ABBREVIATIONS
- 2-HG
2-hydroxyglutarate
- GBM
glioblastoma multiform
- IDH
isocitrate dehydrogenase
- MGMT
O6-methylguanine DNA-methyltransferase
- MRS
magnetic resonance spectroscopy
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCF
neurocognitive function
- NOS
not otherwise specified
- OS
overall survival
- PFS
progression-free survival
- WHO
World Health Organization
The presence of an isocitrate dehydrogenase gene (IDH1 or IDH2) mutation has become one of the most critical biomarkers for molecular classification and prognostication in adult diffuse gliomas.1 Here, we review the translational impact of IDH1/2 mutation on neurosurgical oncology, with a focus on how this emerging knowledge has advanced the precision of our surgical approach to these diseases.
Generally speaking, there are 2 major goals for the initial surgical procedure in a patient with suspected adult diffuse glioma. The first goal is to obtain sufficient tissue for diagnostic classification. In the era preceding the recent World Health Organization (WHO) 2016 revised criteria,2 there were limitations on diagnosis imposed by surgical sampling error. In other words, the extent of surgery and subsequent diagnostic grading of an adult diffuse glioma had been shown to be tightly linked.3,4 Patients who underwent biopsy only, unfortunately, would too often have inaccurate diagnoses, due to so-called undergrading, as there would be insufficient material for pathological review. This undergrading complicated the retrospective analyses of surgical treatment, since biopsy-only diagnoses were frequently inaccurate. However, with the molecular genomic component of classifiers codified into the revised diagnostic criteria (primarily IDH1 mutation and 1p/19q-codeletion, as described below), this scenario has occurred more rarely,5 since less tissue is required for molecular testing.
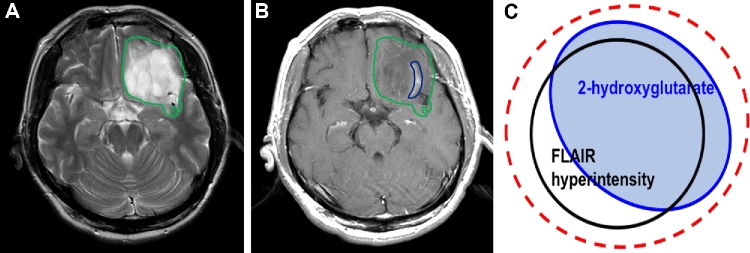
The second goal of surgery, in most cases, is to perform therapeutic cytoreduction to secure a prolonged survival and preservation of neurological function for the patient. For glioblastoma multiform (GBM), this has traditionally meant that “complete resection of enhancement” was the intended surgical goal,6,7 while for lower grade lesions, which are mostly nonenhancing, this has meant “complete resection of T2/FLAIR hyper-intensity”8 (Figure A). The evidence base that supports these surgical strategies requires updating in the context of the new WHO 2016 diagnostic criteria.
FIGURE.
For IDH1 wild-type tumors, the surgical goal is “complete resection of enhancement,” while for IDH1 mutant astrocytomas, which are mostly nonenhancing, the goal is “complete resection of T2/FLAIR hyper-intensity” A and B. Further studies are needed to determine the optimal targeting of radiation therapy for IDH1 mutant gliomas C.
ETIOLOGY AND CLASSIFICATION FOR IDH MUTANT GLIOMAS
Recurrent mutations of the IDH1 gene were initially identified in 12% of grade IV GBM within a broad sequence screen of more than 20,000 genes.9 Subsequently, more focused large sample studies confirmed that IDH1 mutation is found in the majority of secondary GBM, and only rarely found in primary GBM and GBM in children.9-11 In addition, 50% to 80% of lower grade gliomas (categorized as grade II or III by the legacy WHO 2007 criteria) harbored IDH1 mutation.10-13 The IDH1 gene mutation is almost always localized within exon 4 to codon 132 and >90% of alterations are c.395G>A (R132H) substitutions, followed by R132C as the second-most common alteration.10,12,14 Although the frequency is rare, mutations in the homologous gene IDH2 are also found in gliomas categorized as grade II or III by the legacy WHO 2007 criteria, and secondary GBM.11,15 From a classification perspective, the discovery of IDH1 mutation allows the clear distinction between primary GBM, which frequently harbors epidermal growth factor receptor, PTEN loss, and cyclin-dependent kinase inhibitor 2A (CDKN2A) gene, deletions, versus secondary GBM, which harbors IDH1 mutation.10,12,16,17
With the revision of adult diffuse glioma classification, the 2016 WHO Classification of Tumors of the Central Nervous System integrated phenotypic and genotypic parameters.2 For IDH1 mutant gliomas, tumors are grouped as diffuse astrocytic or oligodendroglial tumors. This group was histologically and genetically divided based on the presence of IDH mutations (typically IDH1R132 and IDH2R172) and 1p/19q codeletion. As an additional reinforcement of this molecular classification, astrocytic gliomas containing IDH1 mutation also near-universally contain TP53 and ATRX gene mutation, whereas oligodendrogliomas are IDH1 mutant with 1p/19q codeletion, often with concomitant CIC and FUBP1 gene and TERT promoter mutation.18,19 These co-occurring genetic abnormalities are mutually exclusive in the vast majority of cases.18-22 Accordingly, most tumors are classified as follows: (1) diffuse astrocytoma (grade II) or anaplastic astrocytoma (grade III) or glioblastoma (grade IV); IDH-mutant, -wild type, or not otherwise specified (NOS); (2) oligodendroglioma (grade II) or anaplastic oligodendroglioma (grade III; IDH mutant and 1p/19q-codeleted or NOS). Remaining cases, which are IDH1 wild type, are classified as (1) oligoastrocytoma (grade II), anaplastic oligoastrocytoma (grade III) (NOS); or (2) diffuse midline glioma (H3K27M-mutant). IDH-wild type glioblastoma (about 90% of cases) is known as primary GBM, while IDH mutant glioblastoma (about 10% of cases) corresponds to secondary GBM.2
PROGNOSTIC SIGNIFICANCE OF IDH1 MUTATION IN GLIOMAS
In GBM, Parsons and colleagues9 initially demonstrated that the overall survival (OS) in IDH1-mutant GBM was more than 3-fold longer than that in IDH1 wild-type GBM. Independent groups rapidly replicated the finding that IDH1 mutation is a favorable prognostic biomarker of both progression-free survival (PFS) and OS when compared to IDH1 wild type in low-grade glioma and high-grade glioma.11,13,23 Subsequently, the majority of clinical studies indicated that IDH mutation was an independent prognostic factor in grade II and III gliomas.11,23-29 This evidence indicates that IDH1 mutation is a favorable prognostic factor in adult gliomas. Among these studies, the prospective randomized study NOA-04 revealed IDH1 mutation, hypermethylation of the O6-methylguanine DNA-methyltransferase (MGMT) promoter, age, extent of resection, and oligodendroglial histology are independent prognostic factors in anaplastic gliomas.24 Of note, the impact of IDH1 mutation conferred a stronger risk reduction than 1p/19q codeletion, MGMT promoter methylation, or histology.24 In secondary high-grade gliomas, IDH mutations are also stronger prognostic markers of both PFS and OS than the MGMT promoter methylation status.30 Notably, the prognosis of IDH1 mutant GBM is better than anaplastic astrocytoma without IDH1 mutation.31 Taken together, IDH1 mutation has proven to be a powerful prognostic factor in gliomas, irrespective of tumor grade and histology.
Additional clinical characteristics in the IDH1 mutant gliomas are the tumor location and age distribution of the patients upon presentation. Compared with IDH wild type, IDH1 mutant gliomas were predominantly located in the frontal lobe.32-36 Patients with GBM or anaplastic astrocytoma with IDH1 mutation were significantly younger than that with IDH1 wild type.9,11 Intriguingly, the patient age at diagnosis of grade II IDH1 mutant astrocytoma is nearly identical grade III IDH1 mutant anaplastic astrocytoma. Also, the age of IDH1 mutant GBM was only 4 yr older than that of IDH1 mutant grade II and III astrocytoma.37 These findings highlighted the fact that grading, per se, had not been validated as a prognostic marker within the genomically homogeneous cohorts of IDH1 mutant vs wild-type tumors, and serves as a cautionary note for future analyses. Notably in this regard, Suzuki et al29 classified gliomas that were grades II and III by WHO 2007 criteria on the basis of the presence of IDH1 mutation, TP53 mutation, and 1p/19q codeletion. Accordingly, tumors were classified into 3 groups: type I (IDH1 mutant with 1p/19q codeletion; favorable prognostic group), type II (IDH1 mutant with TP53 mutation; intermediate group), and type III (IDH1 wild type; poor prognostic group).29 Survival difference between grade II and grade III were observed only in type II (astrocytic), but not in type I (oligodendroglial) gliomas,29 findings consistent with the results from large randomized studies of grade II and III oligodendrogliomas.38,39
TREATMENT EVIDENCE FOR IDH1 MUTANT GLIOMAS
Although scant class I evidence exists, accumulating evidence supports the proposal that more extensive surgical resection has a pivotal role in improving survival in adults with glioma. Extensive resection has been demonstrated to be associated with a survival benefit in low-grade glioma and also in GBM (IDH1 wild type).6,8,40,41 Of note, magnetic resonance (MR) imaging studies have demonstrated IDH1 mutant tumors to be rarely located in high-risk (so-called eloquent) areas of the brain, with a typically unilateral pattern of growth, sharp tumor margin, and less contrast enhancement,32,42 implying IDH1 mutant gliomas are relatively more feasible for resection, when compared to their wild-type counterparts. Intriguingly, patients with IDH1 wild-type gliomas also display reduced neurocognitive function (NCF) and lower performance score than those with IDH1 mutant gliomas.43 In addition, glioma tumor volume was not associated with NCF for patients with IDH1 mutant tumors, but was associated decreased NCF in IDH1 wild-type tumors.43 Diffusion-tensor imaging studies demonstrate that IDH mutant GBM have a less invasive phenotype compared to IDH wild-type lesions.44 Interestingly, we found that extensive resection including nonenhancing area prolonged survival in IDH1 mutant anaplastic astrocytoma and glioblastoma. Since IDH1 mutant gliomas were predominantly located in frontal lobe and the less functional disturbance of adjacent normal brain, IDH1 mutant gliomas were also more amenable to maximal resection.35 These findings were consistent with an independent study, on a separate cohort, demonstrating that the gross total resection was associated with extended survival in grade III IDH1 mutant gliomas without 1p/19q codeletion, but not in IDH1 wild-type or IDH1 mutant gliomas with 1p/19q codeletion.45 Altogether, these findings suggest that extensive resection of both enhancing and nonenhancing (T2/FLAIR hyperintense) disease should be considered for IDH1 mutant gliomas, especially astrocytoma, regardless of WHO grade46 (Figure A and B).
To assess for mutant IDH1 noninvasively, several MR techniques including diffusion tensor imaging, relative cerebral blood volume, and magnetic resonance spectroscopy (MRS) have been reported.47-49 MRS can detect 2-hydroxyglutarate (2-HG), which is produced by the IDH mutant enzyme product and is found at levels 100-fold higher in tumors, than that of normal brain.50-58 Additionally, intraoperative technologies to rapidly assess for IDH1 mutation have been established.59-61 Advances in these technologies may allow the surgical strategy to determine the degree of resection to be adjusted intraoperatively during a surgical procedure, based on the IDH1 mutation status of the tumor.
Although there is no level I evidence that radiation therapy extends survival in glioma patients with IDH1 mutation, experimental investigations have revealed that forced IDH1 mutant expression in glioma cells results in increased reactive oxygen species, by inhibiting nicotinamide adenine dinucleotide phosphate (NADPH) production, which promotes sensitivity to radiation therapy.62-64 Indeed, 65% of the total NADPH production capacity in GBM is provided for by wild-type IDH activity and introduction of the IDH1 mutation reduced this capacity by 38%.65 In addition, MRS demonstrated that IDH1 mutation decreased glutathione level compared with those with IDH1 wild type.51 Intriguingly, in ATRX mutant tumors, nonhomologous end joining was impaired and increased sensitivity to DNA damaging agents that induce double-stranded DNA breaks.66 These findings may support the recent clinical data that patients with IDH mutant, 1p/19q non-codeleted (astrocytic) tumors treated with radiotherapy had a longer PFS than those treated with temozolomide, whereas no differences in PFS for patients with IDH mutant, 1p/19q codeleted (oligodendroglial), and IDH wild-type (GBM-like) tumors.67 Recently, MRS detected 2-HG has been piloted in the clinical assessment of treatment response and treatment planning in radiotherapy,56,68,69 indicating the potential for clinical application of noninvasively assessed 2-HG. Further work is needed to determine the optimal targeting of radiation therapy for IDH1 mutant gliomas (Figure C).
CONCLUSION
In conclusion, with the revision of the WHO diagnostic criteria, surgery for adult diffuse gliomas has become even more tightly integrated with radiology and pathology, in both the diagnostic phase as well as the treatment phase of these diseases. Certain cases, namely IDH1 mutant astrocytic gliomas, display a substantial survival benefit in association with maximal resection, regardless of tumor grade under the legacy criteria. Thus, individualization of surgical strategy for patients with IDH1 mutant gliomas has advanced significantly in the modern era.
Disclosures
This work was supported by NIH P50CA165962 and Burroughs-Wellcome CAMS # 1007616.02. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Dunn GP, Andronesi OC, Cahill DP. From genomics to the clinic: biological and translational insights of mutant IDH1/2 in glioma. Neurosurgical Focus. 2013;34(2):E2. [DOI] [PubMed] [Google Scholar]
- 2. Louis DN, Perry A, Reifenberger G et al. . The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 3. Glantz MJ, Burger PC, Herndon JE 2nd et al. . Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991;41(11):1741-1744. [DOI] [PubMed] [Google Scholar]
- 4. Jackson RJ, Fuller GN, Abi-Said D et al. . Limitations of stereotactic biopsy in the initial management of gliomas. Neuro Oncol. 2001;3(3):193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim BY, Jiang W, Beiko J et al. . Diagnostic discrepancies in malignant astrocytoma due to limited small pathological tumor sample can be overcome by IDH1 testing. J Neurooncol. 2014;118(2):405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacroix M, Abi-Said D, Fourney DR et al. . A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190-198. [DOI] [PubMed] [Google Scholar]
- 7. Stummer W, Reulen HJ, Meinel T et al. . Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576; discussion 564-576. [DOI] [PubMed] [Google Scholar]
- 8. Smith JS, Chang EF, Lamborn KR et al. . Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338-1345. [DOI] [PubMed] [Google Scholar]
- 9. Parsons DW, Jones S, Zhang X et al. . An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ichimura K, Pearson DM, Kocialkowski S et al. . IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan H, Parsons DW, Jin G et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597-602. [DOI] [PubMed] [Google Scholar]
- 13. Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002-6007. [DOI] [PubMed] [Google Scholar]
- 14. Pusch S, Schweizer L, Beck AC et al. . D-2-Hydroxyglutarate producing neo-enzymatic activity inversely correlates with frequency of the type of isocitrate dehydrogenase 1 mutations found in glioma. Acta Neuropathol Commun. 2014;2:19. doi:10.1186/2051-5960-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hartmann C, Meyer J, Balss J et al. . Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469-474. [DOI] [PubMed] [Google Scholar]
- 16. Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013;126(6):931-937. [DOI] [PubMed] [Google Scholar]
- 17. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764-772. [DOI] [PubMed] [Google Scholar]
- 18. Arita H, Narita Y, Fukushima S et al. . Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013;126(2):267-276. [DOI] [PubMed] [Google Scholar]
- 19. Killela PJ, Reitman ZJ, Jiao Y et al. . TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013;110(15):6021-6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiao Y, Killela PJ, Reitman ZJ et al. . Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kannan K, Inagaki A, Silber J et al. . Whole-exome sequencing identifies ATRX mutation as a key molecular determinant in lower-grade glioma. Oncotarget. 2012;3(10):1194-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu XY, Gerges N, Korshunov A et al. . Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012;124(5):615-625. [DOI] [PubMed] [Google Scholar]
- 23. Sanson M, Marie Y, Paris S et al. . Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150-4154. [DOI] [PubMed] [Google Scholar]
- 24. Wick W, Hartmann C, Engel C et al. . NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874-5880. [DOI] [PubMed] [Google Scholar]
- 25. Sun H, Yin L, Li S et al. . Prognostic significance of IDH mutation in adult low-grade gliomas: a meta-analysis. J Neurooncol. 2013;113(2):277-284. [DOI] [PubMed] [Google Scholar]
- 26. Killela PJ, Pirozzi CJ, Healy P et al. . Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget. 2014;5(6):1515-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minniti G, Scaringi C, Arcella A et al. . IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J Neurooncol. 2014;118(2):377-383. [DOI] [PubMed] [Google Scholar]
- 28. Eckel-Passow JE, Lachance DH, Molinaro AM et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suzuki H, Aoki K, Chiba K et al. . Mutational landscape and clonal architecture in grade II and III gliomas. Nat Genet. 2015;47(5):458-468. [DOI] [PubMed] [Google Scholar]
- 30. Juratli TA, Kirsch M, Geiger K et al. . The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol. 2012;110(3):325-333. [DOI] [PubMed] [Google Scholar]
- 31. Hartmann C, Hentschel B, Wick W et al. . Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707-718. [DOI] [PubMed] [Google Scholar]
- 32. Lai A, Kharbanda S, Pope WB et al. . Evidence for sequenced molecular evolution of IDH1 mutant glioblastoma from a distinct cell of origin. J Clin Oncol. 2011;29(34):4482-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan W, Zhang W, You G et al. . Correlation of IDH1 mutation with clinicopathologic factors and prognosis in primary glioblastoma: a report of 118 patients from China. PLoS One. 2012;7(1):e30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ellingson BM, Lai A, Harris RJ et al. . Probabilistic radiographic atlas of glioblastoma phenotypes. AJNR Am J Neuroradiol. 2013;34(3):533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beiko J, Suki D, Hess KR et al. . IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16(1):81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun ZL, Chan AK, Chen LC et al. . TERT promoter mutated WHO grades II and III gliomas are located preferentially in the frontal lobe and avoid the midline. Int J Clin Exp Pathol. 2015;8(9):11485-11494. [PMC free article] [PubMed] [Google Scholar]
- 37. Reuss DE, Mamatjan Y, Schrimpf D et al. . IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cairncross G, Berkey B, Shaw E et al. . Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24(18):2707-2714. [DOI] [PubMed] [Google Scholar]
- 39. Buckner JC, Shaw EG, Pugh SL et al. . Radiation plus Procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3-8. [DOI] [PubMed] [Google Scholar]
- 41. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qi S, Yu L, Li H et al. . Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014;7(6):1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wefel JS, Noll KR, Rao G, Cahill DP. Neurocognitive function varies by IDH1 genetic mutation status in patients with malignant glioma prior to surgical resection. Neuro Oncol. 2016;18(12):1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Price SJ, Allinson K, Liu H et al. . Less invasive phenotype found in isocitrate dehydrogenase-mutated glioblastomas than in isocitrate dehydrogenase wild-type glioblastomas: a Diffusion-tensor imaging study. Radiology. 2017;283(1):215-221. [DOI] [PubMed] [Google Scholar]
- 45. Kawaguchi T, Sonoda Y, Shibahara I et al. . Impact of gross total resection in patients with WHO grade III glioma harboring the IDH 1/2 mutation without the 1p/19q co-deletion. J Neurooncol. 2016;129(3):505-514. [DOI] [PubMed] [Google Scholar]
- 46. Taylor JW, Chi AS, Cahill DP. Tailored therapy in diffuse gliomas: using molecular classifiers to optimize clinical management. Oncology. 2013;27(6):504-514. [PubMed] [Google Scholar]
- 47. Tan WL, Huang WY, Yin B, Xiong J, Wu JS, Geng DY. Can diffusion tensor imaging noninvasively detect IDH1 gene mutations in astrogliomas? A retrospective study of 112 cases. AJNR Am J Neuroradiol. 2014;35(5):920-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kickingereder P, Sahm F, Radbruch A et al. . IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci Rep. 2015;5:16238. doi:10.1038/srep16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yamashita K, Hiwatashi A, Togao O et al. . MR imaging-based analysis of glioblastoma multiforme: estimation of IDH1 mutation status. AJNR Am J Neuroradiol. 2016;37(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Andronesi OC, Kim GS, Gerstner E et al. . Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci Transl Med. 2012;4(116):116ra114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pope WB, Prins RM, Albert Thomas M et al. . Non-invasive detection of 2-hydroxyglutarate and other metabolites in IDH1 mutant glioma patients using magnetic resonance spectroscopy. J Neurooncol. 2012;107(1):197-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Choi C, Ganji SK, DeBerardinis RJ et al. . 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elkhaled A, Jalbert LE, Phillips JJ et al. . Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas. Sci Transl Med. 2012;4(116):116ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lazovic J, Soto H, Piccioni D et al. . Detection of 2-hydroxyglutaric acid in vivo by proton magnetic resonance spectroscopy in U87 glioma cells overexpressing isocitrate dehydrogenase-1 mutation. Neuro Oncol. 2012;14(12):1465-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andronesi OC, Rapalino O, Gerstner E et al. . Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013;123(9):3659-3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. de la Fuente MI, Young RJ, Rubel J et al. . Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 2016;18(2):283-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Emir UE, Larkin SJ, de Pennington N et al. . Noninvasive Quantification of 2-Hydroxyglutarate in Human Gliomas with IDH1 and IDH2 Mutations. Cancer Res. 2016;76(1):43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nagashima H, Tanaka K, Sasayama T et al. . Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma. Neuro Oncol. 2016;18(11):1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kanamori M, Kikuchi A, Watanabe M et al. . Rapid and sensitive intraoperative detection of mutations in the isocitrate dehydrogenase 1 and 2 genes during surgery for glioma. J Neurosurg. 2014;120(6):1288-1297. [DOI] [PubMed] [Google Scholar]
- 60. Santagata S, Eberlin LS, Norton I et al. . Intraoperative mass spectrometry mapping of an onco-metabolite to guide brain tumor surgery. Proc Natl Acad Sci USA. 2014;111(30):11121-11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shankar GM, Francis JM, Rinne ML et al. . Rapid intraoperative molecular characterization of glioma. JAMA Oncol. 2015;1(5):662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li S, Chou AP, Chen W et al. . Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2013;15(1):57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang XW, Labussiere M, Valable S et al. . IDH1(R132H) mutation increases U87 glioma cell sensitivity to radiation therapy in hypoxia. Biomed Res Int. 2014;2014:198697. doi:10.1155/2014/198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kessler J, Guttler A, Wichmann H et al. . IDH1(R132H) mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother Oncol. 2015;116(3):381-387. [DOI] [PubMed] [Google Scholar]
- 65. Bleeker FE, Atai NA, Lamba S et al. . The prognostic IDH1 (R132) mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119(4):487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koschmann C, Calinescu AA, Nunez FJ et al. . ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med. 2016;8(328):328ra328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Baumert BG, Hegi ME, van den Bent MJ et al. . Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol. 2016;17(11):1521-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Andronesi OC, Loebel F, Bogner W et al. . Treatment response assessment in IDH-Mutant glioma patients by noninvasive 3D functional spectroscopic mapping of 2-Hydroxyglutarate. Clin Cancer Res. 2016;22(7):1632-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jafari-Khouzani K, Loebel F, Bogner W et al. . Volumetric relationship between 2-hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients. Neuro Oncol. 2016;18(11):1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]