Abstract
In this study, we investigated the effects of NAD(P)H oxidase (NOX) inhibitor VAS2870 (3-benzyl-7-(2-benzoxazolyl)thio-1,2,3-triazolo[4,5-d]pyrimidine) on the histamine-induced elevation of free cytoplasmic calcium concentration ([Ca2+]i) and the secretion of von Willebrand factor (vWF) in human umbilical vein endothelial cells (HUVECs) and on relaxation of rat aorta in response to histamine. At 10 μM concentration, VAS2870 suppressed the [Ca2+]i rise induced by histamine. Inhibition was not competitive, with IC50 3.64 and 3.22 μM at 1 and 100 μM concentrations of histamine, respectively. There was no inhibition of [Ca2+]i elevation by VAS2870 in HUVECs in response to the agonist of type 1 protease-activated receptor SFLLRN. VAS2870 attenuated histamine-induced secretion of vWF and did not inhibit basal secretion. VAS2870 did not change the degree of histamine-induced relaxation of rat aortic rings constricted by norepinephrine. We suggest that NOX inhibitors might be used as a tool for preventing thrombosis induced by histamine release from mast cells without affecting vasorelaxation.
Keywords: histamine, calcium, endothelial cells, NADPH-oxidase, VAS2870, von Willebrand factor, aorta, relaxation
1. Introduction
Histamine plays an important role as chemical mediator in multiple physiological and pathophysiological processes in central and peripheral tissues. Mast cells and basophils are important sources of histamine, which is released from granule stores in response to several stimuli. The pleiotropic effects of histamine are mediated via four G protein-coupled receptor (GPCR) subtypes (H1R–H4R), which differ in their distribution, ligand binding, signaling pathways, and functions [1]. Histamine acts as a full agonist on the receptors, with subtype-specific differences in affinity. H1R is ubiquitously expressed and mediates its effects by Gq/11 activation via phospholipase C (PLC) and to a minor degree by PLA2 and PLD. Activation of H1R leads to an increase of inositol-1,4,5-triphosphate (IP3) and 1,2-diacylglycerol (DAG), associated with an increase of the intracellular Ca2+ concentration, followed by activation of protein kinase C (PKC). Other signaling pathways include the stimulation of adenylyl cyclase via H2R with the formation of cAMP, inducing the generation of NO; the NF-κB cascade leads to a release or increase in the expression of (pro)inflammatory mediators (P-selectin, ICAM-1, VCAM-1, iNOS, IL-1beta, IL-6, TNF-alpha, etc.) [2]. Brain vascular endothelial cells (ECs) express histamine H1 and H2 receptors, which regulate brain capillary permeability. Also, histamine receptor genes Hrh3 and Hrh4, for corresponding H3 and H4 receptors, are expressed in rat brain ECs, which are potentially important for the regulation of blood–brain barrier permeability, including trafficking of immunocompetent cells [3]. H4 receptors play a dominant role in histamine-induced eosinophil adhesion to endothelium [4].
Reactive oxygen species (ROS) in large amounts clearly have detrimental effects on cell physiology, whereas low concentrations of ROS are permanently produced in cells and play a role as signaling molecules. An imbalance in ROS production and defense mechanisms can lead to pathological vascular remodeling. Among the possible sources of ROS within endothelial and/or neighboring cells are mitochondria, NADPH-oxidases, xanthine oxidase, peroxidases, NO-synthases, cytochrome P450, cyclooxygenases, lipoxygenases, monoamine oxidases, and the hemoglobin of red blood cells [5,6,7]. Among these possible sources of ROS, nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) play a central role. NOXs are present in neutrophils, where they mostly determine the non-specific immune response [8]. In the ischemic-reperfusion condition, NOXs substantially determine tissue injury by generating ROS in ECs and smooth muscle cells (SMCs) of blood vessels [9].
In ECs, four NOX isoforms include the superoxide-generating enzymes NOX1, NOX2, and NOX5 and the hydrogen-peroxide-generating enzyme NOX4 [10]. NOX2 is the most studied isoform of NOX, which generates superoxide anion in professional phagocytes and many other cells. In ECs, it is the predominant form, and all four subunits of NOX2 are present [11,12]. It is activated by angiotensin II [13], oxidized low-density lipoproteins [14], cytokines, and growth factors [15]. Superoxide anion generated by NOX2 causes the inactivation of nitric oxide and promotes the development of endothelial dysfunction, hypertension, and inflammation [16]. NOX4 predominantly generates hydrogen peroxide (H2O2). H2O2 produced by endothelial NOX4 potentiates vasorelaxation induced by acetylcholine and histamine [17]. Activators of other NOXs do not affect the activity of NOX4, which is constitutive, since the generation of H2O2 in cells is dependent on the level of expression of NOX4 [18]. NADPH oxidases of the DUOX group (DUOX1 and DUOX2) also generate H2O2 [19,20], though their existence and/or functional significance in ECs and SMCs has not been clearly shown.
Calcium-dependent NOX5 has been implicated in the oxidative damage of ECs in atherosclerosis and hypertension [21]. It is noteworthy that NOX5 is expressed in primates but does not occur naturally in rodents. In transgenic mice expressing human NOX5 in a vascular SMC-specific manner, agonist-induced vasoconstriction was exaggerated, and endothelium-dependent vasorelaxation was attenuated [22].
The influence of ROS on Ca2+ signaling in EC has been demonstrated in several works [23,24,25,26,27,28,29,30]. ROS can activate various calcium channels of the endoplasmic reticulum and plasma membrane, such as InsP3- and ryanodine-sensitive channels, and some cation channels of the TRP superfamily [25,31,32]. NOX-derived ROS are critical for the generation of Ca2+ oscillations in response to histamine in human aortic endothelial cells [33]. Recently, we demonstrated an involvement of two-pore channels in an H2O2-induced increase in the level of calcium ions in the cytoplasm of human umbilical vein endothelial cells (HUVECs) [34]. This could be coupled with exocytosis of the von Willebrand factor (vWF) in these cells in response to H2O2 [35]. The vWF release from EC is induced by superoxide anion [30]. In the present work, we studied the role of NOX in histamine-induced Ca2+ rise and vWF secretion in HUVECs and NOX involvement in rat aorta relaxation in response to histamine. To solve these questions, we used VAS2870 as a tool inhibitor of NOXs [36]. VAS2870 belongs to the triazolopyrimidines, which are regarded as the most specific inhibitors of NOXs [37].
2. Methods
2.1. Reagents
VAS2870, histamine, and SFLLRN were from SigmaAldrich (St. Louis, MO, USA); CalciumGreen/AM and dihydroethidium were from Thermo Fischer Scientific (Waltham, MA, USA). VAS2870 was dissolved at a 10 mM concentration in DMSO. Before being added to the cells, it was dissolved to the required concentrations in physiological salt solution (PSS). DMSO at appropriate concentrations was used as a vehicle control. PSS was added as a vehicle control for histamine and SFLLRN.
2.2. Cell Culture
Human umbilical vein endothelial cells (HUVECs) were isolated according to [38]. The cells were grown in plastic dishes pre-coated with gelatin, using M199 medium with Earl’s salts and 20 mM HEPES containing 20% fetal calf serum (SigmaAldrich), 300 µg/mL endothelial growth supplement, isolated from rabbit brain, 100 µg/mL heparin and gentamicin. We used the cells on early passages (1–4). Accutase® was applied for passaging the cells (SigmaAldrich).
2.3. Measurement of Free Cytoplasmic Calcium Concentration in HUVECs
HUVECs grown in 96-well plates were loaded with 1 µM CalciumGreen/AM dissolved with 0.02% Pluronic F-127 in M199 during 1 h at 37 °C in a CO2 incubator (New Brunswick Scientific, Edison, NJ, USA). Measurement of [Ca2+]i was performed in physiological salt solution (PSS) containing NaCl (145 mM), KCl (5 mM), MgCl2 (1 mM), CaCl2 (1 mM), HEPES (5 mM), and D-glucose (10 mM), at pH 7.4. Fluorescence was registered at 485 nm (excitation) and 530 nm (emission) at 25 °C using a Synergy 4 Microplate Reader (BioTek, Winooski, VT, USA). The changes in [Ca2+]i in HUVECs are presented as the ratio of the increment in F530 and initial F530. Each curve in in the graph is a superposition of three curves recorded simultaneously from three wells in a plate.
2.4. Registration of ROS Generation in HUVECs
Kinetics of ROS generation in HUVECs was registered with the fluorescent indicator dihydroethidium. The fluorescence was measured by a Synergy 4 Microplate Reader (BioTek) with excitation filter 485 nm (bandwidth 20 nm) and emission 620 nm (bandwidth 40 nm). The bandwidth of the emission filter is enough wide to cover a large part of the emission of the oxidation products of DHE [39]. The cells grown in 96-well plates were incubated with different concentrations of VAS2870 or vehicle control (dimethylsulfoxide at final concentrations from 0.006% to 0.2%) during 5 min and then DHE at final concentration 2.5 μM was added. The slight inhibitory effect of DMSO on DHE oxidation at a concentration of 0.2% was taken into account when analyzing the data. The oxidation kinetics of DHE determined by the increase in fluorescence was linear during the 60 min of incubation both in the absence and in presence of VAS2870. The increase in fluorescence in the absence of VAS2870 was taken as 100%. The results are presented as the mean values obtained in three experiments with different cell preparations. In each experiment, the experimental point was a mean value of the fluorescence from six wells in a 96-well plate.
2.5. Measurement of vWF Secretion
HUVECs grown in 48-well plates were incubated in PSS at 30 °C during 5 min with or without 10 μM VAS2870. Then, histamine at final concentration or vehicle were added and the cells were incubated for 30 min. The extracellular fluid from each well was collected in a separate tube and frozen. Later, it was used for the measurement of vWF concentration with a TECHNOZYM vWF:Ag ELISA kit (Technoclone, Vienna, Austria). The values of the secreted vWF are the means of six measurements.
2.6. Registration of Aorta Contraction
Aorta were isolated from male Wistar rats weighing 250–300 g. The rats were anesthetized with 25% urethane (4 mL/kg) and decapitated. All manipulations with the animals were performed in accordance with the guide for the care and use of Laboratory animals of the Bioethics committee of the Koltsov Institute of Developmental Biology and European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. Aorta were cleaned from connective tissue and cut into rings with a width of around 2 mm. Experiments were performed on a four-channel wire myograph (ADInstruments, Bella Vista, New South Wales, Australia) using LabChart 7.3.7 program for data acquisition and analysis. The rings were mounted on the holders in chambers filled with Krebs–Henseleit solution (37°C) perfused with 95% O2/5% СO2 and extended with a force of 1 g. Contractility of the vessel rings and intactness of its endothelium were tested by adding 10−7 M norepinephrine and then 10−5 M carbachol. After washing, norepinephrine and carbachol were added again. Aortic rings with relaxation of at least 50% were used for the experiments.
2.7. Statistics
Data are presented as mean ± SEM. The number of measurements is presented in the legend to the figures. In each case at least three independent experiments were performed with different cell or vessel preparations. Statistical significance was calculated using Excel 2003 and MedCalc 18.9.1 statistical software according the Student-Neuman-Keuls test. The IC50 values were determined with GraphPad Prism 8.0.
3. Results and Discussion
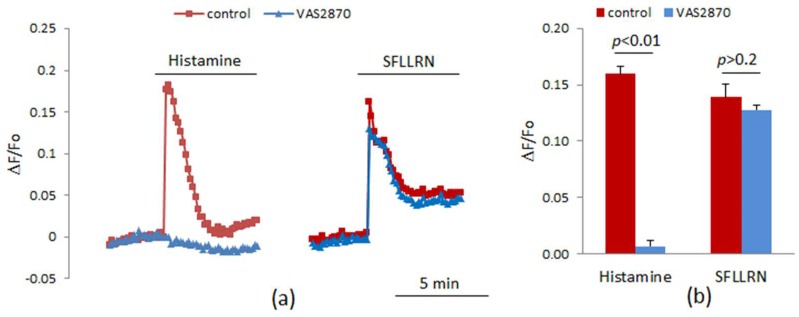
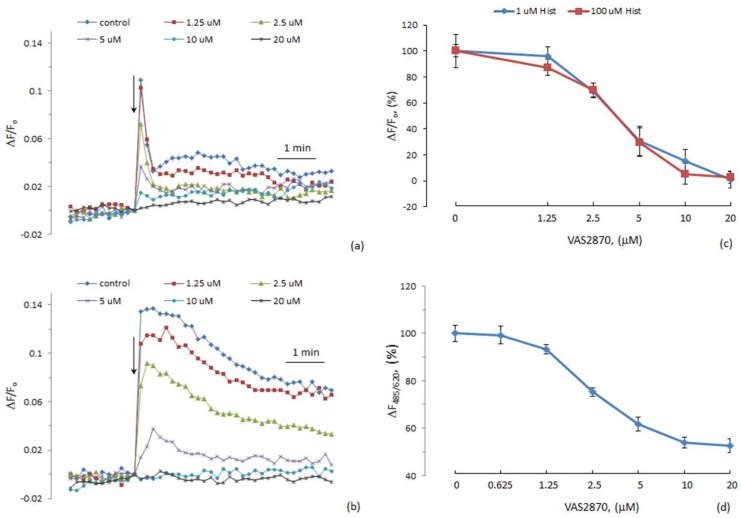
We studied the effect of VAS2870 on [Ca2+]i elevation induced by histamine. At a concentration of 10 μM, VAS2870 almost completely suppressed the calcium signal of 1 mM histamine, while there was no significant inhibition of the action of PAR1 agonist SFLLRN (Figure 1). The effect of VAS2870 was not competitive toward histamine, since wat is observed at a low concentration of VAS2870, which is two orders of magnitude lower than the concentration of histamine. We determined the concentration-dependence curves for the inhibition by VAS2870 of [Ca2+]i elevation at different histamine concentrations (Figure 2c). The IC50 for VAS2870 calculated from these data was 3.64 and 3.22 μM at 1 and 100 μM concentrations of histamine, respectively. These results suggest the existence of a specific mechanism of histamine-induced [Ca2+]i mobilization in HUVECs. The following data provide evidence in favor of this conclusion. It has been shown that two-pore channels activated by NAADP are involved in histamine-induced [Ca2+]i mobilization in HUVEC via H1 receptors, while calcium signaling of thrombin is independent from NAADP [40,41].
Figure 1.
The influence of VAS2870 (10 µM) on calcium signaling in response to histamine (10 μM) and agonist of PAR1 SFLLRN (2 µg/mL). (a) The curves represent the data of one of three experiments with similar results; (b) The bars represent the mean values of [Ca2+]i increase in three independent experiments. [Ca2+]i was measured as a relative increase in CalciumGreen fluorescence. In control experiments, the vehicle, dimethylsulfoxide (DMSO) at a final concentration 0.1%, was added instead of VAS2870.
Figure 2.
The influence of different concentrations of VAS2870 on histamine-induced [Ca2+]i increases and on the generation of reactive oxygen species (ROS) in human umbilical vein endothelial cells (HUVECs). (a,b) The kinetics of [Ca2+]i elevation in HUVECs in response to 1 and 100 μM histamine, respectively. (c) Concentration-dependent curves of the inhibition of [Ca2+]i increases. (d) Concentration-dependence curve of the inhibition of dihydroethidium (DHE) oxidation by VAS2870. Each point in (c,d) is a mean of three values from three independent experiments on different cell preparations. In control experiments, it was demonstrated that DMSO at concentrations on which it was applied to the cells along with VAS2870 did not suppress histamine-induced [Ca2+]i elevation and oxidation of DHE.
VAS2870 in micromolar concentrations is widely used to suppress NOX activity in different types of cells [42,43,44,45]. The IC50 for VAS2870’s effect on NOX activity in a cell-free system with membranes and cytosol from human neutrophils was 10.6 μM, and in intact HL-60 cells the IC50 was 2 μM [43]. However, according to Gatto et al. [46], in neutrophils, ROS generation is inhibited by VAS2870 at submicromolar concentrations with an IC50 of 77 nM. We determined which concentration of VAS2870 suppresses ROS production in HUVECs. For this purpose, dihydroethidium (DHE) was used. VAS2870 reduced the rate of DHE oxidation in HUVECs with an IC50 of 2.47 μM (Figure 2d). At a 20 μM concentration, VAS2870 suppressed nearly half of ROS formation measured with DHE, and its effect reached a plateau. The reason for this might be that oxidation of DHE in cells is caused only partially by superoxide anion [39]. The values of IC50 for the inhibition of ROS generation and suppression of histamine-induced [Ca2+]i elevation in HUVECs were very close, which suggests a link between these processes. VAS2870 is an inhibitor of NADPH-oxidase isoforms NOX1/2/4 [47,48] and does not suppress NOX5 [22]. It has been demonstrated that VAS2870 reverses oxidative stress, which is caused by NOX1/2 activation [49]. We propose that the suppression of histamine-induced [Ca2+]i increase in HUVECs might occur due to the inhibition of NOX2 because this form of NOX is under the control of several intracellular regulatory pathways [9], while the activity of NOX4 is mostly regulated by the level of expression. In addition, it has been shown that increased expression of NOX4 in endothelial cells enhances endothelium-dependent relaxation [17].
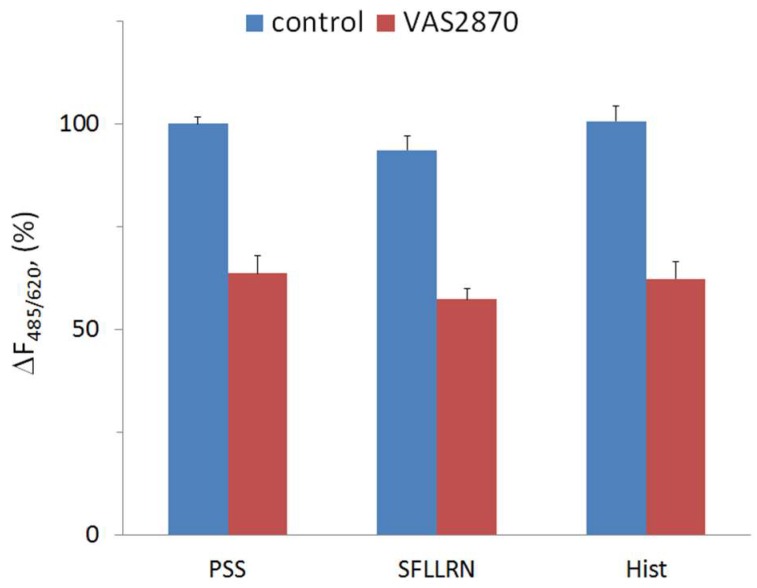
Evidence in favor of NOX involvement in histamine-induced calcium signaling was published quite a long time ago by Hu and co-workers [33], wherein they demonstrated that NOX-derived ROS were critical for generating the Ca2+ oscillations in response to histamine in human aortic ECs. Recently, it was demonstrated that in microglial cells, histamine stimulates ROS formation due to the activation of NOX1 via H1 receptors [50]. We expected that histamine might affect ROS generation in HUVECs. However, there was no increase in the rate of DHE oxidation in the presence of histamine (Figure 3). SFLLRN also did not produce any effect. It might be suggested that either histamine does not stimulate the generation of superoxide anion in HUVECs, or the method with DHE is not sensitive enough to detect the increment in ROS formation due to histamine. It should be mentioned that in [33] 2′,7′-dihydrodichlorofluorescin diacetate was used as a fluorescent probe. Elucidation of the mechanism of NOX involvement in signal transduction from histamine receptors in HUVECs requires further research.
Figure 3.
The influence of SFLLRN and histamine on DHE oxidation in the absence and presence of VAS2870. Physiological salt solution (PSS) was used as a vehicle control for the agonists. Each value is a mean of three values from three independent experiments with different cell preparations. DMSO at final concentration 0.1% was used as a vehicle control for VAS2870. The difference between the values in the absence and presence of VAS2870 was statistically significant (p < 0.01).
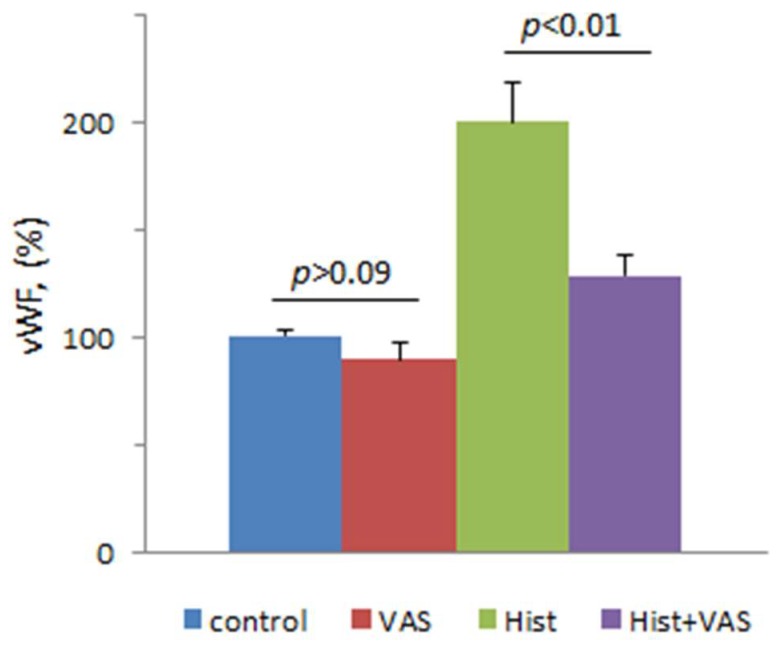
The next task was to study how VAS2870 can affect physiological reactions of ECs activated by histamine, which causes secretion of vWF in HUVECs due to the activation of H1 receptors. This secretion is mediated by the elevation of cytoplasmic calcium ion concentration [40,51]. We showed that, in the presence of VAS2870, the effect of histamine on vWF secretion was attenuated, though VAS2870 did not inhibit the basal secretion of vWF (Figure 4). This result correlates with our data on the inhibition of histamine’s effect on [Ca2+]i by VAS2870.
Figure 4.
The influence of histamine on the secretion of von Willebrand factor (vWF) in the absence or presence of VAS2870. HUVECs were incubated at 30 °C during 5 min with 10 μM VAS2870 or vehicle (DMSO at final concentration 0.1% in the wells of a 48-well plate. Then, 100 μM histamine, or PSS as a vehicle control, were added to the cells, and they were additionally incubated for 30 min. Each value is a mean of the data from three independent experiments.
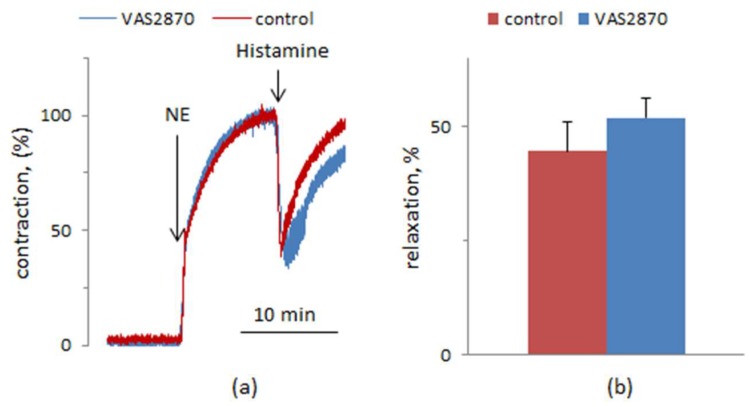
Among the physiological effects of histamine, relaxation of blood vessels is one of the most important, so the next task was to evaluate the influence of VAS2870 on this function. We determined the histamine-induced decrease of the contraction force of rat aortic rings preconstricted with norepinephrine. The rings were preincubated for 30 or 60 min with VAS2870 or vehicle before adding norepinephrine, followed by the addition of histamine. At concentrations of 10 and 100 μM, histamine induced the relaxation of the rings. Surprisingly, there was no attenuation of histamine-induced relaxation after preincubation of the rings with VAS2870 (Figure 5). It can be assumed that in rat aortic ECs VAS2870 does not produce as strong an inhibition of histamine-induced [Ca2+]i rise as in HUVECs. It is known that histamine elevates [Ca2+]i in HUVEC due to its mobilization from endoplasmic reticulum via the channels activated by inositol 1,4,5-trisphosphate and from endolysosomal vesicles via two-pore channels [40,41]. The relative roles of these two calcium signaling mechanisms might be different in ECs from rat aorta and human umbilical vein. Another possible explanation is that the role of calcium ions entering the cytoplasm from different sources is different in the case of vasorelaxation and vWF secretion. It has been demonstrated that the suppression of Ca2+ release from endolysosomal vesicles inhibits vWF secretion induced by histamine [40]. On the other hand, endothelium-dependent vasorelaxation depends on store-operated Ca2+ entry [52].
Figure 5.
Relaxation of rat aortic rings preconstricted by norepinephrine (NE) in response to 100 μM histamine in the absence and presence of VAS2870. VAS2870 at a concentration of 10 μM or vehicle was added to the rings 30 min or 1 h before NE. (a) Typical curves of the contraction and relaxation. (b) The degrees of relaxation in the absence and presence of VAS2870 (n = 10 for control and for VAS2870 from five independent experiments). In each experiment there were measurements from two control and two experimental aortic rings.
In [49], it was demonstrated that VAS2870 improves endothelium-dependent relaxation of spontaneously hypertensive rat (SHR) aortas induced by acetylcholine. The reason for the difference of these data and our results about the lack of VAS2870’s effect on endothelium-dependent vasorelaxation is the increased level of ROS in the aorta of SHR due to the elevated expression of NOX1 and NOX2. The inhibition of their activity by VAS2870 improved impaired the acetylcholine-induced relaxation of spontaneously hypertensive rat aortas [49]. In control normotensive Wistar-Kioto rats, VAS2870 does not affect endothelium-dependent relaxation. There is evidence that VAS2870 can normalize arteriolar flow-induced dilation caused by oxidative stress at hyperinsulinemia [53]. In experiments with murine aorta rings it was demonstrated that their incubation in conditions of hyperglycemia-induced oxidative stress causes impairment of vasodilation mediated by PAR2 agonists, and VAS2870 improved this function [54]. From all these data it can be concluded that VAS2870 is able to improve endothelial-dependent vasorelaxation at pathological state associated with oxidative stress when NOX1 and NOX2 activities are increased, while in normal vessels it does not affect relaxation. This is also supported by our data.
Inhibition of vWF secretion by VAS2870 indicates that ROS produced by NOXs are involved in this reaction. Direct activation of vWF secretion was demonstrated for superoxide anion [30]. We have recently shown that H2O2 induces the exocytosis of vWF in HUVECs [35]. The physiological significance of the stimulation of vWF exocytosis and secretion by ROS is not clear. However, this effect could take place in pathophysiological conditions when hyperactivation of vWF secretion occurs. It was demonstrated that histamine released from mast cells is one of the factors initiating deep vein thrombosis due to excessive secretion of vWF [55]. Thus, it could be suggested that NOX inhibitors might be used as a tool for preventing deep vein thrombosis induced by histamine release from mast cells without affecting vasorelaxation. In fact, the number of pathological states which could be targeted by VAS2870 or other NOX inhibitors together with or alternatively to other pharmaceuticals has been increasing over the past several years [56].
Endothelial activation initiates multiple processes, including hemostasis and inflammation. The molecules that contribute to these processes are co-stored in secretory granules, the Weibel-Palade bodies (WPBs) being the most known and important in ECs. It was previously shown that Ca2+-elevating agonists can deplete the cell of almost all WPBs, whereas cAMP-elevating agonists selectively release a pool of mature WPBs that contain vWF but little or no P-selectin [57]. NOX inhibitors can control the release of granule content from ECs to allow differentiated responses, and the development of selected agonists for tuning the endothelial response is currently an urgent problem [58].
Author Contributions
P.V.A. proposed the project, designed experiments, wrote the paper. E.Y.R., P.P.A., S.K.T., G.Y.M., A.A.T. performed experiments, analyzed data; N.V.G. wrote the paper.
Funding
This research was funded by Russian Science Foundation (grant 18-15-00417) and by Russian Foundation for Basic Research (grant 17-04-01267).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L., Stark H., Thurmond R.L., Haas H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2015;67:601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker R.A., Timmerman H., Leurs R. Histamine receptors: Specific ligands, receptor biochemistry, and signal transduction. Clin. Allergy Immunol. 2002;17:27–64. [PubMed] [Google Scholar]
- 3.Karlstedt K., Jin C., Panula P. Expression of histamine receptor genes Hrh3 and Hrh4 in rat brain endothelial cells. Br. J. Pharmacol. 2013;170:58–66. doi: 10.1111/bph.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grosicki M., Wojcik T., Chlopicki S., Kiec-Kononowicz K. In vitro study of histamine and histamine receptor ligands influence on the adhesion of purified human eosinophils to endothelium. Eur. J. Pharmacol. 2016;777:49–59. doi: 10.1016/j.ejphar.2016.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Mailloux R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturza A., Duicu O.M., Vaduva A., Danila M.D., Noveanu L., Varro A., Muntean D.M. Monoamine oxidases are novel sources of cardiovascular oxidative stress in experimental diabetes. Can. J. Physiol. Pharmacol. 2015;93:555–561. doi: 10.1139/cjpp-2014-0544. [DOI] [PubMed] [Google Scholar]
- 7.Goncharov N.V., Avdonin P.V., Nadeev A.D., Zharkikh I.L., Jenkins R.O. Reactive oxygen species in pathogenesis of atherosclerosis. Curr. Pharm. Des. 2015;21:1134–1146. doi: 10.2174/1381612820666141014142557. [DOI] [PubMed] [Google Scholar]
- 8.Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lassegue B., San Martin A., Griendling K.K. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ. Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond G.R., Sobey C.G. Endothelial NADPH oxidases: Which NOX to target in vascular disease? Trends Endocrinol. Metab. 2014;25:452–463. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Jones S.A., O’Donnell V.B., Wood J.D., Broughton J.P., Hughes E.J., Jones O.T. Expression of phagocyte NADPH oxidase components in human endothelial cells. Am. J. Physiol. 1996;271:H1626–H1634. doi: 10.1152/ajpheart.1996.271.4.H1626. [DOI] [PubMed] [Google Scholar]
- 12.Meyer J.W., Holland J.A., Ziegler L.M., Chang M.M., Beebe G., Schmitt M.E. Identification of a functional leukocyte-type NADPH oxidase in human endothelial cells: A potential atherogenic source of reactive oxygen species. Endothelium. 1999;7:11–22. doi: 10.3109/10623329909165308. [DOI] [PubMed] [Google Scholar]
- 13.Thakur S., Du J., Hourani S., Ledent C., Li J.M. Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J. Biol. Chem. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinloth A., Heermeier K., Raff U., Wanner C., Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J. Am. Soc. Nephrol. 2000;11:1819–1825. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 15.Schroder K. Isoform specific functions of Nox protein-derived reactive oxygen species in the vasculature. Curr Opin Pharmacol. 2010;10:122–126. doi: 10.1016/j.coph.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Konior A., Schramm A., Czesnikiewicz-Guzik M., Guzik T.J. NADPH oxidases in vascular pathology. Antioxid Redox Signal. 2014;20:2794–2814. doi: 10.1089/ars.2013.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray R., Murdoch C.E., Wang M., Santos C.X., Zhang M., Alom-Ruiz S., Anilkumar N., Ouattara A., Cave A.C., Walker S.J., et al. Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo. Arterioscler. Thromb. Vasc. Biol. 2011;31:1368–1376. doi: 10.1161/ATVBAHA.110.219238. [DOI] [PubMed] [Google Scholar]
- 18.Martyn K.D., Frederick L.M., von Loehneysen K., Dinauer M.C., Knaus U.G. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Geiszt M., Witta J., Baffi J., Lekstrom K., Leto T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 20.Ris-Stalpers C. Physiology and pathophysiology of the DUOXes. Antioxid Redox Signal. 2006;8:1563–1572. doi: 10.1089/ars.2006.8.1563. [DOI] [PubMed] [Google Scholar]
- 21.Touyz R.M., Anagnostopoulou A., Camargo L.L., Rios F.J., Montezano A.C. Vascular Biology of Superoxide-Generating NADPH Oxidase 5-Implications in Hypertension and Cardiovascular Disease. Antioxid Redox Signal. 2018 doi: 10.1089/ars.2018.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montezano A.C., De Lucca Camargo L., Persson P., Rios F.J., Harvey A.P., Anagnostopoulou A., Palacios R., Gandara A.C.P., Alves-Lopes R., Neves K.B., et al. NADPH Oxidase 5 Is a Pro-Contractile Nox Isoform and a Point of Cross-Talk for Calcium and Redox Signaling-Implications in Vascular Function. J. Am. Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doan T.N., Gentry D.L., Taylor A.A., Elliott S.J. Hydrogen peroxide activates agonist-sensitive Ca(2+)-flux pathways in canine venous endothelial cells. Biochem J. 1994;297:209–215. doi: 10.1042/bj2970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreher D., Junod A.F. Differential effects of superoxide, hydrogen peroxide, and hydroxyl radical on intracellular calcium in human endothelial cells. J. Cell Physiol. 1995;162:147–153. doi: 10.1002/jcp.1041620118. [DOI] [PubMed] [Google Scholar]
- 25.Hecquet C.M., Ahmmed G.U., Vogel S.M., Malik A.B. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ. Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 26.Siflinger-Birnboim A., Lum H., Del Vecchio P.J., Malik A.B. Involvement of Ca2+ in the H2O2-induced increase in endothelial permeability. Am. J. Physiol. 1996;270:L973–L978. doi: 10.1152/ajplung.1996.270.6.L973. [DOI] [PubMed] [Google Scholar]
- 27.Suresh K., Servinsky L., Reyes J., Baksh S., Undem C., Caterina M., Pearse D.B., Shimoda L.A. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L1467–L1477. doi: 10.1152/ajplung.00275.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volk T., Hensel M., Kox W.J. Transient Ca2+ changes in endothelial cells induced by low doses of reactive oxygen species: Role of hydrogen peroxide. Mol. Cell Biochem. 1997;171:11–21. doi: 10.1023/A:1006886215193. [DOI] [PubMed] [Google Scholar]
- 29.Wesson D.E., Elliott S.J. The H2O2-generating enzyme, xanthine oxidase, decreases luminal Ca2+ content of the IP3-sensitive Ca2+ store in vascular endothelial cells. Microcirculation. 1995;2:195–203. doi: 10.3109/10739689509146767. [DOI] [PubMed] [Google Scholar]
- 30.Vischer U.M., Jornot L., Wollheim C.B., Theler J.M. Reactive oxygen intermediates induce regulated secretion of von Willebrand factor from cultured human vascular endothelial cells. Blood. 1995;85:3164–3172. [PubMed] [Google Scholar]
- 31.Poteser M., Graziani A., Rosker C., Eder P., Derler I., Kahr H., Zhu M.X., Romanin C., Groschner K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588–13595. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 32.Hu Q., Zheng G., Zweier J.L., Deshpande S., Irani K., Ziegelstein R.C. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J. Biol. Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 33.Hu Q., Yu Z.X., Ferrans V.J., Takeda K., Irani K., Ziegelstein R.C. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J. Biol. Chem. 2002;277:32546–32551. doi: 10.1074/jbc.M201550200. [DOI] [PubMed] [Google Scholar]
- 34.Avdonin P.V., Nadeev A.D., Tsitrin E.B., Tsitrina A.A., Avdonin P.P., Mironova G.Y., Zharkikh I.L., Goncharov N.V. Involvement of two-pore channels in hydrogen peroxide-induced increase in the level of calcium ions in the cytoplasm of human umbilical vein endothelial cells. Dokl. Biochem. Biophys. 2017;474:209–212. doi: 10.1134/S1607672917030152. [DOI] [PubMed] [Google Scholar]
- 35.Avdonin P.V., Tsitrina A.A., Mironova G.Y., Avdonin P.P., Zharkikh I.L., Nadeev A.D., Goncharov N.V. Hydrogen Peroxide Stimulates Exocytosis of Von Willebrand Factor in Human Umbilical Vein Endothelial Cells. Biol. Bulletin. 2017;44:531–537. doi: 10.1134/S106235901705003X. [DOI] [Google Scholar]
- 36.Stielow C., Catar R.A., Muller G., Wingler K., Scheurer P., Schmidt H.H., Morawietz H. Novel Nox inhibitor of oxLDL-induced reactive oxygen species formation in human endothelial cells. Biochem. Biophys. Res. Commun. 2006;344:200–205. doi: 10.1016/j.bbrc.2006.03.114. [DOI] [PubMed] [Google Scholar]
- 37.Wind S., Beuerlein K., Eucker T., Muller H., Scheurer P., Armitage M.E., Ho H., Schmidt H.H., Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharmacol. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goncharov N.V., Sakharov I., Danilov S.M., Sakandelidze O.G. Use of collagenase from the hepatopancreas of the Kamchatka crab for isolating and culturing endothelial cells of the large vessels in man. Biull. Eksp. Biol. Med. 1987;104:376–378. doi: 10.1007/BF00842029. [DOI] [PubMed] [Google Scholar]
- 39.Kalyanaraman B., Hardy M., Podsiadly R., Cheng G., Zielonka J. Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch. Biochem Biophys. 2017;617:38–47. doi: 10.1016/j.abb.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esposito B., Gambara G., Lewis A.M., Palombi F., D’Alessio A., Taylor L.X., Genazzani A.A., Ziparo E., Galione A., Churchill G.C., et al. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood. 2011;117:4968–4977. doi: 10.1182/blood-2010-02-266338. [DOI] [PubMed] [Google Scholar]
- 41.Zharkich I.L., Nadeev A.D., Tsitrin E.B., Goncharov N.V., Avdonin P.V. Suppression of Histamine Induced Relaxation of Rat Aorta and Calcium Signaling in Endothelial Cells by Two Pore Channel Blocker trans-NED19 and Hydrogen Peroxide. Biol. Bulletin. 2016;43:365–372. doi: 10.1134/S1062359016030146. [DOI] [PubMed] [Google Scholar]
- 42.Yang J., Li J., Wang Q., Xing Y., Tan Z., Kang Q. Novel NADPH oxidase inhibitor VAS2870 suppresses TGFbetadependent epithelialtomesenchymal transition in retinal pigment epithelial cells. Int. J. Mol. Med. 2018;42:123–130. doi: 10.3892/ijmm.2018.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ten Freyhaus H., Huntgeburth M., Wingler K., Schnitker J., Baumer A.T., Vantler M., Bekhite M.M., Wartenberg M., Sauer H., Rosenkranz S. Novel Nox inhibitor VAS2870 attenuates PDGF-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006;71:331–341. doi: 10.1016/j.cardiores.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 44.Hosseini E., Ghasemzadeh M., Atashibarg M., Haghshenas M. ROS scavenger, N-acetyl-l-cysteine and NOX specific inhibitor, VAS2870 reduce platelets apoptosis while enhancing their viability during storage. Transfusion. 2019 doi: 10.1111/trf.15114. [DOI] [PubMed] [Google Scholar]
- 45.Munhoz A.C., Riva P., Simoes D., Curi R., Carpinelli A.R. Control of Insulin Secretion by Production of Reactive Oxygen Species: Study Performed in Pancreatic Islets from Fed and 48-Hour Fasted Wistar Rats. PLoS One. 2016;11:e0158166. doi: 10.1371/journal.pone.0158166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gatto G.J., Jr., Ao Z., Kearse M.G., Zhou M., Morales C.R., Daniels E., Bradley B.T., Goserud M.T., Goodman K.B., Douglas S.A., et al. NADPH oxidase-dependent and -independent mechanisms of reported inhibitors of reactive oxygen generation. J. Enzyme Inhib. Med. Chem. 2013;28:95–104. doi: 10.3109/14756366.2011.636360. [DOI] [PubMed] [Google Scholar]
- 47.Sancho P., Fabregat I. The NADPH oxidase inhibitor VAS2870 impairs cell growth and enhances TGF-beta-induced apoptosis of liver tumor cells. Biochem. Pharmacol. 2011;81:917–924. doi: 10.1016/j.bcp.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Kleinschnitz C., Grund H., Wingler K., Armitage M.E., Jones E., Mittal M., Barit D., Schwarz T., Geis C., Kraft P., et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wind S., Beuerlein K., Armitage M.E., Taye A., Kumar A.H., Janowitz D., Neff C., Shah A.M., Wingler K., Schmidt H.H. Oxidative stress and endothelial dysfunction in aortas of aged spontaneously hypertensive rats by NOX1/2 is reversed by NADPH oxidase inhibition. Hypertension. 2010;56:490–497. doi: 10.1161/HYPERTENSIONAHA.109.149187. [DOI] [PubMed] [Google Scholar]
- 50.Rocha S.M., Saraiva T., Cristovao A.C., Ferreira R., Santos T., Esteves M., Saraiva C., Je G., Cortes L., Valero J., et al. Histamine induces microglia activation and dopaminergic neuronal toxicity via H1 receptor activation. J. Neuroinflamm. 2016;13:137. doi: 10.1186/s12974-016-0600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lorenzi O., Frieden M., Villemin P., Fournier M., Foti M., Vischer U.M. Protein kinase C-delta mediates von Willebrand factor secretion from endothelial cells in response to vascular endothelial growth factor (VEGF) but not histamine. J. Thromb. Haemost. 2008;6:1962–1969. doi: 10.1111/j.1538-7836.2008.03138.x. [DOI] [PubMed] [Google Scholar]
- 52.Kassan M., Zhang W., Aissa K.A., Stolwijk J., Trebak M., Matrougui K. Differential role for stromal interacting molecule 1 in the regulation of vascular function. Pflugers Arch. 2015;467:1195–1202. doi: 10.1007/s00424-014-1556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahmoud A.M., Ali M.M., Miranda E.R., Mey J.T., Blackburn B.K., Haus J.M., Phillips S.A. Nox2 contributes to hyperinsulinemia-induced redox imbalance and impaired vascular function. Redox Biol. 2017;13:288–300. doi: 10.1016/j.redox.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Daly M., Pulakazhi Venu V.K., Saifeddine M., Mihara K., Kang S., Fedak P.W.M., Alston L.A., Hirota S.A., Ding H., Triggle C.R., et al. Hyperglycaemic impairment of PAR2-mediated vasodilation: Prevention by inhibition of aortic endothelial sodium-glucose-co-Transporter-2 and minimizing oxidative stress. Vascul Pharmacol. 2018;109:56–71. doi: 10.1016/j.vph.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Ponomaryov T., Payne H., Fabritz L., Wagner D.D., Brill A. Mast Cells Granular Contents Are Crucial for Deep Vein Thrombosis in Mice. Circ. Res. 2017;121:941–950. doi: 10.1161/CIRCRESAHA.117.311185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma M.W., Wang J., Dhandapani K.M., Wang R., Brann D.W. NADPH oxidases in traumatic brain injury - Promising therapeutic targets? Redox Biol. 2018;16:285–293. doi: 10.1016/j.redox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cleator J.H., Zhu W.Q., Vaughan D.E., Hamm H.E. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nightingale T.D., McCormack J.J., Grimes W., Robinson C., Lopes da Silva M., White I.J., Vaughan A., Cramer L.P., Cutler D.F. Tuning the endothelial response: Differential release of exocytic cargos from Weibel-Palade bodies. J. Thromb. Haemost. 2018;16:1873–1886. doi: 10.1111/jth.14218. [DOI] [PMC free article] [PubMed] [Google Scholar]