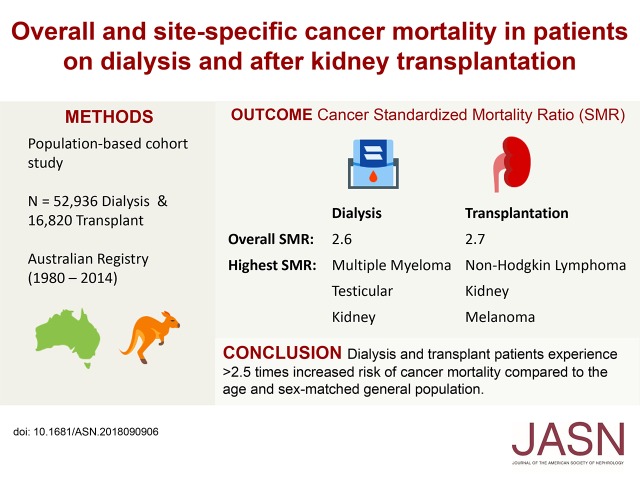
Significance Statement
Patients with ESRD experience a substantially increased incidence of cancer, but few studies have examined patterns of cancer mortality among such patients. This population-based cohort study of Australian patients who initiated dialysis or received a kidney transplant from 1980 to 2014 found that the risk of cancer-related death among 52,936 patients on dialysis and 16,820 kidney transplant recipients was 2.5 times higher than that of age- and sex-matched controls in the general population. For patients on dialysis, increased cancer mortality is primarily from cancers that caused ESRD; among transplant recipients, the increase is largely due to de novo cancers. Additional efforts are required to clarify factors and mechanisms involved in increased cancer risk and to improve early detection and management of cancer in this population.
Keywords: end stage kidney disease, cancer, dialysis, transplantation, mortality
Visual Abstract
Abstract
Background Patients with ESRD have a substantially increased cancer risk, but few studies have examined the patterns of cancer mortality along a patient's journey from dialysis to transplantation.
Methods
We identified all Australian patients on dialysis and patients with transplants from 1980 to 2014 from the Australia and New Zealand Dialysis and Transplant Registry. Using standardized mortality ratios (SMRs), we compared cancer mortality among patients on dialysis and patients with transplants versus the general population (overall and by age, sex, year, and site); we also performed a subgroup analysis excluding patients with preexisting cancers.
Results
We followed 52,936 patients on dialysis and 16,820 transplant recipients for 170,055 and 128,352 patient-years, respectively. There were 2739 cancer deaths among patients on dialysis and 923 cancer deaths among transplant recipients. Overall, cancer SMRs were 2.6 for patients on dialysis and 2.7 for transplant recipients. For patients on dialysis, SMRs were highest for multiple myeloma (30.5), testicular cancer (17.0), and kidney cancer (12.5); for transplant recipients, SMRs were highest for non-Hodgkin lymphoma (10.7), kidney cancer (7.8), and melanoma (5.8). Some 61.0% of patients on dialysis and 9.6% of transplant recipients who experienced cancer death had preexisting cancer. The SMRs for de novo cancer was 1.2 for patients on dialysis and 2.6 for transplant recipients.
Conclusions
Patients on dialysis and transplant recipients experienced >2.5-fold increased risk of cancer death compared with the general population. This increased risk was largely driven by preexisting cancers in patients on dialysis and de novo cancers in patients with transplants.
There is now clear evidence showing that cancer incidence is increased by up to fourfold for patients on dialysis and kidney transplant recipients compared with the general population.1–4 Registry analyses and observational studies indicate that the 5-year cumulative incidence for cancer is about 5%–10% in dialysis and transplant populations.4–7 Although patients on dialysis and patients with transplants experience a greater incidence of cancer compared with the general population, the patterns and outcomes of cancers in these patients may differ from the general population due to the high burden of comorbid medical conditions, such as cardiovascular disease, and the influence of transplantation and immunosuppression.8,9
Given the substantially increased risk of cancer in patients on dialysis and patients with transplants, a clear understanding of the pattern of cancer mortality is needed to identify at-risk patients who may benefit from interventions, such as cancer screening and specific cancer treatment. However, relatively few studies have examined the rates and patterns of cancer mortality in patients on dialysis and patients with transplants and the differences in cancer mortality in a patient’s journey from dialysis to transplantation.8,10,11 Few have examined cancer mortality by age, sex, year, and cancer type or the effect of preexisting cancers on cancer mortality after commencement of dialysis and after kidney transplantation. The primary objective of this study was to assess the pattern of cancer mortality in patients on dialysis and patients after kidney transplantation and compare the rates of overall and site-specific cancer mortality with those of the age- and sex-matched general population.
Methods
Study Population
All patients who commenced dialysis or received a kidney transplant in Australia between 1980 and 2014 were included. We included recipients who had a functioning first allograft and excluded patients who experienced graft failure and returned to dialysis. Patients on dialysis who later received a kidney transplant were included in the dialysis group until the time of transplantation.
Data Collection
Details of patient characteristics, dialysis and transplantation treatment, cause of death, and cancer outcomes for patients on dialysis and patients with transplants were obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. Baseline patient characteristics included age, sex, ethnicity, cause of ESRD, smoking status, and comorbid medical conditions. Data for dialysis and transplantation treatments included the commencement date of RRT, initial dialysis modality, dialysis duration, transplantation date, date of graft failure, and initial transplant immunosuppression.
Ascertainment of Cancer Death
All incident cancers and cancer-related deaths for patients on dialysis or patients with a kidney transplant are recorded in the ANZDATA Registry, including deaths from treatment withdrawal due to cancer. The first diagnosis of nonmelanoma skin cancer is also recorded. Cancers reported to the ANZDATA Registry are coded for site and cell type on the basis of the classification from the International Classification of Disease for Oncology first edition.12 Previous analyses have shown that cancer records within the ANZDATA Registry are robust and accurate, with a high concordance rate when compared with cancer incidence and mortality records reported to the New South Wales Cancer Registry13 and the Australian National Death Index.14 We included all cancer diagnoses and cancer deaths in adult patients (age 20 years old or older) except for nonmelanoma skin cancer, premalignant, or in situ lesions in our analyses. Date and causes of death as recorded in the ANZDATA Registry were also included. Cancer-related deaths for patients on dialysis and patients with transplants were defined on the basis of the reported cause of death or where cancer was reported as contributing to death. Cancer mortality data by age, sex, and year of death for the Australian general population were obtained from the Australian Institute of Health and Welfare.15
Statistical Analyses
Cancer mortality rates for patients on dialysis and patients with transplants were calculated by age, sex, and year of dialysis commencement or transplantation (in 5-year periods). The cumulative incidence rates of death from cancer, cardiovascular disease, and infection for the first 15 years after commencement of dialysis or receiving a kidney transplant were calculated using a nonparametric method,16 which takes into account the competing risk of transplantation in patients on dialysis, graft failure in transplant recipients, and other causes of death. The cumulative incidence of death for each cancer type was calculated to identify the five most common cancers causing death in patients on dialysis and patients with transplants. Standardized mortality ratios (SMRs) and standardized rate differences for all cancers and cancer types were calculated using indirect standardization stratified by age group (20–34, 35–49, 50–64, and over 65 years old), sex, and year of death (1980–2014 in 5-year periods). To examine cancer mortality over time, SMRs stratified by the year of dialysis commencement or transplantation (in 5-year periods) were calculated. A subgroup analysis was performed by excluding patients with preexisting cancers. Preexisting cancers were defined as cancers diagnosed before or within 6 months of commencing dialysis or receiving a kidney transplant. Cancer incidence rates and standardized incidence ratios (SIRs) for cancers diagnosed >6 months after commencing dialysis or receiving a kidney transplant were also calculated for comparison. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
In total, 52,936 patients who commenced dialysis and 16,820 patients who received their first kidney transplant between 1980 and 2014 were identified. The median durations (and interquartile ranges [IQRs]) of follow-up for patients on dialysis and patients with transplants were 2.3 years (IQR, 1.0–4.6) and 6.3 years (IQR, 2.3–12.0), respectively, representing a total of 170,055 and 128,352 patient-years of follow-up, respectively.
Baseline Characteristics
The majority of patients were men (60%), with median ages of 60 years old (IQR, 47–70) for patients on dialysis and 43 years old (IQR, 31–53) for patients with transplants at the time of reaching ESRD (Table 1). More than three quarters were white. Less than 4% of transplant recipients and 10% of patients on dialysis were Indigenous Australians. Among patients on dialysis, the most common causes of ESRD were GN (27.5%) and diabetes mellitus (26.3%). In transplant recipients, GN was the most common cause of ESRD (43.6%) followed by polycystic kidney disease (12.5%) and diabetes mellitus (11.0%).
Table 1.
Baseline characteristics of patients on dialysis and patients with transplants
| Characteristic | Dialysis, n (%) | Transplantation, n (%) |
|---|---|---|
| Total | 52,936 (100) | 16,820 (100) |
| Sex | ||
| Men | 31,224 (59.0) | 10,172 (60.5) |
| Women | 21,712 (41.0) | 6648 (39.5) |
| Age at reaching ESRD, yr | ||
| Under 20 | 1436 (2.7) | 1514 (9.0) |
| 20–34 | 4530 (8.6) | 3770 (22.4) |
| 35–49 | 9688 (18.3) | 5794 (34.5) |
| 50–64 | 16,778 (31.7) | 5205 (31.0) |
| 65 or over | 20,504 (38.7) | 537 (3.2) |
| Ethnicity | ||
| White | 42,234 (79.8) | 14,255 (84.8) |
| Indigenous | 4222 (8.0) | 563 (3.4) |
| Other | 6480 (12.2) | 2002 (11.9) |
| Cause of renal failure | ||
| GN | 14,549 (27.5) | 7331 (43.6) |
| Vascular | 6556 (12.4) | 715 (4.3) |
| Polycystic kidney disease | 3480 (6.6) | 2110 (12.5) |
| Reflux | 2047 (3.9) | 1610 (9.6) |
| Diabetes mellitus | 13,918 (26.3) | 1856 (11.0) |
| Other | 12,386 (23.4) | 3198 (19.0) |
| Comorbidities | ||
| Chronic lung disease | 8045 (16.7) | 1405 (9.3) |
| Coronary artery disease | 20,194 (41.8) | 3220 (21.4) |
| Peripheral vascular disease | 12,389 (25.7) | 1766 (11.7) |
| Cerebrovascular disease | 7836 (16.3) | 1199 (8.0) |
| Diabetes mellitus | 20,549 (42.0) | 4025 (26.1) |
| Preexisting cancer | 5762 (10.9) | 717 (4.3) |
| Smoker (current or former) | 24,444 (52.2) | 5659 (40.8) |
| Initial renal replacement modality | ||
| Hemodialysis | 37,767 (71.3) | 10,738 (63.8) |
| Peritoneal dialysis | 15,169 (28.7) | 4586 (27.3) |
| Transplantation | — | 1496 (8.9) |
| Initial immunosuppression | ||
| Prednisolone | — | 15,653 (94.8) |
| Tacrolimus | — | 6438 (39.0) |
| Cyclosporin | — | 8969 (54.3) |
| Mycophenolate | — | 8242 (49.9) |
| Azathioprine | — | 5576 (33.8) |
—, not applicable.
Cancer Death
There were in total 26,004 and 3168 deaths in the dialysis and transplant cohorts, respectively. Of patients who died during follow-up, 2739 (8.7%) and 923 (16.7%) deaths in patients on dialysis and patients with transplants, respectively, were attributed to cancer. The median age of cancer death among patients on dialysis was 70 years old (IQR, 63–76), and it was 62 years old (IQR, 55–68) for transplant recipients. In patients on dialysis, the median time from commencement of dialysis to cancer death was 2.0 years (IQR, 0.8–4.0), whereas in patients with transplants, the median time from transplantation to cancer death was 8.6 years (IQR, 4.9–13.1).
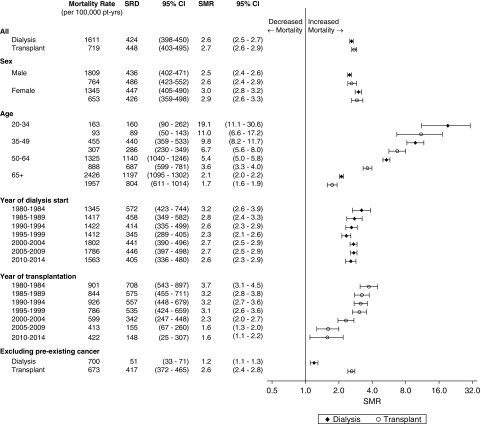
The overall 10-year mortality from any cause was 58.7% in patients on dialysis and 15.6% in transplant recipients. In patients on dialysis, the 10-year cumulative incidence of cancer death was 6.1%; it was 25.4% for cardiovascular deaths, and 32.9% received a kidney transplant (Supplemental Figure 1A). In transplant recipients, the 10-year cumulative incidence of cancer deaths was 4.5%; it was 5.5% for deaths from cardiovascular disease, and 24.6% experienced graft loss and returned to dialysis (Supplemental Figure 1B). The incidence of cancer death in patients on dialysis was greatest in the first few years after commencing dialysis, particularly for deaths from multiple myeloma, whereas in transplant recipients, cancer death increased steadily with time after transplantation (Figure 2 and Supplemental Figure 1). Patients on dialysis had the highest crude cancer mortality rate, with 1611 cancer deaths per 100,000 patient-years, compared with crude cancer mortality rates of 719 cancer deaths per 100,000 patient-years in patients with transplants and 258 cancer deaths per 100,000 patient-years in the general population. Relative to the age- and sex-matched general population, the overall SMRs for cancer death among patients on dialysis and patients with transplants were 2.6 (95% confidence interval [95% CI], 2.5 to 2.7) and 2.7 (95% CI, 2.6 to 2.9), respectively.
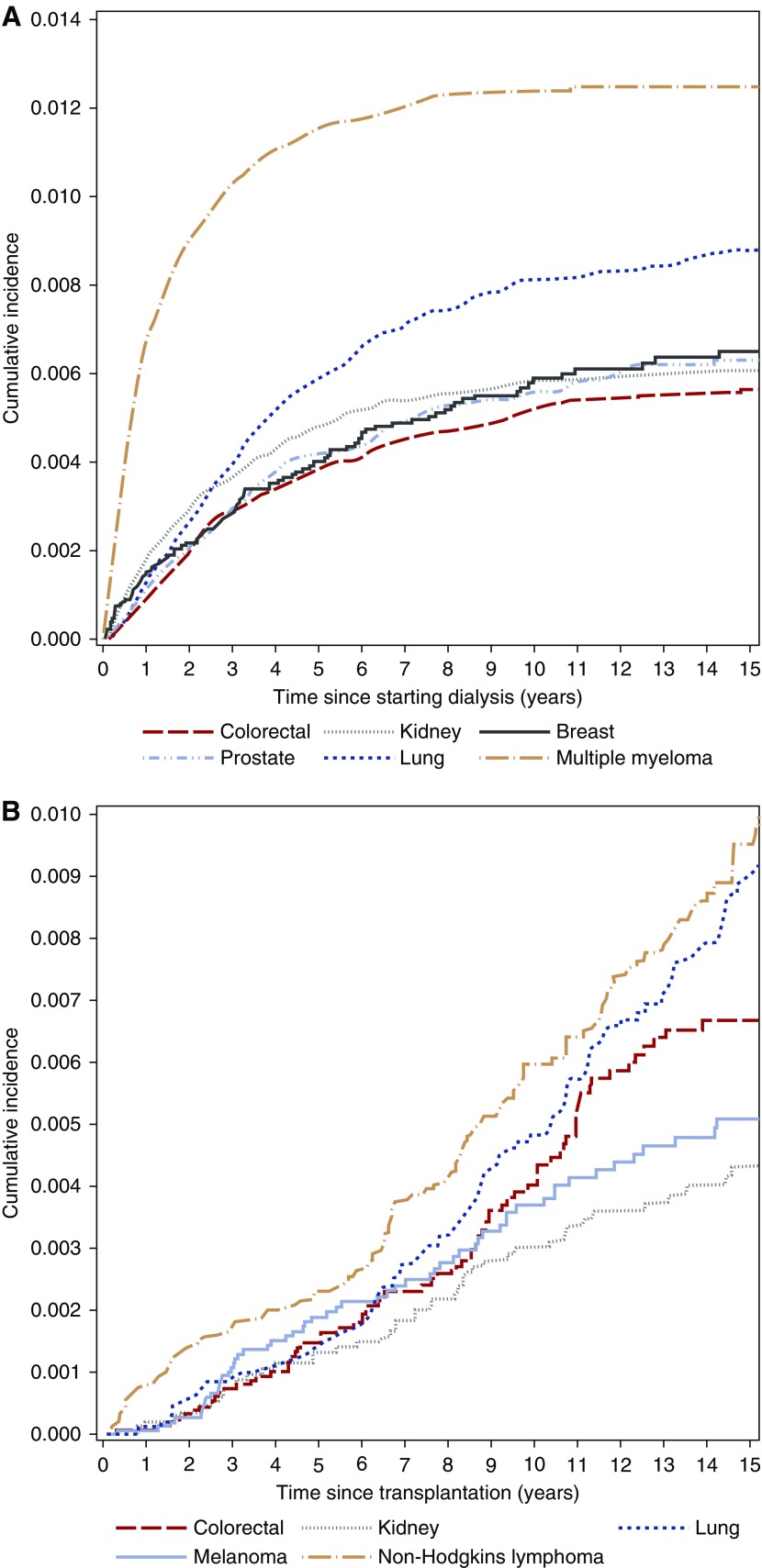
Figure 2.
The cumulative incidence of the most common cancers causing death in patients on dialysis (A) and patients with transplants (B) are different. In patients on dialysis, the most common causes of cancer death were multiple myeloma, lung cancer and kidney cancers. In patients with transplants, the most common causes of cancer death were non-Hodgkin lymphoma, lung cancer and colorectal cancer.
Cancer Deaths by Sex
Men on dialysis and men with transplants experienced higher crude cancer mortality rates than women (P<0.05) (Figure 1). Relative to the age-matched general population, women on dialysis and women with kidney transplants seemed to experience a greater increased risk of cancer-related death than men. Women on dialysis had an SMR of 3.0 (95% CI, 2.8 to 3.2), whereas men on dialysis had an SMR of 2.5 (95% CI, 2.4 to 2.6). Among transplant recipients, women had an SMR of 2.9 (95% CI, 2.6 to 3.3) compared with an SMR of 2.6 (95% CI, 2.4 to 2.9) in men.
Figure 1.
Cancer mortality rate and standardized mortality ratio (SMR) varied by age, sex, year, and preexisting cancer status. Standardized rate differences (SRDs) are presented in comparison with the general population. 95% CI, 95% confidence interval.
Cancer Deaths by Age
Similar to the general population, absolute cancer mortality rates increased with increasing age. The lowest absolute rates were seen in patients ages 20–34 years old (dialysis: 163 cancer deaths per 100,000 patient-years; transplant: 93 cancer deaths per 100,000 patient-years) compared with absolute cancer mortality rates of 2426 and 1957 cancer deaths per 100,000 patient-years in patients on dialysis and patients with transplants ages 65 years old and above, respectively.
Younger patients on dialysis and younger patients with transplants (age 20–34 years old) experienced excess risks of cancer death of 19.1 (SMR; 95% CI, 11.1 to 30.6) and 11.0 times (SMR; 95% CI, 5.5 to 17.2), respectively, whereas older patients on dialysis and older patients with transplants (age over 65 years old) had cancer mortality rates that were 2.1 (SMR; 95% CI, 2.0 to 2.2) and 1.7 times (SMR; 95% CI, 1.6 to 1.9), respectively, those of people in the same age group in the general population (Figure 1).
Cancer Deaths by Year
Cancer mortality rates for patients on dialysis and patients with transplants by year of commencing dialysis or receiving a kidney transplant, respectively, are presented in Figure 1. For patients on dialysis, crude cancer mortality rates seem to have increased for patients starting dialysis in more recent years: from 1345 cancer deaths per 100,000 patient-years in 1980–1984 to 1563 cancer deaths per 100,000 patient-years in 2010–2014 (Figure 1). After standardization for age and sex, the standardized rate difference and SMR of cancer deaths remained unchanged irrespective of the year of dialysis commencement. For transplant recipients, the crude and relative cancer mortality rates (in comparison with the general population) were lower for patients who received a transplant in recent years, with a crude cancer mortality rate of 901 cancers per 100,000 patient-years and an SMR of 3.7 (95% CI, 3.1 to 4.5) for transplant recipients who received a transplant in 1980–1984 compared with a crude cancer mortality rate of 422 cancers per 100,000 patient-years and an SMR of 1.6 (95% CI, 1.1 to 2.2) for transplant recipients who received a transplant in 2010–2014.
Cancer Deaths by Cancer Type
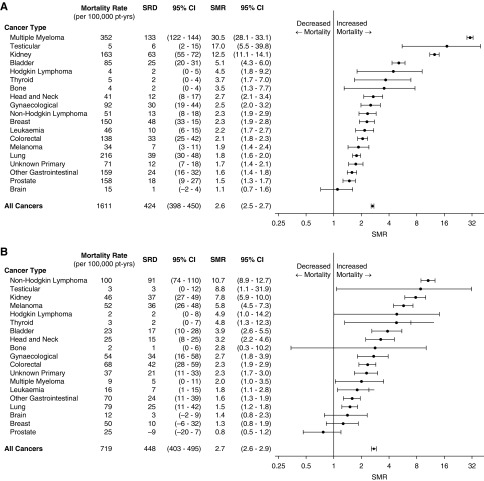
In the dialysis population, the most common cancer deaths occurred in cancers that cause ESRD, such as multiple myeloma (352 per 100,000 patient-years) and kidney cancers (162 per 100,000 patient-years), and cancers that are common in the general population, such as lung cancer (216 per 100,000 patient-years), breast cancer in women (150 per 100,000 patient-years), and prostate cancer (158 per 100,000 patient-years). Accounting for the competing risks of transplantation and noncancer causes of death, the cancer types with the highest 10-year cumulative incidence rates of death were multiple myeloma (1.2%), lung cancer (0.8%), kidney cancers (0.6%), and breast cancer (0.6% in women) (Figure 2A). Relative to the age- and sex-matched general population, the highest cancer SMRs were for multiple myeloma (30.5; 95% CI, 28.1 to 33.1), testicular cancer (17.0; 95% CI, 5.5 to 39.8), and kidney cancers (12.5; 95% CI, 11.1 to 14.1) as shown in Figure 3A.
Figure 3.
In patients on dialysis (A), the greatest standardized mortality ratio (SMR) are in cancers related to ESRD, whereas for patients with transplants (B), the greatest SMR are in cancers relating to immunosuppression. Standardized rate differences (SRDs) are presented in comparison with the general population. Non-Hodgkin lymphoma includes post-transplant lymphoproliferative disorder. Gynecologic cancers include cervical, uterine, and ovarian cancers. 95% CI, 95% confidence interval.
In kidney transplant recipients, lung cancer (79 per 100,000 patient-years); colorectal cancer (68 per 100,000 patient-years); breast cancer in women (50 per 100,000 patient-years); and cancers related to immunosuppression, such as non-Hodgkin lymphoma (100 per 100,000 patient-years), gynecologic cancers including cervical, ovarian, and uterine cancers (54 per 100,000 patient-years), and melanoma (52 per 100,000 patient-years), had the highest absolute rates of cancer death. Accounting for the competing risks of graft failure and noncancer causes of death, the cancer types with the highest 10-year cumulative incidence of death were non-Hodgkin lymphoma (0.6%), lung cancer (0.5%), colorectal cancer (0.4%), and melanoma (0.4%) (Figure 2B). Relative to the general population, the greatest increased risks of cancer death were for non-Hodgkin lymphoma (SMR, 10.7; 95% CI, 8.9 to 12.7), kidney cancers (SMR, 7.8; 95% CI, 5.9 to 10.0), and melanoma (SMR, 5.8; 95% CI, 4.5 to 7.3) as shown in Figure 3B. There was no significant increased risk of cancer death from breast cancer in women (SMR, 1.3; 95% CI, 0.8 to 1.9) or prostate cancer (SMR, 0.8; 95% CI, 0.5 to 1.2).
Cancer Deaths and Preexisting Cancer
In total, there were 5762 (10.9%) patients on dialysis and 717 (4.3%) patients with transplants with a cancer diagnosed before or within 6 months of commencing dialysis or receiving a kidney transplant, respectively. There were 1672 cancer deaths in patients on dialysis and 89 cancer deaths in patients with transplants with a preexisting cancer (Supplemental Figure 2). Most cancer deaths occurring in patients with a preexisting cancer were due to the same type of cancer (1545 [92.4%] in patients on dialysis and 61 [68.5%] in patients with transplants). Of all cancer deaths in patients on dialysis, 61.0% were among those with a preexisting cancer. For transplant recipients, 9.6% of cancer deaths were from patients with a prior cancer history. In patients on dialysis, the median times from starting dialysis to cancer death were 1.1 years (IQR, 0.5–2.6) for patients with preexisting cancers and 3.6 years (IQR, 2.1–5.9) for deaths attributed to de novo cancers, whereas for patients with transplants, the median times from transplantation to cancer death were 3.5 years (IQR, 1.5–8.8) for patients with preexisting cancers and 8.9 years (IQR, 5.5–13.5) for those with de novo cancers.
Among patients on dialysis without a preexisting cancer, the overall cancer mortality rate was 700 per 100,000 patient-years, and the risk of overall cancer death was increased in comparison with the general population but to a lesser extent than when patients with a preexisting cancer were included (SMR, 1.2; 95% CI, 1.1 to 1.3). The 10-year cumulative incidence of de novo cancer death was 2.8%, with the most common cancers being lung (0.6%), colorectal (0.3%), and breast cancer (0.3%) (Supplemental Figure 3A). The greatest increased risks of cancer-related death were for kidney (SMR, 2.4; 95% CI, 1.7 to 3.2), head and neck (SMR, 2.0; 95% CI, 1.4 to 2.7), and bladder cancers (SMR, 1.9; 95% CI, 1.4 to –2.5) (Supplemental Figure 4A). For comparison, the cancer incidence rates and SIRs by cancer type for patients on dialysis are provided in Supplemental Figure 5A. The increased cancer mortality rates for kidney and bladder cancers in patients on dialysis mirror the increased incidence rates of these cancers in this population (kidney cancer: SIR, 3.7; 95% CI, 3.1 to 4.3; bladder cancer: SIR, 2.2; 95% CI, 1.8 to 2.6). However, although the incidence of thyroid cancer seems to be increased (SIR, 2.7; 95% CI, 2.0 to 3.6), mortality from thyroid cancer in patients on dialysis was similar to that of the general population (SMR, 0.9; 95% CI, 0.1 to 3.4).
Among transplant recipients without a prior cancer history, the cancer mortality rate for all cancers was 673 per 100,000 patient-years. The 10-year cumulative incidence of death from de novo cancer in transplant recipients was 4.1%, with the most common cancer deaths occurring with non-Hodgkin lymphoma (0.6%), lung (0.5%), and colorectal cancers (0.4%) (Supplemental Figure 3B). Compared with the general population, transplant recipients without a preexisting cancer experienced a 2.6-fold increased risk of cancer death (SMR; 95% CI, 2.4 to 2.8). SMRs were greatest for non-Hodgkin lymphoma (10.4; 95% CI, 8.6 to 12.4), kidney cancers (6.1; 95% CI, 4.5 to 8.2), and melanoma (5.1; 95% CI, 3.8 to 6.6) (Supplemental Figure 4B). The increased risks of death from non-Hodgkin lymphoma, kidney cancers, and melanoma may reflect the higher incidence rates of these cancers in transplant recipients (Supplemental Figure 5B).
Discussion
In this study of 52,936 patients on dialysis and 16,820 patients with transplants followed for 170,055 and 128,352 patient-years, respectively, an excess risk of cancer-related death of over 2.5 times compared with the age- and sex-matched general population was found. The magnitude of the increased risk varied by age, sex, year, and cancer type. Compared with the general population, women and younger patients experienced increased risks of cancer-related death by at least three times. Younger patients (ages 20–34 years old) on dialysis and younger patients after kidney transplantation experienced more than tenfold increased risks of cancer mortality relative to the general population, whereas older patients (ages over 65 years old) on dialysis and older patients after transplantation experienced increased cancer mortality risks of around 1.5 times. We observed SMRs exceeding ten for virus-related cancers, such as non-Hodgkin lymphoma (including post-transplant lymphoproliferative disorder), in transplant recipients and ESRD-related cancers, such as myeloma, in patients on dialysis. SMRs were not increased for some cancers, such as breast and prostate cancers.
Although there is evidence suggesting increased risk of cancer in patients on dialysis and patients with transplants,2,17,18 relatively few studies have examined the rates and patterns of cancer mortality in patients on dialysis and patients with transplants compared with the general population.10,11,19–21 A recent large cohort study in Ontario, Canada found an increased risk of cancer mortality in solid organ transplant recipients, with an overall cancer SMR of 2.8 and an SMR of 1.9 for de novo cancers.10 A study using data from the Hong Kong Renal Registry found an overall cancer SMR of 2.3 in kidney transplant recipients, with the highest cancer SMR found for non-Hodgkin lymphoma (18.2).19 The increased cancer mortality rates in transplant recipients in these studies are similar to those found in this study. Similarly, a pattern of increased cancer SMR in younger transplant recipients, which decreased with increasing age, was found in these studies, but a difference in cancer mortality by sex was not consistently seen.10,19 However, somewhat conflicting results were observed in an older United States cohort study on patients transplanted between 1990 and 2004. Although a greater risk of cancer death was found in younger kidney transplant recipients ages <50 years old (SMR>1), transplant recipients >60 years old were found to have a lower risk of cancer death (SMR<0.8), with an overall cancer mortality rate that was similar in transplant recipients compared with the general population (SMR=0.96).21 These observed findings may be attributed to the competing risks of death associated with cardiovascular disease, particularly for older recipients. Only one large-scale European registry study has previously explored cancer mortality in patients on dialysis and patients with transplants compared with the general population.11 In this study, cancer mortality was found to be 2.9 times higher in patients on dialysis and 1.7 times higher in patients with kidney transplants, with a lower standardized cancer mortality ratio of 2.2 in patients on dialysis after exclusion of patients with malignancy as the primary cause of renal disease.11 Although prior work has examined the overall risk of cancer death in patients on dialysis and patients with transplants, few studies have quantified both the absolute and relative risks of cancer death over a patient’s journey from ESRD on dialysis to kidney transplantation.
This study has shown that the key contributors to the observed excess risk of cancer death are different between the dialysis and transplanted populations, with variations in cancer risk across cancer types. In patients on dialysis, the increased risk of cancer death seemed to be driven primarily by death from cancers diagnosed before or within 6 months of commencing dialysis, comprising over 61.0% of all cancer deaths. Most of these cancers were cancers that caused ESRD, such as multiple myeloma and cancers of the kidney and urinary tract, and resulted in death in the first few years after commencement of dialysis. When only de novo cancer deaths are considered, the rates of cancer death from multiple myeloma and kidney and bladder cancers were only moderately increased compared with in the general population (SMR of up to 2.4). The high cancer mortality rate seen in patients on dialysis with a preexisting cancer that leads to ESRD, such as multiple myeloma and kidney cancer, and the relatively short time to cancer death (of <2 years) after commencement of dialysis suggest that, despite commencement of dialysis, these patients’ overall prognosis may still be predominantly affected by their underlying malignancy. This is an important consideration for clinicians when considering dialysis and other treatment options for ESRD in these patients.
For transplant recipients, <10% of those with cancer-related death had a prior cancer. The lower rates of cancer death from preexisting cancer in patients with transplants are likely due to the rigorous pretransplant screening and assessment of potential transplant candidates to exclude those with active cancer or a significant risk of cancer recurrence.22 For instance, a previous history of multiple myeloma is generally considered a contraindication to transplantation. However, transplant recipients without a prior cancer still experienced an increased burden of cancer death that varied considerably by cancer type, with excess cancer mortality risks of over ten times for non-Hodgkin lymphoma and over five times for melanoma compared with the general population.
The poor outcomes experienced by transplant recipients for certain cancers may be due to a range of factors, such as the influence of immunosuppression on the aggressiveness of cancer development and the limited available treatment options for these cancers. The association between immunosuppression and cancers associated with viral pathogenesis, such as non-Hodgkin lymphoma and post-transplant lymphoproliferative disorder, has been described in previous studies.23,24 Post-transplantation immunosuppression reduces immune surveillance and can lead to reduced antiviral immunity, resulting in an altered balance between effective antiviral response and oncogenic potential of these viruses.25 Immunosuppressive medications, such as calcineurin inhibitors, also have direct effects on cancer development, such as increasing production of vascular endothelial growth factor and TGF-β, leading to accelerated tumor growth.26 In the setting of transplantation, cancer treatments and response to cancer therapies may also differ from those in the general population. Clinicians may be less aggressive in pursuing cancer therapies and reducing immunosuppression due to concerns of causing graft dysfunction, potential drug-drug interactions of chemotherapy with immunosuppression medication, and nephrotoxicity associated with certain chemotherapy agents. The use of immune checkpoint inhibitors, such as anticytotoxic T lymphocyte antigen 4 inhibitors and antiprogrammed cell death protein 1 inhibitors, has also been reported to cause graft rejection in some transplant recipients due to the upregulation of T cell activation.27
There are several potential limitations to this study. Although patients on dialysis and patients with transplants were found to have overall increased rates of cancer mortality compared with the general population, we were not able to fully explore the factors that contribute to the observed increased risk, such as differences in the stage and aggressiveness of cancer, cancer treatments, and responses to cancer therapy. In addition, cancer mortality rates for patients on dialysis and patients with transplants were compared with the general population through indirect standardization for age, sex, and year of death, and they did not account for other factors that may have contributed to the observed differences in cancer mortality rates, such as smoking. Future studies should explore the risk factors and mechanisms that may lead to increased cancer mortality in patients on dialysis and patients with transplants.
In summary, patients on dialysis and patients after kidney transplantation experienced >2.5-fold greater risk of cancer death than the age- and sex-matched general population. Relative to the general population, cancer mortality rates seem to be the highest in younger patients on dialysis and younger patients with transplants compared with older patients on dialysis and older patients with transplants. In patients on dialysis, the increased risks of cancer death were predominantly due to preexisting cancers related to ESRD, whereas in transplant recipients, the increased cancer death risk is driven by de novo cancers relating to immune deficiency and viral infections. Given the significant mortality associated with cancer in patients with ESRD, additional studies are needed to clarify the risk factors and mechanisms leading to increased cancer risk and improve identification and management of cancer in this at-risk population.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors acknowledge the contributions of all renal units throughout Australia and New Zealand to the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry that make this work possible.
This research was funded by the Better Evidence and Translation in CKD research program (National Health and Medical Research Council Program Grant APP1092957). The ANZDATA Registry is funded by the Australian Organ and Tissue Donation and Transplantation Authority, the New Zealand Ministry of Health, and Kidney Health Australia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018090906/-/DCSupplemental.
Supplemental Figure 1. (A) Cumulative incidence for different causes of death—dialysis; (B) cumulative incidence for different causes of death—transplant.
Supplemental Figure 2. (A) Cumulative incidence for different causes of death—dialysis (excluding preexisting cancer); (B) cumulative incidence for different causes of death—transplant (excluding preexisting cancer).
Supplemental Figure 3. (A) Cumulative incidence of most common cancer deaths—dialysis (excluding preexisting cancers); (B) cumulative incidence of most common cancer deaths—transplant (excluding preexisting cancers).
Supplemental Figure 4. (A) Cancer mortality rate and SMR by cancer type—dialysis (excluding preexisting cancers); (B) cancer mortality rate and SMR by cancer type—transplant (excluding preexisting cancers).
Supplemental Figure 5. (A) Cancer incidence rate and SIR by cancer type—dialysis. (B) Cancer incidence rate and SIR by cancer type—transplant.
References
- 1.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al.: Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 354: 93–99, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Stewart JH, Vajdic CM, van Leeuwen MT, Amin J, Webster AC, Chapman JR, et al.: The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant 24: 3225–3231, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Vajdic CM, McDonald SP, McCredie MRE, van Leeuwen MT, Stewart JH, Law M, et al.: Cancer incidence before and after kidney transplantation. JAMA 296: 2823–2831, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y: Cancer incidence among Canadian kidney transplant recipients. Am J Transplant 7: 941–948, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Collett D, Mumford L, Banner NR, Neuberger J, Watson C: Comparison of the incidence of malignancy in recipients of different types of organ: A UK Registry audit. Am J Transplant 10: 1889–1896, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Chapman JR, Webster AC: Cancer report. In: ANZDATA Registry 2004 Report, edited by Excell L, McDonald S, Adelaide, SA, Australia, Australia and New Zealand Dialysis and Transplant Registry, 2004, pp 99–103 [Google Scholar]
- 7.Butler AM, Olshan AF, Kshirsagar AV, Edwards JK, Nielsen ME, Wheeler SB, et al.: Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996-2009. Am J Kidney Dis 65: 763–772, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Registry ANZDATA: Mortality in End Stage Kidney Disease, Adelaide, Australia, Australia and New Zealand Dialysis and Transplant Registry, 2017 [Google Scholar]
- 9.Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ: Reduction in cardiovascular death after kidney transplantation. Transplantation 89: 851–857, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Acuna SA, Fernandes KA, Daly C, Hicks LK, Sutradhar R, Kim SJ, et al.: Cancer mortality among recipients of solid-organ transplantation in ontario, canada. JAMA Oncol 2: 463–469, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Vogelzang JL, van Stralen KJ, Noordzij M, Diez JA, Carrero JJ, Couchoud C, et al.: Mortality from infections and malignancies in patients treated with renal replacement therapy: Data from the ERA-EDTA registry. Nephrol Dial Transplant 30: 1028–1037, 2015 [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization : International Classification of Diseases for Oncology, Geneva, Switzerland, World Health Organization, 1976 [Google Scholar]
- 13.Webster AC, Supramaniam R, O’Connell DL, Chapman JR, Craig JC: Validity of registry data: Agreement between cancer records in an end-stage kidney disease registry (voluntary reporting) and a cancer register (statutory reporting). Nephrology (Carlton) 15: 491–501, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Sypek MP, Dansie KB, Clayton P, Webster AC, McDonald S: Comparison of cause of death between anzdata and the australian national death index [published online ahead of print March 1, 2018]. Nephrology (Carlton) 10.1111/nep.13250 [DOI] [PubMed] [Google Scholar]
- 15.Australian Institute of Health and Welfare : Australian Cancer Incidence and Mortality (ACIM) Books, Canberra, Australia, Australian Institute of Health and Welfare, 2017 [Google Scholar]
- 16.Kim HT: Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 13: 559–565, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, et al.: Spectrum of cancer risk among US solid organ transplant recipients. JAMA 306: 1891–1901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanik EL, Clarke CA, Snyder JJ, Pfeiffer RM, Engels EA: Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals. J Am Soc Nephrol 27: 1495–1504, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung CY, Lam MF, Chu KH, Chow KM, Tsang KY, Yuen SK, et al.: Malignancies after kidney transplantation: Hong Kong renal registry. Am J Transplant 12: 3039–3046, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Farrugia D, Mahboob S, Cheshire J, Begaj I, Khosla S, Ray D, et al.: Malignancy-related mortality following kidney transplantation is common. Kidney Int 85: 1395–1403, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Kiberd BA, Rose C, Gill JS: Cancer mortality in kidney transplantation. Am J Transplant 9: 1868–1875, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Batabyal P, Chapman JR, Wong G, Craig JC, Tong A: Clinical practice guidelines on wait-listing for kidney transplantation: Consistent and equitable? Transplantation 94: 703–713, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM: Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet 370: 59–67, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Francis A, Johnson DW, Teixeira-Pinto A, Craig JC, Wong G: The incidence and predictors of post-transplant lymphoproliferative disease (PTLD) after kidney transplantation during adulthood and childhood. Nephrol Dial Transplant 33: 881–889, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Dharnidharka VR, Webster AC, Martinez OM, Preiksaitis JK, Leblond V, Choquet S: Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers 2: 15088, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Guba M, Graeb C, Jauch K-W, Geissler EK: Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 77: 1777–1782, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Alhamad T, Venkatachalam K, Linette GP, Brennan DC: Checkpoint inhibitors in kidney transplant Recipients and the potential risk of rejection. Am J Transplant 16: 1332–1333, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.