Circular plasmids are important tools for molecular manipulation in model fungi such as baker’s yeast, yet, in Candida albicans, an important yeast pathogen of humans, prior studies were not able to generate circular plasmids that were autonomous (duplicated without inserting themselves into the chromosome). Here, we found that linearizing circular plasmids with sequences from telomeres, the chromosome ends, allows the plasmids to duplicate and segregate in C. albicans. We used this system to identify chromosomal sequences that facilitate the initiation of plasmid replication (origins) and to show that an ∼100-bp fragment of a C. albicans origin and an origin sequence from a distantly related yeast can both function as origins in C. albicans. Thus, the requirements for plasmid geometry, but not necessarily for origin sequences, differ between C. albicans and baker’s yeast.
KEYWORDS: CaURA3, linear plasmids, replication, replication origins, telomere repeats
ABSTRACT
The ability to generate autonomously replicating plasmids has been elusive in Candida albicans, a prevalent human fungal commensal and pathogen. Instead, plasmids generally integrate into the genome. Here, we assessed plasmid and transformant properties, including plasmid geometry, transformant colony size, four selectable markers, and potential origins of replication, for their ability to drive autonomous plasmid maintenance. Importantly, linear plasmids with terminal telomere repeats yielded many more autonomous transformants than circular plasmids with the identical sequences. Furthermore, we could distinguish (by colony size) transient, autonomously replicating, and chromosomally integrated transformants (tiny, medium, and large, respectively). Candida albicans URA3 and a heterologous marker, ARG4, yielded many transient transformants indicative of weak origin activity; the replication of the plasmid carrying the heterologous LEU2 marker was highly dependent upon the addition of a bona fide origin sequence. Several bona fide chromosomal origins, with an origin fragment of ∼100 bp as well as a heterologous origin, panARS, from Kluyveromyces lactis, drove autonomous replication, yielding moderate transformation efficiency and plasmid stability. Thus, C. albicans maintains linear plasmids that yield high transformation efficiency and are maintained autonomously in an origin-dependent manner.
IMPORTANCE Circular plasmids are important tools for molecular manipulation in model fungi such as baker’s yeast, yet, in Candida albicans, an important yeast pathogen of humans, prior studies were not able to generate circular plasmids that were autonomous (duplicated without inserting themselves into the chromosome). Here, we found that linearizing circular plasmids with sequences from telomeres, the chromosome ends, allows the plasmids to duplicate and segregate in C. albicans. We used this system to identify chromosomal sequences that facilitate the initiation of plasmid replication (origins) and to show that an ∼100-bp fragment of a C. albicans origin and an origin sequence from a distantly related yeast can both function as origins in C. albicans. Thus, the requirements for plasmid geometry, but not necessarily for origin sequences, differ between C. albicans and baker’s yeast.
INTRODUCTION
Plasmids are autonomously replicating extrachromosomal elements that facilitate molecular studies in bacteria as well as in yeasts and other fungi (1). Some yeast species carry circular plasmids (e.g., 2µ in Saccharomyces cerevisiae [2]) or linear plasmids (e.g., killer plasmids in Kluyveromyces lactis [3] and mitochondrial plasmids in Fusarium oxysporum [4, 5]). Plasmid replication requires, among other components, an origin DNA sequence to which the origin recognition complex (ORC) binds. Origins of replication initiation (ORIs) on chromosomes and plasmids appear to have different sequence requirements in different yeast species (6). In S. cerevisiae, autonomously replicating sequences (ARSs; ORIs able to drive plasmid replication) are modular, requiring a minimum of 100 bp that includes an 11-bp ARS consensus sequence (ACS) (7–9) and a T-rich “B element” (10, 11). In most other organisms, the DNA requirements for centromere and ORI function are less well defined: K. lactis requires a 50-bp ACS that is necessary and sufficient for ARS activity (12), and Schizosaccharomyces pombe has no specific ARS consensus but requires a region of >500 bp with multiple A-T hook motifs that binds the ORC (13–15).
In Candida albicans, a common human fungal commensal and an opportunistic pathogen, ORIs have been only partially characterized. C. albicans origins, like those of S. pombe and higher eukaryotes, have longer and less well defined DNA motifs (16). Prior work with S. cerevisiae identified ARSs based on their high transformation efficiency (17, 18). Early studies found that ScARS plasmids with circular or linear geometry could be maintained autonomously for some time (19, 20). Work in C. albicans identified a few sequences that conferred high transformation efficiency on circular plasmids (21–27). However, either the resulting transformants were highly unstable (transient transformants) or the plasmid rapidly integrated into the genome (integrants). The CaURA3 marker used in many of these studies was later found to have an intrinsic weak ARS activity (28), and there was no direct evidence that replication initiated from the inserted sequences.
We previously used a machine learning approach to identify proposed-origins (pro-ORIs) based on ORC binding activity and nucleosome occupancy patterns (28). Four pro-ORIs were shown to be bona fide origins that produced replication bubble structures on nondenaturing 2-dimensional (2-D) DNA gels, thereby providing direct evidence of ORI function (28). Importantly, all four bona fide ORIs also drove plasmid replication on linear (but not circular) plasmids derived from circles carrying long inverted telomere (TEL) repeats separated by a spacer sequence that is cleaved to linearize the plasmid (29). These large plasmids with inverted telomere sequences could work well but were prone to rearrangement during propagation of the circular precursor plasmid in Escherichia coli.
Here, we compared circular and linear plasmids in C. albicans that rely on bona fide ORIs for their maintenance. Linear plasmids were constructed from circles de novo by PCR with primers bearing telomeric repeats prior to transformation. Linear plasmids consistently had higher transformation efficiency, larger numbers of autonomous transformants, and higher mitotic stability than analogous circular plasmids. Transformant colony size was a clear reflection of plasmid stability, with tiny colonies indicative of unstable, transient transformants; medium-size colonies indicative of autonomous transformants with moderate stability levels; and large, smooth colonies indicative of integrants, in which plasmid was inserted at chromosomal positions. We also tested four markers including CaURA3 and CaHIS1 as well as heterologous markers CdARG4 and CmLEU2 (30), which all had different levels of origin-dependent transformation efficiency and maintenance. Finally, we tested bona fide ORIs (28) as well as origin fragments and heterologous origin sequences and found that an ∼100-bp ORI fragment and a K. lactis panARS (31) have moderate origin activity in C. albicans.
RESULTS
Circular CaURA3 plasmids with and without ORIs.
In this study, we investigated the outcomes of transformations with plasmids that differed by selection markers, geometry (circular versus linear), and replication origins. We compared the transformation parameters, including transformation efficiency (number of transformants/µg of DNA), size of the transformant colonies (tiny [<0.4 mm], medium [0.4 to 1.6 mm], and large [>1.6 mm]; Table 1), mitotic stability (proportion of cells that retain the plasmid under selection), and plasmid loss rate (rate of plasmid loss per generation in the absence of selection). We used a set of isogenic strains that differed primarily in the relevant auxotrophy.
TABLE 1.
Properties of different types of transformants obtained with circular and linear plasmids
| Type of colony | Size category | Size (mm in diam) | Lag time (min) | Doubling time (min) | Relevant plasmid(s) | Mitotic stability (%) |
|---|---|---|---|---|---|---|
| Transient | Tiny | ≤0.4 | 174 ± 5 | 856 ± 3 | pCir/pLin-CaURA3 (±ORI410), pCir/pLin-CaHIS1 (±ORI410), pCir/pLin-CmLEU2 (±ORI410), pCir/pLin-CdARG4 (±ORI410) |
0 |
| Autonomous | Medium | 0.4–1.6 | 30 ± 10 | 140 ± 50 | pCir-CaHIS1 (±ORI410), pCir-CdARG4 (±ORI410), pCir-CmLEU2 (+ORI410) |
≤10 |
| Medium | 0.4–1.6 | 28 ± 6 | 42 ± 27 | pLin-CaURA3 (±ORI410), pLin-CaHIS1 (±ORI410), pLin-CdARG4 (±ORI410), pLin-CmLEU2 (±ORI410) |
10–40 | |
| Integrant | Large | 1.6–2.2 | 19 ± 2 | 25 ± 3 | pCir/pLin-CaHIS1 (±ORI410) | 80–100 |
Overall, across the markers and plasmids tested, three types of transformant colonies were evident. Tiny colonies that could not be maintained on selection (see Fig. S1A and B in the supplemental material) with undetectable plasmid retention (mitotic stability, ∼0) indicative of rapid plasmid loss were defined as transient transformants (referred to as transients here) (Table 1). Large, round colonies with short lag time and doubling time (Table 1; Fig. S1C) were defined as integrants based on their high stability under selection (mitotic stability, ∼80 to 100%). Medium colonies (mitotic stability, 1 to 80%) that grew, albeit less well than integrants under selection, with comparatively longer lag and doubling times (Table 1; Fig. S1D), were defined as autonomously replicating transformants (ARS-transformants), assuming that replicating plasmids can be maintained under selection and lost in the absence of selection. Accordingly, colony size can reliably predict the mitotic stability of plasmids and used as a proxy for the number of different transformant types.
Growth curves of transients obtained with pCir-CaURA3-ORI410 (A), transients obtained with pCir-CdARG4-ORI410 (B), integrants obtained with pCir-CdARG4-ORI410 (C), ARS-transformants obtained with pCir-CdARG4-ORI410 (D), and ARS-transformants obtained with pLin-CdARG4-ORI410 (E and F). The growth curves are representative of one colony of each type (the growth parameters averaged for three colonies of each type are listed in Table 1). Download FIG S1, PDF file, 0.08 MB (87.3KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To test the hypothesis that bona fide ORIs drive the autonomous replication of plasmids in C. albicans, we first constructed circular plasmids with the CaURA3 marker similar to those from prior studies (22, 23, 25–27) with and without bona fide ORI410 (28) (Fig. 1A). Transformation efficiency with and without ORI410 was relatively modest (17 and 9 transformants/µg DNA, respectively [Fig. 1B]). Importantly, all selected colonies were tiny (<0.4 mm), with and without inclusion of ORI410 (Fig. 1B). The tiny colonies did not grow upon restreaking or when seeded into liquid cultures (Fig. S1A), which is a characteristic of transients. Thus, as in several prior studies (22, 23, 25, 27), circular CaURA3 plasmids were not maintained autonomously.
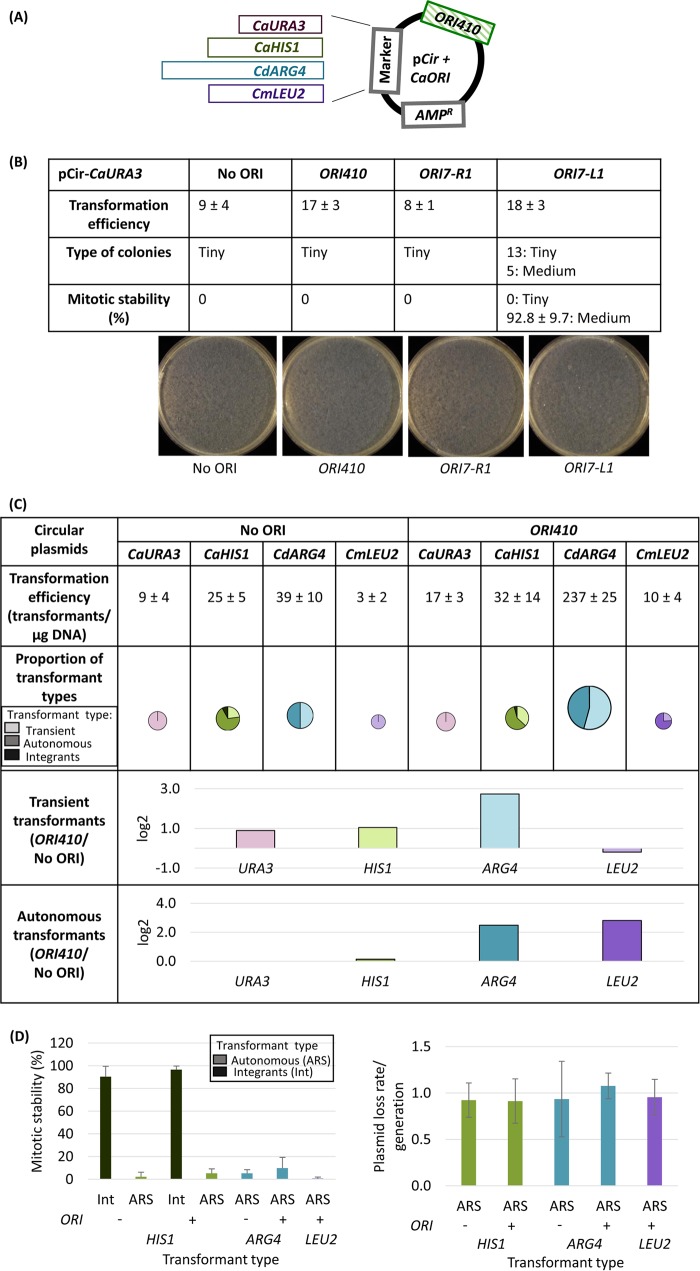
FIG 1.
(A) Map of a circular plasmid showing relative position of selection markers (CaURA3, CaHIS1, CdARG4, and CmLEU2) and ORI sequence. (B) pCir-CaURA3 plasmid with different origin sequences transformed in C. albicans BWP17: transformation efficiency, types of colonies, and their mitotic stability. The transformation efficiency is an average from three independent experiments. (C) Comparison of circular plasmids carrying different selection markers with and without ORI410: transformation efficiency, proportion of different types of transformants, and log2 value of the ratio of average number of transient or autonomous transformants with ORI to that without ORI (ORI410/No ORI). CaURA3, CaHIS1, CdARG4, and CmLEU2 plasmids were transformed in C. albicans BWP17, SN76, SN76, and SN152, respectively. Different markers are represented by different colors, and different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency is an average from three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (D) Mitotic stability (%) of integrants and ARS-transformants and plasmid loss rate/generation for ARS-transformants obtained with different circular plasmids with and without ORI410. The data represent the average from three independent colonies of each type. Int, integrants; ARS, ARS-transformants.
Because this result conflicts with the claim that two sequences, ORI7-R1 and ORI7-L1, drive the replication of a circular CaURA3 plasmid (26), we constructed plasmids with these sequences in pCir-CaURA3. Both of them had modest transformation efficiency (8 and 18 transformants, respectively; Fig. 1B). We obtained only transients for ORI7-R1 with similar transformation efficiency as the no-ORI plasmid; ORI7-L1 gave twice as many transients as no-ORI plasmid and produced a small number of stable transformants (Fig. 1B), indicating that they integrated into the genome. Thus, neither of the two CEN7 flanking sequences yielded autonomous transformants in the context of a circular CaURA3 plasmid (Fig. 1B), consistent with the poor performance of pCir-CaURA3-ORI410. Similar results for pCir-CaURA3 with ORI410, ORI7-L1, and ORI7-R1 were also evident in a second strain background (Table S1).
Transformation efficiency (average from three independent experiments) of pCir-CaURA3 with different origin sequences in C. albicans BWP17 and SN76. Download Table S1, PDF file, 0.1 MB (111.3KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of different selectable markers on circular plasmid.
We next asked if the C. albicans HIS1 (CaHIS1) marker would show better transformation efficiency and mitotic stability than CaURA3, with the goal of obtaining ARS-transformants. However, the CaHIS1 plasmid yielded small numbers of transformants (32 and 25, with and without ORI410, respectively), with a modest increase (∼25%) in transformation efficiency attributable to ORI410 (Fig. 1C). pCir-CaHIS1 ARS-transformants had mitotic stability of <5% and plasmid loss rates of ∼0.9 (Fig. 1D), indicating that they were autonomous but highly unstable. Thus, in addition to transients and integrants (analyzed in more detail below), pCir-CaHIS1 produced a small number of ARS-transformants—a group not detected with pCir-CaURA3 (Fig. 1C).
Because heterologous markers are less likely to integrate into the genome, we tested the CmLEU2 marker from Candida maltosa and the CdARG4 marker from Candida dubliniensis (30). With the addition of ORI410, transformation efficiency of CmLEU2 was increased by ∼3 times (Fig. 1C), and most of them were ARS-transformants with mitotic stability of <5% (Fig. 1D) compared to only transients without ORI410; no large colonies were detected. Thus, CmLEU2 produced a small number of ARS-transformants with low mitotic stability upon addition of ORI410.
In contrast, CdARG4 had a 5-fold-higher transformation efficiency with ORI410 on the plasmid relative to that without the ORI, ∼50% being ARS-transformants (Fig. 1C) that had mitotic stability of ∼10% for those with ORI410 and ∼5% for those without the ORI (Fig. 1D). Thus, CdARG4 with ORI410 yielded more than 100 ARS-transformants/µg of DNA, with an improved mitotic stability (but with the loss rate remaining quite high [Fig. 1D]). However, while ORI410 was required for high transformation efficiency and improved mitotic stability, it was not required for some autonomous plasmid replication. We suggest that the CdARG4 sequence might enable a weak ORI to form on the plasmid (discussed below). Thus, for circular plasmids with all four selectable markers tested, the inclusion of an origin was not sufficient to produce relatively stable autonomously replicating plasmids (low mitotic stability and high loss rate). This indicates that a heterologous marker can drive autonomous replication of a circular plasmid with rare integration events, but they are lost at high frequency.
We also asked if autonomous plasmids were detectable in DNA extracts from the medium colonies (low mitotic stability and high loss rate). Indeed, Southern blotting of DNA from medium colonies (Fig. S2A) detected bands with similar electrophoretic mobility as that of naked circular plasmids. In contrast, in the DNA from a pCir-CdARG4-ORI410 large colony with high mitotic stability (presumed integrant), a larger band was detected along with autonomously replicating plasmid (Fig. S2A), indicating integration in some cells in a population. This is consistent with the idea that large colonies contain integrated plasmid and medium colonies contain autonomously replicating plasmids. Moreover, analysis of the CaHIS1 integrants found gene replacement at the native locus by single crossover (Fig. S3A).
Southern blot of ApaI-digested DNA isolated from different transformants and probed with AMPR. (A) Circular plasmids: pCir-CaHIS1-ORI410 ARS-transformants (lanes 1 and 2), pCir-CdARG4-ORI410 integrant (lane 3), pCir-CdARG4-ORI410 ARS-transformants (lanes 4 and 5), pCir-CaHIS1-ORI410 control plasmid (lane 6), and pCir-CdARG4-ORI410 control plasmid (lane 7). All ARS-transformants gave a band corresponding to their control plasmid, indicating the existence of autonomously replicating plasmids. (B) Linear plasmids: pLin-CaHIS1-ORI410 and ARS-transformants with mitotic stability of 46% and 63% (lanes 1 and 2), pLin-CdARG4-ORI410 ARS-transformant with mitotic stability of 44% (lane 3), pLin-CaHIS1-ORI410 control plasmid (lane 4), and pLin-CdARG4-ORI410 control plasmid (lane 5). pLin-CaHIS1-ORI410 plasmids showed integration, whereas the pLin-CdARG4-ORI410 ARS-transformant gave a band corresponding to its control plasmid, indicating an autonomously replicating plasmid. Int, integrants; ARS, ARS-transformants. Download FIG S2, PDF file, 0.3 MB (334.5KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Schematic showing integration of pCir-CaHIS1-ORI410 into the genome by single crossover at either A or B. The integration was confirmed in three independent integrants by different primer sets indicated in the figure along with the size of PCR product expected. (B) Schematic showing integration of pLin-CaHIS1-ORI410 into the genome by a double-crossover or gene conversion event. The integration was confirmed in three independent integrants by different primer sets indicated in the figure along with the size of the PCR product expected. M, marker; C, parent strain C. albicans SN76; Int, integrants. Download FIG S3, PDF file, 0.2 MB (194.7KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Linear plasmids with telomere ends are maintained autonomously.
Since circular plasmids did not yield high transformation efficiency and high mitotic stability for ARS-transformants, we constructed and transformed linear plasmids, which are known to replicate autonomously in some fungal model organisms (4, 32–35). Since classical methods of producing linear plasmids (29) used for monitoring origin function in C. albicans (28) proved challenging, we designed a new approach in which linear plasmids were constructed from circular plasmids by PCR (details in Materials and Methods) (Fig. 2A) and transformed directly into C. albicans.
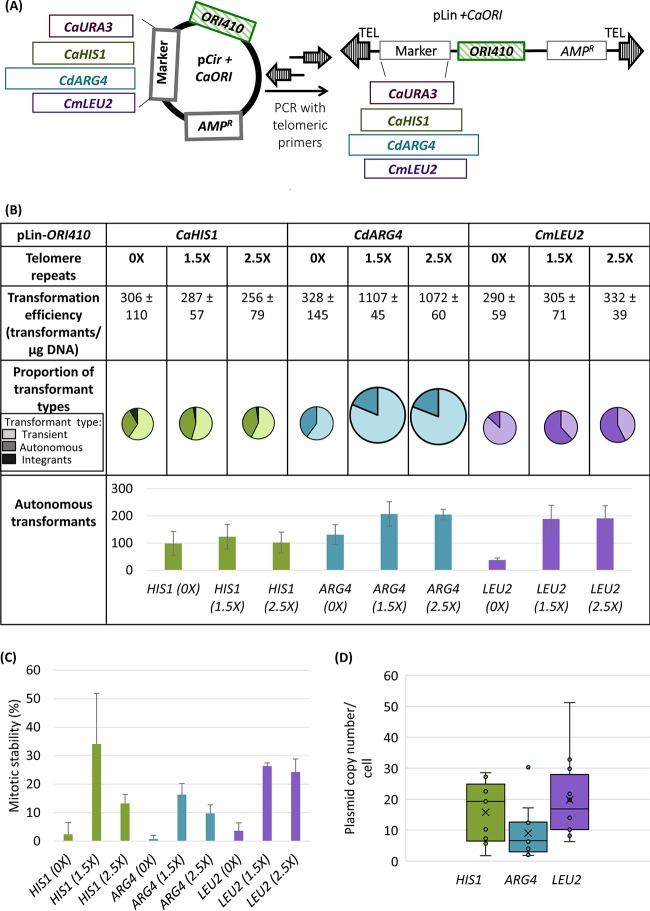
FIG 2.
(A) Schematic of construction of linear plasmid using primers with telomeric repeats at their ends. (B) Comparison of linear plasmids carrying different selection markers and ORI410 with 0×, 1.5×, and 2.5× telomere repeats at its ends: transformation efficiency, proportion of different types of transformants, and the number of autonomous transformants. CaHIS1 and CdARG4 plasmids were transformed in C. albicans SN76, and CmLEU2 plasmids were transformed in C. albicans SN152. Different markers are represented by different colors, and different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency and number of autonomous transformants are an average for three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (C) Mitotic stability (%) of ARS-transformants obtained with different linear plasmids with 0×, 1.5×, and 2.5× telomere repeats. The data represent the average for three independent ARS-transformants. (D) A box plot representing copy number variations of linear plasmids with CaHIS1, CdARG4, or CmLEU2 and ORI410. The data represent the average copy number of nine independent ARS-transformants (accounting for mitotic stability). In the box plot, dots represent different samples, crosses represent mean values, and the horizontal lines represent the medians.
Because telomere sequences are not necessary to be added to linear DNA during transformation in some fungal models (4, 32–34), we asked if the presence and the length of the telomere repeats (34-nt versus 57-nt TEL, i.e., 1.5× versus 2.5× of a single 23-nt C. albicans TEL repeat [36]) affect transformation parameters. Linear plasmids without TEL repeats had a transformation efficiency of ∼300/µg for all three markers tested (CaHIS1, CdARG4, and CmLEU2) with the majority being transients (Fig. 2B). The CaHIS1 linear plasmid without telomere repeats resulted in an increased number of total and ARS-transformants but also an increase in integration events over the corresponding circular plasmid (Fig. 2B versus Fig. 1C; P ≤ 0.025 and P ≤ 0.044 for total and ARS-transformants, respectively, using Student’s t test). Notably, the CmLEU2 linear plasmid without telomere repeats resulted in a much higher transformation efficiency and more ARS-transformants (∼50/µg DNA) than the corresponding circular plasmid (<10/µg DNA [Fig. 2B versus Fig. 1C]; P ≤ 0.007 and P ≤ 0.003 for total and ARS-transformants, respectively, using Student’s t test). The CdARG4 plasmid was an exception yielding similar transformation efficiencies in circular and linear plasmids without telomeres (Fig. 2B versus Fig. 1C). However, all of the ARS-transformants obtained had low mitotic stability (<5%) (Fig. 2C) with an irregular colony shape, indicating that they were not readily maintained in the autonomous state, and higher proportions of cells failed to divide in the colony under selection conditions.
Adding TEL repeats to linear plasmids increased the number of ARS-transformants for both CdARG4 and CmLEU2 plasmids, compared to those without TEL repeats (Fig. 2B; P ≤ 0.043 and P ≤ 0.018 for CdARG4 and CmLEU2 ARS-transformants, respectively, using Student’s t test). In contrast, TEL sequence addition increased the transformation efficiency only for CdARG4 plasmids among the three markers tested. Furthermore, adding TEL repeats increased the mitotic stability of ARS-transformants by 2- to 6-fold (mitotic stability, ∼10 to 35%) for all three markers. Thus, relative to circular plasmids, linearized plasmids with terminal TEL repeats produced more ARS-transformants with higher mitotic stability (Fig. 2B and C), and the ARS-transformants displayed shorter lag time and doubling time (Table 1; see also Fig. S1E and F). In contrast, when 1.5× TEL sequence was included on circular plasmids, there was no significant change in any of the transformation parameters relative to the corresponding circular plasmids (Fig. S4A and B). Thus, it is likely the linear geometry of the plasmids, along with the inclusion of telomere sequence, that resulted in an increase in ARS-transformants and mitotic stability (see Discussion). Since we found no obvious advantage to including 2.5× versus 1.5× TEL repeats, we used plasmids linearized with the 1.5× TEL repeats in all subsequent studies.
(A) Comparison of circular plasmid, linear plasmid (with 1.5× TEL repeats), and circular plasmid (with one 1.5× telomere repeat) using CmLEU2 marker transformed in C. albicans SN152: transformation efficiency, proportion of different types of transformants, and the number of autonomous transformants. Different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency and number of autonomous transformants are an average from three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (B) Mitotic stability (%) of ARS-transformants obtained with plasmids mentioned in panel A. The data represent the average from three independent ARS-transformants of each plasmid. Download FIG S4, PDF file, 0.2 MB (168.4KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We next asked if linear plasmids carrying 1.5× TEL repeats were maintained autonomously. The ARS-transformants obtained exhibited moderate mitotic stability even after three passages, indicating that they were maintained autonomously over a few generations (Table S2). Southern blotting of DNA from a pLin-CdARG4-ORI410 ARS-transformant with moderate mitotic stability (Fig. S2B) showed a single band with electrophoretic mobility similar to that of the naked linear DNA molecule used for transformation. We also recovered pLin-CmLEU2-ORI410 molecules from ARS-transformants in E. coli (Fig. S5), demonstrating autonomous replication in vivo. Copy number of the linear plasmids in ARS-transformants, measured by qPCR, ranged widely (∼2 to 50 per cell, accounting for mitotic stability) (Fig. 2D).
Steps for recovering pLin-CmLEU2-ORI410 autonomously replicating plasmids from C. albicans ARS-transformants in E. coli. Three independent ARS-transformants were analyzed by this method. C, pCir-CmLEU2-ORI410 plasmid control; M, marker. Download FIG S5, PDF file, 0.2 MB (176.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mitotic stability (averaged for three colonies) of ARS-transformants obtained with linear plasmids carrying ORI410 after passaging. Download Table S2, PDF file, 0.1 MB (108.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
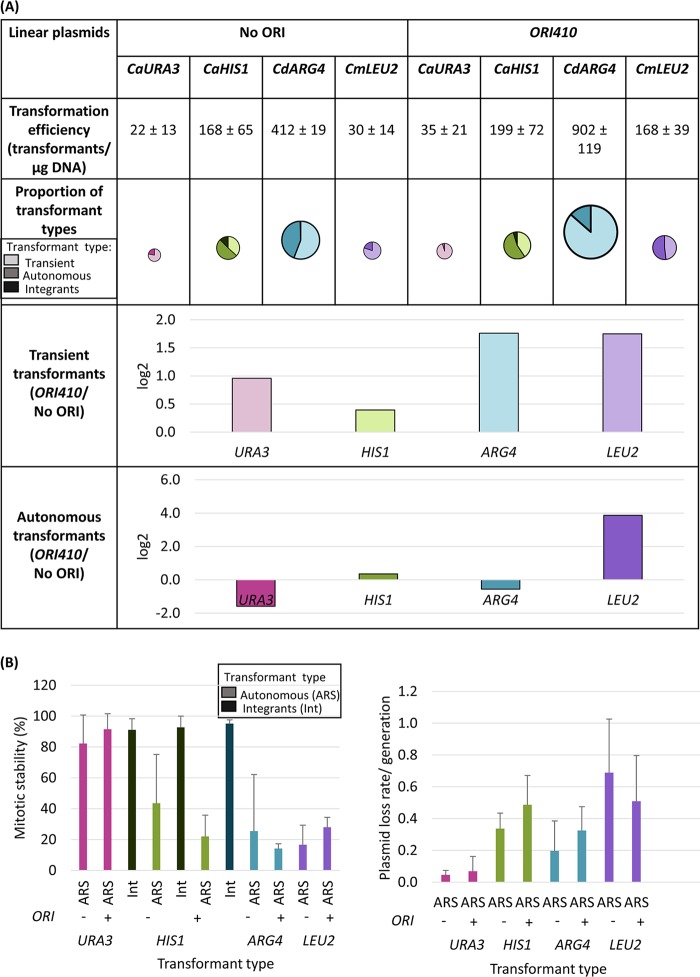
In contrast, CaHIS1 transformants with moderate mitotic stability (45% and 63%) produced larger plasmid-hybridizing bands on Southern blots indicative of genomic integration (Fig. S2B). Further analysis of these CaHIS1 integrants indicated gene replacement at the native locus by either double crossover or a gene conversion event (Fig. S3B). This is consistent with the idea that plasmids with a homologous marker can yield integrants apart from ARS-transformants.
Effect of marker gene and bona fide ORIs on transformation parameters.
We next asked to what extent a bona fide ORI sequence (ORI410) affected the transformation parameters of linear plasmids with different markers. With both homologous markers, CaURA3 and CaHIS1, there were some integration events, initially more frequent for CaHIS1, although when propagated under selection, many of the CaURA3 plasmids integrated (mitotic stability, ∼80 to 100%, and loss rate, <0.1 per generation; Fig. 3B). Furthermore, for CaURA3 and CdARG4, addition of ORI410 had no effect on the number of ARS-transformants (Fig. 3A) but improved plasmid stability compared to circular plasmids (Fig. 3B). This suggests that there may be a cryptic, intrinsic origin activity within CaURA3 and CdARG4 marker fragments (1.3 and 3.1 kb, respectively) that obviates the use of these markers to monitor the contribution of ORIs to plasmid replication and maintenance (discussed below). Similar results for CaHIS1 and CdARG4 were evident in different lab strains (Fig. S6). In contrast, addition of ORI410 on pLin-CmLEU2 resulted in an ∼5-fold increase in transformation efficiency, an ∼14-fold increase in ARS-transformants (Fig. 3A), and improved plasmid stability (Fig. 3B) relative to pLin-CmLEU2 (P ≤ 0.005 and P ≤ 0.025 for total and ARS-transformants, respectively, using Student’s t test), suggesting that CmLEU2 does not carry the intrinsic ARS activity seen on other markers.
FIG 3.
(A) Comparison of linear plasmids carrying different selection markers with and without ORI410: transformation efficiency, proportion of different types of transformants, and log2 value of the ratio of average number of transient or autonomous transformants with ORI to that without ORI (ORI410/No ORI). CaURA3, CaHIS1, CdARG4, and CmLEU2 plasmids were transformed in C. albicans BWP17, SN76, SN76, and SN152, respectively. Different markers are represented by different colors, and different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency is an average from three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (B) Mitotic stability (%) of integrants and ARS-transformants and plasmid loss rate/generation for ARS-transformants obtained with different linear plasmids with and without ORI410. The data represent the average from three independent colonies of each type except for ARS-transformants with CdARG4 and CmLEU2 plasmids, where they represent the average from six independent colonies. Int, integrants; ARS, ARS-transformants.
Comparison of pLin-CaHIS1-ORI410 (A) and pLin-CdARG4-ORI410 (B) transformation efficiency across different C. albicans strains. The transformation efficiency is an average from three independent experiments. Different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). Download FIG S6, PDF file, 0.6 MB (630.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Given that all markers were inserted in the same position on a plasmid, which does not have any obvious origin-promoting sequence features, we tested the hypothesis that some feature required for origin firing is present at higher levels in CaURA3, CaHIS1, and CdARG4 relative to CmLEU2, although many CaHIS1 ARS-transformants integrate into the genome after additional passages. Since the lengths of CaHIS1 and CmLEU2 are similar, it seems unlikely that marker length is an important factor. Interestingly, the AT content of the two markers CmLEU2 (62.3%) and CaHIS1 (63.3%) with higher levels of ORI-dependent ARS-transformants (Fig. 3A) was below the average AT content of the C. albicans genome (66.7%), while the AT content of CaURA3 (68.4%) and CdARG4 (69.1%) was higher than that of the C. albicans genome. Thus, it appears that the markers with cryptic ORI function (CaURA3 and CdARG4) that interferes with bona fide ORI activity have higher AT content. Of note, neither ORIs alone nor sequences on markers with possible cryptic ARSs share any obvious conserved primary sequence motifs. Based on its ORI dependence, CmLEU2 is the most effective of the markers tested for comparing origin activity.
Comparing different bona fide origins and ORI410 fragments.
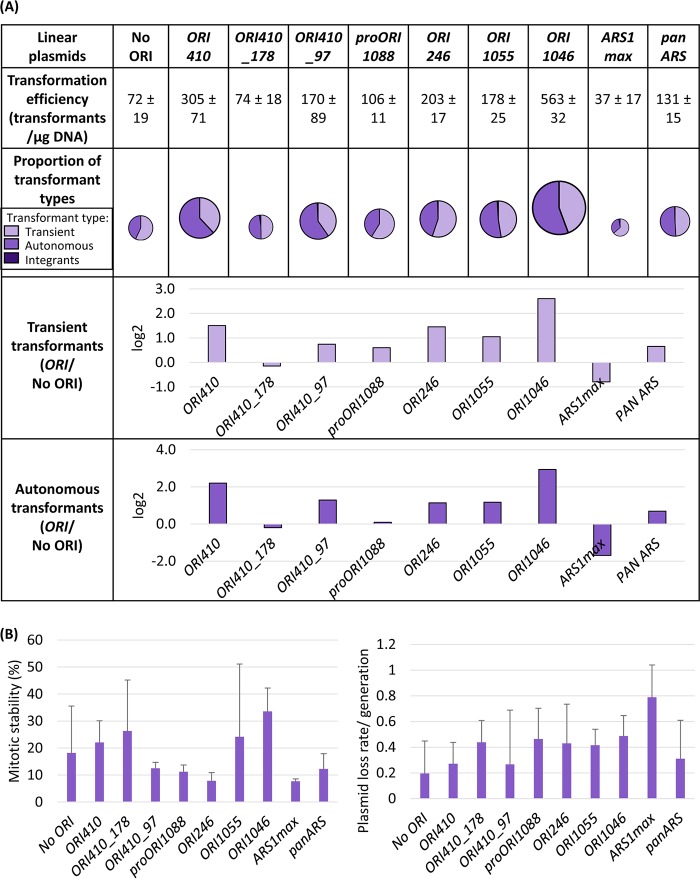
Four ORIs from C. albicans (ORI410 as well as ORI1055, ORI1046, and ORI246), defined previously as bona fide ORIs (28), were inserted into pLin-CmLEU2 to examine their function compared to no-origin plasmids. All four bona fide ORIs yielded high transformation efficiencies (∼150 to 600/µg DNA) and ARS-transformants (∼75 to 300/µg DNA) (Fig. 4A) with moderate mitotic stability (10 to 45%) and plasmid loss rate (0.2 to 0.7 per generation) (Fig. 4B). ORI1046 consistently yielded the highest transformation efficiency and ARS-transformants (∼300/µg DNA). Both negative-control plasmids, pLin-CmLEU2 (No ORI) and that with proORI1088, a genomic ORC binding region that did not produce replication bubble arcs in 2-D gels (28), gave much lower transformation efficiency and ARS-transformants (31 and 44/µg DNA). Thus, all four bona fide ORIs can drive the origin-dependent autonomous replication of pLin-CmLEU2 (Fig. 4A).
FIG 4.
(A) Comparison of linear plasmids carrying CmLEU2 marker with ORI410 fragments, different bona fide ORIs, and heterologous ORIs transformed in C. albicans SN152: transformation efficiency, proportion of different types of transformants, and log2 value of the ratio of average number of transient or autonomous transformants with ORI to that without ORI (ORI/No ORI). Different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency is an average from three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (B) Mitotic stability (%) and plasmid loss rate/generation for ARS-transformants obtained with different linear plasmids mentioned in panel A. The data represent the average from six independent ARS-transformants of each plasmid.
In S. cerevisiae, where the ACS is 11 bp, ARS function is detected only when the transforming fragment is ∼100 bp, including ACS (7, 8, 37, 38). We asked if two small overlapping fragments (178 bp and 97 bp) derived from ORI410 (1.2 kb) (28) were able to retain minimum ARS function in C. albicans. ORI410_97 had 2- to 3-times-higher transformation efficiency (∼170 transformants/µg DNA) and a ∼3-times-higher number of ARS-transformants than the no-ORI control (Fig. 4A). While the number of total and ARS-transformants for ORI410_97 was lower than for the entire ORI410, the ARS-transformants had moderate mitotic stability (10 to 15%) and loss rate (∼0.4 per generation) (Fig. 4B). Thus, an ORI fragment of only ∼100 bp can drive linear plasmid replication and can yield ARS-transformants, which are 2- to 3-fold more stable than an analogous circular plasmid carrying the entire ORI410 fragment.
Heterologous ARSs.
C. albicans centromeres are regional and epigenetic, which contrasts with the point centromeres of S. cerevisiae (16). Since plasmid replication and origin function were difficult to demonstrate in C. albicans, we asked whether heterologous ARS fragments would function in C. albicans. The “panARS,” a 452-bp fragment from the K. lactis genome, functions as an active ORI in a range of Saccharomycotina yeast species with diverse ARS requirements, in some cases, even more efficiently than average homologous ARSs (e.g., Pichia pastoris) (31). The “ARS1max,” an origin from S. cerevisiae, was selected to drive better growth rates and lower plasmid loss rates than the original ARS1 (39). Thus, we tested the ability of both the sequences to direct C. albicans plasmid replication on pLin-CmLEU2 (pLin-CmLEU2+panARS and pLin-CmLEU2+ARS1max) relative to pLin-CmLEU2+ORI410 and pLin-CmLEU2.
The pLin-CmLEU2+panARS plasmid resulted in ∼2- to 3-times-higher numbers of total and ARS-transformants relative to pLin-CmLEU2 (Fig. 4A). The panARS ARS-transformants had moderate mitotic stability (∼10 to 20%) and loss rate (∼0.3 per generation) that were comparable to ORI410_97 (Fig. 4B). This suggests that the sequence requirements of C. albicans origin function are at least partially conserved with those of K. lactis among other Saccharomycotina species. In contrast, pLin-CmLEU2+ARS1max had transformation parameters inferior to those of control plasmid pLin-CmLEU2: lower transformation efficiency, fewer ARS-transformants with the lowest mitotic stability (<8%), and the highest loss rate (∼0.8 per generation) (Fig. 4A and B) detected for any linear plasmid. This supports the idea that sequence requirements for origins in C. albicans (and other yeasts, for example, Pichia pastoris [40]) are distinct from those in S. cerevisiae.
DISCUSSION
Early studies seeking potential origin sequences based on their ability to confer high transformation efficiency usually used circular plasmids with CaURA3 as the selectable (and counterselectable) marker (22, 23, 25, 27). However, most transformants were highly unstable or rapidly integrated into the genome and thus were not useful for autonomous plasmid maintenance. Here we systematically compared four selectable markers in plasmids with circular or linear geometries to monitor the function of ORI sequences. Importantly, an ∼100-bp fragment (28) or the heterologous panARS (31) was sufficient to provide ARS function on a plasmid in C. albicans. This implies that sequence requirements for origin function in C. albicans are shared with distantly related yeasts. Nevertheless, the ability of cryptic ARSs on marker sequences to generate transient transformants implies that the sequence requirements for ARS function (and most likely chromosomal ORI function as well) are dependent on sequence context, possibly AT-richness, and other features that are not yet well understood.
An important insight from this work is that transformant colony size provides a useful preliminary indicator of plasmid mitotic stability. Presumably, colony size reflects the degree to which the plasmid replication and/or segregation enables growth of individual cells in a population under selective conditions. Specifically, in tiny or “pinpoint” colonies (25), plasmids are lost rapidly; in large colonies, plasmids are integrated stably (Table 1). In medium colonies, plasmids are moderately stable (Table 1) because they replicate autonomously, with some cells retaining the plasmid and others losing it. Of note, ARS-transformants with plasmids carrying homologous markers sometimes integrate in subsequent passages (generating larger colony subclones), a property less prevalent with the heterologous markers. Nonetheless, all markers on linear plasmids yield ARS-transformants, which initially can be identified based on colony size.
Circular versus linear plasmids.
In S. cerevisiae, linear plasmids and minichromosomes were used to study chromosome components and to propagate large segments of DNA (41, 42). However, most work was done with circular plasmids that are readily propagated in E. coli; propagation of linearizable plasmids with inverted telomere repeats (29, 43–45) was labor-intensive and subject to recombination of the repeats in E. coli. Here, a simple approach obviates many of these technical challenges by synthesizing linear plasmids from circles immediately prior to transformation (Fig. 2A). Thus, the two plasmid geometries are directly comparable, differing only by the presence or absence of 1.5× TEL repeats.
Does the presence of TEL sequence alone improve the segregation of linear versus circular plasmids? In C. albicans as in S. cerevisiae, adding TEL sequences to linear plasmids improves their stability (46) (Fig. 2C). However, adding TEL sequences does not improve circular plasmid segregation in C. albicans (see Fig. S4 in the supplemental material); by contrast, TEL sequences on circles stabilized ScARS plasmids (47) and antagonized the segregation of ScCEN plasmids (48). Thus, CaTEL sequence function is required for autonomous linear plasmid maintenance and is dependent upon its geometry: in a chromosome end context, but not within a circular context. This supports the idea that interactions between nonterminal TEL DNA and telomeric proteins likely differ between C. albicans and S. cerevisiae and that linear plasmids require telomere ends to remain stable.
In S. cerevisiae, noncentromeric plasmids are retained in the mother cells due to their attachment to the nuclear membrane (49) as well the presence of a diffusion barrier at the bud neck (50). It is tempting to speculate if this is also true for circular plasmids (with or without TEL) in C. albicans. Whether and how the linear plasmids might be better able to segregate to daughter cells remain to be explored.
Effect of selectable markers.
Comparison of the markers found that CaURA3 was not ideal, which explains difficulties in many earlier investigations (25, 26), and addition of LEU2 or HIS1 to CaURA3 plasmids relied on URA3 selection as well (22, 27). Studies selecting for IMH3R or CaADE2 found that putative ARS-transformants integrated at high frequency (21, 25). Sometimes integration events involved and/or altered the putative origin structure (24), and the resulting plasmids were not maintained autonomously. Notably, CaURA3 linear plasmid produced very few ARS-transformants, with or without ORI addition, and these eventually integrated into the genome (Fig. 3). In contrast, ARS-transformants with either CaHIS1, CdARG4, or CmLEU2 were maintained over three passages (Table S2).
We suggest the appearance of transient transformants cannot be used to define origin function on a plasmid, especially when the CaURA3 marker with latent origin activity is used. Therefore, transients seen with ORI7-L1 and -R1 cannot be used to draw conclusions about the function of these chromosomal regions as origins, especially since the published data lack a control plasmid containing the CaURA3 marker without an origin (26). Transient transformants with these origins have been used to postulate that centromere function required a preexisting origin. However, our results showing that these chromosomal regions do not act as origins, together with published neocentromere locations at chromosomal regions that did not contain preexisting origins (51, 52), support a model where kinetochore assembly can convert a nonorigin region to an origin. Furthermore, if many genome sequences can recruit replication factors and provide weak origin function on a plasmid as in S. pombe (14, 53), it is not surprising that sequences within neocentromere regions may recruit origins to new loci. The dramatically increased origin efficiency of the neocentromeric loci is likely due to neocentromere-mediated recruitment of replication initiation activities like Cdc7-Dbf4, which is normally found at wild-type centromeres (54).
Heterologous CdARG4 did not integrate frequently, yet, it gave high numbers of ARS-transformants in the absence of an added ORI. We posit that both CaURA3 and CdARG4 have weak intrinsic ARS activity and that this may compete with a bona fide ORI when both are on a plasmid. This suggests that C. albicans, like S. pombe, has “cryptic origins” (55), i.e., sites that are normally not used for replication initiation yet have the potential to form active replication origins. It also suggests that, once a cryptic origin has been established, it can continue to function, perhaps because, once well established in an ARS-transformant, a weak origin may be more likely to fire in the next cell cycle. The requirements for cryptic ARS function remain elusive. We cannot rule out the possibility that chromatin structure and topological constraints might affect ARS activity.
Why might inefficient ORIs interfere with bona fide ORI activity? In S. cerevisiae, two ORIs in close proximity in the genome interfere with each other (56). Three mechanisms were proposed to explain this: (i) timing of ORI firing might differ such that the nonfiring ORI is replicated passively, (ii) DNA at the two ORIs might interfere topologically (e.g., via altered supercoiling), or (iii) the two origins may compete for a limited number of licensing factors (e.g., ORC-associated proteins) (56). Interestingly, the orientation of ORC sites relative to one another could also be relevant (57), and all six predicted ORC sites (28) on CmLEU2 are oriented in the same direction, while predicted ORC sites (28) on the other three markers were found in both orientations. While mechanisms of CaORI and ScORI firing are likely to differ to some degree, these options may explain the phenomenon in C. albicans as well.
Most organisms do not have highly defined ARS consensus sequences, and it appears that this is the case in C. albicans as well. In S. pombe, ORIs have average AT contents ranging from 72 to 75% (58), with an average of 64% in the genome. CaURA3 and CdARG4 have 68.4% and 69.1%, respectively, with an average of 66.7% AT content in the genome. Furthermore, for all four markers on linear plasmids, the number of poly(A) tracts (≥3 nucleotides, normalized for marker length) correlated well (R2 = 0.85) with the number of transients obtained. This is consistent with the idea that AT-rich sequences and/or poly(A) tracts may attract replication factors and acquire cryptic ORI function. This, in turn, might interfere with bona fide ORI firing on the plasmid by mechanisms like those proposed for S. cerevisiae (56).
Testing origin function.
The linear CmLEU2 plasmid backbone provided the first opportunity to compare the efficiencies of different bona fide ORIs, ORI410-derived fragments (28), and heterologous origins (31, 39). All four bona fide ORIs yielded high numbers of ARS-transformants as well as moderate mitotic stability and loss rates (Fig. 4). We do not know why the 178-bp fragment, ORI410_178, had little or no obvious origin function while a smaller fragment derived from it (ORI410_97) was active. Clearly, DNA primary sequence is not sufficient to confer ORI function. We presume that sequence features together with their context relative to other plasmid components affect ORI activity, which has been seen on ScARS plasmids as well (59). Importantly, the synthetic panARS, which was derived from K. lactis (31), had transformation parameters similar to those of ORI410_97. Thus, the requirements for replication origin function in C. albicans are at least partially conserved with other Saccharomycotina species, and panARS provides a heterologous ORI that should not integrate into the genome. We suggest that it might be possible to whittle down the 452-bp panARS to generate a relatively good heterologous ORI of ∼100 bp.
Summary.
ARS function can be studied in C. albicans using a heterologous marker and a bona fide ORI of as small as ∼100 bp or the heterologous panARS on linear plasmids carrying 1.5× TEL ends. Importantly, linear plasmid conformation greatly facilitates transformation efficiency and mitotic stability. Unexpectedly, the choice of selectable marker has a major effect on the degree to which plasmids are maintained autonomously. To date, CmLEU2 is the single marker that has a low level of intrinsic cryptic origin activity and rare integration events, making it ideal for studying origin activity on a plasmid. The linear plasmids described here fill a major gap in the tools available for conventional molecular manipulations of C. albicans and will facilitate our ability to study molecular aspects of ORI, telomeric, and centromeric structure and function.
MATERIALS AND METHODS
Strains, plasmids, primers, and growth conditions.
Yeast strains and plasmids used are listed in Tables 2 and 3, respectively. Primers used are provided in Table 4.
TABLE 2.
List of strains used in the study
| Strain no. | C. albicans strain | Genotype | Reference | Gene(s) used with |
|---|---|---|---|---|
| YJB-T 45 | BWP17 | ura3::λimm434/ura3::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | 65 | CaURA3 |
| YJB-T 72 | SN76 | ura3-iro1::imm434/ura3-iro1::imm434 his1::hisG/his1::hisG arg4Δ/arg4Δ | 30 | CaHIS1, CdARG4 |
| YJB-T 736 | SN152 | ura3-iro1::imm434/URA3-IRO1 his1::hisG/his1::hisG arg4Δ/arg4Δ leu2Δ/leu2Δ | 66 | CmLEU2 |
| YJB-T 65 | SN95 | ura3-iro1::imm434/URA3-IRO1 his1::hisG/his1::hisG arg4Δ/arg4Δ | 30 | CaHIS1, CdARG4 |
TABLE 3.
List of plasmids used in the study
| Plasmid no. | Description | Reference or source |
|---|---|---|
| BJB-T1 | pGEM-URA3 | 65 |
| BJB-T226 | pGEM-URA3-ORI410 | This study |
| BJB-T2 | pGEM-HIS1 | 65 |
| BJB-T140 | pGEM-HIS1-ORI410 | This study |
| BJB-T391 | pGEM-CdARG4 | This study |
| BJB-T234 | pGEM-CdARG4-ORI410 | This study |
| BJB-T230 | pGEM-CmLEU2 | This study |
| BJB-T231 | pGEM-CmLEU2-ORI410 | This study |
| BJB-T398 | pGEM-CmLEU2-ORI410_178 | This study |
| BJB-T399 | pGEM-CmLEU2-ORI410_97 | This study |
| BJB-T400 | pGEM-CmLEU2-proORI1088 | This study |
| BJB-T401 | pGEM-CmLEU2-ORI246 | This study |
| BJB-T402 | pGEM-CmLEU2-ORI1055 | This study |
| BJB-T403 | pGEM-CmLEU2-ORI1046 | This study |
| BJB-T404 | pGEM-CmLEU2-ARS1max | This study |
| BJB-T405 | pGEM-CmLEU2-panARS | This study |
| BJB-T227 | pGEM-URA3-ORI7-R1 | This study |
| BJB-T228 | pGEM-URA3-ORI7-L1 | This study |
TABLE 4.
List of primers used in the study
| Primer no. |
Primer sequence (5′–3′)a | Purpose |
|---|---|---|
| BP196 | aggcaatagcatttccatctggtttcttgtcgaccatatgGGAACATCTGAAATTGGTTC | Primer to amplify ORI410 to clone in pGEM-CaHIS1 |
| BP197 | gaatactcaagctatgcatccaacgcgttgggagctctccTTGATGATTGGATCGGGTTC | Primer to amplify ORI410 to clone in pGEM-CaHIS1 |
| BP1266 | gcatgctcccggccgccatggccgcgggatGTAACGGCCGCCAGTGTG | Primer to amplify CdARG4 and CmLEU2 for cloning in pGEM and pGEM-ORI410 |
| BP1267 | catccaacgcgttgggagctctcccatatgCCAGTGTGATGGATATCTGCAG | Primer to amplify CdARG4 and CmLEU2 for cloning in pGEM and pGEM-ORI410 |
| BP1262 | CATATGGGAGAGCTCCCAACGCGTTG | Forward primer to amplify pGEM backbone from pGEM-CaHIS1 to clone CdARG4 and CmLEU2 |
| BP1265 | CATATGGGAACATCTGAAATTGGTTCTTTGGTAGATCTGCC | Forward primer to amplify pGEM-ORI410 backbone from pGEM-CaHIS1-ORI410 to clone CdARG4 and CmLEU2 |
| BP1263 | ATCCCGCGGCCATGGCGG | Reverse primer to amplify pGEM backbone from pGEM-CaHIS1 and pGEM-CaHIS1-ORI410 to clone CdARG4 and CmLEU2 |
| BP1246 | GTCGACCTGCAGGCGGCC | Primer to amplify pGEM-CaURA3 to clone ORI |
| BP1247 | GGAGAGCTCCCAACGCGTTG | Primer to amplify pGEM-CaURA3 to clone ORI |
| BP1248 | aatcactagtgcggccgcctgcaggtcgacTTGTAGATTTCAAAAATGCTTC | Primer to clone ORI7-L1 in pGEM-CaURA3 |
| BP1249 | gctatgcatccaacgcgttgggagctctccGATTTGTGTGTGCTTACTAGAG | Primer to clone ORI7-L1 in pGEM-CaURA3 |
| BP1250 | aatcactagtgcggccgcctgcaggtcgacTTGTGTAGTAAAGGGTTGTTG | Primer to clone ORI7-R1 in pGEM-CaURA3 |
| BP1251 | gctatgcatccaacgcgttgggagctctccAGTTAGGAAGAGTATAAATATGTGTAGTC | Primer to clone ORI7-R1 in pGEM-CaURA3 |
| BP1198 | ttctgcagatatccatcacactggcatatgACAAAAAATCATTAGCAAAATATTC | Primer to amplify ORI410_178 to clone in pGEM-CmLEU2 |
| BP1199 | gctatgcatccaacgcgttgggagctctccCCAGTGGAATTTGCAACC | Primer to amplify ORI410_178 to clone in pGEM-CmLEU2 |
| BP1200 | ttctgcagatatccatcacactggcatatgACTTTCAGAAATTGGTTGG | Primer to amplify ORI410_97 to clone in pGEM-CmLEU2 |
| BP1201 | gctatgcatccaacgcgttgggagctctccACACAAAAAATCATTAGCAAAATATTC | Primer to amplify ORI410_97 to clone in pGEM-CmLEU2 |
| BP1214 | ttctgcagatatccatcacactggcatatgAGCAGTTTTAAAATAAATAGGG | Primer to amplify proORI1088 to clone in pGEM-CmLEU2 |
| BP1215 | gctatgcatccaacgcgttgggagctctccTTGGATTATCAAAAAATCATTAG | Primer to amplify proORI1088 to clone in pGEM-CmLEU2 |
| BP1194 | ttctgcagatatccatcacactggcatatgTGTTGCAAAATATGAGTAAAAAAA | Primer to amplify ORI246 to clone in pGEM-CmLEU2 |
| BP1195 | gctatgcatccaacgcgttgggagctctccACAACGGAGGGTAAGGTG | Primer to amplify ORI246 to clone in pGEM-CmLEU2 |
| BP1192 | ttctgcagatatccatcacactggcatatgTGGTTATGTACTTGATCACCC | Primer to amplify ORI1055 to clone in pGEM-CmLEU2 |
| BP1193 | gctatgcatccaacgcgttgggagctctccTACAGAATGAGTAATATACAATGTTTG | Primer to amplify ORI1055 to clone in pGEM-CmLEU2 |
| BP1196 | ttctgcagatatccatcacactggcatatgATATATTTGTGATTCAACCACAC | Primer to amplify ORI1046 to clone in pGEM-CmLEU2 |
| BP1197 | gctatgcatccaacgcgttgggagctctccCAAAAATATCTCGTGAATCTTTTC | Primer to amplify ORI1046 to clone in pGEM-CmLEU2 |
| BP1186 | ttctgcagatatccatcacactggcatatgCACATGTTAAAATAGTGAAGGAG | Primer to amplify ARS1max to clone in pGEM-CmLEU2 |
| BP1187 | gctatgcatccaacgcgttgggagctctccAAAGCTTACATTTTATGTTAGCTG | Primer to amplify ARS1max to clone in pGEM-CmLEU2 |
| BP1188 | ttctgcagatatccatcacactggcatatgTCAACATCTTTGGATAATATCAG | Primer to amplify panARS to clone in pGEM-CmLEU2 |
| BP1189 | gctatgcatccaacgcgttgggagctctccTAGTGCTGATTATGATTTGACG | Primer to amplify panARS to clone in pGEM-CmLEU2 |
| BP1179 | CATATGCCAGTGTGATGGATATCTG | Primer to amplify pGEM-CmLEU2 to clone ORIs |
| BP1180 | GGAGAGCTCCCAACGCGT | Primer to amplify pGEM-CmLEU2 to clone ORIs |
| BP1204 | ACTGGCCGTCGTTTTACA | Primer to amplify linear plasmids without TEL |
| BP1205 | GAATTGTAATACGACTCACTATAGGG | Primer to amplify linear plasmids without TEL |
| BP1252 | CCGTACACCAAGAAGTTAGACATCCGTACACCAActtaagggatccgcatgctcccggccgccatg | Primer to amplify linear plasmids with 1.5× TEL repeat |
| BP1253 | CCGTACACCAAGAAGTTAGACATCCGTACACCAActtaagggatccgggcccaattcgccctatag | Primer to amplify linear plasmids with 1.5× TEL repeat |
| BP1254 | CCGTACACCAAGAAGTTAGACATCCGTACACCAAGAAGTTAGACATCCGTACACCAA
cttaagggatccgcatgctcccggccgccatg |
Primer to amplify linear plasmids with 2.5× TEL repeats |
| BP1255 | CCGTACACCAAGAAGTTAGACATCCGTACACCAAGAAGTTAGACATCCGTACACCAA
cttaagggatccgggcccaattcgccctatag |
Primer to amplify linear plasmids with 2.5× TEL repeats |
| BP1843 | CAAGGCGAGTTACATGATCC | Primer to amplify AMPR for qPCR |
| BP1844 | GGATGGCATGACAGTAAGAG | Primer to amplify AMPR for qPCR |
| BP285 | TTTGTACTTAGCGGCTACCTG | Primer to amplify chromosome 1L CEN for qPCR |
| BP317 | GAAAGAAGTGGGAGGAAAGGG | Primer to amplify chromosome 1L CEN for qPCR |
| BP1869 | CATGTATGGTAATCCAAATGGG | Forward primer that anneals outside 5′ UTR of CaHIS1 |
| BP1870 | AACACGGTGCACCAGTC | Reverse primer that anneals outside 3′ UTR of CaHIS1 |
| BP1841 | GGCTGGCTGGTTTATTGC | Reverse primer that anneals to AMPR gene |
| BP1873 | GGTAATGTAATGGACGAATTGAAG | Forward primer that anneals within CaHIS1 |
| BP1857 | CAACCTGGGTATTGATATGTTG | Reverse primer that anneals to CmLEU2 promoter |
Sequences in lowercase indicate regions homologous to the plasmid.
C. albicans strains were grown at 30°C in YPAD medium (60) or SD minimal medium or SD-Complete medium (60) containing leucine at 170 mg/liter and all other amino acids (Sigma-Aldrich, USA) at 85 mg/liter.
E. coli DH5α was used for all cloning experiments and was grown in LB medium (60) at 37°C with ampicillin (Sigma-Aldrich, USA) at 100 µg/ml.
Cloning of selection markers and ORIs in plasmids.
Selection markers and ORIs were amplified with primers (Table 4) carrying 15- to 40-bp homology to the vector and ∼20-bp homology to the marker or ORI fragment. Amplified vector and insert (1:3 ratio) were assembled in 20 µl Gibson reaction mixture (61) per the manufacturer’s instructions (New England BioLabs [NEB], USA), and 2 µl was transformed into chemically competent E. coli (NEB, USA). Following selection on LB plus ampicillin overnight, recombinants were detected by colony PCR using primers to the vector, outside the cloning sites. Putative positive clones were then confirmed by Sanger sequencing.
Construction of linear plasmids.
Linearizing primers (BP1252, BP1253, BP1254, and BP1255; Table 4) contained (from 5′ and 3′) a 34- or 57-nt telomere sequence (36), AflII and BamHI recognition sites, and then homology to the plasmid AatII site. Linear plasmids were amplified from circular plasmids (Fig. 2A) by two-step PCR using Kappa HiFi HotStart polymerase (Roche, Switzerland). Cycling conditions were as follows: 98°C denaturation step for 30 s, 30 cycles of 98°C (10 s) and 72°C (30 s/kb), and final extension of 72°C for 10 min.
To generate linear plasmids without telomeric ends, the circular plasmids were amplified with primers (BP1204 and BP1205; Table 4) using Phusion polymerase (Thermo Fisher Scientific, USA). Cycling conditions were as follows: 98°C denaturation step for 30 s; 25 cycles of 98°C (10 s), 60°C (30 s), and 72°C for (30 s/kb); and a final extension step at 72°C for 10 min.
Colony PCR.
A small portion of the colony was resuspended in the PCR with Taq polymerase (Hy-Taq Ready Mix, Hy-labs, Israel). Cycling conditions were as follows: 95°C denaturation step for 5 min; 25 cycles of 95°C (30 s), annealing at a primer-dependent temperature (30 s), and 72°C (1 min/kb); and a final extension step at 72°C for 5 min.
High-efficiency transformation of C. albicans.
C. albicans transformation was carried out as described previously (62) with the only difference being that DTT was added at a final concentration of 25 mM and that after a 45-min incubation with lithium acetate (LiAc)-TE, the cells were further incubated with DTT for 1.5 h.
Mitotic stability assay.
Yeast transformants were inoculated into SDC(−AA) (selective) medium and grown overnight at 30°C. The cultures were 10-fold serially diluted, and an appropriate dilution to yield 100 to 200 colonies was plated onto both SDC(−AA) and SDC plates. The plates were incubated at 30°C, and the number of colonies was counted after 2 days. Mitotic stability was calculated as [no. of colonies on SDC(−AA)/no. of colonies on SDC] × 100.
Plasmid loss assay.
Yeast transformants grown overnight in SDC(−AA) for mitotic stability assay were diluted 100-fold into SDC medium and grown overnight at 30°C. The cultures were 10-fold serially diluted, and 5 µl of each dilution was spotted on both SDC(−AA) and SDC plates. The plates were incubated at 30°C for 2 days, and the number of colonies was counted from the highest dilution at which they were well separated. The proportion of cells that retained the plasmid without selection was calculated as [no. of colonies on SDC(−AA)/no. of colonies on SDC] from the same dilution. The plasmid loss rate was then determined as described in the work of Longtine et al. (47).
Southern blotting.
The genomic DNA was extracted from 10-ml overnight-grown cultures in SDC(−AA) as described previously (62). Fifteen to 20 µg genomic DNA was digested overnight with ApaI and run on a 1% agarose gel for 16 to 20 h at 1.4 V/cm. Southern blotting was performed as described previously (63). A PCR fragment of the AMPR gene was used to probe the plasmids on the blot.
qPCR to determine plasmid copy number.
qPCR was carried out with the genomic DNA from autonomous transformants using SYBR green master mix (Bio-Rad, USA) per the manufacturer’s protocol in a Bio-Rad CFX96 Touch real-time PCR detection system. Cycling conditions were as follows: 95°C (3 min), 40 cycles of 95°C (5 s) and 60°C (30 s), and melt curve from 65.0 to 95.0 for 5 s. The AMPR gene was used to determine plasmid copy number, and CEN of chromosome 1 was used as a reference gene. The two primer sets used had similar efficiencies in the reaction; therefore, fold change in the copy number of plasmids was determined relative to the genomic control. Copy number of plasmids was calculated as copy number = (fold change × 2)/mitotic stability.
Growth rate determination.
From fresh transformation plate, three independent colonies per colony size were inoculated into 2 ml SDC(−AA) and grown overnight at 30°C and 250 rpm. Fifty microliters of cell culture was washed with ddH2O and resuspended in 1 ml SDC(−AA); 10 µl was inoculated in 100 µl SDC(−AA) in a 96-well round-bottom sterile polystyrene plate (Corning). For tiny colonies that could not be propagated in liquid medium, three independent colonies were directly inoculated from the plate into 100 µl SDC(−AA). The plate was subsequently incubated at 30°C in a Tecan Infinite F200 Pro (Tecan, Switzerland) microplate incubator/spectrometer with a shaking duration of 900 s, and the OD600 was collected every 15 min over a 24-h period. OD versus time was plotted to generate growth curves.
Recovery of pLin-CmLEU2-ORI410 plasmid from autonomous transformants.
The genomic DNA from the yeast transformants was digested with BamHI (NEB, USA) to cut the linear plasmid at both of the ends, resulting in removal of telomere repeats. The digested DNA was ligated overnight with T4 DNA ligase (Thermo Fisher Scientific, USA). The ligation product was transformed into electrocompetent E. coli (64), and the clones obtained were confirmed by PCR primers flanking the ligation site followed by sequencing.
ACKNOWLEDGMENTS
We thank members of the Berman lab for stimulating discussions throughout the work; Anton Levitan for help with sequence analysis; and Ella Segal, Andre Maicher, Shay Bramson, and Sophia Hirsch for technical assistance. We thank Ella Segal, Laura Burrack, and Amnon Koren for helpful comments on the manuscript.
This work was supported by the Israel Science Foundation (314/13) and by the European Research Council Advanced Award (340087, RAPLODAPT) to J.B. and a PBC postdoctoral fellowship to M.A.T.
REFERENCES
- 1.Griffiths AJF, Wessler SR, Carroll SB, Doebley J. 2015. Introduction to genetic analysis, 11th ed, p 351–392. WH Freeman, New York, NY. [Google Scholar]
- 2.Gerbaud C, Guérineau M. 1980. 2 μm plasmid copy number in different yeast strains and repartition of endogenous and 2 μm chimeric plasmids in transformed strains. Curr Genet 1:219–228. doi: 10.1007/BF00390947. [DOI] [PubMed] [Google Scholar]
- 3.Gunge N, Tamaru A, Ozawa F, Sakaguchi K. 1981. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol 145:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell WA, Kistler HC. 1990. In vivo rearrangement of foreign DNA by Fusarium oxysporum produces linear self-replicating plasmids. J Bacteriol 172:3163–3171. doi: 10.1128/jb.172.6.3163-3171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samac DA, Leong SA. 1988. Two linear plasmids in mitochondria of Fusarium solani f. sp. cucurbitae. Plasmid 19:57–67. doi: 10.1016/0147-619X(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 6.Raghuraman MK, Liachko I. 2016. Sequence determinants of yeast replication origin In Kaplan DL. (ed), The initiation of DNA replication in eukaryotes. Springer International Publishing, Basel, Switzerland. [Google Scholar]
- 7.Diffley JF, Stillman B. 1988. Purification of a yeast protein that binds to origins of DNA replication and a transcriptional silencer. Proc Natl Acad Sci U S A 85:2120–2124. doi: 10.1073/pnas.85.7.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srienc F, Bailey JE, Campbell JL. 1985. Effect of ARS1 mutations on chromosome stability in Saccharomyces cerevisiae. Mol Cell Biol 5:1676–1684. doi: 10.1128/MCB.5.7.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broach JR, Li YY, Feldman J, Jayaram M, Abraham J, Nasmyth KA, Hicks JB. 1983. Localization and sequence analysis of yeast origins of DNA replication. Cold Spring Harb Symp Quant Biol 47(Pt 2):1165–1173. doi: 10.1101/SQB.1983.047.01.132. [DOI] [PubMed] [Google Scholar]
- 10.Bouton AH, Smith MM. 1986. Fine-structure analysis of the DNA sequence requirements for autonomous replication of Saccharomyces cerevisiae plasmids. Mol Cell Biol 6:2354–2363. doi: 10.1128/MCB.6.7.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Celniker SE, Sweder K, Srienc F, Bailey JE, Campbell JL. 1984. Deletion mutations affecting autonomously replicating sequence ARS1 of Saccharomyces cerevisiae. Mol Cell Biol 4:2455–2466. doi: 10.1128/MCB.4.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liachko I, Bhaskar A, Lee C, Chung SC, Tye BK, Keich U. 2010. A comprehensive genome-wide map of autonomously replicating sequences in a naive genome. PLoS Genet 6:e1000946. doi: 10.1371/journal.pgen.1000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang RY, Kelly TJ. 1999. The fission yeast homologue of Orc4p binds to replication origin DNA via multiple AT-hooks. Proc Natl Acad Sci U S A 96:2656–2661. doi: 10.1073/pnas.96.6.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, Chuang RY, Kelly TJ. 2005. DNA replication origins in the Schizosaccharomyces pombe genome. Proc Natl Acad Sci U S A 102:337–342. doi: 10.1073/pnas.0408811102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maundrell K, Hutchison A, Shall S. 1988. Sequence analysis of ARS elements in fission yeast. EMBO J 7:2203–2209. doi: 10.1002/j.1460-2075.1988.tb03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrack LS, Berman J. 2012. Flexibility of centromere and kinetochore structures. Trends Genet 28:204–212. doi: 10.1016/j.tig.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan CS, Tye BK. 1980. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 77:6329–6333. doi: 10.1073/pnas.77.11.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struhl K, Stinchcomb DT, Scherer S, Davis RW. 1979. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A 76:1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray AW, Szostak JW. 1983. Pedigree analysis of plasmid segregation in yeast. Cell 34:961–970. doi: 10.1016/0092-8674(83)90553-6. [DOI] [PubMed] [Google Scholar]
- 20.Dani GM, Zakian VA. 1983. Mitotic and meiotic stability of linear plasmids in yeast. Proc Natl Acad Sci U S A 80:3406–3410. doi: 10.1073/pnas.80.11.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckerman J, Chibana H, Turner J, Magee PT. 2001. Single-copy IMH3 allele is sufficient to confer resistance to mycophenolic acid in Candida albicans and to mediate transformation of clinical Candida species. Infect Immun 69:108–114. doi: 10.1128/IAI.69.1.108-114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon RD, Jenkinson HF, Shepherd MG. 1990. Isolation and nucleotide sequence of an autonomously replicating sequence (ARS) element functional in Candida albicans and Saccharomyces cerevisiae. Mol Gen Genet 221:210–218. [DOI] [PubMed] [Google Scholar]
- 23.Goshorn AK, Grindle SM, Scherer S. 1992. Gene isolation by complementation in Candida albicans and applications to physical and genetic mapping. Infect Immun 60:876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herreros E, García-Sáez MI, Nombela C, Sánchez M. 1992. A reorganized Candida albicans DNA sequence promoting homologous non-integrative genetic transformation. Mol Microbiol 6:3567–3574. doi: 10.1111/j.1365-2958.1992.tb01792.x. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz MB, Cortelyou MW, Miller SM, Lai M, Kirsch DR. 1987. Development of autonomously replicating plasmids for Candida albicans. Mol Cell Biol 7:209–217. doi: 10.1128/MCB.7.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra S, Gomez-Raja J, Larriba G, Dubey DD, Sanyal K. 2014. Rad51-Rad52 mediated maintenance of centromeric chromatin in Candida albicans. PLoS Genet 10:e1004344. doi: 10.1371/journal.pgen.1004344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pla J, Pérez-Diaz RM, Navarro-Garcia F, Sánchez M, Nombela C. 1995. Cloning of the Candida albicans HIS1 gene by direct complementation of a C. albicans histidine auxotroph using an improved double-ARS shuttle vector. Gene 165:115–120. doi: 10.1016/0378-1119(95)00492-O. [DOI] [PubMed] [Google Scholar]
- 28.Tsai HJ, Baller JA, Liachko I, Koren A, Burrack LS, Hickman MA, Thevandavakkam MA, Rusche LN, Berman J. 2014. Origin replication complex binding, nucleosome depletion patterns, and a primary sequence motif can predict origins of replication in a genome with epigenetic centromeres. mBio 5:e01703-14. doi: 10.1128/mBio.01703-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke DT, Carle GF, Olson MV. 1987. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science 236:806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- 30.Noble SM, Johnson AD. 2005. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell 4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liachko I, Dunham MJ. 2014. An autonomously replicating sequence for use in a wide range of budding yeasts. FEMS Yeast Res 14:364–367. doi: 10.1111/1567-1364.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods JP, Goldman WE. 1992. In vivo generation of linear plasmids with addition of telomeric sequences by Histoplasma capsulatum. Mol Microbiol 6:3603–3610. doi: 10.1111/j.1365-2958.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 33.Woods JP, Goldman WE. 1993. Autonomous replication of foreign DNA in Histoplasma capsulatum: role of native telomeric sequences. J Bacteriol 175:636–641. doi: 10.1128/jb.175.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edman JC. 1992. Isolation of telomerelike sequences from Cryptococcus neoformans and their use in high-efficiency transformation. Mol Cell Biol 12:2777–2783. doi: 10.1128/MCB.12.6.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffiths AJ. 1995. Natural plasmids of filamentous fungi. Microbiol Rev 59:673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEachern MJ, Hicks JB. 1993. Unusually large telomeric repeats in the yeast Candida albicans. Mol Cell Biol 13:551–560. doi: 10.1128/MCB.13.1.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoggard T, Liachko I, Burt C, Meikle T, Jiang K, Craciun G, Dunham MJ, Fox CA. 2016. High throughput analyses of budding yeast ARSs reveal new DNA elements capable of conferring centromere-independent plasmid propagation. G3 (Bethesda) 6:993–1012. doi: 10.1534/g3.116.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palzkill TG, Newlon CS. 1988. A yeast replication origin consists of multiple copies of a small conserved sequence. Cell 53:441–450. doi: 10.1016/0092-8674(88)90164-X. [DOI] [PubMed] [Google Scholar]
- 39.Liachko I, Youngblood RA, Keich U, Dunham MJ. 2013. High-resolution mapping, characterization, and optimization of autonomously replicating sequences in yeast. Genome Res 23:698–704. doi: 10.1101/gr.144659.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liachko I, Youngblood RA, Tsui K, Bubb KL, Queitsch C, Raghuraman MK, Nislow C, Brewer BJ, Dunham MJ. 2014. GC-rich DNA elements enable replication origin activity in the methylotrophic yeast Pichia pastoris. PLoS Genet 10:e1004169. doi: 10.1371/journal.pgen.1004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarke L, Carbon J. 1985. The structure and function of yeast centromeres. Annu Rev Genet 19:29–55. doi: 10.1146/annurev.ge.19.120185.000333. [DOI] [PubMed] [Google Scholar]
- 42.Dershowitz A, Snyder M, Sbia M, Skurnick JH, Ong LY, Newlon CS. 2007. Linear derivatives of Saccharomyces cerevisiae chromosome III can be maintained in the absence of autonomously replicating sequence elements. Mol Cell Biol 27:4652–4663. doi: 10.1128/MCB.01246-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins J. 1981. Instability of palindromic DNA in Escherichia coli. Cold Spring Harb Symp Quant Biol 45(Pt 1):409–416. doi: 10.1101/SQB.1981.045.01.055. [DOI] [PubMed] [Google Scholar]
- 44.Guerrini AM, Ascenzioni F, Tribioli C, Donini P. 1985. Transformation of Saccharomyces cerevisiae and Schizosaccharomyces pombe with linear plasmids containing 2 micron sequences. EMBO J 4:1569–1573. doi: 10.1002/j.1460-2075.1985.tb03818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szostak JW, Blackburn EH. 1982. Cloning yeast telomeres on linear plasmid vectors. Cell 29:245–255. doi: 10.1016/0092-8674(82)90109-X. [DOI] [PubMed] [Google Scholar]
- 46.Murray AW. 1984. Chromosome and plasmid behavior in yeast. PhD dissertation. Harvard University, Cambridge, MA. [Google Scholar]
- 47.Longtine MS, Enomoto S, Finstad SL, Berman J. 1992. Yeast telomere repeat sequence (TRS) improves circular plasmid segregation, and TRS plasmid segregation involves the RAP1 gene product. Mol Cell Biol 12:1997–2009. doi: 10.1128/MCB.12.5.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enomoto S, Longtine MS, Berman J. 1994. TEL+CEN antagonism on plasmids involves telomere repeat sequences tracts and gene products that interact with chromosomal telomeres. Chromosoma 103:237–250. doi: 10.1007/BF00352248. [DOI] [PubMed] [Google Scholar]
- 49.Denoth-Lippuner A, Krzyzanowski MK, Stober C, Barral Y. 2014. Role of SAGA in the asymmetric segregation of DNA circles during yeast ageing. Elife 3:e03790. doi: 10.7554/eLife.03790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baldi S, Bolognesi A, Meinema AC, Barral Y. 2017. Heat stress promotes longevity in budding yeast by relaxing the confinement of age-promoting factors in the mother cell. Elife 6:e28329. doi: 10.7554/eLife.28329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burrack LS, Hutton HF, Matter KJ, Clancey SA, Liachko I, Plemmons AE, Saha A, Power EA, Turman B, Thevandavakkam MA, Ay F, Dunham MJ, Berman J. 2016. Neocentromeres provide chromosome segregation accuracy and centromere clustering to multiple loci along a Candida albicans chromosome. PLoS Genet 12:e1006317. doi: 10.1371/journal.pgen.1006317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ketel C, Wang HS, McClellan M, Bouchonville K, Selmecki A, Lahav T, Gerami-Nejad M, Berman J. 2009. Neocentromeres form efficiently at multiple possible loci in Candida albicans. PLoS Genet 5:e1000400. doi: 10.1371/journal.pgen.1000400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Yanagisawa Y, Tsankov AM, Hart C, Aoki K, Kommajosyula N, Steinmann KE, Bochicchio J, Russ C, Regev A, Rando OJ, Nusbaum C, Niki H, Milos P, Weng Z, Rhind N. 2012. Genome-wide identification and characterization of replication origins by deep sequencing. Genome Biol 13:R27. doi: 10.1186/gb-2012-13-4-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Natsume T, Müller CA, Katou Y, Retkute R, Gierliński M, Araki H, Blow JJ, Shirahige K, Nieduszynski CA, Tanaka TU. 2013. Kinetochores coordinate pericentromeric cohesion and early DNA replication by Cdc7-Dbf4 kinase recruitment. Mol Cell 50:661–674. doi: 10.1016/j.molcel.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cotobal C, Segurado M, Antequera F. 2010. Structural diversity and dynamics of genomic replication origins in Schizosaccharomyces pombe. EMBO J 29:934–942. doi: 10.1038/emboj.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brewer BJ, Fangman WL. 1993. Initiation at closely spaced replication origins in a yeast chromosome. Science 262:1728–1731. doi: 10.1126/science.8259517. [DOI] [PubMed] [Google Scholar]
- 57.Coster G, Diffley JFX. 2017. Bidirectional eukaryotic DNA replication is established by quasi-symmetrical helicase loading. Science 357:314–318. doi: 10.1126/science.aan0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Segurado M, de Luis A, Antequera F. 2003. Genome-wide distribution of DNA replication origins at A+T-rich islands in Schizosaccharomyces pombe. EMBO Rep 4:1048–1053. doi: 10.1038/sj.embor.embor7400008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brewer BJ, Fangman WL. 1994. Initiation preference at a yeast origin of replication. Proc Natl Acad Sci U S A 91:3418–3422. doi: 10.1073/pnas.91.8.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (ed). 2003. Current protocols in molecular biology. John Wiley & Sons, Inc, New York, NY. [Google Scholar]
- 61.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 62.Bijlani S, Nahar AS, Ganesan K. 2018. Improved Tet-On and Tet-Off systems for tetracycline-regulated expression of genes in Candida. Curr Genet 64:303–316. doi: 10.1007/s00294-017-0720-9. [DOI] [PubMed] [Google Scholar]
- 63.Askree SH, Yehuda T, Smolikov S, Gurevich R, Hawk J, Coker C, Krauskopf A, Kupiec M, McEachern MJ. 2004. A genome-wide screen for Saccharomyces cerevisiae deletion mutants that affect telomere length. Proc Natl Acad Sci U S A 101:8658–8663. doi: 10.1073/pnas.0401263101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma RC, Schimke RT. 1996. Preparation of electrocompetent E. coli using salt-free growth medium. Biotechniques 20:42–44. doi: 10.2144/96201bm08. [DOI] [PubMed] [Google Scholar]
- 65.Wilson RB, Davis D, Mitchell AP. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181:1868–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vincent BM, Lancaster AK, Scherz-Shouval R, Whitesell L, Lindquist S. 2013. Fitness trade-offs restrict the evolution of resistance to amphotericin B. PLoS Biol 11:e1001692. doi: 10.1371/journal.pbio.1001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth curves of transients obtained with pCir-CaURA3-ORI410 (A), transients obtained with pCir-CdARG4-ORI410 (B), integrants obtained with pCir-CdARG4-ORI410 (C), ARS-transformants obtained with pCir-CdARG4-ORI410 (D), and ARS-transformants obtained with pLin-CdARG4-ORI410 (E and F). The growth curves are representative of one colony of each type (the growth parameters averaged for three colonies of each type are listed in Table 1). Download FIG S1, PDF file, 0.08 MB (87.3KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Transformation efficiency (average from three independent experiments) of pCir-CaURA3 with different origin sequences in C. albicans BWP17 and SN76. Download Table S1, PDF file, 0.1 MB (111.3KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Southern blot of ApaI-digested DNA isolated from different transformants and probed with AMPR. (A) Circular plasmids: pCir-CaHIS1-ORI410 ARS-transformants (lanes 1 and 2), pCir-CdARG4-ORI410 integrant (lane 3), pCir-CdARG4-ORI410 ARS-transformants (lanes 4 and 5), pCir-CaHIS1-ORI410 control plasmid (lane 6), and pCir-CdARG4-ORI410 control plasmid (lane 7). All ARS-transformants gave a band corresponding to their control plasmid, indicating the existence of autonomously replicating plasmids. (B) Linear plasmids: pLin-CaHIS1-ORI410 and ARS-transformants with mitotic stability of 46% and 63% (lanes 1 and 2), pLin-CdARG4-ORI410 ARS-transformant with mitotic stability of 44% (lane 3), pLin-CaHIS1-ORI410 control plasmid (lane 4), and pLin-CdARG4-ORI410 control plasmid (lane 5). pLin-CaHIS1-ORI410 plasmids showed integration, whereas the pLin-CdARG4-ORI410 ARS-transformant gave a band corresponding to its control plasmid, indicating an autonomously replicating plasmid. Int, integrants; ARS, ARS-transformants. Download FIG S2, PDF file, 0.3 MB (334.5KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Schematic showing integration of pCir-CaHIS1-ORI410 into the genome by single crossover at either A or B. The integration was confirmed in three independent integrants by different primer sets indicated in the figure along with the size of PCR product expected. (B) Schematic showing integration of pLin-CaHIS1-ORI410 into the genome by a double-crossover or gene conversion event. The integration was confirmed in three independent integrants by different primer sets indicated in the figure along with the size of the PCR product expected. M, marker; C, parent strain C. albicans SN76; Int, integrants. Download FIG S3, PDF file, 0.2 MB (194.7KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Comparison of circular plasmid, linear plasmid (with 1.5× TEL repeats), and circular plasmid (with one 1.5× telomere repeat) using CmLEU2 marker transformed in C. albicans SN152: transformation efficiency, proportion of different types of transformants, and the number of autonomous transformants. Different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). The transformation efficiency and number of autonomous transformants are an average from three independent experiments. The size of circles showing proportion of transformant types is an approximation of transformation efficiency. (B) Mitotic stability (%) of ARS-transformants obtained with plasmids mentioned in panel A. The data represent the average from three independent ARS-transformants of each plasmid. Download FIG S4, PDF file, 0.2 MB (168.4KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Steps for recovering pLin-CmLEU2-ORI410 autonomously replicating plasmids from C. albicans ARS-transformants in E. coli. Three independent ARS-transformants were analyzed by this method. C, pCir-CmLEU2-ORI410 plasmid control; M, marker. Download FIG S5, PDF file, 0.2 MB (176.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Mitotic stability (averaged for three colonies) of ARS-transformants obtained with linear plasmids carrying ORI410 after passaging. Download Table S2, PDF file, 0.1 MB (108.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of pLin-CaHIS1-ORI410 (A) and pLin-CdARG4-ORI410 (B) transformation efficiency across different C. albicans strains. The transformation efficiency is an average from three independent experiments. Different types of transformants are represented by various shades of a color (lightest shade representing transients, intermediate shade representing ARS-transformants, and darkest shade representing integrants). Download FIG S6, PDF file, 0.6 MB (630.8KB, pdf) .
Copyright © 2019 Bijlani et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.