Abstract
Background
Rapid growth in transcatheter aortic valve replacement (TAVR) demand has translated to inadequate access, reflected by prolonged wait times. Increasing wait times are associated with important adverse outcomes while on the wait‐list; however, it is unknown if prolonged wait times influence postprocedural outcomes. Our objective was to determine the association between TAVR wait times and postprocedural outcomes.
Methods and Results
In this population‐based study in Ontario, Canada, we identified all TAVR procedures between April 1, 2010, and March 31, 2016. Wait time was defined as the number of days between initial referral and the procedure. Primary outcomes of interest were 30‐day all‐cause mortality and all‐cause readmission. Multivariable regression models incorporated wait time as a nonlinear variable, using cubic splines. The study cohort included 2170 TAVR procedures, of which 1741 cases were elective and 429 were urgent. There was a significant, nonlinear relationship between TAVR wait time and post‐TAVR 30‐day mortality, as well as 30‐day readmission. We observed an increased hazard associated with shorter wait times that diminished as wait times increased. This statistically significant nonlinear relationship was seen in the unadjusted model as well as after adjusting for clinical variables. However, after adjusting for case urgency status, there was no relationship between wait times and postprocedural outcomes. In sensitivity analyses restricted to either only elective or only urgent cases, there was no relationship between wait times and postprocedural outcomes.
Conclusions
Wait time has a complex relationship with postprocedural outcomes that is mediated entirely by urgency status. This suggests that further research should elucidate factors that predict hospitalization requiring urgent TAVR while on the wait list.
Keywords: outcome, transcatheter aortic valve implantation, transcatheter aortic valve replacement, wait time
Subject Categories: Aortic Valve Replacement/Transcather Aortic Valve Implantation, Quality and Outcomes, Complications
Clinical Perspective
What Is New?
Increasing wait times are associated with important adverse outcomes while on the wait‐list. However, it is unknown if prolonged wait times influence postprocedural outcomes.
We found that wait time has a nonlinear relationship with postprocedural outcomes.
Counterintuitively, we found an increased hazard with shorter wait times; this relationship was mediated via urgency status.
What Are the Clinical Implications?
Triaging patients while on the wait‐list is an underdeveloped area.
Our results suggest that research on preprocedural queuing and triage should focus on how to better identify patients who may deteriorate to the point of requiring in‐hospital transcatheter aortic valve replacement.
Our work suggests a potential framework, which should have 3 separate stages. Developing risk scores for identifying patients who may die on the wait‐list is the first step.
The second step is to develop risk scores to identify outpatient transcatheter aortic valve replacement candidates who may deteriorate to the point that they require hospitalization, and the third step is to identify those who will need an inpatient transcatheter aortic valve replacement.
Introduction
Transcatheter aortic valve replacement (TAVR) represents a paradigm shift in therapeutic options for severe aortic stenosis. Since being first described in 2002,1, 2 TAVR has rapidly disseminated, with >350 000 procedures performed in >70 countries.1, 2 The growth in TAVR demand has further increased with the recent expansion of TAVR into intermediate‐ and lower‐risk patients, as recommended by clinical practice guidelines in 2017.3 In some jurisdictions, this dramatic increase in demand has overwhelmed current capacity, translating into prolonged wait times.4
Inadequate access, as reflected by increased wait times, has important clinical and policy implications. Previous work from our group estimated the hypothetical impact of increasing wait times on the effectiveness of TAVR by applying discrete event modeling. We found that a wait time of >60 days would negate any potential benefit of receiving TAVR instead of traditional surgical aortic valve replacement.5 Observational data have shown associations between increased wait times and greater mortality4, 6, 7 and all‐cause hospitalization4 while on the wait‐list, as well as declines in functional status8 and quality of life.9 However, there is a paucity of literature on the association between wait time and postprocedural outcomes.
Arnold and colleagues10 found that low pre‐TAVR functional status was a strong predictor of poor post‐TAVR outcomes, including mortality and diminished quality of life. Given that patients are prone to declines in functional status while waiting for TAVR,8, 9, 10 it would be reasonable to hypothesize that prolonged wait times may result in poorer postprocedural outcomes. Accordingly, our objective was to test this hypothesis, by evaluating the association between TAVR wait times and early post‐TAVR outcomes using a population‐level registry of all TAVR procedures in Ontario, Canada.
Methods
This retrospective cohort study was approved by the Institutional Research Ethics Board at Sunnybrook Health Sciences Center, at the University of Toronto (Toronto, Ontario, Canada), before data collation and analysis. The use of anonymized administrative data without patient consent at the Institute for Clinical Evaluative Sciences is allowed in Ontario on the basis of provincial privacy legislation. Analytic methods and study materials will be available to other researchers for purposes of reproducing the results or replicating the procedure. However, individual data will not be available, to be compliant with privacy regulations in Ontario, Canada. Dr Wijeysundera will be responsible for maintaining availability of analytic methods and study materials.
Context
Ontario is the largest province in Canada, with a population of 13.6 million. All residents have universal access to health care and hospital services through a publicly funded healthcare program administered by a single third‐party payer, the Ontario Ministry of Health and Long‐Term Care.
Data Sources
Our study used data collected in the CorHealth Ontario TAVR Registry. The TAVR CorHealth Registry contains demographic, comorbidity, and procedural variables from the 10 hospitals across the province that perform TAVR. These data elements have been validated through selected chart abstractions and core laboratory analyses.
Data from the TAVR CorHealth Registry were linked using encrypted unique patient identifiers to population‐based administrative databases housed at the Institute for Clinical Evaluative Sciences in Toronto, Ontario. We used the Canadian Institute for Health Information Discharge Abstract Database for data on short‐term hospitalizations, as well as to supplement baseline comorbidity and procedural data. Dementia diagnoses were determined through linkage with any of the following 3 administrative databases: Ontario Health Insurance Program physician claims database, Ontario Drug Benefit database, or Canadian Institute for Health Information Discharge Abstract Database. Validated Institute for Clinical Evaluative Sciences–derived databases were used to identify diabetes mellitus,11, 12 heart failure (HF),13, 14 hypertension,15 and chronic obstructive pulmonary disease.16 Medical frailty was determined using the John Hopkins Adjusted Clinical Group Case‐Mix adjustment system (The Johns Hopkins ACG System, version 10).17 Mortality was ascertained via the Registered Persons Database, as were additional demographic variables, such as neighborhood income quintile and rural residence.
Patient Selection and Variable Definitions
We included all TAVR procedures in Ontario between April 1, 2010, and March 31, 2016. Mean and median total TAVR wait times were defined as the interval from referral date to the date of the TAVR procedure. Urgency status was either elective or urgent. Elective patients were defined as patients who were admitted for their TAVR procedure on an elective basis; in contrast, urgent patients were those who required a TAVR procedure during a concurrent hospitalization for declining medical status. Hospitalizations while on the wait‐list were defined as only those that resulted in a discharge home without a TAVR procedure. A hospitalization during the wait period that resulted in a TAVR was counted as an urgent case, with that hospitalization defined as the index hospitalization.
Outcome
Our primary outcomes of interest were post‐TAVR 30‐day all‐cause mortality and 30‐day all‐cause readmission.
Statistical Analysis
To determine the association between wait time and the risk of 30‐day mortality, Cox proportional hazard regression models were developed, applying a robust, sandwich‐type variance estimator to account for homogeneity/clustering of patients within each TAVR center. For all‐cause readmission within 30 days, we developed marginal cause‐specific Cox proportional hazard models, accounting for the competing risk of death. To determine if there was a nonlinear association between wait times and outcomes, we modeled wait time using a cubic spline function with 3 knots at 30, 120, and 240 days. To identify potential mediators of the relationship between wait times and outcomes, we built several sequential models. First, we modeled the unadjusted relationship between wait times and outcomes. Then, we adjusted the model for all baseline clinical variables. Finally, we included TAVR urgency status. We conducted 3 sensitivity analyses. First, using the full cohort, we also included HF hospitalization and all‐cause hospitalization while on the wait‐list. Second, we restricted our cohort to only elective patients and repeated our regression models. Finally, we restricted our cohort and analyses to only urgent patients who underwent a TAVR while hospitalized.
All data analyses were performed using SAS, version 9.4 (SAS Institute Inc, Cary, NC). Statistical significance was considered to be a 2‐sided P<0.05. Consistent with convention, a nonlinear relationship was significant at P<0.1.
Results
The CorHealth registry included a total of 4535 TAVR referrals between April 1, 2010, and March 31, 2016. Of those referred, 2251 led to a TAVR procedure. As seen in Figure 1, after applying additional exclusions, we had a final cohort of 2170 procedures, with 1741 elective procedures (80.2%) and 429 urgent procedures (19.8%).
Figure 1.
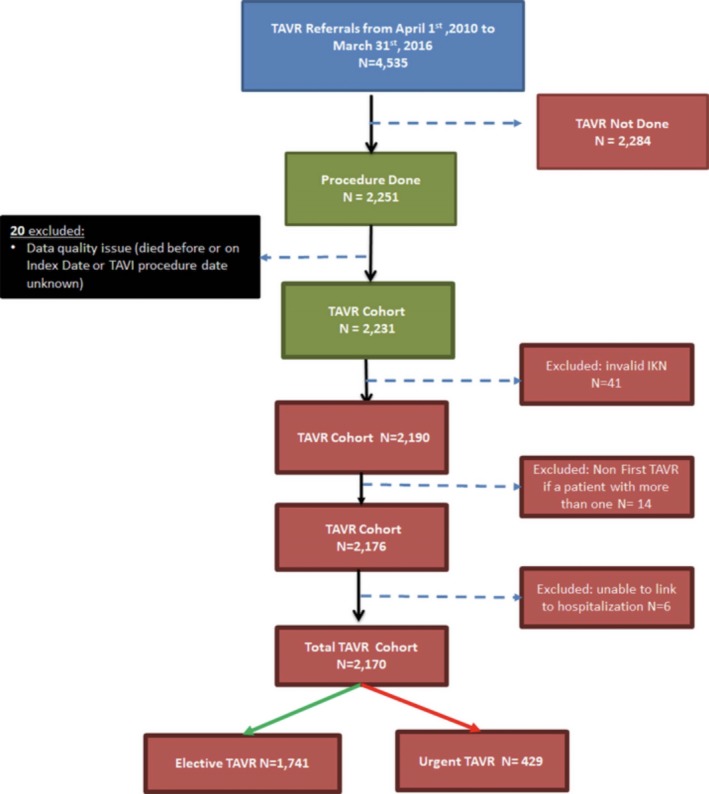
Cohort selection. IKN indicates unique identifier; TAVI, transcatheter aortic valve implantation; TAVR, transcatheter aortic valve replacement.
Baseline Characteristics
Baseline characteristics of the total cohort are found in Table 1. The mean age of our cohort was 81.8 years, with 46% women. The patients who underwent an urgent TAVR had a significantly higher proportion with HF, renal disease, dialysis, cardiac arrhythmia, and prior valve surgery compared with patients who underwent elective TAVR (P<0.05).
Table 1.
Baseline Characteristics
| Characteristic | Elective (N=1741) | Urgent (N=429) | Total (N=2170) | P Value |
|---|---|---|---|---|
| Age, mean±SD, y | 81.9±7.2 | 81.3±8.9 | 81.8±7.6 | 0.15 |
| Sex, female | 804 (46.2) | 192 (44.8) | 996 (45.9) | 0.60 |
| Income quintile | 0.03 | |||
| 1 | 277 (15.9) | 69 (16.1) | 346 (15.9) | |
| 2 | 350 (20.1) | 103 (24) | 453 (20.9) | |
| 3 | 369 (21.2) | 85 (19.8) | 454 (20.9) | |
| 4 | 360 (20.7) | 96 (22.4) | 456 (21.0) | |
| 5 | 379 (21.8) | 73 (16.6) | 450 (20.7) | |
| Rural resident | 189 (10.9) | 68 (15.9) | 257 (11.8) | 0.004 |
| Charlson score, mean±SD | 1.81±1.86 | 2.31±2.05 | 1.91±1.91 | <0.001 |
| Dyslipidemia | 1168 (67.1) | 255 (59.4) | 1423 (65.6) | 0.003 |
| Dementia | 122 (7) | 38 (8.9) | 160 (7.4) | 0.19 |
| DM | 780 (44.8) | 211 (49.2) | 991 (45.7) | 0.10 |
| Hypertension | 1644 (94.4) | 395 (92.1) | 2039 (94.0) | 0.07 |
| HF | 1207 (69.3) | 357 (83) | 1563 (72.0) | <0.001 |
| COPD | 618 (35.5) | 159 (37.1) | 777 (35.8) | 0.54 |
| Malignancy | 118 (6.8) | 23 (5.4) | 141 (6.5) | 0.29 |
| Renal disease | 168 (9.6) | 75 (17.5) | 243 (11.2) | <0.001 |
| Dialysis | 51 (2.9) | 22 (5.1) | 73 (3.4) | 0.02 |
| CAD | 1280 (73.5) | 257 (59.9) | 1537 (70.8) | <0.001 |
| Cardiac arrhythmia/AF | 442 (25.4) | 137 (31.9) | 579 (26.7) | 0.006 |
| CVD | 93 (5.3) | 23 (5.4) | 116 (5.3) | 0.99 |
| Lung disease | 23 (1.3) | 6 (1.4) | 29 (1.3) | 0.9 |
| PVD | 94 (5.4) | 25 (5.8) | 119 (5.5) | 0.73 |
| Frailty* | 351 (20.2) | 103 (24.2) | 454 (20.9) | 0.08 |
| Previous PCI | 617 (35.4) | 121 (28.2) | 738 (34.0) | 0.005 |
| Previous CABG | 424 (24.4) | 97 (22.6) | 521 (24.0) | 0.45 |
| Previous valve surgery | 184 (10.6) | 74 (17.2) | 258 (11.9) | <0.001 |
| TAVR procedure | ||||
| Transfemoral | 1420 (81.6) | 352 (82.1) | 1772 (81.7) | 0.60 |
| Valve in valve | 147 (8.4) | 63 (14.7) | 210 (9.7) | <0.001 |
Data are given as number (percentage) of each group, unless otherwise indicated. AF indicates atrial fibrillation; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DM, diabetes mellitus; HF, heart failure; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; TAVR, transcatheter aortic valve replacement.
Frailty was determined using the John Hopkins Adjusted Clinical Group Case‐Mix adjustment system (The Johns Hopkins ACG System, version 10).
Unadjusted Outcomes
The mean TAVR wait time was 132.5 days, with a median of 107 days. There was substantial variation, as reflected in an interquartile range from 55 to 176 days. There was a significantly shorter wait time in the urgent versus elective patients (mean, 67.4±86.6 versus 148.5±118.5 days; P<0.001). While on the wait‐list, 38.8% of patients had a hospitalization, with 9.1% having a HF hospitalization. These were all hospitalizations in which the patient was successfully discharged home without a concomitant TAVR procedure.
Mean length of hospital stay for the index TAVR hospitalization was 9.8 days (15.1 versus 8.6 days in the urgent versus elective TAVR groups; P<0.001). Furthermore, patients who underwent urgent TAVR had a significantly higher rate of procedural complications, such as acute kidney injury, dialysis, and any bleeding (P<0.001) (Table 2).
Table 2.
Wait Times and Outcomes
| Elective (N=1741) | Urgent (N=429) | Total (N=2170) | P Value | |
|---|---|---|---|---|
| Referral to TAVR, d | ||||
| Mean (SD) | 148.5±118.5 | 67.4±86.6 | 132.5±117.4 | <0.001 |
| Median (IQR) | 124 (72–189) | 36 (14–95) | 107 (55–176) | <0.001 |
| Outcomes on wait‐list for TAVR | ||||
| HF hospitalization on wait‐list | 154 (8.8) | 44 (10.3) | 198 (9.1) | 0.36 |
| All‐cause hospitalization on wait‐list | 728 (41.8) | 114 (26.6) | 842 (38.8) | <0.001 |
| Length of stay, mean±SD, d | ||||
| TAVR procedure date to discharge, mean±SD | 8.6±13.0 | 15.0±25.2 | 9.8±16.4 | <0.001 |
| In‐hospital complication, secondary outcome | ||||
| Pacemaker insertion | 230 (13.2) | 59 (13.8) | 289 (13.3) | 0.767 |
| Stroke/TIA | 34 (2.0) | 8 (1.9) | 42 (1.9) | 0.906 |
| Dialysis | 42 (2.4) | 26 (6.1) | 68 (3.1) | <0.001 |
| Acute kidney injury | 19 (1.1) | 29 (6.8) | 48 (2.2) | <0.001 |
| In hospital bleeding (all types) | 154 (8.8) | 64 (14.9) | 218 (10.0) | <0.001 |
| Post‐TAVR outcomes | ||||
| Mortality within 30 d post‐TAVR | 100 (5.7) | 49 (11.4) | 149 (6.9) | <0.001 |
| Readmission within 30 d post‐TAVR | 252 (14.5) | 87 (20.3) | 339 (15.6) | 0.003 |
Data are given as number (percentage) of each group, unless otherwise indicated. HF indicates heart failure; IQR, interquartile range; TAVR, transcatheter aortic valve replacement; TIA, transient ischemic attack.
For our primary post‐TAVR outcomes, 149 patients (6.9%) and 339 patients (15.6%) died and were readmitted, respectively, within 30 days after the TAVR procedure. There were significantly worse outcomes in the urgent versus elective groups (11.4% versus 5.7% [P<0.001] for mortality and 20.3% versus 14.5% [P=0.003] for readmission) (Table 2).
Wait Time Relationship to Postprocedural Outcomes 30‐Day Mortality
In the unadjusted model, we found a statistically significant relationship between mortality and wait times (P<0.001) that was nonlinear (P<0.08). The relationship was complex, as seen in Figure 2A. In contrast to our original hypothesis, we observed an increased hazard associated with shorter wait times that was attenuated with longer wait times. When adjusted for all clinical variables (see Table S1 for list of variables), this nonlinear relationship persisted (Figure 2B). When urgency status was forced into the model, there was no longer a relationship between wait times and mortality, as seen in Figure 2C (P=0.58 for overall relationship, P=0.77 for linearity). Urgency status was a strong predictor of 30‐day mortality, with urgent patients having a hazard ratio (HR) of 1.80 (95% CI, 1.24–2.62; P=0.002) compared with elective patients.
Figure 2.
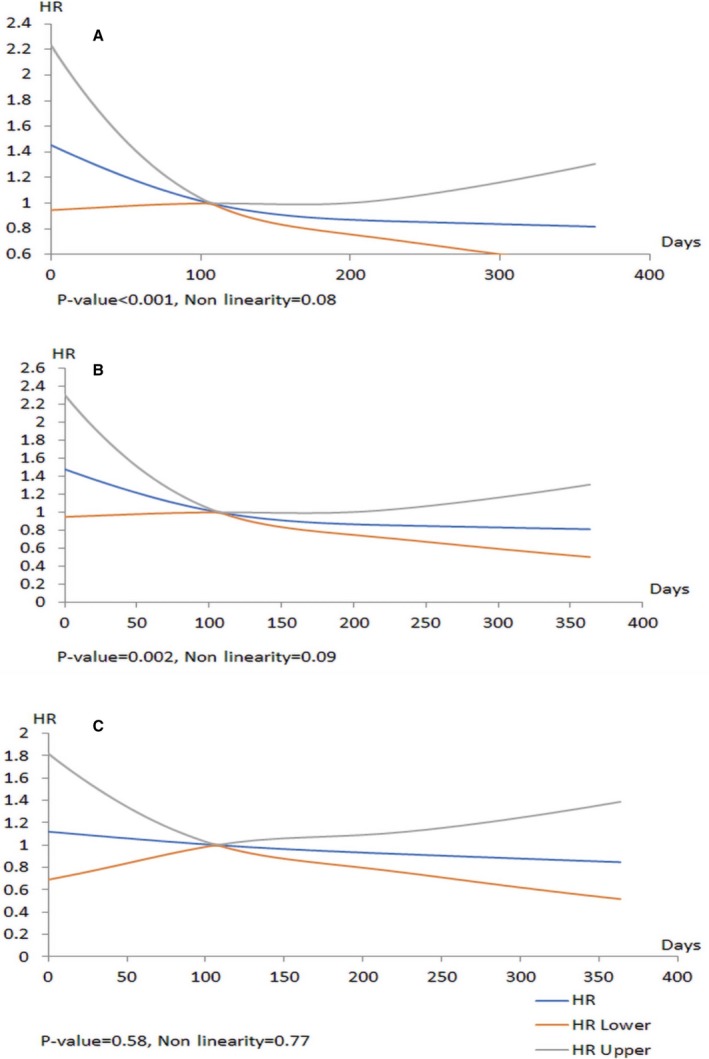
Models for mortality within 30‐day post–transcatheter aortic valve replacement (TAVR). The x axis shows the hazard ratios (HRs) for 30‐day post‐TAVR mortality for the total cohort, and the y axis represents days on the wait‐list. A, Unadjusted data. B, Data adjusted for clinical variables. C, Data adjusted for clinical variables and urgency status.
All‐Cause Readmission at 30 Days
A similar pattern was seen for 30‐day all‐cause readmission, as shown in Figure 3A through 3C. In the unadjusted model (Figure 3A), there was a statistically significant relationship between wait times and readmission, (P=0.01 for overall relationship, P=0.06 for linearity), with a higher hazard associated with shorter wait times. This relationship between wait times persisted when the model was for clinical variables (Figure 3B; Table S2). With the introduction of urgency status in the model (Figure 3C), there was no overall association (P=0.98) between wait time and readmission. Urgent status was a statistically significant predictor of readmission (urgent versus elective HR, 1.35; 95% CI, 1.04–1.75; P=0.02).
Figure 3.
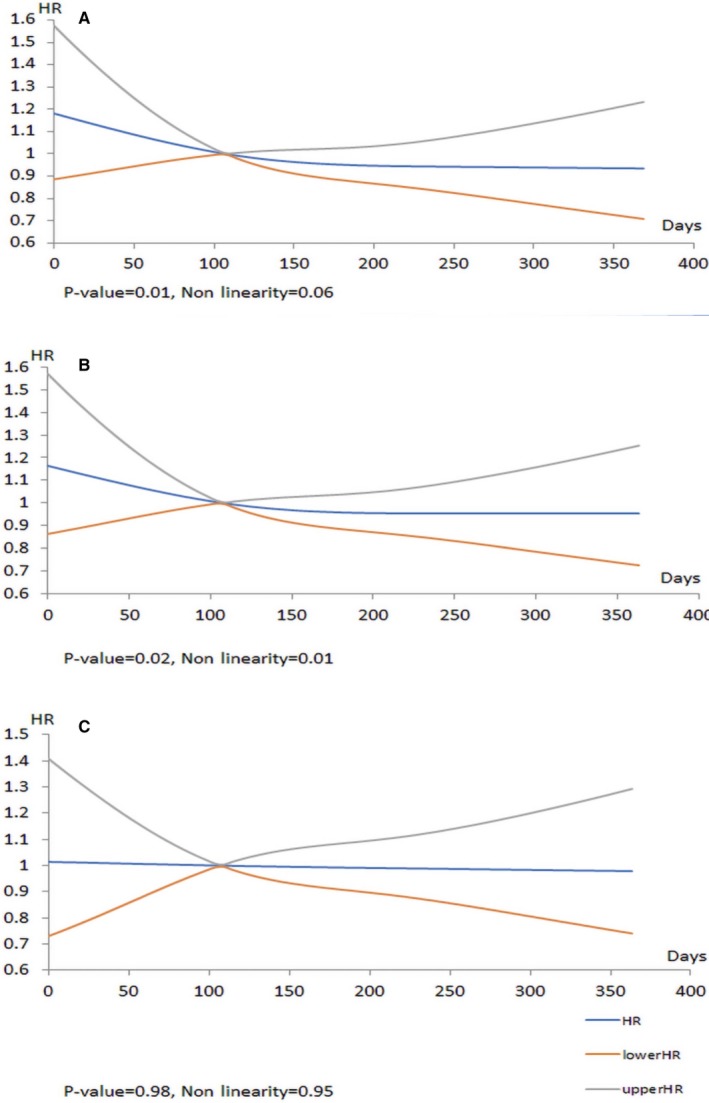
Models for readmission within 30‐day post–transcatheter aortic valve replacement (TAVR). The x axis shows the hazard ratios (HRs) for 30‐day post‐TAVR readmission for the total cohort, and the y axis represents days on the wait‐list. A, Unadjusted data. B, Data adjusted for clinical variables. C, Data adjusted for clinical variables and urgency status.
Sensitivity Analyses
In the full cohort, when HF admission or all‐cause admission while on the wait‐list was introduced into the model, the nonlinear relationship between wait times and outcomes persisted (Figures S1A, S1B and S2A, S2B). This suggests that it is not hospitalization per se that mediates the relationship between TAVR wait times and outcomes but rather a hospitalization that requires an urgent TAVR.
In our second sensitivity analysis, we restricted our models to the 1741 elective patients. In contrast to the significant nonlinear relationship that we found in our primary analysis, in the elective‐only analysis, we found that wait times had no relationship with either 30‐day mortality (HR, 0.99; 95% CI, 0.99–1; P=0.12) or readmission (HR, 1.0; 95% CI, 0.99–1.0; P=0.52). Similarly, when restricted to the 429 urgent patients, there was no relationship between wait times and outcomes (30‐day mortality HR, 1.00; 95% CI, 0.99–1.01; P=0.40; or 30‐day readmission HR, 1.00; 95% CI, 0.99–1.01; P=0.16).
Discussion
In this study of all TAVR referrals in Ontario, we found a statistically significant relationship between TAVR wait time and 30‐day mortality or readmission post‐TAVR procedure that was nonlinear. This relationship was complex, with a counterintuitive higher hazard associated with shorter wait times. This relationship was accounted for almost entirely by urgency status, with urgent patients having worse outcomes. Almost 40% of patients on the wait‐list had a hospitalization while waiting; an additional 20% had a wait‐time hospitalization requiring an urgent in‐hospital TAVR. These urgent inpatients had a greater proportion of high‐risk features. However, despite short wait times, they had worse outcomes. After adjustment for all the higher‐risk clinical variables, this nonlinear relationship nonetheless persisted. Once urgency status was accounted for, there was no longer any relationship between wait times and outcomes, a finding that was robust in multiple sensitivity analyses.
Management of patient wait times is important because wait time reflects the balance between demand for treatment and the capacity to deliver treatment. Longer wait times have a direct relationship with adverse events while waiting,4 including an increase in wait‐time mortality and hospitalization.4, 6, 7 Furthermore, patients undergoing urgent TAVR who require an in‐hospital TAVR require a more prolonged post‐TAVR hospital stay.18, 19 Other studies have found an important relationship between greater wait times and deterioration in functional capacity and quality of life while on the wait‐list, which, in turn, negatively affects post‐TAVR mortality and recovery.8, 9, 10, 20
Given this background, our hypothesis was that longer wait times would translate to worse post‐TAVR outcomes. Counterintuitively, our results suggest the opposite. We found an increased hazard with shorter wait times. This relationship was attenuated when urgency status was introduced into the model, as seen in our primary analysis, and reinforced by our sensitivity analysis in only elective patients. Performing TAVR in acutely decompensated patients as part of a concurrent hospitalization was associated with worse outcomes; such an urgent group had actually shorter TAVR wait times than those for the elective group, which explained the counterintuitive relationship between wait times and postprocedural outcomes.
This is a critical insight that has implications for TAVR wait‐time management. Although several risk models have been developed to predict postprocedure mortality in patients undergoing TAVR to improve patient selection,21, 22, 23, 24 no risk models exist to triage patients on the basis of their level of risk for adverse events while on the wait‐list. Our results suggest that research on preprocedural queuing and triage should focus on how to better identify patients who may deteriorate to the point of requiring in‐hospital TAVR.
Triaging patients while on the wait‐list is an underdeveloped area, but our work suggests a potential framework for such models, which should have 3 separate stages (Figure 4). Developing risk scores for identifying patients who may die on the wait‐list is the first step. The second step is to develop risk scores to identify outpatient TAVR candidates who may deteriorate to the point that they require hospitalization, and the third step is to identify those who will need an inpatient TAVR. This is a subtle, yet important, point. We found that a high proportion of patients will require hospitalization while on the TAVR wait‐list; however, most of these will not result in an urgent TAVR. It is important to identify both these patients who will be hospitalized and, within this group, the subset of patients (≈1 in 5 of the total TAVR population) who will need an urgent TAVR. This work and others suggest that such patients will have a prolonged length of stay and critically worse postprocedural outcomes.
Figure 4.
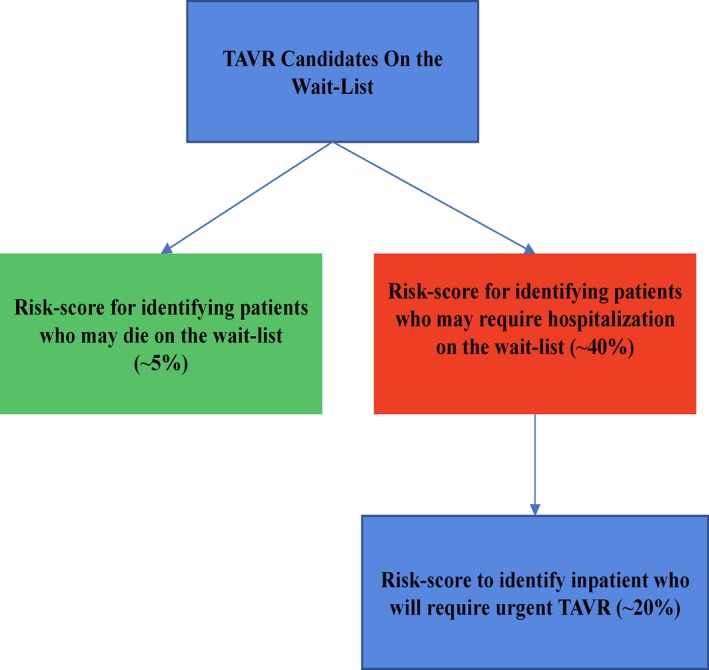
Conceptual framework for triaging patients on the wait‐list. TAVR indicates transcatheter aortic valve replacement.
The findings of this study should be considered in the context of several limitations that merit discussion. First, our models were adjusted for only the factors that are captured in the registry; this excludes potentially important factors, such as left ventricular function, Society of Thoracic Surgeons, and Euro score. As such, we cannot discount the presence of residual confounding. Second, our cohort extended across a time period that included substantial evolutions in TAVR technology and care delivery. Third, we were not able to determine the onset of symptoms, only that of referral. This may be different between patient subgroups, such as rural and urban patients. Finally, ours was an observational study, and we cannot conclude causality in the effect seen with urgency status and its influence on the relationship between wait time and outcomes. Thus, our conclusions should be considered hypothesis generating, and not conclusive.
Conclusion
Wait time has a complex relationship with postprocedural outcomes that appears to be related to urgency status. This suggests that further research is needed to elucidate factors that predict hospitalization requiring urgent TAVR while on the wait‐list.
Sources of Funding
This study is funded by a Grant‐in‐Aid from the Heart and Stroke Foundation of Canada and from an Early Research Award from the Ministry of Research and Innovation of Ontario. This study was supported by the Institute for Clinical Evaluative Science (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of the CIHI. Wijeysundera is supported by a Distinguished Clinical Scientist Award from the Heart and Stroke Foundation of Canada. Fremes is supported in part by the Bernard S. Goldman Chair in Cardiovascular Surgery.
Disclosures
Wijeysundera receives research funding from Medtronic Inc and Edwards Life Sciences. The remaining authors have no disclosures to report.
Supporting information
Table S1. Cox Models for Mortality Within 30‐Day Post‐TAVR Adjusted for Clinical Variables
Table S2. Cox Models for Readmission Within 30‐Day of Post‐TAVR Adjusted for Clinical Variables
Figure S1. Models for mortality within 30‐day of post‐TAVR.
Figure S2. Models for readmission within 30‐day of post‐TAVR.
Acknowledgments
The authors acknowledge that the clinical registry data used in this publication are from participating hospitals through CorHealth Ontario, which serves as an advisory body to the Ontario Ministry of Health and Long‐Term Care (MOHLTC), is funded by the MOHLTC, and is dedicated to improving the quality, efficiency, access, and equity in the delivery of the continuum of adult cardiac, vascular, and stroke services in Ontario, Canada. We thank IMS Brogan Inc for use of its Drug Information Database. Wijeysundera had access to all the study data, takes responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication.
(J Am Heart Assoc. 2019;8:e010407 DOI: 10.1161/JAHA.118.010407.)
References
- 1. Barbanti M, Webb JG, Gilard M, Capodanno D, Tamburino C. Transcatheter aortic valve implantation in 2017: state of the art. EuroIntervention. 2017;13:AA11–AA21. [DOI] [PubMed] [Google Scholar]
- 2. Cribier A. The development of transcatheter aortic valve replacement (TAVR). Glob Cardiol Sci Pract. 2016;2016:e201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falk V, Baumgartner H, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Munoz DR, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2017;52:616–664. [DOI] [PubMed] [Google Scholar]
- 4. Elbaz‐Greener G, Masih S, Fang J, Ko DT, Lauck SB, Webb JG, Nallamothu BK, Wijeysundera HC. Temporal trends and clinical consequences of wait‐times for trans‐catheter aortic valve replacement: a population based study. Circulation. 2018;138:483–493. [DOI] [PubMed] [Google Scholar]
- 5. Wijeysundera HC, Wong WW, Bennell MC, Fremes SE, Radhakrishnan S, Peterson M, Ko DT. Impact of wait times on the effectiveness of transcatheter aortic valve replacement in severe aortic valve disease: a discrete event simulation model. Can J Cardiol. 2014;30:1162–1169. [DOI] [PubMed] [Google Scholar]
- 6. Bainey KR, Natarajan MK, Mercuri M, Lai T, Teoh K, Chu V, Whitlock RP, Velianou JL. Treatment assignment of high‐risk symptomatic severe aortic stenosis patients referred for transcatheter AorticValve implantation. Am J Cardiol. 2013;112:100–103. [DOI] [PubMed] [Google Scholar]
- 7. Nuis RJ, Dager AE, van der Boon RM, Jaimes MC, Caicedo B, Fonseca J, Van Mieghem NM, Benitez LM, Umana JP, O'Neill WW, de Marchena E, de Jaegere PP. Patients with aortic stenosis referred for TAVI: treatment decision, in‐hospital outcome and determinants of survival. Neth Heart J. 2012;20:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forman JM, Currie LM, Lauck SB, Baumbusch J. Exploring changes in functional status while waiting for transcatheter aortic valve implantation. Eur J Cardiovasc Nurs. 2015;14:560–569. [DOI] [PubMed] [Google Scholar]
- 9. Olsson K, Naslund U, Nilsson J, Hornsten A. Experiences of and coping with severe aortic stenosis among patients waiting for transcatheter aortic valve implantation. J Cardiovasc Nurs. 2016;31:255–261. [DOI] [PubMed] [Google Scholar]
- 10. Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodes‐Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ; PARTNER Investigators . Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guttmann A, Nakhla M, Henderson M, To T, Daneman D, Cauch‐Dudek K, Wang X, Lam K, Hux J. Validation of a health administrative data algorithm for assessing the epidemiology of diabetes in Canadian children. Pediatr Diabetes. 2010;11:122–128. [DOI] [PubMed] [Google Scholar]
- 12. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25:512–516. [DOI] [PubMed] [Google Scholar]
- 13. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33:160–166. [PubMed] [Google Scholar]
- 14. Tu K, Chen Z, Lipscombe LL; Canadian Hypertension Education Program Outcomes Research Taskforce . Prevalence and incidence of hypertension from 1995 to 2005: a population‐based study. CMAJ. 2008;178:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tu K, Campbell NR, Chen ZL, Cauch‐Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1:e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 16. Gershon AS, Wang C, Guan J, Vasilevska‐Ristovska J, Cicutto L, To T. Identifying individuals with physician diagnosed COPD in health administrative databases. COPD. 2009;6:388–394. [DOI] [PubMed] [Google Scholar]
- 17. Weiner JP, Abrams C. The Johns Hopkins ACG system, technical reference guide, version 10.0. Health Services Research and Development Center at The Johns Hopkins University, Bloomberg School of Public Health. 2011. https://studylib.net/doc/8365333/the-johns-hopkins-acg%C2%AE-system-technicalreference-guide. Accessed January, 2018.
- 18. Arbel Y, Zivkovic N, Mehta D, Radhakrishnan S, Fremes SE, Rezaei E, Cheema AN, Al‐Nasser S, Finkelstein A, Wijeysundera HC. Factors associated with length of stay following trans‐catheter aortic valve replacement: a multicenter study. BMC Cardiovasc Disord. 2017;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sud M, Qui F, Austin PC, Ko DT, Wood D, Czarnecki A, Patel V, Lee DS, Wijeysundera HC. Short length of stay after elective transfemoral transcatheter aortic valve replacement is not associated with increased early or late readmission risk. J Am Heart Assoc. 2017;6:e005460 DOI: 10.1161/JAHA.116.005460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, Reardon MJ, Yakubov SJ, Adams DH, Cohen DJ; U.S. CoreValve Investigators . Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hermiller JB Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators . Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:343–352. [DOI] [PubMed] [Google Scholar]
- 22. Kotting J, Schiller W, Beckmann A, Schafer E, Dobler K, Hamm C, Veit C, Welz A. German Aortic Valve Score: a new scoring system for prediction of mortality related to aortic valve procedures in adults. Eur J Cardiothorac Surg. 2013;43:971–977. [DOI] [PubMed] [Google Scholar]
- 23. Iung B, Laouenan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau‐Gouge P, Fajadet J, Leprince P, Leguerrier A, Lievre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M; FRANCE 2 Investigators . Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–1023. [DOI] [PubMed] [Google Scholar]
- 24. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry . Development and validation of a risk prediction model for in‐hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Cox Models for Mortality Within 30‐Day Post‐TAVR Adjusted for Clinical Variables
Table S2. Cox Models for Readmission Within 30‐Day of Post‐TAVR Adjusted for Clinical Variables
Figure S1. Models for mortality within 30‐day of post‐TAVR.
Figure S2. Models for readmission within 30‐day of post‐TAVR.


