Abstract
Introduction
Alterations in miR-155 serum levels have been described in inflammatory and infectious diseases. Moreover, a role for miR-155 in aging and age-related diseases was recently suggested. We therefore analyzed a potential age-dependent prognostic value of circulating miR-155 as a serum-based marker in critical illness.
Methods
Concentrations of circulating miR-155 were determined in 218 critically ill patients and 76 healthy controls.
Results
By using qPCR, we demonstrate that miR-155 serum levels are elevated in patients with critical illness when compared to controls. Notably, levels of circulating miR-155 were independent on the severity of disease, the disease etiology, or the presence of sepsis. In the total cohort, miR-155 was not an indicator for patient survival. Intriguingly, when patients were subdivided according to their age upon admission to the ICU into those younger than 65 years, lower levels of miR-155 turned out as a strong marker, indicating patient mortality with a similar accuracy than other markers frequently used to evaluate critically ill patients on a medical ICU.
Conclusion
In summary, the data provided within this study suggest an age-specific role of miR-155 as a prognostic biomarker in patients younger than 65 years. Our study is the first to describe an age-dependent miRNA-based prognostic biomarker in human diseases.
1. Introduction
Sepsis represents a complex pathological process including inflammation, coagulopathy, and deterioration of the patients' hemodynamic state, finally leading to organ failure [1]. Although it was shown that an immediate initiation of anti-infective and supportive therapeutic measures considerably improves the prognosis in critically ill patients [2], the overall sepsis-related mortality remains high. This highlights the need for biomarkers allowing an early possible diagnosis on the one hand and prognosis assessment to guide therapy, on the other [3, 4].
MicroRNAs represent endogenous RNA molecules with a length of ~22 nucleotides [5]. They are created by a complex process leading from pre-miRNAs to the mature miRNA that regulates multiple processes such as cell metabolism, cell growth, and differentiation as well as cell death [5]. miR-155 represents one of the best characterized microRNAs in the context of infection and inflammation. This miRNA is predominantly found in the liver, spleen, and thymus [6] and is involved in immune cell development and the regulation of systemic inflammatory processes [7–9]. Alterations in miR-155 expression were demonstrated in activated immune cells and consequently in many inflammatory diseases such as allergic asthma [8], atopic dermatitis [10], rheumatoid arthritis [11], Crohn's disease [12], and liver injury [13]. In a recently published in vitro study using LPS-induced THP-1 monocytes, it was demonstrated that miR-155 regulated the expression of different proinflammatory mediators [14], arguing for a function in systemic inflammation and infection.
Due to their simple chemical structure and the resulting biological stability, circulating miRNAs were proposed by many authors as biomarkers for several diseases including inflammatory diseases and infections [15]. In particular, many authors proposed circulating miRNAs as serum-based markers in patients with critical illness. Nevertheless, despite intensive research efforts, specific mechanisms or pathogenic factors regulating concentrations of circulating miRNAs in sepsis (and many other diseases) are poorly understood.
Here, we analyzed the diagnostic and prognostic value of miR-155 serum levels in 218 critically ill patients treated on an intensive care unit.
2. Methods
2.1. Study Design and Patient Characteristics
Between 2010 and 2013, 218 patients (see Table 1), consecutively admitted to the Internal Medicine Intensive Care Unit at the University Clinic (RWTH) Aachen, were included. Patients, expected having an intensive care treatment < 3 days, were excluded. After discharge, patients were included into a follow-up by contacting the patients, the patients' relatives, or the primary care physician. Sepsis, severe sepsis, and septic shock were diagnosed based on the criteria published by the ACCP/SCCM Consensus Conference. 76 healthy blood donors (47 males, 29 females; median age 33 years, range 18-67) with normal values for blood counts, C-reactive protein, and liver enzymes served as a control as recently described [16].
Table 1.
Baseline patient characteristics.
| Parameter | All patients | <65 years | >65 years |
|---|---|---|---|
| Number | 218 | 125 | 93 |
| Sex (male/female) | 138/80 | 82/43 | 56/37 |
| Age median (range) (years) | 63 (18-89) | 52 (18-65) | 74 (66-89) |
| APACHE II score median (range) | 17 (2-43) | 15 (2-43) | 19 (5-40) |
| SAPS2 score median (range) | 43.0 (0-79) | 40 (9-79) | 45 (0-72) |
| ICU days median (range) | 7 (1-83) | 7 (1-70) | 7 (1-83) |
| Death during ICU or follow-up (%) | 47.2% | 36% | 51.6% |
| 28 d mortality | 24.8% | 18.2% | 31.3% |
| Ventilation time median (range) (h) | 129 (0.5-1363) | 127 (0.5-928) | 132 (1-1363) |
| Diabetes mellitus (%) | 30.7% | 20.0% | 45.16% |
| Body mass index (BMI) | 26.78 (16.6-86.5) | 26 (16.6-86.5) | 26.12 (19.3-61) |
| Creatinine | 1.3 (0-15) | 1.3 (0.2-15) | 1.35 (0-11.5) |
| Albumin | 27.0 (15.2- 52.2) | 26 (15.2-41) | 28.6 (15.8-52.2) |
| WBC median (range) (×103/μl) | 12.15 (0.1-67.4) | 11.65 (0.1-67.4) | 12.7 (0.1-66.2) |
| CRP median (range) (mg/dl) | 95.5 (<5-230) | 112 (5-230) | 90 (<5-230) |
| Procalcitonin median (range) (μg/l) | 0.7 (0-180.6) | 0.7 (0.06-125.2) | 0.65 (0-180.6) |
| Interleukin-6 median (range) (pg/ml) | 105 (0-83000) | 130 (2-28000) | 100 (0-83000) |
| Tumor necrosis factor median (pg/ml) | 19 (4.9-140) | 19 (4.9-140) | 20 (10-100) |
| Serum lactate (mmol/l) | 1.70 (0-21.9) | 1.5 (0-21.9) | 1.7 (0-20.8) |
| miR-155 median (range) (rel. ex.) | 9.35 (0.1-56.49) | 10.05 (0.17-56.49) | 8.63 (0.1-29.65) |
APACHE: acute physiology and chronic health evaluation; CRP: C-reactive protein; ICU: intensive care unit; SAPS: simplified acute physiology score; WBC: white blood cell count.
2.2. miRNA Isolation [16, 17]
400 μl serum was spiked with miScript miRNA mimic SV40 (Qiagen 2 μM, 1 μl/100 μl serum). 800 μl phenol (Qiazol) and 200 μl chloroform were added to the sample and mixed vigorously for 15 sec followed by an incubation at room temperature for 10 min, followed by centrifugation for 15 min at 12,000 g. The aqueous phase was precipitated with 500 μl 100% isopropanol and 2 μl glycogen (Fermentas, St. Leon-Rot, Germany) o.n. at -20°C. After centrifugation at 4°C for 30 min (12,000 g), the pellets were washed with 70% EtOH and RNA was resuspended in 30 μl RNase-free water (Ambion, Austin, TX). RNA quality was assessed using a NanoDrop spectrophotometer (NanoDrop), and a small RNA assay for Agilent's Bioanalyzer was performed (Agilent Technologies, Böblingen, Germany).
2.3. Quantitative Real-Time PCR [16, 17]
5 μl of extracted total RNA was used for cDNA synthesis using the miScript Reverse Transcriptase Kit (Qiagen). cDNA samples were used for qPCR using the miScript SYBR Green PCR Kit (Qiagen) and miRNA-specific primers (Qiagen) on a qPCR machine (Applied Biosystems 7300 Sequence Detection System, Applied Biosystems, Foster City, CA). Data were generated and analyzed using the SDS 2.3 and RQ manager 1.2 software packages.
2.4. Statistical Analysis [16–19]
Data are given as the median and range using the Mann-Whitney U test, and for multiple comparisons, the Kruskal-Wallis H test was used. Box plot graphics display a statistical summary of the median, quartiles, and ranges. Correlation analyses were performed by using the Spearman correlation tests. Kaplan-Meier curves were used to analyze the overall survival (OS). Optimal cut-off values were established using the well-established Youden index as described before. The prognostic relevance of serum miR-155 was further tested using univariate Cox regression analysis. ROC curves were generated by plotting sensitivity against 1 − specificity. All statistical analyses were performed with SPSS (SPSS 23, Chicago, IL, USA).
3. Results
3.1. miR-155 Serum Levels Are Elevated in Critically Ill Patients
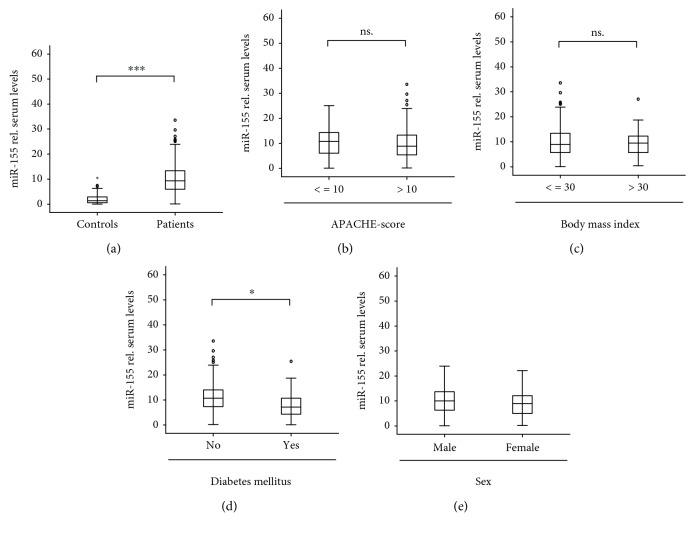
We measured miR-155 serum concentrations in 218 patients upon admission to the ICU and in 76 healthy controls. In these analyses, miR-155 serum concentrations were significantly elevated in critically ill patients (Figure 1(a)). Next, we analyzed whether miR-155 concentrations might reflect the disease severity in critically ill patients. Therefore, we subdivided our cohort of patients into those with a more severe disease state versus a less severe disease state according to APACHE-II score values. Interestingly, both groups displayed similar miR-155 serum concentrations (Figure 1(b)), indicating that circulating miR-155 is independent of the disease severity in critically ill patients.
Figure 1.
Serum miR-155 levels of critically ill patients at ICU admission. (a) qPCR was used to determine the concentrations of circulating miR-155 at admission to the ICU. In this analysis, critically ill patients (n = 218) displayed significantly higher serum levels of miR-155 compared to healthy controls (n = 76). (b) Serum miR-155 concentrations were independent on disease severity. (c) Serum concentrations of miR-155 were measured in patients with/without diabetes mellitus type 2. (d) Serum concentrations of miR-155 independent on the presence of obesity. (e) Serum concentrations of miR-155 did not vary with respect to patients' sex. ∗∗∗ p < 0.001.
It was recently demonstrated that metabolic comorbidities determine the prognosis and treatment outcome of patients treated on a medical ICU. As alterations in miR-155 serum levels have recently been found in metabolic diseases, we next analyzed the impact of preexisting type 2 diabetes or obesity on miR-155 concentrations. Of note, we found significantly lower levels of miR-155 in patients with type 2 diabetes, while miR-155 concentrations were independent on the presence of obesity (Figures 1(c) and 1(d)).
There is increasing evidence for sex differences in inflammatory pathologies. Therefore, we analyzed the levels of circulating miR-155 specifically in male and female patients. Notably, no differences were found in this analysis (Figure 1(e)).
3.2. miR-155 Serum Levels Are Not Affected by the Presence of Sepsis
Based on recent results, suggesting that mir-155 serum concentrations are elevated in sepsis [20], we subdivided our patients into those that fulfilled sepsis 3 criteria (n = 135) and those that did not (patients' characteristics are given in Table 2). Interestingly, no significant differences in circulating miR-155 levels between both subgroups of patients became apparent (Supplementary Figure 1A). To further substantiate this finding, we performed correlation analyses between miR-155 and parameters routinely used to access the presence of sepsis in critically ill patients. In this analysis, serum miR-155 levels were not correlated to pro- or anti-inflammatory C-reactive protein (CRP; r = 0.027, p = 0.770), procalcitonin (PCT; r = 0.133, p = 0.197), interleukin-6 (IL-6; r = 0.084, p = 0.519), interleukin-10 (IL-10; r = −0.002, p = 0.983), or tumor necrosis factor (TNF; r = −0.022, p = 0.990). Next, we hypothesized that miR-155 serum levels might be altered in specific disease etiologies. However, the differences between the various etiologies did not meet the criteria of statistical significance (Supplementary Figure 1B).
Table 2.
Disease etiology of the study population.
| All patients | <65 years | >65 years | |
|---|---|---|---|
| Sepsis critical illness | n = 135 | n = 74 | n = 61 |
| Source of infectionn(%) | |||
| Pulmonary | 71 | 34 | 37 |
| Abdominal | 28 | 17 | 11 |
| Urogenital | 3 | 3 | 0 |
| Other | 33 | 20 | 12 |
| Nonsepsis critical illness n (%) | n = 83 | n = 51 | n = 32 |
| Cardiopulmonary disease | 28 | 13 | 16 |
| Decompensated liver cirrhosis | 12 | 9 | 3 |
| Nonsepsis other | 43 | 29 | 14 |
3.3. miR-155 Serum Concentrations Predict Survival Specifically in Critically Ill Patients Younger than 65 Years
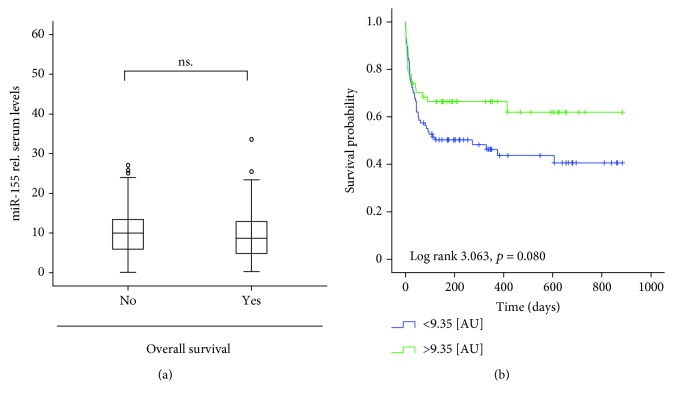
We next tested whether miR-155 serum levels might allow to estimate overall mortality. We therefore compared miR-155 levels in patients that succumbed to death to those in patients that survived in the long-term follow-up. However, no differences between both patient groups became apparent (Figures 2(a) and 2(b)).
Figure 2.
Serum levels of miR-155 are not predictive for patients' overall prognosis. (a) Serum levels of miR-155 were analyzed by qPCR in critically ill patients that survived in the long-term follow-up or succumbed to death. No difference between these groups became apparent. (b) Patients with miR-155 levels below or higher than the median of all patients displayed a similar long-term survival.
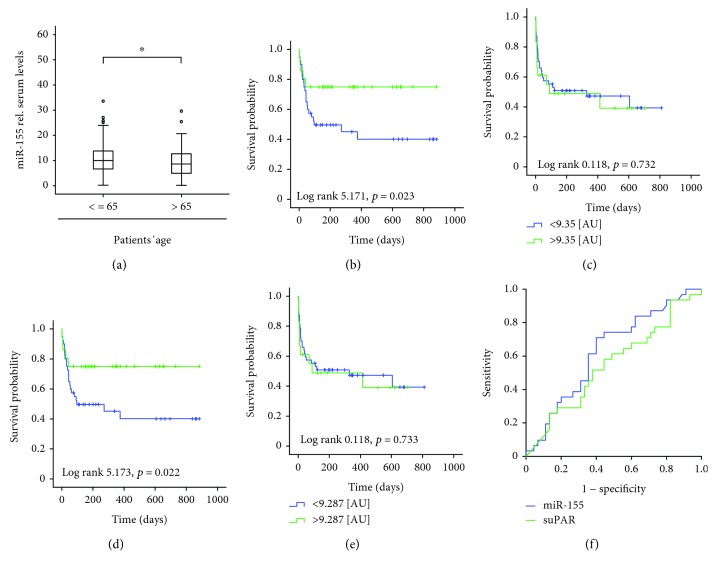
Based on the recent data suggesting that the expression patterns and functions of miRNAs might depend on the patients' age, we next compared concentrations in circulating miR-155 between patients that were older than 65 years on those that were younger at the time point of admission to the ICU. Interestingly, we found statistically significant lower miR-155 levels in the subgroup of the older patients compared to patients < 65 years old (Figure 3(a); patients' characteristics are given in Supplementary Figure 2, Tables 1 and 2). In line, miR-155 levels correlated with patients' age (r = −0.212; p = 0.002). To evaluate the impact of age on the role of miR-155 as a prognostic biomarker in critical illness, we performed Kaplan Meier curve analysis separately in the subgroup of the younger (<65 years) and the older (>65 years) patients. Strikingly, while in the subgroup of patients < 65 years, those with low miR-155 concentrations (below the median of all patients) displayed a significantly impaired overall mortality compared to the other patients (Figure 3(b)), no such effect was observed in the group of the patients > 65 years (Figure 3(c)). To substantiate these findings, the Youden index was used to determine an optimal threshold of miR-155 levels for predicting patients' survival within the group of the patients < 65 years [21]. This analysis revealed that relative miR-155 concentrations of 9.28 (AU) had the best sensitivity and specificity to decide whether a patient will survive or not. Strikingly, patients with miR-155 serum concentrations >9.28 (AU) demonstrated a significantly longer survival compared to patients with lower values (Figures 3(c) and 3(d)). Again, no such effect was seen in the subgroup of the critically ill patients older than 65 years (Figure 3(e)). Consequently, circulating miR-155 demonstrated a significant correlation with the patients' survival time specifically in the group of the young ICU patients (r = 0.443, p = 0.001 vs. r = 0.170, p = 0.330) and differences in mortality between patients with low (51%) vs. high (29%, p = 0.014) serum concentrations of miR-155 were only apparent in this specific collective of patients, while in older patients a similar mortality was observed (56% vs. 62%, n.s.).
Figure 3.
Serum concentrations of ICU predict long-term survival specifically in young ICU patients. (a) miR-155 serum levels in patients younger or older than 65 years. (b, c) Kaplan-Meier curve analysis demonstrating that patients < 65 years (but not older patients) with miR-155 concentrations below the median of all patients had an increased overall mortality. (d) The Youden index was used to calculate the optimal threshold for distinguishing between long-term survivors and patients that did not survive in the group of patients < 65 years old. Kaplan-Meier survival curve analyses revealed that patients with miR-155 concentrations below this threshold had an increased overall mortality. (e) Kaplan-Meier curve analysis was performed in patients > 65 years old, revealing that the mortality of these patients was independent of their miR-155 serum concentration. (f) ROC curve analysis revealing that miR-155 serum levels display a superior prognostic value in critically ill patients younger than 65 years. ∗ p < 0.05.
Finally, we used ROC curve analysis, showing that the prognostic value of miR-155 is similar to that of the APACHE-II score, patient's age, serum creatinine concentration, INR, and suPAR (Figure 3(f); Supplementary Figure 3). In summary, these results imply a novel function of miR-155 serum levels as a prognostic serum-based marker that is only apparent in critically ill patients younger than 65 years.
4. Discussion and Conclusions
miR-155 levels were recently described as diagnostic biomarkers in coronary artery disease [22, 23] and dissection of the ascending aorta [24] and have also been validated as a powerful biomarker in B-cell malignancies [25], esophageal cancer [20], and other malignant diseases [26]. However, studies proving a diagnostic or prognostic role of miR-155 in critical illness are not available yet. Our study reports a novel role of circulating miR-155 in distinguishing critically ill patients from healthy controls and in predicting survival of critically ill patients < 65 years old. Our data rely on a large sequentially recruited cohort comprising 218 critically ill patients that were precisely characterized regarding clinical characteristics. 76 healthy blood donors that were unfortunately not age-matched to the patients were used as controls.
A Chinese study group investigated a cohort of sixty patients and found that, compared to healthy controls, sepsis patients exhibit significantly elevated miR-155 levels, which is positively related to a greater severity of sepsis [20]. Neither could we identify an influence of sepsis on miR-155 levels nor were miR-155 serum levels correlated to disease severity. Interestingly, elevated miR-155 levels were predictive of a severe condition and poor prognosis in sepsis patients [20]. In accordance, our study identifies the prognostic value of miR-155 regarding survival. However, we demonstrate that low miR-155 levels correlate with reduced survival in young critically ill patients including the group of patients with sepsis.
In the pathophysiology of critical illness, life-threatening diseases of diverse origins like infection and shock lead to instant local and systemic physiologic responses involving all major organs [27]. The time course and severity of the host response to damage are determined by both the intensity of the trauma and host factors, and the activation of the first-line inflammatory immune response is sometimes followed by immunosuppression. Prior studies identified miR-155 as an integral part of the initial immune reaction (i.e., activation of macrophages) to diverse inflammation-causing agents. For example, it was shown that miR-155 expression can be initiated upon stimulation with LPS in a human monocytic cell line [28]. Furthermore, activators of inflammation like interferon-β or TNF can provoke miR-155 expression in macrophages and monocytes. In patients with critical illness, the inflammatory mediator LPS is found in higher levels compared to healthy probands [29]. Moreover, LPS and TNF exert profound influence on the systemic inflammatory response caused by infection, trauma, burns or hemorrhagic shock, and pancreatitis [30, 31]. Investigations using on a mouse macrophage/monocyte cell line showed that an increase in TNF levels by LPS results in the upregulation of miR-155 [32]. Conversely, LPS-induced upregulation of miR-155 leads to increased TNF production [32]. Furthermore, studies in a transgenic mouse line overexpressing miR-155 in a B-cell lineage synthetize more TNF when stimulated with LPS and are highly susceptible to septic shock induced by LPS [32]. Thus, a possible pathomechanism in the pathogenesis of critical illness might be the interaction between miR-155 and LPS/TNF. One possible explanation might be that in patients with critical illness, increased LPS levels might lead to the upregulation of miR-155 which then leads to an increase in TNF, contributing to long-term detrimental effects on survival in these patients. It is conceivable that miR-155 raises TNF levels by improving transcript stability through binding to its 3′UTR. Finally, targeting and reduction of expression of SHIP1 phosphatase by miR-155 might explain its proinflammatory effects observed in different pathologies [33]. As another option, miR-155 could influence gene transcripts coding for proteins that are recognized for their ability to suppress TNF-α translation. This pathomechanism will be subject to studies in the future. Furthermore, miR-155 is reported to target and reduce the expression of genes involved in the LPS/TNF such as FADD. Therefore, it will be important to characterize further downstream targets of miR-155 in this context. We demonstrate a significant association of miR-155 levels with the presence of critical illness and miR-155 levels correlated with survival in patients younger than 65 years old. Several investigators have reported that the amount of serum miRNA is age dependent [34] and that circulating microvesicle number, function, and small RNA content vary with age [35]. Importantly, miR-155 has been identified to have a significantly lower abundance in peripheral blood mononuclear cells of older (mean age 65 years old) individuals [36]. These findings could explain the fact that the prognostic value of miR-155 levels is compromised with increased age.
In summary, our data and results from previous studies imply a novel function of miR-155 as a serum-based marker in critically ill patients.
Acknowledgments
This work was supported by a Mildred-Scheel Endowed Professorship from the German Cancer Aid (Deutsche Krebshilfe); the German Research Foundation (DFG) (LU 1360/3-1 and SFB-TRR57/P06); the Interdisciplinary Centre for Clinical Research (IZKF) Aachen, Germany, and the Ernst Jung Foundation Hamburg to TL; a project grant from the German Research Foundation (DFG RO 4317/4-1) to CR; a START grant from the Medical Faculty RWTH Aachen to CR and SR; and a project grant of the German Center for Cardiovascular Diseases (DZHK, B18-005Ext) to MES, CR, ML, and TL.
Abbreviations
- ACCP/SCCM:
American College of Chest Physicians and the Society of Critical Care Medicine
- ALT:
Alanine aminotransferase
- APACHE:
Acute physiology and chronic health evaluation
- APRIL:
A proliferation-inducing ligand
- AST:
Aspartate aminotransferase
- AUC:
Area under the curve
- BMI:
Body mass index
- BNP:
Brain natriuretic peptide
- CRP:
C-reactive protein
- DNA:
Deoxyribonucleic acid
- GFR:
Glomerular filtration rate
- ICU:
Intensive care unit
- IL:
Interleukin
- INR:
International normalized ratio
- LDH:
Lactate dehydrogenase
- miRNA:
Microribonucleic acid
- PCHE:
Pseudocholinesterase
- PCR:
Polymerase chain reaction
- PCT:
Procalcitonin
- RNA:
Ribonucleic acid
- ROC:
Receiver operating characteristic
- SAPS:
Simplified acute physiology score
- SIRS:
Systemic inflammatory response syndrome
- SOFA:
Sequential organ failure assessment
- SuPAR:
Soluble urokinase-type plasminogen activator receptor
- TNF:
Tumor necrosis factor
- WBC:
White blood cell count.
Contributor Information
Christoph Roderburg, Email: croderburg@ukaachen.de.
Tom Luedde, Email: tluedde@ukaachen.de.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Additional Points
Key Messages. miR-155 serum concentrations are elevated in critically ill patients; are independent on the severity of disease, the disease etiology, and the presence of sepsis; do not reflect the overall survival in the total cohort of patients; and predict long-term survival specifically in patients < 65 years old.
Ethical Approval
Patients were included into the study upon providing written informed consent, and the institutional ethics committees approved this consent procedure. The study protocol was conducted in accordance with the ethical standards laid down in the Declaration of Helsinki (ethics committee of the University Hospital Aachen, RWTH-University, Aachen, Germany; reference number EK 150/06).
Disclosure
Frank Tacke and Martina E. Spehlmann share first-authorship. Christoph Roderburg and Tom Luedde share senior-authorship.
Conflicts of Interest
All authors declare that they have no competing interests.
Authors' Contributions
CR, MS, ML, FB, MES, SL, H-JH, CT, FT, and TL designed the study, analyzed the data, and wrote the manuscript. SR, MV, DVC, and FB performed the measurements. CR, FB, and FT performed the statistical analyses. AK and FT collected the data and organized patient recruitment.
Supplementary Materials
Supplementary Figure 1: serum miR-155 concentrations are unaltered in sepsis. (A) miR-155 serum levels were analyzed in patients with or without sepsis. (B) miR-155 was analyzed in different disease etiologies. Supplementary Figure 2: analysis of the cohort with respect to patients' age. (A) miR-155 serum levels were analyzed in patients < 65 years with different disease severities. (B) miR-155 serum levels were analyzed in patients < 65 years with or without sepsis. (C) miR-155 serum levels analyzed in patients < 65 years with different disease etiologies. (D) Serum concentrations of miR-155 were analyzed in patients < 65 years with or without diabetes mellitus type 2. (E) Serum concentrations of miR-155 were analyzed in patients < 65 years with or without obesity. (F) miR-155 serum levels were analyzed in patients > 65 years with different disease severities. (G) miR-155 serum levels were analyzed in patients > 65 years with or without sepsis. (H) miR-155 serum levels analyzed in patients > 65 years with different disease etiologies. (I) Serum concentrations of miR-155 were analyzed in patients > 65 years with or without diabetes mellitus type 2. (J) Serum concentrations of miR-155 were analyzed in patients > 65 years with or without obesity. Supplementary Figure 3. prognostic value of miR-155 serum levels in patients younger than 65 years old. ROC curve analysis was performed. Supplementary Table 1A: disease etiology of the study population (<65 years) Supplementary Table 1B: disease etiology population (>65 years).
References
- 1.Riedemann N. C., Guo R. F., Ward P. A. The enigma of sepsis. The Journal of Clinical Investigation. 2003;112(4):460–467. doi: 10.1172/JCI200319523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davey P. G., Marwick C. Appropriate vs. inappropriate antimicrobial therapy. Clinical Microbiology and Infection. 2008;14(Supplement 3):15–21. doi: 10.1111/j.1469-0691.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- 3.Singer M. Personalizing sepsis care. Critical Care Clinics. 2018;34(1):153–160. doi: 10.1016/j.ccc.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 4.van Engelen T. S. R., Wiersinga W. J., Scicluna B. P., van der Poll T. Biomarkers in sepsis. Critical Care Clinics. 2018;34(1):139–152. doi: 10.1016/j.ccc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Mashima R. Physiological roles of miR-155. Immunology. 2015;145(3):323–333. doi: 10.1111/imm.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques-Rocha J. L., Samblas M., Milagro F. I., Bressan J., Martinez J. A., Marti A. Noncoding RNAs, cytokines, and inflammation-related diseases. The FASEB Journal. 2015;29(9):3595–3611. doi: 10.1096/fj.14-260323. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H., Li J., Gao P., Wang Q., Zhang J. miR-155: a novel target in allergic asthma. International Journal of Molecular Sciences. 2016;17(10) doi: 10.3390/ijms17101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner W., Wein F., Roderburg C., et al. Adhesion of human hematopoietic progenitor cells to mesenchymal stromal cells involves CD44. Cells, Tissues, Organs. 2008;188(1-2):160–169. doi: 10.1159/000112821. [DOI] [PubMed] [Google Scholar]
- 10.Bin L., Leung D. Y. M. Genetic and epigenetic studies of atopic dermatitis. Allergy, Asthma & Clinical Immunology. 2016;12:p. 52. doi: 10.1186/s13223-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su L. C., Huang A. F., Jia H., Liu Y., Xu W. D. Role of microRNA-155 in rheumatoid arthritis. International Journal of Rheumatic Diseases. 2017;20(11):1631–1637. doi: 10.1111/1756-185X.13202. [DOI] [PubMed] [Google Scholar]
- 12.Szűcs D., Béres N. J., Rokonay R., et al. Increased duodenal expression of miR-146a and -155 in pediatric Crohn’s disease. World Journal of Gastroenterology. 2016;22(26):6027–6035. doi: 10.3748/wjg.v22.i26.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel K., Herrera L., Zhou T., et al. The functional role of microRNAs in alcoholic liver injury. Journal of Cellular and Molecular Medicine. 2014;18(2):197–207. doi: 10.1111/jcmm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeiffer D., Rossmanith E., Lang I., Falkenhagen D. miR-146a, miR-146b, and miR-155 increase expression of IL-6 and IL-8 and support HSP10 in an in vitro sepsis model. PLoS One. 2017;12(6, article e0179850) doi: 10.1371/journal.pone.0179850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benz F., Roy S., Trautwein C., Roderburg C., Luedde T. Circulating microRNAs as biomarkers for sepsis. International Journal of Molecular Sciences. 2016;17(1) doi: 10.3390/ijms17010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roderburg C., Luedde M., Vargas Cardenas D., et al. Circulating microRNA-150 serum levels predict survival in patients with critical illness and sepsis. PLoS One. 2013;8(1, article e54612) doi: 10.1371/journal.pone.0054612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roderburg C., Urban G. W., Bettermann K., et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53(1):209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 18.Loosen S. H., Roderburg C., Kauertz K. L., et al. Elevated levels of circulating osteopontin are associated with a poor survival after resection of cholangiocarcinoma. Journal of Hepatology. 2017;67(4):749–757. doi: 10.1016/j.jhep.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Loosen S. H., Benz F., Niedeggen J., et al. Serum levels of S100A6 are unaltered in patients with resectable cholangiocarcinoma. Clinical and Translational Medicine. 2016;5:p. 39. doi: 10.1186/s40169-016-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R., Liao J., Yang M., et al. Circulating miR-155 expression in plasma: a potential biomarker for early diagnosis of esophageal cancer in humans. Journal of Toxicology and Environmental Health Part A. 2012;75(18):1154–1162. doi: 10.1080/15287394.2012.699856. [DOI] [PubMed] [Google Scholar]
- 21.Budczies J., Klauschen F., Sinn B. V., et al. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12, article e51862) doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faccini J., Ruidavets J. B., Cordelier P., et al. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Scientific Reports. 2017;7(1, article 42916) doi: 10.1038/srep42916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roy S., Benz F., Vargas Cardenas D., et al. miR-30c and miR-193 are a part of the TGF-β-dependent regulatory network controlling extracellular matrix genes in liver fibrosis. Journal of Digestive Diseases. 2015;16(9):513–524. doi: 10.1111/1751-2980.12266. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z., Wang Q., Pan J., et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Scientific Reports. 2017;7(1, article 13659) doi: 10.1038/s41598-017-13696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Due H., Svendsen P., Bødker J. S., et al. miR-155 as a biomarker in B-cell malignancies. BioMed Research International. 2016;2016:14. doi: 10.1155/2016/9513037.9513037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou Y., Wang J., Wang X., Shi S., Wang W., Chen Z. Appraising microRNA-155 as a noninvasive diagnostic biomarker for cancer detection: a meta-analysis. Medicine. 2016;95(2, article e2450) doi: 10.1097/MD.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeb A. L., Longnecker D. E. Inhibition of endothelium-derived relaxing factor-dependent circulatory control in intact rats. The American Journal of Physiology. 1992;262(5) Part 2:H1494–H1500. doi: 10.1152/ajpheart.1992.262.5.H1494. [DOI] [PubMed] [Google Scholar]
- 28.Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(5):1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strieter R. M., Kunkel S. L., Bone R. C. Role of tumor necrosis factor-α in disease states and inflammation. Critical Care Medicine. 1993;21(Supplement):S447–S463. doi: 10.1097/00003246-199310001-00006. [DOI] [PubMed] [Google Scholar]
- 31.Juskewitch J. E., Knudsen B. E., Platt J. L., et al. LPS-induced murine systemic inflammation is driven by parenchymal cell activation and exclusively predicted by early MCP-1 plasma levels. The American Journal of Pathology. 2012;180(1):32–40. doi: 10.1016/j.ajpath.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tili E., Michaille J. J., Cimino A., et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-α stimulation and their possible roles in regulating the response to endotoxin shock. Journal of Immunology. 2007;179(8):5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 33.Costinean S., Sandhu S. K., Pedersen I. M., et al. Src homology 2 domain-containing inositol-5-phosphatase and CCAAT enhancer-binding protein beta are targeted by miR-155 in B cells of Emicro-MiR-155 transgenic mice. Blood. 2009;114(7):1374–1382. doi: 10.1182/blood-2009-05-220814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hooten N. N., Fitzpatrick M., Wood W. H., et al. Age-related changes in microRNA levels in serum. Aging. 2013;5(10):725–740. doi: 10.18632/aging.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enjeti A. K., Ariyarajah A., D'Crus A., Seldon M., Lincz L. F. Circulating microvesicle number, function and small RNA content vary with age, gender, smoking status, lipid and hormone profiles. Thrombosis Research. 2017;156:65–72. doi: 10.1016/j.thromres.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Noren Hooten N., Abdelmohsen K., Gorospe M., Ejiogu N., Zonderman A. B., Evans M. K. microRNA expression patterns reveal differential expression of target genes with age. PLoS One. 2010;5(5, article e10724) doi: 10.1371/journal.pone.0010724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: serum miR-155 concentrations are unaltered in sepsis. (A) miR-155 serum levels were analyzed in patients with or without sepsis. (B) miR-155 was analyzed in different disease etiologies. Supplementary Figure 2: analysis of the cohort with respect to patients' age. (A) miR-155 serum levels were analyzed in patients < 65 years with different disease severities. (B) miR-155 serum levels were analyzed in patients < 65 years with or without sepsis. (C) miR-155 serum levels analyzed in patients < 65 years with different disease etiologies. (D) Serum concentrations of miR-155 were analyzed in patients < 65 years with or without diabetes mellitus type 2. (E) Serum concentrations of miR-155 were analyzed in patients < 65 years with or without obesity. (F) miR-155 serum levels were analyzed in patients > 65 years with different disease severities. (G) miR-155 serum levels were analyzed in patients > 65 years with or without sepsis. (H) miR-155 serum levels analyzed in patients > 65 years with different disease etiologies. (I) Serum concentrations of miR-155 were analyzed in patients > 65 years with or without diabetes mellitus type 2. (J) Serum concentrations of miR-155 were analyzed in patients > 65 years with or without obesity. Supplementary Figure 3. prognostic value of miR-155 serum levels in patients younger than 65 years old. ROC curve analysis was performed. Supplementary Table 1A: disease etiology of the study population (<65 years) Supplementary Table 1B: disease etiology population (>65 years).
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.