Abstract
The midcycle surge of LH sets in motion interconnected networks of signaling cascades to bring about rupture of the follicle and release of the oocyte during ovulation. Many mediators of these LH-induced signaling cascades are associated with inflammation, leading to the postulate that ovulation is similar to an inflammatory response. First responders to the LH surge are granulosa and theca cells, which produce steroids, prostaglandins, chemokines, and cytokines, which are also mediators of inflammatory processes. These mediators, in turn, activate both nonimmune ovarian cells as well as resident immune cells within the ovary; additional immune cells are also attracted to the ovary. Collectively, these cells regulate proteolytic pathways to reorganize the follicular stroma, disrupt the granulosa cell basal lamina, and facilitate invasion of vascular endothelial cells. LH-induced mediators initiate cumulus expansion and cumulus oocyte complex detachment, whereas the follicular apex undergoes extensive extracellular matrix remodeling and a loss of the surface epithelium. The remainder of the follicle undergoes rapid angiogenesis and functional differentiation of granulosa and theca cells. Ultimately, these functional and structural changes culminate in follicular rupture and oocyte release. Throughout the ovulatory process, the importance of inflammatory responses is highlighted by the commonalities and similarities between many of these events associated with ovulation and inflammation. However, ovulation includes processes that are distinct from inflammation, such as regulation of steroid action, oocyte maturation, and the eventual release of the oocyte. This review focuses on the commonalities between inflammatory responses and the process of ovulation.
Essential Points
The process of ovulation shares many features with inflammatory responses
Granulosa and theca cells of the follicle cooperate with resident and infiltrating immune cells to produce paracrine mediators of ovulation, many of which are also common to inflammatory responses
Angiogenesis, increased vascular permeability, both vasodilation and vasoconstriction, and edema are essential features of both ovulation and inflammation
Extensive remodeling of the extracellular matrix is stimulated by inflammatory mediators such as steroids, prostaglandins, and cytokines
Coordinated control over proteolysis facilitates follicle rupture and oocyte release while also permitting rapid healing after ovulation and transformation of the ruptured follicle into the corpus luteum
Several disorders of ovulation share common features with dysregulated inflammatory responses
Nearly 40 years ago Bukovsky and Presl (1) proposed that the immune system regulated ovulatory ovarian function. In his landmark paper in 1980, Espey (2) put forth the hypothesis of ovulation as an inflammatory reaction and outlined the similarities in inflammatory processes and ovulation. Classically defined, inflammation is a protective response of a tissue to a harmful stimulus such as irritants, pathogens, or cellular damage (3). This inflammatory response involves chemokine and cytokine release, blood vessel dilation, immune cell infiltration, and localized production of molecular mediators that abrogate the inflammatory stimulus (3). Throughout his review, Espey posed numerous thought-provoking questions about the parallels between inflammation and ovulation, as there is a high degree of analogy between many of these routine inflammatory processes and the ovulatory events that the ovary undergoes in response to an ovulatory LH stimulus. Central to this hypothesis is the role of prostaglandins as mediators of the inflammatory process and the relationship between prostaglandins and ovarian proteolytic activity. For example, while recognizing the critical actions of the LH surge and resulting steroid hormone synthesis in the ovulatory follicle, Espey proposed that prostaglandins also participate in the control of ovulation. In the intervening years, the advent of molecular biology, genetic engineering, and new pharmacological tools have provided insight into many of Espey’s original questions, such as the importance of steroids and prostaglandins in the ovulatory process, the follicular concentrations of other inflammatory eicosanoids such as leukotrienes, and the distribution of immune cells in the follicle wall during ovulation. Despite significant advances, many queries remain unanswered such as the contribution of leukocytes to ovarian prostaglandin and protease production, the impact of serotonin and bradykinin on vascular permeability, factors that activate thecal fibroblasts, among others. Yet, new questions have emerged. This review summarizes advances that address many of the original questions raised by Espey and presents new discoveries and concepts developed since Dr. Espey’s review that relate to hormone signaling and downstream changes associated with inflammatory mediators, the vasculature, and the proteolytic system. The current review focuses on data from the primate, including humans when available, but will use key information from other mammalian species, including rodents and domestic animals, where data are lacking in the primate to fully address the current status of our understanding of the relationship between inflammation and ovulation.
The structure of the ovarian follicle
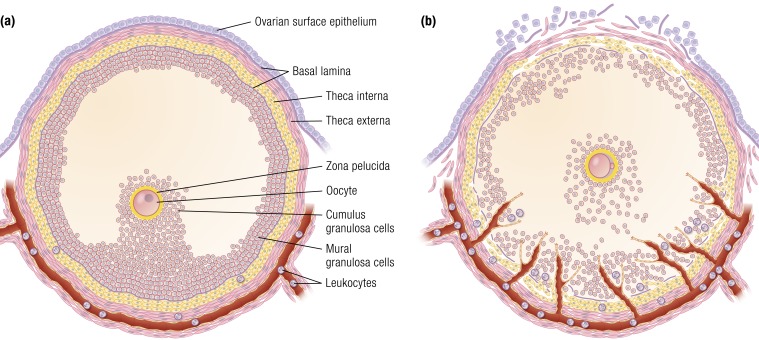
The ovarian follicle is widely understood to be a functional unit, comprised of the oocyte, the granulosa cell compartment, and the theca cell compartment (Fig. 1). As granulosa cells proliferate, they differentiate into three different populations of cells: the cumulus cells that enclose and support the oocyte, antral granulosa cells that are adjacent to the follicular antrum, and basal or mural granulosa cells that are adjacent to the basal lamina that separates the granulosa cell compartment from the thecal cell compartment. The thecal cell compartment contains an inner layer of steroidogenic cells called the theca interna, an outer layer of fibroblast-like theca externa, and a rich vascular network. The theca externa blends into a layer of connective tissue known as the tunica albuginea. The tunica albuginea is separated from the ovarian surface epithelium (OSE) by a basal lamina supporting the ovarian surface epithelial cells, which varies from a single layer of flat to cuboidal to columnar epithelium (4). The blood supply to and from the ovary consists of a single ovarian artery and single ovarian vein, which enter and exit the ovary at the hilus and provide the sole connection between the ovarian vasculature and the systemic circulation. Resident immune cells are present in the theca and stroma. The ovarian circulation delivers additional leukocytes to the ovary, some of which extravagate into the surrounding ovarian tissues to become resident immune cells.
Figure 1.
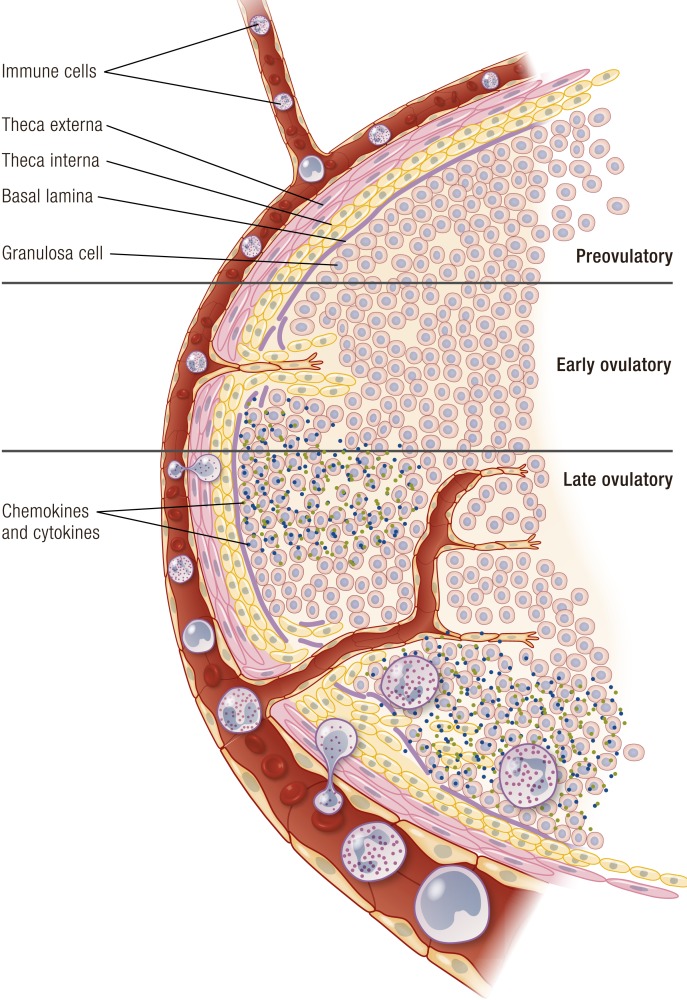
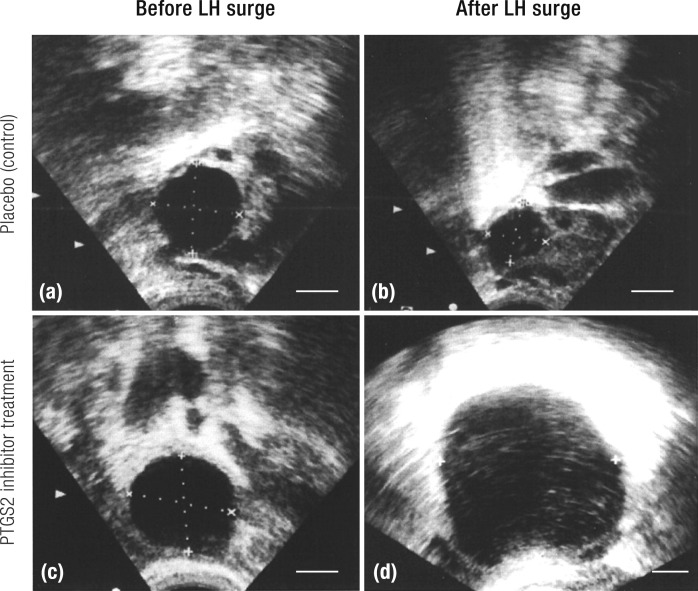
The preovulatory follicle. (a) Preovulatory follicle prior to the LH surge. The oocyte is surrounded by the zona pelucida and cumulus granulosa cells that connect to the mural granulosa cells that line the interior of the follicle. The granulosa cell compartment is separated from the theca cell compartment by a basal lamina. The theca cell compartment is composed of an inner theca interna and an outer theca externa. Unlike the granulosa cell compartment, the theca cell layer is highly vascularized (red). Circulating leukocytes are present in the vessels. The theca externa blends into a layer of connective tissue that is separated from the ovarian surface epithelium by a basal lamina. (b) Preovulatory follicle following LH stimulation immediately prior to ovulation. Disruption of the granulosa cell basal lamina allows extension of vessels into the granulosa cell compartment. Theca cells and leukocytes also enter into the granulosa cell compartment. The cumulus oocyte complex detaches from the surrounding granulosa cells and undergoes cumulus expansion. At the follicular apex (top of image), there is a loss of ovarian surface epithelium, the breakdown of the underlying basal lamina, and a loss of theca cells and granulosa cells. Rupture will occur at the follicle apex.
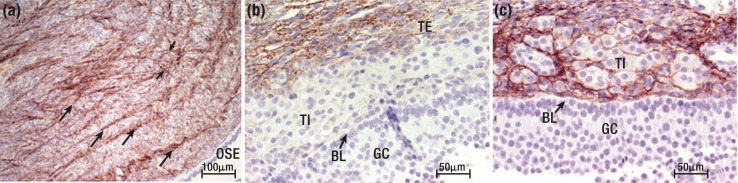
Cells within these compartments are supported by different extracellular matrix (ECM) components. As noted above, the cells of the OSE rest on a basal lamina that separates the OSE from the tunica albuginea. This basal lamina is comprised primarily of laminin, entactin, and heparin sulfate proteoglycans (5–7). Some authors have proposed that the basal lamina contains collagen IV (7). However, Lind et al. (8) did not observe collagen IV in the basal lamina in the human ovary. Immediately below the OSE, the tunica albuginea is comprised of fibroblast-like cells embedded in a dense collagenous connective tissue framework of collagens type I, type III, and type IV, with collagen type I exhibiting a concentric, network-like distribution (8) [Fig. 2(a)]. This network-like distribution extends into the theca externa layer. However, collagen type I is not present in the theca interna [Fig. 2(b)], the basal lamina separating the theca interna and the granulosa cells, or in the granulosa cell layer itself (8). In contrast, collagen type III is found in both the theca externa and the theca interna but is absent from the basal lamina separating the theca interna from the granulosa cell compartment [Fig. 2(c)]. Collagen type IV is found in both the theca interna and the basal lamina (8). The variety of ECM components provides different levels of cellular support to the overall follicle structure (9).
Figure 2.
Collagens in the human ovary and ovulatory follicle. (a) Collagen type I (brown) in the human ovarian capsular stroma, showing a distribution of collagen type I in concentric layers (long arrows) with bundles (short arrows) joining the concentric layers. (b) Collagen type I (brown) in the theca externa (TE) and (c) collagen type III (brown) in the theca interna (TI) of a human preovulatory follicle. Nova Red stain. BL, basal lamina; GC, granulosa cells. [Reproduced with permission from Lind A-K, Weijdegard B, Dahm-Kahler P. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet Gynecol Scand 2006;85(12):1476–1484.]
Structural changes during ovulation
For ovulation to be successful, substantial structural changes at the apex of the follicle must create a breach in the follicle wall to allow release of the oocyte. Disruption of the ECM within each cell layer as well as the breakdown of the basal lamina separating the granulosa and theca cell compartments and the basal lamina at the surface epithelium are all required to weaken the follicle wall and eventually create an opening at the follicle apex, also known as the stigmata. During the ovulatory process there is also a loss of the OSE at the apical region of the ovulatory follicle. Although a detailed time course of changes in the human ovulatory follicle has not been performed, examples from animal models provide insight into the structural changes that occur throughout the ovulatory process.
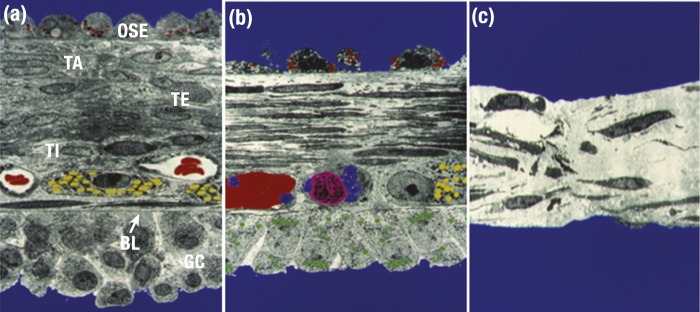
Espey (4) elegantly documented the morphologic changes in the rabbit preovulatory follicle [Fig. 3(a)]. Approximately 1 to 2 hours prior to rupture, the cells of the surface epithelium begin to detach from the ovarian surface overlaying the follicle apex [Fig. 3(b)]. The connective tissue begins to fragment and is degraded. The follicle wall becomes thinner. Fibroblasts in the tunica albuginea and theca externa become elongated and appear to transform from quiescent to motile cells. There is also sloughing of some granulosa cells into the follicular antrum. Immediately prior to ovulation, the surface epithelium is lost, and compaction of the layers of the tunica, theca, and granulosa cell compartments occurs [Fig. 3(c)]. Interestingly, these dynamic changes are restricted to the apical region of the ovulatory follicle. The ECM of the tunica albuginea and theca becomes dissociated and sparse, and it eventually disappears, forming an opening connecting the antral fluid and exterior of the ovary. Although these events have been detailed in the rabbit, similar events are thought to occur in ovaries of nonhuman primates and humans (Fig. 4).
Figure 3.
Structure of the apex of the rabbit preovulatory follicle. Faux-colored electron microscopic images were obtained (a) before the LH surge, (b) 1 to 2 h prior to follicle rupture, and (c) immediately before follicular rupture. (a) Layers of the intact follicular wall. At the apex is a single layer of ovarian surface epithelium (OSE) containing granules with unknown contents (red). Underlying the OSE is the tunica albuginea (TA) and theca externa (TE), with numerous cells and extracellular connective tissue. Capillaries with red blood cells (red) and steroidogenic theca interna cells (TI, containing yellow lipid droplets) are adjacent to the granulosa cell (GC) basal lamina (BL). (b) Changes in the follicular wall following an LH stimulus. Notable changes include loss of many of the OSE, elongation of fibroblasts and thinning of the ECM in the TA and TI, and fewer granulosa cells. Capillaries contain clotted red blood cells (red), platelets (blue), and immune cells (pink). Granulosa cells now contain many lipid droplets (green), consistent with increased steroid hormone synthesis. In (c), which depicts the follicular apex immediately prior to ovulation, no OSE or granulosa cells remain at the apex. Remaining connective tissue is thin and disorganized. [Color micrographs courtesy of Dr. Lawrence Espey.]
Figure 4.
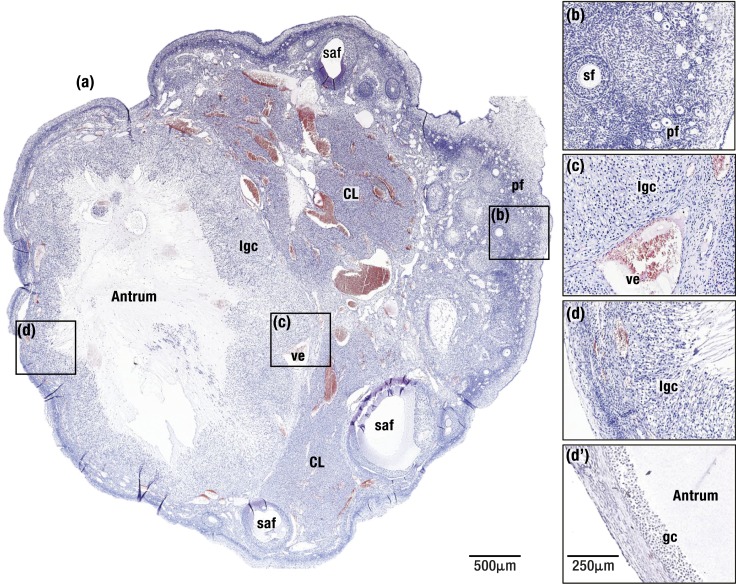
(a) Macaque ovary soon after ovulation. On the left, a recently ovulated follicle (∼8 h after follicle rupture) is seen with the antrum surrounded by luteinizing granulosa cells (lgc). Remainder of the ovary contains two regressing corpora lutea (CL) and several small antral follicles (saf). (b) An enlarged view of the ovarian cortex shows numerous primordial follicles (pf) and a secondary follicle (sf). (c and d) The luteinizing granulosa cell layer (lgc) thickens as cells undergo hypertrophy at both the follicle base (c) and apex (d). In (c), an enlarged vessel (ve) is adjacent to the luteinizing granulosa cell layer. (d′) An example of the apical region of a macaque preovulatory follicle shows the thin cortical stroma and few layers of compact granulosa cells (gc) present prior to the LH surge. Hematoxylin and eosin stain.
Equally dramatic changes also occur elsewhere in the follicle and are essential components of the ovulatory cascade. Decellularization contributes to the formation of the apical rupture site, but elsewhere in the follicle, granulosa and theca cells are retained and begin the process of luteinization. Granulosa cells cease proliferating, begin to enlarge (hypertrophy), and accumulate lipids into droplets that supply cholesterol for steroid hormone synthesis. Focal disruption of the granulosa cell basal lamina, coupled with prostaglandins and vascular growth factors produced by granulosa and theca cells, attract new capillary growth into the previously avascular granulosa cell layer. Vessels, along with theca and other cells of the stroma, invade the granulosa cell layer. Capillaries branch from stromal vessels and form an intersecting network that will eventually contact every granulosa lutein cell (11). Along with expanding vasculature, increased blood flow, and secretion of chemokines and cytokines from granulosa cells, theca cells and resident immune cells trigger a massive infiltration of leukocytes from the circulation, inducing an acute inflammatory response in the ovary. These changes, coupled with enhanced protease activity, likely weaken the follicular wall at the apex, leading to the rupture of the follicle.
The LH surge
Ovulatory events are initiated by a midcycle surge of LH. GnRH is released in pulses from the arcuate nucleus of the hypothalamus and is delivered to the anterior pituitary via a portal circulation (12). Growing antral follicles secrete estrogens, most notably estradiol. High circulating levels of estrogen increase the frequency of GnRH pulses and prime the gonadotropes of the anterior pituitary to release large amounts of LH in response to each pulse of GnRH, resulting in sustained elevated serum levels of LH for ∼24 hours in women and nonhuman primates (13, 14). In this way, the dominant follicle signals its readiness to ovulate through the ability to produce large amounts of estrogen. Ovulatory events in humans and nonhuman primates are initiated when serum LH levels increase above levels present in circulation during most of the reproductive cycle (13, 14). This threshold for LH to trigger ovulation varies between individuals, and peak LH levels can occur after ovulation is complete (13, 14).
LH is rapidly removed from serum. In contrast with LH, human chorionic gonadotropin (hCG), an LH mimic, is cleared slowly from circulation and binds to the LH/hCG receptor (LHCGR) with a higher affinity than LH (15). Accordingly, hCG is often used in place of LH in experimental animal models as well as a substitute for the LH surge to initiate ovulatory events in women undergoing fertility treatments. The response of follicle cells to LHCGR stimulation by LH/hCG is described in detail in “LH-Mediated Immediate Cellular Responses in Follicular Cells” below.
Regional responses in the ovulatory follicle
Ovulatory events are controlled in part by regional responsiveness to LH and paracrine signals produced within the follicle. Receptor distribution may explain how a single endocrine or paracrine mediator can have different actions in different regions of the follicle. Although theca and granulosa cells express LHCGR, highest levels are present in theca and the granulosa cells closest to the basal lamina (16, 17). Remaining granulosa cells, including the cumulus, have low LHCGR levels (16, 17). Indeed, LHCGRs may be expressed in focal areas around the ovulatory follicle, with very limited expression at the follicle apex (18). Progesterone receptors (PGRs) are expressed primarily in mural granulosa cells, whereas cumulus cells and theca cells show little to no expression of PGRs (19–22). Prostaglandin E2 (PGE2), a key paracrine mediator of the LH surge, acts through multiple PGE2 receptors (PTGERs). Certain PTGERs are highly expressed by cumulus cells, whereas other PTGERs are highly expressed by apical granulosa cells, and still other PTGERs are highly expressed by granulosa cells at the follicle base (23). Some paracrine factors, such as vascular endothelial growth factor (VEGF)A, are stored extracellularly, bound to ECM, and subsequently liberated by proteolytic cleavage for action at neighboring endothelial cells (24). Contraction of smooth muscle cells surrounding the follicle and stroma (25–27) may pull the follicle toward the follicle base and stretch the apex, contributing to apical thinning. The endothelins (EDN1 and EDN2) (28–30) and prostaglandins (25, 27, 31) may stimulate the contraction of smooth muscle–like cells surrounding the follicle and eventually facilitate follicle rupture at the apex. Regional remodeling of ECM also plays a key role in ovulatory events. Cumulus cells produce a novel hyaluronic acid-rich ECM as the cumulus oocyte complex (COC) detaches from the mural granulosa cells (32). Focal remodeling of matrix permits new capillaries to reach from the theca interna into the granulosa cell layer (33). Controlled proteolysis contributes to apical thinning as demonstrated by gelatinase activity localized to the follicle apex [Fig. 5 (23, 34)], whereas matrix remodeling elsewhere around the follicle is essential as the follicle transforms into the corpus luteum (34). Throughout the follicle, regional responses abound and are critical for successful ovulation (23, 34).
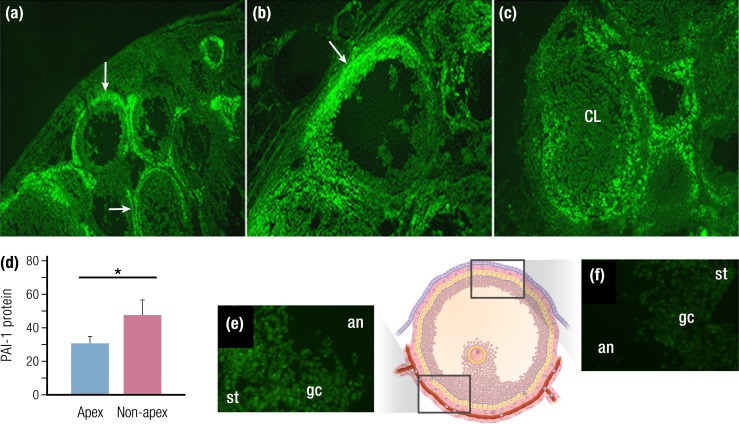
Figure 5.
Regional expression of proteases and protease inhibitors. Gelatinase activity (intense green) is localized at the rat follicle apex as ovulation approaches. (a–c) Gelatinase activity predominates in the theca (a) before hCG administration (arrows), (b) in the apical region of the follicle 12 h after hCG (arrow), and (c) throughout the forming corpus luteum (CL). (d) The PA inhibitor PAI-1 (now known as SERPINE1) protein is lower at the follicle apex than at the follicle base (nonapex) just before ovulation in monkey ovulatory follicles; (e) SERPINE1 protein correlates with higher expression of the PGE2 receptor PTGER1 (green) in granulosa cells (gc) at the follicle base when compared with (f) the apex. an, antrum; st, stroma. (a–c) Gelatinase activity visualized with green fluorescence; (e and f) Alexa Fluor 488. [Panels (a)–(c) adapted with permission from Curry TE Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod 2001;65(3):855–865. Illustration presentation copyright by the Endocrine Society. Panels (d)–(f) adapted with permission from Harris SM, Aschenbach LC, Skinner SM, et al. Prostaglandin E2 receptors are differentially expressed in subpopulations of granulosa cells from primate periovulatory follicles. Biol Reprod 2011;85(5):916–923. Illustration presentation copyright by the Endocrine Society.]
Consequences of the LH surge: timeline of ovulatory events
Ovulation is initiated by an endocrine trigger, the LH surge. Importantly, however, note that although there are commonalities in the LH-induced signaling processes among mammalian species, the timing of these events is different in women and nonhuman primates when compared with many other species. To depict these events specifically in primates, a broad timeline is outlined in Table 1. “First responders” are granulosa and theca cells, which express LHCGRs. Granulosa and theca cells produce cytokines, chemokines, prostaglandins, steroid hormones, and other autocrine and paracrine mediators associated with inflammation (see “LH-Mediated Immediate Cellular Responses in Follicular Cells” and “LH-Stimulated Production of Paracrine Mediators by Follicular Cells”). These mediators, in turn, activate resident immune cells and attract additional immune cells as an essential step in the response (see “Influx of Immune Cells and Their Function in Ovulation”). Proteases produced by both ovarian cells and immune cells weaken the basal lamina and facilitate invasion of vascular endothelial cells and additional immune cells (see “The Role of the Changing Vasculature in Ovulation” and “Proteolytic Changes Associated With Inflammation During Ovulation”). Traditional inflammatory mediators, in concert with follicle-specific stimuli, trigger cumulus expansion, resumption of oocyte meiosis, and detachment of the COC from the basal granulosa cells. The follicle apex experiences vascular constriction, decellularization, and extensive ECM remodeling (see “The Role of the Changing Vasculature in Ovulation” and “Proteolytic Changes Associated With Inflammation During Ovulation”). The remainder of the follicle undergoes angiogenesis, functional differentiation of granulosa and theca cells, tissue remodeling, and contraction (see “The Role of the Changing Vasculature in Ovulation” and “Proteolytic Changes Associated With Inflammation During Ovulation”). Ultimately, these functional and structural changes culminate in both rupture and luteinization of the remainder of the follicle essentially simultaneously.
Table 1.
Timeline of LH-stimulated Ovulatory Events in the Human and Nonhuman Primate Follicle
| Before LH Surge/hCG | 12 h After LH Surge/hCG | 24 h After LH Surge/hCG | 36 h After LH Surge/hCG | |
|---|---|---|---|---|
| Oocyte and cumulus (COC) | Germinal vesicle intact Tight cumulus | Germinal vesicle intact Tight cumulus | Meiosis 1 Cumulus expansion begins | Meiosis 2 Cumulus expansion complete; COC detached from mural granulosa cells |
| Granulosa | Highly proliferative Estrogen synthesis predominates | LH-stimulated activation of signaling cascades Increased PGR and EGFR Progesterone synthesis predominates Granulosa cell proliferation ends Secretion of chemokines, cytokines, EGFR ligands, and VEGFA Secretion of proteases and inhibitors | Granulosa cell hypertrophy Progesterone synthesis predominates Secretion of proteases and inhibitors Basal lamina weakens | Granulosa cell hypertrophy continues Progesterone synthesis predominates Secretion of PGF Increased PGE2 and PGE2 receptors Apical cell loss |
| Theca interna | Androstenedione synthesis predominates | Secretion of progesterone and androstenedione Secretion of proteases and inhibitors | Secretion of proteases and inhibitors | Focal invasion into granulosa cell layer Apical cell loss |
| Vasculature | Small vessels present in theca interna Larger vessels present in theca externa | Stromal vessel dilation | Theca vessels dilated New capillary formation in theca interna and granulosa cell layer | Capillary network formation in granulosa cell layer Increased blood flow at follicle base Reduced blood flow at follicle apex |
| Leukocytes | Resident immune cells present | Leukocytes infiltrate theca and stroma Leukocytes secrete chemokines and cytokines | Secretion of proteases Leukocytes infiltrate granulosa cells | Secretion of proteases |
| Theca externa | Smooth muscle contraction Apical proteolysis, thinning, and cell loss | |||
| Cortical stroma | Tissue integrity weakens | Apical proteolysis, thinning, and cell loss |
Abbreviations: EGFR, epidermal growth factor receptor; PGF, placental growth factor.
LH-Mediated Immediate Cellular Responses in Follicular Cells
Dramatic cellular, hormonal, and structural changes in the ovulatory follicle are initiated by the activation of intertwined, intracellular signaling pathways, all set in motion by the midcycle LH surge in granulosa and theca cells of preovulatory follicles. These signaling transduction cascades lead to the activation and induction of transcription factors that directly control the transcription of a diverse array of genes involved in various aspects of ovulation. Importantly, many of these genes encode proteins that are routinely associated with inflammation, indicating that these LH-activated specific signaling cascades and transcription factors commence preovulatory follicles to undergo complex, yet well-orchestrated processes displaying many similarities to inflammatory processes. In this section of the review, we summarize the previous literature on these LH-activated intracellular signaling cascades, with essential pathways illustrated in Fig. 6 and key LH-induced/activated transcription factors and their downstream genes listed in Table 2 (19, 20, 35–59).
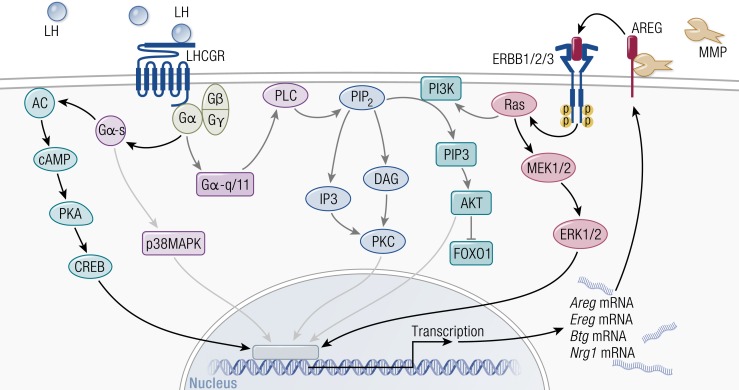
Figure 6.
Signaling pathways activated by the LH surge in ovulatory follicles. LH activates multiple signaling pathways, including protein kinase A (PKA), protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), and p38MAPK. LH also activates the epidermal growth factor receptor (EGFR) signaling pathway through the rapid induction of EGF-like factor expression and shedding of existing EGF-like factors from the membrane. Bold arrows emphasize the importance of PKA and EGFR signaling pathways in granulosa cells of ovulatory follicles. AC, adenylyl cyclase; AKT, Akt/protein kinase B; CREB, cAMP response element binding protein; DAG, 1,2-diacylglycerol; IP3, inositol 1,4,5-triphosphate; MEK, mitogen-activated protein kinase kinase; MMP, matrix metalloproteinase; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; PLC, phospholipase C.
Table 2.
Transcription Factors That Mediate LH Actions in Granulosa Cells of Ovulatory Follicles
| Transcription Factor | Transgenic Mouse Models | Pharmacological Inhibitors | Downstream Regulated Genes | Reproductive Phenotype |
|---|---|---|---|---|
| PGR | Pgr−/− (20, 35–39) | Adamts1 (20, 35), Ctsl (20), Edn2 (35, 38), Prkg2 (35), IL6 (35), Adam8 (36), Snap25 (37), Pparg (35), Hif1a (39), Epas1 [Hif2a (39)], Arnt [hif1b (39)] | Anovulation/infertility (40, 41) | |
| RU486 (19, 42–46), ZK98299 (47, 48), and CDB2914 (38) | Cxcr4 (42–44), Oxt (45), Pacap (48), Xlr5c-like (44), Runx1 (47), Fam110c (49), Edn2 (38), PTGS2 (19), PTGES (19), ABCC4 (19), AKR1C1 (19), AREG (19), EREG (19), FOS (46) | |||
| PPARγ | Ppargflox/flox × Pgrcre/+ (35) | Edn2 (35), IL6 (35), Prkg2 (35) | Reduced ovulation/subfertility (35) | |
| HIFs | Echinomycin (39) | Adamts1 (39), Edn2 (39), Vegfa (39), Cxcr4 (39) | Anovulation (39) | |
| C/EBPα and C/EBPβ | Cebpb−/− (50) | Ptgs2 (50), Cyp19a1 (50) | Infertility (50) | |
| Cebpa flox/flox × Cyp19a1cre (51) | Reduced ovulation/subfertility (51) | |||
| Cebpb flox/flox × Cyp19a1cre (51) | Reduced ovulation/subfertility (51) | |||
| Cebpa flox/flox × Cebpb flox/flox × Cyp19a1cre (51) | Has2 (51), Vcan (51), Runx2 (51), Abcb1b (51), Saa3 (51), Bhmt (51), Emcn (51), Apln (51), StAR (51), Cyp11a1 (51) | Anovulation/infertile (51) | ||
| The remaining genes are listed in Tables 1 and 2 of Ref. (95) | ||||
| RUNX1 and RUNX2 | Cbfbflox/flox × Cyp19cre (52) | End2 (52, 53), Ptgs1 (52, 53), Cxcr4 (52, 53), Sfrp4 (52, 53), Sgk1 (52, 53), Wnt4 (52, 53), Lhcgr (52, 53), Ptgfr (52, 53), Lipg (52, 53), Saa3 (52, 53), Prlr (52, 53), Ccrl2 (52, 53) The remaining genes are listed in figures 5 and 6 of Ref. (52) | Reduced ovulation/subfertility (52) | |
| Cbfbflox/flox × Esr2cre/+ (53) | ||||
| NR5A2 | Nr5a2flox/flox × Amhr2cre (54) | Ptgs2 (54), Cd44 (54), Tnfaip6 (54), C1qbp (54), Sult1e1 (54), Cyp19a1 (54), Nos3 (54), Scarb1 (54), Star (54), Cyp11a1 (54) | Anovulation/infertility (54) | |
| Nr5a2flox/flox × Cyp19a1cre (55) | Pgr (55), Adamts4 (55), Cyp19a1 (55), Scarb1 (55), Ldlr (55), Akr1c18 (55), Star(55) | Anovulation/infertility (55) | ||
| Nr5a2flox/flox × Pgrcre/+ (56) | StAR (56), Cyp11a1 (14), Hsd3b (56) | Infertility due to compromised endometrial decidualization (56) | ||
| NRIP1 | Nrip1−/− (57, 58) | Areg (58), Ptgs2 (58), Has2 (58), Vcan (58), Adamts1 (58), Tnfaip6 (58), Ptx3 (59), Ereg (59) | Infertile (57)/anovulation (58, 59) |
Abbreviations: C/EBP, CCAAT/enhancer-binding protein; HIF, hypoxia-inducible factor; PPARγ, peroxisome proliferator-activated receptor γ.
Immediate downstream mediators of LH action
Intracellular signaling pathways
During the past several decades, the complexity of the LH surge–activated signaling pathways has begun to be elucidated. Studies from several laboratories have demonstrated that LH activates a complex network of intracellular signaling cascades in preovulatory follicles, including protein kinase A (PKA), protein kinase C (PKC), phosphatidylinositol 3-kinase (PI3K), tyrosine kinase–mediated pathways, and their respective downstream MAPKs (see Fig. 6 and detailed specific references below).
It is well known that LH activates adenylate cyclase and increases intracellular cAMP as its primary intracellular signaling molecule (60–62). Increased cAMP leads to the activation of the cAMP-dependent PKA that, in turn, activates the cAMP response element binding protein (CREB) (63, 64). This intracellular signaling pathway (PKA) is widely regarded as the primary pathway mediating LH/hCG action in the preovulatory follicle.
Several studies have also implicated the PKC pathway in LH-induced signaling events. Davis et al. (65) have shown that LH increased inositol triphosphate levels in rat granulosa cells. A recent study by Breen et al. (66) showed that hCG-induced increases in inositol phosphate levels were reduced in granulosa cells of Gα-q/11 mutant mice (Gαqflox/fox × Gα11−/− × Cyp19cre). Importantly, this mutant mouse line showed a reduced ovulation rate and reduced levels of mRNA for PGR and several PGR downstream target genes [e.g., a disintegrin and metalloproteinase [ADAM] with transpondin motifs [Adamts]1, Ctsl1, Edn2, and Prkg2] in granulosa cells of preovulatory follicles (66). Taken together, these studies suggest that the LH surge activates the Gα-q/phospholipase C (PLC)–PKC pathway. Similarly, in differentiated hen granulosa cells LH increased PKC activity within 15 minutes of stimulation (67). Furthermore, the PKC inhibitor GF109203X attenuated LH and 8-bromo-cAMP–induced phosphorylation of ERK and reduced the expression of epidermal growth factor (EGF) family ligands (67), indicating the involvement of the PKC pathway in the EGF network and ERK phosphorylation in differentiated hen granulosa cells. In contrast to these studies, Salvador et al. (64) reported that, in rat granulosa cells, PKCs were already activated even before hCG injection and hCG did not further increase activation in granulosa cells of the preovulatory ovary.
There is evidence that LH activates the PI3K pathway. In the mouse ovary, hCG induced rapid and transient increases in phosphorylation of AKT (68) and FOXO1 (69), both well-known signaling effectors downstream of the PI3K pathway. Fan et al. (68) showed that the phosphorylation of AKT was enhanced in granulosa cells expressing a constitutively active form of KRAS (KrasG12D) (67), implicating RAS as an upstream signaling messenger of the PI3K pathway. In agreement with this concept, hCG transiently increased the level of GTP-bound RAS in the mouse ovary (68).
Previous studies showed that hCG induced transient increases in p38MAPK phosphorylation in preovulatory rat ovaries and that the hCG-induced increase in p38MAPK phosphorylation is not inhibited by the PKA inhibitor H89, indicating that the p38MAPK activation is independent of the PKA pathway (64, 70). In particular, this kinase has been shown to be critical for COC expansion and oocyte maturation, as the inhibition of p38MAPK activity by pharmacological inhibitors and genetic deletion of the p38MAPKα isoform (Mapk14flox/flox × Cyp19cre) resulted in impaired meiotic resumption and cumulus expansion in pig and mouse COCs (71, 72).
In addition to these pathways mentioned above, a series of studies using mouse ovaries showed that the LH-activated cAMP–PKA signaling pathway leads to very rapid activation of the EGF receptor (EGFR)–tyrosine kinase pathway. This concept is based on several observations: (i) the LH surge or hCG stimulates rapid and dramatic increases in EGF-like factors such as AREG, EREG, BTG, and NRG1; (ii) hCG induces phosphorylation of EGFR family members, including EGFR (ERBB1), ERBB2, and ERBB3 in the preovulatory follicle; (iii) hCG-induced EGFR phosphorylation is inhibited by the PKA inhibitor H89; and (iv) granulosa cells of PDE4 null mice (which have reduced levels of LH-induced cAMP) also lack expression of Areg, Ereg, and Btc (63, 73, 74). Similarly, LH surge or hCG stimulates a rapid increase in follicular expression of EGF-like factors AREG and EREG in all mammals studied to date, including humans and nonhuman primates (19, 73, 75–83). Furthermore, Panigone et al. (63) demonstrated that inhibitors for metalloproteases (GM6001 and TAPI-1) abolished LH-induced EGFR phosphorylation within 30 minutes in cultured preovulatory follicle, indicating that the activation of EGFR is also dependent on the action of metalloproteases in shedding the existing and newly synthesized EGF-like factors from the plasma membrane.
The phosphorylation and activation of EGFR by its ligands (e.g., AREG, EREG, BTG, NRG1) stimulates its intrinsic tyrosine kinase activity, which transduces the signal to downstream kinases, notably the RAS–mitogen-activated protein kinase kinase–MAPK1/3 (ERK1/2) pathway in granulosa and cumulus cells (63, 68, 84). Recent studies using mutant mouse models have demonstrated the obligatory role of the activation of EGFR and their key downstream kinases, ERK1 and ERK2, in the ovulatory process, including COC expansion, follicular rupture, and luteinization (84, 85). Double knockout mice for Areg and the hypomorphic allele of Egfr (Areg−/− × Egfrwa2/wa2) showed severely compromised ovulation and COC expansion, resulting in drastic reduction in fecundity (85). Similarly, conditional deletion of ERK1/2 genes in granulosa cells (Erk1−/− × Erk2flox/flox × Cyp19Cre) resulted in sterility owing to the complete blockade of the LH surge–induced events, including oocyte maturation, cumulus cell expansion, ovulation, and luteinization (84).
Studies using these mutant mouse models revealed a number of downstream targets of the EGFR and ERK1/2 signaling pathways, including Ptgs2, Pgr, CCAAT/enhancer-binding protein (CEBP)a, CEBPb, Runx1, and Runx2 (84, 85). As discussed below in detail, transcription factors PGR, CEBPA and CEBPB, and RUNX1 and RUNX2 play essential roles in ovulation by regulating the expression of genes, many of which are associated with inflammation. PTGS2 is considered a rate-limiting enzyme in the production of prostaglandins in ovulatory follicles. Taken together, these studies indicated that the activation of EGFR signaling pathways is an integral part of the LH surge–induced signaling cascades, resulting in the induction/activation of key transcription factors and ovulatory inflammatory mediators.
Key transcriptional regulators of LH action
Specific transcription factors activated or induced by the LH surge in ovulatory follicles directly regulate the transcription of a variety of genes that exert specific actions involved in tissue remodeling, angiogenesis, and inflammatory responses. Among those critical transcriptional factors included and reviewed herein are PGRs, PGR-downstream transcription factors peroxisome proliferator–activated receptor γ (PPARG) and hypoxia-inducible factors (HIFs), CEBPA and CEBPB, core-binding factors (RUNX1 and RUNX2), the liver receptor homolog-1 (NR5A2), and nuclear receptor–interacting protein 1 (NRIP1, formerly known as RIP140) (Table 2).
PGR and its downstream transcription factors, PPARG and HIFs
Progesterone is a key regulator of reproductive events, including ovulation and luteinization (20, 86–89). Additionally, progesterone has been shown to be involved in the inflammatory reaction in various tissues and can act as either a proinflammatory or anti-inflammatory modulator depending on the context in which inflammation occurs (90–93). In the ovary, the LH surge or hCG induces the transient increases in PGR expression in granulosa cells of preovulatory follicles in all species examined to date, including humans and monkeys (19, 21, 22, 45, 94–97). Evidence that PGR is an essential mediator of ovulation was provided by global Pgr knockout mice. Follicles of Pgr knockout mice develop normally but fail to ovulate, and the oocytes remained trapped within transforming corpus lutea even when exogenous gonadotropins were administered (20, 40). Early studies by Robker et al. (20) using this mutant mouse identified two genes that are regulated by PGR: Adamts1 and Ctsl. These genes are highly upregulated by hCG in granulosa cells of wild-type mouse preovulatory follicles, but their expression was reduced in Pgr knockout mice. Both ADAMST1 and CTSL are proteases that can act on ECM proteins to aid in the breakdown of the follicular wall at the time of ovulation. Adamts1 knockout mice were subfertile owing to the compromised follicular development and ovulation (98, 99), suggesting that this PGR-downstream protease acts as a key proteolytic enzyme in the degradation of the follicular wall. ADAMTS1 has been also found to cleave a versican, a hyaluronan-binding ECM proteoglycan, which accumulates in expanding COCs, indicating the involvement of this protease in COC expansion (100, 101). A subsequent study from the same laboratory found another proteolytic enzyme, Adam8, as a PGR-regulated gene in granulosa cells of preovulatory follicles in mice (36). More recent gene-profiling studies using Pgr knockout mice have identified an array of PGR-downstream genes in granulosa cells of ovulatory follicles. Among those included are Snap25, Prkg2, Edn1, Edn2, IL6, Pparg, Tgfb1, CD34, Cxcr4, and the HIFs (Hif1a, Hif2a, and Hif1b) (35, 37–39, 90, 102). Additionally, several laboratories have used pharmacological inhibitors of PGR (e.g., RU486, CDB2914, and ZK98299) to identify genes that are regulated by PGR using human, rat, bovine, or equine granulosa cells. These genes include Pacap, Xlr5c-like, Runx1, Fam110c, CXCR4, and OXT as well as prostaglandin synthases and transporters (PTGS2, PTGES, ABCC4, AKC1) and EGF-like factors (AREG, EREG) (19, 42–45, 47–49, 78). Particularly, CXCR4 was identified to be a downstream gene of PGR in all species examined, including the mouse, rat, cow, horse, and human (39, 42–44). The expression of EGF-like factors (AREG and EREG) were also regulated by PGR in mice and humans (19, 78). These findings indicate that there are common PGR-regulated genes among different species. Considering the species-specific difference in gene expression profile, however, it is also expected that there are species-specific differences in PGR-regulated genes.
As mentioned above, PGR-downstream genes include transcription factors such as PPARG, HIFs, and RUNX1. PPARG has been shown to regulate the transcription of a variety of target genes by binding to specific DNA elements [reviewed in Ref. (103)]. In the mouse ovary, Pparg expression is localized to granulosa cells of growing follicles and is transiently increased by hCG stimulation in granulosa cells of preovulatory follicles (35, 104). Granulosa cell–specific Pparg knockout mice (Ppargflox/flox × PgrCre/+) are subfertile; these animals also showed a significant reduction of Edn2, IL6, and Prkg2 expression, but not Adamts1, indicating that PPARG regulates the expression of a subset of PGR-downstream genes (35).
HIFs are also transcription factors that regulate gene transcription in response primarily to hypoxic conditions and are essential to both angiogenic and inflammation (103). It has been speculated that the preovulatory follicle is hypoxic, owing to the rapid growth of the follicle with minimal vascularization of the granulosa cell compartment during the ovulatory period. Kim et al. (39) have demonstrated that the expression of Hif1a, Hif2a, and Hif1b was upregulated in preovulatory follicles after hCG administration in mice. In that study, blocking the HIF transcriptional activity using echinomycin prevented the rupture of ovulatory follicles and reduced the expression of Adamts1, Edn2, Cxcr4, and Vegfa, demonstrating the critical role of HIFs in regulating the expression of these genes in ovulatory follicles. Importantly, transgenic mice lacking the Edn2 and Adamts1 genes showed defective ovulatory phenotypes (28, 98, 99, 105), similar to PGR knockout mice. Taken together, these studies indicate that PGR and its downstream transcription factors HIFs and PPARG play critical roles in ovulation by regulating the expression of a number of genes that are involved in various aspects of inflammation during the ovulatory process.
CEBPA and CEBPB
Cebpb expression was reported to be induced by hCG stimulation in granulosa cells of preovulatory follicles in both the rat and mouse (84, 106, 107). Further investigation has demonstrated that Cebpa expression also was increased by hCG stimulation in the mouse ovary (51). Originally, Sterneck et al. (50) showed that global Cebpb knockout mice were infertile and failed to ovulate. The use of conditional knockout mouse lines generated using Cyp19Cre showed that the deletion of either Cebpa or Cebpb in granulosa cells resulted in reduced ovulation and fecundity (51). Moreover, the deletion of both Cebpa and Cebpb in granulosa cells (Cebpaflox/flox × Cebpbflox/flox × Cyp19cre;Cebpa/bgc−/−) leads to infertility due to complete blockade of the LH surge–induced ovulatory events, including COC expansion, the rupture of follicles, and luteinization (51). Gene profiling analysis of Cebpa/bgc−/− mice further revealed an array of genes whose expression was affected, including genes associated with progesterone synthesis (e.g., Cyp11a1 and Star), angiogenesis, and endothelial cell function (e.g., Emcn, Apl, Aplr, Nrp1, and Plxnd1) (51). The levels of mRNA for Runx2 were also decreased in Cebpa/bgc−/− mouse ovary (51). Taken together, these studies indicated that CEBPA and CEBPB play a critical role in ovulation by controlling the expression of genes involved in progesterone production and angiogenesis, both of which are associated with ovulatory inflammatory events.
RUNX1 and RUNX2
Core-binding factor (CBF) is a heterodimeric transcription factor complex composed of α and β subunits [reviewed in Ref. (108)]. The α subunit is encoded by one of three Runx genes (RUNX1, RUNX2, and RUNX3), and the β subunit is encoded by a single gene, CBFB. In the ovary, RUNX1 and RUNX2 expression is rapidly induced in granulosa cells of preovulatory follicles after the LH surge or hCG stimulation in rodents and humans (47, 109, 110). Studies using rat granulosa cell cultures identified several genes regulated by RUNX1 or RUNX2, including genes involved in the ovulatory process (e.g., Ptgs2, Hapln1, Rgc32, Btg, Abcb1, and Spp1) (47, 109, 110). Interestingly, RUNX2 is also involved in the downregulation of Runx1, Ptgs2, and Tnfaip6 after ovulation (111). Granulosa cell–specific CBFB knockout mice (Cbfβflox/flox × Cyp19cre and Cbfbflox/flox × Esr2cre/+) exhibited subfertility with compromised ovulation and luteinization (52, 53). Further analysis of granulosa cells from these mutant mouse lines revealed altered expression of genes associated with the inflammatory response (e.g., Edn2 and Ptgs1) as well as genes associated with the corpus luteum (Prlr, Sfrp4, Wnt4, and Sgk1). These findings indicate that RUNX1 and RUNX2 play an important role in the ovulatory process by regulating the expression of many ovulatory genes associated with inflammatory responses, including Ptgs1, Ptgs2, Abcb1, Tnfip6, and Edn2.
NR5A2
Unlike other LH-induced transcription factors mentioned above, NR5A2 (also known as Lhr1) is abundantly expressed in granulosa cells of growing antral follicles before the LH surge in the rodent ovary (112, 113). Yet, granulosa cell–specific deletion of Nr5a2 (Nr5a2flox/flox × Amhr2Cre and Nr5a2flox/flox × Cyp19Cre) resulted in impaired ovulation and luteinization without overt defects in follicular growth (54, 55). These studies showed that several genes affected by deletion of Nr5a2 in granulosa cells are related to steroidogenesis, including Cyp19a1, Star, Scarb1, Ldlr, Cyp11a1, Sultle1, and Akr1c18. In agreement, these mutant animals showed altered levels of estradiol and progesterone and failure to shift from estradiol to progesterone synthesis during the ovulatory period. These mutant animals also showed reduced levels of mRNA for Pgr and Ptgs2, indicating that NR5A2 is involved in regulation of two key intrafollicular paracrine pathways, progesterone and prostaglandins (54, 55), both of which are known to be involved in the inflammatory response in the ovary.
NRIP1
NRIP1 does not bind the DNA directly but instead interacts with other transcription factors and regulates the transcription activity of a variety of genes (114). In the ovary, Nrip1 is expressed in granulosa cells of follicles (57, 58, 115). Nrip1 expression is highest in preovulatory follicles before the LH surge, similar to Nr5a2 (57, 58, 115). However, Nrip1 null mice were infertile and showed defective ovulation and COC expansion without affecting follicular development (57, 58). Further analysis of this mutant mouse showed that the expression of many genes involved in ovulation and COC expansion were decreased such as EGF-like factors (Areg, Ereg, Btc), Adamts1, Ptgs2, Has2, Tnfaip6, Vcan, and Ptx3. Therefore, it is conceivable that NRIP1 interacts with LH-induced or activated transcription factors to regulate the expression of specific ovulatory genes. Indeed, Nautiyal et al. (59) has demonstrated that NRIP1 stimulates mouse Areg promoter reporter activity, and this stimulatory effect of NRIP1 requires CREB or JUN, suggesting that NRIP1 may function as a transcriptional coactivator for CREB and JUN to stimulate transcription of the Areg gene in mouse granulosa cells. Taken together, these findings indicate that NRIP1 is an important transcription regulator controlling the expression of these specific ovulatory genes associated directly or indirectly with inflammation during the ovulatory process.
In summary, the LH surge activates complex intercellular signaling pathways, which transduce extracellular signals to induce or activate specific transcription factors in granulosa cells of ovulatory follicles. Little to nothing is known about the LH surge–activated/induced signaling pathways and transcription factors in humans or nonhuman primates. However, accumulating evidence from studies using mutant mouse models demonstrates that transcription factors mentioned in this review play an essential role in the ovulatory process by directly controlling the transcription of diverse genes encoding transcription regulators, growth factors, and signaling molecules as well as modulators of vascular activity, chemokines, cytokines, and proteases. Thus, these findings indicate that the LH-initiated cellular changes (e.g., activation of signaling pathways and subsequent induction/activation of transcription factors) lead to the production of diverse molecules, many of which are involved in the inflammatory response, facilitating ovulation. The following sections detail the actions of these proteins in inflammatory responses during the ovulatory process. Importantly, note that most of our understanding regarding LH-activated intracellular signaling pathways and transcription factors described herein came from studies using rodent models. Therefore, it is important to determine whether the LH surge utilizes the same or unique signaling pathways and transcription factors in preovulatory follicles of human or nonhuman primates compared with those in mice and to identify their specific roles in the inflammatory response during ovulation in humans and large animals.
LH-Stimulated Production of Paracrine Mediators by Follicular Cells
An immediate consequence of activation of these numerous intracellular signaling cascades is the production of diverse autocrine and paracrine mediators of ovulation. Progesterone and prostaglandins are well established key intrafollicular regulators of ovulation and mediators of inflammatory responses (116). However, additional steroids and eicosanoids are also produced in response to the LH surge. Protein hormones established as regulators of angiogenesis and vascular function are also produced by the LH surge. Increased blood flow is a feature of inflammation (3). This process also facilitates delivery of immune cells to the ovulatory follicle and is an essential component of the ovulatory cascade. These LH-stimulated mediators are discussed with a focus on women and nonhuman primates.
Steroid hormone synthesis in the ovulatory follicle
Production of steroid hormones within the follicle is critical for successful ovulation (117, 118) (Fig. 7). In response to the LH surge, granulosa cells rapidly accumulate cholesterol-containing lipid droplets, providing an accessible source of steroid hormone precursors. LH action via LHCGRs initiates or enhances granulosa cell expression of CYP11A1, STAR, and HSD3B1, which are involved in the early steps of steroidogenesis (119). Granulosa cells express only low levels of CYP17A1, so conversion of progesterone to androgens and estrogens is severely limited. This pattern of expression and activity of enzymes results in synthesis of progesterone as the major steroid hormone product after the LH surge in primates (120). Ovulatory follicles of humans produce both progesterone and 17α-hydroxy-progesterone, which are both present at high concentrations in serum and in follicular fluid (121). Both progesterone and 17α-hydroxy-progesterone have similar affinity for PGRs (122, 123), but progesterone is widely discussed for the sake of simplicity.
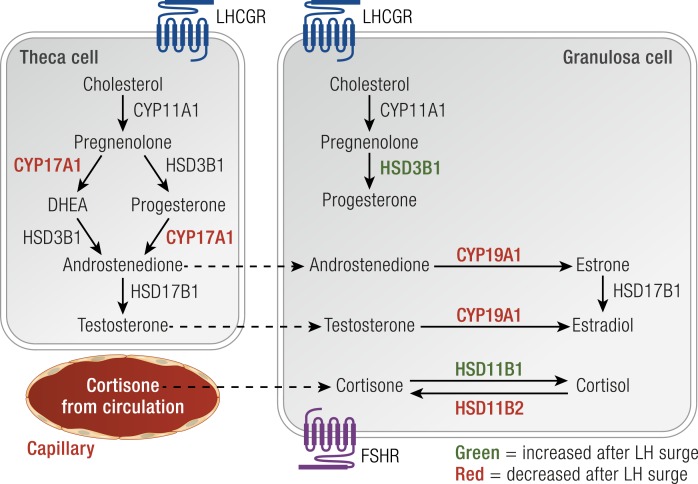
Figure 7.
Granulosa and theca cells cooperate to produce steroid hormones. Before the LH surge, (i) theca cells produce predominantly androgens in response to LH, (ii) androgens diffuse to granulosa cells, and (iii) granulosa cells convert androgens to estrogens in response to FSH. After the LH surge, (i) decreased CYP17A1 expression increases progesterone synthesis and decreases androgen synthesis in theca cells, and (ii) increased HSD3B1 increases progesterone synthesis and declining CYP19A1 decreases estrogen synthesis by granulosa cells. The LH surge also increases HSD11B1 and decreases HSD11B2 in granulosa cells to increases synthesis of cortisol from circulating cortisone. Enzymes shown in green increase after the LH surge. Enzymes shown in red decrease after the LH surge.
Granulosa cells are dependent on androgens provided by theca cells for synthesis of estrogens, but reduced expression of CYP19A1 results in reduced estrogen synthesis by the end of the ovulatory interval. As granulosa cells luteinize, low levels of CYP17A1 (not shown in Fig. 7) act preferentially to convert Δ5 steroids to androgens, with conversion of pregnenolone to dehydroepiandrosterone to androstenedione predominating. The strong preference of granulosa cell CYP17A1 for pregnenolone as a substrate effectively prevents binding of progesterone to the enzyme’s catalytic site, so progesterone is not converted to other sex steroids in granulosa cells of primates, including macaques and women (124). These changes in enzyme expression and activity effectively shift the balance of steroid hormone synthesis from primarily estrogens before the LH surge to primarily progesterone after the LH surge.
Circulating levels of progesterone are very low prior to the LH surge in monkeys and women (14, 80, 121, 125, 126). Within minutes of the LH surge or hCG administration, serum progesterone levels increase. Similarly, follicular fluid levels of progesterone rapidly rise from nanomolar to micromolar levels. In contrast, follicular fluid levels of estradiol and other estrogens are high before the LH surge, peak soon after the LH surge, and then fall as progesterone becomes the major steroid hormone product of the luteinizing follicle. Similarly, follicular fluid concentrations of androstenedione and other androgens peak after the LH surge, then fall to lower levels at the time of ovulation. Circulating levels of estrogens and androgens experience less dramatic shifts in response to the LH surge or hCG, typically of an order of magnitude or less (14, 80, 121, 125, 126).
Synthesis of glucocorticoids and mineralocorticoids within the ovulatory follicle has received considerably less attention. Liquid chromatography/mass spectroscopy analysis of follicular fluid revealed that human and monkey follicles contain high levels of these steroids (121, 127–129). Although aldosterone levels are similar before and after the LH surge, the LH surge leads to follicular fluid accumulation of several glucocorticoids, including cortisol, before follicle rupture. CYP11B1 and CYP11B2 are not expressed by cells of the follicle, indicating that local synthesis of cortisol and aldosterone from cholesterol or pregnenolone does not occur. However, the LH surge increases granulosa cell expression of HSD11B1, which can convert cortisone of adrenal origin to the more active cortisol within the follicle (80, 120, 128, 130). This occurs concomitantly with a decrease in expression of HSD11B2, which converts cortisol to cortisone, which may augment the increase in cortisol observed after the LH surge. The role that cortisol may play in ovulation is not yet known. However, as an anti-inflammatory hormone, cortisol may play a balancing role in response to inflammatory mediators during ovulation, thereby protecting the ovary from damage while allowing quick tissue repair after ovulation (131). This question of cortisol’s actions in the ovulatory process is a potential area for future studies.
Steroid hormone receptors in the ovulatory follicle
Progesterone receptors
The classical nuclear PGR is present in very low to nondetectable levels in granulosa cells of dominant follicles before the LH surge, but granulosa cell PGR expression increases rapidly after the LH surge in women and monkeys (19, 95–97, 132, 133). Additionally, theca cells express modest levels of PGR (132, 133). Elevated intrafollicular progesterone is widely thought to be essential for ovulation in monkeys and women. In macaques, blockade of steroid hormone synthesis during ovarian stimulation prevented ovulation, and replacement with a nonmetabolizable progestin restored ovulation (87). Progestin-containing contraceptives can also prevent ovulation in women. PGR agonists such as levonorgestrel have their primary antiovulatory action at the hypothalamus/anterior pituitary to reduce or prevent the LH surge, whereas PGR antagonists such as ulipristal acetate can blunt the LH surge and also act directly at the ovary to block ovulation (134).
Two forms of PGR, denoted PRA and PRB, are generated from the same gene via usage of subtype-specific promoters (135, 136). In granulosa cells, expression of PRA predominates over PRB both before and after the LH surge (137–139). The importance of PGR in the ovulatory process has been demonstrated by a complete loss of oocyte release in mice lacking PGR discussed above (41) as well as a blockage of ovulation following PGR knockdown by small interfering RNA injected into monkey follicles (140). Further exploration illustrated that ovulatory success was significantly reduced in PRA knockout mice, whereas PRB knockout mice had near-normal rates of ovulation (141), supporting the concept that PRA plays a more fundamental role than does PRB in ovulation.
Membrane progesterone receptors also mediate progesterone action within the ovulatory follicle. PGRMC1 and PGRMC2 are progesterone-binding proteins that cooperate with additional protein partners to generate a cellular response to progesterone [reviewed in Ref. (142)]. The best studied progesterone membrane component in the ovary is PGRMC1. PGRMC1 is expressed by theca and granulosa cells of antral and ovulatory follicles (122, 143). Expression of PGRMC1 by human granulosa cells decreases after the LH surge (80, 122). However, additional studies show relocation of PGRMC1 from intracellular membranes to the plasma membrane in response to surge levels of LH, correlating with increased progestin signaling via PGRMC1 [reviewed in Ref. (142)]. However, deletion of PGRMC1 or PGRMC2 expression in mouse ovarian cells did not alter ovulation rates, suggesting that these receptors may not mediate essential ovulatory actions of progesterone (144, 145).
Androgen receptors
Androgen receptors (ARs) are present in both theca and granulosa cells or primate follicles before and after the LH surge (95, 132, 133, 146–148). Mice with disrupted expression of the AR are subfertile, with a negative impact of AR deletion on follicle health prior to the LH surge as well as actions during the ovulatory interval (149). Blockade of androgen action during the ovulatory period in vivo provides additional support for androgen involvement in ovulation (150, 151). Androgen treatment in mice showed increased expression of key ovulatory proteins, including PTGS2 and AREG (152). In nonhuman primates, androgens did not promote follicle rupture but rather reduced oocyte atresia and promoted oocyte health (87, 153). Optimal androgen concentrations appear to be critical for successful ovulation, with both high and low androgen levels causing ovulatory dysfunction (154).
Androstenedione is the predominant androgen produced in the ovulatory follicle (155). Androstenedione has low affinity for the androgen receptor (156) and most often serves as a substrate for local production of estrogen or more potent androgens, such as testosterone (Fig. 7). Although testosterone binds AR with greater affinity than does androstenedione (156), higher concentrations of androstenedione suggest that both androgens may be ligands for AR in the ovulatory follicle. Elevated androgen levels are associated with polycystic ovarian syndrome (PCOS) (157). Antiandrogens can increase ovulation rates in women with PCOS (158, 159), but this improvement is typically associated with altered endocrine axis function or increased follicle development (160, 161).
Estrogen receptors
The ovary is a target of estrogen action. Two classical estrogen receptors, ESR1 and ESR2 (also known as ERα and ERβ), are expressed in a distinctive manner in the ovary. In the rodent, ESR2 is the predominant estrogen receptor in the ovary, and it is primarily localized to granulosa cells, whereas ESR1 is localized to theca cells and OSE (150, 151, 162). In human and nonhuman primate ovaries, the localization of estrogen receptors is not different from those in rodents, as ESR2 and ESR1 are predominantly localized to granulosa cells and theca cells and OSE (163–165). ESR2 protein is present in granulosa cells throughout the ovulatory interval, but ESR2 mRNA declines rapidly after the LH surge and may contribute to a decline in ESR2 protein after the LH surge and before ovulation (80, 95, 166, 167). There are reports that mRNA for ESR1 is detected in the granulosa cells and ESR2 mRNA in the theca/interstitial cells, but the expression levels of these transcripts are likely minimal, as such physiologically relevant levels of proteins are unlikely to be present (95, 138).
This site- and time-specific ovarian distributions of ESR1 and ESR2 indicate that estradiol may use these two receptor subtypes to regulate different cellular functions in different cells, simultaneously. This idea is supported by the divergent ovarian phenotypes seen in the transgenic mice that are lacking either ESR1 or ESR2. Both mutants are infertile, reflecting the importance of estrogen receptors throughout the reproductive tract. Interestingly, upon gonadotropin stimulation, ESR1-deficient mice ovulated but ESR2-deficient mice did not (168–170). This comparison provides evidence that ESR1 and ESR2 play different roles in ovarian function in mice, with ESR2 having a role in the ovulatory process.
Despite findings involving ESR2-deficient mice, a role for estrogen in human ovulation remains controversial. Gonadotropin-driven ovarian follicular development, oocyte maturation, and fertilization were achieved in women with severely reduced estrogen synthesis due to specific enzyme deficits (171, 172). Severe reduction of nonhuman primate ovarian steroidogenesis by administration of an HSD3B inhibitor did not disrupt follicle growth but did result in ovulation failure and production of oocytes with very poor fertilization rates (87). Progestin replacement restored ovulation (87). However, replacement of androgen or progesterone did not restore normal fertilization rates (87), indicating that estrogen may be important for oocyte health and/or the process of fertilization. Although data from humans and nonhuman primates argue against a critical role for follicular estrogen in the process of primate ovulation, low levels of estrogen may be present in the follicle at concentrations sufficient to activate ESRs and facilitate follicular development and ovulation.
Other steroid receptors and binding proteins
In addition to progesterone and estrogen receptors, numerous other steroid hormone receptors are present in the ovary, but their role in the ovulatory process is unclear. Expression of the classical glucocorticoid receptor (NR3C1) and the classical mineralocorticoid receptor (NR3C2) have been detected in granulosa cells of primate ovulatory follicles (125, 128). The high follicular concentrations of cortisol after the LH surge plus the presence of the cortisol synthesis enzyme HSD11B1 support the concept that cortisol acting through NR3C1 and NR3C2 in granulosa cells may play a role in the ovulatory process. Additionally, the availability of steroid hormones may be influenced by steroid hormone–binding proteins such as albumin, SHBG, and CBG, which are present in human follicular fluid at concentrations similar to levels measured in serum (173, 174). There is little evidence to suggest that steroid-binding proteins are synthesized by follicular cells, so binding proteins are most likely present in follicular fluid as an exudate of serum (130).
In summary, steroid hormone action is a critical component of the ovulatory process. Steroids are necessary for proper regulation of ovulatory blood flow and play a role in regulating proteolysis. Steroid hormones, especially progesterone and cortisol, are also classical immunomodulators (175, 176). Although their action in this regard is typically immunosuppressive, concentrations of steroids in the follicle are much higher than elsewhere in the body. Progesterone has been reported to decrease numbers of selected populations of immune cells in the primate corpus luteum (177). The ability of ovarian steroid hormones to directly regulate immune cell function in the ovulatory follicle remains to be established.
Eicosanoids in the ovulatory follicle
Eicosanoids are signaling molecules derived from membrane phospholipids and are synthesized via a series of enzymatic conversions. In the ovulatory follicle, bioactive eicosanoids are derived primarily from arachidonic acid. The LH surge enhances granulosa cell expression of many key enzymes, leading to enhanced follicular fluid levels of several eicosanoids. Prostaglandins of the E and F series have received the most attention as mediators of ovulation (178, 179).
“Vascular growth regulators are critical for aspects of inflammation....”
In particular, PGE2 has been implicated in mediating LH-induced ovulatory response in the ovary as detailed below in this section. Additional eicosanoids in the follicle include leukotrienes and thromboxanes, which have important roles in inflammatory responses (see below in this section).
Prostaglandins
The essential role of prostaglandins in the process of ovulation is well established. Conversion of arachidonic acid to prostaglandin H2 (PGH2) is the first committed step in prostaglandin synthesis [Fig. 8 (180)]. Two enzymes, PTGS1 and PTGS2, catalyze the peroxidase and cyclooxygenase activities involved in this conversion (181). Granulosa and theca cells express primarily the PTGS2 enzyme (formerly known as cyclooxygenase-2 or COX-2), but PTGS1 (formerly known as COX-1) is also present (182, 183). PTGS2 is widely thought to catalyze the rate-limiting step in synthesis of bioactive prostanoids in the ovarian follicle, and the LH surge rapidly increases expression of PTGS2 in all species examined to date (184). The LH surge stimulates a rapid increase in follicular PGE2 in rodent species (182). However, elevated follicular PGE2 levels occur much later in follicles of primates and larger domestic animal species for reasons explained below.
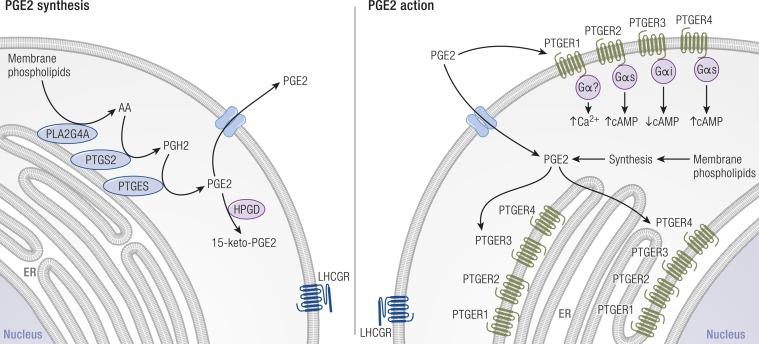
Figure 8.
PGE2 synthesis, receptors, transport, and metabolism in granulosa cells. Left: PGE2 synthesis enzymes (blue) are associated with membranes of the nuclear envelope (not shown) and endoplasmic reticulum (ER). PLA2G4A cleaves arachidonic acid (AA) from membrane phospholipids. PTGS2 converts AA into PGH2. PTGES converts PGH2 into bioactive PGE2. PGE2 is converted to an inactive metabolite (15-keto-PGE2) by HPGD (purple). Right: PGE2 acts via four PGE2 receptors (PTGER1, PTGER2, PTGER3, and PTGER4, green). Each PTGER couples to a subset of G proteins (purple); most frequently used and major intracellular signals are shown for plasma membrane PTGERs. PTGERs can also be located in the membranes of the ER and nucleus; G proteins also couple with PTGERs in these locations (not shown). On both panels, multiple methods of PGE2 transport across the plasma membrane have been proposed (blue) and are discussed in the text. [Adapted with permission from Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update 2015;21(5):652–670. Illustration presentation copyright of the Endocrine Society.]
Follicular levels of both PGE2 and prostaglandin F2α (PGF2α) increase in response to the LH surge in primates (185, 186) and domestic animal species (187, 188). However, studies examining prostaglandin receptors (discussed immediately below) have confirmed that PGE2 is the key ovulatory prostaglandin. The LH surge or hCG increases granulosa cell expression of at least one form of every enzyme involved in the synthesis of PGE2, including the phospholipase A2 PLA2G4A, the peroxidase/cyclooxygenase PTGS2, and the prostaglandin E synthase PTGES (19, 180). Enhanced granulosa cell levels of mRNA, protein, and activity for each of these enzymes, as well as close proximity of enzymes in the intracellular membranes of the nuclear envelope and endoplasmic reticulum, contribute to efficient and rapid conversion of arachidonic acid to PGH2 and then PGE2 [reviewed in Ref. (180)] (Fig. 8). Prostaglandin metabolism is also regulated by the LH surge. The LH surge increases expression of members of the AKR1C family of enzymes, which can synthesize PGF2α from PGH2 or PGE2. It is unknown whether synthesis of PGF2α represents synthesis of an important bioactive prostaglandin or catabolism of the key ovulatory prostaglandin PGE2. LH also alters expression of the primary catabolic enzyme, HPGD, to decrease overall activity and thereby facilitate PGE2 accumulation in the primate follicle (19, 185, 189). The LH surge increases expression of prostaglandin transporters such as ABCC4 and SLCO2A1 (19, 190), which may enhance prostaglandin exit from the granulosa cells and increase the pool of PGE2 able to interact with plasma membrane receptors.
There are species differences in the time interval between the LH surge and PGE production and metabolism. In macaques, PTGS2 expression precedes accumulation of PGE2 by ∼24 hours, suggesting that elevated HPGD activity (resulting in PGE2 catabolism) may delay the rise in follicular PGE2 concentration in monkeys and likely women as well (180). However, the paradigm of LH-regulated PGE2 accumulation is widely thought to be a necessary and rate-limiting step in the process of ovulation in all mammalian species.
Studies in genetically modified mice, as well as ablate-and-replace studies in nonhuman primates, identified PGE2 as the key ovulatory prostaglandin [reviewed in Ref. (180)] and focused attention on PGE2 receptors in follicle cells. The four PTGERs (formerly EP1 through EP4) are seven-transmembrane domain receptors that couple to a variety of G proteins, with each PTGER yielding different intracellular responses to the PGE2 signal. For this reason, expression and location within the follicle of PGE2 receptors has received considerable attention [reviewed in Ref. (191)]. All of the four identified PGE2 receptors are expressed by cells of the primate ovulatory follicle. Granulosa cell expression of each PTGER is increased in response to the LH surge. PTGER1 is expressed primarily by mural granulosa cells and invading vascular endothelial cells. Interestingly, preferential expression of PTGER1 by granulosa cells at the follicle base correlates with increased expression of the protease inhibitor SERPINE1, which may limit protease activity at the follicle base and focus proteolysis at the apex (23) (Fig. 5). PTGER2 is located on mural granulosa cells near the site of follicle rupture, invading vascular endothelial cells, the oocyte, and cumulus granulosa cells. PTGER3 is located on mural granulosa cells and cumulus granulosa cells, whereas PTGER4 expression is limited but is detected in mural granulosa cells, the oocyte, and cumulus granulosa cells. Additionally, PTGER2 has been reported in theca cells from multiple species; PTGER3 and PTGER4 expression has also been reported in bovine theca cells.
Receptors for PGF2α (PTGFR, formerly FP receptors) are also expressed by granulosa cells and theca cells of the ovulatory follicle, and the LH surge increased expression of PTGFR in both follicular cell types in many species (192–195), including humans and nonhuman primates (196, 197). However, mice lacking PTGFR expression ovulate normally (198), and PTGFR in primate granulosa cells may not be coupled to signal transduction molecules (197). Because a functional role for these receptors in ovulation cannot be established, PGF2α may be present in the ovulatory follicle as a catabolic product of PGE2, with PGF2α receptors gaining capacity for signal transduction during the luteal phase (197, 199).
In toto, these findings demonstrate that PGE2 is a well-established mediator of inflammatory responses with an equally well-established role in ovulation. As discussed later in this review, PGE2 and, to a lesser extent, PGF2α are involved in regulation of angiogenesis, blood flow, immune cell function, and tissue remodeling associated with cumulus expansion, follicle wall proteolysis, and formation of the corpus luteum. Other immunomodulatory eicosanoids, such as leukotrienes and thromboxanes, are produced by the ovulatory follicle (200–210), but their roles in ovarian inflammatory responses associated with ovulation remain to be demonstrated.
Vascular growth factors in the ovulatory follicle
The LH surge increases follicular expression of peptides and proteins that play key paracrine roles in ovulation. The most highly studied groups of angiogenesis regulators include the VEGFs and the angiopoietins (ANGTPs). Vascular growth factor families are necessary paracrine mediators of ovulation (116, 211), and vascular changes are a critical component of inflammation as well (212).
VEGF family
Members of the vascular endothelial cell family of growth factors are structurally related and use the same group of receptors to mediate vessel growth and permeability. Vascular growth and remodeling are critical for follicle recruitment and development of follicles to preovulatory size [reviewed in Ref. (213)]. Changes in expression of VEGFs and their receptors occur specifically in response to the LH surge and are essential components of the ovulatory cascade.
VEGFA is the best characterized VEGF and is often referred to as simply VEGF in the older literature. Neutralization of VEGFA action within the follicle by introduction of antibodies, soluble VEGFA receptors, or VEGF trap molecules significantly disrupted ovulation in nonhuman primates (214, 215). The rapid increase in follicular fluid VEGFA levels in response to the ovulatory gonadotropin surge has been measured in many species (216–218), including women and nonhuman primates (215, 219, 220). Multiple isoforms of VEGFA are produced by differential splicing (221). These isoforms have differing interactions with ECM and VEGF receptors (VEGFRs) to yield a variety of responses to promote angiogenesis [reviewed in Ref. (222)]. The LH surge increases VEGFA mRNA and protein in granulosa cells, which then accumulates in follicular fluid (215, 217–220, 223). However, by the time of ovulation, granulosa cell VEGFA mRNA levels are lower than pre-LH surge levels in monkeys and women (80, 224). Longer, matrix-binding forms of VEGFA predominate, but shorter forms that do not bind to the matrix are also produced (224, 225). Theca cells from domestic animal species (216, 217, 225) and primates (224) also express VEGFA, providing an additional source of this key vascular growth factor within the ovulatory follicle.
A critical role for placental growth factor (PGF, formerly known as PlGF) in ovulation and ovulatory angiogenesis was recently demonstrated (224). PGF mRNA and protein levels increase after the ovulatory gonadotropin surge in granulosa cells of monkey ovulatory follicles (224). PGF also accumulated in monkey follicular fluid late in the ovulatory interval (224) and has been quantified in follicular fluid of women undergoing fertility treatments (219, 226).
Additional members of this growth factor family expressed by the ovulatory follicle include VEGFC and VEGFD (formerly known as FIGF). VEGFC and VEGFD are expressed by granulosa cells of ovulatory follicles, with subtle increases in protein levels after the LH surge in primates (227, 228) and rodents (223). These vascular growth factors are most often associated with lymphatic development (229). However, VEGFC and VEGFD can act at vascular endothelial cells to promote new capillary growth (228, 230, 231) and, therefore, may be involved in ovulatory angiogenesis.
Receptors for members of the VEGF family of ligands include the three VEGFRs (FLT1, KDR, and FLT4; also known as VEGFR1, VEGFR2, and VEGFR3) along with coreceptors such as the neuropilins (NRP1 and NRP2) (222). Homodimers and heterodimers between VEGFRs or between a VEGFR and a neuropilin have been described (222). In general, VEGFA utilizes FLT1 and KDR whereas PGF utilizes only FLT1 (222). VEGFC and VEGFD can interact with both KDR and FLT4, depending on the degree of proteolytic processing of the ligand (222). Most studies of ovarian VEGFR expression have relied on whole-follicle or whole-ovary homogenates. These studies demonstrate the presence of FLT1, KDR, and FLT4 in ovarian follicles (217, 218, 223, 225). Detailed study of ovine follicles demonstrated increased expression of KDR in both granulosa and theca cells in response to the LH surge (217). Expression of FLT1, KDR, and FLT4 protein has also been localized to follicular vascular endothelial cells (228, 232). A soluble form of FLT1 is also present in the follicle (219, 233). Soluble FLT1 may bind and therefore sequester VEGF family members, preventing interaction with receptors on cells. Ovarian expression of VEGF coreceptors, the neuropilin receptors, highlight the potential complexity of VEGF signaling within the ovulatory follicle (218, 234). The important actions of VEGF signaling in ovulation is discussed in “The Role of the Changing Vasculature in Ovulation” below.
ANGPTs
ANGPT1 and ANGPT2 (formerly known as ANG1 and ANG2) play important roles in angiogenesis [reviewed in Ref. (235)]. Both ANGPT1 and ANGPT2 mRNA are detected in ovarian follicles, including both granulosa and theca cells, with ANGPT1 and ANGPT2 protein present in follicular fluid from primate (215, 236, 237) and nonprimate (217, 218, 238) species. Regulation of angiogenesis by ANGPT1 and ANGPT2 is generally thought to be determined by the ratio of these vascular growth factors (239). The ratio of ANGPT1/ANGPT2 mRNA in granulosa cells is altered in response to the ovulatory gonadotropin surge in nonhuman primates and large animals, such as monkeys and cows (215, 238). ANGPT1 mRNA levels are dynamic; the ANGPT1 mRNA level is high before the surge, low midway through the ovulatory interval, and high again just before ovulation (215, 238). In these species, granulosa cell ANGPT2 mRNA does not change during the ovulatory interval (215, 238). In contrast, rat granulosa cells have stable levels of Angpt1 mRNA with changing levels of Angpt2 mRNA, such that Angpt2 mRNA peaks after the LH surge and before ovulation (218). Intrafollicular administration of ANGPT2 reduced ovulation in nonhuman primates, indicating a key role for the ANGPT pathway in ovulatory events (240).
The transmembrane receptor TEK (also known as TIE2) mediates the actions of both ANGPT1 and ANGPT2. ANGPT1 is an agonist for TEK, whereas ANGPT2 is an endogenous antagonist for the TEK receptor (241). Expression of the TEK receptor has also been confirmed for whole ovary, whole follicle, and both granulosa and theca cells, yet there is no clear pattern of LH regulation (217, 218, 238). The importance of ANGPTs and their receptors to the process of ovulation is discussed in “The Role of the Changing Vasculature in Ovulation” below.
In summary, expression of key paracrine mediators by the cells of the ovulatory follicle is initiated in response to the LH surge. Steroids and eicosanoids are classic mediators of inflammatory responses. Both progesterone and PGE2 are essential for successful ovulation, with multiple experimental approaches to block synthesis or action leading ovulation failure. These traditional inflammatory mediators are involved in regulation of the vasculature and control of proteolysis, as detailed in “The Role of the Changing Vasculature in Ovulation” and “Proteolytic Changes Associated With Inflammation During Ovulation.” Cortisol is produced by the ovulatory follicle but has received relatively little attention as a paracrine mediator of ovulation. Cortisol is a well-established modulator of inflammatory responses and typically terminates key aspects of the inflammatory cascade, preventing extensive tissue damage (242). Proinflammatory actions of androgens have been linked to cancer progression and chronic inflammatory disorders in men (136, 243, 244), but a relationship between androgens, follicular inflammation, and ovulation has not been established. Further studies are needed to determine whether androgens stimulate or cortisol prevents inflammatory damage to the ovulatory follicle and developing corpus luteum.
Vascular growth regulators are critical for aspects of inflammation, including altered vascular permeability, regulation of blood flow, and local edema. Formation of new vessels and changes in vascular permeability, coupled with increased blood flow to much of the follicle, facilitate delivery of circulating immune cells to the ovulatory follicle as a part of a classic inflammatory response. Yet, a critical aspect of inflammatory processes, and especially inflammation in the ovulatory follicle, is timely termination of vascular changes. Tightly controlled vascular development permits rapid angiogenesis of the ovulatory follicle and luteinization but prevents overvascularization of the corpus luteum. This may occur through the growth-restraining actions of the ANGPTs and other vascular growth regulators. For example, thrombospondins are a family of vascular growth regulators produced within the ovary and, in both ovarian and nonovarian tissues, are most often associated with termination of angiogenesis (245–251). However, specific mechanisms that limit follicular and luteal angiogenesis remain to be identified.
Additional inflammatory mediators have been suggested to play a role in ovulation. Eicosanoids such as leukotrienes and thromboxanes are synthesized by follicular cells and accumulate in follicular fluid after the ovulatory LH surge (201, 204, 207, 210, 252). Limited studies indicate that blockade of leukotriene synthesis can reduce ovulation rates in rodents (203, 205, 206). Given the rapid enzymatic conversions responsible for eicosanoid synthesis and metabolism, identification of key receptors or use of nonmetabolizable analogs are needed to determine which mediators contribute to the ovulatory cascade. Specific cellular targets for the actions of these eicosanoids must also be identified to fully explore their ovulatory role.
The Role of the Changing Vasculature in Ovulation
The ovary is a highly vascularized organ. A single artery/vein pair enters and exits the ovary at the hilus and provides the sole connection between the ovarian vasculature and the systemic circulation. The entering artery branches out into multiple arterioles and then further into capillaries through which oxygen, nutrients, and pituitary hormones are transported to the ovarian tissues (Fig. 9). The capillaries then collect carbon dioxide, metabolic products, and ovarian hormones, and they merge into a system of venules and veins through which ovary-produced substances are transported to the systemic circulation.
Figure 9.
LH-stimulated changes in capillary growth and leukocyte delivery in the ovulatory follicle. Before the LH surge, immune cells are present in the ovarian vasculature (red) and theca interna of the preovulatory follicle (top). In response to the LH surge, capillary growth and leukocyte invasion begin. During the early ovulatory period, vessels in the theca interna are the source for new capillary growth into the granulosa cell layer. New vessels and breakdown of the granulosa cell basal lamina provide points of entry for circulating leukocytes to reach the theca and granulosa cell layers of the follicle (center). Secretion of chemokines and cytokines (granules) by leukocytes and granulosa cells begins (center). By the late ovulatory period, breakdown of the granulosa cell basal lamina continues, extensive capillary networks form, and additional leukocytes are seen in the granulosa cell layer prior to ovulation (bottom). Leukocyte-secreted chemokines and cytokines increase as ovulation approaches.
Previous studies on the vasculature in the ovary have focused on follicle growth (primary-to-small antral transitions) and the corpus luteum, especially luteolysis [reviewed in Refs. (116, 211)]. There is comparatively little information on vascular changes during the interval between the LH surge and follicle rupture when the ovary undergoes an inflammatory response. During this ovulatory interval, changing levels of vascular mediators have been reported (see “LH-Stimulated Production of Paracrine Mediators by Follicular Cells”). In some cases, the potential actions of these mediators seem contradictory. For example, prostaglandins have been implicated in both vasoconstriction and vasodilation, but both of these processes are essential components of the ovulatory cascade (253). Importantly, vascular changes are regional. Vasoconstriction at the apex and elsewhere around the follicle facilitates follicle rupture (28, 30, 254). Elsewhere, angiogenesis, vasodilation, and enhanced permeability predominate to increase blood flow and promote the influx of immune cells, hallmarks of inflammation.
Location of vascular components in the ovary: ovulatory changes
In the primate ovarian follicle, vessels are located in the theca layer but not among the granulosa cells of primary, secondary, antral, or preovulatory follicles (255–257) (Fig. 4). Although more than a half of the ovary is occupied by multiple follicles of different stages and corpora lutea, the vast majority of the literature indicates that vessels are restricted to the interstitium, corpora lutea, and the theca layers of the follicles, regardless of the species examined (255–257).
Vessels, including arteries, veins, arterioles, and venules, can be visualized by routine histological staining in ovarian tissue sections. The classic papers of Corner et al. (257) and Koering (255) describe LH-stimulated changes in the stromal vasculature of the macaque ovulatory follicle. More recently, Kerban et al. (258) documented vascular changes, including edema, hemorrhage, and increased number and area of blood vessels, in equine ovulatory follicles. Enlarged vessels of the theca interna give rise to an expanded capillary network. Stromal infoldings push toward the luteinizing granulosa cells and follicle antrum. However, these reports did not observe new capillaries within the granulosa cell layer (255, 257, 258). The use of immunohistochemical detection of proteins that are uniquely expressed in the endothelial cells has permitted detection of developing capillaries throughout the ovary.
“Within hours after the LH surge...the ovary experiences a rapid influx of leukocytes....”
Von Willebrand factor and CD31 (also known as PECAM1) are used to locate endothelial cells in developing tissues, including the ovary (232, 259–263). More recently, the tissue-clearing CLARITY approach has been used to visualize the enlarging stromal vessels during the ovulatory cascade (264). Immunostaining showed that vascular endothelial cells are primarily present in the theca layers but also in the granulosa cell layer of monkey and rodent follicles immediately prior to follicle rupture, forming branches from vessels located within the theca interna (203, 205, 232, 263, 265). Interestingly, although chains of endothelial cells have recently been demonstrated in the granulosa cell layers of periovulatory follicles (263, 266), these forming capillaries are unlikely to support true vascular flow because they are not connected to both arterial and venous vasculature. However, these new capillaries may enhance follicular access for serum, red blood cells, and leukocytes, because blood cells are present in large numbers within the follicle antrum prior to ovulation (232, 263).
The LH surge increases vessel dilation and permeability
Acute inflammation is characterized in part by edema, with tissue swelling stemming from fluid accumulation. Underlying this anatomical change are dilated vessels and increased permeability at the site of inflammation. Vasodilation and increased permeability are also two primary contributors to the leukocyte extravasation at the site of inflammation (267–270). Prior to ovulation, the ovary undergoes an acute inflammation-like process that is accompanied by an influx of leukocytes. Therefore, it is not surprising that the ovarian blood vessels become dilated (271–273) and their permeability increases prior to ovulation (274–276).
Vasodilation is primarily caused by relaxation of the smooth muscle layer surrounding vessels. Generally, molecules that trigger vasodilation are produced by local cells or resident leukocytes, which then bind to receptors that are expressed on the membranes of smooth muscle cells. In the ovary, LH stimulates granulosa cells and theca cells of large antral follicles to secrete a variety of such molecules. Among them are PGF2α (277, 278), PGE2 (279, 280), and histamine (281). Potential involvement of other important vasodilators such as PGD2 (282–284), vasoactive intestinal peptide (285, 286), and bradykinin (287–289) has yet to be fully explored in ovarian tissues.
Increased permeability of vessel walls allows molecular water, small solutes, or cells to flow out of the vessel. Permeability of a vessel increases when the intercellular junctions connecting endothelial cells become loosened. Either decreased expression of junctional proteins such as connexins, cadherins, occludins, nectins, and claudins or degradation of junctional proteins increases vascular permeability (290). With the ovulatory LH surge, the ovarian follicle swells, and there is an influx of leukocytes; both likely result from increased vascular permeability (259, 291). In support of this concept, LH decreases the expression of junctional proteins in the ovary (see below) but increases matrix metalloproteinase (MMP) expression (292). In a classical study with IV administration of colloidal carbon, increased accumulation of the carbon particles was observed around and inside of preovulatory follicles a few hours after hCG stimulation in rabbits (293), providing direct evidence of increased permeability resulting from gonadotropin stimulation. An ovulatory dose of hCG was also shown to increase rat ovarian capillary permeability by increasing the number of large pores, similar to a classical inflammatory response (294).
The LH surge increases ovarian blood flow
Microspheres have been traditionally used to characterize changing ovarian blood flow through the reproductive cycle (277, 295, 296). Presently, Doppler ultrasound is widely used to provide a noninvasive, albeit indirect, method to measure the rate of blood flow within regions of the ovary. Using these approaches, increased blood flow after the ovulatory LH surge and prior to ovulation has been demonstrated in rabbits (277), rats (297), sheep (298, 299), cows (300, 301), and humans (302). Specifically, studies have shown that systemic administration of LH rapidly increases ovarian blood flow via vasodilation, with blood flow to other organs unaffected (277). Measurements of ovarian blood flow using an advanced color Doppler system or by intravital multiphoton microscopy revealed that blood flow rates are regionally different within an individual ovary. In particular, immediately prior to ovulation, there is a significant change in regional blood flow around the human ovulatory follicle (303), with a marked increase of the flow in the base of the follicle and a concomitant decrease of blood flow at the apex [Fig. 10 (303)]. This regional difference in the blood flow may be the result of active remodeling of the ECM (34, 304). Matrix remodeling can promote angiogenesis by providing space for migrating endothelial cells or by reducing vascularity by destabilizing the matrix supporting existing vessels. Alternatively, increased contractility of the follicles may provide subtle regional pressure to prevent blood flow in apical vessels (28, 30, 254). Active vasoconstriction caused by soluble signaling molecules such as endothelins may also be responsible for reduced blood flow through apical vessels (254). Regardless of the cause, differential blood flow and change in the vascular distribution in the ovary is likely essential for the successful release of a mature oocyte (303). Loss of vasculature at the follicle apex has been suggested as a major cause of the cell death that contributes to the apical thinning (305–308). The apex eventually becomes avascular, theca cells as well as the OSE undergo apoptosis, the follicular wall thins, and the follicles contract, leading to rupture at the apex (309–314). Use of a corrosion casting technique visually shows a rapid and massive increase in vascular volume in the theca layer around mature preovulatory follicles (315, 316). LH-triggered new capillary formation (232, 263, 317) may additionally contribute to the increase of follicular blood flow.
Figure 10.
Blood flow in the human preovulatory follicle. (a and b) Color Doppler and ultrasound (US) images of human ovarian follicles (a) before the LH surge and (b) after the LH surge but before ovulation. For each panel, the left side shows ultrasound with Doppler blood velocity (US + Doppler) and right side shows only Doppler blood velocity. Red represents flow toward the transducer. Blue represents flow away from the transducer. Blood flow is concentrated at the follicle base and is less prominent at the follicle apex as ovulation approaches (b). [Reproduced with permission from Brannstrom M, Zackrisson U, Hagstrom HG, et al. Preovulatory changes of blood flow in different regions of the human follicle. Fertil Steril 1998;69(3):435–442.]
Regulators of ovulatory angiogenesis
LH does not act directly at endothelial cells of vessels to produce ovulatory changes in the ovarian vasculature. Instead, LH acts at theca and granulosa cells of ovulatory follicles where the LHGCR is expressed to regulate production of endocrine and paracrine mediators of vascular remodeling and function (reviewed earlier in “LH-Mediated Immediate Cellular Responses in Follicular Cells” and “LH-Stimulated Production of Paracrine Mediators by Follicular Cells”). LH-induced expression of angiogenesis regulators by granulosa and theca cells is thought to set up a gradient of angiogenic factors that serves as primary drivers of vascular changes in the ovulatory follicle. These ovulatory angiogenic factors are outlined below and summarized in Table 3.
Table 3.
Vascular Regulators in the Ovulatory Follicle
| Pro-Angiogenesis | Anti-Angiogenesis | Blood Flow Regulators |
|---|---|---|
| VEGFA PGF PGE2 ANGPT1 | ANGPT2 THBS1 | PGF2α PGE2 Histamine Progesterone Endothelin1/2 Angiotensin II/Ang-(1–7) |
The VEGF family and ovarian angiogenesis
VEGFA is required for oocyte release as is evident from reports that blockade of VEGFA action severely compromises ovulation (see “LH-Stimulated Production of Paracrine Mediators by Follicular Cells”) (214, 224, 240, 318–323). This compromise of ovulation is likely to result from changes in ovarian blood flow. For example, VEGFA action within the primate follicle is necessary for the normal process of angiogenesis that occurs around the time of ovulation. This is supported by intrafollicular injection studies of a soluble VEGFR or anti-VEGFA antibody that effectively prevented vascular changes associated with ovulation, including formation of capillary networks within the granulosa cell layer (224, 323). In women, support for the proposition that VEGFA increases vascular permeability of the ovulatory follicle is seen in findings that demonstrate a correlation between follicular fluid VEGFA levels and ovarian hyperstimulation syndrome (256, 324, 325).
Recently, PGF was identified as a gonadotropin-regulated vascular growth factor that plays an important role in angiogenesis during the ovulatory interval. PGF is known as a regulator of angiogenesis, in particular endothelial cell proliferation and capillary lengthening [reviewed in Ref. (222)]. PGF action at vascular endothelial cells from monkey ovulatory follicles and human follicular aspirates demonstrated that PGF stimulates endothelial cell proliferation, migration, and the formation of capillary-like sprouts in vitro (223, 225). Furthermore, blockade of PGF action by intrafollicular administration of a PGF-neutralizing antibody in macaque ovulatory follicles demonstrated that PGF is necessary for successful ovulation as well as formation of new capillaries within the granulosa cell layer of luteinizing follicles (224). Overall, these studies suggest that PGF complements the essential ovulatory actions of VEGFA to form the developing vasculature of the ovulatory follicle and young corpus luteum. Finally, synergy between VEGFA and PGF has been reported to promote vascular permeability and angiogenesis in wound healing (326), and such synergism may also be critical for vascular changes in the ovulatory follicle.
VEGFC and VEGFD are also expressed within the primate ovulatory follicle, and proangiogenic actions at primate ovarian endothelial cells have been reported (228). This suggests that VEGFC and VEGFD may also act to promote angiogenesis.
ANGPTs and ovarian angiogenesis
ANGPTs are regulators of blood flow. Additionally, they complement the VEGF system during vascular remodeling as angiogenic regulators (222). The ANGPTs ANGPT1 and ANGPT2 regulate the interaction of vascular endothelial cells with ECM and, therefore, vessel stability [reviewed in Ref. (235)]. A stable vessel resists remodeling. An unstable vessel can be the source of new capillaries or undergo apoptosis, depending on the availability of specific vascular growth regulators. ANGPT1 acts at TEK to increase vessel stability by promoting endothelial cell stability, survival, and attachment to surrounding tissue. In contrast, ANGPT2 is a naturally produced antagonist and acts at TEK to destabilize vessels. Destabilized vessels can regress or, in the presence of vascular growth promotors such as VEGFA, experience new capillary growth.
The ANGPT1/ANGPT2 ratio is a metric of angiogenic potential, with a higher ratio favoring stable vessels and a lower ratio favoring vascular remodeling. Changes in follicular ANGPT1 and ANGPT2 in response to the LH surge are discussed above. Although details vary between mammalian species, in general the ratio of ANGPT1/ANGPT2 expression is low midway through the ovulatory period, consistent with a role in destabilizing vessels to permit vascular remodeling and new capillary formation. The ratio of ANGPT1/ANGPT2 protein in human follicular fluid is lowest in the largest and most mature periovulatory follicles (237), supporting the concept that this ratio favors ovulatory events.
Disruption of the intrafollicular ANGPT system compromises ovulation. The ovulatory failure seen in the monkey follicles injected with ANGPT2 protein (discussed above) may be an outcome of failed angiogenesis and/or altered blood flow (327). Elevated follicular ANGPT2 levels caused structural destruction of the follicle (327), consistent with the concept that vessels were excessively destabilized and unable to respond to appropriate signals to undergo organized angiogenesis associated with ovulation and luteal formation. Dysregulation of the ANGPT system has been implicated in PCOS, a disorder of both antral follicle growth and anovulation [reviewed in Ref. (328)], indicating its importance in follicular growth and ovulation.
Prostaglandins
Although much has been reported regarding prostaglandin regulation of ovarian blood flow, this body of work has focused primarily on the corpus luteum and mechanisms of luteolysis (317). Specifically regarding ovulatory follicles, blockade of prostaglandin synthesis by infusion of indomethacin to ovaries of ewes in estrus caused follicular edema and decreased ovarian blood flow (279). In contrast, in gonadotropin-primed preovulatory rats, indomethacin increased ovarian blood flow after administration of an ovulatory dose of gonadotropin by reducing ovarian vascular resistance and increasing capillary area (329, 330). These and similar studies provide a conflicting view of prostaglandin-regulated vascular changes in the ovulatory follicle. Systemic administration of prostaglandin synthesis inhibitors prevents production of many prostaglandins and, therefore, does not identify the specific prostaglandin involved in changing ovarian vascularity. A pair of studies, however, demonstrated that PGE2 plays a critical role by showing that intrafollicular administration of indomethacin prevented growth of capillaries in the luteinizing follicle, whereas replacement with PGE2 restored follicular angiogenesis and ovulation (263, 331). In vitro studies of ovarian vascular endothelial cells from monkeys and humans showed that PGE2 action via its PTGER1 and PTGER2 receptors stimulates endothelial cell migration and capillary stalk formation (232, 263), with similar ability of PTGER1 and PTGER2 agonists shown to promote angiogenesis in macaque ovulatory follicles in vivo (263). These findings suggest that PGE2 is a key modulator of the vascular changes associated with ovulation in the primate.
Ovarian steroids
Ovarian steroids are powerful vasomodulators in the reproductive tract [reviewed in Ref. (332)]. Both estrogens and progesterone can cause vasodilation, increased blood flow, and induce vascular permeability. In particular, progesterone is established as an important mediator of vascular changes in the ovulatory follicle. Depletion of progesterone with a progesterone synthesis inhibitor in monkeys reduced preovulatory structural luteinization, including vascular remodeling, in response to the ovulatory gonadotropin stimulus (333). Replacement of progestin activity restored follicular angiogenesis (333). Similarly, administration of the PGR antagonist RU486 reduced vascular remodeling associated with ovulation and luteinization in pigs (216). Besides progesterone action directly on vascular smooth muscle (334–336), progesterone may regulate local mediators of vascular remodeling within the ovulatory follicle. A screening study of genes expressed in mice lacking PGR expression showed alteration of expression of the genes linked to angiogenesis such as Edn1 and Hif1a (337). For instance, activation of PGR increases granulosa cell expression of HIF1A and other hypoxia-induced transcription factors in mice, resulting in enhanced expression of VEGFA (39). The PGR antagonist RU486 decreased VEGFA expression in pig follicles, with the RU486 effect primarily at theca cells (216). However, VEGFA expression by granulosa cells of monkey follicles was not altered by blockade of progesterone synthesis or with progestin replacement (215), suggesting that species differences may exist regarding the specific pathway by which progesterone promotes vascular remodeling of the ovulatory follicle. Androgens have also been implicated in control of microvascular dilation, as testosterone reduces the ability of subcutaneous vessels to dilate in response to endothelins (338), but an effect of androgens on ovarian blood flow has not been directly demonstrated.
Other protein vascular mediators
EDN1 and EDN2 are potent regulators of the ovarian vasculature. In response to the ovulatory gonadotropin surge, granulosa cell EDN2 mRNA and total ovarian endothelin protein increase to peak levels just before ovulation in rodent species and humans, whereas expression of endothelin receptors EDNRA and EDNRB are relatively constant through the ovulatory interval (29, 30, 339). EDN2 stimulates both ovarian contraction and oocyte release (30, 38). Endothelin also constricts smooth muscle surrounding ovarian vessels specifically at the follicle apex, perhaps contributing to local hypoxia and loss of cells at the site of follicle rupture (254). Conditional knockout of EDN2 using an ESR2-CRE (Edn2flox/flox × Esr2cre/+) severely compromised oocyte release and fertility, confirming the essential role of granulosa cell–derived EDN2 in the process of ovulation (28). Progesterone is necessary for induction of EDN2 expression as demonstrated by failure of PGR knockout mice to increase EDN2 expression after the ovulatory gonadotropin stimulus (38). Given the timing of EDN2 peak expression and its established roles in ovarian contraction and vasoconstriction, endothelins are likely critical for ovulatory success, at least in part through actions on the vasculature.
The renin–angiotensin system is also present and active in the ovary and, in particular, within the ovulatory follicle (340). The precursor angiotensinogen is produced by the liver, converted to an inactive prohormone by renin, and then converted to the bioactive hormones angiotensin II and a shorter form [Ang-(1–7)] by converting enzymes (341). Follicular expression of angiotensin-converting enzymes and angiotensin receptors increases after the ovulatory gonadotropin surge in cows, with peak follicular fluid levels of Ang-(1–7) reported just prior to ovulation (342, 343). Antagonism of both angiotensin receptors decreased both ovarian blood flow and ovulation rates in rats and cows (344, 345). However, the specific angiotensin receptor (AGTR1, AGTR2, or a member of the MAS family of G protein–coupled receptors) responsible for facilitating ovulation remains uncertain. Derangements of the renin–angiotensin system within the ovary have been reported in women with ovarian hyperstimulation syndrome and PCOS [reviewed in Ref. (340)]. However, the specific mechanisms by which angiotensin receptor agonists regulate ovarian blood flow and ovulatory events are yet to be explored.
In summary, upon the LH surge, the ovary undergoes dramatic vascular changes that include vessel dilation, increased permeability, changes in regional blood flow, and new vessel formation around the follicle and eventually into the follicle. In addition to other consequences of altered blood flow, vascular changes also likely facilitate the infiltration of leukocytes, which contribute to the overall ovarian inflammatory responses associated with ovulation.
Influx of Immune Cells and Their Function in Ovulation
Leukocytes are produced in the bone marrow and released into the bloodstream. Whereas most leukocytes migrate into lymphoid tissues such as the spleen, thymus, or lymph nodes for future activation and release, some circulating leukocytes infiltrate peripheral tissues, including the ovary. They serve as surveillance agents by recognizing inflammatory signals in peripheral tissues and as facilitators of inflammatory processes by secreting chemokines, cytokines, and proteases. Prior to the LH surge, the ovary is populated with a limited number of resident leukocytes [Table 4 (273, 346–360)]. Within hours after the LH surge, however, the ovary experiences a rapid influx of leukocytes that are primarily from circulating blood and spleen (273, 361). In the rat, the ovarian leukocyte population size increases by ∼2 million in 6 hours after a stimulation by an ovulatory dose of hCG (259). This acute leukocyte influx in the ovary may be assisted by the concomitant structural and functional changes that ovarian vasculature undergoes (vessel dilation, enhanced permeability, increased blood flow, and angiogenesis) upon the LH surge.
Table 4.
Leukocyte Species in the Ovary
| Cell Type | Key Ovulatory Functions | References |
|---|---|---|
| Monocytes/macrophages | Stimulate infiltration of inflammatory cells by secreting cytokines and chemokines and removes damaged cells and tissues via phagocytosis | (346–349) |
| Lymphocytes (T cells and B cells) | Stimulate infiltration of inflammatory cells by secreting cytokines and chemokines | (273, 350–352) |
| Mast cells | Stimulate infiltration of inflammatory cells by secreting chemokines and weaken follicular wall by degrading ECM via secreting proteases | (353–356) |
| Natural killer cells | Increase inflammatory response by secreting cytokines and remove damaged cells and promote angiogenesis | (126, 357) |
| Neutrophils | Activated by cytokines, weaken follicular wall by degrading ECM via secreting collagenase, cathepsin, and gelatinase | (358–360) |
Leukocytes as contributors to ovulation
In contrast to the mounting evidence of the influx of leukocytes and their presence in the ovary after the LH surge, whether they play essential roles as mediators of ovulatory processes or play a minor role in ovulation is not clear. However, an overwhelming amount of literature favors their important roles in ovulation as facilitators [reviewed in Ref. (273)]. In support of their role, treatment with neutralizing antibody against neutrophils significantly reduces ovulation in rabbits (362). There have been reports that ovulation can be induced in perfused ovaries, indicating that the post-LH surge influx of leukocytes is not required for ovulation. For example, ovulation was induced in the perfused rabbit and rat ovary (88, 363, 364), suggesting successful ovulation without leukocyte infiltration. However, ovulation efficiency is decreased in this model. It is possible that resident immune cells are sufficient to induce ovulation, but without the influx of additional immune cells, full ovulatory capacity is not achieved. This concept is supported by findings that leukocyte supplementation increases the ovulation rate in in vitro perfused rat ovaries (365). Thus, it is difficult to rule out the involvement of leukocytes or inflammation in ovulation in this and other models. Currently available data, however, weigh more to the significant roles that leukocytes play in the ovulatory processes (see ‘Proteolytic Changes Associated With Inflammation During Ovulation” and “Disorders of Ovulation Related to Inflammation and Immune Cells”).
In the ovaries of rodents (259, 366) and humans (352), leukocytes are primarily localized in the periphery of ovarian follicles, the ovarian interstitium, and corpora lutea where they likely facilitate structural changes by secreting proteases (367). The substrates for those proteases are not restricted to matrix proteins in the ovary but also include mediators of inflammation such as cytokines, chemokines, cell surface receptors, and adhesions molecules, indicating their involvement not only in the digestion of matrices but also in signal activation. Additionally, various types of leukocytes such as monocytes, macrophages, and neutrophils secrete vascular growth factors (368, 369) that may trigger vascular changes, a hallmark of inflammation. In particular, Guimerà et al. (370) showed that macrophages taken from human follicular fluids produce VEGFA upon gonadotropin stimulation in vitro.
The pattern of immune cell trafficking in the ovary
The findings that LH induces leukocyte migration into the ovary raises the question as to how this is accomplished. Leukocytes migrate from a vessel into the surrounding tissue, typically via a classical four-step process: (i) tethering and rolling, (ii) activation, (iii) firm adhesion, and (iv) transmigration into the tissues (371) (Fig. 9). Once a leukocyte is tethered on the endothelial cell wall, it migrates toward the source of an attractant, such as a chemokine. In the ovary, chemokines are produced by granulosa cells, theca cells, and resident leukocytes [Table 5 (126, 372–382)]. For example, basal IL-8 levels in the ovary are low; however, IL-8 synthesis by granulosa cells and theca cells increases rapidly after LH stimulation (383). Increased IL-8 stimulates neutrophils to infiltrate the bovine ovary (378), a clear demonstration of the interplay between ovarian cells and leukocytes in LH-triggered ovarian inflammation. The infiltrating leukocytes may interact with ovarian endothelial cells and other cell types via a multitude of chemokines (346, 384). Specifically, treatment with neutralizing antibodies to either IL-8 or neutrophils significantly reduces ovulation in rabbits (362), signifying the critical roles for these chemokines. Indeed, recent studies in humans show that LH stimulation increases the expression of the chemokines CCL20 and CXCL12 in both granulosa cells and theca cells (126, 381) and in leukocytes (42). CCL20 is a ligand for CCR6, a chemokine receptor present on the cell membrane of dendritic cells and T cells, whereas CXCL12 is a ligand for CXCR4 on T cells and other leukocytes (259). Interestingly, the LH surge induces CXCR4 expression in the granulosa cells of the human ovary (42), indicating an involvement of CXCL12/CXCR4 in the ovulatory inflammatory response and an immune cell–like behavior of the granulosa cells.
Table 5.
Chemokines Produced in Response to LH Surge in the Ovaries of Humans or Nonhuman Primates
| Chemokine | Functions in Ovulation | References |
|---|---|---|
| CCL2 | Secreted by granulosa cells, macrophages, and stromal fibroblasts; attracts T cells, monocytes, basophils, dendritic cells, and natural killer cells | (372–374) |
| CCL20 | Secreted by granulosa cells and theca cells; attracts monocytes, macrophages, dendritic cells, B cells, and T cells | (126, 372) |
| CCL25 | Secreted by theca cells; attracts monocytes, B cells, T cells, and natural killers cells | (375) |
| CSF1 | Secreted by granulosa cells; induces differentiation of monocytes into macrophages | (372, 376) |
| CSF2 | Secreted by granulosa and theca cells; attracts monocytes and macrophages | (376) |
| CXCL1 | Secreted by granulosa cells, monocytes, macrophages, and dendritic cells; attracts neutrophils | (377) |
| CXCL8 | Secreted by granulosa cells, macrophages, and epithelial, endothelial, and mast cells; attracts neutrophils, basophils, and T cells | (378) |
| CXCL10 | Secreted by granulosa cells; attracts T cells, natural killer cells, B cells, dendritic cells, monocytes, and macrophages | (379, 380) |
| CXCL12 | Secreted by granulosa cells, endothelial cells, and stromal fibroblasts; attracts monocytes, macrophages, B cells, T cells, neutrophils, and dendritic cells | (380, 381) |
| CXCL16 | Secreted by granulosa cells; attracts monocytes, B cells, T cells, and natural killer cells | (382) |
A recent study in rats provides a holistic picture of the sequential pattern of leukocyte influx into the ovulatory ovary (259). A comprehensive flow cytometry approach quantitatively recorded temporal changes in leukocyte populations in the ovaries of naturally cycling adults and immature rats that were stimulated to undergo ovulatory follicle development and synchronized ovulation. This treatment resulted in a massive increase of total leukocyte numbers ∼6 hours after the LH surge, but not at any other time point, providing evidence that LH initiates leukocyte infiltration in the ovary (259). Importantly, the pattern of leukocyte infiltration triggered by the LH surge is very similar to acute inflammation caused by infection or physical injury (2, 259). These findings show an essential role for LH in triggering a classical inflammatory response in the ovary as a component of the ovulatory process.
Mechanism of leukocyte infiltration in the ovary
The initiator of an inflammatory event is often a discrete signal that is rapidly amplified by additional signals produced by responding tissues and infiltrating leukocytes. Chemokines, cytokines, and adhesion molecules play key roles in inducing leukocyte infiltration in an inflammatory site. As with inflammation due to infection and injury, these same mediators are produced in the ovary upon the LH surge or stimulation by hCG (259, 385, 386). Cytokines, such as IL-1, IL-6, and others [Table 6 (215, 219, 224, 237, 240, 387–398)], may contribute to the leukocyte infiltration by enhancing permeability of small vessels or increasing expression of cell adhesion molecules in the endothelial cells of the vessels, whereas chemokines (Table 5) act as chemoattractants for leukocytes (399). LH or hCG increased expression of ICAM-1 and E-selectin in the preovulatory ovary (259) that may facilitate the attachment of circulating leukocytes to the ovarian endothelial cells.
Table 6.
Cytokines Produced in Response to LH Surge in the Ovaries of Humans or Nonhuman Primates
| Cytokines | Functions in Ovulation | References |
|---|---|---|
| ANGPT1 | Produced by granulosa cells; stabilizes newly developed capillaries | (215) |
| ANGPT2 | Produced by granulosa cells; destabilizes existing vessel cells and promotes angiogenesis by stimulating endothelial cell migration and proliferation | (215, 237, 240) |
| TNF | Produced by granulosa cells and macrophages; stimulates prostaglandin production and suppresses steroidogenesis by inhibiting the expression of StAR or Cyp11A1 in granulosa cells | (387–389) |
| PGF | Produced by granulosa cells and theca cells; stimulates angiogenesis and attracts monocytes | (224) |
| VEGFA | Produced by granulosa cells and theca cells; stimulates angiogenesis and promotes the survival of newly formed capillaries | (215, 219, 224) |
| TGFB1 | Produced by granulosa cells, theca cells, and oocytes; promotes expansion of the COC complex | (390) |
| IFNA1 and IFNB1 | Produced by dendritic cells, macrophages, and other leukocytes in response to Toll-like receptor activation; promotes expansion of the COC complex by inducing the expressions of Has2, Ptx3, Tnfaip6, and Ptgs2 | (391, 392) |
| IL-1A | Produced by granulosa cells, theca cells, monocytes, and macrophages; induces the expression of gelatinases and nitric oxide synthase in granulosa and theca cells | (393, 394) |
| IL-1B | Produced by granulosa cells, theca cells, and activated macrophages; induces the expression of Cox-2, IL-8, and protease expression | (395–397) |
| IL-6 | Secreted by granulosa cells, theca cells, T cells, and macrophages; stimulates angiogenesis, COC expansion, and steroidogenesis | (393, 396, 398) |
In addition to CCL20 and CXCL12 described above, two additional chemokines, CCL2 and CCL25, are well characterized as mediators of ovarian leukocyte infiltration. CCL2 is a potent chemoattractant for monocytes and also effectively recruits macrophages and T-lymphocytes (400, 401). The major sources of CCL2 are monocytes and macrophages, although several other types of cells such as endothelial and smooth muscle cells of vessels also produce this chemokine (402). Interestingly, studies in humans and rats have demonstrated that CCL2 is involved in all aspects of ovarian function, including follicular development, ovulation, and luteolysis (346, 403–405). Several studies have demonstrated that inhibiting the production of CCL2 in the monocytes/macrophages (406, 407) or the direct neutralization of this molecule results in reduced ovulation, further supporting the role of monocytes/macrophages in ovulation (348). Neutralization of CCL25 inhibited leukocyte infiltration to the ovary and resulted in complete inhibition of ovulation in mice (408). Interestingly, ovarian CCL25 expression is tightly regulated by gonadotropins (409), and ovulatory failure was attributed to the lack of infiltration of a rare CD8α+ T cell population (375, 408).
Although substantial data have been added to the body of knowledge about the species, quantities, localization, and trafficking of the leukocytes brought into the ovary by the LH surge, the specific contributions made by these leukocytes individually and collectively are yet to be further discovered. The complexity of the inflammatory response itself, the involvement of multiple types of immune cells and their interaction with the endocrine system, their regulators (cytokines and chemokines), and their secretory products collectively make it difficult to accurately predict their precise roles. However, the overwhelming data indicate that leukocytes are involved in many different aspects of ovulation by amplifying inflammatory signals via secreting chemokine and cytokines, stimulating tissue remodeling and follicular wall degradation by secreting proteases, inducing vascular changes by secreting and activating VEGFs, and facilitating tissue repair after ovulation. Future studies will discover and further reveal the role of leukocytes in the ovulatory process.
Proteolytic Changes Associated With Inflammation During Ovulation
The dynamic structural changes required for ovulation are postulated to be mediated in part by proteolytic systems that regulate ECM homeostasis of the ovarian follicular connective tissue. These proteolytic systems control ECM turnover and homeostasis, in part by coordinating the activity of MMPs, the plasminogen activator (PA) system, the ADAM/ADAMTS family, and their associated inhibitors. Proteolysis in the ovulatory follicle is driven not only by resident and infiltrating leukocytes (discussed above) but also by granulosa and theca cells, suggesting that structural changes in the follicle result from collaboration between many cell types expressing the requisite proteases. Degradation of the ECM is a delicately balanced process and, as such, the synthesis, activation, and regulation of the MMPs, PAs, and ADAMTS are critical points of ECM homeostasis (410–412). Proteolytic remodeling can be both tissue and protease specific, and it can be influenced by inflammation. In other systems, an inflammatory response serves to recruit leukocytes and these infiltrating leukocytes produce cytokines and chemokines that stimulate protease production. For example, with myocardial infarction, leukocytes stimulate production and release of MMP9, which in turn regulates cytokine and chemokine activity (413). However, in other systems, MMPs have been shown to regulate specific immune processes, such as leukocyte influx and migration as well as macrophage activation. This may occur either by the ability of proteases to release bioactive fragments that may function as chemoattractants for different leukocytes subsets or modulate the activity or function of immune cells (414). Thus, a reciprocal interaction exists where an inflammatory reaction can induce protease action that, in turn, can regulate the pattern, type, and duration of the immune response (413–415).
Proteolytic systems
The MMP system
The MMP family encompasses at least 25 related proteolytic enzymes (410, 411, 416, 417). These enzymes are broadly divided into four classes: the collagenases, gelatinases, stromelysins, and membrane-type enzymes [Fig. 11 (418, 419)]. Additionally, there are a number of family members that are classified outside of these four broad classes. A listing of the MMP family members and their substrates is detailed in a previous review (292). Although there are similarities in the structure of the MMPs, there are also distinct differences in their recognition and specificity for components of the ECM (410–412, 420). For example, the collagenases (MMP1, MMP8, and MMP13) are able to cleave both fibrillar collagens, such as collagen types I, II, III, V, and XI, as well as nonfibrillar collagens such as collagen types IX, XII, and XIV (411). Cleavage of collagen changes its stability and solubility properties, resulting in the denaturation of the collagen molecule into gelatin. Gelatin is susceptible to a wide range of tissue proteases, including the gelatinases (MMP2 and MMP9) and stromelysins (MMP3, MMP7, MMP10, and MMP11) (410, 411). In the monkey and the human, there is an increase in the collagenases (MMP1), the gelatinases (MMP2 and MMP9), and the stromelysins (MMP7 and MMP10) following LH or hCG as described in detail below. Both the gelatinases and stromelysins are capable of degrading major constituents of basement membranes, including type IV collagen, laminin, and fibronectin, components found underlying the OSE and the granulosa cell compartment. The membrane-type MMPs contain a transmembrane domain that anchors these proteases to the plasma membrane (Fig. 11), allowing the extracellular domain to direct proteolysis to the cell surface. In addition to degrading the ECM, the MMPs and especially the stromelysins also exhibit activity toward other MMPs, growth factors, and cytokines such as IGF-binding proteins, EGF, and TNF (also known as TNFα) (412, 421). The ability of these enzymes to cleave binding proteins as well as cleave active extracellular domains of growth factors expands the repertoire of MMP actions to include modulation of growth factor bioavailability during the ovulatory process. This may be a critical role for the MMPs, as Panigone et al. (63) demonstrated that GM6001, a broad-spectrum MMP inhibitor, was able to block the cleavage and shedding of the EGF-like growth factors in mouse preovulatory follicles, thereby blocking EGFR activation and signaling (23). Thus, timely regulation of the balance between release or removal of MMPs is critical for successful tissue remodeling during follicular rupture and oocyte release as well as transformation and repair during follicular remodeling to form the corpus luteum.
Figure 11.
The MMP and ADAMTS families. A general model of the structural organization of the more common MMPs is presented. The MMPs and ADAMTSs contain a signal peptide, a propeptide domain with a sulfhydryl group that must be cleaved for activation, and a catalytic domain that contains the zinc binding site. The MMPs also contain a hinge region and a hemopexin-like domain. The gelatinases contain a fibronectin type II domain whereas certain other MMPs contain a furin-susceptible site that allows intracellular activation. The transmembrane-type MMPs contain a transmembrane linker to a cytoplasmic domain. The ADAMTSs contain a disintegrin domain along with regions of thrombospondin repeats. MT-MMP, membrane-type MMP. [Adapted with permission from Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4(8):617–629; and under a Creative Commons CC-BY 4.0 license from Noel A, Gutierrez-Fernandez A, Sounni NE. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol 2012;3:140. Illustration presentation copyright by the Endocrine Society.]
MMP activity in the extracellular environment is rigorously controlled by endogenous MMP inhibitors (Fig. 11). There are two major classes of MMP inhibitors, the serum-borne and tissue-derived inhibitors (422, 423). The serum-borne inhibitors, which include the macroglobulins, are found in the circulation and have a potent ability to inhibit a broad range of proteases, including the MMPs (412). The macroglobulins are large molecular proteins that include α2-macroglobulin (A2M) and the ovomacroglobulins (e.g., ovostatin), both of which are present in the ovary (412, 424–426). Interestingly, the MMPs, especially MMP1, have an ∼150-fold higher affinity for A2M than type I collagen, suggesting that this class of inhibitors is a major regulator of collagenolysis in serum and tissue fluids (412). It has been proposed that during follicular rupture as capillaries become leaky that serum-borne inhibitors would be present at the site of rupture to control further ECM turnover (412, 424–426). The second group of inhibitors, referred to as tissue inhibitors of metalloproteinases (TIMPs), are produced locally and specifically inhibit MMPs. Originally, the two classes of MMP inhibitors were distinguished as serum borne or tissue derived, based on the site of action (425, 426). Findings that A2M is produced in the ovary and the tissue-derived inhibitors are present in serum (424–426) have blurred the nomenclature distinction between these two different classes of inhibitors.
The expression of TIMPs is induced by LH/hCG in the ovary (292) with an increase in TIMP1 and TIMP2 in the monkey and human (described below). The four members of the TIMP family differ in their regulation and their selectivity for the different MMPs (423, 427, 428). TIMP1 preferentially binds to MMP9, whereas TIMP2 has a high affinity for MMP2. TIMP3 differs from TIMP1 and TIMP2 in that this inhibitor is secreted and then bound to the ECM as opposed to being free in the extracellular fluid (412, 423). The presence of TIMP3 in the ECM has been proposed to provide an additional regulatory stop point by acting at the site of MMP action (429, 430). In addition to their classical ability to regulate MMP action, TIMPs also regulate aspects of cellular physiology independent of the MMP action, including cell growth and steroidogenesis (431, 432), embryo growth and development (433), angiogenesis (434, 435), apoptosis (436), and entry into cell cycle (437).
The ADAM/ADAMTS family
The ADAMTS family includes a class of 19 related secreted metalloendopeptidases (272, 438, 439) (Fig. 11). The ADAMs differ from the ADAMTSs in that ADAMs lack the thrombospondin motifs as well as in their site of action. Most mammalian ADAMs are transmembrane proteins, whereas the ADAMTSs are secreted molecules (439). Another important difference is the ability of many ADAMTSs to bind to the ECM through the involvement of the thrombospondin repeats (440–442). In the monkey and the human, there is a periovulatory increase in ADAMTS1, ADAMTS4, ADAMTS9, and ADAMTS15 discussed below. Similar to the MMP system, the ADAMTSs have diverse actions, including degradation of proteoglycans such as aggrecan, brevican, and versican; processing of procollagens to collagen; antiangiogenic activity; and control of basal lamina remodeling (443, 444). The activity of the ADAMTSs in the extracellular space may be regulated by their interactions with the ECM and by the TIMPs. TIMP3 has been reported to be the major inhibitory TIMP that regulates ADAMTS activity (445, 446). Serum-borne inhibitors such as A2M also act as a substrate for some of the ADAMTSs and represent another endogenous inhibitor of the ADAMTSs (447).
The plasmin/plasminogen activator system
Plasminogen is a glycoprotein that is synthesized in the liver and brain and is enzymatically cleaved by PAs to form the protease plasmin (448, 449) (Fig. 12). Plasmin is a broad-spectrum serine protease that cleaves fibrin and fibrinogen as well as a variety of ECM proteins, including collagen types III, IV, and VI, gelatin, proteoglycans, elastin, fibronectin, laminin, and vitronectin, many of which are components of the ovarian follicle ECM (450, 451). Plasmin can also cleave and activate pro-MMPs (452). Owing to the abundance of plasminogen in body fluids, a relatively small increase in PAs can result in a large increase in plasmin activity (448).
Figure 12.
The plasmin–PA–MMP cascade. The PAs, PLAT or PLAU, are able to cleave plasminogen to plasmin. Plasmin has numerous actions, including activation of the MMP system to bring about matrix breakdown. Plasminogen activator action is regulated by PA inhibitors (SERPINE1 and SERPINB2) wheras MMP activity is regulated in the extracellular environment by the MMP inhibitors, the TIMPs.
The two main PAs are urokinase-type (PLAU) and tissue-type (PLAT). Although PLAU and PLAT are immunologically distinct proteins and the products of independent genes, they share similarities in their basic structures and physiological modes of action (450).
Activation of the plasmin/PA system is initiated by release of PLAT or PLAU from cells in response to external signals such as hormones, growth factors, or cytokines. PLAU is present in the ovary. However, in the monkey and the human, LH or hCG induces only the expression of PLAT (described below). The presence of PLAT or PLAU in extracellular fluids leads to locally restricted extracellular proteolytic activities (453–455). PLAU is able to bind to a specific receptor on the surface of cells; this binding results in concentrating PLAU activity at the cellular surface and enhances activation of plasminogen, thereby facilitating pericellular proteolysis (451, 456, 457). Control of local PA proteolysis is accomplished by the production of specific inhibitors, several of which are induced in the ovary. These inhibitors include PA inhibitor type 1 (SERPINE1), PA inhibitor type 2 (SERPINB2), PA inhibitor type 3 (SERPINA5), and the protease nexin I (451, 458). There are also direct inhibitors of plasmin activity, such as A2M.
Proteolysis in ovulation
Proteolytic systems have been postulated to play a paramount role in follicular rupture. This hypothesis is based on a number of experimental observations, including ECM degradation at the follicular apex, an LH/hCG-induced increase in protease expression and activity, and inhibition of oocyte release when protease action is blocked. As noted above, ovulation involves fragmentation of the collagenous matrix at the follicular apex (4, 459, 460). Biochemical analyses have revealed a decrease in ovarian and follicular collagen after the LH surge, especially at the apex (9, 461, 462). As described below, there is an LH/hCG induction of proteases, and proteolytic activity has been localized at the apex of the ovulatory follicle (Fig. 5). Extracts of rat ovulatory follicles are able to degrade ovarian follicular collagen (463). In further support of the proteases in follicular rupture, administration of synthetic protease inhibitors, including collagenase inhibitors, blocks ovulation in the perfused rat ovary (364). Intrabursal injection of broad-spectrum protease inhibitors blocked ovulation and breakdown of ovarian collagen in a dose-dependent manner (463), and intrabursal administration of antibodies to PLAT also reduced oocyte release (464). Finally, intrafollicular injection of a broad-spectrum metalloprotease inhibitor totally blocked ovulation (465). These observations provide the foundation for the concept that proteolysis acts on the ECM to weaken the follicle apex and bring about ovulation.
The MMP and ADAMTS systems in ovulation
Support for the MMP and ADAMTS systems in follicular rupture is evident from experiments where LH/hCG induce MMP and ADAMTS expression and activity (292, 304, 466–468) and ovulation is inhibited by blocking MMP activity with exogenous chemical MMP inhibitors or antibodies against the MMPs (265, 364, 463–465, 469, 470). Previous reviews have focused on species other than primates (20, 292, 452), so the following section of this review addresses the changes in the MMP system in the monkey and the human during the periovulatory period.
Studies in the monkey examined the expression of key MMPs and TIMPs in granulosa cells collected during the periovulatory period. Chaffin and Stouffer (471) aspirated granulosa cells from the dominant follicle prior to hCG administration (0 hours) or 12, 24, or 36 hours after hCG and examined mRNA expression of MMP1, MMP2, MMP3, MMP7, MMP9, TIMP1, and TIMP2. Within 12 hours after hCG, there was an increase in MMP1, MMP2, MMP7, TIMP1, and TIMP2. Levels of MMP9 mRNA did not increase until immediately prior to ovulation, that is, 36 hours after hCG (471). Expression of MMP3 was low to undetectable and did not change after hCG stimulation, similar to the pattern observed in human granulosa cells (discussed below). Peluffo et al. (465) examined the expression of key proteolytic systems in the rhesus monkey ovary; dominant follicles were collected across the periovulatory period. Administration of hCG rapidly increased mRNA levels for key MMPs (MMP1, MMP10, and MMP19) and ADAMTSs (ADAMTS4, ADAMTS9, and ADAMTS15). ADAMTS1 increased after ovulation in the rhesus monkey (465), which is in contrast to other species, including the human, where ADAMTS1 increases prior to follicular rupture (discussed below). In the mouse, a lack of ADAMTS1 results in subfertility due to a lack of cumulus expansion and impaired ovulation, with mature oocytes remaining trapped in the follicles (20, 101, 468). Overall, there is strong evidence for a post-hCG, preovulatory increase in follicular MMPs, TIMPs, and ADAMTSs in the monkey ovulatory follicle. The importance MMPs in primate ovulation was elegantly demonstrated by intrafollicular injection of GM6001 into preovulatory macaque follicles (465). GM6001 injection at the time of hCG administration resulted in trapped oocytes and the lack of a typical stigmata whereas all the vehicle-injected follicles ovulated. GM6001 injection did not alter luteal progesterone levels or luteal phase length (465). GM6001 may inhibit ovulation via multiple pathways. As noted above, GM6001 was able to block the cleavage and shedding of the EGF-like growth factors, which blocked EGFR activation and signaling (63), and MMPs may facilitate EGFR ligand availability in monkey follicles as well. These finding suggest that GM6001 may act directly to block MMP action on the ECM or activate key intrafollicular signaling pathways and highlight the importance of MMP-driven proteolysis to weaken the follicular wall prior to follicle rupture.
Steroid hormone regulation of MMPs and TIMPs has also been explored in the macaque follicle. Monkeys were treated with the HSD3B inhibitor trilostane alone to block steroid synthesis; additional animals received trilostane in combination with the nonmetabolizable progestin R5020 because blockade of progesterone synthesis blocked ovulation (471). Trilostane administration during hCG treatment decreased granulosa cell levels of the mRNA levels for MMP1, MMP2, MMP7, TIMP1, and TIMP2 compared with hCG alone. Progestin (R5020) replacement during hCG plus trilostane treatment returned MMP1 and TIMP1 mRNAs to control levels (471), suggesting that changes in MMP2, MMP7, and TIMP2 may be mediated by estrogens or androgens. These data provide a key link between changing follicular steroid hormone levels and proteolytic events in the ovulatory cascade.
Dynamic changes in the expression of the MMPs across the periovulatory period in women are less precisely understood, owing at least in part to the inherent challenges of collecting human ovarian tissue. However, significant progress has recently been made. Studies from our group have methodically obtained human ovulatory follicles from women divided into four groups (preovulatory phase, early ovulatory phase, late ovulatory phase, and postovulatory phase) to distinguish between the different stages of the ovulatory process, with hCG administered as the ovulatory trigger to obtain ovarian tissue at more precise stages of ovulation (8, 472, 473). These samples have provided insight into the changes in the ECM (8) (discussed above in the introductory section to this review) as well as changes in expression of gelatinases (473), stromelysins (474), and other MMPs and ADAMTSs (475). Western blot analysis of the entire dominant follicle revealed no change in the protein expression of MMP2, MMP9, or TIMP2 during the ovulatory phases (473). TIMP1 mRNA and protein increased in all ovulatory phases compared with the preovulatory phase, with TIMP1 immunoreactivity seen in both granulosa and theca cell layers (472, 473). McCord et al. (474) explored the changes in the stromelysins (MMP3, MMP10, MMP11) in the granulosa and theca cell compartments of human ovulatory follicles across the periovulatory period. There was a striking increase in granulosa cell levels of MMP10 mRNA between the preovulatory and early ovulatory periods, which remained elevated at the late ovulatory phase. In theca cells, MMP10 mRNA levels increased after hCG but returned to preovulatory levels by the postovulatory period. In contrast to MMP10, levels of MMP11 mRNA decreased ∼70% after hCG administration in both human granulosa and thecal cells. Expression of the other stromelysin, MMP3, was extremely low and did not change throughout the periovulatory period in either granulosa or theca cells (474), similar to the pattern seen in the monkey (471). In additional studies, administration of hCG induced increases in levels of mRNA for MMP1, MMP19, ADAMTS1, and ADAMTS9 in the granulosa cell compartment (475). However, the temporal mRNA expression pattern of the various MMPs and ADAMTSs in granulosa cells differed slightly among the proteases with expression of MMP1, ADAMTS1, and ADAMTS9 mRNA, increasing during the early ovulatory period, whereas expression of MMP19 mRNA was elevated during the late ovulatory period. For the other collagenases, MMP8 and MMP13 mRNA expression was extremely low and did not change throughout the periovulatory period in either granulosa or theca cells. In theca cells, MMP19 was the only protease that was induced by hCG, with mRNA expression increasing during the late ovulatory period and remaining elevated during the postovulatory period (475). Determining the localization of these proteases revealed an overall general pattern with increased staining intensity present in the granulosa and theca cell layers after hCG administration. Thus, the localization correlates with the observations of the mRNA expression, in which these proteases are highly abundant throughout the follicular cell layers before ovulation.
There were similarities in the hCG induction of the proteases between the monkey and the human. For example, there was an early induction of MMP1, MMP10, and ADAMTS9 in both the monkey and the human (465, 474, 475). However, as noted above, ADAMTS1 was induced after ovulation in the monkey (465) but was induced within 12 to 18 hours after hCG in the human (475). Yet, the activation of a number of proteolytic systems is to be expected if degradation of the components of the follicle wall comprised of various types of collagen, fibronectin, and laminin is to take place.
Studies of human ovarian tissues collected across the periovulatory period have been supplemented by examination of human granulosa cells and follicular fluid obtained immediately prior to ovulation during oocyte retrieval for in vitro fertilization (IVF). Follicular fluid was shown to have both MMP2 and MMP9 activity, with MMP2 predominating (476, 477). In women undergoing IVF, MMP9 activity in follicular fluid correlated with a higher rate of fertilization and pregnancy (478, 479). Bilen et al. (480) investigated the relationship between follicular fluid gelatinase, oocyte quality, and fertilization; they reported a positive correlation between MMP9 levels and oocyte quality and fertilization. Additionally, Yang et al. (481) found that human follicular fluid MMP2 activity was associated with improved oocyte maturity and higher fertilization rates. These studies demonstrate an increase in MMP2 and MMP9 during the period immediately preceding ovulation in the human. D’Ascenzo et al. (476) compared the protein levels of MMP2, MMP9, TIMP1, and TIMP2 in the follicular fluid of women undergoing IVF treatment vs those of normally ovulating women. They observed reduced MMP levels in follicular fluid of women undergoing IVF treatment compared with those of normally ovulating women, yet the levels of TIMP1 were increased in follicular fluid from IVF patients compared with normally ovulating women. These differences may be related to the hormonal environment induced by the IVF gonadotropin protocol (476). ADAMTS1 expression has also been positively correlated with IVF outcomes. For example, a significant correlation was reported between ADAMTS1 expression in cumulus cells and the fertilization capacity of the related oocyte (482). In women with PCOS undergoing IVF, ADAMTS1 expression was decreased compared with normally ovulating women (i.e., women undergoing IVF for male and/or tubal factor infertility) and was closely related to lower oocyte recovery, oocyte maturity, and fertilization rate (483).
The plasmin/plasminogen activator system in ovulation
Numerous studies have proposed that the PA system plays a crucial role in the degradation of the follicular wall during the process of oocyte release in rodents (6, 452, 453, 467, 484). For example, in the rat, ovulation is preceded by a transient and cell-specific expression of PLAT and SERPINE1, which is postulated to result in proteolytic activity localized to the surface of the ovary overlaying the ovulatory follicle prior to ovulation (485). In the mouse, PLAU is the most abundant and upregulated PA; however, double-knockout mice lacking both PLAT and PLAU have a 26% decreased ovulation rate (486), suggesting that PA activity contributes to ovulatory proteolysis. The following section focuses on the changes in the human and nonhuman primate.
PA family member expression and activity are highly regulated in the monkey ovulatory follicle. Peluffo et al. (465) demonstrated that whole follicle content of PLAU mRNA increased in response to hCG; differential expression of PLAT or PA inhibitors was not observed at the whole follicle level. Liu et al. (453) demonstrated that following exogenous hCG administration, granulosa cells are the major source of PLAT, as reflected by changes in mRNA and proteolytic activity, whereas the theca interstitial cells are the major source of SERPINE1 (453). Interestingly, the expression of PLAT and SERPINE1 differed, with the peak of SERPINE1 earlier than that of PLAT. When PLAT reached its highest level, the expression of SERPINE1 had already decreased to its lowest level. The result of this discordant expression of PLAT and SERPINE1 may be the generation of high PLAT activity in follicles just before ovulation, thereby facilitating the breakdown of the follicular wall to permit oocyte release (453). Other studies in the macaque, however, have suggested a different spatial relationship between the PA system and SERPINE1. Protein levels for both PLAT and SERPINE1 increased within 12 hours after hCG in granulosa cells and follicular fluid whereas PLAU protein was low or undetectable in granulosa cells and the ovarian stroma (487). However, even in the presence of elevated SERPINE1 protein, there was an increase in proteolytic activity as seen by in situ zymography of casein within 12 hours in the preovulatory follicle. This may reflect a spatial orientation of the PA/PA inhibitor complex or levels of PLAT that exceed those of the inhibitor that allow proteolysis to proceed. In support of this concept, Harris et al. (23) demonstrated lower levels of SERPINE1 protein in the apical region of the preovulatory follicle compared with the basal region, which would allow PA proteolysis to occur at the apex of the follicle while the basal region would be protected.
Early studies in the human reported the presence of low levels of PLAT mRNA and a lack of PLAU mRNA in human granulosa cells collected from IVF patients (488). In concordance with the presence of PLAT mRNA, follicular fluid collected from human preovulatory follicles at the time of IVF contained PA activity (489, 490).
“...LUF formation may be linked to dysregulation of the ovulation-associated inflammatory changes within the ovary.”
In contrast to the PAs, SERPINE1 mRNA and PA inhibitor activity were relatively abundant in the granulosa cells and follicular fluid retrieved at the time of IVF (488, 489). These studies were conducted during a period of burgeoning IVF activity, and there was a strong interest in correlating oocyte quality and IVF outcomes with ovarian proteolytic activity. Milwidsky et al. (491) reported that the fibrinolytic activity of PA was significantly higher in fluids from follicles that contained oocytes that were later found to fertilize in vitro compared with fluids from follicles that contained oocytes that failed to fertilize. Similar to follicular fluid, there was an increase in PLAT antigen in granulosa cell lysates from intermediate and mature COCs compared with granulosa cell lysates from immature COCs (492, 493). There were no correlations in PLAU levels or PA inhibitor levels with the maturity of COCs, leading to the suggestion PLAT is the predominant PA involved in the process leading to follicular rupture in the human (493). Overall, the findings from IVF patient materials suggest that PLAT is increased after hCG administration, and that levels of PLAT in follicular fluid positively correlate with IVF outcomes.
Inflammation and proteolysis
In addition to granulosa and theca cell–derived MMPs, ADAMTs, and PAs, the OSE is also a source of proteolytic enzymes. Yang et al. (7) examined inflammation-induced changes in the proteolytic pathway of human OSE cells exposed to TNF, IL-1A, PGF2α, and LH. In cultured human OSE, there was a marked induction of PLAU and MMP9 protein after TNF or IL-1B treatment, whereas PGF2α or LH treatment had a negligible effect. TNF also stimulated MMP19 protein but did not alter the levels of MMP2, MMP14, TIMP1, or TIMP2 protein expression (292). Using a gene expression profiling of cultured OSE cells treated with IL-1A, Rae et al. (494) revealed an upregulation (>10-fold) of MMP1, MMP8, MMP9, and MMP13 and a modest increase (>5-fold) of MMP3, MMP10, and MMP14.
Fedorcsák et al. (495) proposed that ovarian leukocytes were a source of MMP9 whereas granulosa-lutein cells were a source of TIMPs. In this study, granulosa-lutein cells were separated from leukocytes in aspirates from preovulatory follicles of women undergoing IVF. Peripheral blood–derived mononuclear cells were collected on the day of follicle aspiration from the same women donating follicular fluid. Cells were placed in culture for 48 hours and the conditioned media were subjected to antibody array analysis, which allowed simultaneous detection of multiple MMPs (1–3, 8–10, 13) and TIMPs (1–4). Both granulosa-lutein cells and leukocytes expressed all of the MMPs and all of the TIMPs. However, the authors proposed that granulosa-lutein cells preferentially expressed TIMP1, TIMP2, and TIMP3, whereas leukocytes expressed mostly MMP3, MMP8, MMP9, MMP10, as well as TIMP4; additionally, secretion of MMP1, MMP2, and MMP13 was comparable between the granulosa-lutein cells and leukocytes. These findings provide interesting insight into the potential crosstalk between inflammatory mediators and ovarian cells. As noted above, there may be a reciprocal interaction between inflammation and proteolysis where an inflammatory reaction can induce protease action that in turn can regulate the immune response, or, conversely, protease action may initiate the inflammatory response (413–415). However, the regulation of the proteolytic system in vivo may involve a more coordinated response between leukocytes and ovarian cells. For example, MMP10 is preferentially expressed in cultured leukocytes (495) whereas in vivo MMP10 is highly expressed in both granulosa and theca cells (474).
In summary, the LH surge induces an increase in the expression and activity of proteolytic pathways during the ovulatory process in women and monkeys. As a generalized schematic model, LH or hCG initiates and synchronizes a series of biochemical events that induce the expression and activity of the MMPs, ADAMs/ADAMTSs, and the PAs. Presently, the impact of inflammation on the LH-induced regulation of the protease system is unknown. This provides a future opportunity to unravel whether LH-stimulated induction of the proteases is dependent, independent, or influenced by inflammatory mediators during the ovulatory process.
Of interest, there does not appear to be a single protease that is paramount for ovulation, as mice deficient for single MMPs, ADAMTSs, or PAs continue to ovulate. In many ways this is not unexpected, as the various layers and compartments of the follicle wall contain a complex interweaving of collagens, laminins, fibronectins, and other proteins that should take the combined action of multiple proteases. Indeed, LH or hCG induce the expression of many different protease families. The strongest evidence for protease regulation of ECM turnover has been reported for ADAMTS1-deficient mice. Female mice lacking ADAMTS1 are subfertile and contain large follicles that failed to rupture (20, 99, 101, 468). ADAMTS1 has been shown to accumulate in the COC where it acts to cleave versican, with most cleavage occurring right around the time oocyte release from the follicle occurs (101). Another potential reason that a single protease has not been identified is that in many of the knockout mice there are reports of compensation after protease deletion. For example, deletion of one of the gelatinases, MMP2, results in an increase in the other gelatinase, MMP9 (496). However, evidence from broad-spectrum protease inhibitors indicates that appropriate expression of the proteolytic systems appears to be critical for oocyte maturation, ovulation, fertilization, and subsequent pregnancy.
Disorders of Ovulation Related to Inflammation and Immune Cells
Anovulation is a common cause of infertility in women, affecting up to 40% of infertile women (497). Anovulation of nonovarian origin can result from many causes, including obesity, thyroid disorders, and endometriosis. Primary ovarian insufficiency, also known as premature ovarian failure, leads to an early elimination of follicles capable of reaching ovulatory size. However, several common causes of anovulation of ovarian origin highlight the key role of inflammation and immune cells on the process of ovulation and are discussed below.
Luteinized unruptured follicle syndrome
Luteinized unruptured follicle (LUF) syndrome is defined as absence of follicle rupture and without release of an oocyte. LUFs occur despite normal follicular growth in the follicular phase, a normal hormonal profile throughout the menstrual cycle, and a luteal phase of normal length. The term LUF was first established in 1978 by Marik and Hulka (498), who observed that some infertile patients with normal menstrual cycles failed to produce an ovulatory stigmata or a corpus hemorrhagicum. Currently, the most reliable diagnostic tool for LUFs is repeated examinations with transvaginal ultrasound to detect the growth of the follicle just before ovulation and then observe the failure of follicular collapse or formation of a corpus luteum (499, 500). Laparoscopy after the expected time of ovulation cannot be used to reliably determine whether follicular rupture has occurred because the area over the stigmata may be covered by new surface epithelium soon after rupture (501). The rate of LUF formation in asymptomatic women with normal menstrual cycles is ∼5%, and recurrence in subsequent menstrual cycles is rare (502). However, LUFs are more frequent among infertile women, with >25% of infertile women showing evidence of LUF formation (503). Among infertile women with a documented LUF cycle, evidence of LUFs in subsequent cycles is 80% to 90% (503).
Information from both clinical and research settings suggests that LUF formation may be linked to dysregulation of the ovulation-associated inflammatory changes within the ovary. Some reports link LUFs to negative influences on the synthesis or action of prostaglandins. The general cyclooxygenase inhibitor indomethacin altered the vasculature of the preovulatory sheep follicles, and these structural changes were similar to those observed in LUFs in women (504). Increased blood volume was observed at the apex of sheep LUFs, whereas reduced blood volume was noted at the follicular apex in untreated sheep with normal ovulation (504). As detailed below, pharmacological inhibition of prostaglandin synthesis can induce LUF formation in humans as well. Defects in cytokine production or action may also be an underlying mechanism of LUF. Granulocyte colony-stimulating factor 3 (CSF3) has been linked to LUF formation in infertile women. In a normal ovulatory cycle, CSF3 concentration in peripheral blood increases during late follicular phase (505). In anovulatory women, a single injection of CSF3 during late follicular phase resulted in ovulation in most of the women (506). In women previously diagnosed with LUF syndrome, a single injection of CSF3 before hCG administration in clomiphene citrate treatment cycles reduced the occurrence of LUFs to 4% of cycles as compared with 19% in those women not receiving CSF3 (507). These results suggest that one pathophysiological mechanism leading to LUF formation is a defect in the mobilization and activation of ovarian granulocytes. This hypothesis is further strengthened by the observations that (i) selective neutrophil depletion in a rat model decreases ovulation rate (508), and (ii) administration of leukocytes in an ovarian perfusion system increases ovulation rate (365). However, it is likely that there exist several additional and as yet undiscovered pathophysiological mechanisms underlying the formation of LUFs, as LUFs are present in mice lacking the PGR (41).
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder in women of reproductive age (157). The characteristics of PCOS include ovulatory dysfunction, the presence of polycystic ovaries, and hyperandrogenism (157). However, aspects of metabolic syndrome (e.g., insulin resistance, dyslipidemia, hypertension, obesity) are also common among women with PCOS (157). The prevalence of PCOS is estimated to be between 5% and 10% of women (509–511), with variations depending on factors such as ethnicity and socioeconomic status (512).
Oligo-ovulation or anovulation associated with PCOS is related to dysfunction of the hypothalamic/pituitary–ovarian axis as well as local ovarian mechanisms. The systemic disturbance is due in part to tonic elevation of LH and the absence of an LH surge (513). Hyperinsulinemia is another endocrine hallmark of PCOS (512, 514), and there is indirect evidence that ovulation rates increase concomitant with administration of insulin-sensitizing agents such as metformin (515). Increased ovarian production of androgens in PCOS is multifactorial and due to increased insulin as well as increased theca cell production of androgens in response to the higher tonic LH level (516). Increased activity of the androgen synthesis enzyme CYP17A1 within theca cells has been reported (517). Additionally, overexpression of the V2 isoform of the endosome trafficking protein DENND1A has also shown strong correlation with PCOS (518).
PCOS is a proinflammatory state (519).
“Upon LH stimulation, the ovary exhibits many classical signs of local inflammation....”
A recent meta-analysis concluded that circulating levels of the inflammatory marker C-reactive protein were around twofold higher in women with PCOS when compared with women without PCOS (520). PCOS women are often overweight or obese (509). Obesity has been compared with a chronic inflammatory state, including an increase in proinflammatory cytokines coupled with invasion of macrophages in fat tissue (521). One study showed elevated levels of the inflammatory mediator TNF in women with PCOS and obesity as compared with women with comparable body mass indexes but without PCOS (522). However, a detailed study of follicular fluid from IVF cycles did not detect differences in TNF levels between women with PCOS and women without PCOS (523).
In recent years, gene expression analyses of granulosa cells and ovarian stroma have pointed toward alterations of the inflammatory transcriptome within the ovaries of women with PCOS. In a key study, ovarian expression levels were examined for almost 60 genes hypothesized to be involved in the pathophysiology of PCOS (524). In general, there was low expression of inflammatory-related genes in the central ovarian stroma of women with PCOS, whereas inflammatory-related genes were highly expressed in granulosa cells of women with PCOS. Stromal levels of mRNA for CCL2 and IL8 were decreased in women with PCOS; these cytokines function to attract monocytes, basophils, and neutrophils. The leukocyte marker CD45 was also decreased in ovarian stroma of women with PCOS, which may negatively impact ovulation, further supporting the role of leukocytes and inflammation in follicular rupture (351, 365). IL-1R1 was also underexpressed in stroma, suggesting that stroma of women with PCOS have decreased responses to proinflammatory IL-1 (524). Expression levels of TIMP1, NOS2, and RUNX2 were also decreased in ovarian stroma from women with PCOS; these gene products have also been implicated in ovulation (see “LH-Mediated Immediate Cellular Responses in Follicular Cells” and “Proteolytic Changes Associated With Inflammation During Ovulation”). Highly expressed inflammatory genes in granulosa cells of women with PCOS included IL8, IL1B, and NOS2 (524). Additionally, mRNA levels for LIF and the prostaglandin synthase PTGS2 were upregulated in granulosa cell of women with PCOS (524). Recently, blockade of LIF action in monkey ovulatory follicles was shown to prevent ovulation (525). Examination of the inflammatory transcriptome in granulosa-lutein cells of women with PCOS undergoing IVF also identified dysregulation of inflammatory mediators (526). Granulosa-lutein cell mRNA levels were elevated for IL8, TNF, CCL20, IL6, CXCL1, and CXCR1 in women with PCOS (526). CCL20 is markedly elevated near the time of ovulation during the menstrual cycle in women (126). Ovarian leukocytes contain the CCL20 receptor CCR6, and CCL20 is able to stimulate leukocyte migration (126). Interestingly, increased cytokine levels were even more pronounced in granulosa-lutein cells of patients with obesity and PCOS. Thus, women with PCOS and obesity represent a distinct subpopulation with regard to this proinflammatory state. Granulosa-lutein cells from women with PCOS also showed signs of increased leukocyte infiltration as detected by elevated mRNA and protein levels of the leukocyte marker CD45. Although ovarian leukocytes promote follicular rupture [see above and Ref. (365)], the invasion of these cells into the ovulatory follicle is finely tuned. Aberrations in the timing or the extent of leukocyte invasion may disrupt essential aspects of the ovulatory process. In summary, the dysregulation of these inflammatory mediators may disrupt the carefully orchestrated communication between compartments of the ovary, contributing to ovulation failure.
Ovarian leukocytes of women with PCOS show different cytokine profiles than do leukocytes from women without PCOS. CD4 lymphocytes were isolated from follicular aspirates at IVF and were then stimulated to release cytokines during culture (527). Ovarian lymphocytes from women with PCOS produced elevated amounts of the Th1-produced cytokines IFNG and IL-2 in vitro; follicular fluid levels were also elevated (527). The predominance of Th1 lymphocytes in ovaries of women with PCOS may be linked to the ovarian mechanisms that cause anovulation.
Approximately 30% of women with anovulatory PCOS are resistant to clomiphene citrate to induce ovulation (528). When circulating levels of inflammatory cytokines were compared between clomiphene citrate–resistant and clomiphene citrate–sensitive women with PCOS, it was found that levels of the chemokine CXCL16 were increased in the clomiphene citrate–resistant patients (382). There was also a correlation between CXCL16 and C-reactive protein levels, suggesting that dysregulation of inflammatory mediators can compromise the responsiveness of the ovarian follicle to ovulation-inducing gonadotropins.
Dysregulation of ovarian leukocytes or inflammatory mediators is unlikely to be the root cause of PCOS. However, available data are consistent with dysregulated inflammation as a contributing factor to anovulation in PCOS.
Effects of nonsteroidal anti-inflammatory drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used for relieving pain, lowering fever, and reducing swelling. NSAIDs have broad effects on the body by supressing production of prostaglandins, essential stimulators of inflammation. Because the concept that ovulation is an event with an inflammatory response, concern regarding the effects of NSAIDs on ovulation emerged. As early as 1986, case reports described a correlation between NSAID use and “reversible infertility” in women (529, 530). Prospective studies also examined the effect of NSAIDs on ovulation, using a variety of dosing regimens designed to include the interval between the LH surge and follicle rupture. Killick and Elstein (531) examined the effect of two different nonselective prostaglandin synthase inhibitors, azapropazone and indomethacin, on ovulation. The incidence of LUFs in cycles treated with azapropazone or indomethacin were 50% or 100%, respectively; the rate of LUFs in untreated cycles was only 10%. LUF cycles exhibited typical luteal progesterone levels, indicating that normal luteinization occurred. A major advance came with discovery of multiple forms of prostaglandin synthase and the demonstration that the ovarian follicle contained both the constitutively expressed prostaglandin synthase (PTGS1) and the inducible form (PTGS2). Expression of PTGS2 in the ovulatory follicle increased significantly before ovulation, and follicular expression of PTGS2 was shown to be necessary for ovulation (532, 533) (discussed in detail in “LH-Stimulated Production of Paracrine Mediators by Follicular Cells”). Rofecoxib was the first PTGS2-selective inhibitor used to study the consequences of PTGS2 inhibition on human ovulation (534). Delayed follicular rupture was seen in two-thirds of women who received rofecoxib, whereas ovulation occurred as expected in women treated with placebo (534) (Fig. 13). Meloxicam, a PTGS inhibitor with relative selectivity toward PTGS2, has been studied extensively to determine the ability of this PTGS inhibitor to impact ovulation. Meloxicam treatment resulted in a delay of follicle rupture of ∼5 days when compared with placebo cycles; meloxicam also resulted in a continued increase in follicle size during the treatment period without altering luteal progesterone levels (535). Subsequent studies confirmed dysfunctional ovulation in 50% of cycles with a lower dose of meloxicam and in 90% with a higher dosage (536). Perhaps surprisingly, ovulatory dysfunction was observed in only 25% to 30% of cycles after administration of the highly selective PTGS2 inhibitor celecoxib (537). Meloxicam administration to monkeys for 5 days around the day of the LH surge significantly reduced rates of follicle rupture, oocyte release, and pregnancy, demonstrating that disruption of ovulation and pregnancy prevention can be achieved in well-controlled nonhuman primate studies (538, 539).
Figure 13.
PTGS2 inhibitor prevents follicle rupture in women. (a and c) Ultrasound images show the preovulatory follicle antrum (dark circles) before the LH surge. (b) Placebo treatment (control) results in decreased size of the antrum, indicative of follicle rupture. (d) The PTGS2 inhibitor rofecoxib treatment yields a follicle which continued to enlarge without rupture. [Reproduced with permission from Pall M, Friden BE, Brannstrom M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod 2001;16(7):1323–1328.]
NSAIDs acting through inhibition of PTGS2 clearly compromise ovulation. Formation of LUFs as a result of NSAID administration suggests a role for PTGS2-derived follicular prostaglandins in follicle rupture and LUF syndrome. A role for prostaglandins in luteinization and progesterone production is less certain, with some studies showing no effect of PTGS inhibitors on luteal function and others showing only a modest reduction in luteal progesterone or luteal phase length (180). Most studies of NSAID inhibition of ovulation use doses of NSAIDs that are either at or above the maximum doses typically prescribed. Appropriate dosing with highly effective NSAIDs may ultimately yield ovulation inhibition comparable to currently available emergency contraceptives (537–540).
Overall, findings from human studies highlight that disorders of ovulation correlate with increases in inflammatory markers. Anovulation in women with PCOS, along with the observations that pharmacologically inhibiting the inflammatory response blocks oocyte release, supports the paramount role of inflammation in the ovulatory process in women.
Summary, Conclusions, and Perspectives
Inflammation triggered by pathogens or injury shares significant similarities with inflammation initiated by the LH surge in the ovulatory follicle. This observation led to the proposal that ovulation is, in itself, an inflammatory process (2). Upon LH stimulation, the ovary exhibits many classical signs of local inflammation: production of inflammatory mediators, increased blood flow, leukocyte infiltration, swelling, tissue digestion, and, ultimately, tissue repair. The past three decades of investigation into the mechanism of ovulation have not only strengthened support for Espey’s view that ovulation is an inflammatory process but have also led to a better understanding of how the inflammatory process is regulated in the ovary. Many cells, gene products, and activities that contribute to LH-initiated ovulatory inflammation have been identified, and the roles they play have been illuminated. In many ways these findings have supported Espey’s statement that “the intended question is whether the endocrine system might be operating, at least in those instances where gonadotropins stimulate their specific target tissues, by ‘taking advantage’ of the immunoresponsive capacity inherent in all cells in mammalian systems.”
Granulosa cells and theca cells that express LHCGR are the primary respondents to LH stimulation. Activated LHCGR triggers multiple intracellular signaling pathways (e.g., PKA, PKC, PI3K, MAPK) to collectively increase the expression or activity of key transcription factors (e.g., PGR, PPARG, HIFs, CEBPA, CEBPB, RUNX1, RUNX2, NR5A2, NRIP1). Subsequently, granulosa cells produce a cohort of enzymes (e.g., prostaglandin synthesis enzymes, steroidogenic enzymes, proteases and their inhibitors) that directly or indirectly lead the biochemical reactions necessary for successful release of the mature oocyte. Some products of these biochemical reactions include classical mediators of inflammation, that is, prostaglandins, vascular regulators, cytokines, and chemokines, providing support for Espey’s postulate that gonadotropins stimulate their specific target tissues to “take advantage” of the immunoresponsive capacity inherent in these ovarian cells. In fact, the granulosa cell production of large quantities of these mediators provides quantifiable evidence that the ovulatory cascade resembles many aspects of an inflammatory event. Every cell in the follicle, including granulosa cells, theca cells, endothelial cells, and resident immune cells, expresses receptors for and respond to at least some of these inflammatory mediators. Mounting evidence indicates that these mediators also attract additional inflammatory leukocytes (e.g., neutrophils, monocytes, macrophages, mast cells), which infiltrate the ovary and, in particular, the ovulatory follicle. Invading leukocytes produce additional cytokines and chemokines, amplifying the signals initiated by granulosa cells. The result is timely production and secretion of the final players of ovulation, including prostaglandins, vascular regulators, cytokines, chemokines, proteases, and endothelins. These final players coordinate the overall response, which results in COC expansion, follicle wall degradation, contraction, rupture of the follicle, and, ultimately, release of the cumulus enclosed oocyte. Invasion of immune cells and final ovulatory events are aided by new blood vessels and altered regional blood flow, which is regulated by inflammatory mediators, including prostaglandins, vascular growth factors, and likely steroid hormones. The final step in the inflammatory response is healing. The ovary undergoes a regenerative process after each ovulation, first clotting the ovulatory stigmata, then rapidly repairing the rupture site with fibrous stroma and surface epithelium.
A strength of the inflammatory hypothesis of ovulation is that it is supported by multiple experimental approaches. Techniques of molecular biology and gene expression profiling have identified many of the early events in the ovulatory cascade. Use of genetically modified animal models and intraovarian administration of antagonists collectively have demonstrated critical roles for many inflammatory mediators in the overall process of ovulation. In vivo experiments using leukocyte depletion–replacement approaches have established the importance of each subtype of immune cells in ovulation. Every essential step in the ovulatory inflammatory response has been supported in multiple species using divergent, complementary, and often inventive techniques.
The complexities of the ovulatory process will continue to challenge further investigation of ovulation and the role played by inflammation. Ovulation is a dynamic process, initiated by the LH surge and culminating in oocyte release in a matter of hours. Timing of events within the follicle is critical and, although detailed timeline studies have provided insight into this process in the rodent, such information does not exist in the human. Most experimental strategies make observations at preselected times, but continuous monitoring in future studies will be necessary to fully comprehend the temporal relationships between changes in gene expression, levels of paracrine mediators, invasion of different classes of leukocytes, enzyme activities, and structural changes within the follicle. Substantial progress has been made using reductionist in vitro approaches, but physical and chemical interactions between neighboring cells are likely required for successful ovulation. In the ovulatory follicle, granulosa and theca cells take on roles typically played by resident immune cells in pathogen-driven inflammation. This required involvement of resident, nonimmune cells may be unique to ovulatory inflammation. Finally, redundancy abounds in the ovulatory process. For example, tissue digestion by proteases is essential for follicle rupture and remodeling, but no single MMP has been shown to be critical for ovulation. Redundancy ensures the success of essential biological processes but also significantly complicates demonstration of the requirement for these processes.
Unanswered questions remain regarding the nature of the ovarian inflammatory response and the role of inflammation in ovulation. Immunosuppressive agents might be expected to prevent ovulation, but available data are inconclusive. Progesterone, cortisol, and estrogen typically serve as anti-inflammatory mediators during inflammatory responses. In the context of ovulation, steroids may be responsible for limiting inflammatory damage or ending the inflammatory response. Intrafollicular levels of these steroids are very high, often 100- to 1000-fold higher than found in the systemic circulation. How are these locally high concentrations of steroids compatible with inflammation during ovulation? Steroids, when acting as paracrine mediators, may take on unique and presently unappreciated roles in inflammatory responses. Chemokines and cytokines are produced by many cells during ovarian inflammation. Are these paracrine mediators used to establish communication between cells of the follicle and invading immune cells to coordinate functions? Invasion of immune cells may not occur evenly around the follicle, such that subpopulations of leukocytes are distributed in a manner that contributes to the regional responses in the follicle. For example, if the secretion of proteases from granulosa cells requires stimulation by immune cells, then concentration of leukocytes at the follicle apex may contribute to selection of the rupture site. Inflammation is an essential feature of ovulation, but controlled cessation of the inflammatory response is required to limit destruction and permit healing. What ends the inflammatory response in the ovary? Similarly, repeated inflammatory events are destructive and can result in loss of organ function. How does the ovary protect itself from repeated, cyclical inflammation? Enhanced understanding of the role of inflammation in ovulation and control of this process may lead to harnessing this knowledge to treat anovulatory infertility or prevent ovulation for contraceptive purposes.
Acknowledgments
The authors appreciate the generous assistance of Drs. Bruce Murphy, John Peluso, and Gerald Pepe for critical review of a draft of this manuscript. The technical expertise of Tom Dolan in the preparation of illustrations is greatly appreciated. The authors also acknowledge the assistance of Kathy Rosewell and Sandra Finch in the preparation of the manuscript.
Financial Support: All authors are supported by The Eunice Kennedy Shriver National Institutes of Child Health and Human Development Grant P01 HD071875 and the National Centers for Translational Research in Reproduction and Infertility.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- ADAM
a disintegrin and metalloproteinase
- ADAMTS
ADAM with thrombospondin motif
- A2M
α 2-macroglobulin
- ANGPT
angiopoietin
- CBF
core-binding factor
- CEBP
CCAAT/enhancer-binding protein
- COC
cumulus oocyte complex
- CREB
cAMP response element binding protein
- CSF3
colony-stimulating factor 3
- ECM
extracellular matrix
- EDN1
endothelin-1
- END2
endothelin-2
- EGF
epidermal growth factor
- EGFR
EGF receptor
- hCG
human chorionic gonadotropin
- HIF
hypoxia-inducible factor
- IVF
in vitro fertilization
- LHCGR
LH/hCG receptor
- LUF
luteinized unruptured follicle
- MMP
matrix metalloproteinase
- NRIP1
nuclear receptor–interacting protein 1
- NSAID
nonsteroidal anti-inflammatory drug
- OSE
ovarian surface epithelium
- PA
plasminogen activator
- PCOS
polycystic ovarian syndrome
- PGE2
prostaglandin E2
- PGF
placental growth factor
- PGF2α
prostaglandin F2α
- PGH2
prostaglandin H2
- PGR
progesterone receptor
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PLAT
tissue-type PA
- PLAU
urokinase-type PA
- PPARG
peroxisome proliferator-activated receptor γ
- PRA
PGR A
- PRB
PGR B
- PTGER
PGE2 receptor
- TIMP
tissue inhibitor of metalloproteinase
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
References
- 1. Bukovsky A, Presl J. Ovarian function and the immune system. Med Hypotheses. 1979;5(4):415–436. [DOI] [PubMed] [Google Scholar]
- 2. Espey LL. Ovulation as an inflammatory reaction—a hypothesis. Biol Reprod. 1980;22(1):73–106. [DOI] [PubMed] [Google Scholar]
- 3. Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. [DOI] [PubMed] [Google Scholar]
- 4. Espey LL. Ultrastructure of the apex of the rabbit graafian follicle during the ovulatory process. Endocrinology. 1967;81(2):267–276. [DOI] [PubMed] [Google Scholar]
- 5. Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr Rev. 2001;22(2):255–288. [DOI] [PubMed] [Google Scholar]
- 6. Murdoch WJ, McDonnel AC. Roles of the ovarian surface epithelium in ovulation and carcinogenesis. Reproduction. 2002;123(6):743–750. [DOI] [PubMed] [Google Scholar]
- 7. Yang WL, Godwin AK, Xu XX. Tumor necrosis factor-α-induced matrix proteolytic enzyme production and basement membrane remodeling by human ovarian surface epithelial cells: molecular basis linking ovulation and cancer risk. Cancer Res. 2004;64(4):1534–1540. [DOI] [PubMed] [Google Scholar]
- 8. Lind AK, Weijdegård B, Dahm-Kähler P, Mölne J, Sundfeldt K, Brännström M. Collagens in the human ovary and their changes in the perifollicular stroma during ovulation. Acta Obstet Gynecol Scand. 2006;85(12):1476–1484. [DOI] [PubMed] [Google Scholar]
- 9. Espey LL, Lipner H. Ovulation. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. Vol 1, 2nd ed. New York, NY: Raven Press; 1994:725–780. [Google Scholar]
- 10. Espey LL. Mammalian ovulation: morpho-physiological aspects. In: Motta PM, ed. Microscopy of Reproduction and Development: A Dynamic Approach. Rome, Italy: Antonio Delfino Editore; 1997:143–150. [Google Scholar]
- 11. Brannian JD, Stouffer RL. Cellular approaches to understanding the function and regulation of the primate corpus luteum. Semin Reprod Med. 1991;(4):341–351. [Google Scholar]
- 12. Everett JW. Pituitary and hypothalamus: perspective and overview. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. Vol 1, 2nd ed. New York, NY: Raven Press; 1994:1509–1526. [Google Scholar]
- 13. Direito A, Bailly S, Mariani A, Ecochard R. Relationships between the luteinizing hormone surge and other characteristics of the menstrual cycle in normally ovulating women. Fertil Steril. 2013;99(1):279–285. [DOI] [PubMed] [Google Scholar]
- 14. Weick RF, Dierschke DJ, Karsch FJ, Butler WR, Hotchkiss J, Knobil E. Periovulatory time courses of circulating gonadotropic and ovarian hormones in the rhesus monkey. Endocrinology. 1973;93(5):1140–1147. [DOI] [PubMed] [Google Scholar]
- 15. Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol. 2014;383(1–2):203–213. [DOI] [PubMed] [Google Scholar]
- 16. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129(6):3200–3207. [DOI] [PubMed] [Google Scholar]
- 17. Yung Y, Aviel-Ronen S, Maman E, Rubinstein N, Avivi C, Orvieto R, Hourvitz A. Localization of luteinizing hormone receptor protein in the human ovary. Mol Hum Reprod. 2014;20(9):844–849. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen T, Lee S, Hatzirodos N, Hummitzsch K, Sullivan TR, Rodgers RJ, Irving-Rodgers HF. Spatial differences within the membrana granulosa in the expression of focimatrix and steroidogenic capacity. Mol Cell Endocrinol. 2012;363(1–2):62–73. [DOI] [PubMed] [Google Scholar]
- 19. Choi Y, Wilson K, Hannon PR, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. Coordinated regulation among progesterone, prostaglandins, and EGF-like factors in human ovulatory follicles. J Clin Endocrinol Metab. 2017;102(6):1971–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97(9):4689–4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park OK, Mayo KE. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991;5(7):967–978. [DOI] [PubMed] [Google Scholar]
- 22. Quirk SM, Cowan RG, Harman RM. Progesterone receptor and the cell cycle modulate apoptosis in granulosa cells. Endocrinology. 2004;145(11):5033–5043. [DOI] [PubMed] [Google Scholar]
- 23. Harris SM, Aschenbach LC, Skinner SM, Dozier BL, Duffy DM. Prostaglandin E2 receptors are differentially expressed in subpopulations of granulosa cells from primate periovulatory follicles. Biol Reprod. 2011;85(5):916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Araújo VR, Duarte AB, Bruno JB, Pinho Lopes CA, de Figueiredo JR. Importance of vascular endothelial growth factor (VEGF) in ovarian physiology of mammals. Zygote. 2013;21(3):295–304. [DOI] [PubMed] [Google Scholar]
- 25. Diaz-Infante A Jr, Virutamasen P, Connaughton JF, Wright KH, Wallach EE. In vitro studies of human ovarian contractility. Obstet Gynecol. 1974;44(6):830–838. [PubMed] [Google Scholar]
- 26. Talbot P, Chacon RS. In vitro ovulation of hamster oocytes depends on contraction of follicular smooth muscle cells. J Exp Zool. 1982;224(3):409–415. [DOI] [PubMed] [Google Scholar]
- 27. Virutamasen P, Wright KH, Wallach EE. Monkey ovarian contractility—its relationship to ovulation. Fertil Steril. 1973;24(10):763–771. [DOI] [PubMed] [Google Scholar]
- 28. Cacioppo JA, Lin PP, Hannon PR, McDougle DR, Gal A, Ko C. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci Rep. 2017;7(1):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi DH, Kim EK, Kim KH, Lee KA, Kang DW, Kim HY, Bridges P, Ko C. Expression pattern of endothelin system components and localization of smooth muscle cells in the human pre-ovulatory follicle. Hum Reprod. 2011;26(5):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ko C, Gieske MC, Al-Alem L, Hahn Y, Su W, Gong MC, Iglarz M, Koo Y. Endothelin-2 in ovarian follicle rupture. Endocrinology. 2006;147(4):1770–1779. [DOI] [PubMed] [Google Scholar]
- 31. Martin GG, Talbot P. Drugs that block smooth muscle contraction inhibit in vivo ovulation in hamsters. J Exp Zool. 1981;216(3):483–491. [DOI] [PubMed] [Google Scholar]
- 32. Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. [DOI] [PubMed] [Google Scholar]
- 33. Irving-Rodgers HF, Hummitzsch K, Murdiyarso LS, Bonner WM, Sado Y, Ninomiya Y, Couchman JR, Sorokin LM, Rodgers RJ. Dynamics of extracellular matrix in ovarian follicles and corpora lutea of mice. Cell Tissue Res. 2010;339(3):613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Curry TE Jr, Song L, Wheeler SE. Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation, and early luteal formation in the rat. Biol Reprod. 2001;65(3):855–865. [DOI] [PubMed] [Google Scholar]
- 35. Kim J, Sato M, Li Q, Lydon JP, Demayo FJ, Bagchi IC, Bagchi MK. Peroxisome proliferator-activated receptor γ is a target of progesterone regulation in the preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28(5):1770–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sriraman V, Eichenlaub-Ritter U, Bartsch JW, Rittger A, Mulders SM, Richards JS. Regulated expression of ADAM8 (a disintegrin and metalloprotease domain 8) in the mouse ovary: evidence for a regulatory role of luteinizing hormone, progesterone receptor, and epidermal growth factor-like growth factors. Biol Reprod. 2008;78(6):1038–1048. [DOI] [PubMed] [Google Scholar]
- 37. Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol. 2007;21(10):2487–2502. [DOI] [PubMed] [Google Scholar]
- 38. Palanisamy GS, Cheon YP, Kim J, Kannan A, Li Q, Sato M, Mantena SR, Sitruk-Ware RL, Bagchi MK, Bagchi IC. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol Endocrinol. 2006;20(11):2784–2795. [DOI] [PubMed] [Google Scholar]
- 39. Kim J, Bagchi IC, Bagchi MK. Signaling by hypoxia-inducible factors is critical for ovulation in mice. Endocrinology. 2009;150(7):3392–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56(1–6 Spec No):67–77. [DOI] [PubMed] [Google Scholar]
- 41. Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9(18):2266–2278. [DOI] [PubMed] [Google Scholar]
- 42. Choi Y, Park JY, Wilson K, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. The expression of CXCR4 is induced by the luteinizing hormone surge and mediated by progesterone receptors in human preovulatory granulosa cells. Biol Reprod. 2017;96(6):1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sayasith K, Sirois J. Expression and regulation of stromal cell-derived factor-1 (SDF1) and chemokine CXC motif receptor 4 (CXCR4) in equine and bovine preovulatory follicles. Mol Cell Endocrinol. 2014;391(1–2):10–21. [DOI] [PubMed] [Google Scholar]
- 44. Mishra B, Park JY, Wilson K, Jo M. X-linked lymphocyte regulated gene 5c-like (Xlr5c-like) is a novel target of progesterone action in granulosa cells of periovulatory rat ovaries. Mol Cell Endocrinol. 2015;412:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jo M, Komar CM, Fortune JE. Gonadotropin surge induces two separate increases in messenger RNA for progesterone receptor in bovine preovulatory follicles. Biol Reprod. 2002;67(6):1981–1988. [DOI] [PubMed] [Google Scholar]
- 46. Choi Y, Rosewell KL, Brännström M, Akin JW, Curry TE Jr, Jo M. FOS, a critical downstream mediator of PGR and EGF signaling necessary for ovulatory prostaglandins in the human ovary. J Clin Endocrinol Metab. 2018;103(11):4241–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jo M, Curry TE Jr. Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles. Mol Endocrinol. 2006;20(9):2156–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ko C, In YH, Park-Sarge OK. Role of progesterone receptor activation in pituitary adenylate cyclase activating polypeptide gene expression in rat ovary. Endocrinology. 1999;140(11):5185–5194. [DOI] [PubMed] [Google Scholar]
- 49. Li F, Jang H, Puttabyatappa M, Jo M, Curry TE Jr. Ovarian FAM110C (family with sequence similarity 110C): induction during the periovulatory period and regulation of granulosa cell cycle kinetics in rats. Biol Reprod. 2012;86(6):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11(17):2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fan HY, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol. 2011;25(2):253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson K, Park J, Curry TE Jr, Mishra B, Gossen J, Taniuchi I, Jo M. Core binding factor-β knockdown alters ovarian gene expression and function in the mouse. Mol Endocrinol. 2016;30(7):733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee-Thacker S, Choi Y, Taniuchi I, Takarada T, Yoneda Y, Ko C, Jo M. Core binding factor β expression in ovarian granulosa cells is essential for female fertility. Endocrinology. 2018;159(5):2094–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22(14):1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bertolin K, Gossen J, Schoonjans K, Murphy BD. The orphan nuclear receptor Nr5a2 is essential for luteinization in the female mouse ovary. Endocrinology. 2014;155(5):1931–1943. [DOI] [PubMed] [Google Scholar]
- 56. Zhang C, Large MJ, Duggavathi R, DeMayo FJ, Lydon JP, Schoonjans K, Kovanci E, Murphy BD. Liver receptor homolog-1 is essential for pregnancy. Nat Med. 2013;19(8):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M. The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med. 2000;6(12):1368–1374. [DOI] [PubMed] [Google Scholar]
- 58. Tullet JM, Pocock V, Steel JH, White R, Milligan S, Parker MG. Multiple signaling defects in the absence of RIP140 impair both cumulus expansion and follicle rupture. Endocrinology. 2005;146(9):4127–4137. [DOI] [PubMed] [Google Scholar]
- 59. Nautiyal J, Steel JH, Rosell MM, Nikolopoulou E, Lee K, Demayo FJ, White R, Richards JS, Parker MG. The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary. Endocrinology. 2010;151(6):2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Richards JS, Jonassen JA, Rolfes AI, Kersey K, Reichert LE Jr. Adenosine 3′,5′-monophosphate, luteinizing hormone receptor, and progesterone during granulosa cell differentiation: effects of estradiol and follicle-stimulating hormone. Endocrinology. 1979;104(3):765–773. [DOI] [PubMed] [Google Scholar]
- 61. Mason NR, Marsh R. The effect of LH on cyclic AMP and progesterone in rat ovaries in vivo. Endocr Res Commun. 1975;2(2):167–177. [DOI] [PubMed] [Google Scholar]
- 62. Conti M. Specificity of the cyclic adenosine 3′,5′-monophosphate signal in granulosa cell function. Biol Reprod. 2002;67(6):1653–1661. [DOI] [PubMed] [Google Scholar]
- 63. Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22(4):924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology. 2002;143(8):2986–2994. [DOI] [PubMed] [Google Scholar]
- 65. Davis JS, Weakland LL, West LA, Farese RV. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem J. 1986;238(2):597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Breen SM, Andric N, Ping T, Xie F, Offermans S, Gossen JA, Ascoli M. Ovulation involves the luteinizing hormone-dependent activation of Gq/11 in granulosa cells. Mol Endocrinol. 2013;27(9):1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Woods DC, Johnson AL. Protein kinase C activity mediates LH-induced ErbB/Erk signaling in differentiated hen granulosa cells. Reproduction. 2007;133(4):733–741. [DOI] [PubMed] [Google Scholar]
- 68. Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135(12):2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22(9):2128–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Maizels ET, Mukherjee A, Sithanandam G, Peters CA, Cottom J, Mayo KE, Hunzicker-Dunn M. Developmental regulation of mitogen-activated protein kinase-activated kinases-2 and -3 (MAPKAPK-2/-3) in vivo during corpus luteum formation in the rat. Mol Endocrinol. 2001;15(5):716–733. [DOI] [PubMed] [Google Scholar]
- 71. Yamashita Y, Hishinuma M, Shimada M. Activation of PKA, p38 MAPK and ERK1/2 by gonadotropins in cumulus cells is critical for induction of EGF-like factor and TACE/ADAM17 gene expression during in vitro maturation of porcine COCs. J Ovarian Res. 2009;2(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu Z, Fan HY, Wang Y, Richards JS. Targeted disruption of Mapk14 (p38MAPKα) in granulosa cells and cumulus cells causes cell-specific changes in gene expression profiles that rescue COC expansion and maintain fertility. Mol Endocrinol. 2010;24(9):1794–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. [DOI] [PubMed] [Google Scholar]
- 74. Jin SL, Richard FJ, Kuo WP, D’Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96(21):11998–12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ben-Ami I, Freimann S, Armon L, Dantes A, Strassburger D, Friedler S, Raziel A, Seger R, Ron-El R, Amsterdam A. PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod. 2006;12(10):593–599. [DOI] [PubMed] [Google Scholar]
- 76. Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22(5):1247–1252. [DOI] [PubMed] [Google Scholar]
- 77. Fang L, Cheng JC, Chang HM, Sun YP, Leung PC. EGF-like growth factors induce COX-2-derived PGE2 production through ERK1/2 in human granulosa cells. J Clin Endocrinol Metab. 2013;98(12):4932–4941. [DOI] [PubMed] [Google Scholar]
- 78. Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. [DOI] [PubMed] [Google Scholar]
- 79. Peluffo MC, Ting AY, Zamah AM, Conti M, Stouffer RL, Zelinski MB, Hennebold JD. Amphiregulin promotes the maturation of oocytes isolated from the small antral follicles of the rhesus macaque. Hum Reprod. 2012;27(8):2430–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Høst T, Borup R, Grøndahl ML. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29(5):997–1010. [DOI] [PubMed] [Google Scholar]
- 81. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. [DOI] [PubMed] [Google Scholar]
- 82. Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE Jr, Ko C. Development and application of a rat ovarian gene expression database. Endocrinology. 2004;145(11):5384–5396. [DOI] [PubMed] [Google Scholar]
- 83. Sayasith K, Lussier J, Doré M, Sirois J. Human chorionic gonadotropin-dependent up-regulation of epiregulin and amphiregulin in equine and bovine follicles during the ovulatory process. Gen Comp Endocrinol. 2013;180:39–47. [DOI] [PubMed] [Google Scholar]
- 84. Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324(5929):938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Murdoch WJ, Peterson TA, Van Kirk EA, Vincent DL, Inskeep EK. Interactive roles of progesterone, prostaglandins, and collagenase in the ovulatory mechanism of the ewe. Biol Reprod. 1986;35(5):1187–1194. [DOI] [PubMed] [Google Scholar]
- 87. Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93(5):1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pall M, Mikuni M, Mitsube K, Brännström M. Time-dependent ovulation inhibition of a selective progesterone-receptor antagonist (Org 31710) and effects on ovulatory mediators in the in vitro perfused rat ovary. Biol Reprod. 2000;63(6):1642–1647. [DOI] [PubMed] [Google Scholar]
- 89. Uilenbroek JT, Sánchez-Criado JE, Karels B. Decreased luteinizing hormone-stimulated progesterone secretion by preovulatory follicles isolated from cyclic rats treated with the progesterone antagonist RU486. Biol Reprod. 1992;47(3):368–373. [DOI] [PubMed] [Google Scholar]
- 90. Akison LK, Robertson SA, Gonzalez MB, Richards JS, Smith CW, Russell DL, Robker RL. Regulation of the ovarian inflammatory response at ovulation by nuclear progesterone receptor. Am J Reprod Immunol. 2018;79(6):e12835. [DOI] [PubMed] [Google Scholar]
- 91. Preciado-Martínez E, García-Ruíz G, Flores-Espinosa P, Bermejo-Martínez L, Espejel-Nuñez A, Estrada-Gutiérrez G, Razo-Aguilera G, Granados-Cepeda M, Helguera-Repetto AC, Irles C, Zaga-Clavellina V. Progesterone suppresses the lipopolysaccharide-induced pro-inflammatory response in primary mononuclear cells isolated from human placental blood. Immunol Invest. 2018;47(2):181–195. [DOI] [PubMed] [Google Scholar]
- 92. Santos SJ, Aupperlee MD, Xie J, Durairaj S, Miksicek R, Conrad SE, Leipprandt JR, Tan YS, Schwartz RC, Haslam SZ. Progesterone receptor A-regulated gene expression in mammary organoid cultures. J Steroid Biochem Mol Biol. 2009;115(3-5):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xue XT, Kou XX, Li CS, Bi RY, Meng Z, Wang XD, Zhou YH, Gan YH. Progesterone attenuates temporomandibular joint inflammation through inhibition of NF-κB pathway in ovariectomized rats. Sci Rep. 2017;7(1):15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. García V, Kohen P, Maldonado C, Sierralta W, Munoz A, Villarroel C, Strauss JF 3rd, Devoto L. Transient expression of progesterone receptor and cathepsin-l in human granulosa cells during the periovulatory period. Fertil Steril. 2012;97(3):707–713.e1. [DOI] [PubMed]
- 95. Chaffin CL, Stouffer RL, Duffy DM. Gonadotropin and steroid regulation of steroid receptor and aryl hydrocarbon receptor messenger ribonucleic acid in macaque granulosa cells during the periovulatory interval. Endocrinology. 1999;140(10):4753–4760. [DOI] [PubMed] [Google Scholar]
- 96. Hild-Petito S, Stouffer RL, Brenner RM. Immunocytochemical localization of estradiol and progesterone receptors in the monkey ovary throughout the menstrual cycle. Endocrinology. 1988;123(6):2896–2905. [DOI] [PubMed] [Google Scholar]
- 97. Iwai T, Nanbu Y, Iwai M, Taii S, Fujii S, Mori T. Immunohistochemical localization of oestrogen receptors and progesterone receptors in the human ovary throughout the menstrual cycle. Virchows Arch A Pathol Anat Histopathol. 1990;417(5):369–375. [DOI] [PubMed] [Google Scholar]
- 98. Shozu M, Minami N, Yokoyama H, Inoue M, Kurihara H, Matsushima K, Kuno K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J Mol Endocrinol. 2005;35(2):343–355. [DOI] [PubMed] [Google Scholar]
- 99. Mittaz L, Russell DL, Wilson T, Brasted M, Tkalcevic J, Salamonsen LA, Hertzog PJ, Pritchard MA. Adamts-1 is essential for the development and function of the urogenital system. Biol Reprod. 2004;70(4):1096–1105. [DOI] [PubMed] [Google Scholar]
- 100. Brown HM, Dunning KR, Robker RL, Boerboom D, Pritchard M, Lane M, Russell DL. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol Reprod. 2010;83(4):549–557. [DOI] [PubMed] [Google Scholar]
- 101. Russell DL, Doyle KM, Ochsner SA, Sandy JD, Richards JS. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J Biol Chem. 2003;278(43):42330–42339. [DOI] [PubMed] [Google Scholar]
- 102. Sriraman V, Rudd MD, Lohmann SM, Mulders SM, Richards JS. Cyclic guanosine 5′-monophosphate-dependent protein kinase II is induced by luteinizing hormone and progesterone receptor-dependent mechanisms in granulosa cells and cumulus oocyte complexes of ovulating follicles. Mol Endocrinol. 2006;20(2):348–361. [DOI] [PubMed] [Google Scholar]
- 103. Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M, Evans RM. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Komar CM, Braissant O, Wahli W, Curry TE Jr. Expression and localization of PPARs in the rat ovary during follicular development and the periovulatory period. Endocrinology. 2001;142(11):4831–4838. [DOI] [PubMed] [Google Scholar]
- 105. Cacioppo JA, Oh SW, Kim HY, Cho J, Lin PC, Yanagisawa M, Ko C. Loss of function of endothelin-2 leads to reduced ovulation and CL formation. PLoS One. 2014;9(4):e96115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pall M, Hellberg P, Brännström M, Mikuni M, Peterson CM, Sundfeldt K, Nordén B, Hedin L, Enerbäck S. The transcription factor C/EBP-β and its role in ovarian function; evidence for direct involvement in the ovulatory process. EMBO J. 1997;16(17):5273–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBPβ promoter element. J Biol Chem. 1993;268(29):21931–21938. [PubMed] [Google Scholar]
- 108. Coffman JA. Runx transcription factors and the developmental balance between cell proliferation and differentiation. Cell Biol Int. 2003;27(4):315–324. [DOI] [PubMed] [Google Scholar]
- 109. Hernandez-Gonzalez I, Gonzalez-Robayna I, Shimada M, Wayne CM, Ochsner SA, White L, Richards JS. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20(6):1300–1321. [DOI] [PubMed] [Google Scholar]
- 110. Park ES, Lind AK, Dahm-Kähler P, Brännström M, Carletti MZ, Christenson LK, Curry TE Jr, Jo M. RUNX2 transcription factor regulates gene expression in luteinizing granulosa cells of rat ovaries. Mol Endocrinol. 2010;24(4):846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Park ES, Park J, Franceschi RT, Jo M. The role for runt related transcription factor 2 (RUNX2) as a transcriptional repressor in luteinizing granulosa cells. Mol Cell Endocrinol. 2012;362(1–2):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hinshelwood MM, Repa JJ, Shelton JM, Richardson JA, Mangelsdorf DJ, Mendelson CR. Expression of LRH-1 and SF-1 in the mouse ovary: localization in different cell types correlates with differing function. Mol Cell Endocrinol. 2003;207(1–2):39–45. [DOI] [PubMed] [Google Scholar]
- 113. Falender AE, Lanz R, Malenfant D, Belanger L, Richards JS. Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology. 2003;144(8):3598–3610. [DOI] [PubMed] [Google Scholar]
- 114. Parker M, Leonardsson G, White R, Steel J, Milligan S. Identification of RIP140 as a nuclear receptor cofactor with a role in female reproduction. FEBS Lett. 2003;546(1):149–153. [DOI] [PubMed] [Google Scholar]
- 115. Seo YM, Park JI, Park HJ, Kim SG, Chun SY. Gonadotropin regulation of RIP140 messenger ribonucleic acid expression in the rat ovary. Life Sci. 2007;81(12):1003–1008. [DOI] [PubMed] [Google Scholar]
- 116. Stouffer RL, Xu F, Duffy DM. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12(1):297–307. [DOI] [PubMed] [Google Scholar]
- 117. Andersen CY, Ezcurra D. Human steroidogenesis: implications for controlled ovarian stimulation with exogenous gonadotropins. Reprod Biol Endocrinol. 2014;12(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Gore-Langton RE, Armstrong DT. Follicular steroidogenesis and its control. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. Vol 1, 2nd ed. New York, NY: Raven Press; 1994:571–627. [Google Scholar]
- 119. King SR, LaVoie HA. Gonadal transactivation of STARD1, CYP11A1 and HSD3B. Front Biosci. 2012;17(1):824–846. [DOI] [PubMed] [Google Scholar]
- 120. Xu F, Stouffer RL, Müller J, Hennebold JD, Wright JW, Bahar A, Leder G, Peters M, Thorne M, Sims M, Wintermantel T, Lindenthal B. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17(3):152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Amin M, Simerman A, Cho M, Singh P, Briton-Jones C, Hill D, Grogan T, Elashoff D, Clarke NJ, Chazenbalk GD, Dumesic DA. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. J Clin Endocrinol Metab. 2014;99(4):1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Peluso JJ, Liu X, Gawkowska A, Johnston-MacAnanny E. Progesterone activates a progesterone receptor membrane component 1-dependent mechanism that promotes human granulosa/luteal cell survival but not progesterone secretion. J Clin Endocrinol Metab. 2009;94(7):2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Edwards DP, Altmann M, DeMarzo A, Zhang Y, Weigel NL, Beck CA. Progesterone receptor and the mechanism of action of progesterone antagonists. J Steroid Biochem Mol Biol. 1995;53(1–6):449–458. [DOI] [PubMed] [Google Scholar]
- 124. Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the Δ5 and Δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56(4):789–799. [DOI] [PubMed] [Google Scholar]
- 125. Chaffin CL, Hess DL, Stouffer RL. Dynamics of periovulatory steroidogenesis in the rhesus monkey follicle after ovarian stimulation. Hum Reprod. 1999;14(3):642–649. [DOI] [PubMed] [Google Scholar]
- 126. Al-Alem L, Puttabyatappa M, Rosewell K, Brännström M, Akin J, Boldt J, Muse K, Curry TE Jr. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology. 2015;156(9):3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dehennin L. Cortisol determination in follicular fluid. Fertil Steril. 1990;54(2):366–367. [DOI] [PubMed] [Google Scholar]
- 128. Fru KN, VandeVoort CA, Chaffin CL. Mineralocorticoid synthesis during the periovulatory interval in macaques. Biol Reprod. 2006;75(4):568–574. [DOI] [PubMed] [Google Scholar]
- 129. Sneeringer R, Penzias AS, Barrett B, Usheva A. High levels of mineralocorticoids in preovulatory follicular fluid could contribute to oocyte development. Fertil Steril. 2011;95(1):182–187. [DOI] [PubMed] [Google Scholar]
- 130. Andersen CY. Possible new mechanism of cortisol action in female reproductive organs: physiological implications of the free hormone hypothesis. J Endocrinol. 2002;173(2):211–217. [DOI] [PubMed] [Google Scholar]
- 131. Hillier SG, Tetsuka M. An anti-inflammatory role for glucocorticoids in the ovaries? J Reprod Immunol. 1998;39(1–2):21–27. [DOI] [PubMed] [Google Scholar]
- 132. Horie K, Takakura K, Fujiwara H, Suginami H, Liao S, Mori T. Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum Reprod. 1992;7(2):184–190. [DOI] [PubMed] [Google Scholar]
- 133. Suzuki T, Sasano H, Kimura N, Tamura M, Fukaya T, Yajima A, Nagura H. Immunohistochemical distribution of progesterone, androgen and oestrogen receptors in the human ovary during the menstrual cycle: relationship to expression of steroidogenic enzymes. Hum Reprod. 1994;9(9):1589–1595. [DOI] [PubMed] [Google Scholar]
- 134. Gemzell-Danielsson K, Berger C, Lalitkumar PG. Emergency contraception—mechanisms of action. Contraception. 2013;87(3):300–308. [DOI] [PubMed] [Google Scholar]
- 135. Kraus WL, Montano MM, Katzenellenbogen BS. Cloning of the rat progesterone receptor gene 5′-region and identification of two functionally distinct promoters. Mol Endocrinol. 1993;7(12):1603–1616. [DOI] [PubMed] [Google Scholar]
- 136. Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68(10–13):771–778. [DOI] [PubMed] [Google Scholar]
- 137. Natraj U, Richards JS. Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology. 1993;133(2):761–769. [DOI] [PubMed] [Google Scholar]
- 138. Artimani T, Saidijam M, Aflatoonian R, Amiri I, Ashrafi M, Shabab N, Mohammadpour N, Mehdizadeh M. Estrogen and progesterone receptor subtype expression in granulosa cells from women with polycystic ovary syndrome. Gynecol Endocrinol. 2015;31(5):379–383. [DOI] [PubMed] [Google Scholar]
- 139. Shao R, Markström E, Friberg PA, Johansson M, Billig H. Expression of progesterone receptor (PR) A and B isoforms in mouse granulosa cells: stage-dependent PR-mediated regulation of apoptosis and cell proliferation. Biol Reprod. 2003;68(3):914–921. [DOI] [PubMed] [Google Scholar]
- 140. Bishop CV, Hennebold JD, Kahl CA, Stouffer RL. Knockdown of progesterone receptor (PGR) in macaque granulosa cells disrupts ovulation and progesterone production. Biol Reprod. 2016;94(5):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57(1):339–355. [DOI] [PubMed] [Google Scholar]
- 142. Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction. 2014;147(5):R169–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology. 2006;147(6):3133–3140. [DOI] [PubMed] [Google Scholar]
- 144. Clark NC, Pru CA, Yee SP, Lydon JP, Peluso JJ, Pru JK. Conditional ablation of progesterone receptor membrane component 2 causes female premature reproductive senescence. Endocrinology. 2017;158(3):640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. McCallum ML, Pru CA, Niikura Y, Yee SP, Lydon JP, Peluso JJ, Pru JK. Conditional ablation of progesterone receptor membrane component 1 results in subfertility in the female and development of endometrial cysts. Endocrinology. 2016;157(9):3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hild-Petito S, West NB, Brenner RM, Stouffer RL. Localization of androgen receptor in the follicle and corpus luteum of the primate ovary during the menstrual cycle. Biol Reprod. 1991;44(3):561–568. [DOI] [PubMed] [Google Scholar]
- 147. Tetsuka M, Hillier SG. Differential regulation of aromatase and androgen receptor in granulosa cells. J Steroid Biochem Mol Biol. 1997;61(3–6):233–239. [PubMed] [Google Scholar]
- 148. Hillier SG, Tetsuka M, Fraser HM. Location and developmental regulation of androgen receptor in primate ovary. Hum Reprod. 1997;12(1):107–111. [DOI] [PubMed] [Google Scholar]
- 149. Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149(4):R193–R218. [DOI] [PubMed] [Google Scholar]
- 150. Peluso JJ, Stude D, Steger RW. Role of androgens in hCG-induced ovulation in PMSG-primed immature rats. Acta Endocrinol (Copenh). 1980;93(4):505–512. [DOI] [PubMed] [Google Scholar]
- 151. Mori T, Suzuki A, Nishimura T, Kambegawa A. Evidence for androgen participation in induced ovulation in immature rats. Endocrinology. 1977;101(2):623–626. [DOI] [PubMed] [Google Scholar]
- 152. Yazawa T, Kawabe S, Kanno M, Mizutani T, Imamichi Y, Ju Y, Matsumura T, Yamazaki Y, Usami Y, Kuribayashi M, Shimada M, Kitano T, Umezawa A, Miyamoto K. Androgen/androgen receptor pathway regulates expression of the genes for cyclooxygenase-2 and amphiregulin in periovulatory granulosa cells. Mol Cell Endocrinol. 2013;369(1-2):42–51. [DOI] [PubMed] [Google Scholar]
- 153. Borman SM, Chaffin CL, Schwinof KM, Stouffer RL, Zelinski-Wooten MB. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin surge. Biol Reprod. 2004;71(1):366–373. [DOI] [PubMed] [Google Scholar]
- 154. Walters KA, Handelsman DJ. Role of androgens in the ovary. Mol Cell Endocrinol. 2018;465:36–47. [DOI] [PubMed] [Google Scholar]
- 155. De los Santos MJ, García-Láez V, Beltrán-Torregrosa D, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Pellicer A, Labarta E. Hormonal and molecular characterization of follicular fluid, cumulus cells and oocytes from pre-ovulatory follicles in stimulated and unstimulated cycles. Hum Reprod. 2012;27(6):1596–1605. [DOI] [PubMed] [Google Scholar]
- 156. Ota M, Sato N, Takahashi S, Ono S. Characteristics of the binding component for testosterone and androstenedione in rat liver cytosol. Endocrinol Jpn. 1980;27(3):321–328. [DOI] [PubMed] [Google Scholar]
- 157.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–47. [DOI] [PubMed] [Google Scholar]
- 158. De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83(1):99–102. [DOI] [PubMed] [Google Scholar]
- 159. Paradisi R, Fabbri R, Battaglia C, Venturoli S. Ovulatory effects of flutamide in the polycystic ovary syndrome. Gynecol Endocrinol. 2013;29(4):391–395. [DOI] [PubMed] [Google Scholar]
- 160. Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 161. Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101(12):2622–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Lee S, Kang DW, Hudgins-Spivey S, Krust A, Lee EY, Koo Y, Cheon Y, Gye MC, Chambon P, Ko C. Theca-specific estrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology. 2009;150(8):3855–3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pelletier G, El-Alfy M. Immunocytochemical localization of estrogen receptors α and β in the human reproductive organs. J Clin Endocrinol Metab. 2000;85(12):4835–4840. [DOI] [PubMed] [Google Scholar]
- 164. Yang P, Wang J, Shen Y, Roy SK. Developmental expression of estrogen receptor (ER) α and ERβ in the hamster ovary: regulation by follicle-stimulating hormone. Endocrinology. 2004;145(12):5757–5766. [DOI] [PubMed] [Google Scholar]
- 165. Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol. 2000;165(2):359–370. [DOI] [PubMed] [Google Scholar]
- 166. Hiroi H, Inoue S, Watanabe T, Goto W, Orimo A, Momoeda M, Tsutsumi O, Taketani Y, Muramatsu M. Differential immunolocalization of estrogen receptor α and β in rat ovary and uterus. J Mol Endocrinol. 1999;22(1):37–44. [DOI] [PubMed] [Google Scholar]
- 167. Fitzpatrick SL, Funkhouser JM, Sindoni DM, Stevis PE, Deecher DC, Bapat AR, Merchenthaler I, Frail DE. Expression of estrogen receptor-β protein in rodent ovary. Endocrinology. 1999;140(6):2581–2591. [DOI] [PubMed] [Google Scholar]
- 168. Schomberg DW, Couse JF, Mukherjee A, Lubahn DB, Sar M, Mayo KE, Korach KS. Targeted disruption of the estrogen receptor-α gene in female mice: characterization of ovarian responses and phenotype in the adult. Endocrinology. 1999;140(6):2733–2744. [DOI] [PubMed] [Google Scholar]
- 169. Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors α and β in reproductive tissues. Reproduction. 2003;125(2):143–149. [DOI] [PubMed] [Google Scholar]
- 170. Antal MC, Petit-Demoulière B, Meziane H, Chambon P, Krust A. Estrogen dependent activation function of ERβ is essential for the sexual behavior of mouse females. Proc Natl Acad Sci USA. 2012;109(48):19822–19827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Rabinovici J, Blankstein J, Goldman B, Rudak E, Dor Y, Pariente C, Geier A, Lunenfeld B, Mashiach S. In vitro fertilization and primary embryonic cleavage are possible in 17α-hydroxylase deficiency despite extremely low intrafollicular 17β-estradiol. J Clin Endocrinol Metab. 1989;68(3):693–697. [DOI] [PubMed] [Google Scholar]
- 172. Pellicer A, Miró F, Sampaio M, Gómez E, Bonilla-Musoles FM. In vitro fertilization as a diagnostic and therapeutic tool in a patient with partial 17,20-desmolase deficiency. Fertil Steril. 1991;55(5):970–975. [DOI] [PubMed] [Google Scholar]
- 173. Andersen CY. Levels of steroid-binding proteins and steroids in human preovulatory follicle fluid and serum as predictors of success in in vitro fertilization-embryo transfer treatment. J Clin Endocrinol Metab. 1990;71(5):1375–1381. [DOI] [PubMed] [Google Scholar]
- 174. Ben-Rafael Z, Mastroianni L Jr, Meloni F, Lee MS, Flickinger GL. Total estradiol, free estradiol, sex hormone-binding globulin, and the fraction of estradiol bound to sex hormone-binding globulin in human follicular fluid. J Clin Endocrinol Metab. 1986;63(5):1106–1111. [DOI] [PubMed] [Google Scholar]
- 175. Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol Rev. 1983;75(1):117–138. [DOI] [PubMed] [Google Scholar]
- 176. Van Voorhis BJ, Anderson DJ, Hill JA. The effects of RU 486 on immune function and steroid-induced immunosuppression in vitro. J Clin Endocrinol Metab. 1989;69(6):1195–1199. [DOI] [PubMed] [Google Scholar]
- 177. Bishop CV, Xu F, Molskness TA, Stouffer RL, Hennebold JD. Dynamics of immune cell types within the macaque corpus luteum during the menstrual cycle: role of progesterone. Biol Reprod. 2015;93(5):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y. Prostaglandin E2-induced inflammation: relevance of prostaglandin E receptors. Biochim Biophys Acta. 2015;1851(4):414–421. [DOI] [PubMed] [Google Scholar]
- 179. Martínez-Boví R, Cuervo-Arango J. Intrafollicular treatment with prostaglandins PGE2 and PGF2α inhibits the formation of luteinised unruptured follicles and restores normal ovulation in mares treated with flunixin-meglumine. Equine Vet J. 2016;48(2):211–217. [DOI] [PubMed] [Google Scholar]
- 180. Duffy DM. Novel contraceptive targets to inhibit ovulation: the prostaglandin E2 pathway. Hum Reprod Update. 2015;21(5):652–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38(1):97–120. [DOI] [PubMed] [Google Scholar]
- 182. Wong WY, Richards JS. Evidence for two antigenically distinct molecular weight variants of prostaglandin H synthase in the rat ovary. Mol Endocrinol. 1991;5(9):1269–1279. [DOI] [PubMed] [Google Scholar]
- 183. Duffy DM, Stouffer RL. The ovulatory gonadotrophin surge stimulates cyclooxygenase expression and prostaglandin production by the monkey follicle. Mol Hum Reprod. 2001;7(8):731–739. [DOI] [PubMed] [Google Scholar]
- 184. Richards JS. Sounding the alarm—does induction of prostaglandin endoperoxide synthase-2 control the mammalian ovulatory clock? Endocrinology. 1997;138(10):4047–4048. [DOI] [PubMed] [Google Scholar]
- 185. Duffy DM, Dozier BL, Seachord CL. Prostaglandin dehydrogenase and prostaglandin levels in periovulatory follicles: implications for control of primate ovulation by prostaglandin E2. J Clin Endocrinol Metab. 2005;90(2):1021–1027. [DOI] [PubMed] [Google Scholar]
- 186. Dozier BL, Watanabe K, Duffy DM. Two pathways for prostaglandin F2α synthesis by the primate periovulatory follicle. Reproduction. 2008;136(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sirois J, Doré M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology. 1997;138(10):4427–4434. [DOI] [PubMed] [Google Scholar]
- 188. Liu J, Carrière PD, Doré M, Sirois J. Prostaglandin G/H synthase-2 is expressed in bovine preovulatory follicles after the endogenous surge of luteinizing hormone. Biol Reprod. 1997;57(6):1524–1531. [DOI] [PubMed] [Google Scholar]
- 189. Sayasith K, Bouchard N, Doré M, Sirois J. Cloning of equine prostaglandin dehydrogenase and its gonadotropin-dependent regulation in theca and mural granulosa cells of equine preovulatory follicles during the ovulatory process. Reproduction. 2007;133(2):455–466. [DOI] [PubMed] [Google Scholar]
- 190. Yerushalmi GM, Markman S, Yung Y, Maman E, Aviel-Ronen S, Orvieto R, Adashi EY, Hourvitz A. The prostaglandin transporter (PGT) as a potential mediator of ovulation. Sci Transl Med. 2016;8(338):338ra68. [DOI] [PubMed] [Google Scholar]
- 191. Kim SO, Duffy DM. Mapping PTGERs to the ovulatory follicle: regional responses to the ovulatory PGE2 signal. Biol Reprod. 2016;95(2):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Orlicky DJ, Fisher L, Dunscomb N, Miller GJ. Immunohistochemical localization of PGF2α receptor in the rat ovary. Prostaglandins Leukot Essent Fatty Acids. 1992;46(3):223–229. [DOI] [PubMed] [Google Scholar]
- 193. Bridges PJ, Fortune JE. Regulation, action and transport of prostaglandins during the periovulatory period in cattle. Mol Cell Endocrinol. 2007;263(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 194. Sayasith K, Bouchard N, Doré M, Sirois J. Molecular cloning and gonadotropin-dependent regulation of equine prostaglandin F2α receptor in ovarian follicles during the ovulatory process in vivo. Prostaglandins Other Lipid Mediat. 2006;80(1–2):81–92. [DOI] [PubMed] [Google Scholar]
- 195. Tsai SJ, Wiltbank MC, Bodensteiner KJ. Distinct mechanisms regulate induction of messenger ribonucleic acid for prostaglandin (PG) G/H synthase-2, PGE (EP3) receptor, and PGF2 alpha receptor in bovine preovulatory follicles. Endocrinology. 1996;137(8):3348–3355. [DOI] [PubMed] [Google Scholar]
- 196. Ristimäki A, Jaatinen R, Ritvos O. Regulation of prostaglandin F2α receptor expression in cultured human granulosa-luteal cells. Endocrinology. 1997;138(1):191–195. [DOI] [PubMed] [Google Scholar]
- 197. Kim SO, Markosyan N, Pepe GJ, Duffy DM. Estrogen promotes luteolysis by redistributing prostaglandin F2α receptors within primate luteal cells. Reproduction. 2015;149(5):453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Sugimoto Y, Yamasaki A, Segi E, Tsuboi K, Aze Y, Nishimura T, Oida H, Yoshida N, Tanaka T, Katsuyama M, Hasumoto K, Murata T, Hirata M, Ushikubi F, Negishi M, Ichikawa A, Narumiya S. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277(5326):681–683. [DOI] [PubMed] [Google Scholar]
- 199. Carrasco MP, Asbóth G, Phaneuf S, López Bernal A. Activation of the prostaglandin FP receptor in human granulosa cells. J Reprod Fertil. 1997;111(2):309–317. [DOI] [PubMed] [Google Scholar]
- 200. Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol Cells. 2011;32(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Espey LL, Tanaka N, Okamura H. Increase in ovarian leukotrienes during hormonally induced ovulation in the rat. Am J Physiol. 1989;256(6 Pt 1):E753–E759. [DOI] [PubMed] [Google Scholar]
- 202. Feldman E, Haberman S, Abisogun AO, Reich R, Levran D, Maschiach S, Zuckermann H, Rudak E, Dor J, Tsafriri A. Arachidonic acid metabolism in human granulosa cells: evidence for cyclooxygenase and lipoxygenase activity in vitro. Hum Reprod. 1986;1(6):353–356. [DOI] [PubMed] [Google Scholar]
- 203. Gaytán M, Bellido C, Morales C, Sánchez-Criado JE, Gaytán F. Effects of selective inhibition of cyclooxygenase and lipooxygenase pathways in follicle rupture and ovulation in the rat. Reproduction. 2006;132(4):571–577. [DOI] [PubMed] [Google Scholar]
- 204. Heinonen PK, Punnonen R, Ashorn R, Kujansuu E, Mörsky P, Seppälä E. Prostaglandins, thromboxane and leukotriene in human follicular fluid. Clin Reprod Fertil. 1986;4(4):253–257. [PubMed] [Google Scholar]
- 205. Kurusu S, Jinno M, Ehara H, Yonezawa T, Kawaminami M. Inhibition of ovulation by a lipoxygenase inhibitor involves reduced cyclooxygenase-2 expression and prostaglandin E2 production in gonadotropin-primed immature rats. Reproduction. 2009;137(1):59–66. [DOI] [PubMed] [Google Scholar]
- 206. Mikuni M, Yoshida M, Hellberg P, Peterson CA, Edwin SS, Brännström M, Peterson CM. The lipoxygenase inhibitor, nordihydroguaiaretic acid, inhibits ovulation and reduces leukotriene and prostaglandin levels in the rat ovary. Biol Reprod. 1998;58(5):1211–1216. [DOI] [PubMed] [Google Scholar]
- 207. Priddy AR, Killick SR, Elstein M, Morris J, Sullivan M, Patel L, Elder MG. Ovarian follicular fluid eicosanoid concentrations during the pre-ovulatory period in humans. Prostaglandins. 1989;38(2):197–202. [DOI] [PubMed] [Google Scholar]
- 208. Pridham D, Lei ZM, Chegini N, Rao CV, Yussman MA, Cook CL. Light and electron microscope immunocytochemical localization of 5- and 12-lipoxygenases and cyclooxygenase enzymes in human granulosa cells from preovulatory follicles. Prostaglandins Leukot Essent Fatty Acids. 1990;39(3):231–238. [DOI] [PubMed] [Google Scholar]
- 209. Seo MJ, Oh DK. Prostaglandin synthases: molecular characterization and involvement in prostaglandin biosynthesis. Prog Lipid Res. 2017;66:50–68. [DOI] [PubMed] [Google Scholar]
- 210. Wilken C, Van Kirk EA, Slaughter RG, Ji TH, Murdoch WJ. Increased production of ovarian thromboxane in gonadotropin-treated immature rats: relationship to the ovulatory process. Prostaglandins. 1990;40(6):637–646. [DOI] [PubMed] [Google Scholar]
- 211. Robinson RS, Woad KJ, Hammond AJ, Laird M, Hunter MG, Mann GE. Angiogenesis and vascular function in the ovary. Reproduction. 2009;138(6):869–881. [DOI] [PubMed] [Google Scholar]
- 212. Pober JS, Sessa WC. Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol. 2014;7(1):a016345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. McFee RM, Rozell TG, Cupp AS. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 2012;349(3):635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wulff C, Wilson H, Wiegand SJ, Rudge JS, Fraser HM. Prevention of thecal angiogenesis, antral follicular growth, and ovulation in the primate by treatment with vascular endothelial growth factor Trap R1R2. Endocrinology. 2002;143(7):2797–2807. [DOI] [PubMed] [Google Scholar]
- 215. Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5(12):1115–1121. [DOI] [PubMed] [Google Scholar]
- 216. Mauro A, Martelli A, Berardinelli P, Russo V, Bernabò N, Di Giacinto O, Mattioli M, Barboni B. Effect of antiprogesterone RU486 on VEGF expression and blood vessel remodeling on ovarian follicles before ovulation. PLoS One. 2014;9(4):e95910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Chowdhury MW, Scaramuzzi RJ, Wheeler-Jones CP, Khalid M. The expression of angiogenic growth factors and their receptors in ovarian follicles throughout the estrous cycle in the ewe. Theriogenology. 2010;73(7):856–872. [DOI] [PubMed] [Google Scholar]
- 218. Miyabayashi K, Shimizu T, Kawauchi C, Sasada H, Sato E. Changes of mRNA expression of vascular endothelial growth factor, angiopoietins and their receptors during the periovulatory period in eCG/hCG-treated immature female rats. J Exp Zoolog A Comp Exp Biol. 2005;303(7):590–597. [DOI] [PubMed] [Google Scholar]
- 219. Gutman G, Barak V, Maslovitz S, Amit A, Lessing JB, Geva E. Regulation of vascular endothelial growth factor-A and its soluble receptor sFlt-1 by luteinizing hormone in vivo: implication for ovarian follicle angiogenesis. Fertil Steril. 2008;89(4):922–926. [DOI] [PubMed] [Google Scholar]
- 220. Baskind NE, Orsi NM, Sharma V. Follicular-phase ovarian follicular fluid and plasma cytokine profiling of natural cycle in vitro fertilization patients. Fertil Steril. 2014;102(2):410–418. [DOI] [PubMed] [Google Scholar]
- 221. Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8(11):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Brown HM, Robker RL, Russell DL. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology. 2010;151(11):5446–5455. [DOI] [PubMed] [Google Scholar]
- 224. Bender HR, Trau HA, Duffy DM. Placental growth factor is required for ovulation, luteinization, and angiogenesis in primate ovulatory follicles. Endocrinology. 2018;159(2):710–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Berisha B, Steffl M, Welter H, Kliem H, Meyer HH, Schams D, Amselgruber W. Effect of the luteinising hormone surge on regulation of vascular endothelial growth factor and extracellular matrix-degrading proteinases and their inhibitors in bovine follicles. Reprod Fertil Dev. 2008;20(2):258–268. [DOI] [PubMed] [Google Scholar]
- 226. Tal R, Seifer DB, Grazi RV, Malter HE. Follicular fluid placental growth factor is increased in polycystic ovarian syndrome: correlation with ovarian stimulation. Reprod Biol Endocrinol. 2014;12(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Laitinen M, Ristimäki A, Honkasalo M, Narko K, Paavonen K, Ritvos O. Differential hormonal regulation of vascular endothelial growth factors VEGF, VEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology. 1997;138(11):4748–4756. [DOI] [PubMed] [Google Scholar]
- 228. Kim SO, Trau HA, Duffy DM. Vascular endothelial growth factors C and D may promote angiogenesis in the primate ovulatory follicle. Biol Reprod. 2017;96(2):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Goldman J, Rutkowski JM, Shields JD, Pasquier MC, Cui Y, Schmökel HG, Willey S, Hicklin DJ, Pytowski B, Swartz MA. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21(4):1003–1012. [DOI] [PubMed] [Google Scholar]
- 230. Marconcini L, Marchio S, Morbidelli L, Cartocci E, Albini A, Ziche M, Bussolino F, Oliviero S. c-fos-Induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96(17):9671–9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Witzenbichler B, Asahara T, Murohara T, Silver M, Spyridopoulos I, Magner M, Principe N, Kearney M, Hu JS, Isner JM. Vascular endothelial growth factor-C (VEGF-C/VEGF-2) promotes angiogenesis in the setting of tissue ischemia. Am J Pathol. 1998;153(2):381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Trau HA, Brännström M, Curry TE Jr, Duffy DM. Prostaglandin E2 and vascular endothelial growth factor A mediate angiogenesis of human ovarian follicular endothelial cells. Hum Reprod. 2016;31(2):436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Macias VR, Pinzón C, Fierro F, Vergara M, Martínez D, Rosado A, Gutiérrez CG, Rosales-Torres AM. Identification of soluble forms of vascular endothelial growth factor receptors, sVEGFR-1 and sVEGFR-2, in bovine dominant follicles. Reprod Domest Anim. 2012;47(3):e39–e42. [DOI] [PubMed] [Google Scholar]
- 234. Shimizu T, Jayawardana BC, Nishimoto H, Kaneko E, Tetsuka M, Miyamoto A. Hormonal regulation and differential expression of neuropilin (NRP)-1 and NRP-2 genes in bovine granulosa cells. Reproduction. 2006;131(3):555–559. [DOI] [PubMed] [Google Scholar]
- 235. Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277(5322):48–50. [DOI] [PubMed] [Google Scholar]
- 236. Scotti L, Parborell F, Irusta G, De Zuñiga I, Bisioli C, Pettorossi H, Tesone M, Abramovich D. Platelet-derived growth factor BB and DD and angiopoietin1 are altered in follicular fluid from polycystic ovary syndrome patients. Mol Reprod Dev. 2014;81(8):748–756. [DOI] [PubMed] [Google Scholar]
- 237. Nishigaki A, Okada H, Tsuzuki T, Cho H, Yasuda K, Kanzaki H. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011;96(6):1378–1383. [DOI] [PubMed] [Google Scholar]
- 238. Shimizu T, Berisha B, Schams D, Miyamoto A. Expression of angiopoietin (ANPT)-1, ANPT-2 and their receptors in dominant follicles during periovulatory period in GnRH-treated cow. Reprod Domest Anim. 2007;42(2):221–224. [DOI] [PubMed] [Google Scholar]
- 239. Eklund L, Saharinen P. Angiopoietin signaling in the vasculature. Exp Cell Res. 2013;319(9):1271–1280. [DOI] [PubMed] [Google Scholar]
- 240. Xu F, Hazzard TM, Evans A, Charnock-Jones S, Smith S, Stouffer RL. Intraovarian actions of anti-angiogenic agents disrupt periovulatory events during the menstrual cycle in monkeys. Contraception. 2005;71(4):239–248. [DOI] [PubMed] [Google Scholar]
- 241. Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. [DOI] [PubMed] [Google Scholar]
- 242. Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1–2):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Malinen M, Niskanen EA, Kaikkonen MU, Palvimo JJ. Crosstalk between androgen and pro-inflammatory signaling remodels androgen receptor and NF-κB cistrome to reprogram the prostate cancer cell transcriptome. Nucleic Acids Res. 2017;45(2):619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Schooling CM. Androgen activity and markers of inflammation among men in NHANES III. Am J Hum Biol. 2013;25(5):622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Berisha B, Schams D, Rodler D, Sinowatz F, Pfaffl MW. Expression and localization of members of the thrombospondin family during final follicle maturation and corpus luteum formation and function in the bovine ovary. J Reprod Dev. 2016;62(5):501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Greenaway J, Gentry PA, Feige JJ, LaMarre J, Petrik JJ. Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod. 2005;72(5):1071–1078. [DOI] [PubMed] [Google Scholar]
- 247. Higuchi T, Fujiwara H, Yamada S, Tatsumi K, Kataoka N, Itoh K, Maeda M, Fujita J, Fujii S. Co-expression of integrin-associated protein (IAP/CD47) and its ligand thrombospondin-1 on human granulosa and large luteal cells. Mol Hum Reprod. 1999;5(10):920–926. [DOI] [PubMed] [Google Scholar]
- 248. Masli S, Sheibani N, Cursiefen C, Zieske J. Matricellular protein thrombospondins: influence on ocular angiogenesis, wound healing and immuneregulation. Curr Eye Res. 2014;39(8):759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Osz K, Ross M, Petrik J. The thrombospondin-1 receptor CD36 is an important mediator of ovarian angiogenesis and folliculogenesis. Reprod Biol Endocrinol. 2014;12(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Petrik JJ, Gentry PA, Feige JJ, LaMarre J. Expression and localization of thrombospondin-1 and -2 and their cell-surface receptor, CD36, during rat follicular development and formation of the corpus luteum. Biol Reprod. 2002;67(5):1522–1531. [DOI] [PubMed] [Google Scholar]
- 251. Thomas FH, Wilson H, Silvestri A, Fraser HM. Thrombospondin-1 expression is increased during follicular atresia in the primate ovary. Endocrinology. 2008;149(1):185–192. [DOI] [PubMed] [Google Scholar]
- 252. Brown CG, Poyser NL. Studies on ovarian prostaglandin production in relation to ovulation in the rat. J Reprod Fertil. 1984;72(2):407–414. [DOI] [PubMed] [Google Scholar]
- 253. Murdoch WJ, Hansen TR, McPherson LA. A review–role of eicosanoids in vertebrate ovulation. Prostaglandins. 1993;46(2):85–115. [DOI] [PubMed] [Google Scholar]
- 254. Migone FF, Cowan RG, Williams RM, Gorse KJ, Zipfel WR, Quirk SM. In vivo imaging reveals an essential role of vasoconstriction in rupture of the ovarian follicle at ovulation. Proc Natl Acad Sci USA. 2016;113(8):2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Koering MJ. Cyclic changes in ovarian morphology during the menstrual cycle in Macaca mulatta. Am J Anat. 1969;126(1):73–101. [DOI] [PubMed] [Google Scholar]
- 256. McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L, Haning RV Jr, Connolly DT, Robertson DM. Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet. 1994;344(8917):235–236. [DOI] [PubMed] [Google Scholar]
- 257. Corner GW, Hartman CG, Bartelmez GW. Development, organization, and breakdown of the corpus luteum in the rhesus monkey. Contrib Embryol. 1945;204:119–146. [Google Scholar]
- 258. Kerban A, Doré M, Sirois J. Characterization of cellular and vascular changes in equine follicles during hCG-induced ovulation. J Reprod Fertil. 1999;117(1):115–123. [DOI] [PubMed] [Google Scholar]
- 259. Oakley OR, Kim H, El-Amouri I, Lin PC, Cho J, Bani-Ahmad M, Ko C. Periovulatory leukocyte infiltration in the rat ovary. Endocrinology. 2010;151(9):4551–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Nicholas CR, Haston KM, Pera RA. Intact fetal ovarian cord formation promotes mouse oocyte survival and development. BMC Dev Biol. 2010;10(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Gomez R, Gonzalez-Izquierdo M, Zimmermann RC, Novella-Maestre E, Alonso-Muriel I, Sanchez-Criado J, Remohi J, Simon C, Pellicer A. Low-dose dopamine agonist administration blocks vascular endothelial growth factor (VEGF)-mediated vascular hyperpermeability without altering VEGF receptor 2-dependent luteal angiogenesis in a rat ovarian hyperstimulation model. Endocrinology. 2006;147(11):5400–5411. [DOI] [PubMed] [Google Scholar]
- 262. Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Trau HA, Davis JS, Duffy DM. Angiogenesis in the primate ovulatory follicle is stimulated by luteinizing hormone via prostaglandin E2. Biol Reprod. 2015;92(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Feng Y, Cui P, Lu X, Hsueh B, Möller Billig F, Zarnescu Yanez L, Tomer R, Boerboom D, Carmeliet P, Deisseroth K, Hsueh AJ. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci Rep. 2017;7(1):44810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Reich R, Kohen F, Slager R, Tsafriri A. Ovarian lipoxygenase activity and its regulation by gonadotropin in the rat. Prostaglandins. 1985;30(4):581–590. [DOI] [PubMed] [Google Scholar]
- 266. Fraser HM. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34(3):235–249. [PubMed] [Google Scholar]
- 268. Issekutz AC, Movat HZ. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am J Pathol. 1982;107(3):300–309. [PMC free article] [PubMed] [Google Scholar]
- 269. Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Sherwood ER, Toliver-Kinsky T. Mechanisms of the inflammatory response. Best Pract Res Clin Anaesthesiol. 2004;18(3):385–405. [DOI] [PubMed] [Google Scholar]
- 271. Anteby EY, Hurwitz A, Korach O, Revel A, Simon A, Finci-Yeheskel Z, Mayer M, Laufer N. Human follicular nitric oxide pathway: relationship to follicular size, oestradiol concentrations and ovarian blood flow. Hum Reprod. 1996;11(9):1947–1951. [DOI] [PubMed] [Google Scholar]
- 272. Acosta TJ, Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. 2004;82–83:127–140. [DOI] [PubMed] [Google Scholar]
- 273. Brännström M, Enskog A. Leukocyte networks and ovulation. J Reprod Immunol. 2002;57(1–2):47–60. [DOI] [PubMed] [Google Scholar]
- 274. Koos RD. Increased expression of vascular endothelial growth/permeability factor in the rat ovary following an ovulatory gonadotropin stimulus: potential roles in follicle rupture. Biol Reprod. 1995;52(6):1426–1435. [DOI] [PubMed] [Google Scholar]
- 275. Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. [DOI] [PubMed] [Google Scholar]
- 276. Kitajima Y, Endo T, Manase K, Nishikawa A, Shibuya M, Kudo R. Gonadotropin-releasing hormone agonist administration reduced vascular endothelial growth factor (VEGF), VEGF receptors, and vascular permeability of the ovaries of hyperstimulated rats. Fertil Steril. 2004;81(Suppl 1):842–849. [DOI] [PubMed] [Google Scholar]
- 277. Janson O, Albrecht I, Ahrén K. Effects of prostaglandin F2α on ovarian blood flow and vascular resistance in the pseudopregnant rabbit. Acta Endocrinol (Copenh). 1975;79(2):337–350. [DOI] [PubMed] [Google Scholar]
- 278. Pang CY, Behrman HR. Acute effects of prostaglandin F2α an ovarian and luteal blood flow, luteal gonadotropin uptake in vivo, and gonadotropin binding in vitro. Endocrinology. 1981;108(6):2239–2244. [DOI] [PubMed] [Google Scholar]
- 279. Murdoch WJ, Myers DA. Effect of treatment of estrous ewes with indomethacin on the distribution of ovarian blood to the periovulatory follicle. Biol Reprod. 1983;29(5):1229–1232. [DOI] [PubMed] [Google Scholar]
- 280. Williams TJ, Peck MJ. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977;270(5637):530–532. [DOI] [PubMed] [Google Scholar]
- 281. Oriowo MA, Bevan JA. Characterization of histamine H1 and H2 receptors in the rabbit isolated ovarian artery and vein. J Cardiovasc Pharmacol. 1987;10(1):76–81. [DOI] [PubMed] [Google Scholar]
- 282. Cheng K, Wu TJ, Wu KK, Sturino C, Metters K, Gottesdiener K, Wright SD, Wang Z, O’Neill G, Lai E, Waters MG. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc Natl Acad Sci USA. 2006;103(17):6682–6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55(1):69–102. [DOI] [PubMed] [Google Scholar]
- 284. Lai E, De Lepeleire I, Crumley TM, Liu F, Wenning LA, Michiels N, Vets E, O’Neill G, Wagner JA, Gottesdiener K. Suppression of niacin-induced vasodilation with an antagonist to prostaglandin D2 receptor subtype 1. Clin Pharmacol Ther. 2007;81(6):849–857. [DOI] [PubMed] [Google Scholar]
- 285. Rahmann A, Wienecke T, Hansen JM, Fahrenkrug J, Olesen J, Ashina M. Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia. 2008;28(3):226–236. [DOI] [PubMed] [Google Scholar]
- 286. Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169(3951):1217–1218. [DOI] [PubMed] [Google Scholar]
- 287. Hall JM. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther. 1992;56(2):131–190. [DOI] [PubMed] [Google Scholar]
- 288. Fox RH, Goldsmith R, Kidd DJ, Lewis GP. Bradykinin as a vasodilator in man. J Physiol. 1961;157(3):589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Cherry PD, Furchgott RF, Zawadzki JV, Jothianandan D. Role of endothelial cells in relaxation of isolated arteries by bradykinin. Proc Natl Acad Sci USA. 1982;79(6):2106–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Curry FR, Adamson RH. Tonic regulation of vascular permeability. Acta Physiol (Oxf). 2013;207(4):628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Cavender JL, Murdoch WJ. Morphological studies of the microcirculatory system of periovulatory ovine follicles. Biol Reprod. 1988;39(4):989–997. [DOI] [PubMed] [Google Scholar]
- 292. Curry TE Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24(4):428–465. [DOI] [PubMed] [Google Scholar]
- 293. Gerdes U, Gåfvels M, Bergh A, Cajander S. Localized increases in ovarian vascular permeability and leucocyte accumulation after induced ovulation in rabbits. J Reprod Fertil. 1992;95(2):539–550. [DOI] [PubMed] [Google Scholar]
- 294. Mitsube K, Brännström M, Haraldsson B. Modulation of microvascular permeability in the preovulatory rat ovary by an ovulatory gonadotropin stimulus. Fertil Steril. 2013;99(3):903–909. [DOI] [PubMed] [Google Scholar]
- 295. Dowell RT, Gairola CG, Diana JN. Reproductive organ blood flow measured using radioactive microspheres in diestrous and estrous mice. Am J Physiol. 1992;262(4 Pt 2):R666–R670. [DOI] [PubMed] [Google Scholar]
- 296. Murdoch WJ, Nix KJ, Dunn TG. Dynamics of ovarian blood supply to periovulatory follicles of the ewe. Biol Reprod. 1983;28(4):1001–1006. [DOI] [PubMed] [Google Scholar]
- 297. Varga B, Horváth E, Folly G, Stark E. Study of the luteinizing hormone-induced increase of ovarian blood flow during the estrous cycle in the rat. Biol Reprod. 1985;32(3):480–488. [DOI] [PubMed] [Google Scholar]
- 298. Niswender GD, Reimers TJ, Diekman MA, Nett TM. Blood flow: a mediator of ovarian function. Biol Reprod. 1976;14(1):64–81. [DOI] [PubMed] [Google Scholar]
- 299. Nett TM, McClellan MC, Niswender GD. Effects of prostaglandins on the ovine corpus luteum: blood flow, secretion of progesterone and morphology. Biol Reprod. 1976;15(1):66–78. [DOI] [PubMed] [Google Scholar]
- 300. Shrestha HK, Ginther OJ. Increase in progesterone and luteal blood flow without a luteolytic response after prostaglandin F2α treatment in early luteal-phase heifers. Anim Reprod Sci. 2011;124(1–2):7–11. [DOI] [PubMed] [Google Scholar]
- 301. Siddiqui MA, Ferreira JC, Gastal EL, Beg MA, Cooper DA, Ginther OJ. Temporal relationships of the LH surge and ovulation to echotexture and power Doppler signals of blood flow in the wall of the preovulatory follicle in heifers. Reprod Fertil Dev. 2010;22(7):1110–1117. [DOI] [PubMed] [Google Scholar]
- 302. Zaidi J, Jurkovic D, Campbell S, Collins W, McGregor A, Tan SL. Luteinized unruptured follicle: morphology, endocrine function and blood flow changes during the menstrual cycle. Hum Reprod. 1995;10(1):44–49. [DOI] [PubMed] [Google Scholar]
- 303. Brännström M, Zackrisson U, Hagström HG, Josefsson B, Hellberg P, Granberg S, Collins WP, Bourne T. Preovulatory changes of blood flow in different regions of the human follicle. Fertil Steril. 1998;69(3):435–442. [DOI] [PubMed] [Google Scholar]
- 304. Smith MF, Ricke WA, Bakke LJ, Dow MP, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191(1):45–56. [DOI] [PubMed] [Google Scholar]
- 305. Jiang JY, Macchiarelli G, Tsang BK, Sato E. Capillary angiogenesis and degeneration in bovine ovarian antral follicles. Reproduction. 2003;125(2):211–223. [PubMed] [Google Scholar]
- 306. Murdoch WJ, Colgin DC, Ellis JA. Role of tumor necrosis factor-α in the ovulatory mechanism of ewes. J Anim Sci. 1997;75(6):1601–1605. [DOI] [PubMed] [Google Scholar]
- 307. Murdoch WJ, Wilken C, Young DA. Sequence of apoptosis and inflammatory necrosis within the formative ovulatory site of sheep follicles. J Reprod Fertil. 1999;117(2):325–329. [DOI] [PubMed] [Google Scholar]
- 308. Murdoch WJ, Townsend RS, McDonnel AC. Ovulation-induced DNA damage in ovarian surface epithelial cells of ewes: prospective regulatory mechanisms of repair/survival and apoptosis. Biol Reprod. 2001;65(5):1417–1424. [DOI] [PubMed] [Google Scholar]
- 309. Bjersing L, Cajander S. Ovulation and the mechanism of follicle rupture. III. Transmission electron microscopy of rabbit germinal epithelium prior to induced ovulation. Cell Tissue Res. 1974;149(3):313–327. [DOI] [PubMed] [Google Scholar]
- 310. Oakley OR, Frazer ML, Ko C. Pituitary–ovary–spleen axis in ovulation. Trends Endocrinol Metab. 2011;22(9):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Bakke LJ, Li Q, Cassar CA, Dow MP, Pursley JR, Smith GW. Gonadotropin surge-induced differential upregulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-13) mRNA and protein in bovine preovulatory follicles. Biol Reprod. 2004;71(2):605–612. [DOI] [PubMed] [Google Scholar]
- 312. Bakke LJ, Dow MP, Cassar CA, Peters MW, Pursley JR, Smith GW. Effect of the preovulatory gonadotropin surge on matrix metalloproteinase (MMP)-14, MMP-2, and tissue inhibitor of metalloproteinases-2 expression within bovine periovulatory follicular and luteal tissue. Biol Reprod. 2002;66(6):1627–1634. [DOI] [PubMed] [Google Scholar]
- 313. Murdoch WJ, Gottsch ML. Proteolytic mechanisms in the ovulatory folliculo-luteal transformation. Connect Tissue Res. 2003;44(1):50–57. [PubMed] [Google Scholar]
- 314. Tsafriri A, Reich R. Molecular aspects of mammalian ovulation. Exp Clin Endocrinol Diabetes. 1999;107(1):1–11. [DOI] [PubMed] [Google Scholar]
- 315. Martelli A, Palmerini MG, Russo V, Rinaldi C, Bernabò N, Di Giacinto O, Berardinelli P, Nottola SA, Macchiarelli G, Barboni B. Blood vessel remodeling in pig ovarian follicles during the periovulatory period: an immunohistochemistry and SEM-corrosion casting study. Reprod Biol Endocrinol. 2009;7(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Macchiarelli G, Nottola SA, Palmerini MG, Bianchi S, Maione M, Lorenzo C, Stifano G, Di Marco E, Correr S. Morphological expression of angiogenesis in the mammalian ovary as seen by SEM of corrosion casts. Ital J Anat Embryol. 2010;115(1–2):109–114. [PubMed] [Google Scholar]
- 317. Plendl J. Angiogenesis and vascular regression in the ovary. Anat Histol Embryol. 2000;29(5):257–266. [DOI] [PubMed] [Google Scholar]
- 318. Fraser HM, Dickson SE, Lunn SF, Wulff C, Morris KD, Carroll VA, Bicknell R. Suppression of luteal angiogenesis in the primate after neutralization of vascular endothelial growth factor. Endocrinology. 2000;141(3):995–1000. [DOI] [PubMed] [Google Scholar]
- 319. Wulff C, Wilson H, Rudge JS, Wiegand SJ, Lunn SF, Fraser HM. Luteal angiogenesis: prevention and intervention by treatment with vascular endothelial growth factor trapA40. J Clin Endocrinol Metab. 2001;86(7):3377–3386. [DOI] [PubMed] [Google Scholar]
- 320. Wulff C, Wiegand SJ, Saunders PT, Scobie GA, Fraser HM. Angiogenesis during follicular development in the primate and its inhibition by treatment with truncated Flt-1-Fc (vascular endothelial growth factor TrapA40). Endocrinology. 2001;142(7):3244–3254. [DOI] [PubMed] [Google Scholar]
- 321. Zimmermann RC, Xiao E, Husami N, Sauer MV, Lobo R, Kitajewski J, Ferin M. Short-term administration of antivascular endothelial growth factor antibody in the late follicular phase delays follicular development in the rhesus monkey. J Clin Endocrinol Metab. 2001;86(2):768–772. [DOI] [PubMed] [Google Scholar]
- 322. Hazzard TM, Rohan RM, Molskness TA, Fanton JW, D’Amato RJ, Stouffer RL. Injection of antiangiogenic agents into the macaque preovulatory follicle: disruption of corpus luteum development and function. Endocrine. 2002;17(3):199–206. [DOI] [PubMed] [Google Scholar]
- 323. Hazzard TM, Xu F, Stouffer RL. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol Reprod. 2002;67(4):1305–1312. [DOI] [PubMed] [Google Scholar]
- 324. Gómez R, Lima I, Simón C, Pellicer A. Administration of low-dose LH induces ovulation and prevents vascular hyperpermeability and vascular endothelial growth factor expression in superovulated rats. Reproduction. 2004;127(4):483–489. [DOI] [PubMed] [Google Scholar]
- 325. Soares SR, Gómez R, Simón C, García-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14(4):321–333. [DOI] [PubMed] [Google Scholar]
- 326. Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. [DOI] [PubMed] [Google Scholar]
- 327. Xu F, Stouffer RL. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod. 2005;72(6):1352–1358. [DOI] [PubMed] [Google Scholar]
- 328. Tal R, Seifer DB, Arici A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. Semin Reprod Med. 2015;33(3):195–207. [DOI] [PubMed] [Google Scholar]
- 329. Abisogun AO, Daphna-Iken D, Reich R, Kranzfelder D, Tsafriri A. Modulatory role of eicosanoids in vascular changes during the preovulatory period in the rat. Biol Reprod. 1988;38(4):756–762. [DOI] [PubMed] [Google Scholar]
- 330. Kranzfelder D, Reich R, Abisogun AO, Tsafriri A. Preovulatory changes in the perifollicular capillary network in the rat: role of eicosanoids. Biol Reprod. 1992;46(3):379–385. [DOI] [PubMed] [Google Scholar]
- 331. Kim SO, Harris SM, Duffy DM. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology. 2014;155(4):1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. 1982;55(Suppl 2):32–42. [PubMed] [Google Scholar]
- 333. Chaffin CL, Stouffer RL. Role of gonadotrophins and progesterone in the regulation of morphological remodelling and atresia in the monkey peri-ovulatory follicle. Hum Reprod. 2000;15(12):2489–2495. [DOI] [PubMed] [Google Scholar]
- 334. Press MF, Udove JA, Greene GL. Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol. 1988;131(1):112–124. [PMC free article] [PubMed] [Google Scholar]
- 335. Snijders MP, de Goeij AF, Debets-Te Baerts MJ, Rousch MJ, Koudstaal J, Bosman FT. Immunocytochemical analysis of oestrogen receptors and progesterone receptors in the human uterus throughout the menstrual cycle and after the menopause. J Reprod Fertil. 1992;94(2):363–371. [DOI] [PubMed] [Google Scholar]
- 336. Sahlin L, Masironi B, Akerberg S, Eriksson H. Tissue- and hormone-dependent progesterone receptor distribution in the rat uterus. Reprod Biol Endocrinol. 2006;4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Sriraman V, Sinha M, Richards JS. Progesterone receptor-induced gene expression in primary mouse granulosa cell cultures. Biol Reprod. 2010;82(2):402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Wenner MM, Taylor HS, Stachenfeld NS. Androgens influence microvascular dilation in PCOS through ET-A and ET-B receptors. Am J Physiol Endocrinol Metab. 2013;305(7):E818–E825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Bridges PJ, Jo M, Al Alem L, Na G, Su W, Gong MC, Jeoung M, Ko C. Production and binding of endothelin-2 (EDN2) in the rat ovary: endothelin receptor subtype A (EDNRA)-mediated contraction. Reprod Fertil Dev. 2010;22(5):780–787. [DOI] [PubMed] [Google Scholar]
- 340. Palumbo A, Ávila J, Naftolin F. The ovarian renin–angiotensin system (OVRAS): a major factor in ovarian function and disease. Reprod Sci. 2016;23(12):1644–1655. [DOI] [PubMed] [Google Scholar]
- 341. Gonçalves PB, Ferreira R, Gasperin B, Oliveira JF. Role of angiotensin in ovarian follicular development and ovulation in mammals: a review of recent advances. Reproduction. 2012;143(1):11–20. [DOI] [PubMed] [Google Scholar]
- 342. Tonellotto dos Santos J, Ferreira R, Gasperin BG, Siqueira LC, de Oliveira JF, Santos RA, Reis AM, Gonçalves PB. Molecular characterization and regulation of the angiotensin-converting enzyme type 2/angiotensin-(1-7)/MAS receptor axis during the ovulation process in cattle. J Renin Angiotensin Aldosterone Syst. 2012;13(1):91–98. [DOI] [PubMed] [Google Scholar]
- 343. Acosta TJ, Ozawa T, Kobayashi S, Hayashi K, Ohtani M, Kraetzl WD, Sato K, Schams D, Miyamoto A. Periovulatory changes in the local release of vasoactive peptides, prostaglandin F2α, and steroid hormones from bovine mature follicles in vivo. Biol Reprod. 2000;63(5):1253–1261. [DOI] [PubMed] [Google Scholar]
- 344. Mitsube K, Mikuni M, Matousek M, Zackrisson U, Brännström M. Role of the angiotensin II system in regulation of ovulation and blood flow in the rat ovary. Reproduction. 2003;125(3):425–435. [DOI] [PubMed] [Google Scholar]
- 345. Ferreira R, Oliveira JF, Fernandes R, Moraes JF, Gonçalves PB. The role of angiotensin II in the early stages of bovine ovulation. Reproduction. 2007;134(5):713–719. [DOI] [PubMed] [Google Scholar]
- 346. Dahm-Kähler P, Ghahremani M, Lind AK, Sundfeldt K, Brännström M. Monocyte chemotactic protein-1 (MCP-1), its receptor, and macrophages in the perifollicular stroma during the human ovulatory process. Fertil Steril. 2009;91(1):231–239. [DOI] [PubMed] [Google Scholar]
- 347. Fedorcsák P, Ráki M, Storeng R. Characterization and depletion of leukocytes from cells isolated from the pre-ovulatory ovarian follicle. Hum Reprod. 2007;22(4):989–994. [DOI] [PubMed] [Google Scholar]
- 348. Van der Hoek KH, Maddocks S, Woodhouse CM, van Rooijen N, Robertson SA, Norman RJ. Intrabursal injection of clodronate liposomes causes macrophage depletion and inhibits ovulation in the mouse ovary. Biol Reprod. 2000;62(4):1059–1066. [DOI] [PubMed] [Google Scholar]
- 349. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–133. [DOI] [PubMed] [Google Scholar]
- 350. Omu AE, Al-Azemi MK, Makhseed M, Al-Oattan F, Ismail AA, Al-Tahir S, Al-Busiri N. Differential expression of T-helper cytokines in the peritoneal fluid of women with normal ovarian cycle compared with women with chronic anovulation. Acta Obstet Gynecol Scand. 2003;82(7):603–609. [DOI] [PubMed] [Google Scholar]
- 351. Brannstrom M, Pascoe V, Norman RJ, McClure N. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertil Steril. 1994;61(3):488–495. [PubMed] [Google Scholar]
- 352. Suzuki T, Sasano H, Takaya R, Fukaya T, Yajima A, Date F, Nagura H. Leukocytes in normal-cycling human ovaries: immunohistochemical distribution and characterization. Hum Reprod. 1998;13(8):2186–2191. [DOI] [PubMed] [Google Scholar]
- 353. Szukiewicz D, Pyzlak M, Klimkiewicz J, Szewczyk G, Maslinska D. Mast cell-derived interleukin-8 may be involved in the ovarian mechanisms of follicle growth and ovulation. Inflamm Res. 2007;56(Suppl 1):S35–S36. [DOI] [PubMed] [Google Scholar]
- 354. Hamouzova P, Cizek P, Novotny R, Bartoskova A, Tichy F. Distribution of mast cells in the feline ovary in various phases of the oestrous cycle. Reprod Domest Anim. 2017;52(3):483–486. [DOI] [PubMed] [Google Scholar]
- 355. Kathpalia K, Parshad RK. Mast cell dynamics in relation to ovulation in rat ovary. Indian J Exp Biol. 1990;28(3):287–288. [PubMed] [Google Scholar]
- 356. Krishna A, Jaiswal K. Changes in the mast cell number and degranulation pattern during periovulatory period and after blocking gonadotrophin surge in mice ovarian compartments. Acta Physiol Hung. 1994;82(4):415–421. [PubMed] [Google Scholar]
- 357. Yang Z, Kong B, Mosser DM, Zhang X. TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol. 2011;11(10):1442–1450. [DOI] [PubMed] [Google Scholar]
- 358. Das S, Bates MD, Vince GS, Lewis-Jones I, Gazvani R. Follicular fluid expression of alpha-defensins and their role in ovulation. J Assist Reprod Genet. 2008;25(2-3):83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Akiyama I, Yoshino O, Osuga Y, Shi J, Takamura M, Harada M, Koga K, Hirota Y, Hirata T, Fujii T, Saito S, Kozuma S. The role of bone morphogenetic protein 6 in accumulation and regulation of neutrophils in the human ovary. Reprod Sci. 2014;21(6):772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Ushigoe K, Irahara M, Fukumochi M, Kamada M, Aono T. Production and regulation of cytokine-induced neutrophil chemoattractant in rat ovulation. Biol Reprod. 2000;63(1):121–126. [DOI] [PubMed] [Google Scholar]
- 361. Brännström M, Mayrhofer G, Robertson SA. Localization of leukocyte subsets in the rat ovary during the periovulatory period. Biol Reprod. 1993;48(2):277–286. [DOI] [PubMed] [Google Scholar]
- 362. Ujioka T, Matsukawa A, Tanaka N, Matsuura K, Yoshinaga M, Okamura H. Interleukin-8 as an essential factor in the human chorionic gonadotropin-induced rabbit ovulatory process: interleukin-8 induces neutrophil accumulation and activation in ovulation. Biol Reprod. 1998;58(2):526–530. [DOI] [PubMed] [Google Scholar]
- 363. Viana GE, Pereira VM, Honorato-Sampaio K, Oliveira CA, Santos RA, Reis AM. Angiotensin-(1–7) induces ovulation and steroidogenesis in perfused rabbit ovaries. Exp Physiol. 2011;96(9):957–965. [DOI] [PubMed] [Google Scholar]
- 364. Brännström M, Woessner JF Jr, Koos RD, Sear CH, LeMaire WJ. Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology. 1988;122(5):1715–1721. [DOI] [PubMed] [Google Scholar]
- 365. Hellberg P, Thomsen P, Janson PO, Brännström M. Leukocyte supplementation increases the luteinizing hormone-induced ovulation rate in the in vitro-perfused rat ovary. Biol Reprod. 1991;44(5):791–797. [DOI] [PubMed] [Google Scholar]
- 366. Komatsu K, Manabe N, Kiso M, Shimabe M, Miyamoto H. Changes in localization of immune cells and cytokines in corpora lutea during luteolysis in murine ovaries. J Exp Zoolog A Comp Exp Biol. 2003;296(2):152–159. [DOI] [PubMed] [Google Scholar]
- 367. Webster NL, Crowe SM. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J Leukoc Biol. 2006;80(5):1052–1066. [DOI] [PubMed] [Google Scholar]
- 368. Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181–186. [DOI] [PubMed] [Google Scholar]
- 369. Heissig B, Nishida C, Tashiro Y, Sato Y, Ishihara M, Ohki M, Gritli I, Rosenkvist J, Hattori K. Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration. Histol Histopathol. 2010;25(6):765–770. [DOI] [PubMed] [Google Scholar]
- 370. Guimerà M, Morales-Ruiz M, Jiménez W, Balasch J. LH/HCG stimulation of VEGF and adrenomedullin production by follicular fluid macrophages and luteinized granulosa cells. Reprod Biomed Online. 2009;18(6):743–749. [DOI] [PubMed] [Google Scholar]
- 371. Williams MR, Azcutia V, Newton G, Alcaide P, Luscinskas FW. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32(10):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Kawano Y, Kawasaki F, Nakamura S, Matsui N, Narahara H, Miyakawa I. The production and clinical evaluation of macrophage colony-stimulating factor and macrophage chemoattractant protein-1 in human follicular fluids. Am J Reprod Immunol. 2001;45(1):1–5. [DOI] [PubMed] [Google Scholar]
- 373. Arici A, Oral E, Bukulmez O, Buradagunta S, Bahtiyar O, Jones EE. Monocyte chemotactic protein-1 expression in human preovulatory follicles and ovarian cells. J Reprod Immunol. 1997;32(3):201–219. [DOI] [PubMed] [Google Scholar]
- 374. Nio-Kobayashi J, Kudo M, Sakuragi N, Kimura S, Iwanaga T, Duncan WC. Regulated C-C motif ligand 2 (CCL2) in luteal cells contributes to macrophage infiltration into the human corpus luteum during luteolysis. Mol Hum Reprod. 2015;21(8):645–654. [DOI] [PubMed] [Google Scholar]
- 375. Zhou C, Wu J, Borillo J, Torres L, McMahon J, Bao Y, Lou YH. Transient expression of CC chemokine TECK in the ovary during ovulation: its potential role in ovulation. Am J Reprod Immunol. 2005;53(5):238–248. [DOI] [PubMed] [Google Scholar]
- 376. Jasper MJ, Brännström M, Olofsson JI, Petrucco OM, Mason H, Robertson SA, Norman RJ. Granulocyte-macrophage colony-stimulating factor: presence in human follicular fluid, protein secretion and mRNA expression by ovarian cells. Mol Hum Reprod. 1996;2(8):555–562. [DOI] [PubMed] [Google Scholar]
- 377. Karström-Encrantz L, Runesson E, Boström EK, Brännström M. Selective presence of the chemokine growth-regulated oncogene alpha (GROalpha) in the human follicle and secretion from cultured granulosa-lutein cells at ovulation. Mol Hum Reprod. 1998;4(11):1077–1083. [DOI] [PubMed] [Google Scholar]
- 378. Runesson E, Boström EK, Janson PO, Brännström M. The human preovulatory follicle is a source of the chemotactic cytokine interleukin-8. Mol Hum Reprod. 1996;2(4):245–250. [DOI] [PubMed] [Google Scholar]
- 379. Fainaru O, Amsalem H, Bentov Y, Esfandiari N, Casper RF. CD56brightCD16− natural killer cells accumulate in the ovarian follicular fluid of patients undergoing in vitro fertilization. Fertil Steril. 2010;94(5):1918–1921. [DOI] [PubMed] [Google Scholar]
- 380. Nishigaki A, Okada H, Okamoto R, Shimoi K, Miyashiro H, Yasuda K, Kanzaki H. The concentration of human follicular fluid stromal cell-derived factor-1 is correlated with luteinization in follicles. Gynecol Endocrinol. 2013;29(3):230–234. [DOI] [PubMed] [Google Scholar]
- 381. Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, Chouaib S, Zou W, Machelon V. The chemokine SDF-1/CXCL12 contributes to T lymphocyte recruitment in human pre-ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol. 2005;54(5):270–283. [DOI] [PubMed] [Google Scholar]
- 382. Wang L, Qi H, Baker PN, Zhen Q, Zeng Q, Shi R, Tong C, Ge Q. Altered circulating inflammatory cytokines are associated with anovulatory polycystic ovary syndrome (PCOS) women resistant to clomiphene citrate treatment. Med Sci Monit. 2017;23:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Bukulmez O, Arici A. Leukocytes in ovarian function. Hum Reprod Update. 2000;6(1):1–15. [DOI] [PubMed] [Google Scholar]
- 384. Połeć A, Tanbo T, Fedorcsák P. Cellular interaction regulates interleukin-8 secretion by granulosa-lutein cells and monocytes/macrophages. Am J Reprod Immunol. 2009;61(1):85–94. [DOI] [PubMed] [Google Scholar]
- 385. Bonello N, Jasper MJ, Norman RJ. Periovulatory expression of intercellular adhesion molecule-1 in the rat ovary. Biol Reprod. 2004;71(4):1384–1390. [DOI] [PubMed] [Google Scholar]
- 386. Rohm F, Spanel-Borowski K, Eichler W, Aust G. Correlation between expression of selectins and migration of eosinophils into the bovine ovary during the periovulatory period. Cell Tissue Res. 2002;309(2):313–322. [DOI] [PubMed] [Google Scholar]
- 387. Wang LJ, Brännström M, Robertson SA, Norman RJ. Tumor necrosis factor α in the human ovary: presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil Steril. 1992;58(5):934–940. [DOI] [PubMed] [Google Scholar]
- 388. Sasson R, Winder N, Kees S, Amsterdam A. Induction of apoptosis in granulosa cells by TNFα and its attenuation by glucocorticoids involve modulation of Bcl-2. Biochem Biophys Res Commun. 2002;294(1):51–59. [DOI] [PubMed] [Google Scholar]
- 389. Carlberg M, Nejaty J, Fröysa B, Guan Y, Söder O, Bergqvist A. Elevated expression of tumour necrosis factor α in cultured granulosa cells from women with endometriosis. Hum Reprod. 2000;15(6):1250–1255. [DOI] [PubMed] [Google Scholar]
- 390. Field SL, Dasgupta T, Cummings M, Orsi NM. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol Reprod Dev. 2014;81(4):284–314. [DOI] [PubMed] [Google Scholar]
- 391. Adashi EY. The potential relevance of cytokines to ovarian physiology: the emerging role of resident ovarian cells of the white blood cell series. Endocr Rev. 1990;11(3):454–464. [DOI] [PubMed] [Google Scholar]
- 392. Hertzog PJ, O’Neill LA, Hamilton JA. The interferon in TLR signaling: more than just antiviral. Trends Immunol. 2003;24(10):534–539. [DOI] [PubMed] [Google Scholar]
- 393. Mandrup-Poulsen T, Nerup J, Reimers JI, Pociot F, Andersen HU, Karlsen A, Bjerre U, Bergholdt R. Cytokines and the endocrine system. I. The immunoendocrine network. Eur J Endocrinol. 1995;133(6):660–671. [DOI] [PubMed] [Google Scholar]
- 394. Gérard N, Caillaud M, Martoriati A, Goudet G, Lalmanach AC. The interleukin-1 system and female reproduction. J Endocrinol. 2004;180(2):203–212. [DOI] [PubMed] [Google Scholar]
- 395. Wang XF, Xing FQ, Chen SL. Interleukin-1β expression on ovarian granulosa cells and its clinical implication in women undergoing in vitro fertilization. J First Mil Med Univ. 2002;22(10):934–936. [PubMed] [Google Scholar]
- 396. Machelon V, Emilie D. Production of ovarian cytokines and their role in ovulation in the mammalian ovary. Eur Cytokine Netw. 1997;8(2):137–143. [PubMed] [Google Scholar]
- 397. Dang X, Zhu Q, He Y, Wang Y, Lu Y, Li X, Qi J, Wu H, Sun Y. IL-1β upregulates StAR and progesterone production through the ERK1/2- and p38-mediated CREB signaling pathways in human granulosa-lutein cells. Endocrinology. 2017;158(10):3281–3291. [DOI] [PubMed] [Google Scholar]
- 398. Büscher U, Chen FC, Kentenich H, Schmiady H. Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries; is ovulation a suppressed inflammatory reaction? Hum Reprod. 1999;14(1):162–166. [DOI] [PubMed] [Google Scholar]
- 399. Robker RL, Akison LK, Richards JS, Smith CW, Russell DL. The inflammatory response at ovulation is altered in ovaries of progesterone receptor null (PRKO) mice. Biol Reprod. 2010;83(Suppl 1):95–95. [Google Scholar]
- 400. Krensky AM, Clayberger C. Biology and clinical relevance of granulysin. Tissue Antigens. 2009;73(3):193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Marçal JR, Samuel RO, Fernandes D, de Araujo MS, Napimoga MH, Pereira SA, Clemente-Napimoga JT, Alves PM, Mattar R, Rodrigues V Jr, Rodrigues DB. T-helper cell type 17/regulatory T-cell immunoregulatory balance in human radicular cysts and periapical granulomas. J Endod. 2010;36(6):995–999. [DOI] [PubMed] [Google Scholar]
- 402. Riese J, Niedobitek G, Lisner R, Jung A, Hohenberger W, Haupt W. Expression of interleukin-6 and monocyte chemoattractant protein-1 by peritoneal sub-mesothelial cells during abdominal operations. J Pathol. 2004;202(1):34–40. [DOI] [PubMed] [Google Scholar]
- 403. Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215(1-2):135–141. [DOI] [PubMed] [Google Scholar]
- 404. Wong KH, Negishi H, Adashi EY. Expression, hormonal regulation, and cyclic variation of chemokines in the rat ovary: key determinants of the intraovarian residence of representatives of the white blood cell series. Endocrinology. 2002;143(3):784–791. [DOI] [PubMed] [Google Scholar]
- 405. Dahm-Kähler P, Runesson E, Lind AK, Brännström M. Monocyte chemotactic protein-1 in the follicle of the menstrual and IVF cycle. Mol Hum Reprod. 2006;12(1):1–6. [DOI] [PubMed] [Google Scholar]
- 406. Cohen PE, Zhu L, Pollard JW. Absence of colony stimulating factor-1 in osteopetrotic (csfmop/csfmop) mice disrupts estrous cycles and ovulation. Biol Reprod. 1997;56(1):110–118. [DOI] [PubMed] [Google Scholar]
- 407. Cohen PE, Nishimura K, Zhu L, Pollard JW. Macrophages: important accessory cells for reproductive function. J Leukoc Biol. 1999;66(5):765–772. [DOI] [PubMed] [Google Scholar]
- 408. Zhou C, Wu J, Borillo J, Torres L, McMahon J, Lou YH. Potential roles of a special CD8αα+ cell population and CC chemokine thymus-expressed chemokine in ovulation related inflammation. J Immunol. 2009;182(1):596–603. [PMC free article] [PubMed] [Google Scholar]
- 409. Foster R, Segers I, Smart D, Adriaenssens T, Smitz J, Arce JC, Princivalle M. A differential cytokine expression profile is induced by highly purified human menopausal gonadotropin and recombinant follicle-stimulating hormone in a pre- and postovulatory mouse follicle culture model. Fertil Steril. 2010;93(5):1464–1476. [DOI] [PubMed] [Google Scholar]
- 410. Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4(2):197–250. [DOI] [PubMed] [Google Scholar]
- 411. Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274(31):21491–21494. [DOI] [PubMed] [Google Scholar]
- 412. Woessner JF. Matrix Metalloproteinases and TIMPs. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 413. Deleon-Pennell KY, Altara R, Yabluchanskiy A, Modesti A, Lindsey ML. The circular relationship between matrix metalloproteinase-9 and inflammation following myocardial infarction. IUBMB Life. 2015;67(8):611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414. Smigiel KS, Parks WC. Matrix metalloproteinases and leukocyte activation. Prog Mol Biol Transl Sci. 2017;147:167–195. [DOI] [PubMed] [Google Scholar]
- 415. Korpos E, Wu C, Sorokin L. Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des. 2009;15(12):1349–1357. [DOI] [PubMed] [Google Scholar]
- 416. Kleiner DE Jr, Stetler-Stevenson WG. Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol. 1993;5(5):891–897. [DOI] [PubMed] [Google Scholar]
- 417. Murphy G, Stanton H, Cowell S, Butler G, Knäuper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107(1):38–44. [DOI] [PubMed] [Google Scholar]
- 418. Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. [DOI] [PubMed] [Google Scholar]
- 419. Noël A, Gutiérrez-Fernández A, Sounni NE, Behrendt N, Maquoi E, Lund IK, Cal S, Hoyer-Hansen G, López-Otín C. New and paradoxical roles of matrix metalloproteinases in the tumor microenvironment. Front Pharmacol. 2012;3:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Murphy G, Knäuper V, Cowell S, Hembry R, Stanton H, Butler G, Freije J, Pendás AM, López-Otín C. Evaluation of some newer matrix metalloproteinases. Ann N Y Acad Sci. 1999;878:25–39. [DOI] [PubMed] [Google Scholar]
- 421. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17(1):463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477(1–2):267–283. [DOI] [PubMed] [Google Scholar]
- 423. Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74(2):111–122. [PubMed] [Google Scholar]
- 424. Curry TE Jr, Mann JS, Estes RS, Jones PB. α2-Macroglobulin and tissue inhibitor of metalloproteinases: collagenase inhibitors in human preovulatory ovaries. Endocrinology. 1990;127(1):63–68. [DOI] [PubMed] [Google Scholar]
- 425. Dajee M, Kazansky AV, Raught B, Hocke GM, Fey GH, Richards JS. Prolactin induction of the alpha 2-Macroglobulin gene in rat ovarian granulosa cells: stat 5 activation and binding to the interleukin-6 response element. Mol Endocrinol. 1996;10(2):171–184. [DOI] [PubMed] [Google Scholar]
- 426. Gaddy-Kurten D, Hickey GJ, Fey GH, Gauldie J, Richards JS. Hormonal regulation and tissue-specific localization of alpha 2-macroglobulin in rat ovarian follicles and corpora lutea. Endocrinology. 1989;125(6):2985–2995. [DOI] [PubMed] [Google Scholar]
- 427. Leco KJ, Hayden LJ, Sharma RR, Rocheleau H, Greenberg AH, Edwards DR. Differential regulation of TIMP-1 and TIMP-2 mRNA expression in normal and Ha-ras-transformed murine fibroblasts. Gene. 1992;117(2):209–217. [DOI] [PubMed] [Google Scholar]
- 428. Stetler-Stevenson WG, Krutzsch HC, Liotta LA. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989;264(29):17374–17378. [PubMed] [Google Scholar]
- 429. Leco KJ, Apte SS, Taniguchi GT, Hawkes SP, Khokha R, Schultz GA, Edwards DR. Murine tissue inhibitor of metalloproteinases-4 (Timp-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett. 1997;401(2–3):213–217. [DOI] [PubMed] [Google Scholar]
- 430. Pavloff N, Staskus PW, Kishnani NS, Hawkes SP. A new inhibitor of metalloproteinases from chicken: ChIMP-3. A third member of the TIMP family. J Biol Chem. 1992;267(24):17321–17326. [PubMed] [Google Scholar]
- 431. Fassina G, Ferrari N, Brigati C, Benelli R, Santi L, Noonan DM, Albini A. Tissue inhibitors of metalloproteases: regulation and biological activities. Clin Exp Metastasis. 2000;18(2):111–120. [DOI] [PubMed] [Google Scholar]
- 432. Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298(1):29–32. [DOI] [PubMed] [Google Scholar]
- 433. Satoh T, Kobayashi K, Yamashita S, Kikuchi M, Sendai Y, Hoshi H. Tissue inhibitor of metalloproteinases (TIMP-1) produced by granulosa and oviduct cells enhances in vitro development of bovine embryo. Biol Reprod. 1994;50(4):835–844. [DOI] [PubMed] [Google Scholar]
- 434. Johnson MD, Kim HR, Chesler L, Tsao-Wu G, Bouck N, Polverini PJ. Inhibition of angiogenesis by tissue inhibitor of metalloproteinase. J Cell Physiol. 1994;160(1):194–202. [DOI] [PubMed] [Google Scholar]
- 435. Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157(2):351–358. [DOI] [PubMed] [Google Scholar]
- 436. Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118(5):1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437. Wick M, Bürger C, Brüsselbach S, Lucibello FC, Müller R. A novel member of human tissue inhibitor of metalloproteinases (TIMP) gene family is regulated during G1 progression, mitogenic stimulation, differentiation, and senescence. J Biol Chem. 1994;269(29):18953–18960. [PubMed] [Google Scholar]
- 438. Kuno K, Kanada N, Nakashima E, Fujiki F, Ichimura F, Matsushima K. Molecular cloning of a gene encoding a new type of metalloproteinase-disintegrin family protein with thrombospondin motifs as an inflammation associated gene. J Biol Chem. 1997;272(1):556–562. [DOI] [PubMed] [Google Scholar]
- 439. Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered proteolytic activities of ADAMTS-4 expressed by C-terminal processing. J Biol Chem. 2004;279(11):10109–10119. [DOI] [PubMed] [Google Scholar]
- 441. Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278(11):9503–9513. [DOI] [PubMed] [Google Scholar]
- 442. Kuno K, Matsushima K. ADAMTS-1 protein anchors at the extracellular matrix through the thrombospondin type I motifs and its spacing region. J Biol Chem. 1998;273(22):13912–13917. [DOI] [PubMed] [Google Scholar]
- 443. Blelloch R, Newman C, Kimble J. Control of cell migration during Caenorhabditis elegans development. Curr Opin Cell Biol. 1999;11(5):608–613. [DOI] [PubMed] [Google Scholar]
- 444. Nishiwaki K, Hisamoto N, Matsumoto K. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science. 2000;288(5474):2205–2208. [DOI] [PubMed] [Google Scholar]
- 445. Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J Biol Chem. 2001;276(16):12501–12504. [DOI] [PubMed] [Google Scholar]
- 446. Rodríguez-Manzaneque JC, Westling J, Thai SN, Luque A, Knauper V, Murphy G, Sandy JD, Iruela-Arispe ML. ADAMTS1 cleaves aggrecan at multiple sites and is differentially inhibited by metalloproteinase inhibitors. Biochem Biophys Res Commun. 2002;293(1):501–508. [DOI] [PubMed] [Google Scholar]
- 447. Tortorella MD, Arner EC, Hills R, Easton A, Korte-Sarfaty J, Fok K, Wittwer AJ, Liu RQ, Malfait AM. α2-Macroglobulin is a novel substrate for ADAMTS-4 and ADAMTS-5 and represents an endogenous inhibitor of these enzymes. J Biol Chem. 2004;279(17):17554–17561. [DOI] [PubMed] [Google Scholar]
- 448. Raum D, Marcus D, Alper CA, Levey R, Taylor PD, Starzl TE. Synthesis of human plasminogen by the liver. Science. 1980;208(4447):1036–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449. Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92(2):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Degen SJ, Rajput B, Reich E. The human tissue plasminogen activator gene. J Biol Chem. 1986;261(15):6972–6985. [PubMed] [Google Scholar]
- 451. Ebisch IM, Thomas CM, Wetzels AM, Willemsen WN, Sweep FC, Steegers-Theunissen RP. Review of the role of the plasminogen activator system and vascular endothelial growth factor in subfertility. Fertil Steril. 2008;90(6):2340–2350. [DOI] [PubMed] [Google Scholar]
- 452. Curry TE Jr, Smith MF. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin Reprod Med. 2006;24(4):228–241. [DOI] [PubMed] [Google Scholar]
- 453. Liu YX, Liu K, Feng Q, Hu ZY, Liu HZ, Fu GQ, Li YC, Zou RJ, Ny T. Tissue-type plasminogen activator and its inhibitor plasminogen activator inhibitor type 1 are coordinately expressed during ovulation in the rhesus monkey. Endocrinology. 2004;145(4):1767–1775. [DOI] [PubMed] [Google Scholar]
- 454. Bachmann F, Fletcher AP, Alkjaersig N, Sherry S. Partial purification and properties of the plasminogen activator from pig heart. Biochemistry. 1964;3(10):1578–1585. [DOI] [PubMed] [Google Scholar]
- 455. Ploug J, Kjeldgaard NO. Urokinase an activator of plasminogen from human urine. I. Isolation and properties. Biochim Biophys Acta. 1957;24(2):278–282. [DOI] [PubMed] [Google Scholar]
- 456. Blasi F. Urokinase and urokinase receptor: a paracrine/autocrine system regulating cell migration and invasiveness. BioEssays. 1993;15(2):105–111. [DOI] [PubMed] [Google Scholar]
- 457. Vassalli JD, Baccino D, Belin D. A cellular binding site for the Mr 55,000 form of the human plasminogen activator, urokinase. J Cell Biol. 1985;100(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458. Aertgeerts K, De Bondt HL, De Ranter C, Declerck PJ. A model of the reactive form of plasminogen activator inhibitor-1. J Struct Biol. 1994;113(3):239–245. [DOI] [PubMed] [Google Scholar]
- 459. Bjersing L, Cajander S. Ovulation and the mechanism of follicle rupture. V. Ultrastructure of tunica albuginea and theca externa of rabbit graafian follicles prior to induced ovulation. Cell Tissue Res. 1974;153(1):15–30. [DOI] [PubMed] [Google Scholar]
- 460. Fukumoto M, Yajima Y, Okamura H, Midorikawa O. Collagenolytic enzyme activity in human ovary: an ovulatory enzyme system. Fertil Steril. 1981;36(6):746–750. [DOI] [PubMed] [Google Scholar]
- 461. Morales TI, Woessner JF Jr, Marsh JM, LeMaire WJ. Collagen, collagenase and collagenolytic activity in rat Graafian follicles during follicular growth and ovulation. Biochim Biophys Acta. 1983;756(1):119–122. [DOI] [PubMed] [Google Scholar]
- 462. Murdoch WJ, McCormick RJ. Enhanced degradation of collagen within apical vs. basal wall of ovulatory ovine follicle. Am J Physiol. 1992;263(2 Pt 1):E221–E225. [DOI] [PubMed] [Google Scholar]
- 463. Reich R, Tsafriri A, Mechanic GL. The involvement of collagenolysis in ovulation in the rat. Endocrinology. 1985;116(2):522–527. [DOI] [PubMed] [Google Scholar]
- 464. Tsafriri A, Bicsak TA, Cajander SB, Ny T, Hsueh AJ. Suppression of ovulation rate by antibodies to tissue-type plasminogen activator and α2-antiplasmin. Endocrinology. 1989;124(1):415–421. [DOI] [PubMed] [Google Scholar]
- 465. Peluffo MC, Murphy MJ, Baughman ST, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology. 2011;152(10):3963–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466. Hägglund AC, Ny A, Leonardsson G, Ny T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology. 1999;140(9):4351–4358. [DOI] [PubMed] [Google Scholar]
- 467. Ny T, Wahlberg P, Brändström IJ. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol Cell Endocrinol. 2002;187(1–2):29–38. [DOI] [PubMed] [Google Scholar]
- 468. Russell DL, Brown HM, Dunning KR. ADAMTS proteases in fertility. Matrix Biol. 2015;44–46:54–63. [DOI] [PubMed] [Google Scholar]
- 469. Gottsch ML, Van Kirk EA, Murdoch WJ. Role of matrix metalloproteinase 2 in the ovulatory folliculo-luteal transition of ewes. Reproduction. 2002;124(3):347–352. [DOI] [PubMed] [Google Scholar]
- 470. Ichikawa S, Ohta M, Morioka H, Murao S. Blockage of ovulation in the explanted hamster ovary by a collagenase inhibitor. J Reprod Fertil. 1983;68(1):17–19. [DOI] [PubMed] [Google Scholar]
- 471. Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod. 1999;61(1):14–21. [DOI] [PubMed] [Google Scholar]
- 472. Lind AK. Human ovulation: studies on collagens, gelatinases and tissue inhibitors of metalloproteinases [thesis]. Gothenburg, Sweden: Sahlgrenska Academy, University of Gothenburg; 2006.
- 473. Lind AK, Dahm-Kähler P, Weijdegård B, Sundfeldt And K, Brännström M. Gelatinases and their tissue inhibitors during human ovulation: increased expression of tissue inhibitor of matrix metalloproteinase-1. Mol Hum Reprod. 2006;12(12):725–736. [DOI] [PubMed] [Google Scholar]
- 474. McCord LA, Li F, Rosewell KL, Brännström M, Curry TE. Ovarian expression and regulation of the stromelysins during the periovulatory period in the human and the rat. Biol Reprod. 2012;86(3):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Rosewell KL, Al-Alem L, Zakerkish F, McCord L, Akin JW, Chaffin CL, Brännström M, Curry TE Jr. Induction of proteinases in the human preovulatory follicle of the menstrual cycle by human chorionic gonadotropin. Fertil Steril. 2015;103(3):826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. D’Ascenzo S, Giusti I, Millimaggi D, Marci R, Tatone C, Cardigno Colonna R, Moscarini M, Pavan A, Dolo V, Caserta D. Intrafollicular expression of matrix metalloproteinases and their inhibitors in normally ovulating women compared with patients undergoing in vitro fertilization treatment. Eur J Endocrinol. 2004;151(1):87–91. [DOI] [PubMed] [Google Scholar]
- 477. Nikolettos N, Asimakopoulos B, Tentes L, Schöpper B, al-Hasani S. Matrix metalloproteinases 2 and 9 in follicular fluids of patients undergoing controlled ovarian stimulation for ICSI/ET. In Vivo. 2003;17(2):201–204. [PubMed] [Google Scholar]
- 478. Lee DM, Lee TK, Song HB, Kim CH. The expression of matrix metalloproteinase-9 in human follicular fluid is associated with in vitro fertilisation pregnancy. BJOG. 2005;112(7):946–951. [DOI] [PubMed] [Google Scholar]
- 479. Horka P, Malickova K, Jarosova R, Janatkova I, Zima T, Kalousova M. Matrix metalloproteinases in serum and the follicular fluid of women treated by in vitro fertilization. J Assist Reprod Genet. 2012;29(11):1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480. Bilen E, Tola EN, Oral B, Doguç DK, Günyeli I, Köse SA, Ilhan I. Do follicular fluid gelatinase levels affect fertilization rates and oocyte quality? Arch Gynecol Obstet. 2014;290(6):1265–1271. [DOI] [PubMed] [Google Scholar]
- 481. Yang WJ, Liu FC, Hsieh JS, Chen CH, Hsiao SY, Lin CS. Matrix metalloproteinase 2 level in human follicular fluid is a reliable marker of human oocyte maturation in in vitro fertilization and intracytoplasmic sperm injection cycles. Reprod Biol Endocrinol. 2015;13(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Yung Y, Maman E, Konopnicki S, Cohen B, Brengauz M, Lojkin I, Dal Canto M, Fadini R, Dor J, Hourvitz A. ADAMTS-1: a new human ovulatory gene and a cumulus marker for fertilization capacity. Mol Cell Endocrinol. 2010;328(1–2):104–108. [DOI] [PubMed] [Google Scholar]
- 483. Xiao S, Li Y, Li T, Chen M, Xu Y, Wen Y, Zhou C. Evidence for decreased expression of ADAMTS-1 associated with impaired oocyte quality in PCOS patients. J Clin Endocrinol Metab. 2014;99(6):E1015–E1021. [DOI] [PubMed] [Google Scholar]
- 484. Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta. 2005;1751(1):95–109. [DOI] [PubMed] [Google Scholar]
- 485. Liu YX, Liu XM, Nin LF, Shi L, Chen SR. Serine protease and ovarian paracrine factors in regulation of ovulation. Front Biosci. 2013;18(2):650–664. [DOI] [PubMed] [Google Scholar]
- 486. Leonardsson G, Peng XR, Liu K, Nordström L, Carmeliet P, Mulligan R, Collen D, Ny T. Ovulation efficiency is reduced in mice that lack plasminogen activator gene function: functional redundancy among physiological plasminogen activators. Proc Natl Acad Sci USA. 1995;92(26):12446–12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Markosyan N, Duffy DM. Prostaglandin E2 acts via multiple receptors to regulate plasminogen-dependent proteolysis in the primate periovulatory follicle. Endocrinology. 2009;150(1):435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Jones PB, Muse KN, Wilson EA, Curry TE Jr. Expression of plasminogen activator (PA) and a PA inhibitor in human granulosa cells from preovulatory follicles. J Clin Endocrinol Metab. 1988;67(4):857–860. [DOI] [PubMed] [Google Scholar]
- 489. Jones PB, Vernon MW, Muse KN, Curry TE Jr. Plasminogen activator and plasminogen activator inhibitor in human preovulatory follicular fluid. J Clin Endocrinol Metab. 1989;68(6):1039–1045. [DOI] [PubMed] [Google Scholar]
- 490. Weimer SL, Campeau JD, Marrs RP, Dizerega GS. Alteration of human follicular fluid plasminogen activator activity by ovarian hyperstimulation. J In Vitro Fert Embryo Transf. 1984;1(4):263–266. [DOI] [PubMed] [Google Scholar]
- 491. Milwidsky A, Kaneti H, Finci Z, Laufer N, Tsafriri A, Mayer M. Human follicular fluid protease and antiprotease activities: a suggested correlation with ability of oocytes to undergo in vitro fertilization. Fertil Steril. 1989;52(2):274–280. [DOI] [PubMed] [Google Scholar]
- 492. Deutinger J, Kirchheimer JC, Reinthaller A, Christ G, Tatra G, Binder BR. Elevated tissue type plasminogen activator in human granulosa cells correlates with fertilizing capacity. Hum Reprod. 1988;3(5):597–599. [DOI] [PubMed] [Google Scholar]
- 493. Reinthaller A, Kirchheimer JC, Deutinger J, Bieglmayer C, Christ G, Binder BR. Plasminogen activators, plasminogen activator inhibitor, and fibronectin in human granulosa cells and follicular fluid related to oocyte maturation and intrafollicular gonadotropin levels. Fertil Steril. 1990;54(6):1045–1051. [DOI] [PubMed] [Google Scholar]
- 494. Rae MT, Price D, Harlow CR, Critchley HO, Hillier SG. Glucocorticoid receptor-mediated regulation of MMP9 gene expression in human ovarian surface epithelial cells. Fertil Steril. 2009;92(2):703–708. [DOI] [PubMed] [Google Scholar]
- 495. Fedorcsák P, Polec A, Ráki M, Holm R, Jebsen P, Abyholm T. Differential release of matrix metalloproteinases and tissue inhibitors of metalloproteinases by human granulosa-lutein cells and ovarian leukocytes. Endocrinology. 2010;151(3):1290–1298. [DOI] [PubMed] [Google Scholar]
- 496. Esparza J, Kruse M, Lee J, Michaud M, Madri JA. MMP-2 null mice exhibit an early onset and severe experimental autoimmune encephalomyelitis due to an increase in MMP-9 expression and activity. FASEB J. 2004;18(14):1682–1691. [DOI] [PubMed] [Google Scholar]
- 497.Practice Committee of American Society for Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2012;98(2):302–307. [DOI] [PubMed] [Google Scholar]
- 498. Marik J, Hulka J. Luteinized unruptured follicle syndrome: a subtle cause of infertility. Fertil Steril. 1978;29(3):270–274. [DOI] [PubMed] [Google Scholar]
- 499. Bourne TH, Hagström H, Hahlin M, Josefsson B, Granberg S, Hellberg P, Hamberger L, Collins WP. Ultrasound studies of vascular and morphological changes in the human corpus luteum during the menstrual cycle. Fertil Steril. 1996;65(4):753–758. [PubMed] [Google Scholar]
- 500. Baerwald AR, Adams GP, Pierson RA. Form and function of the corpus luteum during the human menstrual cycle. Ultrasound Obstet Gynecol. 2005;25(5):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Dhont M, Serreyn R, Duvivier P, Vanluchene E, De Boever J, Vandekerckhove D. Ovulation stigma and concentration of progesterone and estradiol in peritoneal fluid: relation with fertility and endometriosis. Fertil Steril. 1984;41(6):872–877. [DOI] [PubMed] [Google Scholar]
- 502. Kerin JF, Kirby C, Morris D, McEvoy M, Ward B, Cox LW. Incidence of the luteinized unruptured follicle phenomenon in cycling women. Fertil Steril. 1983;40(5):620–626. [DOI] [PubMed] [Google Scholar]
- 503. Wang L, Qiao J, Liu P, Lian Y. Effect of luteinized unruptured follicle cycles on clinical outcomes of frozen thawed embryo transfer in Chinese women. J Assist Reprod Genet. 2008;25(6):229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504. Murdoch WJ, Cavender JL. Effect of indomethacin on the vascular architecture of preovulatory ovine follicles: possible implication in the luteinized unruptured follicle syndrome. Fertil Steril. 1989;51(1):153–155. [DOI] [PubMed] [Google Scholar]
- 505. Hock DL, Huhn RD, Kemmann E. Leukocytosis in response to exogenous gonadotrophin stimulation. Hum Reprod. 1997;12(10):2143–2146. [DOI] [PubMed] [Google Scholar]
- 506. Check JH, Vaniver J, Senft D, DiAntonio G, Summers D. The use of granulocyte colony stimulating factor to enhance oocyte release in women with the luteinized unruptured follicle syndrome. Clin Exp Obstet Gynecol. 2016;43(2):178–180. [PubMed] [Google Scholar]
- 507. Shibata T, Makinoda S, Waseda T, Tomizawa H, Fujii R, Utsunomiya T. Granulocyte colony-stimulating factor as a potential inducer of ovulation in infertile women with luteinized unruptured follicle syndrome. Transl Res. 2016;171:63–70. [DOI] [PubMed] [Google Scholar]
- 508. Brännström M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. [DOI] [PubMed] [Google Scholar]
- 509. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745–2749. [DOI] [PubMed] [Google Scholar]
- 510. Asunción M, Calvo RM, San Millán JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85(7):2434–2438. [DOI] [PubMed] [Google Scholar]
- 511. March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544–551. [DOI] [PubMed] [Google Scholar]
- 512. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–697. [DOI] [PubMed] [Google Scholar]
- 513. Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 514. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515. Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 2017;11:CD003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516. Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA. 1995;92(23):10619–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518. McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, Legro RS, Strauss JF III. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Diamanti-Kandarakis E, Paterakis T, Alexandraki K, Piperi C, Aessopos A, Katsikis I, Katsilambros N, Kreatsas G, Panidis D. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod. 2006;21(6):1426–1431. [DOI] [PubMed] [Google Scholar]
- 520. Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–1058.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Pietiläinen KH, Kannisto K, Korsheninnikova E, Rissanen A, Kaprio J, Ehrenborg E, Hamsten A, Yki-Järvinen H. Acquired obesity increases CD68 and tumor necrosis factor-α and decreases adiponectin gene expression in adipose tissue: a study in monozygotic twins. J Clin Endocrinol Metab. 2006;91(7):2776–2781. [DOI] [PubMed] [Google Scholar]
- 522. Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Müller B. Central fat excess in polycystic ovary syndrome: relation to low-grade inflammation and insulin resistance. J Clin Endocrinol Metab. 2005;90(11):6014–6021. [DOI] [PubMed] [Google Scholar]
- 523. Wu R, Fujii S, Ryan NK, Van der Hoek KH, Jasper MJ, Sini I, Robertson SA, Robker RL, Norman RJ. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22(2):527–535. [DOI] [PubMed] [Google Scholar]
- 524. Schmidt J, Weijdegård B, Mikkelsen AL, Lindenberg S, Nilsson L, Brännström M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20(1):49–58. [DOI] [PubMed] [Google Scholar]
- 525. Murphy MJ, Halow NG, Royer PA, Hennebold JD. Leukemia inhibitory factor is necessary for ovulation in female rhesus macaques. Endocrinology. 2016;157(11):4378–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526. Adams J, Liu Z, Ren YA, Wun WS, Zhou W, Kenigsberg S, Librach C, Valdes C, Gibbons W, Richards J. Enhanced inflammatory transcriptome in the granulosa cells of women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2016;101(9):3459–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 527. Qin L, Xu W, Li X, Meng W, Hu L, Luo Z, Wang Y, Luo S, Li S. Differential expression profile of immunological cytokines in local ovary in patients with polycystic ovarian syndrome: analysis by flow cytometry. Eur J Obstet Gynecol Reprod Biol. 2016;197:136–141. [DOI] [PubMed] [Google Scholar]
- 528. Brown J, Farquhar C, Beck J, Boothroyd C, Hughes E. Clomiphene and anti-oestrogens for ovulation induction in PCOS. Cochrane Database Syst Rev. 2009; (4):CD002249. [DOI] [PubMed] [Google Scholar]
- 529. Akil M, Amos RS, Stewart P. Infertility may sometimes be associated with NSAID consumption. Br J Rheumatol. 1996;35(1):76–78. [DOI] [PubMed] [Google Scholar]
- 530. Mendonça LL, Khamashta MA, Nelson-Piercy C, Hunt BJ, Hughes GR. Non-steroidal anti-inflammatory drugs as a possible cause for reversible infertility. Rheumatology (Oxford). 2000;39(8):880–882. [DOI] [PubMed] [Google Scholar]
- 531. Killick S, Elstein M. Pharmacologic production of luteinized unruptured follicles by prostaglandin synthetase inhibitors. Fertil Steril. 1987;47(5):773–777. [DOI] [PubMed] [Google Scholar]
- 532. Hedin L, Gaddy-Kurten D, Kurten R, DeWitt DL, Smith WL, Richards JS. Prostaglandin endoperoxide synthase in rat ovarian follicles: content, cellular distribution, and evidence for hormonal induction preceding ovulation. Endocrinology. 1987;121(2):722–731. [DOI] [PubMed] [Google Scholar]
- 533. Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 534. Pall M, Fridén BE, Brännström M. Induction of delayed follicular rupture in the human by the selective COX-2 inhibitor rofecoxib: a randomized double-blind study. Hum Reprod. 2001;16(7):1323–1328. [DOI] [PubMed] [Google Scholar]
- 535. Bata MS, Al-Ramahi M, Salhab AS, Gharaibeh MN, Schwartz J. Delay of ovulation by meloxicam in healthy cycling volunteers: a placebo-controlled, double-blind, crossover study. J Clin Pharmacol. 2006;46(8):925–932. [DOI] [PubMed] [Google Scholar]
- 536. Jesam C, Salvatierra AM, Schwartz JL, Croxatto HB. Suppression of follicular rupture with meloxicam, a cyclooxygenase-2 inhibitor: potential for emergency contraception. Hum Reprod. 2010;25(2):368–373. [DOI] [PubMed] [Google Scholar]
- 537. Edelman AB, Jensen JT, Doom C, Hennebold JD. Impact of the prostaglandin synthase-2 inhibitor celecoxib on ovulation and luteal events in women. Contraception. 2013;87(3):352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538. Hester KE, Harper MJ, Duffy DM. Oral administration of the cyclooxygenase-2 (COX-2) inhibitor meloxicam blocks ovulation in non-human primates when administered to simulate emergency contraception. Hum Reprod. 2010;25(2):360–367. [DOI] [PubMed] [Google Scholar]
- 539. McCann NC, Lynch TJ, Kim SO, Duffy DM. The COX-2 inhibitor meloxicam prevents pregnancy when administered as an emergency contraceptive to nonhuman primates. Contraception. 2013;88(6):744–748. [DOI] [PubMed] [Google Scholar]
- 540. Group EC; ESHRE CapriWorkshop Group. Emergency contraception. Widely available and effective but disappointing as a public health intervention: a review. Hum Reprod. 2015;30(4):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]