Abstract
Acetylation of the lysine residues in histones and other DNA-binding proteins plays a major role in regulation of eukaryotic gene expression. This process is controlled by histone acetyltransferases (HATs/KATs) found in multiprotein complexes that are recruited to chromatin by the scaffolding subunit transformation/transcription domain-associated protein (TRRAP). TRRAP is evolutionarily conserved and is among the top five genes intolerant to missense variation. Through an international collaboration, 17 distinct de novo or apparently de novo variants were identified in TRRAP in 24 individuals. A strong genotype-phenotype correlation was observed with two distinct clinical spectra. The first is a complex, multi-systemic syndrome associated with various malformations of the brain, heart, kidneys, and genitourinary system and characterized by a wide range of intellectual functioning; a number of affected individuals have intellectual disability (ID) and markedly impaired basic life functions. Individuals with this phenotype had missense variants clustering around the c.3127G>A p.(Ala1043Thr) variant identified in five individuals. The second spectrum manifested with autism spectrum disorder (ASD) and/or ID and epilepsy. Facial dysmorphism was seen in both groups and included upslanted palpebral fissures, epicanthus, telecanthus, a wide nasal bridge and ridge, a broad and smooth philtrum, and a thin upper lip. RNA sequencing analysis of skin fibroblasts derived from affected individuals skin fibroblasts showed significant changes in the expression of several genes implicated in neuronal function and ion transport. Thus, we describe here the clinical spectrum associated with TRRAP pathogenic missense variants, and we suggest a genotype-phenotype correlation useful for clinical evaluation of the pathogenicity of the variants.
Keywords: TRRAP, histone acetylation, de novo variants, intellectual disability, congenital malformations, autism spectrum disorder, neurodevelopmental disorders
Main Text
Post-translational modifications including acetylation, methylation, phosphorylation, and ubiquitination, of core histones directly alter DNA-histone and histone-histone interactions and thus influence nucleosome dynamics.1 Tight regulation of these marks is required by cells for proper gene transcription,2 DNA repair,3 and DNA replication. One major activator of transcription is the acetylation of histone tails, which act by neutralizing the positive charges of lysine residues or by recruiting chromatin remodelers and transcription factors.4 This tightly regulated process is performed by histone acetyltransferases (HATs) and reversed by histone deacetylases (HDACs). There are three major families of HATs: Gcn5-related N-acetyltrasnferase (GNAT), MYST (MOZ, SAS2, SAS3—also known as YBF2—and TIP60), and p300 (EP300-CREBBP).5 The activity and localization of most HATs, such as TIP60 or GCNL5, depend on a multiprotein assembly that contains the scaffolding protein transformation/transcription domain-associated protein (TRRAP).
TRRAP is a large protein of 3,859 amino acids and is conserved from yeast to humans. It is an ataxia-telangiectasia mutated (ATM) related member of the phosphatidylinositol 3-kinase-related kinase (PIKK) family.6 Like other ATM-related members, it contains FAT (FRAP, ATM, and TRRAP) and FATC (FRAP, ATM, and TRRAP, C terminus) domains flanking a PI3/PI4-kinase domain. The kinase domain of TRRAP does not engage in catalytic activity7 but is required for the proper recruitment of HAT complexes.8 TRRAP has been shown to be involved in P53-, E2F-, and c-MYC-dependent gene transcription and oncogenic transformation.6, 9, 10 As stressed in cancer studies, TRRAP plays an important role in cell-cycle regulation. A recurrent somatic TRRAP variant, c.2165C>T p.(Ser722Phe),11 has been identified in melanoma, and the oncogenic potential of TRRAP has been identified in glioblastoma multiforme,12 pancreatic adenocarcinoma,13 and lymphoma.10 Furthermore, Trrap knockout leads to early embryonic lethality in mice through errors in the cell cycle and a failure to arrest at the mitotic checkpoint.14 In mouse embryonic stem cells (ESCs), Trrap is indispensable for self-renewal as well as correct differentiation,15 suggesting an essential role in embryonic development and morphogenesis. Moreover, brain-specific Trrap knockout in mice leads to premature differentiation of neural progenitors and abnormal brain development through a decrease in the expression of cell-cycle regulators. This decreased expression results in brain atrophy and microcephaly.16 TRRAP has previously been associated with neuropsychiatric disorders such as schizophrenia in a few patients.17, 18, 19, 20 We herein provide data showing that TRRAP pathogenic variants are associated with a variable neurodevelopmental disorder.
Through an international collaboration and aided by the web-based tool GeneMatcher,21 we identified 17 distinct missense TRRAP variants with strong clinical and/or molecular evidence for pathogenicity in 24 individuals with neurodevelopmental disorders (Table 1, Figure 1A). These variants were identified either by trio or solo exome sequencing (ES) from research and clinical cohorts. All affected individuals or their guardians gave appropriate consent for research procedures. This study was approved by the CHU de Nantes ethics committee (comité consultatif sur le traitement de l’information en matière de recherche no. 14.556). Methods are described in Table S1.
Table 1.
De Novo TRRAP Variants Identified in 24 Individuals
| cDNA | Protein | Inheritance | CpG | gnomAD | CADD Phred Score (v1.3) | SIFT | PolyPhen2 HVAR | Number of Individuals |
|---|---|---|---|---|---|---|---|---|
| c.2413C>T | p.Leu805Phe | de novo | no | absent | 28.2 | deleterious (0) | probably damaging (0.998) | 1 |
| c.2580C>G | p.Phe860Leu | de novo | no | absent | 27.6 | deleterious (0.03) | possibly damaging (0.867) | 1 |
| c.2678G>T | p.Arg893Leu | apparently de novo | yes | absent | 34 | deleterious (0) | probably damaging (0.986) | 1 |
| c.3093T>G | p.Ile1031Met | de novo | no | absent | 23.4 | deleterious (0.02) | benign (0.308) | 1 |
| c.3104G>A | p.Arg1035Gln | de novo | yes | absent | 23.9 | tolerated (0.09) | benign (0.404) | 1 |
| c.3111C>A | p.Ser1037Arg | de novo | yes | absent | 23.7 | tolerated (0.14) | possibly damaging (0.656) | 1 |
| c.3127G>A | p.Ala1043Thr | de novo | yes | absent | 23.2 | tolerated (0.27) | benign (0.066) | 5 |
| c.3311A>G | p.Glu1104Gly | de novo | no | absent | 24.6 | deleterious (0.04) | probably damaging (0.91) | 1 |
| c.3316G>A | p.Glu1106Lys | de novoa | no | absent | 27.7 | deleterious (0) | possibly damaging (0.816) | 2 |
| c.3331G>T | p.Gly1111Trp | apparently de novo | yes | absent | 34 | deleterious (0) | probably damaging (0.999) | 1 |
| c.3475G>A | p.Gly1159Arg | de novo | no | absent | 33 | deleterious (0) | probably damaging (0.999) | 1 |
| c.5575C>T | p.Arg1859Cys | de novo | yes | absent | 34 | deleterious (0) | probably damaging (0.997) | 1 |
| c.5596T>A | p.Trp1866Arg | de novo | no | absent | 28.7 | deleterious (0) | probably damaging (0.999) | 1 |
| c.5598G>T | p.Trp1866Cys | de novo | no | absent | 33 | deleterious (0) | probably damaging (0.999) | 1 |
| c.5647G>A | p.Gly1883Arg | de novo | yes | absent | 33 | deleterious (0) | probably damaging (1) | 2 |
| c.5795C>T | p.Pro1932Leu | germline mosaicism | yes | absent | 35 | deleterious (0) | probably damaging (0.997) | 2 |
| c.11270G>A | p.Arg3757Gln | de novo | yes | absent | 28.6 | deleterious (0.01) | benign (0.269) | 1 |
The RefSeq transcript used for TRRAP is RefSeq: NM_001244580.1. Apparently de novo was mentioned when paternity and maternity were not checked. a. For one individual with p.(Glu1106Lys), father was unavailable, paternal grandparents were tested and did not carry the variant.
Figure 1.
Genotype-Phenotype Correlation Associated with TRRAP Variants
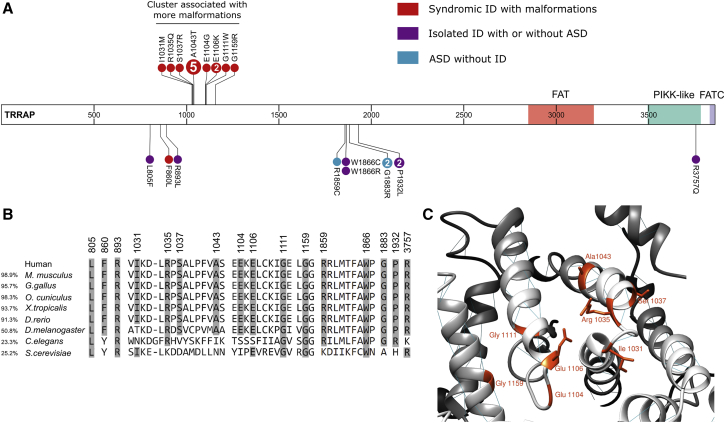
(A) Predicted de novo and apparently de novo variants in affected individuals are represented on the TRRAP protein. The variants in red represent individuals with apparent ID and malformations, the variants in purple represent individuals with isolated ID with or without ASD, and the variants in blue represent individuals with only ASD and an IQ above 70. If more than one individual was heterozygous for the variant, the number of affected individuals is indicated in the circle. Adapted from ProteinPaint.55
(B) Amino acid conservation of each mutated residue. The overall amino acid similarity with the human sequence is shown on the left.
(C) Homology model of human TRRAP (GenBank: NP_001231509.1) predicted by PHYRE2 Protein Fold Recognition Server56 represented by UCSF Chimera.57 Mutated residues in the 1031–1159 cluster are shown. Abbreviations are as follows: FAT—FRAP, ATM, and TRRAP; PIKK-like—phosphatidylinositol 3-kinase-related protein kinase-like; and FATC—FRAP, ATM, and TRRAP C-terminal.
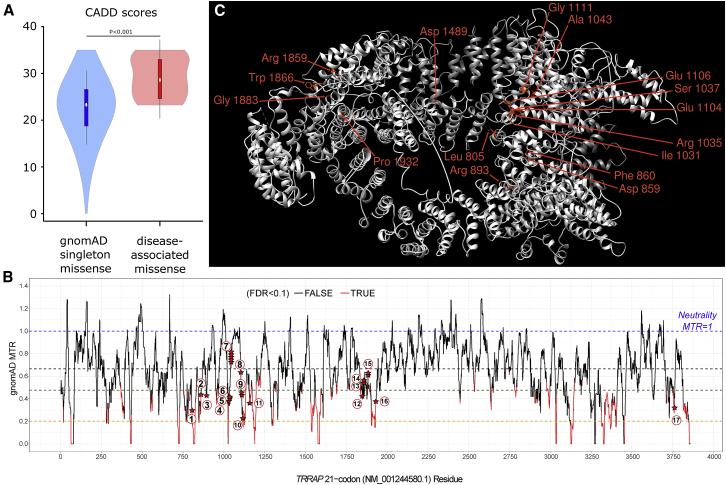
These 17 variants were absent from ExAC and gnomAD22 and were found de novo or apparently de novo (maternity and paternity not checked) in all individuals, except for two sisters who had inherited a variant from a mother with low-level mosaicism (Figure S1) and an individual whose father was unavailable but whose paternal grandparents did not carry the variant. Three variants were recurrently observed: p.Ala1043Thr was identified in five individuals, and p.Glu1106Lys and p.Gly1883Arg were each identified in two individuals. All the variants were predicted to be deleterious by CADD23 (scaled C scores were over 20), and they were variously predicted to be pathogenic by SIFT24 and PolyPhen-2 HVAR.25 As shown in Figure 2A, the 17 variants seen in the individuals we studied had significantly increased CADD scores compared to the scores for singleton missense variants reported in gnomAD.
Figure 2.
TRRAP Sequence Is Intolerant to Missense Variants
(A) CADD scores of the 17 variants identified in affected individuals are compared to scores for gnomAD singleton missense variants. In order to avoid CADD training circularity, we compared the individuals’ variants to variants seen once in gnomAD.
(B) TRRAP missense tolerance ratio (MTR) plot. The MTR is a statistic that quantifies the extent of purifying selection that has been acting specifically against missense variants in the human population. For TRRAP, we adopted the 21-codon sliding window and used exome-sequencing standing-variation data in the gnomAD database, version 2.0. MTR data were downloaded from Missense Tolerance Ratio (MTR) Gene Viewer (see Web Resources). An MTR = 1 (blue dashed line) represents neutrality (i.e., observing the same proportion of missense variants in the window as expected on the basis of the underlying sequence context). Red segments of the MTR plot have achieved exome-wide FDR<0.10 for a significance test of a window’s deviation from MTR = 1. The black dashed line signifies gene-specific median MTR, the brown dashed line signifies gene-specific 25th centile MTR, and the orange dashed line signifies gene-specific fifth centile MTR. The locations of our 23 case-ascertained de novo variants are denoted by red stars along TRRAP’s MTR plot. The 17 different variants are numbered within circles as follows: (1) p.Leu805Phe; (2) p.Phe860Leu; (3) p.Arg893Leu; (4) p.Ile1031Met; (5) p.Arg1035Gln; (6) p.Ser1037Arg; (7) p.Ala1043Thr; (8) p.Glu1104Gly; (9) p.Glu1106Lys; (10) p.Gly1111Trp; (11) p.Gly1159Arg; (12) p.Arg1859Cys; (13) p.Trp1866Arg; (14) p.Trp1866Cys; (15) p.Gly1883Arg; (16) p.Pro1932Leu; and (17) p.Arg3757Gln. We found that de novo variants were significantly enriched in the intolerant 50% of TRRAP’s protein-coding sequence; 18 (78%) of the 23 de novo events affected the most intolerant 50% of the TRRAP sequence (binomial exact test p = 0.01). Strikingly, only the most recurring de novo missense variant (GenBank: NM_001244580.1 p. Ala1043Thr) resided outside of the intolerant TRRAP sequence.
(C) Localization of the mutated TRRAP residues on 3D protein models including 14 out of 17 likely pathogenic variants and two out of six additional variants of unknown significance are shown. The representation of the structure of human TRRAP (GenBank: NP_001231509.1) was predicted by PHYRE2 Protein Fold Recognition Server by comparison to its Saccharomyces cerevisiae ortholog, according to the cryo-EM structure of the SAGA (Spt-Ada-Gcn5-acetyltransferase) and NuA4 coactivator subunit Tra1 present in the protein data bank (PDB: 5OJS). Variants in regions non-homologous to Tra1 are not represented. Structure representation was made with UCSF Chimera.
The 17 variants all occurred at residues conserved among vertebrates (Figure 1B) and in regions depleted in missense variants in gnomAD. Indeed, when we assessed missense tolerance ratios for TRRAP, we observed that most of the 17 variants were in regions intolerant to missense variants (Figure 2B). Nine out of the 17 variants occurred at highly mutable CpG sites, including one within the codon that leads to the recurrent p.Ala1043Thr variant observed in five individuals. Six missense variants with lesser evidence for pathogenicity were found in another six unrelated individuals (individuals 25 to 30 in Table S1). These variants might be deleterious but were not clearly pathogenic: perhaps the inheritance pattern could not be determined; the variant was present in gnomAD or led to another missense change at the same residue as a variant reported in gnomAD; or the variant was located in a less conserved region of TRRAP (Table S2).
Given the number of de novo variants identified, the enrichment for TRRAP de novo variants in our study was calculated as (p = 4.2 × 10−6) on the basis of denovolyzer.26 Nevertheless, the current number of 22 detected de novo variants in TRRAP is not of genome-wide significance (p = 0.08) after correction for the following: (a) ∼19,000 protein-coding genes, (b) 22,898 trios studied, and (c) the underlying mutability of the full-length protein-coding TRRAP transcript. However, this statistical calculation does not take into account the spatial distribution of the variants. Indeed, three-dimensional modeling of human TRRAP structure inferred from the orthologous Saccharomyces cerevisiae protein Tra1 (Figure 2C) suggested a clustering of the variants in different regions of TRRAP. The most important clustering was observed for 13 variants between codons 1031 and 1159. Interestingly, when visualized in 3D, these variants localized near one another (Figure 1C), revealing a domain of TRRAP with a potentially novel specific function, although this domain has not yet been characterized. We performed a statistical clustering analysis comparing the mean distance between observed variants to ten million permutations of random variants, as previously described.27 This analysis revealed a significant clustering of variants along the primary sequence of TRRAP (p value = 9 × 10−8), suggesting a model in which specific domains are affected and haploinsufficiency is unlikely, at least for clustering variants.
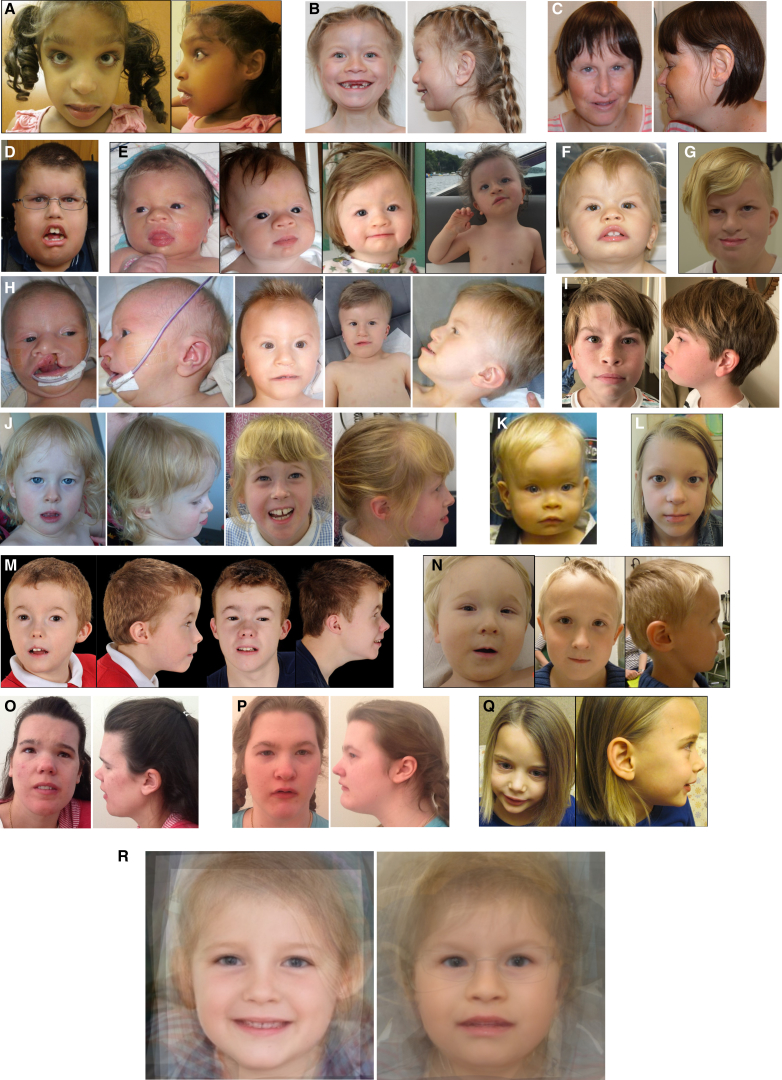
Among the 24 individuals who carried pathogenic variants, 19 presented with facial dysmorphisms. Recurrent features that were noted among these individuals included upslanted palpebral fissures, epicanthus, telecanthus, a wide nasal bridge and ridge, a broad and smooth philtrum, and a thin upper lip (Figure 3). We performed a computer-assisted facial gestalt visualization,28, 29 which highlighted several of these features, particularly for individuals with variants clustering with the recurrent p.Ala1043Thr variant (Figure 3R). All the individuals had developmental delay, although the severity of intellectual disability (ID) was highly variable. Whereas most individuals had apparent ID with markedly impaired basic life functions, some of them presented with mild ID or even no cognitive deficits (Table 2 and Table S3). Peripheral neuropathy was also noted; it was severe in one individual and consisted of lower-limb hyperreflexia in five other individuals.
Figure 3.
Photographs of Individuals with TRRAP Variants
(A) Individual 1 at the age of 8 years. Note the telecanthus, broad nasal bridge, widely spaced eyes, relatively thin upper vermilion, flared eyebrows, and ectropion.
(B) Individual 5 at the age of 8.5 years. Note the wide mouth, thin upper lip, and widely spaced eyes with a wide and depressed nasal bridge.
(C) Individual 6 at the age of 29 years. Note the sparse eyebrows, upslanting palpebral fissures, smooth philtrum, thin upper lip, and low columella.
(D) Individual 9 at the age of 11 years. Note the deeply set eyes, sparse eyebrows, and wide nasal bridge.
(E) Individual 8. Note the telecanthus, low-set ears with upturned earlobes, and, on the fourth picture from the left, the single transverse palmar crease.
(F) Individual 12 at the age of 5 years. Note the prominent forehead, arched eyebrows, short palpebral fissures, epicanthal folds, depressed nasal bridge, and thick upper vermilion.
(G) Individual 13 at the age of 14 years. Note the upslanted palpebral fissures and prominent forehead.
(H) Individual 10 at the ages of 1 month, 16 months, and 3 years. Note the cleft lip and palate, wide mouth, epicanthic fold, prognathism, and supernumerary nipples.
(I) Individual 15 at the age of 12 years. Note the wide nasal bridge and upslanting palpebral fissures.
(J) Individual 19 at the ages of 2.5 years and 8 years. Note folded-down upper eyelid and sparse medial eyebrows.
(K) Individual 16 at the age of 2 years. Note the prominent forehead, epicanthic fold, telecanthus, flat nasal bridge, and low-set ears.
(L) Individual 20 at the age of 10 years. Note the widely spaced eyes, telecanthus, wide nasal bridge and ridge, and thin upper vermilion.
(M) Individual 18. Note the narrow nose, flared eyebrows, almond-shaped eyes with hypoplastic infraorbital ridges, telecanthus, smooth philtrum, and small, low-set, and posteriorly rotated ears.
(N) Individual 21. Note the short palpebral fissures, epicanthal folds, and thin upper vermilion.
(O) Individual 22 at the age of 24 years. Note the broad nasal bridge, deeply set eyes, upslanted palpebral fissures, widely spaced eyes, and posteriorly rotated ears.
(P) Individual 23 at the age of 19 years. Note the deeply set eyes, upslanted palpebral fissures, widely spaced eyes, epicanthal folds, and posteriorly rotated ears.
(Q) Individual 24. Note the smooth philtrum and wide nasal ridge.
(R) Average facial gestalt visualization of nine healthy age- and gender-matched controls on the left; on the right, nine individuals with variants in the 1031–1159 cluster. Facial images are flipped and aligned to preserve bilateral asymmetry.
Table 2.
Clinical Description of Individuals with Variants Inside or Outside the 1031–1159 Cluster
| Symptoms | All Individuals | Cluster 1031–1159 | Variants Outside the Cluster |
|---|---|---|---|
| Global developmental delay | 24/24 (100%) | 13/13 (100%) | 11/11 (100%) |
| Intellectual disability | 17/20 (85%) | 11/11 (100%) | 6/9 (67%) |
| Facial dysmorphisms | 19/24 (79%) | 11/13 (85%) | 8/11 (73%) |
| Autism spectrum disorder | 5/24 (21%) | 0/13 (0%) | 5/11 (45%) |
| Microcephaly (<-2.5 SD) | 7/24 (29%) | 6/13 (46%) | 1/11 (9%) |
| Short stature | 7/23 (30%) | 4/12 (33%) | 3/11 (27%) |
| Hypotonia | 8/24 (33%) | 4/13 (31%) | 4/11 (36%) |
| Feeding difficulties | 8/24 (33%) | 7/13 (54%) | 1/11 (9%) |
| Seizures | 5/24 (21%) | 1/13 (8%) | 4/11 (36%) |
| Cleft lip and palate | 5/24 (21%) | 5/13 (38%) | 0/11 (0%) |
| Cerebellar hypoplasia | 6/18 (33%) | 6/11 (55%) | 0/7 (0%) |
| Cerebral abnormalities | 6/18 (33%) | 6/11 (55%) | 0/7 (0%) |
| Cardiac malformations | 10/15 (66%) | 9/12 (75%) | 1/3 (33%) |
| Renal malformations | 5/17 (29%) | 5/13 (38%) | 0/4 (0%) |
| Genital malformations | 5/24 (21%) | 5/13 (38%) | 0/11 (0%) |
| Hearing impairment | 3/24 (12%) | 3/13 (23%) | 0/11 (0%) |
| Visual impairment | 4/24 (17%) | 3/13 (23%) | 1/11 (9%) |
| Scoliosis | 3/24 (12%) | 3/13 (23%) | 0/11 (0%) |
| Dysplastic nails | 8/24 (33%) | 8/13 (62%) | 0/11 (0%) |
| Lower-limb hyperreflexia | 5/24 (21%) | 1/13 (8%) | 4/11 (36%) |
| Lacrimal-duct aplasia | 3/24 (12%) | 1/13 (8%) | 2/11 (18%) |
| Accessory nipple | 4/24 (17%) | 3/13 (23%) | 1/11 (9%) |
In addition to alteration in cerebral function, some individuals showed brain, cerebellum, heart, kidney, or urogenital malformations. We observed a strong genotype-phenotype correlation (Figure 1A, Table 2); the highest incidence of malformations was seen in 13 individuals whose variants cluster in the region of the predicted protein from codons 1031 to 1159: c.3093T>G (p.Ile1031Met), c.3104G>A (p.Arg1035Gln), c.3111C>A (p.Ser1037Arg), c.3127G>A (p.Ala1043Thr), c.3311A>G (p.Glu1104Gly), c.3316G>A (p.Glu1106Lys), c.3331G>T (p.Gly1111Trp), and c.3475G>A (p.Gly1159Arg). In contrast, individuals with variants residing outside of this region had less malformation and presented mainly with autism spectrum disorder (ASD) and/or ID, sometimes associated with epilepsy. Variants in these individuals were more dispersed along the protein, although some, including c.5575C>T (p.Arg1859Cys), c.5596T>A (p.Trp1866Arg), c.5598G>T (p.Trp1866Cys), c.5647G>A (p.Gly1883Arg), and c.5795C>T (p.Pro1932Leu), apparently aggregated in another region.
13 individuals with variants in the codon 1031–1159 region had global developmental delay and apparent ID, ranging from speech delay and learning difficulties to markedly impaired basic life functions (Table 2 and Table S3). The last available occipitofrontal-circumference measurements revealed microcephaly (ranging from −2.8 to −5 standard deviations [SDs]) in 46% (6/13) of individuals. Cerebral magnetic resonance imaging (MRI) had been performed in 10 out of 13 individuals, and seven of those 10 (70%) showed structural brain anomalies, including cerebellar vermis hypoplasia (6/10), ventricular enlargement (3/10), cortical atrophy (2/10), brainstem atrophy (2/10), polymicrogyria (1/10), focal gliosis (1/10), delayed myelination (1/10), and corpus callosum hypoplasia (1/10). Neurological examination revealed hypotonia in 31% (4/13) of individuals. Only one individual was reported to have epilepsy. Seven individuals (54%) were reported to require feeding exclusively by gastrostomy tube. Among the 10 individuals who were examined by echocardiography, 70% (7/10) had abnormal results, 50% (5/10) had ventricular septal defects, 30% (3/10) had patent ductus arteriosus, 30% (3/10) had patent foramen ovale, 20% (2/10) had pulmonary hypertension, and 20% (2/10) had aortic coarctation. Abdominal ultrasound revealed anomalies in 70% (7/10) of individuals in which it was performed. Abnormal renal morphology, namely multicystic dysplastic kidney, hydronephrosis, a duplicate kidney, and/or a small kidney, was described in 60% (6/10) of individuals, and vesicoureteral reflux was also observed in 30% (3/10) of these individuals. Individual 15 presented with a large left-sided posterolateral congenital diaphragmatic hernia (Table S3). Hernias of the abdominal wall were also found in 23% (3/13) of individuals and included an umbilical hernia, an omphalocele, and an inguinal hernia. Three males (3/6; 50%) had external-genitalia anomalies, including microphallus, hypoplastic scrotum, and cryptorchidism, and two females (2/7; 29%) had a duplicated vagina and/or uterus. Other observed anomalies included dysplastic nails (8/13; 62%), cleft lip and palate (5/13; 38%), clinodactyly of the fifth finger (4/13; 31%), laryngotracheomalacia (3/13), accessory nipple (3/13; 23%), bilateral cutaneous syndactyly of the second and third toe (2/13; 15%), and anomalies of the lacrimal glands (1/13; 8%; see also below with regard to individuals 1 and 19). Four individuals (4/13; 31%) had visual impairment, and three (3/13; 23%) had hearing impairment. Hearing impairment was associated with inner-ear malformations in two cases. Recurrent infections, mainly respiratory and urinary-tract infections, affected three out of 13 (23%) individuals. Individual 9 died at 12 years of age in the context of multiple co-morbidities, including renal failure with acute fluid fluctuations, tracheostomy for severely obstructive laryngotracheomalacia, intermittent supraventricular tachycardia, arterial insufficiency, and polyendocrinopathy (insulin-dependent diabetes, adrenal insufficiency, and hypothyroidism).
Among individuals with variants falling outside of the 1031–1159 region, 5/11 (45%) were diagnosed with ASD, and another three individuals (3/11; 27%) had some findings of ASD but no formal diagnosis. 8/11 (73%) had developmental delay and mild-to-severe ID, and three had speech delay, but their IQs were measured above 70, and two of these IQs were in the normal range. Four individuals (4/11; 36%) had various types of epilepsy, namely absence and tonic-clonic seizures, or Lennox-Gastaut syndrome. The age of seizure onset ranged from 2 to 10 years old. Malformations were infrequent in this group overall, although individual 2 had microcephaly and heart malformations, individual 1 had lacrimal duct aplasia, individual 19 had lacrimal duct aplasia and optic disc colobomas, and individual 21 had a postaxial polydactyly of one hand.
TRRAP-associated chromatin remodeling complexes are generally associated with gene activation,30 which is consistent with their HAT activity. Nevertheless, the NuA4 complex has been shown to have a gene-repression activity necessary for ESC pluripotency.31, 32 This gene-repression activity seems to be independent from its lysine acetyltransferase activity.33 To test the hypothesis that TRRAP variants alter gene expression, we obtained skin fibroblasts from two individuals, individual 1, with p.Leu805Phe, and individual 19, with p.Trp1866Cys and performed next-generation sequencing with technical replicates of RNA (i.e., separately prepared libraries from the same samples). The RNA library preparation and sequencing as well as bioinformatics analysis methods can be found in the Supplemental Data. We found that, in comparison to two typically developing individuals (controls), both individuals with TRRAP variants had remarkably different gene expression patterns (Figure S2A). Interestingly, most differentially expressed genes (DEGs) analyzed with DESeq2 were upregulated in affected individuals compared to controls (Figure S2B). Moreover, the individual with p.Leu805Phe had 619 DEGs; the Log2 fold change (Log2FC) was higher than 2 or lower than −2, and the p value was adjusted for 10% false discovery rate lower than 0.01 (padj) (Supplemental Data, Table S5).
To identify genes with significant expression differences, we performed differential gene expression analysis between the two individuals with TRRAP variants (combined as biological replicates) and two unaffected controls. Gene ontology (GO) enrichment analysis of these genes with the GOrilla web application indicates an enrichment for the adrenergic receptor signaling pathway, genes important for neurological function, and potassium and ATP-sensitive ion transporters (Figure S2B, Supplemental Data, Table S5). The two individuals who were tested carried variants outside the cluster associated with the more syndromic ID; if there are distinct effects on gene regulation, it will be worth comparing gene expression between the two groups. Finally, because it has been shown that TRRAP has direct interactions with different partners not related to the HAT complex, we cannot exclude the possibility that the transcriptome alteration might be caused by a mechanism other than impaired HAT activity. Thus, we highlighted candidate pathways that might be useful for uncovering the pathomechanism of TRRAP variants in future studies.
TRRAP acts as a scaffold in HAT complexes. Although it does not have a direct role in acetylation, we hypothesize that pathogenic effects of variants might be due to dysregulation of acetylation, a major process that has been associated with several neurodevelopmental disorders.34 Pathogenic variants of KAT6B (MIM: 605880) cause both Say-Barber-Biesecker-Young-Simpson syndrome (SBBYSS [MIM: 603736])35, 36, 37 and genitopatellar syndrome (GPS [MIM: 606170]),38, 39 and pathogenic variants in KAT6A and BRPF1 mutations have also been associated with a neurodevelopmental disorder.40, 41, 42 Rubinstein-Taybi syndrome (MIM: 180849 and 613684) is associated with variants in HAT-complex-encoding genes, namely CREBBP and EP300.43, 44, 45, 46 In addition to cognitive impairment, abnormal histone acetylation can also result in behavioral disorders, as evidenced by the associations found between non-syndromic ASD and/or schizophrenia and alterations in several lysine acetyltransferase and lysine deacetylase genes, including BRD1, HDAC4, HDAC6, and HDAC9.34, 47, 48, 49, 50
Variants in TRRAP were associated with neuropsychiatric disorders, including childhood disintegrative disorder,17 schizophrenia,18, 19 and ASD.20 The ASD report included individuals 18 and 19, who had p.Trp1866Arg and p.Trp1866Cys, respectively. We thus confirmed the association with ASD and provide evidence that it can be found either isolated or associated with ID. On the basis of the ExAC dataset alone without studies on neuropsychiatric disorders, TRRAP is in the top five human genes that are most intolerant of missense variants: it has a missense z-score of 10.1.22 Although this study includes only the first 24 identified individuals, a strength of the study is that it was primarily ascertained by sequencing, reducing phenotypic ascertainment bias. Given the highly constrained region of the observed variants coupled with the population constraint and evolutionary conservation, we hypothesize that variants outside of these regions are likely to be associated with prenatal lethality, although we cannot exclude the possibility that milder phenotypes might be underrepresented in current exome datasets. It is worth noting that we exclusively identified missense variants in the affected individuals. Given the loss-of-function (LoF) intolerance of TRRAP in ExAC (pLI = 1.00), we would expect to identify at least some LoF variants if haploinsufficiency of TRRAP was the causal mechanism. In DECIPHER (accessed May 14, 2018), no small or intragenic deletions involving TRRAP have been identified. Thus, when the significant clustering is taken into account, our results suggest that missense variants might act either as gain-of-function or dominant-negative variants and that haploinsufficiency of TRRAP is likely to be prenatally lethal, although we cannot exclude the possibility that an LoF effect of non-clustering variants is associated with a milder phenotype.
TRRAP participates in embryonic development, as demonstrated by its binding with proteins regulating the Notch signaling pathway in fruit fly51, the Ras signaling pathway in C. elegans52, or the Wnt53 signaling pathway in 293T cells.53 Therefore we suspect that TRRAP variants, more especially those falling within the 1031–1159 region, perturb the interactions with at least one of these developmental signaling pathways; such a perturbance would explain the multiple malformations observed in about half of the affected individuals.
In yeast, a series of ∼100 codon deletion mutants in the ortholog tra1 showed reduced or complete loss of viability.54 Most deletions impaired coactivator complex assembly, notably the ones encompassing the homologous 1031–1159 cluster (mutants Δ13–Δ14), as well as the regions homologous to those containing variants p.Leu805Phe, p.Phe860Leu, and p.Arg893Leu (mutants Δ11–Δ12) and the p.Arg3757Gln variant (mutant Δ39). In contrast, mutants Δ21–Δ22 encompassing the region homologous to the cluster associated with fewer malformations (codons 1859–1932) were viable, which might help explain the milder clinical phenotype associated with variants within this cluster. In mice, Trrap knockout leads to early embryonic lethality,14 and a neural-cell-specific conditional Trrap knockout line16 revealed premature differentiation of neural progenitors, depletion of progenitor pools, and a significant reduction in cortical thickness. These mice exhibited striking microcephaly, in agreement with what we observed in half of the individuals in our study cohort, primarily those with variants in the 1031–1159 cluster.
In summary, we report evidence that variants in TRRAP are associated with a pleiotropic neurodevelopmental syndrome with a potential genotype-phenotype correlation. Our functional data highlight an enrichment of genes related to neuronal function and ion transport. This enrichment could underline the pathophysiology of the disease. Future in vitro and in vivo studies on variants inside and outside the main cluster will be required if we are to determine which gene expression changes are connected to which TRRAP-related specific phenotypes.
Declaration of Interests
E.E.E. is on the scientific advisory board of DNAnexus. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue for clinical genetic testing completed at Baylor Genetics Laboratory. K.M., K.R., J.Z., M.D., A.T., A.B., and I.M.W. are employees of GeneDx, Inc. Dr. Goldstein is Founder and holds equity in Pairnomix and Praxis Therapeutics. Dr. Goldstein is not aware of any overlap with Pairnomix or Praxis Therapeutics.
Acknowledgments
We would like to thank all families for participating in this study. This work was supported in part by grants from: the French Ministry of Health and the Health Regional Agency from Poitou-Charentes (HUGODIMS, 2013, RC14_0107) to S.B.; the National Institute of Neurological Disorders and Stroke (The Epilepsy Phenome/Genome Project NS053998; Epi4K NS077364, NS077274, NS077303, and NS077276) to D.L. and D.B.G.; the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grant (HD064667) to D.A.S.; NINDS R35 NS105078 to J.R.L.; the National Human Genome Research Institute (NHGRI) and National Heart, Lung, and Blood Institute (NHLBI) to the Baylor-Hopkins Center for Mendelian Genomics (UM1 HG006542); NHGRI K08 HG008986 to J.E.P.; the Duke Genome Sequencing Clinic to V.S. and J.S.; the intramural research program of the NHGRI (grant HG200328 12) to L.G.B., J.J.J., and J.C.S.; the US National Institute of Mental Health grant R01MH101221 to E.E.E.; the Kids Brain Health Network and Dart NeuroScience to F.B.; and Mining for Miracles, British Columbia Children’s Hospital Foundation, and Genome British Columbia to the CAUSES Study. We thank the Canadian Institutes of Health Research (CIHR) and Fonds de la recherche en santé du Québec (FRSQ) for clinician-scientist awards to P.M.C.; and the Mayo Clinic Center for Individualized Medicine (CIM) for supporting this research through the CIM Investigative and Functional Genomics Program. We are grateful to the members of the Canadian Center for Computational Genomics and the McGill University and Génome Québec Innovation Center for their help in bioinformatics analysis.
Published: February 28, 2019
Footnotes
Supplemental Data can be found with this article online at https://doi.org/10.1016/j.ajhg.2019.01.010.
Contributor Information
Sébastien Küry, Email: sebastien.kury@chu-nantes.fr.
Philippe M. Campeau, Email: p.campeau@umontreal.ca.
Web Resources
DECIPHER, https://decipher.sanger.ac.uk/
Ensembl VEP, http://grch37.ensembl.org/Homo_sapiens/Tools/VEP
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
Missense Tolerance Ratio (MTR) Gene Viewer, http://mtr-viewer.mdhs.unimelb.edu.au/
OMIM, http://www.omim.org/
UniProt, http://www.uniprot.org/uniprot/
Supplemental Data
References
- 1.Bowman G.D., Poirier M.G. Post-translational modifications of histones that influence nucleosome dynamics. Chem. Rev. 2015;115:2274–2295. doi: 10.1021/cr500350x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesh S., Workman J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015;16:178–189. doi: 10.1038/nrm3941. [DOI] [PubMed] [Google Scholar]
- 3.Hunt C.R., Ramnarain D., Horikoshi N., Iyengar P., Pandita R.K., Shay J.W., Pandita T.K. Histone modifications and DNA double-strand break repair after exposure to ionizing radiations. Radiat. Res. 2013;179:383–392. doi: 10.1667/RR3308.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legube G., Trouche D. Regulating histone acetyltransferases and deacetylases. EMBO Rep. 2003;4:944–947. doi: 10.1038/sj.embor.embor941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berndsen C.E., Denu J.M. Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr. Opin. Struct. Biol. 2008;18:682–689. doi: 10.1016/j.sbi.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMahon S.B., Van Buskirk H.A., Dugan K.A., Copeland T.D., Cole M.D. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- 7.Vassilev A., Yamauchi J., Kotani T., Prives C., Avantaggiati M.L., Qin J., Nakatani Y. The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol. Cell. 1998;2:869–875. doi: 10.1016/s1097-2765(00)80301-9. [DOI] [PubMed] [Google Scholar]
- 8.Park J., Kunjibettu S., McMahon S.B., Cole M.D. The ATM-related domain of TRRAP is required for histone acetyltransferase recruitment and Myc-dependent oncogenesis. Genes Dev. 2001;15:1619–1624. doi: 10.1101/gad.900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L.-J., Loewenstein P.M., Green M. Enhanced MYC association with the NuA4 histone acetyltransferase complex mediated by the adenovirus E1A N-terminal domain activates a subset of MYC target genes highly expressed in cancer cells. Genes Cancer. 2017;8:752–761. doi: 10.18632/genesandcancer.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jethwa A., Słabicki M., Hüllein J., Jentzsch M., Dalal V., Rabe S., Wagner L., Walther T., Klapper W., Bohnenberger H., MMML Network Project TRRAP is essential for regulating the accumulation of mutant and wild-type p53 in lymphoma. Blood. 2018;131:2789–2802. doi: 10.1182/blood-2017-09-806679. [DOI] [PubMed] [Google Scholar]
- 11.Wei X., Walia V., Lin J.C., Teer J.K., Prickett T.D., Gartner J., Davis S., Stemke-Hale K., Davies M.A., Gershenwald J.E., NISC Comparative Sequencing Program Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nat. Genet. 2011;43:442–446. doi: 10.1038/ng.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wurdak H., Zhu S., Romero A., Lorger M., Watson J., Chiang C.-Y., Zhang J., Natu V.S., Lairson L.L., Walker J.R. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 2010;6:37–47. doi: 10.1016/j.stem.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Loukopoulos P., Shibata T., Katoh H., Kokubu A., Sakamoto M., Yamazaki K., Kosuge T., Kanai Y., Hosoda F., Imoto I. Genome-wide array-based comparative genomic hybridization analysis of pancreatic adenocarcinoma: identification of genetic indicators that predict patient outcome. Cancer Sci. 2007;98:392–400. doi: 10.1111/j.1349-7006.2007.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herceg Z., Hulla W., Gell D., Cuenin C., Lleonart M., Jackson S., Wang Z.Q. Disruption of Trrap causes early embryonic lethality and defects in cell cycle progression. Nat. Genet. 2001;29:206–211. doi: 10.1038/ng725. [DOI] [PubMed] [Google Scholar]
- 15.Sawan C., Hernandez-Vargas H., Murr R., Lopez F., Vaissière T., Ghantous A.Y., Cuenin C., Imbert J., Wang Z.-Q., Ren B., Herceg Z. Histone acetyltransferase cofactor Trrap maintains self-renewal and restricts differentiation of embryonic stem cells. Stem Cells. 2013;31:979–991. doi: 10.1002/stem.1341. [DOI] [PubMed] [Google Scholar]
- 16.Tapias A., Zhou Z.-W., Shi Y., Chong Z., Wang P., Groth M., Platzer M., Huttner W., Herceg Z., Yang Y.-G., Wang Z.Q. Trrap-dependent histone acetylation specifically regulates cell-cycle gene transcription to control neural progenitor fate decisions. Cell Stem Cell. 2014;14:632–643. doi: 10.1016/j.stem.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A.R., Westphal A., Yang D.Y.J., Sullivan C.A.W., Eilbott J., Zaidi S., Voos A., Vander Wyk B.C., Ventola P., Waqar Z. Neurogenetic analysis of childhood disintegrative disorder. Mol. Autism. 2017;8:19. doi: 10.1186/s13229-017-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Ionita-Laza I., Roos J.L., Boone B., Woodrick S., Sun Y., Levy S., Gogos J.A., Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 2012;44:1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takata A., Xu B., Ionita-Laza I., Roos J.L., Gogos J.A., Karayiorgou M. Loss-of-function variants in schizophrenia risk and SETD1A as a candidate susceptibility gene. Neuron. 2014;82:773–780. doi: 10.1016/j.neuron.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geisheker M.R., Heymann G., Wang T., Coe B.P., Turner T.N., Stessman H.A.F., Hoekzema K., Kvarnung M., Shaw M., Friend K. Hotspots of missense mutation identify neurodevelopmental disorder genes and functional domains. Nat. Neurosci. 2017;20:1043–1051. doi: 10.1038/nn.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: A matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kircher M., Witten D.M., Jain P., O’Roak B.J., Cooper G.M., Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014;46:310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 25.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P., Kondrashov A.S., Sunyaev S.R. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware J.S., Samocha K.E., Homsy J., Daly M.J. Interpreting de novo variation in human disease using denovolyzeR. Curr. Protoc. Hum. Genet. 2015;87:7.25.1–7.25.15. doi: 10.1002/0471142905.hg0725s87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lelieveld S.H., Wiel L., Venselaar H., Pfundt R., Vriend G., Veltman J.A., Brunner H.G., Vissers L.E.L.M., Gilissen C. Spatial clustering of de novo missense mutations identifies candidate neurodevelopmental disorder-associated genes. Am. J. Hum. Genet. 2017;101:478–484. doi: 10.1016/j.ajhg.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferry Q., Steinberg J., Webber C., FitzPatrick D.R., Ponting C.P., Zisserman A., Nellåker C. Diagnostically relevant facial gestalt information from ordinary photos. eLife. 2014;3:e02020. doi: 10.7554/eLife.02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reijnders M.R.F., Janowski R., Alvi M., Self J.E., van Essen T.J., Vreeburg M., Rouhl R.P.W., Stevens S.J.C., Stegmann A.P.A., Schieving J. PURA syndrome: clinical delineation and genotype-phenotype study in 32 individuals with review of published literature. J. Med. Genet. 2018;55:104–113. doi: 10.1136/jmedgenet-2017-104946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murr R., Vaissière T., Sawan C., Shukla V., Herceg Z. Orchestration of chromatin-based processes: Mind the TRRAP. Oncogene. 2007;26:5358–5372. doi: 10.1038/sj.onc.1210605. [DOI] [PubMed] [Google Scholar]
- 31.Chen P.B., Hung J.-H., Hickman T.L., Coles A.H., Carey J.F., Weng Z., Chu F., Fazzio T.G. Hdac6 regulates Tip60-p400 function in stem cells. eLife. 2013;2:e01557. doi: 10.7554/eLife.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fazzio T.G., Huff J.T., Panning B. An RNAi screen of chromatin proteins identifies Tip60-p400 as a regulator of embryonic stem cell identity. Cell. 2008;134:162–174. doi: 10.1016/j.cell.2008.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acharya D., Hainer S.J., Yoon Y., Wang F., Bach I., Rivera-Pérez J.A., Fazzio T.G. KAT-independent gene regulation by Tip60 promotes ESC self-renewal but not pluripotency. Cell Rep. 2017;19:671–679. doi: 10.1016/j.celrep.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tapias A., Wang Z.-Q. Lysine acetylation and deacetylation in brain development and neuropathies. Genomics Proteomics Bioinformatics. 2017;15:19–36. doi: 10.1016/j.gpb.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton-Smith J., O’Sullivan J., Daly S., Bhaskar S., Day R., Anderson B., Voss A.K., Thomas T., Biesecker L.G., Smith P. Whole-exome-sequencing identifies mutations in histone acetyltransferase gene KAT6B in individuals with the Say-Barber-Biesecker variant of Ohdo syndrome. Am. J. Hum. Genet. 2011;89:675–681. doi: 10.1016/j.ajhg.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szakszon K., Salpietro C., Kakar N., Knegt A.C., Oláh É., Dallapiccola B., Borck G. De novo mutations of the gene encoding the histone acetyltransferase KAT6B in two patients with Say-Barber/Biesecker/Young-Simpson syndrome. Am. J. Med. Genet. A. 2013;161A:884–888. doi: 10.1002/ajmg.a.35848. [DOI] [PubMed] [Google Scholar]
- 37.Yilmaz R., Beleza-Meireles A., Price S., Oliveira R., Kubisch C., Clayton-Smith J., Szakszon K., Borck G. A recurrent synonymous KAT6B mutation causes Say-Barber-Biesecker/Young-Simpson syndrome by inducing aberrant splicing. Am. J. Med. Genet. A. 2015;167A:3006–3010. doi: 10.1002/ajmg.a.37343. [DOI] [PubMed] [Google Scholar]
- 38.Campeau P.M., Kim J.C., Lu J.T., Schwartzentruber J.A., Abdul-Rahman O.A., Schlaubitz S., Murdock D.M., Jiang M.-M., Lammer E.J., Enns G.M. Mutations in KAT6B, encoding a histone acetyltransferase, cause Genitopatellar syndrome. Am. J. Hum. Genet. 2012;90:282–289. doi: 10.1016/j.ajhg.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simpson M.A., Deshpande C., Dafou D., Vissers L.E.L.M., Woollard W.J., Holder S.E., Gillessen-Kaesbach G., Derks R., White S.M., Cohen-Snuijf R. De novo mutations of the gene encoding the histone acetyltransferase KAT6B cause Genitopatellar syndrome. Am. J. Hum. Genet. 2012;90:290–294. doi: 10.1016/j.ajhg.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Millan F., Cho M.T., Retterer K., Monaghan K.G., Bai R., Vitazka P., Everman D.B., Smith B., Angle B., Roberts V. Whole exome sequencing reveals de novo pathogenic variants in KAT6A as a cause of a neurodevelopmental disorder. Am. J. Med. Genet. A. 2016;170:1791–1798. doi: 10.1002/ajmg.a.37670. [DOI] [PubMed] [Google Scholar]
- 41.Murray C.R., Abel S.N., McClure M.B., Foster J., 2nd, Walke M.I., Jayakar P., Bademci G., Tekin M. Novel causative variants in DYRK1A, KARS, and KAT6A associated with intellectual disability and additional phenotypic features. J. Pediatr. Genet. 2017;6:77–83. doi: 10.1055/s-0037-1598639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan K., Rousseau J., Littlejohn R.O., Kiss C., Lehman A., Rosenfeld J.A., Stumpel C.T.R., Stegmann A.P.A., Robak L., Scaglia F., DDD Study. CAUSES Study Mutations in the chromatin regulator gene BRPF1 cause syndromic intellectual disability and deficient histone acetylation. Am. J. Hum. Genet. 2017;100:91–104. doi: 10.1016/j.ajhg.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roelfsema J.H., White S.J., Ariyürek Y., Bartholdi D., Niedrist D., Papadia F., Bacino C.A., den Dunnen J.T., van Ommen G.-J.B., Breuning M.H. Genetic heterogeneity in Rubinstein-Taybi syndrome: Mutations in both the CBP and EP300 genes cause disease. Am. J. Hum. Genet. 2005;76:572–580. doi: 10.1086/429130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartholdi D., Roelfsema J.H., Papadia F., Breuning M.H., Niedrist D., Hennekam R.C., Schinzel A., Peters D.J.M. Genetic heterogeneity in Rubinstein-Taybi syndrome: Delineation of the phenotype of the first patients carrying mutations in EP300. J. Med. Genet. 2007;44:327–333. doi: 10.1136/jmg.2006.046698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai A.C., Dossett C.J., Walton C.S., Cramer A.E., Eng P.A., Nowakowska B.A., Pursley A.N., Stankiewicz P., Wiszniewska J., Cheung S.W. Exon deletions of the EP300 and CREBBP genes in two children with Rubinstein-Taybi syndrome detected by aCGH. Eur. J. Hum. Genet. 2011;19:43–49. doi: 10.1038/ejhg.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negri G., Milani D., Colapietro P., Forzano F., Della Monica M., Rusconi D., Consonni L., Caffi L.G., Finelli P., Scarano G. Clinical and molecular characterization of Rubinstein-Taybi syndrome patients carrying distinct novel mutations of the EP300 gene. Clin. Genet. 2015;87:148–154. doi: 10.1111/cge.12348. [DOI] [PubMed] [Google Scholar]
- 47.Xu L.-M., Li J.-R., Huang Y., Zhao M., Tang X., Wei L. AutismKB: An evidence-based knowledgebase of autism genetics. Nucleic Acids Res. 2012;40:D1016–D1022. doi: 10.1093/nar/gkr1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piton A., Jouan L., Rochefort D., Dobrzeniecka S., Lachapelle K., Dion P.A., Gauthier J., Rouleau G.A. Analysis of the effects of rare variants on splicing identifies alterations in GABAA receptor genes in autism spectrum disorder individuals. Eur. J. Hum. Genet. 2013;21:749–756. doi: 10.1038/ejhg.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lang B., Alrahbeni T.M., Clair D.S., Blackwood D.H., International Schizophrenia Consortium. McCaig C.D., Shen S. HDAC9 is implicated in schizophrenia and expressed specifically in post-mitotic neurons but not in adult neural stem cells. Am. J. Stem Cells. 2011;1:31–41. [PMC free article] [PubMed] [Google Scholar]
- 50.Severinsen J.E., Bjarkam C.R., Kiaer-Larsen S., Olsen I.M., Nielsen M.M., Blechingberg J., Nielsen A.L., Holm I.E., Foldager L., Young B.D. Evidence implicating BRD1 with brain development and susceptibility to both schizophrenia and bipolar affective disorder. Mol. Psychiatry. 2006;11:1126–1138. doi: 10.1038/sj.mp.4001885. [DOI] [PubMed] [Google Scholar]
- 51.Gause M., Eissenberg J.C., Macrae A.F., Dorsett M., Misulovin Z., Dorsett D. Nipped-A, the Tra1/TRRAP subunit of the Drosophila SAGA and Tip60 complexes, has multiple roles in Notch signaling during wing development. Mol. Cell. Biol. 2006;26:2347–2359. doi: 10.1128/MCB.26.6.2347-2359.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ceol C.J., Horvitz H.R. A new class of C. elegans synMuv genes implicates a Tip60/NuA4-like HAT complex as a negative regulator of Ras signaling. Dev. Cell. 2004;6:563–576. doi: 10.1016/s1534-5807(04)00065-6. [DOI] [PubMed] [Google Scholar]
- 53.Finkbeiner M.G., Sawan C., Ouzounova M., Murr R., Herceg Z. HAT cofactor TRRAP mediates beta-catenin ubiquitination on the chromatin and the regulation of the canonical Wnt pathway. Cell Cycle. 2008;7:3908–3914. doi: 10.4161/cc.7.24.7354. [DOI] [PubMed] [Google Scholar]
- 54.Knutson B.A., Hahn S. Domains of Tra1 important for activator recruitment and transcription coactivator functions of SAGA and NuA4 complexes. Mol. Cell. Biol. 2011;31:818–831. doi: 10.1128/MCB.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X., Edmonson M.N., Wilkinson M.R., Patel A., Wu G., Liu Y., Li Y., Zhang Z., Rusch M.C., Parker M. Exploring genomic alteration in pediatric cancer using ProteinPaint. Nat. Genet. 2016;48:4–6. doi: 10.1038/ng.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelley L.A., Mezulis S., Yates C.M., Wass M.N., Sternberg M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.