Abstract
Background:
Appropriate postoperative pain management can improve outcomes in patients with esophageal cancer (EC).
Objective:
To compare different combinations of anesthesia and analgesia techniques in patients with EC undergoing open thoracotomy.
Methods:
This randomized, controlled, open-label trial enrolled 100 patients with EC (aged 40–65 years; American Society of Anesthesiologists [ASA] grade I/II) receiving elective surgery at Jiangsu Province Hospital (China) between July 2016 and December 2017. Patients were randomized to 4 groups (n = 25 per group): total intravenous general anesthesia plus patient-controlled intravenous analgesia (TIVA/PCIA); TIVA plus patient-controlled epidural analgesia (TIVA/PCEA); thoracic epidural anesthesia with intravenous general anesthesia plus PCIA (TEA-IVA/PCIA); and TEA-IVA/PCEA (TEA-IVA plus PCEA). Primary outcomes were plasma cortisol level (measured at baseline, 2 h after skin incision, surgery completion, and 24 and 48 h post-surgery) and pain (assessed at 24, 48, and 72 hours post-surgery using a visual analog scale). Secondary outcomes included time to first flatus, hospital stay and treatment costs. Postoperative adverse events (AEs) were analyzed.
Results:
Baseline and operative characteristics were similar between the 4 groups. Plasma cortisol level increased (P <.05 vs baseline) earlier in the TIVA groups (2 h after skin incision) than in the TEA-IVA groups (24 h after surgery). At 48 hours after surgery, plasma cortisol had returned to baseline levels in the PCEA groups but not in the PCIA groups. VAS pain scores at rest and during coughing were lower in the PCEA groups than in the PCIA groups (P <.05). Compared with the PCIA groups, the PCEA groups had shorter time to first flatus and shorter hospital stay, while use of TEA-IVA lowered the costs of intraoperative anesthesia (P <.05). However, the PCEA groups had a higher incidence of nausea, vomiting, and pruritus.
Conclusion:
Thoracic epidural anesthesia/analgesia can reduce the stress response, improve postoperative recovery and reduce hospital stay and costs for patients with EC.
Keywords: hospital stay, pain, patient-controlled epidural analgesia, patient-controlled intravenous analgesia, stress response, thoracic epidural anesthesia, treatment cost
1. Introduction
Esophageal cancer (EC) is the eighth most common cancer worldwide and the sixth most common cause of cancer-related death.[1,2] The incidence of EC is much greater in China than in western countries, and over half of all new cases of EC, each year occurs in China.[3] Esophageal squamous cell carcinoma (ESCC) accounts for approximately 95% of all cases of EC[4] and is usually found in the middle and distal parts of the esophagus.[5] The incidence of EC increases with age to peak in the seventh decade of life.[5,6] Risk factors for ESCC include smoking, alcohol consumption and caustic injury to the esophagus.[5,7] The 5-year survival rate is only 15–25%,[1,2,5] emphasizing the need for improvements to current therapies.
The current treatments for EC include surgery[8,9] and concurrent chemoradiation therapy.[10,11] Surgical management of EC involves a relatively large operative wound, severe postoperative pain, substantial stress responses and the risks of various postoperative complications that prolong hospital stay and increase treatment costs. It is becoming increasingly recognized that the intraoperative anesthetic regimen and the technique used for postoperative analgesia can affect patient recovery and clinical outcomes. In the past, surgery was generally performed under total intravenous general anesthesia (TIVA) while postoperative pain relief was achieved using patient-controlled intravenous analgesia (PCIA). However, in recent decades it has become increasingly evident that combining thoracic epidural anesthesia with intravenous general anesthesia (TEA-IVA) and/or the use of postoperative patient-controlled epidural analgesia (PCEA) can improve patient outcomes after surgery.[12–15] Thoracic epidural anesthesia and/or analgesia have been reported to inhibit the stress response[16] and immune system dysfunction,[16–18] enhance the microcirculation of the gastric tube,[19] decrease postoperative opioid use,[17,20] shorten the stay in the intensive care unit (ICU) or hospital[20–24] and reduce the incidences of pneumonia,[24] postoperative respiratory failure,[25] chronic post-thoracotomy pain syndrome,[26] and anastomotic leakage,[27] although not all studies agree with the latter finding.[28] Furthermore, there is also evidence that perioperative epidural anesthesia can reduce the rate of cancer recurrence and prolong patient survival.[29] However, it has not yet been established which combination of techniques for anesthesia and analgesia meets the medical needs of the patient with regard to optimizing outcomes (such as minimizing hospital stay) without unnecessarily utilizing medical resources that would otherwise increase treatment costs.
Therefore, the aim of this randomized controlled trial was to establish which combination of anesthesia technique (TIVA or TEA-IVA) and postoperative analgesia method (PCIA or PCEA) was most appropriate for use in patients undergoing surgery for EC. To achieve this objective, various combinations of the anesthesia and analgesia techniques were compared with regard to their effects on the stress response, postoperative recovery and treatment costs.
2. Methods
2.1. Study design and participants
This single-center, randomized, controlled, open-label trial enrolled 100 patients with carcinoma of the esophagus or gastric cardia scheduled for elective surgery at Jiangsu Province Hospital (First Affiliated Hospital of Nanjing Medical University, China) between July 2016 and December 2017.
The inclusion criteria were: age 40 to 65 years; weight 45 to 80 kg; diagnosis of gastroesophageal carcinoma was made based on histopathologic analysis of samples obtained during gastroscopy; American Society of Anesthesiologists (ASA) physical status grade I or II; surgical resection was carried out via a standard incision in the left thoracic wall plus an abdominal incision; neither chemotherapy nor radiotherapy were administered before surgery; no previous history of cardiovascular, autoimmune, endocrine system or metabolic disease; normal liver and renal functions; and no history of opiate or non-steroidal anti-inflammatory drug (NSAID) use. The exclusion criteria were as follows: coagulation defects; received a perioperative blood infusion; change in surgical procedure during the operation; transferred to the ICU after surgery with tracheal intubation; and communication impairment.
The study was designed so that the final analysis included 25 patients in each of the 4 study groups (see below). An initial 100 patients, who satisfied the inclusion and exclusion criteria, were enrolled and divided equally into the 4 study groups. A power calculation was not performed.
The same 2 chief surgeons at the Department of Cardiothoracic Surgery carried out all the operations. All patients included in this study provided informed written consent before surgery. The Ethics Committee of Jiangsu Province Hospital approved the study.
2.2. Randomization and patient grouping
The 100 patients were randomized to 4 groups (n = 25 per group): TIVA/PCIA (to receive TIVA during surgery and postoperative PCIA); TIVA/PCEA (to receive TIVA during surgery and postoperative PCEA); TEA-IVA/PCIA (to receive TEA-IVA during surgery and postoperative PCIA); and TEA-IVA/PCEA (to receive TEA-IVA during surgery and postoperative PCEA). For randomization, each patient was assigned with an identifier from 1 to 100, and each identifier was associated one-to-one with a random number generated using SAS 8.0 (SAS Institute, Cary, NC). Then, the random numbers were sorted to generate a random number table. The first 25 patients in the random number table were allocated to the TIVA/PCIA group, the next 25 to the TIVA/PCEA group, the next 25 to the TEA-IVA/PCIA group, and the final 25 to the TEA-IVA/PCEA group. Allocation concealment was achieved using sequentially numbered opaque sealed envelopes. Neither the patient nor the anesthesiologist was aware of which group the patient had been assigned to.
2.3. Method of anesthesia
For patients in the TIVA/PCEA and TEA-IVA/PCEA groups, epidural puncture and catheterization via the clearance between T7–8 was performed before the induction of anesthesia, and successful epidural catheterization was confirmed by assessing the level of analgesia 5 to 10 minutes after the injection of a test dose of lidocaine (1.5%, 3 mL). Each patient was placed in the supine position before anesthesia. For all patients, midazolam (0.05 mg/kg), propofol (1.5 mg/kg), atracurium (0.8 mg/kg), and fentanyl (3 μg/kg) were used for induction of anesthesia. For patients in the TIVA/PCIA and TIVA/PCEA groups, additional fentanyl (6–8 μg/kg) was injected intravenously before incision of the skin, and maintenance of anesthesia was achieved by continuous intravenous infusion of propofol (5 mg/kg/h), atracurium (0.5 mg/kg/h), and remifentanil (0.2 μg/kg/h). For patients in the TEA-IVA/PCIA and TEA-IVA/PCEA groups, additional fentanyl (2 μg/kg) was injected intravenously before incision of the skin. Then, maintenance of anesthesia was achieved by continuous intravenous infusion of propofol (5 mg/kg/h) and atracurium (0.5 mg/kg/h), and ropivacaine (0.25%, 10 mL) was injected into the epidural space every hour during surgery. All patients received tracheal intubation and were connected to a Dräg Fabius GS anesthesia machine (Drägerwerk AG, Lübeck, Germany) during the operation. Intermittent positive pressure ventilation was delivered with a tidal volume of 8 to 10 mL/kg, a respiration rate of 10–12 times/min and an expiration/inspiration ratio of 1:2. A Datex AS/3 multi-function monitor (GE Healthcare, Chicago, IL) was used to continuously monitor the electrocardiogram (ECG), heart rate (HR), peripheral capillary oxygen saturation (SpO2), end-tidal partial pressure of carbon dioxide (PETCO2) and invasive blood pressure to help maintain the stability of the respiratory and circulatory systems. Blood volume was maintained during surgery using infusions of sodium lactate and 6% hydroxyethyl starch (2000–2500 mL).
2.4. Method of analgesia
For patients in the TIVA/PCIA and TEA-IVA/PCIA groups, PCIA was administered using an electronic analgesia pump (ZZB; Nantong Aipu, Nantong, China). The drugs used for analgesia were fentanyl (6 μg/kg), tramadol (12 mg/kg), and ondansetron hydrochloride (16 mg), which were diluted in 0.9% normal saline to a final volume of 100 mL. The analgesia pump settings were: load volume, 3 mL; background dose, 2 mL/h; self-controlled additional dose, 2 mL/time; and lockout time, 15 minutes.
For patients in the TIVA/PCEA and TEA-IVA/PCEA groups, PCEA was administered using an electronic analgesia pump (REHN-11; Jiangsu Rehn Medtech Technology Co. Ltd, Jiangsu, China). The drugs given for analgesia were ropivacaine (0.125%) and fentanyl (2 μg/mL), diluted in 0.9% normal saline to a final volume of 250 mL. The analgesia pump settings were: load volume, 3 mL; background dose, 5 mL/h; self-controlled additional dose, 2 mL/time; and lockout time, 20 minutes.
2.5. Baseline and operative characteristics
The following baseline demographic and clinical characteristics were collected: age, gender, body mass index (BMI), and presence/absence of hypertension and diabetes mellitus. The following operative characteristics were recorded: nasopharyngeal temperature, operation time, blood loss, and urine volume.
2.6. Primary outcome measures
The primary outcome measures were pain score and plasma cortisol level (which was used as an indicator of the stress response). Assigned nurses utilized a visual analog scale (VAS) to determine the pain score according to the facial expression of the patient. The pain score was assessed at 24, 48 and 72 hours after surgery, both at rest and when the patient was actively coughing. The plasma cortisol level was measured at baseline, at 2 hours after skin incision, at completion of surgery, and at 24 hours and 48 hours after surgery. At each time point, blood was collected from an antecubital vein into a heparin-anticoagulated tube, centrifuged at 3000 rpm for 5 minutes and then stored at 4°C until use. The plasma cortisol level was measured by radioimmunoassay.
2.7. Secondary outcome measures
The secondary outcome measures were time to first flatus, duration of hospital stay (before, surgery, after surgery and total), and treatment costs (cost of anesthetic drugs, total cost of anesthesia and cost of postoperative analgesia).
2.8. Adverse events (AEs)
The occurrence of any AEs, including postoperative nausea, vomiting, urinary retention, pruritus, and infection, was recorded.
2.9. Statistical analysis
SPSS 22.0 (IBM Corp., Armonk, NY) was used for the statistical analysis. The data are presented as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for comparisons between different time points within the same group and between different groups; the Bonferroni post-hoc test was used for pairwise comparisons. P <.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study participants
According to the inclusion and exclusion criteria, a total of 82 males and 18 females were included in the final analysis. The baseline demographic and clinical characteristics of the study participants are presented in Table 1. There were no significant differences between groups in patient age, gender, BMI, or prevalence of hypertension or diabetes mellitus (Table 1).
Table 1.
Baseline demographic and clinical characteristics of the study participants.

3.2. Comparison of operative characteristics between groups
Mean operation time ranged from 164.8 to 168.7 minutes, and mean blood loss volume ranged from 249.5 to 261.4 mL (Table 2). There were no significant differences between the 4 groups in nasopharyngeal temperature, operation time, blood loss volume or urine volume (Table 2).
Table 2.
Comparison of operative characteristics between groups.

3.3. Primary outcome measures
3.3.1. Plasma cortisol levels
As shown in Table 3, a significant increase in plasma cortisol level (compared with baseline) was observed at 2 hours after skin incision in the TIVA/PCIA and TIVA/PCEA groups (P <.01). However, the increase in plasma cortisol level was delayed in the TEA-IVA/PCIA and TEA-IVA/PCEA groups, being first observed at 24 hours after surgery in these groups (P <.01; Table 3). Furthermore, the plasma cortisol level at completion of surgery was significantly lower in the TEA-IVA/PCIA group than in the TIVA/PCIA group and significantly lower in the TEA-IVA/PCEA group than in the TIVA/PCIA or TIVA/PCEA groups (P <.05; Table 3). These findings indicate that the use of TEA can reduce the stress response during surgery.
Table 3.
Comparison of plasma cortisol levels between groups.

It was notable that a significant elevation of plasma cortisol level was maintained at 48 hours after surgery in the TIVA/PCIA and TEA-IVA/PCIA groups (P <.01 vs baseline) but not in the TIVA/PCEA and TEA-IVA/PCEA groups (Table 3). Indeed, the plasma cortisol level at 48 hours after surgery was significantly lower in the TIVA/PCEA and TEA-IVA/PCEA groups than in the TIVA/PCIA or TEA-IVA/PCIA groups (P <.05; Table 3). These data suggest that postoperative PCEA can alleviate the stress response during the recovery from surgery.
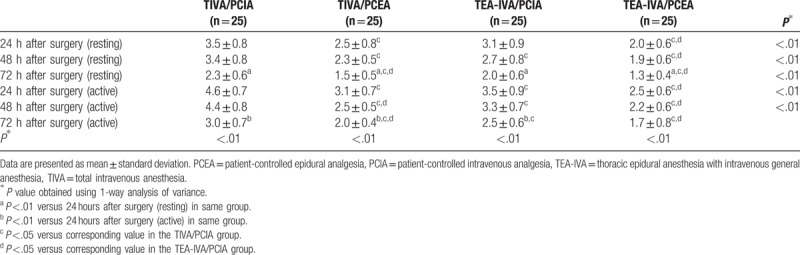
3.3.2. Pain score
In all 4 groups, the VAS score for pain was significantly lower at 72 hours after surgery than at 24 hours after surgery, both at rest and during coughing (P <.01), except for the active pain score in the TEA-IVA/PCEA group (Table 4). These findings suggesting that both postoperative analgesia methods had good analgesic effects. However, the VAS pain scores in the TEA-IVA/PCEA group were significantly lower than those in the TIVA/PCIA and TEA-IVA/PCIA groups at all time points (P <.01), both at rest and during coughing (Table 4). Furthermore, the VAS pain scores in the TIVA/PCEA group were significantly lower than those in the TIVA/PCIA group at all time points, both at rest and during coughing (P <.05; Table 4). In addition, the VAS pain scores in the TIVA/PCEA group were lower than those in the TEA-IVA/PCIA group at 72 hours after surgery in the resting state and at 48 hours and 72 hours after surgery in the active state (P <.05; Table 4). Thus, the analgesic effects of PCEA were greater than those of PCIA.
Table 4.
Comparison of visual analog scale pain scores between groups.
3.4. Secondary outcome measures
3.4.1. Time to first flatus after surgery
The time to first flatus after surgery was significantly shorter in the TIVA/PCEA and TEA-IVA/PCEA groups than in the TIVA/PCIA and TEA-IVA/PCIA groups (P <.05; Table 2). However, the time to first flatus did not differ significantly between the TIVA/PCEA and TEA-IVA/PCEA groups and between the TIVA/PCIA and TEA-IVA/PCIA groups (Table 2).
3.4.2. Hospital stay and treatment costs
The length of hospital stay before surgery did not differ between any of the groups (Table 5). However, the duration of hospital stay after surgery and total hospital stay were significantly shorter in the TIVA/PCEA group than in the TIVA/PCIA and TEA-IVA/PCIA groups (P <.05) and significantly shorter in the TEA-IVA/PCEA group than in the TIVA/PCIA group (P <.05; Table 5). The cost of anesthetic drugs and the total cost of anesthesia were significantly lower in the TEA-IVA/PCIA and TEA-IVA/PCEA groups than in the TIVA/PCIA and TIVA/PCEA groups (P <.05; Table 5). The cost of postoperative analgesia did not differ significantly between groups (Table 5).
Table 5.
Comparison of hospital stay and treatment costs between groups.

3.5. Incidence of postoperative AEs
The incidences of postoperative nausea/vomiting and pruritus were significantly higher in the 2 PCEA groups than in the 2 PCIA groups (P <.05; Table 6). There were no cases of urinary retention during the study period due to the use of urinary catheterization. The incidence of postoperative infection was not significantly different between the 4 groups (Table 6).
Table 6.
Comparison of postoperative adverse events between groups.

4. Discussion
A notable finding of the present study was that the stress response, as measured from changes in plasma cortisol level, was attenuated during surgery and postoperatively by the use of TEA and PCEA, respectively. Furthermore, the level of postoperative pain was lower in patients given PCEA than in those provided with PCIA. In addition, the use of TEA-IVA rather than TIVA lowered the cost of intraoperative anesthesia, and patients receiving PCEA had shorter time to first flatus and shorter hospital stay. However, the PCEA groups had higher incidences of nausea/vomiting and pruritus. Taken together, our novel findings suggest that thoracic epidural anesthesia/analgesia can reduce the stress response, improve postoperative recovery, shorten hospital stay and reduce costs for patients with EC.
Studies focusing on colorectal carcinoma have indicated that stress-inducing factors, such as autoimmune dysfunction, surgical wounds, anesthesia, and postoperative pain, can inhibit cellular immune functions in patients with malignant tumors and thereby promote tumor extension and metastasis.[30–32] Thus, reducing the stress response by optimizing the intraoperative and postoperative management of patients with EC could potentially improve outcomes. Factors likely to contribute to the stress response in patients with EC undergoing open thoracotomy include relatively large surgical wounds and substantial postoperative pain. Thus, the selection of appropriate methods of anesthesia and analgesia could potentially improve postoperative outcomes in these patients.[33] The addition of TEA to general anesthesia and the use of PCEA for postoperative analgesia are the most commonly used methods in current clinical practice,[34] and there is evidence from numerous studies that thoracic epidural anesthesia/analgesia can inhibit the stress response[16] and immune system dysfunction[16–18] in patients treated surgically for EC. The findings of the present study, in which changes in plasma cortisol level were used as an objective indicator of the stress response, were consistent with these previous reports.[16–18] Specifically, we found that the stress response during surgery was reduced by the addition of TEA to the anesthetic protocol, while the stress response after surgery was attenuated by the use of PCEA (rather than PCIA). In this study, 0.25% ropivacaine was administered during TEA, and 0.125% ropivacaine plus 2 μg/mL fentanyl were used for postoperative PCEA. Stimuli during surgery and recovery (including pain) can affect the hypothalamic-pituitary-adrenal (HPA) axis via spinal cord pathways and thereby increase the levels of corticosteroids.[35] We speculate that the use of thoracic epidural anesthesia/analgesia can block the transmission of harmful stimuli from the surgical site to the central nervous system and thus inhibit the increase in plasma cortisol level induced by surgery.
An important observation of the present study was that PCEA appeared to be superior to PCIA with regard to postoperative analgesia, as assessed using VAS pain scores. This would be in agreement with previous research indicating that PCEA can decrease postoperative opioid use.[17,20] The spinal cord is an important pathway for transmitting painful stimuli from the peripheral to the central nervous system and is closely associated with peripheral and central sensitization.[16] The drugs used in PCEA (ropivacaine and fentanyl) can exert analgesic effects via various mechanisms.[36] For example, fentanyl has been reported to activate opioid receptors in pain conduction zones, such as the spinal cord, medulla, midbrain, and thalamus, and thereby increase the pain threshold and inhibit the severity of harmful stimuli.[36] Interestingly, it has been demonstrated in experimental studies that opioids and local anesthetics can exert synergistic analgesic effects when introduced into the spinal canal.[37] The use of PCEA rather than PCIA in our patients was associated with a greater analgesic effect, and we speculate that this contributed to attenuation of the stress response. Thus, thoracic epidural anesthesia/analgesia could be an important component of enhanced recovery after surgery (ERAS) strategies, which are perioperative management methods designed to reduce surgical stress responses and the incidence of complications, shorten hospital stay and promote functional recovery.[38] ERAS was introduced 20 years ago for colorectal surgery but has since been applied to many other surgeries, such as pancreas surgery, thoracoscopic surgery and hepatobiliary surgery[39]. ERAS has recently been linked to decreased in-hospital morality[40]. Thoracic epidural anesthesia (TEA) still represents the standard of reference for the thoracotomic approach,[41] but other locoregional techniques have gained popularity in recent times[42]. Due to lack of adequate evidence, a similar consensus on the best approach has not been reached for esophageal carcinoma undergoing thoracic surgery.
This study also investigated the effects of the different anesthesia and analgesia methods on the recovery of intestinal function, which was assessed using the time to first flatus after surgery. The results showed that the time to first flatus was significantly shorter in the PCEA groups than in the PCIA groups, suggesting that PCEA can improve the recovery of intestinal function after surgery. Interestingly, a previous investigation found that PCEA enhanced the microcirculation of the gastric tube after surgery.[19] We speculate that local anesthetics administered during PCEA block nervous pathways in the abdominal region that respond to tissue damage as well as sympathetic efferent pathways, thereby reducing the neural inhibition of intestinal movement. In addition, the systemic absorption of local anesthetic agents may have other effects that contribute to activation of intestinal smooth muscles.[43] Furthermore, PCEA can reduce the use of opioids,[17,20] which can inhibit gastrointestinal motility and thus hamper the recovery of gastrointestinal functions.[44] A quicker recovery of gastrointestinal functions would facilitate earlier oral intake of food, potentially improving postoperative nutritional status and hence recovery of the patient.
Another advantage of PCEA over PCIA that was detected in our study was a shorter hospital stay. This is in agreement with other published studies reporting that PCEA could reduce the length of stay in the ICU or hospital.[20–24] It was also notable that the use of TEA during general anesthesia reduced the overall cost of the anesthetic procedure. This suggests that the use of thoracic epidural anesthesia and analgesia has the dual benefits of quickening patient recovery and reducing treatment costs.
The incidences of postoperative nausea/vomiting and pruritus were higher in patients receiving PCEA than in those given PCIA. However, the severity of these AEs was mild and all were tolerable, with no notable influence on patient prognosis. The occurrence of nausea and vomiting during PCEA may be associated with the effects of opioids on the central nervous system after systemic absorption from the spinal canal; the vestibular sensory/movement disorders and delayed gastric emptying induced by opioids may be associated with this mechanism. Pruritus is a rather common AE associated with opioid use during PCEA.[45] The mechanism underlying pruritus after opioid administration is still unclear.[46] However, studies have suggested that the effects could be associated with actions of opioids in the central nervous system since pruritus rarely appears after intravenous or muscular injection of higher doses of opioid.
This study has some limitations. First, this was a single-center study with a limited sample size, so the generalizability is unknown. Second, the VAS pain scoring system was objective and may have introduced some bias into the results. Third, inflammatory indicators were not assessed in this study, so the effects on immune system dysfunction were not explored. Fourth, other markers of gastrointestinal function recovery were not examined. Fifth, long-term outcomes such as tumor recurrence and survival were not measured. Additional research is merited to extend our findings.
In conclusion, the novel findings described in this study indicate that thoracic epidural anesthesia/analgesia can reduce the stress response, improve postoperative analgesia, facilitate the recovery of gastrointestinal function, shorten hospital stay and reduce treatment costs for patients with EC. We recommend that thoracic epidural anesthesia and analgesia be adopted as a standard protocol for patients undergoing surgery for EC.
Acknowledgments
The authors wish to thank the study participants for agreeing to take part in this study and the staff at Jiangsu Province Hospital who contributed to the care of these patients.
Author contributions
Conceptualization: Yan Li, Hongquan Dong, Shanbai Tan.
Data curation: Yan Li, Hongquan Dong, Shanbai Tan, Yanning Qian, Wenjie Jin.
Formal analysis: Yan Li, Hongquan Dong, Shanbai Tan, Yanning Qian, Wenjie Jin.
Investigation: Yan Li, Hongquan Dong, Yanning Qian.
Methodology: Yan Li, Hongquan Dong, Shanbai Tan, Yanning Qian, Wenjie Jin.
Project administration: Yan Li, Shanbai Tan, Wenjie Jin.
Resources: Hongquan Dong, Yanning Qian.
Supervision: Yan Li, Yanning Qian, Wenjie Jin.
Visualization: Hongquan Dong, Shanbai Tan.
Writing – original draft: Yan Li, Hongquan Dong.
Writing – review & editing: Yan Li, Hongquan Dong, Shanbai Tan, Yanning Qian, Wenjie Jin.
Footnotes
Abbreviations: AEs = adverse events, BMI = body mass index, EC = esophageal cancer, ERAS = enhanced recovery after surgery, ESCC = esophageal squamous cell carcinoma, HR = heart rate, ICU = intensive care unit, PCIA = patient-controlled intravenous analgesia, TEA-IVA = thoracic epidural anesthesia with intravenous general anesthesia, TIVA = total intravenous general anesthesia, VAS = visual analog scale.
YL and HD are contributed equally to this work.
The Ethics Committee of Jiangsu Province Hospital approved the study.
All patients included in this study provided informed written consent before surgery.
This project was sponsored by the National Natural Science Foundation of China (No.81701375), and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- [2].Di Pardo BJ, Bronson NW, Diggs BS, et al. The global burden of esophageal cancer: a disability-adjusted life-year approach. World J Surg 2016;40:395–401. [DOI] [PubMed] [Google Scholar]
- [3].Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen DJ, Ajani J. An expert opinion on esophageal cancer therapy. Expert Opin Pharmacother 2011;12:225–39. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cummings LC, Cooper GS. Descriptive epidemiology of esophageal carcinoma in the Ohio Cancer Registry. Cancer Detect Prev 2008;32:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mao WM, Zheng WH, Ling ZQ. Epidemiologic risk factors for esophageal cancer development. Asian Pac J Cancer Prev 2011;12:2461–6. [PubMed] [Google Scholar]
- [8].Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 2013;61:330–5. [DOI] [PubMed] [Google Scholar]
- [9].Uzunoglu FG, Reeh M, Kutup A, et al. Surgery of esophageal cancer. Langenbecks Arch Surg 2013;398:189–93. [DOI] [PubMed] [Google Scholar]
- [10].Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- [11].Zhu LL, Yuan L, Wang H, et al. A meta-analysis of concurrent chemoradiotherapy for advanced esophageal cancer. PLoS One 2015;10:e0128616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Feltracco P, Bortolato A, Barbieri S, et al. Perioperative benefit and outcome of thoracic epidural in esophageal surgery: a clinical review. Dis Esophagus 2018;31. [DOI] [PubMed] [Google Scholar]
- [13].Coisel Y, Jourdan A, Conseil M, et al. Esophageal cancer surgery: evolution of pain management, hemodynamics and ventilation practices during 16 years. Ann Fr Anesth Reanim 2014;33:16–20. [DOI] [PubMed] [Google Scholar]
- [14].Aceto P, Congedo E, Cardone A, et al. Postoperative management of elective esophagectomy for cancer. Rays 2005;30:289–94. [PubMed] [Google Scholar]
- [15].Chandrashekar MV, Irving M, Wayman J, et al. Immediate extubation and epidural analgesia allow safe management in a high-dependency unit after two-stage oesophagectomy. Results of eight years of experience in a specialized upper gastrointestinal unit in a district general hospital. Br J Anaesth 2003;90:474–9. [DOI] [PubMed] [Google Scholar]
- [16].Gu CY, Zhang J, Qian YN, et al. Effects of epidural anesthesia and postoperative epidural analgesia on immune function in esophageal carcinoma patients undergoing thoracic surgery. Mol Clin Oncol 2015;3:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fares KM, Mohamed SA, Hamza HM, et al. Effect of thoracic epidural analgesia on pro-inflammatory cytokines in patients subjected to protective lung ventilation during Ivor Lewis esophagectomy. Pain Physician 2014;17:305–15. [PubMed] [Google Scholar]
- [18].Xing CY, Wu MY, Fan HP. Effects of different anesthetic and analgesic protocols on cellular immune function and stress hormone level in patients undergoing lobectomy for esophagus cancer. Nan Fang Yi Ke Da Xue Xue Bao 2010;30:284–7. [PubMed] [Google Scholar]
- [19].Michelet P, Roch A, D’Journo XB, et al. Effect of thoracic epidural analgesia on gastric blood flow after oesophagectomy. Acta Anaesthesiol Scand 2007;51:587–94. [DOI] [PubMed] [Google Scholar]
- [20].Heinrich S, Janitz K, Merkel S, et al. Short- and long term effects of epidural analgesia on morbidity and mortality of esophageal cancer surgery. Langenbecks Arch Surg 2015;400:19–26. [DOI] [PubMed] [Google Scholar]
- [21].Yang M, Ahn HJ, Kim JA, et al. Risk score for postoperative complications in thoracic surgery. Korean J Anesthesiol 2012;63:527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saeki H, Ishimura H, Higashi H, et al. Postoperative management using intensive patient-controlled epidural analgesia and early rehabilitation after an esophagectomy. Surg Today 2009;39:476–80. [DOI] [PubMed] [Google Scholar]
- [23].Nagy K, Muranyi M, Nadas G, et al. Perioperative treatment after esophagogastric surgery. Magy Seb 2001;54:138–43. [PubMed] [Google Scholar]
- [24].Cense HA, Lagarde SM, de Jong K, et al. Association of no epidural analgesia with postoperative morbidity and mortality after transthoracic esophageal cancer resection. J Am Coll Surg 2006;202:395–400. [DOI] [PubMed] [Google Scholar]
- [25].Zingg U, Smithers BM, Gotley DC, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol 2011;18:1460–8. [DOI] [PubMed] [Google Scholar]
- [26].Khoronenko VE, Malanova AS, Baskakov DS, et al. Regional and peripheral blockades for prevention of chronic post-thoracotomy pain syndrome in oncosurgical practice. Khirurgiia (Mosk) 2017;58–63. [DOI] [PubMed] [Google Scholar]
- [27].Michelet P, D’Journo XB, Roch A, et al. Perioperative risk factors for anastomotic leakage after esophagectomy: influence of thoracic epidural analgesia. Chest 2005;128:3461–6. [DOI] [PubMed] [Google Scholar]
- [28].Wang W, Zhao G, Wu L, et al. Risk factors for anastomotic leakage following esophagectomy: impact of thoracic epidural analgesia. J Surg Oncol 2017;116:164–71. [DOI] [PubMed] [Google Scholar]
- [29].Hiller JG, Hacking MB, Link EK, et al. Perioperative epidural analgesia reduces cancer recurrence after gastro-oesophageal surgery. Acta Anaesthesiol Scand 2014;58:281–90. [DOI] [PubMed] [Google Scholar]
- [30].Kirkegaard T, Gogenur M, Gogenur I. Assessment of perioperative stress in colorectal cancer by use of in vitro cell models: a systematic review. PeerJ 2017;5:e4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].van der Bij GJ, Oosterling SJ, Beelen RH, et al. The perioperative period is an underutilized window of therapeutic opportunity in patients with colorectal cancer. Ann Surg 2009;249:727–34. [DOI] [PubMed] [Google Scholar]
- [32].Martinez AB, Ramirez JM, Calvo B, et al. Effect of the Enhanced Recovery After Surgery (ERAS) protocols on the immune response in open vs laparoscopic colorectal surgery. Clin Nutr Espen 2016;12:e34–5. [Google Scholar]
- [33].Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep 2017;7:14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guay J, Nishimori M, Kopp S. Epidural local anaesthetics versus opioid-based analgesic regimens for postoperative gastrointestinal paralysis, vomiting and pain after abdominal surgery. Cochrane Database Syst Rev 2016;7:CD001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Volk T, Schenk M, Voigt K, et al. Postoperative epidural anesthesia preserves lymphocyte, but not monocyte, immune function after major spine surgery. Anesth Analg 2004;98:1086–92. [DOI] [PubMed] [Google Scholar]
- [36].Coda BA, Brown MC, Schaffer R, et al. Pharmacology of epidural fentanyl, alfentanil, and sufentanil in volunteers. Anesthesiology 1994;81:1149–61. [DOI] [PubMed] [Google Scholar]
- [37].Mahon SV, Berry PD, Jackson M, et al. Thoracic epidural infusions for post-thoracotomy pain: a comparison of fentanyl-bupivacaine mixtures vs. fentanyl alone. Anaesthesia 1999;54:641–6. [DOI] [PubMed] [Google Scholar]
- [38].Pirzada MT, Naseer F, Haider R, et al. Enhanced recovery after surgery (ERAS) protocol in stoma reversals. J Pak Med Assoc 2017;67:1674–8. [PubMed] [Google Scholar]
- [39].Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice[J]. J Thorac Dis 2018;10suppl 4:S542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Desai H, Natt B, Kim S, et al. Decreased in-hospital mortality after lobectomy using video-assisted thoracoscopic surgery compared with open thoracotomy. Ann Am Thorac Soc 2017;14:262–6. [DOI] [PubMed] [Google Scholar]
- [41].Loop T. Fast track in thoracic surgery and anaesthesia: update of concepts. Curr Opin Anaesthesiol 2016;29:20–5. [DOI] [PubMed] [Google Scholar]
- [42].Steinthorsdottir KJ, Wildgaard L, Hansen HJ, et al. Regional analgesia for video-assisted thoracic surgery: a systematic review. Eur J Cardiothorac Surg 2014;45:959–66. [DOI] [PubMed] [Google Scholar]
- [43].Zhao J, Mo H. The impact of different anesthesia methods on stress reaction and immune function of the patients with gastric cancer during peri-operative period. J Med Assoc Thai 2015;98:568–73. [PubMed] [Google Scholar]
- [44].Arroyo Plasencia AM, Mowry J, Smith J, et al. In vitro release of fentanyl from transdermal patches in gastric and intestinal fluid. Clin Toxicol (Phila) 2014;52:945–7. [DOI] [PubMed] [Google Scholar]
- [45].Ruan X, Ma L, Couch JP, et al. Intractable pruritus during outpatient epidural hydromorphone infusion: a case report and a focused review of the literature. J Opioid Manag 2015;11:184–90. [DOI] [PubMed] [Google Scholar]
- [46].Hirabayashi M, Doi K, Imamachi N, et al. Prophylactic pentazocine reduces the incidence of pruritus after cesarean delivery under spinal anesthesia with opioids: a prospective randomized clinical trial. Anesth Analg 2017;124:1930–4. [DOI] [PubMed] [Google Scholar]


