Key Teaching Points.
-
•
The premature ventricular complex (PVC) response algorithm can force atrial pacing after the extended postventricular atrial refractory period (PVARP) to avoid initiation of endless loop tachycardia after the subsequent ventricular beat.
-
•
Intrinsic atrial activity that occurs near the end of the extended PVARP and is followed by atrial pacing can result in a proarrhythmic long–short sequence.
-
•
Atrial fibrillation (AF) induced by a vendor-specific PVC response algorithm may be an underappreciated mechanism of AF initiation in patients with an implantable device.
Introduction
New-onset atrial fibrillation (AF) is commonly detected in patients with an implantable cardiac device. Appropriate evaluation of these patients includes a search for “reversible” causes that eventually might preclude the need for rate or rhythm control and, importantly, oral anticoagulation. We present the case of a patient with new-onset AF attributed to a premature ventricular complex (PVC) response algorithm that resulted in atrial pacing-induced long–short sequences. In this case, disabling the PVC response algorithm successfully prevented further AF.
Case report
A 70-year-old man with a history of ischemic cardiomyopathy and left bundle branch block status postimplantation of a cardiac resynchronization therapy device (Quadra Assura, St. Jude Medical, St. Paul, MN) was referred for evaluation of new-onset AF. Although the patient reported significant exertional intolerance, he had no symptoms that were definitely attributed to new-onset AF. The patient’s CHADS-VASc score was 4.
Device interrogation demonstrated a normally functioning device that was programmed DDD 60–110 with 95% biventricular pacing, 1.8% PVCs, and 1.3% AF. A total of 1022 mode switch events were recorded over the preceding 3.5 months. Although 952 of these episodes were <5 minutes in duration, several were longer, with a maximum episode duration of 44 minutes (Supplemental Figure 1). Episode frequency and duration had increased since the previous interrogation.
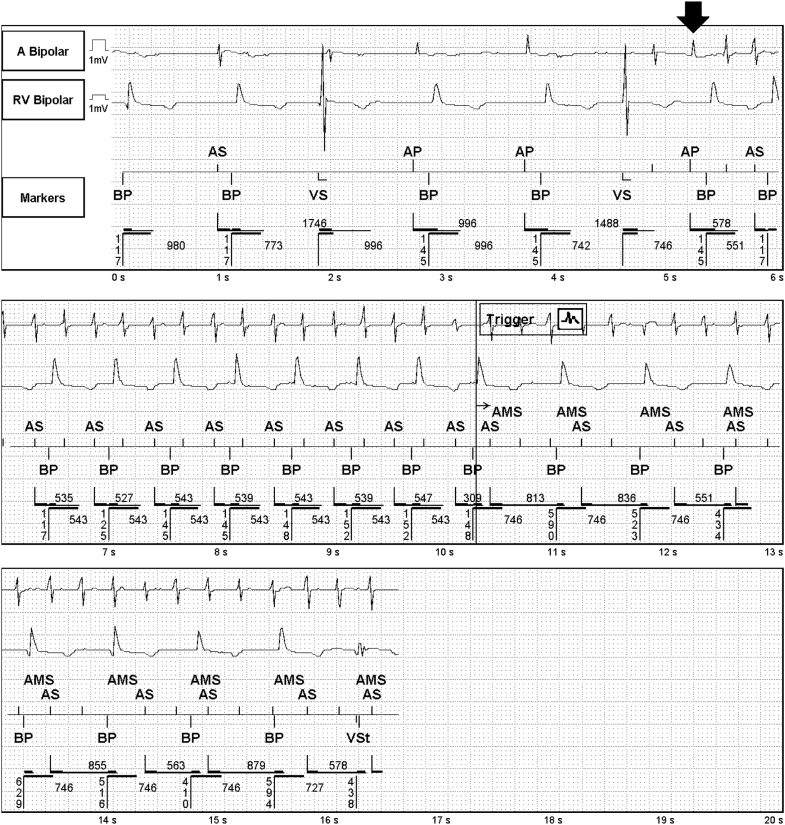
The available episodes of AF were reviewed, and all demonstrated an identical initiation sequence (Figure 1): a PVC followed by an intrinsic atrial depolarization during the postventricular atrial refractory period (PVARP), a closely coupled atrial pacing spike, and then tachycardia. The sequence of intrinsic atrial depolarization during the PVARP followed by an atrial paced event functionally resulted in a long–short sequence triggering a stable tachycardia that ultimately degenerated into AF. With these sequences, the atrial paced event occurred much earlier than would be predicted based on the programmed lower rate of 60 bpm, suggesting a vendor-specific algorithm.
Figure 1.
Representative device electrogram showing how the atrial pace premature ventricular contraction response (arrow) resulted in a long–short sequence and eventually atrial fibrillation due to an intrinsic atrial event occurring late in the extended postventricular atrial refractory period. RV = right ventricle.
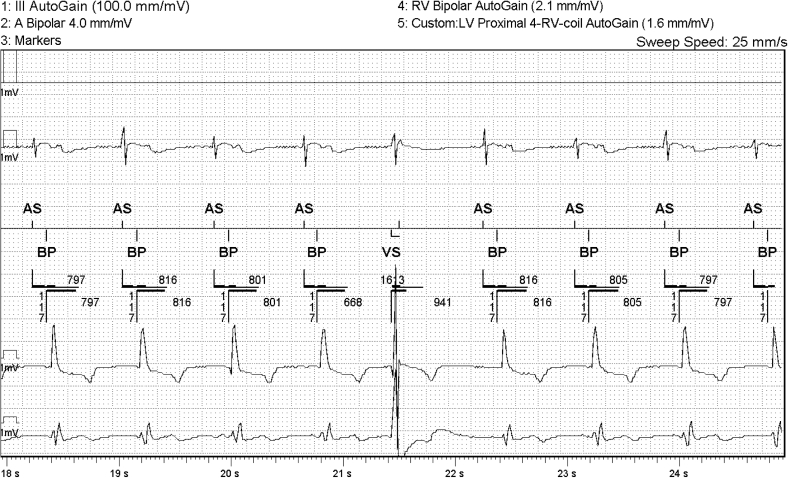
During the initial interrogation, we disabled the PVC response algorithm and noted that atrial pacing was no longer observed after frequent PVCs (Figure 2). We hypothesized that this programming change would prevent further pacing-induced long–short sequences. The patient was discharged from the clinic with follow-up interrogation scheduled for 1 month later.
Figure 2.
After the atrial pace premature ventricular contraction (PVC) response algorithm was disabled, PVCs occurred without closely coupled atrial pacing. Of note, the intrinsic atrial activity early in the extended postventricular atrial refractory period likely would be too early to result in long–short induced atrial fibrillation even if the PVC response had remained on. LV = left ventricle; RV = right ventricle.
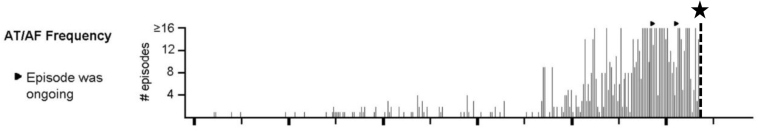
Repeat interrogation performed 1 month after reprogramming revealed a 2.2% PVC burden without any episodes of AF. A graphic depiction of daily AF burden (Figure 3) shows that device reprogramming (denoted by star and dashed line) resulted in prompt resolution of frequent AF episodes. Based on these findings, the mechanism of AF initiation was confirmed to be iatrogenic, due to atrial paced long–short sequences attributed to the PVC response algorithm. Importantly, this identified reversible cause obviated the need for systemic anticoagulation at that time. However, the patient will require close monitoring given his risk for stroke if AF recurs.
Figure 3.
Plot of atrial fibrillation (AF) episode frequency showing an abrupt drop in AF episodes occurring at the time of reprogramming the premature ventricular contraction response algorithm off (star and vertical dashed line). AT = atrial tachycardia.
Discussion
Several algorithms have been developed to mitigate the risk of endless loop tachycardia (ELT) due to retrograde ventriculoatrial (VA) conduction of PVCs. Extension of the PVARP (to 475 ms in this patient’s device1) is commonly used to prevent VA conduction-induced atrial activity from being tracked, which would prompt ventricular pacing and set up the conditions for ELT. However, if no intrinsic atrial activity occurs after the extended PVARP and ventricular pacing occurs, ELT can still be initiated. In this situation, retrograde conduction and ELT can be prevented by forcing atrial pacing after the extended PVARP (atrial pace PVC response) because the AV node will be rendered refractory to retrograde conduction at the time of the ventricular paced (or sensed) event. This specific algorithm forces atrial pacing if intrinsic atrial activity is not detected within the 330 ms after conclusion of the extended PVARP.1
In the current case, the PVC response algorithm resulted in a short interval between the atrial activity late within the PVARP and the PVC response commanded atrial pacing, resulting in a long–short sequence and tachycardia. Although a previous report showed that the patient’s algorithm can induce ventricular arrhythmias via induction of a long–short sequence,2 this represents the first report in the literature of this mechanism of AF initiation. Atrial pacing must occur at a critical time relative to the intrinsic atrial activity in order to set up functional reentry and fibrillatory activity. Thus, intrinsic atrial activity must occur late within the extended PVARP in order for the atrium to be partially refractory at the time of PVC response-induced atrial pacing. Thus, it is unlikely that atrial activity due to PVC-induced VA conduction would set up the critically timed long–short sequence necessary to induce tachycardia unless VA conduction was exceptionally prolonged. The post-PVC atrial activity shown in Figure 2 is early enough within the extended PVARP that it probably would not have initiated tachycardia even if the PVC response had remained on. However, frequent instances of sinus or nonsinus atrial activity late in an extended PVARP have the potential to trigger atrial tachyarrhythmias, as depicted in Figure 1.
The most common response to a PVC (for a device programmed DDD or DDDR) is PVARP extension without atrial pacing. Notably, this is the nominal configuration for St. Jude devices. In the setting of a programmed long AV delay, it is theoretically possible to have a short coupling interval between a premature atrial contraction that falls late in the extended PVARP and the subsequent atrial paced event.
This case underscores the importance of reviewing the initiation of new-onset AF episodes and highlights a novel reversible mechanism of AF in patients with an implantable device.
Conclusion
The PVC response algorithm forces atrial pacing shortly after an extended PVARP and can set up a long–short sequence and AF if intrinsic atrial activity occurs late in the PVARP. This represents the first report of this novel reversible mechanism of AF initiation.
Footnotes
Dr Friedman has received salary support from the NIH; research support from the National Cardiovascular Data Registry, Boston Scientific, Biosense Webster, and Abbott; and educational grants from Boston Scientific, Medtronic, Abbott, and Biotronik. Dr Sun reports consulting for Medtronic, Merit Medical, Biosense Webster, and Abbott. The remaining authors report no disclosures.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2018.11.012.
Appendix. Supplementary data
Visual depiction of the distribution of episode durations for the 1022 mode switch events.
References
- 1.St. Jude Medical Inc; St. Paul, MN: 2018. Merlin™ patient care system help manual. Bradycardia and tachycardia devices, bradycardia parameters. 201584. [Google Scholar]
- 2.El-Damaty A., Gray C., Sharma R., Sapp J. Atrial pace on PVC algorithm inducing ventricular fibrillation. Pacing Clin Electrophysiol. 2012;35:749–751. doi: 10.1111/j.1540-8159.2011.03263.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual depiction of the distribution of episode durations for the 1022 mode switch events.