Abstract
Obesity, and associated metabolic syndrome, have been identified as primary risk factors for the development of knee osteoarthritis (OA), representing nearly 60% of the OA patient population. In this study, we sought to determine the effects of prebiotic fibre supplementation, aerobic exercise, and the combination of the two interventions, on the development of metabolic knee osteoarthritis in a high-fat/high-sucrose (HFS) diet-induced rat model of obesity. Twelve-week-old male Sprague-Dawley rats were randomized into five groups: a non-exercising control group fed a standard chow diet, a non-exercising group fed a HFS diet, a non-exercising group fed a HFS diet combined with prebiotic fibre supplement, an exercise group fed a HFS diet, and an exercise group fed a HFS diet combined with prebiotic fibre supplement. Outcome measures included knee joint damage, percent body fat, insulin sensitivity, serum lipid profile, serum endotoxin, serum and synovial fluid cytokines and adipokines, and cecal microbiota. Prebiotic fibre supplementation, aerobic exercise, and the combination of the two interventions completely prevented knee joint damage that is otherwise observed in this rat model of obesity. Prevention of knee damage was associated with a normalization of insulin resistance, leptin levels, dyslipidemia, gut microbiota, and endotoxemia in the HFS-fed rats.
Introduction
Obesity, and associated metabolic syndrome, have been identified as primary risk factors for the development of knee osteoarthritis (OA)1,2. OA linked to obesity has been defined as a separate “metabolic OA” phenotype3, and this phenotype represents nearly 60% of the OA patient population4. Metabolic OA is associated with large visceral fat depots deposits5 that release inflammatory cytokines/adipokines resulting in low-level systemic inflammation6,7. Exposing rats to a high-fat/high-sucrose (HFS) diet leads to dysbiosis (impaired gut microbiota)8, increased body fat, systemic inflammation, knee joint inflammation9, intramuscular fat deposition8,10, and knee damage in rats within 12-weeks9 (for a detailed review see Collins et al.11, and Courties et al.2).
In many cases, obesity is considered a modifiable risk factor12. Exercise is known as an effective modulator of body fat, systemic inflammation, and blood lipid levels13–16. Similarly, dietary prebiotic fibre supplementation has been shown to decrease body fat and increase metabolic health in rodents17,18 and humans19. For example, exercise has been suggested to enhance the ability of skeletal muscles to use lipids, thus allowing for better control of plasma lipid levels13; while the fermentation of oligofructose (OFS) prebiotic fibre, a non-digestible plant-derived carbohydrate20, by the gut microbiota leads to the production of short-chain fatty acids (SCFA) in the colon, which bind to fatty acid receptors and stimulate hormones associated with a reduction in appetite, delay of gastric emptying, and improvement of insulin sensitivity21,22. However, there are no studies to date that have examined the effects of exercise and dietary intervention in the knee joint during obesity development (spontaneous knee OA in the metabolic OA phenotype). We hypothesize that prebiotic fibre supplementation, an aerobic exercise regime, and the combination of prebiotic fibre and aerobic exercise will prevent OA-like damage in the knee of rats fed a HFS diet by controlling inflammation, dysbiosis, and metabolic syndrome.
In this study, we determined the effects of prebiotic fibre supplementation, aerobic exercise, and the combination of prebiotic fibre and aerobic exercise on the development of metabolic knee OA in a HFS diet-induced rat model of obesity. Our findings, confirmed our hypotheses, indicating that prebiotic fibre, aerobic exercise, and the combination of prebiotic fibre and aerobic exercise prevented the development of OA-like damage that is observed in rats on a HFS diet that are not receiving these interventions. We defined OA-like damage as a disorder that results in structural changes to joint integrity involving cartilage, subchondral bone, menisci, and/or synovium.
Results
Prebiotic fibre and aerobic exercise prevent OA-like damage to the knee of rats exposed to a HFS diet
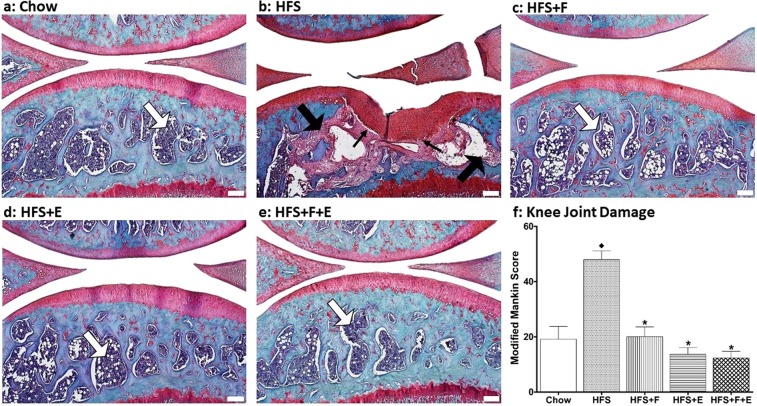
The HFS obesity-inducing diet disrupts knee joint homeostasis of male Sprague Dawley rats resulting in structural damage to the joint (F(4,51) = 22.387, p < 0.001). Our most prominent finding is that the subchondral bone in rats fed a HFS diet was replaced by a fibrotic tissue with consequent collapse of the adjacent cartilage, which is a novel contribution to the literature in this area (Fig. 1). The thick arrow on Fig. 1b indicates that normal bone has been replaced by fibrotic tissue in rats fed a HFS diet. The fibrous tissue extends to the tidemark in some places (Fig. 1b, thin arrow), and the loss of subchondral bone likely leads to the collapse of the bone and the adjacent cartilage. These changes were observed in the medial tibial plate, and medial/posterior femoral condyle of 75% (9 out 12) of rats fed a HFS diet. The other 3 rats presented changes in the subchondral bone, with thickening of the cartilage, but without the collapse (Supplementary Material Fig. 1). We have also observed thickening of the synovium in HFS fed rats when compare to chow rats (Supplementary Material Fig. 2).
Figure 1.
Knee joint integrity. White thick arrows indicate healthy bone marrow. Black thick arrows indicate that the normal marrow has been replaced with a fibrotic marrow. Black thin arrows indicate that the fibrous and granulation tissue extended to the subchondral bone and to the tidemark. ♦Indicates difference from chow group, while *Indicates difference from HFS group. Chow: chow control diet; HFS: high fat/high sucrose diet; F: prebiotic fibre; E: aerobic exercise. Values are means ± 1 SEM (white line, bar = 200 µm).
We found that the prebiotic and exercise interventions, and the combination of the two, fully protected the knee from joint damage (all p < 0.001), despite the intake of a HFS diet (Fig. 1, Table 1).
Table 1.
Knee joint damage, percent body fat, synovial fluid leptin, endotoxin, composite insulin sensitivity index, serum lipid profiles, and cecal microbiota for rats treated in the chow, HFS, HFS + F, HFS + E, HFS + F + E groups at the end of the experimental protocol.
| Chow | HFS | HFS + F | HFS + E | HFS + F + E | Two way Anova | One way Anova | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | E | F * E | Adjusted R2 | p-value | partial η2 | Observed Power | |||||||||
| p-value | partial η2 | p-value | partial η2 | p-value | partial η2 | ||||||||||
| Knee joint integrity | 19.1 ± 4.7 | 47.9 ± 3.2 | 20.1 ± 3.5 | 13.6 ± 2.4 | 12.3 ± 2.4 | <0.001 | 0.361 | <0.001 | 0.539 | <0.001 | 0.318 | 0.666 | <0.001 | 0.637 | 1.000 |
| Percent body fat, % | 19.0 ± 1.1 | 29.9 ± 1.9 | 26.9 ± 1.6 | 26.1 ± 1.6 | 21.6 ± 2.0 | 0.045 | 0.088 | 0.016 | 0.125 | 0.690 | 0.004 | 0.141 | 0.001 | 0.299 | 0.962 |
| SF: leptin, pg/mL | 63.8 ± 8.5 | 251.8 ± 48.7 | 130.5 ± 20.7 | 125.4 ± 24.1 | 113.1 ± 18.3 | 0.045 | 0.092 | 0.019 | 0.125 | 0.098 | 0.064 | 0.188 | 0.001 | 0.304 | 0.959 |
| Endotoxin, EU/ML | 4.3 ± 0.6 | 8.2 ± 0.9 | 3.7 ± 0.6 | 3.4 ± 0.5 | 4.2 ± 0.6 | 0.006 | 0.161 | 0.002 | 0.200 | <0.001 | 0.272 | 0.441 | <0.001 | 0.432 | 0.999 |
| CISI | 0.60 ± 0.08 | 0.25 ± 0.04 | 0.52 ± 0.04 | 0.54 ± 0.07 | 0.56 ± 0.06 | 0.011 | 0.153 | 0.003 | 0.203 | 0.016 | 0.14 | 0.315 | 0.001 | 0.343 | 0.976 |
| Total Cholesterol (mmol/L) | 1.40 ± 0.10 | 1.94 ± 0.10 | 1.69 ± 0.11 | 1.85 ± 0.08 | 1.56 ± 0.08 | 0.005 | 0.179 | 0.244 | 0.033 | 0.792 | 0.002 | 0.156 | 0.001 | 0.36 | 0.956 |
| HDL Cholesterol (mmol/L) | 1.12 ± 0.16 | 1.67 ± 0.09 | 1.70 ± 0.11 | 1.64 ± 0.05 | 1.46 ± 0.06 | 0.359 | 0.019 | 0.108 | 0.058 | 0.208 | 0.036 | 0.044 | 0.001 | 0.297 | 0.96 |
| LDL Cholesterol (mmol/L) | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.02 ± 0.02 | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.230 | 0.033 | 0.567 | 0.008 | 0.930 | <0.001 | 0.026 | 0.768 | 0.035 | 0.148 |
| Non-HDL Cholesterol (mmol/L) | 0.24 ± 0.03 | 0.34 ± 0.05 | 0.22 ± 0.05 | 0.24 ± 0.04 | 0.18 ± 0.03 | 0.031 | 0.160 | 0.091 | 0.102 | 0.512 | 0.016 | 0.183 | 0.052 | 0.248 | 0.67 |
| Triglycerides (mmol/L) | 0.64 ± 0.09 | 1.58 ± 0.16 | 0.79 ± 0.11 | 0.81 ± 0.07 | 0.71 ± 0.07 | <0.001 | 0.310 | <0.001 | 0.291 | 0.002 | 0.207 | 0.501 | <0.001 | 0.543 | 1.000 |
| Ratio: Total Chol/HDL | 1.10 ± 0.03 | 1.17 ± 0.04 | 1.05 ± 0.01 | 1.13 ± 0.02 | 1.06 ± 0.02 | 0.003 | 0.195 | 0.545 | 0.009 | 0.345 | 0.022 | 0.158 | 0.031 | 0.198 | 0.741 |
| Ratio: Tryg/HDL | 0.50 ± 0.06 | 1.03 ± 0.21 | 0.48 ± 0.03 | 0.50 ± 0.04 | 0.49 ± 0.05 | 0.022 | 0.122 | 0.032 | 0.107 | 0.025 | 0.117 | 0.240 | 0.002 | 0.296 | 0.941 |
| Cecal Microbiota | |||||||||||||||
| Bacteroides/Prevotella | 11.91 ± 1.48 | 4.26 ± 0.39 | 13.75 ± 2.46 | 5.95 ± 0.49 | 12.78 ± 2.26 | <0.001 | 0.344 | 0.835 | 0.001 | 0.437 | 0.014 | 0.306 | <0.001 | 0.346 | 0.988 |
| Bifidobacterium | 0.08 ± 0.02 | 0.26 ± 0.05 | 11.01 ± 1.91 | 0.21 ± 0.05 | 10.87 ± 1.40 | <0.001 | 0.649 | 0.934 | <0.001 | 0.973 | <0.001 | 0.625 | <0.001 | 0.680 | 1.000 |
| Enterobacteriaceae | 0.16 ± 0.03 | 0.85 ± 0.19 | 0.82 ± 0.13 | 0.52 ± 0.10 | 0.34 ± 0.08 | 0.412 | 0.015 | 0.003 | 0.178 | 0.551 | 0.008 | 0.139 | 0.001 | 0.292 | 0.956 |
| Lactobacillus | 3.65 ± 0.63 | 0.36 ± 0.11 | 3.07 ± 0.56 | 0.26 ± 0.07 | 2.37 ± 0.32 | <0.001 | 0.552 | 0.224 | 0.033 | 0.368 | 0.018 | 0.532 | <0.001 | 0.569 | 1.000 |
| C. coccoides (cluster XIV) | 7.20 ± 0.43 | 7.99 ± 0.63 | 8.07 ± 1.12 | 9.97 ± 0.67 | 8.03 ± 1.02 | 0.301 | 0.024 | 0.279 | 0.027 | 0.260 | 0.029 | 0.013 | 0.256 | 0.097 | 0.397 |
| C. leptum (cluster IV) | 14.08 ± 0.97 | 15.36 ± 1.70 | 3.49 ± 1.04 | 12.58 ± 1.14 | 4.27 ± 0.72 | <0.001 | 0.615 | 0.410 | 0.015 | 0.147 | 0.047 | 0.598 | <0.001 | 0.640 | 1.000 |
| C. cluster I | 1.23 ± 0.18 | 1.19 ± 0.24 | 0.50 ± 0.04 | 0.86 ± 0.07 | 0.44 ± 0.04 | <0.001 | 0.296 | 0.141 | 0.049 | 0.309 | 0.024 | 0.285 | <0.001 | 0.360 | 0.992 |
| C. cluster XI | 0.11 ± 0.01 | 0.30 ± 0.07 | 0.18 ± 0.05 | 0.28 ± 0.07 | 0.08 ± 0.02 | 0.006 | 0.157 | 0.264 | 0.028 | 0.499 | 0.010 | 0.128 | 0.017 | 0.208 | 0.810 |
| Roseburia | 0.66 ± 0.32 | 0.24 ± 0.08 | 1.98 ± 0.24 | 0.14 ± 0.05 | 1.91 ± 0.32 | <0.001 | 0.624 | 0.679 | 0.004 | 0.936 | <0.001 | 0.599 | <0.001 | 0.577 | 1.000 |
| Methanobrevibacter | 0.01 ± 0.00 | 0.01 ± 0.00 | <0.001 | 0.01 ± 0.00 | 0.00 ± 0.00 | <0.001 | 0.592 | 0.980 | <0.001 | 0.111 | 0.057 | 0.575 | <0.001 | 0.594 | 1.000 |
| Akkemansia muciniphila | 1.08 ± 0.50 | 1.25 ± 0.26 | 0.21 ± 0.11 | 1.21 ± 0.25 | 0.14 ± 0.06 | <0.001 | 0.407 | 0.786 | 0.002 | 0.934 | <0.001 | 0.367 | 0.001 | 0.300 | 0.963 |
| Faecalibacterium prausnitzii | 0.31 ± 0.05 | 0.95 ± 0.23 | 0.21 ± 0.08 | 0.52 ± 0.11 | 0.23 ± 0.05 | <0.001 | 0.248 | 0.139 | 0.049 | 0.107 | 0.058 | 0.26 | 0.001 | 0.314 | 0.973 |
| Collinsella aerofaciens | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.0.01 | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.301 | 0.024 | 0.200 | 0.037 | 0.118 | 0.055 | 0.047 | 0.185 | 0.112 | 0.462 |
Values are means ± SEM. Cecal microbiota data are shown as means ± SEM expressed as the relative abundance (%) of bacterial taxa per total bacteria (16S rRNA gene copies of microbial group/total bacteria 16S rRNA gene copies). Chow: chow control diet; HFS: high fat/high sucrose diet; F: prebiotic fibre; E: aerobic exercise. HDL: high density lipoprotein; LDL: low density lipoprotein; Chol: cholesterol. Trig: triglycerides.
The combination of prebiotic fibre and aerobic exercise prevents increases in percent body fat in rats exposed to a HFS diet
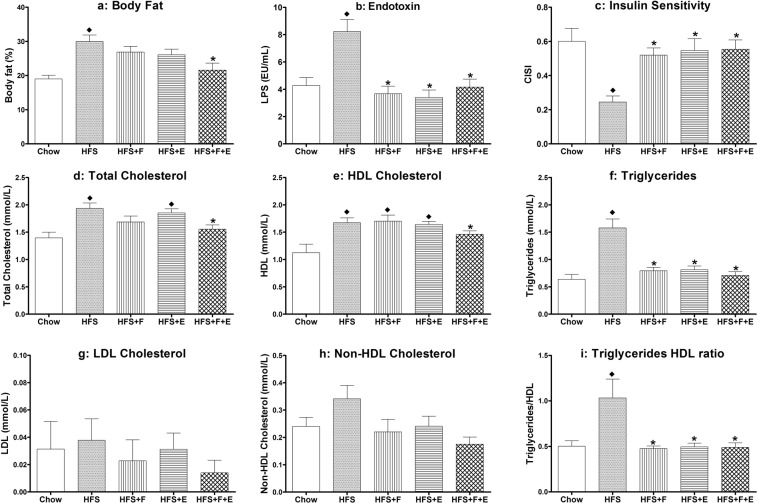
Absolute percent body fat in rats in the HFS group was 11% higher than rats rats fed a standard chow control diet (p = 0.002, Fig. 2), and 8% higher than HFS fed rats that received prebiotic fibre supplementation and undertook aerobic exercise at the same time (HFS + F + E; p = 0.013). There were no statistically significant differences for percent body fat between the chow, HFS combined with prebiotic fibre supplementation (HFS + F), HFS combined with aerobic exercise (HFS + E), and HFS + F + E group animals (Fig. 2, Table 1). Additionally, percent body fat had a positive association with knee joint damage (Table 2).
Figure 2.
Metabolic profile. Body fat, endotoxin, composite insulin sensitivity index, serum lipid profile for rats in the chow, HFS, HFS + F, HFS + E, HFS + F + E groups at the end of the experimental protocol. ♦Indicates difference from chow group, while *indicates difference from HFS group. Chow: chow control diet; HFS: high fat/high sucrose diet; F: prebiotic fibre; E: aerobic exercise. Values are means ± 1 SEM.
Table 2.
Relationship between knee joint damage (Modified Mankin Score), rat metabolic profile and cecal microbiota.
| Knee Joint Damage | Knee Joint Damage | ||||
|---|---|---|---|---|---|
| r | p-value | r | p-value | ||
| Percent body fat | 0.327 | 0.014 | Bacteroides/Prevotella | −0.328 | 0.013 |
| SF: leptin | 0.451 | 0.001 | Bifidobacterium | −0.320 | 0.016 |
| Serum: leptin | 0.389 | 0.003 | Enterobacteriaceae | 0.210 | 0.121 |
| Endotoxin | 0.455 | <0.001 | Lactobacillus | −0.125 | 0.359 |
| CISI | −0.327 | 0.010 | C. coccoides (cluster XIV) | −0.020 | 0.885 |
| Total cholesterol | 0.343 | 0.012 | C. leptum (cluster IV) | 0.436 | 0.001 |
| HDL-cholesterol | 0.230 | 0.087 | C. cluster I | 0.226 | 0.094 |
| LDL-cholesterol | 0.264 | 0.052 | C. cluster XI | 0.206 | 0.127 |
| Non-HDL cholesterol | 0.399 | 0.015 | Roseburia | −0.294 | 0.028 |
| Triglycerides | 0.546 | <0.001 | Methanobrevibacter | 0.237 | 0.079 |
| Ratio: Total Chol/HDL | 0.220 | 0.116 | Akkemansia muciniphila | 0.307 | 0.021 |
| Ratio: Trig/HDL | 0.330 | 0.017 | Faecalibacterium prausnitzii | 0.457 | <0.001 |
| Collinsella aerofaciens | 0.008 | 0.953 | |||
r: Pearson’s correlation coefficient; SF: synovial fluid; CISI: composite insulin sensitivity index; HDL: high density lipoprotein; LDL: low density lipoprotein; Chol: cholesterol. Trig: triglycerides.
On average, body mass in the beginning of the experimental protocol was 469 g per rat, and it increased for all groups of rats during the experimental period. HFS fed rats had an increase in body mass of 56% from the baseline measurements, while chow, HFS + F, HFS + E, and HFS + F + E rats increased 36%, 41%, 34%, and 32%, respectively (F(4,51) = 13.830, p < 0.001).
Prebiotic fibre and aerobic exercise prevent increases in local and systemic leptin concentration levels in rats exposed to a HFS diet
Rats in the HFS group had higher leptin levels in knee synovial fluid than did rats in the chow, HFS + F, HFS + E, and HFS + F + E groups (p = 0.001, p = 0.034, p = 0.040, p = 0.014, respectively), indicating that the interventions prevented the increases in synovial fluid leptin levels detected in the untreated obese animals (Table 1). Serum leptin levels exhibited the same pattern as the synovial fluid leptin. The HFS group animals had higher serum leptin levels than did the chow group rats (p = 0.006). Prebiotic fibre, aerobic exercise, and the combination of both prevented increases in serum leptin in rats on the HFS diet (p = 0.010, p = 0.038, and p = 0.019, respectively, Supplementary Material Table 2). Additionally, synovial fluid and serum leptin exhibited a positive association with knee joint damage (Table 2).
Overall, the other 26 biomarkers assessed in the synovial fluid and serum did not differ between groups (Supplementary Material Table 2).
Prebiotic fibre and aerobic exercise prevent decreases in insulin sensitivity of rats exposed to a HFS diet
HFS fed rats had decreased whole body insulin sensitivity compared to rats fed a chow control diet (p = 0.001). Prebiotic fibre, aerobic exercise, and the combination of the two normalized insulin sensitivity to levels found in the chow group rats (p = 0.004, p = 0.011, p = 0.003, compared to HFS group, respectively; Fig. 2, Table 1). Insulin sensitivity exhibited a negative association with knee joint damage (Table 2).
Prebiotic fibre and aerobic exercise improve serum lipid profile of rats fed a HFS diet
The HFS diet led to increased serum total cholesterol, HDL-cholesterol, triglycerides, and the triglycerides to HDL-cholesterol ratio compared to rats fed a chow diet (Fig. 2). The combined intervention of prebiotic fibre and exercise prevented these increases in serum total cholesterol and HDL-cholesterol levels (Table 1, Fig. 2). Prebiotic fibre supplementation, exercise, and the combination of the two prevented increases in serum triglyceride levels and increases in the serum triglycerides to HDL-cholesterol ratio (Fig. 2, Table 1). Triglycerides levels exhibited a strong positive association with knee joint damage, while total cholesterol and non-HDL-cholesterol exhibited a moderate positive association with knee joint damage, and HDL- and LDL-cholesterol exhibited weak positive associations with knee joint damage (Table 2).
Prebiotic fibre and aerobic exercise prevent increases in serum endotoxin in rats exposed to a HFS diet
The HFS diet led to increased serum endotoxin levels (p = 0.002) compared to chow fed rats. Prebiotic fibre, aerobic exercise, and the combination of exercise and prebiotic fibre prevented these increases (p < 0.001, p < 0.001, and p < 0.001, respectively), maintaining values similar to those in chow fed control rats (Fig. 2b, Table 1). Endotoxin levels exhibited a positive association with knee joint damage (Table 2).
Prebiotic fibre, but not exercise, prevents microbial dysbiosis in rats fed a HFS diet
The cecal microbiota composition of the HFS rats showed a decrease in Bacteroides/Prevotella (p = 0.048), Lactobacillus (p < 0.001), and Faecalibacterium prausnitzii (p = 0.024) compared to chow fed rats that was prevented by prebiotic fibre supplementation, but not by exercise; and an increase in Enterobacteriaceae (p = 0.010), that was prevented by the combination of prebiotic fibre and exercise (Fig. 3, Table 1). Prebiotic fibre supplementation, and the combination of prebiotic fibre and aerobic exercise, shifts the microbiota towards a decrease in Clostridium leptum (cluster IV), Clostridium cluster I, Methanobrevibacter, and Akkemansia muciniphila; and an increase in Bifidobacterium and Roseburia compared to the cecal microbiota of HFS rats (p < 0.05, Fig. 3, Table 1). Bacteroides/Prevotella, Bifidobacterium and Roseburia exhibited a negative association with knee joint damage, while Clostridium leptum (cluster IV), Akkemansia muciniphila and Faecalibacterium prausnitzii exhibited a positive association with knee joint damage (Table 2).
Figure 3.
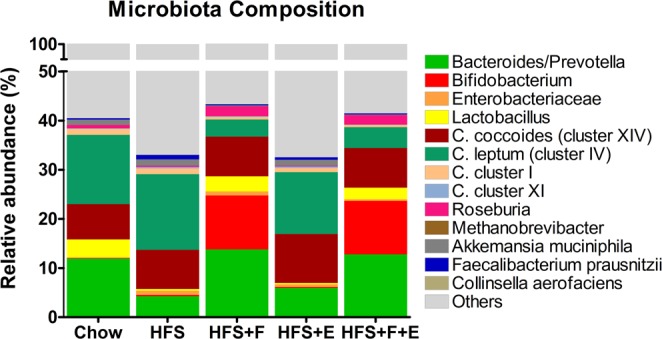
Microbiota composition. Cecal matter microbiota composition at the end of the experimental protocol. Values are mean relative abundance of cecal microbiota. Microbial abundance was measured as 16S rRNA gene copies per 20 ng DNA and reported here as relative abundance (%) of bacterial taxa per total bacteria. Chow: chow control diet; HFS: high fat/high sucrose diet; F: prebiotic fibre; E: aerobic exercise.
Discussion
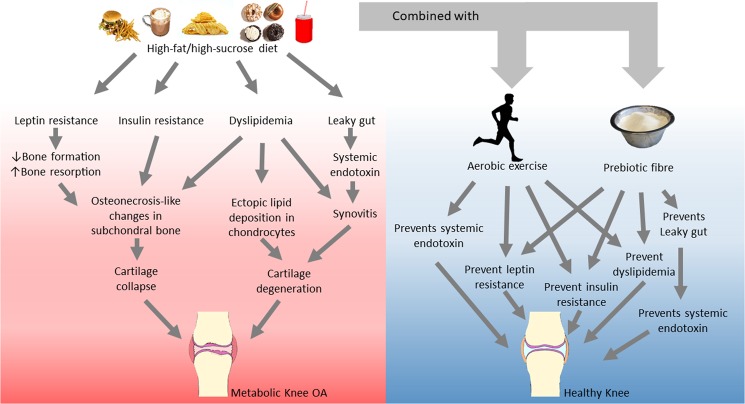
We showed that prebiotic fibre supplementation and aerobic exercise protect against the development of knee joint damage in rats fed a HFS diet. The maintenance of knee joint integrity achieved with prebiotic fibre and aerobic exercise was accompanied by a maintenance of metabolic and gut microbiota homeostasis. It appears that the obesity-related metabolic dysregulation and gut microbiota dysbiosis might be strongly linked to the metabolic OA phenotype in the Sprague Dawley rat model (see schematic in Fig. 4).
Figure 4.
Diagram summarizing the findings of the present study. Model for the link between HFS diet and metabolic knee OA, and the pathways which prebiotic fibre and aerobic exercise might have activated to prevent damage of the knee joint in Sprague Dawley rats fed a HFS diet. Photographs are under Public Domain or CC0 Creative Commons License.
Majority of the damage detected in the knee joint of HFS fed rats occurred in the subchondral bone, and the structural changes observed in this study are similar to changes reported in an emu femoral head model of osteonecrosis23, suggesting a potential initiating role for subchondral bone changes in OA pathogenesis associated with the HFS diet. Such a role has been advanced previously in the literature as a result of reduced blood supply and compromised nutrient exchange to the bone2,3.
The metabolic OA phenotype is associated with hyperglycaemia, insulin resistance, and leptin resistance, which in turn are linked to bone formation/structure2,3,24–27. Hyperglycaemia and insulin resistance combined with increased leptin levels may have resulted in some of the changes observed in the subchondral bone of the HFS animals. Additionally, altered lipid metabolism in patients has also been associated with OA development in metabolic disease3,28. Rats fed the HFS diet had an altered lipid metabolism, as indicated by the increase in serum triglyceride levels and serum triglycerides to HDL-cholesterol ratio, and we detected a strong association between triglycerides levels and knee joint damage (Table 2).
The prebiotic oligofructose fibre supplementation used in our study prevented dyslipidemia, thereby potentially contributing to the maintenance of knee joint homeostasis. It has been shown that drugs that lower lipid levels inhibit OA development in a mouse model of atherosclerosis29. Elevated plasma cholesterol levels trigger oxidative stress in mitochondria and degradative changes in chondrocytes that, in combination, are thought to lead to OA development and progression30. Moreover, mouse models of hypercholesterolemia-induced OA are characterized by changes in subchondral bone architecture that are rescued when lipid-lowering drugs are administrated30. It has been suggested that hypercholesterolemia induces oxidation and deposition of lipids in tissues, resulting in damage to cartilage similar to that seen in atherosclerosis31, while osteonecrosis might occur because of a narrowing of the vasculature by lipids.
Obesity has been associated with increased gut permeability, allowing bacterial lipopolysaccharides from Gram-negative bacteria to translocate into the blood (endotoxemia), triggering systemic inflammation and insulin resistance21,32. It has been shown that improvements in glucose tolerance in rodents after prebiotic fibre supplementation are related to changes in the microbiota33,34. We made similar observations regarding changes in microbiota. Increases in the inflammation-associated Enterobacteriaceae35 were prevented by the combination of prebiotic fibre and exercise only. Prebiotic fibre supplementation also increased Bifidobacterium compared to HFS fed rats. Bifidobacterium has been shown to decrease intestinal permeability, upregulate expression of tight junction proteins and reduce IL-6 and TNF-α in cell lines and in rodents36. Given that oligofructose is known to increase the abundance of Bifidobacterium, it is possible that the prebiotic fibre reduced serum endotoxin levels via this mechanism. Furthermore, certain lactobacilli strains have been associated with health-promoting properties, including attenuated weight gain and improved gut barrier function37, which suggest that the HFS fed animals may have increased gut permeability due in part to the decrease in lactobacilli abundance.
Moreover, in agreement with our finding, it has been shown in a mouse model of high-fat diet–induced obesity that prebiotic fibre supplementation has a protective effect against trauma- induced OA. Manipulation of the gut microbiota is thought to represent a viable disease-modifying therapeutic strategy for OA associated with obesity38.
The aerobic exercise used in this study consisted of a progressive treadmill training of up to 150 minutes week. This amount of exercise corresponds to the recommended minimal physical activity guidelines for humans39, and has been shown to be safe for Sprague Dawley knees40. Aerobic exercise in our rats was found to modulate insulin and leptin levels, similar to what has been observed for the prebiotic fibre supplementation. Aerobic exercise in humans is also associated with decreased plasma leptin and insulin levels41. Thus, maintenance of insulin homeostasis in the exercising rats may have prevented increases in leptin and leptin resistance in rats fed a HFS diet, despite similar body fat percentages in HFS + E and HFS rats. Therefore, aerobic exercise may have helped maintain bone turnover homeostasis, thereby preventing the development of OA-like damage in the knees of rats fed the HFS diet.
Aerobic exercise also prevented increases in serum triglyceride levels and serum triglycerides to HDL-cholesterol ratio observed in rats exposed to a HFS diet. These results agree with findings from human studies. For example, it has been shown that there is a dose-response relationship between physical activity and improvements in triglycerides and HDL-cholesterol levels in previously sedentary people42. However, the mechanisms underlying these improvements are unclear. By preventing dyslipidemia, aerobic exercise may have contributed to the maintenance of knee joint integrity in the HFS rats.
Aerobic exercise also prevented endotoxemia in rats fed a HFS diet. However, the pathway might not be the same as that suggested for the prebiotic fibre intervention. Endotoxin, an enteric bacteria-derived molecule, is recognized by Toll-like receptors (TLRs) on immune cells, adipocytes, and cells in several organs, such as Kupffer cells in the liver, and when in excess, triggers an inflammatory response43. The capacity of Kupffer cells to remove endotoxin might be important in the prevention of metabolic knee OA. Aerobic exercise has been shown to improve the removal of endotoxins by Kupffer cells44, thereby contributing to the reduction of endotoxemia in rats fed a HFS diet, and possibly preventing the OA-like damage observed in the rat knee.
While prebiotic fibre supplementation and aerobic exercise prevented the development of knee damage, and affected the metabolic outcome variables to a similar extent, exercise did not affect the gut microbiota, while the prebiotic fibre intervention did. Therefore, it appears that the prebiotic intervention affected the microbiota, and as a consequence, the host secondarily via the fermentation of the prebiotic fiber, short chain fatty acids21 (not assessed in our study). In contrast, the exercise protocol primarily affected host parameters to diminish the impact of the HFS diet and the altered microbiota. Thus, the exercise protocol appeared to impact the metabolic profile and the gut leaky syndrome by increasing the uptake of endotoxins by Kupffer cells44. Aerobic exercise combined with prebiotic fibre supplementation was not more effective as each intervention alone (Fig. 4). Therefore, it appears that the mechanisms involved for each intervention complement each other but are not additive. Therefore, the impact of the HFS diet on a sedentary host is different than its impact on a host that is exercising and enhancing the resistance of host systems. Exercise is expect to contribute to tissue repair, enhance the metabolism of glucose and fat, and enhance the release of exercise-associated regulatory molecules from the brain to counteract diet-induced factors45. It is known that mechanical loading of tissues, such as cartilage, ligaments, tendons, and skin, can lead to resistance to catabolic mediators46 or their expression47. Therefore, exercise-induced tissue loading may result in the maintenance of tissue integrity. The non-exercised rats in this study had reduced tissue loads compared to exercised rats, and their systems might have been at an increased risk for alteration caused by the HFS diet. It appears that exercise and prebiotic fibre supplementation impact HFS fed differently. Providing both interventions simultaneously may lead to a reinforcement of resistance to the adverse effects of the HFS diet over 12 weeks.
In summary, a HFS diet led to development of knee joint damage in rats that was associated with changes in the metabolic profile in these animals. We provide novel information on the prevention of metabolic OA using a prebiotic fibre and/or an aerobic exercise intervention (Fig. 4). We suggest that the preventive effects of the prebiotic fibre and aerobic exercise interventions are associated with the leptin and insulin pathways, as well as with lipid metabolism and the leaky gut syndrome. Effectively addressing the prevention of metabolic osteoarthritis will require the engagement of public health programmes. Low cost, non-invasive treatments strategies, such as prebiotic fibre supplementation and aerobic exercise should be a priority.
Methods
Animals and intervention protocol
Forty-eight male, 12-week old, Sprague Dawley rats, fed a high-fat/high-sucrose diet (HFS, 20% of total weight as fat, 50% sucrose, 20% protein, and 10% from fibre and micronutrients; custom Diet #102412, Dyets, United States) were randomized into a sedentary (HFS, n = 12), aerobic exercise (HFS + E, n = 12), prebiotic fibre supplementation (HFS + F, n = 12), or aerobic exercise combined with prebiotic fibre supplementation (HFS + F + E, n = 12) group for 12 weeks. Eight chow-fed (standard chow diet control group, 5% of total weight as fat, 47.5% carbohydrates (only 4% from sucrose), 25% protein, 12.5% from fibre and micronutrients, and 10% moisture; Lab Diet 5001, United States, n = 8), age- and sex-matched animals were included as controls. The aerobic exercise intervention consisted of a progressive moderate treadmill training program for 12 weeks, up to 30 minutes per day, 5 days a week as described previously (Supplementary Material Table 1)40,48. The prebiotic fibre was supplemented in the diet at a dose of 10% (wt/wt, Orafti P95, BENEO-Orafti, Germany)18,49. Calculation of sample size was performed using G*Power Software (version 3.0.10, Germany)50. Data for sample size calculations were based on a previous study with HFS and control rats9. All experiments were approved by the University of Calgary Life and Environmental Sciences Animal Care Committee, and all methods were conducted in accordance with the animal welfare regulations and guidelines at the University of Calgary.
Body composition
Three days after completing the 12 week intervention protocol, rats were lightly anaesthetized with isoflurane and body composition was measured using Dual X-ray absorptiometry (DXA) with software for small animals (Hologic ODR 4500; Hologic, Bedford, MA, USA). The mean of three repeat scans for each animal were used for analysis.
Body mass was measured at the beginning of each week. Body mass for each animal was normalized to that of week 1 (familiarization week) and was expressed as the percent increase in body mass from that initial value.
Blood glucose and insulin
One day after the end of the intervention period, following 16 h of food deprivation, rats were given an oral gavage of 2 g/kg glucose. Blood was collected at 0, 15, 30, 60, and 120 min post-gavage via tail nick in a chilled tube for insulin analysis. Blood glucose was measured immediately with a blood glucose meter (OneTouch Verio and Blood Glucose Monitoring System, Lifescan, Switzerland). Whole body insulin sensitivity was determined using proxy measures from the glucose tolerance tests (composite insulin sensitivity index – CISI)51.
Blood lipid profile and endotoxin
One week prior to the start of the 12 week intervention protocol (acclimatization week), following 16 h of food deprivation, blood was collected via tail nick to evaluate the baseline values for cytokines/adipokines (details below). Four days after completing the 12 week intervention protocol, and following 16 h of food deprivation, rats were anaesthetized with isoflurane and cardiac blood samples were collected. Serum was analyzed for lipid profile (total cholesterol, LDL-cholesterol, HDL-cholesterol, and triglycerides) using colorimetric assays (Calgary Lab Service, Calgary, AB, Canada), cytokines/adipokines (details below), and for endotoxin. Concentration of blood endotoxin was measured using the Pierce LAL Chromogenic Endotoxin Quantification assay (Thermo Fisher Scientific, MA, USA) according to manufacturer directions.
Blood and synovial fluid cytokines and adipokines
The right knee joints were opened to collect synovial fluid shortly after sacrifice using the Whatman chromatography paper method52. Samples were weighed, diluted and centrifuged40. Serum and synovial fluid cytokines and adipokines were quantified using a Rat 27 Multiplex Discovery Assay with Luminex®xMAP technology (Eotaxin, EGF, Fractalkine, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, IL-17A, IL-18, IP-10/CXCL10, GRO/KC, IFN-γ, TNF-α, G-CSF, GM-CSF, MCP-1, leptin, LIX, MIP-1α, MIP-2, RANTES, VEGF; Eve Technologies, AB, Canada).
Cecal matter microbiota
Microbiota analysis was carried out using 16rRNA qPCR as per our previous work53. Cecal samples were collected at euthanasia for cecal microbiota analysis. DNA was extracted from the cecal samples using the MP Biomedicals Fast DNA Spin Kit for Feces (MP Biomedicals, Lachine, QC, Canada). Amplification and detection were conducted in 96-well plates with SYBR Green 2 × qPCR Master Mix (BioRad). Purified template DNA from reference strains was used to generate standard curves for each primer set using 10-fold serial dilutions of DNA. Standard curves were normalized to copy number of 16S rRNA genes using reference strain genome size and 16S rRNA gene copy number values. Total bacteria were also measured. Primer selection for qPCR was based on providing broad coverage of the total microbial signal in rats and those microbes of relevance to an obese rat model53.
Knee joint histology
The left knee was harvested by cutting the femur and tibia/fibula 2 cm above and below the joint line, respectively. Muscles were removed and joints were fixed in a 10% neutral buffered formalin solution for 14 days at room temperature. Knees were then decalcified, dehydrated in a graded series of alcohols, embedded in paraffin wax, and stored at room temperature until sectioning40. Serial sagittal plane sections of 10 µm thickness were obtained, and mounted. Subsequently, every second section was stained sequentially with haematoxylin, fast green and safranin-O40. Two independent, blinded graders scored all histological sections using a Modified Mankin Histology Scoring System54, and the Osteoarthritis Research Society International’s (OARSI) histologic55 sub-scores for bone changes, synovial thickening and menisci for each joint. Only the worst defect quantified per joint was used for the statistical analyses. The total Modified Mankin Score for each animal was defined as the sum of all individual OARSI sub-scores and the Modified Mankin Score.
Statistical analysis
All data are presented as means ± 1 SEM. Levene’s test for equality of variance was conducted on all outcomes. A one way ANOVA with the five experimental groups as the main factor was performed using Bonferroni post hoc testing to determine differences between groups. A 2-way ANOVA was used to determine the main effects of prebiotic fibre supplementation, aerobic exercise, and their interaction, on the HFS diet. Changes in serum cytokines and adipokines from pre- to post-intervention were determined using a repeated measures ANOVA with time as the within-subject factor and experimental grouping as the between-subject factor. To further explore the changes in serum cytokines and adipokines pre- and post-intervention, data were normalized relative to the pre-intervention values, and a one way ANOVA with experimental grouping as the main factor was performed using Bonferroni post hoc testing. To measure the association between knee joint damage, the metabolic variables, and cecal microbiota data, the Pearson Correlation Coefficient was calculated. Analyses were done using SPSS V22.0 software (SPSS, Chicago, IL, USA). Data were considered statistically significant at p < 0.05, two-tailed.
Supplementary information
Acknowledgements
This work was supported by the Canadian Institutes of Health Research # RT736475 (WH) and MOP 115076, the Canada Research Chair Programme (WH), the Alberta Innovates Health Solutions Osteoarthritis Team Grant (DAH, WH), Alberta Innovates Health Solutions, Killam Foundation (WH), Alberta Innovates (JLR), and the Ministry of Education, Brazil (CAPES Foundation Grant 13157-13-2, JLR). The authors thank Fernando Diefenthaeler, Kristine Lee, Ruth-Anne Seerattan, Seong-won Han, Shuyue Liu, and Venus Joumaa for technical contributions to this paper.
Author Contributions
J.L.R. was responsible for design of the study, execution of the study, data collection, data analysis, interpretation of data, drafting the manuscript, revising the manuscript, and approving the final submitted version of the manuscript under W.H. supervision. K.H.C. and M.B. contributed to data collection, interpretation of data, and critically revising the manuscript and approving the final submitted version. D.A.H. and R.A.R. contributed to interpretation of data, critically revising the manuscript, and approving the final submission. W.H. contributed to study design, interpretation of the data, critically revising the manuscript, and approving the final submission.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40601-x.
References
- 1.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23:1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Courties A, Sellam J, Berenbaum F. Metabolic syndrome-associated osteoarthritis. Curr Opin Rheumatol. 2017;29:214–222. doi: 10.1097/BOR.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 3.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8:729–737. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 4.Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self-reported osteoarthritis: data from the Third National Health and Nutrition Examination Survey. Am J Manag Care. 2002;8:S383–391. [PubMed] [Google Scholar]
- 5.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23:22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Collins KH, et al. A High-Fat High-Sucrose Diet Rapidly Alters Muscle Integrity, Inflammation and Gut Microbiota in Male Rats. Sci Rep. 2016;6:37278. doi: 10.1038/srep37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthritis Cartilage. 2015;23:957–965. doi: 10.1016/j.joca.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Collins KH, et al. High-fat high-sucrose diet leads to dynamic structural and inflammatory alterations in the rat vastus lateralis muscle. J Orthop Res. 2016;34:2069–2078. doi: 10.1002/jor.23230. [DOI] [PubMed] [Google Scholar]
- 11.Collins KH, et al. Obesity, Metabolic Syndrome, and Musculoskeletal Disease: Common Inflammatory Pathways Suggest a Central Role for Loss of Muscle Integrity. Front Physiol. 2018;9:112. doi: 10.3389/fphys.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell A, Teichtahl AJ, Wluka AE, Cicuttini FM. Obesity: a preventable risk factor for large joint osteoarthritis which may act through biomechanical factors. Br J Sports Med. 2005;39:4–5. doi: 10.1136/bjsm.2004.011841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earnest CP, et al. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc. 2013;88:259–270. doi: 10.1016/j.mayocp.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeffery RW, et al. Long-term maintenance of weight loss: current status. Health Psychol. 2000;19:5–16. doi: 10.1037/0278-6133.19.Suppl1.5. [DOI] [PubMed] [Google Scholar]
- 15.Conti, F. F. et al. Positive effect of combined exercise training in a model of metabolic syndrome and menopause: autonomic, inflammatory and oxidative stress evaluations. Am J Physiol Regul Integr Comp Physiol, ajpregu.00076.02015, 10.1152/ajpregu.00076.2015 (2015). [DOI] [PubMed]
- 16.Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 17.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cluny NL, Eller LK, Keenan CM, Reimer RA, Sharkey KA. Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats. Obesity (Silver Spring) 2015;23:769–778. doi: 10.1002/oby.21017. [DOI] [PubMed] [Google Scholar]
- 19.Nicolucci AC, et al. Prebiotics Reduce Body Fat and Alter Intestinal Microbiota in Children Who Are Overweight or With Obesity. Gastroenterology. 2017;153:711–722. doi: 10.1053/j.gastro.2017.05.055. [DOI] [PubMed] [Google Scholar]
- 20.Gibson GR, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura YK, Omaye ST. Metabolic diseases and pro- and prebiotics: Mechanistic insights. Nutr Metab (Lond) 2012;9:60. doi: 10.1186/1743-7075-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conzemius MG, Brown TD, Zhang Y, Robinson RA. A new animal model of femoral head osteonecrosis: one that progresses to human-like mechanical failure. J Orthop Res. 2002;20:303–309. doi: 10.1016/S0736-0266(01)00108-5. [DOI] [PubMed] [Google Scholar]
- 24.Klein GL. Insulin and bone: Recent developments. World J Diabetes. 2014;5:14–16. doi: 10.4239/wjd.v5.i1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srikanthan P, et al. Insulin resistance and bone strength: findings from the study of midlife in the United States. J Bone Miner Res. 2014;29:796–803. doi: 10.1002/jbmr.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shanbhogue VV, Finkelstein JS, Bouxsein ML, Yu EW. Association Between Insulin Resistance and Bone Structure in Nondiabetic Postmenopausal Women. J Clin Endocrinol Metab. 2016;101:3114–3122. doi: 10.1210/jc.2016-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan M, Zhang J, Yang H, Sun Y. The role of leptin in osteoarthritis. Medicine (Baltimore) 2018;97:e0257. doi: 10.1097/MD.0000000000010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baudart P, Louati K, Marcelli C, Berenbaum F, Sellam J. Association between osteoarthritis and dyslipidaemia: a systematic literature review and meta-analysis. RMD Open. 2017;3:e000442. doi: 10.1136/rmdopen-2017-000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gierman LM, et al. Osteoarthritis development is induced by increased dietary cholesterol and can be inhibited by atorvastatin in APOE*3Leiden.CETP mice–a translational model for atherosclerosis. Ann Rheum Dis. 2014;73:921–927. doi: 10.1136/annrheumdis-2013-203248. [DOI] [PubMed] [Google Scholar]
- 30.Farnaghi S, et al. Protective effects of mitochondria-targeted antioxidants and statins on cholesterol-induced osteoarthritis. Faseb J. 2017;31:356–367. doi: 10.1096/fj.201600600R. [DOI] [PubMed] [Google Scholar]
- 31.Sevin G, Yasa M, Akcay DY, Kirkali G, Kerry Z. Different responses of fluvastatin to cholesterol-induced oxidative modifications in rabbits: evidence for preventive effect against DNA damage. Cell Biochem Funct. 2013;31:325–332. doi: 10.1002/cbf.2903. [DOI] [PubMed] [Google Scholar]
- 32.Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cani PD, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 34.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012;107:601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:119–129. doi: 10.1016/j.chom.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Ling X, Linglong P, Weixia D, Hong W. Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS One. 2016;11:e0161635. doi: 10.1371/journal.pone.0161635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20:15632–15649. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schott, E. M. et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight3, 10.1172/jci.insight.95997 (2018). [DOI] [PMC free article] [PubMed]
- 39.De Souto Barreto P. Time to challenge public health guidelines on physical activity. Sports Med. 2015;45:769–773. doi: 10.1007/s40279-015-0326-7. [DOI] [PubMed] [Google Scholar]
- 40.Rios JL, et al. Quantifying the Effects of Different Treadmill Training Speeds and Durations on the Health of Rat Knee Joints. Sports Med Open. 2018;4:15. doi: 10.1186/s40798-018-0127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Essig DA, Alderson NL, Ferguson MA, Bartoli WP, Durstine JL. Delayed effects of exercise on the plasma leptin concentration. Metabolism. 2000;49:395–399. doi: 10.1016/S0026-0495(00)90396-2. [DOI] [PubMed] [Google Scholar]
- 42.Aadahl M, Kjaer M, Jørgensen T. Associations between overall physical activity level and cardiovascular risk factors in an adult population. Eur J Epidemiol. 2007;22:369–378. doi: 10.1007/s10654-006-9100-3. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Komine S, et al. Exercise training enhances in vivo clearance of endotoxin and attenuates inflammatory responses by potentiating Kupffer cell phagocytosis. Sci Rep. 2017;7:11977. doi: 10.1038/s41598-017-12358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips C, Baktir MA, Srivatsan M, Salehi A. Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci. 2014;8:170. doi: 10.3389/fncel.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ballyns JJ, Bonassar LJ. Dynamic compressive loading of image-guided tissue engineered meniscal constructs. J Biomech. 2011;44:509–516. doi: 10.1016/j.jbiomech.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 47.Natsu-Ume T, et al. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10:396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 48.Boldt, K. R., Rios, J. L., Joumaa, V. & Herzog, W. Force properties of skinned cardiac muscle following increasing volumes of aerobic exercise in rats. J Appl Physiol (1985), 10.1152/japplphysiol.00631.2017 (2018). [DOI] [PMC free article] [PubMed]
- 49.Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity (Silver Spring) 2014;22:763–771. doi: 10.1002/oby.20632. [DOI] [PubMed] [Google Scholar]
- 50.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 52.Seifer DR, et al. Novel synovial fluid recovery method allows for quantification of a marker of arthritis in mice. Osteoarthritis Cartilage. 2008;16:1532–1538. doi: 10.1016/j.joca.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bomhof MR, Paul HA, Geuking MB, Eller LK, Reimer RA. Improvement in adiposity with oligofructose is modified by antibiotics in obese rats. Faseb J. 2016;30:2720–2732. doi: 10.1096/fj.201600151R. [DOI] [PubMed] [Google Scholar]
- 54.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. doi: 10.2106/00004623-197153030-00009. [DOI] [PubMed] [Google Scholar]
- 55.Gerwin N, Bendele AM, Glasson S, Carlson CS. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the rat. Osteoarthritis Cartilage. 2010;18(Suppl 3):S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.