Abstract
Background
More intense and longer‐lasting heat events are expected in the United States as a consequence of climate change. This study aimed to project the potential changes in maternal heat exposure during early pregnancy (3–8 weeks post conception) and the associated burden of congenital heart defects (CHDs) in the future.
Methods and Results
This study expanded on a prior nationwide case‐control study that evaluated the association between CHDs and maternal heat exposure during early pregnancy in summer and spring. We defined multiple indicators of heat exposure, and applied published odds ratios obtained for the matching season of the baseline (1995–2005) into the projection period (2025–2035) to estimate potential changes in CHD burden throughout the United States. Increases in maternal heat exposure were projected across the United States and to be larger in the summer. The Midwest will potentially have the highest increase in summer maternal exposure to excessively hot days (3.42; 95% CI, 2.99–3.88 per pregnancy), heat event frequency (0.52; 95% CI, 0.44–0.60) and heat event duration (1.73; 95% CI, 1.49–1.97). We also found large increases in specific CHD subtypes during spring, including a 34.0% (95% CI, 4.9%–70.8%) increase in conotruncal CHD in the South and a 38.6% (95% CI, 9.9%–75.1%) increase in atrial septal defect in the Northeast.
Conclusions
Projected increases in maternal heat exposure could result in an increased CHD burden in certain seasons and regions of the United States.
Keywords: climate change, congenital heart defects, maternal heat exposure, pregnancy, projection
Subject Categories: Epidemiology, Pregnancy, Pediatrics, Women
Clinical Perspective
What Is New?
High‐resolution climate projections suggest future increases in maternal heat exposure during early pregnancy across the United States.
The burden of congenital heart defects across the United States may increase as a result of climate change.
The estimated impact on the burden of congenital heart defects varies across regions and seasons, as well as under different definitions of heat exposure.
What Are the Clinical Implications?
Pregnant women should be cautious of extreme heat exposure, especially during early pregnancy.
Increases in the occurrences and severity of extreme heat events would increase the need for medical preparedness and care for congenital heart defects.
Introduction
For decades, pregnant women were overlooked as a potential vulnerable group during ambient heat events, despite evidence from animal research and population studies.1, 2, 3 Prior studies have reported a positive relationship between extreme heat exposure and adverse reproductive outcomes, including preterm birth and low birth weight.4, 5, 6 More importantly, 2 recent studies have reported an association between maternal heat exposure during early pregnancy and an increased odds of congenital heart defects (CHDs),1, 7 the most common birth defects grouping and a leading cause of infant morbidity and mortality in the United States.8, 9, 10
Maternal heat exposure during early pregnancy may directly cause fetal cell death or interfere with protein synthesis via heat‐shock proteins and induce severe fetal malformations as observed in animal studies.11 As global temperatures continue to rise, more intense, frequent, and longer‐lasting heat events are expected.12, 13, 14 Significant gaps remain in understanding the potential impact of climate change on maternal heat exposures and the associated CHD burden. Existing projection studies frequently focus on common diseases such as cardiovascular and respiratory diseases; however, potential effects of temperature on pregnancy outcomes were not typically examined.15, 16, 17, 18 Currently, there remains a lack of studies projecting changes in heat exposure during pregnancy or heat‐related CHD burden. Additionally, most prior heat‐health projection studies either have been limited to diseases other than reproductive outcomes and small geographic areas or have used weather projections from low‐resolution global climate models that result in elevated uncertainty and heat exposure misclassification. Moreover, most of these studies used average ambient temperature as a single heat definition.
Lin et al (2018)7 reported associations between CHDs and maternal heat exposure during the critical gestational period (3–8 weeks post conception) in multiple US regions during spring and summer. Their findings revealed an elevated risk based on CHD subtypes in spring and in regions with larger temperature variations, or lower average temperature. The present study was designed to expand on these findings by projecting the US nationwide changes in maternal heat exposure during early pregnancy and changes in CHD burden for the years 2025 to 2035. We utilized multiple heat exposure metrics that were based on spatially and temporally high‐resolution weather projections. Generally, all weather‐related diseases are preventable. Understanding maternal heat exposure and the associated CHD burden under future climate scenarios will assist in guiding public health practitioners to develop early warning and preparedness programs to modify behaviors with the aim of reducing CHD burden.
Methods
The health data used in this study are available from Lin et al (2018). The high‐resolution weather projection data are available from coauthors at the US Environmental Protection Agency, and the R Project for Statistical Computing code developed for the study is available from the corresponding author, upon request.
Study Design
This study was based on NBDPS (National Birth Defects Prevention Study), a multisite, large population‐based case‐control study in the United States that investigated risk factors for major structural birth defects.19, 20 The NBDPS spanned multiple regions including the South (Arkansas and Texas), West (California), Midwest (Iowa), Southeast (North Carolina and Georgia), Northeast (New York), and Southwest (Utah) regions, covering ≈482 000 births per year from 1997 through 2007. As previously published,7, 19, 20 each CHD case (fetus or liveborn) was identified from the state's birth defects surveillance system in each NBDPS site and reviewed by clinical geneticists adhering to specific criteria.21, 22 The controls consisted of nonmalformed live‐born infants, randomly selected from either birth certificates or hospitals in the same surveillance area as the cases.
The present study included (1) obtaining baseline (1995–2005) conditions, such as the odds ratio (OR) of CHDs and populations at risk; (2) simulating the potential changes in ambient temperature and subsequent maternal heat exposure in a future projection period (2025–2035); and (3) predicting changes in the associated CHD burden between 2 periods. This study was approved by the NBDPS Data Sharing Committee and Institutional Review Boards at each NBDPS site. No informed consent was required.
Baseline Conditions of the Population
Odds ratios
ORs associated with each unit increase in maternal heat exposure were obtained from our prior study.7 We examined major CHD subtypes, including conotruncal heart defects, left or right ventricular outflow tract obstruction, and septal heart defects (ventricular septal defect [VSD] and atrial septal defect [ASD]). Regions with significantly elevated ORs were examined to estimate the potential changes in CHD burden.
Number of CHDs and increase of live births
The number of CHDs at baseline, used as the base for projection, were estimated from annual reports published by the National Birth Defects Prevention Network (https://www.nbdpn.org/ar.php). The number of live births in the United States for the baseline and projection periods was obtained from the National Vital Statistics Reports (https://www.cdc.gov/nchs/nvss/births.htm), and the US Census Bureau,23 respectively. The ratio of the number of live births during the projection period to that at baseline (ratio ≈1.05) was used to adjust for increases in live births.
Temperature and Exposure Simulations
Dynamic downscaling
The daily maximum temperature data were dynamically downscaled (using a comprehensive physics‐based weather model rather than establishing statistical relationships) from the Coupled Model Inter‐comparison Project, Phase 5 of the Intergovernmental Panel on Climate Change.14 We used climate projections from the ModelE2‐R obtained from the National Aeronautics and Space Administration/Goddard Institute for Space Studies24 and dynamically downscaled to high‐resolutions using the Weather Research and Forecasting model25 following methods from our previously published work.26 By using dynamic downscaling, the temporal and spatial discretization were increased, allowing us to create hourly meteorologic fields over North America in 36×36‐km grid cells for the baseline (1995–2005) and the future period (2025–2035) that were each represented by 11 years of modeled data. Data for the baseline represent the climatic conditions that were typical during this period. The projection period followed Representative Concentration Pathway 6.0, which exhibits modest warming in 2030 compared with 2000.27 The data for the projection period were assumed to be equally plausible in any given year following the warming scenario. The dynamic downscaling produced hourly temperature estimates at each 36×36‐km Weather Research and Forecasting grid cell.
Exposure definition
We derived the daily maximum temperature (T max‐cell) in each 36×36‐km grid cell using the hourly temperature estimates within each grid cell. The maximum, median, and minimum of T max‐cell in each region were used to represent the regional daily maximum temperature (T max‐region). According to demographic statistics from the United Nations Statistics Division, there is only a slight seasonal variation in the number of live births in the United States28; therefore, we assumed the number of live births is uniformly distributed throughout the year. Accordingly, to evaluate the average heat exposure per pregnancy, we assumed 1 conception per day. To be consistent with our previous study,7 we focused on the average heat exposure during the critical gestational period (3–8 weeks after conception) for pregnant women who have at least 1 day of this critical period overlapping with spring or summer. For each pregnancy and region, we defined (1) the count of excessively hot days (EHD) as the number of days with T max‐region exceeding either the 90th (ie, EHD90) or 95th (ie, EHD95) percentile for the same season of the baseline period; (2) the frequency of extreme heat events (EHE) as the number of occurrences of at least 3 consecutive EHD90 days (ie EHE90) or 2 consecutive EHD95 days (ie EHE95); (3) the duration of EHE as the number of days for the longest EHE within the 42‐day period. We calculated these exposure metrics based on multiple definitions of regional daily maximum temperature and excessively hot days and averaged each metric for the season as described previously.29
Prediction of CHD Burden Changes
The change in heat exposure was calculated between the baseline and projection periods. For each region and season, instead of estimating a single average heat exposure metric for each period and a single indicator of change, we also calculated the 95%CIs following the bootstrap framework, resampling 1000 times from daily heat exposure metrics that were used to estimate the average exposure for the 2 periods. We paired these calculations with associated regional ORs and assessed the change in the number of CHDs as:
where Count0 represents the seasonal number of CHDs during the baseline period. We calculated the seasonal estimates of cases by multiplying the annual reported number by the proportion of days per season in a year. ΔExposure is the change in heat exposure computed from the bootstrap procedure. Ratio represents the relative change in live births over the projection period compared with the baseline.
Results
We projected increases in temperature in all regions across both seasons, regardless of the definition of regional daily maximum temperature (Figure 1). We projected greater temperature increases during the summer in the Midwest (Iowa), Southeast (North Carolina/Georgia), and Northeast (New York) compared with other regions. We also projected larger variations of temperature for the South (Arkansas/Texas) and West (California) during the summer and for the Midwest and Northeast in spring, compared with other regions. We further projected potential changes in maternal heat exposure over the projection period by season, region, and definition of extreme heat exposures, as presented in Tables 1 and 2 and Figure 2. We projected general increases in maternal heat exposure in most situations with larger increases in summer than spring, especially when using the EHE90 definition. In summer, the Midwest region was projected to have the highest increase in maternal exposure to excessively hot days, as well as in the frequency and duration of exposure to EHE90, followed by the Northeast and South regions. In spring, maternal heat exposure was projected to increase the most in the South and Southwest, followed by the Northeast and Midwest regions. In terms of heat exposure definitions, the regional maximum of the grid‐cell‐level daily maximum temperature may not reflect the potential severity of heat exposure increase. We found that heat exposures were projected to increase greatly when using regional median or minimum of grid‐cell‐level daily maximum temperature for all regions, except the Midwest and Southeast in summer, and for the South, West, and Southwest regions in spring.
Figure 1.
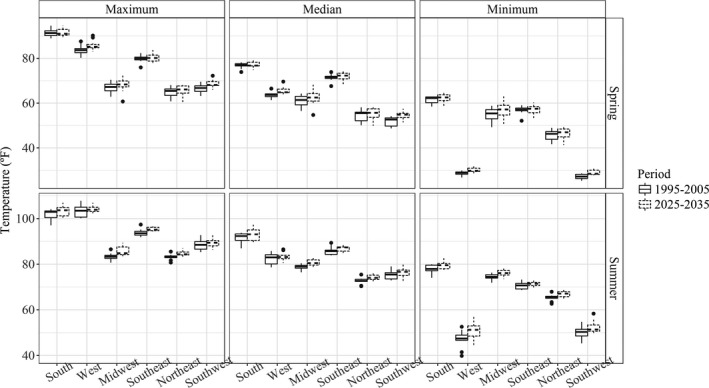
Comparing temperature range (°F) between the baseline (1995–2005) and the projection (2025–2035) periods by season and geographic region.
Table 1.
Projected Increase in Maternal Heat Exposure During Early Pregnancy by Different Metrics and Region (2025–2035 Versus 1995–2005) in the United States in Summer (per Pregnancy)
| Regions | Maximum Temperature Criteriona | EHE 90 | EHE 95 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHD Counts | EHE Frequency | EHE Duration | EHD Counts | EHE Frequency | EHE Duration | ||||||||
| Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | ||
| South (AR/TX) | Maximum | 1.08 | 0.57–1.58 | 0.19 | 0.11–0.26 | 1.05 | 0.72–1.37 | 0.71 | 0.36–1.06 | 0.06 | −0.02–0.14 | 0.34 | 0.12–0.57 |
| Median | 2.15 | 1.57–2.74 | 0.26 | 0.17–0.35 | 1.29 | 0.88–1.67 | 1.58 | 1.19–1.99 | 0.23 | 0.15–0.31 | 1.17 | 0.88–1.44 | |
| Minimum | 2.90 | 2.38–3.41 | 0.35 | 0.26–0.44 | 1.19 | 0.94–1.47 | 1.61 | 1.28–1.94 | 0.33 | 0.25–0.41 | 0.63 | 0.44–0.83 | |
| West (CA) | Maximum | −0.14 | −0.58–0.29 | −0.04 | −0.12–0.04 | 0.08 | −0.21–0.35 | −0.29 | −0.62–0.02 | −0.16 | −0.24–−0.08 | −0.17 | −0.39–0.02 |
| Median | −0.10 | −0.58–0.32 | −0.16 | −0.24–−0.08 | 0.31 | 0.02–0.58 | 0.01 | −0.29–0.28 | −0.17 | −0.25–−0.10 | 0.23 | 0.01–0.42 | |
| Minimum | 1.43 | 0.94–1.91 | 0.21 | 0.13–0.28 | 1.00 | 0.67–1.31 | 1.13 | 0.77–1.47 | 0.16 | 0.08–0.23 | 0.83 | 0.58–1.07 | |
| Midwest (IA) | Maximum | 4.27 | 3.75–4.80 | 0.74 | 0.65–0.83 | 2.66 | 2.43–2.93 | 3.50 | 3.11–3.93 | 0.85 | 0.74–0.96 | 1.86 | 1.68–2.05 |
| Median | 3.42 | 2.99–3.88 | 0.52 | 0.44–0.60 | 1.73 | 1.49–1.97 | 2.95 | 2.59–3.34 | 0.66 | 0.56–0.76 | 1.80 | 1.63–2.00 | |
| Minimum | 2.09 | 1.68–2.50 | 0.20 | 0.12–0.28 | 0.97 | 0.74–1.19 | 1.96 | 1.64–2.25 | 0.49 | 0.40–0.57 | 1.05 | 0.91–1.20 | |
| Southeast (NC/GA) | Maximum | 2.45 | 1.96–2.95 | 0.17 | 0.09–0.24 | 1.19 | 0.91–1.50 | 0.67 | 0.36–1.00 | 0.19 | 0.12–0.26 | 0.49 | 0.30–0.68 |
| Median | 1.20 | 0.73–1.69 | 0.20 | 0.12–0.29 | 0.05 | −0.22–0.35 | 0.46 | 0.20–0.75 | 0.22 | 0.14–0.31 | 0.13 | −0.01–0.28 | |
| Minimum | 0.84 | 0.44–1.25 | 0.05 | −0.02–0.11 | −0.56 | −0.79–−0.34 | −0.16 | −0.42–0.12 | 0.10 | 0.03–0.17 | −0.27 | −0.45–−0.10 | |
| Northeast (NY) | Maximum | 2.01 | 1.62–2.41 | 0.18 | 0.10–0.26 | 0.85 | 0.66–1.04 | 1.28 | 1.00–1.57 | 0.51 | 0.43–0.59 | 0.82 | 0.69–0.96 |
| Median | 2.66 | 2.27–3.07 | 0.43 | 0.34–0.51 | 1.21 | 1.01–1.41 | 1.76 | 1.42–2.06 | 0.56 | 0.47–0.65 | 0.80 | 0.65–0.96 | |
| Minimum | 2.29 | 1.93–2.69 | 0.67 | 0.58–0.75 | 1.32 | 1.17–1.50 | 0.86 | 0.62–1.11 | 0.06 | −0.02–0.15 | 0.24 | 0.11–0.36 | |
| Southwest (UT) | Maximum | 0.86 | 0.35–1.36 | 0.21 | 0.12–0.30 | 0.30 | −0.02–0.59 | 0.19 | −0.16–0.52 | 0.03 | −0.06–0.11 | −0.03 | −0.28–0.19 |
| Median | 2.06 | 1.57–2.55 | 0.18 | 0.10–0.25 | 0.97 | 0.64–1.30 | 1.29 | 0.92–1.63 | 0.36 | 0.27–0.45 | 0.29 | 0.06–0.49 | |
| Minimum | 1.76 | 1.26–2.24 | 0.20 | 0.12–0.28 | 0.62 | 0.27–0.96 | 1.44 | 1.08–1.77 | 0.31 | 0.23–0.40 | 0.77 | 0.54–0.99 | |
EHD indicates excessively hot day; EHE, extreme heat event.
Maximum, median, or minimum grid‐cell daily maximum temperature T max‐cell was used to represent the regional daily maximum temperature, T max‐region.
Table 2.
Projected Increase in Maternal Heat Exposure During Early Pregnancy by Different Metrics and Region (2025–2035 Versus 1995–2005) in the United States in Spring (per Pregnancy)
| Regions | Maximum Temperature Criteriona | EHE 90 | EHE 95 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHD Counts | EHE Frequency | EHE Duration | EHD Counts | EHE Frequency | EHE Duration | ||||||||
| Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | Increase | 95% CI | ||
| South (AR/TX) | Maximum | 1.35 | 0.85–1.85 | 0.14 | 0.06–0.21 | 1.37 | 1.08–1.66 | 1.12 | 0.79–1.48 | 0.21 | 0.12–0.30 | 0.60 | 0.41–0.78 |
| Median | 1.96 | 1.48–2.45 | 0.25 | 0.18–0.33 | 1.26 | 0.95–1.59 | 1.90 | 1.56–2.27 | 0.24 | 0.18–0.32 | 1.18 | 0.94–1.43 | |
| Minimum | 1.21 | 0.79–1.61 | 0.07 | 0.00–0.14 | 0.62 | 0.41–0.84 | 0.80 | 0.55–1.07 | 0.11 | 0.05–0.17 | 0.37 | 0.20–0.55 | |
| West (CA) | Maximum | 0.62 | 0.19–1.05 | −0.03 | −0.11–0.04 | 0.46 | 0.17–0.73 | 0.47 | 0.14–0.80 | 0.10 | 0.03–0.17 | 0.27 | 0.05–0.49 |
| Median | 0.63 | 0.18–1.06 | 0.05 | −0.04–0.13 | 0.27 | 0.01–0.51 | 0.15 | −0.11–0.42 | −0.01 | −0.07–0.06 | 0.25 | 0.05–0.44 | |
| Minimum | 1.82 | 1.38–2.24 | 0.30 | 0.23–0.37 | 0.96 | 0.68–1.24 | 0.54 | 0.25–0.82 | 0.03 | −0.03–0.09 | 0.28 | 0.08–0.48 | |
| Midwest (IA) | Maximum | 1.53 | 1.06–1.98 | 0.28 | 0.19–0.37 | 1.14 | 0.92–1.34 | 1.41 | 1.10–1.72 | 0.38 | 0.28–0.47 | 0.83 | 0.65–0.98 |
| Median | 0.95 | 0.48–1.36 | 0.26 | 0.18–0.33 | 0.49 | 0.25–0.71 | 1.30 | 0.95–1.61 | 0.24 | 0.15–0.33 | 0.79 | 0.61–0.95 | |
| Minimum | 1.27 | 0.78–1.68 | 0.17 | 0.09–0.24 | 0.34 | 0.09–0.57 | 1.09 | 0.78–1.37 | 0.10 | 0.01–0.18 | 0.68 | 0.51–0.85 | |
| Southeast (NC/GA) | Maximum | 1.18 | 0.69–1.66 | 0.15 | 0.08–0.23 | 0.51 | 0.21–0.80 | 0.60 | 0.31–0.88 | 0.17 | 0.11–0.24 | 0.30 | 0.12–0.48 |
| Median | 0.84 | 0.41–1.27 | 0.12 | 0.05–0.18 | 0.57 | 0.32–0.84 | 0.32 | 0.08–0.56 | 0.19 | 0.12–0.26 | 0.09 | −0.04–0.22 | |
| Minimum | 0.57 | 0.23–0.93 | 0.08 | 0.03–0.13 | 0.13 | −0.07–0.32 | 0.24 | 0.02–0.45 | 0.24 | 0.18–0.31 | 0.21 | 0.07–0.34 | |
| Northeast (NY) | Maximum | 1.41 | 0.99–1.80 | 0.14 | 0.06–0.20 | 0.91 | 0.71–1.11 | 0.82 | 0.56–1.07 | 0.24 | 0.16–0.32 | 0.49 | 0.34–0.62 |
| Median | 1.29 | 0.87–1.67 | 0.10 | 0.02–0.18 | 0.43 | 0.23–0.62 | 0.74 | 0.48–0.99 | 0.18 | 0.09–0.25 | 0.44 | 0.30–0.58 | |
| Minimum | 0.51 | 0.12–0.88 | 0.06 | −0.01–0.12 | 0.28 | 0.09–0.46 | 0.01 | −0.20–0.23 | −0.12 | −0.19–−0.05 | −0.05 | −0.18–0.07 | |
| Southwest (UT) | Maximum | 1.37 | 0.88–1.83 | 0.33 | 0.25–0.42 | 0.46 | 0.17–0.75 | 0.41 | 0.09–0.71 | 0.10 | 0.02–0.17 | −0.06 | −0.28–0.15 |
| Median | 2.18 | 1.68–2.67 | 0.27 | 0.20–0.34 | 1.07 | 0.75–1.37 | 1.16 | 0.82–1.46 | 0.40 | 0.32–0.48 | 0.24 | 0.02–0.44 | |
| Minimum | 2.46 | 1.92–2.93 | 0.35 | 0.27–0.42 | 1.07 | 0.73–1.39 | 1.53 | 1.20–1.84 | 0.42 | 0.34–0.49 | 0.76 | 0.53–0.97 | |
EHD indicates excessively hot day; EHE, extreme heat event.
Maximum, median, or minimum grid‐cell daily maximum temperature T max‐cell was used to represent the regional daily maximum temperature, T max‐region.
Figure 2.
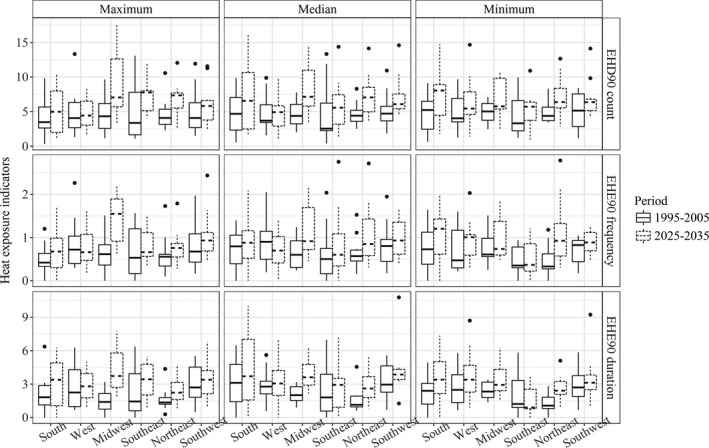
Comparing maternal heat exposure by different heat indicators and areas in summer between baseline (1995–2005) and projection (2025–2035) periods. EHD indicates excessively hot day; EHE, extreme heat event.
Because we previously observed positive associations between maternal heat exposure and CHD, we further estimated the potential associated changes in CHD burden in this study. The estimated CHD burden may not change substantially between 2 periods in the West, with fewer than 20 additional cases projected in most scenarios. However, across other regions, hundreds to thousands of additional CHD cases (increases of up to 62%; Figure 3) over the projection period were estimated.
Figure 3.
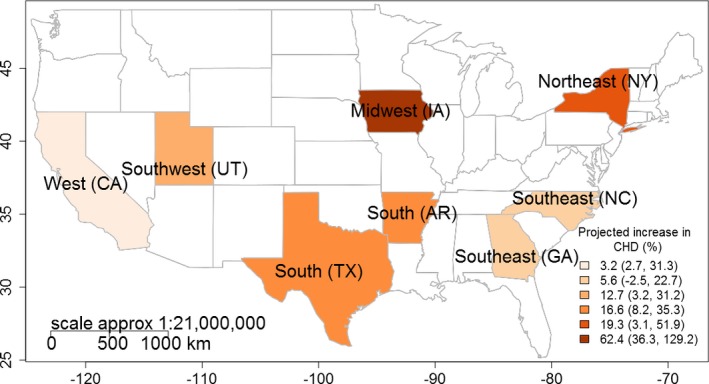
Projected increase in congenital heart defect cases (%) over the projection (2025–2035) period.
We projected larger changes in the CHD burden with EHE95 as shown in Table 3. Although the Midwest was projected to have the greatest relative increase in the burden of certain CHD subtypes (Figure 3), the South and Northeast regions have the largest number of projected CHD increases. In the South, potential increases in the frequency of exposure to spring EHE95 may be related to a 12.3% (95% CI, 5.9%–18.9%) increase in the number of total CHDs, a 19.7% (95% CI, 7.4%–33.5%) increase in conotruncal heart defects, and an 18.9% (95% CI, 6.7%–32.6%) increase in VSD. As a result, there may be ≈2607 additional total CHDs, 301 additional conotruncal heart defects, and 1573 additional VSD cases estimated over the projection period. Moreover, projected increases in the duration of exposure to spring EHE95 may be associated with a 34.0% (95% CI, 4.9%–70.8%) increase in conotruncal heart defects, and a 35.3% (95% CI, 6.2%–72.1%) increase in VSDs. The duration of similar exposures in the Northeast (Figure 3) may result in a 38.6% (95% CI, 9.9%–75.1%) increase in ASDs, a 17.9% (95% CI, 7.2%–30.1%) increase in septal heart defects, and a 23.4% (95% CI, 9.9%–39.0%) increase in VSDs. We also projected that septal defects may increase by 33.9% (95% CI, 14.9%–56.8%) because of maternal exposure to an increased number of excessively hot days, resulting in 2555 additional cases over the projection period. Similar changes were projected with EHE90.
Table 3.
Projected Increase in Congenital Heart Defect Burden in United States by Region, Season, and Heat Definition Based on the Previous Positive Findings (2025–2035 Versus 1995–2005)a
| Region | Exposure | Criteria | CHD: OR (95% CI) | Projected Increase in Cases (%) | Baseline Cases for the Seasonb | Projected Increase in Cases (Total N)c | |
|---|---|---|---|---|---|---|---|
| Increase | 95% CI | ||||||
| South (AR/TX) | Spring EHE95 frequency | Maximum | Total: 1.32 (95% CI, 1.04–1.67) | 11.1 | 5.8–16.6 | 21 263 | 2363 (23 626) |
| Median | 12.3 | 5.9–18.9 | 2607 (23 870) | ||||
| Minimum | 8.2 | 5.4–11.0 | 1739 (23 002) | ||||
| Maximum | Conotruncal: 1.72 (1.10–2.69) | 17.4 | 7.0–28.7 | 1525 | 265 (1790) | ||
| Median | 19.7 | 7.4–33.5 | 301 (1826) | ||||
| Minimum | 11.4 | 6.0–17.0 | 174 (1699) | ||||
| Maximum | VSD: 1.67 (1.07–2.62) | 16.6 | 6.4–28.0 | 8334 | 1387 (9721) | ||
| Median | 18.9 | 6.7–32.6 | 1573 (9907) | ||||
| Minimum | 11.0 | 5.7–16.7 | 918 (9252) | ||||
| Spring EHE95 duration | Maximum | Conotruncal: 1.23 (1.00–1.51) | 18.7 | 4.9–34.1 | 1525 | 285 (1810) | |
| Median | 34.0 | 4.9–70.8 | 519 (2044) | ||||
| Minimum | 13.2 | 4.9–22.1 | 202 (1727) | ||||
| Maximum | VSD: 1.24 (1.01–1.52) | 19.3 | 5.5–34.6 | 8334 | 1605 (9939) | ||
| Median | 35.3 | 6.2–72.1 | 2942 (11 276) | ||||
| Minimum | 13.6 | 5.3–22.4 | 1130 (9464) | ||||
| West (CA) | Summer EHD90 counts | Maximum | RVOTO: 1.17 (1.00–1.37) | 2.7 | 0.5–4.9 | 95 | 3 (98) |
| Median | 3.2 | 1.5–4.9 | 3 (98) | ||||
| Minimum | 31.3 | 4.9–64.4 | 30 (125) | ||||
| Midwest (IA) | Summer EHD95 counts | Maximum | Septal: 1.25 (1.04–1.51) | 129.2 | 20.4–344.2 | 1194 | 1543 (2737) |
| Median | 102.7 | 17.8–254.1 | 1227 (2421) | ||||
| Minimum | 62.4 | 13.3–135.0 | 745 (1939) | ||||
| Summer EHE95 frequency | Maximum | Septal: 1.71 (1.09–2.69) | 65.2 | 12.9–142.4 | 1194 | 779 (1973) | |
| Median | 49.4 | 11.1–101.4 | 590 (1784) | ||||
| Minimum | 36.3 | 9.4–70.0 | 433 (1627) | ||||
| Southeast (NC/GA) | Summer EHE90 duration | Maximum | VSD: 1.14 (1.01–1.29) | 22.7 | 6.2–42.1 | 3071 | 696 (3767) |
| Median | 5.6 | 5.0–6.3 | 173 (3244) | ||||
| Minimum | −2.5 | −9.0–4.3 | −77 (2994) | ||||
| Northeast (NY) | Spring EHD90 counts | Maximum | Septal: 1.18 (1.05–1.34) | 32.5 | 12.4–58.5 | 7532 | 2447 (9979) |
| Median | 29.8 | 11.7–52.9 | 2245 (9777) | ||||
| Minimum | 14.2 | 7.6–22.0 | 1073 (8605) | ||||
| Spring EHE90 duration | Maximum | ASD: 1.50 (1.07–2.11) | 51.9 | 11.6–107.4 | 3801 | 1973 (5774) | |
| Median | 24.9 | 8.0–44.7 | 948 (4749) | ||||
| Minimum | 17.5 | 6.9–29.2 | 663 (4464) | ||||
| Maximum | Septal: 1.20 (1.03–1.39) | 23.9 | 7.8–41.7 | 7532 | 1802 (9334) | ||
| Median | 13.5 | 6.3–20.9 | 1016 (8548) | ||||
| Minimum | 10.4 | 5.8–15.0 | 782 (8314) | ||||
| Maximum | VSD: 1.27 (1.06–1.52) | 30.5 | 10.7–53.8 | 3732 | 1138 (4870) | ||
| Median | 16.3 | 7.6–25.7 | 608 (4340) | ||||
| Minimum | 12.1 | 6.6–17.9 | 453 (4185) | ||||
| Spring EHD95 counts | Maximum | Septal: 1.39 (1.13–1.72) | 37.3 | 16.0–63.5 | 7532 | 2812 (10 344) | |
| Median | 33.9 | 14.9–56.8 | 2555 (10 087) | ||||
| Minimum | 5.4 | 5.1–5.7 | 405 (7937) | ||||
| Spring EHE95 duration | Maximum | ASD: 1.87 (1.11–3.16) | 42.5 | 10.4–84.1 | 3801 | 1614 (5415) | |
| Median | 38.6 | 9.9–75.1 | 1468 (5269) | ||||
| Minimum | 1.8 | −0.7–4.4 | 70 (3871) | ||||
| Maximum | Septal: 1.30 (1.05–1.62) | 19.3 | 7.5–32.8 | 7532 | 1452 (8984) | ||
| Median | 17.9 | 7.2–30.1 | 1350 (8882) | ||||
| Minimum | 3.6 | 4.7–2.5 | 272 (7804) | ||||
| Maximum | VSD: 1.44 (1.11–1.88) | 25.4 | 10.4–42.8 | 3732 | 948 (4680) | ||
| Median | 23.4 | 9.9–39.0 | 874 (4606) | ||||
| Minimum | 3.1 | 1.8–4.4 | 116 (3848) | ||||
| Southwest (UT) | Spring EHE95 duration | Maximum | Conotruncal: 1.34 (1.00–1.81) | 3.2 | 1.4–4.9 | 180 | 6 (186) |
| Median | 12.7 | 4.9–21.3 | 23 (203) | ||||
| Minimum | 31.2 | 4.9–65.1 | 56 (236) | ||||
| Summer EHE95 frequency | Maximum | LVOTO: 1.53 (1–2.35) | 6.2 | 4.9–7.5 | 293 | 18 (311) | |
| Median | 22.4 | 4.9–42.9 | 66 (359) | ||||
| Minimum | 19.9 | 4.9–37.3 | 58 (351) | ||||
ASD indicates atrial septal defect; CHD, congenital heart defect; EHD, excessively hot day; EHE, extreme heat event; LVOTO, left ventricular outflow tract obstruction; OR, odds ratio; RVOTO, right ventricular outflow tract obstruction; VSD, ventricular septal defect.
Projections were made where significant OR was observed at baseline.
Average annual number of cases for this season over 1995–2005.
Projected increase of cases for this season over 2025–2035, accounting for 4.9% birth increase.
As demonstrated in Tables 1, 2 through 3, although we projected larger increases in maternal heat exposure in the summer, we found more projected CHD increases in spring, especially for the South and Northeast regions. In addition to seasonal variation, we also observed differences between CHD burden projections using different definitions of heat exposure. For the Midwest, Southeast, and Northeast regions, larger increases in CHD burden were projected when regional daily maximum temperature was represented by the maximum of grid‐cell‐level daily maximum temperature, while for other regions, CHD increases were projected to be larger with the median or minimum temperature.
Discussion
Projected Increase in Maternal Heat Exposure
Our estimates suggest nationwide increases in maternal heat exposure during early pregnancy across the United States by 2030. We observed that regardless of the definition (EHE 90 or EHE 95; maximum, median, or minimum) used for heat exposure, increases were projected for pregnant women in all regions and in both spring and summer, as well as for all exposure metrics (number of excessively hot days, frequency, and duration of EHE). These increases were projected to be stronger in the Midwest and Northeast regions in summer. We also found that populations in the South and Southwest regions may experience similar increases in heat exposure in spring. Although few studies have assessed the impact of climate change on maternal heat exposure, our estimates are consistent with prior findings of the association between increased ambient heat events and climate change. The global coupled climate model ensembles suggest that several regions in the Northern Hemisphere, including the United States, may experience increased extreme heat severity in the 21st century.30 Specific atmospheric circulation patterns in the United States that are intensified by ongoing increases in greenhouse gases are expected to produce more future extreme heat events.30
In addition, the US Census Bureau has projected a continuous increase in births across the nation through 2030. It is estimated that the number of pregnant women may increase nationwide by 5.0%, meaning 4.2 million would be affected annually.23 The potential increases in both the number of pregnant women and maternal heat exposure suggest an alarming effect that climate change may have on reproductive health.
Projected Increase in CHD Burden
Nationwide increases in CHD burden were also projected. We found substantial variability in projected changes in regional CHD burden potentially attributable to maternal heat exposure. We projected that climate change could impose a greater impact on pregnant women in the South, Northeast, and Midwest regions compared with other regions, with greater increases in CHD burden projected for these 3 regions. Large spatial differences in ambient temperature and temperature ranges may be the most plausible explanation, as suggested by Lin et al.7 In this study, we projected a larger variation for daily maximum temperature in these regions. Large temperature variation was previously suggested to be associated with a higher risk of multiple health outcomes, such as respiratory diseases, CHD, and mortality.31, 32 Additionally, differences in heat acclimatization among pregnant women from different regions of the United States may be another reason for the spatial variation of CHD increase. Loughnan et al33 and Lin et al7 suggested an increased heat susceptibility for pregnant women in the Northeast and colder areas in general due to insufficient physical or behavioral adaptation. Moreover, it is possible that spatial differences in other factors, such as land coverage, human activity patterns, residential proximity to heavy traffic or industrial emissions, and farming density among the different regions may also contribute to spatial variations in CHDs.34, 35 The 2009 National Climate Assessment suggested that potential increases in summer heat events in the midwestern United States along with local socioeconomic compositions could significantly increase the risk of morbidity.36, 37
We projected higher increases in CHD burden for spring and for certain CHD subtypes (conotruncal and septal defects) compared with summer and other CHD subtypes, aligning with our prior findings at baseline.7 Although no previous study has clarified the reasoning behind the seasonal difference in the CHD increase, some have suggested higher health hazards of heat exposure in transitional seasons, such as in spring.33, 38, 39 A plausible explanation is the poor acclimation (ie, pregnant women do not quickly adapt biologically and behaviorally to warming weather).33, 40 The underlying reasoning for differences by subtype remains unknown. A potential possibility is that these subtypes have larger sample sizes compared with others, increasing the statistical power to detect an effect. Prior studies also found that these defects are more susceptible to environmental triggers.1, 41, 42
In terms of variation in CHD burden projections, our study also suggested a potential influence of the definition of heat exposure. As there is no gold standard to define heat exposure indicators, we used multiple definitions, EHE90 or EHE95, maximum, median, or minimum, in which 3 further EHE definitions were used, including the number of excessively hot days and frequency and duration of pregnancy exposure to EHE. Among these indicators, we projected larger increases in heat exposure with EHE90, but found more and larger increases in CHD burden with EHE95. In addition, we also projected larger increases in CHD burden for the Midwest, Southeast, and Northeast regions with “maximum” compared with “median” and “minimum” criteria. Therefore, EHE95 and “maximum” might be sensitive indicators to predict CHD burden related to global warming. Although no previous studies compared these definitions, our study provides insight for choosing optimal heat exposure indicators that can better capture the potential impact of climate change on human reproductive health.
Strengths and Uncertainties
To our knowledge, this is the first study to estimate future changes in maternal heat exposure and its associated burden on CHDs. In contrast to numerous studies that project heat‐related disease burden across small areas or based on low‐resolution global climate data,15, 16, 17 we dynamically downscaled the original weather projections to a local and hourly scale to increase both the temporal and spatial resolutions in this nationwide study. Additionally, our projections used different heat indicators to capture a more comprehensive picture of CHD burden increase associated with climate change and to confirm the robustness of our projections.
However, our findings need to be interpreted cautiously due to uncertainties inherent in our methods. First, our projections assumed that previously identified associations between maternal heat exposure and CHD were true. We were not able to exclude the possibility of Type I error attributable to residual confounding in our previous study. However, we attempted to minimize the confounding bias through the following approaches:
To the best of our knowledge, our previous study has the most comprehensive list of potential confounders among the existing heat‐CHD studies. For example, we screened and examined multiple major confounders including maternal race/ethnicity, education level, body mass index, infant sex, family history of CHD, and maternal lifestyle in our analysis.
We also conducted multiple sensitivity analyses by comparing the results after excluding potential confounders, such as pregestational diabetes mellitus, multiple birth, and preterm birth to the original results, and found no significant changes.
We rechecked all results using a Bayesian analysis approach to address positive findings potentially due to chance.
Our previous findings regarding specific CHD types associated with maternal heat exposure were consistent with most prior studies.1, 42
Second, we assumed the heat‐CHD association would remain constant over time, while gradual population heat adaptation was not considered. There is no approach available to estimate the heat adaptation rate for future pregnancies. However, a study from New York City suggested a 4.6% decrease in the risk of heat‐related mortality per decade over the 20th century.43 When the same rate was applied to the current study, CHD burden projections would be reduced by 1‐(1‐0.046)3 (≈13%). However, the total increasing trend in the burden of heat‐related CHD was still not significantly altered. Additionally, we projected only for situations in which associated elevated ORs were observed in the baseline period. Since the heat exposures were projected to increase in most regions, it is possible that areas with statistically insignificant findings may evolve to be significant over time. For example, although few associations were observed with EHE95 in the Midwest, 18 of 30 (60%) OR estimates were >1.0.7 The percentage of insignificant increases of CHD (OR >1) related to maternal heat exposure was 53% for the South, 47% for the Southeast, 73% for the Northeast, 53% for the Southwest, and 67% for the West, suggesting a possibility of much larger numbers of CHD increases in the future than we expected. Therefore, CHD increases attributable to increased pregnancy heat exposure might be a major concern in the United States.
It must be noted that our results are based on climate projections downscaled from a single climate scenario. Although our baseline and future projections each spanned 11 years, this may not account for the full range of internal variability in the Earth's climate system. Additionally, there is significant uncertainty in human emissions of carbon dioxide and other greenhouse gases, as well as the extent to which society will act to mitigate climate change. Other climate scenarios and models may differ in the spatial distribution and magnitude of warming and the resultant increases in projected cases of CHDs.
Although we adjusted for the potential increase in live births, we assumed that maternal demographic composition such as race and ethnicity would remain constant with the baseline. However, the US Census Bureau suggested approximately a 10% increase in the Hispanic population between the projection and baseline periods.23 We reran the analysis based on this estimate and found relative to the “white non‐Hispanic” group, the OR of total CHD for “black non‐Hispanic,” “Hispanic,” and “Other races” was 1.08 (95% CI, 0.96–1.21), 1.07 (95% CI, 0.98–1.17) and 1.13 (95% CI, 0.97–1.31), respectively. This suggests that changes in maternal demographic characteristics might not substantially modify CHD burden projections in the current study.
Our findings indicate a potential adverse impact, that is, increased burden of CHDs, of global warming. Our results provide useful metrics and maps for policy makers involved in preparedness and resource allocation for climate change adaptation. Even with significant actions taken to mitigate climate change, temperatures are likely to continue to increase later in the 21st century.44 Thus, our estimations reflect an outcome that should be relevant (regardless of the climate scenario and model) and a trend that will continue toward the end of the 21st century.
In conclusion, our findings reveal a potential nationwide increase in future maternal heat exposure in the United States. Exposure increases may be larger in summer, especially for the Midwest, followed by the Northeast and South regions. However, increases in the CHD burden can occur more in spring, especially in the South and Northeast regions for septal and conotruncal CHDs. We also found that EHE95 and maximum temperature might be sensitive indicators to predict the CHD burden associated with increasing occurrences and severity of extreme heat events. Our findings and geographic vulnerability maps provide useful information for policy makers in preparing and allocating resources for adapting to climate change.
Sources of Funding
This study was supported by ES021359 from the National Institutes of Health (NIH) and U38EH000184‐05 from the Centers for Disease Control and Prevention of the United States. The first author was also supported by the National Natural Science Foundation of China (#81473064 and 81773543).
Disclosures
None.
Acknowledgments
The authors thank the support from the NBDPS and all participants. We thank Peter Egeghy, Megan Mallard, and Lindsay Stanek from the US Environmental Protection Agency and referees from NBDPS for their comments and suggestions in internal reviews. We thank the California Department of Public Health, Maternal Child and Adolescent Health Division, for providing data from California. We thank Dr Wayne R. Laurence and Dr Emily K. D'Angelo for their help in language edits. The views expressed in this paper are those of the authors and do not necessarily represent the views or policies of the US Environmental Protection Agency, the Centers for Disease Control and Prevention, or the California Department of Public Health.
Investigators and Staff of the National Birth Defects Prevention Study (as shown on the NBDPS website on December 3, 2018): Charlotte Hobbs (Arkansas), Gary Shaw, Suzan Carmichael (California), Jennita Reefhuis, Sarah Tinker (Georgia), Paul Romitti (Iowa), Marlene Anderka (Massachusetts), Charlotte Druschel, Erin Bell, Marilyn Browne (New York), Andy Olshan, Robert Meyer (North Carolina), Mark Canfield, Peter Langlois (Texas), Marcia Feldkamp, Lorenzo Botto, Spotlight‐Researchers (Utah).
(J Am Heart Assoc. 2019;8:e010995 DOI: 10.1161/JAHA.118.010995)
This article was handled independently by Mark Russell, MD as a guest editor. The editors had no role in the evaluation of the manuscript or in the decision about its acceptance.
Contributor Information
Yuantao Hao, Email: haoyt@mail.sysu.edu.cn.
Shao Lin, Email: slin@albany.edu.
the National Birth Defects Prevention Study:
Charlotte Hobbs, Suzan Carmichael, Jennita Reefhuis, Sarah Tinker, Marlene Anderka, Charlotte Druschel, Erin Bell, Andy Olshan, Robert Meyer, Mark Canfield, Peter Langlois, and Lorenzo Botto
References
- 1. Auger N, Fraser WD, Sauve R, Bilodeau‐Bertrand M, Kosatsky T. Risk of congenital heart defects after ambient heat exposure early in pregnancy. Environ Health Perspect. 2017;125:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Weather Service . Heat safety tips and resources. Available at: https://www.weather.gov/safety/heat. Accessed November 1, 2018.
- 3. WHO, World Meteorological Organization . Heatwaves and health: guidance on warning‐system development. 2015.
- 4. He J‐R, Liu Y, Xia X‐Y, Ma W‐J, Lin H‐L, Kan H‐D, Lu J‐H, Feng Q, Mo W‐J, Wang P, Xia H‐M, Qiu X, Muglia LJ. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001–2011). Environ Health Perspect. 2016;124:1100–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strand LB, Barnett AG, Tong S. Maternal exposure to ambient temperature and the risks of preterm birth and stillbirth in Brisbane, Australia. Am J Epidemiol. 2011;175:99–107. [DOI] [PubMed] [Google Scholar]
- 6. Lawlor DA, Leon DA, Smith GD. The association of ambient outdoor temperature throughout pregnancy and offspring birthweight: findings from the Aberdeen children of the 1950s cohort. BJOG. 2005;112:647–657. [DOI] [PubMed] [Google Scholar]
- 7. Lin S, Lin Z, Ou Y, Soim A, Shrestha S, Lu Y, Sheridan S, Luben TJ, Fitzgerald E, Bell E, Shaw GM, Reefhuis J, Langlois PH, Romitti P, Feldkamp ML, Malik S, Pantea C, Na S; the National Birth Defects Prevention Study . Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects—a large US population‐based, case‐control study. Environ Int. 2018;118:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 9. Reller MD, Strickland MJ, Riehle‐Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989–2002. Birth Defects Res A Clin Mol Teratol. 2006;76:706–713. [DOI] [PubMed] [Google Scholar]
- 11. Bennett GD. Hyperthermia: malformations to chaperones. Birth Defects Res Part B Dev Reprod Toxicol. 2010;89:279–288. [DOI] [PubMed] [Google Scholar]
- 12. Institute of Medicine . Global Climate Change and Extreme Weather Events: Understanding the Contributions to Infectious Disease Emergence: Workshop Summary. Washington, DC: The National Academies Press; 2008. DOI: 10.17226/12435. [DOI] [PubMed] [Google Scholar]
- 13. Lau N‐C, Nath MJ. A model study of heat waves over North America: meteorological aspects and projections for the twenty‐first century. J Clim. 2012;25:4761–4784. [Google Scholar]
- 14. IPCC . Climate Change 2014: Mitigation of Climate Change. Cambridge, England: Cambridge University Press; 2015. [Google Scholar]
- 15. Knowlton K, Lynn B, Goldberg RA, Rosenzweig C, Hogrefe C, Rosenthal JK, Kinney PL. Projecting heat‐related mortality impacts under a changing climate in the New York City region. Am J Public Health. 2007;97:2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Bi P, Hiller JE. Projected burden of disease for Salmonella infection due to increased temperature in Australian temperate and subtropical regions. Environ Int. 2012;44:26–30. [DOI] [PubMed] [Google Scholar]
- 17. Lin S, Hsu W‐H, Van Zutphen AR, Saha S, Luber G, Hwang S‐A. Excessive heat and respiratory hospitalizations in New York State: estimating current and future public health burden related to climate change. Environ Health Perspect. 2012;120:1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andersson A, Meier HEM, Ripszam M, Rowe O, Wikner J, Haglund P, Eilola K, Legrand C, Figueroa D, Paczkowska J, Lindehoff E, Tysklind M, Elmgren R. Projected future climate change and Baltic Sea ecosystem management. Ambio. 2015;44:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, Jenkins MM, Langlois PH, Newsome KB, Olshan AF, Romitti PA, Shapira SK, Shaw GM, Tinker SC, Honein MA. The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res A Clin Mol Teratol. 2015;103:656–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, Costa P, Druschel C, Hobbs CA, Romitti PA, Langlois PH, Edmonds LD. The National Birth Defects Prevention Study. Public Health Rep. 2001;116:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler‐Noreuil KM, Moore CA. Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67:193–201. [DOI] [PubMed] [Google Scholar]
- 22. Botto LD, Lin AE, Riehle‐Colarusso T, Malik S, Correa A. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79:714–727. [DOI] [PubMed] [Google Scholar]
- 23. US Census Burea . Population projections. Available at: https://www.census.gov/data/tables/2014/demo/popproj/2014-summary-tables.html. Accessed August 15, 2016.
- 24. Schmidt GA, Kelley M, Nazarenko L, Ruedy R, Russell GL, Aleinov I, Bauer M, Bauer SE, Bhat MK, Bleck R, Canuto V, Chen Y‐H, Cheng Y, Clune TL, Del Genio A, de Fainchtein R, Faluvegi G, Hansen JE, Healy RJ, Kiang NY, Koch D, Lacis AA, LeGrande AN, Lerner J, Lo KK, Matthews EE, Menon S, Miller RL, Oinas V, Oloso AO, Perlwitz JP, Puma MJ, Putman WM, Rind D, Romanou A, Sato M, Shindell DT, Sun S, Syed RA, Tausnev N, Tsigaridis K, Unger N, Voulgarakis A, Yao M‐S, Zhang J. Configuration and assessment of the GISS ModelE2 contributions to the CMIP5 archive. J Adv Model Earth Syst. 2014;6:141–184. [Google Scholar]
- 25. Skamarock WC, Klemp JB. A time‐split nonhydrostatic atmospheric model for weather research and forecasting applications. J Comput Phys. 2008;227:3465–3485. [Google Scholar]
- 26. Otte TL, Nolte CG, Otte MJ, Bowden JH. Does nudging squelch the extremes in regional climate modeling? J Clim. 2012;25:7046–7066. [Google Scholar]
- 27. van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J‐F, Masui T, Meinshausen M, Nakicenovic N, Smith SJ, Rose SK. The representative concentration pathways: an overview. Clim Change. 2011;109:5. [Google Scholar]
- 28. United Nations Statistics Division . Live births by month of birth. Available at: http://data.un.org/Data.aspx?d=POP&f=tableCode%3A55. Accessed July 5, 2018.
- 29. Van Zutphen AR, Lin S, Fletcher BA, Hwang S‐A. A population‐based case‐control study of extreme summer temperature and birth defects. Environ Health Perspect. 2012;120:1443–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meehl GA, Tebaldi C. More intense, more frequent, and longer lasting heat waves in the 21st century. Science. 2004;305:994–997. [DOI] [PubMed] [Google Scholar]
- 31. Lin S, Insaf TZ, Luo M, Hwang S‐A. The effects of ambient temperature variation on respiratory hospitalizations in summer, New York State. Int J Occup Environ Health. 2012;18:188–197. [DOI] [PubMed] [Google Scholar]
- 32. Guo Y, Gasparrini A, Armstrong BG, Tawatsupa B, Tobias A, Lavigne E, Coelho MS, Pan X, Kim H, Hashizume M, Honda Y, Guo YL, Wu CF, Zanobetti A, Schwartz JD, Bell ML, Overcenco A, Punnasiri K, Li S, Tian L, Saldiva P, Williams G, Tong S. Temperature variability and mortality: a multi‐country study. Environ Health Perspect. 2016;124:1554–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loughnan M, Tapper N, Loughnan T. The impact of “unseasonably” warm spring temperatures on acute myocardial infarction hospital admissions in Melbourne, Australia: a city with a temperate climate. J Environ Public Health. 2014;2014:483785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Callaghan TV, Bjorn LO, Chernov Y, Chapin T, Christensen TR, Huntley B, Ims RA, Johansson M, Jolly D, Jonasson S, Matveyeva N, Panikov N, Oechel W, Shaver G, Schaphoff S, Sitch S. Effects of changes in climate on landscape and regional processes, and feedbacks to the climate system. Ambio. 2004;33:459–468. [DOI] [PubMed] [Google Scholar]
- 35. Thompson LG. Climate change: the evidence and our options. Behav Anal. 2010;33:153–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ebi KL, Meehl GA. The heat is on: climate change and heatwaves in the Midwest. Citeseer 2007;1–8–21.
- 37. U S Global Change Research Program . Global Climate Change Impacts in the United States. Cambridge, England: Cambridge University Press; 2009. [Google Scholar]
- 38. Fitzgerald EF, Pantea C, Lin S. Cold spells and the risk of hospitalization for asthma: New York, USA 1991–2006. Lung. 2014;192:947–954. [DOI] [PubMed] [Google Scholar]
- 39. Saez M, Sunyer J, Castellsague J, Murillo C, Anto JM. Relationship between weather temperature and mortality: a time series analysis approach in Barcelona. Int J Epidemiol. 1995;24:576–582. [DOI] [PubMed] [Google Scholar]
- 40. Schifano P, Leone M, De Sario M, DeDonato F, Bargagli AM, DIppoliti D, Marino C, Michelozzi P. Changes in the effects of heat on mortality among the elderly from 1998–2010: results from a multicenter time series study in Italy. Environ Health. 2012;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen EK‐C, Zmirou‐Navier D, Padilla C, Deguen S. Effects of air pollution on the risk of congenital anomalies: a systematic review and meta‐analysis. Int J Environ Res Public Health. 2014;11:7642–7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agay‐Shay K, Friger M, Linn S, Peled A, Amitai Y, Peretz C. Ambient temperature and congenital heart defects. Hum Reprod. 2013;28:2289–2297. [DOI] [PubMed] [Google Scholar]
- 43. Petkova EP, Gasparrini A, Kinney PL. Heat and mortality in New York City since the beginning of the 20th century. Epidemiology. 2014;25:554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collins M, Knutti R, Arblaster J, Dufresne J‐L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, others. Long‐term climate change: projections, commitments and irreversibility. In: Climate Change 2013. Cambridge University Press; 2013:1029–1136.


