Abstract
Myosin light chain 2 (MYL2) gene encodes the myosin regulatory light chain (RLC) simultaneously in heart ventricles and in slow-twitch skeletal muscle. Using transgenic mice with cardiac-specific expression of the human R58Q-RLC mutant, we sought to determine whether the hypertrophic cardiomyopathy phenotype observed in papillary muscles (PMs) of R58Q mice is also manifested in slow-twitch soleus (SOL) muscles. Skinned SOL muscles and ventricular PMs of R58Q animals exhibited lower contractile force that was not observed in the fast-twitch extensor digitorum longus muscles of R58Q vs. wild-type–RLC mice, but mutant animals did not display gross muscle weakness in vivo. Consistent with SOL muscle abnormalities in R58Q vs. wild-type mice, myosin ATPase staining revealed a decreased proportion of fiber type I/type II only in SOL muscles but not in the extensor digitorum longus muscles. The similarities between SOL muscles and PMs of R58Q mice were further supported by quantitative proteomics. Differential regulation of proteins involved in energy metabolism, cell–cell interactions, and protein–protein signaling was concurrently observed in the hearts and SOL muscles of R58Q mice. In summary, even though R58Q expression was restricted to the heart of mice, functional similarities were clearly observed between the hearts and slow-twitch skeletal muscle, suggesting that MYL2 mutated models of hypertrophic cardiomyopathy may be useful research tools to study the molecular, structural, and energetic mechanisms of cardioskeletal myopathy associated with myosin RLC.—Kazmierczak, K., Liang, J., Yuan, C.-C., Yadav, S., Sitbon, Y. H., Walz, K., Ma, W., Irving, T. C., Cheah, J. X., Gomes, A. V., Szczesna-Cordary, D. Slow-twitch skeletal muscle defects accompany cardiac dysfunction in transgenic mice with a mutation in the myosin regulatory light chain.
Keywords: cardioskeletal myopathy, muscle proteomics, R58Q-RLC mutation, X-ray diffraction
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease of the heart muscle that is most commonly caused by mutations in sarcomeric muscle proteins (1). HCM is characterized by left ventricular hypertrophy, myofibrillar disarray, fibrosis, and severe contractile dysfunction, leading to compromised heart performance and sudden cardiac death (2). Dominant pathogenic variants in HCM genes are the most common causes of HCM (3), and the 2 most common genes responsible for approximately one half of the cases of familial HCM are MYH7 (myosin heavy chain 7) and myosin- binding protein C (MYBPC3) (2). Although mutations in myosin light chain 2 (MYL2) encoding the ventricular myosin regulatory light chain (RLC) are rare, they are often implicated in malignant HCM outcomes (4–7).
Although HCM primarily affects cardiac muscles, functional abnormalities of the skeletal muscle, with the absence of neurogenic defects, were observed in patients with HCM (8). Infantile fiber type I hypotrophy with concurrently occurring severe onset of cardiomyopathy was previously reported in several Dutch and Italian families (9). Shortly after birth, the patients experienced progressive skeletal muscle myopathy and ultimately died of heart failure between 4 and 6 mo of age due to the consequences of cardiomyopathy of a dilated, hypertrophic, restrictive, or noncompaction origin. More than a decade later, genetic studies have linked this progressive cardioskeletal myopathy and heart failure observed in Dutch and Italian patients to the mutated MYL2 gene (10) that simultaneously expresses the myosin RLC protein in heart ventricles and in slow-twitch skeletal muscle (11).
Myosin RLC is a major regulatory subunit of cardiac myosin and a modulator of Ca2+ and tropomyosin-troponin–regulated muscle contraction and heart function (12). It is positioned at the head-rod junction of the myosin heavy chain (MHC) and, together with the myosin essential light chain, stabilizes the α-helical neck region of the myosin head (13). To address the mechanisms responsible for triggering cardiac disease, several RLC mutations have been investigated in humanized mouse models expressing the human ventricular RLC mutant proteins (14–21). This study focuses on the R58Q (arginine-to-glutamine) mutation shown by population studies to cause HCM and sudden cardiac death that occurred in families of various ethnic origins (5–7). Characterization of α-MHC–driven transgenic expression of R58Q in mice exhibited cardiac hypertrophy, histopathologic changes, fibrosis, and diastolic dysfunction (15, 16), consistent with human HCM phenotypes that were not observed in transgenic wild-type (WT) mice expressing the human ventricular RLC [Accession No. P10916; National Center for Biotechnology Information (NCBI), Bethesda, MD, USA]. The molecular basis of heart pathology associated with the R58Q mutation has been studied with a wide range of research tools, including investigations of structure–function effects in R58Q-mutant reconstituted cardiac muscle preparations (22–26) and transgenic hearts of R58Q mice (15, 16, 27–29). Combined results suggest that the mutation imposes significant perturbations in the motor function of myosin head, leading to its reduced ability to interact with actin and generate force.
The study question addressed here was whether the R58Q-RLC mouse model of HCM also manifests the slow-twitch skeletal muscle abnormalities that are evidenced in R58Q vs. WT hearts (15, 16). The results of our multilevel investigation show that the HCM-related functional, structural, and energetic changes observed in R58Q hearts are also present in soleus (SOL) muscles but not in the fast-twitch extensor digitorum longus (EDL) muscle of R58Q-HCM mice. Remarkably, differential regulation of proteins involved in energy metabolism, cell–cell interactions, and protein–protein signaling were concurrently observed in the hearts and SOL muscles of R58Q mice according to quantitative proteomics. Therefore, cardiac remodeling observed in R58Q hearts is echoed in slow-twitch skeletal muscles by virtue of a multitude of abnormal protein–protein and cellular interactions.
MATERIALS AND METHODS
Transgenic mice
The present study conforms to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH; Bethesda, MD, USA] publication no. 85–23, revised 2011]. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Miami Miller School of Medicine (Assurance No. A-3224-01, effective November 24, 2015). Euthanasia of mice was achieved through inhalation of CO2 followed by cervical dislocation. The generation and characterization of transgenic WT and R58Q mice used in this study have been previously described (15). Previously produced transgenic WT L2 expressing the human ventricular RLC (Accession No. P10916; NCBI) and the Arg58→Gln mutation, R58Q L9, were used (15, 16).
Ventricular and SOL muscle expression of human WT (MYL2) and R58Q mutation
Relative expression of human ventricular/slow-twitch skeletal WT and R58Q mutant RLC (MYL2 gene) was assessed in SOL muscles and in the hearts of transgenic WT-L2 and R58Q-L9 mice by using mass spectrometry (Supplemental Fig. S1). SDS-PAGE bands of myosin purified from the hearts or SOL muscles of mice were digested with trypsin, then reduced and alkylated. Digested peptides were analyzed by mass spectrometry, and protein identification was determined using X! Tandem (The Global Proteome Machine Organization; https://www.thegpm.org/TANDEM/index.html). The relative amount of human protein was determined by the amount of human-specific peptide (AGGANSN) relative to the total amount of mouse (IEGGSSN) and human-specific RLC peptides (21).
Steady-state force development and force-pCa relationship
Left ventricular papillary muscles (PMs), SOL muscles, and EDL muscles were isolated from 6- to 9-mo-old R58Q [2 males (M) and 2 females (F)] and WT (3M and 1F) mice and dissected into small muscle bundles (2–3 mm in length and 0.5–1 mm in diameter) in ice-cold pCa 8 solution that contained 30 mM 2,3-butanedione monoxime and 15% glycerol. After dissection, the bundles were transferred to pCa 8 solution [10−8 M Ca2+, 1 mM Mg2+, 7 mM EGTA, 2.5 mM MgATP2−, 15 mM creatine phosphate, 20 mM (3-N-morpholino)-propanesulfonic acid], ionic strength adjusted to 150 mM with K+-propionate, and pH adjusted to 7.00, mixed with 50% glycerol, and incubated for 1 h on ice. The strips were then transferred to a fresh pCa8/50% glycerol, v/v (storage solution) mixed with 1% Triton X-100 (MilliporeSigma, Burlington, MA, USA) for 24 h at 4°C. Finally, the fibers were transferred to a fresh storage solution and kept at −20°C for 5–10 d (19–21). Before the experiment, muscle bundles were dissected into strips of ∼1.5 mm in length and ∼100 μm in diameter, attached to a Güth force transducer, freshly skinned in pCa 8 solution with 1% Triton X-100 (∼30 min), rinsed thoroughly in pCa 8 solution, and their sarcomere length adjusted to 2.1–2.3 µm. The force-pCa measurements were performed at 21°C in solutions of increasing Ca2+ concentrations from pCa 8 to 4, each containing the ATP regeneration system. The force-pCa curves were analyzed by using the Hill equation, and the maximal tension (pCa 4) was expressed in kilonewton per square meters with the cross-sectional area assumed to be circular (19–21).
In vivo assessment of skeletal muscle phenotype
To evaluate the in vivo neuromuscular performance of mice, the limb grip strength test using the Grip Strength Test equipment from Bioseb (model Bio-GS3; Pinellas Park, FL, USA) was employed. The test is based on the natural tendency of the mouse to grasp a bar or grid when it is suspended by the tail and gently pulled backward (30). The grip strength of the forelimb or all 4 limbs of each mouse was measured by allowing the mouse to grasp a wire screen as it was being pulled by the tail horizontally relative to the force meter. The peak resistance force was recorded at the time the animal released the grip as it was pulled away from the device (30). To reduce procedure-related variability, an average of 4 repeated peak force measurements for the same animal were analyzed. The strength of the forelimb muscles was also evaluated by using a hanging wire test (31). Mice were gently placed near the wire to allow them to utilize their forelimbs to suspend their body weight on a wire stretched ∼20 cm above a foam pillow. The hanging time (in seconds) was recorded until the animal fell off the wire. A score of 0 was assigned if the mouse fell off immediately, and 60 s was the timeout period. Three trials were performed for each mouse. Mice that exhibited an unexpected behavior such as balancing properly on the wire or those that refused to hang by the forelimbs were excluded from the experiment.
Small angle X-ray diffraction studies
Equatorial X-ray diffraction patterns were collected from freshly skinned left ventricular PMs and SOL muscle strips mounted in a X-ray chamber using the small angle instrument on the BioCAT beamline 18-D at the Advanced Photon Source, Argonne National Laboratory (Argonne, IL, USA) as previously described (20, 21). The following mice were used for measurements in PMs: WT, 6M and 14F (5–7 mo old); and R58Q, 4M and 11F (4–8 mo old). For measurements in SOL muscle, 4M and 8F (5–6 mo old) were used for WT, and 2M and 6F (4 mo old) were used for R58Q. As previously described, simultaneous force and diffraction pattern measurements were performed in serial pCa (8, 7, 6, 5.4, 5.2, and 4) solutions (21). Force and length were monitored by using a digital controller (model 600A; Aurora Scientific, Aurora, ON, Canada). Diffraction patterns were collected only at the plateau phase of force developing during contraction in each activation pCa solution. Equatorial small-angle X-ray diffraction patterns were collected on a CCD-based X-ray detector (Mar 165; Rayonix, LLC, Evanston, IL, USA). The position of the 1,0 and 1,1 equatorial X-ray reflections and the resulting distance d1,0 was converted to the center-to-center distance [interfilament lattice spacing (IFS)] between 2 adjacent thick filaments by multiplying by 2/√3. Intensities of the 1,0 and 1,1 equatorial X-ray reflections were determined from nonlinear least square fits to 1-dimensional projections of the integrated intensity along the equator. Data were analyzed independently by 3 people, and the results were averaged as previously described.
Fiber typing and determination of cross-sectional areas
SOL and EDL muscles were dissected from R58Q and WT mice (three 7–9-mo-old mice/each genotype) and embedded in Optimal Cutting Temperature (O.C.T.) Compound (Tissue-Tek, Torrance, CA, USA). They were snap-frozen in liquid nitrogen and stored in −80°C until needed. Frozen blocks were placed in a cryostat at −20°C and left for 30 min before processing. The midsections of muscle were cut into 10-µm thick sections and used for fiber typing according to the protocol available from Washington University School of Medicine, Neuromuscular Disease Center (St. Louis, MO, USA). Briefly, two 10-μm serial cut sections were incubated at room temperature in preincubation solution: 1) pH 4.31 (0.2 M barbital acetate) for 5 min; or 2) pH 10.20 (0.02 M sodium barbital, 0.036 M CaCl2) for 15 min. After rinsing with H2O, the sections were incubated in ATP-containing solution (3.6 mM ATP, 27 mM sodium barbital, 18 mM CaCl2) for 25 min (acidic preincubated slides) or for 15 min (alkali preincubated slides). The sections were then washed in 1% CaCl2 for 10 min and transferred to 2% CoCl2 for 10 min, washed in 5 mM sodium barbital, and rinsed with distilled water. The reactions were developed with 2% ammonium sulfide for 20–30 s, washed with 5 exchanges of water, dehydrated in ascending ethanol, and cleared with xylene. The sections were mounted by using Canada balsam and examined for the number (and percentage) of each fiber type. Cross-sectional areas were measured for at least 250 cells per animal by using ImageJ software (NIH).
Proteomics experiments
Hearts and SOL muscles from three 8-mo-old male R58Q, and three 7-mo-old male WT mice were collected for proteomic studies. Protein isolation, sample processing, and data analysis were performed as previously described (20, 32). Statistically significant changes in protein expression in the hearts (Supplemental Table S1) or SOL muscles (Supplemental Table S2) of R58Q vs. WT mice are shown with the positive fold change (FC) values indicating the up-regulated proteins and the negative FC indicating the proteins that were down-regulated in the mutant mouse model. Briefly, 20 mg of powdered muscle tissue was solubilized in 8 M urea containing protease and phosphatase inhibitors (MilliporeSigma). The concentration of the protein lysates was determined by using the Bio-Rad RC/DC method (Bio-Rad, Hercules, CA, USA), and 100 µg of protein from each muscle sample was digested with trypsin (trypsin gold sequencing grade; Promega, Madison, WI) after treatment with Tris(2-carboxyethyl)phosphine and iodoacetamide. After digestion, muscle samples were each labeled separately with different isotopic tandem mass Tag labeling (TMT) variants (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions and then combined. The comparison between 3 heart and SOL WT and 3 heart and SOL R58Q samples was conducted by using TMT 6-plex, which allows the direct comparison between 6 different samples in the same liquid chromatography tandem mass spectrometry analysis. Each set of TMT labeling was performed as previously described (20, 32). Briefly, TMT-labeled peptides were fractionated by using ion exchange columns (SCX SpinTips; Protea Biosciences, Morgantown, WV, USA). Elution of peptides from the SCX columns was performed in a stepwise manner using 20, 60, 80, 125, 150, 200, 400, and 500 mM ammonium formate in 10% acetonitrile at pH 3. Each eluted fraction was desalted by using SDB columns (GL Sciences, Tokyo, Japan), dried using a speed vacuum, and suspended in 5% acetonitrile, 0.1% TFA. Each desalted fraction was then analyzed by liquid chromatography tandem mass spectrometry on a Thermo Scientific Q ExactivePlus Orbitrap Mass Spectrometer with an attached Proxeon Nanospray source and a Waters UPLC (Waters, Milford, MA, USA) as previously described (20, 32). Scaffold Q+ (v.4.4.1; Proteome Software, Portland, OR, USA) was used to quantitate labeling-based quantitation (TMT) peptide and protein identifications. Peptide identifications were accepted if they could be established at >96.0% probability by the Scaffold Local false discovery rate (FDR) algorithm. Protein identifications were accepted if they could be established at >99.0% probability to achieve an FDR <1.0% and contained at least 2 identified peptides. Protein probabilities were assigned according to the Protein Prophet algorithm (33). Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. Normalization was performed iteratively (across samples and spectra) on intensities, as described in Oberg et al. (34). Medians were used for averaging. Spectra data were log-transformed, pruned of those matched to multiple proteins, and weighted by using an adaptive intensity-weighting algorithm. For the heart, of 165,645 spectra in the experiment at the given thresholds, 112,792 (68%) were included in quantitation. For SOL muscles, of 114,845 spectra in the experiment at the given thresholds, 73,558 (64%) were included in quantitation. Differentially expressed proteins were determined by applying the Mann-Whitney test with an unadjusted significance level of P < 0.05 corrected by the Bonferroni correction (an adjustment made to P values when several dependent or independent statistical tests are being performed simultaneously on a single dataset). The Protein Analysis Through Evolutionary Relationships (PANTHER) database (http://pantherdb.org) was used to determine the molecular and biologic processes of the differentially expressed proteins.
Validation of proteomics data
To confirm the up-regulation of desmin in the hearts (n = 4 mice per group) and SOL muscle (n = 3 mice/group) of R58Q vs. WT mice, as well as the up-regulation of the slow-skeletal myosin heavy chain (MYH4) in the SOL muscle of R58Q mice, SDS-PAGE was performed by using 15% (desmin) or 4–20% (MYH4) polyacrylamide gels. Immunoblotting was performed with a goat monoclonal antibody specific for desmin (sc-7559; Santa Cruz Biotechnology, Dallas, TX, USA), followed by a secondary donkey anti-goat antibody conjugated with IRDye 800CW (Li-Cor, Lincoln, NE, USA). For MYH4, a mouse monoclonal antibody (clone NOQ7.5.4D; M8421; MilliporeSigma) followed by goat anti-mouse antibody conjugated with the fluorescent dye IR 800 Red were used. Membranes were also probed with glyceraldehyde-3-phosphate dehydrogenase (MilliporeSigma) or C-terminal RLC (CT-1) antibody specific for myosin RLC (19, 21), that served as loading controls. Band intensities were quantified by using ImageJ software.
Statistical analysis
All values are shown as means ± sem or sd. Statistically significant differences between 2 groups were determined by using an unpaired Student’s t test, with significance defined as P < 0.05. Comparisons between multiple groups were performed by using 1- or 2-way ANOVA (Prism 7.03; GraphPad Software, La Jolla, CA, USA).
RESULTS
To study the origin of R58Q-induced cardiac remodeling echoed by similar effects on the structure and function of slow-twitch skeletal (SOL) muscle, the hearts and SOL muscle preparations of previously generated transgenic R58Q and WT mice (15) were subjected to force measurements and small-angle X-ray diffraction, followed by a quantitative proteomics approach. The data were separately collected for male and female mice; if there were no differences between sexes, the results were pooled.
Force development in skinned PMs and SOL muscles of mice
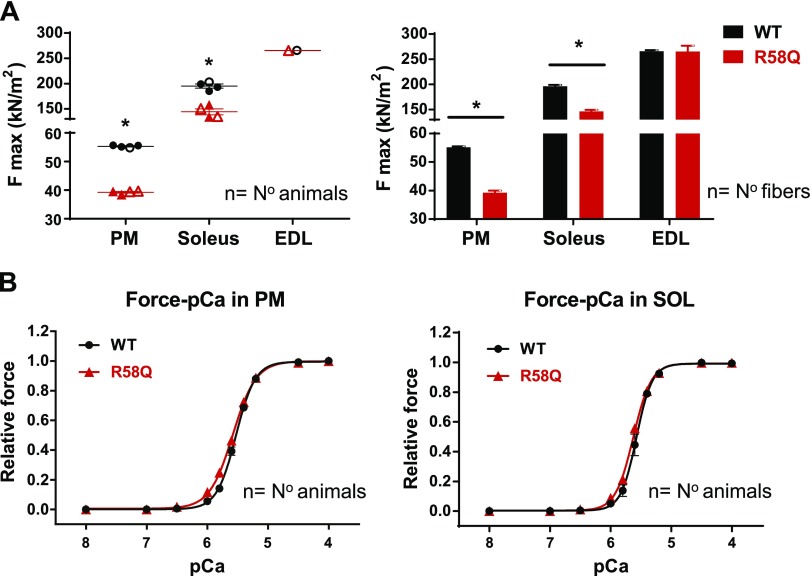
Measurements of steady-state force generation were performed in R58Q vs. WT mice using chemically skinned PMs and SOL muscle strips with the EDL muscle serving as a negative control. As shown in Fig. 1 and Table 1, a significantly lower maximal isometric force (at pCa 4) per cross-section of muscle was observed in R58Q vs. WT mice in both PMs (∼30% lower than WT) and SOL muscles (∼25% lower than WT) (P < 0.001); no differences between the genotypes were observed in maximal isometric force for EDL muscles. All 3 types of muscle (PM, SOL, and EDL) showed no statistically significant changes in the Ca2+ sensitivity of force in R58Q vs. WT mice. The force data in PMs are consistent with the previously established direction of changes in heart preparations of R58Q vs. WT mice (15, 16). The new measurements in SOL and control EDL muscles from the same R58Q vs. WT mice clearly indicate the functional similarities between the cardiac and slow-twitch skeletal muscles. Therefore, the effect of HCM mutation on the contractile force properties of cardiac muscle could also be observed in SOL muscles but not in EDL muscles.
Figure 1.
Force (A) and Ca2+ sensitivity (B) in skinned PMs and slow-twitch skeletal SOL muscles from 6- to 9-mo-old R58Q and WT mice. For measurements in PMs and SOL muscles, 2F and 2M R58Q and 1F and 3M WT mice were used. For measurements in EDL muscles (negative control), 1F for R58Q (10 fibers) and 1F WT (10 fibers) were used. The exact number of animals and fibers used in contractile measurements is listed in Table 1. Data are average of n ± sem animals (fibers for EDL). *P < 0.05 (Student’s t test).
TABLE 1.
Force measurements in skinned PMs, SOL muscles, and EDL muscles of R58Q vs. WT mice
| Force measurement | |||||
|---|---|---|---|---|---|
| System | Genotype | Fmax (kN/m2) | pCa50 | Hill coefficient | Animals, n (fibers) |
| PM | WT | 55.3 ± 0.2 | 5.53 ± 0.02 | 2.8 ± 0.1 | 4 (29) |
| R58Q | 39.2 ± 0.3** | 5.59 ± 0.02 | 2.3 ± 0.1 | 4 (33) | |
| SOL | WT | 195 ± 3.9 | 5.58 ± 0.03 | 3.9 ± 0.3 | 4 (30) |
| R58Q | 144.2 ± 5.9** | 5.63 ± 0.02 | 3.1 ± 0.2 | 4 (29) | |
| EDL | WT | 265.8 ± 2.5 | 5.59 ± 0.02 | 3.4 ± 0.1 | 1 (10) |
| R58Q | 265.7 ± 11.2 | 5.56 ± 0.02 | 3.9 ± 0.2 | 1 (10) | |
Data are the average ± sem animals (fibers for EDL) for force measurements. **P < 0.001 (Student’s t test).
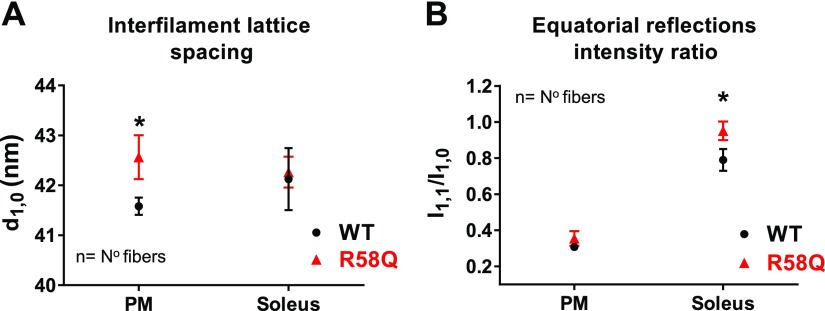
Sarcomeric structure in skinned PMs and SOL muscles by small-angle X-ray diffraction
To determine the structural effects of the R58Q mutation on the interfilament lattice spacing (d1,0 or IFS) in PMs and SOL fibers, freshly skinned muscles from R58Q and WT mice were studied by using X-ray diffraction as previously described (21). In this experiment, we measured the position of the 1,0 and 1,1 equatorial X-ray reflections, and the resulting d1,0 spacing was converted to the center-to-center distance (IFS) between 2 adjacent thick filaments. The intensity ratio of 1,1 to 1,0 equatorial X-ray reflections (I1,1/I1,0) denotes the ratio of the integrated intensity of the 1,1 equatorial reflection representing density in the plane containing both thick and thin filaments to that of the 1,0 equatorial X-ray reflection representing density in the plane containing only thick filaments (35). Changes in I1,1/I1,0 are commonly used as measures of shifts in mass, presumably cross-bridges, from the region of the thick filament to that of the thin filament. Both parameters (d1,0 and I1,1/I1,0) provide important metrics of sarcomeric structure and were shown previously to be sensitive to other amino acid substitutions in RLC and the myosin essential light chain proteins (19–21).
We found that the R58Q mutation significantly increased the IFS in PMs, from 48.01 ± 0.20 nm (WT) to 49.15 ± 0.51 nm (R58Q) under relaxing (pCa 8) conditions (Fig. 2A and Table 2). This finding implied an R58Q-mediated movement of the adjacent thick filaments farther away from each other and, therefore, from the thin filaments, leading to potentially reduced contractile force, which has been observed in PM force measurements (Fig. 1A). Comparison of d1,0 in SOL of R58Q vs. WT muscles showed no difference between the genotypes (Fig. 2A). The I1,1/I1,0 showed larger values for R58Q than for WT in both PMs and SOL muscles, but a statistically significant difference between R58Q and WT mice was only observed in SOL muscles (Fig. 2B and Table 2).
Figure 2.
IFS (d1,0; A) and I1,1/I1,0 (B) in skinned PM and SOL muscle fibers from R58Q vs. WT mice under relaxation (pCa 8) conditions. The following mice were used for measurements in PMs: WT, 6M and 14F (5–7 mo old); R58Q, 4M and 11F (4–8 mo-old). For measurements in SOL muscle, 4M and 8F (5–6 mo old) were used for WT and 2M and 6F (4 mo old) were used for R58Q (Table 2). IFS and I1,1/I1,0 were established for sarcomere length (SL) of 2.1 µm. Both d1,0 (IFS) and I1,1/I1,0 appeared higher in R58Q mice compared with WT mice. Statistical significance between the genotypes was observed for measurement of d1,0 in PMs (P = 0.015) and for I1,1/I1,0 (P = 0.05) in SOL muscles. Data are the average ± sem fibers. *P < 0.05 (Student’s t test).
TABLE 2.
Small-angle X-ray diffraction study (under relaxation, pCa 8) in PM and SOL muscles of R58Q vs. WT mice
| Small-angle X-ray diffraction study | ||||||
|---|---|---|---|---|---|---|
| System | Genotype | d1,0 (nm) | IFS (nm) | Fibers, n (animals) | I1,1/I1,0 | Fibers, n (animals) |
| PM | WT | 41.58 ± 0.18 | 48.01 ± 0.20 | 46 (16) | 0.36 ± 0.05 | 10 (4) |
| R58Q | 42.56 ± 0.44* | 49.15 ± 0.51* | 18 (10) | 0.43 ± 0.05 | 12 (5) | |
| SOL | WT | 42.13 ± 0.62 | 48.64 ± 0.72 | 17 (8) | 0.79 ± 0.06 | 12 (4) |
| R58Q | 42.27 ± 0.31 | 48.8 ± 0.36 | 17 (5) | 0.95 ± 0.06* | 13 (3) | |
Data are the average ± sem fibers for small-angle X-ray diffraction study. *P < 0.05 (Student’s t test).
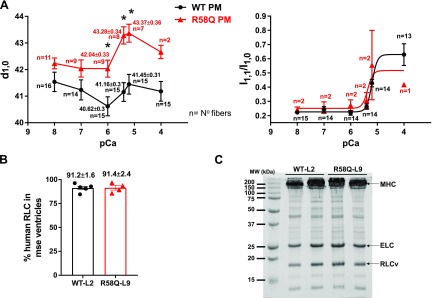
Simultaneous force and X-ray diffraction pattern measurements, assessed in PMs of R58Q and WT mice at serial pCa (8, 7, 6, 5.4, 5.2, and 4) solutions, are pictured in Fig. 3A. As for the experiment conducted in pCa 8, the sarcomere length of muscle was adjusted to 2.1 μm and measured by using He-Ne laser diffraction (21). Significant increases in d1,0 at 3 Ca2+-activation solutions (pCa, 6, 5.4, and 5.2) were observed in the PMs of R58Q compared with WT mice. These data are in accord with the measurement of lower contractile force in PMs of R58Q vs. WT mice (Fig. 1A) and are consistent with the interpretation of the striated muscle length-tension curve in the modeling study of Williams et al. (36). The I1,1/I1,0–pCa relationship follows the force-pCa curve with increasing I1,1/I1,0 as the calcium concentration rises. However, technical difficulties prevented us from the comparison of equal “n” for R58Q and WT muscles, and no significant differences between the R58Q vs. WT samples were detected. Direct assessment of the human cardiac RLC (in percentages) in the ventricles of WT-L2 and R58Q-L9 mice according to mass spectrometry showed the expression of 91.2 ± 1.6 (n = 6 samples) for WT and 91.4 ± 2.4 (n = 4) for R58Q (Fig. 3B). This assessment is in accord with the published values of transgenic RLC expression determined in the atria of WT-L2 and R58Q-L9 mice by SDS-PAGE (15). The representative SDS-PAGE image of myosin purified from the heart ventricles of WT and R58Q mice is shown in Fig. 3C.
Figure 3.
A) IFS and I1,1/I1,0 as a function of pCa (8, 7, 6, 5.4, 5.2, and 4) in skinned PM fibers from left ventricles of 5–7-mo-old R58Q (2F, 4M) and WT (4F, 3M) mice. The sarcomere length (SL) was adjusted to 2.1 μm. Data are the average ± sem fibers, *P < 0.05 (Student’s t test). B). Mass spectrometry data (average ± sd) showing expression of the human RLC in the ventricles of WT (n = 6 samples) and R58Q (n = 4 samples) mice. C) Representative SDS-PAGE image of myosin purified from heart ventricles of WT and R58Q mice.
Skeletal fiber-type remodeling in SOL muscles of R58Q vs. WT mice
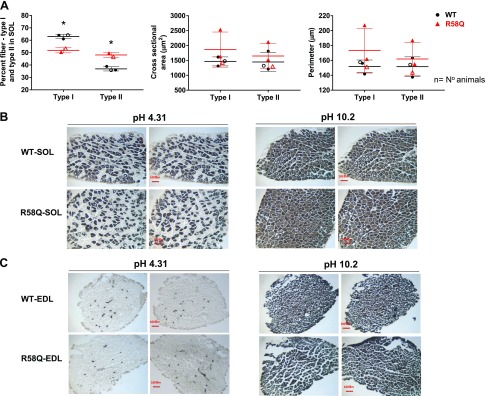
Histologic staining for myosin ATPase activity was performed in SOL vs. EDL muscles of mice to examine whether SOL muscles of HCM mice undergo changes in fiber type composition (proportion) compared with age- and sex-matched WT mice (Fig. 4). The ratio of fiber type I/ II was calculated from the images of cross-sections of SOL muscles preincubated at pH 4.31 or 4.53, in which fiber type I exhibit dark-gray staining compared with very-light-gray staining of fiber type II. At basic pH = 10.2, the preincubation of fiber type I resulted in gray-stained muscles compared with dark-gray fiber type II staining. We found that the proportion of fiber type I to type II in SOL muscles of R58Q mice (analyzed from multiple sections of n = 2 animals) was 52–48%, which was significantly lower than the ratio established for WT mice, 63–37% (n = 3 animals) (P = 0.0022 by 1-way ANOVA). The measurement of the cross-sectional area and myocyte perimeter revealed no differences between fiber type I and type II of SOL-R58Q vs. SOL-WT. No differences between the genotypes were found in EDL-stained muscles. In conclusion, myosin ATPase staining data suggest differences in SOL muscle remodeling between R58Q and WT mice, with a decreased proportion of fiber type I to type II occurring in R58Q vs. WT mice. No differences between the genotypes were observed in EDL control muscles.
Figure 4.
Myosin ATPase staining of SOL and EDL muscles from R58Q and WT mice. A) Proportion of fiber type I to type II and cell size in SOL muscles of WT (black) and R58Q (red) mice. Each data point represents the average ± sd (2–3 animals/group). Points originate from quantification of 13–15 slides per animal. *P < 0.05 (1-way ANOVA). B, C) Representative images of cross-sections of SOL (B) or EDL (C) muscles stained at pH 4.31 (left panels) and 10.20 (right panels). At pH = 4.31, fiber type I exhibits dark-gray staining vs. very light gray–stained fiber type II. At pH 10.20, the opposite is true, and fiber type I staining is represented by gray color, whereas fiber type II is represented by dark gray.
In vivo skeletal muscle strength assessment
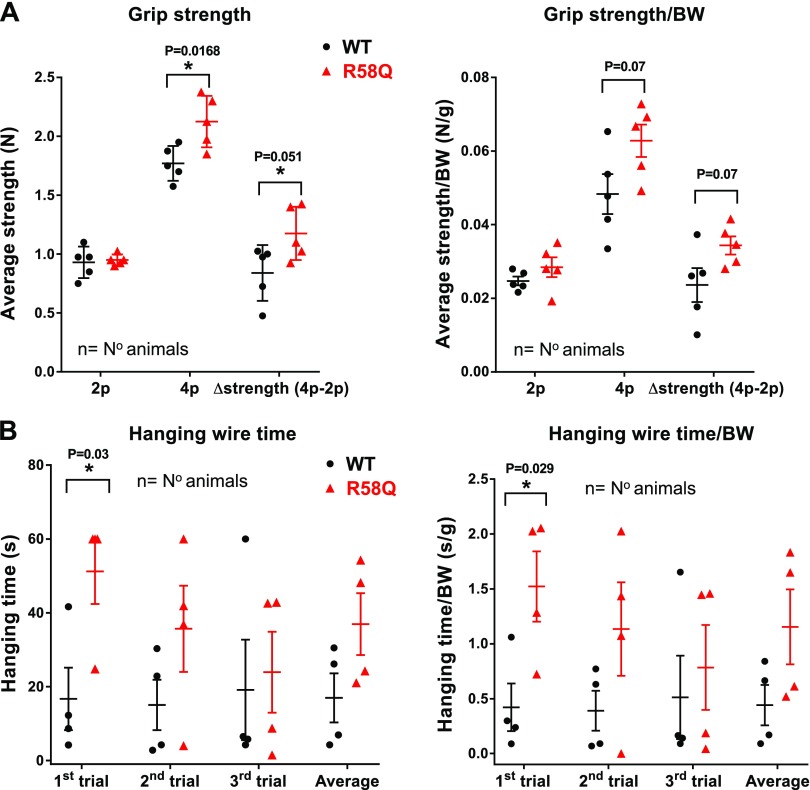
To investigate a potential in vivo skeletal muscle phenotype in the R58Q transgenic mouse model, we assessed the skeletal muscle strength in ∼7-mo-old male R58Q mice vs. age and sex-matched WT mice with 2 different tests: the limb grip strength test and the hanging wire test (30, 31). With the grip strength meter, the maximal resistance the animal exerts was measured, and the maximum strength (in newtons) was recorded in 4 independent measurements for each mouse (Fig. 5A). Mice were allowed to grasp the iron grid, first using their forelimbs and then using all 4 limbs. The peak tension was recorded when either the forelimbs or all 4 legs were released from the iron grid. In the hanging wire test (Fig. 5B), the animals were lifted by their tails and placed hanging from a wire with their front paws. The hanging time (in seconds) was recorded until they fell off the wire. Three consecutive trials were performed, with a maximum time of 60 s. As shown in Fig. 5A, there was a significant difference in the grip strength of all 4 limbs compared with WT mice. When adjusted by body weight, this tendency remained the same, although not statistically significant. The forelimb strength was additionally tested by the wire-hanging ability of the mice (31). In the wire-hanging test, significant differences were found between the 2 genotypes during the first trial, with the transgenic R58Q mice hanging for a significantly longer time compared with WT animals. In the second and third trials, the tendency remained the same, but the differences between the genotypes were not significant. The tendency of the R58Q mutant to display a higher maximum strength compared with WT was most likely due to a decreased ratio of fiber type I /fiber type II in the R58Q SOL muscle (Fig. 4) that would be expected to result in more gross skeletal muscle strength in the mutant.
Figure 5.
Muscle strength assessment in ∼7-mo-old R58Q mutant male mice compared with ∼7-mo-old WT male mice. A) Grip strength expressed in newtons (N) (left) and grip strength in N/BW (body weight) in newtons per gram (right) for forelimbs (2p), 4 limbs (4p), and Δstrength (4p-2p), which is the difference in grip strength between 4 limbs and forelimbs for both genotypes. Each point represents 1 animal subjected to 4 independent measurements of force. B) Total time that the mice can spend hanging from a wire with their 4 paws before the first fall (left), and the ratio between the wire-hanging time and the animal body weight (right). Each point represents 1 measurement per animal in 3 consecutive trails. Data are the average ± sem; relevant significance values by Student’s t test are depicted.
Quantitative proteomics
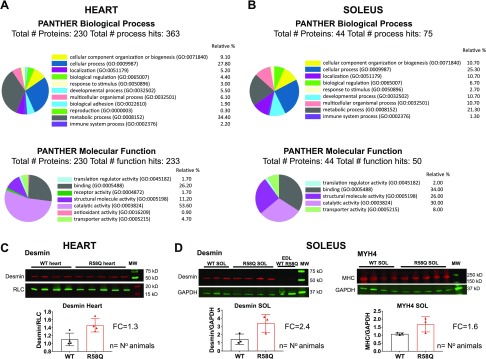
To gain insight into molecular mechanisms underlying the pathogenesis of the R58Q-induced cardioskeletal myopathy, a TMT quantitative proteomic approach was used in the hearts and SOL muscles of R58Q mice, and the protein expression profiles were compared with those of control WT mice (Table 3 and Supplemental Tables S1 and S2). The largest amount of proteins exhibiting R58Q-associated changes in both the hearts (Fig. 6A) and SOL muscles (Fig. 6B) included proteins involved in metabolic processes, calcium-handling machinery, and the cytoskeletal structural proteins.
TABLE 3.
Differentially expressed proteins in the hearts and SOL muscles of R58Q mice compared with WT RLC animals
| Protein |
Gene_Mouse |
UniProt ID | MW kDa | FC R58Q vs. WT |
|
|---|---|---|---|---|---|
| Heart | SOL | ||||
| Metabolic process/proteins related to enzyme activity/transport | |||||
| Hemopexinaa | HEMO | Q91X72 | 51 | 1.40 | 1.37 |
| Protein disulfide-isomerase A3a | PDIA3 | P27773 | 57 | 1.33 | 1.28 |
| 78 kDa glucose-regulated proteina | GRP78 | P20029 | 72 | 1.21 | 1.19 |
| Hexokinase-1 | HXK1 | P17710 | 108 | 1.15 | |
| Hexokinase-2 | E9Q5B5 | E9Q5B5 | 99 | −1.11 | |
| Serum albumina | ALBU | P07724 | 69 | 1.12 | 1.17 |
| Very-long-chain specific acyl-CoA dehydrogenase, mitochondrialb | ACADV | P50544 | 71 | −1.14 | −1.12 |
| Glycogen phosphorylase, muscle formb | PYGM | Q9WUB3 | 97 | −1.12 | −1.1 |
| Calcium-binding proteins | |||||
| Calreticulina | CALR | P14211 | 48 | 1.41 | 1.24 |
| Cluster of annexin A1a | ANXA1 | P10107 | 39 | 1.53 | |
| Cluster of annexin A2a | ANXA2 | P07356 | 39 | 1.33 | |
| Annexin A5a | ANXA5 | P48036 | 36 | 1.45 | |
| Annexin A6a | ANXA6 | P14824 | 76 | 1.11 | |
| Annexin A7a | ANXA7 | Q07076 | 50 | 1.22 | |
| Annexina | F8WIT2 | F8WIT2 | 75 | 1.20 | |
| Calsequestrin-2b | CASQ2 | O09161 | 48 | −1.12 | −1.46 |
| Ryanodine receptor 2b | RYR2 | E9Q401 | 565 | −1.11 | |
| Ryanodine receptor 1b | K3W4M2 | K3W4M2 | 565 | −1.12 | |
| Sarcomeric/contractile and cytoskeletal proteins | |||||
| α-Actinin-4 | ACTN4 | P57780 | 105 | 1.23 | |
| α-Actinin-2 | ACTN2 | Q9JI91 | 104 | −1.21 | |
| Desmina | DESM | P31001 | 53 | 1.46 | 1.13 |
| Xin actin-binding repeat-containing protein 2a | XIRP2 | Q4U4S6 | 428 | 1.56 | |
| Xin actin-binding repeat-containing protein 1a | E9QQ93 | E9QQ93 | 124 | 1.17 | |
| Cysteine and glycine-rich protein 3a | CSRP3 | P50462 | 21 | 1.31 | 1.27 |
| Prelamin-A/Ca | LMNA | P48678 | 74 | 1.39 | 1.31 |
| Tropomyosin α-4 chain | TPM4 | Q6IRU2 | 28 | 1.43 | |
| Tropomyosin α-3 chain | E9Q5J9 | E9Q5J9 | 33 | 1.57 | |
| Tropomyosin β chain | TPM2 | P58774 | 33 | 1.47 | |
| Filamin-B | FLNB | Q80X90 | 278 | 1.21 | |
| Filamin-C | D3YW87 | Q80X90 | 287 | 1.21 | −1.1 |
| Cell growth/transcription/chaperones | |||||
| Nucleolina | NUCL | P09405 | 77 | 1.21 | 1.33 |
| Heterogeneous nuclear RNP A2/B1a | ROA2 | O88569 | 37 | 1.16 | 1.35 |
| Protein AHNAKa | E9Q616 | E9Q616 | 604 | 1.21 | 1.27 |
| Heterogeneous nuclear RNP L (fragment)a | G5E924 | G5E924 | 67 | 1.18 | 1.18 |
| Galectin-1a | LEG1 |
P16045 |
15 | 1.38 | 1.42 |
FC <1 indicate proteins that were significantly down-regulated in R58Q vs. WT muscles, whereas FC >1 indicate proteins that were significantly up-regulated in R58Q vs. WT muscles, with significance defined as P < 0.05. A Mann-Whitney U test was used to calculate the Bonferroni-corrected significance values (P < 0.00005 for SOL and P < 0.00002 for heart proteins). a,bProteins simultaneously up-regulated (a) and down-regulated (b) in the hearts and SOL muscles of R58Q vs.WT mice.
Figure 6.
Classification scheme of differentially expressed proteins in the hearts (A) and SOL muscles (B) of R58Q compared with WT mice using Panther database. The molecular and biologic processes of the differentially expressed proteins for which comprehensive data are available in the database included 230 of the 274 proteins for cardiac tissue and 44 of 61 for SOL muscle. C, D) Validation of proteomic data in the hearts and SOL muscles of R58Q vs. WT mice (D). Western blot of lysates prepared from 3 to 4 animals per group were probed with desmin (heart and SOL) or slow-skeletal myosin heavy chain-4 (MYH4) (SOL) antibodies (C, D). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or CT-1 (specific for myosin RLC) antibodies were used as loading controls. Band intensities were quantified by using ImageJ, and the ratios of desmin/GAPDH or desmin/RLC as well as MYH4/GAPDH were calculated. Each point represents averaged measurements (2–5) per animal. Note the up-regulation of desmin in heart (FC = 1.3 ± 0.2) and SOL (FC = 2.4 ± 0.8) muscles, and MYH4 in SOL (FC = 1.6 ± 0.4) of R58Q vs. WT mice. Data are the average ± sd.
Cardiac remodeling in R58Q vs. WT mice
The analysis of the results showed that of 1619 TMT-labeled proteins identified with high confidence (FDR, 0.62%), 274 were differentially regulated in the hearts of R58Q vs. WT mice (Supplemental Table S1). In the biologic processes class, the majority of proteins were involved in metabolic (34%) and cellular (28%) processes (Fig. 6A). The most prominent biologic processes displaying changes in R58Q hearts were those responsible for cellular organization and biogenesis. In the group of proteins involved in the energy production, the majority were down-regulated in R58Q vs. WT hearts. In the molecular function class, catalytic activity processes formed the largest group of proteins (54%), followed by binding (∼26%) and structural molecular activity (∼11%) proteins.
Myocardial insult or exposure to chronic pathologic stress (e.g., cardiomyopathy-causing mutation) often involves a shift in the cellular fuel system from fatty acid to glucose metabolism (37). In agreement with this notion, there was −1.16-fold down-regulation of NADH dehydrogenase 1, subunit C2, and other subunits of this complex in the hearts of R58Q vs. WT mice (Supplemental Table S1). Up-regulation of proteins involved in glycolysis, hexokinase-1 (1.15-fold), phosphoglycerate kinase 1 (1.15-fold), triosephosphate isomerase (1.21-fold), and phosphoglycerate mutase 1 (1.31-fold) supported reprogramming of cardiac metabolism in R58Q hearts that occurred in response to pathologic hypertrophy and a fatty acid to glucose metabolism pathway shift. These changes, combined with down-regulation of 3-ketoacyl-CoA thiolase (−1.2-fold) and acetyl-CoA acetyltransferase, mitochondrial (−1.26-fold) that are involved in fatty acids β-oxidation represent a reduced capacity for mitochondrial oxidative metabolism in R58Q vs. WT hearts. Consistent with the energy starvation hypothesis that has been believed to contribute to the transition from functionally compensated hypertrophy to heart failure, key proteins involved in Krebs cycle and oxidative phosphorylation (mitochondrial ATP synthase subunit α, β, and subunit d, fumarate hydratase and aconitate hydratase) were all down-regulated in R58Q vs. WT hearts. Another important enzyme involved in energy metabolism, creatine kinase S-type, mitochondrial, was also −1.24-fold down-regulated in R58Q vs. WT mice. Up-regulation of collagen type VI, CO6A1, and CO6A2 (1.12-fold) and a cardiomyopathy-associated protein, XIRP2 (1.56-fold), in R58Q vs. WT mice confirmed the R58Q-induced insult in HCM mice.
In the group of Ca2+-binding proteins, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2, ryanodine receptor 2, calsequestrin-2, calreticulin, haptoglobin, EF-hand domain-containing protein D2, annexin A1, cluster of annexin A2, and annexin A5 were differentially expressed in R58Q vs. WT hearts, suggesting that Ca2+ handling might be altered in HCM mice (15, 16). In addition, up-regulation of sarcomeric/contractile proteins, titin (1.07-fold), tropomyosin α-4 chain (1.43-fold), and nebulin-related–anchoring protein (1.38-fold), were observed in R58Q vs. WT hearts (Supplemental Table S1). Likewise, cytoskeletal proteins involved in the formation/maintenance of cellular structures, desmin (1.46-fold), α-actinin-4 (1.23-fold), talin-1 (1.26-fold), myotilin (1.65-fold), cofilin-1 and cofilin-2 (1.4- and 1.25-fold), transgelin-2 (1.49-fold), elongation factor 1-γ (1.13-fold), elongation factor 1-α 1 (1.55-fold), plakophilin 2 (1.19-fold), and myosin-9 (1.19-fold) were all up-regulated in the hearts of R58Q vs. WT mice. The up-regulation of desmin that plays a role in maintaining sarcomeric structures linking them to the sarcolemmal cytoskeleton and the mitochondria, in the hearts of R58Q vs. WT mice was confirmed by SDS-PAGE (Fig. 6C).
We also observed an up-regulation of the several proteins of the ubiquitin/proteasome system family: 26S proteasome non-ATPase regulatory subunit 1 and 3 (1.17- and 1.16-fold), ubiquitin-conjugating enzyme E2 L3 (1.2-fold), and cluster of ubiquitin-like modifier-activating enzyme 1 (1.15-fold). The main role of the ubiquitin/proteasome system is the maintenance of protein homeostasis by degradation of damaged, misfolded, and mutant proteins by proteolysis, but its altered activity (increased or decreased) has also been reported in humans and rodent models of cardiac hypertrophy (serving as “cardiac protein quality control”) (38, 39).
Soleus muscle remodeling in R58Q vs. WT mice
The analysis of the results shows that of 1029 TMT-labeled proteins identified with high confidence (FDR, 0.81%), 61 were differentially regulated in SOL muscles of R58Q vs. WT mice. In accord with proteomic analyses of the hearts, the most prominent Biologic Processes altered in SOL muscles of R58Q vs. WT included metabolic (21%) and cellular (25%) processes (Fig. 6B). In addition, proteins associated with cellular component organization or biogenesis (10%), localization (10%), developmental process (10%), and multicellular organismal processes (10%) were differentially expressed in SOL muscle of R58Q vs. WT mice. In the molecular function class, and similar to the hearts, proteins involved in catalytic activity (30%), binding (34%), and structural molecular activity (26%) formed the largest group of differentially expressed proteins in SOL muscles of R58Q vs. WT mice.
As in the hearts, SOL muscles of R58Q mice exhibited differential expression of proteins related to energy metabolism and muscle contractile activity compared with WT SOL muscles (Table 3 and Supplemental Table S2). Up-regulation of proteins involved in enzymatic activity and muscle energy metabolism was observed, and they included cytochrome c, a mitochondrial electron transport chain component (1.47-fold), and cytochrome c oxidase subunit 5B, the last enzyme in the respiratory electron transport chain of mitochondria (1.51-fold). The group of down-regulated proteins included voltage-dependent anion-selective channel protein 3, which is involved in the transport of ions and metabolites through the outer membrane of mitochondria (−1.16-fold). The l-lactate dehydrogenase B-chain LDH, an enzyme responsible for the conversion of pyruvate to lactate (−1.28-fold), and hexokinase-2, a protein involved in glycolysis (−1.1-fold), were down-regulated in SOL muscles of R58Q vs. WT mice. The proteins involved in fatty acid β-oxidation, long-chain-fatty-acid CoA ligase 1 (−1.18-fold) and very-long-chain specific acyl-CoA dehydrogenase (−1.12 fold), malate dehydrogenase [a key protein involved in Krebs cycle (−1.14-fold)], glycogen phosphorylase (PYGM) [essential for carbohydrate metabolism and glycogenolysis (−1.1-fold)], and glycogen (starch) synthase (GYS) (−1.13-fold), were all down-regulated in SOL muscles of R58Q vs. WT mice. Because the skeletal muscle is the major site for glycogen storage and glucose disposal for muscle contraction, the down-regulation of these proteins involved in glycogen metabolism [PYGM, glycogen (starch) synthase] in SOL muscle of R58Q vs. WT animals suggests a potential functional impairment of skeletal muscle that may underlie the myopathy phenotype.
Interestingly, the majority of differentially expressed cytoskeletal and contractile proteins were up-regulated in SOL muscle of R58Q vs. WT mice (Supplemental Table S2). A 2.09-fold up-regulation of myosin-4 (MYH4), the skeletal muscle myosin heavy chain-4, was observed in SOL muscles of R58Q vs. WT mice, and this result was validated by immunoblotting (Fig. 6D). The up-regulation of several regulators of biologic processes playing a role in proteolytic activity, inflammation, transport, and membrane–cytoskeleton interactions was observed in SOL muscles of R58Q mice vs. WT mice. These included serpin B6 (1.45-fold), annexin (1.20-fold), vimentin (1.39-fold), and galectin-1 (1.42-fold). Similar to the heart, desmin, a component of intermediate filaments, was 1.13-fold up-regulated in the SOL muscle of R58Q vs. WT mice, and this result was confirmed according to SDS-PAGE. In addition, several proteins reportedly associated with myogenesis were up-regulated in SOL muscles of R58Q mice vs. WT mice. Among them was nucleolin, which has been found to be expressed on the surface of developing myotubes (1.33-fold); tropomyosin α-3 chain (1.57-fold); and calreticulin (1.24-fold). Other up-regulated proteins in the SOL muscle of R58Q vs. WT mice included the BAG family molecular chaperone regulator 3 (1.21-fold) that interacts with the ATPase domain of heat shock protein 70 and can modulate major biologic processes and cytoskeleton organization.
Similarities in R58Q-mediated remodeling between the heart and SOL muscle of mice
Up-regulation of proteins involved in enzymatic activity and muscle energy metabolism was observed in the hearts and SOL muscles of R58Q vs. WT mice (Fig. 6 and Table 3). Cardiac muscle had 274 differentially expressed proteins of the 1619 proteins detected (16.9%), whereas SOL muscle had 61 differentially expressed proteins of the 1029 proteins detected (5.9%). Of the 61 proteins that were differentially expressed in SOL muscle, 21 proteins (34.4%) were altered in the same direction as differentially expressed proteins in cardiac tissue, and 3 proteins (4.9%) were altered in different directions.
Simultaneously up-regulated proteins in the hearts and SOL muscles of R58Q vs. WT mice included hemopexin (∼1.4-fold), protein disulfide-isomerase A3 (∼1.3-fold), and 78 kDa glucose-regulated protein (∼1.2-fold) (Table 3). Hemopexin, also known as β-1B-glycoprotein, belongs to the hemopexin family of proteins; this family is involved in scavenging the heme released by the turnover of heme proteins (e.g., hemoglobin), and thus they play a protective role against oxidative damage (40). Protein disulfide-isomerase A3 catalyzes the rearrangement (formation and breakage) of the S-S bonds, and thus the enzyme acts to catalyze the protein-folding pathway that occurs in specialized cellular compartments (41). Likewise, 78 kDa glucose-regulated protein, up-regulated in both the hearts and SOL muscle of R58Q vs. WT mice, is involved in protein folding and degradation of misfolded proteins. It plays a role in facilitating the assembly of multimeric protein complexes inside the endoplasmic reticulum. Conversely, a down-regulation of proteins involved in mitochondrial fatty acid β-oxidation pathway [e.g., very-long-chain specific acyl-CoA dehydrogenase, mitochondrial (−1.1-fold)] was observed in both types of muscles in R58Q vs. WT mice. Moreover, down-regulation of long-chain-fatty-acid–CoA ligase 1 (−1.1- and −1.2-fold for heart and SOL, respectively) and the muscle form of glycogen phosphorylase (−1.1-fold), an important allosteric enzyme in carbohydrate metabolism, was noted in both types of muscles in R58Q vs. WT mice. Remarkably, proteins involved in transcription activation (nucleolin, heterogeneous nuclear RNP A2/B1) and cell growth (cysteine and glycine-rich protein 3) were all simultaneously up-regulated in the hearts and SOL muscles of R58Q vs. WT mice. The up-regulation of desmin simultaneously in the hearts (1.46-fold) and SOL muscles (1.13-fold) of R58Q vs. WT mice was confirmed by immunoblotting (Fig. 6C). These data clearly indicate that similar changes were occurring in muscle myogenesis and cell contraction mechanisms in both the hearts and SOL muscles of HCM R58Q mice.
DISCUSSION
HCM is one of the most common inherited cardiac disorders, with a prevalence of 1:625–1:344 in the general adult population (2, 3), and there is currently no therapy targeting the underlying pathophysiology of HCM. Although it largely affects the function and morphology of the heart, functional abnormalities in the skeletal muscle fiber type I were observed in patients with HCM (8, 9). This study aimed to elucidate the effects of HCM-linked R58Q mutation in the myosin regulatory light chain (MYL2 gene) on the structure, function, and proteomic landscape of the heart and the slow-twitch skeletal muscle. This is because similar to other sarcomeric genes (e.g., MYH7, MYL3, TNNC1), MYL2 expresses the myosin RLC protein simultaneously in heart ventricles and in the slow-twitch skeletal muscle. The results of our multilevel investigation showed that R58Q-induced cardiac remodeling is echoed by similar effects on the structure–function and energetics of slow-twitch SOL muscle but not EDL muscle.
To study the origin of R58Q-induced cardiac remodeling manifested concurrently in the hearts and in slow-twitch skeletal muscle of R58Q mice, we first examined whether the α-MHC promoter–driven expression of R58Q in the heart of mice (15) was also observed in their SOL muscles. Mass spectrometry data showed no expression of human RLC peptides in the SOL muscle of WT mice and a very low (2.71% ± 3.83) expression in R58Q SOL muscles, whereas native mouse RLC peptides (DTFAALGR) were easily detected in SOL muscles of both WT and R58Q mice (Supplemental Fig. S1A–C). Consistent with the latter results, no R58Q-containing peptides such as DTFAALGQ(58)VNVK were found to be present in either WT or R58Q SOL muscles. Mass spectrometry data were supported by Western blotting (Supplemental Fig. S1D) exhibiting no human cardiac RLC protein present in the blood serum of WT/R58Q mice. Likewise, no mRNA of human cardiac RLC (MYL2 gene) was found in SOL or EDL muscles of WT/R58Q mice according to quantitative PCR (Supplemental Fig. S1E). These data suggest that the observed detrimental phenotypes associated with the R58Q mutation in SOL muscle are not due to the direct expression of the mutant protein in the slow-twitch skeletal muscle and therefore must be of a different origin.
Structural and functional similarities between SOL muscles and PMs of R58Q mice were fully supported by quantitative proteomics showing differential expression of proteins involved in energy metabolism, cell–cell interactions, and protein–protein signaling concurrently in the hearts and SOL muscles of R58Q mice (Table 3). The energy requirements of cardiac muscle are similar to those of slow-twitch skeletal muscles, with a strong dependence on mitochondrial energy conservation (9). We observed enzymes involved in metabolic pathways that were similarly affected in the hearts and SOL muscles of R58Q vs. WT mice. Relative to WT, hemopexin, disulfide-isomerase A3, and 78 kDa glucose-related proteins were all up-regulated in the hearts and SOL muscles of R58Q mice, whereas glycogen phosphorylase (muscle form), very-long-chain specific acyl-CoA dehydrogenase (mitochondrial), and long-chain-fatty-acid-CoA ligase 1 were down-regulated. Deficiency of very-long-chain acyl-CoA dehydrogenase (VLCAD), which catalyzes the first step in β-oxidation of long-chain fatty acids, has been shown to manifest infantile (cardiac defects with HCM) and adolescent (intermittent skeletal rhabdomyolysis) phenotypes (42). It has been suggested that mutations in almost any protein component of the sarcomere and many cytoskeletal proteins could cause myopathy (43). For example, there is direct evidence that among the dominantly inherited or sporadic myofibrillar myopathies, there is a genetically distinct subgroup (desmin myopathy) characterized by pathogenic mutations in the desmin gene and by frequent cardiac conduction defects (44).
A reduction in left ventricular heart function sets in motion a series of metabolic events that lead to compromised function of slow-twitch skeletal muscle. This scenario is most likely due to a similar sarcomeric protein composition between the cardiac and SOL muscle, and the changes initiated in the heart are communicated through cell–cell interactions and protein–protein signaling to the slow-twitch skeletal muscle echoing cardiac dysfunction in SOL muscles. The finding that 34.4% of the differentially expressed proteins in SOL muscles were altered in the same direction as the differentially expressed proteins in cardiac tissue supports this proposed mechanism. Consistent with this mechanism, proteins involved in transcription activation and cell growth were simultaneously up-regulated in the hearts and SOL muscles of R58Q vs. WT mice (Table 3). Cysteine and glycine-rich protein 3 (CSRP3) gene encoding the muscle LIM protein (MLP) was ∼1.3-fold up-regulated in the hearts and SOL muscles of R58Q mice. MLP can form large oligomers that may affect protein–protein interactions and thus be involved in the structural and functional alterations observed concurrently in PMs and SOL muscles of R58Q vs. WT mice. CSRP3 has been shown to be involved in cardiomyopathy disease, and the up-regulation of MLP simultaneously in the hearts and SOL muscle of R58Q mice suggests aberrant protein signaling, possibly involving the Z discs, in which MLP has been found in abundance (45).
Similar changes between the hearts and SOL muscles of R58Q animals were observed in calcium-binding and cytoskeletal proteins and protein chaperones. Calmodulin, a multifunctional Ca2+-binding messenger protein, was found to be significantly up-regulated in SOL muscles (1.37-fold) (Supplemental Table S2) and also up-regulated in the hearts (1.16-fold, close to statistical significance) of R58Q mice. Consistent with the proposed protein–protein signaling theory, calmodulin could be involved in a calcium signal transduction pathway between the heart and SOL muscle by modifying its interactions with various target proteins such as kinases or phosphatases (46, 47). Calmodulin mediates many important processes such as inflammation, metabolism, apoptosis, and the immune response (48), and its overexpression in transgenic mice has also been shown to contribute to cardiac hypertrophy by a calcineurin-dependent pathway (49). Galectin-1, another simultaneously up-regulated protein in the hearts and SOL muscle of R58Q mice (Table 3) and a family member of β-galactoside–binding proteins, is a 15-kDa protein that is present inside and outside cells and thus can play a role in intracellular and extracellular processes (50). Members in the galectin family have been suggested to be useful biomarkers and are up-regulated in decompensated heart failure and have been associated with activation of fibroblasts and macrophages, which are hallmarks of cardiac remodeling (51). Galectin-1 has been shown to be elevated shortly after myocardial infarction (50) and was also shown to prevent pathology and improve skeletal muscle function in mdx mice (52). Up-regulation of galectin-1 simultaneously in the hearts and SOL muscles of R58Q mice could be of compensatory nature to muscle injury similar to its proposed function to serve as a new therapeutic protein in the treatment of Duchenne muscular dystrophy.
As we observed earlier, the HCM-related defects in the hearts of R58Q mice mostly involved alterations in myocardial morphology and a reduced ability of myosin to generate contractile force (15). In vivo abnormalities included occurrences of diastolic dysfunction in R58Q vs. WT mice (15, 16), hallmark features of human HCM. According to clinical reports, the R58Q-induced HCM was manifested by left ventricular hypertrophy, abnormal ECG findings, and multiple cases of premature sudden cardiac death (5–7, 53). The functional and structural phenotypes found in R58Q-positive patients were close to those observed in transgenic R58Q mice (15, 16). The current report reveals that in addition to the heart, functional and structural remodeling observed in R58Q hearts is echoed in slow-twitch skeletal muscles by virtue of a multitude of abnormal protein–protein and cellular interactions. Interestingly, combined involvement of cardiac and SOL muscles in the manifestation of mutant-induced defects bears relevance to the previously described phenotype associated with MYL2 mutations responsible for cardiac and slow-twitch skeletal muscle pathophysiology, leading to cardioskeletal myopathy in patients (9, 10). The most severe MYL2 mutation was the splice site mutation in intron 6 (IVS6-1) that was predicted to lead to the C-terminal truncation of the RLC protein and its C-terminal amino acid sequence changes (10, 11). The homozygous appearance of IVS6-1 led to the early death of infants (4–6 mo of age) due to dilated, hypertrophic, or noncompaction cardiomyopathy that was paralleled by muscle type I hypotrophy and abnormalities in skeletal myofibril organization (9–11). Our current data indicate that transgenic mice expressing the R58Q mutation in the heart also exhibit similar functional defects in slow-twitch skeletal muscles compared with WT mice. Future studies will include testing of slow-twitch skeletal muscles in the newly reported RLC-D94A mouse model of dilated cardiomyopathy (21). It is anticipated that a strong dilated cardiomyopathy phenotype observed in D94A mice will be recapitulated in the functional and structural assessments of SOL muscle from these mice.
In summary, our collective multilevel data on transgenic R58Q animals show that mutations in MYL2 may trigger pathologic remodeling in both the heart and slow-twitch skeletal muscle, thus leading to cardioskeletal myopathy as observed previously in young patients with mutations in MYL2 (10). In the current study, we found that despite the absence of the R58Q mutation in the slow-twitch skeletal muscle of R58Q mice, the HCM-mediated adverse heart remodeling in transgenic R58Q mice was echoed in the SOL muscle of transgenic humanized HCM-RLC mouse models. These models may be considered as a useful research tool to study the molecular, energetic, and cellular mechanisms of a dual cardioskeletal myopathy associated with the MYL2 gene.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Clemer Abad (Mouse Behavioral Core, University of Miami) for technical assistance. This work was supported by U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grants HL123255 (to D.S.-C.) and HL096819 (to A.V.G.), NIH National Institute of General Medical Sciences Grant 9P41-GM103622 (to T.C.I.), and American Heart Association Grants 15PRE23020006 (to C.C.-Y.) and 17PRE33650085 (to S.Y.). This research used resources from the Advanced Photon Source, a U.S. Department of Energy Office of Science User Facility operated for the Department of Energy Office of Science by Argonne National Laboratory under contract DE-AC02-06CH11357. Mass spectrometry was conducted with the use of the Q-Exactive Plus at the University of California, Davis Proteomic Core Facility. The authors declare no conflicts of interest.
Glossary
- CSRP3
cysteine and glycine-rich protein 3
- d1,0
distance between 1,0 and 1,1 equatorial X-ray reflections
- EDL
extensor digitorum longus
- FC
fold change
- FDR
false discovery rate
- HCM
hypertrophic cardiomyopathy
- I1,1/I1,0
intensity ratio of 1,1 to 1,0 equatorial X-ray reflections
- IFS
interfilament lattice spacing
- MYBPC3
myosin binding protein C
- MHC
myosin heavy chain
- MLP
muscle LIM protein
- MYH7
myosin heavy chain 7
- MYL2/3
myosin light chain 2/3
- PM
papillary muscle
- RLC
regulatory light chain of myosin
- SOL
soleus muscle
- TMT
tandem mass Tag
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Kazmierczak, A. V. Gomes, T. C. Irving, and D. Szczesna-Cordary designed the research; K. Kazmierczak, J. Liang, C.-C. Yuan, S. Yadav, Y. H. Sitbon, K. Walz, W. Ma, and J. X. Cheah performed the experiments; K. Kazmierczak, C.-C. Yuan, K. Walz, W. Ma, A. V. Gomes, T. C. Irving, and D. Szczesna-Cordary analyzed the data; and K. Kazmierczak, A. V. Gomes, T. C. Irving, and D. Szczesna-Cordary wrote the manuscript.
REFERENCES
- 1.Seidman C. E., Seidman J. G. (1998) Molecular genetic studies of familial hypertrophic cardiomyopathy. Basic Res. Cardiol. 93(Suppl 3), 13–16 [DOI] [PubMed] [Google Scholar]
- 2.Marian A. J., Braunwald E. (2017) Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121, 749–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfares A. A., Kelly M. A., McDermott G., Funke B. H., Lebo M. S., Baxter S. B., Shen J., McLaughlin H. M., Clark E. H., Babb L. J., Cox S. W., DePalma S. R., Ho C. Y., Seidman J. G., Seidman C. E., Rehm H. L. (2015) Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet. Med. 17, 880–888; erratum: 319 [DOI] [PubMed] [Google Scholar]
- 4.Poetter K., Jiang H., Hassanzadeh S., Master S. R., Chang A., Dalakas M. C., Rayment I., Sellers J. R., Fananapazir L., Epstein N. D. (1996) Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat. Genet. 13, 63–69 [DOI] [PubMed] [Google Scholar]
- 5.Flavigny J., Richard P., Isnard R., Carrier L., Charron P., Bonne G., Forissier J. F., Desnos M., Dubourg O., Komajda M., Schwartz K., Hainque B. (1998) Identification of two novel mutations in the ventricular regulatory myosin light chain gene (MYL2) associated with familial and classical forms of hypertrophic cardiomyopathy. J. Mol. Med. (Berl.) 76, 208–214 [DOI] [PubMed] [Google Scholar]
- 6.Kabaeva Z. T., Perrot A., Wolter B., Dietz R., Cardim N., Correia J. M., Schulte H. D., Aldashev A. A., Mirrakhimov M. M., Osterziel K. J. (2002) Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 10, 741–748 [DOI] [PubMed] [Google Scholar]
- 7.Mörner S., Richard P., Kazzam E., Hellman U., Hainque B., Schwartz K., Waldenström A. (2003) Identification of the genotypes causing hypertrophic cardiomyopathy in northern Sweden. J. Mol. Cell. Cardiol. 35, 841–849 [DOI] [PubMed] [Google Scholar]
- 8.Caforio A. L., Rossi B., Risaliti R., Siciliano G., Marchetti A., Angelini C., Crea F., Mariani M., Muratorio A. (1989) Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: evidence of subclinical myogenic myopathy. J. Am. Coll. Cardiol. 14, 1464–1473 [DOI] [PubMed] [Google Scholar]
- 9.Barth P. G., Wanders R. J., Ruitenbeek W., Roe C., Scholte H. R., van der Harten H., van Moorsel J., Duran M., Dingemans K. P. (1998) Infantile fibre type disproportion, myofibrillar lysis and cardiomyopathy: a disorder in three unrelated Dutch families. Neuromuscul. Disord. 8, 296–304 [DOI] [PubMed] [Google Scholar]
- 10.Weterman M. A., Barth P. G., van Spaendonck-Zwarts K. Y., Aronica E., Poll-The B. T., Brouwer O. F., van Tintelen J. P., Qahar Z., Bradley E. J., de Wissel M., Salviati L., Angelini C., van den Heuvel L., Thomasse Y. E., Backx A. P., Nürnberg G., Nürnberg P., Baas F. (2013) Recessive MYL2 mutations cause infantile type I muscle fibre disease and cardiomyopathy. Brain 136, 282–293 [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z., Huang W., Liang J., Szczesna-Cordary D. (2016) Molecular and functional effects of a splice site mutation in the MYL2 gene associated with cardioskeletal myopathy and early cardiac death in infants. Front. Physiol. 7, 240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szczesna D. (2003) Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr. Drug Targets Cardiovasc. Haematol. Disord. 3, 187–197 [DOI] [PubMed] [Google Scholar]
- 13.Geeves M. A. (2002) Stretching the lever-arm theory. Nature 415, 129–131 [DOI] [PubMed] [Google Scholar]
- 14.Szczesna-Cordary D., Guzman G., Zhao J., Hernandez O., Wei J., Diaz-Perez Z. (2005) The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J. Cell Sci. 118, 3675–3683 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y., Xu Y., Kerrick W. G. L., Wang Y., Guzman G., Diaz-Perez Z., Szczesna-Cordary D. (2006) Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J. Mol. Biol. 361, 286–299 [DOI] [PubMed] [Google Scholar]
- 16.Abraham T. P., Jones M., Kazmierczak K., Liang H.-Y., Pinheiro A. C., Wagg C. S., Lopaschuk G. D., Szczesna-Cordary D. (2009) Diastolic dysfunction in familial hypertrophic cardiomyopathy transgenic model mice. Cardiovasc. Res. 82, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerrick W. G. L., Kazmierczak K., Xu Y., Wang Y., Szczesna-Cordary D. (2009) Malignant familial hypertrophic cardiomyopathy D166V mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice. FASEB J. 23, 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang W., Liang J., Kazmierczak K., Muthu P., Duggal D., Farman G. P., Sorensen L., Pozios I., Abraham T. P., Moore J. R., Borejdo J., Szczesna-Cordary D. (2014) Hypertrophic cardiomyopathy associated Lys104Glu mutation in the myosin regulatory light chain causes diastolic disturbance in mice. J. Mol. Cell. Cardiol. 74, 318–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan C. C., Muthu P., Kazmierczak K., Liang J., Huang W., Irving T. C., Kanashiro-Takeuchi R. M., Hare J. M., Szczesna-Cordary D. (2015) Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc. Natl. Acad. Sci. USA 112, E4138–E4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C. C., Kazmierczak K., Liang J., Kanashiro-Takeuchi R., Irving T. C., Gomes A. V., Wang Y., Burghardt T. P., Szczesna-Cordary D. (2017) Hypercontractile mutant of ventricular myosin essential light chain leads to disruption of sarcomeric structure and function and results in restrictive cardiomyopathy in mice. Cardiovasc. Res. 113, 1124–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan C. C., Kazmierczak K., Liang J., Zhou Z., Yadav S., Gomes A. V., Irving T. C., Szczesna-Cordary D. (2018) Sarcomeric perturbations of myosin motors lead to dilated cardiomyopathy in genetically modified MYL2 mice. Proc. Natl. Acad. Sci. USA 115, E2338–E2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg M. J., Kazmierczak K., Szczesna-Cordary D., Moore J. R. (2010) Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc. Natl. Acad. Sci. USA 107, 17403–17408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kampourakis T., Ponnam S., Irving M. (2018) Hypertrophic cardiomyopathy mutation R58Q in the myosin regulatory light chain perturbs thick filament-based regulation in cardiac muscle. J. Mol. Cell. Cardiol. 117, 72–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karabina A., Kazmierczak K., Szczesna-Cordary D., Moore J. R. (2015) Myosin regulatory light chain phosphorylation enhances cardiac β-myosin in vitro motility under load. Arch. Biochem. Biophys. 580, 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szczesna D., Ghosh D., Li Q., Gomes A. V., Guzman G., Arana C., Zhi G., Stull J. T., Potter J. D. (2001) Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure, Ca2+ binding, and phosphorylation. J. Biol. Chem. 276, 7086–7092 [DOI] [PubMed] [Google Scholar]
- 26.Szczesna-Cordary D., Guzman G., Ng S. S., Zhao J. (2004) Familial hypertrophic cardiomyopathy-linked alterations in Ca2+ binding of human cardiac myosin regulatory light chain affect cardiac muscle contraction. J. Biol. Chem. 279, 3535–3542 [DOI] [PubMed] [Google Scholar]
- 27.Greenberg M. J., Watt J. D., Jones M., Kazmierczak K., Szczesna-Cordary D., Moore J. R. (2009) Regulatory light chain mutations associated with cardiomyopathy affect myosin mechanics and kinetics. J. Mol. Cell. Cardiol. 46, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mettikolla P., Calander N., Luchowski R., Gryczynski I., Gryczynski Z., Zhao J., Szczesna-Cordary D., Borejdo J. (2011) Cross-bridge kinetics in myofibrils containing familial hypertrophic cardiomyopathy R58Q mutation in the regulatory light chain of myosin. J. Theor. Biol. 284, 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L., Muthu P., Szczesna-Cordary D., Kawai M. (2013) Diversity and similarity of motor function and cross-bridge kinetics in papillary muscles of transgenic mice carrying myosin regulatory light chain mutations D166V and R58Q. J. Mol. Cell. Cardiol. 62, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonetto A., Andersson D. C., Waning D. L. (2015) Assessment of muscle mass and strength in mice. Bonekey Rep. 4, 732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Haraguchi S., Koda T., Hashimoto K., Nakagawara A. (2011) Muscle atrophy and motor neuron degeneration in human NEDL1 transgenic mice. J. Biomed. Biotechnol. 2011, 831092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomes A. V., Kazmierczak K., Cheah J. X., Gilda J. E., Yuan C. C., Zhou Z., Szczesna-Cordary D. (2015) Proteomic analysis of physiological versus pathological cardiac remodeling in animal models expressing mutations in myosin essential light chains. J. Muscle Res. Cell Motil. 36, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesvizhskii A. I., Keller A., Kolker E., Aebersold R. (2003) A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 75, 4646–4658 [DOI] [PubMed] [Google Scholar]
- 34.Oberg A. L., Mahoney D. W., Eckel-Passow J. E., Malone C. J., Wolfinger R. D., Hill E. G., Cooper L. T., Onuma O. K., Spiro C., Therneau T. M., Bergen H. R., III (2008) Statistical analysis of relative labeled mass spectrometry data from complex samples using ANOVA. J. Proteome Res. 7, 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Millman B. M. (1998) The filament lattice of striated muscle. Physiol. Rev. 78, 359–391 [DOI] [PubMed] [Google Scholar]
- 36.Williams C. D., Salcedo M. K., Irving T. C., Regnier M., Daniel T. L. (2013) The length-tension curve in muscle depends on lattice spacing. Proc. Biol. Sci. 280, 20130697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karwi Q. G., Uddin G. M., Ho K. L., Lopaschuk G. D. (2018) Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5, 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang D., Zong C., Koag M. C., Wang Y., Drews O., Fang C., Scruggs S. B., Ping P. (2011) Proteome dynamics and proteome function of cardiac 19S proteasomes. Mol. Cell Proteomics 10, M110 006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zolk O., Schenke C., Sarikas A. (2006) The ubiquitin-proteasome system: focus on the heart. Cardiovasc. Res. 70, 410–421 [DOI] [PubMed] [Google Scholar]
- 40.Tolosano E., Altruda F. (2002) Hemopexin: structure, function, and regulation. DNA Cell Biol. 21, 297–306 [DOI] [PubMed] [Google Scholar]
- 41.Gruber C. W., Cemazar M., Heras B., Martin J. L., Craik D. J. (2006) Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem. Sci. 31, 455–464 [DOI] [PubMed] [Google Scholar]
- 42.Vianey-Saban C., Divry P., Brivet M., Nada M., Zabot M. T., Mathieu M., Roe C. (1998) Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin. Chim. Acta 269, 43–62 [DOI] [PubMed] [Google Scholar]
- 43.Marston S. B., Hodgkinson J. L. (2001) Cardiac and skeletal myopathies: can genotype explain phenotype? J. Muscle Res. Cell Motil. 22, 1–4 [DOI] [PubMed] [Google Scholar]
- 44.Dalakas M. C., Park K. Y., Semino-Mora C., Lee H. S., Sivakumar K., Goldfarb L. G. (2000) Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N. Engl. J. Med. 342, 770–780 [DOI] [PubMed] [Google Scholar]
- 45.Vafiadaki E., Arvanitis D. A., Papalouka V., Terzis G., Roumeliotis T. I., Spengos K., Garbis S. D., Manta P., Kranias E. G., Sanoudou D. (2014) Muscle lim protein isoform negatively regulates striated muscle actin dynamics and differentiation. FEBS J. 281, 3261–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bers D. M. (2011) Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm 8, 1101–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryder J. W., Lau K. S., Kamm K. E., Stull J. T. (2007) Enhanced skeletal muscle contraction with myosin light chain phosphorylation by a calmodulin-sensing kinase. J. Biol. Chem. 282, 20447–20454 [DOI] [PubMed] [Google Scholar]
- 48.Gu R., Ding M., Shi D., Huang T., Guo M., Yu L., Hu J., Huang W., Liao H. (2018) Calcium/calmodulin-dependent protein kinase IV mediates IFN-γ-Induced immune behaviors in skeletal muscle cells. Cell. Physiol. Biochem. 46, 351–364 [DOI] [PubMed] [Google Scholar]
- 49.Obata K., Nagata K., Iwase M., Odashima M., Nagasaka T., Izawa H., Murohara T., Yamada Y., Yokota M. (2005) Overexpression of calmodulin induces cardiac hypertrophy by a calcineurin-dependent pathway. Biochem. Biophys. Res. Commun. 338, 1299–1305 [DOI] [PubMed] [Google Scholar]
- 50.Al-Salam S., Hashmi S. (2014) Galectin-1 in early acute myocardial infarction. PLoS One 9, e86994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Boer R. A., Yu L., van Veldhuisen D. J. (2010) Galectin-3 in cardiac remodeling and heart failure. Curr. Heart Fail. Rep. 7, 1–8; erratum: 9, 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Ry P. M., Wuebbles R. D., Key M., Burkin D. J. (2015) Galectin-1 protein therapy prevents pathology and improves muscle function in the mdx mouse model of Duchenne muscular dystrophy. Mol. Ther. 23, 1285–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard P., Charron P., Carrier L., Ledeuil C., Cheav T., Pichereau C., Benaiche A., Isnard R., Dubourg O., Burban M., Gueffet J. P., Millaire A., Desnos M., Schwartz K., Hainque B., Komajda M.; EUROGENE Heart Failure Project (2003) Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107, 2227–2232; erratum: 109, 3258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.