Abstract
Cyanobacteria (blue-green algae) are photosynthetic bacteria that under favorable environmental conditions produce secondary metabolites (cyanotoxins) which are harmful to the environment, including humans. The mass proliferation of harmful cyanobacteria is termed CyanoHABs. CyanoHABs can adapt to different climatic fluctuations, therefore, understanding their dynamics in freshwater systems is crucial. Variation in climatic and hydrological processes, changing land use and economic growth all influence the occurrence and distribution of CyanoHABs. There have been inadequate CyanoHAB studies at local scales, therefore their occurrence and dynamics cannot be generalized. This study reviews and synthesizes cases of CysnoHAB occurrence, magnitude, and timing and how these are linked with climatic and hydrological variations in the United Republic of Tanzania. In this study, a scoping review approach was adopted. Research articles, reports, and databases were consulted. The most common species of toxin-producing cyanobacteria were identified in different water bodies in Tanzania, as well as the record of mass fatality of birds (Lesser Flamingo) in Lake Manyara, which in almost all cases occurred during dry years. While previous studies on CyanoHAB dynamics and their links to climate, hydrological, and environmental changes have not been undertaken in Tanzania, there are studies in Lake Victoria and Tanganyika. Therefore, there should be an immediate response from water users, managers, researchers, and water authorities to address and actively engage in monitoring and managing the risks associated with CyanoHABs in Tanzania.
Keywords: Environmental science, Hydrology, Atmospheric science
1. Introduction
Cyanobacteria (blue-green algae) are oxygenic photosynthetic bacteria that occur naturally in fresh, brackish, and marine waters, and terrestrial environments (Codd et al., 2005b). Apart from their function as primary producers (Moorehead et al., 2011), cyanobacteria are now a worldwide problem (Pham and Utsumi, 2018) because they can form massive blooms that produce a wide range of toxins (Codd, 2000; Codd et al., 2005a,b; Zamyadi et al., 2012 and Suzane, 2016). Over many years cyanobacteria were not regarded as water-borne pathogens (Codd et al., 2005a,b), but recently the US Environmental Protection Agency (EPA, 2014) listed cyanobacteria on the Contaminant Candidate List (CCL), meaning that is an issue of public health concern. According to the World Health Organization (2003), 60% (worldwide) of samples from freshwater systems investigated had a mass occurrences of toxin-producing cyanobacteria.
The multiple environmental and health impacts of cyanobacteria have attracted researchers from different fields (Sanseverino et al., 2016). One example is the development of models (Guven and Howard, 2006) studying occurrence, extent, and timing, while others have demonstrated the dynamics of cyanobacteria toxins in aquatic systems (Rodgers, 2008). Ho and Michalak (2015) for example, outlined several metrics for examining cyanobacterial proliferation. They include abundance, remotely-sensed/spectral metrics, species-specific, toxicity, and qualitative metrics.
Harmful cyanobacteria, or CyanoHABs, are now a problem of global environmental concern and efforts are being taken to prevent, predict, minimizes, and suppress their occurrences (Gatz, 2018). Triggers for CyanoHABs have been studied (Kubiak et al., 2016; Paerl and Otten, 2016; Reichwaldt and Ghadouani, 2012; Schmidt et al., 2014). These studies have demonstrated a scientific understanding of the dynamics of cyanobacteria. Despite all these efforts, geographical variation in CyanoHABs is not well explored, including the links between triggers and CyanoHAB proliferation. In the tropics, Tanzania is an example of insufficient CyanoHAB. Of the few studies consulted, the particular reviews suggest the dominance of both Microcystis and Cylindrospermopsis species in the tropics, and their occurrence is throughout the year, unlike temperate regions in which their occurrences are confined to the warm summer months. In nearby Lake Victoria, blooms of cyanobacteria have been observed since 1980 which are associated with massive fish kills (Ndlela et al., 2016). Some reviews recommend preventive measures to be taken to combat cyanobacteria occurrences in major lakes (Ndlela et al., 2016).
Climate and hydrological variations are considered as important factors promoting the occurrence and dominance of CyanoHABs in the aquatic environment (Ogashawara et al., 2014; Reichwaldt and Ghadouani, 2012; Havens et al., 2016). Other studies (e.g. Ndlela et al., 2016; Wells et al., 2015) suggests that little is known regarding climate variability and changes in cyanobacteria dynamics in Africa. In addition there can be a conflict between the observation and experimental data. On the other hand, of the few studies, genetic characterization of cyanobacteria isolated from Africa and Europe demonstrated the variation of cyanobacteria from different geographical regions (Haande et al., 2008; Harke et al., 2016). A study of Sinoven (2009) suggested that generalization of CyanoHAB dynamics should be avoided because local climate and weather strongly impact contribution to an occurrence, as well as their extent. Sometimes cyanobacteria can withstand or adapt to changes or climatic fluctuations (El-Shehawy et al., 2012). Studies (e.g. Lugomela et al., 2006; Nonga et al., 2011; Mdegela et al., 2011; Fyumagwa et al., 2013; and Kihwele et al., 2014) in the United Republic of Tanzania have demonstrated the occurrence of toxin producing-cyanobacteria in specific regions. However, these studies are not sufficient to conclusively judge CyanoHABs dynamics and their link with climatic and hydrological variation, because most of them are event-driven, for example, post-mortem of Flamingo mortality in Lake Manyara. In this particular study, our goal was to review and synthesize cases of CyanoHABs (occurrence, extent, and timing) and how they have been linked with climate, hydrological variations and or environmental (nutrients, land use etc.) changes in the United Republic of Tanzania. It will also to provides the status as it stands to water managers, researchers, and policymakers to elucidate and plan the best management practices in water resources.
2. Main text
2.1. Study area description
The United Republic of Tanzania lies within 1–12 °S and 29–40 °E. Tanzania is blessed with a range of natural resources, for example, 6.4% of the country's area is water bodies (Lake Victoria in the North, Tanganyika to the west and Nyasa (Lake Malawi) to the south-west) and to the east lies the Indian Ocean (Basalirwa et al., 1999). A large population depends on agriculture, including livestock and fisheries (Drakenberg et al., 2016). Regarding the climate, there is a temporal variation in both temperature and rainfall, and the trend is consistent (New et al., 2006). Tanzania's climate varies from tropical (along with the coast) to temperate (in the highlands) and there are two rainfall distribution types (unimodal and bimodal) (FAO, 2016). A well-detailed climate classification over Tanzania can be depicted from the previous climate studies (Kottek et al., 2006; and Peel et al., 2007). Several weather systems are responsible for the observed climatic variation, including thunderstorms, Intertropical Convergence Zone (ITCZ) as it moves south and north and, tropical cyclones (which pool moisture from Congo forest), Sea Surface Temperature (SST) which enhance easterly to northeasterly winds resulting into moisture influx over land (Mbululo and Nyihirani, 2012; Kijazi and Reason, 2009; and Mafuru and Guirong, 2018).
In Tanzania , no reports of fish kills or harm to domestic animals, including humans are directly linked to specific cyanotoxins. Guidelines and standards for algal toxin are yet to be established (Miraji et al., 2016). Therefore, in view of the above one can hypothesize that, if business, as usual, continues under a current climate change (warming, increase in carbon dioxide) CyanoHABs are likely to compromise water dependencies and in turn negatively affect our adaptation strategies.
In the current review, we considered all published articles and reports on HABs incidences in Tanzania and how they have been associated with climate and hydrological variations. A wide range of limnological, environmental and scientific databases was also consulted for elucidating the theoretical aspects of HABs in the environment. There was no restriction on time of publications and or languages. Google search engine, webs, and journal databases were tools used in the current review.
2.2. Factors influencing the proliferation of harmful algal blooms
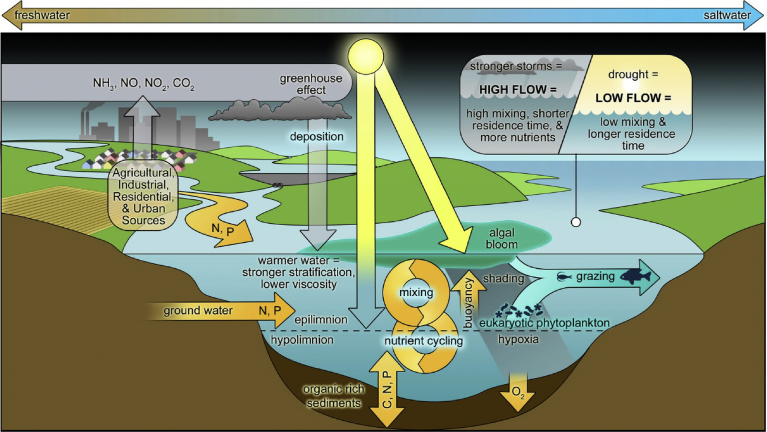
There is no single factor that can describe the formation of cyanobacteria bloom (Te and Gin, 2011) but rather a complex set of interactions (Fig. 1) of several environmental factors (Wells et al., 2015) and optimal conditions should be met (Berger and Gobler, 2008; Descy and Sarmento, 2008). Factors such as light intensity and duration, temperature, nutrient availability, episodic hydrological events (droughts and floods events), bio-physiological and chemical characteristics and ecosystem structure are responsible for bloom formation (Moore et al., 2008; Paul, 2008; Reichwaldt and Ghadouani, 2012; Merel et al., 2013; Hazen and Sawyer, 2015; Ndlela et al., 2016). Few have studied the impact or the mechanism that co-exist between cyanobacteria and carbon dioxide (CO2) (Visser et al., 2016). Most studies suggest the use of chlorophyll-a as a proxy measure of algal blooms (C. Anderson et al., 2015). Other studies (Elliott, 2012; Reichwaldt and Ghadouani, 2012; and Moore et al., 2008) have demonstrated the influences of major weather shifts on freshwater HABs dynamics. For example, a positive/negative correlation that exists between rainfall intensity and length of dry periods which in turn determine the occurrence of cyanobacteria blooms (Paerl et al., 2016). These studies establish how individual weather phenomena directly or indirectly influence CyanoHAB proliferation. In the same line of argument, Anderson (2014) suggest case studies especially events that mimic future climate scenarios if studied can be more informative than doing single parameters.
Fig. 1.
Schematic diagram of interactive physical, chemical and biological controls on harmful algal bloom formation and proliferation along the freshwater-to-marine continuum. Adapted with permission from “Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum”, by Paerl et al. (2018). Environmental Sciences & Technology, 52, 5519–5529. https://doi.org/10.1021/acs.est.7b05950. Copyright (2018) American Chemical Society.
Some studies have also looked into single or few factors and their influences on HABs. The factors are not standing alone because sometimes they may interact; therefore multifactor analysis should be done to critically highlight at what state (say mixing and pH) favours the growth or at what level (temperature, flushing, and nutrient inputs) CyanoHABs decrease. Others for example (Shen et al., 2012), concluded a link between environmental factors and how they influence HABs dynamics is neither well documented nor explained. Although there have been efforts in the understanding of HABs such as a recent scientific consensus (Heisler et al., 2008) highlight some doubts which need to be cleared.
Many studies have used methanol for the extraction of cyanotoxins (Codd, 2000; Fyumagwa et al., 2013; Kim et al., 2009; Mbukwa et al., 2012; Merel et al., 2013; Metcalf and Codd, 2014). The approach was challenged by a recent study (Visser et al., 2016) which question the validity of the previous findings. This is still debatable.
2.3. Findings on cyanobacteria (HABs) in Tanzania
In Tanzania, there have been reports on species of cyanobacteria and their toxins (Harke et al., 2016) in different water bodies but few studied harmful algal blooms and their link with climate and hydrology. Metcalf et al. (2012) reported species of Oscillatoria amphibian and Oscillatoria formosa (0.125 ng mg-1 of dry weight) on their study on the analysis of microcystins and microcystins genes in 60–170 years herbarium specimen of cyanobacteria which were collected in an aquatic and terrestrial environment in Zanzibar during 1988. As noted earlier, available literature asserts the occurrence of toxic strains of cyanobacteria and their impacts in some parts of the United Republic of Tanzania. To date, the only documented reports/incidences in Tanzania is the mass fatality of Lesser Flamingos in saline lakes in Arusha and Manyara Region (Fyumagwa et al., 2013; Kihwele et al., 2014; Kotut et al., 2006; Lugomela et al., 2006; Nonga et al., 2011).
Following the mass fatalities of Flamingos in three soda lakes (Embakaai crater, Lake Natron and Lake Manyara in Arusha Region) the year 2000, 2002, and 2004, samples of both water and tissue were collected and analyzed for cyanotoxins. Same as a post-mortem study of Fyumagwa et al. (2013), which revealed the impact was due to cyanobacterial toxins (Anatoxin-a and Microcystins). In these studies, no detailed analysis of the environmental factors responsible for the mortality rate was conducted. In this particular study , the findings (for example pH) were more abstract (values not reported). The author (Fyumagwa et al., 2013) made a deduced that varying pH was due to prolonged drought out of no data, but it was good to speculate that anthropogenic activities could have triggered proliferation of harmful blooms. This implies that if there was regular monitoring of all environmental parameters in the lake, it would have facilitated tracking the influences of individual causal effects on HABs.
Some others studies (Kihwele et al., 2014; Krienitz et al., 2016; Lugomela et al., 2006) in Lake Manyara found species of Cylindrospermum (701 cells/ml) and Microcystis (6043 cells/ml). These studies suggest repeated and unpredictable episodes of lesser flamingo fatalities which are attributed to the exposure to cyanotoxins and that the toxification may be spread to other East African Lakes. Increased water abreaction, tourism developments, and degradation of the catchment were found to be negatively contributing to the ecohydrological health of Lake Manyara (Kihwele et al., 2014; Krienitz et al., 2016; Lugomela et al., 2006). According to Kihwele et al. (2014), the change was constant with seasonal variability (more pronounced in the recent decade) although the study could not demonstrate the magnitude of change and climate variability.
Several studies (Ndlela et al., 2016; Sinha et al., 2012; Paerl and Paul, 2012) reported species of Microcystis, Anabaena, Cylindrospermopsis raciborskii and Plantolyngbya (Sekadende et al., 2005; Miles et al., 2013) in Lake Victoria. Another study, for example, Rumisha and Nehemia (2013) on feeding selectivity of wild and cultured Oreochromis niloticus (Nile tilapia) registered high concentration of Microcystis, Anabaena flos-aquae, and Lingbya circumcreta species in Lake water than in fish ponds. Changes in water quality in Lake Victoria is attributed to anthropogenic activities (Silsbe et al., 2006). Other parts of the country, for example in Morogoro, Microcystis species were identified in ponds on the study of the effects of ponds management and prevalence of intestinal parasites (Mdegela et al., 2011). Another recent study by Mushi (2015) which compared pristine, urban and agricultural fields also found species of cyanobacteria, including toxin-producing specie of Cylindrospermopsis.
In Lake Babati in the central part of Tanzania, Aphanizomenon species, strains of Chrysopsorum ovalisporum were identified, and in Lake Rukwa in the Southeastern highlands , strains of Sphaerospemopsis aphanizomenides and Sphaerospermopsis renifomis were identified (Cire, 2016). In all these studies there was no direct link established between physiochemical parameters and bloom occurrences. Along similar lines, species of Anabaena flos-aqua were reported to occur annually from October to November in Lake Tanganyika (Codd et al., 2005a). Communicating variations in the Lake Tanganyika, a study of Paul (2008) reported that cyanobacteria biomass dominated the lake over other phytoplankton during the period of March to April 2001 than how it was in 1975. The study suggests that in Lake Tanganyika climate changes (warming) has been the driving factor which was also observed by Paerl (2014). On a global scale. Paerl (2014) and (Paerl et al., 2016) stated that climate change and hydrological modification may require modifying management strategies for controlling cyanoHABs.
2.4. General and predicted climate changes in Tanzania
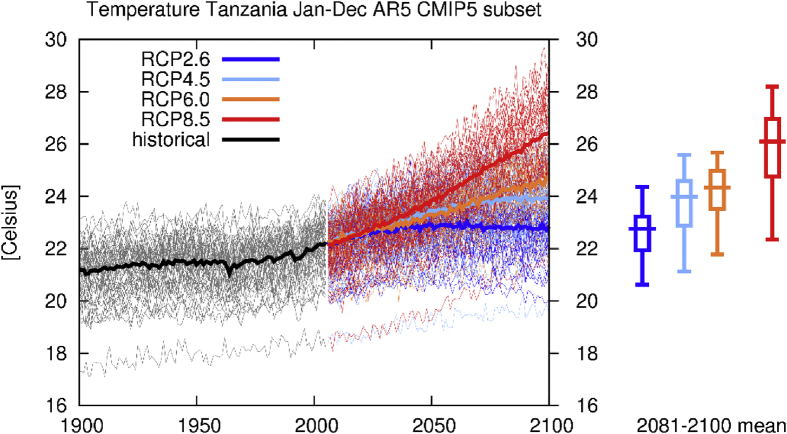
Temperature is the most studied climate parameter when addressing CyanoHAB dynamics. In Tanzania, evidence and projection of an increase in temperature can be deduced from models (as in Fig. 2) and empirical studies (New et al., 2006; World Wide Fund For Nature, 2006). To further highlight this, in Tanzania there are climate change hotspots (Niang et al., 2014) which are Lake Tanganyika (directly linked with cyanobacteria dynamics and decrease in fish productivity) and Mountain Kilimanjaro (a good indicator of global warming). Changes in climate (for example warming) has been attributed to the occurrence of blooms (Paul, 2008). While studies on climate variability and cyanobacteria dynamics are limited (Ndlela et al., 2016), existing ones (for example in Lake Tanganyika) indicate chlorophyll-a concentrations are constant throughout a year with the alteration of dry and wet seasons (De Senerpont Domis et al., 2013). Chlorophyll-a is used as a proxy for cyanobacterial biomass estimation in marine and freshwaters (Dörnhöfer and Oppelt, 2016). A study by Niang et al. (2014), which was based on more than 90 years of observation suggests that increased stratification, nutrient fluxes and decreased productivity was due to recent increases in surface temperatures. The effects have negatively affected the catches of sardines by 30–50% (Drakenberg et al., 2016). Predicted changes in climate (World Wide Fund For Nature, 2006) in the region require measures such as finding out the influence of both temperature and rainfall has to nutrients increases in the water bodies (Paerl et al., 2016; and Paerl et al., 2018).
Fig. 2.
Projected changes in air temperatures (°C) for different climate scenarios in Tanzania (Data generated from “KNMI Climate Explorer,”).
2.5. Specific observations in selected areas
2.5.1. Manyara, Arusha
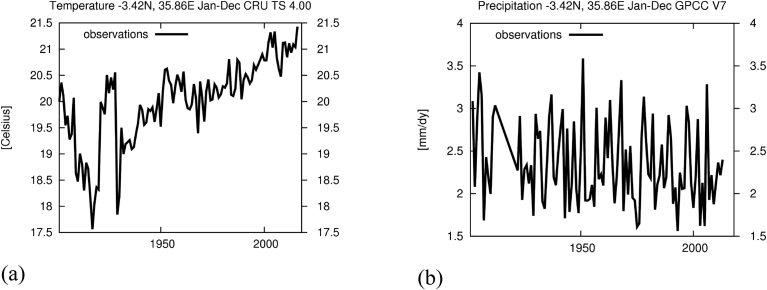
Plankton dynamics are system- (weather and hydrological) specific, for example, temporal variability in precipitation can be an important driver of the seasonal development of plankton (De Senerpont Domis et al., 2013). Indirectly, increases in precipitation and warming are likely to impact water reservoirs stratification (Harke et al., 2016). The National Adaptation Programme (The United Republic of Tanzania, 2007) specifically for Lake Manyara emphasizes an increase in frequencies of floods, drought and land degradation which reduces the frequency of recreational and tourism activities. The report further argues that it is the hydrological conditions in Lake Manyara that influence birds' (Flamingo) breeding patterns. Among the studied climate parameters in relation to algal blooms is temperature (Wells et al., 2015). Generally, Manyara for example, temperature indicates an increasing trend (Fig. 3a) which were also demonstrated by New et al. (2006) and insignificant variations in precipitation (Fig. 3b), which is a key driving factor of CyanoHABs.
Fig. 3.
(a). An increasing air temperature trend (observed) (from Climate Research Unit TS 4.00) and (b) Observed Precipitation (mm/day) from Global Precipitation and Climate Centre version 7 (GPPCC V7) for Manyara (Data generated from “KNMI Climate Explorer,”).
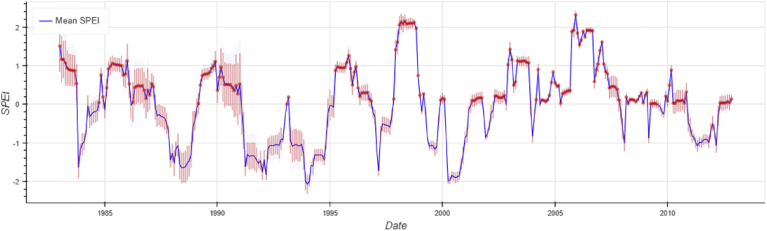
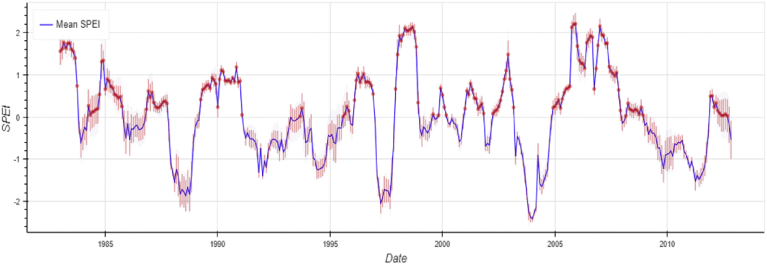
Standardized Precipitation and Evapotranspiration Index (SPEI) (Fig. 4) obtained from Water Cycle Integrator (WCI) portal (available at http://wci.earth2observe.eu/) generally indicate drought conditions (negative values of SPEI) for most of the years (for example 1994, 2000, 2004, 2008). Some post-mortem studies in the same years (Lugomela et al., 2006; Nonga et al., 2011; Kihwele et al., 2014) may confirm whether Lesser Flamingos were exposed to cyanotoxins. The hypothesis needs to be tested whether weather and hydrological changes contributed significantly in the changing of the concentration of CyanoHABs in the Lake Manyara which could have resulted in the deaths of birds. Case study analysis is an appropriate approach to investigate these events.
Fig. 4.
Standardized Precipitation and Evapotranspiration Index (SPEI) for Manyara (Generated from “eartH2Observe Water Cycle Integrator,” available on http://wci.earth2observe.eu/).
2.6. Morogoro
2.6.1. Climatic and hydrological variation
It is widely accepted that local climate and weather variation, climate change, eutrophication, and hydrological variations are key drivers of CyanoHABs (Paul, 2008). ITCZ and El Ńino Southern Oscillation (ENSO) cause greater than average rainfall in Tanzania in the short rainfall season (October to December) while the cold phase (LA Ńina) causes a dry condition than the average and these can work synergistically (GLOWSFIU, 2014b and Kijazi and Reason, 2009). A study by Paavola (2008) on drought in Morogoro region enumerated all the drought years which indicates that Morogoro is a drought-prone area. Mean climatic conditions, for example, temperatures (New et al., 2006) of Morogoro agrees with a threshold of more than 20–35 °C air temperature (Elliott, 2012 and Sinha et al., 2012) which is the optimal temperature for CyanoHAB formations. However, a study by Baig et al. (2017) demonstrated a threshold of 24–28 °C (water temperature), where the highest peak of cyanobacteria was observed. Moreover, the same study noted a decline of cyanobacteria blooms and chlorophyll-a when temperatures were 30 °C. Another factor in Morogoro, drought (Paavola, 2008) can be depicted (negative values of standardized precipitation index (SPEI)) from Fig. 5, has the characteristics to prolong periods of surface temperatures, stabilizing water columns and increasing nutrient concentration indirectly (Reichwaldt and Ghadouani, 2012).
Fig. 5.
Standardized Precipitation and Evapotranspiration Index (SPEI) in Morogoro (Data point generated from “eartH2Observe Water Cycle Integrator,” available on http://wci.earth2observe.eu/.
2.7. Hydrological variations
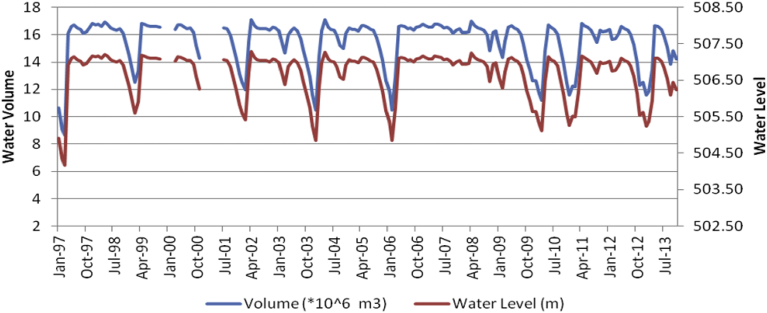
In Mindu Dam a Man-made Dam situated in the Ngerengere River Catchment, Morogoro Urban, there has been periodic maintenance of floodgates and other infrastructure to ensure that gates do not get jammed in the event of heavy rainfall and river flows (GLOWSFIU, 2014b). The water level peaks (which is an indicator of hydrological variations) in Fig. 6 depicts some dry and wet years in Morogoro, which indicate there could be severe drought and wet years which was also reported by Paavola (2008). The dry and wet peaks in the (SPEI) in Fig. 5 also indicates negative values for dry and positive values for wet years which corroborates with water levels peaks variations in Mindu Dam (Fig. 6). These variations are useful in describing the formation of algal blooms as highlighted in the framework of Reichwaldt and Ghadouani, 2012 and Zhang et al. (2016). Lack of monitoring both climate, hydrological and other environmental variables make it impossible to study and ultimately manage CyanoHAB variations in this study area.
Fig. 6.
Variations of water levels (m) and volume (m3) in Mindu Dam for a period of 1997–2013 (data from WAMI/RUVU, 2013)
It is widely accepted that evapotranspiration can assist forecasting the hydrology and climate systems hence ecological dynamics (Yang et al., 2016). Land use characteristics (residential, agriculture, industrial, forest and undeveloped area) can also be a factor in determining the severity of bloom formation (Shayo et al., 2017). These hydrological variations in the Morogoro Urban suggest optimal conditions for the proliferation of CyanoHABs. A study of Yanda and Munishi (2007) on hydrological and land use/cover change analysis for the Ruvu River (Uluguru) and Sigi River (East Usambara) watersheds both in Morogoro which looked at the historical flow data confirmed sedimentation in the catchment due to land use. The same study found a match between the flows and rainfall variations, but also predominance declining flows in both the dry and wet seasons. These seasonal variations in flow are a very important predictor for the formation of cyanobacteria in the catchment.
2.8. Water quality studies in Morogoro Urban areas
Morogoro Urban is prone to pollution due to anthropogenic activities (Sigala et al., 2017). Numerous studies (Ngoye and Machiwa, 2004; Ngonyani and Nkotagu, 2007; Mero, 2011; GLOWSFIU, 2014a) suggest significant levels of water pollution when compared with other studies (Merel et al., 2013; Hazen and Sawyer, 2015; Glibert, 2017) they are optimal conditions for the algal bloom proliferation. Overall, the findings challenge water authorities on how HABs are perceived in the study area. This is also heightened by the facts that HABs have the ability to adapt to a changing environment (D. Anderson, 2012). A recent study by Mushi (2015) found the occurrence of Cyanobacteria species in Morogoro (pristine, agriculture and urban). According to Mushi (2015) there was no significant variation of the microbial community (Cyanobacteria inclusive) during the entire periods of the study. The claim leaves many questions, for example, predicting blooms in the study area. One would argue that the author's claim was based on limited data (short time period) and, therefore, this needs to be re-examined and evaluated.
2.9. Social-economic impacts of HABs
Several studies (Sanseverino et al., 2017; Suzane, 2016) have indicated how CyanoHABs are associated with a wide range of economic impacts on human health, fishery, tourism, and recreational use, and monitoring and management costs. Cyanobacteria exert toxic effects on human, animals, fish, birds, and other phytoplankton that are associated with (WHO, 1999; Chen et al., 2016). Human health, for example, several studies (e.g. EPA, 2014; Backer, 2002; Elliott, 2012; Chen et al., 2016; Wolf and Klaiber, 2017) have indicated acute effects such as neurotoxicity, hyper-toxicity, diarrhoea and amnesia, abdominal pain, headache, altered pulse, respiratory failure, cardiac arrests, and even death. The principle behind is that cyanobacteria release toxins as their defense mechanism, when grazed, disturbed, and or in competition with phytoplankton (Reichwaldt and Ghadouani, 2012). This is still debatable.
Tanzania has favorable environmental conditions (ecological, hydro-geological, and physical), and social economic activities (agriculture, industries, etc.) that support occurrence, and proliferation of CyanoHABs. A good example is the availability of several small to large standing and non-standing surface freshwater bodies (FAO, 2016). On the other hand, research activities and awareness of harmful algal blooms are lagging (Ndlela et al., 2016). Because of its heavy use of nutrients in food supplies, aquaculture can also trigger HABs proliferation. According to the Ministry of Livestock and Fisheries Development (The United Republic of Tanzania, 2013), Tanzania aquaculture fish farmers increased from 3347 to 17511 in between the year 2000 and 2013 with a corresponding increase in ponds from 4000 to 19930 and landed fish production from 200 tons to 2989.5 tons (The United Republic of Tanzania, 2013).
Several researchers (Bushaw-Newton and Sellner, 2012; Grover, 2006; Merel et al., 2013; Rastogi et al., 2015; Sanseverino et al., 2016) have directly linked diarrhea among symptoms of exposure to cyanotoxins. This is important because diarrhea is among the leading waterborne diseases in Tanzania (IHME, n.d.). The current study does not intend to confirm that diarrhea in the study area is caused by cyanotoxins, but it is a hypothesis which needs to be tested. In summary, social-economic activities in Tanzania can be one among the route of exposure to CyanoHABs.
2.10. Management of algal blooms
A review by Ndlela et al. (2016) focused on Africa noted that regarding research on CyanoHABs, little has been done. However, efforts have been made to at least formulate a global network to discuss and deliberate on matters related to CyanoHABs and their toxin risk management (Codd et al., 2005a). Further, to mitigate CyanoHABs, some solutions (technical and policies) are in place (in a global perspective); for example, land and water management, water treatment and blooms control (Berger and Gobler, 2008; Hazen and Sawyer, 2015; Shayo et al., 2011). Monitoring of CyanoHABs, treatment of algal toxins in drinking, recreational, fish farms, and irrigation waters can significantly minimize health impacts associated with their formation and dominance. Algal bloom management should be proactive because HABs are capable to adapt to a variety of climatic and other environmental conditions (Wolf and Klaiber, 2017) that promote their ability to outcompete other phytoplankton (O'Neil et al., 2012). According to Anderson (2012), case studies (e.g. heavy rainfall or drought) that mimic climate change scenarios should be used to understand HABs responses. Suzane (2016) also suggest the use of case studies to quantify the social economic impact of HABs.
Managing CyanoHABs in the context of climate and hydrological variations is challenging (Paerl et al., 2016). Future studies should focus on how climate and hydrological variations affect nutrients dynamics. If nutrient reduction can significantly reduce HABs events (Paerl et al., 2018) then understanding the linkage between climate, hydrological variation, and other environmental factors are essential. There is a global and regional initiative for studying and management of HABs, for example, a global network for cyanobacteria blooms and toxin risk management (CYANONET) in which the United Republic of Tanzania is a member (Codd et al., 2005a). The project is intended for awareness, prevention and mitigating algal blooms. However, Tanzania, like many other countries policies, regulation, and guidelines on HABs management are yet to established (Miraji et al., 2016). Since there have been reports of HABs species and cyanotoxins in the study area, the opportunity for nation-wide monitoring, prevention, prediction, minimizing and suppression of HABs should be undertaken.
3. Conclusions
CyanoHABs species are widely distributed in the study area and anecdotal observations suggest that they vary with seasons (dry and wet). No sufficient limnological data exist to perform time series analysis for getting a clear understanding of CyanoHABs dynamics in the region. In Tanzania the fields of CyanoHAB ecology, monitoring and management are in their nascent stage. There are however documentable cases that link seasonal variations of CyanoHABs, especially in Lakes Victoria and Tanganyika. Most of the studies are event-driven, for example, post-mortem studies although climate, hydrological variations (especially drought years and temperatures), and environmental conditions support their occurrence. Therefore, research and or academic institutions working on CyanoHABs in the country should be assessed and developed/empowered. Likewise, regular monitoring and documenting, and prediction of CyanoHABs dynamics in the regions should be considered. Future studies should focus on awareness and occurrence of CyanoHABs, epidemiological studies, toxicity occurrence and levels in the food web and timing. Due to the distribution of water bodies in varying localities (Dams, Rivers, Lakes, Ponds, and Ocean) in Tanzania, it would be wise to conduct a spatiotemporal survey of CyanoHABs and their link with environmental stressors. It is also encouraged to revisit the current management options, for example, water treatment plants for assessing CyanoHABs control efficiencies. Adapting to the risks associated with CyanoHABs occurrences, case studies that mimic future climate change scenarios (e.g. drought and heavy rains) will be more informative. Technological improvement such as the application of remote sensing for monitoring HABs should also be utilized. Policy and guidelines for dealing with algal blooms are yet to be formulated that also consider effects of climate, hydrological, and environmental (e.g. nutrients, and metals) conditions on HABs proliferations.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the University of Venda, Limpopo, South Africa, grant number SES/17/ERM/14.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We acknowledge the support of data support from KNMI, and WCI, Wami Ruvu Basin Authority. Thanks to Professor Hans W. Paerl, Kenan Professor of Marine and Environmental Sciences, the University of North Carolina at Chapel Hill (UNC-CH), Institute of Marine Sciences and the three anonymous reviewers for their comment and suggestions which improved the quality of this paper.
References
- Anderson C., Moore S., Tomlinson M., Silke J., Cusack C. Chapter 17 – living with harmful algal blooms in a changing world: strategies for modeling and mitigating their effects in coastal marine ecosystems. Coast. Mar. Hazards Risks Disasters. 2015 [Google Scholar]
- Anderson D. HABs in a changing world: a perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climatic and environmental change. Harmful Algae. 2012;2014(2012):3–17. http://www.ncbi.nlm.nih.gov/pubmed/26640829 Retrieved from. [PMC free article] [PubMed] [Google Scholar]
- Backer L.C. Cyanobacterial harmful algal blooms (CyanoHABs): developing a public health response. Lake Reservoir Manag. 2002;18(1):20–31. [Google Scholar]
- Baig S.A., Huang L., Sheng T., Lv X., Yang Z., Qasim M. Impact of climate factors on cyanobacterial dynamics and their interactions with water quality in South Taihu Lake, China. Chem. Ecol. 2017;33(1):76–87. [Google Scholar]
- Basalirwa C.P.K., Odiyo J.O., Mngodo R.J., Mpeta E.J. The climatological regions of Tanzania based on the rainfall characteristics. Int. J. Climatol. 1999;19(1):69–80. [Google Scholar]
- Berger P., Gobler C. Vol. 2008. 2008. (Cyanobacterial Harmful Algal Blooms : Chapter 9 : Causes, Prevention, and Mitigation Workgroup Report). [Google Scholar]
- Bushaw-Newton K.L., Sellner K.G. Detrimental effects of harmful algal blooms. Harmful Algae. 2012;20:71–80. [Google Scholar]
- Chen L., Chen J., Zhang X., Xie P. A review of reproductive toxicity of microcystins. J. Hazard Mater. 2016;301:381–399. doi: 10.1016/j.jhazmat.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Cire S. A review of the phylogeny, ecology and toxin production of bloom-forming Aphanizomenon spp. and related species within the Nostocales (cyanobacteria) 2016;54:21–43. doi: 10.1016/j.hal.2015.09.007. [DOI] [PubMed] [Google Scholar]
- Codd G.A. Cyanobacterial toxins, the perception of water quality, and the prioritization of eutrophication control. Ecol. Eng. 2000;16(1):51–60. [Google Scholar]
- Codd G.A., Azevedo S.M.F.O., Bagchi S.N., Burch M.D., Carmichael W.W., Harding W.R. HP-VI Technical Document in Hydrology N°76 UNESCO Working Series SC-2005/WS/55. Paris. 2005. CYANONET a global network for cyanobacterial bloom and toxin risk management. [Google Scholar]
- Codd G.A., Louise M.F., Metcalf J.S. Cyanobacterial toxins: risk management for health protection. Toxicol. Appl. Pharmacol. 2005;203(3 SPEC. ISS.):264–272. doi: 10.1016/j.taap.2004.02.016. [DOI] [PubMed] [Google Scholar]
- De Senerpont Domis L.N., Elser J.J., Gsell A.S., Huszar V.L.M., Ibelings B.W., Jeppesen E. Plankton dynamics under different climatic conditions in space and time. Freshw. Biol. 2013;58(3):463–482. [Google Scholar]
- Descy J.-P., Sarmento H. Microorganisms of the East African great lakes and their response to environmental changes. Freshw. Rev. 2008;1(1):59–73. [Google Scholar]
- Dörnhöfer K., Oppelt N. Remote sensing for lake research and monitoring – recent advances. Ecol. Indicat. 2016;64:105–122. [Google Scholar]
- Drakenberg O., Ek G., Fernqvist K.W. 2016. Environmental and Climate Change Policy Brief.http://sidaenvironmenthelpdesk.se/wordpress3/wp-content/uploads/2013/04/Environmental-and-Climate-Change-Policy-Brief-Tanzania-160530.pdf Retrieved from. [Google Scholar]
- eartH2Observe Water Cycle Integrator. (n.d.). Retrieved March 1, 2018, from https://wci.earth2observe.eu/.
- El-Shehawy R., Gorokhova E., Fernández-Piñas F., del Campo F.F. Global warming and hepatotoxin production by cyanobacteria: what can we learn from experiments? Water Res. 2012;46(5):1420–1429. doi: 10.1016/j.watres.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Elliott J.A. Is the future blue-green? A review of the current model predictions of how climate change could affect pelagic freshwater cyanobacteria. Water Res. 2012;46(5):1364–1371. doi: 10.1016/j.watres.2011.12.018. [DOI] [PubMed] [Google Scholar]
- EPA . 2014. Cyanobacteria and Cyanotoxins: Information for Drinking Water Systems. Fact Sheet. EPA-810F11001. [Google Scholar]
- FAO . 2016. AQUASTAT - FAO’s Information System on Water and Agriculture.http://www.fao.org/nr/water/aquastat/countries_regions/TZA/index.stm Retrieved October 9, 2018, from. [Google Scholar]
- Fyumagwa R.D., Bugwesa Z., Mwita M., Kihwele E.S., Nyaki A., Mdegela H., Mpanduji D.G. Cyanobacterial toxins and bacterial infections are the possible causes of mass mortality of lesser flamingos in Soda lakes in northern Tanzania. Res. Opin. Anim. Vet. Sci. ROAVS. 2013;3(3):80–85. [Google Scholar]
- Gatz L. 2018. Freshwater Harmful Algal Blooms: Causes, Challenges, and Policy Considerations.https://fas.org/sgp/crs/misc/R44871.pdf Retrieved from. [Google Scholar]
- Glibert P.M. Eutrophication, harmful algae and biodiversity — challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017;124(2):591–606. doi: 10.1016/j.marpolbul.2017.04.027. [DOI] [PubMed] [Google Scholar]
- GLOWSFIU . 2014. Climate, Forest Cover, and Water Resources Vulnerability Wami/Ruvu Basin. Tanzania. North Miami, Florida 33181, USA. [Google Scholar]
- GLOWSFIU . 2014. Water Quality Survey, Ruvu River Basin, Tanzania.www.globalwaters.net North Miami, Florida 33181, USA. Retrieved from. [Google Scholar]
- Grover V.I. 2006. Water: Global Common and Global Problems.http://books.google.com/books?hl=en&lr=&id=yFJgabX6P6UC&pgis=1 Retrieved from. [Google Scholar]
- Guven B., Howard A. A review and classification of the existing models of cyanobacteria. Prog. Phys. Geogr. 2006;30(1):1–24. [Google Scholar]
- Haande S., Rohrlack T., Ballot A., Røberg K., Skulberg R., Beck M., Wiedner C. Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, cyanobacteria) isolates from Africa and Europe. Harmful Algae. 2008;7(5):692–701. [Google Scholar]
- Harke M.J., Steffen M.M., Gobler C.J., Otten T.G., Wilhelm S.W., Wood S.A., Paerl H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae. 2016;54:4–20. doi: 10.1016/j.hal.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Havens K.E., Fulton R.S., Beaver J.R., Samples E.E., Colele J. Effects of climate variability on cladoceran zooplankton and cyanobacteria in a shallow subtropical lake. J. Plankton Res. 2016;38(3):418–430. [Google Scholar]
- Hazen, Sawyer . 2015. Harmful Algal Blooms.hazenandsawyer.com Retrieved from. [Google Scholar]
- Heisler J., Glibert P.M., Burkholder J.M., Anderson D.M., Cochlan W., Dennison W.C. Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae. 2008;8(1):3–13. doi: 10.1016/j.hal.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.C., Michalak A.M. Challenges in tracking harmful algal blooms: a synthesis of evidence from Lake Erie. J. Gt. Lakes Res. 2015;41(2):317–325. [Google Scholar]
- IHME. Tanzania | Institute for Health Metrics and Evaluation Measuring what Matters. (n.d.). Retrieved November 8, 2018, from http://www.healthdata.org/tanzania.
- Kihwele E.S., Lugomela C., Howell K.M. Temporal changes in the lesser flamingos population ( Phoenicopterus minor ) in relation to phytoplankton abundance in Lake Manyara, Tanzania. Open journal of ecology Tanzania. Open J. Ecol. 2014;4:145–161. [Google Scholar]
- Kijazi A., Reason C. Analysis of the 2006 floods over northern Tanzania. Int. J. Climatol. 2009;29(December 2007):955–970. [Google Scholar]
- Kim I.S., Huong Nguyen G., Kim S., Lee J., Yu H.-W. Evaluation of methods for cyanobacterial cell lysis and toxin (Microcystin-LR) extraction using chromatographic and mass spectrometric analyses. Eng. Res. 2009;14(4):250–254. [Google Scholar]
- KNMI Climate Explorer. (n.d.). Retrieved March 1, 2018, from http://climexp.knmi.nl/start.cgi.
- Kottek M., Grieser J., Beck C., Rudolf Bruno, Rubel F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006;15(3):259–263. [Google Scholar]
- Kotut K., Ballot A., Krienitz L. Toxic cyanobacteria and their toxins in standing waters of Kenya: implication for water resource use. J. Water Health. 2006;4(SUPPL. 2):3–18. [PubMed] [Google Scholar]
- Krienitz L., Krienitz D., Dadheech P.K., Hubener T., Kotut K., Luo W. Food algae for lesser flamingos: a stocktaking. Hydrobiologia. 2016;775(1):21–50. [Google Scholar]
- Kubiak K., Mazur A., Kotlarz J. Monitoring cyanobacteria blooms in freshwater lakes using remote sensing methods. Pol. J. Environ. Stud. 2016;25(1):27–35. [Google Scholar]
- Lugomela C., Pratap H.B., Mgaya Y.D. Cyanobacteria blooms-A possible cause of mass mortality of lesser flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae. 2006;5(5):534–541. [Google Scholar]
- Mafuru K.B., Guirong T. 2018. Assessing Prone Areas to Heavy Rainfall and the Impaction of the Upper Warm Temperature Anomaly during March – May Rainfall Season in Tanzania, 2018(May 2015) [Google Scholar]
- Mbukwa E.A., Msagati T.A.M., Mamba B.B. Quantitative variations of intracellular microcystin-LR, -RR and -YR in samples collected from four locations in Hartbeespoort Dam in North West Province (South Africa) during the 2010/2011 summer season. Int. J. Environ. Res. Public Health. 2012;9(10):3484–3505. doi: 10.3390/ijerph9103484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbululo Y., Nyihirani F. Climate characteristics over southern highlands Tanzania. Atmos. Clim. Sci. 2012;02(04):454–463. [Google Scholar]
- Mdegela R.H., Omary A.N., Mathew C., Nonga H.E. Effect of pond management on prevalence of intestinal parasites in nile tilapia (oreochromis niloticus) under small scale fish farming systems in Morogoro, Tanzania. Livest. Res. Rural Dev. 2011 http://lrrd.cipav.org.co/lrrd23/6/mdeg23127.htm Retrieved from. [Google Scholar]
- Merel S., Walker D., Chicana R., Snyder S., Baurès E., Thomas O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013;59(59):303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Mero R. University of Zimbabwe; 2011. Assessment of Water Quality and Spartial Distribution of the Major Pollutants in the Ngerengere River Catchment, Tanzania.http://www.waternetonline.org/download//data/download/00000076/Rose-thesis-27-7-11.pdf Retrieved from. [Google Scholar]
- Metcalf J.S., Beattie K.A., Purdie E.L., Bryant J.A., Irvine L.M., Codd G.A. Analysis of microcystins and microcystin genes in 60 – 170-year-old dried herbarium specimens of cyanobacteria. Harmful Algae. 2012;15:47–52. [Google Scholar]
- Metcalf J.S., Codd G.A. Cyanobacterial toxins (cyanotoxins) in water: a review of current knowledge. Found. Water Res. 2014;44(February 2004):47. [Google Scholar]
- Miles C.O., Sandvik M., Nonga H.E., Rundberget T., Wilkins A.L., Rise F. Identification of microcystins in a Lake Victoria cyanobacterial bloom using LC – MS with thiol derivatization. Toxicon. 2013;70:21–31. doi: 10.1016/j.toxicon.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Miraji H., Othman O., Ngassapa N., Mureithi W. vol. 2016. Scientifica Hindawi Publishing Corporation; 2016. pp. 1–6. (). Research trends in emerging contaminants on the aquatic environments of Tanzania). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S.K., Trainer V.L., Mantua N.J., Parker M.S., Laws E.A., Backer L.C. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health. 2008;12:1–9. doi: 10.1186/1476-069X-7-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorehead K., Capelli B., Cysewski G.R. Cyanotech Corporation; Kailua-Kona, Hawaii: 2011. Nature ’ S Superfood. [Google Scholar]
- Mushi D. Technical University Braunschweig; TUB: 2015. Bacterial Community Structure and Composition of Tropical River and Drinking Water – Insights from Deep Sequencing and Correlation to Environmental Drivers.http://digisrv-1.biblio.etc.tu-bs.de:8080/docportal/servlets/MCRFileNodeServlet/DocPortal_derivate_00041788/Diss_Mushi_Douglas.pdf;jsessionid=857820AB4190FA0BD41D242D84EA2E9F Retrieved from. [Google Scholar]
- Ndlela L.L., Oberholster P.J., Van Wyk J.H., Cheng P.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae. 2016;60:11–26. doi: 10.1016/j.hal.2016.10.001. [DOI] [PubMed] [Google Scholar]
- New M., Hewitson B., Stephenson D.B., Tsiga A., Kruger A., Manhique A. Evidence of trends in daily climate extremes over southern and west Africa. J. Geophys. Res. Atmos. 2006;111(14) [Google Scholar]
- Ngonyani C.J., Nkotagu H.H. Study of nutrient pollutants and their impacts on the water quality of the Mindu reservoir at Morogoro municipality.pdf. Tanzania J. Eng. Technol. 2007;1(3):138–148. [Google Scholar]
- Ngoye E., Machiwa J.F. The influence of land-use patterns in the Ruvu river watershed on water quality in the river system. Phys. Chem. Earth. 2004;29(15–18 SPEC.ISS.):1161–1166. [Google Scholar]
- Niang I., Ruppel O.C., Abdrabo M.A., Essel A., Lennard C., Padgham J., Urquhart P. Cambridge University Press; Cambridge, United Kingdom and New York, NY, USA: 2014. Africa. in: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects, Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change.https://ipcc-wg2.gov/AR5/images/uploads/WGIIAR5-Chap22_FINAL.pdf Retrieved from. [Google Scholar]
- Nonga H.E., Sandvik M., Miles C.O., Lie E., Mdegela R.H., Mwamengele G.L. Possible involvement of microcystins in the unexplained mass mortalities of lesser flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia. 2011;678(1):167–178. [Google Scholar]
- O'Neil J.M., Davis T.W., Burford M.A., Gobler C.J. The rise of harmful cyanobacteria blooms: the potential roles of eutrophication and climate change. Harmful Algae. 2012;14:313–334. [Google Scholar]
- Ogashawara I., Zavattini J., Tundisi J. The climatic rhythm and blooms of cyanobacteria in a tropical reservoir in São Paulo, Brazil. J. Biol. 2014;74(1):72–78. doi: 10.1590/1519-6984.17412. [DOI] [PubMed] [Google Scholar]
- Paavola J. Livelihoods, vulnerability and adaptation to climate change in Morogoro, Tanzania. Environ. Sci. Pol. 2008;11(7):642–654. [Google Scholar]
- Paerl H.W. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life. 2014;4:988–1012. doi: 10.3390/life4040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl H.W., Gardner W.S., Havens K.E., Joyner A.R., McCarthy M.J., Newell S.E. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae. 2016;54:213–222. doi: 10.1016/j.hal.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Paerl H.W., Otten T.G. Duelling ‘CyanoHABs’: unravelling the environmental drivers controlling dominance and succession among diazotrophic and non-Nitrogen -fixing harmful cyanobacteria. Environ. Microbiol. 2016;18(2):316–324. doi: 10.1111/1462-2920.13035. [DOI] [PubMed] [Google Scholar]
- Paerl H.W., Otten T.G., Kudela R. Mitigating the expansion of harmful algal blooms across the freshwater-to-marine Continuum. Environ. Sci. Technol. 2018;52:5519–5529. doi: 10.1021/acs.est.7b05950. [DOI] [PubMed] [Google Scholar]
- Paerl H.W., Paul V.J. Climate change: links to global expansion of harmful cyanobacteria. Water Res. 2012;46(5):1349–1363. doi: 10.1016/j.watres.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Paul V.J. Global warming and cyanobacterial harmful algal blooms. Adv. Exp. Med. Biol. 2008;619:239–257. doi: 10.1007/978-0-387-75865-7_11. [DOI] [PubMed] [Google Scholar]
- Peel M.C., Finlayson B.L., Mcmahon T.A. Updated world map of the K oppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007;11:1633–1644. www.hydrol-earth-syst-sci.net/11/1633/2007/ Retrieved from. [Google Scholar]
- Pham T.-L., Utsumi M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018;213:520–529. doi: 10.1016/j.jenvman.2018.01.077. [DOI] [PubMed] [Google Scholar]
- Rastogi R., Madamwar D., Incharoensakdi A. Bloom dynamics of cyanobacteria and their toxins: environmental health impacts and mitigation strategies. Front. Microbiol. 2015;1254 doi: 10.3389/fmicb.2015.01254. 6(November) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichwaldt E.S., Ghadouani A. Effects of rainfall patterns on toxic cyanobacterial blooms in a changing climate: between simplistic scenarios and complex dynamics. Water Res. 2012;46(5):1372–1393. doi: 10.1016/j.watres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- Rodgers J.H. 2008. Algal Toxins in Pond Aquaculture.http://www2.ca.uky.edu/wkrec/AlgalToxinsPond.pdf (No. SRAC Pub No 4605) Retrieved from. [Google Scholar]
- Rumisha C., Nehemia A. Feeding selectivity of wild and pond-cultured nile tilapia (Oreochromis niloticus) in the lake Victoria basin in Mara, Tanzania. Afr. J. Aquat. Sci. 2013;38(sup1):55–60. [Google Scholar]
- Sanseverino I., António D.C., Loos R., Lettieri T. 2017. Cyanotoxins: Methods and Approaches for Their Analysis and Detection.http://publications.jrc.ec.europa.eu/repository/bitstream/JRC106478/kjna28624enn.pdf Retrieved from. [Google Scholar]
- Sanseverino I., Conduto D., Pozzoli L., Dobricic S., Lettieri T. 2016. Algal Bloom and its Economic Impact. Italy. [Google Scholar]
- Schmidt J.R., Wilhelm S.W., Boyer G.L. The fate of microcystins in the environment and challenges for monitoring. Toxins. 2014;6(12):3354–3387. doi: 10.3390/toxins6123354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekadende B.C., Lyimo T.J., Kurmayer R. Microcystin production by cyanobacteria in the Mwanza Gulf (Lake Victoria, Tanzania) Hydrobiologia. 2005;543(1):299–304. [Google Scholar]
- Shayo S.D., Lugomela C., Machiwa J.F. Influence of land use patterns on some limnological characteristics in the south-eastern part of Lake Victoria, Tanzania. Taylors & Fransis. 2011;14(3):246–251. [Google Scholar]
- Shayo S.D., Lugomela C., Machiwa J.F. Influence of land use patterns on some limnological characteristics in the south-eastern part of Lake Victoria, Tanzania Influence of land use patterns on some limnological characteristics in the south-eastern part of Lake Victoria, Tanzania. Aquat. Ecosys. Health Manag. 2017;14(3):246–251. [Google Scholar]
- Shen L., Xu H., Guo X. Satellite remote sensing of harmful algal blooms (HABs) and a potential synthesized framework. Sensors. 2012;12(6):7778–7803. doi: 10.3390/s120607778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigala S., Mabiki F., Styrishave B., Mdegela R. Pollution by endocrine disrupting estrogens in aquatic ecosystems in Morogoro urban and peri-urban areas in Tanzania. Afr. J. Environ. Sci. Technol. 2017;11(2):122–131. [Google Scholar]
- Silsbe G.M., Hecky R.E., Guildford S.J., Mugidde R. Variability of chlorophyll a and photosynthetic parameters in a nutrient-saturated tropical great lake. Limnol. Oceanogr. 2006;51(5):2052–2063. https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/lo.2006.51.5.2052 Retrieved from. [Google Scholar]
- Sinha R., Pearson L.A., Davis T.W., Burford M.A., Orr P.T., Neilan B.A. Increased incidence of Cylindrospermopsis raciborskii in temperate zones - is climate change responsible? Water Res. 2012;46(5):1408–1419. doi: 10.1016/j.watres.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Sinoven K. Encyclopedia of Microbialogy. Elsevier; 2009. Cyanobacterial toxins; pp. 290–307. [Google Scholar]
- Suzane M. Algal blooms. In: Suzane M., editor. Biological and Environmental Hazards, Risks, and Disasters. Elsevier Inc; 2016. [Google Scholar]
- Te S.H., Gin K.Y.H. The dynamics of cyanobacteria and microcystin production in a tropical reservoir of Singapore. Harmful Algae. 2011;10(3):319–329. [Google Scholar]
- The United Republic of Tanzania . Division of Environment; Dar es Salaam: 2007. The United Republic of Tanzania. National Adaptation Programme of Action (NAPA) [Google Scholar]
- The United Republic of Tanzania . 2013. Basic Data for Livestock and Fisheries Sectors.https://africaopendata.org/dataset/944c8373-f0b0-4c5e-ba80-ece7e8c8874e/resource/b0f1f3e4-efdb-44ff-be51-757349c838a7/download/livestock-and-fisheries-basic-data-1.pdf Dar es Salaam. Retrieved from. [Google Scholar]
- Visser P.M., Verspagen J.M.H., Sandrini G., Stal L.J., Matthijs H.C.P., Paerl H.W., Huisman J. How rising CO2 and global warming may stimulate harmful cyanobacterial blooms. Harmful Algae. 2016;54:145–159. doi: 10.1016/j.hal.2015.12.006. [DOI] [PubMed] [Google Scholar]
- WAMI/RUVU . 2013. 2012/2013 Annual Basin Hydrological Report. Morogoro. [Google Scholar]
- Wells M.L., Trainer V.L., Smayda T.J., Karlson B.S.O., Trick C.G., Kudela R.M. Harmful algal blooms and climate change: learning from the past and present to forecast the future. Harmful Algae. 2015;49:68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 1999. Algae and Cyanobacteria in Fresh Water; pp. 136–158. [Google Scholar]
- Wolf D., Klaiber H.A. Bloom and bust: toxic algae’s impact on nearby property values. Ecol. Econ. 2017;135:209–221. [Google Scholar]
- World Health Organization . Vol 1. Coastal and Fresh Waters; Geneva: 2003. http://www.who.int/water_sanitation_health/bathing/srwe2full.pdf (Guidelines for Safe Recreational Water). 1, 219. Retrieved from. [Google Scholar]
- World Wide Fund For Nature Climate change impacts on East Africa: a review of the scientific literature. East Africa Climate Impacts. 2006 [Google Scholar]
- Yanda P.Z., Munishi P.K.T. 2007. Hydrologic and Landuse/cover Change Analysis for the Ruvu River (Uluguru) and (Sigi River (East Usambara) Watersheds.http://www.easternarc.or.tz/groups/webcontent/documents/pdf/RuvuandSigihydrologicalandlandusecha.pdf Dar es Salaam. Retrieved from. [Google Scholar]
- Yang Z., Zhang Q., Hao X. Evapotranspiration trend and its relationship with precipitation over the loess plateau during the last three decades. Adv. Meteorol. 2016;2016 [Google Scholar]
- Zamyadi A., Ho L., Newcombe G., Bustamante H., Prévost M. Fate of toxic cyanobacterial cells and disinfection by-products formation after chlorination. Water Res. 2012;46(5):1524–1535. doi: 10.1016/j.watres.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shi K., Liu J., Deng J., Qin B., Zhu G., Zhou Y. Meteorological and hydrological conditions driving the formation and disappearance of black blooms, an ecological disaster phenomena of eutrophication and algal blooms. Sci. Total Environ. 2016;569–570:1517–1529. doi: 10.1016/j.scitotenv.2016.06.244. [DOI] [PubMed] [Google Scholar]