Abstract
The incidence of coccidioidomycosis, also known as Valley Fever, is increasing in the Southwestern United States and Mexico. Despite considerable efforts, a vaccine to protect humans from this disease is not forthcoming. The aim of this project was to isolate and phylogenetically compare bacterial species that could serve as biocontrol candidates to suppress the growth of Coccidioides immitis, the causative agent of coccidioidomycosis, in eroded soils or in areas close to human settlements that are being developed. Soil erosion in Coccidioides endemic areas is leading to substantial emissions of fugitive dust that can contain arthroconidia of the pathogen and thus it is becoming a health hazard. Natural microbial antagonists to C. immitis, that are adapted to arid desert soils could be used for biocontrol attempts to suppress the growth of the pathogen in situ to reduce the risk for humans and animals of contracting coccidioidomycosis. Bacteria were isolated from soil samples obtained near Bakersfield, California. Subsequently, pairwise challenge assays with bacterial pure cultures were initially performed against Uncinocarpus reesii, a non-pathogenic relative of C. immitis on media plates. Bacterial isolates that exhibited strongly antifungal properties were then re-challenged against C. immitis. Strongly anti-C. immitis bacterial isolates related to Bacillus subtilis and Streptomyces spp. were isolated, and their antifungal spectrum was investigated using a selection of environmental fungi.
Keywords: Coccidioides immitis, Valley Fever, biocontrol, microbial antagonists, challenge assays, arid soils
1. Introduction
Valley Fever, also known as coccidioidomycosis, is an infection caused by the ascomycete fungal pathogens Coccidioides spp. The dimorphic C. immitis and C. posadasii are endemic to soils of American deserts and semi-arid regions where they can live as soil saprophytes. C. immitis is thought to be endemic to the Southern San Joaquin Valley, whereas C. posadasii is predominantly localized in Southern Arizona, but it has also been detected in other states of the Southwestern United States, such as Texas, Nevada, New Mexico, and areas in Mexico and South America [1,2]. Recently, C. immitis was isolated from Washington State and the expansion of its range due to climate change is being discussed [3,4,5]. Although Coccidioides spp. are considered to be maintained in rodent reservoirs [6,7], arthroconidia of the pathogen can become airborne when they grow as a soil saprophyte following soil disruption. When inhaled, these dormant forms of the pathogen can infect the lung of humans and animals, primarily mammals [8,9].
Epidemiological studies documented that the number of reported cases of coccidioidomycosis in the United States increased from 1200 in 1995 to more than 20,000 in 2011, including about 5000 cases in California, and more than 3000 documented deaths were noted nationwide where the disease was an underlying or contributing cause [10]. In 2016 and 2017, prevalent cases reached a similar number [11,12]. Coccidioidomycosis is widely underdiagnosed and therefore underreported. It is estimated that more than 200,000 cases of coccidioidomycosis occur annually in the United States alone [13,14]. Kern County in the Southern San Joaquin Valley of California is a well-known highly endemic area for C. immitis with a generally high annual incidence of more than 2000 cases in 2016 (42% of all reported cases in California) [12]. With growing numbers of elderly and immunosuppressed persons in the United States, the number of coccidioidomycosis related deaths will probably increase, resulting in higher costs to the health care system and increased human suffering [15]. Given the significant disease burden, it is surprising that coccidioidomycosis is still considered an ‘orphan disease’ [16,17]. Furthermore, it should be noted that the development of a vaccine to protect humans from coccidioidomycosis has been unsuccessful so far, despite considerable efforts and promising initial results [18].
Although much is known about the physiology of C. immitis and how it causes disease [19,20], many fundamental gaps in our understanding of the ecology of this pathogenic fungus persist. It is known that the pathogen is adapted to loamy sands with elevated salt concentrations, and that it is able to withstand high surface temperatures (>40 °C) [9,21], unlike other microorganisms that share the Lower Sonoran Life Zone with arid to semi-arid soils. There are few published data available about the distribution of C. immitis growth sites in California [22,23] and even fewer on the diversity of bacteria, fungi, and other microorganisms in these types of soils [24,25]. C. immitis and also C. posadasii growth sites are often detected in nutrient poor, arid soils with increased pH and electrical conductivity, extreme environments where surface soil temperature easily reach > 60 °C during the summer (e.g., alkali sinks, dry lakes, and salt bush areas). In these desert environments, the pathogen likely encounters less antagonism from other soil microbes [9]. It is unknown how soil microorganisms interact with Coccidioides spp. in their natural habitat and whether there is any antibiosis or how the microbial community, including the pathogen, reacts to seasonal changes or to human influence (e.g., agriculture, pollution, and disturbance of soil due to construction). Egeberg et al. [25] showed that C. immitis is able to grow on Sabouraud medium supplemented with 8% NaCl or CaCl2, in contrast to two bacterial and one fungal antagonist.
In the complex soil environment, microbial diversity and the presence or absence of potential plant, animal, and human pathogens are influenced by soil physical and chemical parameters, but also factors such as seasonal influences and climate change, soil disturbance, diversity of plant growth, presence or absence of root exudates, and pollutants [26,27,28,29,30,31] impact microbial diversity. A very significant factor is also the interaction between microbial organisms through synergism and antagonism [25,32]. Microbial antagonism is common in soil and might explain the absence of Coccidioides spp. in soils that theoretically could support their growth based on certain physical and chemical parameters. Biological parameters, such as the presence of plants, are important for the establishment of microbial populations as well. The influence of plant root secretions on soil borne microbial communities as a powerful selective force has been confirmed [33,34,35,36,37]. Antimicrobial compounds have repeatedly been implicated in the antagonism of Streptomyces and Bacillus species against soil fungi. Some strains of Bacillus subtilis have been described as antifungal bacterial agents. A list of antifungal substances produced by Streptomyces, Bacillus and other bacteria has been published [38], and some may also inhibit the growth of Coccidioides spp., but this has never been investigated. In agriculture, bacterial antagonists have been successfully used to protect plants from certain plant pathogens, especially fungi [39,40,41,42], but biocontrol of fungi that can cause disease in humans is uncommon. Fungal-bacterial interactions (FBI) influence microbial community diversity and have been studied predominantly in terrestrial systems [43,44,45,46]. Only two microbial strains that showed antagonistic properties to C. immitis in vitro have been identified in the past: a strain related to Bacillus subtilis and a strain of Penicillium janthinellum [25].
Our project focused on the isolation and phylogenetic comparison of anti-C. immitis bacterial isolates from loamy soils collected Northeast and Southwest of Bakersfield, California, by performing pairwise FBI on artificial media plates. The aim of this study was to obtain anti-C. immitis bacterial isolates that are heat- and salt tolerant and adapted to grow in arid soils. These bacterial isolates could be candidates for the development of a biocontrol method to suppress the pathogen in natural soils on land destined for development without negatively influencing most other soil fungi.
2. Materials and Methods
2.1. Sampling Sites
For the isolation of potential anti-C. immitis bacterial species, two sampling sites (Figure 1) were chosen that were similar in soil texture and physical and chemical parameters to sites identified in a previous study to support the growth of C. immitis. Site A is a non-agricultural field located Southwest of Bakersfield close to the Buena Vista Aquatic Recreational Area that has tested positive for the presence of the pathogen in two consecutive years (data not shown). Soil physical and chemical parameters obtained from the websoilsurvey database of the United States Department for Agriculture (USDA) are similar to other sites around Kern County where the pathogen has been detected [20]. Site B is close to a C. immitis positive site known as Sharktooth Hill [21,47] and is located Northeast of Bakersfield, near the California Living Museum (CALM). Both sites are partially covered with different species of grasses (predominantly invasive Bromus spp.) and other native and non-native annuals. Site descriptions and soil parameters are listed in Table 1.
Figure 1.
(A) Soils at site A (Cole’s Levee Road [CLR]) appearing dry and low in organic matter. (B) Site B (near the California Living Museum [CALM]) showing evidence of fossil digging.
Table 1.
Soil physical and chemical parameters obtained from the United States Department for Agriculture (USDA) websoilsurvey database for our two sampling sites and reference site.
| Sampling Sites | Reference Site | ||
|---|---|---|---|
| Site A (Cole’s Levee Rd.) | Site B (across CALM) | Sharktooth Hill | |
| Soil Parameters | |||
| coordinates | 119° 13″ 60.0′ W, 35° 14″ 08.0′ N | 118°513″ 14.1′ W 35° 25″ 50.3′ N | 118° 54″ 37.0′ W, 35° 28″ 20.0′ N |
| vegetation | grasses and herbs (native and non-native) | grasses and herbs (native and non-native) | grasses and herbs (native and non-native) |
| soil type | Garces loam | Chanac clay loam | Pleito-Trigo-Chanac complex |
| landform | fan remnants | fan remnants | fan remnants/stream terraces |
| parent material | alluvium derived from granitoid | alluvium derived from mixed | alluvium derived from mixed |
| (soil map unit symbols) | 180 | 130 | 205 |
| drainage class | well drained | well drained | well drained |
| maximum salinity (mmhos/cm) | 8–16 | 0–2 | 0–2 |
| Physical Parameters | |||
| Surface texture | clay loam | clay loam | gravelly clay loam |
| Clay (%) | 25.5 | 31 | 33.5 |
| Silt (%) | 36.5 | 33.6 | 36.5 |
| Sand (%) | 38 | 35.4 | 30 |
| Available water capacity (cm/cm) | 0.21 | 0.17 | 0.16 |
| Available water supply (0-25 cm) | 5.04 | 4.25 | 3.69 |
| Organic matter (%) | 0.98 | 0.75 | 1.5 |
| Water content (15 bar) | 16.7 | 18.2 | 17.2 |
| Water content (1/3 bar) | 30.9 | 30.1 | 29.3 |
| Sat. hydraulic conductivity (Ksat) (micrometers/s) | 8.37 | 9 | 2.82 |
| Chemical Parameters | |||
| pH | 8.5 | 7.9 | 7.8 |
| CaCO3 (%) | 3 | 3 | 0 |
| Cation exchange capacity (CEC7) (milliequivalents/100 grams) | 20.6 | 24.4 | 24.3 |
| Gypsum (%) | 0 | 0 | 0 |
| Sodium adsorption ratio (SAR) | 2 | 0 | 0 |
| Electrical conductivity (decisiemens/m) | 5 | 0 | 0.5 |
Environmental fungi were isolated from outside air at the California State University Bakersfield (CSUB) campus.
2.2. Soil Sampling
Soil samples (~20 g, 5–7 cm depth) were collected using aseptic techniques and placed into sterile containers (Thermo Scientific* Samco* Wide-Mouth Bio-Tite* Specimen Containers, Fisher Scientific, Pittsburgh, PA, USA). They were transported to the laboratory on ice and processed for bacterial isolation on the same day.
2.3. Isolation of Bacteria and Fungi
To obtain bacterial isolates, soil samples were diluted 1:100,000 in a 10-fold dilution series, and aliquots of 100 µL were plated onto low nutrient R2A medium (Difco) that was supplemented with 10% soil extract (SE) (3 replicate plates). Soil extract was produced by autoclaving 200 g of soil from the original sampling sites with 200 mL of distilled water. After autoclaving, the soil slurry settled overnight, and the supernatant was harvested and used as medium supplement. Soil extract was included to provide the growing microorganisms with a diversity of nutrients and trace elements from their natural habitat. It has been shown in previous research that the addition of soil extract to growth media can significantly increase the diversity of growing bacteria by providing additional nutrients and growth factors [48]. The plates were incubated for two weeks at room temperature under aerobic conditions. All colony morphology types that resembled spore forming Bacillus and Streptomyces related species were transferred onto new media plates until pure cultures were obtained. The Gram behavior of the bacterial isolates was determined by using the Gram stain method [49]. We focused on Gram positive spore formers, because these species are more likely to withstand harsh environmental conditions compared to most Gram-negative species. Spore-forming microbes can easily be harvested and stored and are therefore good candidates for biocontrol attempts.
To obtain fungal isolates from airborne spores, R2A+SE plates were exposed to the outside air at the CSU Bakersfield campus for 10 min. These plates were also incubated for up to 2 weeks at room temperature under aerobic conditions. Different fungal colonies were re-streaked onto new media plates until pure cultures were obtained.
2.4. Challenge Assays
All bacterial pure cultures, isolated from our soil samples, were initially screened for antifungal activities against Uncinocarpus reesii which has been considered a non-pathogenic strain of C. immitis by some researchers [50,51,52]. All anti-U. reesii bacterial isolates were subsequently challenged for anti-C. immitis activity at the Monterey Public Health Laboratory in Salinas, California. Fungal colonies were grown in the center of R2A+SE plates and allowed to grow to about 1 cm diameter at 30 °C, before two bacterial isolates were inoculated at either site of the fungus in a simple streak (distance 1 cm). Plates were incubated for one week at 30 °C and then evaluated for a visible zone of inhibition between the fungus and the bacterial isolates. The challenge assays were scored visually as follows:
Strongly antifungal: an inhibition zone of several mm appeared between fungi and bacteria. The fungus was unable to overgrow the bacteria.
Weakly antifungal: the density of the fungal mycelium was lower toward the bacterial area, whereas all bacteria free space on the plate was covered with a thick fungal mycelium. The fungal growth adjacent to the bacterial growth was also slowed down considerably.
Not antifungal: the whole plate was evenly covered with fungal hyphae [53,54].
All anti-C. immitis bacterial isolates, from the screening described above, were then challenged for antagonistic effects against different environmental fungi using the method described above to investigate the spectrum of antagonism (broad or narrow). The mechanisms of antibiosis were not investigated.
U. reesii was purchased from the American Type Culture Collection (ATCC, # 34533), and a C. immitis isolate was obtained from the laboratory of the County of Kern Public Health Services Department, Bakersfield, CA (patient specimen KC12672). A PCR fragment (475 bp) of this isolate obtained with primer pair EC3/EC100 (see Section 2.6) was 99% related to an 18S rDNA fragment from C. immitis in the GenBank nucleotide database (Accession # MH863096) of the National Center of Bioinformatics (NCBI).
2.5. Heat and Salt Tolerance of Anti-Coccidioides Bacterial Isolates
All anti-C. immitis bacterial isolates obtained in this study were investigated for their ability to tolerate extreme environmental conditions, such as increased salt concentrations and increased temperature. The bacteria were grown on R2A+SE supplemented with 10% and 20% sodium chloride or with 0.5% borax (disodium tetraborate), a compound that is often found in Coccidioides endemic habitats in California and which is known for its antimicrobial properties [55]. These plates were incubated at 30 °C under aerobic conditions. Furthermore, the bacterial isolates were investigated for their abilities to tolerate 40 °C, 50 °C, and 63 °C on R2A+SE medium. All plates were incubated for one week and then evaluated for growth.
2.6. DNA Extraction from Bacterial and Fungal Pure Cultures and PCR
The phylogenetic relationship of bacterial isolates was investigated using DNA extraction followed by PCR targeting the ribosomal gene. DNA from anti-fungal bacterial isolates and from environmental fungi was extracted with the MoBio Microbial DNA isolation kit (MoBio, Solana, CA, USA) according to the manufacturer’s protocol. Fragments of the 16S rRNA gene from bacterial isolates resembling members of the actinomycetes based on visual observations were amplified using primer pair 243F/1378R following the procedure described in [56]. Bacterial isolates that did not resemble strains of actinomycetes were amplified with primer pair 8F/1492R [57,58]. DNA from fungal isolates was amplified using primer pair NSA3/NLC2 [59]. Amplification reactions were carried out in a 25 µL reaction volume containing 12.5 µL 2x GoTaq Green MasterMix (Promega, Madison, WI), 1.5 µL of each primer (250 nmol), 1.5 µL of DNA template (10-100 nmol), 7.5 µl of sterile ddH2O using a C1000 Touch Thermal Cycler (BioRad, USA) and were based on the protocols referenced earlier. Briefly, for primer pair 243F/1378R an initial denaturation step of 5 min at 94 °C was followed by 35 cycles which consisted of 1 min at 94 °C, 1 min at 63 ºC, and 1 min at 72 °C with a final elongation at 72 °C for 10 min. For primer pair 8F/1492R an initial denaturation at 94 °C for 4 min was followed by 35 cycles which consisted of 30 sec at 94 °C, 30 sec at 50 °C and 1 min at 72 °C, followed by a final elongation at 72 °C for additional 10 min. The amplification protocol using fungal DNA targeting for 18S rDNA followed the steps as described in detail by Martin and Rygiewicz [59]. The correct size of all PCR products was verified on 2% agarose gels in 1X Tris, borate, EDTA (TBE) buffer with a PCR marker for comparison (exACTGene, Fisher Scientific, Pittsburgh, PA). Gels were stained with SYBRsafe (Invitrogen, Carlsbad, CA, USA) and documented using the Universal Hood II Gel documentation system (BioRad, Hercules, CA, USA).
2.7. Sequencing and Phylogenetic Analysis of rDNA Fragments
In preparation of sequencing, PCR products from microbial pure cultures were purified using the Qiagen PCR purification kit (Qiagen, USA) according to the manufacturer’s instructions. All rDNA fragments were sequenced at the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida. All retrieved sequences were compared to entries in the nucleotide database GenBank using the Basic Local Alignment Search Tool (BLAST) (www.ncbi.nlm.nih.gov). Finally, a phylogenetic tree was assembled which included bacterial rDNA sequences from anti-fungal bacterial isolates together with their closest matches in the GenBank nucleotide database with support of the software MEGA 7 [60].
3. Results
3.1. Isolation of Bacterial and Fungal Pure Cultures
Spore forming bacterial species such as Streptomyces spp. and Bacillus spp. were recognizable on the original isolation plates based on their colony morphology and made up a considerable fraction of the overall colony morphology types. The plate count on R2A+SE medium revealed approximately 15.6 × 105 cfu/mL, predominantly bacteria. About 4 % of the colonies visually represented Streptomyces spp. or related species based on their colony morphology (rigid and round colonies, powdery surface due to spore formation, earthy smell, and release of pigments into the surrounding medium). Fungal species were observed on the plates as well, predominantly belonging to the genus Penicillium, as revealed by colony color (mostly turquoise green) and microscopy of conidia characteristic for this genus [61]. Coccidioides spp. did not grow on any plates.
Overall, 100 bacterial isolates were obtained in pure culture from both sampling sites (60 isolates from sampling site A [CLR], and 40 from site B [across CALM]). As mentioned earlier, we focused on the isolation of Gram positive, spore forming bacterial species such as Streptomyces and Bacillus species, because these organisms are better adapted to harsh conditions in their natural habitat because of their ability to form spores. Gram negative, non-spore forming species were also part of the natural bacterial community in these soils (presented by predominantly pink colonies on original plates). Furthermore, 13 fungal isolates were obtained from the air at CSUB campus.
Antagonistic effects between some bacterial colonies and some fungi were observed on the original media plates (Figure 2).
Figure 2.
Visible zone of inhibition between bacterial colonies and fungal colonies (samples from site 1 [CLR]). (A) A large bacterial colony related to Bacillus sp. is inhibiting the growth of a Penicillium sp. Small white colonies were identified as members of the Streptomyces genus which indicates that these bacteria are among the dominant members of the cultivable soil microbiota at our sampling sites. Results for site 2 were similar (not shown). (B) A small bacterial colony identified as Streptomyces sp. is inhibiting a Penicillium sp. The zones of inhibition are indicated by a white arrow.
3.2. Challenge Assays
Of the 100 bacterial cultures obtained from both sampling sites, 35 inhibited the growth of U. reesii in challenge assays on R2A+SE. These isolates predominantly belonged to different species of Streptomyces, but also some members of the genus Bacillus showed anti-U. reesii properties. From these 35 anti-U. reesii bacterial isolates, eight inhibited C. immitis (Table 2). These anti-C. immitis bacterial isolates were subsequently challenged against different environmental fungi identified as members of the Pleosporales, Hypocreales, Chaetothyriales and Eurotiales. Differences in the spectrum of their anti-fungal effects were observed. Generally, bacterial isolates related to the Bacillaceae showed a broader spectrum of antifungal behavior compared to members of the Actinobacteria. Some of the isolated Streptomyces species were only antifungal against the Onygenales U. reesii and C. immitis. Examples of challenge assay results are presented in Figure 3.
Table 2.
Challenge assays of bacterial isolates against environmental fungal isolates revealed differences in antifungal spectra.
| Isolate # | Fungal ID | GenBank Accession # or ATCC # | Similarity (%) | Challenge Assays: Bacteria against Fungi | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | Firmicutes | ||||||||||
| IV-4 | IV-7 | IV-18 | BA-3 | T-4b | IV-1A | BA-35 | BA-36 | ||||
| KVF4 | Alternaria alternata | AY154682 | 96 | neg | neg | neg | neg | neg | pos | pos | pos |
| LDF1 | Aspergillus versicolor | KJ082097 | 99 | neg | neg | pos | neg | neg | neg | pos | pos |
| LDF2 | Aspergillus keveii | MF004311 | 98 | neg | neg | neg | neg | neg | pos | pos | pos |
| ZMF2 | Lewia infectoria | AY154691 | 98 | neg | pos | neg | neg | neg | pos | pos | pos |
| AKF4 | Aspergillus keveii | MF004311 | 98 | neg | neg | neg | neg | neg | pos | pos | pos |
| AKFB | Penicillium citrinum | KM491892 | 99 | neg | pos | neg | neg | neg | pos | neg | pos |
| 482-2 | Penicillium gladioli | DQ339568 | 92 | neg | neg | neg | neg | neg | neg | pos | pos |
| 426-1 | Penicillium gladioli | DQ339568 | 92 | neg | neg | neg | neg | neg | pos | pos | pos |
| 435-2 | Penicillium gladioli | DQ339568 | 92 | neg | neg | neg | neg | neg | pos | pos | pos |
| N26-6 | Peniophora sp. | HQ608067 | 98 | neg | neg | neg | neg | neg | neg | neg | neg |
| ZMF-1 | Fusarium proliferatum | LT841264 | 97 | neg | neg | neg | neg | neg | pos | pos | pos |
| AKF7 | Fusarium accuminatum | KJ019024 | 98 | neg | neg | neg | neg | neg | neg | neg | neg |
| U. reesii | Uncinocarpus reesii | ATCC34534 | 100 | pos | pos | pos | pos | pos | pos | pos | pos |
| C. immitis | Coccidioides immitis | MH863096 | 99 | pos | pos | pos | pos | pos | pos | pos | pos |
Figure 3.
Results of challenge assays on R2A+SE medium. (A) Bacterial isolate # F, closely related to Streptomyces anulatus (AB184199), showed antifungal activity against U. reesii. A distinct zone of inhibition (ZOI) is visible. (B) Isolate # BA-35, closely related to Bacillus subtilis (JN641290), showed antifungal activity against C. immitis. No anti-C. immitis activity was observed for isolate I-BA-2 (a Streptomyces sp. that was antifungal against U. reesii).
3.3. Phylogenetic Relationships of Bacterial Isolates with Anti U. reesii and Anti-C. Immitis Properties
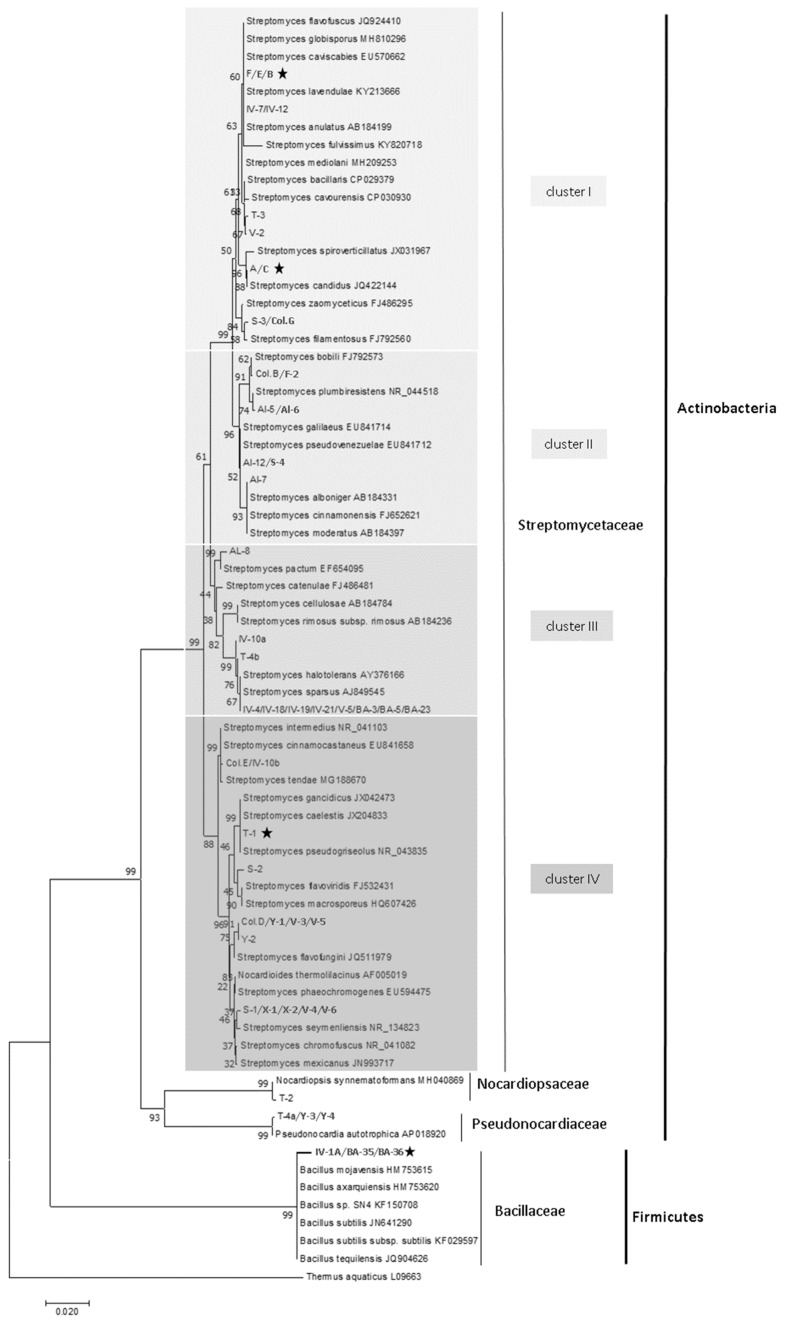
We were able to phylogenetically describe all anti-fungal bacterial species by sequencing about 1100 bp of the 16S rRNA gene obtained with primer pairs 243F/1378R or 8F/1492R. The majority (87.5 %) of the bacterial isolates belonged to different members of the genus Streptomyces. The fragment size of 1100 bp did not allow us to identify our bacterial isolates to the species level but it was sufficient to phylogenetically compare them. The phylogenetic analysis revealed four main clusters within the family Streptomycetaceae with bacterial isolates related to S. flavofuscus and S. filamentosus (cluster I), S. plumbiresistens and S. moderatus (cluster II), S. catenulae and S. sparsus (cluster III), S. intermedius and S. mexicanus (cluster IV). Strongly anti-C. immitis isolates were closely related to S. candidus (cluster I). The most prevalent Streptomyces isolates with anti-U. reesii properties were related to S. seymenliensis (cluster IV), but they did not possess anti-C. immitis properties. The remaining 12.5% of the bacterial isolates with antifungal behavior were members of the genera Bacillus, Pseudonocardia and Nocardiopsis. The strongly anti-C. immitis isolates that were phylogenetically related to Bacillus spp. were closely related to B. subtilis and other Bacillaceae that had been isolated from the Mojave Desert Mexico, and Saudi Arabia, such as B. mojavensis, B. tequilensis, and B. axarquiensis. A phylogenetic analysis of all 16S rRNA sequences together with closest matches from the GenBank database is presented in Figure 4. The Genbank accession numbers of anti-Coccidioides bacterial rDNA sequences are KF638402-KF638416.
Figure 4.
Phylogenetic relationship between Gram positive antifungal bacterial isolates obtained in this study and other species belonging to the genera Streptomyces, Pseudonarcdia and Bacillus based on a ~1100 bp fragment of the 16S rRNA gene. Bacterial isolates belonging to the Streptomycetaceae can be divided into four main clusters with closest matches in the GenBank nucleotide database. The evolutionary history was inferred using the Neighbor-Joining method [62]. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) are shown next to the branches [63]. The tree is rooted using a sequence from Thermus aquaticus as outgroup. A star indicates bacterial isolates which were anti-U. reesii and anti-C. immitis. Evolutionary analyses were conducted in MEGA7 [64].
3.4. Heat and Salt Tolerance of Anti-Coccidioides Bacterial Isolates
The bacterial isolates varied in their response to different growth conditions in the laboratory. Many isolates tolerated temperatures up to 50 °C, but only one isolate, related to a Bacillus spp., grew at 63 °C. Many Streptomyces species formed colonies on R2A+SE medium supplemented with 0.5% borate, in contrast to Bacillus species. Not all bacterial isolates with anti-C. immitis activities tolerated these extreme conditions (Table 3).
Table 3.
Colony morphologies and closest matches in the GenBank nucleotide database, as well as tolerance to increased temperature, borate and NaCl concentrations for all bacterial isolates investigated are shown.
| Isolate ID | Colony Morphology on R2A+SE | Closest Match in GenBank with Similarity (%) and Accession # | R2A+SE, Incubated at Different Temperatures | R2A+SE, Supplemented with Salt | ||||
|---|---|---|---|---|---|---|---|---|
| 40 °C | 50 °C | 63 °C | Borate (0.5%) | NaCl (10%) | NaCl (20%) | |||
| BA-35 | large, white-tan, dull, flat, irregular margin | Bacillus subtilis, 99%, CP024961 | pos | pos | neg | neg | pos | neg |
| BA-36 | large, white-tan, dull, flat, irregular margin | Bacillus subtilis, 99%, CP024961 | pos | pos | neg | neg | pos | neg |
| IV-1A | large, white-tan, dull, flat, irregular margin | Bacillus subtilis, 99%, CP024961 | pos | pos | few colonies | neg | pos | neg |
| V-2 | medium size, grey | Streptomyces cavourensis, 98 %, CP030930 | pos | neg | neg | neg | neg | neg |
| T-3 | large size, white | Streptomyces cavourensis, 97 %, CP030930 / Streptomyces bacillaris, 98%, CP029379 | pos | neg | neg | pos | neg | neg |
| IV-10a | large size, white | Streptomyces halotolerans, 97%, AY376199 | pos | neg | neg | neg | neg | neg |
| IV-7 | medium size, purple | Streptomyces lavendulae, 99%, KY213666 | pos | neg | neg | neg | neg | neg |
| IV-12 | medium size, white | Streptomyces fulvissimus, 99%, KY820718 | pos | neg | neg | neg | neg | neg |
| T-4b | large size, white | Streptomyces halotolerans, 97%, AY376199 | pos | pos | neg | neg | neg | neg |
| V-5 | small size, white | Streptomyces sparsus, 97%, KM999546 | pos | neg | neg | pos | pos | neg |
| IV-4 | medium size, white | Streptomyces sparsus, 97%, KM999546 | pos | neg | neg | few colonies | pos | neg |
| IV-18 | medium size, white | Streptomyces sparsus, 98%, KM999546 | pos | neg | neg | pos | pos | neg |
| IV-21 | medium size, white | Streptomyces sparsus, 99%, KM999546 | pos | neg | neg | few colonies | pos | neg |
| IV-19 | medium size, white | Streptomyces sparsus, 99%, KM999546 | pos | neg | neg | pos | pos | neg |
| BA-3 | small size, white | Streptomyces sparsus, 98%, KM999546 | pos | neg | neg | pos | pos | neg |
| BA-5 | medium size, white | Streptomyces sparsus, 99%, KM999546 | pos | neg | neg | pos | pos | neg |
| BA-23 | medium size, white | Streptomyces sparsus, 99%, KM999546 | pos | neg | neg | pos | pos | neg |
| Y-2 | medium size, purple | Streptomyces flavofungini, 94%, JQ511979 | pos | neg | neg | neg | neg | neg |
| Col.B | small size, white | Streptomyces bobili, 99%, FJ792573 | pos | neg | neg | neg | neg | neg |
| T-2 | large size, white | Nocardiopsis synnemataformans, 99%, MH040869 | pos | neg | neg | pos | neg | neg |
| T-4a | tiny size, white | Pseudonocardia autotrophica, 99%, AP018920 | pos | neg | neg | neg | neg | neg |
4. Discussion
Interest in biological control has recently increased due to public concerns regarding the use of chemicals in the environment, especially in agriculture where it is used to suppress plant pathogens and to increase harvest. The need to find non-chemical alternatives has fueled investigation on natural microbial antagonists to a variety of pathogens [65,66,67].
In our research, we did not anticipate isolating novel antibiotic producing bacterial species or antifungal compounds. Instead, the focus of our efforts was to identify suitable members of the soil microbial community that show potential to inhibit the pathogenic fungus C. immitis and are also able to withstand adverse conditions in alluvium-derived, arid, and salinic soils that are typical for Coccidioides spp. habitats in the Southwestern United States and Mexico.
In this study, several Streptomyces, Pseudonocardia, Nocardiopsis, and Bacillus species with anti-C. immitis activity were isolated from two different locations near Bakersfield, Kern County, California. A selection of these bacterial isolates was further investigated regarding their antifungal spectrum against a variety of other fungal species to assess their suitability as potential selective biocontrol agents against the pathogen C. immitis. Among these bacterial isolates was only one (IV-1A, phylogenetically related to B. subtilis) that showed growth above 50 °C and tolerance to increased salinic conditions, characteristics that allowed it to thrive when other soil microbes are inhibited. However, this bacterial isolate inhibited most other soil fungi in pairwise FBI in this study and therefore it might be harmful for beneficial soil fungi. Several antifungal isolates related to Streptomyces species were able to tolerate salinic conditions as well but most stopped growing when temperature was increased to 50 °C (exception: isolate T-4b). Among these isolates were several that showed a narrow spectrum of antibiosis, some only inhibited C. immitis, which makes them good candidates for selective biocontrol. Based on early laboratory studies, it has been suspected that Coccidioides spp., as weak competitors, favor environments with reduced diversity, such as alkaline and salinic soils where fewer potential antagonists are established [24,68,69].
Arid and semi-arid environments, such as the soils investigated in this study, are often dominated by spore formers such as fungi and bacteria that are related to actinomycetes and Bacillus species which can survive heat stress and desiccation in their dormant forms. Different Streptomyces species have been found to be associated with arid soils, often established within the rhizosphere of certain desert plants where they contribute to overall plant growth and health [34,70,71,72]. We have shown in this study that Streptomyces spp. were abundant in soils of our study sites which are known to support the growth of C. immitis [21,24,68], and which might explain its spotty distribution.
Various methods to detect fungal growth inhibition in vitro, such as the pairwise challenge assays performed in this study, have been successfully developed and applied in the past [53,54,73,74]. In vitro screening assays, such as these FBI, can be useful in selecting microbial antagonists for in vivo experiments. However, direct extrapolations from results obtained by these assays to different levels of complexity such as ecosystems should be made carefully. Because of the ‘great plate count anomaly’ [75], and the selection of only one type of medium in this study, we were not able to isolate all microbial species with anti-C. immitis properties that reside in these types of soils. However, we were able to obtain several anti-C. immitis bacterial isolates within the Actinobacteria and Firmicutes (Figure 4). Only two microbial strains that are antagonistic to C. immitis have been described in the past: one strain related to Bacillus subtilis, and a second related to Penicillium janthinellum. However, neither organism inhibited C. immitis in laboratory experiments when incubation conditions reached 40 °C and when salinity was increased, mimicking environmental conditions of the soil during the summer months [24]. Furthermore, it should be noted that fungal inhibition can be lost by interactions with other microbes in a complex soil environment [45].
Antifungal activity is a relatively common characteristic among soil bacteria and fungi that compete for space and nutrients. This confers an ecological advantage in environments that support the growth of mixed microbial communities. Bacteria that show antifungal capabilities in vitro may or may not be active antagonists in the soil, while those being non-antifungal in vitro are generally also inactive in their natural environment, as has been shown by other researchers [46,76]. In addition, soil temperature, pH, moisture, and application of phosphate fertilizers are known factors that influence antibiotic production and activity [77,78,79,80]. An established microbial organism isolated from a site that could also support the growth of C. immitis, is likely a more suitable candidate for a biocontrol approach to inhibit the growth of the pathogen, compared to an exotic organism that may or may not adapt to the existing chemical and physical conditions in a particular soil. Furthermore, to support the establishment of antifungal biocontrol agents, Cretoiu et al. [81] showed that chitin amendment supported the growth of antifungal bacteria that produced the enzyme chitinase and increased soil suppressiveness toward plant pathogens. Therefore, amendment of soils to be treated with nutrients and growth factors to support the antagonist to the pathogen should be considered for a successful biocontrol approach.
Coccidioidomycosis is a well-documented occupational hazard in California. Furthermore, epidemiological studies have established that the burden of this disease is a significant problem in prisons located within endemic areas of California. Microbial antagonists to C. immitis, such as B. subtilis or Streptomyces spp., could be considered in a biocontrol approach to suppress the growth of the pathogen in endemic areas of risk (e.g., construction sites and areas surrounding prisons) [82,83]. Choosing a mixture of anti-Coccidioides microbial species that are members of the microbial community (which naturally reside in these types of soils and enhance their presence) in order to keep negative impacts on other members of the soil microbial community and local plants and animals to a minimum, could be proposed. Any risks need to be carefully investigated before biocontrol procedures are attempted in areas outside controlled conditions in the laboratory. Bacillus spp. and Streptomyces spp. like those isolated in this study can be used as probiotics in future studies to suppress the growth of C. immitis in soils of California. However, it has to be confirmed in situ that these antagonists will also be effective against the pathogen in its natural habitat and that the benefit of a biocontrol approach will outweigh any potential negative influences on other soil inhabitants, as well as plants, animals, and humans who reside in the area [84,85]. Furthermore, it should be considered that Coccidioides strains might vary in their ability to withstand microbial antagonism. Ideally, several Coccidioides isolates should be obtained from sites to be treated to confirm the antifungal abilities of any microbial antagonist to the pathogen before tests are conducted in situ.
5. Conclusions
Spraying water to reduce dust prior and during constructions in arid and semiarid environments is common but could enhance the growth of Coccidioides spp. in the soil. Adding microbial antagonists, such as those described in this study, to the water and spraying this mixture several weeks prior to the development of an area might be an alternative method to reduce the risk of contracting coccidioidomycosis. Furthermore, areas near schools and recreation sites that are known growth areas of the pathogen could be treated in early spring to suppress the growth of Coccidioides spp.
Our results might be of interest to other researchers who focus on different soil borne pathogens that cause similar infectious diseases as Valley Fever, such as blastomycosis and histoplasmosis which, together with coccidioidomycosis, are the major pulmonary mycoses of humans, because these fungal pathogens can also grow as soil saprophytes [86,87]. Future work with anti-Coccidioides bacterial isolates may include the characterization of genes involved in anti-fungal production and investigations on the mode of action of these anti-fungal compounds.
Acknowledgments
We acknowledge Maynard Moe and Isolde Francis for critical comments on the manuscript.
Author Contributions
All authors contributed substantially to the work reported. Conceptualization, Data Curation, Methodology, Project Administration, Funding Acquisition, Supervision, and Writing-original Draft Preparation, A.L.; Investigation and Validation, A.L., J.D.B., K.N.C., S.D.M., A.H.V., A.K.P. and G.G.; Visualization, A.L., G.G.; Writing–Review & Editing, A.L., J.D.B., A.K.P., G.G.
Funding
This research was funded by The National Science Foundation (NSF) grant HRD-0331537, and the CSUB Student Research Scholarship (SRS 2016). The APC was funded by the same grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Fisher M.C., Koenig G., White T., San-Blas G., Negroni R., Alvarez I.G., Wanke B., Taylor J.W. Biogeographic range expansion into South America by Coccidioides immitis mirrors New World patterns of human migration. Proc. Natl. Acad. Sci. USA. 2001;98:4558–4562. doi: 10.1073/pnas.071406098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher M.C., Koenig G.L., White T.J., Taylor J.W. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia. 2002;94:73–84. doi: 10.1080/15572536.2003.11833250. [DOI] [PubMed] [Google Scholar]
- 3.Litvintseva A.P., Marsden-Haug N., Hurst S., Hill H., Gade L., Driebe E.M., Ralston C., Roe C., Barker B.M., Goldoft M., et al. Valley Fever: Finding new places for an old disease: Coccidioides immitis found in Washington State soil associated with recent human infection. Clin. Infect. Dis. 2014;60:e1–e3. doi: 10.1093/cid/ciu681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict K., Thompson G.R., III, Deresinski S., Chiller T. Mycotic infections acquired outside areas of known endemicity, United States. Emerg. Infect. Dis. 2015;11:1935. doi: 10.3201/eid2111.141950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorris M.E., Cat L.A., Zender C.S., Treseder K.K., Randerson J.T. Coccidioidomycosis dynamics in relation to climate in the southwestern United States. GeoHealth. 2018;2:6–24. doi: 10.1002/2017GH000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmons C.W. Isolation of Coccidioides from soil and rodents. Publ. Health Rep. 1942:109–111. doi: 10.2307/4583988. [DOI] [Google Scholar]
- 7.del Rocío Reyes-Montes M., Pérez-Huitrón M.A., Ocaña-Monroy J.L., Frías-De-León M.G., Martínez-Herrera E., Arenas R., Duarte-Escalante E. The habitat of Coccidioides spp. and the role of animals as reservoirs and disseminators in nature. BMC Infect. Dis. 2016;16:550. doi: 10.1186/s12879-016-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown J., Benedict K., Park B.J., Thompson G.R., III Coccidioidomycosis: Epidemiology. Clin. Epidemiol. 2013;5:185. doi: 10.2147/CLEP.S34434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher F.S., Bultman M.W., Johnson S.M., Papagianis D., Zaborsky E. Coccidioides niches and habitat parameters in the Southwestern United States. Ann. N. Y. Acad. Sci. 2007;1111:47–72. doi: 10.1196/annals.1406.031. [DOI] [PubMed] [Google Scholar]
- 10.Center of Disease Control and Prevention (CDC Feature 24/7 Saving Lives, Protecting People) Valley Fever: Awareness is Key. [(accessed on 16 July 2017)]; Available online: http://www.cdc.gov/Features/ValleyFever/
- 11.Tabnak F., Knutson K., Cooksey G., Nguyen A., Vugia D. Epidemiologic Summary of Coccidioidomycosis in California. California Department of Public Health, Center for Infectious Diseases, Division of Communicable Disease Control; Sacramento, CA, USA: 2017. [(accessed on 23 July 2017)]. Available online: https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/CocciEpiSummary2016.pdf. [Google Scholar]
- 12.Cooksey Sondermeyer G., Nguyen A., Knutson K., Tabnak F., Benedict K., McCotter O., Jain S., Vugia D. Notes from the field: Increase in coccidioidomycosis—California, 2016. Morb. Mortal. Wkly. Rep. 2017;66:833. doi: 10.15585/mmwr.mm6631a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckley M. The Fungal Kingdom: Diverse and Essential Roles in Earth’s Ecosystem. American Society for Microbiology (ASM); Proceedings of the ASM Conference Proceedings; Washington, DC, USA. 23–26 July 2008; [PubMed] [Google Scholar]
- 14.Freedman M., Jackson B.R., McCotter O., Benedict K. Coccidioidomycosis Outbreaks, United States and Worldwide, 1940–2015. Emerg. Infect. Dis. 2018;24:417. doi: 10.3201/eid2403.170623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondermeyer G., Lee L., Gilliss D., Tabnak F., Vugia D. Coccidioidomycosis-associated hospitalizations, California, USA, 2000–2011. Emerg. Infect. Dis. 2013;19:1590. doi: 10.3201/eid1910.130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galgiani J. Fungal Diseases, a Threat to Humans, Animals, and Plant Health. National Academies Press; Washington, DC, USA: 2011. Geography, climate, dust, and disease: Epidemiology of Valley Fever (coccidioidomycosis) and ways it might be controlled; pp. 196–207. Workshop Summary. [Google Scholar]
- 17.Seidel D., Durán Graeff L.A., Vehreschild M.J., Wisplinghoff H., Ziegler M., Vehreschild J.J., Liss B., Hamprecht A., Köhler P., Racil Z., et al. FungiScope™—Global Emerging Fungal Infection Registry. Mycoses. 2017;60:508–516. doi: 10.1111/myc.12631. [DOI] [PubMed] [Google Scholar]
- 18.Hector R.F., Rutherford G.W., Tsang C.A., Erhart L.M., McCotter O., Anderson S.M., Komatsu K., Tabnak F., Vugia D.J., Yang Y., et al. The public health impact of coccidioidomycosis in Arizona and California. Int. J. Environ. Res. Publ. Health. 2011;8:1150–1173. doi: 10.3390/ijerph8041150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swatek F.E. Ecology of Coccidioides immitis. Mycopathol. Mycol. Appl. 1970;40:1–12. doi: 10.1007/BF02051479. [DOI] [PubMed] [Google Scholar]
- 20.Lacy G.H., Swatek F.E. Soil Ecology of Coccidioides immitis at Amerindian Middens in California. Appl. Microbiol. 1974;27:379–388. doi: 10.1128/am.27.2.379-388.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egeberg R.O., Ely F. Coccidioides immitis in the soil of the Southern SanJoaquin Valley. Am. J. Med. Sci. 1956;231:151–154. doi: 10.1097/00000441-195602000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Lauer A., Baal J.D., Baal J.C., Verma M., Chen J.M. Detection of Coccidioides immitis in Kern County, California, by multiplex PCR. Mycologia. 2012;104:62–69. doi: 10.3852/11-127. [DOI] [PubMed] [Google Scholar]
- 23.Ranzoni V. Fungi isolated in culture from soils of the Sonoran Desert. Mycologia. 1968;60:356–371. doi: 10.1080/00275514.1968.12018577. [DOI] [PubMed] [Google Scholar]
- 24.Orr G.F. Some fungi isolated with Coccidioides immitis from soils of endemic areas in California. Bull. Torrey Bot. Club. 1968;95:424–431. doi: 10.2307/2483474. [DOI] [Google Scholar]
- 25.Egeberg R.O., Elconin A.E., Egeberg M.C. Effect of salinity and temperature on Coccidioides immitis and three antagonistic soil saprophytes. J. Bacteriol. 1964;88:473–476. doi: 10.1128/jb.88.2.473-476.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul E.A. Soil Microbiology, Ecology and Biochemistry. Academic Press; Cambridge, MA, USA: 2014. [Google Scholar]
- 27.Castro H.F., Classen A.T., Austin E.E., Norby R.J., Schadt C.W. Soil microbial community responses to multiple experimental climate change drivers. Appl. Environ. Microbiol. 2010;76:999–1007. doi: 10.1128/AEM.02874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jurburg S.D., Nunes I., Stegen J.C., Le Roux X., Priemé A., Sørensen S.J., Salles J.F. Autogenic succession and deterministic recovery following disturbance in soil bacterial communities. Sci. Rep. 2017;7:45691. doi: 10.1038/srep45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bardgett R.D., Van Der Putten W.H. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- 30.Burns R.G. Enzyme activity in soil: Location and a possible role in microbial ecology. Soil Biol. Biochem. 1982;14:423–427. doi: 10.1016/0038-0717(82)90099-2. [DOI] [Google Scholar]
- 31.Kirchman D.L. Processes in Microbial Ecology. Oxford University Press; Oxford, UK: 2018. [Google Scholar]
- 32.De Boer W., Folman L.B., Summerbell R.C., Boddy L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005;29:795–811. doi: 10.1016/j.femsre.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Köberl M., Müller H., Ramadan E.M., Berg G. Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0024452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duineveld B.M., Kowalchuk G.A., Keijzer A., van Elsas J.D., van Veen J.A. Analysis of bacterial communities in the rhizosphere of Chrysanthemum via Denaturing Gradient Gel Electrophoresis of PCR-amplified 16S rRNA as well as DNA fragments coding for 16S rRNA. Appl. Environ. Microbiol. 2001;67:172–178. doi: 10.1128/AEM.67.1.172-178.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basil A.J., Strap J.L., Knotek-Smith H.M., Crawford D.L. Studies on the microbial populations of the rhizosphere of big sagebrush (Artemisia tridentata) J. Ind. Microbiol. Biotechnol. 2004;31:278–288. doi: 10.1007/s10295-004-0140-y. [DOI] [PubMed] [Google Scholar]
- 36.Junaid J.M., Dar N.A., Bhat T.A., Bhat A.H., Bhat M.A. Commercial biocontrol agents and their mechanism of action in the management of plant pathogens. Int. J. Mod. Plant Anim. Sci. 2013;1:39–57. [Google Scholar]
- 37.Kerr J. Bacterial inhibition of fungal growth and pathogenicity. Microb. Ecol. Health Dis. 1999;11:129–142. doi: 10.1080/089106099435709. [DOI] [Google Scholar]
- 38.Kathiravan M.K., Salake A.B., Chothe A.S., Dudhe P.B., Watode R.P., Mukta M.S., Gadhwe S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012;20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 39.Duijff B.J., Recorbet G., Bakker P.A.H., Loper J.E., Lemanceau P. Microbial antagonism at the root level is involved in the suppression of Fusarium wilt by the combination of non-pathogenic Fusarium oxysporum Fo47 and Pseudomonas putida WCS358. Biol. Control. 1999;89:1073–1079. doi: 10.1094/PHYTO.1999.89.11.1073. [DOI] [PubMed] [Google Scholar]
- 40.De Boer W., Gunnewiek P.J., Lafeber P., Janse J.D., Spit B.E., Woldendorp J.W. Anti-fungal properties of chitinolytic dune soil bacteria. Soil Biol. Biochem. 1998;30:193–203. doi: 10.1016/S0038-0717(97)00100-4. [DOI] [Google Scholar]
- 41.De Boer W., Verheggen P., Klein Gunnewiek P.J.A., Kowalchuk G.A., van Veen J.A. Microbial community composition affects soil fungistasis. Appl. Environ. Microbiol. 2003;69:835–844. doi: 10.1128/AEM.69.2.835-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas D., Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- 43.Nazir R., Warmink J.A., Boersma H., Van Elsas J.D. Mechanisms that promote bacterial fitness in fungal-affected soil microhabitats. FEMS Microbiol. Ecol. 2009;71:169–185. doi: 10.1111/j.1574-6941.2009.00807.x. [DOI] [PubMed] [Google Scholar]
- 44.Frey-Klett P., Burlinson P., Deveau A., Barret M., Tarkka M., Sarniguet A. Bacterial-fungal interactions: Hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiol. Mol. Biol. Rev. 2011;75:583–609. doi: 10.1128/MMBR.00020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haq I.U., Zhang M., Yang P., van Elsas J.D. Advances in Applied Microbiology. Volume 89. Academic Press; Cambridge, MA, USA: 2014. The interactions of bacteria with fungi in soil: Emerging concepts; pp. 185–215. [DOI] [PubMed] [Google Scholar]
- 46.De Boer W. Upscaling of fungal–bacterial interactions: From the lab to the field. Curr. Opin. Microbiol. 2017;37:35–41. doi: 10.1016/j.mib.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Kemp R. Master’s Thesis. California State College; Long Beach, CA, USA: 1974. Soil Isolation and Molecular Identification of Coccidioides immitis. [Google Scholar]
- 48.Sørheim R., Torsvik V.L., Goksøyr J. Phenotypical divergences between populations of soil bacteria isolated on different media. Microbiol. Ecol. 1989;17:181–192. doi: 10.1007/BF02011852. [DOI] [PubMed] [Google Scholar]
- 49.Gram C. Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten. Fortschritte der Medizin. 1884;2:185–189. [Google Scholar]
- 50.Pan S., Sigler L., Cole G.T. Evidence for a phylogenetic connection between Coccidioides immitis and Uncinocarpus reesii (Onygenaceae) Microbiology. 1994;140:1481–1494. doi: 10.1099/00221287-140-6-1481. [DOI] [PubMed] [Google Scholar]
- 51.Sharpton T.J., Stajich J.E., Rounsley S.D., Gardner M.J., Wortman J.R., Jordar V.S., Maiti R., Kodira C.D., Neafsey D.E., Zeng Q., et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009 doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkland T.N. Identification and characterization of genes found in Coccidioides spp. but absent in nonpathogenic Onygenales. bioRxiv. 2018:413906. doi: 10.1101/413906. [DOI] [Google Scholar]
- 53.Harris R.N., James T.Y., Lauer A., Simon M.A., Patel A. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. Ecohealth. 2006;3:53–56. doi: 10.1007/s10393-005-0009-1. [DOI] [Google Scholar]
- 54.Becker M.H., Walke J.B., Murrill L., Woodhams D.C., Reinert L.K., Rollins-Smith L.A., Burzynski E.A., Umile T.P., Minbiole K.P.C., Belden L.K. Phylogenetic distribution of symbiotic bacteria from Panamanian amphibians that inhibit growth of the lethal fungal pathogen Batrachochytrium dendrobatidis. Mol. Ecol. 2015;24:1628–1641. doi: 10.1111/mec.13135. [DOI] [PubMed] [Google Scholar]
- 55.Saita K., Nagaoka S., Shirosaki T., Horikawa M., Matsuda S., Ihara H. Preparation and characterization of dispersible chitosan particles with borate crosslinking and their antimicrobial and antifungal activity. Carbohydr. Res. 2012;349:52–58. doi: 10.1016/j.carres.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 56.Heuer H., Krsek M., Baker P., Smalla K., Wellington E.M.H. Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl. Environ. Microbiol. 1997;63:3233–3241. doi: 10.1128/AEM.69.2.835-844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards U., Rogall T., Blocker H., Emde M., Bottger E. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stackebrandt E., Liesack W. Nucleic acids and classification. In: Goodfellow M., O’Donnell A.G., editors. Handbook of New Bacterial Systematics. Academic Press; London, UK: 1993. pp. 152–189. [Google Scholar]
- 59.Martin K.J., Rygiewicz P.T. Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol. 2005;5:28. doi: 10.1186/1471-2180-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–174. [Google Scholar]
- 62.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 63.Hedges S.B. The number of replications needed for accurate estimation of the bootstrap P value in phylogenetic studies. Mol. Biol. Evol. 1992;9:366–369. doi: 10.1093/oxfordjournals.molbev.a040725. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Altieri M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999;74:19–31. doi: 10.1016/S0167-8809(99)00028-6. [DOI] [Google Scholar]
- 66.Bianchi F.J.A., Booij C.J.H., Tscharntke T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B. 2006;273:1715–1727. doi: 10.1098/rspb.2006.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shennan C. Biotic interactions, ecological knowledge and agriculture. Philos. Trans. R. Soc. B. 2008;363:717–739. doi: 10.1098/rstb.2007.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elconin A.F., Egeberg R.O., Egeberg M. Significance of soil salinity on the ecology of Coccidioides immitis. J. Bacteriol. 1964;87:500–503. doi: 10.1128/jb.87.3.500-503.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swatek F.E., Omieczynski D.T., Plunkett O.A. Coccidiodes immitis in California. In: Ajello L., editor. Coccidioidomycosis, Papers from the Second Symposium on Coccidioidimycosis. University of Arizona Press; Tucson, AZ, USA: 1967. pp. 255–265. [Google Scholar]
- 70.Broadbent P., Baker K.F., Waterworth Y. Bacteria and actinomycetes antagonistic to fungal root pathogens in Australian soils. Aust. J. Biol. Sci. 1971;24:925–944. doi: 10.1071/BI9710925. [DOI] [PubMed] [Google Scholar]
- 71.Crawford D.L., Lynch J.M., Whipps J.M., Ousley M.A. Isolation and characterization of actinomycete antagonists of a fungal root pathogen. Appl. Environ. Microbiol. 1993;59:3899–3905. doi: 10.1128/aem.59.11.3899-3905.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chowdhury S.P., Schmid M., Hartmann A., Tripathia A.K. Diversity of 16S-rRNA and nifH genes derived from rhizosphere soil and roots of an endemic drought tolerant grass, Lasiurus sindicus. Eur. J. Soil Biol. 2009;45:114–122. doi: 10.1016/j.ejsobi.2008.06.005. [DOI] [Google Scholar]
- 73.Lauer A., Simon M.A., Banning J.L., André E., Duncan K., Harris R.N. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia. 2007;1:630–640. doi: 10.1643/0045-8511(2007)2007[630:CCBFTE]2.0.CO;2. [DOI] [Google Scholar]
- 74.Nandhini S.U., Sudha S., Jeslin V.A., Manisha S. Isolation, identification and extraction of antimicrobial compounds produced by Streptomyces spp. from terrestrial soil. Biocatal. Agric. Biotechnol. 2018;15:317–321. doi: 10.1016/j.bcab.2018.06.024. [DOI] [Google Scholar]
- 75.Staley J.T., Konopka A. Measurements of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Ann. Rev. Microbiol. 1985;39:321–346. doi: 10.1146/annurev.mi.39.100185.001541. [DOI] [PubMed] [Google Scholar]
- 76.Raijmakers J.M., Vlami M., de Souza J.T. Antibiotic production by bacterial biocontrol agents. Antonie van Leeuwenhoek. 2002;81:537–547. doi: 10.1023/A:1020501420831. [DOI] [PubMed] [Google Scholar]
- 77.Shanahan P., O’Sullivan D.J., Simpson P., Glennon J.D., O’Gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl. Environ. Microbiol. 1992;58:353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ownley B.H., Weller D.M., Thomashow L.S. Influence of in situ and in vitro pH on suppression of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79. Phytopathology. 1992;82:178–184. [Google Scholar]
- 79.Georgakopoulos D., Hendson M., Panopoulos N.J., Schroth M.N. Cloning of a phenazine biosynthetic locus of Pseudomonas aureofaciens PGS12 and analysis of tis expression in vitro with the ice nucleation reporter gene. Appl. Environ. Microbiol. 1994;60:2931–2938. doi: 10.1128/aem.60.8.2931-2938.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Duffy B.K., Defago G. Environmental factors modulating antibiotic and siderophores biosynthesis by Pseudomonas flourescens biocontrol strains. Appl. Environ. Microbiol. 1999;65:2429–2438. doi: 10.1128/aem.65.6.2429-2438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cretoiu M.S., Korthals G., Visser J., van Elsas J.D. Chitin amendment raises the suppressiveness of soil towards plant pathogens and modulates the actinobacterial and oxalobacteriaceal communities in an experimental agricultural field. Appl. Environ. Microbiol. 2013;28:01361. doi: 10.1128/AEM.01361-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.California Department of Public Health, Occupational Health Branch, Preventing Work-Related Valley Fever (Coccidioidomycosis) [(accessed on 20 October 2018)];2018 Available online: https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/OHB/Pages/Cocci.aspx.
- 83.Wall D.H., Nielsen U.N., Six J. Soil biodiversity and human health. Nature. 2015;528:69. doi: 10.1038/nature15744. [DOI] [PubMed] [Google Scholar]
- 84.Bajsa N., Morel M.A., Braña V., Castro-Sowinski S. The effect of agricultural practices on resident soil microbial communities: Focus on biocontrol and biofertilization. Mol. Microb. Ecol. Rhizosphere. 2013;2:687–700. [Google Scholar]
- 85.Wheeler C., Lucas K.D., Mohle-Boetani J.C. Rates and Risk Factors for Coccidioidomycosis among Prison Inmates, California, USA, 2011. Emerg. Infect. Dis. 2015;21:70–75. doi: 10.3201/eid2101.140836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.De Medeiros Muniz M., Pizzini C.V., Peralta J.M., Reiss E., Zancopé-Olivera R.M. Genetic diversity of Histoplasma capsulatum strains isolated from soil, animals, and clinical specimens in Rio de Janeiro State, Brazil, by a PCR-based Random Amplified Polymorphic DNA assay. J. Clin. Microbiol. 2001;39:4487–4494. doi: 10.1128/JCM.39.12.4487-4494.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burgess J.W., Schwan W.R., Volk T.J. PCR-based detection of DNA from the human pathogen Blastomyces dermatidis in natural soil samples. Med. Mycol. 2006;44:741–748. doi: 10.1080/13693780600954749. [DOI] [PubMed] [Google Scholar]