Abstract
Mitosis requires extensive rearrangement of cellular architecture and of subcellular structures so that replicated chromosomes can bind correctly to spindle microtubules and segregate towards opposite poles. This process originates two new daughter nuclei with equal genetic content and relies on highly-dynamic and tightly regulated phosphorylation of numerous cell cycle proteins. A burst in protein phosphorylation orchestrated by several conserved kinases occurs as cells go into and progress through mitosis. The opposing dephosphorylation events are catalyzed by a small set of protein phosphatases, whose importance for the accuracy of mitosis is becoming increasingly appreciated. This review will focus on the established and emerging roles of mitotic phosphatases, describe their structural and biochemical properties, and discuss recent advances in understanding the regulation of phosphatase activity and function.
Keywords: mitosis, phosphatases, cell division cycle 25 (CDC25), protein phosphatase 1 (PP1), protein phosphatase 2A (PP2A), kinetochores, microtubules, chromosomes
1. Introduction
Protein phosphorylation is the most profuse post-translational modification in eukaryotes. It plays a critical role in controlling protein function, activity, and molecular interactions. The phosphorylation state of a given protein is established by the balance of activities between protein kinases and protein phosphatases. Kinases catalyze the transfer of a phosphate group to tyrosine (Tyr), threonine (Thr), or serine (Ser) residues, while protein phosphatases are responsible for the counteracting hydrolytic removal of the phosphate moiety. Phosphoproteomic analyses revealed that protein phosphorylation occurs primarily at Ser and Thr residues (83% and 15% of sites, respectively), with Tyr phosphorylation accounting for only up to 2% of the total phosphoproteome [1]. The importance and regulatory role of protein kinases is well established, whereas protein phosphatases were long regarded as passive housekeeping enzymes. However, it is now evident that phosphatases are also subjected to stringent control and assume equally critical roles in the regulation of protein function and signaling responses. The human genome contains 189 known and predicted protein phosphatase genes [2]. Their evolutionary history can be traced back to a core catalytic domain that evolved through a series of gene duplication events and by adopting the use of regulatory subunits and/or fusion with novel functional modules or domains [2]. Thus, contrasting with kinases, whose catalytic domains are commonly from a single protein structural fold [3], protein phosphatase activity is associated with numerous different protein folds and catalytic mechanisms [4,5]. Based on substrate specificity, protein phosphatases are typically classified as serine/threonine phosphatases, tyrosine phosphatases, or as dual-specificity phosphatases [6].
1.1. Serine/Threonine Phosphatases
Serine/threonine phosphatases are distributed through three broad families in accordance to their primary structure and catalytic mechanism: the phosphoprotein phosphatase (PPP), the protein phosphatase metal-dependent (PPM), and the haloacid dehydrogenases (HAD) families [5]. The most abundant serine/threonine phosphatases in eukaryotic cells are protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) [6]. Given their vital importance for cell cycle progression, PP1 and PP2A will be discussed in greater detail. These phosphatases are members of the PPP family, which also includes PP2B, PP4, PP5, PP6, and PP7. All PPPs are phosphomonoesterases (EC3.1.3) and share a similar core catalytic domain, with two metal ions at the active site. This bimetallic center, typically a Zn::Fe in native enzymes or a Mn::Mn pair in recombinant PPPs, is bridged by a water molecule that acts as a nucleophile in the hydrolysis of phosphorylated Ser (pSer) and pThr substrates [6,7,8,9]. The core catalytic domains of all PPPs exhibit modest substrate specificity when assayed in vitro. Unlike most kinases, PPPs fail to recognize a consensus sequence surrounding or adjacent to the phosphorylated residue [10,11,12,13,14]. Tight substrate selectivity is, however, achieved in a cellular context through association with specific regulatory subunits, which also ensure the control of the phosphatase subcellular localization and modulate overall activity [4,6]. Despite overall similarities, PP1 and PP2A catalytic subunits show some, albeit limited, intrinsic substrate specificity in vitro due to structural variations in their catalytic sites. Protein phosphatase 2A, for instance, is inhibited by subnanomolar concentrations of okadaic acid, due to hydrophobic residues in its catalytic cleft that bind tightly to okadaic acid, whereas PP1 inhibition requires 100-fold higher concentrations [15,16]. Furthermore, isolated catalytic subunits of PP1 and PP2A show distinct preferences for different subunits of phosphorylase b kinase [17]. However, there is compelling evidence that PP1 and PP2A do not exist as free catalytic subunits in cells but operate as multimeric holoenzyme complexes composed of the catalytic subunit and by one or more regulatory and scaffolding subunits that control the phosphatase specificity and activity under physiological settings.
1.1.1. Protein Phosphatase 1
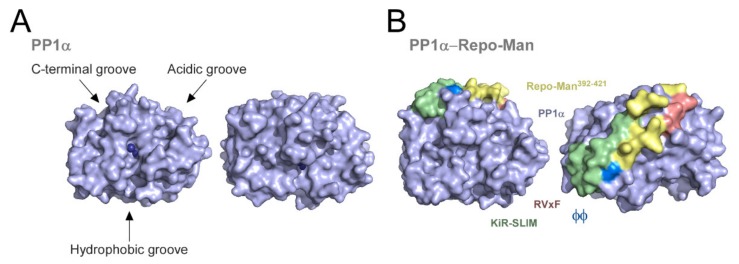
A PP1 holoenzyme contains one of four isoforms of the PP1 catalytic subunit—PP1α, PP1β, PP1γ1, or PP1γ2 (alternative splicing variant of PP1γ only expressed in testis) and one or two of the numerous regulatory subunits [18,19,20,21]. Catalytic subunits of PP1 show extreme phylogenetic and functional conservation. This is illustrated by the ability of any human PP1 isoform to rescue PP1/Glc7 loss in budding yeast [22]. Biochemically, PP1α, PP1β, PP1γ1, or PP1γ2 are identical enzymes [5,6,23] with extensive functional overlap, although some specific functions have been attributed to PP1γ2 in testis and to PP1β in some tissues [20,21,24]. Protein phosphatase 1 isoforms adopt a compact α/β fold, with a β sandwich wedged between two α-helical domains [25,26]. The catalytic site resides at the intersection of three potential substrate/adaptor binding regions: the hydrophobic, acidic, and C-terminal grooves. These are shallow and converge at the catalytic center, forming a Y-shaped surface feature (Figure 1A) [5].
Figure 1.
Structure of PP1α catalytic subunit and its interaction with the regulatory subunit, RepoMan. (A) Surface representation of the three-dimensional (3D) structure of PP1α catalytic subunit. Manganese atoms in the active site are represented as dark blue spheres and lie at the Y-shaped intersection of the three substrate-binding grooves. Protein Data Bank (PDB) accession identification (ID) for the structure: 4MOV. (B) Surface representation of the three-dimensional structure of the PP1α catalytic subunit in complex with Repo-Man392–421. The PP1α catalytic subunit is represented in light blue and the Repo-Man392–421 peptide is represented in yellow. The interaction is mediated by RVxF (red), ΦΦ (blue) and KiR-SLiM (green) motifs on Repo-Man392–421 that bind to specific pockets on the PP1α surface. PDB accession ID 5IOH.
More than 200 regulatory subunits of PP1 have been described. These are generically designated as PP1-interacting proteins (PIPs) and its diversity provides the cell with a wide array of different PP1 holoenzymes to meet specificity and regulatory requirements [27,28]. PIPs are mostly structurally unrelated proteins whose interaction with PP1 is frequently mediated by short linear motifs (SLiMs) that bind to the surface grooves of PP1 (Figure 1B) [29,30,31,32]. Several distinct PP1-binding motifs have now been characterized, enabling a huge combinatorial potential to generate unique PP1 holoenzymes with specific properties [6,27,28]. The most frequent and best characterized PP1-binding SLiM is the RVxF-motif. This motif conforms to the consensus sequence [K/R]x(0–1)[V/I]x[F/W] and is present in nearly 70% of identified PIPs [33,34]. Structural insights revealed that the RVxF-motif docks into a hydrophobic pocket on the PP1 surface through contacts that are primarily mediated by the hydrophobic side-chains of valine (Val) and phenylalanine (Phe) [35]. Additional examples of PP1-binding SLiMs include the SILK, MyPhoNE, and SpiDoC motifs [27,28]. The binding of PIPs to PP1 can also occur through highly structured segments, such as leucine-rich repeats or ankyrin repeats, respectively present on SDS22 and MYPT1 regulatory subunits [36,37,38]. Different PP1-binding motifs associate with different regions of the phosphatase surface. Therefore, a given PP1:PIP interaction might be mediated by several PP1-binding motifs to provide a higher affinity and enhance the complex stability. Importantly, although the same PP1 surface can establish contact with different PIPs, the interaction occurs in a highly specific manner due to the unique combination of PP1-binding motifs in each PIP.
Crystallography studies show that binding to PIPs does not impose a discernible conformational change on the PP1 catalytic domain. Instead, most PIPs limit PP1 activity towards specific substrates through a combination of functional domains involved in PP1-anchoring, PP1-inhibition, substrate-recruitment, and/or subcellular-targeting [6,27,28]. Therefore, controlled dephosphorylation can be achieved by a multitude of mechanisms. Some PIPs, such as spinophilin or protein phosphatase 1 nuclear-targeting subunit (PNUTS), bind to the PP1 C-terminal groove and sterically hinder the access to substrates recruited through this region [39,40]. Nuclear inhibitor of protein phosphatase 1 (NIPP1), on the other hand, defines PP1 substrate selectivity through a polybasic stretch in its PP1-anchoring domain, which dramatically changes the electrostatic charge distribution of the NIPP1:PP1 surface, and by a N-terminus forkhead-associated (FHA) domain that mediates the recruitment of specific substrates [41]. Other PIPs target PP1 to specific subcellular compartments through domains that may also be involved in substrate recruitment. This raises the local concentration of PP1 and enables the dephosphorylation of resident substrates. The regulatory subunit inhibitor-2 (I-2) binds to the acidic and hydrophobic grooves of PP1. This positions the IDoHA motif in contact with the catalytic center, obstructing its access to substrates and causing the release of metals required for catalysis [42]. Other PIPs, such as inhibitor-1 (I-1), CPI-17, and MYPT1, associate with PP1 in a phospho-dependent manner and may inhibit its activity by posing as pseudosubstrates or act as anchoring subunits [26,43,44,45]. Several heterotrimeric PP1 holoenzymes have been identified where PP1 associates with one targeting- or substrate-specifying PIP and one inhibitory PIP. The I-1, I-2, inhibitor-3 (I-3), and CPI-17 are examples of inhibitory PIPs that are able to interact with various PP1-PIPs dimers. The crystallographic structure of PP1 bound to spinophilin, and to I-2 has revealed major structural rearrangements in both regulatory subunits to assemble the heterotrimeric complex [46,47]. The RVxF motif of spinophilin has a higher affinity for the hydrophobic pocket on PP1 and displaces the RVxF motif of I-2, which remains, however, anchored to PP1 through a SILK motif. The IDoHA motif of I-2 dislodges the ∂-helix of spinophilin. These rearrangements enhance the flexibility of the holoenzyme, which is thought to increase its ability to bind substrates or provide new protein interaction sites that were previously concealed and thereby mediate access to additional targets. In summary, amongst the vast repertoire of identified PIPs, there are subcellular/substrate-specifiers, inhibitors, and substrates that also act as inhibitory regulators of PP1. Their combinatorial association with each of the PP1 catalytic subunits provides the cell with a multitude of PP1 holoenzymes with unique structural and biochemical properties to enable the spatiotemporally controlled dephosphorylation of specific targets under physiological conditions.
1.1.2. Protein Phosphatase 2A
The PP2A catalytic subunit has two isoforms (PP2ACα and PP2ACβ) that share 97% of their sequence identity with each other [48,49]. Like other PPPs, PP2A catalytic subunits operate as members of discrete holoenzyme complexes to achieve substrate specificity and controlled activity in cells. PP2A holoenzymes are predominantly heterotrimers consisting of a catalytic subunit (C) bound to a scaffold subunit (A), which in turn recruits a variety of mutually exclusive regulatory subunits (B) with distinct substrate and subcellular specificities. The human genome encodes two scaffolding subunits that share 87% of their sequence identity with each other (PP2AAα and PP2Aaβ isoforms), and 16 regulatory subunits grouped into four families according to their primary sequence and structure: B55 (PR55/B), B56 (PR61/B′), B72 (PR72/B′′), and Striatin (PR93/B′′′) [50,51].
The PP2A scaffold subunit contains 15 tandem helical HEAT (huntingtin-elongation-A subunit-TOR, target of rapamycin) repeats assembled into an elongated, horseshoe-shaped structure [52] (Figure 2A). PP2A(C) specifically binds to the conserved rim of HEAT repeats 11–15 to form the heterodimeric core enzyme [15]. Although the PP2A catalytic subunit shares extensive sequence similarity with other PPPs, the majority of the latter fail to associate with PP2AAα or PP2AAβ because the interaction is mediated by specific amino acids that have been replaced by nonconserved residues in PP1, PP2B, PP5, and PP7 [5]. Roughly one-third of PP2A catalytic subunits are present in mammalian cells in the form of heterodimers with a scaffolding subunit [53]. Whether this PP2A heterodimeric complex is physiologically relevant or merely represents a readily available intermediate towards holoenzyme assembly remains unclear.
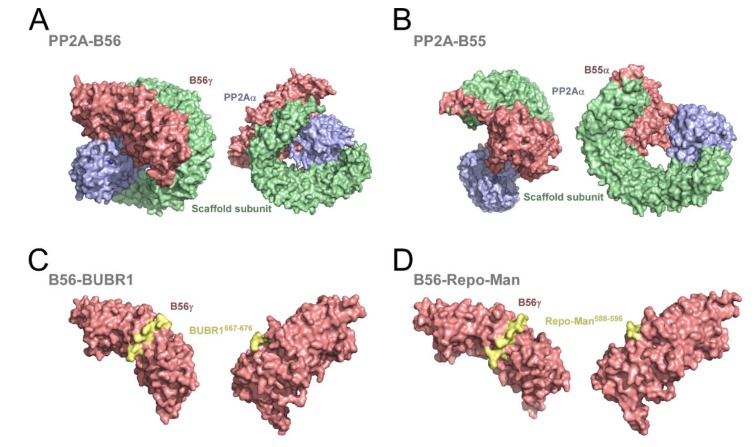
Figure 2.
Structure of PP2Aα holoenzymes. (A,B) Surface representation of the three-dimensional structure of the PP2Aα catalytic subunit (light blue) in complex with the scaffold subunit and the regulatory subunits, B56γ (A) or B55α (B). The PP2A catalytic subunit is represented in light blue with manganese atoms in the active site represented as dark blue spheres. The scaffold subunit is represented in green and the regulatory subunits are depicted in light red. Protein Data Bank (PDB) accession ID 2NPP (A) and 3DW8 (B). (C,D) Surface representation of the three-dimensional structure of B56γ (light red) interacting with BUBR1667–676 and Repo-Man588–596 peptides (yellow). Both peptides encompass a LxxIxE motif that binds to a conserved basic pocket at the concave surface of B56γ. PDB accession ID 5K6S (C) and 5SW9 (D).
The association of a regulatory B subunit to the core enzyme provides substrate specificity as well as spatially and temporally controlled functions [54]. Structural analysis revealed that the B56 subunit extensively interacts with both the scaffold and the catalytic subunit (Figure 2A). The B56 subunit contains eight HEAT-like repeats and is structurally similar to the scaffold subunit. The convex surface of B56 binds to HEAT repeats 2–8 of the scaffold subunit, whereas the concave surface is tilted toward the active site pocket of the catalytic subunit to define the substrate-recognizing surface (Figure 2A) [55,56]. The B55 subunit on the other hand adopts a WD40 fold. It comprises a seven-bladed β propeller and a protruding β-hairpin handle that respectively bind to the HEAT repeats 3–7 and 1–2 of the scaffold subunit [57]. Contrasting with the B56 subunit, B55 makes few contacts with the catalytic subunit and the interaction with substrates is mediated by the acidic central groove of the β propeller (Figure 2B). Thus, notwithstanding evident structural differences and their specific interactions with the scaffold subunit, both B55 and B56 generate PP2A holoenzymes in which the substrate-binding site is located on the top face of the regulatory subunit at a position that is proximal to the active site of the catalytic domain [5] (Figure 2A,B). Different B subunits generate different substrate-recognition interfaces. The PP2A core enzyme is only able to efficiently dephosphorylate the microtubule-binding protein, Tau, when in complex with the B55 subunit [57]. Two lysine-rich sequences in Tau are predicted to bind to the acidic groove on the top face of the β propeller. The residues on B55 required for the interaction have been mapped, but further studies will be required to establish the precise Tau sequences that contact with B55 [57]. Additional critical insight into the B55 substrate recognition mechanism was recently provided through a combination of phosphoproteome screens and kinetic modelling. The strategy allowed the global identification of B55 mitotic substrates, most of which conform to the cyclin-dependent kinase 1 (CDK1) phosphorylation signature [58]. This specificity of B55 is provided by a bipartite positively charged polybasic motif located upstream and downstream of the CDK1 site that acts as a binding determinant for the acidic surface of B55 [58]. Notably, substrates that are dephosphorylated more rapidly by PP2A-B55 are generally more basic than substrates that are dephosphorylated at slower rates [58]. This strongly suggests that the cooperative electrostatic interactions between the negatively charged residues in B55 and the positively charged amino acids on the polybasic motif control the dephosphorylation kinetics [58,59]. Moreover, PP2A-B55 exhibits a preference towards pThr over pSer residues that is not determined by electrostatic interactions with B55, as it has also been observed in other PP2A holoenzymes [60,61,62,63]. This preference seems to reflect instead an inherent property of the PP2A catalytic subunit. It is possible that the additional methyl side group of threonine could affect the binding affinity or even the catalytic reaction [64].
Recent studies shed new light on the mechanisms by which PP2A-B56 holoenzymes engage with some of its substrates. The B56 subfamily in humans comprises five isoforms (α, β, γ, δ, and ε) with no discernible differences in their substrate binding pockets [51]. These subunits recognize SLiMs conforming to the [LMFI]xx[ILV]xE sequence, henceforward referred to as the LxxIxE motif [65,66,67,68]. Structural analysis of B56γ interaction with the kinetochore protein, BubR1, or with the nuclear scaffolding protein, Repo-Man, revealed that the LxxIxE motif binds to a conserved basic pocket between the third and the fourth HEAT repeats on the concave surface of B56γ [66,67] (Figure 2C,D). Peptide phage display of intrinsically disordered regions of the human proteome identified 126 ligands of B56 and confirmed substantial specificity overlap between PP2A-B56α and PP2A-B56γ holoenzymes as expected from the highly conserved LxxIxE binding surface in all B56 isoforms [68]. Nevertheless, B56 isoforms were recently shown to bind differentially to LxxIxE motifs on mitotic proteins [69]. This apparent selectivity is proposed to allow B56α and B56γ to localize at discrete subcellular compartments and control separate mitotic processes. Thus, although functional redundancy has been attributed to B56 isoforms, some sub-functionalization appears to occur under certain circumstances. The molecular features underlying B56 isoform-specific interactions during mitosis are discussed in Section 2.4.
1.2. Tyrosine Phosphatases
Although recurrently underrepresented in global phosphoproteomes, tyrosine phosphorylation plays a critical role in numerous signal transduction pathways that underlie a broad spectrum of fundamental physiological processes. Protein tyrosine phosphatases (PTPs) are defined by the active-site signature motif HCX5R, in which the cysteine residue acts as a nucleophile essential for catalysis [70,71]. Protein tyrosine phosphatases are categorized as classical tyrosine-specific phosphatases (CTSPs) or as dual-specificity phosphatases (DUSPs) [4,72,73]. Contrasting with PPP catalytic subunits, PTPs are selective enzymes that exhibit striking substrate specificity in a cellular context. Hence, instead of occurring in vivo in complex with a multitude of regulatory subunits, PTPs comprise a range of structurally distinct catalytic units that are encoded by nearly 100 genes in the human genome [70,74,75]. The use of alternative promoters, alternative splicing, and post-translational modifications introduce further structural diversity [70]. The catalytic domain of classical PTPs (CTSPs) comprises approximately 280 residues and is defined by the HCX5R motif that functions as a phosphate-binding loop at the active site and by a mobile general acid/base loop (WPD) [4]. Despite relatively large sequence variations in the X5 segment, the P-loop conformation is strictly conserved and ensures that the cysteine and arginine residues remain in close proximity to efficiently remove the phosphate group from the substrate tyrosine. The sulphur atom of the cysteine thiolate serves as a nucleophile and attacks the phosphorus of the phosphotyrosyl target, whereas arginine contributes to substrate binding and stabilizes the cysteine-phosphate intermediate [76,77]. This intermediate is resolved by an activated water molecule aided by an aspartate in the WPD loop [76,77]. Dual-specificity phosphatases are the largest sub-group of PTPs with approximately 70 members [70]. These phosphatases also encompass a typical HCX5R signature motif in their active site, but have smaller catalytic domains and are more structurally diverse than the CTSPs. Notably, the active site pocket of DUSPs is wider and shallower, which allows them to accommodate pSer and pThr residues as well as pTyr residues [78]. Dual-specificity phosphatases share the same catalytic mechanism as the CTSPs [78]. Although DUSPs do not possess a WPD loop, a conserved aspartate residue in the corresponding position is proposed to act as a general acid/base during catalysis [75]. With the notable exception of the inhibitory phosphorylation of CDK1 on Tyr15, reversible tyrosine phosphorylation was long thought to play a minor role in mitosis. However, SFKs, ABL1, EGFR, and PKM2 are examples of PTPs whose inhibition was shown to compromise mitotic accuracy [79,80,81,82]. Furthermore, the master mitotic regulator, polo-like kinase 1 (PLK1), was recently shown to be phosphorylated on Tyr217 during mitosis [83]. This phosphorylation occurs within the kinase activation segment and is proposed to repress the activity of selected pools of PLK1 during cell division [83]. Hence, available new evidence suggests that mitotic pTyr signaling may be more prevalent than previously appreciated. Nevertheless, a comprehensive and mechanistic knowledge of mitotic events controlled by tyrosine phosphorylation remains limited and the only PTPs with well-established essential roles in mitosis are the dual-specificity phosphatases, cell division cycle 14 (CDC14) and cell division cycle 25 (CDC25) [84,85,86]. Phosphatases of the CDC25 family have evolutionarily conserved functions in controlling key transitions between cell cycle phases. CDC14, on the other hand, is a key mitotic exit phosphatase in budding yeast, but its relevance for mitotic exit in metazoans remains ill-defined. Instead, a growing body of evidence indicates that mitotic exit in animal cells relies primarily on regulatory networks orchestrated by PP1 and PP2A phosphatases [86].
CDC25 Phosphatases
The human genome encodes three CDC25 phosphatases: CDC25A, CDC25B, and CDC25C [87,88,89]. The three isoforms are ~60% identical in their C-terminal catalytic domain, but diverge considerably in their N-terminal regulatory region [90,91]. All three CDC25 phosphatases act at various points of the cell cycle to control the activity of specific CDK-Cyclin subpopulations [92,93,94]. Not surprisingly, CDC25 phosphatases are often overexpressed in cancer cells [90]. Structural characterization of CDC25A and CDC25B catalytic domains revealed a near identical folding that is clearly distinct from that of any PTP [95,96]. The small catalytic domains adopt a rhodanese-like structure with a central five-stranded parallel β-sheet sandwiched by three α-helices from below and two α-helices from above. The active sites containing the HCX5R signature are flat and shallow and lack auxiliary loops or obvious features for mediating substrate recognition. Rather, this seems to rely on three hotspot residues (R488, R492, and Y497 on CDC25B) that form a substrate docking site remotely located from the catalytic cysteine [91,97,98]. In line with that, the primary sequence surrounding the site of dephosphorylation does not seem to contribute significantly for substrate recognition [99]. For instance, CDC25A and CDC25B phosphatases exhibit a clear preference towards phosphorylated CDK2 (on Thr14 and Tyr15) in complex with Cyclin A (CDK2-pThr14pTyr15-CyclinA) over artificial substrates or phosphorylated peptides that harbor the same target sequence [91]. Both catalytic domain constructs and respective full-length phosphatases exhibit the same affinity for CDK2-pThr14pTyr15-CyclinA, indicating that all the elements required for substrate recognition are in the catalytic domain. Interestingly, CDC25C is significantly less active towards CDK2-pThr14pTyr15-CyclinA, but highly efficient in dephosphorylating CDK1-pThr14pTyr15-CyclinB [100]. Such differential reactivity is attributed to differences in the C-terminal tail of each CDC25 phosphatase. The C-terminus corresponds to the least conserved region of the catalytic domain and remains undefined in crystal structures, presumably due to its intrinsic flexibility or disordered nature. Nevertheless, two arginine residues present in the C-terminal tail of CDC25A and CDC25B, but absent from CDC25C were shown to dramatically enhance the affinity for CDK2-pThr14pTyr15-CyclinA [91]. The catalytic mechanism of CDC25 phosphatases is very similar to the well-established mechanism of PTPs [91,101,102]. The cysteine in the active site forms a transient covalent phospho-cysteine. Departure of the leaving group and formation of the intermediate are facilitated by protonation of the oxyanion by aspartate acting as the catalytic acid. In a second chemical step, water serves as a nucleophile in the hydrolysis of the phospho-enzyme intermediate assisted by the aspartate, which now acts as a base to activate the water molecule. Extensive experimental evidence confirmed this general mechanism for CDC25 phosphatases, but the identity of the catalytic acid remains to be unambiguously established [91,103,104,105].
2. Roles of Protein Phosphatases in Mitosis
The unidirectional transitions between the phases of the cell cycle is governed by reversible phosphorylation and proteolysis. The highest occupancy of phosphorylation sites is observed in mitosis. Over 32,000 protein phosphorylation/dephosphorylation events occur as cells go into, progress through, and exit from mitosis [106,107,108,109]. Such an extensive network of dynamic phosphorylations underlies the physical events that distribute one copy of the genome to each daughter cell and their subsequent resumption to an interphase state. Mitotic entry in animal cells involves dramatic cellular and structural reorganization, including cell rounding, disassembly of the nuclear envelope, condensation of chromatin into tightly packed chromosomes, and formation of the microtubule-based mitotic spindle. These events occur in concert with regulatory and surveillance pathways to enable the attachment of chromosomes to microtubules from opposite spindle poles and are primarily driven by elevated activity of the CDK1-Cyclin B complex. This attachment configuration allows sister chromatids to be pulled towards opposite poles during mitotic exit, hence providing the same genetic content to progeny cells. To safeguard the accuracy of chromatid partitioning, cells only commit to irreversibly exit mitosis when sister kinetochores of each chromosome attain proper amphitelic attachments. Chromosome segregation and decondensation, cytokinesis, and reassembly of interphase cell structures are the major events defining mitotic exit, all of which require inactivation of CDK1 as well as spatially and temporally confined removal of mitotic phosphorylations to occur. These are respectively driven by the degradation of Cyclin B and controlled activity of protein phosphatases.
2.1. Mitotic Entry: CDC25 Phosphatases Set up the Commitment
Activation of CDK1-Cyclin B has long been established as the trigger that drives the transition into mitosis. The activity of CDK1 throughout the cell cycle is regulated by the quantity and subcellular localization of Cyclin B and by the phosphorylation status of CDK1 itself. Expression of Cyclin B increases during the G2 phase of the cell cycle, thus allowing formation of the CDK1-Cyclin B complex before mitotic entry [110]. The activity of CDK1-Cyclin B is however repressed by inhibitory phosphorylations on CDK1 Thr14 and Tyr15 catalyzed by WEE1 and MYT1 kinases [111,112]. These residues are in the CDK1 catalytic domain and their phosphorylation induces conformational changes in the ATP-binding loop that render the kinase inactive. Dephosphorylation of pThr14 and pTyr15 catalyzed by CDC25 dual-specificity phosphatases is considered the rate-limiting step in the transition from G2 into mitosis (Figure 3A). In human cells, all three CDC25 isoforms contribute for mitotic entry. The CDC25B isoform is responsible for the initial activation of CDK1-Cyclin B at centrosomes during late G2 [113,114,115]. The kinase is subsequently translocated into the nucleus, where it becomes completely activated by CDC25C at the onset of mitosis [116]. The CDC25A phosphatase is proposed to activate CDK1-Cyclin B complexes that trigger chromosome condensation [113,117,118,119]. Partially active CDK1-Cyclin B directly stimulates CDC25B and CDC25C activities and promotes the inhibition of WEE1 and MYT1 kinases, hence establishing both a positive and a double-negative feedback loop that drives swift and sustained CDK1 activation and the irreversible transition to mitosis (Figure 3A) [120,121,122,123,124].
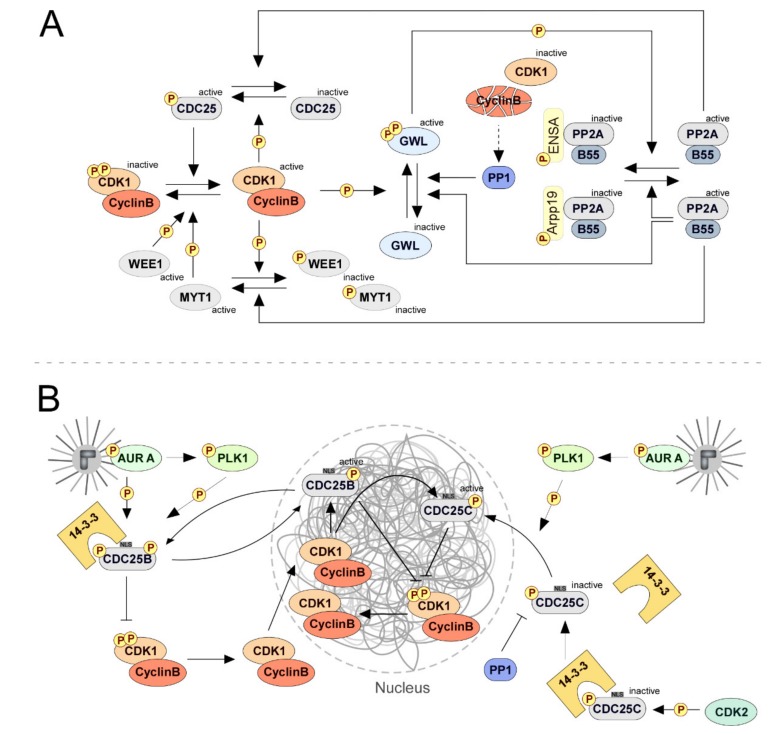
Figure 3.
Molecular circuitry controlling mitotic entry. (A) CDC25 phosphatases dephosphorylate and activate CDK1-Cyclin B to promote mitotic entry. CDC25 activity antagonizes the inhibitory phosphorylations on CDK1 (Thr14 and Tyr15) catalyzed by MYT1 and WEE1. Active CDK1-Cyclin B respectively inhibits and promotes MYT1/WEE1 and CDC25 activities, establishing both a double negative feedback loop and a positive feedback loop. This regulatory circuitry defines a bistable trigger that ensures irreversible transition into mitosis. CDK1-Cyclin B further promotes the activation of GWL kinase, which subsequently phosphorylates ENSA and Arpp19. The phosphorylated forms of these endosulfines bind to PP2A-B55 and inhibit the phosphatase activity towards CDK1-Cyclin B substrates, including MYT1, WEE1, and CDC25. (B) The CDC25B and CDC25C isoforms become active during late G2 to drive CDK1-Cyclin B activation. During interphase, CDC25B and CDC25C are kept inactive by phosphorylation and are retained in the cytoplasm due to binding to 14-3-3 proteins. The activity of CDC25B is proposed to be responsible for the initial activation of CDK1-Cyclin B at centrosomes. Activation of CDC25B is triggered by Aurora A- and PLK1-dependent phosphorylations on the phosphatase N-terminus, which also promote CDC25B shuttling between the cytoplasm and the nucleus. Following activation, the centrosomal pool of CDK1-Cyclin B is translocated to the nucleus, where it enhances CDC25C activity, initiating an amplification loop that drives the cell into mitosis. The inhibitory phosphorylation on CDC25C is removed by PP1 phosphatase upon dissociation of the 14-3-3 protein. This concurs with PLK1-mediated phosphorylation of the CDC25C N-terminus to promote the phosphatase nuclear import and directly stimulate the phosphatase catalytic activity.
In addition to CDK1-Cyclin B, multiple other mechanisms contribute to regulate CDC25 phosphatases (Figure 3B). These include changes in CDC25 protein levels and subcellular localization, inhibitory phosphorylations, and binding to regulatory proteins. The CDC25A isoform is predominantly expressed in G1 and subjected to rapid turnover during interphase as a result of SCFβTRCP ubiquitin-mediated degradation [125]. The phosphatase becomes, however, stabilized at the G2/M transition upon CDK1-Cyclin B-mediated phosphorylation on Ser17 and Ser115 [118,126], thus contributing to the phosphatase pool required to fully activate CDK1-Cyclin B. The levels of CDC25A decrease rapidly at the end of mitosis due to APC/C-Cdh1-mediated targeting for proteasomal degradation [125]. The levels of CDC25B begin to accumulate during mid S-phase, peak at mitotic entry, and decrease as a result of SCFβTRCP-dependent degradation at the metaphase-anaphase transition [114,127,128,129,130]. Contrasting with the other isoforms, CDC25C protein levels remain constant throughout the cell cycle [92]. However, the phosphatase is kept inactive during interphase due to Ser216 phosphorylation. This phosphorylation can be catalyzed by a number of kinases, including Chk1, Chk2, C-Tak, CaMKII, and PKA [131,132,133,134,135,136], and generates a binding site for the 14-3-3 family of proteins [137,138,139]. The interaction of CDC25C with 14-3-3 masks a nuclear localization signal, resulting in cytoplasmic sequestration of the phosphatase and consequently in the inhibition of mitotic entry [92,119,140]. Moreover, studies with Xenopus oocytes indicate that pSer216 might also directly suppress CDC25C catalytic activity independently of 14-3-3 binding [141]. Similarly, phosphorylation of CDC25B on the equivalent residue (Ser323) by p38 MAPK or pEG3 promotes binding to 14-3-3 and thereby cytoplasmic retention of the phosphatase during interphase. Interestingly, 14-3-3 also binds to CDC25A following phosphorylation of the latter on Thr505 by CHK1. This, however, does not affect the phosphatase nuclear localization but rather impairs its interaction with CDK1-Cyclin B to control proper timing of mitotic onset [142].
Mitotic kinases have also been shown to operate as active regulators of CDC25 phosphatases (Figure 3B). Centrosome-localized Aurora A phosphorylates CDC25B on Ser353 during prophase. Although the molecular underpinnings remain elusive, this phosphorylation correlates with the relocalization of Cyclin B to the nucleus and CDK1 activation during mitotic entry [143,144]. Aurora A is also implicated in promoting timely activation of CDC25C, though indirectly through PLK1 [145]. While the requirement of PLK1 activity for mitotic entry upon the G2 DNA-damage checkpoint is well established, its relevance during unperturbed cell cycles has long remained controversial [146,147,148,149,150,151]. Recent studies have now demonstrated that complete inhibition of PLK1 delays mitotic entry even in the absence of DNA-damage events. PLK1 is rapidly activated in late G2 shortly before CDK1-Cyclin B and is dependent on Aurora A and CDK1-Cyclin A activities [152,153,154]. Polo-like kinase 1 subsequently phosphorylates the CDC25C N-terminal region on multiple sites, promoting its nuclear import [155,156] and possibly directly stimulating the phosphatase catalytic activity [146,149]. Therefore, Aurora A- and PLK1-mediated control of CDC25 phosphatases represent critical events in the molecular circuitry that ensures robust CDK1-Cyclin B activation and mitotic commitment (Figure 3B).
Data from Xenopus oocytes revealed that prompt CDC25C activation further requires the activity of PP1 phosphatase (Figure 3B). During mitotic entry, CDK2 activity promotes the dissociation of 14-3-3 protein from CDC25C, exposing the inhibitory phosphorylation on Ser287 (corresponding to Ser216 in the human CDC25C orthologue) for PP1-mediated removal [157]. This enables the initial activation of CDC25C and a consequent increment in CDK1-Cyclin B activity. CDK1-Cyclin B subsequently phosphorylates CDC25C on Ser285 and enhances the recruitment of PP1 to CDC25C, hence accelerating S287 dephosphorylation and thereby warranting prompt and sustained phosphatase activation [141].
Along with increased CDK1-Cyclin B activity, the inhibition of PP2A-B55 phosphatase is essential for mitotic compliance (Figure 3A). B55-type regulatory subunits confer specificity of PP2A towards CDK1 phosphosites, which suggests that PP2A-B55 holoenzymes might represent a critical opposing activity to CDK1-Cyclin B [57]. Accordingly, PP2A-B55 mediated removal of numerous CDK1-generated phosphorylations was shown to be critical for mitotic exit [58,63,158,159,160,161,162,163,164]. In order to prevent premature dephosphorylation of CDK1-Cyclin B substrates and ensure mitotic commitment, PP2A-B55 activity is repressed by the Ser/Thr kinase Greatwall (GWL) at the G2/M transition (Figure 3A). Two key studies using Xenopus egg extracts uncovered the underlying biochemical pathway. GWL phosphorylates Arpp19 and ENSA, two small and unstructured proteins of the Endosulfine family. Phosphorylation occurs on specific serine residues (Ser62 in Arpp19 and Ser67 in ENSA) within a highly conserved FDSpGDY motif and allows Arpp19 and ENSA to bind PP2A-B55 in a highly specific manner, causing its near-complete inhibition [165,166]. This mechanism was also shown to be essential for mitotic entry in flies, starfish oocytes, and in human cultured cells [167,168,169,170,171,172]. A notable exception was observed in mouse embryonic fibroblasts, where the control of PP2A-B55 by GWL seems to be dispensable for the G2/M transition, but nevertheless required after nuclear envelope breakdown to prevent mitotic collapse [173,174]. As expected, and by opposition to PP2A-B55, GWL activity is undetectable throughout interphase, abruptly increases at the G2/M transition, and remains elevated until anaphase onset [169,171,175]. Activation of GWL is triggered by CDK1-Cyclin B-mediated phosphorylation of two conserved residues located in the kinase presumptive activation loop. This primes GWL to intramolecularly autophosphorylate its C-terminal tail and stabilize the kinase in its active conformation [175,176,177]. In frame of the G2/M signaling circuitry, the inhibition of PP2A–B55 orchestrated by GWL has been proposed to increase the levels of activating phosphorylation events on CDC25 phosphatases and inhibitory phosphorylation events on WEE1 and MYT1 [86,178] (Figure 3A).
2.2. Sister Chromatid Cohesion: PP2A-B56 Holds Them Together
Mitotic entry is accompanied by the condensation of DNA into chromosomes, each comprised of two sister-chromatids held together from DNA replication until anaphase onset by proteinaceous ring-like cohesin complexes [179,180] (Figure 4A). These are composed by heterodimers of SMC1 and SMC3 bound to the α-Kleisin subunit, SCC1/MCD1/RAD21, forming a closed tripartite structure that encircles the replicated DNA [181]. The Kleisin subsequently binds to SCC3/SA, which when associated with the heterodimer, WAP1-PDS5, forms a subcomplex that regulates the maintenance of cohesion on chromatin [182,183] (Figure 4B). During prophase, cohesin is displaced from chromosome arms by WAP1, possibly through disruption of the SMC3-SCC1 interface (Figure 4A,B) [179,184,185,186,187,188]. Prior to mitotic entry, however, cohesin rings are insensitive to WAP1 as a result of SMC3 acetylation by ECO1/2 acetyltransferases [189,190,191,192,193]. Acetylation of SMC3 during DNA replication promotes the recruitment of Sororin, which antagonizes WAP1 by impairing its binding to PDS5 [194,195]. Notably, loss of protection against WAP1 during early mitosis does not involve SMC3 deacetylation. It is instead elicited through extensive phosphorylation of Sororin and SCC3/SA2 by Aurora B, CDK1, and PLK1 [196,197,198,199,200,201,202,203]. Aurora B- and CDK1-dependent phosphorylation of Sororin causes its dissociation from PDS5, hence consenting WAP1 binding to PDS5 (Figure 4A,B) [199,201]. Moreover, WAP1-mediated removal of cohesin further requires phosphorylation of SCC3/SA2 by PLK1. The underlying mechanism remains, however, unknown. Loss of cohesion from chromosome arms in prophase seems to occur exclusively in metazoan cells, where is thought to facilitate sister DNA decatenation and allow timely cohesin reloading in the subsequent cell cycle [204,205,206,207]. Cohesion must, however, persist at centromeres until the kinetochores of all chromosomes are correctly attached to microtubules of opposite spindle poles. Centromeric cohesin facilitates this process by promoting the back-to-back arrangement of sister kinetochores and grants resistance to microtubules pulling forces so that separation can only occur upon mitotic checkpoint silencing [208,209,210,211].
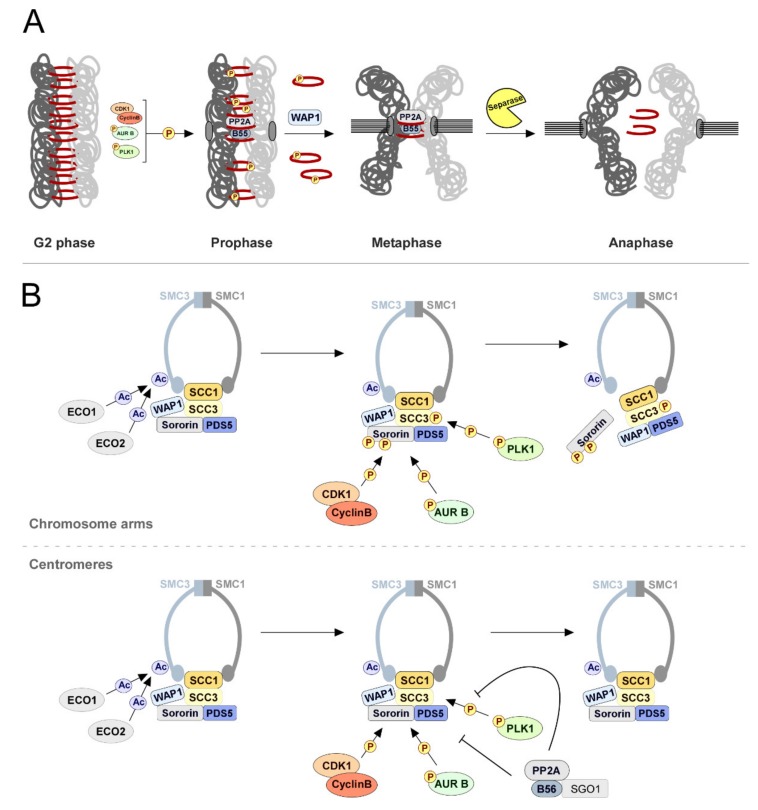
Figure 4.
PP2A-B56 maintains centromeric cohesion until anaphase onset. (A) Following DNA replication, sister chromatids are held together by cohesin-ring complexes. During prophase, CDK1, PLK1, and Aurora B trigger WAP1-mediated removal of cohesin from chromosome arms. Centromere cohesion is protected from WAP1 by the activity of PP2-B56 bound to SGO1. Centromeric PP2-B56 antagonizes the phosphorylations catalyzed by the mitotic kinases, hence maintaining cohesion at centromeres until kinetochores of all chromosomes are correctly attached to microtubules of opposite spindle poles. At this stage, Separase catalyzes the proteolytic degradation of cohesin complexes, abolishing cohesion and enabling sister chromatid separation in anaphase. (B) During G2, cohesin rings are insensitive to WAP1 by Sororin recruited to acetylated SMC3. The presence of Sororin prevents WAPl binding to PDS5. Following mitotic entry, Aurora B- and CDK1-dependent phosphorylation of Sororin causes its dissociation, thus consenting WAP1 binding to PDS5. WAP1-mediated removal of cohesin also requires phosphorylation of SCC3 by PLK1. At centromeres, PP2A-B56 in complex with SGO1 counteracts Aurora B, CDK1, and PLK1 destabilizing phosphorylations and thereby prevents the dissociation of cohesin complexes by WAP1.
Centromeres are protected against WAP1-dependent cohesin removal by the activity of SGO1-associated PP2A-B56 [199,212,213,214,215]. Recruitment of PP2A-B56 to centromeres of mammalian cells is mediated both by SGO1 and SGO2 [216,217,218]. Although SGO2 is required for cohesion protection during meiosis, it seems to be dispensable for this function in mitotic cells, where instead SGO1 assumes primary relevance [212,213,215,216,217,219,220,221,222,223]. Structural analysis of an N-terminal fragment of human SGO1 bound to PP2A-B56γ reveals a bipartite interaction between SGO1 and the regulatory and catalytic subunits of the PP2A-B56γ holoenzyme [218]. SGO1 N-terminus forms a parallel coiled-coil homodimer whose N- and C-terminal ends bind to PP2AC and B56γ, respectively. Each binding interface contains a hydrophobic core as well as peripheral hydrogen bonds and salt bridges. Binding of SGO1 to PP2A-B56γ does not seem to impose significant alterations in PP2AC structure or catalytic activity. In a cellular context, this interaction likely places PP2A-B56γ in close proximity to Sororin and SCC3/SA2 to counteract Aurora B, CDK1, and PLK1 destabilizing phosphorylations and thereby prevent the dissociation of centromeric cohesin complexes (Figure 4B). Several lines of evidence concur in support of this model: binding of PP2A-B56γ to SGO1 is required for protection of centromeric cohesion [215,218], SGO1 physically interacts with cohesin complexes during mitosis through SCC1–SCC3/SA2 heterodimers, and an unphosphorylatable version of Sororin prevents chromatid separation that would normally follow SGO1 depletion [199].
2.3. To Build a Spindle: A PP1, PP2A, PP4, and PP6 Groundwork
Accurate segregation of chromosomes relies on the formation of a bipolar spindle structure [224]. This requires the maturation and separation of duplicated centrosomes to originate two independent microtubule-organizing centers correctly positioned at opposite poles of the cell. Centrosome maturation occurs when cells prepare to enter mitosis and results from a dramatic expansion in the organized matrix of pericentriolar material (PCM) surrounding mother centrioles and a concomitant increase in its microtubule nucleation capacity [225,226,227]. Expanded PCM serves as a catalytic scaffold for γ-tubulin ring complexes (γ-TuRC) to mediate microtubule nucleation [228,229] and its assembly involves the recruitment of numerous PCM proteins orchestrated by PLK1 and Aurora A kinases [227,230]. The Ser/Thr phosphatase PP4 is predominantly enriched at mitotic centrosomes of metazoan cells and its activity was shown to be required for γ-tubulin accumulation and microtubule nucleation in Drosophila and Caenorhabditis elegans embryos, as well as in human cultured cells. The mechanism by which PP4 promotes the maturation of centrosomes remains unknown, but the phosphatase appears to be necessary for proper centrosomal localization of PLK1 [231] and activation of the the Aurora A-CEP192 complex [232]. Conversely, disruption of PP4 in MEFs has no impact on γ-tubulin levels, but leads to unstable contacts between PCM and microtubules minus-ends [233].
Regulation of microtubule dynamics at the centrosomes and spindle poles relies in part on the activity of the microtubule-severing Katanin complex [234,235], whose centrosomal localization is mediated by the interaction of its p60 subunit with the Dynein-motor regulator NDEL1 [236]. Binding of p60 Katanin to NDEL1 increases during mitotic entry as a result of NDEL1 phosphorylation at Ser198, Thr219, and Ser231 by CDK1-Cyclin B [236]. These phosphosites are directly antagonized by PP4 phosphatase, which limits the accumulation of Katanin activity at centrosomes and thereby promotes the stabilization of microtubule connections [233]. This is important to enable organized outgrowing of microtubules into a functional mitotic spindle, a process that additionally requires discrete activities of PP2A and PP6 phosphatases. Interestingly, in C. elegans, PP4 directly dephosphorylates the Katanin catalytic subunit homologue MEI-1 to promote microtubule-severing activity in meiosis [237,238].
Plus-end growth of microtubules emanating from centrosomes relies on the activity of the microtubule-polymerase ch-TOG/XMAP215/MSPS [239], whose mechanism was recently uncovered in Xenopus egg extracts [240]. The C-terminus of XMAP215 directly interacts with γ-tubulin whereas the N-terminus TOG domains bind to soluble α/β-tubulin dimers to endorse their assembly onto γ-TuRCs [240]. The number of microtubules that grow out from centrosomes is limited by the activity of the microtubule depolymerizing kinesin MCAK/XKCM1/KLP-7 [239,241]. In C. elegans embryos, the regulatory subunit, PR72/RSA-1, targets PP2A to centrosomes through a direct interaction with the PCM-associated RSA-2 protein. Centrosomal PP2A-PR72/RSA-1 promotes microtubule outgrowth and spindle stability by controlling KLP-7 and the spindle assembly factor, TPX2/TPXL-1. The PP2A-PR72/RSA-1 complex restricts the levels of KLP7 through an unknown mechanism and directly mediates the accumulation of TPX2/TPXL-1 at centrosomes, thus increasing the rate of microtubules’ nucleation as well as the length of centrosomal microtubules [242]. No obvious RSA-2 orthologues have been identified so far and the requirement of PP2A-PR72 for spindle formation in other metazoans remains to be demonstrated. In fact, TPX2 displays limited centrosomal localization in vertebrate cells, accumulating preferentially on spindle microtubules [243,244], where it plays a critical role in recruiting and promoting Aurora A activity required for chromatin-driven spindle assembly [245,246,247]. Following nuclear envelope breakdown, the RanGTP that is produced by the chromatin-associated GEF RCC1 relieves TPX2 from inhibitory interactions with Importin-α/β [246,248,249]. This licenses TPX2 to bind and allosterically activate Aurora A [243,250,251,252]. The TPX2-Aurora A complex oligomerizes and binds to the RHAMM-γ-TuRC complex, which becomes competent for microtubule nucleation after Aurora A-mediated phosphorylation of the γ-TuRC adaptor subunit, NEDD1 [253,254,255]. Interestingly, microtubule-associated TPX2-Aurora A is more active in the purlieu of spindle poles. This is owed to the spatially controlled activity of PP6 phosphatase and is critical for the stability of the assembling spindle [256]. In addition to allosteric regulation by TPX2 binding, dimerization-mediated T-loop autophosphorylation (Thr288) further enhances Aurora A activation [257,258,259]. Moreover, the interaction with TPX2 protects the activating phosphorylation from the antagonizing action of several phosphatases, including PP1 and PP2A [250,251,252,260,261]. The Ser/Thr phosphatase, PP6, is, however, able to recognize and dephosphorylate the T-loop of the TPX2-complexed form of Aurora A and its depletion was shown to impair proper spindle assembly [256,262]. Although PP6 is uniformly distributed through the cytoplasm of mitotic cells, its activity towards TPX2-Aurora A is inhibited in the vicinity of centrosomes by action of PLK1 [263]. Following CDK1-dependent priming, PLK1 binds to and phosphorylates the PP6-regulatory subunit, PP6R2, at multiple sites [263]. These phosphorylations repress PP6 activity possibly by preventing its interaction with substrates. Because PLK1 is particularly enriched at centrosomes, PP6-mediated dephosphorylation of TPX2-Aurora A complexes located on proximal microtubules is repressed, thus ensuring maximal Aurora A activation at spindle poles and moderate activation on distal spindle microtubules [259,264,265]. This enhances Aurora A-catalyzed phosphorylation of transforming acidic coiled-coil (TACC) proteins at spindle poles, which enables TACC-ch-TOG complexes to efficiently bind to and stabilize the minus-ends of nascent microtubules and, in this way, promote growth of microtubules from the centrosome [241,266,267,268,269,270,271,272,273,274,275]. Hence, coordinated activities of PLK1, PP6, and Aurora A favor the centrosomes as dominant sites of spindle assembly in mitosis.
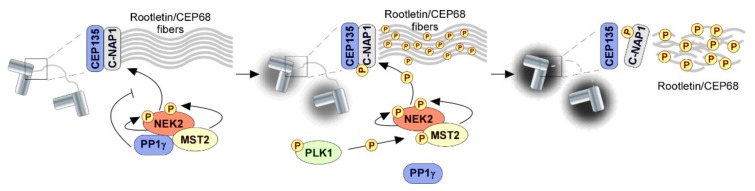
Setting two independent microtubule-organizing centers at opposite poles of a mitotic cell requires the separation of duplicated centrosomes, which typically occurs before nuclear envelope breakdown in organisms that undergo an open mitosis [276]. Centrosome splitting during late G2 involves the dissolution of a fibrous proteinaceous linker that holds the duplicated centrosomes together, a process elicited by an increase in NEK2A kinase activity and controlled by PP1 phosphatase [277,278,279,280] (Figure 5). The primary target of NEK2A is C-Nap1, which is docked to the proximal ends of parental centrioles through the centriolar protein, CEP135, and serves as a platform for the assembly of Rootletin/CEP68 fibers [281,282,283,284,285]. Data from STED microscopy and 3D-STORM analysis suggest that Rootletin/CEP68 filaments coming from each centriole are entwined into a web-like structure that probably underpins the flexible nature of the centrosomal linker [286]. Multisite phosphorylation of C-NAP1 by NEK2A abrogates C-NAP1 interaction with CEP135, hence causing its displacement from centrosomes [287]. This concurs with NEK2A-mediated phosphorylation of Rootletin and of associated proteins to ultimately disrupt the linker structure and enable centrosome disjunction (Figure 5) [281,287,288,289,290]. The kinase activity of NEK2A is tightly regulated to prevent premature centrosome separation, whose occurrence was shown to result in aberrant nuclear positioning and incorrect spindle orientation [291,292,293]. Activation of NEK2A occurs through homodimerization-induced autophosphorylation of the activation loop [294,295,296]. The kinase is also phosphorylated in its non-catalytic domain by sterile-20 like kinase MST2, which significantly enhances its localization at centrosomes [297]. However, NEK2A activity towards C-NAP1, and possibly other substrates, is kept at residual levels up until late G2 by action of PP1γ, which binds to the NEK2A-MST2-hSAV1 complex through an RVxF motif on the NEK2A C-terminus [298,299,300]. Within the complex, PP1γ antagonizes NEK2A phosphorylations on C-NAP1, thus maintaining the linker integrity and, consequently, the centrosomes tethered [299]. Whether PP1γ or PP1α dephosphorylates the NEK2A T-loop to directly inactivate the kinase remains unclear [300,301]. Loss of centrosome cohesion is triggered by the activation of PLK1, which subsequently phosphorylates MST2 on Ser15, Ser18, and Ser516, and disrupts PP1γ from the complex (Figure 5) [297]. The mechanism by which these phosphorylations abolish PP1γ interaction with NEK2A is currently unknown. The absence of PP1γ leaves NEK2A-mediated phosphorylations of linker proteins unopposed, thereby allowing pre-mitotic resolution of centrosomes and consequently minimizing the rate of erroneous interactions that the mitotic spindle might establish with chromosomes [302,303,304,305].
Figure 5.
PP1 prevents premature separation of centrosomes. Centrosome splitting during late G2 involves the dissolution of the Rootletin/CEP68 linker that holds duplicated centrosomes together. This process is orchestrated by NEK2A-mediated phosphorylation of C-Nap1 and of linker proteins. Phosphorylation of C-NAP1 abrogates its interaction with CEP135 docked on the proximal ends of parental centrioles, thus causing C-NAP1 displacement from centrosomes. Phosphorylation of Rootletin and of associated proteins disrupts the linker structure and enables centrosome disjunction. PP1γ suppresses NEK2A activity up until late G2, thereby preventing premature resolution of centrosomes. During interphase, PP1γ bound to NEK2A in complex with MST2 is able to efficiently antagonize phosphorylations catalyzed by the kinase. Increasing activity of PLK1 in late G2 results in MST2 phosphorylation and consequential dissociation of PP1γ from the complex. This renders NEK2A phosphorylations unopposed, hence allowing pre-mitotic resolution of centrosomes.
Phosphatases not only control the assembly of the mitotic spindle, but also its orientation. The position that the spindle assumes in mitosis defines the axis of cell division and plays a key role in cell fate determination in tissues. Depletion of PP4 from progenitor cells of the mouse neocortex causes premature differentiation into neurons and severe defects in cortical layering and brain cytoarchitecture [306]. Biochemical and functional studies demonstrated that PP4-mediated dephosphorylation of NDEL1 at CDK1 sites promotes its binding to LIS1-Dynein to efficiently generate microtubule pulling forces that orient the spindle in parallel to the neuroepithelial surface [306]. This mechanism was shown to be critical at the onset of neurogenesis to ensure extensive symmetric cell division required for the expansion of the progenitor pool [306]. The orientation of the mitotic spindle is also regulated upstream by the distribution of the conserved Gαi-LGN-NuMA module at the cell cortex [307,308,309,310]. Cortical NuMA recruits Dynein complexes that interact with the plus ends of astral microtubules and generate pulling forces to orient the spindle apparatus [311,312,313]. Hence, the localization of NuMA must be tightly controlled, as both insufficient and excessive levels at the cortex impair accurate spindle orientation [312,314]. The association of NuMA with the cell cortex is dynamically modulated by concerted action of CDK1-Cyclin B, Aurora A, PLK1, and several phosphatases. Prior to anaphase onset, phosphorylation of NuMA by Aurora A directs it from the spindle poles to the cortex [315,316], whereas phosphorylation by PLK1 and CDK1-Cyclin B displaces NuMA from the cortex [317,318]. Importantly, phosphorylation of Thr2055 by CDK1 is continuously antagonized by PP2A activity. This results in moderate levels of cortical NuMA during metaphase to ensure accurate spindle positioning and contributes to the enhancement of its cortical recruitment following CDK1 inactivation, which is required to sustain robust spindle elongation in anaphase [317,319]. Protein phosphatase 1-α in complex with its regulatory subunit Repo-Man also promotes cortical accumulation of NuMA in anaphase. This occurs, however, through a separate pathway that is kept in check during metaphase by p37/UBXN2B, a cofactor of the p97 AAA ATPase [320]. Although exactly how p37/UBXN2B limits PP1α-Repo-Man activity towards NuMA is unknown, this balance is critical to prevent excessive Dynein-dependent forces and concomitant spindle orientation defects. Future studies are also expected to cast new light on the mechanism by which PP1α-Repo-Man controls NuMA cortical localization. Moreover, at least in C. elegans, PP6 was also shown to positively regulate pulling forces during anaphase by promoting the cortical localization of LGN and NuMA homologues [316,321]. However, the underlying mechanism remains elusive.
2.4. Kinetochore-Microtubule Attachments: PP1 and PP2A-B56 Keep Them Stable
To ensure faithful genome partitioning, sister kinetochores must become attached to microtubules emanating from opposite spindle poles. This attachment configuration is termed amphitelic or bioriented and enables the dividing cell to segregate sister chromatids towards opposite sides during anaphase and in this way distribute one copy of each chromosome per daughter nucleus. Amphitelic attachments assume an end-on arrangement with the plus-end of microtubules embedded within the kinetochore. This is believed to provide sufficient stability to sustain the force released by the depolymerisation of kinetochore-associated microtubules during anaphase [322]. However, the initial contacts occurring between kinetochores and the spindle microtubules are asynchronous and stochastic, which implies that amphitelic attachments are often preceded by immature or erroneous microtubule interactions. Following nuclear envelope breakdown, kinetochores might establish lateral (kinetochores interact with the side of microtubules), syntelic (both kinetochores bind microtubules from the same spindle pole), or merotelic attachments (one kinetochore binds microtubules that originate from both spindle poles) [303,323,324,325,326,327,328]. Consequently, accurate chromosome segregation requires the destabilization of the inadequate kinetochore-microtubule interactions and the formation of robust amphitelic end-on attachments, events that are orchestrated by the coordinated activities of molecular motors, kinases, and phosphatases [329,330,331].
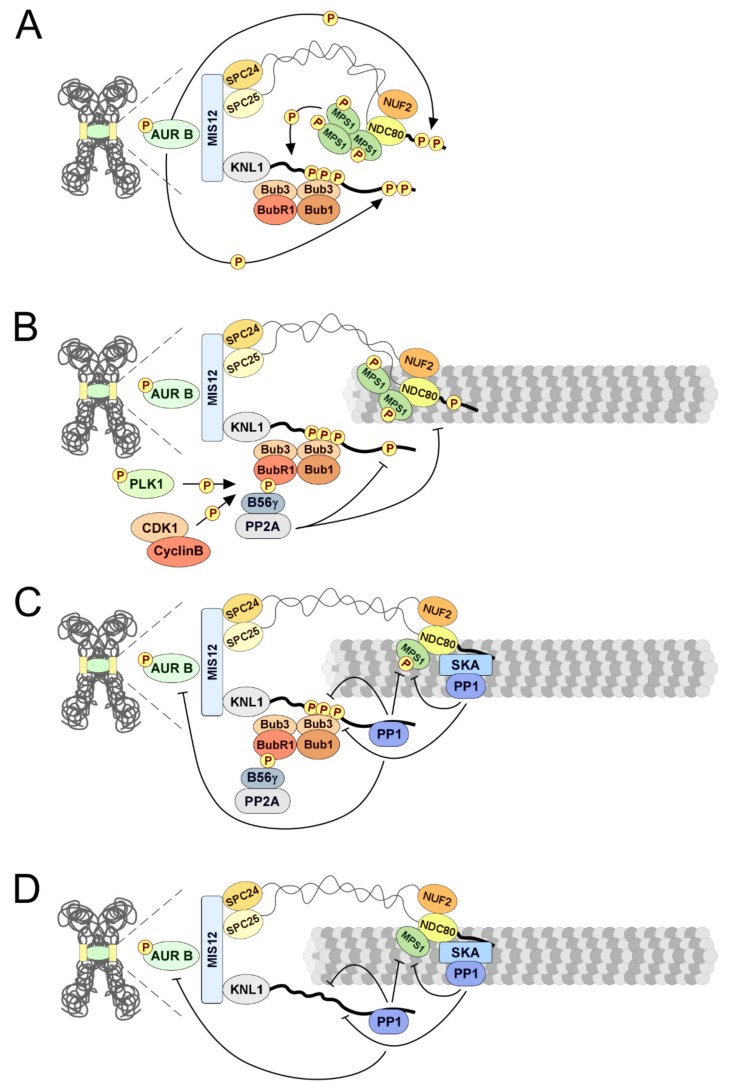
A central player in the formation of stable end-on attachments is the NDC80 complex at the outer-kinetochore, which directly binds microtubules through the CH domain and the N-terminal tail of NDC80/HEC1 [332,333,334]. The CH domain encompasses several positively charged residues that contact both α- and β-tubulin at the intra- and inter-tubulin interfaces [333,334,335]. The highly basic nature of the structurally disordered N-terminal tail further increases the affinity for microtubules by directly interacting with negatively charged E-hooks of tubulin subunits [335]. This binding arrangement and the oligomerization of the NDC80 complex confers the kinetochore the capacity to maintain a processive association with dynamic microtubules and in this way couple chromosome movement to microtubule depolymerisation/polymerization [327,335]. The interaction between NDC80 and tubulin is, however, destabilized by Aurora B-mediated phosphorylation of multiple residues on the NDC80 N-terminal tail. The negatively charged phosphates neutralize the intrinsic positive charge of the NDC80 N-terminus, significantly reducing its binding affinity for microtubules [332,333,334,335,336,337,338,339,340]. Notably, this destabilization mechanism acts preferentially on incorrect kinetochore-microtubules attachments. Although the basis for this selectivity is not fully understood, the prevailing model is that the level of tension across centromeres and/or kinetochores plays an important role in controlling the capacity of Aurora B to phosphorylate NDC80 [341,342,343,344]. Aurora B is the catalytic subunit of the chromosome passenger complex (CPC) that also comprises INCENP, Survivin, and Borealin as scaffold subunits. Survivin and Borealin mediate CPC recruitment to centromeres, which subsequently promotes Aurora B T-loop activation through trans-autophosphorylation [345,346,347,348,349,350,351,352]. Microtubules responsible for amphitelic attachments impose tension or alterations on the kinetochore architecture so that the distance between centromeric active Aurora B and the outer kinetochore increases enough to preclude NDC80 phosphorylation (Figure 6A–D) [353,354,355,356]. On the other hand, erroneous attachments fail to produce sufficient tension, thus leaving NDC80 within reach of Aurora B activity to ultimately cause microtubule detachment [342,357,358,359]. Because unattached kinetochores are tensionless, this error correction mechanism might seemingly represent a problem for the formation of initial end-on microtubule attachments (Figure 6A). In that respect, it is important to consider that the early contacts that kinetochores establish with microtubules are predominantly lateral and that PP2A-B56 at outer-kinetochores was shown to play a critical role in antagonizing Aurora B dependent phosphorylations (Figure 6B). The interaction of kinetochores with the side of microtubules is mediated by the molecular motors, Dynein and CENP-E, and are therefore impervious to the phosphorylation state of NDC80 [303,324,325,327,328,360]. Dynein minus-end motor activity transports chromosomes towards the spindle pole, exposing the kinetochores to a higher density of microtubules that might favor direct end-on capture [361,362,363,364]. As spindle microtubules become more stable and detyrosinated, CENP-E motility increases to promote the movement of the persisting laterally attached chromosomes towards the equator [325,365,366,367,368,369]. Once it reaches microtubule ends, CENP-E converts from a lateral transporter into a microtubule tip-tracker that tethers kinetochores to the dynamic microtubule tips, thus directly contributing for the formation and stability of end-on attachments [370,371,372]. Importantly, lateral attachments exert intermediate levels of tension that increase the distance between Aurora B and the outer-kinetochore. This concurs with the dephosphorylation of the NDC80 N-terminal tail by PP2A-B56γ to allow the formation of stable end-on attachments [360,373,374,375] (Figure 6B–D). The recruitment of PP2A-B56γ to the outer-kinetochore is directly mediated by a LxxIxE motif within the KARD domain of BUBR1 [66,69,373,374,375]. A conserved pocket in the B56γ regulatory subunit recognizes and binds to the LxxIxE motif [65,66,67,68] (Figure 2C). Phosphorylations within and around the LxxIxE motif, respectively catalyzed by CDK1 and PLK1, significantly strengthen the interaction [66,67,373,374,376,377]. Notably, although the binding pocket is conserved in all B56 isoforms, BubR1 binds preferentially to B56γ and possibly to B56δ and presents limited association with B56α and B56ε, which instead display preference for centromeric SGO2 [69,378,379,380]. Similarly, several other mitotic proteins harboring LxxIxE motifs were shown to bind preferentially to B56γ over B56α [69]. A possible explanation for B56 differential binding, and therefore localization, was recently attributed to specific differences in a small loop at the C-terminus of each B56 isoform. The identity of four critical amino acids within the loop seems to modulate its capacity to repress or allow binding to LxxIxE motifs [69]. The residues, Glu405, Pro409, Val412, and Ala413, define the EPVA loop of B56α and B56ε subunits, which not only hampers binding to LxxIxE motifs, but is also critical to enable B56α and B56ε interaction with SGO2. Remarkably, replacing the EPVA signature amino acids by the corresponding residues of the B56γ isoform (Thr405, Lys409, His412, and Gly413) enhanced B56α ability to bind BUBR1 and other LxxIxE-containing proteins [69]. Although structural details on how the C-terminal loop controls B56 specificity remain unknown, these observations clearly demonstrate sub-functionalization between the B56 isoforms. Whether such specialization allows PP2A activity to be differently regulated in specific subcellular compartments represents an alluring possibility that could be addressed in future studies.
Figure 6.
Kinetochore PP2A-B56 and PP1 promote the stability of microtubule attachments and efficient Spindle Assembly Checkpoint silencing in animal cells. (A,B) In the absence of microtubules, MPS1 kinase efficiently accumulates at kinetochores through direct binding to the NDC80 complex. This enables MPS1 trans-autoactivation and instates SAC signaling. MPS1 phosphorylates several MELT motifs on KNL1, generating docking sites for the hierarchical recruitment of SAC proteins required for MCC assembly. This also enables the recruitment of PP2A-B56γ to kinetochores, following PLK1-mediated phosphorylation of BUBR1 KARD motif. PP2A-B56γ bound to BUBR1 has critical roles in allowing the initial binding of microtubules to NDC80 and in establishing SAC responsiveness. The activity of PP2A-B56γ-BUBR1 antagonizes microtubule-destabilizing phosphorylations on the NDC80 N-terminal tail. These phosphorylations are catalyzed by centromeric Aurora B and predominate at tensionless kinetochores to enable the correction of erroneous attachments. Dephosphorylation of the NDC80 N-terminal tail by PP2A-B56-BUBR1 provides NDC80 an opportunity to bind microtubules. (C,D) As microtubules occupancy increases, kinetochore tension or alterations on the kinetochore architecture place outer-kinetochore proteins further apart from the Aurora B zone of influence. This renders PP2A-B56γ-BUBR1 activity largely unopposed, which ultimately results in complete dephosphorylation of NDC80 and in the formation of stable end-on attachments. At this point, several pools of PP1γ accumulate at the kinetochore to preserve the stability of bioriented attachments and promote prompt SAC silencing. The recruitment of PP1γ is mediated in part by its interaction with PP1-binding motifs on the KNL1 N-terminus. PP1γ-KNL1 dephosphorylates phospho-MELTs, causing the dissociation of SAC proteins from kinetochores and consequently ceasing MCC assembly. Binding of PP1γ to KNL1 is limited at unattached or tensionless kinetochores due to Aurora B-mediated phosphorylation of both PP1-docking motifs, which significantly represses their ability to interact with the phosphatase. These phosphorylations are counteracted by the activity of PP2A-B56γ bound to BUBR1. Thus, as end-on attachments are established, the KNL1 N-terminus becomes inaccessible to Aurora B and PP2A-B56γ ensures efficient recruitment of PP1γ. Increasing levels of PP1γ-KNL1 further suppress Aurora B activity at kinetochores, hence enhancing the stability of microtubule attachments. PP1γ-KNL1 is also proposed to directly antagonize the activating T-loop autophosphorylation of Aurora B, which likely favors the recruitment of the SKA complex to dephosphorylated NDC80. The SKA complex directly binds PP1γ through its SKA1 subunit and independently of KNL1, thus representing an additional pathway to deliver PP1γ to kinetochores of bioriented chromosomes. Importantly, because MPS1 and microtubules have partially overlapping binding surfaces on the NDC80 complex, the formation of stable end-on attachments imposes a substantial reduction in the amount of MPS1 at kinetochores. Residual MPS1 molecules remaining on bioriented kinetochores are dephosphorylated and inactivated by PP1. These mechanisms concur to efficiently avert SAC function at the top of the signaling cascade.
Because BUBR1 associates with KNL1 in the absence of microtubules, the binding of PP2A-B56γ to BUBR1 places the phosphatase in the close proximity of NDC80 to efficiently counteract Aurora B destabilizing activity and enable initial end-on microtubule interactions [373,374,375,381] (Figure 6A,B). As microtubule occupancy increases, the outer-kinetochore is further pulled away from Aurora B zone of influence and attachments stability concomitantly increases. At this point, PP1 activity at kinetochores increases to silence the mitotic checkpoint (as discussed in the following section) and ensure that mature end-on microtubule attachments remain stabilized [360,382,383,384] (Figure 6C,D). Recruitment of PP1 to kinetochores is mediated in part by SILK and RVSF motifs on KNL1 N-terminus [382]. At tensionless kinetochores, both PP1-docking motifs are phosphorylated by Aurora B, which significantly represses their ability to interact with PP1 [382] (Figure 6A). As end-on attachments mature, KNL1 N-terminus is no longer accessible to Aurora B and PP2A-B56γ dephosphorylates SILK and RVSF to allow PP1 binding (Figure 6B–D).
The PP1γ-KNL1 complex further suppresses kinetochore Aurora B activity, hence enhancing the stability of amphitelic attachments [385,386]. Disrupting the PP1γ-KNL1 interaction destabilizes kinetochore-microtubule attachments on metaphase-aligned chromosomes while failing to cause a discernible impact on chromosome congression [360,382]. Thus, although PP1γ-KNL1 activity seems to be dispensable for initial microtubule binding, it does contribute to preserve attachment stability following biorientation. In that respect, PP1γ-KNL1 antagonizes the activating T-loop autophosphorylation of Aurora B [385,387,388] and enables the recruitment of the SKA complex to the KMN network [389,390] (Figure 6C,D). The SKA complex at the kinetochore-microtubule interface is proposed to enhance the microtubule-binding affinity and end-tracking ability of the Ndc80 complex [391]. This increases the stability of attached microtubule bundles and maintains the kinetochore associated with dynamic microtubules, thus enhancing the ability of end-on attachments to sustain load-bearing force and couple chromosome movement to microtubules plus-end dynamics [389,391,392,393,394,395,396,397,398,399]. Aurora B-mediated phosphorylation of the SKA complex reduces its binding affinity for NDC80, which might provide a safety strategy to minimize premature stabilization of erroneous attachments during early prometaphase [389,391,396,400,401]. As chromosomes congress and PP1γ-KNL1 activity increases, SKA recruitment is triggered and the complex becomes maximally enriched at amphitelically attached kinetochores [394,396,400,402]. In C. elegans, timely association of the SKA complex with bioriented kinetochores is facilitated by dephosphorylation of the NDC80 N-terminal tail [389]. Whether PP1γ-KNL1 cooperates with PP2A-B56γ to dephosphorylate Aurora B phosphosites on the NDC80 tail remains unclear, as well as PP1γ-KNL1 aptitude to directly target Aurora B-mediated phosphorylations of the SKA complex. Interestingly, the SKA complex directly binds PP1γ through the C-terminal domain (CTD) of its SKA1 subunit and independently of KNL1, thus representing an additional pathway to increment PP1γ at kinetochores of bioriented chromosomes [403]. Although PP1γ-SKA significantly contributes to timely inactivation of the mitotic checkpoint, its role in kinetochore-microtubule attachments is unclear [403]. The accumulation of PP1 on bioriented kinetochores can be further endorsed by spindle motors. The budding yeast Kinesin 5, CIN8, associates with PP1/GL7 through a canonical RVxF motif [404]. Kinetochore localization of CIN8 dramatically increases during metaphase in a microtubule-dependent manner and is mediated by a direct interaction with the DAM1 complex, a functional analog of the SKA complex in vertebrates [393,395,404]. Binding to DAM1 places the PP1/GL7-CIN8 complex near the N-terminus of NDC80, likely resulting in dephosphorylation of its N-terminal tail. This increases the affinity of NDC80 for microtubules, and consequently, the stability and tension required for robust attachments [404]. Human cells engage in a similar strategy, but differ in the choice of motor, as the human Kinesin-5 orthologue does not localize at kinetochores. Instead, PP1γ binds to RVxF motifs on the plus-end directed motors, KIF18A [405,406] and CENP-E [407]. Pools of PP1γ-KIF18A and PP1γ-CENP-E accumulate at the ends of kinetochore-attached microtubules as cells progress from prometaphase to metaphase, thus providing a localized delivery of PP1γ to oppose NDC80 phosphorylation and promote attachment stability [407,408]. Importantly, Aurora A-mediated phosphorylation of CENP-E on its PP1-docking motif averts premature binding of PP1γ. This phosphorylation is maximal on polar chromosomes and is required for CENP-E-powered congression along kinetochore-microtubule bundles [407]. As chromosomes move away from Aurora A, dephosphorylation of CENP-E takes place, enabling PP1γ recruitment. Although it is presently uncertain which phosphatase pool dephosphorylates the PP1-docking motif on CENP-E, this was shown to be essential for the formation of stable end-on attachments and biorientation of chromosomes that congressed from the spindle poles [407].
Several other PIPs have been shown to act as important kinetochore-targeting factors of PP1, but their precise impact on microtubule attachment has been difficult to discern. For instance, PP1 bound to MYPT1 was shown to antagonize the phosphorylated T-loop of PLK1, which consequently renders the kinase inactive [409,410]. Contrasting with the previously described PP1-PIP complexes, MYPT1-PP1 associates with kinetochores preferentially during early mitosis and depends on CDK1-Cyclin A activity [45]. CDK1-Cyclin A-mediated phosphorylation of MYPT1 promotes its interaction with PLK1, hence dampening PLK1 activity and therefore the stability of kinetochore-microtubule attachments [45]. This is thought to generate a temporally-defined microtubule-destabilizing environment to facilitate the correction of erroneous attachments typically produced during early prometaphase [411]. The observation that PLK1 activity is suppressed prior to chromosome biorientation seems somehow difficult to reconcile with its requirement for initial end-on attachments. However, it is becoming increasingly clear that PLK1 modulates the stability of kinetochore-microtubule interactions through multiple kinetochore/centromere pools and possibly responding to different inputs [373,376,412,413,414,415,416,417,418,419,420,421,422,423,424]. The conserved regulatory subunit, SDS22/PPP1R7, has been reported to be essential for the activity of multiple PP1 isoforms at kinetochores. Depletion of SDS22/PPP1R7 in human cells perturbs kinetochore-microtubule attachments and results in chromosome alignment defects [385,386]. Paradoxically, SDS22/PPP1R7 does not seem to localize at kinetochores and PP1 fails to dephosphorylate its kinetochore substrates while bound to SDS22/PPP1R7 [425]. Critical insight into this apparent discrepancy came from the observation that SDS22 and I3 transiently act as chaperones during PP1 biogenesis [426]. Binding of SDS22 and I3 stabilizes newly-synthetized PP1 in an inactive state until it is under control of substrate specifier PIPs [425,426,427,428]. The p97 AAA-ATPase complex subsequently catalyzes the disassembly of the heterotrimeric complex, which enables PP1 to associate with one of its numerous adaptor subunits and form a functional holoenzyme [426].
2.5. Silencing the Spindle Assembly Checkpoint: PP1 and PP2A-B56 Shut It Up
To ensure that each daughter nucleus receives one copy of the genome, chromosome segregation can only occur after all sister-kinetochore have established stable amphitelic attachments. Timely control of anaphase onset is achieved through the spindle assembly checkpoint (SAC), a conserved signaling pathway primarily instated by unattached/tensionless kinetochores that inhibits the ubiquitin-dependent proteolysis of Securin and Cyclin B. Degradation of Securin triggers the cleavage of centromeric cohesion by the protease Separase to enable sister chromatid separation, whereas reduced levels of Cyclin B lead to inactivation of CDK1, which consequently reverts the cell into an interphase state. According to the prevailing mechanistic model of SAC signaling, unattached/tensionless kinetochores catalyze the assembly of a diffusible protein tetramer known as mitotic checkpoint complex (MCC) that binds to the E3 ubiquitin ligase anaphase promoting complex/cyclosome (APC/C) and thereby prevents it from targeting Securin and Cyclin B for degradation [429,430,431,432]. A weakened SAC signaling was shown to allow anaphase onset in the presence of one or few unattached chromosomes, which resulted in varied levels of aneuploidy [433,434,435,436]. On the other hand, failing to switch off the SAC in a timely manner delays the cells in metaphase, thus increasing the likelihood of merotelic attachments and cohesion fatigue, errors that often lead to lagging chromosomes and chromatin bridges [436,437]. Hence, to safeguard against genome imbalances, the SAC must be robust enough so that a single unattached kinetochore is sufficient to halt the transition to anaphase, but also highly responsive to allow swift mitotic exit upon formation of stable microtubule attachments [384,438,439]. Activation and strength of SAC signaling is driven by the activity of multiple mitotic kinases, with monopolar spindle 1 (MPS1) assuming the leading role [440,441]. Unsurprisingly, counteracting phosphatases play a critical role in extinguishing the checkpoint signal and ensure timely cell cycle progression.
Monopolar spindle 1 associates with unattached kinetochores and phosphorylates KNL1 on its MELT repeats, creating docking sites for the hierarchical recruitment of additional SAC proteins necessary for MCC assembly [442,443,444,445,446] (Figure 6A). Heterodimers of BUB1-BUB3 bind to phosphorylated MELTs and recruit BUBR1-BUB3 through hetero-dimerization [447]. Monopolar spindle 1 phosphorylates KNL1-associated Bub1 to promote the binding of MAD1-MAD2 complexes [448,449,450,451,452,453]. Subsequently, MPS1-mediated phosphorylation of MAD1 dramatically accelerates the structural conversion of MAD2 into a conformer that binds to CDC20, the rate-limiting step for MCC assembly [450,451]. These multi-target phosphorylations along the SAC signaling cascade render the checkpoint highly responsive to variations in MPS1 activity [454]. Activation of MPS1 is triggered during early mitosis by T-loop trans-autophosphorylation. The CH domains of NDC80 and NUF2 directly mediate the recruitment of MPS1 to unattached kinetochores [455,456], which likely favors the kinase trans-autoactivation [457,458,459,460,461,462]. Kinetochore tethering of MPS1 and its activity are enhanced by Aurora B, PLK1, and CDK1, but the underlying mechanisms remain largely unknown [463,464,465,466,467,468,469,470]. On the other hand, MPS1 promotes centromeric accumulation of Aurora B via the BUB1-H2A-SGO1 axis and was shown to directly foster Aurora B activity, thus setting a positive feedback loop that ensures prompt and robust activation of both kinases at the onset of mitosis [471,472].
Once all kinetochores attach to microtubules and become stably bioriented, the SAC must be silenced to permit APC/C activation and initiate anaphase. This primarily entails mechanisms that prevent and revert the phosphorylations catalyzed by MPS1. The region of the NDC80 CH domain that interacts with MPS1 partially overlaps with the microtubule binding interface [455], which significantly restrains MPS1 ability to associate with kinetochores at a high microtubule occupancy. This biochemical competition imposes a severe reduction on the amount of MPS1 present at kinetochores once stable end-on attachments are established [455,456] (Figure 6A–D). This concurs with increased kinetochore localization of PP1, which in Drosophila cells was shown to dephosphorylate the T-loop of MPS1 to ensure the inactivation of residual MPS1 molecules remaining at kinetochores and thereby efficiently avert the phosphorylation of SAC substrates during metaphase [473] (Figure 6C,D). PP2A-B55 was also shown to dephosphorylate MPS1 in MEFs [174], but the functional relevance of the targeted residue remains unclear [460,465].
Suppressing MPS1 activity at bioriented kinetochores is not sufficient to promptly silence the SAC, as the phosphorylations carried out to instate the checkpoint still need to be reversed. This task has been almost consensually attributed to PP1 [379,442,474,475,476,477,478,479,480]. Several PP1-PIP complexes were shown to contribute for SAC silencing. PP1γ bound to KNL1 is particularly well positioned, as it lies adjacent to the site of MCC production. Impairing PP1γ interaction with KNL1 prevents the dephosphorylation of MELT motifs and delays exit from mitosis in different organisms [379,442,478,479,480,481]. Human cells lacking the PP1γ-KNL1 complex and incubated with microtubule depolymerizing drugs were able to remain in mitosis even after MPS1 inhibition, suggesting that PP1γ directly targets the phospho-MELTs to cease the recruitment of SAC proteins and consequently silence the SAC [379]. Efficient binding of PP1γ to KNL1 relies on the dephosphorylation of SILK and RVxF motifs by PP2A-B56γ in complex with BUBR1, which is itself indirectly recruited to KNL1 upon MELT phosphorylation [379,382,442,443,444] (Figure 6A–C). This negative feedback loop enables the checkpoint to prime its own inhibition, thus rendering signaling quenching highly responsive to microtubule binding [380]. Prompt SAC silencing following end-on attachment is further ensured by pools of PP1γ delivered to kinetochores through microtubules [403,478,482] (Figure 6C,D). In fission yeast, DIS2/PP1 bound to the kinesin-8 motor KLP5-KLP6 heterodimer cooperates with SPC7/KNL1-associated DIS2/PP1 in switching off the SAC [478]. However, the kinetics of checkpoint silencing mediated by each pathway differs considerably, with DIS2-SPC7 acting firstly under low microtubule occupancy, while DIS2-KLP5-KLP6 appears to operate during later time points as attachments stabilize [478,482]. Preventing PP1γ binding to the KIF18A orthologue in human cells delays the transition to anaphase, but whether this is caused by inefficient SAC silencing is unclear as the activity of PP1γ-KIF18A is required for the stability of kinetochore-microtubule attachments [406,408]. Likewise, disruption of the interaction between PP1γ and SKA1 through deletion of SKA1 CTD results in a prolonged metaphase delay [403]. Notably, replacing the CTD by a PP1-binding motif or by a direct fusion to PP1γ largely restores timely anaphase onset, suggesting that the recruitment of PP1γ mediated by the SKA complex is important to efficiently silence the SAC at bioriented kinetochores [403]. However, SKA1 CTD has been shown to be required for the stability of kinetochore-microtubule attachments [391,401], which calls for some caution in ascribing a direct role for PP1γ-SKA1 in switching off the SAC. It is possible that PP1γ-SKA1 might instead counteract destabilizing phosphorylations on NDC80 and thereby indirectly promote SAC silencing by increasing the stability of end-on attachments [384]. In line with that, the SKA complex was shown to directly interact with multiple regions of NDC80 [389,390,399,483,484,485]. On the other hand, this also means that PP1γ is favorably positioned to dephosphorylate and inactivate MPS1 when robust microtubule attachments are established [384]. It is therefore important to determine which specific phosphorylations are targeted by each pool of PP1γ present at kinetochores. This will be critical to better understand their role and how the activities of PP1γ-KNL1 and PP1γ complexes recruited through microtubules are coordinated to ensure efficient SAC silencing. In that respect, recent structural data have shown that PP1-docking motifs in KNL1 overlap extensively with domains that also mediate binding to microtubules, and, at least in vitro, these are mutually exclusive [486]. This hints that in mammalian cells, as described for fission yeast [478,482], PP1γ-KNL1 operates mainly at a low microtubule occupancy earlier in mitosis and sets up the recruitment of microtubule-dependent pools of PP1γ to bioriented kinetochores. This would be consistent with the observation that KNL1 mutants defective in PP1γ binding fail to switch off the SAC in nocodazole [379], while exhibiting only a modest metaphase delay under unperturbed conditions [464], whereas loss of the PP1γ-SKA1 complex results in a prominent SAC-dependent metaphase arrest, but only marginally affects mitotic exit when MPS1 is inhibited in the absence of microtubules [384,403].
Interestingly, PP2A-B56γ bound to BUBR1 might also directly contribute to dephosphorylate phospho-MELT motifs [487] and was recently shown to antagonize MPS1-mediated phosphorylation of BUB1 Thr461 [452]. This phosphorylation stimulates MAD1 binding [451,452,453] and appears to be only transiently required for SAC initiation immediately upon nuclear envelope breakdown [452]. Dephosphorylation of BUB1 Thr461 is triggered during early prometaphase concomitantly with the recruitment of PP2A-B56γ to BUBR1. Phosphorylation of BUB1 Thr461 is virtually undetectable in metaphase or after a drug-elicited prometaphase arrest, suggesting that BUB1 dephosphorylation by PP2A-B56γ is impervious to kinetochore attachment status [452]. Thus, MPS1 and PP2A-B56γ-BUBR1 establish an incoherent feedforward loop that restricts BUB1 Thr461 phosphorylation to the period of time required to assemble PP2A-B56γ-BUBR1 at kinetochores [452]. Because this transient pulse in BUB1 Thr461 phosphorylation is not affected by microtubule occupancy, it was proposed to function as a biochemical timer defining the minimum length of time the cell spends in mitosis [452]. Bub1 further promotes APC/C inhibition through an MCC-independent mechanism. Kinetochore BUB1 acts as a scaffold for PLK1-mediated phosphorylation of the APC/C co-activator, CDC20. Although the mechanistic underpinnings remain elusive, this phosphorylation was shown to inhibit CDC20 ability to bind and activate the APC/C [488]. The inhibitory phosphorylation was proposed to be removed by PP2A-B56γ-BUBR1 [488]. However, CDC20 phosphorylation remained more stable in cells incubated with okadaic acid than in cells depleted of all B56 isoforms, suggesting that PP1 or additional phosphatases might contribute to dephosphorylate CDC20 and enable APC/C activation [489,490]. Further inhibition of CDC20 is promoted by CDK1-Cyclin B, which paradoxically also phosphorylates the APC/C to ensure its proper ubiquitination activity [64,489,490,491,492]. PP2A-B55 has been implicated in opposing the inhibiting and activating CDK1 phosphorylations on CDC20 and APC/C, respectively [64]. The intrinsic preference of PP2A-B55 towards pThr over pSer is proposed to provide a short period of time in which dephosphorylated CDC20 is able to interact with phosphorylated and functional APC/C [64]. However, the APC/C-CDC20 is known to be active prior to reactivation of PP2A-B55 [493], which in fact requires APC/C-CDC20 dependent repression of GWL activity. Hence, removal of CDK1 inhibitory phosphorylations on CDC20 likely relies on the activity of other phosphatases, an undertaking recently attributed to PP1γ-KNL1 in C. elegans [491].
2.6. Mitotic Exit: PP1 and PP2A-B55 Clear the Way
Once the SAC is switched off and APC/C-CDC20-dependent degradation of Securin and Cyclin B occurs, the cell becomes irreversibly committed to exit mitosis. Resumption of an interphase state requires remodeling of the spindle architecture, reassembly of the functional nuclear envelope, and decondensation of chromatin [86]. This extensive structural reorganization relies not only on the inactivation of CDK1, but also on the removal of mitotic phosphorylations, an assignment that in animal cells is primarily led by PP2A-B55 and PP1 phosphatases [58,86]. A key event during anaphase is the reorganization of spindle microtubules into a structure referred to as the central spindle. This is assembled between the segregating chromosomes to define the position of the cleavage furrow, thus ensuring spatial and temporal coordination of cytokinesis with genome partitioning [86,494,495,496,497]. Formation of the central spindle requires PRC1, a microtubule-bundling protein that stabilizes and organizes microtubules in an antiparallel overlapped fashion and promotes anaphase spindle elongation [498]. Phosphorylation of PRC1 by CDK1-Cyclin B prevents its microtubules’ bundling activity before anaphase [499,500,501,502]. Timely PRC1 dephosphorylation is catalyzed by PP2A-B55 [164] to enable PRC1 interaction with the kinesin motor KIF4 and its consequent translocation to the plus ends of antiparallel interdigitating microtubules [498,503] (Figure 7A). Furthermore, PP2A-B55-mediated dephosphorylation promotes PRC1 homodimerization that is required for its microtubule bundling activity and thereby for the assembly of the central spindle [164,502,504,505] (Figure 7A).
Figure 7.
PP2A-B55 and PP1 activities orchestrate mitotic exit. (A) Formation of the central spindle during anaphase relies on the activity of PRC1. Phosphorylation of PRC1 by CDK1-Cyclin B precludes its microtubule bundling activity. Dephosphorylation of PRC1 is catalyzed by PP2A-B55 during anaphase and enables PRC1 interaction with the kinesin motor KIF4, its consequent translocation to the plus ends of antiparallel interdigitating microtubules, and the protein homodimerization required for its microtubule bundling activity and thereby for the assembly of the central spindle. (B) Reformation of the nuclear envelope and associated nuclear pore complexes around chromatin occurs at the end of mitosis. This requires removal of the multiple phosphorylations that were catalyzed during mitotic entry to promote the disassembly of the nuclear envelope and of nuclear pore complexes. During late anaphase/telophase, PP2A-B55-mediated dephosphorylation of BAF restores its interaction with chromatin and with components of the inner nuclear membrane (INM). PP2A-B55 might also target Lamin and several nucleoporins to direct their assembly into the nuclear envelope. Multiple pools of PP1 cooperate with PP2A-B55 to promote nuclear envelope reformation. AKAP149 localizes PP1 on the assembling nuclear envelope, where it dephosphorylates Lamin B to promote its polymerization and accelerate nuclear lamina assembly. Repo-Man targets PP1γ to the periphery of late anaphase chromosomes. The N-terminus of Repo-Man directly mediates the recruitment of Importin-β to control early nuclear envelope formation and nucleoporin deposition.
Reformation of the nuclear envelope and associated nuclear pore complexes around chromatin occurs at the end of mitosis to establish functional interphase nuclei [506,507,508]. Disassembly of the nuclear envelope and of nuclear pore complexes at mitotic entry is triggered by a myriad of phosphorylations catalyzed by several mitotic kinases (Figure 7B). Phosphorylation of Lamins by CDK1 promotes depolarization of the nuclear lamina, the filamentous protein meshwork underlying the nuclear membranes [509,510]. Cyclin-dependent kinase 1 further cooperates with PLK1 in the phosphorylation of several nucleoporins to elicit disintegration of nuclear pore complexes [511,512,513,514,515,516,517,518]. Moreover, phosphorylation of the chromatin binding protein, BAF, by VRK reduces its affinity for chromatin and towards the LEM family of inner nuclear membrane proteins interacting with the nuclear lamina [519,520,521]. Loss of the BAF-mediated link between chromatin and the inner nuclear membrane at mitotic entry is proposed to contribute to nuclear envelope disassembly. Phosphorylations on BAF are however removed during mitotic exit by action of PP2A-B55 (Figure 7B). This restores BAF interaction with chromatin and with components of the nuclear periphery, which was shown to be required for timely nuclear envelope reformation in worms, flies, and human cells [522,523]. Furthermore, recent findings in Drosophila cells suggest that PP2A-B55 might also target Lamin and NUP107 to direct their assembly into the nuclear envelope during mitotic exit [523]. Further evidence supporting PP2A-B55 involvement in nuclear pore reformation come from the observation that the phosphatase dephosphorylates NUP153 (as well as NUP107) to promote its recruitment to chromatin in human cells [58] (Figure 7B).
Several findings indicate that PP1 is equally important for reassembly of functional nuclear envelopes. Binding to AKAP149 localizes PP1 on the assembling nuclear envelope, where it dephosphorylates Lamin B to promote its polymerization and accelerates nuclear lamina assembly [524,525,526] (Figure 7B). The regulatory subunit, Repo-Man, targets PP1γ to the periphery of late anaphase chromosomes, where the initial reassembly events take place [508,527,528,529]. There, the N-terminus of Repo-Man directly mediates the recruitment of Importin-β to control early nuclear envelope formation and nucleoporin deposition [528,530,531,532,533,534] (Figure 7B). Binding of Importin-β to Repo-Man does not require PP1γ catalytic activity and is repressed during mitosis by CDK1 phosphorylations on the Repo-Man N-terminus [528]. Localized activity of PP1 seems, however, to be required for later steps of nuclear pore complex formation. One subset of the nucleoporins that is controlled by Importin-β is the NUP107–NUP160 complex [535], which is recruited to chromatin by the nuclear pore complex assembly factor, ELYS/MEL-28 [536,537,538,539,540]. This requires RanGTP-dependent relief of NUP107–NUP160 nucleoporins from an inhibitory association with importin-β, most likely docked at the vicinity of ELYS/MEL-28 by Repo-Man [527,528,529]. This results in the formation of chromatin-bound pre-pores onto which additional nucleoporins are subsequently assembled, a process that in C. elegans was recently shown to require the contribution of PP1c/GSP-2 bound to ELYS/MEL-28 [541]. However, direct evidence for dephosphorylation events catalyzed by PP1c/GSP-2 and a mechanistic understanding of its role in the reassembly of nuclear pore complexes are still missing.
As the nuclear envelope reassembles, the segregated chromatin decondenses to re-establish an interphase structure compatible with DNA replication and transcription [542]. This relies on the activity of PP1γ, which, prior to its accumulation at the periphery of late anaphase chromosomes, is homogenously distributed over chromatin during early stages of anaphase. This initial recruitment of PP1γ to chromatin is also mediated by the regulatory subunit, Repo-Man, although through a separate C-terminal module that is dispensable for the peripheral localization [528,543,544]. Analogously to inhibitory phosphorylations on the Repo-Man N-terminal domain, phosphorylation of the C-terminus chromatin-binding module by CDK1 and Aurora B prevents Repo-Man binding to histones before anaphase onset [528,545]. PP1γ-Repo-Man antagonizes the mitotic phosphorylation of Thr3 and Ser10 on histone H3, respectively catalyzed by Haspin and Aurora B [528,546,547]. Dephosphorylation of Thr3 allows the translocation of the CPC from chromosomes to the central spindle, whereas dephosphorylation of Ser10 triggers decondensation and enables the re-association of HP1 to H3K9me3 after mitosis [546,548]. Intriguingly, depletion of Repo-Man fails to cause a dramatic impairment of chromatin decondensation [528]. Because the recruitment of PP1γ to anaphase chromatin is also mediated by Ki-67 [549,550], it is possible that the pool of PP1γ-Ki-67 compensates for the loss of Repo-Man [542]. However, the exact contribution of PP1γ-Ki-67 holoenzyme for chromatin decondensation remains to be demonstrated. Another chromatin targeting factor for PP1γ is the regulatory subunit, PNUTS. Binding of PP1γ to PNUTS through a canonical RVxF motif was shown to enhance chromatin decondensation in vitro [551]. However, the association of PP1γ-PNUTS with chromatin is only detected during telophase, after H3 Ser10 dephosphorylation and nuclear envelope reformation [551]. Therefore, the precise targets of PP1γ-PNUTS and its relevance for chromatin decondensation are still elusive.
3. Spatiotemporal Control of Phosphatase Activities
The accuracy of mitosis requires tight spatiotemporal control of phosphatase activities. Although PP1 does contribute to mitotic exit progression, the bulk of CDK1-mediated phosphorylations are removed by PP2A when in complex with B55 [58]. Thus, PP2A-B55 must be kept inhibited from nuclear envelope breakdown to anaphase onset for the sake of mitotic compliance, becoming then reactivated to orchestrate the major events underlying anaphase and mitotic exit. As mentioned in Section 2.1, repression of PP2A-B55 is triggered by CDK1-mediated activation of GWL, which subsequently phosphorylates ENSA and Arpp19 to promote their inhibitory binding to PP2A-B55 [165,166,176,177] (Figure 3A). Both inhibitors are high affinity substrates of PP2A-B55, but because their dephosphorylation rate is orders of magnitude lower than CDK1-phosphorylated targets, this efficiently restrains PP2A-B55 activity in what is known as “unfair competition” [552]. Degradation of Cyclin B upon transition into anaphase initiates the cascade that activates PP2A-B55. Decreasing CDK1-Cyclin B activity is proposed to render PP1 active to dephosphorylate and partially inactivate GWL [553,554,555,556] (Figure 3A). This means that the phosphorylations that are ultimately removed from ENSA and Arpp19 by PP2A-B55 now fail to be efficiently replaced, hence allowing PP2A-B55 to target CDK1-phosphorylated substrates [552]. In that respect, PP2A-B55 further dephosphorylates GWL to ensure its complete inactivation and thereby maintain elevated PP2A-B55 activity to promote mitotic exit [553,554,555,556] (Figure 3A).
Importantly, active PP2A-B55 seems to follow a timely defined dephosphorylation program so that events in anaphase, telophase, and cytokinesis are carefully coordinated and regulated to ensure a successful cell division [58,164]. It is absolutely critical that dephosphorylation of PRC1 and of other spindle proteins occurs before the dephosphorylation of Lamins and of nucleoporins, so that the nuclear envelope and nuclear pores can only assemble and import proteins once chromosomes are successfully segregated [58,59]. The timing of substrate dephosphorylation during mitotic exit is determined by the net basic charge of the two polybasic regions upstream and downstream of the CDK1-phosphorylated site. Spindle proteins have pronounced polybasic regions and are therefore readily selected and dephosphorylated by PP2A-B55 early in anaphase, whereas nuclear envelope proteins comprise an inferior number of basic residues and are therefore dephosphorylated at lower rates. This differential catalytic efficiency enables PP2A-B55 to achieve temporal dephosphorylation of CDK1 sites during mitotic exit and, thereby, coordinate the segregation of chromosomes with nuclear envelope reformation [58]. Moreover, the intrinsic preference of the PP2A catalytic subunit towards pThr over pSer residues provides an additional regulatory element to ensure ordered dephosphorylation of substrates during mitotic exit [63,64]. Early anaphase events seem to be predominantly driven by pThr dephosphorylation, whereas pSer-bearing substrates are dephosphorylated with slower dynamics, thus contributing for sequential execution of the mitotic exit program [64].
The mechanism controlling PP1-mediated inactivation of GWL remains, however, somewhat contentious. It has been proposed that PP1 activity is globally repressed in mitosis by a CDK1 inhibitory phosphorylation on its C-terminus [557,558,559] and by binding to I-1, an inhibitory PIP whose interaction with PP1 is promoted by a PKA-dependent phosphorylation residue [560]. Notably, both phosphorylations are targeted by PP1 itself. Thus, declining CDK1-Cyclin B activity following SAC silencing is thought to be sufficient to allow PP1 autodephosphorylation and I-1 dephosphorylation, thereby resulting in increased activation of PP1 to levels that drive mitotic exit [553,554,555,556,560]. This sequential activation of PP1 and PP2A-B55 occurring in animal cells resembles a phosphatase relay reported in fission yeast, which also operates as a timer for the orderly dephosphorylation of mitotic substrates at the end of division [559]. Interestingly, not all PP1 molecules are inhibited by CDK1 during mitosis [561] and, therefore, are likely to be involved in the phosphatase relay. In fact, the activity of multiple PP1 holoenzymes is known to be required for the stability of kinetochore-microtubule attachments and efficient SAC silencing, events that occur before APC/C-CDC20-triggered degradation of Cyclin B. It would be interesting to identify which specific pools of PP1 are under control of CDK1-Cyclin B to elucidate how these engage with GWL to instate the phosphatase relay and to understand the mechanism that selectively renders some PP1 holoenzymes impervious to this inhibition. The activity of PP1-PIP complexes operating in mitosis is, however, locally regulated, frequently by controlling PP1 interaction with regulatory subunits or substrates. This generally entails the phosphorylation of RVxF PP1-binding motifs by Aurora B, which abrogates the PIP ability to bind PP1 [562]. This regulatory strategy enables the cell to tightly control the levels of PP1 at centromeres and at kinetochores in response to microtubule attachment status [562,563] (Figure 6A–D). The inhibitory phosphorylations on the KNL1 PP1-docking motif are removed by BUBR1-PP2A-B56, which allows PP1γ to associate with KNL1. This comprises another phosphatase relay, now at kinetochores and required for efficient SAC silencing and timely anaphase onset.
The activity of PP2A-B56 at kinetochores is modulated to avert deleterious dephosphorylation during early mitosis, when correction of erroneous attachments is often required. This is accomplished by BOD1, a small protein that shares sequence similarity with ENSA and Arpp19, and that specifically binds to PP2A-B56 when phosphorylated by CDK1 [564]. BOD1-mediated inhibition of PP2A-B56 is maximal at early prometaphase, possibly to prevent premature dephosphorylation of the NDC80 N-terminus and of KNL1 PP1-docking motifs [565]. As the cell progresses through mitosis, relief of PP2A-B56 from the inhibitory interaction with BOD1 is essential for the formation and stabilization of end-on attachments. The mechanism responsible for BOD1 dephosphorylation remains, however, undetermined.
4. Concluding Remarks and Future Directions
In the past decades, we have witnessed the emergence of protein phosphatases as critical regulators of mitosis. In recent years, the list of identified substrates and regulators has increased considerably, as has our understanding of phosphatases’ roles in mitotic progression.
Structural and biochemical determinants of phosphatase holoenzyme assembly and specificity have been unveiled. Research has made important advances in the functional characterization of protein phosphatases and has generated critical molecular knowledge to better understand their spatiotemporal control. Importantly, these achievements have provided new and renovated perspectives on the regulation of mitotic events, whose accurate execution is absolutely critical for genomic stability.
Nonetheless, enduring unanswered questions deserve further investigation. It remains relevant to dissect the regulatory network underlying the activity of CDC25 isoforms. The field will also benefit from a comprehensive identification of the precise substrates that are targeted by specific Ser/Thr phosphatase holoenzymes and of the upstream signaling inputs controlling the subcellular localization and activity of the different holoenzymes. These have been hampered by the inability to routinely tackle specific interactions of phosphatases with substrates or regulators. Further structural insights into SLiM-mediated interactions might reveal critical mechanisms to enable the disruption of specific phosphatase complexes in a way that is compatible with systematic studies. Furthermore, the development of specific inhibitors targeting phosphatase-SLiM contacts should also fuel their potential use as therapeutic strategies in disease. This might represent a valuable alternative, or complement, to protein kinase inhibitors that are often associated with on-target resistance.
Acknowledgments
The authors would like to thank Claudio Sunkel (i3S/IBMC), Mariana Osswald (i3S/IBMC), Nelson Leça (i3S/IBMC) and Ana Pinto (i3S/IBMC) for critical reading of the manuscript and helpful suggestions.
Author Contributions
Writing—original draft preparation, C.C. and M.M.; writing—review and editing, C.C.; supervision, C.C.; project administration, C.C.; funding acquisition, C.C.
Funding
This research was funded by project Norte Portugal Regional Operational Program (NORTE 2020) Norte-01-0145-FEDER-000029—Advancing Cancer Research: From basic knowledge to application, under the PORTUGAL 2020 Partnership Agreement through the European Regional Development Fund, and by National Funds through Fundação para a Ciência e a Tecnologia under the project IF/01755/2014. C.C. is supported by the FCT investigator program (IF/01755/2014) and M.M. is supported by an FCT PhD fellowship (SFRH/BD/123306/2016).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Olsen J.V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Chen M.J., Dixon J.E., Manning G. Genomics and evolution of protein phosphatases. Sci. Signal. 2017;10:eaag1796. doi: 10.1126/scisignal.aag1796. [DOI] [PubMed] [Google Scholar]
- 3.Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 4.Andersen J.N., Mortensen O.H., Peters G.H., Drake P.G., Iversen L.F., Olsen O.H., Jansen P.G., Andersen H.S., Tonks N.K., Møller N.P. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 2001;21:7117–7136. doi: 10.1128/MCB.21.21.7117-7136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y. Serine/Threonine Phosphatases: Mechanism through Structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Brautigan D.L., Shenolikar S. Protein serine/threonine phosphatases: Keys to unlocking regulators and substrates. Annu. Rev. Biochem. 2018;87:921–964. doi: 10.1146/annurev-biochem-062917-012332. [DOI] [PubMed] [Google Scholar]
- 7.Mitić N., Miraula M., Selleck C., Hadler K.S., Uribe E., Pedroso M.M., Schenk G. Catalytic mechanisms of metallohydrolases containing two metal ions. Adv. Protein Chem. Struct. Biol. 2014;97:49–81. doi: 10.1016/bs.apcsb.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Heroes E., Rip J., Beullens M., Van Meervelt L., De Gendt S., Bollen M. Metals in the active site of native protein phosphatase-1. J. Inorg. Biochem. 2015;149:1–5. doi: 10.1016/j.jinorgbio.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Verbinnen I., Ferreira M., Bollen M. Biogenesis and activity regulation of protein phosphatase 1. Biochem. Soc. Trans. 2017;45:89–99. doi: 10.1042/BST20160154. [DOI] [PubMed] [Google Scholar]
- 10.McNall S.J., Fischer E.H. Phosphorylase phosphatase. Comparison of active forms using peptide substrates. J. Biol. Chem. 1988;263:1893–1897. [PubMed] [Google Scholar]
- 11.Agostinis P., Goris J., Pinna L.A., Marchiori F., Perich J.W., Meyer H.E., Merlevede W. Synthetic peptides as model substrates for the study of the specificity of the polycation-stimulated protein phosphatases. Eur. J. Biochem. 1990;189:235–241. doi: 10.1111/j.1432-1033.1990.tb15482.x. [DOI] [PubMed] [Google Scholar]
- 12.Agostinis P., Derua R., Sarno S., Goris J., Merlevede W. Specificity of the polycation-stimulated (type-2A) and ATP, Mg-dependent (type-1) protein phosphatases toward substrates phosphorylated by P34cdc2 kinase. Eur. J. Biochem. 1992;205:241–248. doi: 10.1111/j.1432-1033.1992.tb16774.x. [DOI] [PubMed] [Google Scholar]
- 13.Imaoka T., Lynham J.A., Haslam R.J. Purification and characterization of the 47,000-dalton protein phosphorylated during degranulation of human platelets. J. Biol. Chem. 1983;258:11404–11414. [PubMed] [Google Scholar]
- 14.Mumby M.C., Russell K.L., Garrard L.J., Green D.D. Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J. Biol. Chem. 1987;262:6257–6265. [PubMed] [Google Scholar]
- 15.Xing Y., Xu Y., Chen Y., Jeffrey P.D., Chao Y., Lin Z., Li Z., Strack S., Stock J.B., Shi Y. Structure of Protein Phosphatase 2A Core Enzyme Bound to Tumor-Inducing Toxins. Cell. 2006;127:341–353. doi: 10.1016/j.cell.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Barr F.A., Elliott P.R., Gruneberg U. Protein phosphatases and the regulation of mitosis. J. Cell Sci. 2011;124:2323–2334. doi: 10.1242/jcs.087106. [DOI] [PubMed] [Google Scholar]
- 17.Ingebritsen T.S., Cohen P. Protein phosphatases: Properties and role in cellular regulation. Science. 1983;221:331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- 18.Okano K., Heng H., Trevisanato S., Tyers M., Varmuza S. Genomic organization and functional analysis of the murine protein phosphatase 1c γ (Ppp1cc) gene. Genomic organization and functional analysis of the murine protein phosphatase 1c γ (Ppp1cc) gene. Genomics. 1997;45:211–215. doi: 10.1006/geno.1997.4907. [DOI] [PubMed] [Google Scholar]
- 19.Boens S., Szekér K., Van Eynde A., Bollen M. Interactor-guided dephosphorylation by protein phosphatase-1. Methods Mol. Biol. 2013;1053:271–281. doi: 10.1007/978-1-62703-562-0_16. [DOI] [PubMed] [Google Scholar]
- 20.Sinha N., Pilder S., Vijayaraghavan S. Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PLoS ONE. 2012;7:e47623. doi: 10.1371/journal.pone.0047623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinha N., Puri P., Nairn A.C., Vijayaraghavan S. Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biol. Reprod. 2013;89:128. doi: 10.1095/biolreprod.113.110239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibbons J.A., Kozubowski L., Tatchell K., Shenolikar S. Expression of human protein phosphatase-1 in Saccharomyces cerevisiae highlights the role of phosphatase isoforms in regulating eukaryotic functions. J. Biol. Chem. 2007;282:21838–21847. doi: 10.1074/jbc.M701272200. [DOI] [PubMed] [Google Scholar]
- 23.Alessi D.R., Street A.J., Cohen P., Cohen P.T. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur. J. Biochem. 1993;213:1055–1066. doi: 10.1111/j.1432-1033.1993.tb17853.x. [DOI] [PubMed] [Google Scholar]
- 24.Liu R., Correll R.N., Davis J., Vagnozzi R.J., York A.J., Sargent M.A., Nairn A.C., Molkentin J.D. Cardiac-specific deletion of protein phosphatase 1β promotes increased myofilament protein phosphorylation and contractile alterations. J. Mol. Cell. Cardiol. 2015;87:204–213. doi: 10.1016/j.yjmcc.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egloff M.P., Cohen P.T., Reinemer P., Barford D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J. Mol. Biol. 1995;254:942–959. doi: 10.1006/jmbi.1995.0667. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg J., Huang H.B., Kwon Y.G., Greengard P., Nairn A.C., Kuriyan J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature. 1995;376:745–753. doi: 10.1038/376745a0. [DOI] [PubMed] [Google Scholar]
- 27.Bollen M., Peti W., Ragusa M.J., Beullens M. The extended PP1 toolkit: Designed to create specificity. Trends Biochem. Sci. 2010;35:450–458. doi: 10.1016/j.tibs.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heroes E., Lesage B., Görnemann J., Beullens M., Van Meervelt L., Bollen M. The PP1 binding code: A molecular-lego strategy that governs specificity. FEBS J. 2013;280:584–595. doi: 10.1111/j.1742-4658.2012.08547.x. [DOI] [PubMed] [Google Scholar]
- 29.Roy J., Cyert M.S. Cracking the phosphatase code: Docking interactions determine substrate specificity. Sci. Signal. 2009;2:re9. doi: 10.1126/scisignal.2100re9. [DOI] [PubMed] [Google Scholar]
- 30.Marsh J.A., Dancheck B., Ragusa M.J., Allaire M., Forman-Kay J.D., Peti W. Structural diversity in free and bound states of intrinsically disordered protein phosphatase 1 regulators. Structure. 2010;18:1094–1103. doi: 10.1016/j.str.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davey N.E., Van Roey K., Weatheritt R.J., Toedt G., Uyar B., Altenberg B., Budd A., Diella F., Dinkel H., Gibson T.J. Attributes of short linear motifs. Mol. Biosyst. 2012;8:268–281. doi: 10.1039/C1MB05231D. [DOI] [PubMed] [Google Scholar]
- 32.Davey N.E., Cyert M.S., Moses A.M. Short linear motifs–ex nihilo evolution of protein regulation. Cell Commun. Signal. 2015;13:43. doi: 10.1186/s12964-015-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakula P., Beullens M., Ceulemans H., Stalmans W., Bollen M. Degeneracy and function of the ubiquitous RVXF-motif that mediates binding to protein phosphatase-1. J. Biol. Chem. 2003;278:18817–18823. doi: 10.1074/jbc.M300175200. [DOI] [PubMed] [Google Scholar]
- 34.Meiselbach H., Sticht H., Enz R. Structural analysis of the protein phosphatase 1 docking motif: Molecular description of binding specificities identifies interacting proteins. Chem. Biol. 2006;13:49–59. doi: 10.1016/j.chembiol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Egloff M.P., Johnson D.F., Moorhead G., Cohen P.T., Cohen P., Barford D. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16:1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirano K., Phan B.C., Hartshorne D.J. Interactions of the subunits of smooth muscle myosin phosphatase. J. Biol. Chem. 1997;272:3683–3688. doi: 10.1074/jbc.272.6.3683. [DOI] [PubMed] [Google Scholar]
- 37.Ceulemans H., Stalmans W., Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays. 2002;24:371–381. doi: 10.1002/bies.10069. [DOI] [PubMed] [Google Scholar]
- 38.Terrak M., Kerff F., Langsetmo K., Tao T., Dominguez R. Structural basis of protein phosphatase 1 regulation. Nature. 2004;429:780–784. doi: 10.1038/nature02582. [DOI] [PubMed] [Google Scholar]
- 39.Ragusa M.J., Dancheck B., Critton D.A., Nairn A.C., Page R., Peti W. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat. Struct. Mol. Biol. 2010;17:459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choy M.S., Hieke M., Kumar G.S., Lewis G.R., Gonzalez-DeWhitt K.R., Kessler R.P., Stein B.J., Hessenberger M., Nairn A.C., Nairn Peti W., et al. Understanding the antagonism of retinoblastoma protein dephosphorylation by PNUTS provides insights into the PP1 regulatory code. Proc. Natl. Acad. Sci. USA. 2014;111:4097–4102. doi: 10.1073/pnas.1317395111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Connell N., Nichols S.R., Heroes E., Beullens M., Bollen M., Peti W., Page R. The molecular basis for substrate specificity of the nuclear NIPP1: PP1 holoenzyme. Structure. 2012;20:1746–1756. doi: 10.1016/j.str.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hurley T.D., Yang J., Zhang L., Goodwin K.D., Zou Q., Cortese M., Dunker A.K., DePaoli-Roach A.A. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J. Biol. Chem. 2007;282:28874–28883. doi: 10.1074/jbc.M703472200. [DOI] [PubMed] [Google Scholar]
- 43.Barford D., Das A.K., Egloff M.P. The structure and mechanism of protein phosphatases: Insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 1998;27:133–164. doi: 10.1146/annurev.biophys.27.1.133. [DOI] [PubMed] [Google Scholar]
- 44.Eto M., Kirkbride J., Elliott E., Lo S.H., Brautigan D.L. Association of the tensin N-terminal protein-tyrosine phosphatase domain with the α isoform of protein phosphatase-1 in focal adhesions. J. Biol. Chem. 2007;282:17806–17815. doi: 10.1074/jbc.M700944200. [DOI] [PubMed] [Google Scholar]
- 45.Dumitru A.M.G., Rusin S.F., Clark A.E., Kettenbach A.N., Compton D.A. Cyclin A/Cdk1 modulates Plk1 activity in prometaphase to regulate kinetochore-microtubule attachment stability. Elife. 2017;6:e29303. doi: 10.7554/eLife.29303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terry-Lorenzo R.T., Elliot E., Weiser D.C., Prickett T.D., Brautigan D.L., Shenolikar S. Neurabins recruit protein phosphatase-1 and inhibitor-2 to the actin cytoskeleton. J. Biol. Chem. 2002;277:46535–46543. doi: 10.1074/jbc.M206960200. [DOI] [PubMed] [Google Scholar]
- 47.Dancheck B., Ragusa M.J., Allaire M., Nairn A.C., Page R., Peti W. Molecular Investigations of the Structure and Function of the Protein Phosphatase 1−Spinophilin−Inhibitor 2 Heterotrimeric Complex. Biochemistry. 2011;50:1238–1246. doi: 10.1021/bi101774g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arino J., Woon C.W., Brautigan D.L., Miller T.B., Johnson G.L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc. Natl. Acad. Sci. USA. 1988;85:4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janssens V., Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/bj3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seshacharyulu P., Pandey P., Datta K., Batra S.K. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nasa I., Kettenbach A.N. Coordination of Protein Kinase and Phosphoprotein Phosphatase Activities in Mitosis. Front. Cell Dev. Biol. 2018;6:30. doi: 10.3389/fcell.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Groves M.R., Hanlon N., Turowski P., Hemmings B.A., Barford D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell. 1999;96:99–110. doi: 10.1016/S0092-8674(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 53.Kremmer E., Ohst K., Kiefer J., Brewis N., Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: Abundant expression of both forms in cells. Mol. Cell. Biol. 1997;17:1692–1701. doi: 10.1128/MCB.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slupe A.M., Merrill R.A., Strack S. Determinants for substrate specificity of protein phosphatase 2A. Enzyme Res. 2011;2011:398751. doi: 10.4061/2011/398751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho U.S., Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2006;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 56.Xu Y., Xing Y., Chen Y., Chao Y., Lin Z., Fan E., Yu J.W., Strack S., Jeffrey P.D., Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 57.Xu Y., Chen Y., Zhang P., Jeffrey P.D., Shi Y. Structure of a Protein Phosphatase 2A Holoenzyme: Insights into B55-Mediated Tau Dephosphorylation. Mol. Cell. 2008;31:873–885. doi: 10.1016/j.molcel.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cundell M.J., Hutter L.H., Bastos R.N., Poser E., Holder J., Mohammed S., Novak B., Barr F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016;214:539–554. doi: 10.1083/jcb.201606033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereira G., Schiebel E. Mitotic exit: Determining the PP2A dephosphorylation program. J. Cell Biol. 2016;214:499–501. doi: 10.1083/jcb.201608019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinna L.A., Donella A., Clari G., Moret V. Preferential dephosphorylation of protein bound phosphorylthreonine and phosphorylserine residues by cytosol and mitochondrial “casein phosphatase”. Biochem. Biophys. Res. Commun. 1976;70:1308–1315. doi: 10.1016/0006-291X(76)91045-7. [DOI] [PubMed] [Google Scholar]
- 61.Deana A.D., Marchiori F., Meggio F., Pinna L.A. Dephosphorylation of synthetic phosphopeptides by protein phosphatase-T, a phosphothreonyl protein phosphatase. J. Biol. Chem. 1982;257:8565–8568. [PubMed] [Google Scholar]
- 62.Deana A.D., Pinna L.A. Identification of pseudo ‘phosphothreonyl-specific’ protein phosphatase T with a fraction of polycation-stimulated protein phosphatase 2A. Biochim. Biophys. Acta. 1988;968:179–185. doi: 10.1016/0167-4889(88)90006-7. [DOI] [PubMed] [Google Scholar]
- 63.Godfrey M., Touati S.A., Kataria M., Jones A., Snijders A.P., Uhlmann F. PP2ACdc55 Phosphatase Imposes Ordered Cell-Cycle Phosphorylation by Opposing Threonine Phosphorylation. Mol. Cell. 2017;65:393–402. doi: 10.1016/j.molcel.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hein J.B., Hertz E.P.T., Garvanska D.H., Kruse T., Nilsson J. Distinct kinetics of serine and threonine dephosphorylation are essential for mitosis. Nat. Cell Biol. 2017;19:1433–1440. doi: 10.1038/ncb3634. [DOI] [PubMed] [Google Scholar]
- 65.Hertz E.P.T., Kruse T., Davey N.E., Lopéz-Méndez B., Sigurðsson J.O., Montoya G., Olsen J.V., Nilsson J. A Conserved Motif Provides Binding Specificity to the PP2A-B56 Phosphatase. Mol. Cell. 2016;63:686–695. doi: 10.1016/j.molcel.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Wang Z., Yu T., Yang H., Virshup D.M., Kops G.J., Lee S.H., Zhou W., Li X., Xu W., et al. Crystal structure of a PP2A B56-BubR1 complex and its implications for PP2A substrate recruitment and localization. Protein Cell. 2016;7:516–526. doi: 10.1007/s13238-016-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X., Bajaj R., Bollen M., Peti W., Page R. Expanding the PP2A interactome by defining a B56-specific SLiM. Structure. 2016;24:2174–2181. doi: 10.1016/j.str.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu C.G., Chen H., Guo F., Yadav V.K., Mcilwain S.J., Rowse M., Choudhary A., Lin Z., Li Y., Gu T., et al. PP2A-B′ holoenzyme substrate recognition, regulation and role in cytokinesis. Cell Discov. 2017;3:17027. doi: 10.1038/celldisc.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallardi G., Allan L.A., Crozier L., Saurin A.T. A molecular explanation for PP2A-B56 isoform specificity during mitosis. bioRxiv. 2018 doi: 10.1101/425579. [DOI] [Google Scholar]
- 70.Tonks N.K. Protein tyrosine phosphatases: From genes, to function, to disease. Nat. Rev. Mol. Cell. Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 71.Tiganis T., Bennett A.M. Protein tyrosine phosphatase function: The substrate perspective. Biochem. J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alonso A., Sasin J., Bottini N., Friedberg I., Friedberg I., Osterman A., Godzik A., Hunter T., Dixon J., Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Andersen J.N., Jansen P.G., Echwald S.M., Mortensen O.H., Fukada T., Del Vecchio R., Tonks N.K., Møller N.P. A genomic perspective on protein tyrosine phosphatases: Gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004;18:8–30. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- 74.Sacco F., Perfetto L., Castagnoli L., Cesareni G. The human phosphatase interactome: An intricate family portrait. FEBS Lett. 2012;586:2732–2739. doi: 10.1016/j.febslet.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S.J., Ryu S.E. Structure and catalytic mechanism of human protein tyrosine phosphatome. BMB Rep. 2012;45:693–699. doi: 10.5483/BMBRep.2012.45.12.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barford D., Flint A.J., Tonks N.K. Crystal structure of human protein tyrosine phosphatase 1B. Science. 1994;263:1397–1404. doi: 10.1126/science.8128219. [DOI] [PubMed] [Google Scholar]
- 77.Jia Z., Barford D., Flint A.J., Tonks N.K. Structural basis for phosphotyrosine peptide recognition by protein tyrosine phosphatase 1B. Science. 1995;268:1754–1758. doi: 10.1126/science.7540771. [DOI] [PubMed] [Google Scholar]
- 78.Yuvaniyama J., Denu J.M., Dixon J.E., Saper M.A. Crystal structure of the dual specificity protein phosphatase VHR. Science. 1996;272:1328–1331. doi: 10.1126/science.272.5266.1328. [DOI] [PubMed] [Google Scholar]
- 79.Wee P., Shi H., Jiang J., Wang Y., Wang Z. EGF stimulates the activation of EGF receptors and the selective activation of major signaling pathways during mitosis. Cell Signal. 2015;27:638–651. doi: 10.1016/j.cellsig.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Y., Li X., Yang W., Hawke D.H., Zheng Y., Xia Y., Aldape K., Wei C., Guo F., Chen Y., et al. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Mol. Cell. 2014;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hitosugi T., Kang S., Vander Heiden M.G., Chung T.W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G.Z., et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsumura S., Hamasaki M., Yamamoto T., Ebisuya M., Sato M., Nishida E., Toyoshima F. ABL1 regulates spindle orientation in adherent cells and mammalian skin. Nat. Commun. 2012;3:626. doi: 10.1038/ncomms1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Caron D., Byrne D.P., Thebault P., Soulet D., Landry C.R., Eyers P.A., Elowe S. Mitotic phosphotyrosine network analysis reveals that tyrosine phosphorylation regulates Polo-like kinase 1 (PLK1) Sci. Signal. 2016;9:rs14. doi: 10.1126/scisignal.aah3525. [DOI] [PubMed] [Google Scholar]
- 84.Queralt E., Uhlmann F. Cdk-counteracting phosphatases unlock mitotic exit. Curr. Opin. Cell Biol. 2008;20:661–668. doi: 10.1016/j.ceb.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trinkle-Mulcahy L., Lamond A.I. Mitotic phosphatases: No longer silent partners. Curr. Opin. Cell Biol. 2006;18:623–631. doi: 10.1016/j.ceb.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 86.Wurzenberger C., Gerlich D.W. Phosphatases: Providing safe passage through mitotic exit. Nat. Rev. Mol. Cell. Biol. 2011;12:469–482. doi: 10.1038/nrm3149. [DOI] [PubMed] [Google Scholar]
- 87.Galaktionov K., Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: Evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 88.Sadhu K., Reed S.I., Richardson H., Russell P. Human homolog of fission yeast cdc25 mitotic inducer is predominantly expressed in G2. Proc. Natl. Acad. Sci. USA. 1990;87:5139–5143. doi: 10.1073/pnas.87.13.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagata A., Igarashi M., Jinno S., Suto K., Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991;3:959–968. [PubMed] [Google Scholar]
- 90.Kristjánsdóttir K., Rudolph J. Cdc25 phosphatases and cancer. Chem. Biol. 2004;11:1043–1051. doi: 10.1016/j.chembiol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Rudolph J. Cdc25 phosphatases: Structure, specificity, and mechanism. Biochemistry. 2007;46:3595–3604. doi: 10.1021/bi700026j. [DOI] [PubMed] [Google Scholar]
- 92.Boutros R., Lobjois V., Ducommun B. CDC25 phosphatases in cancer cells: Key players? Good targets? Nat. Rev. Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 93.Aressy B., Ducommun B. Cell cycle control by the CDC25 phosphatases. Anticancer Agents Med. Chem. 2008;8:818–824. doi: 10.2174/187152008786847756. [DOI] [PubMed] [Google Scholar]
- 94.Lavecchia A., Di Giovanni C., Novellino E. CDC25 phosphatase inhibitors: An update. Mini Rev. Med. Chem. 2012;12:62–73. doi: 10.2174/138955712798868940. [DOI] [PubMed] [Google Scholar]
- 95.Fauman E.B., Cogswell J.P., Lovejoy B., Rocque W.J., Holmes W., Montana V.G., Piwnica-Worms H., Rink M.J., Saper M.A. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. doi: 10.1016/S0092-8674(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds R.A., Yem A.W., Wolfe C.L., Deibel M.R.Jr., Chidester C.G., Watenpaugh K.D. Crystal structure of the catalytic subunit of Cdc25B required for G2/M phase transition of the cell cycle. J. Mol. Biol. 1999;293:559–568. doi: 10.1006/jmbi.1999.3168. [DOI] [PubMed] [Google Scholar]
- 97.Sohn J., Kristjánsdóttir K., Safi A., Parker B., Kiburz B., Rudolph J. Remote hot spots mediate protein substrate recognition for the Cdc25 phosphatase. Proc. Natl. Acad. Sci. USA. 2004;101:16437–16441. doi: 10.1073/pnas.0407663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sohn J., Buhrman G., Rudolph J. Kinetic and structural studies of specific protein-protein interactions in substrate catalysis by Cdc25B phosphatase. Biochemistry. 2007;46:807–818. doi: 10.1021/bi061257y. [DOI] [PubMed] [Google Scholar]
- 99.Rudolph J., Epstein D.M., Parker L., Eckstein J. Specificity of natural and artificial substrates for human Cdc25A. Anal. Biochem. 2001;289:43–51. doi: 10.1006/abio.2000.4906. [DOI] [PubMed] [Google Scholar]
- 100.Wilborn M., Free S., Ban A., Rudolph J. The C-terminal tail of the dual-specificity Cdc25B phosphatase mediates modular substrate recognition. Biochemistry. 2001;40:14200–14206. doi: 10.1021/bi015638h. [DOI] [PubMed] [Google Scholar]
- 101.Denu J.M., Dixon J.E. A catalytic mechanism for the dual-specific phosphatases. Proc. Natl. Acad. Sci. USA. 1995;92:5910–5914. doi: 10.1073/pnas.92.13.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Denu J.M., Stuckey J.A., Saper M.A., Dixon J.E. Form and function in protein dephosphorylation. Cell. 1996;87:361–364. doi: 10.1016/S0092-8674(00)81356-2. [DOI] [PubMed] [Google Scholar]
- 103.Chen W., Wilborn M., Rudolph J. Dual-specific Cdc25B phosphatase: In search of the catalytic acid. Biochemistry. 2000;39:10781–10789. doi: 10.1021/bi000909u. [DOI] [PubMed] [Google Scholar]
- 104.Rudolph J. Catalytic mechanism of Cdc25. Biochemistry. 2002;41:14613–14623. doi: 10.1021/bi0263513. [DOI] [PubMed] [Google Scholar]
- 105.McCain D.F., Catrina I.E., Hengge A.C., Zhang Z.Y. The catalytic mechanism of Cdc25A phosphatase. J. Biol. Chem. 2002;277:11190–11200. doi: 10.1074/jbc.M109636200. [DOI] [PubMed] [Google Scholar]
- 106.Sharma K., D’Souza R.C., Tyanova S., Schaab C., Wiśniewski J.R., Cox J., Mann M. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell Rep. 2014;8:1583–1594. doi: 10.1016/j.celrep.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 107.Kuilman T., Maiolica A., Godfrey M., Scheidel N., Aebersold R., Uhlmann F. Identification of Cdk targets that control cytokinesis. EMBO J. 2015;34:81–96. doi: 10.15252/embj.201488958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCloy R.A., Parker B.L., Rogers S., Chaudhuri R., Gayevskiy V., Hoffman N.J., Ali N., Watkins D.N., Daly R.J., James D.E., et al. Global Phosphoproteomic Mapping of Early Mitotic Exit in Human Cells Identifies Novel Substrate Dephosphorylation Motifs. Mol. Cell Proteom. 2015;14:2194–2212. doi: 10.1074/mcp.M114.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burgess A., Vuong J., Rogers S., Malumbres M., O’Donoghue S.I. SnapShot: Phosphoregulation of Mitosis. Cell. 2017;169:1358. doi: 10.1016/j.cell.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 110.Pines J., Hunter T. Isolation of a human cyclin cDNA: Evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- 111.Parker L.L., Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 112.Morgan D.O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 113.Lindqvist A., Källström H., Lundgren A., Barsoum E., Rosenthal C.K. Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1–Cdk1 at the centrosome. J. Cell Biol. 2005;171:35–45. doi: 10.1083/jcb.200503066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gabrielli B.G., De Souza C.P., Tonks I.D., Clark J.M., Hayward N.K., Ellem K.A. Cytoplasmic accumulation of cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J. Cell Sci. 1996;109:1081–1093. doi: 10.1242/jcs.109.5.1081. [DOI] [PubMed] [Google Scholar]
- 115.De Souza C.P., Ellem K.A., Gabrielli B.G. Centrosomal and cytoplasmic Cdc2/cyclin B1 activation precedes nuclear mitotic events. Exp. Cell Res. 2000;257:11–21. doi: 10.1006/excr.2000.4872. [DOI] [PubMed] [Google Scholar]
- 116.Gabrielli B.G., Clark J.M., McCormack A.K., Ellem K.A.O. Ultraviolet light-induced G2 phase cell cycle checkpoint blocks cdc25-dependent progression into mitosis. Oncogene. 1997;15:749–758. doi: 10.1038/sj.onc.1201254. [DOI] [PubMed] [Google Scholar]
- 117.Molinari M., Mercurio C., Dominguez J., Goubin F., Draetta G.F. Human Cdc25 A inactivation in response to S phase inhibition and its role in preventing premature mitosis. EMBO Rep. 2000;1:71–79. doi: 10.1093/embo-reports/kvd018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mailand N., Podtelejnikov A.V., Groth A., Mann M., Bartek J., Lukas J. Regulation of G2/M events by Cdc25A through phosphorylation-dependent modulation of its stability. EMBO J. 2002;21:5911–5920. doi: 10.1093/emboj/cdf567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boutros R., Dozier C., Ducommun B. The when and wheres of CDC25 phosphatases. Curr. Opin. Cell Biol. 2006;18:185–191. doi: 10.1016/j.ceb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 120.Watanabe N., Arai H., Nishihara Y., Taniguchi M., Watanabe N., Hunter T., Osada H. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl. Acad. Sci. USA. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Booher R.N., Holman P.S., Fattaey A. Human Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activity. J. Biol. Chem. 1997;272:22300–22306. doi: 10.1074/jbc.272.35.22300. [DOI] [PubMed] [Google Scholar]
- 122.Nakajima H., Toyoshima-Morimoto F., Taniguchi E., Nishida E. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 2003;278:25227–25280. doi: 10.1074/jbc.C300126200. [DOI] [PubMed] [Google Scholar]
- 123.O’Farrell P.H. Triggering the all-or-nothing switch into mitosis. Trends Cell Biol. 2001;11:512–519. doi: 10.1016/S0962-8924(01)02142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lindqvist A., Rodríguez-Bravo V., Medema R.H. The decision to enter mitosis: Feedback and redundancy in the mitotic entry network. J. Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Donzelli M., Squatrito M., Ganoth D., Hershko A., Pagano M., Draetta G.F. Dual mode of degradation of Cdc25 A phosphatase. EMBO J. 2002;21:4875–4884. doi: 10.1093/emboj/cdf491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jinno S., Suto K., Nagata A., Igarashi M., Kanaoka Y., Nojima H., Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garner-Hamrick P.A., Fisher C. Antisense phosphorothioate oligonucleotides specifically down-regulate cdc25B causing S-phase delay and persistent antiproliferative effects. Int. J. Cancer. 1998;76:720–728. doi: 10.1002/(SICI)1097-0215(19980529)76:5<720::AID-IJC18>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 128.Kieffer I., Lorenzo C., Dozier C., Schmitt E., Ducommun B. Differential mitotic degradation of the CDC25B phosphatase variants. Oncogene. 2007;26:7847–7858. doi: 10.1038/sj.onc.1210596. [DOI] [PubMed] [Google Scholar]
- 129.Baldin V., Cans C., Knibiehler M., Ducommun B. Phosphorylation of human CDC25B phosphatase by CDK1-cyclin A triggers its proteasome-dependent degradation. J. Biol. Chem. 1997;272:32731–32734. doi: 10.1074/jbc.272.52.32731. [DOI] [PubMed] [Google Scholar]
- 130.Kanemori Y., Uto K., Sagata N. β-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc. Natl. Acad. Sci. USA. 2005;102:6279–6284. doi: 10.1073/pnas.0501873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furnari B., Rhind N., Russell P. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 132.Furnari B., Blasina A., Boddy M.N., McGowan C.H., Russell P. Cdc25 inhibited in vivo and in vitro by checkpoint kinases Cds1 and Chk1. Mol. Biol. Cell. 1999;10:833–845. doi: 10.1091/mbc.10.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kumagai A., Guo Z., Emami K.H., Wang S.X., Dunphy W.G. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J. Cell Biol. 1998;142:1559–1569. doi: 10.1083/jcb.142.6.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng C.Y., Graves P.R., Ogg S., Thoma R.S., Byrnes M.J., 3rd., Wu Z., Stephenson M.T., Piwnica-Worms H. C-TAK1 protein kinase phosphorylates human Cdc25C on serine 216 and promotes 14-3-3 protein binding. Cell Growth Differ. 1998;9:197–208. [PubMed] [Google Scholar]
- 135.Zeng Y., Forbes K.C., Wu Z., Moreno S., Piwnica-Worms H., Enoch T. Replication checkpoint requires phosphorylation of the phosphatase Cdc25 by Cds1 or Chk1. Nature. 1998;395:507–510. doi: 10.1038/26766. [DOI] [PubMed] [Google Scholar]
- 136.Duckworth B.C., Weaver J.S., Ruderman J.V. G2 arrest in Xenopus oocytes depends on phosphorylation of Cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA. 2002;99:16794–16799. doi: 10.1073/pnas.222661299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peng C.Y., Graves P.R., Thoma R.S., Wu Z., Shaw A.S., Piwnica-Worms H. Mitotic and G2 Checkpoint Control: Regulation of 14-3-3 Protein Binding by Phosphorylation of Cdc25C on Serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 138.Sanchez Y., Wong C., Thoma R.S., Richman R., Wu Z., Piwnica-Worms H., Elledge S.J. Conservation of the Chk1 Checkpoint Pathway in Mammals: Linkage of DNA Damage to Cdk Regulation Through Cdc25. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 139.Kumagai A., Yakowec P.S., Dunphy W.G. 14-3-3 proteins act as negative regulators of the mitotic inducer Cdc25 in Xenopus egg extracts. Mol. Biol. Cell. 1998;9:345–354. doi: 10.1091/mbc.9.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hermeking H., Benzinger A. 14-3-3 proteins in cell cycle regulation. Semin. Cancer Biol. 2006;16:183–192. doi: 10.1016/j.semcancer.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 141.Margolis S.S., Perry J.A., Weitzel D.H., Freel C.D., Yoshida M., Haystead T.A., Kornbluth S. A role for PP1 in the Cdc2/Cyclin B-mediated positive feedback activation of Cdc25. Mol. Biol. Cell. 2006;17:1779–1789. doi: 10.1091/mbc.e05-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen M.S., Ryan C.E., Piwnica-Worms H. Chk1 kinase negatively regulates mitotic function of Cdc25A phosphatase through 14-3-3 binding. Mol. Cell. Biol. 2003;23:7488–7497. doi: 10.1128/MCB.23.21.7488-7497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dutertre S., Cazales M., Quaranta M., Froment C., Trabut V., Dozier C., Mirey G., Bouché J., Theis-Febvre N., Schmitt E., et al. Phosphorylation of CDC25B by Aurora-A at the centrosome contributes to the G2–M transition. J. Cell Sci. 2004;117:2523–2531. doi: 10.1242/jcs.01108. [DOI] [PubMed] [Google Scholar]
- 144.Cazales M., Schmitt E., Montembault E., Dozier C., Prigent C., Ducommun B. CDC25B phosphorylation by Aurora A occurs at the G2/M transition and is inhibited by DNA damage. Cell Cycle. 2005;4:1233–1238. doi: 10.4161/cc.4.9.1964. [DOI] [PubMed] [Google Scholar]
- 145.Seki A., Coppinger J.A., Jang C.Y., Yates J.R., Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kumagai A., Dunphy W.G. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;237:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 147.Qian Y.W., Erikson E., Maller J.L. Purification and cloning of a protein kinase that phosphorylates and activates the polo-like kinase Plx1. Science. 1998;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 148.Karaïskou A., Jessus C., Brassac T., Ozon R. Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci. 1999;112:3747–3756. doi: 10.1242/jcs.112.21.3747. [DOI] [PubMed] [Google Scholar]
- 149.Roshak A.K., Capper E.A., Imburgia C., Fornwald J., Scott G., Marshall L.A. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 2000;12:405–411. doi: 10.1016/S0898-6568(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 150.van Vugt M.A., Brás A., Medema R.H. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol. Cell. 2004;15:799–811. doi: 10.1016/j.molcel.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 151.Lobjois V., Froment C., Braud E., Grimal F., Burlet-Schiltz O., Ducommun B., Bouche J.P. Study of the docking-dependent PLK1 phosphorylation of the CDC25B phosphatase. Biochem. Biophys. Res. Commun. 2011;410:87–90. doi: 10.1016/j.bbrc.2011.05.110. [DOI] [PubMed] [Google Scholar]
- 152.Gheghiani L., Loew D., Lombard B., Mansfeld J., Gavet O. PLK1 activation in late G2 sets up commitment to mitosis. Cell Rep. 2017;19:2060–2073. doi: 10.1016/j.celrep.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 153.Qi F., Chen Q., Chen H., Yan H., Chen B., Xiang X., Liang C., Yi Q., Zhang M., Cheng H., et al. WAC Promotes Polo-like Kinase 1 Activation for Timely Mitotic Entry. Cell Rep. 2018;24:546–556. doi: 10.1016/j.celrep.2018.06.087. [DOI] [PubMed] [Google Scholar]
- 154.Vigneron S., Sundermann L., Labbé J.C., Pintard L., Radulescu O., Castro A., Lorca T. Cyclin A-Cdk1-dependent phosphorylation of Bora is the triggering factor promoting mitotic entry. Dev. Cell. 2018;45:637–650. doi: 10.1016/j.devcel.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 155.Toyoshima-Morimoto F., Taniguchi E., Nishida E. Plk1 promotes nuclear translocation of human Cdc25C during prophase. EMBO Rep. 2002;3:341–348. doi: 10.1093/embo-reports/kvf069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lobjois V., Jullien D., Bouché J.P., Ducommun B. The polo-like kinase 1 regulates CDC25B-dependent mitosis entry. Biochim. Biophys. Acta. 2009;1793:462–468. doi: 10.1016/j.bbamcr.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 157.Margolis S.S., Walsh S., Weiser D.C., Yoshida M., Shenolikar S., Kornbluth S. PP1 control of M phase entry exerted through 14-3-3-regulated Cdc25 dephosphorylation. EMBO J. 2003;22:5734–5745. doi: 10.1093/emboj/cdg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Clarke P.R., Hoffmann I., Draetta G., Karsenti E. Dephosphorylation of Cdc25-C by a type-2A protein phosphatase: Specific regulation during the cell cycle in Xenopus egg extracts. Mol. Biol. Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mayer-Jaekel R.E., Ohkura H., Ferrigno P., Andjelkovic N., Shiomi K., Uemura T., Glover D.M., Hemmings B.A. Drosophila mutants in the 55 kDa regulatory subunit of protein phosphatase 2A show strongly reduced ability to dephosphorylate substrates of p34cdc2. J. Cell Sci. 1994;107:2609–2616. doi: 10.1242/jcs.107.9.2609. [DOI] [PubMed] [Google Scholar]
- 160.Mochida S., Hunt T. Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature. 2007;449:336–340. doi: 10.1038/nature06121. [DOI] [PubMed] [Google Scholar]
- 161.Castilho P.V., Williams B.C., Mochida S., Zhao Y., Goldberg M.L. The M Phase Kinase Greatwall (Gwl) Promotes Inactivation of PP2A/B55δ, a Phosphatase Directed Against CDK Phosphosites. Mol. Biol. Cell. 2009;20:4777–4789. doi: 10.1091/mbc.e09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mochida S., Ikeo S., Gannon J., Hunt T. Regulated activity of PP2A-B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schmitz M.H.A., Held M., Janssens V., Hutchins J.R.A., Hudecz O., Ivanova E., Goris J., Trinkle-Mulcahy L., Lamond A.I., Poser I., et al. Live-cell imaging RNAi screen identifies PP2A-B55α and importin-β1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 2010;12:886–893. doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cundell M.J., Bastos R.N., Zhang T., Holder J., Gruneberg U., Novak B., Barr F.A. The BEG (PP2A-B55/ENSA/Greatwall) pathway ensures cytokinesis follows chromosome separation. Mol. Cell. 2013;52:393–405. doi: 10.1016/j.molcel.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gharbi-Ayachi A., Labbé J.C., Burgess A., Vigneron S., Strub J.M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. The Substrate of Greatwall Kinase, Arpp19, Controls Mitosis by Inhibiting Protein Phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- 166.Mochida S., Maslen S.L., Skehel M., Hunt T. Greatwall Phosphorylates an Inhibitor of Protein Phosphatase 2A That Is Essential for Mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- 167.Archambault V., Zhao X., White-Cooper H., Carpenter A.T.C., Glover D.M. Mutations in Drosophila Greatwall/Scant Reveal Its Roles in Mitosis and Meiosis and Interdependence with Polo Kinase. PLoS Genet. 2007;3:e200. doi: 10.1371/journal.pgen.0030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Burgess A., Vigneron S., Brioudes E., Labbé J.C., Lorca T., Castro A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA. 2010;107:12564–12569. doi: 10.1073/pnas.0914191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hara M., Abe Y., Tanaka T., Yamamoto T., Okumura E., Kishimoto T. Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat. Commun. 2012;3:1059. doi: 10.1038/ncomms2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lorca T., Bernis C., Vigneron S., Burgess A., Brioudes E., Labbé J.C., Castro A. Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J. Cell Sci. 2010;123:2281–2291. doi: 10.1242/jcs.064527. [DOI] [PubMed] [Google Scholar]
- 171.Voets E., Wolthuis R.M. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle. 2010;9:3591–3601. doi: 10.4161/cc.9.17.12832. [DOI] [PubMed] [Google Scholar]
- 172.Yu J., Fleming S.L., Williams B., Williams E.V., Li Z., Somma P., Rieder C.L., Goldberg M.L. Greatwall kinase: A nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 2004;164:487–492. doi: 10.1083/jcb.200310059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Álvarez-Fernández M., Sánchez-Martinez R., Sanz-Castillo B., Can P.P., Sanz-Flores M., Trakala M., Ruiz-Torres M., Lorca T., Castro A., Malumbres M. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc. Natl. Acad. Sci. USA. 2013;110:17374–17379. doi: 10.1073/pnas.1310745110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Diril M.K., Bisteau X., Kitagawa M., Caldez M.J., Wee S., Gunaratne J., Lee S.H., Kaldis P. Loss of the Greatwall Kinase Weakens the Spindle Assembly Checkpoint. PLoS Genet. 2016;12:e1006310. doi: 10.1371/journal.pgen.1006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yu J., Zhao Y., Li Z., Galas S., Goldberg M.L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 176.Blake-Hodek K.A., Williams B.C., Zhao Y., Castilho P.V., Chen W., Mao Y., Yamamoto T.M., Goldberg M.L. Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell. Biol. 2012;32:1337–1353. doi: 10.1128/MCB.06525-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vigneron S., Gharbi-Ayachi A., Raymond A.A., Burgess A., Labbé J.C., Labesse G., Monsarrat B., Lorca T., Castro A. Characterization of the mechanisms controlling Greatwall activity. Mol. Cell. Biol. 2011;31:2262–2275. doi: 10.1128/MCB.00753-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao Y., Haccard O., Wang R., Yu J., Kuang J., Jessus C., Goldberg M.LL. Roles of Greatwall Kinase in the Regulation of Cdc25 Phosphatase. Mol. Biol. Cell. 2008;19:1317–1327. doi: 10.1091/mbc.e07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Peters J.M., Tedeschi A., Schmitz J. The cohesin complex and its roles in chromosome biology. Genes Dev. 2008;22:3089–3114. doi: 10.1101/gad.1724308. [DOI] [PubMed] [Google Scholar]
- 180.Nasmyth K., Haering C.H. Cohesin: Its roles and mechanisms. Annu. Rev. Genet. 2009;43:525–558. doi: 10.1146/annurev-genet-102108-134233. [DOI] [PubMed] [Google Scholar]
- 181.Haering C.H., Löwe J., Hochwagen A., Nasmyth K. Molecular architecture of SMC proteins and the yeast cohesion complex. Mol. Cell. 2002;9:773–788. doi: 10.1016/S1097-2765(02)00515-4. [DOI] [PubMed] [Google Scholar]
- 182.Gandhi R., Gillespie P.J., Hirano T. Human WapI is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr. Biol. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kueng S., Hegemann B., Peters B.H., Lipp J.J., Schleiffer A., Mechtler K., Peters J.M. WapI controls the dynamic association of cohesion with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 184.Chan K.L., Roig M.B., Hu B., Beckouët F., Metson J., Nasmyth K. Cohesin’s DNA exit gate is distinct from its entrance gate and is regulated by acetylation. Cell. 2012;150:961–974. doi: 10.1016/j.cell.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Buheitel J., Stemmann O. Prophase pathway-dependent removal of cohesin from human chromosomes requires opening of the Smc3–Scc1 gate. EMBO J. 2013;32:666–676. doi: 10.1038/emboj.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chatterjee A., Zakian S., Hu X.W., Singleton M.R. Structural insights into the regulation of cohesion establishment by Wpl1. EMBO J. 2013;32:677–687. doi: 10.1038/emboj.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ouyang Z., Zheng G., Song J., Borek D.M., Otwinowski Z., Brautigam C.A., Tomchick D.R., Rankin S., Yu H. Structure of the human cohesin inhibitor Wapl. Proc. Natl. Acad. Sci. USA. 2013;110:11355–11360. doi: 10.1073/pnas.1304594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Eichinger C.S., Kurze A., Oliveira R.A., Nasmyth K. Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis. EMBO J. 2013;32:656–665. doi: 10.1038/emboj.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ben-Shahar T.R., Heeger S., Lehane C., East P., Flynn H., Skehel M., Uhlmann F. Eco1-dependent cohesin acetylation during establishment of sister chromatid cohesion. Science. 2008;321:563–566. doi: 10.1126/science.1157774. [DOI] [PubMed] [Google Scholar]
- 190.Rowland B.D., Roig M.B., Nishino T., Kurze A., Uluocak P., Mishra A., Beckouët F., Underwood P., Metson J., Imre R., et al. Building sister chromatid cohesion: Smc3 acetylation counteracts an antiestablishment activity. Mol. Cell. 2009;33:763–774. doi: 10.1016/j.molcel.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 191.Sutani T., Kawaguchi T., Kanno R., Itoh T., Shirahige K. Budding yeast Wpl1 (Rad61)-Pds5 complex counteracts sister chromatid cohesion-establishing reaction. Curr. Biol. 2009;19:492–497. doi: 10.1016/j.cub.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 192.Ünal E., Heidinger-Pauli J.M., Kim W., Guacci V., Onn I., Gygi S.P., Koshland D.E. A molecular determinant for the establishment of sister chromatid cohesion. Science. 2008;321:566–569. doi: 10.1126/science.1157880. [DOI] [PubMed] [Google Scholar]
- 193.Zhang J., Shi X., Li Y., Kim B.J., Jia J., Huang Z., Yang T., Fu X., Jung S.Y., Wang Y., et al. Acetylation of Smc3 by Eco1 is required for S phase sister chromatid cohesion in both human and yeast. Mol. Cell. 2008;31:143–151. doi: 10.1016/j.molcel.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 194.Lafont A.L., Song J., Rankin S. Sororin cooperates with the acetyltransferase Eco2 to ensure DNA replication-dependent sister chromatid cohesion. Proc. Natl. Acad. Sci. USA. 2010;107:20364–20369. doi: 10.1073/pnas.1011069107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Nishiyama T., Ladurner R., Schmitz J., Kreidl E., Schleiffer A., Bhaskara V., Bando M., Shirahige K., Hyman A.A., Mechtler K., et al. Sororin mediates sister chromatid cohesion by antagonizing WapI. Cell. 2010;143:737–749. doi: 10.1016/j.cell.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 196.Dreier M.R., Bekier M.E., 2nd., Taylor W.R. Regulation of sororin by Cdk1-mediated phosphorylation. J. Cell Sci. 2011;124:2976–2987. doi: 10.1242/jcs.085431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Giménez-Abián J.F., Sumara I., Hirota T., Hauf S., Gerlich D., de la Torre C., Ellenberg J., Peters J.M. Regulation of sister chromatid cohesion between chromosome arms. Curr. Biol. 2004;14:1187–1193. doi: 10.1016/j.cub.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 198.Hauf S., Roitinger E., Koch B., Dittrich C.M., Mechtler K., Peters J.M. Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol. 2005;3:e69. doi: 10.1371/journal.pbio.0030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Liu H., Rankin S., Yu H. Phosphorylation-enabled binding of SGO1-PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat. Cell Biol. 2013;15:40–49. doi: 10.1038/ncb2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Losada A., Hirano M., Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16:3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nishiyama T., Sykora M.M., Huis P.J., Mechtler K., Peters J.M. Aurora B and Cdk1 mediate WapI activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc. Natl. Acad. Sci. USA. 2013;110:13404–13409. doi: 10.1073/pnas.1305020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Sumara I., Vorlaufer E., Stukenberg P.T., Kelm O., Redemann N., Nigg E.A., Peters J.M. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell. 2002;9:515–525. doi: 10.1016/S1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 203.Zhang N., Panigrahi A.K., Mao Q., Pati D. Interaction of Sororin protein with Polo-like kinase 1 mediates resolution of chromosomal arm cohesion. J. Biol. Chem. 2011;286:41826–41837. doi: 10.1074/jbc.M111.305888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang L.H., Mayer B., Stemmann O., Nigg E.A. Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J. Cell Sci. 2010;123:806–813. doi: 10.1242/jcs.058255. [DOI] [PubMed] [Google Scholar]
- 205.Deardorff M.A., Wilde J.J., Albrecht M., Dickinson E., Tennstedt S., Braunholz D., Mönnich M., Yan Y., Xu W., Gil-Rodríguez M.C., et al. RAD21 mutations cause a human cohesinopathy. Am. J. Hum. Genet. 2012;90:1014–1027. doi: 10.1016/j.ajhg.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Haarhuis J.H., Elbatsh A.M.O., van den Broek B., Camps D., Erkan H., Jalink K., Medema R.H., Rowland B.D. WAPL-mediated removal of cohesin protects against segregation errors and aneuploidy. Curr. Biol. 2013;23:2071–2077. doi: 10.1016/j.cub.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 207.Tedeschi A., Wutz G., Huet S., Jaritz M., Wuensche A., Schirghuber E., Davidson I.F., Tang W., Cisneros D.A., Bhaskara V., et al. WapI is an essential regulator of chromatin structure and chromosome segregation. Nature. 2013;501:564–568. doi: 10.1038/nature12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 209.Tanaka T., Fuchs J., Loidl J., Nasmyth K. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat. Cell Biol. 2000;2:492–499. doi: 10.1038/35019529. [DOI] [PubMed] [Google Scholar]
- 210.Haarhuis J.H.I., Elbatsh A.M.O., Rowland B.D. Cohesin and its regulation: On the logic of X-shaped chromosomes. Dev. Cell. 2014;31:7–18. doi: 10.1016/j.devcel.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 211.Morales C., Losada A. Establishing and dissolving cohesion during the vertebrate cell cycle. Curr. Opin. Cell Biol. 2018;52:51–57. doi: 10.1016/j.ceb.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 212.McGuinness B.E., Hirota T., Kudo N.R., Peters J.M., Masmyth K. Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol. 2005;3:e86. doi: 10.1371/journal.pbio.0030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kitajima T.S., Sakuno T., Ishiguro K., Iemura S., Natsume T., Kawashima S.A., Watanabe Y. Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature. 2006;441:46–52. doi: 10.1038/nature04663. [DOI] [PubMed] [Google Scholar]
- 214.Riedel C.G., Katis V.L., Katou Y., Mori S., Itoh T., Helmhart W., Gálová M., Petronczki M., Gregan J., Cetin B., et al. Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature. 2006;441:53–61. doi: 10.1038/nature04664. [DOI] [PubMed] [Google Scholar]
- 215.Tang Z., Shu H., Qi W., Mahmood N.A., Mumby M.C., Yu H. PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev. Cell. 2006;10:575–585. doi: 10.1016/j.devcel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 216.Rivera T., Losada A. Shugoshin regulates cohesion by driving relocalization of PP2A in Xenopus extracts. Chromosoma. 2009;118:223–233. doi: 10.1007/s00412-008-0190-4. [DOI] [PubMed] [Google Scholar]
- 217.Tanno Y., Kitajima T.S., Honda T., Ando Y., Ishiguro K., Watanabe Y. Phosphorylation of mammalian Sgo2 by Aurora B recruits PP2A and MCAK to centromeres. Genes Dev. 2010;24:2169–2179. doi: 10.1101/gad.1945310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xu Z., Cetin B., Anger M., Cho U.S., Helmhart W., Nasmyth K., Xu W. Structure and function of the PP2A-shugoshin interaction. Mol. Cell. 2009;35:426–441. doi: 10.1016/j.molcel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gutiérrez-Caballero C., Cebollero L.R., Pendás A.M. Shugoshins: From protectors of cohesion to versatile adaptors at the centromere. Trends Genet. 2012;28:351–360. doi: 10.1016/j.tig.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 220.Huang H., Feng J., Famulski J., Rattner J.B., Liu S.T., Kao G.D., Muschel R., Chan G.K., Yen T.J. Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J. Cell Biol. 2007;177:413–424. doi: 10.1083/jcb.200701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kitajima T.S., Hauf S., Ohsugi M., Yamamoto T., Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting Shugoshin localization. Curr. Biol. 2005;15:353–359. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 222.Llano E., Gómez R., Gutiérrez-Caballero C., Herrán Y., Sánchez-Martín M., Vázquez-Quiñones L., Hernández T., de Alava E., Cuadrado A., Barbero J.L., et al. Shugoshin-2 is essential for the completion of meiosis but not for mitotic cell division in mice. Genes Dev. 2008;22:2400–2413. doi: 10.1101/gad.475308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lee J., Kitajima T.S., Tanno Y., Yoshida K., Morita T., Miyano T., Miyake M., Watanabe Y. Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat. Cell Biol. 2008;10:42–52. doi: 10.1038/ncb1667. [DOI] [PubMed] [Google Scholar]
- 224.Wittmann T., Hyman A., Desai A. The spindle: A dynamic assembly of microtubules and motors. Nat. Cell Biol. 2001;3:E28–E34. doi: 10.1038/35050669. [DOI] [PubMed] [Google Scholar]
- 225.Mennella V., Agard D.A., Huang B., Pelletier L. Amorphous no more: Subdiffraction view of the pericentriolar material architecture. Trends Cell Biol. 2014;24:188–197. doi: 10.1016/j.tcb.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Woodruff J.B., Wueseke O., Hyman A.A. Pericentriolar material structure and dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130459. doi: 10.1098/rstb.2013.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Conduit P.T., Wainman A., Raff J.W. Centrosome function and assembly in animal cells. Nat. Rev. Mol. Cell Biol. 2015;16:611–624. doi: 10.1038/nrm4062. [DOI] [PubMed] [Google Scholar]
- 228.Stearns T., Kirschner M. In vitro reconstitution of centrosome assembly and function: The central role of γ-tubulin. Cell. 1994;76:623–637. doi: 10.1016/0092-8674(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 229.Sunkel C.E., Gomes R., Sampaio P., Perdigão J., González C. γ-tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J. 1995;14:28–36. doi: 10.1002/j.1460-2075.1995.tb06972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lattao R., Kovács L., Glover D.M. The Centrioles, Centrosomes, Basal Bodies, and Cilia of Drosophila melanogaster. Genetics. 2017;206:33–53. doi: 10.1534/genetics.116.198168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sumiyoshi E., Sugimoto A., Yamamoto M. Protein phosphatase 4 is required for centrosome maturation in mitosis and sperm meiosis in C. elegans. J. Cell Sci. 2002;115:1403–1410. doi: 10.1242/jcs.115.7.1403. [DOI] [PubMed] [Google Scholar]
- 232.Martin-Granados C., Philp A., Oxenham S.K., Prescott A.R., Cohen P.T. Depletion of protein phosphatase 4 in human cells reveals essential roles in centrosome maturation, cell migration and the regulation of Rho GTPases. Int. J. Biochem. Cell Biol. 2008;40:2315–2332. doi: 10.1016/j.biocel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 233.Toyo-oka K., Mori D., Yano Y., Shiota M., Iwao H., Goto H., Inagaki M., Hiraiwa N., Muramatsu M., Wynshaw-Boris A., et al. Protein phosphatase 4 catalytic subunit regulates Cdk1 activity and microtubule organization via NDEL1 dephosphorylation. J. Cell Biol. 2008;180:1133–1147. doi: 10.1083/jcb.200705148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.McNally F.J., Vale R.D. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. doi: 10.1016/0092-8674(93)90377-3. [DOI] [PubMed] [Google Scholar]
- 235.McNally F. Capturing a ring of samurai. Nat. Cell Biol. 2000;2:E4–E7. doi: 10.1038/71385. [DOI] [PubMed] [Google Scholar]
- 236.Toyo-Oka K., Sasaki S., Yanon Y., Mori D., Kobayashi T., Toyoshima Y.Y., Tokuoka S.M., Ishii S., Shimizu T., Muramatsu M., et al. Recruitment of katanin p60 by phosphorylated NDEL1, an LIS1 interacting protein, is essential for mitotic cell division and neuronal migration. Hum. Mol. Genet. 2005;14:3113–3128. doi: 10.1093/hmg/ddi339. [DOI] [PubMed] [Google Scholar]
- 237.Han X., Gomes J.E., Birmingham C.L., Pintard L., Sugimoto A., Mains P.E. The role of protein phosphatase 4 in regulating microtubule severing in the Caenorhabditis elegans embryo. Genetics. 2009;181:933–943. doi: 10.1534/genetics.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gomes J.E., Tavernier N., Richaudeau B., Formstecher E., Boulin T., Mains P.E., Dumont J., Pintard L. Microtubule severing by the katanin complex is activated by PPFR-1-dependent MEI-1 dephosphorylation. J. Cell Biol. 2013;202:431–439. doi: 10.1083/jcb.201304174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Srayko M., Kaya A., Stamford J., Hyman A.A. Identification and characterization of factors required for microtubule growth and nucleation in the early C. elegans embryo. Dev. Cell. 2005;9:223–226. doi: 10.1016/j.devcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 240.Thawani A., Kadzik R.S., Petry S. XMAP215 is a microtubule nucleation factor that functions synergistically with the γ-tubulin ring complex. Nat. Cell Biol. 2018;20:575–585. doi: 10.1038/s41556-018-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kinoshita K., Noetzel T.L., Pelletier L., Mechtler K., Drechsel D.N., Schwager A., Lee M., Raff J.W., Hyman A.A. Aurora A phosphorylation of TACC3/maskin is required for centrosome-dependent microtubule assembly in mitosis. J. Cell Biol. 2005;170:1047–1055. doi: 10.1083/jcb.200503023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schlaitz A.L., Srayko M., Dammermann A., Quintin S., Wielsch N., MacLeod I., de Robillard Q., Zinke A., Yates J.R., 3rd., Müller-Reichert T., et al. The C. elegans RSA complex localizes protein phosphatase 2A to centrosomes and regulates mitotic spindle assembly. Cell. 2007;128:115–127. doi: 10.1016/j.cell.2006.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kufer T.A., Silljé H.H., Körner R., Gruss O.J., Meraldi P., Nigg E.A. Human TPX2 is required for targeting Aurora-A kinase to the spindle. J. Cell Biol. 2002;158:617–623. doi: 10.1083/jcb.200204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ozlü N., Srayko M., Kinoshita K., Habermann B., O’toole E.T., Müller-Reichert T., Schmalz N., Desai A., Hyman A.A. An essential function of the C. elegans ortholog of TPX2 is to localize activated aurora A kinase to mitotic spindles. Dev. Cell. 2005;9:237–248. doi: 10.1016/j.devcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 245.Wittmann T., Wilm M., Karsenti E., Vernos I. TPX2, A novel xenopus MAP involved in spindle pole organization. J. Cell Biol. 2000;149:1405–1418. doi: 10.1083/jcb.149.7.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gruss O.J., Carazo-Salas R.E., Schatz C.A., Guarguaglini G., Kast J., Wilm M., Le Bot N., Vernos I., Karsenti E., Mattaj I.W. Ran induces spindle assembly by reversing the inhibitory effect of importin α on TPX2 activity. Cell. 2001;104:83–93. doi: 10.1016/S0092-8674(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 247.Meunier S., Vernos I. Acentrosomal Microtubule Assembly in Mitosis: The Where, When, and How. Trends Cell Biol. 2016;26:80–87. doi: 10.1016/j.tcb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 248.Kalab P., Heald R. The RanGTP gradient—A GPS for the mitotic spindle. J. Cell Sci. 2008;121:1577–1586. doi: 10.1242/jcs.005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cavazza T., Vernos I. The RanGTP Pathway: From Nucleo-Cytoplasmic Transport to Spindle Assembly and Beyond. Front Cell Dev. Biol. 2016;3:82. doi: 10.3389/fcell.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Bayliss R., Sardon T., Vernos I., Conti E. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell. 2003;12:851–862. doi: 10.1016/S1097-2765(03)00392-7. [DOI] [PubMed] [Google Scholar]
- 251.Eyers P.A., Erikson E., Chen L.G., Maller J.L. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. 2003;13:691–697. doi: 10.1016/S0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 252.Tsai M.Y., Wiese C., Cao K., Martin O., Donovan P., Ruderman J., Prigent C., Zheng Y. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 2003;5:242–248. doi: 10.1038/ncb936. [DOI] [PubMed] [Google Scholar]
- 253.Groen A.C., Cameron L.A., Coughlin M., Miyamoto D.T., Mitchison T.J., Ohi R. XRHAMM functions in ran-dependent microtubule nucleation and pole formation during anastral spindle assembly. Curr. Biol. 2004;14:1801–1811. doi: 10.1016/j.cub.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 254.Scrofani J., Sardon T., Meunier S., Vernos I. Microtubule nucleation in mitosis by a RanGTP-dependent protein complex. Curr. Biol. 2015;25:131–140. doi: 10.1016/j.cub.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 255.Pinoyl R., Scrofani J., Vernos I. The role of NEDD1 phosphorylation by Aurora A in chromosomal microtubule nucleation and spindle function. Curr. Biol. 2013;23:143–149. doi: 10.1016/j.cub.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 256.Zeng K., Bastos R.N., Barr F.A., Gruneberg U. Protein phosphatase 6 regulates mitotic spindle formation by controlling the T-loop phosphorylation state of Aurora A bound to its activator TPX2. J. Cell Biol. 2010;191:1315–1332. doi: 10.1083/jcb.201008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zorba A., Buosi V., Kutter S., Kern N., Pontiggia F., Cho Y.J., Kern D. Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2. Elife. 2014;3:e02667. doi: 10.7554/eLife.02667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Cyphers S., Ruff E.F., Behr J.M., Chodera J.D., Levinson N.M. A water-mediated allosteric network governs activation of Aurora kinase A. Nat. Chem. Biol. 2017;13:402–408. doi: 10.1038/nchembio.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ruff E.F., Muretta J.M., Thompson A.R., Lake E.W., Cyphers S., Albanese S.K., Hanson S.M., Behr J.M., Thomas D.D., Chodera J.D., et al. A dynamic mechanism for allosteric activation of Aurora kinase A by activation loop phosphorylation. eLife. 2018;7:e32766. doi: 10.7554/eLife.32766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Andrésson T., Ruderman J.V. The kinase Eg2 is a component of the Xenopus oocyte progesterone-activated signaling pathway. EMBO J. 1998;17:5627–5637. doi: 10.1093/emboj/17.19.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Walter A.O., Seghezzi W., Korver W., Sheung J., Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000;19:4906–4916. doi: 10.1038/sj.onc.1203847. [DOI] [PubMed] [Google Scholar]
- 262.Rusin S.F., Schlosser K.A., Adamo M.E., Kettenbach A.N. Quantitative phosphoproteomics reveals new roles for the protein phosphatase PP6 in mitotic cells. Sci. Signal. 2015;8:rs12. doi: 10.1126/scisignal.aab3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kettenbach A.N., Schlosser K.A., Lyons S.P., Nasa I., Gui J., Adamo M.E., Gerber S.A. Global assessment of its network dynamics reveals that the kinase Plk1 inhibits the phosphatase PP6 to promote Aurora A activity. Sci. Signal. 2018;11:eaaq1441. doi: 10.1126/scisignal.aaq1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Dodson C.A., Bayliss R. Activation of Aurora-A kinase by protein partner binding and phosphorylation are independent and synergistic. J. Biol. Cell. 2012;287:1150–1157. doi: 10.1074/jbc.M111.312090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Joukov V., De Nicolo A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal. 2018;11:eaar4195. doi: 10.1126/scisignal.aar4195. [DOI] [PubMed] [Google Scholar]
- 266.Tournebize R., Popov A., Kinoshita K., Ashford A.J., Rybina S., Pozniakovsky A., Mayer T.U., Walczak C.E., Karsenti E., Hyman A.A. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nat. Cell Biol. 2000;2:13–19. doi: 10.1038/71330. [DOI] [PubMed] [Google Scholar]
- 267.Lee M.J., Gergely F., Jeffers K., Peak-Chew S.Y., Raff J.W. Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behavior. Nat. Cell Biol. 2001;3:643–649. doi: 10.1038/35083033. [DOI] [PubMed] [Google Scholar]
- 268.Gergely F. Centrosomal TACCtics. Bioessays. 2002;24:915–925. doi: 10.1002/bies.10162. [DOI] [PubMed] [Google Scholar]
- 269.Giet R., McLean D., Descamps S., Lee M.J., Raff J.W., Prigent C., Glover D.M. Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules. J. Cell Biol. 2002;156:437–451. doi: 10.1083/jcb.200108135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Gergely F., Draviam V.M., Raff J.W. The ch-TOG/XMAP215 protein is essential for spindle pole organization in human somatic cells. Genes Dev. 2003;17:336–341. doi: 10.1101/gad.245603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Peset I., Seiler J., Sardon T., Bejarano L.A., Rybina S., Vernos I. Function and regulation of Maskin, a TACC family protein, in microtubule growth during mitosis. J. Cell Biol. 2005;170:1057–1066. doi: 10.1083/jcb.200504037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Barros T.P., Kinoshita K., Hyman A.A., Raff J.W. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 2005;170:1039–1046. doi: 10.1083/jcb.200504097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.LeRoy P.J., Hunter J.J., Hoar K.M., Burke K.E., Shinde V., Ruan J., Bowman D., Galvin K., Ecsedy J.A. Localization of human TACC3 to mitotic spindles is mediated by phosphorylation on Ser558 by Aurora A: A novel pharmacodynamic method for measuring Aurora A activity. Cancer Res. 2007;67:5362–5370. doi: 10.1158/0008-5472.CAN-07-0122. [DOI] [PubMed] [Google Scholar]
- 274.Peset I., Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–388. doi: 10.1016/j.tcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 275.Burgess S.G., Mukherjee M., Sabir S., Joseph N., Gutiérrez-Caballero C., Richards M.W., Huguenin-Dezot N., Chin J.W., Kennedy E.J., Pfuhl M., et al. Mitotic spindle association of TACC3 requires Aurora-A-dependent stabilization of a cryptic α-helix. EMBO J. 2018;37:e97902. doi: 10.15252/embj.201797902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tanenbaum M.E., Medema R.H. Mechanisms of centrosome separation and bipolar spindle assembly. Dev. Cell. 2010;19:797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 277.Ghosh S., Paweletz N., Schroeter D. Cdc2-independent induction of premature mitosis by okadaic acid in HeLa cells. Exp. Cell Res. 1998;242:1–9. doi: 10.1006/excr.1998.4115. [DOI] [PubMed] [Google Scholar]
- 278.Meraldi P., Nigg E.A. Centrosome cohesion is regulated by a balance of kinase and phosphatase activities. J. Cell Sci. 2001;114:3749–3757. doi: 10.1242/jcs.114.20.3749. [DOI] [PubMed] [Google Scholar]
- 279.Agircan F.G., Schiebel E., Mardin B.R. Separate to operate: Control of centrosome positioning and separation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369:20130461. doi: 10.1098/rstb.2013.0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Wang G., Jiang Q., Zhang C. The role of mitotic kinases in coupling the centrosome cycle with the assembly of the mitotic spindle. J. Cell Sci. 2014;127:4111–4122. doi: 10.1242/jcs.151753. [DOI] [PubMed] [Google Scholar]
- 281.Fry A.M., Mayor T., Meraldi P., Stierhof Y.D., Tanaka K., Nigg E.A. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle regulated protein kinase Nek2. Cell Biol. 1998;141:1563–1574. doi: 10.1083/jcb.141.7.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yang J., Liu X., Yue G., Adamian M., Bulgakov O., Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J. Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bahe S., Stierhof Y.D., Wilkinson C.J., Leiss F., Nigg E.A. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J. Cell Biol. 2005;171:27–33. doi: 10.1083/jcb.200504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Graser S., Stierhof Y.D., Nigg E.A. Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J. Cell Sci. 2007;120:4321–4331. doi: 10.1242/jcs.020248. [DOI] [PubMed] [Google Scholar]
- 285.Yang J., Adamian M., Li T. Rootletin interacts with C-Nap1 and may function as a physical linker between the pair of centrioles/basal bodies in cells. Mol. Biol. Cell. 2006;17:1033–1040. doi: 10.1091/mbc.e05-10-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Vlijm R., Li X., Panic M., Rüthnick D., Hata S., Herrmannsdörfer F., Kuner T., Heilemann M., Engelhardt J., Hell S.W., et al. STED nanoscopy of the centrosome linker reveals a CEP68-organized, periodic rootletin network anchored to a C-Nap1 ring at centrioles. Proc. Natl. Acad. Sci. USA. 2018;115:E2246–E2253. doi: 10.1073/pnas.1716840115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hardy T., Lee M., Hames R.S., Prosser S.L., Cheary D.M., Samant M.D., Schultz F., Baxter J.E., Rhee K., Fry A.M. Multisite phosphorylation of C-Nap1 releases it from Cep135 to trigger centrosome disjunction. J. Cell Sci. 2014;127:2493–2506. doi: 10.1242/jcs.142331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Faragher A.J., Fry A.M. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol. Biol. Cell. 2003;14:2876–2889. doi: 10.1091/mbc.e03-02-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Bahmanyar S., Kaplan D.D., Deluca J.G., Giddings T.H., Jr., O’Toole T.E., Winey M., Salmon E.D., Casey P.J., Nelson W.J., Barth A.I. beta-Catenin is a Nek2 substrate involved in centrosome separation. Genes Dev. 2008;22:91–105. doi: 10.1101/gad.1596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.He R., Huang N., Bao Y., Zhou H., Teng J., Chen J. LRRC45 is a centrosome linker component required for centrosome cohesion. Cell Rep. 2013;4:1100–1107. doi: 10.1016/j.celrep.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 291.Zhang Y., Foreman O., Wigle D.A., Kosari F., Vasmatzis G., Salisbury J.L., van Deursen J., Galardy P.J. USP44 regulates centrosome positioning to prevent aneuploidy and suppress tumorigenesis. J. Clin. Investig. 2012;122:4362–4374. doi: 10.1172/JCI63084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Nam H.J., van Deursen J.M. Cyclin B2 and p53 control proper timing of centrosome separation. Nat. Cell Biol. 2014;16:538–549. doi: 10.1038/ncb2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Decarreau J., Wagenbach M., Lynch E., Halpern A.R., Vaughan J.C., Kollman J., Wordeman L. The tetrameric kinesin Kif25 supresses pre-mitotic centrosome separation to establish proper spindle orientation. Nat. Cell Biol. 2017;19:384–390. doi: 10.1038/ncb3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fry A.M., Arnaud L., Nigg E.A. Activity of the human centrosomal kinase, Nek2, depends on an unusual leucine zipper dimerization motif. J. Biol. Chem. 1999;274:16304–16310. doi: 10.1074/jbc.274.23.16304. [DOI] [PubMed] [Google Scholar]
- 295.Rellos P., Ivins F.J., Baxter J.E., Pike A., Nott T.J., Parkinson D.M., Das S., Howell S., Fedorov O., Shen Q.Y., et al. Structure and regulation of the human Nek2 centrosomal kinase. J. Biol. Chem. 2007;282:6833–6842. doi: 10.1074/jbc.M609721200. [DOI] [PubMed] [Google Scholar]
- 296.Croasdale R., Ivins F.J., Muskett F., Daviter T., Scott D.J., Hardy T., Smerdon S.J., Fry A.M., Pfuhl M. An undecided coiled coil: The leucine zipper of Nek2 kinase exhibits atypical conformational exchange dynamics. J. Biol. Chem. 2011;286:27537–27547. doi: 10.1074/jbc.M110.196972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mardin B.R., Lange C., Baxter J.E., Hardy T., Scholz S.R., Fry A.M., Schiebel E. Components of the Hippo pathway cooperate with Nek2 kinase to regulate centrosome disjunction. Nat. Cell Biol. 2010;12:1166–1176. doi: 10.1038/ncb2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Helps N.R., Luo X., Barker H.M., Cohen P.T. NIMA-related kinase 2 (Nek2), a cell-cycle-regulated protein kinase localized to centrosomes, is complexed to protein phosphatase 1. Biochem. J. 2000;349:509–518. doi: 10.1042/bj3490509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mardin B.R., Agircan F.G., Lange C., Schiebel E. Plk1 controls the Nek2A-PP1γ antagonism in centrosome disjunction. Curr. Biol. 2011;21:1145–1151. doi: 10.1016/j.cub.2011.05.047. [DOI] [PubMed] [Google Scholar]
- 300.Eto M., Elliot E., Prickett T.D., Brautigan D.L. Inhibitor-2 regulates protein phosphatase-1 complexed with NimA-related kinase to induce centrosome separation. J. Biol. Chem. 2002;277:44013–44020. doi: 10.1074/jbc.M208035200. [DOI] [PubMed] [Google Scholar]
- 301.Mi J., Guo C., Brautigan D.L., Larner J.M. Protein phosphatase-1alpha regulates centrosome splitting through Nek2. Cancer Res. 2007;67:1082–1089. doi: 10.1158/0008-5472.CAN-06-3071. [DOI] [PubMed] [Google Scholar]
- 302.Hames R.S., Wattam S.L., Yamano H., Bacchieri R., Fry A.M. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J. 2001;20:7117–7127. doi: 10.1093/emboj/20.24.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Magidson V., O’Connell C.B., Lončarek J., Paul R., Mogilner A., Khodjakov A. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell. 2011;146:555–567. doi: 10.1016/j.cell.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Silkworth W.T., Nardi I.K., Paul R., Mogilner A., Cimini D. Timing of centrosome separation is important for accurate chromosome segregation. Mol. Biol. Cell. 2012;23:401–411. doi: 10.1091/mbc.e11-02-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Kaseda K., McAinsh A.D., Cross R.A. Dual pathway spindle assembly increases both the speed and the fidelity of mitosis. Biol. Open. 2012;1:12–18. doi: 10.1242/bio.2011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Xie Y., Jüschke C., Esk C., Hirotsune S., Knoblich J.A. The phosphatase PP4c controls spindle orientation to maintain proliferative symmetric divisions in the developing neocortex. Neuron. 2013;79:254–265. doi: 10.1016/j.neuron.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Siller K.H., Doe C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 308.di Pietro F., Echard A., Morin X. Regulation of mitotic spindle orientation: An integrated view. EMBO Rep. 2016;17:1106–1130. doi: 10.15252/embr.201642292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Seldin L., Macara I. Epithelial spindle orientation diversities and uncertainties: Recent developments and lingering questions. F1000Research. 2017;6:984. doi: 10.12688/f1000research.11370.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Bergstralh D.T., Dawney N.S., St. Johnston D. Spindle orientation: A question of complex positioning. Development. 2017;144:1137–1145. doi: 10.1242/dev.140764. [DOI] [PubMed] [Google Scholar]
- 311.Nguyen-Ngoc T., Afshar K., Gönczy P. Coupling of cortical dynein and G α proteins mediates spindle positioning in Caenorhabditis elegans. Nat. Cell Biol. 2007;9:1294–1302. doi: 10.1038/ncb1649. [DOI] [PubMed] [Google Scholar]
- 312.Kotak S., Busso C., Gönczy P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 2012;199:97–110. doi: 10.1083/jcb.201203166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Laan L., Pavin N., Husson J., Romet-Lemonne G., van Duijn M., López M.P., Vale R.D., Jülicher F., Reck-Peterson S.L., Dogterom M. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 2012;148:502–514. doi: 10.1016/j.cell.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Du Q., Macara I.G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–516. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 315.Gallini S., Carminati M., De Mattia F., Pirovano L., Martini E., Oldani A., Asteriti I.A., Guarguaglini G., Mapelli M. NuMA Phosphorylation by Aurora-A Orchestrates Spindle Orientation. Curr. Biol. 2016;26:458–469. doi: 10.1016/j.cub.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 316.Kotak S., Afshar K., Busso C., Gönczy P. Aurora A kinase regulates proper spindle positioning in C. elegans and human cells. J. Cell Sci. 2016;129:3015–3025. doi: 10.1242/jcs.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kotak S., Busso C., Gönczy P. NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function. EMBO J. 2013;32:2517–2529. doi: 10.1038/emboj.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Sana S., Keshri R., Rajeevan A., Kapoor S., Kotak S. Plk1 regulates spindle orientation by phosphorylating NuMA in human cells. Life Sci. Alliance. 2018;1:e201800223. doi: 10.26508/lsa.201800223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zheng Z., Wan Q., Meixiong G., Du Q. Cell cycle-regulated membrane binding of NuMA contributes to efficient anaphase chromosome separation. Mol. Biol. Cell. 2014;25:606–619. doi: 10.1091/mbc.e13-08-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Lee B.H., Schwager F., Meraldi P., Gotta M. p37/UBXN2B regulates spindle orientation by limiting cortical NuMA recruitment via PP1/Repo-Man. J. Cell Biol. 2018;217:483–493. doi: 10.1083/jcb.201707050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Afshar K., Werner M.E., Tse Y.C., Glotzer M., Gönczy P. Regulation of cortical contractility and spindle positioning by the protein phosphatase 6 PPH-6 in one-cell stage C. elegans embryos. Development. 2010;137:237–247. doi: 10.1242/dev.042754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Musacchio A., Desai A. A molecular view of kinetochore assembly and function. Biology. 2017;6:5. doi: 10.3390/biology6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Cimini D., Howell B., Maddox P., Khodjakov A., Degrassi F., Salmon E.D. Merotelic Kinetochore Orientation Is a Major Mechanism of Aneuploidy in Mitotic Mammalian Tissue Cells. J. Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Tanaka K., Mukae N., Dewar H., Breugel M., James E.K., Prescott A.R., Anthony A., Tanaka T.U. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature. 2005;434:987–994. doi: 10.1038/nature03483. [DOI] [PubMed] [Google Scholar]
- 325.Kapoor T.M., Lampson M.A., Hergert P., Cameron L., Cimini D., Salmon E.D., McEwen B.F., Khodjakov A. Chromosomes Can Congress to the Metaphase Plate Before Biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Thompson S.L., Compton D.A. Chromosome missegregation in human cells arises through specific types of kinetochore–microtubule attachment errors. Proc. Natl Acad. Sci. USA. 2011;108:17974–17978. doi: 10.1073/pnas.1109720108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Foley E.A., Kapoor T.M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat. Rev. Mol. Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Barisic M., Aguiar P., Geley S., Maiato H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat. Cell Biol. 2014;16:1249–1256. doi: 10.1038/ncb3060. [DOI] [PubMed] [Google Scholar]
- 329.Bakhoum S.F., Thompson S.L., Manning A.L., Compton D.A. Genome stability is ensured by temporal control of kinetochore-microtubule dynamics. Nat. Cell Biol. 2009;11:27–35. doi: 10.1038/ncb1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Bakhoum S.F., Genovese G., Compton D.A. Deviant Kinetochore Microtubule Dynamics Underlie Chromosomal Instability. Curr. Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zaytsev A.V., Grishchuk E.L. Basic mechanism for biorientation of mitotic chromosomes is provided by the kinetochore geometry and indiscriminate turnover of kinetochore microtubules. Mol. Biol. Cell. 2015;26:3985–3998. doi: 10.1091/mbc.E15-06-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Cheeseman I.M., Chappie J.S., Wilson-Kubalek E.M., Desai A. The Conserved KMN Network Constitutes the Core Microtubule-Binding Site of the Kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 333.Wei R.R., Al-Bassam J., Harrison S.C. The Ndc80/HEC1 complex is a contact point for kinetochore-microtubule attachment. Nat. Struct. Mol. Biol. 2006;14:54–59. doi: 10.1038/nsmb1186. [DOI] [PubMed] [Google Scholar]
- 334.Ciferri C., Pasqualato S., Screpanti E., Varetti G., Santaguida S., Reis G., Maiolica A., Polka J., DeLuca J.G., de Wulf P., et al. Implications for Kinetochore-Microtubule Attachment from the Structure of an Engineered Ndc80 Complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Alushin G.M., Ramey V.H., Pasqualato S., Ball D.A., Grigorieff N., Musacchio A., Nogales E. The Ndc80 kinetochore complex forms oligomeric arrays along microtubules. Nature. 2011;467:805–810. doi: 10.1038/nature09423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.DeLuca J.G., Gall W.E., Ciferri C., Cimini D., Musacchio A., Salmon E.D. Kinetochore Microtubule Dynamics and Attachment Stability Are Regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 337.Guimarães G.J., Dong Y., McEwen B.F., DeLuca J.G. Kinetochore-Microtubule Attachment Relies on the Disordered N-Terminal Tail Domain of Hec1. Curr. Biol. 2008;18:1778–1784. doi: 10.1016/j.cub.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Miller S.A., Johnson M.L., Stukenberg P.T. Kinetochore Attachments Require an Interaction between Unstructured Tails on Microtubules and Ndc80Hec1. Curr. Biol. 2008;18:1785–1791. doi: 10.1016/j.cub.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Zaytsev A.V., Sundin L.J.R., DeLuca K.F., Grishchuk E.L., DeLuca J.G. Accurate phosphoregulation of kinetochore–microtubule affinity requires unconstrained molecular interactions. J. Cell Biol. 2014;206:45–59. doi: 10.1083/jcb.201312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zaytsev A.V., Mick J.E., Maslennikov E., Nikashin B., DeLuca J.G., Grishchuk E.L. Multisite phosphorylation of the NDC80 complex gradually tunes its microtubule-binding affinity. Mol. Biol. Cell. 2015;26:1829–1844. doi: 10.1091/mbc.E14-11-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Tanaka T.U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E., Stark M.J.R., Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora Kinase-INCENP) Complex Promotes Chromosome Bi-orientation by Altering Kinetochore-Spindle Pole Connections. Cell. 2002;108:317–329. doi: 10.1016/S0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 342.Liu D., Vader G., Vromans M.J.M., Lampson M.A., Lens S.M.A. Sensing Chromosome Bi-Orientation by Spatial Separation of Aurora B Kinase from Kinetochore Substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Krenn V., Musacchio A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front. Oncol. 2015:5. doi: 10.3389/fonc.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Lampson M.A., Cheeseman I.M. Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol. 2011;21:133–140. doi: 10.1016/j.tcb.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Bishop J.D., Schumacher J.M. Phosphorylation of the Carboxyl Terminus of Inner Centromere Protein (INCENP) by the Aurora B Kinase Stimulates Aurora B Kinase Activity. J. Biol. Chem. 2002;277:27577–27580. doi: 10.1074/jbc.C200307200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Honda R., Korner R., Nigg E.A. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 2003;14:3325–3341. doi: 10.1091/mbc.e02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Yasui Y., Urano T., Kawajiri A., Nagata K., Tatsuka M., Saya H., Furukawa K., Takahashi T., Izawa I., Inagaki M. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J. Biol. Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- 348.Sessa F., Mapelli M., Ciferri C., Tarricone C., Areces L.B., Schneider T.R., Stukenberg P.T., Musacchio A. Mechanism of Aurora B Activation by INCENP and Inhibition by Hesperadin. Mol. Cell. 2005;18:379–391. doi: 10.1016/j.molcel.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 349.Kelly A.E., Sampath S.C., Maniar T.A., Woo E.M., Chait B.T., Funabiki H. Chromosomal Enrichment and Activation of the Aurora B Pathway Are Coupled to Spatially Regulate Spindle Assembly. Dev. Cell. 2007;12:31–43. doi: 10.1016/j.devcel.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang E., Ballister E.R., Lampson M.A. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. J. Cell Biol. 2011;194:539–549. doi: 10.1083/jcb.201103044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Zaytsev A.V., Segura-Peña D., Godzi M., Calderon A., Ballister E.R., Stamatov R., Mayo A.M., Peterson L., Black B.E., Ataullakhanov F.I., et al. Bistability of a coupled Aurora B kinase-phosphatase system in cell division. Elife. 2016;5:e10644. doi: 10.7554/eLife.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Ruppert F.G., Samejima K., Platani M., Molina O., Kimura H., Jeyaprakash A.A., Ohta S., Earnshaw W.C. HP1α targets the chromosomal passenger complex for activation at heterochromatin before mitotic entry. EMBO J. 2018:e97677. doi: 10.15252/embj.201797677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Maresca T.J., Salmon E.D. Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J. Cell Biol. 2009;184:373–381. doi: 10.1083/jcb.200808130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Uchida K.S., Takagaki K., Kumada K., Hirayama Y., Noda T., Hirota T. Kinetochore stretching inactivates the spindle assembly checkpoint. J. Cell Biol. 2009;184:383–390. doi: 10.1083/jcb.200811028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Wan X., O’Quinn R.P., Pierce H.L., Joglekar A.P., Gall W.E., DeLuca J.G., Carroll C.W., Liu S., Yen T.J., McEwen B.F., et al. Protein Architecture of the Human Kinetochore Microtubule Attachment Site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Maresca T.J., Salmon E.D. Welcome to a new kind of tension: Translating kinetochore mechanics into a wait-anaphase signal. J. Cell Sci. 2010;123:825–835. doi: 10.1242/jcs.064790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Lampson M.A., Renduchitala K., Khodjakov A., Kapoor T.M. Correcting improper chromosome–spindle attachments during cell division. Nat. Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- 358.Pinsky B.A., Kung C., Shokat K.M., Biggins S. The Ipl1-Aurora protein kinase activates the spindle checkpoint by creating unattached kinetochores. Nat. Cell Biol. 2006;8:78–83. doi: 10.1038/ncb1341. [DOI] [PubMed] [Google Scholar]
- 359.Welburn J.P., Vleugel M., Liu D., Yates J.R., Lampson M.A., Fukagawa T., Cheeseman I.M. Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface. Mol. Cell. 2010;38:383–392. doi: 10.1016/j.molcel.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Shrestha R.L., Conti D., Tamura N., Braun D., Ramalingam R.A., Cieslinski K., Ries J., Draviam V.M. Aurora-B kinase pathway controls the lateral to end-on conversion of kinetochore-microtubule attachments in human cells. Nat. Commun. 2017;8:150. doi: 10.1038/s41467-017-00209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Li Y., Yu W., Liang Y., Zhu X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res. 2007;17:701–712. doi: 10.1038/cr.2007.65. [DOI] [PubMed] [Google Scholar]
- 362.Yang Z., Tulu U.S., Wadsworth P., Rieder C.L. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 2007;17:973–980. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Vorozhko V.V., Emanuele M.J., Kallio M.J., Stukenberg P.T., Gorbsky G.J. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma. 2008;117:169–179. doi: 10.1007/s00412-007-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Monda J.K., Cheeseman I.M. The kinetochore–microtubule interface at a glance. J. Cell Sci. 2018;131:jcs214577. doi: 10.1242/jcs.214577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Cai S., O’connell C.B., Khodjakov A., Walczak C.E. Chromosome congression in the absence of kinetochore fibres. Nat. Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Barisic M., Sousa R.S., Tripathy S.K., Magiera M.M., Zaytsev A.V., Pereira A.L., Janke C., Grishchuk E.L., Maiato H. Microtubule detyrosination guides chromosomes during mitosis. Science. 2015;348:799–803. doi: 10.1126/science.aaa5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Iemura K., Tanaka K. Chromokinesin Kid and kinetochore kinesin CENP-E differentially support chromosome congression without end-on attachment to microtubules. Nat. Commun. 2015;6:6447. doi: 10.1038/ncomms7447. [DOI] [PubMed] [Google Scholar]
- 368.Barisic M., Maiato H. The tubulin code: A navigation system for chromosomes during mitosis. Trends Cell Biol. 2016;26:766–775. doi: 10.1016/j.tcb.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Itoh G., Ikeda M., Iemura K., Amin M.A., Kuriyama S., Tanaka M., Mizuno N., Osakada H., Haraguchi T., Tanaka K. Lateral attachment of kinetochores to microtubules is enriched in prometaphase rosette and facilitates chromosome alignment and bi-orientation establishment. Sci. Rep. 2018;8:3888. doi: 10.1038/s41598-018-22164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Lombillo V.A., Nislow C., Yen T.J., Gelfand V.I., McIntosh J.R. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J. Cell Biol. 1995;128:107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Gudimchuk N., Vitre B., Kim Y., Kiyatkin A., Cleveland D.W., Ataullakhanov F.I., Grishchuk E.L. Kinetochore kinesin CENP-E is a processive bi-directional tracker of dynamic microtubule tips. Nat. Cell Biol. 2013;15:1079–1088. doi: 10.1038/ncb2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Shrestha R.L., Draviam V.M. Lateral to end-on conversion of chromosome-microtubule attachment requires kinesins CENP-E and MCAK. Curr. Biol. 2013;23:1514–1526. doi: 10.1016/j.cub.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Suijkerbuijk S.J., Vleugel M., Teixeira A., Kops G.J. Integration of kinase and phosphatase activities by BUBR1 ensures formation of stable kinetochore-microtubule attachments. Dev. Cell. 2012;23:745–755. doi: 10.1016/j.devcel.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 374.Kruse T., Zhang G., Larsen M.S.Y., Lischetti T., Streicher W., Nielsen T.K., Bjørn S.P., Nilsson J. Direct binding between BubR1 and B56-PP2A phosphatase complexes regulate mitotic progression. J. Cell Sci. 2013;126:1086–1092. doi: 10.1242/jcs.122481. [DOI] [PubMed] [Google Scholar]
- 375.Xu P., Raetz E.A., Kitagawa M., Virshup D.M., Lee S.H. BUBR1 recruits PP2A via the B56 family of targeting subunits to promote chromosome congression. Biol. Open. 2013;2:479–486. doi: 10.1242/bio.20134051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Elowe S., Hümmer S., Uldschmid A., Li X., Nigg E.A. Tension-sensitive Plk1 phosphorylation on BubR1 regulates the stability of kinetochore–microtubule interactions. Genes Dev. 2007;21:2205–2219. doi: 10.1101/gad.436007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Huang H., Hittle J., Zappacosta F., Annan R.S., Hershko A., Yen T.J. Phosphorylation sites in BubR1 that regulate kinetochore attachment, tension, and mitotic exit. J. Cell Biol. 2008;183:667–680. doi: 10.1083/jcb.200805163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Rivera T., Ghenoiu C., Rodríguez-Corsino M., Mochida S., Funabiki H., Losada A. Xenopus Shugoshin 2 regulates the spindle assembly pathway mediated by the chromosomal passenger complex. EMBO J. 2012;31:1467–1479. doi: 10.1038/emboj.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Nijenhuis W., Vallardi G., Teixeira A., Kops G.J.P.L., Saurin A.T. Negative feedback at kinetochores underlies a responsive spindle checkpoint signal. Nat. Cell Biol. 2014;16:1257–1264. doi: 10.1038/ncb3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Meppelink A., Kabeche L., Vromans M.J., Compton D.A., Lens S.M. Shugoshin-1 balances Aurora B kinase activity via PP2A to promote chromosome bi-orientation. Cell Rep. 2015;11:508–515. doi: 10.1016/j.celrep.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Foley E.A., Maldonado M., Kapoor T.M. Formation of stable attachments between kinetochores and microtubules depends on the B56-PP2A phosphatase. Nat. Cell Biol. 2011;13:1265–1271. doi: 10.1038/ncb2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Liu D., Vleugel M., Backer C.B., Hori T., Fukagawa T., Cheeseman I.M., Lampson M.A. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Vallardi G., Cordeiro M.H., Saurin A.T. A kinase-phosphatase network that regulates kinetochore-microtubule attachments and the SAC. Prog. Mol. Subcell. Biol. 2017;56:457–484. doi: 10.1007/978-3-319-58592-5_19. [DOI] [PubMed] [Google Scholar]
- 384.Saurin A.T. Kinase and phosphatase cross-talk at the kinetochore. Front. Cell Dev. Biol. 2018;6:62. doi: 10.3389/fcell.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Posch M., Khoudoli G.A., Swift S., King E.M., DeLuca J.G., Swedlow J.R. Sds22 regulates aurora B activity and microtubule–kinetochore interactions at mitosis. J. Cell Biol. 2010;131:61–74. doi: 10.1083/jcb.200912046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Wurzenberger C., Held M., Lampson M.A., Poser I., Hyman A.A., Gerlich D.W. Sds22 and Repo-Man stabilize chromosome segregation by counteracting Aurora B on anaphase kinetochores. J. Cell Biol. 2012;198:173–183. doi: 10.1083/jcb.201112112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Murnion M.E., Adams R.R., Callister D.M., Allis C.D., Earnshaw W.C., Swedlow J.R. Chromatin-associated protein phosphatase 1 regulates aurora-B and histone H3 phosphorylation. J. Biol. Chem. 2001;276:26656–26665. doi: 10.1074/jbc.M102288200. [DOI] [PubMed] [Google Scholar]
- 388.Rosasco-Nitcher S.E., Lan W., Khorasanizadeh S., Stukenberg P.T. Centromeric Aurora-B activation requires TD-60, microtubules, and substrate priming phosphorylation. Science. 2008;319:469–472. doi: 10.1126/science.1148980. [DOI] [PubMed] [Google Scholar]
- 389.Cheerambathur D.K., Prevo B., Hattersley N., Lewellyn L., Corbett K.D., Oegema K., Desai A. Dephosphorylation of the Ndc80 tail stabilizes kinetochore-microtubule attachments via the Ska complex. Dev. Cell. 2017;41:424–437. doi: 10.1016/j.devcel.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Janczyk P.Ł., Skorupka K.A., Tooley J.G., Matson D.R., Kestner C.A., West T., Pornillos O., Stukenberg P.T. Mechanism of Ska recruitment by Ndc80 complexes to kinetochores. Dev. Cell. 2017;41:438–449. doi: 10.1016/j.devcel.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Schmidt J.C., Arthanari H., Boeszoermenyi A., Dashkevich N.M., Wilson-Kubalek E.M., Monnier N., Markus M., Oberer M., Milligan R.A., Bathe M., et al. The kinetochore-bound Ska1 complex tracks depolymerizing microtubules and binds to curved protofilaments. Dev. Cell. 2012;23:968–980. doi: 10.1016/j.devcel.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Hanisch A., Silljé H.H., Nigg E.A. Timely anaphase onset requires a novel spindle and kinetochore complex comprising Ska1 and Ska2. EMBO J. 2006;25:5504–5515. doi: 10.1038/sj.emboj.7601426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Gaitanos T.N., Santamaria A., Jeyaprakash A.A., Wang B., Conti E., Nigg E.A. Stable kinetochore–microtubule interactions depend on the Ska complex and its new component Ska3/C13Orf3. EMBO J. 2009;28:1442–1452. doi: 10.1038/emboj.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Raaijmakers J.A., Tanenbaum M.E., Maia A.F., Medema R.H. RAMA1 is a novel kinetochore protein involved in kinetochore-microtubule attachment. J. Cell Sci. 2009;122:2436–2445. doi: 10.1242/jcs.051912. [DOI] [PubMed] [Google Scholar]
- 395.Welburn J.P., Grishchuk E.L., Backer C.B., Wilson-Kubalek E.M., Yates III J.R., Cheeseman I.M. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev. Cell. 2009;16:374–385. doi: 10.1016/j.devcel.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Jeyaprakash A.A., Santamaria A., Jayachandran U., Chan Y.W., Benda C., Nigg E.A., Conti E. Structural and functional organization of the Ska complex, a key component of the kinetochore-microtubule interface. Mol. Cell. 2012;46:274–286. doi: 10.1016/j.molcel.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 397.Monda J.K., Whitney I.P., Tarasovetc E.V., Wilson-Kubalek E., Milligan R.A., Grishchuk E.L., Cheeseman I.M. Microtubule Tip Tracking by the Spindle and Kinetochore Protein Ska1 Requires Diverse Tubulin-Interacting Surfaces. Curr. Biol. 2017;27:3666–3675. doi: 10.1016/j.cub.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Auckland P., Clarke N.I., Royle S.J., McAinsh A.D. Congressing kinetochores progressively load Ska complexes to prevent force-dependent detachment. J. Cell Biol. 2017;216:1623–1638. doi: 10.1083/jcb.201607096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Helgeson L.A., Zelter A., Riffle M., MacCoss M.J., Asbury C.L., Davis T.N. Human Ska complex and Ndc80 complex interact to form a load-bearing assembly that strengthens kinetochore–microtubule attachments. Proc. Nat. Acad. Sci. USA. 2018;115:2740–2745. doi: 10.1073/pnas.1718553115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Chan Y.W., Jeyaprakash A.A., Nigg E.A., Santamaria A. Aurora B controls kinetochore–microtubule attachments by inhibiting Ska complex–KMN network interaction. J. Cell Biol. 2012;196:563–571. doi: 10.1083/jcb.201109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Abad M.A., Medina B., Santamaria A., Zou J., Plasberg-Hill C., Madhumalar A., Jayachandran U., Redli P.M., Rappsilber J., Nigg E.A., et al. Structural basis for microtubule recognition by the human kinetochore Ska complex. Nat. Commun. 2014;5:2964. doi: 10.1038/ncomms3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Redli P.M., Gasic I., Meraldi P., Nigg E.A., Santamaria A. The Ska complex promotes Aurora B activity to ensure chromosome biorientation. J. Cell Biol. 2016;215:77–93. doi: 10.1083/jcb.201603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Sivakumar S., Janczyk P.Ł., Qu Q., Brautigam C.A., Stukenberg P.T., Yu H., Gorbsky G.J. The human SKA complex drives the metaphase-anaphase cell cycle transition by recruiting protein phosphatase 1 to kinetochores. Elife. 2016;5:e12902. doi: 10.7554/eLife.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Suzuki A., Gupta A., Long S.K., Evans R., Badger B.L., Salmon E.D., Biggings S., Bloom K. A Kinesin-5, Cin8, Recruits Protein Phosphatase 1 to Kinetochores and Regulates Chromosome Segregation. Curr. Biol. 2018;28:2697–2704. doi: 10.1016/j.cub.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.De Wever V., Nasa I., Chamousset D., Lloyd D., Nimick M., Xu H., Trinkle-Mulcahy L., Moorhead G.B. The human mitotic kinesin KIF18A binds protein phosphatase 1 (PP1) through a highly conserved docking motif. Biochem. Biophys. Res. Commun. 2014;453:432–437. doi: 10.1016/j.bbrc.2014.09.105. [DOI] [PubMed] [Google Scholar]
- 406.Häfner J., Mayr M.I., Möckel M.M., Mayer T.U. Pre-anaphase chromosome oscillations are regulated by the antagonistic activities of Cdk1 and PP1 on Kif18A. Nat. Commun. 2014;5:4397. doi: 10.1038/ncomms5397. [DOI] [PubMed] [Google Scholar]
- 407.Kim Y., Holland A.J., Lan W., Cleveland D.W. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142:444–455. doi: 10.1016/j.cell.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Kim H., Stumpff J.K. Kif18A promotes Hec1 dephosphorylation to coordinate chromosome alignment with kinetochore microtubule attachment. bioRxiv. 2018 doi: 10.1101/304147. [DOI] [Google Scholar]
- 409.Totsukawa G., Yamakita Y., Yamashiro S., Hosoya H., Hartshorne D.J., Matsumura F. Activation of myosin phosphatase targeting subunit by mitosis-specific phosphorylation. J. Cell Biol. 1999;144:735–744. doi: 10.1083/jcb.144.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Yamashiro S., Yamakita Y., Totsukawa G., Goto H., Kaibuchi K., Ito M., Hartshorne D.J., Matsumura F. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev. Cell. 2008;14:787–797. doi: 10.1016/j.devcel.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Kabeche L., Compton D.A. Cyclin A regulates kinetochore microtubules to promote faithful chromosome segregation. Nature. 2013;502:110–113. doi: 10.1038/nature12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Li H., Liu X.S., Yang X., Wang Y., Wang Y., Turner J.R., Liu X. Phosphorylation of CLIP-170 by Plk1 and CK2 promotes timely formation of kinetochore–microtubule attachments. EMBO J. 2010;29:2953–2965. doi: 10.1038/emboj.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Bader J.R., Kasuboski J.M., Winding M., Vaughan P.S., Hinchcliffe E.H., Vaughan K.T. Polo-like kinase 1 is required for recruitment of dynein to kinetochores during mitosis. J. Biol. Chem. 2011:jbc-M111. doi: 10.1074/jbc.M111.226605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Hood E.A., Kettenbach A.N., Gerber S.A., Compton D.A. Plk1 regulates the kinesin-13 protein Kif2b to promote faithful chromosome segregation. Mol. Biol. Cell. 2012;23:2264–2274. doi: 10.1091/mbc.e11-12-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Maia A.R., Garcia Z., Kabeche L., Barisic M., Maffini S., Macedo-Ribeiro S., Cheeseman I.M., Compton D.A., Kaverina I., Maiato H. Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore–microtubule attachments. J. Cell Biol. 2012;199:285–301. doi: 10.1083/jcb.201203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Kakeno M., Matsuzawa K., Matsui T., Akita H., Sugiyama I., Ishidate F., Nakano A., Takashima S., Goto H., Inagaki M., et al. Plk1 phosphorylates CLIP-170 and regulates its binding to microtubules for chromosome alignment. Cell Struct Funct. 2014;39:45–59. doi: 10.1247/csf.14001. [DOI] [PubMed] [Google Scholar]
- 417.Shao H., Huang Y., Zhang L., Yuan K., Chu Y., Dou Z., Jin C., Garcia-Barrio M., Liu X., Yao X. Spatiotemporal dynamics of Aurora B-PLK1-MCAK signaling axis orchestrates kinetochore bi-orientation and faithful chromosome segregation. Sci. Rep. 2015;5:12204. doi: 10.1038/srep12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Nishino M., Kurasawa Y., Evans R., Lin S.H., Brinkley B.R., Yu-Lee L.Y. NudC is required for Plk1 targeting to the kinetochore and chromosome congression. Curr. Biol. 2006;16:1414–1421. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 419.Amin M.A., Itoh G., Iemura K., Ikeda M., Tanaka K. CLIP-170 is required to recruit PLK1 to kinetochores during early mitosis for chromosome alignment. J. Cell Sci. 2014:jcs-150755. doi: 10.1242/jcs.150755. [DOI] [PubMed] [Google Scholar]
- 420.Ehlen A., Martin C., Julien M., Miron S., Theillet F.X., Boucherit V., Duchambon P., Marjou A.E., Justin S.Z., Carreira A. Proper chromosome alignment depends on BRCA2 phosphorylation by PLK1. bioRxiv. 2018 doi: 10.1101/265934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J.J., Hoffmann M., Rettig W.J., Kraut N., Peter J.M. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr. Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 422.Liu D., Davydenko O., Lampson M.A. Polo-like kinase-1 regulates kinetochore–microtubule dynamics and spindle checkpoint silencing. J. Cell Biol. 2012;198:491–499. doi: 10.1083/jcb.201205090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Moutinho-Santos T., Conde C., Sunkel C.E. POLO ensures chromosome bi-orientation by preventing and correcting erroneous chromosome–spindle attachments. J. Cell Sci. 2012;125:576–583. doi: 10.1242/jcs.092445. [DOI] [PubMed] [Google Scholar]
- 424.Lera R.F., Potts G.K., Suzuki A., Johnson J.M., Salmon E.D., Coon J.J., Burkard M.E. Decoding Polo-like kinase 1 signaling along the kinetochore–centromere axis. Nat. Chem. Biol. 2016;12:411–418. doi: 10.1038/nchembio.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Eiteneuer A., Seiler J., Weith M., Beullens M., Lesage B., Krenn V., Musacchio A., Bollen M., Meyer H. Inhibitor-3 ensures bipolar mitotic spindle attachment by limiting association of SDS22 with kinetochore-bound protein phosphatase-1. EMBO J. 2014;33:2704–2720. doi: 10.15252/embj.201489054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Weith M., Seiler J., van den Boom J., Kracht M., Hülsmann J., Primorac I., Garcia J.P., Kaschani F., Kaiser M., Musacchio A., et al. Ubiquitin-independent disassembly by a p97 AAA-ATPase complex drives PP1 holoenzyme formation. Mol. Cell. 2018;72:766–777. doi: 10.1016/j.molcel.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 427.Lesage B., Beullens M., Pedelini L., Garcia-Gimeno M.A., Waelkens E., Sanz P., Bollen M. A complex of catalytically inactive protein phosphatase-1 sandwiched between Sds22 and inhibitor-3. Biochemistry. 2007;46:8909–8919. doi: 10.1021/bi7003119. [DOI] [PubMed] [Google Scholar]
- 428.Cheng Y.L., Chen R.H. Assembly and quality control of protein phosphatase 1 holoenzyme involve Cdc48-Shp1 chaperone. J. Cell Sci. 2015;128:1180–1192. doi: 10.1242/jcs.165159. [DOI] [PubMed] [Google Scholar]
- 429.London N., Biggins S. Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell. Biol. 2014;15:736–747. doi: 10.1038/nrm3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Musacchio A. The Molecular Biology of Spindle Assembly Checkpoint Signaling Dynamics. Curr. Biol. 2015;25:1002–1018. doi: 10.1016/j.cub.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 431.Joglekar A.P. A Cell Biological Perspective on Past, Present and Future Investigations of the Spindle Assembly Checkpoint. Biology. 2016;5:44. doi: 10.3390/biology5040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Corbett K.D. Molecular Mechanisms of Spindle Assembly Checkpoint Activation and Silencing. Prog. Mol. Subcell. Biol. 2017;56:429–455. doi: 10.1007/978-3-319-58592-5_18. [DOI] [PubMed] [Google Scholar]
- 433.Michel L.S., Liberal V., Chatterjee A., Kirchwegger R., Pasche B., Gerald W., Dobles M., Sorger P.K., Murty V.V., Benezra R. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature. 2001;409:355–359. doi: 10.1038/35053094. [DOI] [PubMed] [Google Scholar]
- 434.Dai W., Wang Q., Liu T., Swamy M., Fang Y., Xie S., Mahmood R., Yang Y.M., Xu M., Rao C.V. Slippage of mitotic arrest and enhanced tumor development in mice with BubR1 haploinsufficiency. Cancer Res. 2004;64:440–445. doi: 10.1158/0008-5472.CAN-03-3119. [DOI] [PubMed] [Google Scholar]
- 435.Iwanaga Y., Chi Y.H., Miyazato A., Sheleg S., Haller K., Peloponese J.M. Jr., Li Y., Ward J.M., Benezra R., Jeang K.T. Heterozygous deletion of mitotic arrest-deficient protein 1 (MAD1) increases the incidence of tumors in mice. Cancer Res. 2007;67:160–166. doi: 10.1158/0008-5472.CAN-06-3326. [DOI] [PubMed] [Google Scholar]
- 436.Schvartzman J.M., Sotillo R., Benezra R. Mitotic chromosomal instability and cancer: Mouse modelling of the human disease. Nat. Rev. Cancer. 2010;10:102–115. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Gorbsky G.J. Cohesion fatigue. Curr. Biol. 2013;23:986–988. doi: 10.1016/j.cub.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 438.Rieder C.L., Cole R.W., Khodjakov A., Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Dick A.E., Gerlich D.W. Kinetic framework of spindle assembly checkpoint signalling. Nat. Cell Biol. 2013;15:1370–1377. doi: 10.1038/ncb2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Liu X., Winey M. The MPS1 family of protein kinases. Annu. Rev. Biochem. 2012;81:561–585. doi: 10.1146/annurev-biochem-061611-090435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Pachis S.T., Kops G.J.P.L. Leader of the SAC: Molecular mechanisms of Mps1/TTK regulation in mitosis. Open Biol. 2018;8:180109. doi: 10.1098/rsob.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.London N., Ceto S., Ranish J.A., Biggins S. Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr. Biol. 2012;22:900–906. doi: 10.1016/j.cub.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Shepperd L.A., Meadows J.C., Sochaj A.M., Lancaster T.C., Zou J., Buttrick G.J., Rappsilber J., Hardwick K.G., Millar J.B. Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr. Biol. 2012;22:891–899. doi: 10.1016/j.cub.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Yamagishi Y., Yang C.H., Tanno Y., Watanabe Y. MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat. Cell. Biol. 2012;14:746–752. doi: 10.1038/ncb2515. [DOI] [PubMed] [Google Scholar]
- 445.Primorac I., Weir J.R., Chiroli E., Gross F., Hoffmann I., van Gerwen S., Ciliberto A., Musacchio A. Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife. 2013;2:e01030. doi: 10.7554/eLife.01030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Vleugel M., Omerzu M., Groenewold V., Hadders M.A., Lens S.M.A., Kops G.J.P.L. Sequential multisite phospho-regulation of KNL1-BUB3 interfaces at mitotic kinetochores. Mol. Cell. 2015;57:824–835. doi: 10.1016/j.molcel.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 447.Overlack K., Primorac I., Vleugel M., Krenn V., Maffini S., Hoffmann I., Kops G.J., Musacchio A. A molecular basis for the differential roles of Bub1 and BubR1 in the spindle assembly checkpoint. Elife. 2015;4:e05269. doi: 10.7554/eLife.05269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.London N., Biggins S. Mad1 kinetochore recruitment by Mps1-mediated phosphorylation of Bub1 signals the spindle checkpoint. Genes Dev. 2014;28:140–152. doi: 10.1101/gad.233700.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Mora-Santos M.D., Hervas-Aguilar A., Sewart K., Lancaster T.C., Meadows J.C., Millar J.B. Bub3-Bub1 Binding to Spc7/KNL1 Toggles the Spindle Checkpoint Switch by Licensing the Interaction of Bub1 with Mad1-Mad2. Curr. Biol. 2016;26:2642–2650. doi: 10.1016/j.cub.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 450.Faesen A.C., Thanasoula M., Maffini S., Breit C., Müller F., van Gerwen S., Bange T., Musacchio A. Basis of catalytic assembly of the mitotic checkpoint complex. Nature. 2017;542:498–502. doi: 10.1038/nature21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Ji Z., Gao H., Jia L., Li B., Yu H. A sequential multi-target Mps1 phosphorylation cascade promotes spindle checkpoint signaling. Elife. 2017;6:e22513. doi: 10.7554/eLife.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Qian J., García-Gimeno M.A., Beullens M., Manzione M.G., Van der Hoeven G., Igual J.C., Heredia M., Sanz P., Gelens L., Bollen M. An Attachment-Independent Biochemical Timer of the Spindle Assembly Checkpoint. Mol. Cell. 2017;68:715–730. doi: 10.1016/j.molcel.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 453.Zhang G., Kruse T., López-Méndez B., Sylvestersen K.B., Garvanska D.H., Schopper S., Nielsen M.L., Nilsson J. Bub1 positions Mad1 close to KNL1 MELT repeats to promote checkpoint signalling. Nat. Commun. 2017;8:15822. doi: 10.1038/ncomms15822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Ciliberto A., Hauf S. Micromanaging checkpoint proteins. Elife. 2017;6:e25001. doi: 10.7554/eLife.25001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Hiruma Y., Sacristan C., Pachis S.T., Adamopoulos A., Kuijt T., Ubbink M., von Castelmur E., Perrakis A., Kops G.J. CELL DIVISION CYCLE. Competition between MPS1 and microtubules at kinetochores regulates spindle checkpoint signaling. Science. 2015;348:1264–1267. doi: 10.1126/science.aaa4055. [DOI] [PubMed] [Google Scholar]
- 456.Ji Z., Gao H., Yu H. CELL DIVISION CYCLE. Kinetochore attachment sensed by competitive Mps1 and microtubule binding to Ndc80C. Science. 2015;348:1260–1264. doi: 10.1126/science.aaa4029. [DOI] [PubMed] [Google Scholar]
- 457.Kang J., Chen Y., Zhao Y., Yu H. Autophosphorylation-dependent activation of human Mps1 is required for the spindle checkpoint. Proc. Natl. Acad. Sci. USA. 2007;104:20232–20237. doi: 10.1073/pnas.0710519105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Mattison C.P., Old W.M., Steiner E., Huneycutt B.J., Resing K.A., Ahn N.G., Winey M. Mps1 activation loop autophosphorylation enhances kinase activity. J. Biol. Chem. 2007;282:30553–30561. doi: 10.1074/jbc.M707063200. [DOI] [PubMed] [Google Scholar]
- 459.Jelluma N., Brenkman A.B., McLeod I., Yates J.R., 3rd., Cleveland D.W., Medema R.H., Kops G.J. Chromosomal instability by inefficient Mps1 auto-activation due to a weakened mitotic checkpoint and lagging chromosomes. PLoS ONE. 2008;3:e2415. doi: 10.1371/journal.pone.0002415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Tyler R.K., Chu M.L., Johnson H., McKenzie E.A., Gaskell S.J., Eyers P.A. Phosphoregulation of human Mps1 kinase. Biochem. J. 2009;417:173–181. doi: 10.1042/BJ20081310. [DOI] [PubMed] [Google Scholar]
- 461.Wang W., Yang Y., Gao Y., Xu Q., Wang F., Zhu S., Old W., Resing K., Ahn N., Lei M., et al. Structural and mechanistic insights into Mps1 kinase activation. J. Cell. Mol. Med. 2009;13:1679–1694. doi: 10.1111/j.1582-4934.2008.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Hewitt L., Tighe A., Santaguida S., White A.M., Jones C.D., Musacchio A., Green S., Taylor S.S. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1-C-Mad2 core complex. J. Cell Biol. 2010;190:25–34. doi: 10.1083/jcb.201002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Santaguida S., Vernieri C., Villa F., Ciliberto A., Musacchio A. Evidence that Aurora B is implicated in spindle checkpoint signalling independently of errorcorrection. EMBO J. 2011;30:1508–1519. doi: 10.1038/emboj.2011.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Saurin A.T., van der Waal M.S., Medema R.H., Lens S.M., Kops G.J. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat. Commun. 2011;2:316. doi: 10.1038/ncomms1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Morin V., Prieto S., Melines S., Hem S., Rossignol M., Lorca T., Espeut J., Morin N., Abrieu A. CDK-dependent potentiation of MPS1 kinase activity is essential to the mitotic checkpoint. Curr. Biol. 2012;22:289–295. doi: 10.1016/j.cub.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 466.Nijenhuis W., von Castelmur E., Littler D., De Marco V., Tromer E., Vleugel M., van Osch M.H., Snel B., Perrakis A., Kops G.J. A TPR domain-containing N-terminal module of MPS1 is required for its kinetochore localization by Aurora B. J. Cell Biol. 2013;201:217–231. doi: 10.1083/jcb.201210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Conde C., Osswald M., Barbosa J., Moutinho-Santos T., Pinheiro D., Guimarães S., Matos I., Maiato H., Sunkel C.E. Drosophila Polo regulates the spindle assembly checkpoint through Mps1-dependent BubR1phosphorylation. EMBO J. 2013;32:1761–1777. doi: 10.1038/emboj.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.von Schubert C., Cubizolles F., Bracher J.M., Sliedrecht T., Kops G.J.P.L., Nigg E.A. Plk1 and Mps1 Cooperatively Regulate the Spindle Assembly Checkpoint in Human Cells. Cell Rep. 2015;12:66–78. doi: 10.1016/j.celrep.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 469.O’Connor A., Maffini S., Rainey M.D., Kaczmarczyk A., Gaboriau D., Musacchio A., Santocanale C. Requirement for PLK1 kinase activity in the maintenance of a robust spindle assembly checkpoint. Biol. Open. 2016;5:11–19. doi: 10.1242/bio.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Ikeda M., Tanaka K. Plk1 bound to Bub1 contributes to spindle assembly checkpoint activity during mitosis. Sci Rep. 2017;7:8794. doi: 10.1038/s41598-017-09114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Jelluma N., Brenkman A.B., van den Broek N.J., Cruijsen C.W., van Osch M.H., Lens S.M., Medema R.H., Kops G.J. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 2008;132:233–246. doi: 10.1016/j.cell.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 472.van der Waal M.S., Saurin A.T., Vromans M.J., Vleugel M., Wurzenberger C., Gerlich D.W., Medema R.H., Kops G.J., Lens S.M. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep. 2012;13:847–854. doi: 10.1038/embor.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Moura M., Osswald M., Leça N., Barbosa J., Pereira A.J., Maiato H., Sunkel C.E., Conde C. Protein Phosphatase 1 inactivates Mps1 to ensure efficient Spindle Assembly Checkpoint silencing. Elife. 2017;6:e25366. doi: 10.7554/eLife.25366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Sassoon I., Severin F.F., Andrews P.D., Taba M.R., Kaplan K.B., Ashford A.J., Stark M.J., Sorger P.K., Hyman A.A. Regulation of Saccharomyces cerevisiae kinetochores by the type 1 phosphatase Glc7p. Genes Dev. 1999;13:545–555. doi: 10.1101/gad.13.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Bloecher A., Tatchell K. Defects in Saccharomyces cerevisiae protein phosphatase type I activate the spindle/kinetochore checkpoint. Genes Dev. 1999;13:517–522. doi: 10.1101/gad.13.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Pinsky B.A., Nelson C.R., Biggins S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 2009;19:1182–1187. doi: 10.1016/j.cub.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Vanoosthuyse V., Hardwick K.G. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Meadows J.C., Shepperd L.A., Vanoosthuyse V., Lancaster T.C., Sochaj A.M., Buttrick G.J., Hardwick K.G., Millar J.B. Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev. Cell. 2011;20:739–750. doi: 10.1016/j.devcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Rosenberg J.S., Cross F.R., Funabiki H. KNL1/Spc105 recruits PP1 to silence the spindle assembly checkpoint. Curr. Biol. 2011;21:942–947. doi: 10.1016/j.cub.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Espeut J., Cheerambathur D.K., Krenning L., Oegema K., Desai A. Microtubule binding by KNL-1 contributes to spindle checkpoint silencing at the kinetochore. J. Cell Biol. 2012;196:469–482. doi: 10.1083/jcb.201111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Zhang G., Lischetti T., Nilsson J. A minimal number of MELT repeats supports all the functions of KNL1 in chromosome segregation. J. Cell Sci. 2014;127:871–884. doi: 10.1242/jcs.139725. [DOI] [PubMed] [Google Scholar]
- 482.Tang N.H., Toda T. Alp7/TACC recruits kinesin-8-PP1 to the Ndc80 kinetochore protein for timely mitotic progression and chromosome movement. J. Cell Sci. 2015;128:354–363. doi: 10.1242/jcs.160036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Zhang G., Kelstrup C.D., Hu X.W., Kaas Hansen M.J., Singleton M.R., Olsen J.V., Nilsson J. The Ndc80 internal loop is required for recruitment of the Ska complex to establish end-on microtubule attachment to kinetochores. J. Cell Sci. 2012;125:3243–3253. doi: 10.1242/jcs.104208. [DOI] [PubMed] [Google Scholar]
- 484.Zhang Q., Sivakumar S., Chen Y., Gao H., Yang L., Yuan Z., Yu H., Liu H. Ska3 Phosphorylated by Cdk1 Binds Ndc80 and Recruits Ska to Kinetochores to Promote Mitotic Progression. Curr. Biol. 2017;27:1477–1484. doi: 10.1016/j.cub.2017.03.060. [DOI] [PubMed] [Google Scholar]
- 485.Zhang Q., Chen Y., Yang L., Liu H. Multitasking Ska in Chromosome Segregation: Its Distinct Pools Might Specify Various Functions. Bioessays. 2018;40:1700176. doi: 10.1002/bies.201700176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Bajaj R., Bollen M., Peti W., Page R. KNL1 Binding to PP1 and Microtubules Is Mutually Exclusive. Structure. 2018;26:1327–1336. doi: 10.1016/j.str.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Espert A., Uluocak P., Bastos R.N., Mangat D., Graab P., Gruneberg U. PP2A-B56 opposes Mps1 phosphorylation of Knl1 and thereby promotes spindle assemblycheckpoint silencing. J. Cell Biol. 2014;206:833–842. doi: 10.1083/jcb.201406109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Jia L., Li B., Yu H. The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20phosphorylation. Nat. Commun. 2016;7:10818. doi: 10.1038/ncomms10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Craney A., Kelly A., Jia L., Fedrigo I., Yu H., Rape M. Control of APC/C-dependent ubiquitin chain elongation by reversible phosphorylation. Proc. Natl. Acad. Sci. USA. 2016;113:1540–1545. doi: 10.1073/pnas.1522423113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Labit H., Fujimitsu K., Bayin N.S., Takaki T., Gannon J., Yamano H. Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J. 2012;31:3351–3362. doi: 10.1038/emboj.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Kim T., Lara-Gonzalez P., Prevo B., Meitinger F., Cheerambathur D.K., Oegema K., Desai A. Kinetochores accelerate or delay APC/C activation by directing Cdc20 to opposing fates. Genes Dev. 2017;31:1089–1094. doi: 10.1101/gad.302067.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Lee S.J., Rodriguez-Bravo V., Kim H., Datta S., Foley E.A. The PP2AB56 phosphatase promotes the association of Cdc20 with APC/C in mitosis. J. Cell Sci. 2017;130:1760–1771. doi: 10.1242/jcs.201608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Nilsson J. Protein phosphatases in the regulation of mitosis. J. Cell Biol. 2018;218:395–409. doi: 10.1083/jcb.201809138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Neef R., Gruneberg U., Kopajtich R., Li X., Nigg E.A., Sillje H., Barr F.A. Choice of Plk1 docking partners during mitosis and cytokinesis is controlled by the activation state of Cdk1. Nat. Cell Biol. 2007;9:436–444. doi: 10.1038/ncb1557. [DOI] [PubMed] [Google Scholar]
- 495.Petronczki M., Glotzer M., Kraut N., Peters J.M. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the Rho GEF Ect2 to the central spindle. Dev. Cell. 2007;12:713–725. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 496.Santamaria A., Neef R., Eberspächer U., Eis K., Husemann M., Mumberg D., Prechtl S., Schulze V., Siemeister G., Wortmann L., et al. Use of the novel Plk1 inhibitor ZK-thiazolidinone to elucidate functions of Plk1 in early and latestages of mitosis. Mol. Biol. Cell. 2007;18:4024–4036. doi: 10.1091/mbc.e07-05-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Wolfe B.A., Takaki T., Petronczki M., Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiatecleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Walczak C.E., Shaw S.L. A MAP for bundling microtubules. Cell. 2010;142:364–367. doi: 10.1016/j.cell.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 499.Jiang W., Jimenez G., Wells N.J., Hope T.J., Wahl G.M., Hunter T., Fukunaga R. PRC1: A human mitotic spindle-associated CDK substrate protein required for cytokinesis. Mol. Cell. 1998;2:877–885. doi: 10.1016/S1097-2765(00)80302-0. [DOI] [PubMed] [Google Scholar]
- 500.Mollinari C., Kleman J.P., Jiang W., Schoehn G., Hunter T., Margolis R.L. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 2002;157:1175–1186. doi: 10.1083/jcb.200111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501.Mishima M., Pavicic V., Grüneberg U., Nigg E.A., Glotzer M. Cell cycle regulation of central spindle assembly. Nature. 2004;430:908–913. doi: 10.1038/nature02767. [DOI] [PubMed] [Google Scholar]
- 502.Zhu C., Lau E., Schwarzenbacher R., Bossy-Wetzel E., Jiang W. Spatiotemporal control of spindle midzone formation by PRC1 in human cells. Proc. Natl. Acad. Sci. USA. 2006;103:6196–6201. doi: 10.1073/pnas.0506926103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Zhu C., Jiang W. Cell cycle-dependent translocation of PRC1 on the spindle by Kif4 is essential for midzone formation and cytokinesis. Proc. Natl. Acad. Sci. USA. 2005;102:343–348. doi: 10.1073/pnas.0408438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Bieling P., Telley I.A., Surrey T. A minimal midzone protein module controls formation and length of antiparallel microtubule overlaps. Cell. 2010;142:420–432. doi: 10.1016/j.cell.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 505.Subramanian R., Wilson-Kubalek E.M., Arthur C.P., Bick M.J., Campbell E.A., Darst S.A., Milligan R.A., Kapoor T.M. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell. 2010;142:433–443. doi: 10.1016/j.cell.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.de Castro I.J., Gokhan E., Vagnarelli P. Resetting a functional G1 nucleus after mitosis. Chromosoma. 2016;125:607–619. doi: 10.1007/s00412-015-0561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Schellhaus A.K., De Magistris P., Antonin W. Nuclear Reformation at the End of Mitosis. J. Mol. Biol. 2016;428:1962–1985. doi: 10.1016/j.jmb.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 508.LaJoie D., Ullman K.S. Coordinated events of nuclear assembly. Curr. Opin. Cell Biol. 2017;46:39–45. doi: 10.1016/j.ceb.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 509.Peter M., Nakagawa J., Dorée M., Labbé J.C., Nigg E.A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell. 1990;61:591–602. doi: 10.1016/0092-8674(90)90471-P. [DOI] [PubMed] [Google Scholar]
- 510.Heald R., McKeon F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell. 1990;61:579–589. doi: 10.1016/0092-8674(90)90470-Y. [DOI] [PubMed] [Google Scholar]
- 511.Macaulay C., Meier E., Forbes D.J. Differential mitotic phosphorylation of proteins of the nuclear pore complex. J. Biol. Chem. 1995;270:254–262. doi: 10.1074/jbc.270.1.254. [DOI] [PubMed] [Google Scholar]
- 512.Favreau C., Worman H.J., Wozniak R.W., Frappier T., Courvalin J.C. Cell cycle-dependent phosphorylation of nucleoporins and nuclear pore membrane protein Gp210. Biochemistry. 1996;35:8035–8044. doi: 10.1021/bi9600660. [DOI] [PubMed] [Google Scholar]
- 513.Mansfeld J., Güttinger S., Hawryluk-Gara L.A., Panté N., Mall M., Galy V., Haselmann U., Mühlhäusser P., Wozniak R.W., Mattaj I.W., et al. The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol. Cell. 2006;22:93–103. doi: 10.1016/j.molcel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 514.Glavy J.S., Krutchinsky A.N., Cristea I.M., Berke I.C., Boehmer T., Blobel G., Chait B.T. Cell-cycle-dependent phosphorylation of the nuclear pore Nup107-160 subcomplex. Proc. Natl. Acad. Sci. USA. 2007;104:3811–3816. doi: 10.1073/pnas.0700058104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Laurell E., Beck K., Krupina K., Theerthagiri G., Bodenmiller B., Horvath P., Aebersold R., Antonin W., Kutay U. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell. 2011;144:539–550. doi: 10.1016/j.cell.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 516.Linder M.I., Köhler M., Boersema P., Weberruss M., Wandke C., Marino J., Ashiono C., Picotti P., Antonin W., Kutay U. Mitotic Disassembly of Nuclear Pore Complexes Involves CDK1- and PLK1-MediatedPhosphorylation of Key Interconnecting Nucleoporins. Dev. Cell. 2017;43:141–156. doi: 10.1016/j.devcel.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.de Castro I.J., Gil R.S., Ligammari L., Di Giacinto M.L., Vagnarelli P. CDK1 and PLK1 coordinate the disassembly and reassembly of the nuclear envelope in vertebrate mitosis. Oncotarget. 2017;9:7763–7773. doi: 10.18632/oncotarget.23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Martino L., Morchoisne-Bolhy S., Cheerambathur D.K., Van Hove L., Dumont J., Joly N., Desai A., Doye V., Pintard L. Channel Nucleoporins Recruit PLK-1 to Nuclear Pore Complexes to Direct Nuclear Envelope Breakdown in C. elegans. Dev. Cell. 2017;43:157–171. doi: 10.1016/j.devcel.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Nichols R.J., Wiebe M.S., Traktman P. The vaccinia-related kinases phosphorylate the N’ terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol. Biol. Cell. 2006;17:2451–2464. doi: 10.1091/mbc.e05-12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Margalit A., Brachner A., Gotzmann J., Foisner R., Gruenbaum Y. Barrier-to-autointegration factor—A BAF fling little protein. Trends Cell Biol. 2007;17:202–208. doi: 10.1016/j.tcb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 521.Gorjánácz M., Klerkx E.P., Galy V., Santarella R., López-Iglesias C., Askjaer P., Mattaj I.W. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in post-mitotic nuclear envelope assembly. EMBO J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Asencio C., Davidson I.F., Santarella-Mellwig R., Ly-Hartig T.B., Mall M., Wallenfang M.R., Mattaj I.W., Gorjánácz M. Coordination of kinase and phosphatase activities by Lem4 enables nuclear envelope reassembly during mitosis. Cell. 2012;150:122–135. doi: 10.1016/j.cell.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 523.Mehsen H., Boudreau V., Garrido D., Bourouh M., Larouche M., Maddox P.S., Swan A., Archambault V. PP2A-B55 promotes nuclear envelope reformation after mitosis in Drosophila. J. Cell Biol. 2018;217:4106–4123. doi: 10.1083/jcb.201804018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Thompson L.J., Bollen M., Fields A.P. Identification of protein phosphatase 1 as a mitotic lamin phosphatase. J. Biol. Chem. 1997;272:29693–29697. doi: 10.1074/jbc.272.47.29693. [DOI] [PubMed] [Google Scholar]
- 525.Steen R.L., Martins S.B., Tasken K., Collas P. Recruitment of protein phosphatase 1 to the nuclear envelope by A-kinase anchoring protein AKAP149 is a prerequisite for nuclear lamina assembly. J. Cell Biol. 2000;150:1251–1262. doi: 10.1083/jcb.150.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Steen R.L., Beullens M., Landsverk H.B., Bollen M., Collas P. AKAP149 is a novel PP1 specifier required to maintain nuclear envelope integrity in G1 phase. J. Cell Sci. 2003;116:2237–2246. doi: 10.1242/jcs.00432. [DOI] [PubMed] [Google Scholar]
- 527.Güttinger S., Laurell E., Kutay U. Orchestrating nuclear envelope disassembly and reassembly during mitosis. Nat. Rev. Mol. Cell Biol. 2009;10:178–191. doi: 10.1038/nrm2641. [DOI] [PubMed] [Google Scholar]
- 528.Vagnarelli P., Ribeiro S., Sennels L., Sanchez-Pulido L., de Lima Alves F., Verheyen T., Kelly D.A., Ponting C.P., Rappsilber J., Earnshaw W.C. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev. Cell. 2011;21:328–342. doi: 10.1016/j.devcel.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Ungricht R., Kutay U. Mechanisms and functions of nuclear envelope remodelling. Nat. Rev. Mol. Cell Biol. 2017;18:229–245. doi: 10.1038/nrm.2016.153. [DOI] [PubMed] [Google Scholar]
- 530.Zhang C., Hutchins J.R., Mühlhäusser P., Kutay U., Clarke P.R. Role of importin-beta in the control of nuclear envelope assembly by Ran. Curr. Biol. 2002;12:498–502. doi: 10.1016/S0960-9822(02)00714-5. [DOI] [PubMed] [Google Scholar]
- 531.Harel A., Chan R.C., Lachish-Zalait A., Zimmerman E., Elbaum M., Forbes D.J. Importin beta negatively regulates nuclear membrane fusion and nuclear pore complex assembly. Mol. Biol. Cell. 2003;14:4387–4396. doi: 10.1091/mbc.e03-05-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Walther T.C., Askjaer P., Gentzel M., Habermann A., Griffiths G., Wilm M., Mattaj I.W., Hetzer M. RanGTP mediates nuclear pore complex assembly. Nature. 2003;424:689–694. doi: 10.1038/nature01898. [DOI] [PubMed] [Google Scholar]
- 533.Clarke P.R., Zhang C. Spatial and temporal coordination of mitosis by Ran GTPase. Nat. Rev. Mol. Cell Biol. 2008;9:464–477. doi: 10.1038/nrm2410. [DOI] [PubMed] [Google Scholar]
- 534.Rotem A., Gruber R., Shorer H., Shaulov L., Klein E., Harel A. Importin beta regulates the seeding of chromatin with initiation sites for nuclear pore assembly. Mol. Biol. Cell. 2009;20:4031–4042. doi: 10.1091/mbc.e09-02-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 535.Hoelz A., Debler E.W., Blobel G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011;80:613–643. doi: 10.1146/annurev-biochem-060109-151030. [DOI] [PubMed] [Google Scholar]
- 536.Fernandez A.G., Piano F. MEL-28 is downstream of the Ran cycle and is required for nuclear-envelope function and chromatin maintenance. Curr. Biol. 2006;16:1757–1763. doi: 10.1016/j.cub.2006.07.071. [DOI] [PubMed] [Google Scholar]
- 537.Galy V., Askjaer P., Franz C., López-Iglesias C., Mattaj I.W. MEL-28, a novel nuclear-envelope and kinetochore protein essential for zygotic nuclear-envelopeassembly in C. elegans. Curr. Biol. 2006;16:1748–1756. doi: 10.1016/j.cub.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 538.Rasala B.A., Orjalo A.V., Shen Z., Briggs S., Forbes D.J. ELYS is a dual nucleoporin/kinetochore protein required for nuclear pore assembly and proper cell division. Proc. Natl. Acad. Sci. USA. 2006;103:17801–17806. doi: 10.1073/pnas.0608484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Franz C., Walczak R., Yavuz S., Santarella R., Gentzel M., Askjaer P., Galy V., Hetzer M., Mattaj I.W., Antonin W. MEL-28/ELYS is required for the recruitment of nucleoporins to chromatin and postmitotic nuclear pore complex assembly. EMBO Rep. 2007;8:165–172. doi: 10.1038/sj.embor.7400889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Gillespie P.J., Khoudoli G.A., Stewart G., Swedlow J.R., Blow J.J. ELYS/MEL-28 chromatin association coordinates nuclear pore complex assembly and replication licensing. Curr. Biol. 2007;17:1657–1662. doi: 10.1016/j.cub.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Hattersley N., Cheerambathur D., Moyle M., Stefanutti M., Richardson A., Lee K.Y., Dumont J., Oegema K., Desai A. A Nucleoporin Docks Protein Phosphatase 1 to Direct Meiotic Chromosome Segregation and Nuclear Assembly. Dev. Cell. 2016;38:463–477. doi: 10.1016/j.devcel.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 542.Antonin W., Neumann H. Chromosome condensation and decondensation during mitosis. Curr. Opin. Cell. Biol. 2016;40:15–22. doi: 10.1016/j.ceb.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 543.Trinkle-Mulcahy L., Andersen J., Lam Y.W., Moorhead G., Mann M., Lamond A.I. Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability. J. Cell. Biol. 2006;172:679–692. doi: 10.1083/jcb.200508154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Vagnarelli P., Hudson D.F., Ribeiro S.A., Trinkle-Mulcahy L., Spence J.M., Lai F., Farr C.J., Lamond A.I., Earnshaw W.C. Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat. Cell. Biol. 2006;8:1133–1142. doi: 10.1038/ncb1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Qian J., Beullens M., Lesage B., Bollen M. Aurora B defines its own chromosomal targeting by opposing the recruitment of the phosphatase scaffold Repo-Man. Curr. Biol. 2013;23:1136–1143. doi: 10.1016/j.cub.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 546.Qian J., Lesage B., Beullens M., Van Eynde A., Bollen M. PP1/Repo-man dephosphorylates mitotic histone H3 at T3 and regulates chromosomal aurora B targeting. Curr. Biol. 2011;21:766–773. doi: 10.1016/j.cub.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 547.Qian J., Beullens M., Huang J., De Munter S., Lesage B., Bollen M. Cdk1 orders mitotic events through coordination of a chromosome-associated phosphatase switch. Nat. Commun. 2015;6:10215. doi: 10.1038/ncomms10215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Fischle W., Tseng B.S., Dormann H.L., Ueberheide B.M., Garcia B.A., Shabanowitz J., Hunt D.F., Funabiki H., Allis C.D. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature. 2005;438:1116–1122. doi: 10.1038/nature04219. [DOI] [PubMed] [Google Scholar]
- 549.Booth D.G., Takagi M., Sanchez-Pulido L., Petfalski E., Vargiu G., Samejima K., Imamoto N., Ponting C.P., Tollervey D., Earnshaw W.C., et al. Ki-67 is a PP1-interacting protein that organises the mitotic chromosome periphery. Elife. 2014;3:e01641. doi: 10.7554/eLife.01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Kumar G.S., Gokhan E., De Munter S., Bollen M., Vagnarelli P., Peti W., Page R. The Ki-67 and RepoMan mitotic phosphatases assemble via an identical, yet novel mechanism. Elife. 2016;5:e16539. doi: 10.7554/eLife.16539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Landsverk H.B., Mora-Bermúdez F., Landsverk O.J., Hasvold G., Naderi S., Bakke O., Ellenberg J., Collas P., Syljuåsen R.G., Küntziger T. The protein phosphatase 1 regulator PNUTS is a new component of the DNA damage response. EMBO Rep. 2010;11:868–875. doi: 10.1038/embor.2010.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 552.Williams B.C., Filter J.J., Blake-Hodek K.A., Wadzinski B.E., Fuda N.J., Shalloway D., Goldberg M.L. Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. Elife. 2014;3:e01695. doi: 10.7554/eLife.01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Heim A., Konietzny A., Mayer T.U. Protein phosphatase 1 is essential for Greatwall inactivation at mitotic exit. EMBO Rep. 2015;16:1501–1510. doi: 10.15252/embr.201540876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Ma S., Vigneron S., Robert P., Strub J.M., Cianferani S., Castro A., Lorca T. Greatwall dephosphorylation and inactivation upon mitotic exit is triggered by PP1. J. Cell Sci. 2016;129:1329–1339. doi: 10.1242/jcs.178855. [DOI] [PubMed] [Google Scholar]
- 555.Rogers S., Fey D., McCloy R.A., Parker B.L., Mitchell N.J., Payne R.J., Daly R.J., James D.E., Caldon C.E., Watkins D.N., et al. PP1 initiates the dephosphorylation of MASTL, triggering mitotic exit and bistability in human cells. J. Cell Sci. 2016;129:1340–1354. doi: 10.1242/jcs.179754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Ren D., Fisher L.A., Zhao J., Wang L., Williams B.C., Goldberg M.L., Peng A. Cell cycle-dependent regulation of Greatwall kinase by protein phosphatase 1 and regulatory subunit 3B. J. Biol. Chem. 2017;292:10026–10034. doi: 10.1074/jbc.M117.778233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Dohadwala M., e Silva E.D.C., Hall F.L., Williams R.T., Carbonaro-Hall D.A., Nairn A.C., Greengard P., Berndt N. Phosphorylation and inactivation of protein phosphatase 1 by cyclin-dependent kinases. Proc. Natl. Acad. Sci. USA. 1994;91:6408–6412. doi: 10.1073/pnas.91.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Kwon Y.G., Lee S.Y., Choi Y., Greengard P., Nairn A.C. Cell cycle-dependent phosphorylation of mammalian protein phosphatase 1 by cdc2 kinase. Proc. Natl. Acad. Sci. USA. 1997;94:2168–2173. doi: 10.1073/pnas.94.6.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Grallert A., Boke E., Hagting A., Hodgson B., Connolly Y., Griffiths J.R., Smith D.L., Pines J., Hagan I.M. A PP1–PP2A phosphatase relay controls mitotic progression. Nature. 2015;517:94–98. doi: 10.1038/nature14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Wu J.Q., Guo J.Y., Tang W., Yang C.S., Freel C.D., Chen C., Nairn A.C., Kornbluth S. PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 2009;11:644–651. doi: 10.1038/ncb1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.Olsen J.V., Vermeulen M., Santamaria A., Kumar C., Miller M.L., Jensen L.J., Gnad F., Cox J., Jensen T.S., Nigg E.A., et al. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci. Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 562.Nasa I., Rusin S.F., Kettenbach A.N., Moorhead G.B. Aurora B opposes PP1 function in mitosis by phosphorylating the conserved PP1-binding RVxF motif in PP1 regulatory proteins. Sci. Signal. 2018;11:eaai8669. doi: 10.1126/scisignal.aai8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Hengeveld R.C., Vromans M.J., Vleugel M., Hadders M.A., Lens S.M. Inner centromere localization of the CPC maintains centromere cohesion and allows mitotic checkpoint silencing. Nat. Commun. 2017;8:15542. doi: 10.1038/ncomms15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564.Porter I.M., Schleicher K., Porter M., Swedlow J.R. Bod1 regulates protein phosphatase 2A at mitotic kinetochores. Nat. Commun. 2013;4:2677. doi: 10.1038/ncomms3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 565.Schleicher K., Porter M., ten Have S., Sundaramoorthy R., Porter I.M., Swedlow J.R. The Ndc80 complex targets Bod1 to human mitotic kinetochores. Open Biol. 2017;7:170099. doi: 10.1098/rsob.170099. [DOI] [PMC free article] [PubMed] [Google Scholar]