Abstract
A highly convergent synthesis of archazolid B, a potent and highly selective V-ATPase inhibitor, is described. A relay ring-closing metathesis reaction was used to form the 24-membered macrocyclic lactone, whereas the sensitive cis-triene moiety of the archazolids was assembled with a modified Stille coupling.
The archazolids are a family of unsaturated polyketides with low nanomolar inhibitory activity and excellent selectivity against mammalian V-ATPases (Scheme 1).1 The isolation of archazolids A (1) and B (2) from the myxobacterium Archangium gephyra and the constitutions of these natural products were reported by Höfle et al. several years ago.2 In 2006, Menche and coworkers disclosed the relative and absolute configurations of 1 and 2, which were obtained through careful analysis of 13C-1H coupling constants combined with degradation studies.1b Shortly thereafter, this impressive exercise in structure elucidation was matched by a total synthesis of archazolid A.3 The structure of archazolid C (3), the β-glucoside of archazolid A isolated from the myxobacterium Cystobacter violaceus, was also disclosed in 2007.4 We now report an efficient total synthesis of archazolid B, the least stable and least abundant member of the family.
Scheme 1.
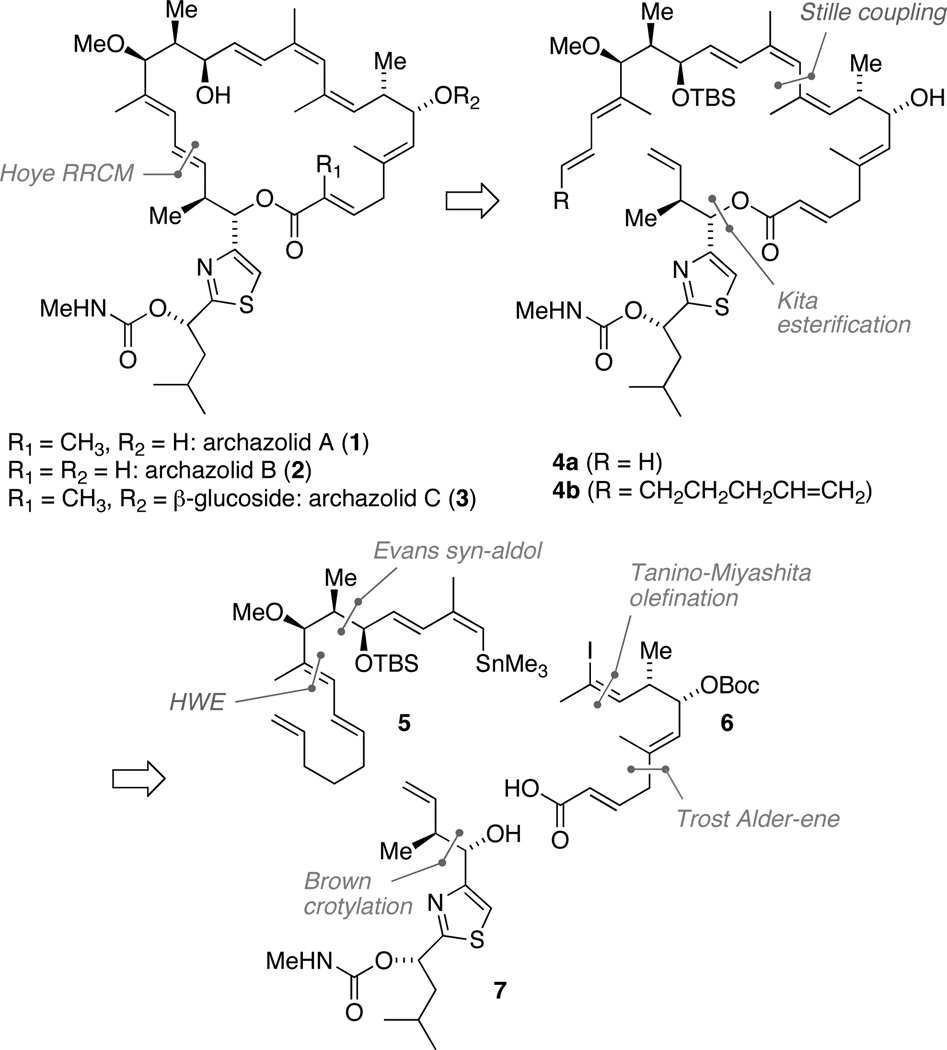
Retrosynthetic analysis of the archazolids.
The archazolids attracted our interest due to their exceptional bioactivity and unusual structural features. Their 24-membered macrolactone ring includes a rare cis-triene moiety, whose chemistry we were familiar with from our previous work on highly unsaturated pyrone polyketides.5 From the outset, our synthetic plan was governed by a desire to install the (Z,Z,E)-triene unit as late as possible to avoid potentially troublesome (cyclo)isomerizations. As our plan for archazolid B unfolded, however, we found it more appealing to close the 24-membered macrolactone through ring-closing metathesis (RCM), rather than intramolecular cross-coupling reactions (Scheme 1).6,7 This strategy would confine protecting group operations and oxidation state adjustments to a minimum. We had doubts however, whether a “simple” RCM involving diene 4a (R = H) would initiate at the more electron rich diene moiety rather than at the other terminal alkene, which would presumably result in an unproductive excision of an unsaturated δ-lactone. Therefore, we decided to promote the desired initiation through relay-ring closing metathesis (RRCM) using 4b as a precursor [R = (CH2)3CH=CH2].8 Once the corresponding, relatively stable Ru vinyl alkylidene would form it would be expected to react with the remaining terminal double bond faster than with any of the more substituted double bonds present.
Further retrosynthetic disconnection of 4b as shown in Scheme 1 yielded three building blocks: stannane 5, iodide 6, and thiazole 7, corresponding to the northwestern, northeastern, and southern regions of archazolid B, respectively. These could be assembled using the reactions shown in Scheme 2.
Scheme 2.
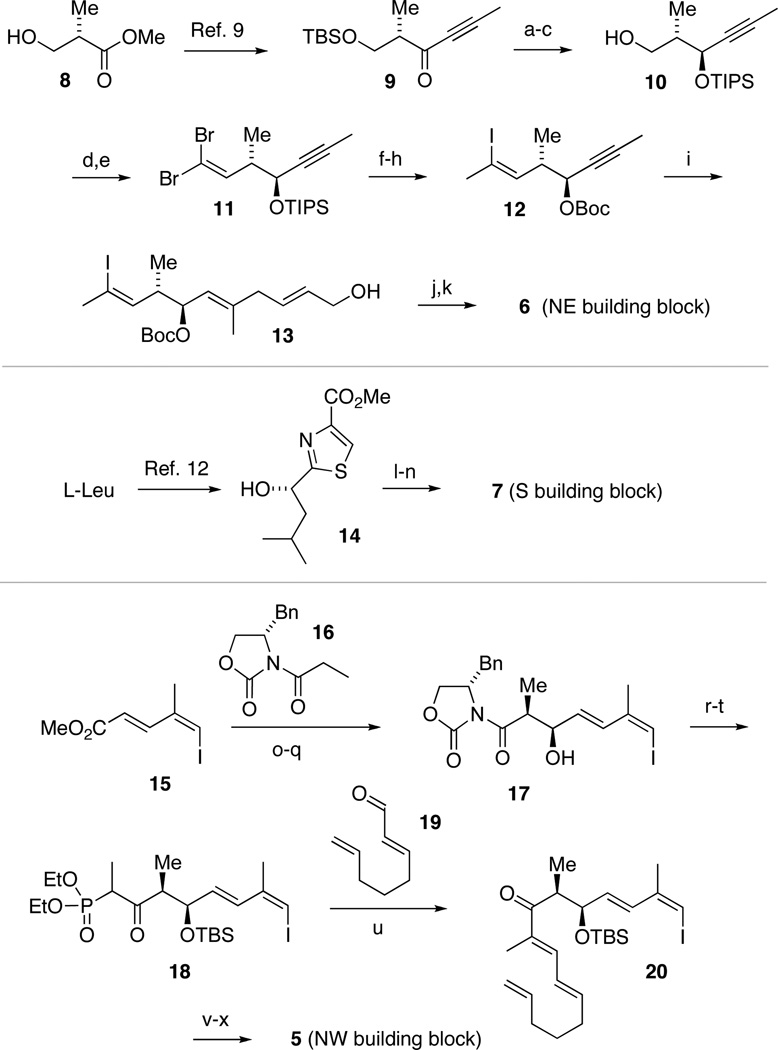
Preparation of the building blocks.
Reagents and conditions: (a) (S)-Alpine Borane, THF, 40 °C (89%, >20:1 d.r.); (b) TIPSCl, Imidazole, DMAP, CH2Cl2 (93%); (c) HOAc, THF/H2O (97%); (d) Dess-Martin periodinane, NaHCO3, CH2Cl2; (e) PPh3, CBr4, K2CO3, CH2Cl2 (75% for 2 steps); (f) MeLi, CuI, Et2O; I2 (77%); (g) TBAF, THF; (h) Boc2O, pyridine, DMAP (99% for 2 steps); (i) RuCp(MeCN)3PF6, 3-buten-1-ol, acetone (88%); (j) Dess-Martin periodinane, NaHCO3, CH2Cl2; (k) NaClO2, NaH2PO4, 2-methyl-2-butene, tBuOH, H2O (99% for 2 steps); (l) CDI, MeNH2 (88%); (m) DIBAL-H, PhCH3, THF (80%); (n) (−)-MeOB(Ipc)2, KOtBu, nBuLi, trans-2-butene, THF (90%); (o) DIBAL-H, CH2Cl2 (96%); (p) Dess-Martin periodinane, NaHCO3, CH2Cl2; (q) 16, Bu2BOTf, Et3N, CH2Cl2 (76% for 2 steps); (r) MeONHMe • HCl, Me3Al, THF (80%); (s) TBSCl, Imidazole, CH2Cl2 (89%); (t) nBuLi, diethylethylphosphonate, THF (95%); (u) 19, Ba(OH)2, 40:1 THF/H2O (79%); (v) NaBH4, MeOH (92%); (w) Me3OBF4, proton sponge, CH2Cl2 (89%); (x) nBuLi, Me3SnCl, THF.
Our northeastern building block 6 was prepared from the known ynone 9, easily available in 3 steps from (S)-Roche ester (8) (Scheme 2).9 A highly diastereoselective reduction of 9 with (S)-alpine borane gave a propargylic alcohol, which was protected as the triisoproylsilyl ether and selectively desilylated to give primary alcohol 10. Subsequent oxidation and olefination yielded dibromoalkene 11. Installation of a (Z)-vinyl iodide using the method of Tanino and Miyashita,10 followed by exchange of the secondary silyl protecting group for a tert-butoxycarbonate, furnished compound 12. By comparison, attempted Stork-Zhao olefination proceeded with inferior yield and stereoselectivity. Compound 12 underwent a highly selective Trost Alder-ene reaction with 3-butenol to afford triene 13 in good yield.11 To proceed with high regioselectivity, this reaction required the presence of a coordinating carbonate (or MOM) protecting group. Two-step oxidation of allylic alcohol 13 then gave carboxylic acid 6 in nearly quantitative yield.
The southern thiazole building block 7 was elaborated from the known hydroxyalkyl thiazolecarboxylate 14 (available from leucine in 6 steps)12 via carbamoylation, followed by chemoselective reduction and Brown crotylation13 to efficiently yield multi-gram quantities of this fragment.3
The synthesis of the remaining northwestern building block 5 started from iododienoate 15, which can be obtained in three steps from propargyl alcohol.14 Reduction followed by oxidation gave an aldehyde that underwent an efficient Evans syn-aldol addition with the boron enolate of benzyl oxazolidinone 16 to afford 17.15 Conversion into the corresponding Weinreb amide, followed by silyl protection of the secondary alcohol and a phosphonate Claisen reaction gave β-keto phosphonate 18, which underwent a Horner-Wadsworth-Emmons reaction with enal 19 to afford dienone 20. A highly diastereoselective reduction with NaBH4 followed by etherification and iodine-tin exchange gave our building block 5.16
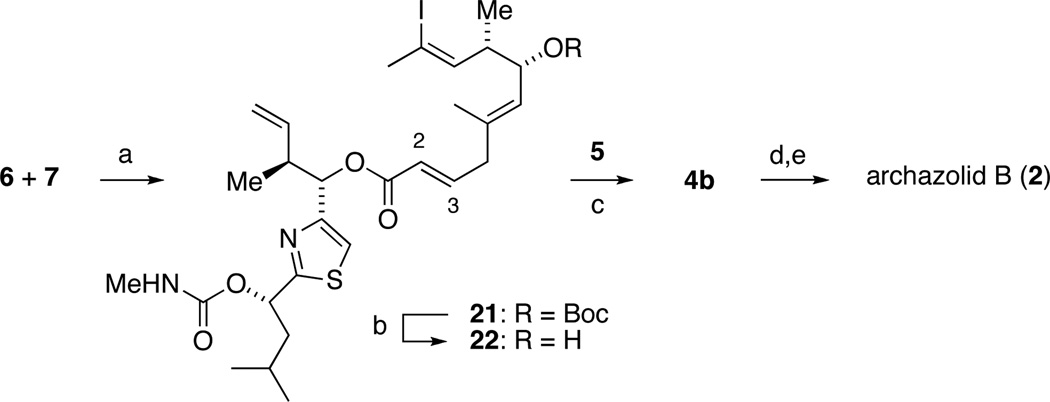
Esterification of 7 with 6 required Ru-catalyzed activation of 6 as the acyl ketene acetal according to Kita, 17 since all base-mediated methods led to the migration of the C2-C3-double bond. Subsequent thermal deprotection of the Boc group gave iodide 22, which underwent a modified Liebeskind coupling with vinyl stannane 5 to yield the acyclic metathesis precursor 4b.18 It is interesting to note that both palladium and copper were required to promote this cross-coupling. The relay ring-closing metathesis using Grubbs’ second generation catalyst proceeded as planned to afford the macrocycle in 27% yield. Finally, careful deprotection of the base-sensitive macrolactone using aqueous formic acid gave archazolid B.
In summary, we have reported a highly convergent and stereoselective synthesis of archazolid B, which proceeds in only 19 steps (longest linear sequence) starting from (S)-Roche ester and in less than 40 total steps. Our synthesis of archazolid B includes several transition metal-catalyzed steps, including three very different reactions promoted by Ru catalysts. The first two of these demonstrate how well Ru- catalyzed reactions interface with other transition metal-catalyzed reactions operating on halogenated substrates. The final Ru-catalyzed ring-closing metathesis, by contrast, underscores the usefulness of relay tactics for the synthesis of highly unsaturated macrolactones where several potential initiation sites exist. Further elaboration of our general strategy should give rise to archazolids A and C, as well as a multitude of structurally simplified and biologically interesting analogs.
Supplementary Material
Scheme 3.
Completion of the synthesis.
Reagents and conditions: (a) [RuCl2(cymene)]2, ethoxyacetylene; then 7, TsOH (54%); (b) SiO2, 125 °C (66%); (c) 5, Pd(PPh3)4, CuTC, DMF (32%; 92% based on recovered 6); (d) Grubbs’ II, PhCH3 (27%); (e) 3:6:1 formic acid/THF/H2O (84%).
Acknowledgment
This work was supported by National Institutes of Health Grant R01 GM067636. We thank Dr. Steven Diver (University of Buffalo) for helpful discussions.
Footnotes
Supporting Information Available: Experimental procedures and compound characterization data. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.(a) Sasse F, Steinmetz H, Höfle G, Reichenbach H. J. Antibiot. 2003;56:520–525. doi: 10.7164/antibiotics.56.520. [DOI] [PubMed] [Google Scholar]; (b) Hassfeld J, Farès C, Steinmetz H, Carlomagno T, Menche D. Org. Lett. 2006;8:4751–4754. doi: 10.1021/ol061831y. [DOI] [PubMed] [Google Scholar]
- 2.Höfle G, Reichenbach H, Sasse F, Steinmetz H. 41 42 951 C1. German Patent DE. 1993
- 3.Menche D, Hassfeld J, Li J, Rudolph S. J. Am. Chem. Soc. 2007;129:6100–6101. doi: 10.1021/ja071461o. [DOI] [PubMed] [Google Scholar]
- 4.Menche D, Hassfeld J, Steinmetz H, Huss M, Wieczorek H, Sasse F. Eur. J. Org. Chem. 2007;7:1196–1202. [Google Scholar]
- 5.Miller AK, Trauner D. Synlett. 2006;14:2295–2316. [Google Scholar]
- 6.Grubbs RH. Tetrahedron. 2004;60:7117–7140. [Google Scholar]
- 7.For recent examples of RCM in macrolide synthesis, see: Maleczka RE, Jr, Terrell LR, Geng F, Ward JS., III Org. Lett. 2002;4:2841–2844. doi: 10.1021/ol0262284. Va P, Roush WR. J. Am. Chem. Soc. 2006;128:15960–15961. doi: 10.1021/ja066663j. Fürstner A, Nevado C, Tremblay M, Chevrier C, Teply F, Aïssa C, Waser M. Angew. Chem. Int. Ed. 2006;45:5837–5842. doi: 10.1002/anie.200601860.
- 8.(a) Hoye TR, Jeffrey CS, Tennakoon MA, Wang J, Zhao H. J. Am. Chem. Soc. 2004;126:10210–10211. doi: 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]; (b) Wallace DJ. Angew. Chem. Int. Ed. 2005;44:1912–1915. doi: 10.1002/anie.200462753. [DOI] [PubMed] [Google Scholar]; (c) Wang X, Bowman EJ, Bowman BJ, Porco JA. Angew. Chem. Int. Ed. 2004;43:3601–3605. doi: 10.1002/anie.200460042. [DOI] [PubMed] [Google Scholar]
- 9.Trost BM, Gunzner JL. J. Am. Chem. Soc. 2001;123:9449–9450. doi: 10.1021/ja011424b. [DOI] [PubMed] [Google Scholar]
- 10.Tanino K, Arakawa K, Satoh M, Iwata Y, Miyashita M. Tetrahedron Lett. 2006;47:861–864. [Google Scholar]
- 11.Trost BM, Toste FD. Tetrahedron Lett. 1999;40:7739–7743. [Google Scholar]
- 12.Schmidt U, Gleich P, Griesser H, Utz R. Synthesis. 1986;12:992–998. [Google Scholar]
- 13.Brown HC, Bhat KS. J. Am. Chem. Soc. 1986;108:5919–5923. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 14.Beaudry CM, Trauner D. Org. Lett. 2002;4:2221–2224. doi: 10.1021/ol026069o. [DOI] [PubMed] [Google Scholar]
- 15.Evans DA, Black WC. J. Am. Chem. Soc. 1993;115:4497–4513. [Google Scholar]
- 16.Relative stereochemistry was confirmed by analysis of corresponding acetonide using 13C chemical shifts and coupling constants in 1H NMR ( Rychnovsky SD, Skalitzky DJ. Tetrahedron Lett. 1990;31:945–948. .) See Supporting Information.
- 17.Kita Y, Maeda H, Omori K, Okuno T, Tamura Y. Synlett. 1993;1:273–274. [Google Scholar]
- 18.(a) Farina V, Kapadia S, Krishnan B, Wang C, Liebeskind LS. J. Org. Chem. 1994;59:5905–5911. [Google Scholar]; (b) Allred GD, Liebeskind LS. J. Am. Chem. Soc. 1996;118:2748–2749. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.