Abstract
In this study, we demonstrated the successful transformation of two pea (Pisum sativum L.) cultivars using Agrobacterium rhizogenes, whereby transgenic roots in the resulting composite plants showed expression of the gene encoding the green fluorescent protein. Subsequent to infection with A. rhizogenes, approximately 70%–80% of pea seedlings developed transgenic hairy roots. We found out that the transgenic roots can be efficiently nodulated by Rhizobium leguminosarum bv. viciae and infected by the arbuscular mycorrhizal (AM) fungus Rhizophagus irregularis. The morphology of nodules in the transgenic roots was found to be identical to that of nodules observed in wild-type roots, and we also observed the effective induction of markers typical of the symbiotic association with AM fungi. The convenient protocol for highly efficient A. rhizogenes-mediated transformation developed in this study would be a rapid and effective tool for investigating those genes involved in the development of the two types of symbioses found in pea plants.
Keywords: Agrobacterium rhizogenes, Composite plants, Mycorrhizae, Pea transformation, Nodulation
Introduction
Pea (Pisum sativum L.) is one of the most agriculturally important legumes and is a potential target for crop improvement. In addition, large collections of pea mutants are available for the elucidation of gene function in the regulation of many processes in this plant (Jacobsen & Feenstra, 1984; Postma, Jacobsen & Feenstra, 1988; Kneen, Weeden & Larue, 1994; Borisov et al., 2007; Yang et al., 2017). The experimental approaches for such analyses are mainly based on complementation tests for mutants, as well as ectopic expression of transgenes or gene silencing mediated via the expression of hairpin RNA. However, in comparison with other legumes such as Lotus japonicus and Medicago truncatula (Schauser et al., 1999; Ané et al., 2002; Limpens et al., 2003; Madsen et al., 2003; Arrighi, 2006; Gonzalez-Rizzo, Crespi & Frugier, 2006; Heckmann et al., 2006; Tirichine et al., 2006; Smit et al., 2007; Floss et al., 2013; Yano et al., 2017), the technique of transformation necessary for such experiments, as well as for crop improvement, has not been widely applied for pea plants.
Since pea plants are involved in symbiotic associations with rhizobial bacteria and mycorrhizal fungi, they potentially constitute a source for addressing important questions relating to plant–microbe interactions. Previous investigations based on the analysis of pea mutants impaired in symbiosis development have yielded new insights into the role of individual genes regulating this process in legume plants, and the ongoing rapid development of methods for large-scale gene expression analyses has resulted in the identification of multiple genes, the expressions of which are specific for, or substantially enhanced by, symbiosis. Accordingly, analysis of these genes in transgenic plants may considerably enhance our understanding of the regulatory mechanisms controlling symbiosis. Currently, pea is again becoming an object of increasing research activity, and thus there is heightened demand for an efficient transformation protocol for this plant.
Although a few attempts using Agrobacterium tumefaciens have succeeded in transforming pea plants, these approaches still require further improvement due to the duration of these procedures, low efficiency, and the frequent fertility of transformed plants (De Kathen, 1990; Puonti-Kaerlas, Eriksson & Engström, 1990; Schroeder et al., 1993). In order to address these problems, one potential approach is the development of hairy root transformation procedures using Agrobacterium rhizogenes to generate composite plants that are characterized by transformed roots but unaltered shoots (Jensen et al., 1986; Hansen et al., 1989). This type of transformation is particularly useful for generating genetically transformed roots in a short period of time and for studying processes involved in the development of symbiotic structures on roots. The approaches used for this type of transformation are mainly based on the application of A. rhizogenes to wounded epicotyls or hypocotyls, with subsequent co-cultivation with bacteria.
Indeed, there have been a few previous studies in which the successful production of hairy roots in composite pea plants has been reported (Hohnjec et al., 2003; Clemow et al., 2011); however, in all cases, the most critical step is the efficiency of Agrobacterium infection resulting in callus formation and the subsequent generation of transformed roots. In this study, we describe a convenient protocol for highly efficient A. rhizogenes-mediated transformation of P. sativum. The composite pea plants generated using this procedure are characterized by transformed roots that are morphologically similar to those of normal roots and can be successfully nodulated by the bacterium Rhizobium leguminosarum bv. viciae or infected by the fungus Rhizophagus irregularis.
Materials & Methods
Plant material and bacterial strains
Surface-sterilized seeds of the Pisum sativum cultivars Finale and Frisson were treated with concentrated sulfuric acid for 5 min, washed five times with a large volume of sterile water, and then germinated on agar plates in the dark at 21–23 °C. After germination for 4 days, the seedlings were transferred to the light and placed into sterile plastic containers filled with Jensen medium and subsequently grown for further 3–4 days in a growth chamber at 21 °C and 60% humidity under 16 h/8 h light/dark cycle. The Agrobacterium rhizogenes strains ARqua1 (Quandt, 1993) and AR1193 (Hansen et al., 1989) and the RCAM1026 (WDCM 966) strain of Rhizobium leguminosarum bv. viciae were grown at 28 °C in Tryptone Yeast medium (Sambrook et al., 1989) containing the appropriate antibiotics.
Transformation procedure
In order to induce the formation of hairy roots, the roots of P. sativum seedlings were excised in the hypocotyl region (3–4 mm below the point of cotyledon attachment). The root hypocotyls were then treated with A. rhizogenes ARqua 1 or AR1193 containing appropriate plasmids. For plant transformation, we used the pB7WGD plasmid carrying the gusA/GFP reporter gene construct driven by the CaMV35S promoter, which was introduced into the A. rhizogenes strains by electroporation. Electroporation of A. rhizogenes was performed using GenePulser Xcell (Bio-Rad Laboratories, Hercules, CA, USA) in 1 mm cuvette with voltage −2,500 V, capacitance 25 µF, resistance 200 ohms. The wounded root tip of the seedling was touched to the A. rhizogenes growing on a plate. Plants were placed on Jensen agar in plastic jars (Duchefa, Haarlem, The Netherlands), and the cut root tips were covered with wet wool and foil. The seedlings were co-cultivated with A. rhizogenes for 10–14 days at 21 °C (16 h/8 h light/dark) until a visible callus had developed. Emerging roots were examined using an epifluorescence stereo microscope, and transformed hairy roots were identified and selected based on GFP expression. Following the removal of primary roots, the seedlings were transferred to Emergence medium containing 150 mg/mL of cefotaxime and incubated for 3–4 days. Thereafter, the plants were transferred into pots of vermiculite saturated with Jensen medium containing 1.5 M M NH4NO3, grown for 2 days in a growth chamber at 21 °C and 60% humidity under a 16 h/8 h light/dark cycle, and then inoculated with Rhizobium strains (OD600 = 0.5). The pots were then placed in large plastic bags and plants were incubated under high humidity conditions for at least 1 week and then under normal conditions. The plants were watered as needed by adding the Jensen solution with 1.5 M M NH4NO3.
RNA extraction and quantitative reverse transcription PCR (qRT-PCR)
RNA extraction was performed as described previously (Leppyanen et al., 2017). RNA (from 1 to 2.5 µg) was used for cDNA synthesis with the RevertAid Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA) for 1 h at 42 °C followed by heating to 95 °C, 5 min. Aliquots of the cDNA were diluted 1:10 for qPCR analysis. qRT-PCR analysis was performed using a CFX-96 real-time PCR detection system incorporating a C1000 thermal cycler (Bio-Rad Laboratories). All reactions were done in triplicate and averaged. Cycle threshold (CT) values were obtained with the accompanying software and data were analyzed with the 2 − ΔΔCt method (Livak & Schmittgen, 2001). The relative expression was normalized against the constitutively expressed Ubiquitin gene. RT-PCR for GFP gene expression analysis was performed using Phusion Flash High-Fidelity PCR Master Mix (Thermo Fisher Scientific). All primer pairs (Table S1) were designed using Vector NTI program and produced by Evrogen (Moscow, Russia; http://www.evrogen.com).
Mycorrhizal colonization
Rhizophagus irregularis (BEG144, International Bank of Glomeromycota (Dijon, France)) was kindly provided by Prof. Dirk Redecker as a soil–root-based inoculum from onion (Allium cepa L.) pot cultures. To obtain mycorrhizal pea plants, pea seedlings were placed in a nurse pot system with chives (Allium schoenoprasum L.) as nurse plants and grown in a growth chamber under conditions as previously described (Shtark et al., 2016). A mineral substrate, silica-rich marl, was used as the growth substrate. Mycorrhizal colonization was determined according to (Trouvelot, Kough & Gianinazzi-Pearson, 1986). Three parameters were considered: M%—intensity of internal colonization of the root system (reflects the proportion of the root length colonized by the fungus), A%—arbuscule abundance in mycorrhizal root fragments (characterizes the functional state of the fungus), V%—vesicle and/or spore abundance in mycorrhizal root fragments. 150 random root pieces (1 cm) were scored per replicate (not less than 750 cm/genotype).
Histochemical staining and microscopy
The root segments and nodules were collected and stained with X-Gluc (0.2 M Na2PO4, pH7.0; 0.5 M EDTA, pH8.0; 20 mM K ferricyanide; 20 mM K ferrocyanide; 20 mM X-Gluc; 0.01% Triton X-100) for GUS staining. Tissues were incubated in staining due overnight at 37 °C. In order to assess roots mycorrhizal colonization, roots were stained with Sheaffer Black Ink at room temperature as described previously (Vierheilig et al., 1998). Photographs were obtained using an Olympus BX51 microscope (Olympus Optical Co. Europa GmbH, Hamburg, Germany) equipped with a ColorView II digital camera and analySIS FIVE analytical software (Olympus Soft Imaging Solution GmbH, Hamburg, Germany).
Results
Generation of pea composite plants and subsequent nodulation
In order to select for transformed hairy roots and quantify the efficiency of transformation, we introduced a plasmid carrying a fusion with the gusA/GFP reporter gene driven by the CaMV35S promoter and subsequently examined hairy roots and nodules for green fluorescent protein (GFP) or β-glucuronidase (GUS) staining.
To obtain the composite plants, young etiolated seedlings of the two cultivars Finale and Frisson were used for transformation with the ARqua1 or AR1193 strain of Agrobacterium rhizogenes carrying the gusA/GFP reporter construct. Using both A. rhizogenes strains, root transformation was induced with satisfactory efficiency, although differences were observed in number of induced roots (Table 1). Consistent with the findings of Clemow et al. (2011), we found that AR1193 was a more effective strain for hairy root transformation. In the procedure used in the present study, the plants were incubated in containers filled with Jensen medium placed within ventilated plastic box (Fig. 1A). Seedlings with one to two internodes are quite susceptible to transformation and were freshly sectioned in the hypocotyl region to remove the remainder of the root. In our transformation protocol, the wounded root tip of each seedling was touched to growing on a plate A. rhizogenes containing a plasmid that harbored the gusA/GFP reporter. Following transformation, the pea seedlings were incubated in jars and the root tips were maintained under high-humidity conditions (Fig. 1B).
Table 1. Agrobacterium rhizogenes-mediated transformation of Pisum sativum cv. Frisson using the ARqua 1 and AR1193 strains of A. rhizogenes.
| Total amount of plants | Number of transformed composite plantsa | Total number of hairy roots | Number of hairy roots per transformed composite plant | |
|---|---|---|---|---|
| Agrobacterium rhizogenes Arqua1 | 114 (10)b | 80 (10)b | 130 | 1,6 |
| Agrobacterium rhizogenes AR1193 | 62 (5)b | 50 (5) | 88 | 1,8 |
Notes.
Transformed composite plants that gave rise to at least one hairy root per plant.
The number of independent experiments is indicated within brackets.
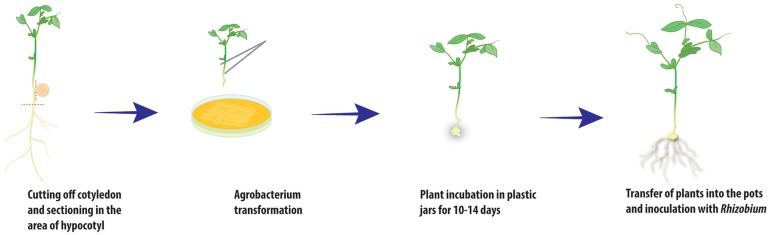
Figure 1. Procedure of transformation.
(A) Pea seedlings were incubated in containers filled with Jensen medium placed within ventilated plastic box. (B) Incubation of pea seedlings in jars after transformation. (C) Callus formation on wounded sites of young cv. Frisson pea seedlings treated with A. rhizogenes. (D) General view of pea seedling after incubation on an antibiotic. The arrows indicate the callus formation.
After 10–14 days, calli started to develop at the wound sites in seedling of the cultivar Frisson (Fig. 1C), whereas the initiation of callus development was slightly more prolonged (16–18 days) for seedlings of the Finale cultivar. Approximately 70%–80% of the wound sites gave rise to callus formation and the subsequent development of transformed roots. At this stage, a number of primary roots appeared (Fig. 1C). These primary roots were removed prior to antibiotic treatment in order to stimulate development of the transformed roots. We found that incubation on an antibiotic-containing medium prevented excessive growth of A. rhizogenes and the development of root abnormalities (Fig. 1D). After incubation, the plants were subsequently transferred to pots saturated with a low-nitrate nutrient solution to prevent nitrogen starvation (Figs. 2 and 3A). Since the seedlings were cultivated under axenic conditions, they may also be used to obtain the cultivated hairy roots at this stage.
Figure 2. Scheme of A. rhizogenes-mediated transformation of pea plants.
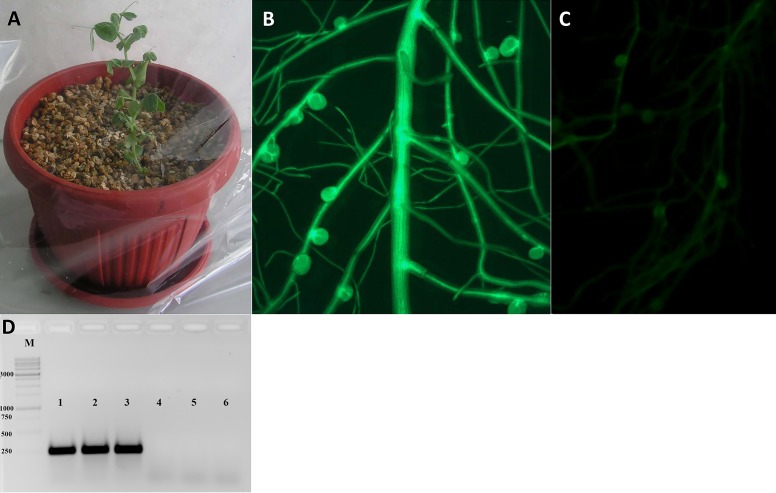
Figure 3. Analysis of transformed pea plants after R. leguminosarum inoculation.
(A) Pea plants incubated under high humidity conditions following their transfer to pots. (B) The plant marker GFP was visualized in transgenic roots and nodules using an epifluorescence microscope. (C) Non-transformed roots of pea plants. (D) The expression of GFP gene in transgenic nodules of P. sativum cv. Frisson using RT-PCR (1–3), as a control the nodules of non-transformed plants were used (4–6).
After transferring to pots and cultivating for a few days, the plants were inoculated with R. leguminosarum bv. viciae RIAM1026 and we observed that root nodules generally appeared after 2–3 weeks. In order to maintain high humidity conditions after transfer, we placed the pots into large plastic packages in which they were retained for at least 1 week, because it was easy to ventilate the plants during cultivation (Fig. 3A). After 2–3 weeks, we were able to visualize the emergence of nodules on transformed roots using an epifluorescence stereo microscope (Fig. 3B). We found that the efficiency of nodule formation varied depending on the cultivar used, with cv. Finale showing a high level of nodule formation, whereas comparatively fewer nodules developed on the transformed roots of cv. Frisson plants. GFP staining was used as a simple marker to score for transformed roots 3 weeks after infection with R. leguminosarum. We found that approximately 70% of the inoculated plants had at least one transformed root, and that composite plants had an average of two to three such roots, thereby indicating the high frequency of transformation achieved using this protocol, which will greatly facilitate studies involving gene function analysis.
Studying the possibility of mycorrhization for transformed roots in pea
In the present study, we also developed approaches for inoculation of transgenic hairy roots with the AM fungus R. irregularis using a nurse-plant system. As nurse plants, we used four young onion seedlings grown in pots containing Hoagland medium-saturated substrate for 4 weeks. After cultivation on antibiotic-containing medium, the transformed pea seedlings were transferred to the pots containing these nurse plants.
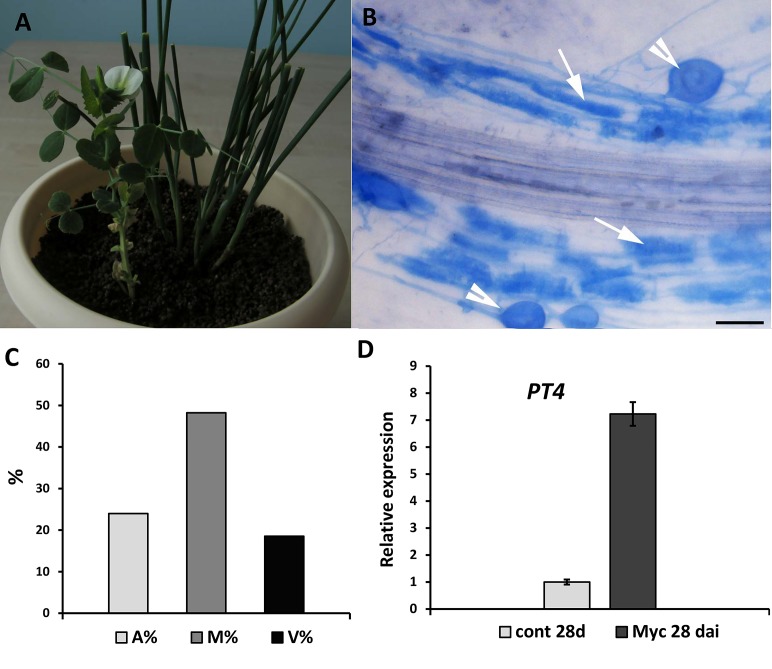
In order to maintain the high humidity conditions after transfer, we placed the pots into large plastic packages for at least 1 week, similar to the procedure used for rhizobial inoculation (Fig. 4A). After 4 weeks of growth, we assessed the efficiency of symbiosis development with AM fungi and detected highly effective inoculation (Figs. 4B and 4C). Expression levels of marker typically associated with AM fungus symbiosis development, namely, the PT4 gene encoding the mycorrhiza-inducible inorganic phosphate transporter, confirmed induction of the symbiotic association (Fig. 4D). These findings indicate that the protocol we describe here may be useful for the analysis of another type of symbiosis in composite pea plants with transformed roots.
Figure 4. Inoculation of pea plants with the AM fungus R. irregularis using a nurse-plant system.
(A) General view of pea plants in a nurse-plant system during mycorrhizal colonization. (B) Arbuscular mycorrhizal fungi colonize efficiently GUS-transformed pea roots in a nurse plant system. White arrows indicate cells with arbuscules, white arrowheads point at vesicles. Scale bars: a = 50 µm. (C) Three parameters were considered: M%, intensity of internal colonization of the root system, A%, arbuscule abundance in mycorrhizal root fragments and V%, vesicle and/or spore abundance in mycorrhizal root fragments. (D) Expression of PT4 gene encoding mycorrhiza-inducible inorganic phosphate transporter in non-inoculated (control) and inoculated (Myc) transgenic roots (28 days after inoculation). mRNA levels were normalized against Ubiquitin, and values were calculated as ratios relative to non-inoculated root expression level.
Discussion
In recent years multiple studies have led to the development of sufficiently effective approaches for pea transformation using A. tumefaciens (Bean et al., 1997; Polowick, Quandt & Mahon, 2000; Wisniewski & Brewin, 2000; Schneider et al., 2002; Pniewski & Kapusta, 2005; Svabova, Smykal & Griga, 2005; Krejčí et al., 2007; Sehgal, Bhat & Raina, 2008; Aftabi, Teressa Negawo & Hassan, 2017). However, due to the duration of these procedures, the alternative hairy root transformation approaches using A. rhizogenes require further development.
A. rhizogenes- mediated transformation has previously been described for a few legume species, initially being applied for the expression of soybean genes in Lotus corniculatus (Jensen et al., 1986; Jørgensen et al., 1988; Hansen et al., 1989). Subsequently, approaches were described for transformation and regeneration of the model legume plant Lotus japonicus (Handberg & Stougaard, 1992; Stiller et al., 1997). Hairy root transformation was also developed for another model legume, Medicago truncatula (Boisson-Dernier et al., 2001; Gourion et al., 2013), and protocols for hairy root transformation have been adapted for other legume plants, including Vigna aconitifolia (Lee et al., 1993), Trifolium repens and Trifolium pratense (Díaz et al., 1989; Díaz, Spaink & Kijne, 2000), Glycine max (Cheon et al., 1993), Vicia hirsute (Quandt, 1993), Phaseolus vulgaris L. (Estrada-Navarrete et al., 2006) and peanut Arachis hypogea (Sinharoy et al., 2009). Furthermore, previous studies have also reported the successful production of hairy roots in composite pea plants (Hohnjec et al., 2003; Clemow et al., 2011).
In an effort to develop approaches for highly efficient A. rhizogenes- mediated hairy root transformation in pea, we have previously treated the needle-wounded hypocotyls of peas with an inoculum of A. rhizogenes (Osipova et al., 2012) as it was described (Hohnjec et al., 2003). However, although transformed pea roots and nodules were obtained and enabled us to investigate certain pea transgenes, this approach did not result in highly efficient transformation. Accordingly, in the present study, we sought to enhance the efficiency of transformation by inducing callus formation on the wounded sites of freshly sectioned hypocotyls of pea seedling via incubation with A. rhizogenes.
Using our novel methodology for hairy root induction in pea, we demonstrate the successful transformation of P. sativum by the ARqua 1 and AR1193 strains of A. rhizogenes, whereby transgenic roots in the composite plants showed expression of the genes encoding the green fluorescent protein and GUS under the control of the CaMV35S promoter. Subsequent to infection with A. rhizogenes, approximately 70% to 80% of pea seedlings developed transgenic hairy roots. Since the seedlings were cultivated under axenic conditions, they may also be used to obtain the cultivated hairy roots.
We observed that these transgenic roots can be efficiently nodulated by the rhizobial bacterium R. leguminosarum and infected by the AM fungus R. irregularis. Morphologically, the nodules that developed on transgenic roots were found to be identical to those characterizing wild-type roots. We also demonstrate that a supply of nitrogen is important for enhancing the growth of transformed plants following their transfer from rich media to pots. Furthermore, we also verified the development of a symbiosis with AM fungi via the effective induction of markers typically associated with this process. We thus demonstrate an approach for the comparatively rapid generation of symbiotic structures on transgenic pea roots, and accordingly consider that our protocol can be employed as a rapid and efficient tool for studying the genes involved in plant–microbe interactions in P. sativum.
Conclusions
A convenient protocol for highly efficient A. rhizogenes-mediated transformation of pea P. sativum has been developed. In pea composite plants, the hairy roots can be nodulated successfully by R. leguminosarum bv. viciae bacteria or infected by R. irregularis fungi.
Supplemental Information
Acknowledgments
We are very grateful to Dr. Lene H. Madsen (Department of Molecular Biology and Genetics, University of Aarhus, Denmark) for the A. rhizogenes strain AR1193 that has been kindly provided for experiments.
Funding Statement
This work was financially supported by the Russian Science Foundation (grant 17-76-30016). The research was performed using equipment of the Core Centrum “Genomic Technologies, Proteomics and Cell Biology” in ARRIAM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Irina V. Leppyanen conceived and designed the experiments, performed the experiments, prepared figures and/or tables, approved the final draft.
Anna N. Kirienko performed the experiments, contributed reagents/materials/analysis tools, prepared figures and/or tables, approved the final draft.
Elena A. Dolgikh conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are available in Supplementary File 1.
References
- Aftabi, Teressa Negawo & Hassan (2017).Aftabi M, Teressa Negawo A, Hassan F. Improved protocol for agrobacterium-mediated transformation of pea (Pisum sativum) Molecular Biology. 2017;7:1–6. doi: 10.4172/2168-9547.1000202. [DOI] [Google Scholar]
- Ané et al. (2002).Ané J, Lévy J, Thoquet P, Kulikova O, De Billy F, Penmetsa V, Kim D, Debellé F, Rosenberg C, Cook DR, Bisseling T, Huguet T, Dénarié J, Biologie L De, Umr C Genetic and cytogenetic mapping of DMI1, DMI2, and DMI3 genes of medicago truncatula involved in nod factor transduction, nodulation, and mycorrhization. Society. 2002;15:1108–1118. doi: 10.1094/MPMI.2002.15.11.1108. [DOI] [PubMed] [Google Scholar]
- Arrighi (2006).Arrighi J-F. The medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiology. 2006;142:265–279. doi: 10.1104/pp.106.084657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean et al. (1997).Bean SJ, Gooding PS, Mullineaux PM, Davies DR. A simple system for pea transformation. Plant Cell Reports. 1997;16:513–519. doi: 10.1007/BF01142315. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier et al. (2001).Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG. Agrobacterium rhizogenes—transformed roots of medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Molecular Plant-Microbe Interactions. 2001;14:695–700. doi: 10.1094/MPMI.2001.14.6.695. [DOI] [PubMed] [Google Scholar]
- Borisov et al. (2007).Borisov AY, Danilova TN, Koroleva TA, Kuznetsova EV, Madsen L, Mofett M, Naumkina TS, Nemankin TA, Ovchinnikova ES, Pavlova ZB, Petrova NE, Pinaev AG, Radutoiu S, Rozov SM, Rychagova TS, Shtark OY, Solovov II, Stougaard J, Tikhonovich IA, Topunov AF, Tsyganov VE, Vasil’chikov AG, Voroshilova VA, Weeden NF, Zhernakov AI, Zhukov VA. Regulatory genes of garden pea (Pisum sativum L.) controlling the development of nitrogen-fixing nodules and arbuscular mycorrhiza: a review of basic and applied aspects. Applied Biochemistry and Microbiology. 2007;43:237–243. doi: 10.1134/S0003683807030027. [DOI] [PubMed] [Google Scholar]
- Cheon et al. (1993).Cheon CI, Lee NG, Siddique AB, Bal AK, Verma DP. Roles of plant homologs of Rab1p and Rab7p in the biogenesis of the peribacteroid membrane, a subcellular compartment formed de novo during root nodule symbiosis. The EMBO Journal. 1993;12:4125–4135. doi: 10.1002/j.1460-2075.1993.tb06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemow et al. (2011).Clemow SR, Clairmont L, Madsen LH, Guinel FC. Reproducible hairy root transformation and spot-inoculation methods to study root symbioses of pea. Plant Methods. 2011;7 doi: 10.1186/1746-4811-7-46. Article 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kathen (1990).De Kathen A. Agrobacterium tumefaciens-mediated transformation of Pisum sativum L. using binary and cointegrate vectors. Plant Cell Reports. 1990:276–279. doi: 10.1007/BF00232301. [DOI] [PubMed] [Google Scholar]
- Díaz et al. (1989).Díaz CL, Melchers LS, Hooykaas PJJ, Lugtenberg BJJ, Kijne JW. Root lectin as a determinant of host–plant specificity in the Rhizobium–legume symbiosis. Nature. 1989;338:579–581. doi: 10.1038/338579a0. [DOI] [Google Scholar]
- Díaz, Spaink & Kijne (2000).Díaz CL, Spaink HP, Kijne JW. Heterologous rhizobial lipochitin oligosaccharides and chitin oligomers induce cortical cell divisions in red clover roots, transformed with the pea lectin gene. Molecular Plant-Microbe Interactions. 2000;13:268–276. doi: 10.1094/MPMI.2000.13.3.268. [DOI] [PubMed] [Google Scholar]
- Estrada-Navarrete et al. (2006).Estrada-Navarrete G, Alvarado-Affantranger X, Olivares J-E, Díaz-Camino C, Santana O, Murillo E, Guillén G, Sánchez-Guevara N, Acosta J, Quinto C, Li D, Gresshoff PM, Sánchez F. Agrobacterium rhizogenes transformation of the phaseolus spp.: a tool for functional genomics. Molecular Plant-Microbe Interactions. 2006;19:1385–1393. doi: 10.1094/MPMI-19-1385. [DOI] [PubMed] [Google Scholar]
- Floss et al. (2013).Floss DS, Schmitz AM, Starker CG, Gantt JS, Harrison MJ. Gene silencing in Medicago truncatula roots using RNAi. Methods in Molecular Biology. 2013;1069:163–177. doi: 10.1007/978-1-62703-613-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo, Crespi & Frugier (2006).Gonzalez-Rizzo S, Crespi M, Frugier F. The medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with sinorhizobium meliloti. The Plant Cell Online. 2006;18:2680–2693. doi: 10.1105/tpc.106.043778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourion et al. (2013).Gourion B, Bourcy M, Cosson V, Ratet P. Protocols for growing plant symbioses; rhizobia. In: Maathuis FJM, editor. Plant mineral nutrients: methods and protocols. Humana Press; Totowa: 2013. pp. 61–75. [DOI] [PubMed] [Google Scholar]
- Handberg & Stougaard (1992).Handberg K, Stougaard J. Lotus japonicus, an autogamous, diploid legume species for classical and molecular genetics. The Plant Journal. 1992;2:487–496. doi: 10.1111/j.1365-313X.1992.00487.x. [DOI] [Google Scholar]
- Hansen et al. (1989).Hansen J, Jørgensen J-E, Stougaard J, Marcker KA. Hairy roots—a short cut to transgenic root nodules. Plant Cell Reports. 1989;8:12–15. doi: 10.1007/BF00735768. [DOI] [PubMed] [Google Scholar]
- Heckmann et al. (2006).Heckmann AB, Lombardo F, Miwa H, Perry JA, Bunnewell S, Parniske M, Wang TL, Downie JA. Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiology. 2006;142:1739–1750. doi: 10.1104/pp.106.089508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnjec et al. (2003).Hohnjec N, Perlick AM, Pühler A, Küster H. The medicago truncatula sucrose synthase gene MtSucS1 is activated both in the infected region of root nodules and in the cortex of roots colonized by arbuscular mycorrhizal fungi. Molecular Plant-Microbe Interactions. 2003;16:903–915. doi: 10.1094/MPMI.2003.16.10.903. [DOI] [PubMed] [Google Scholar]
- Jacobsen & Feenstra (1984).Jacobsen E, Feenstra WJ. A new pea mutant with efficient nodulation in the presence of nitrate. Plant Science Letters. 1984;33:337–344. doi: 10.1016/0304-4211(84)90025-7. [DOI] [Google Scholar]
- Jensen et al. (1986).Jensen JS, Marcker KA, Otten L, Schell J. Nodule-specific expression of a chimaeric soybean leghaemoglobin gene in transgenic Lotus corniculatus. Nature. 1986;321:669–674. doi: 10.1038/321669a0. [DOI] [Google Scholar]
- Jørgensen et al. (1988).Jørgensen JE, Stougaard J, Marcker A, Marcker KA. Root nodule specific gene regulation: analysis of the soybean nodulin N23 gene promoter in heterologous symbiotic systems. Nucleic Acids Research. 1988;16:39–50. doi: 10.1093/nar/16.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneen, Weeden & Larue (1994).Kneen BE, Weeden NF, Larue TA. Non-nodulating mutants of Pisum Sativum (L.) cv. sparkle. Journal of Heredity. 1994;85:129–133. doi: 10.1093/oxfordjournals.jhered.a111410. [DOI] [Google Scholar]
- Krejčí et al. (2007).Krejčí P, Matušková P, Hanáček P, Reinöhl V, Procházka S. The transformation of pea (Pisum sativum L.): applicable methods of Agrobacterium tumefaciens-mediated gene transfer. Acta Physiologiae Plantarum. 2007;29:157–163. doi: 10.1007/s11738-006-0020-3. [DOI] [Google Scholar]
- Lee et al. (1993).Lee NG, Stein B, Suzuki H, Verma DPS. Expression of antisense nodulin-35 RNA in Vigna aconitifolia transgenic root nodules retards peroxisome development and affects nitrogen availability to the plant. Plant Journal. 1993;3:599–606. doi: 10.1046/j.1365-313X.1993.03040599.x. [DOI] [PubMed] [Google Scholar]
- Leppyanen et al. (2017).Leppyanen IV, Shakhnazarova VY, Shtark OY, Vishnevskaya NA, Tikhonovich IA, Dolgikh EA. Receptor-Like Kinase LYK9 in Pisum sativum L. Is the CERK1-like receptor that controls both plant immunity and AM symbiosis development. International Journal of Molecular Sciences. 2017;19 doi: 10.3390/ijms19010008. Article 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens et al. (2003).Limpens E, Franken C, Smit P, Willemse J, Bisseling T, Geurts R. LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science. 2003;302:630–633. doi: 10.1126/science.1090074. [DOI] [PubMed] [Google Scholar]
- Livak & Schmittgen (2001).Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madsen et al. (2003).Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J. A receptor kinase gene of the LysM type is involved in legumeperception of rhizobial signals. Nature. 2003;425:637–640. doi: 10.1038/nature02045. [DOI] [PubMed] [Google Scholar]
- Osipova et al. (2012).Osipova MA, Mortier V, Demchenko KN, Tsyganov VE, Tikhonovich IA, Lutova LA, Dolgikh EA, Goormachtig S. Wuschel-related homeobox5 gene expression and interaction of CLE peptides with components of the systemic control add two pieces to the puzzle of autoregulation of nodulation. Plant Physiology. 2012;158:1329–1341. doi: 10.1104/pp.111.188078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pniewski & Kapusta (2005).Pniewski T, Kapusta J. Efficiency of transformation of Polish cultivars of pea (Pisum sativum L.) with various regeneration capacity by using hypervirulent Agrobacterium tumefaciens strains. Journal of Applied Genetics. 2005;46:139–147. [PubMed] [Google Scholar]
- Polowick, Quandt & Mahon (2000).Polowick PL, Quandt J, Mahon JD. The ability of pea transformation technology to transfer genes into peas adapted to western Canadian growing conditions. Plant Science. 2000;153:161–170. doi: 10.1016/s0168-9452(99)00267-8. [DOI] [PubMed] [Google Scholar]
- Postma, Jacobsen & Feenstra (1988).Postma JG, Jacobsen E, Feenstra WJ. Three pea mutants with an altered nodulation studied by genetic analysis and grafting. Journal of Plant Physiology. 1988;132:424–430. doi: 10.1016/S0176-1617(88)80056-7. [DOI] [Google Scholar]
- Puonti-Kaerlas, Eriksson & Engström (1990).Puonti-Kaerlas J, Eriksson T, Engström P. Production of transgenic pea (Pisum sativum L.) plants by Agrobacterium tumefaciens—mediated gene transfer. Theoretical and Applied Genetics. 1990;80:246–252. doi: 10.1007/BF00224394. [DOI] [PubMed] [Google Scholar]
- Quandt (1993).Quandt H-J. Transgenic root nodules of vicia hirsuta: a fast and efficient system for the study of gene expression in indeterminate-type nodules. Molecular Plant-Microbe Interactions. 1993;6:699–706. doi: 10.1094/MPMI-6-699. [DOI] [Google Scholar]
- Sambrook et al. (1989).Sambrook J, Russell DW, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. [DOI] [Google Scholar]
- Schauser et al. (1999).Schauser L, Roussis A, Stiller J, Stougaard J. A plant regulator controlling development of symbiotic root nodules. Nature. 1999;402:191–195. doi: 10.1038/46058. [DOI] [PubMed] [Google Scholar]
- Schneider et al. (2002).Schneider A, Walker SA, Sagan M, Duc G, Ellis THN, Downie JA. Mapping of the nodulation loci sym9 and sym10 of pea (Pisum sativum L.) Theoretical and Applied Genetics. 2002;104:1312–1316. doi: 10.1007/s00122-002-0896-2. [DOI] [PubMed] [Google Scholar]
- Schroeder et al. (1993).Schroeder HE, Schotz AH, Wardley-Richardson T, Spencer D, Higgins T. Transformation and regeneration of two cultivars of pea (Pisum sativum L.) Plant Physiology. 1993;101:751–757. doi: 10.1104/pp.101.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal, Bhat & Raina (2008).Sehgal D, Bhat V, Raina SN. Advent of diverse dna markers to decipher genome sequence polymorphism. In: Kirti PB, editor. Handbook of new technologies for genetic improvement of legumes. Boca Raton; CRC Press: 2008. p. c2008. [Google Scholar]
- Shtark et al. (2016).Shtark OY, Sulima AS, Zhernakov AI, Kliukova MS, Fedorina JV, Pinaev AG, Kryukov AA, Akhtemova GA, Tikhonovich IA, Zhukov VA. Arbuscular mycorrhiza development in pea (Pisum sativum L.) mutants impaired in five early nodulation genes including putative orthologs of NSP1 and NSP2. Symbiosis. 2016;68:129–144. doi: 10.1007/s13199-016-0382-2. [DOI] [Google Scholar]
- Sinharoy et al. (2009).Sinharoy S, Saha S, Chaudhury SR, DasGupta M. Transformed hairy roots of arachis hypogea: a tool for studying root nodule symbiosis in a non-infection thread legume of the aeschynomeneae tribe. Molecular Plant-Microbe Interactions. 2009;22:132–142. doi: 10.1094/MPMI-22-2-0132. [DOI] [PubMed] [Google Scholar]
- Smit et al. (2007).Smit P, Limpens E, Geurts R, Fedorova E, Dolgikh E, Gough C, Bisseling T. Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiology. 2007;145:183–191. doi: 10.1104/pp.107.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiller et al. (1997).Stiller J, Martirani L, Tuppale S, Chian R-J, Chiurazzi M, Gresshoff PM. High frequency transformation and regeneration of transgenic plants in the model legume Lotus japonicus. Journal of Experimental Botany. 1997;48:1357–1365. doi: 10.1093/jxb/48.7.1357. [DOI] [Google Scholar]
- Svabova, Smykal & Griga (2005).Svabova L, Smykal P, Griga M. Agrobacterium-mediated transformation of Pisum sativum in vitro and in vivo. Biologia Plantarum. 2005;49(3):361–370. doi: 10.1007/s10535-005-0009-6. [DOI] [Google Scholar]
- Tirichine et al. (2006).Tirichine L, James EK, Sandal N, Stougaard J. Spontaneous root-nodule formation in the model legume Lotus japonicus: a novel class of mutants nodulates in the absence of rhizobia. Molecular Plant-Microbe Interactions. 2006;19:373–382. doi: 10.1094/MPMI-19-0373. [DOI] [PubMed] [Google Scholar]
- Trouvelot, Kough & Gianinazzi-Pearson (1986).Trouvelot A, Kough J, Gianinazzi-Pearson V. Mesure du taux de mycorhization VA d’un systeme radiculaire. Recherche de methodes d’estimation ayant une significantion fonctionnelle. In: Gianinazzi-Pearson V, et Gianinazzi S, editors. Physiological and genetical aspects of mycorrhizae. INRA edition; Paris: 1986. [Google Scholar]
- Vierheilig et al. (1998).Vierheilig H, Coughlan AP, Wyss URS, De Recherche C. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Applied and Environmental Microbiology. 1998;64:5004–5007. doi: 10.1128/aem.64.12.5004-5007.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski & Brewin (2000).Wisniewski JP, Brewin NJ. Construction of transgenic pea lines with modified expression of diamine oxidase and modified nodulation responses with exogenous putrescine. Molecular Plant-Microbe Interactions. 2000;13:922–928. doi: 10.1094/MPMI.2000.13.9.922. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang C, Bueckert R, Schoenau J, Diederichsen A, Zakeri H, Warkentin T. Evaluation of growth and nitrogen fixation of pea nodulation mutants in western Canada. Canadian Journal of Plant Science. 2017;97:1121–1129. doi: 10.1139/cjps-2016-0383. [DOI] [Google Scholar]
- Yano et al. (2017).Yano K, Aoki S, Liu M, Umehara Y, Suganuma N, Iwasaki W, Sato S, Soyano T, Kouchi H, Kawaguchi M. Function and evolution of a Lotus japonicus AP2/ERF family transcription factor that is required for development of infection threads. DNA Research. 2017;24:193–203. doi: 10.1093/dnares/dsw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are available in Supplementary File 1.