Abstract
Transferring the zebrafish larvae on an imaging platform has long been performed manually by the use of forceps or through mechanical pumping. These methods induce detrimental damages to the fragile bodies of zebrafish larvae during the transportation. To address this issue, in this work we are devising a light driven technique to transport zebrafish larvae within a microfluidic environment. In particular, an optomotor behavioral response of the zebrafish larvae was controlled through the computer animated moving gratings for their transportation within a microfluidics chamber. It was observed that with an optimum grating frequency of 1.5 Hz and a grating width ratio of 1:1, a 5 days-post fertilization zebrafish larva can be transported within minimum and maximum time periods of 0.63 and 2.49 s, respectively. This proposed technique can be utilized towards multi-automatic transportation of zebrafish larvae within the microfluidic environment as well as the zebrafish core facility.
INTRODUCTION
Zebrafish as an animal model has been extensively used for the study of vertebrate biomechanics, developmental biology, pharmacology, toxicology, genetical study, and cardiovascular development due to their experimental friendly characteristics.1,2 To access the importantly internal organs of this animal model, a high resolution imaging is required for which the zebrafish larvae are anesthetized and transported onto the imaging platform.3 Conventionally, zebrafish larvae are transported from the breeding environment to the imaging platform by means of mechanical pumping within the narrow capillary tubes or microfluidic channels.4,5 Mechanical pumping of these anesthetized zebrafish larvae may induce serious damage to their fragile body, and this requires an alternative noninvasive methodology for larvae transportation. Studies have been established that to survive in a hostile environment and to find favorable environments to collect their foods, juvenile zebrafish larvae gather sensory and visual cues just 3 days post to their fertilization.6–8 Visuomotor behaviors in a larval zebrafish can be broadly classified in two major categories such as optokinetic reflex (OKR) and optomotor response (OMR). OKR can be defined as a robust eye movement corresponding to a moving stimulus in the surrounding of zebrafish larvae, whereas OMR can be defined as the tendency of zebrafish larvae to follow the translational whole-field motion to stabilize their position in the flow fields.9,10 Although visuomotor behaviors of zebrafish larvae have been extensively studied and made considerable progress towards understanding neural activity dynamics towards their innate responses, yet a little attention has been devoted towards exploring its application side. As moving grating can produce innate optomotor behavioral response among juvenile zebrafish larvae and the larvae can swim along the direction of perceived motion,11 we took advantage of this by the use of computer-animated displays for their transportation within a microfluidic device.
EXPERIMENTAL SETUP AND PROTOCOLS
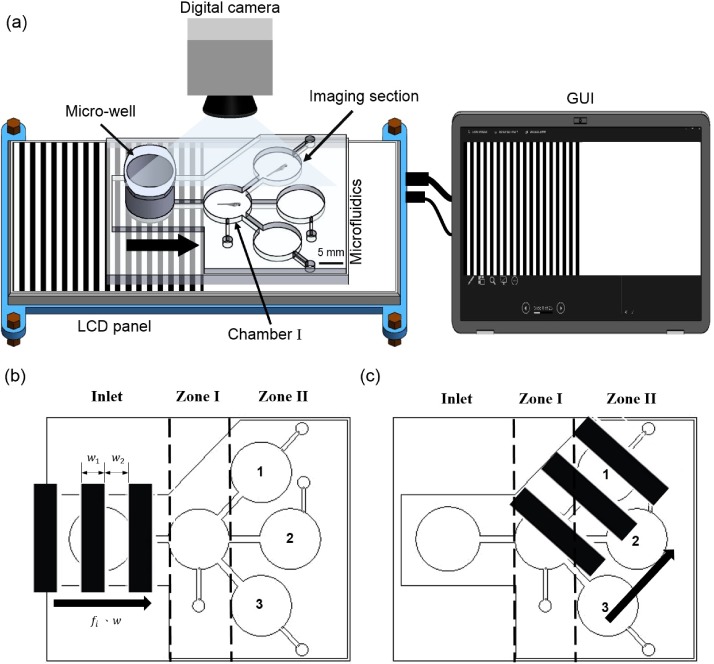
To fabricate the microfluidic device, a series of micromachining and casting was employed. To generate moving gratings, a liquid crystal display projector was used and the microfluidic device designed for the experiment was mounted on it (Fig. 1). It can be noted that the microfluidic device consists of a microwell, a chamber, and three imaging sections. The experiment was designed to transport the larvae into different imaging sections from the breeding chamber via the chamber I. To avoid terminological confusion, the different sections of the microfluidic device were coined as inlet, zone I, and zone II. The moving gratings were generated inclined or horizontal according to the required direction of transportation. Considering that the visuomotor response is developed only after 3 days-post fertilization (d. p. f.),8 5 d. p. f. zebrafish larvae were considered in this study. To generate the moving gratings on the bottom of the microfluidic device, a custom made GUI was developed. To observe the response of the zebrafish larvae corresponding to the artificial stimuli, a digital camera was employed and the acquired videos were analyzed through the ImageJ software.12 To realize the statistical significance, an independent sample student's t-test was performed.
FIG. 1.
Schematic illustration of the experimental setup. The moving gratings were generated through the LED panel and the larvae were transported from the inlet to zone I and to the imaging section of zone II. The direction of motion is indicated by black arrow.
RESULTS AND DISCUSSIONS
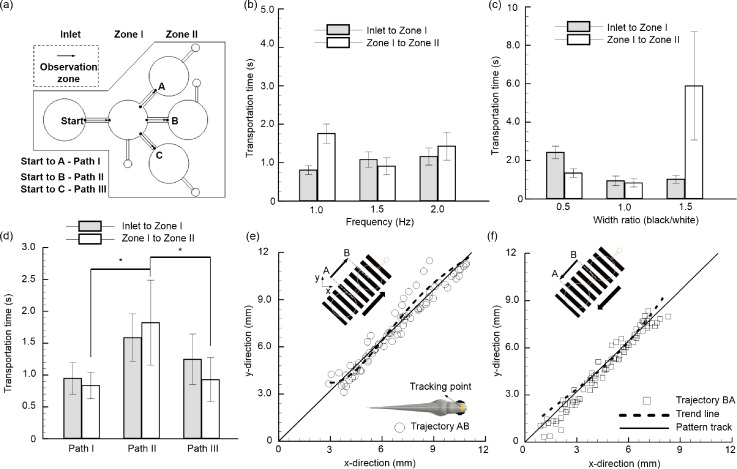
To test the efficacy of the proposed system and to optimize the device parameters, experiments were designed and conducted. As depicted in Fig. 2(a), three test paths, namely, path I, path II, and path III, were tested to verify that whether the transportation of larvae was truly a response to generated gratings and was not due to the microfluidic design or natural innate behaviors of larvae. As the distance traversed by a juvenile zebrafish larva in a given time indicates the effectiveness of the generated stimulus,11 the grating features such as temporal frequency and width ratio were tested for effective transportation [Figs. 2(b) and 2(c)]. From the experiment, it was observed that strong optomotor responses were evoked in all the used zebrafish larvae corresponding to the movies with drifting gratings. However, by averaging over several trials (n = 5), we could detect behavioral responses of the larvae which tend to respond well under the conditions where the moving grating has a temporal frequency of 1.5 Hz and grating width ratio of 1:1. The obtained results are in good accordance with the following literature that highlights the inability of zebrafish larvae to follow the fast-moving stimuli due to an aliasing issue.8 With the optimized grating parameters, i.e., temporal frequency of 1.5 Hz and grating width ratio of 1:1, the minimum and maximum transportation time periods for 5 d. p. f. zebrafish larvae are accounted as 0.63 and 2.49 s, respectively. In detail, it took 0.95 ± 0.25 s and 0.84 ± 0.21 s, respectively, for the larvae to move from inlet to test zone I and test zone I to test zone II through path I. Similar results were obtained when the larvae were transported via path III which has a similar channel design as path I. However, it took a relatively larger time period to transfer the larvae via path II, as the transportation time for inlet to test zone I and test zone I to test zone II were recorded as 1.59 ± 0.38 s and 1.82 ± 0.67 s, respectively. These results further illustrate that the zebrafish larvae respond well to the stimuli generated in an inclined manner from their side rather than generated horizontally from their front as the average transportation time recorded for zone I to zone II via path I and path II were quantified as 0.84 ± 0.21 s and 1.82 ± 0.67 s, respectively. This behavioral response of the larvae can be described by shedding some light on the larvae's central visual field which is located around 45° from the tip of larvae's rostrum.13 The position of zebrafish larvae's eyes allows them to capture the stimulus generated either from its left or right side, and they reacted better towards the gratings generated with an angle rather than horizontal. A statistical significance (p-value < 0.05) between the three different paths between zone I to zone II further highlights that the feasibility of this proposed method towards the transportation of larvae in a competitive time span.
FIG. 2.
Transportation of zebrafish larvae from the inlet to test zone I and zone II through the proposed light driven technique. (a) Schematic illustration of the microfluidic device describes the different paths used during experiment. Transportation time for 5 d. p. f. zebrafish larvae: (b) corresponding to different gratings frequency and (c) corresponding to different grating width ratio. (d) Transportation time for 5 d. p. f. zebrafish larvae through different paths (path I, path II, and path III) with an optimum grating frequency of 1.5 Hz and a grating width ratio of 1:1. Error bars denote one standard deviation over five zebrafish larvae. Transportation of larvae (e) was achieved from the starting point “A” to the destination point “B” within zone I and zone II. The inset picture illustrates the tracking point which was selected between both the eyes of the larvae. Transportation of larvae (f) was achieved from the destination point “B” to the starting point “A.” The tracking points plotted were collected from 5 individual larvae. The correlation coefficient (R2) values for both the cases were quantified as 0.94 and 0.97, respectively. It highlights the linearity of the obtained data sets highlighting that the larvae swim along the direction of the motion of gratings.
Although from the results it was observed that the fish traversed along the direction of generated stimulus, it might be possible that they were traversing naturally either due to the microfluidic design or due to their natural response. An additional experiment was conducted to illustrate that the larvae truly follow the generated stimulus. As illustrated in Figs. 2(e) and 2(f), a test was conducted so that the larvae can return to its starting point “A” from its destination point “B” through the moving gratings in both the directions. The mid-point between both the eyes was selected as a tracking point and its positions were recorded throughout the motions for 5 individual larvae, and the results are plotted in the horizontal plane. It was observed that all the fishes follow a straight path from their starting point to the destination point and return back to the starting point in a straight path as the correlation coefficient (R2) between all the data points were accounted as 0.94 and 0.97, respectively. This high R2 value further depicts the linearity in the obtained data sets and therefore highlights that the larvae swam vigorously along with the direction of gratings. As the minimum transportation time with proposed technique was accounted only as 0.63 s, it can be effectively used to transport the zebrafish larvae within the microfluidics. On top of that, this device can be integrated with any of the currently other existing devices with a minimum energy budget to transport the larvae in a non-invasive manner without the use of anesthesia which potentially hinders larvae's natural behaviors.
ACKNOWLEDGMENTS
This study was supported through the Ministry of Science and Technology of Taiwan under Contract No. MOST 105-2628-E-006-006-MY3 (to Chia-Yuan Chen). This work would not be possible without the facility provided by Center for Micro/Nano Science and Technology, National Cheng Kung University.
References
- 1. Asnani A. and Peterson R. T., Dis. Models Mech. 7(7), 763–767 (2014). 10.1242/dmm.016170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dooley K. and Zon L. I., Curr. Opin. Genet. Dev. 10(3), 252–256 (2000). 10.1016/S0959-437X(00)00074-5 [DOI] [PubMed] [Google Scholar]
- 3. Mani K., Chien T.-C. C., Panigrahi B., and Chen C.-Y., Sci. Rep. 6, 36385 (2016). 10.1038/srep36385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang T. Y., Pardo-Martin C., Allalou A., Wahlby C., and Yanik M. F., Lab Chip 12(4), 711–716 (2012). 10.1039/C1LC20849G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin X., Wang S., Yu X., Liu Z., Wang F., Li W. T., Cheng S. H., Dai Q., and Shi P., Lab Chip 15(3), 680–689 (2015). 10.1039/C4LC01186D [DOI] [PubMed] [Google Scholar]
- 6. Schmitt E. A. and Dowling J. E., J. Comp. Neurol. 404(4), 515–536 (1999). [DOI] [PubMed] [Google Scholar]
- 7. Wolf S., Dubreuil A. M., Bertoni T., Böhm U. L., Bormuth V., Candelier R., Karpenko S., Hildebrand D. G., Bianco I. H., and Monasson R., Nat. Commun. 8(1), 651 (2017). 10.1038/s41467-017-00310-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neuhauss S. C., Dev. Neurobiol. 54(1), 148–160 (2003). 10.1002/neu.10165 [DOI] [PubMed] [Google Scholar]
- 9. Orger M. B., Curr. Biol. 26(9), R377–R385 (2016). 10.1016/j.cub.2016.03.054 [DOI] [PubMed] [Google Scholar]
- 10. Roeser T. and Baier H., J. Neurosci. 23(9), 3726–3734 (2003), available at https://www.ncbi.nlm.nih.gov/pubmed/12736343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orger M. B., Smear M. C., Anstis S. M., and Baier H., Nat. Neurosci. 3(11), 1128 (2000). 10.1038/80649 [DOI] [PubMed] [Google Scholar]
- 12. Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., and Schmid B., Nat. Methods 9(7), 676–682 (2012). 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kubo F., Hablitzel B., Dal Maschio M., Driever W., Baier H., and Arrenberg A. B., Neuron 81(6), 1344–1359 (2014). 10.1016/j.neuron.2014.02.043 [DOI] [PubMed] [Google Scholar]