mercuric reductase, one to mercury resistance operon, one to copper homeostasis:copper tolerance and one to cadmium resistance. No gene associated with virulence, toxins or bacteriocins has been found [38]. There were 193 genes (6.7%) identified as ORFans. The remaining 481 (16.8%) were annotated as hypothetical proteins. The distribution of genes into COGs functional categories of strain AT3 with its nearest neighbours' species is presented in Table 3 and Fig. 4. The numbers of orthologous proteins shared between genomes is shown in Table 4. The genome of the strain was deposited in EMBL-EBI (European Molecular Biology Laboratory–European Bioinformatics Institute) under accession number OEJM0000000. The digital DNA-DNA hybridization values of strain AT3 compared to its closely related species ranged from 21.5% with A. muris to 33.7% with R. champanellensis and are shown in Table 5. The average amino acid identity between the strain AT3 and closely related species was evaluated (Table 6). These values ranged from 47% with R. flavefaciens to 68.5% with A. colihominis.
Fig. 3.
Graphical circular map of genome of strain AT3. From outside to centre: Genes on forward strand coloured by COGs categories (only genes assigned to COGs), genes on reverse strand coloured by COGs categories (only gene assigned to COGs), RNA genes (tRNAs green, rRNAs red), GC content and GC skew. COGs, Clusters of Orthologous Groups database; rRNA, ribosomal RNA; tRNA, transfer RNA.
Table 3.
Number of genes associated with 26 general COGs functional categories of strain AT3T
| Code | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Description |
|---|---|---|---|---|---|---|---|---|---|
| J | 151 | 141 | 144 | 149 | 153 | 148 | 151 | 134 | Translation |
| A | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | RNA processing and modification |
| K | 201 | 222 | 191 | 282 | 256 | 195 | 181 | 138 | Transcription |
| L | 106 | 161 | 116 | 201 | 294 | 147 | 124 | 119 | Replication, recombination and repair |
| B | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | Chromatin structure and dynamics |
| D | 24 | 29 | 25 | 28 | 36 | 22 | 31 | 29 | Cell cycle control, mitosis and meiosis |
| Y | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Nuclear structure |
| v | 42 | 69 | 48 | 98 | 123 | 60 | 80 | 49 | Defence mechanisms |
| T | 85 | 84 | 111 | 124 | 119 | 85 | 127 | 57 | Signal transduction mechanisms |
| M | 93 | 121 | 116 | 160 | 109 | 107 | 149 | 107 | Cell wall/membrane biogenesis |
| N | 8 | 45 | 49 | 5 | 37 | 57 | 9 | 8 | Cell motility |
| Z | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Cytoskeleton |
| w | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Extracellular structures |
| U | 14 | 23 | 20 | 44 | 47 | 13 | 17 | 17 | Intracellular trafficking and secretion |
| O | 58 | 53 | 56 | 56 | 62 | 59 | 66 | 45 | Posttranslational modification, protein turnover, chaperones |
| X | 5 | 16 | 6 | 22 | 22 | 25 | 16 | 15 | Mobilome: prophages, transposons |
| C | 148 | 149 | 126 | 109 | 111 | 99 | 108 | 82 | Energy production and conversion |
| G | 255 | 236 | 272 | 307 | 219 | 179 | 144 | 105 | Carbohydrate transport and metabolism |
| E | 227 | 236 | 191 | 193 | 135 | 157 | 126 | 113 | Amino acid transport and metabolism |
| F | 85 | 74 | 66 | 71 | 53 | 51 | 54 | 51 | Nucleotide transport and metabolism |
| H | 77 | 78 | 71 | 64 | 45 | 33 | 87 | 49 | Coenzyme transport and metabolism |
| I | 59 | 46 | 40 | 41 | 40 | 43 | 61 | 58 | Lipid transport and metabolism |
| P | 83 | 101 | 93 | 99 | 59 | 66 | 64 | 51 | Inorganic ion transport and metabolism |
| Q | 33 | 28 | 16 | 19 | 21 | 21 | 34 | 24 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 249 | 284 | 245 | 292 | 296 | 236 | 295 | 182 | General function prediction only |
| S | 156 | 199 | 170 | 175 | 181 | 161 | 139 | 125 | Function unknown |
COGs, Clusters of Orthologous Groups database.
1, Strain AT3; 2, Anaerotruncus colihominis; 3, Hydrogenoanaerobacterium saccharovorans; 4, Ruthenibacterium lactatiformans; 5, Acutalibacter muris; 6, Acetanaerobacterium elongatum; 7, Ruminococcus flavefaciens; 8, Ruminococcus champanellensis.
Fig. 4.
Graphical circular map of chromosome. From outside to centre: Genes on forward strand coloured by COGs categories (only genes assigned to COGs), genes on reverse strand coloured by COGs categories (only gene assigned to COGs), RNA genes (tRNAs green, rRNAs red), GC content and GC skew. COGs, Clusters of Orthologous Groups database; rRNA, ribosomal RNA; tRNA, transfer RNA.
Table 4.
Numbers of orthologous proteins shared between genomes (upper right), average percentage similarity of nucleotides corresponding to orthologous protein shared between genomes (lower left) and numbers of proteins per genome (bold)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 | 2868 | 1240 | 1158 | 965 | 843 | 978 | 813 | 808 |
| 2 | 73.7% | 3656 | 1183 | 6433 | 3823 | 987 | 806 | 823 |
| 3 | 61.3% | 63.0% | 2932 | 954 | 855 | 1028 | 814 | 818 |
| 4 | 65.7% | 64.3% | 60.7% | 3908 | 818 | 847 | 847 | 758 |
| 5 | 64.5% | 62.5% | 59.8% | 63.8% | 3823 | 831 | 756 | 764 |
| 6 | 64.2% | 63.9% | 64.8% | 63.3% | 62.5% | 2746 | 815 | 816 |
| 7 | 62.4% | 60.8% | 62.4% | 59.9% | 60.5% | 62.3% | 3089 | 843 |
| 8 | 64.4% | 63.5% | 62.1% | 63.8% | 63.5% | 63.7% | 66.0% | 2356 |
1, Strain AT3; 2, Anaerotruncus colihominis; 3, Hydrogenoanaerobacterium saccharovorans; 4, Ruthenibacterium lactatiformans; 5, Acutalibacter muris; 6, Acetanaerobacterium elongatum; 7, Ruminococcus flavefaciens; 8, Ruminococcus champanellensis.
Table 5.
Pairwise comparison of strain AT3 with other species using GGDC, formula 2 (DDH estimates based on identities/HSP length)a
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 26.0% ± 2.4 | 26.4% ± 2.4 | 27.1% ± 2.4 | 21.5% ± 2.4 | 22.1% ± 2.4 | 22.6% ± 2.4 | 33.7% ± 2.4 |
| 2 | 100 | 27.3% ± 2.4 | 24.8% ± 2.4 | 21.1% ± 2.4 | 20.6% ± 2.4 | 36.0% ± 2.5 | 35.9% ± 2.4 | |
| 3 | 100 | 32.6% ± 2.45 | 34.3% ± 2.5 | 19.4% ± 2.4 | 28.0% ± 2.5 | 35.5% ± 2.5 | ||
| 4 | 100 | 19.1% ± 2.3 | 27.1% ± 2.4 | 27.7% ± 2.5 | 34.1% ± 2.5 | |||
| 5 | 100 | 22.0% ± 2.4 | 34.5% ± 2.5 | 22.0% ± 2.4 | ||||
| 6 | 100 | 34.1% ± 2.5 | 33.7% ± 2.5 | |||||
| 7 | 100 | 21.1% ± 2.4 | ||||||
| 8 | 100 |
DDH, DNA-DNA hybridization; GGDC, Genome-to-Genome Distance Calculator; HSP, high-scoring segment pairs; rRNA, ribosomal RNA.
1, Strain AT3; 2, Anaerotruncus colihominis; 3, Hydrogenoanaerobacterium saccharovorans; 4, Ruthenibacterium lactatiformans; 5, Acutalibacter muris; 6, Acetanaerobacterium elongatum; 7, Ruminococcus flavefaciens; 8, Ruminococcus champanellensis.
Confidence intervals indicate inherent uncertainty in estimating DDH values from intergenomic distances based on models derived from empirical test data sets. These results are consistent with 16S rRNA and phylogenomic analyses as well as GGDC results.
Table 6.
Average amino acid identity values of strain AT3 compared to its phylogenetically close neighbours
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 | 100 | 68.5% ± 18.5 | 54.0% ± 16.4 | 49.9% ± 15.6 | 48.1% ± 15.2 | 51.8% ± 16.5 | 47.0% ± 15.5 | 48.7% ± 15.6 |
| 2 | 100 | 52.9% ± 16.4 | 50.1% ± 16.7 | 46.9% ± 15.3 | 50.3% ± 16.2 | 45.9% ± 15.5 | 48.1% ± 15.5 | |
| 3 | 100 | 48.7% ± 15.6 | 46.4% ± 15.4 | 53.2% ± 16.6 | 47.3% ± 16.1 | 48.8% ± 16.1 | ||
| 4 | 100 | 47.2% ± 15.3 | 47.4% ± 15.6 | 44.2% ± 15.1 | 46.7% ± 14.8 | |||
| 5 | 100 | 46.4% ± 15.1 | 45.2% ± 15.0 | 46.4% ± 14.9 | ||||
| 6 | 100 | 47.3% ± 15.9 | 48.8% ± 15.8 | |||||
| 7 | 100 | 54.7% ± 17.9 | ||||||
| 8 | 100 |
1, Strain AT3; 2, Anaerotruncus colihominis; 3, Hydrogenoanaerobacterium saccharovorans; 4, Ruthenibacterium lactatiformans; 5, Acutalibacter muris; 6, Acetanaerobacterium elongatum; 7, Ruminococcus flavefaciens; 8, Ruminococcus champanellensis.
Discussion
When we compared the strain AT3 with the closest relative species differed by the catalase production, we found that his genome G+C content (63.7 vs. 41.9 to 54.6 for A. colihominis, H. saccharovorans, R. lactatiformans, A. muris, A. elongatum, R. flavefaciens and R. champanellensis, its closest relative species). The strain AT3 strain is phylogenetically close to A. colihominis but differs from it in Gram staining; A. colihominis is Gram staining positive while the new strain is Gram staining negative. The anteiso-C15:0 (29%) is one of the major cellular fatty acid for the new strain, but it is absent from A. colihominis.
On the basis of the phenotypic, chemotaxonomic, genomic and phylogenetic characteristics, a novel bacterium isolated from the stool sample of a morbidly obese Frenchman after bariatric surgery, termed Anaerotruncus massiliensis sp. nov., is proposed. The type strain is strain AT3T = CSURP P2007T = DSM 100567T. A preliminary report of main characteristics was previously published as a new species announcement [39], but the present study reports the full characterization of this putative new species.
Description of Anaerotruncus massiliensis sp. nov.
Anaerotruncus massiliensis (mas.si.li.en'sis, L. fem. adj., massiliensis, ’of Massilia,’ the Latin name of Marseille) is a Gram-negative-staining, catalase positive, oxidase negative, nonmotile non–spore-forming and anaerobic rod 0.3 μm wide and 2.9 μm long. Sheep's blood Columbia agar–grown colonies were opalescent with a mean diameter 0.5 to 1.5 mm after 72 hours' incubation in anaerobic atmosphere. Its optimum growth temperature was 37°C, pH tolerance ranges from pH 6 to 8.5 and maximal saltness for growth is 5 g/L of NaCl concentration. Using API strips, glycerol, erythritol, adonithol, methyl-βd-xylopyranoside, galactose, glucose, mannose, sorbitol, esculin, trehalose, inulin, glycogen, gentiobiose, tagatose and potassium 5-cetogluconate were positive. Using 50CH, glucose, maltose, mannose, trehalose, rhamnose were positive and aesculin and gelatin hydrolase was observed with API 20A strip; alkaline phosphatase, esterase (C-4), esterase lipase (C-8), acid phosphatase, naphthol-AS-BI-phosphohydrolase and α-glucosidase were positive with API ZYM strip; indole production, nitrate reduction, alkaline phosphatase, arginine arylamidase, leucyl glycine arylamidase, phenylalanine arylamidase, leucine arylamidase, pyroglutamic acid arylamidase, tyrosine arylamidase, alanine arylamidase, glycine arylamidase, histidine arylamidase, glutamyl glutamic acid arylamidase and serine arylamidase were positive with rapid ID 32 A strip. Its major cellular fatty acids are; anteiso-C15:0, C16:0 and C14:0. The genome of this strain is 3 145 950 bp long with 63.6% G+C content. The type strain, AT3T (= CSURP P2007T = DSM 100567T) was isolated from the stool sample of a French morbidly obese man after bariatric surgery. The 16S rRNA gene sequence and whole-genome shotgun sequence of strain AT3 were deposited in GenBank with accession numbers LN866995 and OEJM00000000, respectively.
Conflict of Interest
None declared.
Acknowledgements
The authors thank the Xegen Company (www.xegen.fr) for automating the genomic annotation process. Financial support received from the French government through the Agence Nationale pour la Recherche (ANR), including the ‘Programme d’Investissement d’Avenir’ under the reference Méditerranée Infection 10-IAHU-03. This work was supported by Région Provence-Alpes-Côte d'Azur and European funding FEDER PRIMMI (Fonds Européen de Développement Régional–Plateformes de Recherche et d’Innovation Mutualisées Méditerranée Infection).
References
- 1.Cecil J.E., Tavendale R., Watt P., Hetherington M.M., Palmer C.N.A. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359(24):2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 2.Frayling T.M., Timpson N.J., Weedon M.N., Zeggini E., Freathy R.M., Lindgren C.M. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fruh S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J Am Assoc Nurse Pract. 2017 Oct;29(S1):S3–S14. doi: 10.1002/2327-6924.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 6.Million M., Maraninchi M., Henry M., Armougom F., Richet H., Carrieri P. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes. 2012;36(6):817–825. doi: 10.1038/ijo.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Kong L.-C., Tap J., Aron-Wisnewsky J., Pelloux V., Basdevant A., Bouillot J.-L. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H., DiBaise J.K., Zuccolo A., Kudrna D., Braidotti M., Yu Y. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lagier J.-C., Hugon P., Khelaifia S., Fournier P.-E., La Scola B., Raoult D. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev. 2015 Jan;28(1):237–264. doi: 10.1128/CMR.00014-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawson P.A., Song Y., Liu C., Molitoris D.R., Vaisanen M.-L., Collins M.D. Anaerotruncus colihominis gen. nov., sp. nov., from human faeces. Int J Syst Evol Microbiol. 2004;54(Pt 2):413–417. doi: 10.1099/ijs.0.02653-0. [DOI] [PubMed] [Google Scholar]
- 12.Togo A.H., Durand G., Khelaifia S., Armstrong N., Robert C., Cadoret F. Fournierella massiliensis, gen. nov., sp. nov., a new human-associated member of the family Ruminococcaceae. Int J Syst Evol Microbiol. 2017;67:1393–1399. doi: 10.1099/ijsem.0.001826. [DOI] [PubMed] [Google Scholar]
- 13.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.-E., Rolain J.M. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin Infect Dis. 2009;49(4):543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 14.Drancourt M., Bollet C., Carlioz A., Martelin R., Gayral J.P., Raoult D. 16S ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol. 2000 Oct;38(10):3623–3630. doi: 10.1128/jcm.38.10.3623-3630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson J.D., Higgins D.G., Gibson T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diop K., Diop A., Khelaifia S., Robert C., Pinto F.D., Delerce J. Characterization of a novel Gram-stain-positive anaerobic coccus isolated from the female genital tract: Genome sequence and description of Murdochiella vaginalis sp. nov. Microbiology Open. 2018;7(3):e00570. doi: 10.1002/mbo3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Citron D.M., Ostovari M.I., Karlsson A., Goldstein E.J. Evaluation of the E test for susceptibility testing of anaerobic bacteria. J Clin Microbiol. 1991;29(10):2197–2203. doi: 10.1128/jcm.29.10.2197-2203.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matuschek E., Brown D.F.J., Kahlmeter G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin Microbiol Infect. 2014;20(4):O255–O266. doi: 10.1111/1469-0691.12373. [DOI] [PubMed] [Google Scholar]
- 21.Togo A.H., Khelaifia S., Lagier J.-C., Caputo A., Robert C., Fournier P.-E. Noncontiguous finished genome sequence and description of Paenibacillus ihumii sp. nov. strain AT5. New Microbes New Infect. 2016;10:142–150. doi: 10.1016/j.nmni.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song L., Dong X. Hydrogenoanaerobacterium saccharovorans gen. nov., sp. nov., isolated from H2-producing UASB granules. Int J Syst Evol Microbiol. 2009 Feb 1 [cited 2018 Nov 9];59(2):295–299. doi: 10.1099/ijs.0.000349-0. [DOI] [PubMed] [Google Scholar]
- 23.Shkoporov A.N., Chaplin A.V., Shcherbakova V.A., Suzina N.E., Kafarskaia L.I., Bozhenko V.K. Ruthenibacterium lactatiformans gen. nov., sp. nov., an anaerobic, lactate-producing member of the family Ruminococcaceae isolated from human faeces. Int J Syst Evol Microbiol. 2016 Aug 1;66(8):3041–3049. doi: 10.1099/ijsem.0.001143. [DOI] [PubMed] [Google Scholar]
- 24.Lagkouvardos I., Pukall R., Abt B., Foesel B.U., Meier-Kolthoff J.P., Kumar N. The Mouse Intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016 Oct;1(10) doi: 10.1038/nmicrobiol.2016.131. [DOI] [PubMed] [Google Scholar]
- 25.Chen S. Acetanaerobacterium elongatum gen. nov., sp. nov., from paper mill waste water. Int J Syst Evol Microbiol. 2004 Nov 1;54(6):2257–2262. doi: 10.1099/ijs.0.63212-0. [DOI] [PubMed] [Google Scholar]
- 26.Sijpesteijn A.K. Cellulose-decomposing bacteria from the rumen of cattle. Antonie Van Leeuwenhoek. 1949;15(1):49–52. [Google Scholar]
- 27.Chassard C., Delmas E., Robert C., Lawson P.A., Bernalier-Donadille A. Ruminococcus champanellensis sp. nov., a cellulose-degrading bacterium from human gut microbiota. Int J Syst Evol Microbiol. 2012 Jan;62(Pt 1):138–143. doi: 10.1099/ijs.0.027375-0. [DOI] [PubMed] [Google Scholar]
- 28.Lechner M., Findeiss S., Steiner L., Marz M., Stadler P.F., Prohaska S.J. Proteinortho: detection of (co-)orthologs in large-scale analysis. BMC Bioinformatics. 2011;12:124. doi: 10.1186/1471-2105-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramasamy D., Mishra A.K., Lagier J.-C., Padhmanabhan R., Rossi M., Sentausa E. A polyphasic strategy incorporating genomic data for the taxonomic description of novel bacterial species. Int J Syst Evol Microbiol. 2014;64(2):384–391. doi: 10.1099/ijs.0.057091-0. [DOI] [PubMed] [Google Scholar]
- 30.Auch A.F., von Jan M., Klenk H.-P., Göker M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genomic Sci. 2010;2(1):117–134. doi: 10.4056/sigs.531120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kent W.J. BLAT–the BLAST-like alignment tool. Genome Res. 2002 Apr;12(4):656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier-Kolthoff J.P., Göker M., Spröer C., Klenk H.-P. When should a DDH experiment be mandatory in microbial taxonomy? Arch Microbiol. 2013 Jun;195(6):413–418. doi: 10.1007/s00203-013-0888-4. [DOI] [PubMed] [Google Scholar]
- 33.Konstantinidis K.T., Tiedje J.M. Towards a genome-based taxonomy for prokaryotes. J Bacteriol. 2005 Sep 15;187(18):6258–6264. doi: 10.1128/JB.187.18.6258-6264.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-R L.M., Konstantinidis K.T. Bypassing cultivation to identify bacterial species: culture-independent genomic approaches identify credibly distinct clusters, avoid cultivation bias, and provide true insights into microbial species. Microbe Mag. 2014 Mar 1;9(3):111–118. [Google Scholar]
- 35.Rainey F.A. Family VIII. Ruminococcaceae fam. nov. In: De Vos P., Garrity G.M., Jones D., Krieg N.R., Ludwig W., Rainey F.A., editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Vol. 3. Springer; New York: 2010. pp. 1016–1043. [Google Scholar]
- 36.Gupta S.K., Padmanabhan B.R., Diene S.M., Lopez-Rojas R., Kempf M., Landraud L. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother. 2014;58(1):212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conway K.R., Boddy C.N. ClusterMine360: a database of microbial PKS/NRPS biosynthesis. Nucleic Acids Res. 2013;41(Database issue):D402–D407. doi: 10.1093/nar/gks993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overbeek R., Olson R., Pusch G.D., Olsen G.J., Davis J.J., Disz T. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) Nucleic Acids Res. 2014;42(Database issue):D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togo A.H., Valero R., Delerce J., Raould D., Million M. “Anaerotruncus massiliensis,” a new species identified from human stool from an obese patient after bariatric surgery. New Microbes New Infect. 2016 Nov;14:56–57. doi: 10.1016/j.nmni.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]