Abstract
Objective
To determine the association of a neurologist visit with health care use and cost outcomes for patients with incident epilepsy.
Methods
Using health care claims data for individuals insured by United Healthcare from 2001 to 2016, we identified patients with incident epilepsy. The population was defined by an epilepsy/convulsion diagnosis code (ICD codes 345.xx/780.3x, G40.xx/R56.xx), an antiepileptic prescription filled within the succeeding 2 years, and neither criterion met in the 2 preceding years. Cases were defined as patients who had a neurologist encounter for epilepsy within 1 year after an incident diagnosis; a control cohort was constructed with propensity score matching. Primary outcomes were emergency room (ER) visits and hospitalizations for epilepsy. Secondary outcomes included measures of cost (epilepsy related, not epilepsy related, and antiepileptic drugs) and care escalation (including EEG evaluation and epilepsy surgery).
Results
After participant identification and propensity score matching, there were 3,400 cases and 3,400 controls. Epilepsy-related ER visits were more likely for cases than controls (year 1: 5.9% vs 2.3%, p < 0.001), as were hospitalizations (year 1: 2.1% vs 0.7%, p < 0.001). Total medical costs for epilepsy care, nonepilepsy care, and antiepileptic drugs were greater for cases (p ≤ 0.001). EEG evaluation and epilepsy surgery occurred more commonly for cases (p ≤ 0.001).
Conclusions
Patients with epilepsy who visited a neurologist had greater subsequent health care use, medical costs, and care escalation than controls. This comparison using administrative claims is plausibly confounded by case disease severity, as suggested by higher nonepilepsy care costs. Linking patient-centered outcomes to claims data may provide the clinical resolution to assess care value within a heterogeneous population.
As payment becomes increasingly tied to high-value neurologic care, it is imperative that we use rigorous measurement of quality and cost.1 This is particularly relevant to the care of patients with epilepsy, a disease with 1% prevalence in the United States, complex medical needs, and high economic burden.2,3 Total annual direct health care costs per person with epilepsy are estimated at $10,000 to $48,000,4 with indirect costs projected to be substantially higher.5
Epilepsy is defined as at least 1 unprovoked seizure with an elevated risk of seizure recurrence.6 Therefore, epilepsy encompasses a heterogeneous population of patients with a broad range of etiologies and severities.2 Practice guidelines advise that many patients presenting with seizures can initially be managed in the primary care setting.7 However, early expert care may be warranted for patients with persistent seizures.8 Prior study suggests that patients with epilepsy may benefit from specialized care,9 but the full effect of neurologists has not been well characterized.
With the current implementation of the Medicine Access and CHIP Reauthorization Act and heightened interest in alternative payment models,10 careful consideration of clinical outcomes and cost is necessary to determine how to maximize the value of neurologist care. We, the American Academy of Neurology Health Services Research Subcommittee, chose to investigate value using administrative claims data, which has previously been used to assess the role of neurologists in other diseases such as stroke and headache.11–13 This study, funded by the American Academy of Neurology, aims to determine the association of a neurologist visit on health care use and cost outcomes for patients with incident epilepsy using a large, private insurance claims dataset.
Methods
Data source and study population
We performed a retrospective analysis using data from the OptumInsight Clinformatics Data Mart (Optum.com, Eden Prairie, MN), a database of inpatient medical, outpatient medical, pharmacy, and laboratory administrative claims for individuals insured by United Healthcare, from 2001 to 2016. This database includes deidentified information for ≈12 to 14 million annual covered lives, for a total of ≈73 million unique lives over the study period.
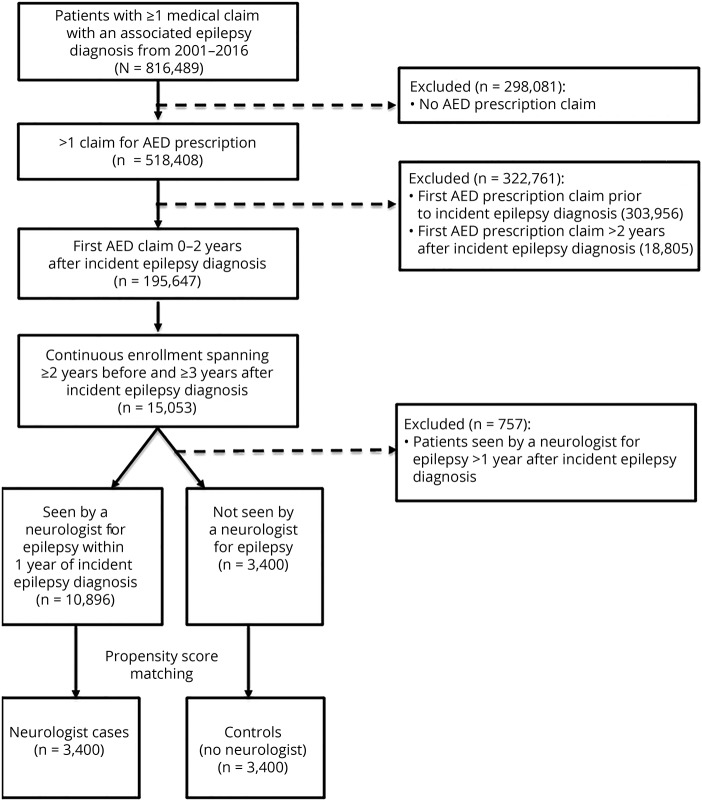
We identified adult patients with incident epilepsy defined by (1) an ICD diagnosis code for epilepsy or convulsion (345.xx/780.3x or G40.xx/R56.xx), (2) a prescription filled for an antiepileptic medication at the time of diagnosis or in the subsequent 2 years, and (3) neither an epilepsy-related diagnosis code nor an antiepileptic prescription in the preceding 2 years. This definition of epilepsy, which includes both diagnostic code and antiepileptic medication, is based on validated criteria for administrative claims data (positive predictive value 84%).14,15 Patients were excluded if they were not enrolled in insurance coverage for 5 continuous years, with at least 2 years of prediagnosis data and at least 3 years of follow-up data. In addition, to have a sufficient follow-up period to assess neurologist influence on longer-term outcomes, patients were excluded if their visit to a neurologist occurred >1 year after their incident diagnosis (figure 1).
Figure 1. Study flow diagram.
AED = antiepileptic drug.
Exposure
The exposure of interest was a neurologist encounter for epilepsy (primary or secondary ICD diagnosis code). A neurologist was identified by provider category code or the National Uniform Claim Committee taxonomy code in the Optumlab data. The case patients were compared to the population of patients with incident epilepsy who did not see a neurologist for epilepsy.
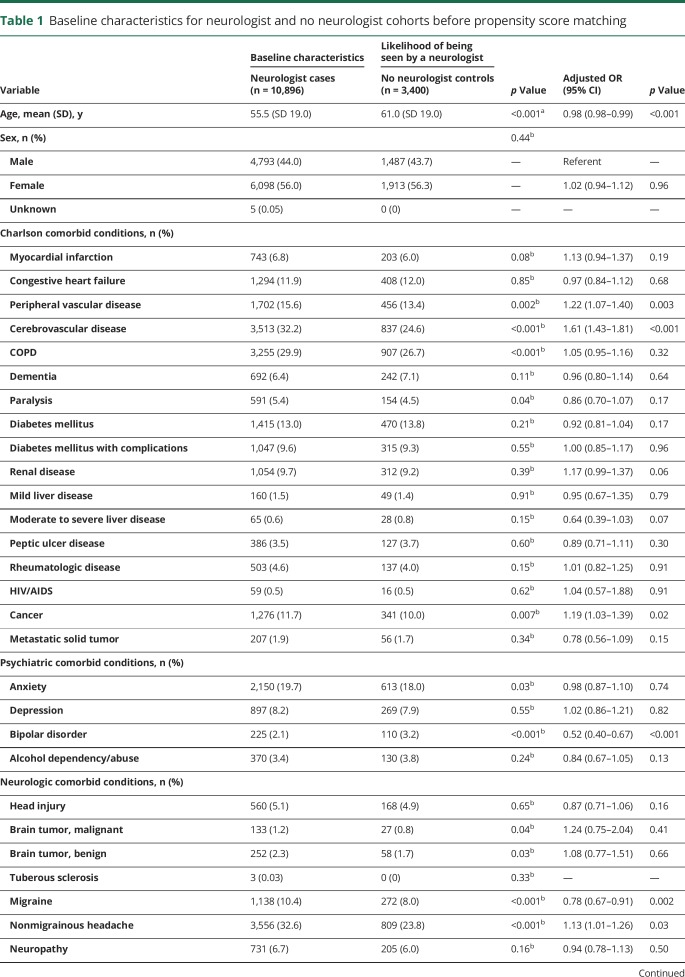
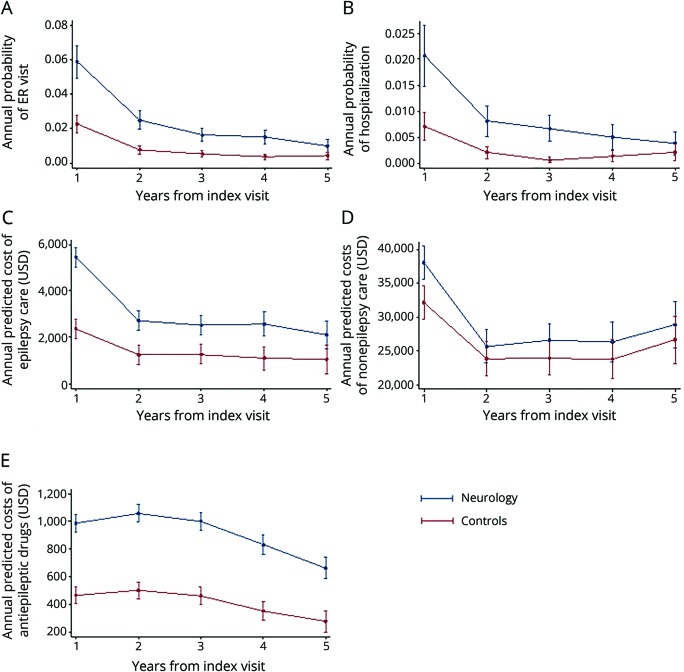
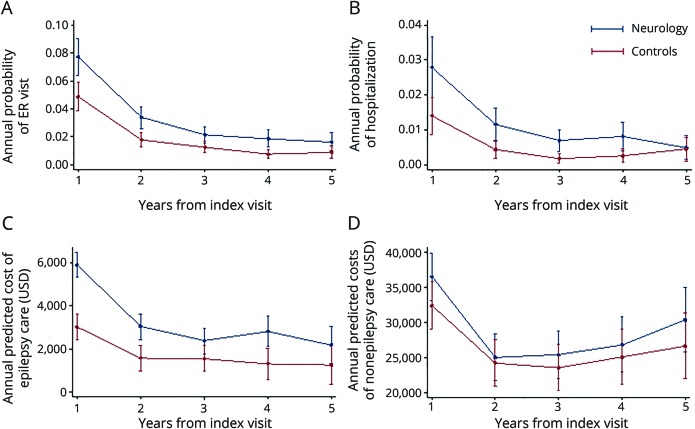
To adjust for pretreatment observable differences between cases and controls, a subsample was created through propensity score modeling.16 A propensity score was calculated from multilevel logistic regression to estimate the probability of not being seen by a neurologist, conditional on matching variables that included the following: age, sex, 17 Charlson Comorbidity Index categories, preexisting psychiatric comorbid conditions (anxiety: ICD codes 293.84, 300.0x; depression: 296.2x, 296.30, 296.31, 296.32, 296.33, 296.34, 296.35, 296.36, 296.82, 311.00; bipolar disorder: 296.0–296.16, 296.4–296.81, 296.83–296.89, 301.13; alcohol dependence/abuse: 303.9x, 305.0x), preexisting neurologic comorbid conditions (head injury: 850.xx–854.xx; malignant brain tumor: 191.xx, 192.1, 198.3; benign brain tumor: 225.0, 225.2, 237.5, 237.6, 239.6; tuberous sclerosis: 759.5; migraine headache: 346.xx; nonmigrainous headache: 307.81, 339.xx, 784.0; neuropathy: 356.xx, 357.xx [excluding 357.0 and 357.81]; chronic pain: 053.10, 053.11, 053.12, 053.13, 333.94, 337.2x, 338.0.338.2x, 338.3, 338.4, 350.1, 353.6, 722.8x, 723.1, 423.2, 423.3, 423.4, 724.03, 724.1, 724.2, 724.3, 724.4, 724.5, 729.1, 729.2, 780.96; of note, cerebrovascular disease was captured as a Charlson comorbidity), total medical expenditures in the 2 years before diagnosis, medical service use in the 2 years before incident diagnosis (hospitalization, skilled nursing facility admission, emergency room [ER] visit, neurologist visit), psychiatric treatment in the 2 years before incident diagnosis (psychiatrist visit claims, antidepressant medication by Optum pharmacy claim category), benefit design (exclusive provider organization, health maintenance organization, preferred provider organization, indemnity, point of service, other), days of available claims data before incident diagnosis (eligibility time), setting of incident epilepsy diagnosis (hospital, skilled nursing facility, ER, clinic), year of incident diagnosis, and a random region-level intercept defined by hospital service area (Dartmouth Atlas of Health Care, dartmouthatlas.org) to account for geographic variation in neurologist availability. A 1:1 nearest-neighbor match was performed without replacement. Baseline characteristics before propensity score matching are summarized in table 1.
Table 1.
Baseline characteristics for neurologist and no neurologist cohorts before propensity score matching
Lag time variables
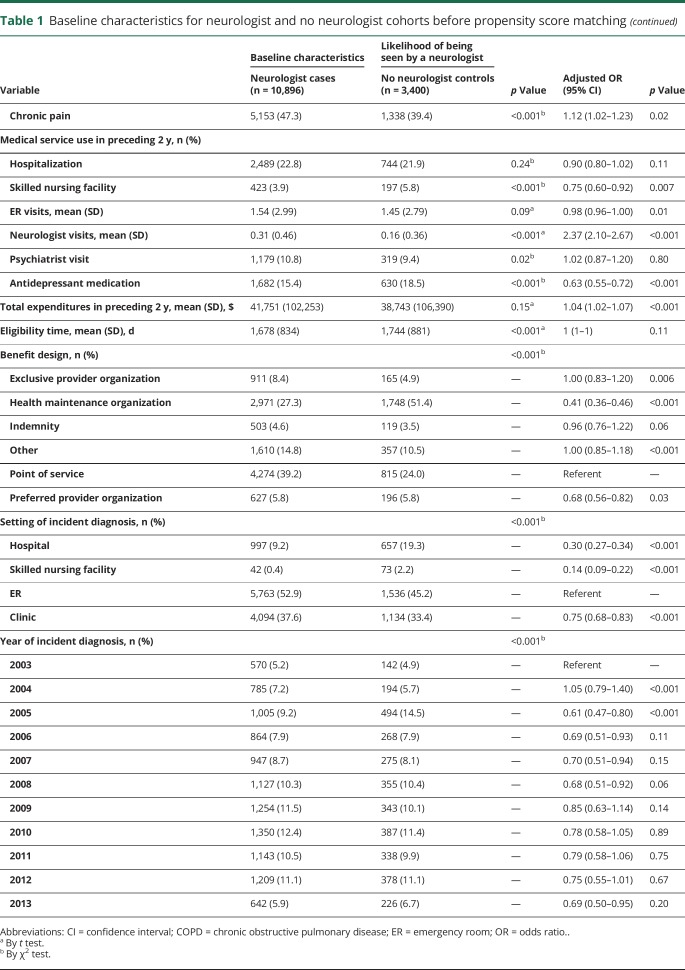
For the cases, the index date was defined as the first neurology encounter after the incident epilepsy diagnosis. Each control patient was then assigned an index date that corresponded to his/her matched case. The length of time between the incident epilepsy diagnosis and the index date (the lag time, capped at 1 year by exclusion criterion) and the health care use within that period varied from patient to patient. Therefore, all analyses were adjusted for relevant lag time variables, including the following: length of lag time, diagnosis of status epilepticus or intractable epilepsy (by ICD diagnosis code), ER visits (epilepsy related, trauma/injury related), hospitalization for epilepsy, medical costs (epilepsy related, not epilepsy related, antiepileptic medication), antiepileptic prescription, care for comorbid conditions (bone health evaluation, antidepressant prescription, psychiatric care), and occurrence of epilepsy-specific care (EEG, presurgical evaluation, intracranial electrode implantation, epilepsy surgery, vagal nerve stimulation [VNS]). For women of childbearing age, defined as 18 to 44 years, valproic acid prescription was also included (table 2).
Table 2.
Lag time variables
Outcomes
Our primary outcomes were ER visits and hospitalizations for epilepsy (primary ICD diagnosis code).
Cost outcomes included epilepsy-related expenditures (antiepileptic medications, head CT, brain MRI, EEG, epilepsy surgical procedures, epilepsy outpatient encounters, epilepsy hospital encounters), non–epilepsy-related expenditures, and antiepileptic drug costs. Antiepileptic drug use was assessed by medication continuation after initial prescription, days of medication supply (measured by summation of all antiepileptic daily doses prescribed over the year), and prescription of generation 2 antiepileptic drugs (included generation 1 drugs were carbamazepine, clonazepam, ethosuximide, ethotoin, mephobarbital, methsuximide, phenobarbital, phenytoin, primidone, and valproic acid; included generation 2 drugs were brivaracetam, clobazam, eslicarbazepine, ezogabine, felbamate, gabapentin, lacosamide, lamictal, levetiracetam, oxcarbazepine, perampanel, pregabalin, rufinamide, tiagabine, topiramate, vigabatrin, and zonisamide).
Quality of care was examined through measures of care escalation, including use of EEG monitoring, epilepsy presurgical evaluation (brain PET, brain SPECT, fMRI, Wada), epilepsy surgery, VNS, and antiepileptic therapy augmentation for breakthrough seizures (defined as an increase in antiepileptic drug dose or new antiepileptic prescription ≤30 days after an ER visit). We also measured attention to psychiatric disorders (antidepressant medication prescription and psychiatrist visits), bone health evaluation (vitamin D monitoring and dual-energy x-ray absorptiometry scan), and specific consideration of women of childbearing age (valproic acid prescription). Presence of intractable epilepsy (345.01, 345.11, 345.41, 345.51, 345.61, 345.71, 345.81, 345.91, G40.01x, G40.11x, G40.21x, G40.31x, G40.A1x, G40.B1x, G40.41x, G40.803, G40.804, G40.813, G40.814, G40.823, G40.824, G40.91x) and status epilepticus (345.3x, G40.001, G40.011, G40.101, G40.111, G40.201, G40.211, G40.301, G40.311, G40.A01, G40.A11, G40.B01, G40.B11, G40.401, G40.411, G40.501, G40.801, G40.803, G40.811, G40.813, G40.821, G40.823, G40.901, G40.911) was noted, as well as presentations to the ER for trauma or injury (800.xx–829.xx, 830.xx–839.xx, 840.xx–848.xx, 850.xx–854.xx, 860.xx–869.xx, 870.xx–897.xx, 910.xx–924.xx, 940.xx-–949.xx, 959.xx, E810.x–E825.x, S00–S99, T07–T14, T20–T32).
Sensitivity analyses
We evaluated variations on the definition of epilepsy and the window of continuous insurance coverage to investigate the effect on outcomes. These secondary analyses were (1) the requirement of 2 epilepsy diagnosis codes (increased diagnostic specificity), (2) only 1 year of prediagnosis data and only 2 years of follow-up data (increased generalizability), and (3) no restriction on data before the incident epilepsy diagnosis code (prevalent epilepsy).
Statistical analysis
Descriptive statistics were calculated to summarize demographics and clinical characteristics of the cases and controls. Covariate balance was examined before and after propensity score matching with χ2 tests for categorical variables and t tests for continuous variables. Separate regression models (logistic regression for binary outcomes and linear regression for continuous outcomes) were built to estimate the association between neurologist visit and each individual outcome over time. Specifically, each outcome was estimated per year (e.g., total expenditures in years 1–5 as measured from index date) for each patient. Multilevel regression models were fit for each outcome after adjustment for patient age, lag time variables, and baseline covariates that were still unbalanced after matching (chronic obstructive pulmonary disease, preexisting anxiety diagnosis, preexisting chronic pain, eligibility time, and setting of incident epilepsy diagnosis) and including a random participant-level intercept. The influence of neurologist visits over time on each outcome was estimated with average marginal effects, with the random intercept set at its mean. Two-sided p values were reported and were considered significant at p < 0.05. Statistical analyses were performed with SAS 9.4 (SAS institute, Cary, NC) and STATA 14 (StataCorp, College Station, TX).
All results presented are adjusted for unbalanced baseline covariates, patient age, and lag time variables and are reported as predicted probabilities, which may be interpreted as adjusted outcomes over time comparing patients who did and did not have a neurologist visit.
Standard protocol approvals, registrations, and patient consents
This study used deidentified data and was determined to be exempt from review by the University of Michigan Institutional Review Board.
Data availability
The full dataset, OptumInsight Clinformatics Data Mart, is available through Optum (Optum.com).
Results
Demographic and clinical characteristics
We identified a total of 10,896 patients who saw a neurologist at or after their incident epilepsy diagnosis and 3,400 patients who did not see a neurologist for epilepsy. After propensity score matching, 3,400 cases (neurologist) and 3,400 controls (no neurologist) remained. Baseline characteristics of the study groups are presented in table 3. At the time of diagnosis, some small differences persisted between cases and controls: cases were more likely to have chronic obstructive pulmonary disease (30.0% vs 26.7%, p = 0.002), a diagnosis of anxiety (20.6% vs 18.0%, p = 0.008), and a diagnosis of chronic pain (43.7% vs 39.4%, p < 0.001). Compared to controls, cases were more frequently diagnosed in clinic (35.5% vs 33.4%, p = 0.007) than at an ER or inpatient facility. Lastly, cases were eligible for insurance coverage for a shorter length of time before incident diagnosis (mean 1,680 days vs 1,744, p = 0.002). Medical use and total medical expenditures in the 2 years preceding epilepsy diagnosis were similar between the 2 cohorts.
Table 3.
Baseline characteristics for neurologist and no neurologist cohorts after propensity score matching
Cost: Acute care use
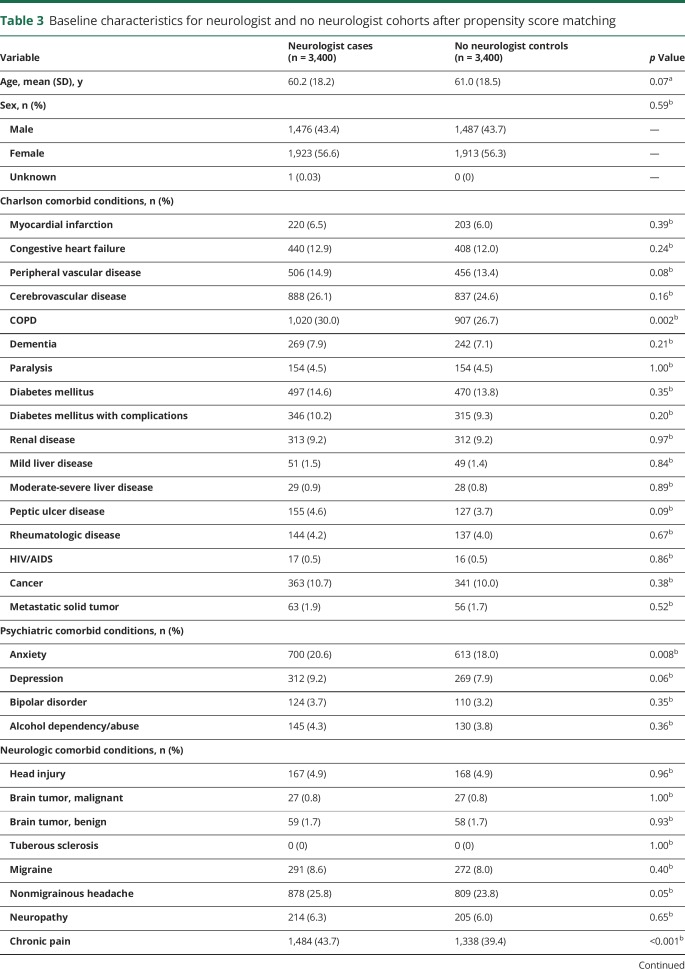
ER visits for epilepsy were more likely for cases than controls (predicted probabilities year 1: 5.9% vs 2.3%, p < 0.001), and the magnitude of difference decreased over time (year 5: 1.0% vs 0.4%, p < 0.001) (figure 2A). A similar pattern was observed with the number of ER visits for epilepsy (predicted visits year 1: 0.14 vs 0.07, p < 0.001; year 5: 0.04 vs 0.02, p < 0.03). Hospitalization for epilepsy was also higher for cases than controls (predicted probabilities year 1: 2.1% vs 0.7%, p < 0.001); this difference also decreased over time (year 5: 0.4% vs 0.2%, p < 0.001) (figure 2B).
Figure 2. Health care use for epilepsy and costs of care.
Predicted (A) epilepsy-related emergency room (ER) visits, (B) epilepsy-related hospitalizations, (C) epilepsy medical costs, (D) nonepilepsy medical costs, and (E) antiepileptic drugs costs each year for cases compared to controls with 95% confidence intervals. USD = US dollars.
Cost: Total medical expenditures
Epilepsy-related total medical costs were higher for cases than controls (predicted costs year 1: $5,464 vs $2,364, p < 0.001), and costs declined for both cohorts through the study period (year 5: $2,111 vs $1,051, p < 0.001) (figure 2C). Total cost of nonepilepsy care was similarly greater for cases than controls (year 1: $38,082 vs $32,135; year 5: $28,861 vs $26,638, p = 0.001) (figure 2D). Antiepileptic drug costs remained substantially higher for cases throughout the study period (p < 0.001) (figure 2E).
Quality of care: Measures of standard evaluation and care escalation
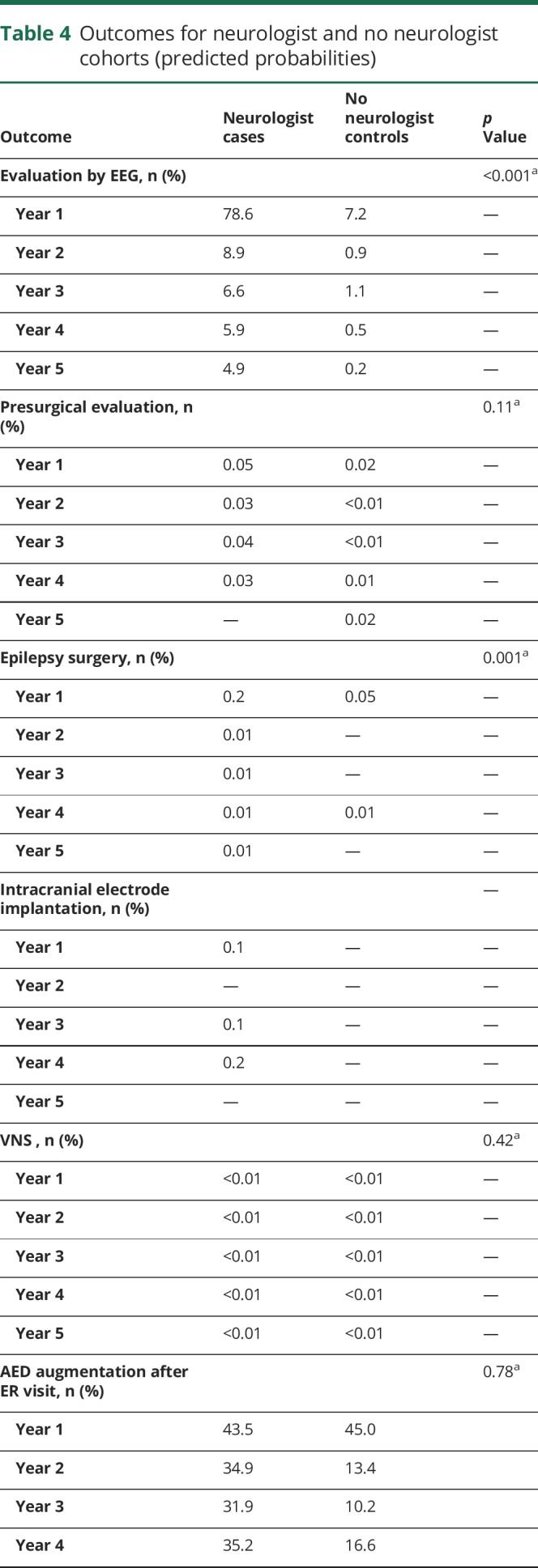
Evaluation by EEG was much more common for cases than controls, most notably in the first year (predicted probabilities year 1: 78.6% vs 7.2%, p < 0.001) (table 4). Completion of epilepsy surgery was more common for cases than controls (year 1: 0.2% vs 0.05%, p = 0.001), although surgery was rare in both cohorts, occurring in total for only 47 cases (1.4%) and 16 controls (0.5%). There were no differences between cohorts in probability of presurgical evaluation, intracranial electrode implantation, use of VNS, or augmentation of antiepileptic medication after an ER visit for seizure.
Table 4.
Outcomes for neurologist and no neurologist cohorts (predicted probabilities)
Quality of care: Attention to epilepsy comorbid conditions and women of childbearing age
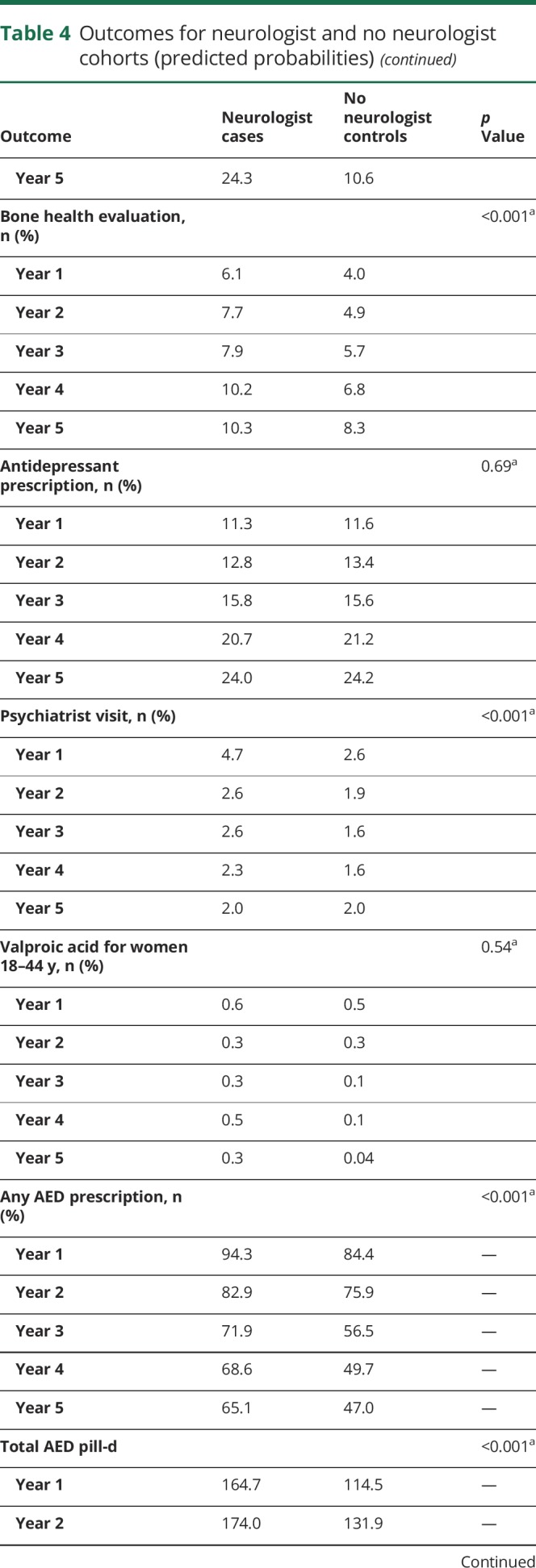
Cases had a higher rate of bone health evaluation, as measured by vitamin D testing and dual-energy x-ray absorptiometry scan (predicted probabilities year 1: 6.1% vs 4.0%; year 5: 10.3% vs 8.3%, p < 0.001). Antidepressant prescription did not differ between cohorts; however, cases more commonly had psychiatrist visits, particularly in the first year (year 1: 4.7% vs 2.6%, p < 0.001). Valproic acid prescription for women of childbearing age did not differ between cohorts.
Quality of care: Antiepileptic drug use patterns
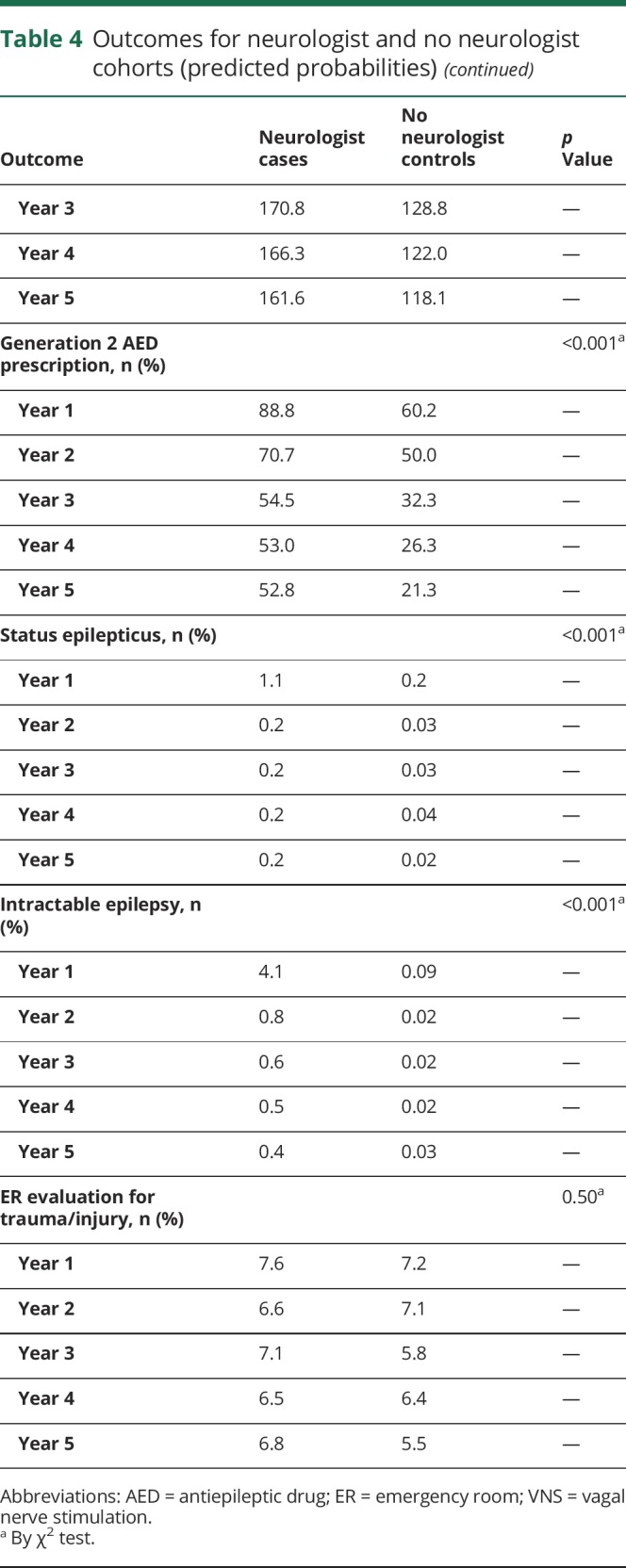
Cases were more likely to be continued on antiepileptic medication after initial prescription compared to controls (predicted probabilities year 1: 94.3% vs 84.4%, p < 0.001); this proportion declined over time for both groups (year 5: 65.1% vs 47.0%, p < 0.001). Among patients who were prescribed antiepileptic drugs, cases had higher total days of antiepileptic medication supply than controls (p < 0.001). Prescription of generation 2 antiepileptic medications was more likely for cases than controls (p < 0.001).
Measures of disease severity and trauma/injury
Status epilepticus and intractable epilepsy were more likely for cases than controls (p < 0.001). Presentations to the ER for trauma or injury were similar between cohorts.
Sensitivity analyses
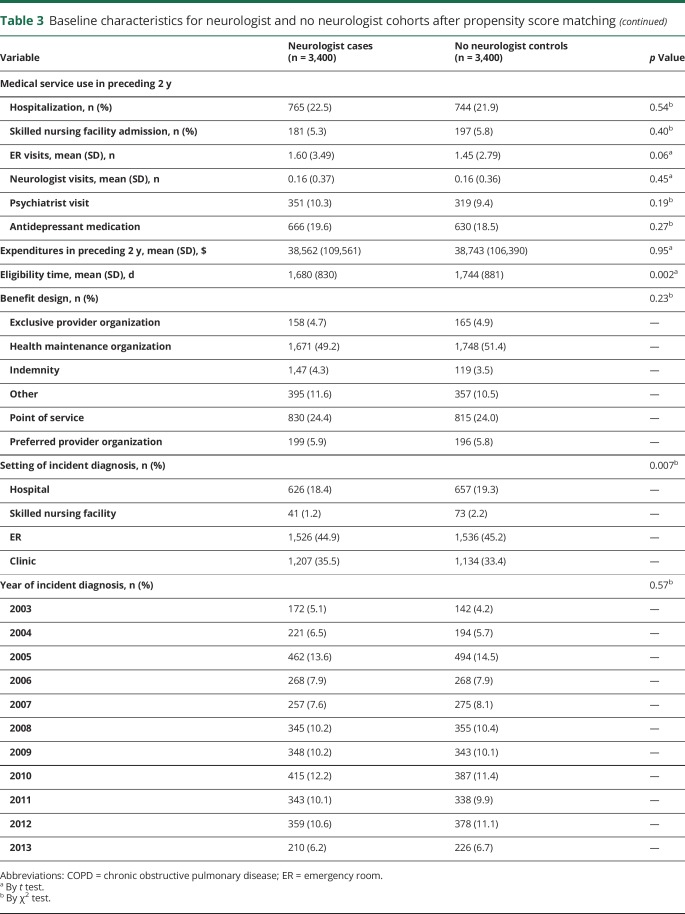
Alterations in the definition of our population, both those more restrictive and those less restrictive (including epilepsy prevalence), led to no significant changes in our primary outcomes. The requirement of 2 epilepsy diagnoses resulted in higher rates of care use overall and narrowed the magnitude of difference between patient cohorts. Total epilepsy-related costs remained greater for cases; however, total non–epilepsy-related costs were no longer significantly greater for cases than controls (figure 3).
Figure 3. Health care use for epilepsy and medical costs: sensitivity analysis.
For the population defined with the requirement of 2 epilepsy diagnosis codes, predicted (A) epilepsy-related emergency room (ER) visits, (B) epilepsy-related hospitalizations, (C) epilepsy medical costs, and (D) nonepilepsy medical costs each year for cases compared to controls with 95% confidence intervals. USD = US dollars.
Discussion
In a large cohort of privately insured patients, we found that patients with incident epilepsy who visited a neurologist had higher subsequent epilepsy-related ER visit and hospitalization rates compared to patients who did not see a neurologist. This difference was greatest in the first year and decreased over time. A similar pattern was observed for epilepsy and nonepilepsy medical costs, with higher costs for patients who visited a neurologist, most notably in the first year.
There are multiple ways to interpret the finding of higher epilepsy-related health care use and cost among cases than controls. The first is that neurologist involvement directly leads to increased ER visits and hospitalizations with their associated costs. A prior investigation of patients with acute stroke found that neurologist management was associated with more extensive testing (however, these patients also had improved outcomes)17; it is a possibility that neurologist management of patients with incident epilepsy increases costs without improving all clinical outcomes. A second explanation is that patients referred to a neurologist have more severe disease or differ from nonreferred patients in other ways that increase use and cannot be measured. While we adjusted for a comprehensive set of variables through application of a 43-factor propensity score, adjustment for remaining dissimilarities, and consideration of lag time events, there are undoubtedly unmeasured differences. Residual confounding is most strongly suggested by our observation that nonepilepsy medical costs, an outcome that should be largely outside of neurologist influence, were also higher for cases than controls. It is conceivable that neurologist involvement encourages patients to seek additional health care directly through referral to other specialists and testing and/or indirectly through resultant proximity to care facilities and providers. However, it is more likely that patients with complicated illness are directed to neurologists for care.
Additional findings further support the notion of confounding by disease severity. The most compelling argument is that when we applied a more stringent disease definition (2 epilepsy diagnostic codes) in sensitivity analysis, the difference in epilepsy care use and cost between cohorts contracted, and nonepilepsy care costs became comparable. Several other factors also suggest that the case cohort is a more medically complex population (although these may have alternative explanations). Considerable comorbidity burden has been described for patients with incident epilepsy, most notably concomitant neurologic disease and psychiatric disorders,2,18 and we found more frequent psychiatrist visits for cases than controls. Characterization of antiepileptic use revealed both a greater proportion of the cases continued on antiepileptic drugs after initial diagnosis and higher total days of drug supply each year, suggestive of recurrent or refractory seizures. Both status epilepticus and intractable epilepsy were more common within the case cohort. Lastly, nearly every measurable indicator of comorbidity was higher among cases, which cautions that this cohort is likely sicker in unmeasured ways as well. Both of these factors are potential confounders by disease severity that are difficult to address with claims data.
The results of our study suggest that even rigorously adjusted claims data are likely inadequate to assess the value of care in epilepsy. The importance and difficulty of proper risk adjustment have been highlighted in recent investigations of claims data for stroke patients and patients with headache.12,19 Several epilepsy-specific considerations make measurement of care quality particularly elusive for this patient population. Classic measures of disease severity such as epilepsy etiology, seizure frequency, and seizure type20 are not readily abstracted from insurance claims. Expenditures are known to be concentrated in patients with drug-resistant epilepsy,5,21 yet claims-based identification of this subpopulation is challenging.22 Epilepsy is a chronic condition, typically managed incrementally over a patient's lifetime, and therefore difficult to capture in a several-year study window. Even selection of clinical outcomes is not straightforward. While patients, providers, and payers generally prefer to avoid hospitalizations, not all hospitalizations for epilepsy are adverse outcomes. Frequently, patients are electively admitted to the hospital to clarify their diagnosis or to receive a therapeutic intervention such as epilepsy surgery. Some important patient outcomes for epilepsy management such as seizure freedom and antiepileptic drug tolerability cannot be assessed through claims data. Moreover, certain features of the illness are nearly impossible to disentangle for interpretation. Does a diagnosis of intractable epilepsy indicate severe underlying disease, insufficient management, or differences in coding behaviors? Does therapy escalation indicate physician responsiveness, severe underlying disease, or both?
Nonetheless, closer examination of how epilepsy-related expenditures differed between cases and controls suggests some potential benefits of neurologist care and additional opportunities to measure quality and value. Recent studies have proposed that antiepileptic medications are a major driver of epilepsy-related spending, accounting for 8% to 77% of direct costs.21 We observed that antiepileptic medication costs were substantially higher for cases than controls; however, there may be unmeasured benefit from such spending. Cases were more commonly prescribed generation 2 antiepileptic drugs. While newer medications are pricier than older drugs and may not be more effective for seizures,23 it is generally accepted that newer medications are better tolerated and associated with decreased morbidity.24 Antiepileptic medication typically should not be discontinued until several years of seizure freedom have elapsed,25 and recent studies have shown that retention rates for certain generation 2 antiepileptic drugs are greater than those for certain older medications.26,27 In our study, continued treatment with an antiepileptic drug was more common for cases than controls. While beyond the scope of this study, identification of drug-resistant patients and quantification of indirect costs should be included in future investigations. We also found that cases more frequently underwent diagnostic evaluation and surgery; therefore, neurologists appear to be important facilitators of targeted therapies such as epilepsy surgery. However, the benefits of surgery may not be realized for several years.28 Other holistic care aspects, including cases receiving bone health evaluation more frequently than controls and a greater proportion of cases being seen by psychiatrists, could be further studied to determine whether fracture risk or treatment for mood disorders is more favorable for cases than controls. Additional morbidity associated with long-term use of certain antiepileptic drugs such as metabolic syndrome, neuropathy, cerebellar atrophy, gingival hyperplasia, weight gain, hepatotoxity, and anemia could be studied with a longer observation period. Furthermore, consideration of use and cost outcomes over a longer follow-up period is particularly important because the tendency for patients with epilepsy to be eligible for disability and therefore receive insurance through Medicare or Medicaid is high. A reduction in long-term medical costs and even disability payments for this population could have dramatic effects on future federal and state expenditures.
Measurement of patient-centered metrics is a critical component of comprehensive assessment of the value of neurologist care. A randomized controlled trial would be optimal for comparing neurologist care to no neurologist care and could allow collection of rich, multidimensional clinical data. However, the ethical standing of randomizing a patient with complex, highly morbid, treatable disease to nonexpert care is questionable. Another potentially more feasible way to answer this research question is through the linking of claims data to patient registry data, which ideally would include patient-centered metrics such as quality of life, seizure freedom, mood, and return to work.
The typical challenges associated with administrative claims data apply to this study. We analyzed data collected primarily for billing purposes and therefore lacked fine clinical resolution. In addition, there are likely undescribed differences in how neurologists code encounters compared to nonneurologists. Retrospectively identifying patients with epilepsy is difficult; patients may have seizures from conditions other than epilepsy (e.g., hypoglycemia or alcohol withdrawal) or may have convulsive episodes due to alternative pathophysiology (e.g., psychogenic nonepileptic spells or syncope). Claims data have limited clinical details to allow the determination of correct alternative diagnosis. In this way, we may have failed to measure additional benefit of neurologist care. Selection bias due to referral is inherent in any valuation of specialist care; however, an extensive collection of potential confounding factors was considered through application of propensity score matching. In fact, our risk adjustment strategy was substantially more robust and comprehensive than the current proposals to measure physician value, which would also use administrative data. In addition, control patients were not required to have seen a nonneurologist provider for epilepsy care after incident diagnosis, while case patients were required to see a neurologist for epilepsy care by inclusion criterion. However, a substantial proportion of controls received epilepsy-related care in the 5 years after the index date: 83.5% of controls in year 1 (compared to 94.3% of cases) and 24.7% of controls in year 5 (compared to 31% of cases). As noted, using hospitalization as an outcome is complicated because not all hospitalizations for epilepsy are adverse outcomes; some hospitalizations may represent an appropriate care escalation. Several important measures relating to women of childbearing age such as folic acid prescription, contraception use, and pregnancy complications could not be evaluated because of limitations in claims data. Lastly, the generalizability of our findings may be limited by the use of data from a single, private insurer and by the small percentage of patients who met study criteria.
ER visits, hospitalizations, and medical costs were greater for patients with incident epilepsy who visited a neurologist compared to those who did not. However, our results suggest that this comparison may be confounded by other factors such as disease severity and that claims data have limited ability to characterize the value of epilepsy care provided by physicians. This study has important ramifications for neurologists and other specialists in an era of quality payment programs and alternative payment model development. New payment models should be cautious with penalties until better risk adjustment strategies are available or linkage of patient registry data becomes possible.
Glossary
- ER
emergency room
- ICD
International Classification of Diseases
- VNS
vagal nerve stimulation
Author contributions
Dr. Hill and Dr. Lin contributed to study design, data analysis and interpretation, and manuscript drafting and revision. Dr. Burke and Dr. Kerber contributed to study design and conceptualization, data analysis and interpretation, and manuscript revision. Dr. Skolarus, Dr. Esper, and Mr. Magliocco contributed to study conceptualization and manuscript revision. Dr. Callaghan contributed to study design and conceptualization, data analysis and interpretation, and manuscript revision.
Study funding
Dr. Hill is supported by the Mirowski Family Fund and the Family of Jonathan Rothberg. Dr. Burke is supported by NIH K08 NS082597 and R01 MD008879. Dr. Kerber is supported by NIH/National Center for Research Resources K23 RR024009, Agency for Healthcare Research and Quality (AHRQ) R18 HS017690, NIH/National Institute on Deafness and Other Communication Disorders R01 DC012760, and AHRQ R18 HS022258. Dr. Skolarus is supported by NIH R01 MD008879, U01 AG032947, and R01 MD011516. Dr. Callaghan is supported by NIH K23 NS079417 and Veterans Affairs Clinical Science Research and Development Merit CX001504. The study was proposed and executed by the American Academy of Neurology Health Services Research Subcommittee. The study was funded by the American Academy of Neurology.
Disclosure
C. Hill, C. Lin, J. Burke, and K. Kerber report no disclosures relevant to the manuscript. L. Skolarus performed consulting for Bracket Global. G. Esper is a consultant for NeuroOne, Inc. He performs medicolegal consultations. B. Magliocco reports no disclosures relevant to the manuscript. B. Callaghan receives research support from Impeto Medical Inc. He performs medical consultations for Advance Medical, consults for a Patient-Centered Outcomes Research Institutegrant, consults for the immune tolerance network, and performs medical legal consultations. Go to Neurology.org/N for full disclosures.
References
- 1.Gorelick PB. Adaptation of neurological practice and policy to a changing US health-care landscape. Lancet Neurol 2016;15:444–450. [DOI] [PubMed] [Google Scholar]
- 2.England MJ, Liverman CT, Schultz AM, Strawbridge LM; Institute of Medicine Committee on Public Health Dimensions of the Epilepsies. Epilepsy Across the Spectrum: Promoting Health and Understanding. Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 3.Gooch CL, Pracht E, Borenstein AR. The burden of neurological disease in the United States: a summary report and call to action. Ann Neurol 2017;81:479–484. [DOI] [PubMed] [Google Scholar]
- 4.Begley CE, Durgin TL. The direct cost of epilepsy in the United States: a systematic review of estimates. Epilepsia 2015;56:1376–1387. [DOI] [PubMed] [Google Scholar]
- 5.Begley CE, Famulari M, Annegers JF, et al. The cost of epilepsy in the United States: an estimate from population-based clinical and survey data. Epilepsia 2000;41:342–351. [DOI] [PubMed] [Google Scholar]
- 6.Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- 7.Labiner DM, Bagic AI, Herman ST, Fountain NB, Walczak TS, Gumnit RJ. Essential services, personnel, and facilities in specialized epilepsy centers: revised 2010 guidelines. Epilepsia 2010;51:2322–2333. [DOI] [PubMed] [Google Scholar]
- 8.Desforges JF, Scheuer ML, Pedley TA. The evaluation and treatment of seizures. N Engl J Med 1990;323:1468–1474. [DOI] [PubMed] [Google Scholar]
- 9.Ney JP, Johnson B, Knabel T, Craft K, Kaufman J. Neurologist ambulatory care, health care utilization, and costs in a large commercial dataset. Neurology 2016;86:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clough JD, McClellan M. Implementing MACRA. JAMA 2016;315:2397. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell JB, Ballard DJ, Whisnant JP, Ammering CJ, Samsa GP, Matchar DB. What role do neurologists play in determining the costs and outcomes of stroke patients? Stroke 1996;27:1937–1943. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan BC, Burke JF, Kerber KA, et al. The association of neurologists with headache health care utilization and costs. Neurology 2018;90:e525–e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ney JP, Sico JJ, Klein BC, Magliocco B, Callaghan BC, Esper GJ. Association of neurologist care with headache expenditures: a population-based, longitudinal analysis. Cephalalgia 2018. 38:1876–1884. [DOI] [PubMed] [Google Scholar]
- 14.Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag 2005;8:1–14. [DOI] [PubMed] [Google Scholar]
- 15.Moura LM, Price M, Cole AJ, Hoch DB, Hsu J. Accuracy of claims-based algorithms for epilepsy research: revealing the unseen performance of claims-based studies. Epilepsia 2017;58:683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Matchar DB, Hoff-Lindquist J, Samsa GP, Horner RD. VA Stroke Study: neurologist care is associated with increased testing but improved outcomes. Neurology 2003;61:792–796. [DOI] [PubMed] [Google Scholar]
- 18.Martin RC, Faught E, Richman J, et al. Psychiatric and neurologic risk factors for incident cases of new-onset epilepsy in older adults: data from U.S. Medicare beneficiaries. Epilepsia 2014;55:1120–1127. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz J, Wang Y, Qin L, et al. Incorporating stroke severity into hospital measures of 30-day mortality after ischemic stroke hospitalization. Stroke 2017;48:3101–3107. [DOI] [PubMed] [Google Scholar]
- 20.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strzelczyk A, Reese JP, Dodel R, Hamer HM. Cost of epilepsy: a systematic review. Pharmacoeconomics 2008;26:463–476. [DOI] [PubMed] [Google Scholar]
- 22.Burneo JG, Shariff SZ, Liu K, Leonard S, Saposnik G, Garg AX. Disparities in surgery among patients with intractable epilepsy in a universal health system. Neurology 2016;86:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beghi E, Atzeni L, Garattini L. Economic analysis of newer antiepileptic drugs. CNS Drugs 2008;22:861–875. [DOI] [PubMed] [Google Scholar]
- 24.French JA, Gazzola DM. New generation antiepileptic drugs: what do they offer in terms of improved tolerability and safety? Ther Adv Drug Saf 2011;2:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camfield P, Camfield C. When is it safe to discontinue AED treatment? Epilepsia 2008;49:25–28. [DOI] [PubMed] [Google Scholar]
- 26.Arif H, Buchsbaum R, Pierro J, et al. Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch Neurol 2010;67:408–415. [DOI] [PubMed] [Google Scholar]
- 27.Zeng QY, Fan TT, Zhu P, et al. Comparative long-term effectiveness of a monotherapy with five antiepileptic drugs for focal epilepsy in adult patients: a prospective cohort study. PLoS One 2015;10:e0131566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Picot MC, Jaussent A, Neveu D, et al. Cost-effectiveness analysis of epilepsy surgery in a controlled cohort of adult patients with intractable partial epilepsy: a 5-year follow-up study. Epilepsia 2016;57:1669–1679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full dataset, OptumInsight Clinformatics Data Mart, is available through Optum (Optum.com).