Abstract
Mechanisms of hippocampus-related memory formation are time-of-day-dependent. While the circadian system and clock genes are related to timing of hippocampal mnemonic processes (acquisition, consolidation, and retrieval of long-term memory [LTM]) and long-term potentiation (LTP), little is known about temporal gating mechanisms. Here, the role of the neurohormone melatonin as a circadian time cue for hippocampal signaling and memory formation was investigated in C3H/He wildtype (WT) and melatonin-receptor-knockout (MT1/2−/−) mice. Immunohistochemical and immunoblot analyses revealed the presence of melatonin receptors on mouse hippocampal neurons. Temporal patterns of time-of-day-dependent clock gene protein levels were profoundly altered in MT1/2−/− mice compared to WT animals. On the behavioral level, WT mice displayed better spatial learning efficiency during daytime as compared to nighttime. In contrast, high error scores were observed in MT1/2−/− mice during both, daytime and nighttime acquisition. Day-night difference in LTP, as observed in WT mice, was absent in MT1/2−/− mice and in WT animals, in which the sympathetic innervation of the pineal gland was surgically removed to erase rhythmic melatonin synthesis. In addition, treatment of melatonin-deficient C57BL/6 mice with melatonin at nighttime significantly improved their working memory performance at daytime. These results illustrate that melatonin shapes time-of-day-dependent learning efficiency in parallel to consolidating expression patterns of clock genes in the mouse hippocampus. Our data suggest that melatonin imprints a time cue on mouse hippocampal signaling and gene expression to foster better learning during daytime.
Keywords: LTP, CREB, clock gene, ganglionectomy, SCN, radial arm, behavior, circadian
Introduction
Almost every reoccurring aspect in physiology and behavior has a temporal facet that is of circadian (circa: about; dies: day) nature. Since the ability to anticipate and control circadian events represents a striking evolutionary advantage, endogenous clocks have evolved in parallel with life. Mechanistically, these circadian clocks constitute a transcriptional/posttranslational feedback loop1. In mammals, the expression of the clock genes Period (Per1–3) and Cryptochrome (Cry1,2) is driven by binding of homo/heterodimers of the clock gene protein products BMAL1 and CLOCK to E-box promoter elements on Per and Cry genes1. The circadian master oscillator, located in the hypothalamic suprachiasmatic nucleus (SCN)2, adjusts remote, light-blind cells, tissues, or organs via neuronal and/or humoral output. Thereby, body functions are temporally coordinated and physiological incompatible events are separated within the omnipresent circadian environment.
Since 1973, learning and memory have been known to be modulated through time-of-day dependent mechanisms3. Ever since, the presence of a circadian rhythm in memory acquisition, consolidation and retrieval has been demonstrated in numerous species, ranging from invertebrates (Aplysia:4; fruit fly:5), to vertebrates (Zebrafish:6; rodents:3,7) including the human8 (for review see:9,10). However, despite these widespread and common observations in circadian gating of memory formation, the mechanisms underlying time-of-day-dependent dynamics in learning efficiency are far from being understood.
In mammals, a central structure for long-term memory (LTM) formation is the hippocampus11–14, with the plasticity of long-term-potentiation (LTP) mirroring hippocampal LTM13,15. It is well described that hippocampal learning efficiency depends on the time-of-day10,16–19. Notably, this temporal coordination depends on the integrity of the SCN20 that shows a higher activity during daytime, independent of whether an animal is day- or night-active21. Despite this, in night-active laboratory mice, disparate findings for the time-of-day window for eased learning are reported10. This raises the question, if additional factors are involved in shaping the time-of-day dependent efficiency in memory formation.
Molecular mechanisms that efficiently drive and execute the encryption of memory traces into LTM are common and evolutionary highly conserved in both, fish and mammals9. In zebrafish, the pineal hormone melatonin, which is secreted exclusively during nighttime, is of critical importance for the circadian modulation in retention of LTM. Zebrafish display enhanced performance, if the task is acquired during daytime, coinciding with the animal’s active phase6. Disrupting melatonin synthesis in zebrafish by pinealectomy, abolishes this rhythm in day-night learning6.
In mammals, little is known about the role of melatonin within principal mechanisms of time-of-day-dependent modulation of LTM formation and/or retrieval. We therefore attempted to pinpoint the importance of circadian timing for learning efficiency and elucidate the role of melatonin in dynamic mnemonic processes in the hippocampus. Our experimental approach was based on previously published evidence that
transcripts for melatonin receptors (MT1 and MT2) are present in rat hippocampus 22,
in rat brain, both, MT1 and MT2 antibodies labelled all hippocampal subfields 23,
day-night difference in LTP is absent in both, MT1,2−/− mice 24, and melatonin-deficient C57BL/6 mice 25,
melatonin inhibits the cAMP-signaling pathway 16,26, notably also in the hippocampus 27, and (v) WT mice display a higher cocaine-induced conditioned place preference during daytime, than during nighttime, with the difference being abolished following pinealectomy 28.
Our results indicate that decoding of the nighttime melatonin signal is required for both, the maintenance of a proper phase-relationship in hippocampal signaling and for an efficient time-of-day-dependent gating of memory processing. Moreover, our data support that the circadian clock in the SCN gates time-of-day-dependent learning via the night-time-restricted impact of melatonin on hippocampal circuity.
Materials and methods
Animals.
All animal experiments were conducted as approved by the Policy on the Use of Animals in Neuroscience Research, the Policy on Ethics of the Society for Neuroscience, the Federal Guidelines and the European Communities Council Directive (89/609/EEC), and the local veterinary administration (approval file number: FU/1045). MT1−/−-, MT2−/−-, and MT1/2−/−-mice (all knockout mice were a kind gift from Dr. D.R. Weaver, Worcester, USA), and wildtype (WT) littermates were bred back onto a melatonin proficient C3H/He background16. C3H/He wildtype (WT) mice, C57BL/6 mice, and WT mice that were subjected to a bilateral surgical removal of their superior cervical ganglia (WT-SCGX) were purchased from Charles Rivers (Sulzbach, Germany).
The here additionally used C57BL/6 mice lack an appreciable endogenous melatonin rhythm29,30, due to a nucleotide difference in an intron that creates a new splice acceptor, and incorporation of a pseudoexon, leading to a stop codon and truncation of the protein of the rate-limiting enzyme of the melatonin synthesis, arylalkylamine N-acetyltransferase (AANAT)31. However, MT receptors in C57BL/6 mice are functional as melatonin inhibits (i) hippocampal LTP27, and (ii) CREB phosphorylation in the SCN32, and also (iii) shifts the activity phase of this strain33. Thus, C57BL/6 mice endow a retained capacity to interpret the melatonin signal and were therefore used in some additional experiments in the presence of an artificially simulated nocturnal melatonin surge.
All animals were aged 8–12 weeks during experimentation. Mice were maintained under a standard 12:12 light/dark (LD) cycle, with 12 h light (daytime: 250 lux; onset [“ON”] defined as Zeitgeber Time [ZT] 0) and 12 h darkness (nighttime: dim red light <10 lux, >680 nm). Animals were kept under constant room temperature with food and water available ad libitum. Tissue sampling was carried out with animals sacrificed under deep anesthesia at ZT 2, 6, 8, 10, 12, 14, 18, and 22 (3–5 animals per time point, unless indicated otherwise), as previously described18,34.
Immunohistochemistry.
Immunohistochemical analyses were performed in the mouse hippocampus and SCN as described previously34. In brief, deeply anesthetized mice (Ketamine 100 mg/kg, Xylazine 10 mg/kg) were flushed transcardially with saline, followed by perfusion with a paraformaldehyde solution (4% in 0.02 M phosphate buffered saline). Brains were post-fixed and sliced into 12 μm thick sections in the coronal plane on a freezing microtome. Sections were incubated overnight at 4°C with according antibodies (Tab.1). Immunoreactions were visualized with standard ExtrAvidin-biotin labelling method (Vector Laboratories, Peterborough, UK) using 0.05% 3.3´-diaminobenzidine. Analyses of clock gene protein levels were performed as described34. Briefly, cryo-protected brains were cut into 40-μm-thick sections in the coronal plane on a freezing microtome. To reduce nonspecific labeling in the immunohistochemical analyses, free floating sections were incubated for 1 h at room temperature in 0.01 M phosphate buffered saline, containing 0.3% triton, 1% bovine serum albumin, and 2% normal goat serum prior to an overnight incubation at 48°C with clock gene protein antibodies (Tab.1). Immunoreactions were visualized with a standard ExtrAvidin-biotin labeling method (Vector Laboratories, Peterborough, UK, USA), using 0.05% 3.3-diaminobenzidine, as described earlier34.
Tab. 1:
List of used antibodies
|
Antibody |
Host | Company | IHC | WB | |
|---|---|---|---|---|---|
| Primary antibody | MT1 | rabbit | Sengupta et al., 2011 | 1:500 | - |
| MEL-1-A-R (MT1) | goat | Santa Cruz #SC13186 | 1:200 | 1:500 | |
| MEL-1-B-R (MT2) | goat | Santa Cruz #SC13177 | 1:200 | 1:500 | |
| MT1 | rabbit | Alomone #AMR-031 | 1:250 | - | |
| MT2 | rabbit | Alomone #AMR-032 | 1:250 | - | |
| MAP2 | mouse | BD Biosciences #566320 | 1:500 | - | |
| ß-Actin | mouse | Sigma-Aldrich #A5316 | - | 1:40000 | |
| pCREB(Ser133) | rabbit | Millipore #06519 | 1:1000 | - | |
| PER1 | rabbit | kind gift from S.M. Reppert, University of Massachusetts Medical School, Worchester, USA | 1:5000 | - | |
| PER2 | rabbit | Alpha Diagnostic #PER21-A | 1:250 | - | |
| CRY1 | rabbit | Alpha Diagnostic #CRY11-A | 1:50 | - | |
| CRY2 | rabbit | Alpha Diagnostic #CRY21-A | 1:250 | - | |
| CLOCK | rabbit | Affinity BioReagents #PA1–520 | 1:500 | - | |
| BMAL1 | rabbit | Affinity BioReagents #PA1–523 | 1:500 | - | |
| Secondary antibody | Alexa Fluor 488 anti-rabbit/mouse | goat | Molecular Probes #A-11008/A-11001 | 1:200 | - |
| Alexa Fluor 568 anti-rabbit/mouse | goat | Molecular Probes #A-11011/A-11004 | 1:200 | - | |
| Anti-rabbit biotinylated antibody | goat | Vector #BP910050 | 1:600 | - | |
| Anti-mouse IgG HRP | goat | Dako #P0447 | - | 1:40000 | |
| Anti-rabbit IgG HRP | goat | Santa Cruz #SC2054 | - | 1:40000 | |
For immunofluorescence analyses a protocol was adapted as described18,19,34. Briefly, tissue slices were pre-incubated for 1 h at room temperature in PBS, 5% NGS (Sigma), and primary antibodies were applied at 4°C for 24 h in PBS plus 5% normal goat serum. Alexa Fluor 488 goat anti-mouse secondary antibody was used at a 1:200 dilution (Molecular Probes, Göttingen, Germany; Tab.1). Adjacent sections, not treated with the primary antibody, were run for each animal in parallel. For immunofluorescence double labelling, sections were incubated overnight at 4°C in the appropriate antibody cocktail containing 1% bovine serum albumin, 0.1% Triton X-100 in 0.1 M PBS. After 3 washes in 0.1 M PBS, sections were incubated with Alexa 488- or 568-conjugated anti-mouse IgG (2 h, room temperature, in 0.1 M PBS), washed again, and incubated with Alexa 568- or 488-conjugated anti-rabbit IgG (2 h, room temperature, in 0.1 M PBS), respectively. After rinsing with 0.1 M PBS, the sections were mounted in fluorescent mounting medium (Dako, Hamburg, Germany). Fluorescent images were acquired using an Axio-Cam digital camera mounted on a Zeiss microscope (Carl Zeiss, Jena, Germany). Single fluorescent images of the same section were digitally superimposed.
For semiquantitative densitometric analyses of the immunoreactions, images were digitized with an Axiocam system (Zeiss, München, Germany; 1,030 × 1,030 pixel, 8-bit color depth), using NIH ImageJ software (Image Processing and Analysis in Java, developer Wayne Rasband), as described previously18,19,34. Briefly, background staining was defined as the lower threshold, and was kept constant for all sections processed with an antibody in a single experiment. The relative intensity of the nuclear and the cytoplasmic immunoreaction in the entire hippocampal formation was assessed separately as gray scale units above background. Staining was time- and genotype dependent (see Results). In addition, sub-regions of the hippocampal formation (CA1, CA3, and dentate gyrus [DG]) were selected and analyzed individually, as described34, and the relative optical density (rel. O.D.) to background staining was measured within selected areas. In immunofluorescent images the corrected total cell fluorescence (CTCF = Integrated Density of cell ROI – [Area of ROI x Mean fluorescence background]) was measured35,36. Subsequently, values were averaged from three to four sections per animal.
Immunoblotting.
Western blots of extracts taken from excised mice hippocampi, or punched out SCN, respectively, were performed with slight modifications to a previous protocol37. Briefly, tissue samples were sonicated in NuPAGE® LDS sample buffer (Invitrogen, Carlsbad, USA) (10% Glycerol, 141 mM Tris Base, 106 mM Tris HCl, 2% LDS, 0.51 mM EDTA, 0.22 mM SERVA® Blue G250, 0.175 mM Phenol Red, 100 mM DTT, pH 8.5) and proteins were denatured by heating and separated electrophoretically using NuPAGE® Novex 4–12% Bis-Tris gels according to the manufacturer’s instructions (Invitrogen, Carlsbad, USA), and transferred onto a PVDF membrane, using the iBlotTM Semi-Dry Blotting System (Invitrogen, Carlsbad, USA). Prior to incubation with primary antibodies (Tab. 1), membranes were blocked with RotiBlock® (Roth, Karlsruhe, Germany) for 1 hour at room temperature. Subsequently, membranes were incubated with secondary antibodies (Tab. 1) for 1 hour at room temperature. Signals were detected using Immobilon Western Chemoluminescent HRP Substrate (Millipore, Billerica, USA), digitized using the ChemiDoc XRS System (BioRad, München, Germany) and analyzed using a luminescence system (Quantity One, ChemiDoc XRS, Bio-Rad, Hercules, CA, USA). The optical intensity of target signals on a given Western blot was normalized to the optical intensity of the actin signal on the same blot. The normalized signal intensities were then expressed as relative signal intensities (rel. O.D.). In separate control experiments with MT antibodies, membranes were preincubated for 1hour with corresponding blocking peptides (Santa Cruz, Heidelberg, Germany).
Real-Time PCR.
Whole hippocampus samples and SCN tissue punches from WT, MT1−/−-, MT2−/−-, and MT1/2−/−-mice were rapidly isolated from deeply anaesthetized mice upon decapitation and frozen on dry ice. Subsequently, total RNA was extracted using minispin columns Absolutely RNA miniprep kit (Stratagene, La Jolla, USA) as described earlier38, and amplified using primers for mPer1, mPer2, mCry1, mCry2, mClock, mBmal1, and mHprt, as described earlier34. Primers for MT1- and MT2- melatonin receptors were designed, according to reported mouse sequences (GeneBank accession number given in brackets):
mMtnr1a (MT1) [NM_008639.2]
forward : 5’-CTC AAT GCC ACT CAG CA-3’ (25–41)
reverse: 5’-GAG CTT CTT GTT GCG GTA-3’(183–166)
mMtnr1b (MT2) [NM_145712.2]
forward : 5’-ATC CCT AAC TGC TGT GA-3’ (115–131)
reverse: 5’-AGC TTG CGG TTC CTG A-3’ (308–293)
In brief, Real-Time PCR was performed using a LightCycler 1.5 (Roche Diagnostics GmbH, Mannheim, Germany) and a LightCycler FastStart DNA MasterPLUS SYBRGreen I kit (Roche Diagnostics GmbH, Mannheim, Germany), according to the instructions of the manufacturer, and as described previously34. A 10 min initial denaturation step at 95°C was followed by 40 amplification cycles of 10 sec denaturation at 95°C, 10 sec of elongation at 60°C, and 10s elongation at 75°C. To ensure the specificity of the PCR amplicon, a temperature controlled melting curve analysis was performed at the end of the PCR reaction. As expected, each melting curve exhibited a single peak, corresponding to the expected specific amplification product. To confirm the specificity of the PCR reaction products were separated on ethidium bromide stained agarose gel (2% w/v). For technical reasons, clock gene mRNA expression could not be analyzed in MT1/2−/− mice.
Behavioral analyses.
Locomotor activity of animals was analyzed routinely by an infrared sensor system, as described34 (see technical description at www.infra-emotion.de). Accumulated averaged activity of a given animal was analyzed within 1 hour time bins.
An 8-arm radial maze task was used to test for day/night differences of a specialized form of short-term memory (STM), known as working memory39, in addition to the long-term-memory (LTM) component of the reference memory. STM refers to a temporary storage of information from an animal’s environment, to enhance performance in a given task in the near future. STM can be improved by repetitive exercise over several days, as measurable by the decreasing number of errors in successive trials. This improvement reflects a hippocampus-dependent learning process that results in the long-term storage of strategic information as a reference memory. Upon retrieval of the latter STM performance improves during task exposure40.
To assess LTM performance, WT, MT1−/−, MT2−/−, MT1/2−/−, WT-SCGX, C57BL/6 and melatonin-treated C57BL/6 mice were trained in a food-baited 8-arm radial maze testing procedure, as described previously34. Briefly, food-deprived animals were trained for 5 consecutive days (1 trial/day), starting either at ZT2 or at ZT14, for daytime and nighttime training, respectively. A trial was completed, when the animal had eaten all rewards, or after 15 minutes, whichever came first. An entry to an arm was counted, when the mouse had entered with all four paws. An error was recorded, when an animal had either re-entered an arm it had visited previously, or if it did not eat the food pellet. Errors were plotted as percentage of the maximum error score. Experiments during the dark phase were performed under dim red light. Experimenters were blinded to the mouse`s genotype during the behavioral testing.
For the melatonin substitution experiments, C57BL/6 mice were treated with melatonin (10 mg/100 ml water; dose according to calculations elaborated in41, added to their drinking water at nighttime only, starting 3 days before behavioral experiments and lasting throughout the food-rewarded radial arm maze test.
Anxiety was analyzed at ZT2 in the elevated plus test as described earlier34,42, by measuring the time an animal spent in the open arm.
Mice can react extremely sensitive to changes of the handling persons, particularly to changes in odorant stimuli43,44. This may explain why the number of errors, particularly on the initial day of training differs between different experiments. In order to still be able to compare individual experiments, some of the obtained data are presented in a different context as percent of the maximal mean error value.
Electrophysiology.
Acute hippocampal slices were prepared 2 hours prior to LTP recordings at ZT2, or ZT14, respectively, according to previously described procedures45,46. The entire duration of the LTP experiment from slice preparation to the final recording lasted strictly between ZT12-ZT16, or ZT22-ZT2, respectively. In brief, concentric bipolar microelectrodes were used to stimulate the Schaffer collaterals at a frequency of 0.033 Hz. Stimulation was adjusted to elicit a fEPSP with a slope of ~40–50% of maximum for LTP recordings. After 20 minutes of baseline stimulation, LTP was induced by applying theta-burst stimulation (TBS), consisting of 4 pulses at 100 Hz per burst, which were repeated 10 times in a 200-ms interval (5 Hz). Three such trains served to induce LTP. Basic synaptic transmission and presynaptic properties were analyzed via input-output (IO) measurements and paired pulse facilitation by applying a pair of two stimuli at an interstimulus interval (ISI) of 40 ms. The IO measurements were performed by application of a defined value of current (0–100 μA in steps of 10 μA).
Statistics.
Statistical analyses were performed using OriginPro 7.5 SR4 (OriginLab Corporation, Northampton, MA, USA). Group means within each genotype were compared with One-way analysis of variance (ANOVA) with Bonferroni´s multiple comparison test, to estimate differences between examined time points. The impact of the genotype in the behavioral experiments was assessed by with a Two-way ANOVA with Bonferroni´s multiple comparison test. An unpaired t-test was performed to compare two different conditions at a distinct time point.
Results
MT receptor expression in the mouse hippocampus.
We initially analyzed MT receptor mRNA expression and MT receptor protein levels in mouse hippocampus (Fig. 1, Suppl. Fig. 1). MT1 and MT2 receptor transcripts could be demonstrated by RT-PCR (Suppl. Fig. 1 A,B), lining up to data previously obtained in the rat22,47. In addition, we show that with RT-PCR for the MT1 or the MT2 receptors, signals were absent in hippocampal extracts derived from MT1−/− and MT2−/− mice, respectively (Suppl. Fig. 1A,B).
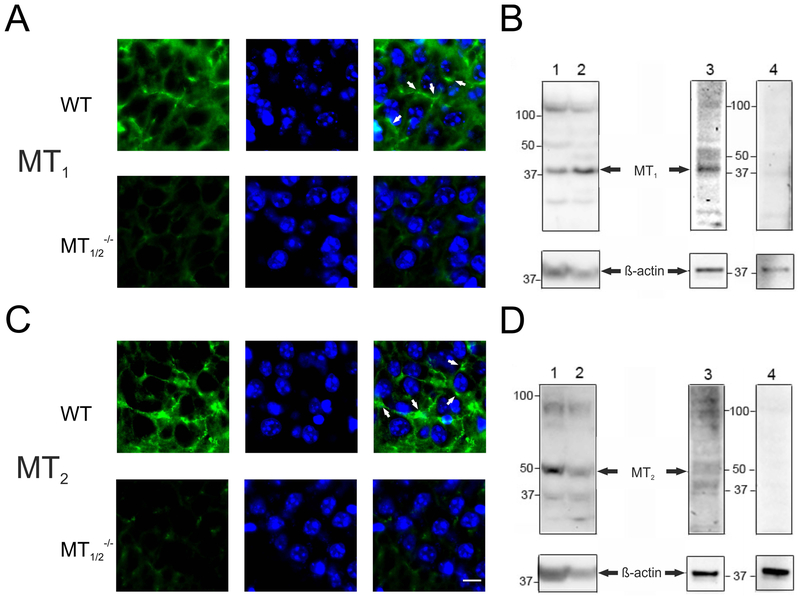
Fig. 1:
Melatonin-receptors expression in the hippocampus of WT mice. Representative immunohistochemical staining for melatonin-receptors (green, left column) and DAPI (blue, middle column) and merged images (right column: white arrows exemplify the membranous MT receptor signal) in the hippocampus of WT (A) and MT1/2−/− (C) mice. Western blots (B, D) show the expression of melatonin-receptor proteins in the mouse hippocampus (lanes 1,3) and SCN (lanes 2). The arrows point to the protein bands corresponding to the size of MT1 (B: ~37kDa), or MT2 (D: ~37kDa). The prominent band, sized ~48 kDa may be the glycosylated form of the receptor52. Signals in hippocampal extracts were abolished upon preincubation of MT antibodies with corresponding antigenic peptides (lanes 4). β-actin served as loading control. Scale bar in lower right picture: 10 μm. Used antibodies SC13186 and SC13177 (see Tab. 1).
Notably, melatonin acts on the level of the expressed receptor proteins. During the course of experiments, 3 different MT receptor antibodies were used (Tab.1). Using antibodies from Santa Cruz, both MT receptors could be detected by immunofluorescence in hippocampal subfields of WT mice (Fig.1 A,C). Co-localization with the nuclear marker DAPI confirmed the presence of high-affinity G-protein-coupled MT receptors exclusively outside the nucleus, presumably on membranes of mouse hippocampal principal neurons (Fig.1 A,C). Signals were notably absent in MT1,2−/− mice hippocampus (Fig. 1A,C). As these antibodies were discontinued, we had to switch during the course of experiments to antibodies purchased from Alomone (Tab. 1; Suppl. Fig. 2). While these antibodies gave a similar signal as obtained with Santa Cruz antibodies, they labelled next to hippocampal subfields also the meninges (Suppl. Fig. 2). The additionally seen positive signals in MT1,2−/− mice hippocampus is explained by us and others to be due to the fact that these mice are functional knockout animals48,49, with only a replacement of exon 1 of both the MTs. Exon 1 encodes the 5´untranslated region and the coding region through the first cytoplasmic loop of MTs. Exon 2 of both, the MT1 and the MT2, encode the rest of the coding region and the 3´untranslated region remained unaltered in here used MT1,2−/− mice. Thus, it is possible that the expression of exon 2-based truncated MT receptor proteins, which notably have no biological activity48,49, still leads to false positive immunohistochemical signals in the MT1,2−/− mice, as the antibodies may be raised against a peptide, translated from this exon.
In addition, a MT1 receptor antibody, previously validated in mouse retina50, yielded a specific signal in WT mouse hippocampus. MT1 receptor localized within the hippocampal formation in CA1, CA3 and the DG (Suppl. Fig. 1C). Co-localization with the neuronal marker MAP2 confirmed the presence of the MT receptors exclusively outside the nucleus on membranes of mouse hippocampal principal neurons. Notably, using this MT receptor antibody, signals were absent in MT1/2−/− mice (Suppl. Fig. 1C).
Using the Santa Cruz MT receptor antibodies, Western blots of lysates derived from WT mouse hippocampus (Fig.1B,D, lane 1,3), revealed bands corresponding to the predicted molecular weights for MT1 (Fig. 1B: about 37 kDa47, indicated by arrows) and for MT2 (Fig.1D: about 37 kDa26, indicated by arrows). Bands of identical size as observed in hippocampal extracts were detected in a tissue, with reported high density of MTs, the SCN51 (Fig. 1B,D, lane 2). No signals were detected in Western blots with MT1 and MT2 receptor antibodies when they were pre-incubated with the corresponding antigenic peptides (Fig. 1B,D, lane 4). With both MT receptor antibodies, additional sized bands were evident in Western blots. While these bands are possibly unspecific, the band sized 48 kDa may likely representing a glycosylated form of the receptor52 (Fig. 1B,D, lanes 1–3). The reason for the very low background signals, present in the immunofluorescence images of MT1/2−/− mice hippocampus (Fig. 1A,C) may be caused by unspecific binding of Santa Cruz MT receptor antibodies (see additional bands in Western blots in Fig. 1 B,D).
Dynamics in hippocampal clock gene expression in WT and MT−/− mice.
In WT mouse hippocampus and DG, the temporal patterns of clock gene protein levels for PER1, PER2, CRY2, CLOCK and BMAL1, and of corresponding clock gene mRNA expression levels, respectively, showed a highly dynamic time-of-day-dependent pattern (Fig. 2A,B; Fig. 3; Suppl. Fig. 3,4; Suppl. Tab. 1, 2) confirming our earlier data34. In MT1/2−/− mice, clock gene protein levels showed remarkable differences in temporal dynamics, as compared to WT mice (Fig. 2 B,C; Suppl. Fig. 3,4; Suppl. Tab 1,2), with the notable exception of PER1 (Figs. 2,3, Suppl. Fig. 3).
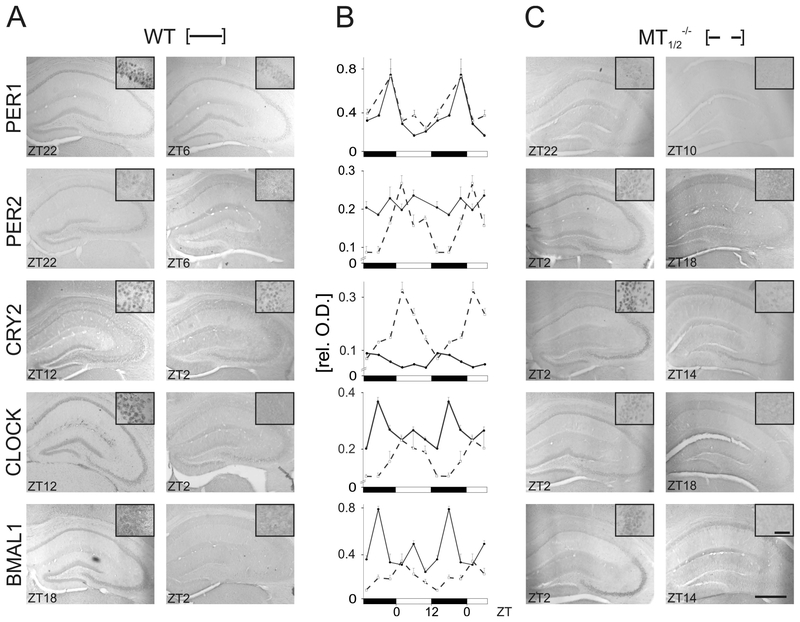
Fig. 2:
Clock-gene protein levels in the hippocampus of WT and MT1/2−/− mice. Representative immunohistochemical images of peak (left columns) and trough (right columns) clock protein expression in coronal hippocampal sections for both, WT (A) and MT1/2−/− mice (C). Time points are indicated as ZT values. Insets are exemplary images from the CA3 region taken with a higher magnification. Scale bars in lower right picture: 200 μm, in inset 10 μm. (B) Semiquantitative densitometric analysis of immunohistochemical signals compiled form DG, CA1 and CA3 region, expressed as mean relative optical densities (rel. O.D.) of all three regions together for the time-of-day-dependent levels of clock gene proteins, PER1, PER2, CRY2, CLOCK, and BMAL1, in the hippocampus of WT (●, solid lines) and MT1/2−/− mice (□, dashed lines). Values (n = 3/group with 3 sections/animal) are expressed as mean ± SEM. For significances see text and Suppl. Tabs. 1–3. Data in B are double-plotted against Zeitgeber time (ZT) for clarity reasons.
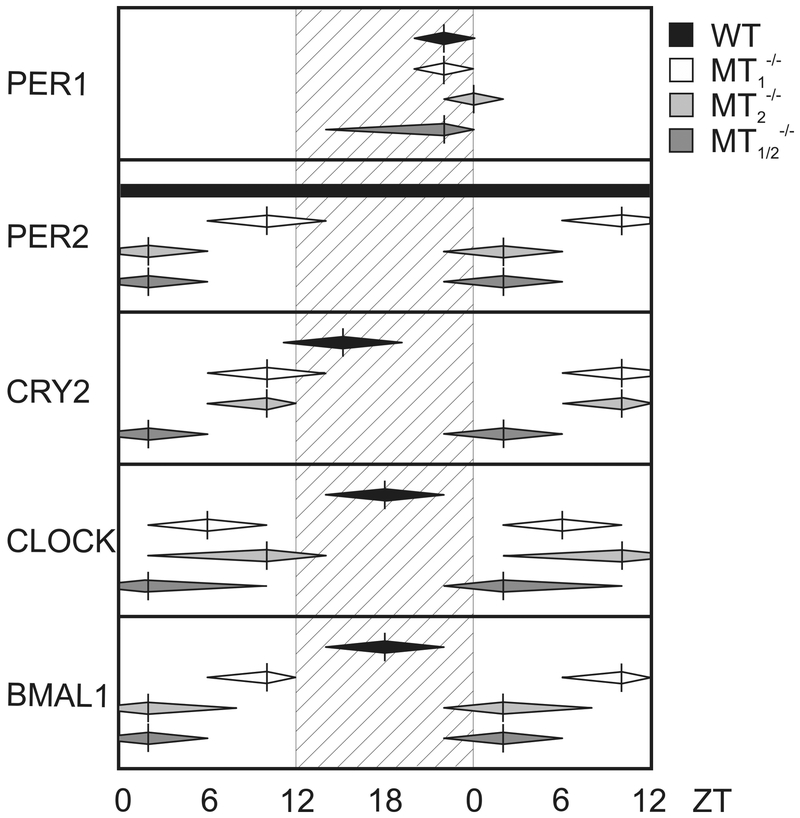
Fig. 3:
Schematic overview of the phase-relationship of peak expression of clock gene proteins in the hippocampus of WT, MT1−/−, MT2−/−, and MT1/2−/− mice. Vertical bars represent times of peak levels, with approximate times of the rise to, and the decline from maximal clock gene protein levels estimated by eye fitting on the basis of the original data sets and indicated by the horizontal extend of the deltoids.
PER2 expression in MT1/2−/− mice was rhythmic, with a peak during the early day (ZT2) and a trough during the first half of the night (ZT14-ZT18), in contrast to a constant expression level of PER2 in WT animals (Figs. 2,3, Suppl. Fig. 3). The amplitude of CRY2 expression levels was significantly higher in MT1/2−/− mice compared to WT mice where the peak expression phase advanced from early night (ZT14) to early day (ZT2) (Figs. 2,3, Suppl. Fig. 3). Likewise, the peak expression level of CLOCK protein was dampened and phase-advanced in MT1/2−/− mice from mid-night (ZT18) to early-day (ZT4) as compared to WT animals (Figs. 2, 3, Suppl. Fig. 3). Rhythmic BMAL1 expression was greatly reduced in MT1/2−/− mice, as compared to WT, with the expression peak shifted from mid-night (ZT18) in WT to early day (ZT2) in MT1/2−/− mice (Figs. 2,3, Suppl. Fig. 3).
The comparison of immunohistochemical signal intensities in the hippocampus of WT, MT1−/−, MT2−/−-, and MT1/2−/− mice showed no significant difference between hippocampal subregions by visual inspection (Suppl. Fig. 3). However, while overall rhythms in subregions showed the same time-phase relationship in all mice strains analysed, statistical analyses revealed some significant differences between subregions in WT (PER2: P < 0.01; CRY2: P < 0.01) and in MT1/2−/− mice (PER2: 0.05; CRY2: P < 0.01; BMAL1: P < 0.0001) (see also Suppl. Tab. 10), which we account to subtle amplitude differences at individual time-points investigated.
Comparing expression of clock genes mPer2, mCry2, mClock, and mBmal1 mRNA in mouse hippocampus revealed that the phase relationship for peak values was greatly altered when only one of the melatonin receptors was knocked out compared to patterns in WT animals (MT1−/−, MT2−/−; Suppl. Fig. 4). The notable exception was the clock gene mPer1, where transcriptional dynamics remained unaltered in MT1−/− and MT2−/− mice compared to WT animals (Suppl. Fig. 4). This indicates that both melatonin receptors may be likely involved in the time-of-day-dependent modulation of clock gene expression in mouse hippocampus.
Our analyses revealed peak and trough values for clock gene protein levels for PER1, PER2, CRY2, CLOCK, and BMAL1 in the SCN of WT mice as reported earlier1,53,54 (Suppl. Fig. 5A). Dynamics in clock gene protein levels in MT1/2−/− mice was similar to WT animals (Suppl. Fig. 5B). Transcription dynamics of clock gene mRNAs in WT mouse SCN (Suppl. Fig. 5C) was as reported earlier1,53,54. Notably, the day/night pattern in clock gene mRNA expression in the SCN of both MT1−/− and MT2−/− mice was identical to WT (Suppl. Fig. 5C; for technical reasons, clock gene mRNA expression could not be analyzed in the SCN of MT1/2−/− mice).
In summary, the absence of melatonin receptor signaling is correlated with a major disturbance in patterns of clock gene mRNA expression and clock gene protein levels in the mouse hippocampus (Figs. 2,3, Suppl. Figs. 3,4; Suppl. Tab. 1,2). Notably, in mouse SCN the lack of MT receptors does not affect patterns of clock gene mRNA expression and clock gene protein levels (Suppl. Figs. 5).
Melatonin determines time-of-day-dependent magnitude in LTP.
Hippocampal LTP, a correlate of learning and memory13,15,45 has a higher magnitude in WT mice at nighttime compared to daytime25. This day/night difference in synaptic weight is absent in melatonin-receptor deficient mice27 and in the melatonin-deficient C57BL/6 strain25. We initially confirmed the fundamental presence of a day-night difference in LTP magnitude in WT mice (Fig. 4A; ZT2, LTP elevation 224±6.8%; ZT14: LTP elevation 161±15.7%) and its absence in MT1/2−/− mice (Fig. 4B; ZT2, LTP elevation 167±10.6%; ZT14, LTP elevation 156±13.7%). Next, we measured LTP in WT-SCGX mice, which do not synthetize melatonin55. Successful SCGX treatment was confirmed by documentation of ptosis, miosis and enophthalmus (Suppl. Fig. 6). In WT-SCGX mice, the day-night difference in LTP magnitude between ZT2 and ZT14 recordings was abrogated (Fig. 4C; ZT2: LTP elevation 168±9.7%; ZT14: LTP elevation 188±4.8%). These observations underline a functional significance for melatonin in circadian synaptic weight in mouse hippocampus. There was no significant difference between ZT2 and ZT14 in input-output strength or paired pulse facilitation (Suppl. Fig. 7), suggesting that melatonin does not interfere with baseline synaptic function, but only with activity-dependent synaptic plasticity.
Fig. 4:
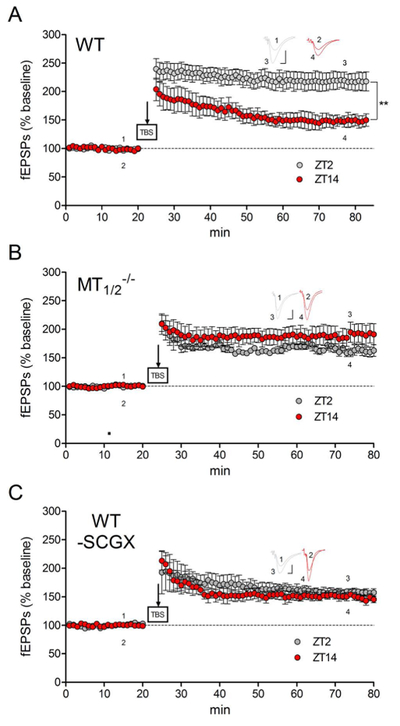
Daytime-dependent hippocampal synaptic plasticity in WT, MT1/2−/− and WT-SCGX mice. Long-term potentiation (LTP) was induced in mouse hippocampal brain slices at ZT2 (gray circles) and ZT14 (red circles). (A) In WT mice, a significant difference was observed between ZT2 (n=8) and ZT14 (n=6) (**= p=0.038; t-test). This change was abolished in MT1/2−/− mice (B; p ≥ 0.05; t-test; n=3 at ZT2 and ZT14, respectively) and in WT-SCGX mice (C; p ≥ 0.05; t-test; n=3 at ZT2 and ZT14, respectively). Insets in A-C show representative field potential waveforms, indicated by numbers at the respective time points, scale bars: 0.5mV, 5ms.
Endogenous CREB phosphorylation in the hippocampus of WT and MT−/− mice.
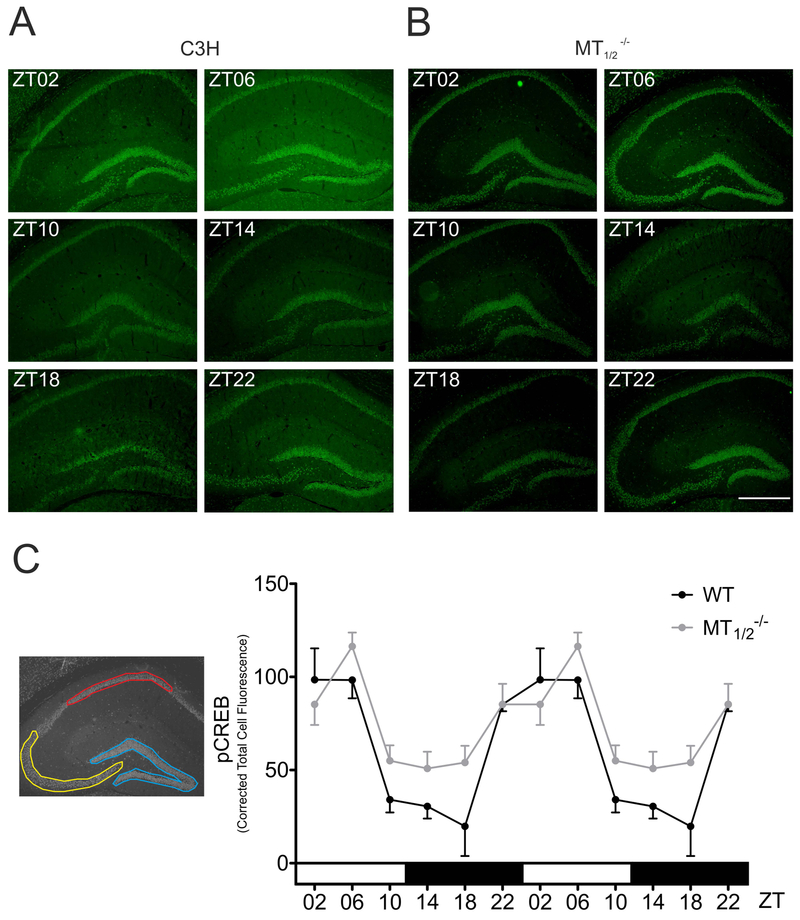
Semiquantitative densitometric analysis demonstrated increased CREB phosphorylation in WT mouse hippocampus and DG as the night progresses from trough levels at ZT 18 to reach peak values at midday (ZT06) (Fig. 5A,C, Suppl. Tab. 3; ZT2 vs ZT10, ZT14, P < 0.05; ZT2 vs ZT18, P < 0.01; ZT06 vs ZT10, ZT14: P < 0.05; ZT06 vs ZT18: P < 0.01; ZT18 vs ZT22: P < 0.05; 1-Way ANOVA with Bonferroni´s multiple comparison test), substantiating our previously published Western Blot data18. A distinct rhythm was present in hippocampal CREB phosphorylation of MT1/2−/− mice (Fig. 5B,C; Suppl. Tab.3; ZT06 vs ZT10, ZT14, ZT18, P < 0.05, One-Way ANOVA), which differed significantly from the rhythm observed in WT animals (Suppl. Tab. 3; genotype: P < 0.05; time: P < 0.0001, Two-Way ANOVA). Hippocampal pCREB immunoreaction of C57/BL/6 mice was evidently diverged between ZT10 and ZT18 (Fig. 5C, Suppl. Tab. 3; P < 0.05; One-way ANOVA with Bonferroni´s multiple comparison test). Notably, in C57BL/6 mice peak levels in pCREB were phase delayed by 4 hours as compared to WT animals (Suppl. Fig. 8).
Fig. 5:
Time-of-day-dependent CREB phosphorylation in mouse hippocampus. Representative immunohistochemical images of pCREB signal in the hippocampus of WT (A) and MT1/2−/− mice (B) at indicated ZTs. Scale bar: 500μm. (C) Semiquantitative densitometric analysis of immunohistochemical signals pooled over hippocampal subregions DG (blue), CA1 (red) and CA3 (yellow), expressed as Corrected Total Cell Fluorescence (CTCF) in WT and MT1/2−/− (all n = 3) mice. Both genotypes show a significant time-of-day-dependent rhythm in CREB phosphorylation (WT: P ≤ 0.001; MT1/2−/−: P ≤ 0.01) and are significantly different to each other (*: P<0.05, Two-way ANOVA). Values are expressed as mean ± SEM. For clarity reasons, data are double-blotted against Zeitgeber time (ZT).
Melatonin impacts hippocampus-dependent learning.
In the hippocampus-dependent spatial learning test of the food-baited 8-arm radial maze test, WT animals acquired the task independently of whether they were trained during the day or during the night (Figs. 6,7 and Suppl. Tab. 4). In line with earlier studies18,34, improved learning occurred during daytime (Fig. 6A). In all three MT receptor deficient mouse lines (MT1−/−, MT2−/−, MT1/2−/−, respectively; Fig. 6D; Suppl. Fig. 9; Suppl. Tabs. 5–7) as well as in melatonin-deficient C57BL/6 mice (Suppl. Fig. 10; Suppl. Tab. 8), daytime learning was severely compromised compared to WT mice. Of note, the performance of mouse strains tested in the radial maze did not depend on their overall activity since no differences were observed between animals when locomotor activity was analyzed in 1 hour time bins under the current experimental conditions (Suppl. Fig. 11).
Fig. 6:
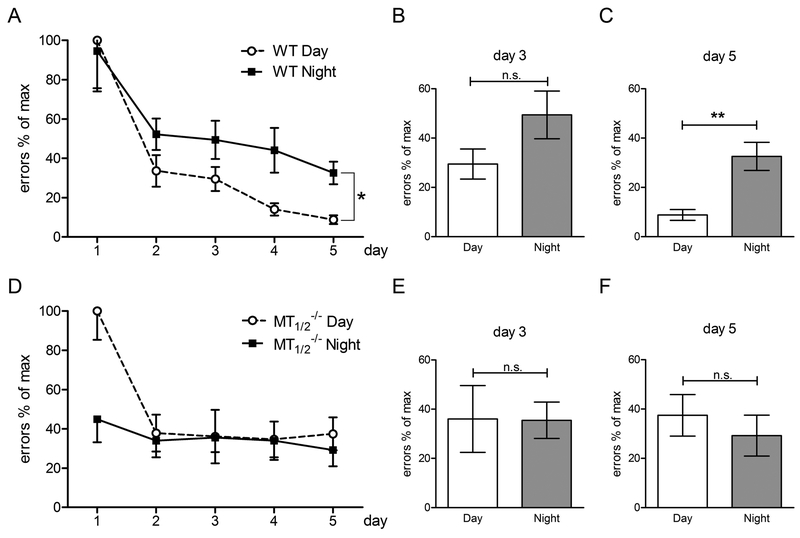
Spatial learning ability of WT (A, B, C) and MT1/2−/− (D, E ,F) mice. Illustrated is the number of errors (mean ± SEM) of WT (A) and MT1/2−/− mice (B) in the 8-arm radial maze test over 5 consecutive days, tested during daytime (Day) and nighttime (Night)(WT n = 9; MT1/2−/− n = 9; *= P ≤ 0.05 Two-way ANOVA). Comparison of the radial arm maze performance between daytime and nighttime at day 3 and day 5 of WT (B, C) and MT1/2−/− (E, F) mice (** = P ≤ 0.01; t-test unpaired). Values are expressed as mean ± SEM.
Fig. 7:
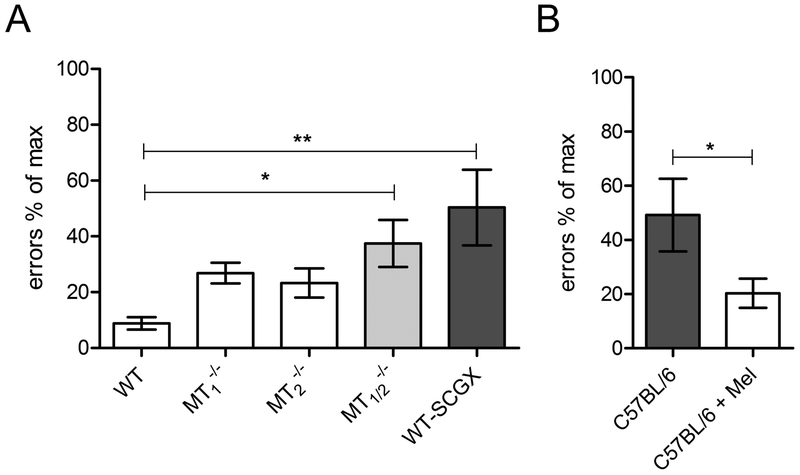
Comparative analysis of errors investigated across mouse strains. Illustrated is the number of errors of (A) WT mice (WT, MT1−/−, MT2−/−, MT1/2−/−, WT-SCGX) and (B) C57BL/6 mice ± melatonin during daytime training in the food-rewarded 8-arm radial maze test on day 5. (*= P ≤ 0.05; ** = P ≤ 0.01; One-way ANOVA with Dunnett´s Post-Test; unpaired t-test; WT: n = 9; MT1−/−: n = 10; MT2−/−: n = 8; MT1/2−/−: n = 8; C57BL/6 n = 7; C57BL/6 + Melatonin: n = 7; WT-SCGX: n = 8). Note that nighttime melatonin treatment increased the performance of C57BL/6 mice (B) in the 8-arm radial maze test. Values are expressed as mean ± SEM. Datasets of the radial arm maze performance during daytime on day 5 (see Figs. 6, Suppl. Figs. 8,9) were replotted here as % of maximal number of errors for a comparison across genotypes.
WT mice.
The number of errors during daytime learning (ZT2) decreased in WT mice progressively from day 1 to day 5 of training (Fig. 6A; day 1 vs. days 2 and 3. P < 0.01; day 1 vs. days 4, 5: P < 0.0001). A significant difference in the radial arm reward task was evident between day 1 and day 5 of training within nighttime performance (Fig. 6A; P < 0.01; Suppl. Tab. 4) and between daytime and nighttime learning (Fig. 6A, Suppl. Tab. 4; P < 0.05; Two-Way ANOVA). In conclusion, memory consolidation in WT mice occurs during both, daytime and nighttime with better retrieval of reference memory into STM in WT mice during daytime training (Fig. 6A), which is in line with earlier reports18,34.
MT1/2−/− mice did not show any improvement in STM during nighttime testing throughout the 5 days of training sessions (Fig. 6D; Suppl. Tab. 7). In daytime performance, STM improvement was evident between day 1 and days 2–5 (in all cases P < 0.05; One-Way ANOVA with Bonferroni´s multiple comparison test). However, no further amelioration was evident after day 2 of training (Fig. 6D; Suppl. Tab. 7). Furthermore, no difference between daytime and nighttime learning was evident in MT1/2−/− mice (Fig. 6 E,F) on day 3 and day 5 of learning. Remarkably, the number of errors observed in MT1/2−/− mice at ZT2 and at ZT14 of training day 5 resembled results obtained in WT mice trained at ZT14 (compare Fig. 6A with 6D) and was significantly different from results obtained in WT mice trained at ZT2 (Fig. 6A; Suppl. Tab. 7).
In MT1−/− mice, STM did not improve with training during daytime (Suppl. Fig. 9A, Suppl. Tab. 8). In contrast, during nighttime training, STM improved progressively (Suppl. Fig. 9A; day 1 vs. day 3: P < 0.05; day 1 vs day 4, 5: P < 0.001; day 2 vs. day 4, 5: P < 0.05; 1-Way ANOVA with Bonferroni´s multiple comparison test; Suppl. Tab. 5). While daytime learning was significantly altered from nighttime learning at day 1 and day 2 (P < 0.0001, P < 0.05, respectively; Two-way ANOVA; Suppl. Tab. 5), animals performed equivalent during day and night at training day 4 and 5 (Suppl. Fig. 9A; Suppl. Tab. 5).
In MT2−/− mice, STM performance during daytime was significantly different between day 1 and day 4 of learning (Suppl. Fig. 9B; day 1 vs day 4: P < 0.05; One-Way ANOVA with Bonferroni´s multiple comparison test; Suppl. Tab. 6). For nighttime training, a significant increase in STM performance became apparent from the second day of training onwards (day 1 vs. day 2: P < 0.001; day 1 vs day 3–5: P < 0.0001; day 2 vs day 3: P < 0.05; day 2 vs day 4, 5: P < 0.001; One-Way ANOVA with Bonferroni´s multiple comparison test; Suppl. Fig. 9B; Suppl. Tab. 6). While daytime learning differed significantly from nighttime learning at day 1 (P < 0.05, Two-way ANOVA; Suppl. Tab. 6), no apparent difference in performance was observed after day 2 (Suppl. Fig. 9B; Suppl. Tab. 6).
C57BL/6 mice.
Learning during daytime did not improve in melatonin-deficient C57BL/6 mice (Suppl. Fig. 10; 1-Way ANOVA with Bonferroni´s multiple comparison test, Suppl. Tab. 8). Of note, after day 5 of training, the number of errors in C57BL/6 mice was similar to that of MT1/2−/−, and WT-SCGX mice (Fig. 7).
Melatonin-treated C57BL/6 mice.
In in C57BL/6 mice, which were treated with melatonin during nighttime, significantly improved daytime learning was witnessed in comparison to untreated animals, (Suppl. Fig. 10; P < 0.05, Two-Way ANOVA; Suppl. Tab. 8). On the final day of training, melatonin treated C57BL/6 mice showed similarly low number of errors as WT mice (Fig. 7).
In the elevated plus maze test conducted at ZT2, no significant differences were observed between WT, MT1−/−, MT2−/− and MT1/2−/− mice (Suppl. Tab. 9). Hence, we conclude that results obtained in the food-rewarded radial arm maze test are not associated with significant changes in anxiety-related behavior.
Taken together, these results show that on the final day of training, no day/night differences in learning were seen in all three melatonin receptor-deficient mouse lines (MT1−/−, MT2−/−, MT1/2−/−), as well as in WT-SCGX mice and in C57BL/6 mice. Notably, MT1/2−/−, WT-SCGX and C57BL/6 mice made significantly more errors compared to intact melatonin-proficient WT and compared to melatonin-treated C57BL/6 mice (Fig. 7).
Discussion
We tested the effects of melatonin on time-of-day-dependent synaptic plasticity and learning. Our results suggest that melatonin receptor signaling shapes time-of-day-dependent learning efficiency and affects underlying events such as hippocampal gene transcription and CREB phosphorylation, which are associated with learning.
In particular, we show that
MT1 and MT2 melatonin receptors are expressed in WT mouse brain structures that are critically involved in learning and memory,
the rhythmic expression of clock genes in WT hippocampus and in the DG is greatly altered in MT1/2−/− mice,
day/night difference in hippocampal LTP in WT mice is absent in MT1/2−/− and in WT-SCGX animals,
time-of-day-dependent deflections in hippocampal pCREB levels are significantly different in the absence of a melatonin signal,
day/night difference in learning in WT animals is absent in MT1/2−/− mice and in WT-SCGX mice,
substitution of melatonin in melatonin-deficient C57BL/6 mice improves daytime learning.
On the basis of our data, we hypothesize that melatonin influences signaling events in hippocampal cells, which underlie mechanisms of time-of-day-dependent learning efficiency in mice.
Various specific effects of melatonin on hippocampal signaling have been described in rodents in the past 27,56–58, suggesting a fundamental role of MTs in the hippocampus. While 125I-melatonin receptorautoradiography did not detect melatonin binding sites in the hippocampus of various species 51,59,60, MT receptor mRNA has been detected in rat hippocampus 22,61. Using different MT-antibodies and sensitive immunofluorescence, we show the presence of MTs on WT mouse hippocampal cells, confirming earlier data obtained in the rat 23. These data can potentially provide a structural basis for the effects of melatonin on hippocampal signaling and learning as outlined below.
-
Clock gene mRNA expression and clock gene protein levels become greatly disturbed in mouse hippocampus with the inability to interpret the nocturnal melatonin message in MT1/2−/− mice compared to WT animals. On the contrary, neither clock gene protein levels in the SCN, nor SCN-driven day/night activity patterns of locomotor activity (Suppl. Fig. 11) were different in MT1/2−/− animals compared to WT animals. This indicates a direct mechanistic impact of melatonin on hippocampal cells via both G-protein-coupled melatonin receptors, the MT1 and the MT2 receptor, respectively. Interestingly, it was shown in the Siberian hamster that melatonin can rapidly increase mPer1 and mBmal1 mRNA expression 62, despite lacking a functional MT2 receptor 63. How hippocampal cells dissociate between the earlier shown distinct MT2-mediated effect on LTP 27, and MT1-mediated effects on hippocampal clock gene expression and learning, is yet unknown. To this end, a rhythmic and temporally coordinated clock gene expression was reported to be indispensable for proper hippocampal functioning 18–20,34.
The suggested possible link between time-of-day-dependent learning efficiency, clock genes and melatonin is greatly substantiated by our findings that the altered day/night pattern in clock gene protein levels in the hippocampus of MT1/2−/− mice is correlated to deficits in memory processing. More specifically, these data show that it is not only the presence (or absence) of a given clock gene that is important for a coordinated cognitive performance, but also the overall phase relation of the rhythmic clock gene protein levels, i.e. the phase of peaks and troughs. This work shows a 12 hour phase-shift in peak values for PER2, CRY2, CLOCK, and BMAL1 into the daytime period in MT1/2−/− mice, annihilating at the same time differences between day- and nighttime performances in the radial arm maze.
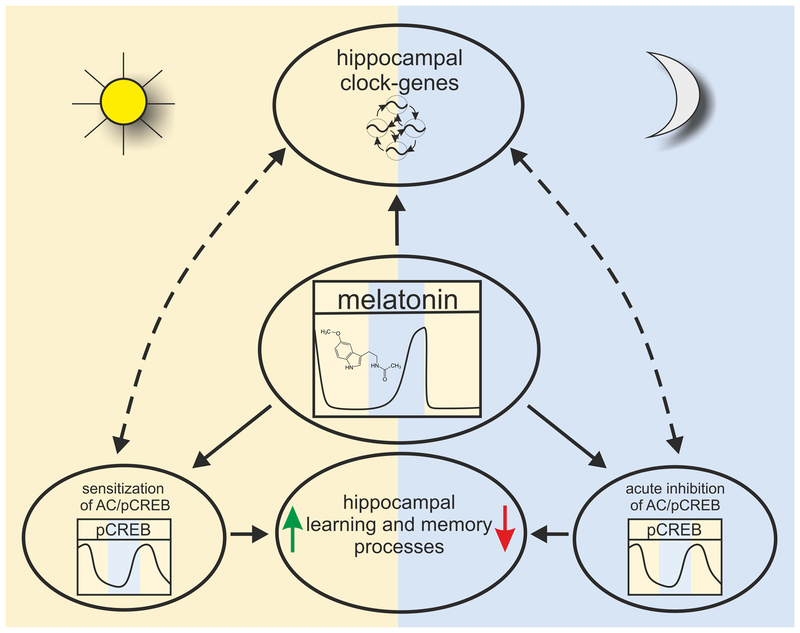
We therefore hypothesize that the temporally restricted impact of melatonin on hippocampal signaling during nighttime functions as a temporal conductor for rhythmic clock gene expression, which in turn is a prerequisite for the improved learning efficiency during daytime as compared to nighttime (Fig. 8).
WT mice exhibited a clear day/night difference in LTP. Melatonin profile in C3H mice rises during the second half of the dark period, peaks prior to the end of the dark period and then drops rapidly to low daytime values 30,64. As melatonin can inflict on LTP 27, it is likely that LTP levels alter dependent on the relative time of slice preparation and recordings in relation to the melatonin cycle. Still, independent of experimental design, day/night differences in hippocampal excitability vanished in MT1/2−/− mice as shown in previous reports 27 and confirmed in the present study. Our novel data from WT-SCGX mice provide additional supportive evidence that melatonin is involved in the time-of-day-dependent variations in LTP magnitude in the mouse hippocampus.
-
Phosphorylation of the ‘memory molecule’ CREB is a critical element for efficient memory formation 11,15,65–67. Time-of-day-dependent fluctuations in CREB phosphorylation are directly linked to cycling memory reconsolidation and maintenance 17,19. The fact that the endogenous rhythm in CREB phosphorylation in MT1/2−/− mice hippocampus remained almost unaltered in phase compared to the rhythm in WT animals implies that melatonin does not directly affect the time when this important transcription factor for memory formation is phosphorylated. However, the overall amplitude of the pCREB rhythm in MT1/2−/− mice is significantly dampened as compared to WT animals. It is well known that melatonin acutely inhibits cAMP accumulation 68 and CREB phosphorylation 16,69. As an additional effect, chronic melatonin exposure (>4h) leads to a time-dependent sensitization of the adenylyl cyclase in pars tuberalis cells 16,68,69. In WT mouse hippocampus, CREB phosphorylation rises as the nocturnally elevated melatonin surge progresses, reaching peak values only several hours after the onset of daytime. Consequently, the persistent nocturnal melatonin impact on mouse hippocampal cells may lead - likewise as observed in pars tuberalis cells 16 - to a sensitization of the adenylyl cyclase. We hypothesize that the less prominent pCREB rhythm in MT1/2−/− mice compared to WT animals may be the reason for a weakened memory-related gene expression: in WT animals, pCREB-induced hippocampal gene expression would be enhanced by the melatonin-initiated sensitization of the adenylyl cyclase, exactly at times, when animals learn better, i.e. during daytime.
In C57BL/6 mice, the pCREB rhythm is phase-advanced by approximately 4 hours compared to WT and MT1/2−/− mice. As C57BL/6 mice do not produce considerable amounts of melatonin 30,64, the suggested sensitization of hippocampal adenylyl cyclase by melatonin may not take place, which potentially explains why spatial learning during daytime is poor in C57BL/6 mice. A likewise explanation may be valid for low daytime learning in MT1/2−/− and in WT-SCGX mice. Our suggestion is supported by the finding that substitution of melatonin to C57BL/6 mice significantly improved learning, up to the level seen in WT animals (Fig. 7).
Several reports demonstrated that performance of mice in acquisition, recall, and extinction of hippocampus-based memory is better during daytime than nighttime, even under conditions of constant darkness 18,70 (for review see: 10). The rhythm in hippocampal spatial learning was severely affected in MT1/2−/− mice as they lack the WT-specific improvement of memory formation during daytime. Both MT receptors are involved in this effect, as already mice with a knockout of only a single MT receptor, the MT1−/− or the MT2−/−, show compromised daytime learning.
Fig. 8:
Working model for the possible mechanistic role of melatonin within hippocampal learning and memory processes.
When training was performed during daytime (at ZT2), no significant amelioration in STM was observed over the 5 consecutive days of testing in C57BL/6 mice. This behavior was similar to the daytime memory performance observed in MT1/2−/− mice. However, when C57BL/6 mice were treated with melatonin during nighttime, daytime learning was significantly improved. Since C57BL/6 mice lack melatonin and therefore also N-acetylserotonin (NAS) but behaved similar to NAS-proficient MT1/2−/− mice, this observed effect of the pineal hormone is not related to the melatonin precursor NAS, which is known to enhance cognition via a circadian activation of TrkB receptors71. Moreover, diminished learning in C57BL/6 mice was rescued by the sole substitution of melatonin and was therefore independent of NAS.
It still has to be delineated, if melatonin unfolds its impact on memory processes in mice by inhibiting STM during the second half of the night or if the hormone promotes better retrieval during daytime, or possibly both. It may well be that better learning during daytime in night-active animals is linked to the well-documented enhanced memory consolidation during sleep72,73. Sleep is characterized by cyclic occurrence of rapid-eye-movement (REM) and non-REM sleep. The latter includes slow-wave sleep (SWS). SWS prevails in the first part of the night (early sleep), whereas REM sleep dominates the second half (late sleep), which is critical for memory consolidation74,75. As long as REM sleep can be established, learning can even be rescued from interference76. Thus, interference with the REM phase will likely lead to declined learning. Reiteration during the early night on the other hand can improve learning, possibly via use- or experience-dependent processes or enhancement72,73. In addition, better learning during daytime in night-active animals is also linked to a temporally gated role of PER1 within memory formation18,19,34.
Across phylogeny the achievement of an optimal cognitive performance is vital for survival. Within the molecular mechanisms that regulate the circadian nature of memory formation, circadian clock gene expression18,34 and the MAPK/pCREB cascade17,19 are central players in mouse hippocampus. Here, we add melatonin to these modulatory impacts on hippocampal circuity (Fig. 8). The pineal hormone seems to affect hippocampal gene expression, LTP and time-of-day-dependent differences in memory performance. In an evolutionary conserved manner, melatonin seems to be involved in mental performance in mice, similar to Zebrafish6. Clarifying the role of melatonin within hippocampus-dependent learning and memory will foster our understanding of circadian rhythm organization in cognition. Lastly, it was shown that disturbance of the hippocampal circuity severely affects sleep and cognition in fish77 and in humans78. In the light of an increased awareness of the link between sleep-wake disturbances in a 24-h society and cognitive and neurodegenerative disorders in humans9,79, a better understanding of how the circadian clock modulates hippocampal circuity and the role of melatonin within this mechanism have great merit.
Supplementary Material
Acknowledgements:
The authors thank A. Konoplev for expert technical assistance, S. Lesny, E. Maronde, N. Molotkov, M. Öser, and O. Rawashdeh for help with initial experiments, M. Schwab for continuous support, J. Hernesniemi for his kind help and support in preparing this manuscript, and T.P. Puma for editing the manuscript and grateful help. This work was supported by grants from the UZH Forschungskredit (FK 15–05), the EMDO foundation (872) and the Heidi Demetriades Foundation to A. Zemmar, from the National Institute of Health (EY026291) to G. Tosini, and by the Henan Provincial People´s Hospital Outstanding Talents Founding Grant Project.
References
- 1.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002; 418:935–941. [DOI] [PubMed] [Google Scholar]
- 2.Reppert S, Klein DC, Moore R. Suprachiasmatic Nucleus: The Mind’s Clock: Oxford University Press; 1991. [Google Scholar]
- 3.Davies JA, Navaratnam V, Redfern PH. A 24-hour rhythm in passive-avoidance behaviour in rats. Psychopharmacologia. 1973; 32:211–214. [DOI] [PubMed] [Google Scholar]
- 4.Lyons LC, Rawashdeh O, Katzoff A, et al. Circadian modulation of complex learning in diurnal and nocturnal Aplysia. Proceedings of the National Academy of Sciences. 2005; 102:12589–12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons LC, Roman G. Circadian modulation of short-term memory in Drosophila. Learn. Mem 2009; 16:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawashdeh O, Borsetti NH de, Roman G, et al. Melatonin suppresses nighttime memory formation in zebrafish. Science. 2007; 318:1144–1146. [DOI] [PubMed] [Google Scholar]
- 7.Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. Journal of Biological Rhythms. 2004; 19:312–324. [DOI] [PubMed] [Google Scholar]
- 8.Wright KP, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. AJP: Regulatory, Integrative and Comparative Physiology. 2002; 283:R1370–7. [DOI] [PubMed] [Google Scholar]
- 9.Gerstner JR, Lyons LC, Wright KP, et al. Cycling Behavior and Memory Formation. Journal of Neuroscience. 2009; 29:12824–12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snider KH, Sullivan KA, Obrietan K. Circadian Regulation of Hippocampal-Dependent Memory: Circuits, Synapses, and Molecular Mechanisms. Neural Plasticity. 2018; 2018:7292540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002; 108:689–703. [DOI] [PubMed] [Google Scholar]
- 12.Pastalkova E, Serrano P, Pinkhasova D, et al. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006; 313:1141–1144. [DOI] [PubMed] [Google Scholar]
- 13.Whitlock JR, Heynen AJ, Shuler MG, et al. Learning induces long-term potentiation in the hippocampus. Science. 2006; 313:1093–1097. [DOI] [PubMed] [Google Scholar]
- 14.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993; 361:31–39. [DOI] [PubMed] [Google Scholar]
- 15.Abel T, Nguyen PV, Barad M, et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997; 88:615–626. [DOI] [PubMed] [Google Scholar]
- 16.Gall C von, Garabette ML, Kell CA, et al. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002; 5:234–238. [DOI] [PubMed] [Google Scholar]
- 17.Eckel-Mahan KL, Phan T, Han S, et al. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat Neurosci. 2008; 11:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawashdeh O, Jilg A, Jedlicka P, et al. PERIOD1 coordinates hippocampal rhythms and memory processing with daytime. Hippocampus. 2014; 24:712–723. [DOI] [PubMed] [Google Scholar]
- 19.Rawashdeh O, Jilg A, Maronde E, et al. Period1 gates the circadian modulation of memory-relevant signaling in mouse hippocampus by regulating the nuclear shuttling of the CREB kinase pP90RSK. J. Neurochem 2016; 138:731–745. [DOI] [PubMed] [Google Scholar]
- 20.Phan TX, Phan TH, Chan GC-K, et al. The diurnal oscillation of MAP (mitogen-activated protein) kinase and adenylyl cyclase activities in the hippocampus depends on the suprachiasmatic nucleus. J. Neurosci 2011; 31:10640–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Challet E Minireview: Entrainment of the Suprachiasmatic Clockwork in Diurnal and Nocturnal Mammals. Endocrinology. 2007; 148:5648–5655. [DOI] [PubMed] [Google Scholar]
- 22.Musshoff U, Riewenherm D, Berger E, et al. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002; 12:165–173. [DOI] [PubMed] [Google Scholar]
- 23.Lacoste B, Angeloni D, Dominguez-Lopez S, et al. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015; 58:397–417. [DOI] [PubMed] [Google Scholar]
- 24.Larson J, Jessen RE, Uz T, et al. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neuroscience Letters. 2006; 393:23–26. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long-term potentiation. Journal of Biological Rhythms. 2005; 20:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reppert SM, Godson C, Mahle CD, et al. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proceedings of the National Academy of Sciences. 1995; 92:8734–8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang LM, Suthana NA, Chaudhury D, et al. Melatonin inhibits hippocampal long-term potentiation. Eur. J. Neurosci 2005; 22:2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtuncu M, Arslan AD, Akhisaroglu M, et al. Involvement of the pineal gland in diurnal cocaine reward in mice. Eur. J. Pharmacol 2004; 489:203–205. [DOI] [PubMed] [Google Scholar]
- 29.Ebihara S, Marks T, Hudson DJ, et al. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986; 231:491–493. [DOI] [PubMed] [Google Scholar]
- 30.Gall C von, Lewy A, Schomerus C, et al. Transcription factor dynamics and neuroendocrine signalling in the mouse pineal gland: a comparative analysis of melatonin-deficient C57BL mice and melatonin-proficient C3H mice. Eur. J. Neurosci 2000; 12:964–972. [DOI] [PubMed] [Google Scholar]
- 31.Roseboom PH, Namboodiri MAA, Zimonjic DB, et al. Natural melatonin `knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase1. Molecular Brain Research. 1998; 63:189–197. [DOI] [PubMed] [Google Scholar]
- 32.Gall C von, Duffield GE, Hastings MH, et al. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J. Neurosci 1998; 18:10389–10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubocovich ML, Hudson RL, Sumaya IC, et al. Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res. 2005; 39:113–120. [DOI] [PubMed] [Google Scholar]
- 34.Jilg A, Lesny S, Peruzki N, et al. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010; 20:377–388. [DOI] [PubMed] [Google Scholar]
- 35.Dias GP, Bevilaqua MCdN, da Luz ACDS, et al. Hippocampal biomarkers of fear memory in an animal model of generalized anxiety disorder. Behavioural brain research. 2014; 263:34–45. [DOI] [PubMed] [Google Scholar]
- 36.Grossi M, Morgunova M, Cheung S, et al. Lysosome triggered near-infrared fluorescence imaging of cellular trafficking processes in real time. Nat Commun. 2016; 7:10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maronde E, Pfeffer M, Olcese J, et al. Transcription factors in neuroendocrine regulation: Rhythmic changes in pCREB and ICER levels frame melatonin synthesis. Journal of Neuroscience. 1999; 19:3326–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karolczak M, Burbach GJ, Sties G, et al. Clock gene mRNA and protein rhythms in the pineal gland of mice. Eur. J. Neurosci 2004; 19:3382–3388. [DOI] [PubMed] [Google Scholar]
- 39.Baddeley AD, Hitch G. Working Memory In: Bower GH, ed. The psychology of learning and motivation: Advances in research and theory. New York: Academic Press; 1974;47–89. [Google Scholar]
- 40.Touzani K, Puthanveettil SV, Kandel ER. Consolidation of learning strategies during spatial working memory task requires protein synthesis in the prefrontal cortex. Proceedings of the National Academy of Sciences. 2007; 104:5632–5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olcese JM, Cao C, Mori T, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. Journal of Pineal Research. 2009; 47:82–96. [DOI] [PubMed] [Google Scholar]
- 42.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protocols 2007; 2:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999; 835:18–26. [DOI] [PubMed] [Google Scholar]
- 44.Deacon RMJ. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc. 2006; 1:936–946. [DOI] [PubMed] [Google Scholar]
- 45.Delekate A, Zagrebelsky M, Kramer S, et al. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc. Natl. Acad. Sci. U.S.A 2011; 108:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tews B, Schönig K, Arzt ME, et al. Synthetic microRNA-mediated downregulation of Nogo-A in transgenic rats reveals its role as regulator of synaptic plasticity and cognitive function. Proc. Natl. Acad. Sci. U.S.A 2013; 110:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994; 13:1177–1185. [DOI] [PubMed] [Google Scholar]
- 48.Liu C, Weaver DR, Jin X, et al. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997; 19:91–102. [DOI] [PubMed] [Google Scholar]
- 49.Jin X, Gall C von, Pieschl R, et al. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol. 2003; 23:1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sengupta A, Baba K, Mazzoni F, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS ONE. 2011; 6:e24483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weaver DR, Stehle JH, Stopa EG, et al. Melatonin receptors in human hypothalamus and pituitary: Implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab. 1993; 76:295–301. [DOI] [PubMed] [Google Scholar]
- 52.Rivera-Bermúdez MA, Masana MI, Brown GM, et al. Immortalized cells from the rat suprachiasmatic nucleus express functional melatonin receptors. Brain Research. 2004; 1002:21–27. [DOI] [PubMed] [Google Scholar]
- 53.Hastings MH, Reddy AB, Garabette M, et al. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found Symp. 2003; 253:203–17; discussion 102–9, 218–22, 281–4. [DOI] [PubMed] [Google Scholar]
- 54.Ko C, Takahashi J. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006; 15 Spec No 2:7. [DOI] [PubMed] [Google Scholar]
- 55.Reiter RJ, Rudeen PK, Banks AF, et al. Acute effects of unilateral or bilateral superior cervical ganglionectomy on rat pineal N-acetyltransferase activity and melatonin content. Experientia. 1979; 35:691–692. [DOI] [PubMed] [Google Scholar]
- 56.El-Sherif Y, Tesoriero J, Hogan MV, et al. Melatonin regulates neuronal plasticity in the hippocampus. J Neurosci Res. 2003; 72:454–460. [DOI] [PubMed] [Google Scholar]
- 57.Collins DR, Davies SN. Melatonin blocks the induction of long-term potentiation in an N-methyl-D-aspartate independent manner. Brain Res. 1997; 767:162–165. [DOI] [PubMed] [Google Scholar]
- 58.Ruby NF, Hwang CE, Wessells C, et al. Hippocampal-dependent learning requires a functional circadian system. Proc. Natl. Acad. Sci. U.S.A. 2008; 105:15593–15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weaver DR, Carlson LL, Reppert SM. Melatonin receptors and signal transduction in melatonin-sensitive and melatonin-insensitive populations of white-footed mice (Peromyscus leucopus). Brain Res. 1990; 506:353–357. [DOI] [PubMed] [Google Scholar]
- 60.Masson-Pévet M, Gauer F. Seasonality and melatonin receptors in the pars tuberalis in some long day breeders. Biol Signals. 1994; 3:63–70. [DOI] [PubMed] [Google Scholar]
- 61.Wan Q, Man HY, Liu F, et al. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999; 2:401–403. [DOI] [PubMed] [Google Scholar]
- 62.Ikeno T, Nelson RJ. Acute melatonin treatment alters dendritic morphology and circadian clock gene expression in the hippocampus of Siberian Hamsters. Hippocampus. 2014:n/a. [DOI] [PubMed] [Google Scholar]
- 63.Weaver DR, Liu C, Reppert SM. Nature’s knockout: The Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol. 1996; 10:1478–1487. [DOI] [PubMed] [Google Scholar]
- 64.Vivien-Roels B, Malan A, Rettori MC, et al. Daily variations in pineal melatonin concentrations in inbred and outbred mice. Journal of Biological Rhythms. 1998; 13:403–409. [DOI] [PubMed] [Google Scholar]
- 65.Bourtchuladze R, Frenguelli B, Blendy J, et al. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994; 79:59–68. [DOI] [PubMed] [Google Scholar]
- 66.Silva AJ, Kogan JH, Frankland PW, et al. CREB and memory. Annu. Rev. Neurosci 1998; 21:127–148. [DOI] [PubMed] [Google Scholar]
- 67.Mizuno M, Yamada K, Maekawa N, et al. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002; 133:135–141. [DOI] [PubMed] [Google Scholar]
- 68.Hazlerigg DG, Gonzalez-Brito A, Lawson W, et al. Prolonged exposure to melatonin leads to time-dependent sensitization of adenylate cyclase and down-regulates melatonin receptors in pars tuberalis cells from ovine pituitary. Endocrinology. 1993; 132:285–292. [DOI] [PubMed] [Google Scholar]
- 69.McNulty S, Ross AW, Barrett P, et al. Melatonin regulates the phosphorylation of CREB in ovine pars tuberalis. J. Neuroendocrinol 1994; 6:523–532. [DOI] [PubMed] [Google Scholar]
- 70.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav. Brain Res 2002; 133:95–108. [DOI] [PubMed] [Google Scholar]
- 71.Jang S-W, Liu X, Pradoldej S, et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. U.S.A 2010; 107:3876–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maquet P The role of sleep in learning and memory. Science. 2001; 294:1048–1052. [DOI] [PubMed] [Google Scholar]
- 73.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Psychol. 2006; 57:139–166. [DOI] [PubMed] [Google Scholar]
- 74.Rasch B, Born J. About sleep’s role in memory. Physiol. Rev 2013; 93:681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rechtschaffen A, Kales A. A manual of standardized terminology: Techniques and scoring system for sleep stages of human subjects. Bethesda, MD: U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
- 76.McDevitt EA, Duggan KA, Mednick SC. REM sleep rescues learning from interference. Neurobiol Learn Mem. 2015; 122:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elbaz I, Foulkes NS, Gothilf Y, et al. Circadian clocks, rhythmic synaptic plasticity and the sleep-wake cycle in zebrafish. Front Neural Circuits. 2013; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ly M, Adluru N, Destiche DJ, et al. Fornix Microstructure and Memory Performance Is Associated with Altered Neural Connectivity during Episodic Recognition. J Int Neuropsychol Soc. 2016; 22:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takahashi JS, Hong H-K, Ko CH, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008; 9:764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.