Abstract
BACKGROUND
Despite the advent of biological drugs, conventional therapy continues to be used in moderate to severe inflammatory bowel disease (MS-IBD). This study hypothesized that as a standard of treatment and the primary alternative to biologics, conventional therapy should present robust effectiveness results in IBD outcomes.
AIM
To investigate the effectiveness of conventional therapy for MS-IBD.
METHODS
A systematic review with no time limit was conducted in July 2017 through the Cochrane Collaboration, MEDLINE, and LILACS databases. The inclusion criteria encompassed meta-analyses, systematic reviews, randomized clinical trials, observational and case-control studies concerning conventional therapy in adult patients with MS-IBD, including Crohn’s disease (CD) and ulcerative colitis (UC). Corticosteroids (prednisone, hydrocortisone, budesonide, prednisolone, dexamethasone), 5-aminosalicylic acid (5-ASA) derivatives (mesalazine and sulfasalazine) and immunosuppressants [azathioprine (AZA), methotrexate (MTX), mycophenolate, cyclosporine, tacrolimus, 6-mercaptopurine (6-MP)] were considered conventional therapy. The exclusion criteria were sample size below 50; narrative reviews; specific subpopulations (e.g., pregnant women, comorbidities); studies on postoperative IBD; and languages other than English, Spanish, French or Portuguese. The primary outcome measures were clinical remission (induction or maintenance), clinical response and mucosal healing. As secondary outcomes, fecal calprotectin, hospitalization, death, and surgeries were analyzed. The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation criteria.
RESULTS
The search strategy identified 1995 citations, of which 27 were considered eligible (7 meta-analyses, 20 individual studies). For induction of clinical remission, four meta-analyses were selected (AZA and 6-MP showed no advantage over placebo, MTX or 5-ASA in CD; MTX showed no statistically significant difference versus placebo, 6-MP, or 5-ASA in UC; tacrolimus was superior to placebo for UC in two meta-analyses). Only one meta-analysis evaluated clinical remission maintenance, showing no statistically significant difference between MTX and placebo, 5-ASA, or 6-MP in UC. AZA and 6-MP had no advantage over placebo in induction of clinical response in CD. Three meta-analyses showed the superiority of tacrolimus vs placebo for induction of clinical response in UC. The clinical response rates for cyclosporine were 41.7% in randomized controlled trials (RCTs) and 55.4% in non-RCTs for UC. For induction of mucosal healing, one meta-analysis showed a favorable rate with tacrolimus versus placebo for UC. For secondary outcomes, no meta-analyses specifically evaluated fecal calprotectin, hospitalization or death. Two meta-analyses were retrieved evaluating colectomy rates for tacrolimus and cyclosporine in UC. Most of the twenty individual studies retrieved contained a low or very low quality of evidence.
CONCLUSION
High-quality evidence assessing conventional therapy in MS-IBD treatment is scarce, especially for remission maintenance, mucosal healing and fecal calprotectin.
Keywords: Inflammatory bowel diseases, Steroids, Sulfasalazine, Mesalamine, Azathioprine, Methotrexate, Mycophenolic acid, Cyclosporine, Tacrolimus, 6-Mercaptopurine
Core tip: Despite the advent of biological drugs, conventional therapy continues to be used in moderate to severe inflammatory bowel disease (MS-IBD), especially in countries where biologics are not covered by insurance. In this systematic review, the effectiveness of conventional therapy for MS-IBD is assessed. There are few studies concerning objective outcomes, especially for remission maintenance, mucosal healing and fecal calprotectin. Additionally, studies are mainly of very low or low quality. As conventional therapy is usually the main therapy for MS-IBD and biologics are used in patients who fail to respond to conventional drugs, robust studies are required to further our understanding of the effectiveness of conventional therapy because it is prescribed to many IBD patients.
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are the two main disease categories of inflammatory bowel disease (IBD), a group of idiopathic chronic inflammatory conditions affecting the digestive system[1]. Patients with IBD frequently present a lifelong relapsing and remitting course that has a negative impact on health and quality of life, often resulting in long-term sequelae[2]. Most cases, particularly in CD, are moderate to severe at diagnosis, with a tendency for disease activity to fluctuate over time[3]. CD can progress from pure inflammatory lesions to destructive complications such as intestinal perforation, strictures, abscesses and fistula formation, which may result in irreversible bowel damage leading to loss of gastrointestinal tract function and disability that may require hospitalizations and surgical treatment[4,5].
Symptoms of active UC or relapse include bloody diarrhea with or without mucus, abdominal pain and fecal urgency. This disease presents a cyclical course, including phases of exacerbation and remission, with a variable degree of intensity. Patients with extensive or severe inflammation may experience acute complications, such as toxic megacolon and severe bleeding[6,7]. It is expected that up to 19% of patients with UC have severe disease at the time of diagnosis[8]. In Brazil, a country located in a low prevalence area of IBD, 27% and 32% of UC patients presented severe and moderate disease, respectively[9]. The main goal of treatment for IBD is to achieve and maintain disease remission, prevent complications, hospitalization and surgery, and improve health-related quality of life[1,10]. According to Lichtenstein et al[11], for moderate to severe CD, daily prednisone is indicated until resolution of symptoms and resumption of weight gain. Azathioprine (AZA) and 6-mercaptopurine (6-MP) are recommended for the maintenance of steroid-induced remission, and parenteral methotrexate (MTX) is indicated for steroid-dependent and steroid-refractory disease. Patients who are refractory to these agents can be treated with biological therapy, such as infliximab (IFX), adalimumab, certolizumab pegol, ustekinumab and vedolizumab[11]. The conventional therapy for inpatients with severe active UC includes intravenous steroids and monotherapy with intravenous cyclosporin. For patients with steroid-dependent disease or those who are refractory to steroids or immunomodulators, a biological therapy should be considered[2]. In addition to clinical remission, endoscopic remission, expressed as mucosal healing, has become an important endpoint in IBD[12]. This outcome has been correlated with a reduction in surgeries and hospitalizations[13]. Another endpoint recommended by current IBD guidelines is the level of fecal calprotectin, a noninvasive biomarker that has been used to evaluate disease activity in IBD[1,2,13]. The level of this biomarker can be correlated with macroscopic and histological inflammation, as detected by colonoscopy and biopsies[14-17].
Despite the emergence of biological therapy, conventional therapy continues to be prescribed in moderate to severe IBD (MS-IBD), particularly in countries where biologics are not covered by insurance[18,19]. As a standard of treatment and the primary alternative to biologics, conventional therapy should present robust effectiveness results in IBD outcomes. This systematic review aims to investigate data on the efficacy of conventional therapy for MS-IBD.
MATERIALS AND METHODS
Search strategy
A systematic literature review was conducted until July 2017 through MEDLINE databases (via PubMed), Latin American and Caribbean Literature on Health Sciences (LILACS), and The Cochrane Library. The following strategy was applied to the PubMed database and adapted for other databases, according to the specialties of each one: [“Inflammatory Bowel Diseases” (Mesh) AND (“moderate” OR “severe”)] AND [“Steroids” (Mesh) OR “Prednisone” (Mesh) OR “Prednisolone” (Mesh) OR “Hydrocortisone” (Mesh) OR “Budesonide” (Mesh) OR “Dexamethasone” (Mesh) OR “Sulfasalazine” (Mesh) OR “Mesalamine” (Mesh) OR “Azathioprine” (Mesh) OR “Methotrexate” (Mesh) OR “Mycophenolic Acid” (Mesh) OR “Cyclosporine” (Mesh) OR “Tacrolimus” (Mesh) OR “6-Mercaptopurine” (Mesh)]. The systematic review was executed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement[20,21].
Eligibility criteria
Studies were considered eligible if they met the following criteria: (1) Meta-analysis, systematic reviews, randomized clinical trials (RCTs), observational or case-control studies; (2) studied conventional therapy in adult patients with MS-IBD, including CD or UC; and (3) comparative or single arm studies. Conventional therapy included corticosteroids (prednisone, hydrocortisone, budesonide, prednisolone, dexamethasone), 5-aminosalicylic acid (5-ASA) derivatives (mesalazine and sulfasalazine) and immunosuppressants (AZA, MTX, mycophenolate, cyclosporine, tacrolimus, 6-MP). Studies evaluating the maintenance of remission in quiescent disease were considered eligible only if they presented information about the disease severity prior to the remission period.
Exclusion criteria were as follows: sample size below 50, narrative review, specific subpopulations (e.g., pregnant women, comorbidities), studies on postoperative IBD, and languages other than English, Spanish, French or Portuguese. No time limits were applied. The quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation criteria for each selected study[22].
Data extraction
Two independent reviewers conducted the search in databases using the predefined strategy and selected the studies. In cases without a consensus, a third reviewer was consulted about the eligibility and was responsible for the final decision. The following information was extracted from each selected study: first author name, journal and year of publication, place where the study was conducted, follow-up period, sample size, disease characteristics, study outcomes, and quality of evidence.
Study outcomes
The primary outcome measures were clinical remission (induction or maintenance), clinical response and mucosal healing. As secondary outcomes, fecal calprotectin, hospitalization, death and surgeries were assessed. All outcomes were classified by whatever definition was used in the individual study. The criterion for considering the outcome as induction or maintenance was based on the description of the individual study. If not specified in the article, induction was used for follow-up of up to 12 wk, and maintenance was applied after this period.
RESULTS
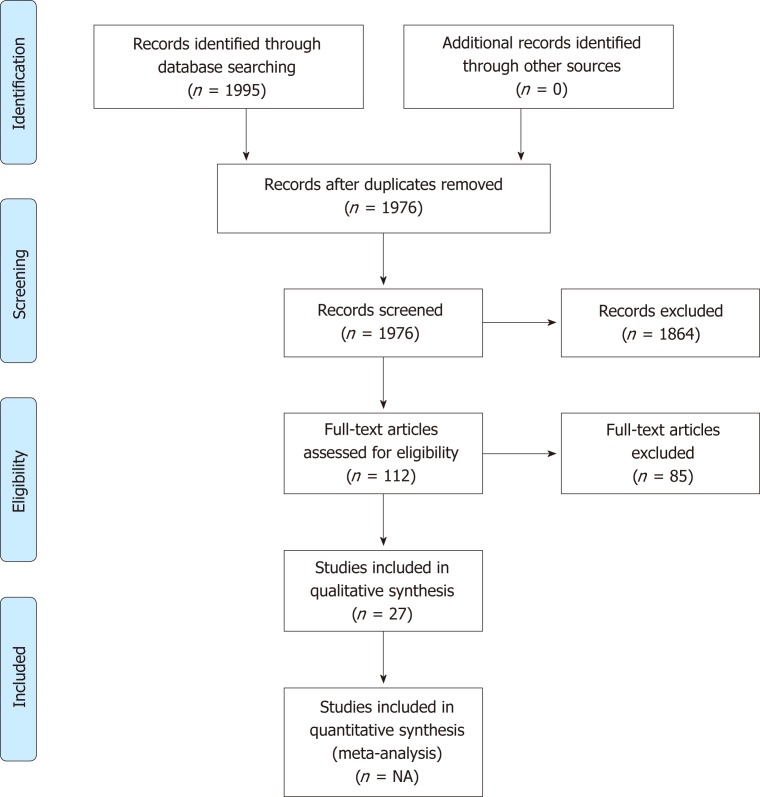
The search strategy identified 1995 citations from three databases. After removal of duplicates and exclusion by titles and abstracts, 112 studies were fully reviewed. Eighty-five studies did not meet eligibility criteria, and 27 were considered eligible (7 meta-analyses, 20 individual studies), as presented in Figure 1.
Figure 1.
Study flow diagram of the article selection procedure. NA: Not applicable.
Meta-analysis for primary outcomes: Qualitative review
Induction of clinical remission in Crohn’s disease: In Chande et al[23], AZA and 6-MP showed no advantage over placebo [risk ratio (RR): 1.23; 95% confidence interval (CI): 0.97-1.55], MTX (RR: 1.13; 95%CI: 0.85-1.49) or 5-ASA (RR: 1.24; 95%CI: 0.80-1.91).
Induction of clinical remission in ulcerative colitis: Chande et al[24], evaluated MTX versus placebo (RR: 0.96; 95%CI: 0.58-1.59), 6-MP (RR: 0.74; 95%CI: 0.43-1.29), and 5-ASA (RR: 2.33; 95%CI: 0.66-3.64) in UC, with no statistically significant difference. Baumgart et al[25], and Lasa et al[26], indicated numerical superiority of tacrolimus versus placebo for induction of clinical remission in UC [odds ratio (OR): 2.27; 95%CI: 0.35-14.75; RR: 0.91; 95%CI: 0.82-1.00, respectively], but the results did not reach statistical significance due to the small number of enrolled patients.
Maintenance of clinical remission in Crohn’s disease: No meta-analysis was found concerning the maintenance of clinical remission in CD.
Maintenance of clinical remission in ulcerative colitis: Only one meta-analysis fulfilled the eligibility criteria for clinical remission maintenance, and that analysis showed no statistically significant difference between MTX and placebo (RR: 0.64; 95%CI: 0.28-1.45), 5-ASA (RR: 1.12; 95%CI: 0.06-20.71) or 6-MP (RR: 0.22; 95%CI: 0.03-1.45) in UC[27].
Induction of clinical response in Crohn’s disease: Induction of clinical response was evaluated in CD for AZA and 6-MP; neither demonstrated any advantage over placebo (RR: 1.26; 95%CI: 0.98-1.62)[23].
Induction of clinical response in ulcerative colitis: Komaki et al[28], Baumgart et al[25], and Lasa et al[26] showed the superiority of tacrolimus versus placebo for clinical response in UC (RR: 4.61; 95%CI: 2.09-10.17; OR: 8.66; 95%CI: 1.79-42.00; RR: 0.58; 95%CI: 0.45-0.73, respectively). Narula et al[29], compared IFX versus cyclosporine in patients with UC. The clinical response rates for cyclosporine and IFX were 41.7% vs 43.8% in RCTs and 55.4% vs 74.8% in non-RCTs (OR: 2.96; 95%CI: 2.12-4.14).
Maintenance of clinical response in Crohn’s disease and ulcerative colitis: No meta-analysis was found concerning the maintenance of clinical response in CD or UC.
Mucosal healing: For mucosal healing induction in UC, one meta-analysis showed a favorable mucosal healing rate with tacrolimus versus placebo (RR: 0.59; 95%CI: 0.46-0.74) in a 12-wk horizon analysis[26]. When compared to IFX in CD, AZA was not favorable for induction of mucosal healing during a follow-up period of 26 wk[23]. The results of the retrieved meta-analyses, as well as their assessed quality, are presented according to primary outcome in Tables 1 2 3 4 5 6 and 7.
Table 1.
Meta-analyses included for induction of clinical remission in Crohn’s disease
| Study | Intervention | Comparator | Follow-up | n | Induction of clinical remission, RR (95%CI) | Quality of evidence |
| Chande et al[23] | AZA or 6-MP | Placebo | 6 wk-9 mo | 380 | RR 1.23 (0.97-1.55)1 | Moderate |
| AZA or 6-MP | MTX | 6 wk-9 mo | 143 | RR 1.13 (0.85-1.49)1 | Low | |
| AZA or 6-MP | 5-ASA | 6 wk-9 mo | 156 | RR 1.24 (0.80-1.91)1 | Very low | |
| AZA | IFX | 26 wk | 399 | RR 0.66 (0.51-0.87)1 | Moderate |
Clinical remission as measured by individual study with a validated outcome (e.g., Crohn’s Disease Activity Index score < 150 points or a Harvey-Bradshaw Index score < 3). 5-ASA: 5-aminosalicylic acid; 6-MP: 6-mercaptopurine; AZA: Azathioprine; CI: Confidence interval; IFX: Infliximab; MTX: Methotrexate; n: Number of patients; RR: Risk ratio.
Table 2.
Meta-analyses included for induction of clinical remission in ulcerative colitis
| Study | Intervention | Comparator | Follow-up | n | Induction of clinical remission, RR (95%CI) or OR (95%CI) | Quality of evidence |
| Chande et al[24] | MTX | Placebo | 36 wk | 67 | RR 0.96 (0.58-1.59)1 | Low |
| MTX | 6-MP | 30 wk | 26 | RR 0.74 (0.43-1.29)2 | Very low | |
| MTX | 5-ASA | 30 wk | 20 | RR 2.33 (0.66-3.64) 2 | Very low | |
| Baumgart et al[25] | Tacrolimus | Placebo | 2 wk | 63 | OR 2.27 (0.35-14.75)3 | High |
| Lasa et al[26] | Tacrolimus | Placebo | 12 wk | 127 | RR 0.91 (0.82-1.00)4 | High |
Clinical remission defined as a Mayo clinic score ≤ 3 (or Mayo score of ≤ 2 without sigmoidoscopy results);
Remission defined as prednisone stopped and Mayo Clinic Score < 7;
Clinical remission [defined as Disease Activity Index (DAI) < 2 with no individual subscore > 1];
Remission defined by individual studies by Truelove-Witts Score, Modified Clinical Activity Index, clinical variables and progression to colectomy, Colitis Activity Index, Lichtiger score, Mayo score for mucosal healing or DAI Score. 5-ASA: 5-aminosalicylic acid; 6-MP: 6-mercaptopurine; CI: Confidence interval; MTX: Methotrexate; n: Number of patients; OR: Odds ratio; RR: Risk ratio; DAI: Disease Activity Index.
Table 3.
Meta-analyses included for maintenance of clinical remission in ulcerative colitis
| Study | Intervention | Comparator | Follow-up | n | Maintenance of clinical remission, RR (95%CI) | Quality of evidence |
| Wang et al[27] | MTX | Placebo | 36 wk | 32 | RR 0.64 (0.28-1.45)1 | Low |
| MTX | 5-ASA | 76 wk | 9 | RR 1.12 (0.06-20.71)2 | Very low | |
| MTX | 6-MP | 76 wk | 18 | RR 0.22 (0.03-1.45)2 | Very low |
Maintenance of remission defined as a Mayo Clinic score of < 3 and off steroids.
Maintenance of remission defined as relapse within 76 wk (defined as 7 or more in the Mayo Clinic Score). 5-ASA: 5-aminosalicylic acid; 6-MP: 6-mercaptopurine; CI: Confidence interval; MTX: Methotrexate; n: Number of patients; RR: Risk ratio.
Table 4.
Meta-analyses included for induction of clinical response in Crohn’s disease
| Study | Intervention | Comparator | Follow-up | n | Clinical response, RR (95%CI) | Quality of evidence |
| Chande et al[23] | AZA or 6-MP | Placebo | 6 wk-9 mo | 434 | RR 1.26 (0.98-1.62)1 | Moderate |
Clinical response considered the outcomes of remission and improvement and varied from study to study making exact comparisons across studies difficult. Therefore, the definition of improvement or remission used in each study was used for data extraction. 6-MP: 6-mercaptopurine; AZA: azathioprine; CI: Confidence interval; n: Number of patients; RR: Risk ratio.
Table 5.
Meta-analyses included for induction of clinical response in ulcerative colitis
| Study | Intervention | Comparator | Follow-up | n | Clinical response, RR (95%CI) or OR (95%CI) | Quality of evidence |
| Komaki et al[28] | Tacrolimus | Placebo | 2 wks (RCT) | 103 | RR 4.61 (2.09-10.17)1 | High |
| Baumgart et al[25] | Tacrolimus | Placebo | 2 wk | 63 | OR 8.66 (1.79-42.00)2 | High |
| Lasa et al[26] | Tacrolimus | Placebo | 12 wk | 127 | RR 0.58 (0.45-0.73)3 | High |
| Narula et al[29] | IFX | Cyclosporine | 3 mo (RCT) | 412 | 43.8% (IFX); 41.7% (C) OR 1.08 (0.73-1.60)4 | Low |
| IFX | Cyclosporine | 3 mo (non RCT) | 801 | 74.8% (IFX); 55.4% (C) OR 2.96 (2.12-4.14)5 | Very low |
Clinical response defined as reduction in Disease Activity Index (DAI) ≥ 4 with improvement of all categories;
Clinical response (partial or complete response) based on the DAI score. A complete response was defined as complete resolution of all symptoms with a zero scored for all assessments of the DAI. A partial response was defined as a reduction of more than four points on the DAI with improvement in all categories, but not a complete response;
Clinical response defined according to each individual study, and not shown by the meta-analysis;
Definitions from individual studies: Failure to respond to treatment was combined end point of the absence of clinical response at day 7 (based on Lichtiger score < 10 and at least 3 points less than baseline), relapse between day 7 and 98, absence of steroid-free remission at day 98, severe adverse event leading to treatment interruption, colectomy; response based on Powell-Tuck index ≤ 3; doubling of the Crohn's and ulcerative colitis questionnaire-32 score at 3 mo;
Definitions from individual studies: Failure to respond including ongoing or worsening symptoms of bloody diarrhea, abdominal pain and persistently elevated inflammatory markers, or the development of a complication (perforation, severe hemorrhage, toxic megacolon); good response to treatment was decrease in stool frequency (< 6/d), little or no blood in feces, absence of complications; clinical remission, as per clinical symptom questionnaire used locally at this institution; being discharged from hospital without surgery or additional rescue therapy; physician global assessment of patient response—those deemed medical failure were treated with colectomy; steroid-free clinical remission (mild or inactive based on the Montreal severity score) plus no need for second rescue therapy or colectomy; modified Truelove and Witts Severity Index score decrease ≥ 4points; remission based on Colitis Activity Index ≤ 4 within 4 wk; in 5 studies, treatment response was not reported. C: Cyclosporine; CI: Confidence interval; IFX: Infliximab; n: Number of patients; OR: Odds ratio; RCT: Randomized clinical trial; RR: Risk ratio.
Table 6.
Meta-analyses included for induction of mucosal healing in Crohn’s disease
| Study | Intervention | Comparator | Follow-up | n | Mucosal healing, RR (95%CI) | Quality of evidence |
| Chande et al[23] | AZA | IFX | 26 wk | 214 | RR 0.55 (0.33-0.94)1 | Moderate |
Mucosal healing was defined as the absence of mucosal ulceration at week 26 in patients who had confirmed mucosal ulceration at baseline. AZA: Azathioprine; CI: Confidence interval; IFX: Infliximab; n: Number of patients; RR: Risk ratio.
Table 7.
Meta-analyses included for induction of mucosal healing in ulcerative colitis
| Study | Intervention | Comparator | Follow-up | n | Mucosal healing, RR (95% CI) | Quality of evidence |
| Lasa et al[26] | Tacrolimus | Placebo | 12 wk | 127 | RR 0.59 (0.46-0.74)1 | High |
Mucosal healing was defined as by the Mayo score for mucosal healing. CI: Confidence interval; n: Number of patients; RR: Risk ratio.
Meta-analysis for secondary outcomes: Qualitative review
For secondary outcomes, no meta-analysis was found to evaluate fecal calprotectin, hospitalization or death specifically. For colectomy, two meta-analyses for UC were retrieved. As shown in Table 8, the first revealed a 0% colectomy rate in both the tacrolimus and placebo arms[28]. In Narula et al[29], colectomy rates at 3 mo in RCTs did not achieve a significant difference between cyclosporine and IFX (OR: 1.00; 95%CI: 0.64-1.59), with pooled 3-mo colectomy rates of 26.6% for IFX and 26.4% for cyclosporine. Among non-RCTs, the pooled 3-mo colectomy rate was 24.1% for IFX and 42.5% for cyclosporine (pooled OR: 0.53; 95%CI: 0.22-1.28; no significant difference between the two groups). Colectomy rates at 12 mo did not show any significant difference between the two groups in RCTs (OR: 0.76; 95%CI: 0.51-1.14). The 12-mo colectomy rate was significantly lower for IFX in non-RCTs (20.7% for IFX vs 36.8% for cyclosporine; pooled OR: 0.42; 95%CI: 0.22-0.83).
Table 8.
Meta-analyses included for inflammatory bowel disease-related surgeries in ulcerative colitis
| Study | Intervention | Comparator | Follow-up | N | Colectomy rate %, or OR (95% CI) | Quality of evidence |
| Komaki et al[28] | Tacrolimus | Placebo | 2 wk (RCT) | 103 | 0% | High |
| Narula et al[29] | IFX | Cyclosporine | 3 mo (RCT) | 385 | 26.6% (IFX); 26.4% (C) OR 1.00 (0.64-1.59) | Low |
| IFX | Cyclosporine | 3 mo (non RCT) | 478 | 24.1% (IFX); 42.5% (C) OR 0.53 (0.22-1.28) | Very low | |
| IFX | Cyclosporine | 12 mo (RCT) | 415 | 34.4% (IFX); 40.8% (C) OR 0.76 (0.51-1.14) | Low | |
| IFX | Cyclosporine | 12 mo (non RCT) | 854 | 20.7% (IFX); 36.8% (C) OR 0.42 (0.22-0.83) | Very low |
C: Cyclosporine; CI: Confidence interval; IFX: Infliximab; n: Number of patients; OR: Odds ratio; RCT: Randomized clinical trial.
Individual studies: Qualitative review
Twenty individual studies were included in this systematic review[30-49]. They were mainly in UC, with small sample sizes and short follow-up. Therapies included cyclosporine, 5-ASA, tacrolimus, corticosteroids, AZA, and 6-MP (Tables 9 10 11 12 13 and 14). The primary outcomes were evaluated, but the majority of studies had retrospective cohorts with low or very low levels of evidence. As a secondary outcome, IBD-related surgeries were the only outcome where data were available (Tables 15 and 16).
Table 9.
Individual studies included for induction of clinical remission in Crohn’s disease
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Induction of clinical remission |
| Thomsen et al[45] | Denmark, France, United Kingdom, Norway, Italy, Spain, Portugal, Greece, South Africa, Austria, Australia, and Ireland | Budesonide | Mesalamine | RCT | 8 wk | 182 | 69% (budesonide) 45% (mesalamine) (P = 0.001)1 |
| Budesonide | Mesalamine | RCT | 16 wk | 182 | 62% (budesonide) 36% (mesalamine) (P < 0.001)1 | ||
| Pavez et al[41] | Chile | IFX | AZA | RCT | 26 wk | 508 | 0.44 (event rate IFX); 0.3 (event rate AZA)2 |
Clinical remission defined as Crohn’s Disease Activity Index (CDAI) ≤ 150;
Clinical remission defined as CDAI less than 150 in patients who did not receive budesonide at a daily dose greater than 6 mg, or systemic corticosteroids for at least 3 wk. AZA: Azathioprine; IFX: Infliximab; n: Number of patients; RCT: Randomized clinical trial; CDAI: Crohn’s Disease Activity Index.
Table 10.
Individual studies included for induction of clinical remission in ulcerative colitis
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Induction of clinical remission |
| Schmidt et al[30] | Germany | Tacrolimus | - | Retrospective cohort | 24 mo | 58 | 51%1 |
| Tacrolimus with purine analogues | - | Retrospective cohort | 24 mo | 79 | 82%1 | ||
| Llaó et al[31] | Spain | IV corticosteroids | - | Retrospective cohort | 3 d | 110 | 52%2 |
| IV corticosteroids | - | Retrospective cohort | 7 d | 110 | 75% 2 | ||
| Campbell et al[35] | UK | Cyclosporine | - | Retrospective cohort | Acute phase | 76 | 74%3 |
| Sood et al[33] | India | AZA | Placebo | RCT | 1 yr | 83 | 56% (AZA); 40% (placebo)4 |
| Prieux-Klotz et al[37] | France | AZA or 6-MP | Retrospective cohort | 38 mo | 80 | 61.2%5 | |
| Yamamoto et al[38] | Japan | Tacrolimus | Anti-TNF | Retrospective | 12 wk | 100 | 40% (tacrolimus); 28% (anti-TNF); P = 0.296 |
| Ogata et al[39] | Japan | Tacrolimus | Placebo | RCT | 2 wk | 62 | 9.4% (tacrolimus); 0% (placebo); P = 0.2387 |
| Tacrolimus | Placebo | RCT | 12 wk | 21 | 28.6% (tacrolimus)7 | ||
| Hyde et al[44] | United Kingdom | Hydrocortisone | - | Retrospective cohort | 5 d | 216 | 61%8 |
| Cyclosporine | - | Retrospective cohort | 4.5 d | 50 | 56%8 | ||
| Kjeldsen et al[43] | Denmark | Prednisolone | - | Retrospective cohort | 6 wk | 51 | 47% (severe disease); 80% (moderate disease)9 |
| Meyers et al[42] | United States | ACTH | Hydrocortisone | RCT | 10 d | 66 | 44% (ACTH); 41% (Hydrocortisone)10 |
Clinical remission defined by a Lichtiger score ≤ 3;
Clinical remission defined as mild activity or inactive disease according to the Montreal severity score, with no need for rescue treatment at day 7 after starting intravenous CS;
Response defined as a reduction of bowel frequency to fewer than three daily and a C-reactive protein < 45 mg/L;
Clinical remission defined as clinical improvement with absent of symptoms of active disease (rectal bleeding, bowel frequency) with sigmoidoscopic appearance of grade 0-1 and normal histological pattern;
Clinical remission defined as a partial Mayo Clinic score ≤ 2 without any clinical subscore > 1;
Clinical remission defined as a score of 0 in the clinical section (both stool frequency and rectal bleeding);
Clinical remission was defined as a total DAI score ≤ 2 with an individual subscore of 0 or 1;
Clinical remission defined as bowel frequency less than three stools per day, no visible blood, no fever or pain;
Remission was assessed in accordance with a modified Truelove and Witts index;
Remission defined as patient receiving no therapy or only prophylactic sulfasalazine. 6-MP: 6-mercaptopurine; ACTH: Adrenocorticotrophic hormone; AZA: Azathioprine; CI: Confidence interval; IV: Intravenous; MTX: Methotrexate; n: Number of patients; RCT: Randomized clinical trial; TNF: Tumor necrosis factor; DAI: Disease Activity Index.
Table 11.
Individual studies included for maintenance of clinical remission in ulcerative colitis
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Maintenance of clinical remission |
| Sood et al[32] | India | AZA | - | Retrospective cohort | 12 mo | 111 | 91%1 |
| AZA | - | Retrospective cohort | 24 mo | N/A | 88%1 | ||
| AZA | - | Retrospective cohort | 36 mo | N/A | 76%1 | ||
| AZA | - | Retrospective cohort | 48 mo | N/A | 53%1 | ||
| AZA | - | Retrospective cohort | 60 mo | N/A | 38%1 | ||
| Campbell et al[35] | United Kingdom | Cyclosporine | - | Retrospective cohort | 1 yr | 76 | 35%2 |
| Cyclosporine | - | Retrospective cohort | 3 yr | N/A | 10%2 | ||
| Arts et al[36] | Belgium | Cyclosporine | - | Retrospective cohort | 1 yr | 34 | 27.8%3 |
| Cyclosporine | - | Retrospective cohort | 3 yr | 5 | 50%3 | ||
| Hyde et al[44] | United Kingdom | Cyclosporine | - | Retrospective cohort | 19 mo | 50 | 40%4 |
| Meyers et al[42] | United States | ACTH | Hydrocortisone | RCT | 1 yr | 66 | 37.5% (ACTH); 23.5% (hydrocortisone)5 |
Remission was defined as absence of symptoms of active disease as rectal bleeding and normal bowel frequency and hence no need for steroids for at least 6 mo;
Maintenance of remission defined as absent of disease relapse;
The study does not present the exact definition considered for clinical remission;
Remission defined as bowel frequency less than three stools per day, no visible blood, no fever or pain;
Therapeutic success was considered as a clinical remission, defined by the absence of all symptoms and the reduction of the frequency of bowel movements to two or less per day. ACTH: Adrenocorticotrophic hormone; AZA: Azathioprine; n: Number of patients; N/A: Not available; RCT: Randomized clinical trial.
Table 12.
Individual studies included for induction or maintenance of clinical response in Crohn’s disease
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Clinical response |
| Chun et al[46] | United States | ACTH | Hydrocortisone | RCT | 10 d | 88 | 82% (ACTH; 67%-92%); 93% (hydrocortisone; 84%-99%)1 |
Clinical response evaluated by Present-Korelitz Index. ACTH: Adrenocorticotrophic hormone; n: Number of patients; RCT: Randomized clinical trial.
Table 13.
Individual studies included for induction or maintenance of clinical response in ulcerative colitis
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Clinical response |
| Arts et al[36] | Belgium | Cyclosporine | - | Retrospective cohort | 9 d | 86 | 83.7%1 |
| Prieux-Klotz et al[37] | France | AZA or 6-MP | - | Retrospective cohort | 38 mo | 80 | 70%2 |
| Yamamoto et al[38] | Japan | Tacrolimus | Anti-TNF | Retrospective | 12 wk | 100 | 62% (tacrolimus); 64% (anti-TNF); P > 0.993 |
| Ogata et al[39] | Japan | Tacrolimus | Placebo | RCT | 2 wk | 62 | 50% (tacrolimus); 13.3% (placebo); P = 0.0034 |
| Van Assche et al[48] | Belgium | Cyclosporine 4 mg/kg | Cyclosporine 2 mg/kg | RCT | 2 wk | 73 | 84.2% (4 mg/kg); 85.7% (2 mg/kg)5 |
| Oshitani et al[47] | Japan | Prednisolone | Methylprednisolone | Retrospective cohort | 7-14 d | 71 | 82% (prednisolone); 82% (methylprednisolone)6 |
Response defined as colectomy avoided;. 2Clinical response defined by a decrease in the partial Mayo score of at least 3 points and 30% with a rectal bleeding Mayo subscore ≤ 1;
Clinical response was defined as a decrease of at least 2 points in the clinical section (stool frequency and/or rectal bleeding);
Clinical response was defined as improvement in all Disease Activity Index subscores;
Clinical response was defined as a score of less than 10 at day 8 with a drop of ≥ 3 as compared with baseline;
Clinical response considered as at least one of: decreased blood in stools compared with previous findings; soft or normal stool; no nocturnal defecation. 6-MP: 6-mercaptopurine; AZA: Azathioprine; n: Number of patients; RCT: Randomized clinical trial; TNF: Tumor necrosis factor.
Table 14.
Individual studies included for mucosal healing in ulcerative colitis
| Study | Country | Intervention | Comparator | Study design | Follow-up | N | Mucosal healing |
| Prieux-Klotz et al[37] | France | AZA or 6-MP | - | Retrospective cohort | 38 mo | 80 | 43.7%1 |
| Yamamoto et al[38] | Japan | Tacrolimus | Anti-TNF | Retrospective | 12 wk | 73 | 32% (tacrolimus); 28% (anti-TNF); P = 0.862 |
| Ogata et al[39] | Japan | Tacrolimus | Placebo | RCT | 2 wk | 62 | 43.8% (tacrolimus); 13.3% (placebo); P = 0.0123 |
| Tacrolimus | Placebo | RCT | 12 wk | 21 | 85.7% (tacrolimus)3 | ||
| Oshitani et al[47] | Japan | Prednisolone | Methylprednisolone | Retrospective cohort | 6 wk | 71 | 78% (prednisolone); 82% (methylprednisolone)4 |
Endoscopic mucosal healing was defined by endoscopic Mayo score of 0 or 1 and ulcerative colitis endoscopic index of severity ≤ 2;
Endoscopic healing was defined as an endoscopic score of 0 or 1;
Mucosal healing was defined as mucosal appearance subscore of 0 or 1;
Endoscopic change considers endoscopic remission (no ulcers, no erosion, no friability) and endoscopic improvement (ulcers, erosion and friability decreased compared with previous findings). 6-MP: 6-mercaptopurine; AZA: Azathioprine; n: Number of patients; RCT: Randomized clinical trial; TNF: Tumor necrosis factor.
Table 15.
Individual studies included for surgeries related to Crohn’s disease
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Colectomy |
| Chun et al[46] | United States | ACTH | Hydrocortisone | RCT | 3 yr | 88 | 28% (both groups) |
ACTH: Adrenocorticotrophic hormone; n: number of patients; RCT: Randomized clinical trial.
Table 16.
Individual studies included for surgeries related to ulcerative colitis
| Study | Country | Intervention | Comparator | Study design | Follow-up | n | Colectomy |
| Schmidt et al[30] | Germany | Tacrolimus | - | Retrospective cohort | 24 mo | 58 | 22% |
| Tacrolimus with purine analogues | - | Retrospective cohort | 24 mo | 79 | 18% | ||
| Llaó et al[31] | Spain | IV corticosteroids | - | Retrospective cohort | 7 d | 110 | 15% |
| Moskovitz et al[34] | Belgium | Cyclosporine | - | Retrospective cohort | 9.3 d | 142 | 16.9% |
| Cyclosporine | - | Retrospective cohort | 1 yr | N/A | 37% | ||
| Cyclosporine | - | Retrospective cohort | 4 yr | N/A | 59% | ||
| Cyclosporine | - | Retrospective cohort | 6 yr | N/A | 84% | ||
| Cyclosporine | - | Retrospective cohort | 7 yr | N/A | 88% | ||
| Campbell et al[35] | UK | Cyclosporine | - | Retrospective cohort | 7 yr | 58% | |
| Arts et al[36] | Belgium | Cyclosporine | - | Retrospective cohort | 9 d | 86 | 16.3% |
| Cyclosporine | - | Retrospective cohort | 1 yr | 45 | 36% | ||
| Cyclosporine | - | Retrospective cohort | 3 yr | 13 | 45% | ||
| Yamamoto et al[38] | Japan | Tacrolimus | Anti-TNF | Retrospective | 12 wk | 100 | 10% (tacrolimus); 16% (anti-TNF); P = 0.55 |
| Cheifetz et al[40] | United States | Cyclosporine | Retrospective cohort | 4 wk | 71 | 15% | |
| Cyclosporine | Retrospective cohort | 1 yr | 71 | 39% | |||
| Cyclosporine | Retrospective cohort | 2 yr | 71 | 42% | |||
| Cyclosporine | Retrospective cohort | 5 yr | 71 | 46% | |||
| Gustavsson et al[49] | Sweden | Corticosteroid | Retrospective cohort | 3 mo | 45 (moderate) | 8.9% | |
| Corticosteroid | Retrospective cohort | 3 mo | 61 (severe) | 45.9% | |||
| Corticosteroid | Retrospective cohort | 20 yr | 41 (moderate) | 48.8% | |||
| Corticosteroid | Retrospective cohort | 20 yr | 33 (severe) | 33.3% | |||
| Van Assche et al[48] | Belgium | Cyclosporine 4 mg/kg | Cyclosporine 2 mg/kg | RCT | 2 wk | 73 | 13.1% (4 mg/kg); 8.6% (2 mg/kg) |
| Hyde et al[44] | United Kingdom | Hydrocortisone | - | Retrospective cohort | 5 d | 216 | 15.7% |
| Cyclosporine | - | Retrospective cohort | 19 mo | 50 | 16% | ||
| Kjeldsen et al[43] | Denmark | Prednisolone | - | Retrospective cohort | 8 mo | 51 | 42% (severe disease); 13% (moderate disease) |
IV: Intravenous; n: Number of patients; N/A: Not available; RCT: Randomized clinical trial; TNF: Tumor necrosis factor.
DISCUSSION
This systematic review aimed to study data on the effectiveness of conventional therapy for MS-IBD. Despite being a very broad theme, the objective was to understand the panorama of available evidence about conventional treatment and its qualities, more than to evaluate the individual efficacy of each drug.
The choice of outcomes was based on the currently most relevant outcomes: Clinical remission and response (induction and maintenance), mucosal healing, fecal calprotectin, hospitalization, death and surgeries. Mucosal healing is considered a more objective goal than clinical remission for evaluating inflammatory disease activity in patients with IBD, and it should be measured in both clinical trials and medical practice to evaluate the management of IBD[50]. In clinical trials on IBD, this endpoint has been defined as complete absence of ulcerative lesions or by specific endoscopic scores such as the Simple Endoscopic Score for CD and the CD Endoscopic Index of Severity in CD or Mayo 1 or 0 for UC[51]. Mucosal healing can alter the natural history of IBD by reducing the frequency of hospitalization and the lifetime risk for surgery and colorectal cancer, in addition to being associated with disease remission[15,50]. In addition, there is a current consensus in the regulatory and academic environment that clinical studies in IBD need an imaging endpoint, such as mucosal healing, with or without histopathology[52]. In this systematic review, only two meta-analyses were retrieved that evaluated mucosal healing[23,26] and four individual studies[37-39,47,53], all for patients with UC. This paucity of available studies supports our claim that there is a lack of data assessing the effectiveness of conventional therapy for mucosal healing.
Despite the advantages of using mucosal healing as an outcome measure, it is usually associated with invasive and costly procedures, which can be barriers, especially for developing countries[14]. Thus, fecal calprotectin has been suggested as a surrogate marker for assessing mucosal healing[15]. In general, biomarkers (wide range of substances present in blood, stool, or urine) play important roles in research: reduce placebo response; select subjects with symptoms directed by specific inflammatory processes; predict the clinical relapse likelihood; identify patients with mucosal healing; provide clinical disease activity indexes; follow disease activity[54]. Fecal calprotectin is probably an alternative marker for assessing IBD disease activity, especially for UC[16]. In the present study, no eligible studies evaluating fecal calprotectin were found.
Colectomy rates were reported often in studies, mainly for UC, and low rates may reflect clinical improvement, as well as reduction of resource utilization vs those who have to undergo colectomy. Death was not an outcome assessed directly as a study objective, perhaps because studies did not have a long enough follow-up period to evaluate this endpoint. Hospitalization was also not explored in the studies we retrieved. Positive results were observed for tacrolimus in the treatment of UC. The drug presents good results for induction and maintenance of remission, mucosal healing and risk reduction of surgical treatment, and in some analyses, it is superior to IFX. On the other hand, tacrolimus is very uncommonly used in clinical practice and very rarely referenced by treatment guidelines. Therefore, we believe that tacrolimus use should be reviewed by IBD consensus.
The main limitations of this study are the wide range of eligible drugs, the considerable number of outcomes and the variety of ways to measure these endpoints. Several instruments are used in individual studies for measuring clinical disease activity in CD (CD Activity Index, Harvey Bradshaw Index, Van Hess or Dutch Index, Therapeutic Goals Score, International Organization of Inflammatory Bowel-Disease-Oxford Index) and for evaluating and measuring endoscopic response to therapy (CD Endoscopic Index of Severity, Rutgeerts Endoscopic Index)[54]. For UC, the usual instruments for measuring clinical disease activity are Truelove and Witts Score, Lichtiger Score, Powell-Tuck Index, Clinical Activity Index, Mayo Score, Sutherland Index, Physician Global Assessment. These instruments generally include measurements of stool frequency, presence of blood, endoscopic findings, abdominal pain and gastrointestinal symptoms, laboratory findings, extraintestinal manifestations, temperature, physician’s global assessment and patient functional evaluation[54]. To circumvent the problem of the variety of instruments for the assessment of illness severity at baseline and response to treatment measurement, we applied the indexes and definitions as used in each individual study.
Some studies cited in treatment guidelines and used as a source of evidence were excluded from this review. The reasons varied but were mainly because the studies contained different disease severities or specific subpopulations, such as those in the postoperative period. Furthermore, studies with no disease severity specification were excluded, according to eligibility criteria. Therefore, only studies in which the disease was explicitly moderate to severe were considered. In this way, some major works may have been excluded. It is important to note that some negative results of conventional therapy in moderate to severe disease do not mean that immunosuppressants have no function in IBD. The exclusion of studies with mild disease and those which did not specify the disease severity may have skewed our results against them. An example is the use of AZA and 6-MP in corticosteroid-dependent patients, where such medications may be useful especially for remission maintenance. Overall, little high-quality evidence is available on conventional therapy for MS-IBD patients to robustly assess their effectiveness in this patient population, which did not encompass all available medications, for all pathologies and with all relevant outcomes for response and prognosis. This review suggests that conventional therapy for MS-IBD does not have scientific evidence of quality that supports its use as a standard for MS-IBD.
In conclusion, there are few studies evaluating objective outcomes in MS-IBD with conventional therapy, especially for remission maintenance, mucosal healing and fecal calprotectin. Additionally, the quality of existing studies is mainly very low or low. As conventional therapies are usually the main treatment for MS-IBD, robust researches are required to enhance the evidence on their effectiveness because they are currently prescribed to many IBD patients.
ARTICLE HIGHLIGHTS
Research background
Inflammatory bowel disease (IBD) frequently present a lifelong relapsing and remitting course with negative impact on health and quality of life, besides long-term sequelae. IBD main treatment goal is the achievement and maintenance of disease remission. Conventional therapies are indicated for patients with moderate to severe disease, despite the advent of biological drugs. Some relevant outcomes, such as clinical remission and endoscopic remission has been correlated with surgeries and hospitalizations reduction.
Research motivation
Conventional therapy continues to be used in moderate to severe IBD (MS-IBD) especially in countries where biologics are not covered by insurance. Thus, extensive knowledge on the efficacy and safety of conventional therapy is necessary.
Research objectives
This systematic review aims to investigate data on the efficacy of conventional therapy for MS-IBD.
Research methods
A systematic review was conducted through the Cochrane Collaboration, MEDLINE, and LILACS databases searching for studies concerning conventional therapy in adult patients with MS-IBD, including Crohn’s disease (CD) and ulcerative colitis (UC). Corticosteroids (prednisone, hydrocortisone, budesonide, prednisolone, dexamethasone), 5-aminosalicylic acid (5-ASA) derivatives (mesalazine and sulfasalazine) and immunosuppressants [azathioprine (AZA), methotrexate (MTX), mycophenolate, cyclosporine, tacrolimus, 6-mercaptopurine (6-MP)] were considered conventional therapy. Primary outcome measures were clinical remission (induction or maintenance), clinical response and mucosal healing.
Research results
For induction of clinical remission, AZA and 6-MP showed no advantage over placebo, MTX or 5-ASA in CD; MTX showed no statistically significant difference versus placebo, 6-MP, or 5-ASA in UC; tacrolimus was superior to placebo for UC in two meta-analyses. One meta-analysis evaluated clinical remission maintenance, showing no statistically significant difference between MTX and placebo, 5-ASA, or 6-MP in UC. AZA and 6-MP had no advantage over placebo in induction of clinical response in CD. Three meta-analyses showed the superiority of tacrolimus versus placebo for induction of clinical response in UC. The clinical response rates for cyclosporine were 41.7% in randomized controlled trials (RCTs) and 55.4% in non-RCTs for UC. For induction of mucosal healing, one meta-analysis showed a favorable rate with tacrolimus versus placebo for UC. For secondary outcomes, no meta-analyses specifically evaluated fecal calprotectin, hospitalization or death. Two meta-analyses were retrieved evaluating colectomy rates for tacrolimus and cyclosporine in UC. Most of the twenty individual studies retrieved contained a low or very low quality of evidence.
Research conclusions
High-quality evidence assessing conventional therapy in MS-IBD treatment is scarce, especially for remission maintenance, mucosal healing and fecal calprotectin.
Research perspectives
From this systematic review, it could be seen, that further studies with high quality and real-world evidence are needed to prove the effectiveness of conventional therapy in MS-IBD.
ACKNOWLEDGEMENTS
We thank SENSE Company Brazil for conducting the literature search and for providing medical writing support in developing drafts of this manuscript. This support was funded by Takeda Pharmaceuticals, Brazil. The authors were responsible for analysis and interpretation of data; critical revision of the manuscript for important intellectual content, final approval of the version to be published; and commitment to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Adérson Omar Mourão Cintra Damião has been a speaker for Takeda, Abbvie, and Janssen; has been an advisory board member for Takeda; and has received conference grants from Janssen, Takeda and Abbvie. Matheus Freitas Cardoso de Azevedo and Alexandre de Sousa Carlos have received research grants from Takeda, Janssen, and Abbvie. Marcela Yumi Wada, Taciana Valéria Marcolino Silva and Flávio de Castro Feitosa work at Takeda Pharmaceuticals, Brazil.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Peer-review started: December 21, 2018
First decision: January 6, 2019
Article in press: February 15, 2019
P- Reviewer: de’Angelis GL, Eleftheriadis NP, M’Koma AE, S- Editor: Yan JP L- Editor: A E- Editor: Huang Y
Contributor Information
Adérson Omar Mourão Cintra Damião, Department of Gastroenterology, University of São Paulo School of Medicine, São Paulo 05403-000, Brazil.
Matheus Freitas Cardoso de Azevedo, Department of Gastroenterology, University of São Paulo School of Medicine, São Paulo 05403-000, Brazil.
Alexandre de Sousa Carlos, Department of Gastroenterology, University of São Paulo School of Medicine, São Paulo 05403-000, Brazil.
Marcela Yumi Wada, Department of Medical Affairs, Takeda Pharmaceuticals, São Paulo 04709-011, Brazil.
Taciana Valéria Marcolino Silva, Department of Medical Affairs, Takeda Pharmaceuticals, São Paulo 04709-011, Brazil.
Flávio de Castro Feitosa, Department of Medical Affairs, Takeda Pharmaceuticals, São Paulo 04709-011, Brazil. flavio.feitosa@takeda.com.
References
- 1.Bernstein CN, Eliakim A, Fedail S, Fried M, Gearry R, Goh KL, Hamid S, Khan AG, Khalif I, Ng SC, Ouyang Q, Rey JF, Sood A, Steinwurz F, Watermeyer G, LeMair A Review Team: World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J Clin Gastroenterol. 2016;50:803–818. doi: 10.1097/MCG.0000000000000660. [DOI] [PubMed] [Google Scholar]
- 2.Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, Kucharzik T, Molnár T, Raine T, Sebastian S, de Sousa HT, Dignass A, Carbonnel F European Crohn’s and Colitis Organisation [ECCO] Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. [DOI] [PubMed] [Google Scholar]
- 3.Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:942–951. doi: 10.3109/00365521.2015.1014407. [DOI] [PubMed] [Google Scholar]
- 4.Mantzaris GJ, Viazis N, Polymeros D, Papamichael K, Bamias G, Koutroubakis IE. Clinical profiles of moderate and severe Crohn's disease patients and use of anti-tumor necrosis factor agents: Greek expert consensus guidelines. Ann Gastroenterol. 2015;28:417–425. [PMC free article] [PubMed] [Google Scholar]
- 5.Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn's disease: Time for a change. Gut. 2011;60:1754–1763. doi: 10.1136/gutjnl-2011-300934. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 7.Delmondes LM, Nunes MO, Azevedo AR, Oliveira MM, Coelho LE, Torres-Neto JD. Clinical and Sociodemographic Aspects of Inflammatory Bowel Disease Patients. Gastroenterology Res. 2015;8:207–215. doi: 10.14740/gr649w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crohn’s Colitis Foundation of America. 2014. Inflammatory bowel diseases. New York, NY; pp. 1–24. [Google Scholar]
- 9.Souza MM, Belasco AGS, Aguilar-Nascimento JE. Perfil epidemiológico dos pacientes portadores de doença inflamatória intestinal do estado de Mato Grosso. Rev Bras Coloproctol. 2008;28:324–328. [Google Scholar]
- 10.Orlando A, Guglielmi FW, Cottone M, Orlando E, Romano C, Sinagra E. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis. 2013;45:986–991. doi: 10.1016/j.dld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein GR, Hanauer SB, Sandborn WJ Practice Parameters Committee of American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465–83; quiz 464, 484. doi: 10.1038/ajg.2008.168. [DOI] [PubMed] [Google Scholar]
- 12.Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969–977. doi: 10.1016/j.dld.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 14.Mooiweer E, Severs M, Schipper ME, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: A plea for deep remission. J Crohns Colitis. 2015;9:50–55. doi: 10.1093/ecco-jcc/jju003. [DOI] [PubMed] [Google Scholar]
- 15.Ikhtaire S, Shajib MS, Reinisch W, Khan WI. Fecal calprotectin: Its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. 2016;51:434–446. doi: 10.1007/s00535-016-1182-4. [DOI] [PubMed] [Google Scholar]
- 16.Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ, Deng FH, Nie B, Jiang B. Meta-analysis: Fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20:1407–1415. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 17.Vieira A, Fang CB, Rolim EG, Klug WA, Steinwurz F, Rossini LG, Candelária PA. Inflammatory bowel disease activity assessed by fecal calprotectin and lactoferrin: Correlation with laboratory parameters, clinical, endoscopic and histological indexes. BMC Res Notes. 2009;2:221. doi: 10.1186/1756-0500-2-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health (Brazil) Portaria SAS/MS no 861, de 04 de novembro de 2002. Protocolo clínico e diretrizes terapêuticas: Retocolite ulcerativa. Sulfasalazina, Mesalazina, Hidrocortisona, Prednisona, Azatioprina, 6-Mercaptopurina, Ciclosporina. Brasília: Ministério da Saúde;; 2002. [Google Scholar]
- 19.Ministry of Health (Brazil) Portaria SAS/MS no 966, de 2 de outubro de 2014. Protocolo Clínico e Diretrizes Terapêuticas: Doença de Crohn. Brasília: Ministério da Saúde;; 2014. [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chande N, Townsend CM, Parker CE, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2016;10:CD000545. doi: 10.1002/14651858.CD000545.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chande N, Wang Y, MacDonald JK, McDonald JW. Methotrexate for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2014;(8):CD006618. doi: 10.1002/14651858.CD006618.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baumgart DC, Macdonald JK, Feagan B. Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. Cochrane Database Syst Rev. 2008;(3):CD007216. doi: 10.1002/14651858.CD007216. [DOI] [PubMed] [Google Scholar]
- 26.Lasa J, Olivera P. Efficacy of tacrolimus for induction of remission in patients with moderate-to-severe ulcerative colitis: A systematic review and meta-analysis. Arq Gastroenterol. 2017;54:167–172. doi: 10.1590/s0004-2803.201700000-15. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, MacDonald JK, Vandermeer B, Griffiths AM, El-Matary W. Methotrexate for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015:CD007560. doi: 10.1002/14651858.CD007560.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komaki Y, Komaki F, Ido A, Sakuraba A. Efficacy and Safety of Tacrolimus Therapy for Active Ulcerative Colitis; A Systematic Review and Meta-analysis. J Crohns Colitis. 2016;10:484–494. doi: 10.1093/ecco-jcc/jjv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narula N, Marshall JK, Colombel JF, Leontiadis GI, Williams JG, Muqtadir Z, Reinisch W. Systematic Review and Meta-Analysis: Infliximab or Cyclosporine as Rescue Therapy in Patients With Severe Ulcerative Colitis Refractory to Steroids. Am J Gastroenterol. 2016;111:477–491. doi: 10.1038/ajg.2016.7. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt KJ, Müller N, Dignass A, Baumgart DC, Lehnert H, Stange EF, Herrlinger KR, Fellermann K, Büning J. Long-term Outcomes in Steroid-refractory Ulcerative Colitis Treated with Tacrolimus Alone or in Combination with Purine Analogues. J Crohns Colitis. 2016;10:31–37. doi: 10.1093/ecco-jcc/jjv175. [DOI] [PubMed] [Google Scholar]
- 31.Llaó J, Naves JE, Ruiz-Cerulla A, Marín L, Mañosa M, Rodríguez-Alonso L, Cabré E, Garcia-Planella E, Guardiola J, Domènech E. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8:1523–1528. doi: 10.1016/j.crohns.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Sood A, Midha V, Sood N, Bansal M. Long term results of use of azathioprine in patients with ulcerative colitis in India. World J Gastroenterol. 2006;12:7332–7336. doi: 10.3748/wjg.v12.i45.7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sood A, Midha V, Sood N, Kaushal V. Role of azathioprine in severe ulcerative colitis: One-year, placebo-controlled, randomized trial. Indian J Gastroenterol. 2000;19:14–16. [PubMed] [Google Scholar]
- 34.Moskovitz DN, Van Assche G, Maenhout B, Arts J, Ferrante M, Vermeire S, Rutgeerts P. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:760–765. doi: 10.1016/j.cgh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: A long-term experience. Eur J Gastroenterol Hepatol. 2005;17:79–84. doi: 10.1097/00042737-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Arts J, D'Haens G, Zeegers M, Van Assche G, Hiele M, D'Hoore A, Penninckx F, Vermeire S, Rutgeerts P. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10:73–78. doi: 10.1097/00054725-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Prieux-Klotz C, Nahon S, Amiot A, Sinayoko L, Galéano-Cassaz C, Chaussade S, Coriat R, Lahmek P, Abitbol V. Rate and Predictors of Mucosal Healing in Ulcerative Colitis Treated with Thiopurines: Results of a Multicentric Cohort Study. Dig Dis Sci. 2017;62:473–480. doi: 10.1007/s10620-016-4374-0. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Shimoyama T, Umegae S, Matsumoto K. Tacrolimus vs. anti-tumour necrosis factor agents for moderately to severely active ulcerative colitis: A retrospective observational study. Aliment Pharmacol Ther. 2016;43:705–716. doi: 10.1111/apt.13531. [DOI] [PubMed] [Google Scholar]
- 39.Ogata H, Kato J, Hirai F, Hida N, Matsui T, Matsumoto T, Koyanagi K, Hibi T. Double-blind, placebo-controlled trial of oral tacrolimus (FK506) in the management of hospitalized patients with steroid-refractory ulcerative colitis. Inflamm Bowel Dis. 2012;18:803–808. doi: 10.1002/ibd.21853. [DOI] [PubMed] [Google Scholar]
- 40.Cheifetz AS, Stern J, Garud S, Goldstein E, Malter L, Moss AC, Present DH. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol. 2011;45:107–112. doi: 10.1097/MCG.0b013e3181e883dd. [DOI] [PubMed] [Google Scholar]
- 41.Pavez CO, Ruiz MIM, Bretón AI, Palma SR, Barrio PDB, Candia RB. Terapia para la enfermedad de Crohn: infliximab, azatioprina o combinación. Gastroenterol latinoam. 2011;22:277–280. [Google Scholar]
- 42.Meyers S, Sachar DB, Goldberg JD, Janowitz HD. Corticotropin versus hydrocortisone in the intravenous treatment of ulcerative colitis. A prospective, randomized, double-blind clinical trial. Gastroenterology. 1983;85:351–357. [PubMed] [Google Scholar]
- 43.Kjeldsen J. Treatment of ulcerative colitis with high doses of oral prednisolone. The rate of remission, the need for surgery, and the effect of prolonging the treatment. Scand J Gastroenterol. 1993;28:821–826. doi: 10.3109/00365529309104016. [DOI] [PubMed] [Google Scholar]
- 44.Hyde GM, Thillainayagam AV, Jewell DP. Intravenous cyclosporin as rescue therapy in severe ulcerative colitis: Time for a reappraisal? Eur J Gastroenterol Hepatol. 1998;10:411–413. doi: 10.1097/00042737-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen OO, Cortot A, Jewell D, Wright JP, Winter T, Veloso FT, Vatn M, Persson T, Pettersson E. A comparison of budesonide and mesalamine for active Crohn's disease. International Budesonide-Mesalamine Study Group. N Engl J Med. 1998;339:370–374. doi: 10.1056/NEJM199808063390603. [DOI] [PubMed] [Google Scholar]
- 46.Chun A, Chadi RM, Korelitz BI, Colonna T, Felder JB, Jackson MH, Morgenstern EH, Rubin SD, Sacknoff AG, Gleim GM. Intravenous corticotrophin vs. hydrocortisone in the treatment of hospitalized patients with Crohn's disease: A randomized double-blind study and follow-up. Inflamm Bowel Dis. 1998;4:177–181. doi: 10.1097/00054725-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Oshitani N, Matsumoto T, Jinno Y, Sawa Y, Hara J, Nakamura S, Arakawa T, Kitano A, Kuroki T. Prediction of short-term outcome for patients with active ulcerative colitis. Dig Dis Sci. 2000;45:982–986. doi: 10.1023/a:1005589428082. [DOI] [PubMed] [Google Scholar]
- 48.Van Assche G, D'Haens G, Noman M, Vermeire S, Hiele M, Asnong K, Arts J, D'Hoore A, Penninckx F, Rutgeerts P. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125:1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 49.Gustavsson A, Halfvarson J, Magnuson A, Sandberg-Gertzén H, Tysk C, Järnerot G. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol. 2007;102:2513–2519. doi: 10.1111/j.1572-0241.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 50.Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 51.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 52.Gottlieb K, Travis S, Feagan B, Hussain F, Sandborn WJ, Rutgeerts P. Central Reading of Endoscopy Endpoints in Inflammatory Bowel Disease Trials. Inflamm Bowel Dis. 2015;21:2475–2482. doi: 10.1097/MIB.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 53.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Sands BE, Abreu MT, Ferry GD, Griffiths AM, Hanauer SB, Isaacs KL, Lewis JD, Sandborn WJ, Steinhart AH. Design issues and outcomes in IBD clinical trials. Inflamm Bowel Dis. 2005;11 Suppl 1:S22–S28. doi: 10.1097/01.mib.0000184849.38816.39. [DOI] [PubMed] [Google Scholar]