Abstract
Introduction:
Few studies examine how depression and substance use interact to affect HIV control.
Methods:
In 14,380 persons with HIV (PWH), we used logistic regression and generalized estimating equations to evaluate how symptoms of depression interact with alcohol, cocaine, opioid, and methamphetamine use to affect subsequent retention in care, maintaining an active prescription for ART, and consistent virologic suppression.
Results:
Among PWH with no or mild depressive symptoms, heavy alcohol use had no association with virologic suppression (OR 1.00 [0.95–1.06]); among those with moderate or severe symptoms, it was associated with reduced viral suppression (OR 0.80 [0.74–0.87]). We found no interactions with heavy alcohol use on retention in care or maintaining ART prescription or with other substances for any outcome.
Discussion:
These results highlight the importance of treating moderate or severe depression in PWH, especially with comorbid heavy alcohol use, and support multifaceted interventions targeting alcohol use and depression.
Keywords: alcohol, HIV, depression, illicit drug use, viral suppression
INTRODUCTION:
Persons with HIV (PWH) consistently adhering to antiretroviral therapy (ART) and sustaining virologic suppression have an almost normal lifespan.1, 2 In order to achieve virologic suppression, PWH must proceed through the HIV care continuum: diagnosis of HIV, linkage to HIV care, retention in care, and receipt of and adherence to ART.3 However, a substantial proportion of PWH linked to care (15–25%) are not retained in care, and of those who are, a significant subset (9–24%) fail to achieve or maintain virologic suppression.4–7 PWH who fail to suppress their viral load represent a missed opportunity to optimize individual health and prevent new HIV infections and are thus a priority for intervention.
Depression and misuse of substances, such as alcohol, opioids, cocaine, and methamphetamine are highly prevalent, modifiable risk factors for poor HIV outcomes. In a nationally representative sample, 36% of PWH in care screened positive for depression.8 Depression is associated with decreased retention in HIV care,9, 10, decreased adherence to ART,11, 12, and failure to achieve virologic suppression.12–19
Similarly, nearly 50% of PWH in care report substance use,20, 21 with 8 to 27% reporting heavy or hazardous alcohol use,20, 22, 23 and 10 to 40% reporting use of an illicit drug other than marijuana.8, 20, 23, 24 Alcohol misuse is associated with decreased retention in care, and decreased utilization of and adherence to ART, with a more pronounced effect for hazardous drinking.25–29 Use of cocaine, opioids, and methamphetamine are associated with decreased receipt of and adherence to ART, as well as worse virologic response to ART in some studies.24, 29–41 Further, alcohol misuse is highly comorbid with depression in PWH,32, 42–45 as are use of cocaine, opioids, and methamphetamine.32, 45–47
Both depression and substance use reduce engagement at multiple steps in HIV care and engender behaviors that impede the ability of PWH to ultimately achieve virologic suppression.36, 48 Furthermore, comorbid depression and substance use may work synergistically in reducing engagement or promoting behaviors that impede the continuum of care. For example, use of alcohol and other substances may have direct effects on HIV replication and immune system activation,49–51 which may render PWH who use substances particularly vulnerable to interruptions in ART as a result of comorbid depression. However, relatively few studies have examined the specific ways in which depression and substance use interact to affect care continuum outcomes.11, 52, 53 Our objective for this study was to identify how symptoms of depression interact with alcohol, cocaine, illicit opioid, and methamphetamine use to affect three care continuum outcomes in PWH: retention in care, having an active prescription for ART, and achievement of virologic suppression.
METHODS:
Study Population:
We studied patients enrolled in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS), a racially diverse cohort of PWH engaged in routine HIV medical care across 8 clinical US sites. CNICS has been described in detail elsewhere.54 Briefly, the CNICS data repository integrates longitudinal data including comprehensive clinical information from outpatient and inpatient encounters, demographic, clinical, medication, laboratory, and socioeconomic data obtained from each site’s electronic health record and other institutional data sources.54 Additionally, approximately every 6 months, as part of routine clinical care visits, a majority of the cohort completes a patient-reported outcome (PRO) computer-assisted survey, which includes the Patient Health Questionnaire 9 (PHQ-9),55 the Alcohol Use Disorders Identification Test consumption questions (AUDIT-C),56, 57 and the National Institute on Drug Abuse modified Alcohol, Smoking And Substance Involvement Screening Test (NIDA-ASSIST).58, 59
Our study sample consisted of all individuals with a PRO completed between Feb 17, 2005 (when PROs were first collected in the cohort) and July 31, 2016, to allow for at least a year of potential observation afterward (although individuals included in the sample may not have been retained in care). Individuals could appear multiple times in the sample if they completed multiple PROs, and were included whether or not they were previously established in care or receiving ART. We excluded PROs that fell within 90 days of a previously completed PRO for an individual.
Outcome Definitions:
Outcomes for a particular individual and PRO were defined relative to the time the PRO was recorded. We used the Institute of Medicine (IOM) definition of retention in care: at least two HIV primary care visits at least 90 days apart within the year following the PRO.60 We defined an active prescription for ART as a prescription for any ART at any time between 150 and 180 days after the PRO by pharmacy records. As almost all CNICS subjects take ART, we did not restrict to ART-naïve; having an active prescription for ART principally reflects continued receipt of ART. We chose a 30-day window so that a gap of a few days between prescriptions would not be counted as lacking an active prescription; we lagged the outcome as antidepressants take a few months to reach full effect,61 and other studies of depression and ART have looked at receipt and adherence ranging from 1 to 15 months, with a number looking at outcomes at 6 months.12, 62–66 We defined consistent virologic suppression as having all viral loads drawn in the subsequent year <200 copies/mL.67 No subjects were censored for the outcomes of retention in care or active prescription for ART; whether or not subjects died or stopped following in clinic, their outcomes were defined based on the criteria above. Subjects were not included in the analysis of virologic suppression if they were retained in care but had no viral loads drawn in the year following a PRO; we made the conservative assumption that subjects who had no viral loads checked within the subsequent year and were not retained in care had unsuppressed viral loads.
We evaluated each of the three outcomes for all subjects, rather than nesting them (i.e., evaluating ART receipt only in those retained in care, and evaluating virologic suppression only in those on ART). Over the course of year, the outcomes do not nest neatly: the overwhelming majority of CNICS subjects are on ART even without meeting the IOM definition of retention in care, and a large proportion also achieve virologic suppression without being retained in care. Our analysis of virologic suppression therefore reflects failures to suppress viral load due to all upstream drivers (engagement in care, receipt of ART, and adherence).
Independent Variables:
The PHQ-9 asked about symptoms over the prior 2 weeks, the NIDA-ASSIST asked about behaviors in the past 3 months, and the AUDIT-C asked about behaviors in the preceding year. Consistent with prior studies, we dichotomized symptoms of depression as “moderate-severe” - a score of ≥10 PHQ-9 – vs. “no-mild” – a score of <10 on the PHQ-9.55 We categorized alcohol use as “none” – a score of 0 on the AUDIT-C – “moderate” - a score of 1 or 2 in women or 1–3 in men - and “heavy” – a score of ≥3 in women or ≥4 in men, consistent with recommended scoring.57 We defined recent use of cocaine, opioids, and methamphetamine as any reported recreational use in the preceding 3 months on the NIDA-ASSIST; opioid use includes abuse of prescription opioids but not opioids prescribed by a medical provider.
We also adjusted for clinic site, age, sex, race, and whether subjects were men who have sex with men (MSM), which have been associated in prior studies with retention in care, receipt of ART, and virologic suppression.68–71 Because subjects who have been enrolled for longer in the cohort may be more likely to continue in care and comply with therapy, we additionally adjusted for time since enrollment in the cohort, as has been done in prior CNICS studies.25
In a secondary analysis, we considered use of antidepressants, defined as an active prescription at the time of PRO completion for any of the following: a selective serotonin reuptake inhibitor, serotonin-norepinephrine reuptake inhibitor, tricyclic antidepressant, serotonin modulator and stimulator, monoamine oxidase inhibitor, bupropion, or nefazodone. Antidepressants may have been prescribed by any provider, not just HIV providers; CNICS captures prescriptions by other providers, such as psychiatrists, in the health care networks of CNICS sites.
Statistical Methods:
We built a logistic model for each of our three outcomes, with indicator variables for recent symptoms of depression, moderate or heavy alcohol use, cocaine use, opioid use, and methamphetamine use as well as product terms for depressive symptoms and each of the four substances. We adjusted for age and time since enrollment in the cohort, both as restricted quadratic splines with knots at the 20th, 40th, 60th, and 80th percentiles.72 We additionally adjusted for clinic site, sex, race, and whether subjects were men who have sex with men (MSM). To account for repeated measurements on subjects, we used generalized estimating equations; because outcomes were defined based on the 6–12 months following a PRO and our sample included repeated PROs as close as 90 days apart, we used an autoregressive-1 correlation structure and robust standard errors.73
Because some outcomes for virologic suppression were missing (subjects who were retained in care but had no viral load measured in the subsequent year), we weighted the logistic model for virologic suppression with inverse probability of observation weights.74 We constructed the weights using a logistic regression model with the same covariates and interaction terms as in the main model. Use of inverse probability of observation weights relaxes the assumption that viral load measurements are missing completely at random and instead assumes they are missing at random conditional on covariates in the weight model.
For missing PRO components, we performed multiple imputation by chained equations75 with 10 imputations based on demographic characteristics, the three care continuum outcomes, and responses to the PRO question on the previous and subsequent evaluation (when there was a previous or subsequent evaluation). We imputed the PROs at the levels of individual questions (e.g., the 9 individual PHQ questions) rather than the aggregate score. We fit our models on each of the 10 imputed datasets, and report the pooled estimates.76 We re-ran the models with 20 imputations to make sure our estimates were stable. We assessed collinearity among model covariates by calculating variance inflation factors averaged across imputations.77
Because symptoms of depression are a modifiable risk factor with respect to the care continuum, we conducted a secondary analysis in which we stratified depressive symptoms according to whether subjects were concurrently on antidepressant therapy. We also conducted post-hoc analyses in which we further explored the relationship between depressive symptoms and alternative measures related to retention in care: number of scheduled visits, number of kept visits, proportion of kept visits, and 6-month visit constancy over a year (the number of 6-month intervals in the following year in which a patient had at least one visit - which could take values 0, 1, or 2).78 These analyses mirrored the covariates and imputation scheme of the primary analyses.
We tested for departures from multiplicative interaction (i.e., sub- or super-multiplicativity) between substance use and depressive symptoms on care continuum outcomes by assessing whether the product terms for moderate-severe symptoms of depression with each of the substances were significantly different than zero on the log scale. Because five interactions for each of three outcomes induces a large number of comparisons, we used the Quasi Information Criterion (QIC)79, 80 a global test of model fit for each outcome to account for multiple comparisons. The QIC is analogous to the Aikake Information Criterion, representing a trade-off between goodness-of-fit and model complexity, and is appropriate for use with GEEs. If the mean QIC (across imputations) for a model with interaction terms was higher (worse) than for the corresponding model without interactions, we concluded that there was not compelling evidence of a departure from multiplicative interaction. We used a global test rather than a Bonferroni or other multiple comparison adjustments because global tests are generally more efficient and sensitive than multiple comparison adjustment which can greatly increase the type II error rate.81, 82
All statistical analyses were conducted in R version 3.4.0,83 using the packages mice,75 geepack,84 geem,85 and car.86
RESULTS:
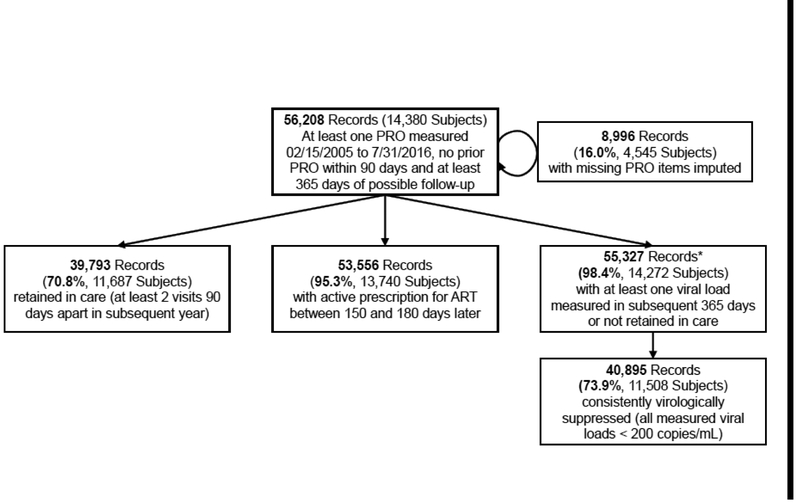
Our study sample comprised 14,380 individuals who completed 56,208 PROs. A plurality of subjects were white (47.5%) and the majority were male (83.0%) (Table I). Of all completed PROs, 8,996 (16.0%) were missing one or more PRO components, and had values imputed (Figure 1). Overall, 11,420 (22.0%) of 51,838 PROs with a complete PHQ-9 score indicated moderate-severe symptoms of depression. Among PROs with moderate-severe symptoms of depression, the prevalence of recent substance use was: moderate alcohol, 30.5%; heavy alcohol, 32.2%; cocaine, 9.8%; opioids, 4.3%; and methamphetamine, 13.9%. Among PROs with no-mild symptoms of depression, prevalence of recent substance use was: moderate alcohol 34.9%; heavy alcohol, 28.6%; cocaine, 5.2%; opioids, 1.4%; and methamphetamine, 6.3%.
Table I:
Characteristics of Study Population Based on Encounters from Feb 17, 2005 to July 31, 2016 in which a PHQ-9 was Completed*
| Initial Visit | All Visits | |||
|---|---|---|---|---|
|
Moderate-Severe Symptoms of Depression (PHQ-9 ≥10) |
No-Mild Symptoms of Depression (PHQ-9 <10) |
Moderate-Severe Symptoms of Depression (PHQ-9 ≥ 10) |
No-Mild Symptoms of Depression (PHQ-9 < 10) |
|
| All, % (n) | 25.5% (3,522) | 74.5% (10,265) | 22% (11,420) | 78% (40,418) |
| Age, mean [IQR] | 43.0 [35.6–50.2] | 44.7 [36.4–52.4] | 45.6 [39–52.3] | 46.6 [38.9–54.1] |
| Female | 18.2% (641) | 16.6% (1,700) | 18.7% (2,139) | 16.8% (6,802) |
| Race, %(n) | ||||
| Other | 5.1% (180) | 4.7% (478) | 4.1% (471) | 3.8% (1,527) |
| MSM, % (n) | 68.5% (2414) | 65.8% (6,756) | 67.8% (7,740) | 67% (27,078) |
| Years since enrollment in cohort, mean [IQR] | 3.5 [0.3–6] | 4.4 [0.5–7.6] | 5.5 [1.5–8.6] | 6.4 [2–10.1] |
| On antidepressant therapy, %(n) | 32.3% (1,139) | 17.4% (1,783) | 40.8% (4,664) | 22% (8,898) |
| Alcohol Use, %(n†) | ||||
| Heavy | 37.1% (1,240) | 31.9% (3,165) | 32.2% (3,487) | 28.6% (11,136) |
| Recent Cocaine Use, % (n†) | 12.6% (425) | 6.6% (659) | 9.8% (1,078) | 5.2% (2,036) |
| Recent Opioid Use, % (n†) | 5.0% (164) | 1.9% (178) | 4.3% (462) | 1.4% (546) |
| Recent Methamphetamine Use, % (n†) | 18.5% (620) | 8.0% (796) | 13.9% (1,518) | 6.3% (2,462) |
Not all subjects completed the PRO questions for alcohol, cocaine, opioid, and methamphetamine use, so the denominators are less than the total numbers of subjects. IQR = Interquartile range
Figure 1:
Consort Diagram
*53,213 (94.7% of the total sample) records had at least one viral load drawn in the subsequent year. Of those that had no viral load drawn, 2,114 (3.8% of the total sample) were not subsequently retained in care - these were assumed to be not consistently virologically suppressed.
472 subjects (0.8%) died within one year of completing a PRO. Of those, 148 (31%) met the definition for retention in care prior to death, 441 (93%) had an active ART script 5–6 months after the index PRO, and 251 (53%) had all viral loads subsequent to the PRO suppressed. In the fitted models no covariate (except splines for age and time since enrollment in cohort) had an average variable inflation factor greater than 8, indicating minimal collinearity among covariates.
Retention in Care:
Following 39,739 PROs (70.8% of the study sample) subjects were retained in care in the subsequent year. There were no statistically significant departures from multiplicativity for the relative odds of retention in care associated with symptoms of depression and substance use (Table II). These associations were consistent when symptoms of depression were stratified by concurrent use of antidepressants (Table III).
Table II:
Effect of Symptoms of Depression and Substance Misuse on HIV Continuum of Care
| Substance Use | Symptoms of Depression (n) |
Retention in Care, AOR, 95% Cl |
Receipt of ART, AOR, 95% Cl |
Virologic Suppression, AOR, 95% CI |
|---|---|---|---|---|
| No Substance Use | No-Mild (17,544) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Moderate-Severe (4,315) | 1.20 [1.11–1.29] | 0.97 [0.87–1.08] | 0.90 [0.84–0.96] | |
| Moderate Alcohol Use | No-Mild (17,281) | 0.85 [0.81–0.90] | 0.99 [0.91–1.08] | 1.02 [0.97–1.08] |
| Moderate-Severe (4,154) | 1.09 [1.00–1.19] | 0.89 [0.78–1.01] | 0.86 [0.79–0.93] | |
| Heavy Alcohol Use | No-Mild (14,268) | 0.79 [0.74–0.83] | 0.90 [0.82–0.99] | 1.00 [0.95–1.06] |
| Moderate-Severe (4,439) | 0.93 [0.85–1.01] | 0.85 [0.75–0.96] | 0.80 [0.74–0.87]* | |
| Recent Cocaine Use | No-Mild (2,899) | 1.01 [0.93–1.11] | 0.81 [0.71–0.93] | 0.82 [0.75–0.90] |
| Moderate-Severe (1,437) | 1.26 [1.08–1.47] | 0.86 [0.70–1.05] | 0.76 [0.66–0.87] | |
| Recent Opioid Use | No-Mild (912) | 1.12 [0.95–1.32] | 1.11 [0.87–1.41] | 0.89 [0.77–1.03] |
| Moderate-Severe (664) | 1.07 [0.88–1.30] | 0.96 [0.71–1.30] | 0.87 [0.73–1.03] | |
| Recent Meth-amphetamine Use | No-Mild (3,131) | 0.95 [0.87–1.05] | 1.00 [0.88–1.13] | 0.72 [0.65–0.78] |
| Moderate-Severe (1,950) | 1.15 [1.00–1.32] | 0.93 [0.77–1.11] | 0.62 [0.54–0.70] |
Models adjusted for clinic site, age, sex, race, whether subjects were men who have sex with men (MSM), and time since enrollment in the cohort. AOR = adjusted odds ratio, CI = confidence interval.
The association between substance use and the outcome in the presence of moderate-severe symptoms of depression is significantly lower than would be expected from the associations with substance use and symptoms of depression alone (p-value for interaction is 0.019).
Table III:
Effects of Symptoms of Depression, Use of Antidepressants, and Substance Misuse on HIV Continuum of Care
| Substance Use | Symptoms of Depression |
Concurrent Antidepressant Use (n) |
Retention in Care, AOR, 95% Cl |
Receipt of ART, AOR, 95% CI |
Virologic Suppression, AOR, 95% Cl |
|---|---|---|---|---|---|
| Moderate Alcohol Use | No-Mild | No (13,357) | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes (4,187) | 1.28 [1.18–1.40] | 2.53 [2.03–3.15] | 1.13 [1.03–1.23] | ||
| Moderate-Severe | No (2,514) | 1.19 [1.08–1.31] | 0.97 [0.85–1.11] | 0.85 [0.78–0.92] | |
| Yes (1,801) | 1.45 [1.29–1.63] | 2.20 [1.78–2.72] | 1.09 [0.98–1.21] | ||
| Moderate Alcohol Use | No-Mild | No (13,901) | 0.85 [0.80–0.90] | 1.04 [0.95–1.14] | 1.04 [0.98–1.11] |
| Yes (3,380) | 1.15 [1.05–1.27] | 2.00 [1.59–2.51] | 1.08 [0.99–1.19] | ||
| Moderate-Severe | No (2,533) | 1.08 [0.97–1.20] | 0.87 [0.76–1.00] | 0.76 [0.69–0.84]* | |
| Yes (1,621) | 1.34 [118–1.51] | 2.02 [1.50–2.72] | 1.14 [1.01–1.28] | ||
| Heavy Alcohol Use | No-Mild | No (11,648) | 0.79 [0.74–0.84] | 0.93 [0.84–1.03] | 1.00 [0.94–1.07] |
| Yes (2,620) | 1.05 [0.94–1.16] | 2.00 [1.57–2.55] | 1.14 [1.03–1.27] | ||
| Moderate-Severe | No (2,824) | 0.92 [0.83–1.03] | 0.84 [0.72–0.98] | 0.74 [0.67–0.82]† | |
| Yes (1,615) | 1.12 [0.98–1.271 | 1.85 [1.46–2.331 | 0.99 [0.88–1.111 | ||
| Recent Cocaine Use | No-Mild | No (2,274) | 1.03 [0.92–1.14] | 0.83 [0.72–0.96] | 0.79 [0.71–0.88] |
| Yes (625) | 1.21 [0.99–1.48] | 1.71 [1.17–2.51] | 1.00 [0.82–1.21] | ||
| Moderate-Severe | No (946) | 1.30 [1.07–1.58] | 0.83 [0.65–1.05] | 0.73 [0.61–0.86] | |
| Yes (491) | 1.42 [1.12–1.801 | 2.06 [1.32–3.231 | 0.91 [0.74–1.121 | ||
| Recent Opioid Use | No-Mild | No (690) | 1.06 [0.87–1.30] | 1.06 [0.81–1.38] | 0.86 [0.72–1.03] |
| Yes (222) | 1.58 [1.16–2.17] | 3.76 [1.67–8.46] | 1.09 [0.82–1.46] | ||
| Moderate-Severe | No (446) | 0.97 [0.76–1.24] | 0.99 [0.68–1.44] | 0.85 [0.69–1.06] | |
| Yes (218) | 1.52 [1.13–2.061 | 2.10 [1.32–3.331 | 0.97 [0.75–1.261 | ||
| Recent Methamphetamine Use | No-Mild | No (2,584) | 0.97 [0.87–1.07] | 1.02 [0.89–1.16] | 0.71 [0.64–0.79] |
| Yes (547) | 1.15 [0.94–1.42] | 2.45 [1.75–3.44] | 0.80 [0.65–0.98] | ||
| Moderate-Severe | No (1,440) | 1.18 [1.00–1.40] | 0.97 [0.79–1.19] | 0.60 [0.52–0.70] | |
| Yes (510) | 1.30 [1.03–1.65] | 1.91 [1.31–2.79] | 0.73 [0.59–0.92] |
Models adjusted for clinic site, age, sex, race, whether subjects were men who have sex with men (MSM), and time since enrollment in the cohort.AOR = adjusted odds ratio, CI = confidence interval. *,†The association between substance use and the outcome in the presence of symptoms of depression given antidepressant use is significantly than would be expected from the associations with substance use and symptoms of depression alone.
P-value for interaction is 0.016.
P-value for interaction is 0.040.
Moderate-severe symptoms of depression (in the absence of substance use) were associated with higher odds of retention (OR 1.20, 95% confidence interval [CI]: 1.11–1.29) (Table II). In contrast, both moderate and heavy alcohol use were associated with lower odds of subsequent retention in care (OR for moderate use vs. no use 0.85, 95% CI: 0.81–0.90, OR for heavy use 0.79, 95% CI: 0.74–0.83). Cocaine, opioid, and methamphetamine use were not associated with retention in care.
In light of finding that symptoms of depression were associated with higher odds of retention in care, we conducted post-hoc analyses to further explore the relationship between depression and attendance at HIV primary care visits. In the year following a PRO indicating moderate-severe symptoms of depression, subjects had an average of 4.9 scheduled HIV primary care visits and 4.2 kept visits vs. 3.4 scheduled visits and 3.2 kept visits following a PRO indicating no-mild symptoms of depression. In multivariate analyses, subjects with moderate-severe symptoms of depression had on average 10% (95% CI: 7–13%) more scheduled visits in the subsequent year, and despite having lower odds of keeping an appointment (OR 0.88, 95% CI: 0.84–0.92), they had on average 8% more kept visits (95% CI: 6–11%) and were more likely to have a kept appointment in the two following six-month intervals (OR 1.15, 95% CI: 1.09–1.23).
Receipt of ART:
Following 53,556 PROs (95.3% of the study sample) subjects had an active prescription for ART 150 – 180 days later. There was no evidence of non-multiplicative interactions between depression and substance use overall nor in models that considered antidepressant use.
There was no association between moderate-severe symptoms of depression and having an active prescription for ART (OR 0.97, 95% CI: 0.87–1.08). Heavy alcohol use and cocaine use were associated with lower odds of subsequently having an active prescription for ART (OR for heavy alcohol use vs. no use 0.90, 95% CI: 0.82–0.99, OR for cocaine 0.81, 95% CI: 0.71–0.93] (Table II). When depressive symptoms were stratified by antidepressant use, both use of antidepressants with no-mild symptoms of depression (OR 2.53, 95% CI: 2.03–3.15) and use of antidepressants with moderate-severe symptoms of depression symptoms (OR 2.20, 95% CI: 1.78–2.72) were associated with increased odds of subsequently having an active prescription for ART relative to no use of antidepressants with no-mild symptoms (Table III).
Virologic Suppression:
Following 40,895 PROs (73.9% of the 55,327 PROs where at least one viral load was measured or the subject was not retained in care over the following year) all viral loads were <200 in the subsequent year. There was significant super-multiplicative interaction between depression and heavy alcohol use for the odds of virologic suppression. With no-mild symptoms of depression, heavy alcohol use had no independent association with virologic suppression (OR 1.00, 95% CI: 0.95–1.06). However, reporting both moderate-severe symptoms of depression and heavy alcohol use was associated with 0.80 times the odds of virologic suppression (95% CI: 0.74–0.87) compared to reporting neither. This was lower than the association expected if each exposure acted independently on a multiplicative scale (the ratio of odds ratios for the interaction term was 0.89, 95% CI: 0.81–0.98, p=0.019). After stratifying depressive symptoms according to concurrent use of antidepressants, the super-multiplicative interaction between heavy alcohol use and moderate-severe symptoms of depression persisted only in the absence of antidepressant use (Table III). In the absence of antidepressant use there was also a significant interaction between moderate alcohol use and moderate-severe symptoms of depression. Interactions with cocaine, opioid, or methamphetamine use were all non-significant.
Moderate-severe symptoms of depression were independently associated with lower odds of subsequently achieving virologic suppression (OR 0.90, 95% CI: 0.84–0.96), as were cocaine use (OR 0.82, 95% CI: 0.75–0.90) and methamphetamine use (OR 0.72, 95% CI: 0.65–0.78) (Table II). Neither moderate nor heavy alcohol use nor opioid use had a significant association with virologic suppression.
DISCUSSION:
In a longitudinal study of PWH in medical care, the association between heavy alcohol use and consistent virologic suppression during the subsequent year depended on concurrent symptoms of depression. With no-mild symptoms of depression, heavy alcohol use had no association with subsequent virologic suppression. However, the combination of both moderate-severe symptoms of depression and heavy alcohol use was associated with lower odds of subsequent virologic suppression than would be expected based on their independent associations. Our secondary analysis suggested that the interaction between symptoms of depression and heavy alcohol use was present only in PWH not taking antidepressants. Cocaine, opioid, and methamphetamine use did not interact super- or sub multiplicatively with symptoms of depression when estimating the odds of virologic suppression, and no substance interacted super- or sub-multiplicatively with symptoms of depression for the odds of either retention in care or receipt of ART. These results highlight the need for antidepressant treatment and multifaceted interventions that target alcohol misuse and depression simultaneously.
Few studies have examined the interaction of depression and substance misuse on the HIV care continuum, and they have all been cross-sectional. Two studies in the HIV Research Network, with 10,284 and 5,119 subjects, did not find significant interactions between any mental illness (of which 84–88% were depression) and illicit drug use in either receipt of ART or virologic suppression, consistent with our results.36, 52 A study of 1,710 women with HIV found a significant interaction between probable depression, by the Center for Epidemiologic Studies Depression Scale (CES-D) scale, and recent illicit drug use in lowering the odds of receiving ART,35 whereas we found no significant interaction. This difference may be due to the fact that the study included only women, or to the different assessment tools used to measure depression; in specific populations, the Pearson correlation between PHQ-9 and CES-D scores has ranged from 0.77 to 0.85.87–89 To our knowledge, no prior studies have evaluated the interaction between alcohol use and depression in PWH.
The interaction between symptoms of depression and heavy alcohol use raises mechanistic questions. Poor adherence to ART is the most cited potential mechanism for the effect of depression on virologic suppression, although in several studies depression has been associated with decreased virologic suppression even after adjusting for self-reported adherence.13 Additionally, depression may affect the timing of ART initiation and interruptions to ART.13, 90 Depression has also been associated with high-risk sexual behaviors,31, 91 which may predispose to superinfection with multiple strains of HIV, increasing the risk of resistance.91 Alcohol likely has similar effects on adherence, timing of ART, and high-risk behaviors.26, 31, 92 Furthermore, in vitro studies and studies of simian immunodeficiency virus suggest that alcohol may have more direct effects on viral burden by increasing viral replication and promoting chronic, systemic immune activation.49 Lastly, both depression and alcohol misuse are associated with psychosocial comorbidities that can impair engagement in care.36, 48 Alcohol and depression may work synergistically in worsening adherence, or it may be that the direct biological effects of alcohol have a more pronounced impact when layered onto poor adherence to ART engendered by depression. The interaction may also represent a confluence of psychosocial factors or that alcohol use patterns in patients with depression differ from use in non-depressed patients in a manner not captured by the AUDIT-C. It is important to note that CNICS measures of depressive symptoms and alcohol use reflect recent trends; as they are measured concurrently, we cannot infer temporal directionality regarding the mechanism of interaction.
Regardless of the mechanism, our results have several implications. Our findings that the interaction between depression and heavy alcohol use persisted in patients not on antidepressants reinforces the imperative of diagnosing and treating depression, especially in the setting of alcohol misuse. Our results also argue for the use of multifaceted interventions that target both depression and alcohol misuse. Integrated interventions that target both psychiatric and substance use disorders simultaneously are more successful than multiple separate interventions,48, 93 and are most effective if they target both clinical and psychosocial aspects of disease in a multidisciplinary fashion.48, 94
Our findings regarding the main effects of depression and substance use on virologic suppression and receipt of ART are largely consistent with the published literature. We found that depression and use of cocaine or methamphetamine were associated with lower odds of virologic suppression, and that cocaine use and heavy alcohol use were associated with lower odds of being on ART. Other longitudinal studies have found that depression and use of cocaine are associated with failure to achieve virologic suppression,12–19, 30, 33 whereas studies of the association between alcohol, opioid, or methamphetamine use and viral load have had mixed results.24, 33, 38, 40, 95–97 Other studies have also found illicit drug use (including cocaine use and intravenous opioid use) to be associated with lower odds of PWH in care being on ART,24, 34, 35, 52 while finding no such association for depression.35, 36, 52
Existing literature on retention in care is more mixed, owing in part to multiple definitions of retention. We found that moderate and heavy alcohol use were associated with decreased retention in care, and found no association of retention in care with either cocaine, opioid, or methamphetamine use. Further, we found that symptoms of depression were associated with increased retention in care. A previous study in the CNICS cohort found, as we did, that heavy alcohol use (as defined by the AUDIT-C) is associated with decreased retention in care,25 although other studies with alcohol misuse abstracted from the medical record have not found such an association.98, 99 In contrast to our findings, prior studies have generally found illicit substance use (both all substances in aggregate, and cocaine and opioids individually) to be associated with worse retention in care,98–100 and have either found depression to be associated with decreased retention in care,9 or found no association.98, 99 With the exception of the analysis done in CNICS, these studies have all used different definitions for retention in care than we did: having a visit in all four 3-month intervals over a year, not being lost to follow-up for 60 days, or visit constancy (the number of intervals in which subjects had at least one visit). Our discordant findings, particularly the association between depressive symptoms and increased retention in care, may be due in part to the fact that the lOM’s definition (at least two HIV care visits at least 90 days apart within the subsequent year60) depends on the total number of kept visits. In our sample, patients with moderate-severe symptoms of depression had more primary care visits scheduled, and, despite having lower odds of keeping an appointment, had more kept visits. This may reflect the fact that depression is associated with higher health services utilization,101 and in a population of PWH in care, a higher depressive symptom burden may prompt more primary care visits. With respect to substance misuse, as many CNICS subjects have been in care for years and we use repeated observations in our study, those who have managed to negotiate HIV care while using illicit drugs likely contribute more observations than those who drop out of care.54 Furthermore, our exposure for drug use (any reported use in the past three months) may represent a different pattern of use than in other studies, many of which use a shorter time frame or restrict to IV drug use.98–100
Our study has a number of potential limitations. First, depression and substance use were based on patient reported instruments, and are subject to possible underreporting. However, the instruments are all validated, and electronic self-report permits integration into care and decreases underreporting of risk behaviors due to social desirability bias.102 Second, we used the PHQ-9 to categorize depression rather than criteria from the Diagnostic and Statistical Manual of Mental Disorders, which differs from some prior studies. However, the PHQ-9 cutoff of 10 has high sensitivity and specificity relative to a clinical diagnosis,55 and current depression symptom burden is arguably more important to HIV outcomes than a clinical diagnosis in which symptoms may or may not be controlled. Third, we combined moderate and severe depression into a single exposure, which might overlook nonlinear associations with depression severity, as have been found in sexual risk behaviors among MSM.103 Fourth, it is possible that some patients leave the cohort to establish care at another clinical site, and are incorrectly categorized as not retained. To skew our results, such transitions would have to be more or less likely for PWH with depression or substance misuse. Fifth, although our analysis accounts for repeated measures within individuals, we do not account for the cumulative effect or duration of depressive symptoms or substance use. Sixth, we do not consider possible mediation of the effect of substance use by depressive symptoms or consider bidirectional effects (e.g. alcohol use increasing depressive symptoms which in turn might increase alcohol use). Seventh, we do not adjust for overall disease severity and comorbidities, which may confound the relationship between depression, substance use, and HIV control outcomes. Finally, we used logistic regression with a common outcome, so odds ratios should not be assumed to estimate risk ratios; odds ratios will generally be greater in magnitude than risk ratios when the risk of the outcome is >10%.
Our study also has a number of strengths. The CNICS cohort comprises patients in routine HIV care and is roughly representative of patients in care for HIV in the United States with both geographic and clinical diversity. Furthermore, within CNICS, PROs are incorporated into regular clinical care. Thus our results are likely to generalize to patients in care for HIV in the United States.104, 105 Additionally, because the PROs are collected via computer-assisted self-interview, social desirability is less likely to influence patients’ responses to sensitive questions like recent illicit substance use. Lastly, we used robust statistical analyses to account for repeated measurements and missing data.
CONCLUSIONS:
Depression and misuse of substances play a significant role in preventing PWH from sustaining HIV control. We found that symptoms of depression and heavy alcohol use interact to lower the chances of achieving virologic suppression, yielding a more detrimental effect of heavy alcohol use in patients with moderate-severe symptoms of depression than in those without. When considering antidepressant use, the interaction persisted only in those not on antidepressants. These results underscore the importance of recognizing and treating depression in PWH, particularly among persons with heavy alcohol use, and suggest that deploying integrated, multifaceted interventions that target both depression and alcohol misuse could greatly benefit a subset of PWH at high risk for poor outcomes.
Acknowledgments
This work was supported by two NIAAA research grants (U01 AA020793, U24 AA020801).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS:
Disclosure of potential conflicts of interest
• Anthony T. Fojo declares that he has no conflict of interest
• Catherine R. Lesko declares that she has no conflict of interest
• Keri L. Calkins declares that she has no conflict of interest
• Richard D. Moore declares that he has no conflict of interest
• Mary E. McCaul declares that she has no conflict of interest
• Heidi E. Hutton declares that she has no conflict of interest
• William C. Mathews declares that he has no conflict of interest
• Heidi Crane declares that she has no conflict of interest
• Katerina Christopoulos has been scientific advisory board member for Roche Pharmaceuticals and a community advisory board member for Gilead Sciences Inc.
• Karen Cropsey declares that she has no conflict of interest
• Michael J. Mugavero declares that he has no conflict of interest
• Kenneth Mayer declares that he has no conflict of interest
• Brian W. Pence declares that he has no conflict of interest
• Bryan Lau declares that he has no conflict of interest
• Geetanjali Chander declares that she has no conflict of interest
Research involving human participants and/or animals:
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
REFERENCES:
- 1.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 2.Okulicz JF, Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, et al. Closing the Gap: Increases in Life Expectancy among Treated HIV-Positive Individuals in the United States and Canada. PLoS ONE. 2013;8(12):e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med. 2015;175(4):588–96. [DOI] [PubMed] [Google Scholar]
- 4.Ghiam MK, Rebeiro PF, Turner M, Rogers WB, Bebawy SS, Raffanti SP, et al. Trends in HIV Continuum of Care Outcomes over Ten Years of Follow-Up at a Large HIV Primary Medical Home in the Southeastern United States. AIDS Res Hum Retroviruses. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colasanti J, Kelly J, Pennisi E, Hu Y-J, Root C, Hughes D, et al. Continuous Retention and Viral Suppression Provide Further Insights Into the HIV Care Continuum Compared to the Cross-sectional HIV Care Cascade. Clinical Infectious Diseases. 2016;62(5):648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yehia BR, Stephens-Shields AJ, Fleishman JA, Berry SA, Agwu AL, Metlay JP, et al. The HIV Care Continuum: Changes over Time in Retention in Care and Viral Suppression. PLoS One. 2015;10(6):e0129376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, et al. Retention Among North American HIV-Infected Persons in Clinical Care, 2000–2008. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2013;62(3):356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric Disorders and Drug Use Among Human Immunodeficiency Virus-Infected Adults in the United States. Archives of General Psychiatry. 2001;58(8):721. [DOI] [PubMed] [Google Scholar]
- 9.Zuniga JA, Yoo-Jeong M, Dai T, Guo Y, Waldrop-Valverde D. The Role of Depression in Retention in Care for Persons Living with HIV. AIDS Patient Care STDS. 2016;30(1):34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krumme AA, Kaigamba F, Binagwaho A, Murray MB, Rich ML, Franke MF. Depression, adherence and attrition from care in HIV-infected adults receiving antiretroviral therapy. Journal of Epidemiology and Community Health. 2015;69(3):284–9. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011;58(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horberg MA, Silverberg MJ, Hurley LB, Towner WJ, Klein DB, Bersoff-Matcha S, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):38490. [DOI] [PubMed] [Google Scholar]
- 13.Hartzell JD, Janke IE, Weintrob AC. Impact of depression on HIV outcomes in the HAART era. Journal of Antimicrobial Chemotherapy. 2008;62(2):246–55. [DOI] [PubMed] [Google Scholar]
- 14.Parienti JJ, Massari V, Descamps D, Vabret A, Bouvet E, Larouze B, et al. Predictors of virologic failure and resistance in HIV-infected patients treated with nevirapine- or efavirenz-based antiretroviral therapy. Clin Infect Dis. 2004;38(9):1311–6. [DOI] [PubMed] [Google Scholar]
- 15.Anastos K, Schneider MF, Gange SJ, Minkoff H, Greenblatt RM, Feldman J, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39(5):537–44. [PubMed] [Google Scholar]
- 16.Barfod TS, Gerstoft J, Rodkjaer L, Pedersen C, Nielsen H, Moller A, et al. Patients’ answers to simple questions about treatment satisfaction and adherence and depression are associated with failure of HAART: a cross-sectional survey. AIDS Patient Care STDS. 2005;19(5):317–25. [DOI] [PubMed] [Google Scholar]
- 17.Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartzell JD, Spooner K, Howard R, Wegner S, Wortmann G. Race and mental health diagnosis are risk factors for highly active antiretroviral therapy failure in a military cohort despite equal access to care. J Acquir Immune Defic Syndr. 2007;44(4):411–6. [DOI] [PubMed] [Google Scholar]
- 19.Pence BW, Miller WC, Gaynes BN, Eron JJ Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–66. [DOI] [PubMed] [Google Scholar]
- 20.Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, et al. Prevalence and Predictors of Substance Use Disorders Among HIV Care Enrollees in the United States. AIDS Behav. 2017;21(4):1138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durvasula R, Miller TR. Substance Abuse Treatment in Persons with HIV/AIDS: Challenges in Managing Triple Diagnosis. Behavioral Medicine. 2014;40(2):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chander G, Josephs J, Fleishman JA, Korthuis PT, Gaist P, Hellinger J, et al. Alcohol use among HIV-infected persons in care: results of a multi-site survey. HIV Med. 2008;9(4):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crane HM, McCaul ME, Chander G, Hutton H, Nance RM, Delaney JAC, et al. Prevalence and Factors Associated with Hazardous Alcohol Use Among Persons Living with HIV Across the US in the Current Era of Antiretroviral Treatment. AIDS Behav. 2017;21(7):1914–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cofrancesco J Jr., Scherzer R, Tien PC, Gibert CL, Southwell H, Sidney S, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS. 2008;22(3):357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, et al. Heavy Alcohol Use Is Associated With Worse Retention in HIV Care. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;73(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43(4):411–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29(7):1190–7. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez A, Barinas J, O’Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Curr HIV/AIDS Rep. 2011;8(4):223–34. [DOI] [PubMed] [Google Scholar]
- 30.Rasbach DA, Desruisseau AJ, Kipp AM, Stinnette S, Kheshti A, Shepherd BE, et al. Active cocaine use is associated with lack of HIV-1 virologic suppression independent of nonadherence to antiretroviral therapy: use of a rapid screening tool during routine clinic visits. AIDS Care. 2013;25(1):109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66(6):769–89. [DOI] [PubMed] [Google Scholar]
- 32.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The Effect of Mental Illness, Substance Use, and Treatment for Depression on the Initiation of Highly Active Antiretroviral Therapy among HIV-Infected Individuals. AIDS Patient Care and STDs. 2008;22(3):233–43. [DOI] [PubMed] [Google Scholar]
- 33.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17(5):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kallas EG, McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, et al. Drug Use and Receipt of Highly Active Antiretroviral Therapy among HIV-Infected Persons in Two U.S. Clinic Cohorts. PLoS ONE. 2011;6(4):e18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cook JA, Grey DD, Burke-Miller JK, Cohen MH, Vlahov D, Kapadia F, et al. Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug Alcohol Depend. 2007;89(1):74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Himelhoch S, Chander G, Fleishman JA, Hellinger J, Gaist P, Gebo KA. Access to HAART and utilization of inpatient medical hospital services among HIV-infected patients with co-occurring serious mental illness and injection drug use. Gen Hosp Psychiatry. 2007;29(6):518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21(5):575–82. [DOI] [PubMed] [Google Scholar]
- 38.Fairbairn N, Kerr T, Milloy MJ, Zhang R, Montaner J, Wood E. Crystal methamphetamine injection predicts slower HIV RNA suppression among injection drug users. Addict Behav. 2011;36(7):762–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188(12):1820–6. [DOI] [PubMed] [Google Scholar]
- 40.Mehta SH, Lucas G, Astemborski J, Kirk GD, Vlahov D, Galai N. Early immunologic and virologic responses to highly active antiretroviral therapy and subsequent disease progression among HIV-infected injection drug users. AIDS Care. 2007;19(5):637–45. [DOI] [PubMed] [Google Scholar]
- 41.Azar P, Wood E, Nguyen P, Luma M, Montaner J, Kerr T, et al. Drug use patterns associated with risk of non-adherence to antiretroviral therapy among HIV-positive illicit drug users in a Canadian setting: a longitudinal analysis. BMC Infectious Diseases. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braithwaite RS, Fang Y, Tate J, Mentor SM, Bryant KJ, Fiellin DA, et al. Do Alcohol Misuse, Smoking, and Depression Vary Concordantly or Sequentially? A Longitudinal Study of HIV-Infected and Matched Uninfected Veterans in Care. AIDS Behav. 2016;20(3):566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan LE, Saitz R, Cheng DM, Libman H, Nunes D, Samet JH. The impact of alcohol use on depressive symptoms in human immunodeficiency virus-infected patients. Addiction. 2008;103(9):1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghebremichael M, Paintsil E, Ickovics JR, Vlahov D, Schuman P, Boland R, et al. Longitudinal association of alcohol use with HIV disease progression and psychological health of women with HIV. AIDS Care. 2009;21(7):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruggles KV, Fang Y, Tate J, Mentor SM, Bryant KJ, Fiellin DA, et al. What are the Patterns Between Depression, Smoking, Unhealthy Alcohol Use, and Other Substance Use Among Individuals Receiving Medical Care? A Longitudinal Study of 5479 Participants. AIDS Behav. 2017;21(7):2014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galvan FH, Burnam MA, Bing EG. Co-occurring psychiatric symptoms and drug dependence or heavy drinking among HIV-positive people. J Psychoactive Drugs. 2003;35 Suppl 1:153–60. [DOI] [PubMed] [Google Scholar]
- 47.Rabkin JG, Johnson J, Lin SH, Lipsitz JD, Remien RH, Williams JB, et al. Psychopathology in male and female HIV-positive and negative injecting drug users: longitudinal course over 3 years. AIDS. 1997;11(4):507–15. [DOI] [PubMed] [Google Scholar]
- 48.Brunette MF, Mueser KT. Psychosocial interventions for the long-term management of patients with severe mental illness and co-occurring substance use disorder. J Clin Psychiatry. 2006;67 Suppl 7:10–7. [PubMed] [Google Scholar]
- 49.Nelson S, Bagby GJ. Alcohol and HIV Infection. Trans Am Clin Climatol Assoc. 2011;122:244–53. [PMC free article] [PubMed] [Google Scholar]
- 50.Kapadia F, Vlahov D, Donahoe RM, Friedland G. The Role of Substance Abuse in HIV Disease Progression: Reconciling Differences from Laboratory and Epidemiologic Investigations. Clinical Infectious Diseases. 2005;41(7):1027–34. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Ho W-Z. Drugs of abuse and HIV infection/replication: Implications for m other-fetus transmission. Life Sciences. 2011;88(21–22):972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chander G, Himelhoch S, Fleishman JA, Hellinger J, Gaist P, Moore RD, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21(5):655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blashill AJ, Bedoya CA, Mayer KH, O’Cleirigh C, Pinkston MM, Remmert JE, et al. Psychosocial Syndemics are Additively Associated with Worse ART Adherence in HIV-Infected Individuals. AIDS Behav. 2015;19(6):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 57.Reinert DF, Allen JP. The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res. 2007;31(2):185–99. [DOI] [PubMed] [Google Scholar]
- 58.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039–47. [DOI] [PubMed] [Google Scholar]
- 59.National Institute on Drug Abuse (NIDA). Clinician’s Screening Tool for Drug Use in General Medical Settings 2010. [Available from: https://www.drugabuse.gov/nmassist/.
- 60.Ford MA, Spicer CM, Institute of Medicine (U.S.). Committee to Review Data Systems for Monitoring HIV Care Monitoring HIV care in the United States : indicators and data systems. Washington, D.C.: National Academies Press; 2012. xxii, 329 p. p. [PubMed] [Google Scholar]
- 61.Machado-Vieira R, Baumann J, Wheeler-Castillo C, Latov D, Henter ID, Salvadore G, et al. The Timing of Antidepressant Effects: A Comparison of Diverse Pharmacological and Somatic Treatments. Pharmaceuticals (Basel). 2010;3(1):19–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Himelhoch S, Moore RD, Treisman G, Gebo KA. Does the presence of a current psychiatric disorder in AIDS patients affect the initiation of antiretroviral treatment and duration of therapy? J Acquir Immune Defic Syndr. 2004;37(4):1457–63. [DOI] [PubMed] [Google Scholar]
- 63.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. [DOI] [PubMed] [Google Scholar]
- 64.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38(4):432–8. [DOI] [PubMed] [Google Scholar]
- 65.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21(9):1175–83. [DOI] [PubMed] [Google Scholar]
- 66.Walkup J, Wei W, Sambamoorthi U, Crystal S. Antidepressant treatment and adherence to combination antiretroviral therapy among patients with AIDS and diagnosed depression. Psychiatr Q. 2008;79(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. In: Department of Health and Human Services, editor. [Google Scholar]
- 68.Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of Adult Retention in HIV Care: A Systematic Review. AIDS Behav. 2018;22(3):752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheehan DM, Fennie KP, Mauck DE, Maddox LM, Lieb S, Trepka MJ. Retention in HIV Care and Viral Suppression: Individual- and Neighborhood-Level Predictors of Racial/Ethnic Differences, Florida, 2015. AIDS Patient Care and STDs. 2017;31(4):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, Jacobson LP, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56(8):1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yehia BR, Rebeiro P, Althoff KN, Agwu AL, Horberg MA, Samji H, et al. Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. 2015;68(4):413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ Jr. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 74.Mansournia MA, Altman DG. Inverse probability weighting. Bmj. 2016:i189. [DOI] [PubMed] [Google Scholar]
- 75.Sv Buuren, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations inR. Journal of Statistical Software. 2011;45(3). [Google Scholar]
- 76.Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, N.J. ;: Wiley-Interscience; 2004. xxix, 287 p. p. [Google Scholar]
- 77.O’Brien RM. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Quality & Quantity. 2007;41(5):673–90. [Google Scholar]
- 78.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni M-L, Gardner LI, et al. Measuring Retention in HIV Care. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2012;61(5):574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–5. [DOI] [PubMed] [Google Scholar]
- 80.Hin LY, Wang YG. Working-correlation-structure identification in generalized estimating equations. Stat Med. 2009;28(4):642–58. [DOI] [PubMed] [Google Scholar]
- 81.Lefkopoulou M, Ryan L. Global tests for multiple binary outcomes. Biometrics. 1993;49(4):975–88. [PubMed] [Google Scholar]
- 82.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 83.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: 2014. [Google Scholar]
- 84.Halekoh U, Højsgaard S, Yan J. The R Package geepack for Generalized Estimating Equations. Journal of Statistical Software. 2006;15(2). [Google Scholar]
- 85.McDaniel LS, Henderson NC, Rathouz PJ. Fast Pure R Implementation of GEE: Application of the Matrix Package. R J. 2013;5(1):181–7. [PMC free article] [PubMed] [Google Scholar]
- 86.Fox J, Weisberg S, Fox J. An R companion to applied regression. 2nd ed Thousand Oaks, Calif: SAGE Publications; 2011. xxii, 449 p. p. [Google Scholar]
- 87.Chilcot J, Chin WY, Choi EPH, Chan KTY, Wong CKH. The Psychometric Properties of the Center for Epidemiologic Studies Depression Scale in Chinese Primary Care Patients: Factor Structure, Construct Validity, Reliability, Sensitivity and Responsiveness. Plos One. 2015;10(8):e0135131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Milette K, Hudson M, Baron M, Thombs BD. Comparison of the PHQ-9 and CES-D depression scales in systemic sclerosis: internal consistency reliability, convergent validity and clinical correlates. Rheumatology. 2010;49(4):789–96. [DOI] [PubMed] [Google Scholar]
- 89.Amtmann D, Kim J, Chung H, Bamer AM, Askew RL, Wu S, et al. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li X, Margolick JB, Conover CS, Badri S, Riddler SA, W itt MD, et al. Interruption and discontinuation of highly active antiretroviral therapy in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2005;38(3):320–8. [PubMed] [Google Scholar]
- 91.Berg CJ, Michelson SE, Safren SA. Behavioral Aspects of HIV Care: Adherence, Depression, Substance Use, and HIV-Transmission Behaviors. Infectious Disease Clinics of North America. 2007;21(1):181–200. [DOI] [PubMed] [Google Scholar]
- 92.Cook RL, Zhou Z, Kelso-Chichetto NE, Janelle J, Morano JP, Somboonwit C, et al. Alcohol consumption patterns and HIV viral suppression among persons receiving HIV care in Florida: an observational study. Addict Sci Clin Pract. 2017;12(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morisano D, Babor TF, Robaina KA. Co-Occurrence of Substance use Disorders with other Psychiatric Disorders: Implications for Treatment Services. Nordic Studies on Alcohol and Drugs. 2017;31(1):5–25. [Google Scholar]
- 94.Drake RE, Mueser KT, Brunette MF. Management of persons with co-occurring severe mental illness and substance use disorder: program implications. World Psychiatry. 2007;6(3):131–6. [PMC free article] [PubMed] [Google Scholar]
- 95.Kalichman SC, Grebler T, Amaral CM, McNerney M, White D, Kalichman MO, et al. Viral suppression and antiretroviral medication adherence among alcohol using HIV-positive adults. Int J Behav Med. 2014;21(5):811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fabris P, Tositti G, Manfrin V, Giordani MT, Vaglia A, Cattelan AM, et al. Does alcohol intake affect highly active antiretroviral therapy (HAART) response in HIV-positive patients? J Acquir Immune Defic Syndr. 2000;25(1):92–3. [DOI] [PubMed] [Google Scholar]
- 97.Conen A, Wang Q, Glass TR, Fux CA, Thurnheer MC, Orasch C, et al. Association of alcohol consumption and HIV surrogate markers in participants of the swiss HIV cohort study. J Acquir Immune Defic Syndr. 2013;64(5):472–8. [DOI] [PubMed] [Google Scholar]
- 98.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10(5):299–305. [DOI] [PubMed] [Google Scholar]
- 99.Ulett KB, Willig JH, Lin HY, Routman JS, Abroms S, Allison J, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni M-L, Cunningham CO. Type and Pattern of Illicit Drug Use and Access to Health Care Services for HIV-Infected People. AIDS Patient Care and STDs. 2007;21(s1):S-68–S-76. [DOI] [PubMed] [Google Scholar]
- 101.Simon GE, VonKorff M, Barlow W. Health care costs of primary care patients with recognized depression. Arch Gen Psychiatry. 1995;52(10):850–6. [DOI] [PubMed] [Google Scholar]
- 102.Fairley CK, Sze JK, Vodstrcil LA, Chen MY. Computer-assisted self interviewing in sexual health clinics. Sex Transm Dis. 2010;37(11):665–8. [DOI] [PubMed] [Google Scholar]
- 103.O’Cleirigh C, Newcomb ME, Mayer KH, Skeer M, Traeger L, Safren SA. Moderate levels of depression predict sexual transmission risk in HIV-infected MSM: a longitudinal analysis of data from six sites involved in a “prevention for positives” study. AIDS Behav. 2013;17(5):1764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lesko CR, Cole SR, Hall HI, Westreich D, Miller WC, Eron JJ, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009–11. Int J Epidemiol. 2016;45(1):140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lesko CR, Buchanan AL, Westreich D, Edwards JK, Hudgens MG, Cole SR. Generalizing Study Results: A Potential Outcomes Perspective. Epidemiology. 2017;28(4):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]