Abstract
Background
We aimed to determine whether cerebral white matter hyperintensities (WMHs) can distinguish stroke survivors susceptible to rapid kidney function decline from intensive blood pressure (BP) lowering.
Methods and Results
The SPS3 (Secondary Prevention of Small Subcortical Strokes) trial randomized participants with recent lacunar stroke to systolic BP targets of 130 to 149 and <130 mm Hg. We included 2454 participants with WMH measured by clinical magnetic resonance imaging at baseline and serum creatinine measured during follow‐up. We tested interactions between BP target and WMH burden on the incidence of rapid kidney function decline (≥30% decrease from baseline estimated glomerular filtration rate at 1‐year follow‐up) and recurrent stroke. Rapid kidney function decline incidence was 11.0% in the lower‐BP‐target arm and 8.1% in the higher‐target arm (odds ratio=1.40; 95% CI=1.07–1.84). Odds ratio for rapid kidney function decline between lower‐ and higher‐target groups ranged from 1.26 in the lowest WMH tertile (95% CI, 0.80–1.98) to 1.71 in the highest tertile (95% CI, 1.05–2.80; P for interaction=0.65). Overall incidence of recurrent stroke was 7.9% in the lower‐target arm and 9.6% in the higher‐target arm (hazard ratio=0.80; 95% CI, 0.63–1.03). Hazard ratio for recurrent stroke in the lower‐target group was 1.13 (95% CI, 0.73–1.75) within the lowest WMH tertile compared with 0.73 (95% CI, 0.49–1.09) within the highest WMH tertile (P for interaction=0.04).
Conclusions
Participants with higher WMH burden appeared to experience greater benefit from intensive BP lowering in prevention of recurrent stroke. By contrast, intensive BP lowering increased the odds of kidney function decline, but WMH burden did not significantly distinguish this risk.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00059306.
Keywords: blood pressure, creatinine, estimated glomerular filtration rate, kidney, lacunar stroke, secondary prevention of small subcortical strokes, white matter disease
Subject Categories: Cerebrovascular Disease/Stroke, Nephrology and Kidney, Hypertension, Magnetic Resonance Imaging (MRI), Clinical Studies
Clinical Perspective
What Is New?
Among survivors of lacunar stroke, persons with high white matter hyperintensity burden are most likely to benefit from intensive blood pressure control to prevent recurrent stroke.
White matter hyperintensity burden did not significantly distinguish risk of kidney function decline after intensive blood pressure lowering.
What Are the Clinical Implications?
White matter hyperintensities may contribute to the assessment of the benefit and harm of intensive blood pressure control after lacunar stroke.
Introduction
The ideal blood pressure (BP) target for prevention of recurrent stroke remains an issue of active discussion. New hypertension guidelines recommend a target systolic BP (SBP) of <130 mm Hg in this population.1 The SPS3 (Secondary Prevention of Small Subcortical Strokes) randomized trial demonstrated that BP lowering to <130 mm Hg, compared with 130 to 149 mm Hg, significantly reduced the risk for intracerebral hemorrhage by 60%, but the 19% reduction in all stroke recurrence failed to reach statistical significance.2 On the other hand, there is great concern that aggressive BP lowering can result in large decreases in kidney function, measured by estimated glomerular filtration rate (eGFR). Higher rates of kidney function decline and acute kidney injury with intensive BP lowering have been demonstrated in SPS3 as well as in other large, randomized trials of BP lowering for primary cardiovascular disease prevention.3, 4, 5
The clinical significance of such decreases in kidney function remains an issue of active investigation. Observational data show that decreases in eGFR are strongly associated with higher end‐stage renal disease risk.6 In a clinical trial setting, even small decreases in eGFR from intensive BP lowering were associated with progression to end‐stage renal disease.7 On the other hand, previous reports from SPS3 and SPRINT (Systolic Blood Pressure Intervention Trial) showed that decreases in eGFR that are specific to the setting of intensive BP lowering may not portend increased risk for further kidney injury or adverse cardiovascular events, but, rather, they represent a hemodynamic phenomenon.5, 8 Many experts remain concerned that associations of even small increases in creatinine after intensive BP lowering with poor renal outcomes raise concern for ongoing ischemic injury.7, 9 The best strategy for identifying persons at highest risk for kidney function decline with intensive BP lowering is unknown. It is possible that kidney function decline from intensive BP lowering is a result of impaired small‐vessel autoregulation in the kidney. This would imply that persons with greater severity of small vessel disease may be at higher risk for kidney function decline from intensive BP lowering. On the other hand, given that these patients are likely at higher risk of cardiovascular events, they may also derive the most benefit from intensive BP lowering.10
White matter hyperintensities (WMHs) are thought to be markers of small vessel disease in the brain.11 WMHs are known to have higher prevalence in the setting of hypertension and advanced age. They have also been associated with increased risk of stroke‐impaired autoregulation of cerebral vasculature.10, 12, 13 Given the anatomical and vasoregulatory similarities between the brain and kidney and the known association between WMHs and chronic kidney disease,14, 15 we hypothesized that those with greater burden of WMHs likely have corresponding small‐vessel disease in the kidney. Consequently, they could be at higher risk of rapid decreases in eGFR after intensive BP lowering. To date, such a relationship has not been investigated.
We conducted a secondary analysis of the SPS3 randomized trial to determine whether the presence of WMHs could identify participants at highest risk of kidney function decline from intensive BP lowering. Given that persons with high WMH burden are at higher risk for stroke recurrence, we were also interested in understanding whether these persons may also derive more benefit from intensive BP lowering for secondary prevention of stroke.
Methods
Study Design
The SPS3 trial was a 2×2 factorial trial that randomized participants with recent lacunar stroke to evaluate the effect of an intensive BP target of <130 mm Hg compared with SBP 130 to 149 mm Hg for the prevention of recurrent stroke.16 Participants were also randomized to an antiplatelet regimen of aspirin and clopidogrel or aspirin and placebo. The BP trial was open label using the PROBE (prospective randomized open blinded end‐point) study design.17 Details of the trial design have been published.16 The study is registered on ClinicalTrials.gov under NCT00059306.
Participants
The SPS3 trial enrolled 3020 patients with recent lacunar stroke. Patients with eGFR <40 mL/min/1.73 m2 were excluded from enrollment. After randomization to an antiplatelet arm and a BP arm, participants were followed at least once per month until achievement of the assigned target BP, then every 3 months for a mean of 3.7 years. In our cross‐sectional analysis and our analysis of recurrent stroke, we excluded 53 participants. Fifty of these participants had no WMH score; 3 participants had no creatinine measure at baseline, leaving a total of 2967 participants available for analysis. Our longitudinal analysis of kidney outcomes excluded an additional 513 participants missing a serum creatinine measure at 1 year after randomization, leaving 2454 included participants. All participants signed informed consent, and the trial was approved by all appropriate institutional review boards.
Kidney Function
We estimated kidney function from serum creatinine measures using the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.18 Serum creatinine measures were obtained from all SPS3 participants at a prerandomization visit and annual follow‐up visits until the study end date.16
Neuroimaging
Patients underwent magnetic resonance imaging for clinical management when presenting with the index stroke or recurrent stroke. WMHs were evaluated visually on fluid‐attenuated inversion recovery images using the Age‐Related White Matter Changes, or Wahlund, scale by 4 readers unaware of clinical information.19 This semiquantitative scale rates WMH severity from 0 to 3 in 5 predefined areas of each brain hemisphere for a total possible range of 0 to 30.20 Inter‐rater agreement among readers was good to excellent on a sample of 40 magnetic resonance imagings: (Cohen's kappa=0.64–0.89; 77–89% agreement).
Intervention
Participants were randomized at least 2 weeks after index stroke to open‐label SBP targets of 130 to 149 or <130 mm Hg. Randomization was stratified by clinical center and baseline hypertensive status and had a permuted‐block design with variable block size. Details regarding BP measurement have been published previously.16
Outcomes
Our primary outcome of interest was incidence of rapid kidney function decline, defined as a reduction in eGFR equal to or greater than 30% between the visit before randomization and the visit 1 year afterward. We focused on the first year after randomization because this was the period of medication intensification, as we have published.5 We also analyzed the mean change in eGFR during the first year of follow‐up as a secondary kidney outcome. Finally, as a secondary outcome of interest, we analyzed the SPS3 primary end point of all stroke to contextualize the risk for rapid kidney function decline with the relative reduction in stroke risk from aggressive BP lowering. This included ischemic stroke, defined as a focal neurological deficit persisting for longer than 24 hours with an absence of hemorrhage, and intracranial hemorrhage, defined by hemorrhage in intracerebral, subdural, epidural, or subarachnoid locations on neuroimaging. Strokes were identified and adjudicated by board‐certified neurologists blinded to assigned SBP target and achieved BP. Other details on outcome adjudication have been published.16
Covariates
Clinical and demographic characteristics were compared at baseline and included in multivariable models. These included age, sex, and race; smoking status, SBP, body mass index, total cholesterol, high‐density lipoprotein cholesterol, self‐reported history of diabetes mellitus or previous lacunar stroke, hypertension, use of statins, use of antihypertensive medications, and, separately, use of angiotensin converting enzyme inhibitors or angiotensin receptor blockers. Hypertension was defined as an average prerandomization SBP greater than 140 mm Hg or a definite history of hypertension being treated with an antihypertensive.
Statistical Analysis
First, we summarized baseline clinical and demographic characteristics of participants by WMH score tertile. Differences in distribution of categorical variables and means of continuous variables were tested using the chi‐squared and ANOVA tests, respectively.
Next, we estimated the associations of WMH score with baseline kidney function. This was done using unadjusted and multivariable adjusted linear regression.
Then, to evaluate the association of WMHs with 1‐year kidney function decline, we compared incidence of rapid kidney function decline and mean 1‐year kidney eGFR decline in higher versus lower BP arms, stratified by tertile of WMHs. We tested for interaction between BP target and WMH score using logistic regression for the binary outcome of rapid kidney function decline and linear regression for the continuous outcome of 1‐year change in kidney function.
We assessed whether WMH burden modified the effect of intensive BP lowering on recurrent stroke by stratification according to WMH tertile and interaction analyses using Cox proportional hazards models for the SPS3 primary outcome of recurrent stroke.
All analyses were conducted using the Stata (version 14.1; StataCorp LP, College Station, TX) statistical software package. The data, analytical methods, and study materials are available to other researchers upon request for purposes of reproducing the results or replicating the procedure.
Results
Baseline Characteristics
Among the 2967 participants included in our cross‐sectional and recurrent stroke analyses, mean age was 62.8±10.8 years with a mean eGFR of 80.7±18.7 mL/min/1.73 m2 (Table 1). Distribution of WMH score was right‐skewed, with a median score of 4 (interquartile range, 2–8). Sixty‐three percent of participants were male, 51% were white, 31% had a history diabetes mellitus, and 75% had a history of hypertension. Persons in the highest tertile of WMH were older (mean age, 67.3±10.8 years), were more likely to have hypertension (82%), and had lower eGFR (74.8±18.3). History of previous lacunar stroke (16%) and eGFR <60 mL/min/1.73 m2 (24%) were also most prevalent in the highest WMH tertile. Participants excluded from our cross‐sectional and stroke analyses were more likely to be male, more likely to be white, had lower eGFR, and had a higher proportion of persons with history of lacunar stroke before the presenting stroke compared with included (Table S1). The 566 participants excluded in our analysis of kidney outcomes had a lower SBP, a smaller proportion of Hispanics and former smokers, and a higher proportion of blacks, whites, current smokers, and persons with history of lacunar stroke before the presenting stroke compared with included (Table S2).
Table 1.
Baseline Clinical and Demographic Characteristics of Secondary Prevention of Small Subcortical Strokes Trial Participants by Tertile of WMH Score
| Characteristic | Lowest Tertile (n=1210) | Middle Tertile (n=937) | Highest Tertile (n=820) | Total (N=2967) | ||||
|---|---|---|---|---|---|---|---|---|
| No. or Mean | (%) or (SD) | No. or Mean | (%) or (SD) | No. or Mean | (%) or (SD) | No. or Mean | (%) or (SD) | |
| Age, y | 59.3 | (10.0) | 63.3 | (10.1) | 67.3 | (10.8) | 62.8 | (10.8) |
| Male* | 785 | (64.9) | 591 | (63.1) | 485 | (59.1) | 1861 | (62.7) |
| Race† | ||||||||
| White | 635 | (52.5) | 483 | (51.5) | 386 | (47.1) | 1504 | (50.7) |
| Hispanic | 373 | (30.8) | 273 | (29.1) | 257 | (31.3) | 903 | (30.4) |
| Black | 170 | (14.0) | 160 | (17.1) | 156 | (19.0) | 486 | (16.4) |
| Other | 32 | (2.6) | 21 | (2.2) | 21 | (2.6) | 74 | (2.5) |
| Smoking | ||||||||
| Never | 506 | (41.8) | 344 | (36.7) | 325 | (39.6) | 1175 | (39.6) |
| Former | 458 | (37.9) | 384 | (41.0) | 343 | (41.8) | 1185 | (39.9) |
| Current | 246 | (20.3) | 209 | (22.3) | 152 | (18.5) | 607 | (20.5) |
| Diabetes mellitus | 407 | (33.6) | 330 | (35.2) | 252 | (30.7) | 989 | (33.3) |
| History of hypertension† | 832 | (68.8) | 716 | (76.4) | 676 | (82.4) | 2224 | (75.0) |
| Antihypertensive use† | 971 | (80.2) | 802 | (85.6) | 739 | (90.1) | 2512 | (84.7) |
| ACE inhibitor or ARB use | ||||||||
| At baseline† | 765 | (63.2) | 636 | (67.9) | 587 | (71.6) | 1988 | (67.0) |
| At 1‐year follow‐up | 688 | (67.9) | 569 | (73.9) | 504 | (75.1) | 1761 | (71.8) |
| Statin use | 832 | (68.8) | 662 | (70.7) | 549 | (67.0) | 2043 | (68.9) |
| Previous lacunar stroke† | ||||||||
| Yes | 61 | (5.0) | 107 | (11.4) | 132 | (16.1) | 300 | (10.1) |
| Missing | 2 | (0.2) | 1 | (0.1) | 0 | (0.0) | 3 | (0.1) |
| Total cholesterol, mg/dL | 189.8 | (48.3) | 187.1 | (47.1) | 185.9 | (45.4) | 187.9 | (47.2) |
| High‐density lipoprotein cholesterol, mg/dL | 44.7 | (19.6) | 45.4 | (20.1) | 46.6 | (14.2) | 45.4 | (18.5) |
| Systolic blood pressure, mm Hg† | 140.1 | (17.3) | 143.2 | (18.9) | 147.3 | (20.2) | 143.0 | (18.9) |
| Body mass index, kg/m2 † | 29.7 | (7.9) | 29.1 | (6.2) | 28.1 | (5.8) | 29.1 | (6.9) |
| eGFR, mL/min/1.73 m2 † | 84.8 | (18.2) | 80.6 | (19.0) | 74.8 | (18.3) | 80.7 | (18.9) |
| eGFR <60, mL/min/1.73 m2 | 114 | (9.4) | 145 | (15.5) | 195 | (23.8) | 454 | (15.3) |
| eGFR 60 to 90, mL/min/1.73 m2 | 583 | (48.2) | 473 | (50.5) | 445 | (54.3) | 1501 | (50.6) |
| eGFR >90, mL/min/1.73 m2 | 513 | (42.4) | 319 | (34.0) | 180 | (22.0) | 1012 | (34.1) |
| WMH score† | 1.8 | (1.0) | 5.4 | (1.1) | 11.0 | (2.9) | 5.5 | (4.1) |
| Randomized to systolic blood pressure target <130 mm Hg | 626 | (51.7) | 471 | (50.3) | 381 | (46.5) | 1478 | (49.8) |
Differences in distribution of categorical variables and means of continuous variables tested using chi‐squared and ANOVA, respectively. Follow‐up data from 2454 participants with baseline WMH score and creatinine measure at 1‐year follow‐up. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; WMH, white matter hyperintensity.
P for statistical test <0.05.
P<0.0005.
WMHs and eGFR at Baseline
WMH score had a significant inverse association with baseline kidney function. Unadjusted linear regression demonstrated that each point higher in WMH score corresponded to 1.01 (95% CI, 0.85–1.17) mL/min/1.73 m2 lower in eGFR, on average. Adjustment for age, sex, and race slightly attenuated the association. After further adjustment for additional traditional chronic kidney disease risk factors and antihypertensive therapy, each point higher in WMH score corresponded to 0.36 (95% CI, 0.18–0.50) mL/min/1.73 m2 lower baseline eGFR.
WMHs and Kidney Function Decline After Intensive BP Control
In the first year after randomization, 100 of the 1236 (8.1%) included participants in the higher‐target arm experienced rapid kidney function decline, compared with 134 of the 1218 (11.0%) in the lower‐target arm (odds ratio=1.40; 95% CI, 1.07–1.84). These findings were similar to those of previous analyses.5
The effect of intensive BP lowering on rapid kidney function decline appeared to intensify with increasing WMH score, suggesting an interaction between BP target and WMH score (FigureA). Specifically, within the lowest WMH tertile, there were 26% higher odds of rapid kidney function decline in the lower‐target group compared with the higher‐target group, whereas the odds were 34% higher in the lower‐target group compared with the higher‐target group within the middle WMH tertile, and the odds were 71% higher in the lower‐target group compared with the higher‐target group within the highest WMH tertile (Table 2). A test for interaction, however, did not indicate statistically significant interaction by WMH score (P=0.65). Similarly, average kidney function decline in the lower‐target group was 1.49 (95% CI, −0.35 to 3.33) mL/min/1.73 m2 faster than in the higher‐target group among persons in the lowest WMH tertile, whereas average decline was 2.17 (95% CI, −0.12 to 4.46) mL/min/1.73 m2 faster in the lower‐target group among persons in the middle WMH tertile and 2.56 (95% CI, −0.36 to 4.77) mL/min/1.73 m2 faster in the lower‐target group among persons in the highest WMH tertile. A formal test for interaction did not indicate statistically significant interaction by WMH on mean kidney function decline (P=0.17).
Figure 1.
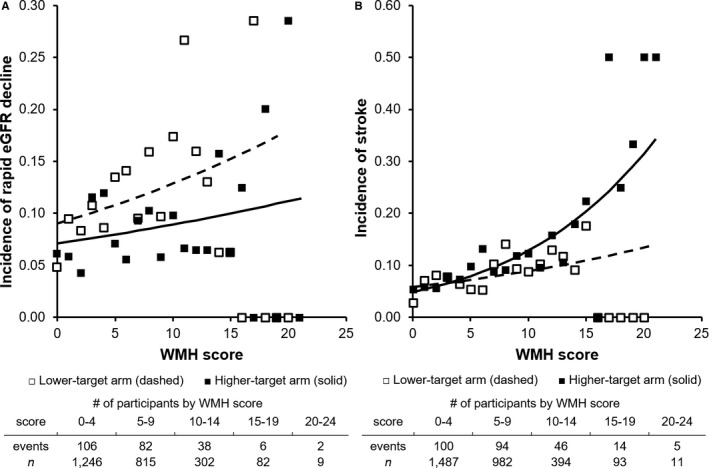
Effect of intensive vs usual systolic blood pressure target on rapid eGFR decline and recurrent stroke among persons across the range of brain magnetic resonance imaging WMH scores. Plotted lines depict predicted rates of rapid kidney function decline (A) and recurrent stroke (B) in higher‐target (solid line) and lower‐target (dashed line) arms. Markers depict observed rates of rapid decline and recurrent stroke in higher target (white squares) and lower‐target (black square) groups within each WMH score. Predictions based on logistic regression (rapid decline) and Cox regression (recurrent stroke) models. eGFR indicates estimated glomerular filtration rate; WMH, white matter hyperintensity.
Table 2.
Association of Usual Versus Intensive SBP Target With Rapid eGFR Decline in the First Year by WMH Tertile Among 2454 SPS3 Trial Participants
| SBP Target, mm Hg | Events | Odds Ratio | (95% CI) | P Value* | ||
|---|---|---|---|---|---|---|
| Lowest WMH tertile (n=1013) | ||||||
| 130 to 149 | 36/498 | 7.2% | Referent | |||
| <130 | 46/515 | 9.0% | 1.26 | (0.80–1.98) | 0.32 | |
| Medium WMH tertile (n=770) | ||||||
| 130 to 149 | 33/381 | 8.7% | Referent | |||
| <130 | 44/389 | 11.3% | 1.34 | (0.84–2.16) | 0.22 | |
| Highest WMH tertile (n=671) | ||||||
| 130 to 149 | 31/357 | 8.7% | Referent | |||
| <130 | 44/314 | 14.0% | 1.71 | (1.05–2.80) | 0.03 | |
P for interaction=0.65. eGFR indicates estimated glomerular filtration rate; SBP, systolic blood pressure; SPS3, Secondary Prevention of Small Subcortical Strokes; WMH, white matter hyperintensity.
P value for OR within WMH tertile.
WMHs and Stroke After Intensive BP Control
Overall incidence of recurrent stroke was 7.9% in the lower‐target arm and 9.6% in the higher‐target arm (hazard ratio=0.80; 95% CI, 0.63–1.03). These findings were similar to those in past analyses.2 Persons in the highest WMH tertile had the highest overall stroke incidence (12.3%), but they also had the largest absolute reduction in stroke incidence from intensive BP lowering (3.4%; Table 3). Within the lowest WMH tertile, the hazard ratio for stroke recurrence in the lower BP target arm compared with the higher‐target arm was 1.13 (95% CI, 0.73–1.75) compared with a hazard ratio of 0.73 (95% CI, 0.49–1.09) within the highest WMH tertile. Kaplan–Meier plots of stroke within tertiles of WMH score are depicted in Figure S1. A test for interaction indicated significant interaction between WMH score and the intensive BP intervention for the outcome of stroke (P=0.04), that is, intensive BP lowering provided greater stroke prevention in those with higher WMH burden (FigureB).
Table 3.
Effect of Intensive Blood Pressure Lowering on Recurrent Stroke, Stratified by WMH Score in 2967 Participants of Secondary Prevention of Small Subcortical Strokes Trial
| Systolic Blood Pressure Target, mm Hg | Events | Hazard Ratio | (95% CI) | P Value* | ||
|---|---|---|---|---|---|---|
| Lowest WMH tertile (n=1210) | ||||||
| 130 to 149 | 37/584 | 6.3% | Referent | |||
| <130 | 44/626 | 7.0% | 1.13 | (0.73–1.75) | 0.589 | |
| Medium WMH tertile (n=937) | ||||||
| 130 to 149 | 45/466 | 9.7% | Referent | |||
| <130 | 32/471 | 6.8% | 0.67 | (0.43–1.06) | 0.089 | |
| Highest WMH tertile (n=820) | ||||||
| 130 to 149 | 61/439 | 13.9% | Referent | |||
| <130 | 40/381 | 10.5% | 0.73 | (0.49–1.09) | 0.123 | |
Included participants analyzed based on intention to treat. Median follow‐up durations in the lowest, medium, and highest WMH tertiles were 3.7, 3.3, and 3.4 years, respectively. P for interaction=0.04. WMH indicates white matter hyperintensity.
P value for hazard ratio within WMH tertile.
Discussion
In our secondary analyses of the SPS3 trial, we found that higher white matter disease burden was independently associated with lower baseline eGFR. We also found that degree of WMH burden, a marker of cerebral small vessel disease, may identify persons at highest risk for rapid kidney function decline from intensive BP lowering; however, this interaction was not statistically significant. Additionally, we observed that a higher WMH score distinguished persons who derive the highest benefit from aggressive BP lowering to reduce risk for recurrent stroke. Our findings suggest that WMH, a marker of microvascular disease in the brain, may identify subgroups of persons with different benefits and risk from intensive BP lowering. Specifically, although persons with a higher burden of microvascular disease may be at higher risk for rapid kidney function decline from aggressive BP lowering, they also appear most likely to benefit in secondary stroke prevention.
This study supports previous literature describing an independent association between cerebrovascular disease and lower kidney function levels.14 In our study, this association was demonstrated to be independent of age, sex, race, and other vascular risk factors. This study also contributes to a much smaller body of literature investigating the relationship between cerebrovascular disease and change in kidney function over time, here, in the context of BP lowering.21, 22
We and others have shown that intensive BP lowering results in increased risk for rapid kidney function decline compared with higher BP target.3, 4, 5 Our study extends these findings by showing that WMHs, a marker of cerebral small‐vessel disease, may identify persons most likely to have creatinine elevation with intensive BP lowering. To our knowledge, this is 1 of very few studies to investigate the effectiveness of intensive BP lowering for prevention of stroke within subgroups defined by level of white matter disease in the brain.11, 23 Several previous studies have demonstrated an elevated risk of incident and recurrent stroke in those with higher levels of white matter disease.10 Our analysis showed those with higher levels of white matter disease may have a greater reduction in stroke recurrence from intensive BP lowering. The larger absolute benefit may be explained by this population's higher absolute risk.
Taken together, our findings could be explained by a common mechanism, such as impaired small‐vessel autoregulation in the brain and kidney among persons with higher burden of WMH. Both the brain and kidney are perfused by low‐resistance vascular beds with small vessels that rely on strict autoregulation to manage hemodynamic stress from large supplying arteries.15, 24 It is plausible that WMHs identify a vascular phenotype with less autoregulatory reserve to manage long‐term hemodynamic stress, whether it is from hypertension or intensive BP lowering.
Alternatively, the findings of greater stroke prevention and possibly greater increase in kidney function decline could be explained by the effects of renin‐angiotensin system blockade in the kidney and cerebral vasculature. Participants in higher WMH tertiles of our study had more‐prevalent use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers at baseline and 1‐year follow‐up (Table 1). We have previously shown that angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers were commonly added to achieve BP targets in SPS3.5 Excessive renin‐angiotensin system pathway blockade has been shown to be harmful to kidney function in previous trials.25, 26 On the other hand, renin‐angiotensin system blockade has also been shown to have protective effects in cerebral tissue during ischemic injury.27, 28 This could explain greater stroke prevention from intensive BP lowering at the expense of kidney function in our population of nonproteinuric persons with elevated stroke risk.
We do not believe that our findings can be attributed to the association between higher WMH burden and lower eGFR, because we have previously reported that the effect of a lower BP target increasing eGFR decline is more pronounced at higher baseline eGFR levels.5 This makes it unlikely that participants with higher WMH burden had more kidney function decline from intensive BP lowering because of their baseline eGFR, which we found in this study to be lower.
The clinical significance of rapid kidney function decline in the context of intensive BP lowering remains uncertain. Our previous work showed no association of rapid eGFR function decline with adverse outcomes in the setting of intensive BP lowering.5 It has also been demonstrated that intensive BP lowering was not associated with higher levels of tubular kidney injury markers in a primary prevention trial of intensive BP lowering, even among those with increases in serum creatinine.8, 29 This suggests that rapid kidney function decline after intensive BP lowering may primarily represent a hemodynamic effect. It has also been demonstrated that most acute kidney injury in a trial of intensive BP lowering is mild and reversible.30 Nevertheless, we believe this outcome is important because rapid kidney function decline may cause clinicians to withdraw potentially beneficial antihypertensive therapy.
The randomized‐controlled design of SPS3 is a key strength of this study, which facilitated investigation into the benefit and harm of intensive BP control. Investigating WMH as a predictor of kidney function decline is novel. It is also very generalizable to the clinical assessment of stroke patients and their prognosis. Additionally, our longitudinal outcome of kidney function decline offers more‐dynamic information about the relationship between cerebrovascular disease and kidney function than is available from previously utilized cross‐sectional analyses. Our outcome of 1‐year kidney function decline may have been too soon after enrollment to observe statistically significant interactions between WMHs and intensive BP lowering with respect to kidney function decline. Additionally, the exclusion of 566 participants in our longitudinal analysis introduces the possibility of bias that could also have affected our results. Despite these limitations and the lack of statistical significance in some of our results, we believe these results of our analyses serve well to generate hypotheses about shared mechanisms of brain and kidney vascular injury.
Future research should be directed at further determining the significance of cerebrovascular disease regarding renal and cardiovascular outcomes. Other identifiable features of small‐vessel cerebrovascular disease, including silent brain infarcts or cerebral microbleeds, may prove to be useful predictors of renal and cardiovascular outcomes. With longer follow‐up than available in the present study, the association of white matter hyperintensities and other markers of small‐vessel disease in the brain with long‐term kidney function decline could also be further elucidated. In addition, studying the effect of intensive BP lowering in a population with proteinuria may offer additional insight toward its relative benefit and harm in the context of different cardiovascular risk profiles.
In summary, higher WMH burden was associated with lower baseline eGFR, which supports the notion of shared vascular injury mechanisms. Burden of WMHs might be able identify persons at increased risk of rapid kidney function decline from intensive BP lowering, though this interaction was not statistically significant. Interestingly, those with high WMH burden were also most likely to benefit from intensive BP lowering to prevent stroke recurrence. Larger studies are required to replicate these findings.
Sources of Funding
This study was supported by a grant (U01NS38529) from the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH‐NINDS; to Benavente). This study was also supported by grant R25MD006832 from National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH‐NIMHD; to Ikeme), grant R01AG046206 from the National Institutes of Health/National Institute on Aging, AHA Established Investigator Award 17IEA33410161 (to Peralta), and the University of California, San Francisco Dean's Office Resource Allocation Program for Trainees (RAPtr).
Disclosures
Dr Shlipak has served on Scientific Advisory Boards for Cricket Health and for Tai Diagnostics. Dr Peralta has served as a consultant for Cricket Health, Inc. and Vital Labs, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics of Secondary Prevention of Small Subcortical Strokes Trial Participants Included and Excluded in Cross‐Sectional Analysis
Table S2. Baseline Characteristics of Secondary Preventions of Small Subcortical Strokes Trial Participants Included and Excluded in Longitudinal Analysis
Figure S1. Kaplan–Meier estimates of probability of recurrent stroke among persons randomized to higher vs lower BP target, stratified by WMH tertile.
(J Am Heart Assoc. 2019;8:e010091 DOI: 10.1161/JAHA.118.010091.)
References
- 1. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KAS, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 2. SPS3 Study Group , Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE, Szychowski JM. Blood‐pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ACCORD Study Group , Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peralta CA, McClure LA, Scherzer R, Odden MC, White CL, Shlipak M, Benavente O, Pergola P. Effect of intensive versus usual blood pressure control on kidney function among individuals with prior lacunar stroke: a post hoc analysis of the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial. Circulation. 2016;133:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, Cheung AK, Coresh J. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–835. [DOI] [PubMed] [Google Scholar]
- 7. Ku E, Bakris G, Johansen KL, Lin F, Sarnak MJ, Campese VM, Jamerson K, Gassman JJ, Smogorzewski M, Hsu CY. Acute declines in renal function during intensive BP lowering: implications for future ESRD risk. J Am Soc Nephrol. 2017;28:2794–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malhotra R, Craven T, Ambrosius WT, Killeen AA, Haley WE, Cheung AK, Chonchol M, Sarnak MJ, Parikh CR, Ix JH, Shlipak MG. The effect of intensive blood pressure lowering on kidney tubule injury among participants with CKD in the SPRINT trial. J Am Soc Nephrol. 2017;28:67. [Google Scholar]
- 9. Palmer BF. Renal dysfunction complicating the treatment of hypertension. N Engl J Med. 2002;347:1256–1261. [DOI] [PubMed] [Google Scholar]
- 10. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S; American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Functional Genomics and Translational Biology, and Council on Hypertension . Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. [DOI] [PubMed] [Google Scholar]
- 12. Matsushita K, Kuriyama Y, Nagatsuka K, Nakamura M, Sawada T, Omae T. Periventricular white matter lucency and cerebral blood flow autoregulation in hypertensive patients. Hypertension. 1994;23:565–568. [DOI] [PubMed] [Google Scholar]
- 13. de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. A follow‐up study of blood pressure and cerebral white matter lesions. Ann Neurol. 1999;46:827–833. [DOI] [PubMed] [Google Scholar]
- 14. Toyoda K. Cerebral small vessel disease and chronic kidney disease. J Stroke. 2015;17:31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney‐brain axis. J Am Soc Nephrol. 2013;24:353–363. [DOI] [PubMed] [Google Scholar]
- 16. Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, Coffey C, McClure LA, Szychowski JM, Conwit R, Heberling PA, Howard G, Bazan C, Vidal‐Pergola G, Talbert R, Hart RG; SPS3 Investigators . The Secondary Prevention of Small Subcortical Strokes (SPS3) study. Int J Stroke. 2011;6:164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end‐point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End‐Point. Blood Press. 1992;1:113–119. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benavente OR, Pearce LA, Bazan C, Roldan AM, Catanese L, Bhat Livezey VM, Vidal‐Pergola G, McClure LA, Hart RG; SPS3 Investigators . Clinical‐MRI correlations in a multiethnic cohort with recent lacunar stroke: the SPS3 trial. Int J Stroke. 2014;9:1057–1064. [DOI] [PubMed] [Google Scholar]
- 20. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P; European Task Force on Age‐Related White Matter Changes . A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. [DOI] [PubMed] [Google Scholar]
- 21. Peng Q, Sun W, Liu W, Liu R, Huang Y; CASISP Study Group . Longitudinal relationship between chronic kidney disease and distribution of cerebral microbleeds in patients with ischemic stroke. J Neurol Sci. 2016;362:1–6. [DOI] [PubMed] [Google Scholar]
- 22. Helmer C, Stengel B, Metzger M, Froissart M, Massy ZA, Tzourio C, Berr C, Dartigues JF. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology. 2011;77:2043–2051. [DOI] [PubMed] [Google Scholar]
- 23. Anderson C, Sato S, Delcourt C, Arima H, Zhang S, Salman RR, Stapf C, Woo D, Flaherty M, Vagal A, Wang J, Chalmers J. OS 34‐04 safety of early intensive blood pressure lowering in cerebral small vessel disease and chronic kidney disease in acute intracerebral hemorrhage. J Hypertens. 2016;34(Suppl 1 ‐ ISH 2016 Abstract Book):e396–e397. [Google Scholar]
- 24. Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro‐cardiovascular risk. Hypertens Res. 2009;32:115–121. [DOI] [PubMed] [Google Scholar]
- 25. Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, Leehey DJ, McCullough PA, O'Connor T, Palevsky PM, Reilly RF, Seliger SL, Warren SR, Watnick S, Peduzzi P, Guarino P; VA NEPHRON‐D Investigators . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369:1892–1903. [DOI] [PubMed] [Google Scholar]
- 26. ONTARGET Investigators , Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 27. Ito T, Yamakawa H, Bregonzio C, Terron JA, Falcon‐Neri A, Saavedra JM. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. [DOI] [PubMed] [Google Scholar]
- 28. Fujii K, Weno BL, Baumbach GL, Heistad DD. Effect of antihypertensive treatment on focal cerebral infarction. Hypertension. 1992;19:713–716. [DOI] [PubMed] [Google Scholar]
- 29. Zhang WR, Craven TE, Malhotra R, Cheung AK, Chonchol M, Drawz P, Sarnak MJ, Parikh CR, Shlipak MG, Ix JH; SPRINT Research Group . Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: a case‐control study. Ann Intern Med. 2018;169:610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, Umanath K, Rahbari‐Oskoui F, Porter AC, Pisoni R, Lewis CE, Lewis JB, Lash JP, Katz LA, Hawfield AT, Haley WE, Freedman BI, Dwyer JP, Drawz PE, Dobre M, Cheung AK, Campbell RC, Bhatt U, Beddhu S, Kimmel PL, Reboussin DM, Chertow GM; SPRINT Research Group . Effects of intensive blood pressure treatment on acute kidney injury events in the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2018;71:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Secondary Prevention of Small Subcortical Strokes Trial Participants Included and Excluded in Cross‐Sectional Analysis
Table S2. Baseline Characteristics of Secondary Preventions of Small Subcortical Strokes Trial Participants Included and Excluded in Longitudinal Analysis
Figure S1. Kaplan–Meier estimates of probability of recurrent stroke among persons randomized to higher vs lower BP target, stratified by WMH tertile.


