Abstract
Background
Epicardial adipose tissue (EAT) is in immediate apposition to the underlying myocardium and, therefore, has the potential to influence myocardial systolic and diastolic function or myocardial geometry, through paracrine or compressive mechanical effects. We aimed to review the association between volumetric EAT and markers of myocardial function and geometry.
Methods and Results
PubMed, Medline, and Embase were searched from inception to May 2018. Studies were included only if complete EAT volume or mass was reported and related to a measure of myocardial function and/or geometry. Meta‐analysis and meta‐regression were used to evaluate the weighted mean difference of EAT in patients with and without diastolic dysfunction. Heterogeneity of data reporting precluded meta‐analysis for systolic and geometric associations. In the 22 studies included in the analysis, there was a significant correlation with increasing EAT and presence of diastolic dysfunction and mean e′ (average mitral annular tissue Doppler velocity) and E/e′ (early inflow / annular velocity ratio) but not E/A (ratio of peak early (E) and late (A) transmitral inflow velocities), independent of adiposity measures. There was a greater EAT in patients with diastolic dysfunction (weighted mean difference, 24.43 mL; 95% confidence interval, 18.5–30.4 mL; P<0.001), and meta‐regression confirmed the association of increasing EAT with diastolic dysfunction (P=0.001). Reported associations of increasing EAT with increasing left ventricular mass and the inverse correlation of EAT with left ventricular ejection fraction were inconsistent, and not independent from other adiposity measures.
Conclusions
EAT is associated with diastolic function, independent of other influential variables. EAT is an effect modifier for chamber size but not systolic function.
Keywords: diastolic function, epicardial fat, systolic dysfunction
Subject Categories: Computerized Tomography (CT), Magnetic Resonance Imaging (MRI), Echocardiography
Clinical Perspective
What Is New?
Increasing epicardial adipose tissue volume is associated with diastolic dysfunction, independent of other markers of adiposity.
Epicardial adipose tissue is an effect modifier for left ventricle chamber geometry.
Epicardial adipose tissue is not associated with systolic function.
What Are the Clinical Implications?
Epicardial adipose tissue may represent an important target for therapy associated with diastolic dysfunction.
Introduction
Epicardial adipose tissue (EAT) has been widely studied as a potential contributor to cardiovascular pathological characteristics. Much of this research has focused on its effect on coronary atherosclerosis,1 but there are unique properties of EAT that may lead to an effect on myocardial function. EAT shares direct anatomic contact with the myocardium without fascial interruption2 and, therefore, may exhibit local compressive forces, resulting in alteration of myocardial function and geometry. In addition, the shared blood supply of the coronary circulation to both the myocardium and surrounding EAT may predispose paracrine effects on the neighboring myocardium with such inflammatory cytokines as MCP‐1 (monocyte chemoattractant), interleukin‐β, interleukin‐6, tumor necrosis factor‐α, and leptin.2 Persisting inflammation may lead to collagen deposition and subsequent impaired left ventricular (LV) relaxation and further effects on diastolic and systolic function. Furthermore, there is an association between EAT and release of free fatty acids, as well as their myocardial consumption.3 The relationship between obesity, visceral fat, and EAT may also explain effects on myocardial function, chamber size, and mass.
Several methods have been used for measurement of EAT, including echocardiography, cardiac computed tomography (CT), and cardiac magnetic resonance imaging (MRI). Echocardiography may overestimate or underestimate total EAT volume because of single‐plane assessment and the effects of probe angulation on linear measurement. Single‐slice area measurements on CT or MRI are also limited by being only single‐plane measures. Recently, we have demonstrated the superiority of volumetric EAT assessment in comparison to 2‐dimensional linear echocardiographic EAT thickness.4 We, therefore, sought the association of full‐volume quantification of EAT (assessed by cardiac CT or cardiac MRI) with myocardial function, as assessed by transthoracic echocardiography, full R‐R interval cardiac CT, or cardiac MRI.
Methods
Search Method
We conducted this systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement, and the trial was registered with PROSPERO (CRD 42017038400). The search was conducted in MEDLINE, EMBASE, and PubMed databases, ending in March 2018. References of eligible articles were hand searched for additional articles. Searches were restricted to human studies, and conference abstracts were included. A study search flowchart is presented in Figure 1, and the specific search term strategy is given in Table S1. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Figure 1.
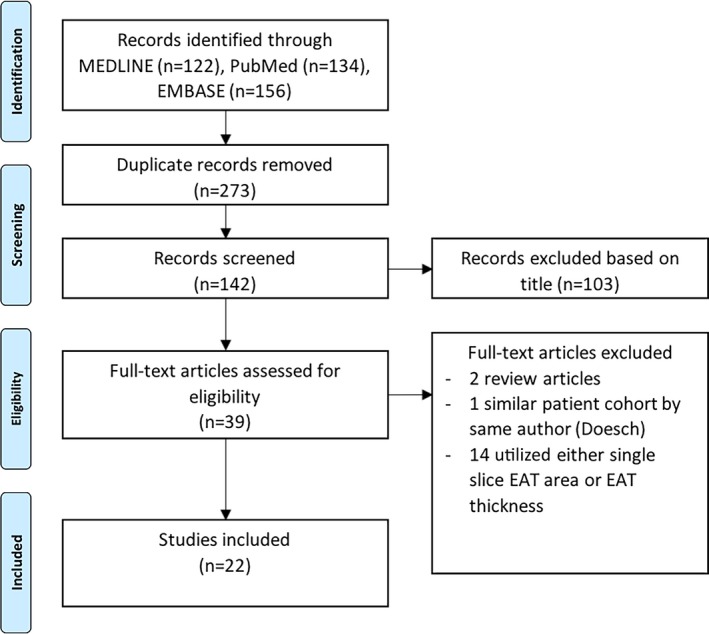
Search strategy. EAT indicates epicardial adipose tissue.
Our inclusion criteria were as follows: patients undergoing cardiac CT (CT angiography or calcium score) or MRI with volumetric assessment of EAT (either volume or mass), with cardiac imaging for assessment of myocardial function parameters (full cardiac cycle cardiac CT or MRI or echocardiography), or measurement of myocardial geometry (LV mass, LV volumes, and left atrium size) by validated methods. Assessment of diastolic function was restricted to studies using echocardiography. Exclusion criteria included the following: any study with linear measurement of EAT thickness, single‐slice area measures of EAT, measures of myocardial lipid content not differentiated from EAT, and measurement of paracardial adipose tissue (ie, fat beyond the parietal pericardium). Two authors (N.N. and R.G.M.) independently reviewed the abstracts from the search to meet the inclusion criteria, and discrepancies were resolved by consensus. Probable overlap of the patient cohort with a similar study led to exclusion of the smaller study.5
Evaluation of Full‐Volume EAT
EAT was regarded as adipose tissue enclosed within the visceral pericardium, and mean values (indexed and nonindexed) were recorded.
Evaluation of Cardiac Function
Included studies measured myocardial performance based on echocardiography or MRI. Measures of diastolic function included the following: transmitral flow for peak early (E) and late (A) inflow velocities and their ratio (E/A); deceleration time; septal, lateral, and/or average myocardial annular velocities on tissue Doppler imaging (e′); early inflow/annular velocity ratio (E/e′); pulmonary vein flow to calculate the time difference between the atrial reversal wave and mitral A‐wave duration; and the isovolumic relaxation time. Diastolic class grade was recorded if reported: normal, grade 1 (impaired relaxation), grade 2 (pseudonormal), and grade 3 (restrictive). Measures of systolic performance assessed included LV ejection fraction, cardiac output, stroke volume, and global longitudinal strain, if recorded. Measures of cardiac structure included LV mass, LV end‐diastolic and end‐systolic volumes, and left atrial size.
Statistical Analysis
Data on univariable correlations are presented because this was the most consistent measure seen in included studies. Where multivariable regression was performed, adjusted study estimates and model covariates are reported. Meta‐analysis was performed for the weighted mean difference in EAT volume between groups with and without diastolic dysfunction. Meta‐regression of weighted mean difference as an effect size and the combined mean EAT in included studies were performed with the moment‐based estimate of between‐study variance and a permutation test using 1000 Monte Carlo simulations to moderate for potentially spurious results, as previously described.6 Precision of pooled estimates is reported as 95% confidence intervals, and heterogeneity is reported by the I2 statistic. The Newcastle Ottawa Scale was used to assess risk of bias (Tables S2 and S3). Statistical analysis was performed using StataMP 14.0 (StataCorpLP, College Station, TX).
Results
Study Selection
A brief outline summary of the 22 studies (18 published and 4 conference papers) included in this review is presented in Table 1.3, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28
Table 1.
Study Characteristics
| First Author | Year | Country | Study Type | Population | Sample Size | EAT Method | EAT Value |
|---|---|---|---|---|---|---|---|
| Bakkum8 | 2015 | the Netherlands | Cross‐sectional | Suspected CAD | 208 | PET‐CT | 113.8±48.1 cm3 |
| Cavalcante9 | 2012 | United States | Cross‐sectional | Self‐referred screening | 110 | MDCT |
Men, 101±51 cm3
Women, 67±40 cm3 |
| Al Chekakie7 | 2010 | United States | Case‐control | AF and controls | 273 | MDCT |
Sinus rhythm, 76.1±36.3 mL; AF, 101.6±44.1 mL |
| Doesch11 | 2012 | Germany | Case‐control | Established CAD |
158 Cases and 40 controls |
MRI |
Control, 31±8 g/m2; CAD, 29±10 g/m2; CAD and EF <50%, 26±8 g/m2; CAD and EF >50%, 36±11 g/m2 |
| Doesch12 | 2013 | Germany | Case‐control | DCM |
112 Cases and 48 controls |
MRI |
Control, 62.1±14.4 g; DCM, 47.2±15.2 g; control, 66±15.3 mL; DCM, 50.2±16.2 mL; control, 31.7±5.6 g/m2; DCM, 24±7.5 g/m2; control, 33.5±6.4 mL/m2; DCM, 25.5±8 mL/m2 |
| Doesch10 | 2010 | Germany | Case‐control |
CHF (LVEF <35%) (ICM=36; DCM=30) |
66 Cases and 31 controls |
MRI |
Control, 71±13 mL; CHF, 46±11 mL; control, 36±5 mL/m2; CHF, 24±5 mL/m2; control, 67±13 g; CHF, 43±11 g; control, 34±4 g/m2; CHF, 22±5 g/m2 |
| Ede13 | 2014 | Turkey | Cross‐sectional | Suspected CAD | 106 | MDCT | 38±31 cm3 |
| Faustino14, a | 2011 | Portugal | Cross‐sectional | Not specified | 78 | MDCT | Threshold of 44.1 mL defined by ROC curve (72% sensitivity and 50% specificity) for diastolic dysfunction |
| Fernando15, a | 2015 | United States | Cross‐sectional | AF before ablation | 20 | MRI | 125.7±56.7 mL |
| Fontes‐Carvalho16 | 2014 | Portugal | Cross‐sectional | Postmyocardial infarction | 225 | MDCT | 113.6±43.2 cm3 |
| Fox17 | 2009 | United States | Cross‐sectional | Substudy of Framingham | 997 | MDCT | Women, 108±41 cm3; men, 136.5±54.4 cm3 |
| Hachiya18 | 2014 | Japan | Cross‐sectional | Suspected CAD | 134 | MDCT | 77.1±29.6 cm3/m2 |
| Khawaja19 | 2011 | Unites States | Cross‐sectional | Suspected CAD | 381 | MDCT |
Normal LVEF, 114.5±98.5 cm3; LVEF <55%, 83.5±67.1 cm3 |
| Konishi20 | 2012 | Japan | Cross‐sectional | Suspected CAD | 229 | MDCT |
Diastolic dysfunction, 184±61 cm3; normal function, 154±58 cm3 |
| Lai21 | 2015 | Taiwan | Cross‐sectional | Self‐referred screening | 318 | MDCT | 80.6±33 mL |
| Liu22 | 2011 | United States | Cross‐sectional | Blacks | 1402 | MDCT |
Men, 79.8±37.1 mL; women, 67.1±29.0 mL |
| Longenecker23, a | 2016 | Cross‐sectional | Patients with HIV | 46 HIV+ and 23 HIV− | MDCT |
HIV+ with DD, median of 120 (74–143) mL; HIV+ with normal function, median of 72 (54–100) mL; HIV−, not specified |
|
| Ng24 | 2016 | Australia | Cross‐sectional | Suspected CAD | 130 | MDCT |
Total, 97.5±43.7 cm3; men, 103.7±39.5 cm3; women, 90.9±47.4 cm3 |
| Ruberg3 | 2010 | United States | Cross‐sectional | Obese with metabolic syndrome |
28 Cases and 18 controls |
MRI |
Controls, 85±66 mL; subjects, 161±88 mL; controls, 1.1±0.7 mL/g; subjects, 2.0±1.1 mL/g |
| Vanni25, a | 2015 | Italy | Case‐control | Not specified |
19 NAFLD and 9 controls |
MRI |
NAFLD, 228.1±112.9 mL; controls, 66.8±25.2 mL |
| Vural26 | 2014 | Turkey | Case‐control | Suspected CAD | 63 | CACS | 137±56 cm3 |
| Wu27 | 2015 | Taiwan | Cross‐sectional | Compensated CHF |
50 Cases and 20 controls |
MRI | Control, 45.8 (39.4–50.3) mL; CHF+VT/VF, 51.5 (46.6–59.8) mL); CHF and no VT/VF, 44.0 (33.9–48.3) mL |
| Yamashita28, a | 2012 | Japan | Cross‐sectional | Suspected CAD | 286 | MDCT | EAT, 71.6±37.9 (10.5–179.9) mL |
Values are mean±SD or mean (range). AF indicates atrial fibrillation; CACS, coronary artery calcium score; CAD, coronary artery disease; CHF, congestive heart failure; DCM, dilated cardiomyopathy; DD, diastolic dysfunction; EAT, epicardial adipose tissue; ICM, ischemic cardiomyopathy; LVEF, left ventricular ejection fraction; MDCT, multidetector computed tomography; MRI, magnetic resonance imaging; NAFLD, nonalcoholic fatty liver disease; PET‐CT, positron emission tomography–computed tomography; ROC, receiver operating characteristic; VT/VF, ventricular tachycardia/ventricular fibrillation.
This is a conference abstract.
Association of EAT With LV Diastolic Function
There were 11 studies that investigated the relationship between EAT and diastolic parameters, with 5 specifying adherence to an iteration of the American Society of Echocardiography diastolic guidelines.29 EAT was associated with diastolic parameters, including peak mitral annular tissue Doppler velocities (e′ septal, e′ lateral, or e′ mean) and transmitral flow (early [E] and late [A] diastolic peak flow velocities and their ratio [E/A]) (Table 2).9, 13, 14, 15, 16, 18, 20, 21, 22, 23, 24, 29, 30, 31, 32 Although some studies did perform comprehensive Doppler measures, such as isovolumic relaxation times, deceleration times, and pulmonary vein Doppler, the association with EAT individually with each parameter was not described. The classification of patients with diastolic dysfunction was available in 5 studies. Most patients (26%–38% of total cohort) had grade 1 diastolic dysfunction, with fewer qualifying as grade ≥2 (2%–28%).
Table 2.
EAT and Diastolic Function
| First Author | Diastolic Function Reference | Subgroup Characteristics | Diastolic Parameter Correlations | Multivariable Regression Comments | |||
|---|---|---|---|---|---|---|---|
| DD | Normal Function | E/A | e′ | E/e′ | |||
| Cavalcante9 | ASE29 |
Grade 1 (n=29, 26%) Grade 2 (n=11, 10%) |
n=70, 64% |
Averaged 0.44a |
0.34a |
Multivariate model outcomes of grade 1 or higher DD, mean e′, and mean E/e′: EAT was an independent predictor (model included 10‐y Framingham Risk Score, metabolic syndrome, subclinical CAD, and LV mass index), β range, −0.02 to 0.04 (all P<0.05). Indexed EAT was found to increase clinical model for prediction of DD (adjusted R 2=0.16 vs 0.24; P=0.004) and mean e′ (adjusted R 2=0.17 vs 0.27; P=0.001) (ie, indexed EAT represents 8%–10% of the variation of predictors for DD |
|
| Ede13 | Lang et al32 |
Grade 1 (n=39, 37%) Grade 2 (n=10, 9%) Grade 3 (n=2, 2%) |
n=55, 52% | −0.404 | |||
| Faustino14, b | Not specified | 46 Patients with DD and EAT >44.1 mL | 32 Patients with no DD and EAT <44.1 mL |
EAT not significant on multivariable regression (results and covariates not reported). Relationship of EAT with DD by ROC AUC of 0.66 (P=0.02) |
|||
| Fernando15, b | Not specified |
EAT=164±118 mL (E/E′ >15) |
EAT=114±54 mL (E/E′ <15) |
−0.48a | 0.22 | On multivariable regression adjusted for age, BMI, LA volume, hypertension, and CAD, EAT associated with abnormal myocardial relaxation (OR, not specified; P=0.04) | |
| Fontes‐Carvalho16 | ASE29 |
EAT=116.7±67.9 cm3
Grade 1 (n=57, 28%) Grade 2 (n=58, 28%) Grade 3 (n=10, 5%) |
EAT=93.0±52.3 cm3
n=80 (39%) |
e′ Septal, −0.26a
e′ lateral, −0.28a |
0.25a | On multivariable regression adjusted for hypertension, age, sex, and other markers of adiposity (SAT, VAT, waist/height ratio, and fat mass %), EAT remained significantly predictive of E/e′ (β, 0.19 [0.06–0.32]; P<0.01), as did e′ septal and e′ lateral | |
| Hachiya18 | ASE29 | −0.05 | −0.31a | 0.24a | Definition of diastolic dysfunction not specified. On different multivariate models, e′ inversely correlated with EAT (standardized β range, −0.30 to −0.36; all P<0.05) but not E/e′ (standardized β, 0.23; P=0.06), except when adjusted for age, sex, and BMI (model 1) and medication use (model 2) (standardized β range, 0.25–0.31; all P<0.05) | ||
| Konishi20 | Defined as E/e′ >10 |
EAT=184±61 cm3
n=141 (62%) |
EAT=154±58 cm3
n=88 (38%) |
0.21a | On multivariable regression with age, hypertension, male sex, diabetes mellitus, and abdominal obesity, there was an independent effect of EAT on DD: OR, 2.09 (1.15–3.79; P=0.02) for EAT per 100 cm3 | ||
| Lai21 | Lang et al32 |
EAT=86.79±31.77 n=100 |
EAT=67.32±31.95 n=218 |
−0.38a | 0.284a | On multivariable regression adjusted for age, sex, BMI, systolic blood pressure, LV mass index, hypertension, diabetes mellitus, hyperlipidemia, and smoking, EAT was significantly associated with E/A (β, −0.002)a and diastolic dyssynchrony (β, 0.197)a | |
| Gottdiener et al31 |
Men, −0.12)a
women, −0.12a |
On multivariable linear regression adjusted for age, height, smoking, alcohol, blood pressure, eGFR, hemoglobin, total physical activity score, medications, VAT, and weight, E/A no longer became significant (regression co‐efficient, −0.01±0.02 [P=0.41] in women and −0.0±0.02 [P=0.64] in men) (described as pericardial fat volume) | |||||
| Longenecker23, b | Not specified |
Grade 1 (n=29 [HIV+, n=19; HIV−, n=10]) Grade 2 (n=2 [HIV+, n=1; HIV−, n=2]) |
n=38 (HIV+) n=26 and n=12 (HIV−) |
−0.392a | On multivariable regression adjusted for age, BMI, and sex, EAT remained independently associated with diastolic dysfunction (OR, 1.35; 95% CI, 1.02–1.79) per 10‐mL increase (described as pericardial fat volume) | ||
| Ng24 | Not specified |
e′ Septal, −0.263)a; e′ lateral, −0.285a |
|||||
| Vural26 | Alnabhan et al30 |
EAT=164.4±54 cm3
Grade 1 (n=24, 38%) Grade 2 (n=4, 6%) Grade 3 (n=1, 1.5%) |
EAT=114.1±46.6 cm3
n=34 (56%) |
−0.437a | On multivariable regression adjusted for age, blood pressure, BMI, waist circumference, and cholesterol, EAT was an independent predictor of DD (OR, 1.03 [1.01–1.06]; P=0.006). ROC‐derived optimal cutoff for DD, 129.6 cm3 (ROC curve, 0.758) | ||
Correlations represent the correlation co‐efficient.
Values are mean±SD or mean (range). ASE indicates American Society of Echocardiography; AUC, area under the curve; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; DD, diastolic dysfunction; e′, average mitral annular tissue Doppler velocity; E/e′, early inflow / annular velocity ratio; E/A, ratio of peak early (E) and late (A) transmitral inflow velocities; EAT, epicardial adipose tissue; eGFR, estimated glomerular filtration rate; LA, left atrial; LV, left ventricular; OR, odds ratio; ROC, receiver operating characteristic; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
P value for univariate correlation is significant at <0.05.
Study is a conference abstract.
In the 5 studies that measured differences in EAT between groups, EAT was significantly greater in the diastolic dysfunction group compared with patients with normal diastolic function (weighted mean difference, 24.4 mL; 95% confidence interval, 18.5–30.4 mL; P<0.001; I2=28%) (Figure 2).15, 16, 20, 21, 23, 26 Meta‐regression, performed evaluating the weighted mean difference (effect size) against the mean EAT volume, demonstrated a nominally increasing presence of diastolic dysfunction with increasing EAT values (β=0.17, SEE=0.09, P=0.06). This was statistically significant after Monte Carlo permutation testing, P=0.001 (Figure 3).
Figure 2.
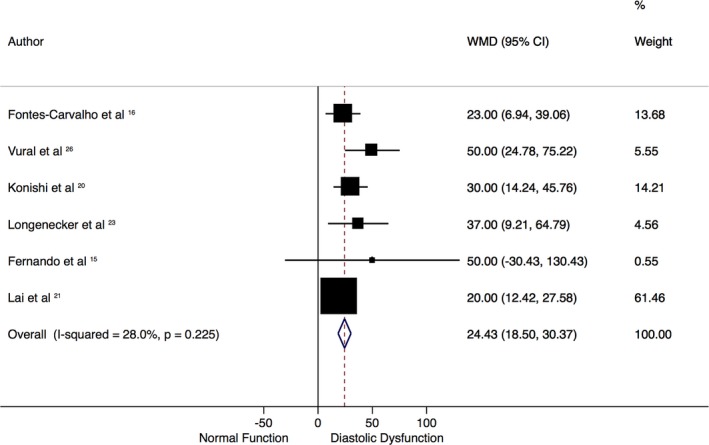
Mean difference of epicardial adipose tissue (EAT) volume in patients with and without diastolic dysfunction. Forest plot demonstrates the weighted mean difference (WMD; in mL) of EAT in studies with and without diastolic dysfunction, according to a random‐effect model. Those with diastolic dysfunction have significantly greater EAT volumes. There is mild heterogeneity, as seen by the I2 statistic of 28%. CI indicates confidence interval.
Figure 3.
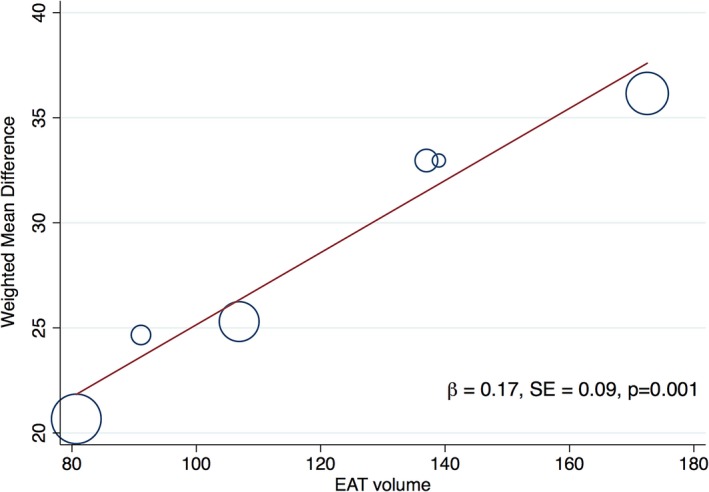
Meta‐regression of the effect of increasing epicardial adipose tissue (EAT) volume on the weighted mean difference (effect size) of EAT in patients with and without diastolic dysfunction. Meta‐regression bubble plot depicts increasing differences in mean EAT volume in patients with diastolic dysfunction as EAT increases. Circles represent the weight of each study. β coefficient is from meta‐regression with associated SEE; P value is from Monte‐Carlo testing (1000 simulations) and demonstrates a significant association (P=0.001).
Mean E/e′ values were positively correlated with EAT (r value range, 0.21–0.34; P<0.05), and mean e′ values were inversely correlated (r value range, −0.26 to −0.44; P<0.05); in all but one study, no consistent association was seen with the E/A ratio (r value range, −0.40 to 0.08). Increasing EAT was an independent predictor of diastolic dysfunction, e′ and E/e′ independent of age, sex, and measures of adiposity (Table 2). No independent association was identified with the E/A ratio. In 6 studies, hypertension was also an adjusted covariate in the model, and increasing EAT remained a predictor of altered diastolic parameters.
Association of EAT With Systolic Function
Of 10 studies describing the association of EAT with systolic parameters, LV function was evaluated with MRI in 5 and echocardiography in 4 (Table 3).3, 10, 11, 16, 18, 19, 22, 27 One study reported associations between EAT and global longitudinal strain, a subclinical measure of myocardial function.24 Only one described an independent effect of EAT on LV ejection fraction (LVEF) by echocardiography.19 No univariable correlation with LVEF was reported in the MRI studies.10, 11, 12 Of the 6 studies reporting multivariable regression analysis, an independent association with LVEF was observed in 2 studies: one study was performed in patients with established coronary artery disease (CAD) stratified by LVEF and compared with normal controls (hazard ratio, 0.48; 95% confidence interval, 0.28–0.68; P<0.01),11 and the other study was performed in patients undergoing investigation for suspected CAD with reduced LVEF compared with normal LVEF (values not reported).19
Table 3.
EAT and Systolic Function
| First Author | Method | Group | EAT Value | Systolic Measure | r Value (Univariate) | Multivariable Regression Comment |
|---|---|---|---|---|---|---|
| Doesch11 | MRI |
CAD and EF >50% (n=44) CAD and EF <50% (n=114) Combined CAD (n=158) Controls (n=40) |
36±11 g/m2
26±8 g/m2 29±10 g/m2 31±8 g/m2 |
LVEF |
0.171 0.137 0.574a Not specified |
On multivariable regression adjusted for BMI, NYHA classes I and III, atrial fibrillation, LV‐EDVI, LV‐ESVI, LV‐EDD, LVRI, and LGE%, LVEF was an independent predictor of indexed EAT (HR, 0.478 [0.28–0.675]; P<0.01)b |
| Doesch12 | MRI |
Control (n=48) DCM (n=112) |
31.7±5.6 g/m2
24±7.5 g/m2 |
LVEF LVEF |
0.069 0.085 |
No correlation with LVEF and EAT (P=0.37) |
| Fontes‐Carvalho16 | Echocardiography | LVEF | −0.07 | |||
| Hachiya18 | Echocardiography | LVEF | 0.22a | Significant association on multivariate regression models adjusted for hypertension, diabetes mellitus, dyslipidemia, previous CAD or revascularization, and medication use (standardized β range, 0.16–0.22; all P<0.05) but not adjusted for age, sex, or BMI (standardized β, 0.13; P>0.05) | ||
| Khawaja19 | Echocardiography |
Normal (n=321) EF <55% (n=60) EF 35%–55% (n=43) EF <35% (n=17) |
114.5±98.5 cm3
83.5±67.1 cm3 96.0±73.9 cm3 52.2±29.7 cm3 |
Multivariate analysis revealed LVEF and triglyceride levels predicted EAT (values and covariates not reported) | ||
| Liu22 | Echocardiography |
Women Men |
LVEF LVEF |
−0.04 0.03 |
Not significant on multivariable regression in either sex (adjusted for age, height, smoking, alcohol, blood pressure, eGFR, hemoglobin, total physical activity score, medications, VAT, and weight: regression coefficient, −0.3±0.4 [P=0.51] in women and 0.2±0.6 [P=0.72] in men). Note: described as pericardial fat volume. | |
| Ruberg3 | MRI | Obese |
CO SV LVEF |
−0.46a
Inversea Not correlated |
Values are normalized to LV mass (mL/g) | |
| Control |
CO SV LVEF |
Not correlated Not correlated Not correlated |
||||
| Wu27 | MRI | LVEF | Not correlated |
Values are mean±SD or r value correlation coefficients, unless otherwise stated. BMI indicates body mass index; CAD, coronary artery disease; CO, cardiac output; DCM, dilated cardiomyopathy; EAT, epicardial adipose tissue; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LGE%, percentage of late gadolinium enhancement; LV, left ventricular; LV‐EDD, LV end‐diastolic diameter; LV‐EDVI, LV end‐diastolic volume index; LV‐ESVI, left ventricular end‐systolic volume index; LVRI, LV remodeling index; MRI, magnetic resonance imaging; NYHA, New York Heart Association; SV, stroke volume; VAT, visceral adipose tissue.
P<0.05.
Directly quoted values from source article.
The only consistent feature across all studies appeared to be a relative decrease in EAT as LVEF decreased. In studies that included control groups (ie, normal LVEF), no association of EAT with EF was identified in the control group. One study demonstrated a significant inverse correlation with EAT (normalized to LV mass) with cardiac output and stroke volume (but not LVEF)3 in obese patients (r value, −0.46) but not in corresponding controls.
In studies focusing specifically on patients with reduced LVEF, EAT was reduced compared with those with preserved LVEF. Doesch et al11 demonstrated that patients with CAD and preserved LVEF had greater EAT (36±11 g/m2) than normal controls without CAD (31±8 g/m2), and both had greater EAT than patients with CAD with LVEF <50% (28±8 g/m2; P<0.01). A population with presumed ischemic cardiomyopathy (CAD with reduced LVEF) also reported a stepwise decrease in EAT volume with reducing grades of LVEF.19 This stepwise decrease was not found in a different study by Doesch et al12 in patients with dilated cardiomyopathy against normal controls, although EAT was reduced overall compared with normal controls.
In the study related to strain analysis,24 there was a positive correlation with EAT and impaired 3‐dimensional global longitudinal strain (r=0.601, P<0.001) that remained significant on multivariable regression (standardized β=0.512, P<0.001), independent of markers of obesity and diabetes mellitus.
Association of EAT With Chamber Measures
There were 14 studies with data relating to a measure of myocardial geometry. All modalities of echocardiography, CT, and MRI were represented, with most values indexed to body surface area, unless otherwise specified. Some studies avoided indexation because body weight or other adiposity measures were used in regression models and, therefore, raw measures were used to prevent collinearity.
The most often reported univariable correlation coefficient was for EAT and LV mass or indexed mass and was always statistically significantly positively correlated in the diseased patient group (not controls), with ranges from r=0.19 to r=0.42 (P<0.05). Only studies by Doesch et al11,12 measured LV end‐diastolic diameter and found a consistent association with EAT (r value range, 0.22–0.42; P<0.05). Similar findings were seen for LV end‐diastolic and end‐systolic volume. Left atrial size was measured either as volume or diameter and demonstrated significant univariable associations with EAT (Table 4).*
Table 4.
EAT and Chamber Geometry
| Author | Modality | Subgroup | LV‐EDD | LA Size (Diameter/Volume) | LVEDMI | LV‐EDVI | LV‐ESVI | LVRI | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Bukkam8 | CT | 0.42a , b | On multivariable regression adjusted for traditional cardiovascular risk factors, CACS and BMI, EAT was not a significant predictor of LV mass in obese patients, but only in nonobese patients (β=0.23, P<0.001) | ||||||
| Cavalcante9 | Echocardiography | 0.41a | Measure not included in multivariate analysis | ||||||
| Al Chekakie7 | CT and echocardiography | 0.25/0.24 | |||||||
| Doesch11 | MRI |
EF <50% (n=44) EF >50% (n=114) Combined (n=158) |
0.076 0.011 0.272a |
0.336a
0.305a 0.019 |
0.201a
0.043 0.16a |
0.089 0.056 0.262a |
0.137 0.202 0.344a |
On multivariable regression including LVEF, BMI, NYHA classes I and III, atrial fibrillation, LV‐EDVI, LV‐ESVI, LV‐EFF, LVRI, and LGE%, best correlates to indexed EAT were LVEF, BMI, LV‐ESVI (HR, 0.48; P<0.01), and LV‐EDD (HR, −0.238; P=0.01). In subgroup analysis by EF <50% or >50%, full model not described; however, no association with LVEDMI in LVEF >50% but association seen in LVEF >50% (HR, 0.105; P=0.01) | |
| Doesch12 | MRI |
Control (n=48) DCM (n=112) |
0.01 0.22a |
0.346a
0.417a |
0.007 0.251a |
0.0001 0.239a |
0.204 0.116 |
Increased EAT mass with increasing LVEDMI in DCM, but less values than healthy control group. Greater mass seen in DCM with hypertrophy vs nonhypertrophy (31.7±5.6 vs 24.4±7.1 g/m2; P=0.01). On multivariable regression only, LVEDMI independently correlated with indexed EAT, as was seen in healthy controls (adjusted for age and BMI [value not reported]). | |
| Doesch10 | MRI |
Control CHF |
NR 0.42a |
0.36a
0.59a |
Increased EAT mass in CHF with increasing LVEDMI; however, higher levels of EAT in controls compared with CHF (34±4 vs 22±5 g/m2; P<0.01). On multivariate regression adjusted for LVEF, LV‐EDD, RVEF, and LVEDMI, only LVEDMI independently associated with indexed EAT (P=0.0001) | ||||
| Fox17 | MRI |
Women Men |
0.28a
0.37a |
0.35a
,
c
0.19a , c |
0.2a
,
c
0.07c |
On multivariable regression adjusted for age, height, smoking, alcohol, menopause, hormone replacement therapy, blood pressure, hypertension therapy, and weight, only in women, LVM (adjusted regression coefficient, 1.66; P=0.01), and in men, LA diameter (adjusted regression coefficient, 0.8; P=0.002) were independent predictors of pericardial fat volume | |||
| Hachiya18 | Echocardiography | 0.28a | Measure not included in multivariate analysis | ||||||
| Konishi20 | Echocardiography | 0.32a | 0.23a | Measure not included in multivariate analysis | |||||
| Liu22 | Echocardiography |
Women Men |
0.3a
0.11 |
0.24a
,
c
0.21a , c |
On multivariable regression adjusted for age, height, smoking, alcohol, blood pressure, eGFR, hemoglobin, total physical activity score, medications, VAT, and weight, only in women, LVM (adjusted regression coefficient, 4.1±1.8; P=0.03) and LA diameter (adjusted regression coefficient, 0.4±0.2; P=0.03) were independent predictors of pericardial fat volume | ||||
| Ng24 | Echocardiography | −0.09 | 0.08 | ||||||
| Ruberg3 | MRI | Not | |||||||
| Vanni25, d | MRI | Cases | 0.46a , e |
Inversely correlated with EF No other analysis specified |
|||||
| Yamashita28, d | CT | 0.25a |
Values are mean±SD or r value correlation coefficients, unless otherwise stated. BMI indicates body mass index; CACS, coronary artery calcium score; CHF, congestive heart failure; CT, computed tomography; DCM, dilated cardiomyopathy; EAT, epicardial adipose tissue; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LA, left atrial; LGE%, percentage of late gadolinium enhancement; LV, left ventricular; LV‐EDVI, LV end‐diastolic volume index; LV‐EDD, LV end‐diastolic diameter; LVEF, LV ejection fraction; LVEDMI, LV end‐diastolic mass index; LV‐EDVI, LV end‐diastolic volume index; LV‐ESVI, LV end‐systolic volume index; LVRI, LV remodeling index; MRI, magnetic resonance imaging; NR, not reported; NYHA, New York Heart Association; RVEF, right ventricular EF; VAT, visceral adipose tissue.
P < 0.05.
Value is for LV mass on CT, nonindexed and time in cardiac cycle not specified.
Represents a nonindexed measure.
Study is a conference abstract.
Value is for end‐systolic LV diameter.
An inconsistent association was seen with measures of adiposity in relation to EAT and cardiac structure. In patients with reduced LVEF, indexed EAT appears to be associated with indexed LV end‐diastolic mass independent of BMI (Table 4).10, 11, 12 One study assessing patients with suspected CAD and normal LVEF demonstrated that EAT correlated best with LV mass (nonindexed) in the nonobese cohort only (β=0.23, P<0.001).8 Finally, in 2 observational studies, an independent association of EAT with LV mass (nonindexed), adjusted for body weight, was only seen in women (Table 4).17, 22
Discussion
This review of 21 studies has demonstrated the emerging body of work relating EAT to myocardial structure and function. Increasing EAT is associated with the following: (1) an increasing prevalence of diastolic dysfunction; (2) a concomitant increase in LV mass; and (3) no consistent association with markers of systolic function. However, these correlations were no more than moderate; no coefficient exceeded 0.50.
Protective Functions of EAT
EAT has a high fatty acid content and can both release and scavenge excess free fatty acids to regulate myocardial energy production.2 In addition, EAT secretes anti‐inflammatory cytokines, such as adiponectin, adrenomedullin, and omentin, which have antiatherogenic effects; EAT also regulates vascular tone and cardiac remodelling.33 There is a thermogenic role for EAT in providing heat for the myocardium in times of hypoxic or ischemic stress.33 However, the presence of numerous proinflammatory cytokines within EAT may lead to a potential imbalance of harmful versus protective cytokines and disruption of myocardial function. Higher levels of these molecules (eg, tumor necrosis factor‐α, interleukin‐6, interleukin‐1, and MCP‐1) are seen in patients with CAD or heart failure. It is uncertain whether the trigger for the imbalance of cytokines is a cause of the pathological characteristics or a consequence, and a potential reciprocal or bidirectional role has been proposed.2
EAT and Diastolic Dysfunction
Adipose tissue can modulate the cardiovascular system by mechanisms including sympathetic activation, adipokine secretion, and myocardial oxidative stress.34, 35 EAT is regarded as a visceral fat depot. Visceral fat is metabolically active and is a determinant of diastolic function.36 The adipokines within EAT can all affect diastolic function through persistent inflammation and subsequent collagen turnover,37 impaired microvascular relaxation, or a direct toxic effect on the myocardium.38, 39 The loss of protective effects of adiponectin can also modify diastolic function.40
Mechanical effects may arise from myocardial compression of EAT because it lies within a fixed pericardial sac,17 inducing a similar mechanism as pericardial constriction. Hachiya et al demonstrated an independent correlation of EAT with aortic pulse pressure as another mechanism of diastolic dysfunction that may be mediated by the association of EAT with aortic stiffness and, therefore, increased pulse wave velocity and early wave reflection.18 Increased pressure in late systole may cause slower LV relaxation and subsequent diastolic dysfunction, as well as compromise coronary perfusion, especially if there is underlying CAD leading to impaired LV relaxation.41
EAT is associated with obesity, which itself is independently associated with diastolic dysfunction.42 Obese patients often have elevated EAT volumes,17 and indexed EAT has modest incremental value for diastolic dysfunction over traditional covariates, such as metabolic syndrome, subclinical CAD, and LV mass index.9 Although the results from our analysis demonstrate that EAT had an independent effect on diastolic function parameters over adiposity measures, adiposity measures varied considerably and included BMI, bioimpedence testing, area of visceral adipose tissue or subcutaneous adipose tissue, or indexed EAT, which accounts for body weight. This heterogeneity needs further explanation to adequately isolate the effect of obesity and EAT on diastolic function. The lack of an association of EAT with E/A ratio may be confounded by the effects of age, proportion of patients with CAD, measurement in patients with normal LVEF, and the U‐shaped relationship of E/A ratio with diastolic function that makes it difficult to assess without the addition of other variables.43
The evaluation of diastolic function is challenging and influenced by a patient's filling status, the presence of CAD, diabetes mellitus, obesity, as well as “normal” changes seen in the ageing patient. Although most studies aim to account for these factors in multivariable regression models, no more than association can be interpreted, and causality cannot be proved. Statistically, there may be implications of collinearity of obesity measures and EAT in multivariable models.
EAT and Systolic Dysfunction
Our study noted weak and inconsistent associations of EAT and systolic parameters. In the single study that evaluated EAT and longitudinal strain as a marker of subclinical myocardial dysfunction, there was a strong association noted independent of confounders, such as obesity and diabetes mellitus.24 This is a notable finding; however, causality remains unproved and requires further assessment in larger‐scale studies as a possible marker of the syndrome of heart failure with preserved ejection fraction. Various hypotheses have been developed to relate EAT and systolic function. In studies of patients with ischemic and dilated cardiomyopathy, there has been a consistent signal of reducing EAT with reducing LVEF, with less EAT also seen compared with normal controls or those with normal LVEF.10, 11, 12, 19 As myocardium becomes progressively dysfunctional, the role of EAT as a source of energy or cytokine homeostasis may become less necessary, contributing to EAT depletion. Conversely, in obese patients, there was no association with EAT (normalized to cardiac mass) and LVEF, and there was a negative correlation with MRI‐derived cardiac output as EAT increased.3 The proposed mechanism is from mechanical restriction of myocardial expansion from EAT in diastole that may lead to less ventricular filling and, therefore, reduced cardiac output.3 A further mechanism may involve the effects of a direct cytokine release, as seen in patients with decompensated heart failure, but no studies have applied this in the context of EAT volume.
EAT and Chamber Measures
Postmortem and experimental studies44, 45 have demonstrated a constant ratio of epicardial fat/ventricular myocardium, regardless of underlying pathological characteristics of hypertrophy, ischemia, or normal muscle. Furthermore, the increase in fat mass parallels LV hypertrophy, although healthy controls have higher quantities of EAT.10 Similar findings are seen when evaluating the LV remodeling index (ratio of mass/end‐diastolic volume), where an inverse correlation is noted with LVEF and the EAT/LV remodeling index ratio. LVEF is inversely correlated with EAT and linearly correlated with LV remodeling index, suggesting that remodeling is not compensated by an adequate increase in EAT.10
Obesity has shown a positive relationship with increased LV mass and EAT, yet the impact of obesity on myocardial geometry may outweigh the local effects of ectopic fat because associations attenuated after adjustment for other adiposity measures, including body weight.17 From a mechanistic perspective, the association of EAT with central obesity and visceral adipose tissue might result in greater LV afterload and subsequent increased LV output, therefore leading to LV remodeling.8 As LV remodeling progresses, LV diameter, volume, and mass increase, which may then deplete EAT stores12 and result in a vicious cycle of reduced protective benefits on the heart and further dysfunction. However, the independent association of EAT with LV mass is limited to nonobese subjects.8 Associations of EAT with the incidence of CAD have been described in nonobese people46 and could contribute to the so‐called obesity paradox.47
Limitations
We acknowledge several limitations in our study. EAT measurement by different modalities may lead to differences between studies. Some reported EAT indexed to Body Surface Area (BSA) (therefore accounting for weight), and some reported raw values using weight as a covariate in multivariable models. Such normalization, as opposed to normalization to height, may obscure the contribution of obesity to differences in chamber volumes and mass, which are associated with EAT. Not all studies adjusted for hypertension in multivariable models, which is also associated with obesity and diastolic function. Variations in the reference literature on measures of diastolic function also lead to difficulties with comparing studies. The differences in regional location of EAT were not available in most studies and, therefore, the effect of EAT distribution was not assessable. The level of heterogeneity and variable study end points precluded detailed meta‐analysis.
Conclusions
Despite small and heterogeneous studies, there is clear evidence of a consistent effect of volumetric EAT on myocardial diastolic function and chamber measurements; however, robust data are lacking to make causal inferences. These findings are observed despite adjustment for common confounders, such as adiposity. No consistent effect is seen with respect to systolic parameters. Further longitudinal studies are necessary to generate quantitative summary measures as well as develop potential targets for treatment.
Sources of Funding
Nerlekar is supported by a scholarship from the National Medical Health and Research Council and the National Heart Foundation. Brown is supported by an Early Career Fellowship from Monash University.
Disclosures
None.
Supporting information
Table S1. Example MEDLINE Search Strategy
Table S2. Newcastle—Ottawa Scale for Assessment of Cross‐sectional Studies
Table S3. Newcastle—Ottawa Scale for Assessment of Case Control Studies
(J Am Heart Assoc. 2018;7:e009975 DOI: 10.1161/JAHA.118.009975.)
Note
References
- 1. Nerlekar N, Brown AJ, Muthalaly RG, Talman A, Hettige T, Cameron JD, Wong DTL. Association of epicardial adipose tissue and high‐risk plaque characteristics: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e006379 DOI: 10.1161/JAHA.117.006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. [DOI] [PubMed] [Google Scholar]
- 3. Ruberg FL, Chen Z, Hua N, Bigornia S, Guo Z, Hallock K, Jara H, LaValley M, Phinikaridou A, Qiao Y, Viereck J, Apovian CM, Hamilton JA. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring). 2010;18:1116–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nerlekar N, Baey YW, Brown AJ, Muthalaly RG, Dey D, Tamarappoo B, Cameron JD, Marwick TH, Wong DT. Poor correlation, reproducibility, and agreement between volumetric versus linear epicardial adipose tissue measurement: a 3D computed tomography versus 2D echocardiography comparison. JACC Cardiovasc Imaging. 2018;11:1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doesch C, Suselbeck T, Leweling H, Fluechter S, Haghi D, Schoenberg SO, Borggrefe M, Papavassiliu T. Bioimpedance analysis parameters and epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure. Obesity (Silver Spring). 2010;18:2326–2332. [DOI] [PubMed] [Google Scholar]
- 6. Harbord RM, Higgins JPT. Meta‐regression in Stata. Stata J. 2008;8:493–519. [Google Scholar]
- 7. Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol. 2010;56:784–788. [DOI] [PubMed] [Google Scholar]
- 8. Bakkum MJ, Danad I, Romijn MA, Stuijfzand WJ, Leonora RM, Tulevski II, Somsen GA, Lammertsma AA, van Kuijk C, van Rossum AC, Raijmakers PG, Knaapen P. The impact of obesity on the relationship between epicardial adipose tissue, left ventricular mass and coronary microvascular function. Eur J Nucl Med Mol Imaging. 2015;42:1562–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavalcante JL, Tamarappoo BK, Hachamovitch R, Kwon DH, Alraies MC, Halliburton S, Schoenhagen P, Dey D, Berman DS, Marwick TH. Association of epicardial fat, hypertension, subclinical coronary artery disease, and metabolic syndrome with left ventricular diastolic dysfunction. Am J Cardiol. 2012;110:1793–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doesch C, Haghi D, Fluchter S, Suselbeck T, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue in patients with heart failure. J Cardiovasc Magn Reson. 2010;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doesch C, Haghi D, Suselbeck T, Schoenberg SO, Borggrefe M, Papavassiliu T. Impact of functional, morphological and clinical parameters on epicardial adipose tissue in patients with coronary artery disease. Circ J. 2012;76:2426–2434. [DOI] [PubMed] [Google Scholar]
- 12. Doesch C, Streitner F, Bellm S, Suselbeck T, Haghi D, Heggemann F, Schoenberg SO, Michaely H, Borggrefe M, Papavassiliu T. Epicardial adipose tissue assessed by cardiac magnetic resonance imaging in patients with heart failure due to dilated cardiomyopathy. Obesity (Silver Spring). 2013;21:E253–E261. [DOI] [PubMed] [Google Scholar]
- 13. Ede H, Erkoc MF, Okur A, Erbay AR. Impaired aortic elasticity and diastolic functions are associated with findings of coronary computed tomographic angiography. Med Sci Monit. 2014;20:2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faustino AP, Paiva L, Mota P, Costa M, Leito‐Marques A. Pericardial fat, a new marker of impaired left ventricle diastolic dysfunction. Eur J Heart Fail Suppl. 2011;10:S248. [Google Scholar]
- 15. Fernando RS, Syed MA, Wilber D, Singh S, Teme T, Rabbat M. Epicardial adipose tissue volume by cardiac magnetic resonance imaging predicts abnormal myocardial relaxation in patients with atrial fibrillation. J Cardiovasc Magn Reson. 2015;17:P352. [Google Scholar]
- 16. Fontes‐Carvalho R, Fontes‐Oliveira M, Sampaio F, Mancio J, Bettencourt N, Teixeira M, Rocha Goncalves F, Gama V, Leite‐Moreira A. Influence of epicardial and visceral fat on left ventricular diastolic and systolic functions in patients after myocardial infarction. Am J Cardiol. 2014;114:1663–1669. [DOI] [PubMed] [Google Scholar]
- 17. Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, Levy D, Larson MG, D'Agostino RB Sr, O'Donnell CJ, Manning WJ. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hachiya K, Fukuta H, Wakami K, Goto T, Tani T, Ohte N. Relation of epicardial fat to central aortic pressure and left ventricular diastolic function in patients with known or suspected coronary artery disease. Int J Cardiovasc Imaging. 2014;30:1393–1398. [DOI] [PubMed] [Google Scholar]
- 19. Khawaja T, Greer C, Chokshi A, Chavarria N, Thadani S, Jones M, Schaefle K, Bhatia K, Collado JE, Shimbo D, Einstein AJ, Schulze PC. Epicardial fat volume in patients with left ventricular systolic dysfunction. Am J Cardiol. 2011;108:397–401. [DOI] [PubMed] [Google Scholar]
- 20. Konishi M, Sugiyama S, Sugamura K, Nozaki T, Matsubara J, Akiyama E, Utsunomiya D, Matsuzawa Y, Yamashita Y, Kimura K, Umemura S, Ogawa H. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol. 2012;59:344–351. [DOI] [PubMed] [Google Scholar]
- 21. Lai YH, Hou CJ, Yun CH, Sung KT, Su CH, Wu TH, Yang FS, Hung TC, Hung CL, Bezerra HG, Yeh HI. The association among MDCT‐derived three‐dimensional visceral adiposities on cardiac diastology and dyssynchrony in asymptomatic population. BMC Cardiovasc Disord. 2015;15:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu J, Fox CS, Hickson DA, May WL, Ding J, Carr JJ, Taylor HA. Pericardial fat and echocardiographic measures of cardiac abnormalities: the Jackson Heart Study. Diabetes Care. 2011;34:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Longenecker CAK, Serhal M, Kinley B, Labbato D, McComsey GA. Diastolic function correlates with pericardial fat [fat around the heart] and vascular remodeling in HIV. Conference on Retroviruses and Opportunistic Infections (CROI) February 22‐25 Boston, MA; 2016. [Google Scholar]
- 24. Ng AC, Goo SY, Roche N, van der Geest RJ, Wang WY. Epicardial adipose tissue volume and left ventricular myocardial function using 3‐dimensional speckle tracking echocardiography. Can J Cardiol. 2016;32:1485–1492. [DOI] [PubMed] [Google Scholar]
- 25. Vanni EM, Faletti R, Morello M, Mezzabotta L, Battisti G, Frea S, Cannillo M, Mosso E, Rosso C, Bergamasco L, Rizzetto M, Bugianesi E. Incerased epicardial fat and early signs of impaired diastolic and systolic left ventricular function in non‐diabetic, normotensive patients with nonalcoholic fatty liver disease. J Hepatol. 2015;62:S745. [Google Scholar]
- 26. Vural M, Talu A, Sahin D, Elalmis OU, Durmaz HA, Uyanik S, Dolek BA. Evaluation of the relationship between epicardial fat volume and left ventricular diastolic dysfunction. Jpn J Radiol. 2014;32:331–339. [DOI] [PubMed] [Google Scholar]
- 27. Wu CK, Tsai HY, Su MY, Wu YF, Hwang JJ, Tseng WY, Lin JL, Lin LY. Pericardial fat is associated with ventricular tachyarrhythmia and mortality in patients with systolic heart failure. Atherosclerosis. 2015;241:607–614. [DOI] [PubMed] [Google Scholar]
- 28. Yamashita KO, Ebara S, Yamamoto MH, Obara C. Increased epicardial adipose tissue are associated with left ventricular diastolic dysfunction. J Am Coll Cardiol. 2012;59:E1349. [Google Scholar]
- 29. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 30. Alnabhan N, Kerut EK, Geraci SA, McMullan MR, Fox E. An approach to analysis of left ventricular diastolic function and loading conditions in the echocardiography laboratory. Echocardiography. 2008;25:105–116. [DOI] [PubMed] [Google Scholar]
- 31. Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. [DOI] [PubMed] [Google Scholar]
- 32. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 33. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3:e000582 DOI: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vincent HK, Powers SK, Stewart DJ, Shanely RA, Demirel H, Naito H. Obesity is associated with increased myocardial oxidative stress. Int J Obes Relat Metab Disord. 1999;23:67–74. [DOI] [PubMed] [Google Scholar]
- 35. Falcao‐Pires I, Castro‐Chaves P, Miranda‐Silva D, Lourenco AP, Leite‐Moreira AF. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov Today. 2012;17:880–889. [DOI] [PubMed] [Google Scholar]
- 36. Canepa M, Strait JB, Milaneschi Y, AlGhatrif M, Ramachandran R, Makrogiannis S, Moni M, David M, Brunelli C, Lakatta EG, Ferrucci L. The relationship between visceral adiposity and left ventricular diastolic function: results from the Baltimore Longitudinal Study of Aging. Nutr Metab Cardiovasc Dis. 2013;23:1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, Patle AK, Baugh JA, McDonald KM. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–1682. [DOI] [PubMed] [Google Scholar]
- 38. Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC Jr. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kankaanpaa M, Lehto HR, Parkka JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab. 2006;91:4689–4695. [DOI] [PubMed] [Google Scholar]
- 40. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation. 2009;119:1661–1670. [DOI] [PubMed] [Google Scholar]
- 41. Buckberg GD, Fixler DE, Archie JP, Hoffman JI. Experimental subendocardial ischemia in dogs with normal coronary arteries. Circ Res. 1972;30:67–81. [DOI] [PubMed] [Google Scholar]
- 42. Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–443. [DOI] [PubMed] [Google Scholar]
- 43. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 44. Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol. 2004;13:313–316. [DOI] [PubMed] [Google Scholar]
- 45. Company JM, Booth FW, Laughlin MH, Arce‐Esquivel AA, Sacks HS, Bahouth SW, Fain JN. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J Appl Physiol (1985). 2010;109:1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iwayama T, Nitobe J, Watanabe T, Ishino M, Tamura H, Nishiyama S, Takahashi H, Arimoto T, Shishido T, Miyashita T, Miyamoto T, Toyama S, Sadahiro M, Kubota I. Role of epicardial adipose tissue in coronary artery disease in non‐obese patients. J Cardiol. 2014;63:344–349. [DOI] [PubMed] [Google Scholar]
- 47. Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and prevalence of cardiovascular diseases and prognosis—the obesity paradox updated. Prog Cardiovasc Dis. 2016;58:537–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Example MEDLINE Search Strategy
Table S2. Newcastle—Ottawa Scale for Assessment of Cross‐sectional Studies
Table S3. Newcastle—Ottawa Scale for Assessment of Case Control Studies


