Abstract
Supplemental Digital Content is available in the text
Storage lesion of red blood cells (RBCs) is a well-recognized process characterized by complex morphological and functional changes.1–3 Those changes deteriorate the life-saving quality of stored blood with a reported increase in mortality for some categories of patients receiving “old” versus “new” blood.4 The importance of RBC cation gradients (K+ and Na+) dissipation in the process of storage lesion has been recently highlighted.2 Here we report a previously unrecognized nonselective cation channel in human RBCs (patch-clamp) activated whenever extracellular Ca2+ is removed and very likely contributing to the cation gradients dissipation when opened. In view of the existence of such a channel the use of non-Ca2+-chelating anticoagulants like heparin, preventing channel opening, can reduce cation gradients dissipation and help limit and delay RBCs storage lesion.
Blood was obtained from healthy donors after giving an informed consent in compliance with the ethical requirements of all involved institutions. Further details on the experimental methods are provided in the supplemental material (Supplemental Digital Content).
Experiments were performed with a chip-based planar patch-clamp setup (NPC-16 Patchliner, Nanion Technologies, Munich, Germany). Using a Cs+-based internal solution and a tetraethylammonium chloride (TEACl)-based external solution, application of 2 mM CaCl2 in the external solution, followed by a CaCl2-free external solution, leads to an increase in the membrane conductance (Fig. 1A). The I/V curve of the Ca2+-blocked current (Fig. 1B) showing a significant outward part and a negative reversal potential, was largely compatible with the blocked current being an outward cation current (carried by Cs+ as the predominant ion in the internal solution). However, a contribution by an inward anion conductance could not be excluded. Both DIDS-sensitive and DIDS-insensitive Cl− currents were reported in human RBCs.5 Thus to examine the probability that the Ca2+-blocked current might possibly be carried by anions we substituted Cl− in the external solution with impermeant anions. The Ca2+ block persisted (Fig. 1C and D) pointing to the cationic nature of the blocked current.
Figure 1.
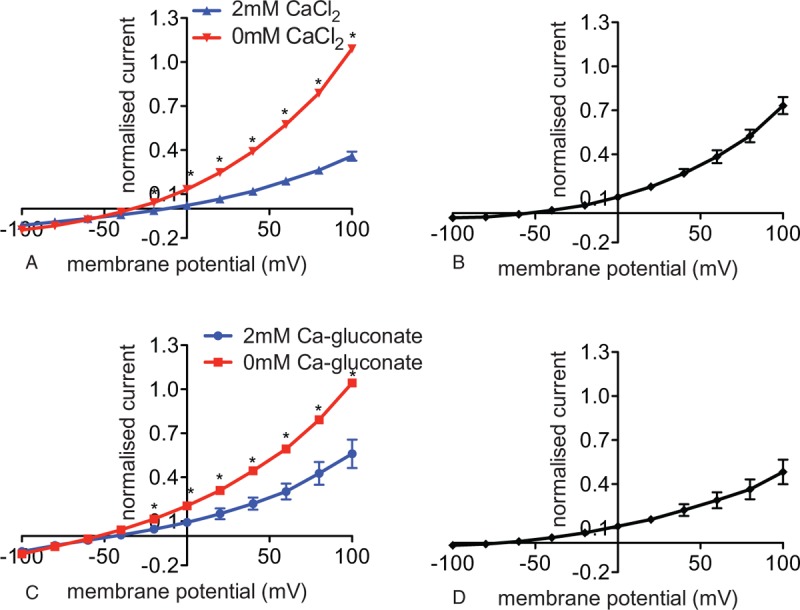
Detection and characterization of a CBC in red blood cells of healthy adults. Whole cell patch clamp recordings in a Cs+-based internal and a tetraethylammonium chloride-based external solutions. (A) I/V curves with 2 mM CaCl2 (blue) and 0 mM CaCl2 (red) in the external solution (n = 5 (3) with n being the number of cells and in brackets the number of donors). Reversal potential for the I/V curve recorded in 2 mM CaCl2 external solution (blue) is −13 mV and in 0 mM CaCl2 in the external solution (red) −33 mV. (B) I/V curve of the CBC—the current recorded in 2 mM CaCl2-external solution was subtracted from the current recorded in 0 mM CaCl2-external solution. Whole cell patch clamp recordings in a Cs+-based internal and a TEANO3-based external solution devoid of Cl−. (C) I/V curves with 2 mM Ca gluconate (blue) and 0 mM Ca gluconate (red) in the external solution (n = 4 (1) with n being the number of cells and in brackets the number of donors). Reversal potential for the I/V curve recorded in 2 mM Ca gluconate in the external solution (blue) is −44 mV and in 0 mM CaCl2 in the external solution (red) is −54 mV. (D) I/V curve of the CBC—the current recorded in 2 mM Ca gluconate-external solution was subtracted from the current recorded in 0 mM Ca gluconate-external solution. Currents were elicited by voltage steps from −100 to 100 mV for 500 milliseconds in 20 mV increments at Vh = −30 mV. Measurements were performed at room temperature. Data are presented as mean ± standard error of the mean and as normalized currents (Supplemental Fig. 2, Supplemental Digital Content, gives currents in absolute values, in pAs). Significance is assessed with a paired Student t test and set at P < 0.05. For better visualization, a significance anywhere below P < 0.05 is denoted with 1 star. CBC = Ca2+-blocked current.
It should be noted that due to the high ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA) in the internal solution, all the above measurements were done in the absence of intracellular Ca2+-dependent functions, including the absence of possible Ca2+-activated Cl− channels similar to those in mice.6
We further characterized the Calcium-inhibited Cation Channel (CiCC) and examined if the current was blocked by Ca2+ also in physiological conditions (Fig. 2). Using a K+-based internal and a Na+-based external solutions, we applied successively 2 mM CaCl2, 0 mM CaCl2, 2 mM CaCl2, and 20 mM CaCl2-external solutions. Similar to the experiments with the Cs+- and TEACl-based solutions, the current increased after changing from a 2 mM CaCl2 to a 0 mM CaCl2 external solution (Fig. 2A–C). However, the current failed to decrease significantly when the 0 mM CaCl2 solution was changed again to a 2 mM CaCl2 solution (Fig. 2A–C). It was only after the 2 mM CaCl2 was replaced by a 20 mM CaCl2-external solution that the current significantly decreased (Fig. 2A–C).
Figure 2.
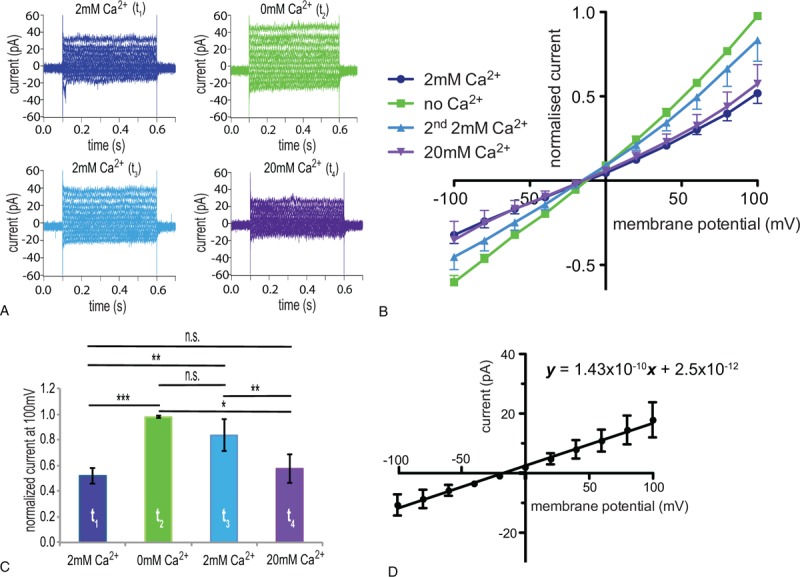
Verification and further characterization in physiological solutions of a Ca2+-blocked current in RBCs of healthy adults. Whole cell patch clamp recordings in physiological (a K+-based internal and a Na+-based external) solutions. (A) Raw current traces from a representative RBC in an external solution containing 2 mM CaCl2 at t1 (dark blue), 0 mM CaCl2 at t2 (green), 2 mM CaCl2 at t3 (light blue), and 20 mM CaCl2 at t4 (violet). (B) I/V curves in 2 mM CaCl2 (t1) (dark blue), 0 mM CaCl2 (t2) (green), 2 mM CaCl2 (2nd application) (t3) (light blue), and 20 mM CaCl2 (t4) (violet)-external solutions (n = 7 (3) with n being the number of cells and in brackets the number of donors). (C) Bar chart of the normalized current at 100 mV with the successive application of 2 mM CaCl2, 0 mM CaCl2, 2 mM CaCl2, and 20 mM CaCl2-external solutions; colors match the conditions in A and B. (D) I/V curve of the Ca2+-blocked current (the current recorded in 2 mM CaCl2-external solution at t1 was subtracted from the current recorded in 0 mM CaCl2-external solution at t2 in physiological solutions). In order to determine the whole cell conductance based on the slope of the I/V curve (143 pS), the current is given in absolute values, which results, due to cell-to-cell variations, in bigger error bars (standard error of mean) compared with normalized currents. Currents were elicited by voltage steps from −100 to 100 mV for 500 milliseconds in 20 mV increments at Vh = −30 mV. Measurements were performed at room temperature. Data are presented as mean ± standard error of the mean. Significance is assessed with a paired Student t test and set at P < 0.05. Stars are used as follows: ∗∗ for P < 0.01, and ∗∗∗ for P < 0.001; n.s. stands for nonsignificant. RBC = red blood cell.
The I/V curves for the Ca2+-blocked current in Cs+- and tetraethylammonium (TEA)-based solutions (Fig. 1B and D) and in physiological solutions (Fig. 2D) revealed that the Ca2+-blocked channel is a voltage-independent nonselective cation channel conducting Cs+ as well as Na+ and K+. It has a slight preference for K+ over Na+ as in physiological solutions the I/V curve of the blocked current, rather than crossing the x-axis at 0 mV, is shifted to potentials closer to the K+ reversal potential and the outward current carried by K+ is bigger than the inward current carried by Na+.
Knowing the role of divalent cations and especially Ca2+ in the formation and maintenance of gigaseals, the observed increase in conductance after changing a Ca2+ containing with a Ca2+-free external solution, could be mistaken for a worsening of the gigaseal and the appearance of leak currents. This might explain why the channel was not reported so far.
A channel blocked by extracellular Ca2+ is not without a precedent. Nonselective monovalent cation currents blocked by extracellular Ca2+ have been described in epithelial cells of frog skin7 and toad urinary bladder7 as well as in chicken and rabbit intestine8 with a suggested function in volume homeostasis.7 Trying to speculate on the identity of the reported channel made us consider the Piezo channels. Piezo1 has been reported in RBCs9 and has an important role in volume regulation with mutations of the channel causative for hereditary xerocytosis characterized by decreased RBC cation content and cell dehydration.10 However, the mechanical (stretch- and pressure-induced) activation of the channel11 and its recently discovered voltage sensitivity12 argue against such an assumption.
Going beyond electrophysiological experiments we were able to give further evidence for the existence of the CiCC by detecting cation gradients dissipation in RBCs of blood anticoagulated with a Ca2+-chelating anticoagulant (ethylenediaminetetraacetic acid [EDTA]) versus a non-Ca2+-chelating anticoagulant (heparin) (Fig. 3). We observed a decrease in the Na+ plasma concentration (Fig. 3B) and an increase in the K+ plasma concentration (Fig. 3C) in freshly drawn blood from healthy donors collected in EDTA versus heparin vacutainers consistent with the existence of a Ca2+-blocked nonselective cation channel which, when opened in the presence of a Ca2+ chelator, generates/contributes to the observed ion imbalance. Furthermore, the estimated flux of monovalent cations trough the channel which is 4.3 mM/min (details of calculation to be found in Supplemental Material, Supplemental Digital Content) is in full agreement, both in time and magnitude, with the cation imbalance measured by us minutes after blood withdrawal (Fig. 3).
Figure 3.
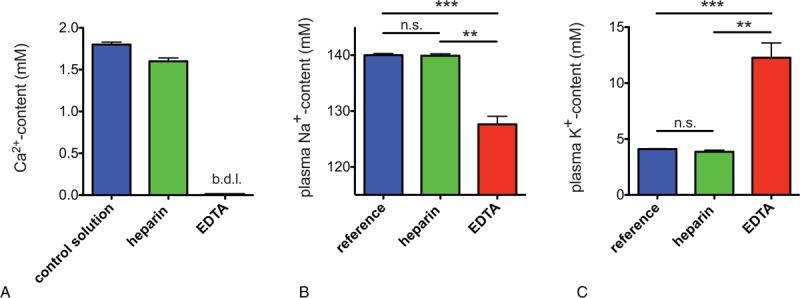
Blood plasma ion content of healthy adults in heparin and EDTA. (A) Ca2+ content of a control aqueous nonbuffered 1.8 mM CaCl2 solution filled in heparin and EDTA vacutainers. The abbreviation b.d.l. denotes “below detection limit.” (B) Na+ plasma content, (C) K+ plasma content of blood anticoagulated with heparin and EDTA. Measurements in heparin and EDTA were performed on blood of healthy adults (n = 3) collected in heparin and EDTA vacutainers, respectively, and reference values were taken from Liappis.14 Error bars represent standard error of the mean and stars denote significances as follows: n.s. for not significant, ∗∗ for P < 0.01 and ∗∗∗ for P < 0.001. It is worthwhile to mention that even though the driving force for Na+ to get into the cell was higher in heparin compared with EDTA (due to the additional Na+ coming from the Na-heparin salt itself) it was in EDTA that we detected a higher Na+ influx (lower Na+ plasma content) (B). Vice versa, even though the driving force for K+ to get out of the cell was lower in EDTA compared with heparin (due to the additional K+ coming from the K3EDTA salt itself) it was in EDTA that we detected a higher K+ outflux (higher K+ plasma content) (C). EDTA = ethylenediaminetetraacetic acid.
A channel in the membrane of RBCs being opened or unblocked by the removal of Ca2+ may have significant consequences. Blood for transfusion and for a variety of laboratory tests is all supplied with anticoagulants. The most commonly used anticoagulants at present are heparin, the tripotassium or trisodium salts of EDTA, and trisodium citrate (or the citrate-based citrate phosphate dextrose-adenine [CPDA]).13 While heparin interferes with coagulation by forming a complex with antithrombin III in the plasma to prevent thrombin formation, CPDA and especially EDTA act by chelating Ca2+ which is essential for the initiation and advancing of coagulation.13 Ca2+ removal reduces proteolysis and is further beneficial as it is believed to help suppress Gardos channel activity and maintain RBCs hydration as well as for preventing ATP consumption by the Ca2+ ATPase and calpain activation in stored cells. Taking into consideration CiCC, however, the benefits of Ca2+ removal should be reconsidered.
RBCs dissipation of ion gradients and increases in osmotic fragility, decreases in glucose and increases in lactate levels, and decreases in pH over time occurring in stored blood1 are only a small part of the detrimental RBCs storage lesion.1–3 Although there is a wide, evidence-reinforced, consensus that during storage ATP is decreased and reactive oxygen species are increased with a consequent RBC membrane oxidation and disruption of the cytoskeleton, aggregation of band 3 and release of vesicles,2 the sequence of changes and a possible trigger of the storage lesion are not quite clear. The cation leak, especially that present immediately,2 supported also by our results, could be a generator and an important contributor to various aspects of storage lesion such as early morphological changes, increases in scrambling and phosphatidylserine exposure; vesiculation and oxidation.2 In RBCs collected or stored in solutions supplemented with Ca2+ chelators, the cation gradient dissipation may be helped and enhanced by the opening of the CiCC. In view of the importance of the reduction of the cation leak for delaying and limiting storage lesion, we consider essential to draw the attention to CiCC. Although our description is limited to functional evidence and is lacking a molecular identification of the channel, the consequences from its existence could be easily prevented by control of extracellular Ca2+.
Supplementary Material
Footnotes
Citation: Petkova-Kirova P, Hertz L, Makhro A, Danielczok J, Huisjes R, Llaudet-Planas E, Mañú-Pereira MdM, Vives Corrons J-L, van Wijk R, Bogdanova A, Kaestner L. A Previously Unrecognized Ca2+-inhibited Nonselective Cation Channel in Red Blood Cells. HemaSphere, 2018;00:00. http://dx.doi.org/10.1097/HS9.0000000000000146
Funding/support: The research leading to these results has received funding from the European Seventh Framework Program under grant agreement number 602121 (CoMMiTMenT) and the European Framework “Horizon 2020” under grant agreement number 675115 (RELEVANCE).
Disclosure: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–59. [DOI] [PubMed] [Google Scholar]
- 2.Flatt JF, Bawazir WM, Bruce LJ. The involvement of cation leaks in the storage lesion of red blood cells. Front Physiol [Internet]. 2014;5: Available from: http://journal.frontiersin.org/Journal/10.3389/fphys.2014.00214/Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tissot J-D, Bardyn M, Sonego G, et al. The storage lesions: from past to future. Transfus Clin Biol. 2017;24:277–284. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sun J, Solomon SB, et al. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huber SM, Gamper N, Lang F. Chloride conductance and volume-regulatory nonselective cation conductance in human red blood cell ghosts. Pflug Arch Eur J Physiol. 2001;441:551–558. [DOI] [PubMed] [Google Scholar]
- 6.Skals M, Jensen UB, Ousingsawat J, et al. Escherichia coli alpha-hemolysin triggers shrinkage of erythrocytes via K(Ca)3.1 and TMEM16A channels with subsequent phosphatidylserine exposure. J Biol Chem. 2010;285:15557–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Driessche W, Desmedt L, Simaels J. Blockage of Na+ currents through poorly selective cation channels in the apical membrane of frog skin and toad urinary bladder. Pflug Arch. 1991;418:193–203. [DOI] [PubMed] [Google Scholar]
- 8.Heinz M, Krattenmacher R, Hoffmann B, et al. Different modes of electrogenic Na+ absorption in the coprodeum of the chicken embryo: role of extracellular Ca2+. J Comp Physiol B Biochem Syst Environ Physiol. 1991;161:363–370. [DOI] [PubMed] [Google Scholar]
- 9.Zarychanski R, Schulz VP, Houston BL, et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood. 2012;120:1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badens C, Guizouarn H. Advances in understanding the pathogenesis of the red cell volume disorders. Br J Haematol. 2016;174:674–685. [DOI] [PubMed] [Google Scholar]
- 11.Coste B, Mathur J, Schmidt M, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroni M, Servin-Vences MR, Fleischer R, et al. Voltage gating of mechanosensitive PIEZO channels. Nat Commun. 2018;9:1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greer JP, Foerster J, Rodgers GM, et al. Wintrobe's Clinical Hematology. 12th ed.Wolters Kluwer/Lippincott, Williams & Wilkins, Philadelphia: 2009. [Google Scholar]
- 14.Liappis N. Sodium-, potassium- and chloride-concentrations in the serum of infants, children and adults. Monatsschr Kinderheilkd. 1972;120:138–142. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


