Abstract
Human-restricted Salmonella enterica serovar Typhi (S. Typhi) is the causative agent of typhoid fever—a life-threatening disease of great global health significance, particularly in the developing world. Ty21a is an oral live-attenuated vaccine that protects against the development of typhoid disease in part by inducing robust T cell responses, among which multifunctional CD8+ cytotoxic T lymphocytes (CTL) play an important role. Following Ty21a vaccination, a significant component of adult CTL have shown to be targeted to S. Typhi antigen presented by the conserved major histocompatibility complex (MHC) class Ib molecule, human leukocyte antigen-E (HLA-E). S. Typhi challenge studies have shown that baseline, multifunctional HLA-E responsive T cells are associated with protection from, and delayed onset of, typhoid disease. However, despite the overwhelming burden of typhoid fever in school-aged children, and due to limited availability of pediatric samples, incomplete information is available regarding these important HLA-E-restricted responses in children, even though studies have shown that younger children may be less likely to develop protective cell mediated immune (CMI) responses than adults following vaccination. To address this gap, we have studied this phenomenon in depth by using mass cytometry to analyze pediatric and adult T cell responses to HLA-E-restricted S. Typhi antigen presentation, before and after Ty21a vaccination. Herein, we show variable responses in all age strata following vaccination among T effector memory (TEM) and T effector memory CD45RA+ (TEMRA) cells based on conventional gating analysis. However, by utilizing the dimensionality reduction tool tSNE (t-distributed Stochastic Neighbor Embedding), we are able to identify diverse, highly multifunctional gut-homing- TEM and TEMRA clusters of cells which are more abundant in adult and older pediatric participants than in younger children. These findings highlight a potential age-associated maturation of otherwise conserved HLA-E restricted T cell responses. Such insights, coupled with the marked importance of multifunctional T cell responses to combat infection, may better inform future pediatric vaccination strategies against S. Typhi and other infectious diseases.
Keywords: T cell response, dimensionality reduction, Ty21a, multifunctionality, pediatric immunology, Salmonella typhi, HLA-E restricted responses, typhoid
Introduction
Salmonella enterica serovar Typhi (S. Typhi) is a human-restricted obligate-intracellular pathogen and is the causative agent of typhoid fever, which causes around 21-million illnesses and over 200,000 deaths per year (1–4). The pathogen is spread via contaminated food and water, and much of the disease burden lies on the developing world with significant morbidity associated among school-aged children (1, 3, 5–9). Challenge studies have shown that robust, multifunctional baseline CD8+ T cell responses to S. Typhi antigen presentation are associated with protection from, and/or delayed onset of typhoid disease (10, 11). Therefore, a successful attenuated typhoid vaccine should aim to induce these cell-mediated immunological (CMI) responses. Indeed, the oral live-attenuated Ty21a vaccine has been shown to induce CMI in approximately two-thirds of adult recipients (12–14).
Because typhoid fever is a disease that disproportionally impacts children in the developing world, successful vaccination strategies require protecting pediatric populations in endemic regions. Unfortunately, limited information has been reported addressing potential differences between pediatric and adult T cell responses to vaccination, especially regarding oral, live-attenuated vaccines. Previous studies in healthy volunteers have shown that the percentage of total T cells (CD3+) and CD8+ T cells in peripheral blood are stable throughout life (15–17). However, in general peripheral naïve T cell populations are higher in younger children, with memory T cell phenotypes increasing throughout life (15–20). Previous work from our group has shown that following mitogenic stimulation, CD8+ T cell activation—as defined by CD69 expression—increases throughout adolescence, plateauing by the age of 15-years-old (17). Further, we identified significantly lower mitogen-induced CD8+ TEM multifunctional effector responses in younger children compared to adults (17). Interestingly, studies have suggested that younger children may be less likely than older children and adults to develop lasting, protective CMI following vaccination (21, 22), and field trial data of Ty21a showed lower efficacy in children aged 9–14 than those aged 15 or older (4, 23, 24).
HLA-E is a highly conserved non-classical class I MHC, capable of presenting the MHC leader peptide to CD94/NKG2 as a means of regulating natural killer cell function (25). In addition to this innate, inhibitory presentation of self-antigens, HLA-E can present a limited repertoire of peptides from a variety of viral and bacterial pathogens to CD8+ T cells, leading to activation of adaptive immune responses separate from those that require classical HLA restriction (26–29). Previous work from our laboratory identified seven HLA-E binding peptides, derived from the S. Typhi GroEL sequence, that elicit cytotoxic CD8+ T lymphocyte responses following Ty21a vaccination—and that this restricted response makes up ~30% of the total CD8+ T cell response (30). Further studies have found that Ty21a vaccination is capable of inducing multifunctional S. Typhi-reactive HLA-E restricted CD8+ TEM and TEMRA in adults for up to 2 years post-vaccination (31). Additionally, we have shown HLA-E restricted responders show an earlier post-Ty21a immunization peak than those responding to S. Typhi infected autologous B-lymphoblastoid cell line (B-LCL) (32). Finally, the presence of baseline S. Typhi-responsive HLA-E-restricted T cells is associated with delayed onset of disease in a low-dose human S. Typhi challenge model (10).
In this study we utilized an established S. Typhi-infected HLA-E-restricted antigen presentation model (10, 11, 30–32) to explore the variability of CD8+ T cell responses among pediatric and adult Ty21a vaccine recipients. To explore these responses in depth we used a comprehensive mass cytometry panel which enables us to characterize T cell memory, activation, and proliferation, as well as the expression of homing molecules and the production of multiple effector cytokines and chemokines. Additionally, we used a variety of dimensionality reduction techniques to characterize multifunctional responders, as well as to further explore distinct Ty21a-responsive populations within our participant cohorts. Herein we describe how Ty21a vaccination elicits age-associated differences among HLA-E restricted CD8+ T cell effector responses, multifunctionality, and homing potential, and discuss how these differences may influence future pediatric S. Typhi vaccination strategies.
Materials and Methods
Participants and Isolation of PBMC
PBMC were collected before Ty21a vaccination, and between 14 and 42 days following Ty21a vaccination, from 18 healthy pediatric [6–17 years of age at the time of enrollment, receiving Ty21a vaccination for medically indicated reasons (i.e., travel to endemic regions)] and 13 healthy adult (20–65 years of age at the time of enrollment) volunteers, as listed in Table 1, following recruitment from the Baltimore-Washington area and the University of Maryland at Baltimore campus. These studies were approved by the University of Maryland at Baltimore Institutional Review Board (IRB) and were carried out in accordance with the Declaration of Helsinki. Written and informed consent was obtained from all adult participants, as well as written informed consent from the parents of any participant under the age of 18 years old, and assent from the pediatric participants, prior to the conduct of any study procedures. PBMC were isolated immediately following blood collection by density gradient centrifugation and stored in liquid nitrogen following standard cryopreservation techniques (33, 34) until used in the assays. Where cell numbers permitted, B-lymphoblastoid cells generated from PBMC were HLA-E typed by DNA exon sequencing at the University of Oklahoma Health Sciences Center's Sequence-Based Typing facility directed by Dr. William Hildebrand.
Table 1.
Participant Ty21a vaccinees listed by age (years), sex, race, and HLA-E haplotype.
| Age (Years) | Sex | Race | HLA-E Haplotype |
|---|---|---|---|
| 6 | Male | African American | *01 |
| 13 | Female | African American | Heterozygous |
| 13 | Female | African American | Heterozygous |
| 13 | Male | Caucasian | *03 |
| 14 | Male | Hispanic | *03 |
| 14 | Female | Caucasian | Heterozygous |
| 14 | Female | Caucasian | Heterozygous |
| 15 | Female | Caucasian | n/a |
| 15 | Female | Caucasian | *01 |
| 15 | Female | Caucasian | n/a |
| 16 | Male | Caucasian | *01 |
| 16 | Male | Caucasian | n/a |
| 16 | Male | Caucasian | *03 |
| 16 | Male | African American | Heterozygous |
| 16 | Male | Caucasian | Heterozygous |
| 17 | Female | Caucasian | *01 |
| 17 | Male | Caucasian | Heterozygous |
| 17 | Male | Caucasian/Hispanic | Heterozygous |
| 20 | Female | African American | *01 |
| 25 | Female | African American | *01 |
| 27 | Female | African American | *01 |
| 33 | Female | Caucasian | Heterozygous |
| 36 | Male | Caucasian | *01 |
| 41 | Female | Caucasian | *03 |
| 43 | Male | African American | *01 |
| 43 | Male | African American | Heterozygous |
| 44 | Female | African American | *01 |
| 51 | Male | African American | Heterozygous |
| 62 | Male | n/a | *03 |
| 65 | Male | n/a | Heterozygous |
| 65 | Male | n/a | *03 |
n/a, not available.
Preparation of HLA-E-Restricted Target Cells
HLA class I-defective B cell line transfected with HLA-E fused to the HLA-A2 leader peptide (721.221.AEH), allowing for expression of the HLA-E*01:01 allele on the cell surface (35), was thawed in complete 1640 RPMI media (Gibco, Carlsbad, CA), supplemented with 100 U/mL penicillin (Sigma), 100 μg/mL streptomycin (Sigma, St. Louis, MO), 50 μg/mL gentamicin (Gibco), 2 mM L-glutamine (Gibco), 2.5 mM sodium pyruvate (Gibco), 10 mM HEPES buffer (Gibco), non-essential amino acids (Lonza, Basel, Switzerland), and 10% fetal bovine serum (Gemini Bioproducts, West Sacramento, CA) and allowed to expand. HLA-E restricted targets were infected in RPMI without antibiotics for 3 h at 37°C with wild-type S. Typhi strain ISP1820 (wt S. Typhi) at a multiplicity of infection (MOI) of 7:1 (3.5 × 107 wt S. Typhi as determined by OD600: 5.0 × 106 721.221.AEH cells). Uninfected targets were used as controls. Following the 3 h incubation, all cells are washed twice and incubated at 37°C, 5% CO2 overnight in cRPMI supplemented with 0.3% gentamycin. Expression of wt S.Typhi antigens on live, infected targets (and not on uninfected targets) was confirmed by conventional flow cytometry with LIVE/DEAD™ yellow cell stain (ThermoFisher Scientific, Waltham, MA) and BacTrace® FITC-labeled anti-Salmonella CSA-1 antibody (SeraCare, Milford, MA). Cells were subsequently gamma-irradiated (6,000 rad) and re-suspended in fresh cRPMI before being used as targets in co-culture with effector cells.
In vitro Stimulation
PBMC were thawed and rested overnight at 37°C, 5% CO2 in cRPMI. After the overnight rest, cells were washed and resuspended in cRPMI at a concentration of 1 × 106 cells/500 μL in 5 mL in cell culture tubes. Irradiated target cells were incubated with PBMC at an effector-to-stimulator ratio of 5:1 in the presence of 3 μL/mL anti-CD107a monoclonal antibody (mAb) conjugated to 151Eu (Fluidigm, South San Francisco, CA) for 2 h at 37°C in 5% CO2. After the 2 h incubation, GolgiStop (containing monensin), and GolgiPlug (containing brefeldin A) from BD (San Jose, CA) were added at 0.5 μL/mL to all tubes and cultures were maintained at 37°C in 5% CO2 overnight.
Surface and Intracellular Labeling and Mass Cytometry Analysis
Co-cultured PBMC were centrifuged and incubated with anti-CD45 (Fluidigm South San Francisco, CA) monoclonal antibodies (mAbs) for barcoding. Pediatric samples were stained with CD45-154Sm and adult samples were stained with CD45-156Gd for 30 min at 4°C. Cells were then washed once with flow cytometry buffer [1x PBS, Quality Biological, Gaithersburg, MD), 0.1% sodium azide (Sigma), 2% fetal bovine serum (Gemini Bioproducts)] and once with serum-free RPMI (Gibco) before being combined into their barcoded layout. Like-stimulated adult (CD45-156Gd) and pediatric (CD45-154Sm) PBMC were combined into a single tube for downstream staining. Mass cytometry staining was performed as described in the Materials and Methods section “Mass Cytometry Measurements” from McArthur et al. (36, 37) using the monoclonal antibodies listed in Table 2. Briefly, cells were labeled with metal-tagged antibodies against specific surface and intracellular targets, cisplatin as a viability marker, and iridium as a DNA intercalator for cell identification before preparation and running by the UMB flow cytometry and mass cytometry core in a CyTOF instrument (Fluidigm). Mass cytometry data were analyzed using WinList version 9.0.1 (Verity Software House, Topsham, ME) following debarcoding of the files, based on whether they were tagged with 154Sm (pediatric)- or 156Gd (adult)-labeled CD45, with Premium Cytobank (Cytobank, Inc, Santa Clara, CA). Net responses were calculated by subtracting T cell responses to uninfected HLA-E-restricted antigen presenting cells from responses to infected HLA-E-restricted targets. tSNE analysis was run in R using the Cytofkit package in biocLite (38). Gating of individual tSNE clusters was performed using WinList version 9.0.1.
Table 2.
Mass cytometry panel showing antibody target, stable metal isotope (or other label), antibody clone, and a brief description of the target function.
| Target | Stable metal isotope | Clone | Description |
|---|---|---|---|
| CD14 | 114 Cd (Qdot) | TüK4 | Monocyte marker |
| CD19 | 114 Cd (Qdot) | SJ25-C1 | B cell marker |
| IL-4 | 142 Nd | MP425D2 | Tc/h2 effector cytokine |
| CXCR5 | Biotin | RF8B2 | Follicular homing chemokine receptor |
| Biotin | 143 Nd | 1D4-C5 | |
| α4β7 | FITC | ACT-1 | Gut-homing integrin |
| FITC | 144 Nd | FIT22 | |
| CD8 | 146 Nd | RPA-T8 | Cytotoxic T lymphocyte marker |
| IL-6 | 147 Sm | MQ213A5 | Proinflammatory proliferation-associated cytokine |
| CCR4 | 149 Sm | L291H4 | Chemokine homing to the skin |
| MIP-1β | 150 Nd | D21-1351 | NK and monocyte recruiting chemokine |
| CD107a | 151 Eu | H4A3 | Degranulation marker |
| TNFα | 152 Sm | Mab11 | Proinflammatory cytokine |
| CD62L | 153 Eu | DREG-56 | Lymphoid-tissue homing selectin |
| CD45 | 154 Sm | HI30 | Pan-leukocyte barcoding marker |
| CD27 | 155 Gd | L128 | TNF superfamily—costimulatory molecule |
| CD45 | 156 Gd | HI30 | Pan-leukocyte barcoding marker |
| IL-2 | 158 Gd | MQ1-17H12 | Induction of T cell differentiation and proliferation |
| CD69 | 162 Dy | FN50 | Activation marker |
| CXCR3 | 163 Dy | G025H7 | Chemokine homing to sites of inflammation |
| IL-17A | 164 Dy | N49-653 | Tc/h17 effector cytokine |
| IFNγ | 165 Ho | B27 | Proinflammatory cytokine |
| IL-10 | 166 Er | JES3-9D7 | Anti-inflammatory cytokine |
| CD154 (CD40L) | 168 Er | 24-31 | Co-stimulatory molecule; Tfh induction of B cell maturation |
| CD45RA | 169 Tm | HI-100 | T cell memory marker |
| CD3 | 170 Er | UCHT1 | TCR co-receptor (T cell marker) |
| Granzyme B | 171 Yb | GB11 | Secreted cytotoxic effector molecule |
| IL-21 | 172 Yb | 3A3-N2 | Tfh effector cytokine (germinal center formation) |
| ICOS (CD278) | 173 Yb | C398.4A | Tfh co-stimulatory marker (B cell help) |
| CD4 | 174 Yb | SK3 | Helper T lymphocyte marker |
| PD-1* | 175 Lu | EH12.2H7 | T cell exhaustion/activation marker |
| CCR6 | 176 Yb | G034E3 | Chemokine homing to mucosal surfaces |
| Cell I.D. (DNA) | 191/193 Ir | n/a | DNA intercalator |
| Viability | 194/195 Pt | n/a | Viability stain |
Primary surface antibodies are filled with blue, secondary surface antibodies with green, and intracellular antibodies with orange (*anti-PD-1 is in both the surface and intracellular antibody mixes). Barcoding antibodies are shaded in yellow, and anti-CD107a, which is added during stimulation to best account for the active cycling between the surface and intracellular vesicles, is highlighted in purple.
Statistical Analyses
All analyses were performed using GraphPad Prism version 7.0c. Unpaired t-tests, paired t-tests, z-scores, and/or chi squared tests were performed depending on the analysis as indicated. P-values of < 0.05 were considered significant.
Results
CD8+ T Cell Populations Before and After Ty21a Vaccination in Different Age Strata
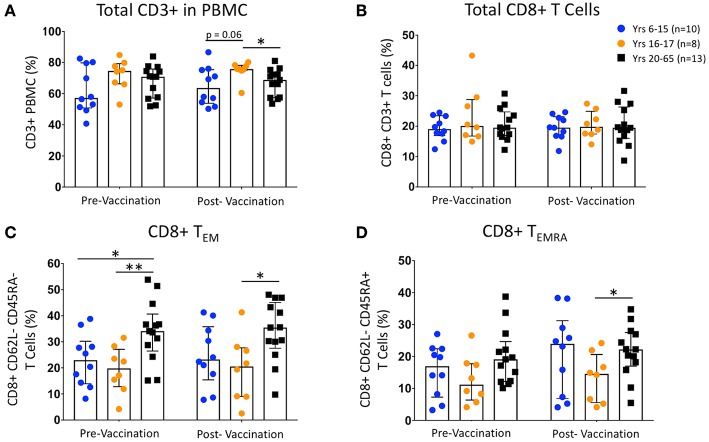
Unstimulated PBMC from pre- and 14 to 42-days post-Ty21a vaccinated healthy pediatric and adult participants were labeled with metal-conjugated mAbs and analyzed on a mass cytometer. Percentages of total T cells (CD3+ CD14− CD19−), total CD8+ T cells (CD4− CD8+), CD8+ TEM (CD62L− CD45RA−), and TEMRA (CD62L− CD45RA+) cells showed no changes between vaccination states within either 6–15 year-old children (n = 10), 16–17 year-old children (n = 8), or adult (20–65 years old; n = 13) participants (Figures 1A–D). CD62L−/lo populations are capable of defining effector memory populations similar to CCR7 expression, and the identification of T memory subsets using CD62L and CD45RA is well-established (39). While no differences were observed comparing pre- and post-vaccinated pediatric age groups, we note that, as has been previously reported by us and others (16, 17, 19, 20, 40, 41), the percentage of CD8+ TEM are lower in children than in adults (Figure 1C). Further, while we see no differences within groups following vaccination, among unstimulated T cell populations divided by both sex and age (Supplemental Figures 1A–D), we observed that the greatest differences among the CD8+ TEM are between pediatric males (ages 6–17; n = 10) and females (ages 11–17; n = 8), and adult males (ages 36–65; n = 7), regardless of vaccination status (Supplemental Figure 1C). Finally, the percentages of CD8+ TEM and TEMRA populations are not significantly different, nor does vaccination alter those percentages, among participants with different HLA-E haplotypes (Supplemental Figures 1E,F).
Figure 1.
Change in CD8 T cell Populations Following Ty21a Vaccination. Scatter plots showing the percentages of (A) pre- and post-vaccination CD3+ T cells, (B) total CD8+ T cells, (C) CD8+ T effector memory (TEM; CD45RA- CD62L-), and (D) CD8+ T EMRA (TEMRA; CD45RA+ CD62L-) populations among 6–15 year-old pediatric (n = 10), 16–17 year-old pediatric (n = 8), and adult (n = 13) participants (media). Bars represent medians with whiskers indicating interquartile ranges. Statistics were analyzed by unpaired t-test (*p < 0.05; **p < 0.01).
Change in Activated CD8+ T Cell Populations Following Ty21a Vaccination
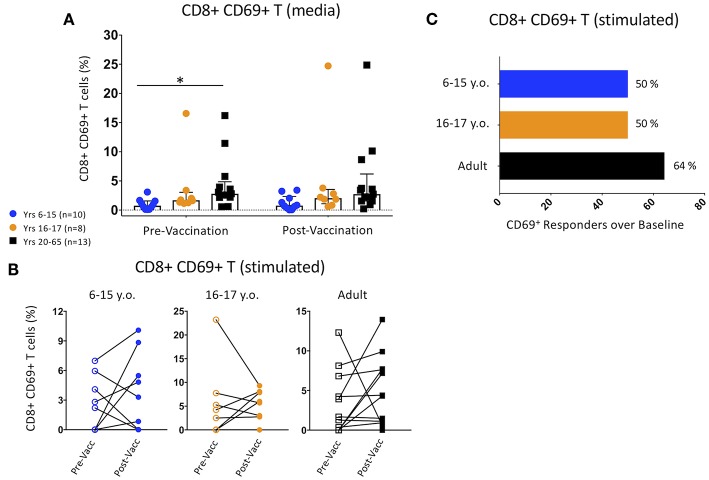
CD69 expression was used to define activated CD8+ T cell populations among participants pre- and post-vaccination. Unstimulated adult PBMC pre-vaccination showed significantly higher CD69 expression than 6–15 year-old pediatric participants, and trended toward significance within the post-Ty21a time point (Figure 2A). These differences were maintained between pediatric females and adult males but were not seen among other gender and age divisions (Supplemental Figure 2A). There was no change in CD69 expression between pre- and post-vaccination in any of the age groups (Figure 2A).
Figure 2.
Changes in Activated CD8+ CD69+ T cell Populations Following Ty21a Vaccination. (A) Scatter plot showing the percentages of CD69+ CD8+ T cells among pre- and post-Ty21a unstimulated PBMC (media). Bars represent medians with whiskers indicating interquartile ranges. (B) Connected dot plots showing each participants' pre- and post-Ty21a CD8+ CD69+ expression levels following co-culture with S. Typhi-infected HLA-E restricted antigen presenting target cells. Data divided among 6–15 year-old pediatric (n = 10), 16–17 year-old pediatric (n = 8), and adult (n = 13) participants. (C) Bars representing the proportion of CD8+ CD69+ responders over baseline levels (cut-off of 0.1%) following co-culture with S. Typhi-infected HLA-E restricted antigen presenting target cells. Statistics were analyzed by unpaired t-test. (*p < 0.05).
We then explored whether Ty21a vaccination induced higher levels of S. Typhi-responsive activation among different age groups by co-culturing PBMC obtained pre- or post-vaccination with S. Typhi-infected HLA-E-restricted target cells. None of the age-associated changes among the pre- and post-vaccination activated CD8+ T cell percentages were significant (Figure 2B). However, when using a z-score between events collected in S. Typhi-infected vs. non-infected targets with a p < 0.05 and a cutoff of 0.1%, over baseline, we observed a trend for higher percentage of adult responders than were observed in either pediatric age group (Figure 2C).
No significant differences were observed between the percentages of CD69+ T cells at baseline or following vaccination based on sex or age (Supplemental Figure 2B). Further, the z-score between events collected in S. Typhi-infected vs. non-infected targets with a p < 0.05 and a cutoff of 0.1%, over baseline, did not show significant differences among gender- and age-parsed populations, despite some trends observed in the percentages of responders (particularly among pediatric females), likely due to insufficient numbers of participants in each group (Supplemental Figure 2C). Interestingly, while there were no differences in unstimulated CD8+ activation percentages among pre- and post-vaccination HLA-E haplotypes (Supplemental Figure 2D), we observed significant differences following stimulation with S. Typhi-infected targets between HLA-E*01:01 or heterozygous individuals and HLA-E*01:03 before immunization (Supplemental Figure 2E), However, when dividing the populations into HLA-E haplotypes, we observed that HLA-E*01:03 participants pre-vaccination show almost no baseline activation (as defined by CD69 expression), yet nearly all of these participants show >0.1% increases in the percentage of CD69+ CD8+ T cells over baseline following vaccination, suggesting a possible Ty21a-induced change in activation threshold among these HLA-E mismatched individuals (Supplemental Figures 2E,F).
CD8+ TEM Responses Following Ty21a Vaccination
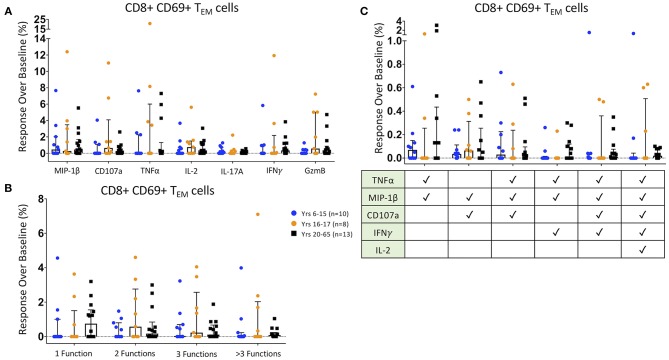
We then analyzed the functionality of HLA-E-restricted antigen-responsive CD8+ TEM cells. We identified increases in MIP-1β, CD107a, TNFα, IL-2, IL-17A, IFNγ, and Granzyme B post-Ty21a vaccination responses to HLA-E restricted S. Typhi antigen presentation over pre-vaccination responses (response over baseline) in many individual participants. There were no significant age-associated differences observed among individual effector functions (Figure 3A). Additionally, among observed mono- and multi-functional activation states -as defined by the increased expression of one or more of the aforementioned effector functions identified with the FCOM function in Winlist- the percentage of responses over baseline showed no significant overall age-associated differences (Figure 3B). Finally, we analyzed specific multifunctional responses over baseline in the HLA-E restricted co-culture model, which were shown in a previous S. Typhi challenge study to be associated with protection from developing, and/or delayed onset of, disease (10, 11). Among these six multifunctional populations, as identified with FCOM, there were no significant differences among age groups (Figure 3C).
Figure 3.
CD8+ T Effector Memory Responses. Scatter plots showing (A) the responses over baseline percentages of CD8+ CD69+ TEM producing/expressing MIP1β, CD107a, TNFα, IL-2, IL-17A, IFNγ, and Granzyme B (GzmB); (B) CD8+ CD69+ TEM observed mono-, bi-, tri-, and >3-functional populations; and (C). specific CD8+ CD69+ TEM selected multifunctional responses following co-culture with HLA-E restricted S. Typhi infected antigen presenting targets. These MF populations were selected based on those shown in a previous S. Typhi challenge study to be associated with protection from developing disease (10, 11). Bars represent medians with whiskers indicating interquartile ranges. Results are shown for the following populations: 6–15 year-old pediatric (n = 10), 16–17 year-old pediatric (n = 8), and adult (n = 13) participants. Scatter plot statistics were analyzed by unpaired t-test. (*p < 0.05).
Similarly, no significant differences among CD8+ TEM effectors were observed when dividing the participants by sex or as age, although Granzyme B+ expression trended toward significance comparing pediatric and adult male participants to female pediatric participants (Supplemental Figure 3A). Interestingly, the observed monofunctional CD8+ TEM population among adult males was significantly higher than either of the female age-groups (Supplemental Figure 3B), likely due to the observed higher Granzyme B expression in this group. Further, when comparing the specific, multifunctional populations associated with protection, we observed that males—especially pediatric males—were more likely to have higher percentages of HLA-E restricted S. Typhi-responsive CD107a/MIP-1β double-positive cells following vaccination than pediatric females (Supplemental Figure 3C). Among the HLA-E haplotype divided populations, no significant individual CD8+ TEM effector responses-over-baseline were seen (Supplemental Figure 3D). HLA-E*01:03 exhibited significantly higher monofunctional responses than heterozygous participants (Supplemental Figure 3E), but there were no significant differences in Ty21a-induced multifunctional populations, including those which has been associated with protection in previous studies (10, 11) (Supplemental Figure 3F), among the HLA-E haplotypes.
Taken together, we observed few significant response-over-baseline trends associated with Ty21a vaccination among HLA-E restricted S. Typhi-responsive CD8+ TEM, based on traditional TC1 and TC17 effector molecules. The trends that were observed were likely due to the wide dispersion of the responses in individual volunteers seen within age groups, sex, and HLA-E haplotypes.
CD8+ TEMRA Responses Following Ty21a Vaccination
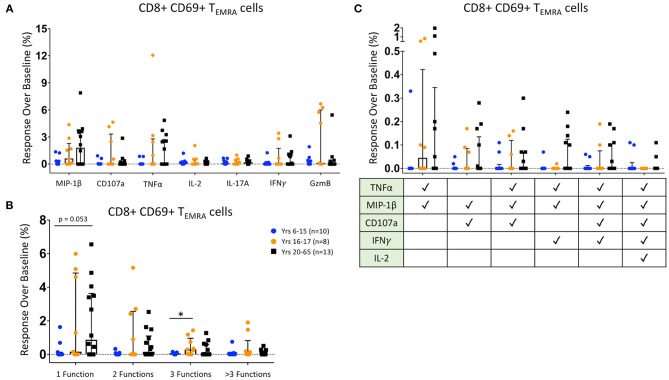
We then analyzed the functionality of HLA-E-restricted antigen-responsive CD8+ TEMRA cells. We identified increases over baseline of HLA-E-restricted CD8+ TEMRA expression of MIP-1β, CD107a, TNFα, IL-2, IL-17A, IFNγ, and Granzyme B among many Ty21a-vaccinated participants. Interestingly, MIP-1β and TNFα responses were significantly higher among CD8+ TEMRA from adults than in our younger pediatric participants, and Granzyme B expression was significantly higher in 16–17 year-old participants than among either adults or younger pediatric vaccines (Figure 4A). Further, when analyzing functional states, we found that younger pediatric vaccines show significantly fewer observed mono- and bi-functional CD8+ TEMRA than both adult and older adolescent participants, while 16–17 year-old individuals show significantly greater tri-functional populations than the younger participants (Figure 4B). There were no significant differences across age groups in the induction of defined protection-associated multifunctional populations; however, adults trended toward a greater percentages of TNFα/MIP-1β/IFNγ triple-positive CD8+ TEMRA than either pediatric group (Figure 4C).
Figure 4.
CD8+ T Effector Memory RA Responses. Scatter plots showing (A) the responses over baseline percentages of CD8+ CD69+ TEMRA producing/expressing MIP1β, CD107a, TNFα, IL-2, IL-17A, IFNγ, and Granzyme B (GzmB); (B) CD8+ CD69+ TEMRA observed mono-, bi-, tri-, and >3-functional populations; and (C). specific CD8+ CD69+ TEMRA selected multifunctional responses following co-culture with HLA-E restricted S. Typhi infected antigen presenting targets. These MF populations were selected based on those shown in a previous S. Typhi challenge study to be associated with protection from developing disease (10, 11). Bars represent medians with whiskers indicating interquartile ranges. Results are shown for the following populations: 6–15 year-old pediatric (n = 10), 16–17 year-old pediatric (n = 8), and adult (n = 13) participants. Scatter plot statistics were analyzed by unpaired t-test. (*p < 0.05).
As with the CD8+ effector memory cells, we divided our participant by sex and age. Adult males showed significantly greater percentages of MIP-1β and TNFα, and pediatric males showed significantly more Granzyme B expression, when compared to pediatric females (Supplemental Figure 4A). These low percentages of functional CD8+ TEMRA in pediatric females was also seen in the analysis of observed mono- and multifunctional responses over baseline, where their monofunctional responses were significantly lower than adults of both genders, and their bifunctional responses were lower than adult males (Supplemental Figure 4B). Among defined protection-associated multifunctional populations, adult males have significantly higher percentages of TNFα/MIP-1β/IFNγ triple-positive CD8+ TEMRA than both male and female pediatric participants (Supplemental Figure 4C). Further, the adult males also have significantly greater TNFα/MIP-1β/CD107a/IFNγ responsiveness than pediatric females (Supplemental Figure 4C). There were, however, no significantly distinct effector responses among the various HLA-E haplotypes (Supplemental Figure 4D). Both homozygous haplotypes showed greater monofunctional responses over baseline compared to the heterozygous participants (Supplemental Figure 4E). Among the specific multifunctional subsets, the HLA-E*01:03 quintuple-positive response was significantly higher than the heterozygous participants; however, the significance of these findings is unclear given the relatively small number of individuals in this cohort and the low magnitude of responses (Supplemental Figure 4F).
Taken together, the CD8+ TEMRA response over baseline to HLA-E restricted S. Typhi-infected target cells are variable among individuals but in general show more significant age- and gender-associated differences than observed with TEM, particularly between adult males and pediatric females.
Protection-Associated Multifunctional T Cells With Granzyme B Expression
The S. Typhi challenge study that found a correlation of certain multifunctional T cell responses and protection did not include Granzyme B expression (10, 11). However, because it is likely that the expression of cytotoxic Granzyme B would aid in defining the protective capabilities of multifunctional T cells, the current studies also included Granzyme B expression to complement the analyses of “protection-associated” multifunctional responses previously described for both CD69+CD8+ TEM and TEMRA (Supplemental Figure 5). Interestingly, the analyses of Granzyme B production identified significantly greater percentages of TNFα/MIP-1β/GzmB triple-positive by PBMC from adult participants, as well as TNFα/MIP-1β/CD107a/IFNγ/GzmB quintuple-positive TEM cells in adult and 16–17 year-old pediatric participants than those observed in the 6–15 year-old participants (Supplemental Figure 5A). TEMRA responsiveness shows fewer significant differences with the addition of Granzyme B than without (Supplemental Figure 5B). Similar to previous observations, the bulk of these differences appear to be between adult males and pediatric females, among TEM (Supplemental Figure 5C) and TEMRA (Supplemental Figure 5D). There were no significant differences between HLA-E haplotypes with Granzyme B-added to the study of TEM multifunctional populations (Supplemental Figure 5E). However, HLA-E*01:01 participants showed a significantly higher percentage of TNFα/MIP-1β /IFNγ/GzmB quadruple-positive TEMRA than heterozygous participants. Of note, no HLA-E*01:03 participants were considered vaccine responders (Supplemental Figure 5F).
tSNE Analysis of Activated CD8+ TEM Populations
Activated (CD69+) CD8+ TEM cell data were exported and processed on the Cytofkit package available in the R biocLite module. Sixteen clusters were identified following tSNE visualization using the ClusterX unbiased clustering method on the following markers: IL-4, CXCR5, α4β7, IL-6, CCR4, MIP-1β, CD107a, TNFα, IL-2, CXCR3, IL-17A, IFNγ, IL-10, CD154 (CD40L), Granzyme B, IL-21, ICOS, PD-1, and CCR6 (Supplemental Figure 6A). Individual participants were parsed into pre- and post-Ty21a vaccinated age groups (6–15, 16–17, and 20–65 years old) and cells were pooled into a single cluster analysis. To ensure that the proportions of the various clusters were representative of the various age and treatment groups, similar numbers of cells from each of the participants and time points were included in the tSNE analyses shown in Figure 5. Of note, the younger pediatric participants clearly showed much fewer cells in clusters 2, 3, 8, and 9 compared to the older pediatric and adults at both pre- and post-vaccine timepoints (Figure 5A). Importantly, older pediatric and adult tSNE populations are very similar. Thus, all downstream analysis was focused on comparing the responses of 6–15 year-old participants with those observed in 16–65 year-old participants.
Figure 5.
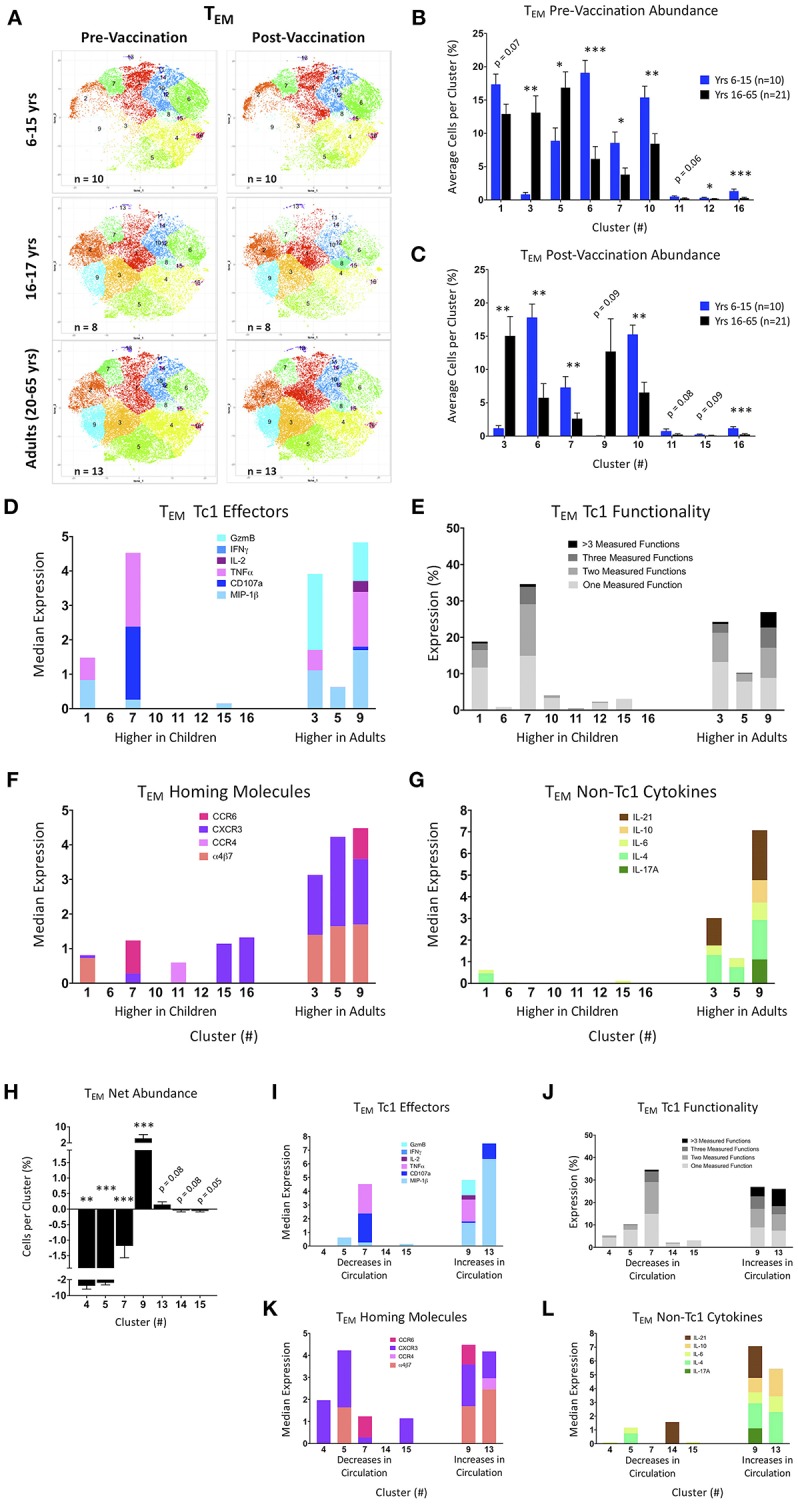
tSNE analysis of activated CD8+ T effector memory populations. (A) tSNE maps of pre- and post-Ty21a vaccinated 6–15 year old, 16–17 year old, and adult participants. (B,C) Significantly different clusters between age groups (6–15 year old and 16 years and over) represented by bar graphs identifying the average percent of cells per volunteer per cluster in pre-Ty21a (B) and postTy21a (C) immunization timepoints. (D–G) Functions of significantly different tSNE clusters from (B,C), divided into: (D) median expression of Tc1 effector functions MIP1β, CD107a, TNFα, IL-2, IFNγ, and Granzyme B (GzmB). (E) Winlist FCOM-analysis determined Tc1 mono- and multifunctional cytokine expression. (F) median expression of homing markers, and (G) median expression of non-Tc1 associated cytokines. (H) Clusters showing significant net changes in the percent of cells following Ty21a vaccination. (I–L) Functions of significantly different tSNE clusters from (H), divided into: (I). median expression of Tc1 effector functions. MIP1β, CD107a, TNFα, IL-2, IFNγ, and Granzyme B (GzmB), (J) Winlist FCOM-analysis determined Tc1 mono- and multifunctional cytokine expression, (K) median expression of homing markers, and (L) median expression of non-Tc1 associated cytokines.
Clusters were then analyzed based on the average percentage of cells per cluster, split by age group, and vaccination status (Figures 5B,C). Of note, clusters 11 through 16 have much fewer percentage of cells per cluster than clusters 1 through 10. Adults show significantly higher percentages of cells in clusters 3 and 5 pre-vaccination, whereas younger children have higher percentages of cells in clusters 6, 7, 10, 12, and 16 (Figure 5B). Following Ty21a vaccination, clusters 3 and 9 are significantly more abundant in adults, while clusters 6, 7, 10, and 16 remain significantly higher among younger children (Figure 5C). Interestingly, although the tSNE graphs show some clusters (e.g., 2 and 9) that appear different between age groups, they approached (p = 0.15), but did not reach statistically significant differences between groups, likely due to over-representation in these clusters of a few individuals. We then analyzed the median expression of conventional cytotoxic CD8+ T cell (TC1) effectors in each significant cluster, thus characterizing the phenotype of the cells within the clusters that are differentially represented among age groups. Through this analysis, it became clear that clusters 3, 7, and 9 are made up of cells capable of co-expressing several TC1 functions (Figure 5D). By gating and analyzing each cluster with FCOM in Winlist, we are able to identify the percentage of cells that express one or more of each of the six TC1 functions. This downstream supervised analysis identified clusters 3, 7, and 9, as those that contain the greatest percentage of multifunctional CD8+ CD69+ TEM (Figure 5E). In addition to conventional TC1 effectors, we also studied the homing characteristics of the cells in each cluster. This analysis showed that clusters with significantly higher percentages of adult cells also contain cells with high median expression of the gut-homing molecule integrin α4β7, as well as inflammation-associated homing chemokine CXCR3 and, in the case of cluster 9, the mucosal-homing chemokine CCR6 (Figure 5F). We also explored the median expression of non-TC1 cytokines within our CD8+ CD69+ TEM and found that multifunctional cluster 9 contained nearly all of the IL-17A producing cells, though cluster 3 was found, together with cluster 9, to produce the TC17-associated cytokine IL-21 (Figure 5G). Of note, both of these clusters are more represented by adult cells.
We next explored significant changes in cluster abundance following Ty21a vaccination by identifying the net change in the percentage of cells per cluster (Figure 5H). Of note, only 16–65 year old participants showed any significant differences in TEM clusters following Ty21a vaccination. These significant clusters were then analyzed as in Figures 5D–G, here based on whether they increased or decreased from circulation following vaccination (Figures 5I–L). Cluster 9, which increases in adults following vaccination, is made up of multifunctional TEM, capable of expressing multiple Tc1 and Tc17 effectors, and a variety of homing molecules. With the exception of cluster 7, the clusters that decrease following Ty21a vaccination tend to contain TEM that are less functional based on the effectors measured in these experiments.
We then performed supervised gating and FCOM analyses of the most multifunctional clusters to explore the percentages of the multifunctional populations associated with protection as described previously. Interestingly, cluster 2 seemed to be populated by many of the more protective phenotypes with or without Granzyme B included in the analyses (Supplemental Figures 7A,B), but cluster 9 is also representative of the Granzyme B-containing populations (Supplemental Figure 7B). In addition to TC1 effectors, non-TC1 effectors, and homing markers, we also explored expression of follicular-associated molecules such as CXCR5, activation molecules CD154 (CD40L) and ICOS, as well as the exhaustion/activation marker PD-1. Interestingly, ICOS [which has been shown to be important for maintenance of TC17 in mice (42)] was present in many clusters (Supplemental Figure 7C). Further, PD-1 was present in the highly multifunctional cluster 9, and CXCR5 is seen in the low-abundance multifunctional cluster 13. Finally, in addition to dividing by age, we also parsed activated CD8+ TEM tSNE maps based on gender and HLA-E haplotype to determine the presence of Ty21a associated relationships. Marked differences were observed in the dominance of various clusters between males and females, whether pre- or post-vaccination (Supplemental Figure 7D). Of note, clusters 2, 3, 5, and 13 are more highly represented in males, whereas cluster 8 is overrepresented in females, many of those from the vaccinated pool (Supplemental Figures 7D,E). Marked differences were observed in the dominance of various clusters between unmatched HLA-E*01:03 and both HLA-E*01:01 and heterozygous, whether pre- or post-vaccination (Supplemental Figure 7F). Unmatched HLA-E*01:03 participants showed a low average number of cells in several clusters, particularly in the more multifunctional clusters (Supplemental Figures 7F,G).
tSNE Analysis of Activated CD8+ TEMRA Populations
Activated (CD69+) CD8+ TEMRA were analyzed using the same methods and clustering markers as utilized for the TEM data above (Supplemental Figure 6B) and generated 19 distinct clusters. To ensure that the proportions of the various clusters were representative of the various age and treatment groups, similar numbers of cells from each of the participants and time points were included in the tSNE analyses shown in Figure 6. The pre- and post-vaccine tSNE maps of younger pediatric participants are rather sparse compared with the older pediatric and adult maps, particularly within clusters 1, 2, 3, 5, and 7 (Figure 6A). Again, due to their similarity, 16–17 year-olds were combined with adults for downstream comparisons to the 6–15 year-old participants.
Figure 6.
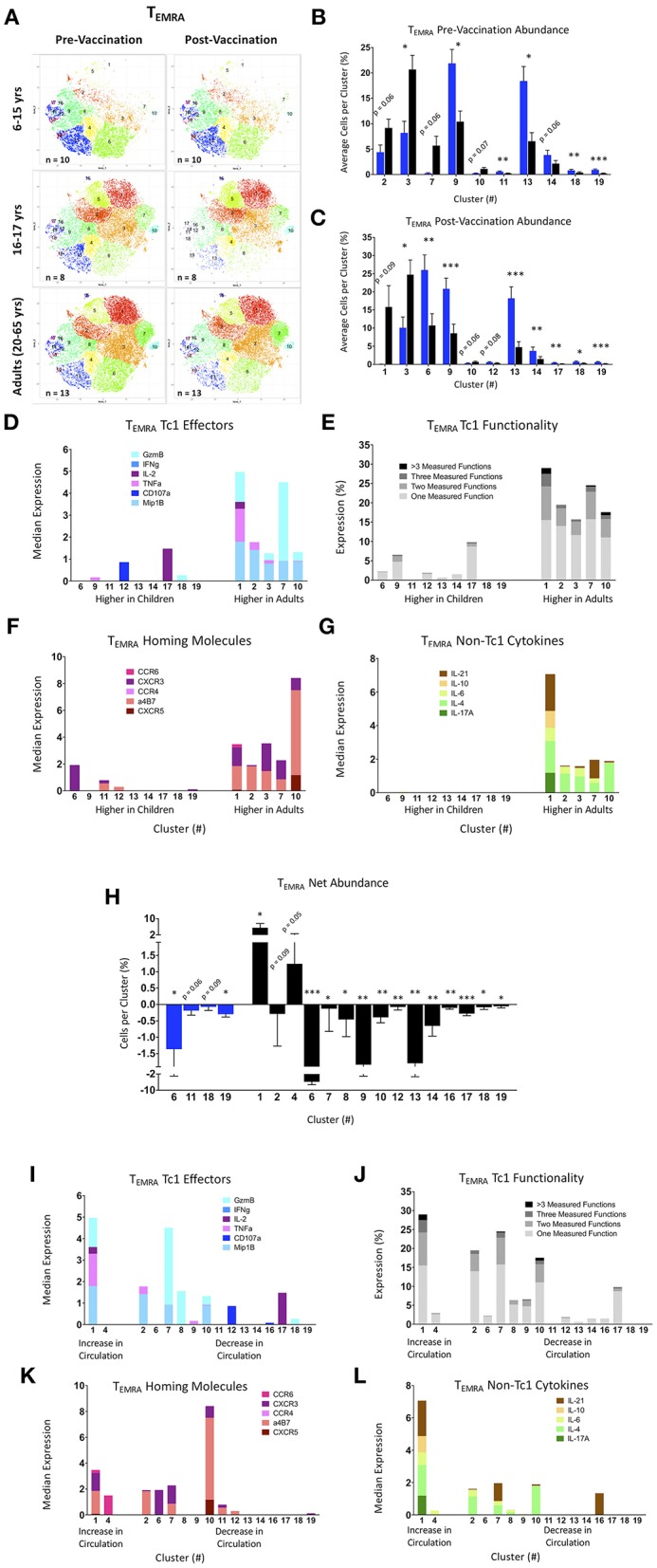
tSNE analysis of activated CD8+ T effector memory RA (TEMRA) populations. (A) tSNE maps of pre- and post-Ty21a vaccinated 6–15 year old, 16–17 year old, and adult participants. (B,C) Significantly different clusters between age groups (6–15 year old and 16 years and over) represented by bar graphs identifying the average percent of cells per volunteer per cluster in pre-Ty21a (B) and post-Ty21a (C) immunization timepoints. (D–G) Functions of significantly different tSNE clusters from (B,C), divided into: (D) median expression of Tel effector functions MIP1β, CD107a, TNFα, IL-2, IFNγ, and Granzyme B (GzmB), (E) Winlist FCOM-analysis determined Tc1 mono- and multifunctional cytokine expression, (F) median expression of homing markers, and (G) median expression of non-Tc1 associated cytokines. (H) Clusters showing significant net changes in the percent of cells following Ty21a vaccination. (I–L) Functions of significantly different tSNE clusters from (H), divided into: (I) median expression of Tc1 effector functions MIP1β, CD107a, TNFα, IL-2, IFNγ, and Granzyme B (GzmB), (J) Winlist FCOM-analysis determined Tc1 mono- and multifunctional cytokine expression, (K) median expression of homing markers, and (L) median expression of non-Tc1 associated cytokines.
As with the TEM, clusters were analyzed based on average percentage of cells per cluster, split between age group, and vaccination status (Figures 6B,C). Here, clusters 10 through 12, and 15 through 19 have much fewer percentage of cells per cluster than clusters 1 through 9, 13, and 14. Among the pre-Ty21a time-point, clusters 9, 11, 13, 18, and 19 are significantly more abundant in children, while only cluster 3 is significantly higher in adults (with clusters 7 and 10 trending toward significance; Figure 6B). Following Ty21a vaccination, clusters 6, 9, 13, 14, 17, 18, and 19 are all made up of a significantly higher percentage of pediatric TEMRA, while cluster 3 again is the only population significantly higher in adults (with clusters 1 and 10 trending toward significance; Figure 6C). As discussed above regarding the TEM data, although the tSNE graphs show striking differences in certain clusters (e.g., 1 and 5), only trends which did reach statistical significance (p < 0.05), or trend toward significance (p < 0.1), were observed for differences in these clusters between age groups. Only post-Ty21a cluster 1 exhibited a close trend toward a significant difference (p = 0.09). We then analyzed the median expression of conventional TC1 effectors in each cluster. We observed that clusters made up mostly of adult TEMRA are more diverse (Figure 6D), and more multifunctional, as determined by FCOM analyses (Figure 6E). Exploration of the median expression of homing molecules show that the more multifunctional clusters more abundant in adults contain cells capable of homing to the gut (integrin α4β7 expression) and sites of inflammation (CXCR3 expression; Figure 6F). Further, TEMRA capable of producing non-Tc1 cytokines, including cluster 1 which contains cells capable of producing IL-17A, previously reported to play an important role in protection against S. Typhi, are only seen in clusters higher in adults (Figure 6G) (10, 11, 32). Interestingly, clusters that are more frequently observed in younger participants show little-to-no median expression of TC1 effectors, gut-homing molecules, or non-TC1 cytokines.
We also explored significant changes in cluster abundance following Ty21a vaccination by identifying net changes in the percentage of cells per cluster (Figure 6H). Interestingly, only two clusters significantly increased in circulation following vaccination, and those were only found 16–65 year old participants. The significant clusters were analyzed based on Tc1 effector, Tc1 multifunctionality, homing, and non-Tc1 cytokines, here based on whether they increased or decreased from circulation following vaccination (Figures 6I–L). Cluster 1, which increases in adults following vaccination, is capable of expressing multiple Tc1 and Tc17 effectors, integrin α4β7, and CXCR3, and shows high multifunctional potential. The TEMRA clusters that decrease following Ty21a vaccination are more variable in their functional and homing characteristics, but are not of the same magnitude as seen in cluster 1.
Similar to the TEM analysis, we explored multifunctional populations which have been associated with protection, and identified cluster 5 as containing the greatest percentage of “protective phenotypes” with and without the addition of Granzyme B as one of the parameters (Supplemental Figures 8A,B). Additionally, we explored non-TC1 effectors expression of CXCR5, CD154 (CD40L), ICOS, and PD-1 among our CD8+ CD69+ TEMRA. ICOS was less frequently observed among TEMRA than among TEM, but it was present in multifunctional clusters 1 and 15 (Supplemental Figure 8C). Exhaustion and activation marker PD-1 is also abundant within cluster 1. Interestingly, cluster 10 contains nearly all of the CXCR5 observed among the TEMRA, a very small cluster by number of cells, present mostly in older adolescents and adults, that also contain cells expressing MIP1-β, IL-4, and abundant integrin α4β7 (Figures 6A–F and Supplemental Figure 8A). In the analyses of the TEMRA tSNE maps divided by gender, we observed that females have fewer numbers of cells than males in multifunctional clusters 2, 3, and 5 (Supplemental Figures 8D,E). Finally, we observed that mismatched HLA-E*01:03 participants had a very low average number of cells for many clusters, especially multifunctional clusters 1, 2, 3, and 5 (Supplemental Figures 8F,G).
Discussion
The oral live-attenuated Ty21a S. Typhi vaccine protects against the development of typhoid disease, at least in part, by inducing robust T cell responses. Challenge studies with wild-type S. Typhi have shown that S. Typhi-antigen-responsive HLA-E-restricted CD8+ T cells are associated with protection from, and/or delayed onset of, typhoid disease (10, 11), and thus are likely important for effective vaccination. However, there is no information on the function and magnitude of these restricted CD8+ T cells within pediatric Ty21a recipients. In this study we compared pediatric and adult S. Typhi-antigen-responsive HLA-E-restricted CD8+ T cells, pre- and post- Ty21a vaccination, to better understand the impact of age and gender on the immunity elicited by Ty21a vaccination.
This study confirms and extends previous observation by us and others that children have a lower proportion of CD8+ TEM cells than adults, while the proportions of CD8+ TEMRA show fewer age dependent traits (15–17, 19, 20). Interestingly, by dividing our participants by sex as well as age, we show that adult males have the greatest proportion of CD8+ TEM, particularly compared to pediatric males and females. Further, we show that Ty21a vaccination does not significantly impact the overall proportions of total T cells, CD8+ T cells, CD8+ TEM, or CD8+ TEMRA, regardless of age, sex, or HLA-E haplotype.
CD69 expression increases following T cell receptor (TCR) and costimulatory molecule engagement, and it is thereby a useful marker to explore T cell activation. In this study, we show that unstimulated cells from adults, particularly adult males, have higher baseline percentages of CD69+ CD8+ T cells than children in the absence of in vitro stimulation. These percentages do not change in Ty21a vaccinated individuals. Further, when looking at CD8+ T cell activation over baseline following in vitro stimulation with a S. Typhi-infected cell line expressing HLA-E in the absence of classical class Ia HLA molecules, the global response to vaccination is observed in variable numbers of individuals, and the responses are of varying magnitude, independently of age or sex. Both HLA-E*01:01 and heterozygous participants showed baseline activation following S. Typhi antigen presentation, possibly the result of previous exposure to HLA-E-restricted activation of cross-reactive antigens. In contrast, HLA-E*01:03 participants had no positive CD69+ CD8+ T cell responses at baseline, likely due to their mismatch with the HLA-E*01:01-homozygous B-LCL cell line used. However, nearly all Ty21a-vaccinated HLA-E*01:03 participants showed positive activated CD8+ T cells after vaccination, suggesting the S. Typhi-infected targets express antigens/peptides which can be presented by both HLA-E*01:01 and HLA-E*01:03 molecules. Interestingly, a recent publication showed distinct peptide repertoires between the HLA-E allelic variants (43), although these studies did not explore the S. Typhi peptides shown in our lab to mediate at least some of the S. Typhi-HLA-E-restricted responses (30). Further studies will be needed to fully understand these observations.
Previous work from our lab has shown that S. Typhi-responsive CMI, including HLA-E-restricted T CMI, might play an important role in protection from typhoid disease (10, 11, 30–32). Further, multiple studies have shown that multifunctional T cell responses are critical for protection against pathogens, including S. Typhi (10, 11, 44–46). Thus, we investigated how Ty21a vaccination influences CD8+ TEM and TEMRA responses to S. Typhi-infected HLA-E restricted targets across age-groups, sex, and among HLA-E mismatched recipients. Our data show that CD8+ TEM responses over baseline are variable among all groups, with respect to individual effector molecules (e.g., IFN-γ, TNF-α) and multifunctionality. However, in general, CD8+ TEMRA responses over baseline tend to be stronger in adults than children, particularly in adult males. These data broaden our understanding of how Ty21a vaccination influences T cell responses depending on the age and sex of the individuals through a conserved means of antigen presentation. However, the variability among individuals suggested that conventional “supervised” analyses of only a single or a handful of activation molecules at a time might not be sufficient to uncover the complex immune responses at play. Thus, we developed a 35+ parameter mass cytometry panel to explore in depth the fine granularity of the HLA-E-restricted immune responses elicited by Ty21a immunization. However, it was not possible to evaluate such a complex set of key phenotypic, activation, and homing molecular markers with conventional flow cytometry analytical tools. This prompted us to use dimensionality reduction tools in an “unsupervised” fashion to further dissect these responses. Specifically, here we utilized tSNE to identify multifunctional populations within activated (CD69+) CD8+ TEM and TEMRA subsets and to extrapolate their pre- and post-Ty21a immunization relative abundance across age, sex, and HLA-E haplotype.
These results showed that highly multifunctional, gut-homing clusters are more frequently populated by adults and older pediatric participants, regardless of vaccination state. Of note, the follicular homing molecule CXCR5, a phenotypic marker used to identify circulating T follicular/helper cells (cTFH), was present in very few clusters despite recent studies identifying CXCR5+ CD8+ T cells to be of interest in other intracellular infections (47). This may be due to the previously observed early 3–7 day peak seen in the appearance of cTFH in circulation following exposure to infectious organisms or vaccination (36), whilst in our studies PBMC were collected 14–42 days after Ty21a immunization. Alternatively, or in addition, CD8+ TFH might be less responsive to bacterial pathogens -even intracellular pathogens such as S. Typhi. Future investigations will be required to address these possibilities. Further, we confirmed the observations utilizing conventional gating of TEMRA responses; namely that these cells are less functional in circulation in younger children. Finally, using downstream gating of both T memory subsets, we observed that phenotypes previously described to be associated with protection from the development of typhoid disease are present in the most multifunctional clusters, particularly among those most abundant in adults and older pediatric participants. Interestingly, many of the clusters identified by tSNE seem to decrease in abundance following Ty21a vaccination. Previous work from our laboratory has shown fluctuations in circulating effector T cells following S. Typhi challenge or Ty21a immunization, likely due to homing and accumulation in the gut followed by a rebound of S. Typhi-specific cells in circulation (10, 11, 32), but the kinetics and magnitude of a potential homing to the gut in children remains unclear. This is largely due to the cell number limitations related to the difficulty in obtaining serial blood draws in this population. tSNE analysis allowed us to identify differences among groups that would otherwise have been very difficult, if not impossible to uncover. In fact, our results provide additional data in support of the contention that dimensionality reduction tools will be ever more critical in future studies as high multiparametric data becomes more extensive and commonplace.
Taken together, these data show that post-Ty21a S. Typhi-antigen-responsive HLA-E-restricted CD8+ TEMRA show the greatest differences between age groups, with adults and older pediatric recipients exhibiting higher percentages of multifunctional cells as observed by both conventional gating and tSNE analysis. These results suggest the possibility of a decrease in efficacy among children vaccinated with Ty21a; however, the limited scope of this antigen presentation system merits further investigation into canonical classical class Ia MHC-restricted S. Typhi antigen presentation models among pediatric vaccinees. The present observations of decreased multifunctional T-cell potential in children, present until late teenage years, could prove of great importance in the development of new, effective oral live attenuated vaccines for use in pediatric populations.
Author Contributions
MR, MM, and MS designed the study, analyzed the data, and wrote the manuscript. MR performed the experiments. WC and RB contributed to the design, collected and processed the clinical samples, and helped draft the manuscript. LM provided statistical expertise.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to the volunteers who allowed us to perform this study. We also thank Ms. Regina Harley, Dr. Paula Bernal, and Ms. Catherine Storrer for excellent technical assistance. This work was supported, in part, by NIAID, NIH, DHHS federal research grants R01 AI036525, and U19 AI082655 [Cooperative Center for Human Immunology (CCHI)] to MS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, or the National Institutes of Health.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2019.00257/full#supplementary-material
References
- 1.Bhutta ZA, Threlfall J. Addressing the global disease burden of typhoid fever. JAMA. (2009) 302:898–9. 10.1001/jama.2009.1259 [DOI] [PubMed] [Google Scholar]
- 2.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. (2004) 82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Crump JA, Mintz ED. Global trends in typhoid and paratyphoid Fever. Clin Infect Dis. (2010) 50:241–6. 10.1086/649541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine MM. Typhoid Fever Vaccines. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plokin's Vaccines. Philadelphia, PA: Elsevier, Inc; (2018). p. 1114–44. 10.1016/B978-0-323-35761-6.00061-4 [DOI] [Google Scholar]
- 5.Antillón M, Warren JL, Crawford FW, Weinberger DM, Kürüm E, Pak GD, et al. The burden of typhoid fever in low- and middle-income countries: a meta-regression approach. PLoS Negl Trop Dis. (2017) 11:e0005376. 10.1371/journal.pntd.0005376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhuiyan TR, Choudhury FK, Khanam F, Saha A, Sayeed MA, Salma U, et al. Evaluation of immune responses to an oral typhoid vaccine, Ty21a, in children from 2 to 5 years of age in Bangladesh. Vaccine. (2014) 32:1055–60. 10.1016/j.vaccine.2014.01.001 [DOI] [PubMed] [Google Scholar]
- 7.Bhutta ZA. Impact of age and drug resistance on mortality in typhoid fever. Arch Dis Child. (1996) 75:214–7. 10.1136/adc.75.3.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks WA, Hossain A, Goswami D, Nahar K, Alam K, Ahmed N, et al. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis. (2005) 11:326–9. 10.3201/eid1102.040422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine. (2013) 31:452–60. 10.1016/j.vaccine.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 10.Fresnay S, McArthur MA, Magder L, Darton TC, Jones C, Waddington CS, et al. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J Transl Med. (2016) 14:62. 10.1186/s12967-016-0819-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fresnay S, McArthur MA, Magder LS, Darton TC, Jones C, Waddington CS, et al. Importance of Salmonella Typhi-responsive CD8+ T cell immunity in a human typhoid fever challenge model. Front Immunol. (2017) 8:208. 10.3389/fimmu.2017.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. (2007) 45(Suppl. 1):S15–9. 10.1086/518140 [DOI] [PubMed] [Google Scholar]
- 13.Sztein MB. Is a human CD8 T-cell vaccine possible, and if so, what would it take? CD8 T-cell-mediated protective immunity and vaccination against enteric bacteria. Cold Spring Harb Perspect Biol. (2017). 10:a029546. 10.1101/cshperspect.a029546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sztein MB, Salerno-Goncalves R, McArthur MA. Complex adaptive immunity to enteric fevers in humans: lessons learned and the path forward. Front Immunol. (2014) 5:516. 10.3389/fimmu.2014.00516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4(+) versus effector memory and terminally differentiated memory cells in CD8(+) compartment. Mech Ageing Dev. (2006) 127:274–81. 10.1016/j.mad.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 16.Tollerud DJ, Ildstad ST, Brown LM, Clark JW, Blattner WA, Mann DL, et al. T-cell subsets in healthy teenagers: transition to the adult phenotype. Clin Immunol Immunopathol. (1990) 56:88–96. 10.1016/0090-1229(90)90172-M [DOI] [PubMed] [Google Scholar]
- 17.Rudolph ME, McArthur MA, Barnes RS, Magder LS, Chen WH, Sztein MB. Differences between pediatric and adult T cell responses to in vitro Staphylococcal enterotoxin B stimulation. Front Immunol. (2018) 9:498. 10.3389/fimmu.2018.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. (2014) 192:2689–98. 10.4049/jimmunol.1302064 [DOI] [PubMed] [Google Scholar]
- 19.Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. (2014) 159:814–28. 10.1016/j.cell.2014.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. (2009) 133:95–107. 10.1016/j.clim.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 21.Arifuzzaman M, Rashu R, Leung DT, Hosen MI, Bhuiyan TR, Bhuiyan MS, et al. Antigen-specific memory T cell responses after vaccination with an oral killed cholera vaccine in Bangladeshi children and comparison to responses in patients with naturally acquired cholera. Clin Vaccine Immunol. (2012) 19:1304–11. 10.1128/CVI.00196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson B. Humoral and cell-mediated immune responses in children and adults after 1 and 2 doses of varicella vaccine. J Infect Dis. (2008) 197(Suppl. 2):S143–6. 10.1086/522130 [DOI] [PubMed] [Google Scholar]
- 23.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. (1987) 1:1049–52. 10.1016/S0140-6736(87)90480-6 [DOI] [PubMed] [Google Scholar]
- 24.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. (1990) 336:891–4. 10.1016/0140-6736(90)92266-K [DOI] [PubMed] [Google Scholar]
- 25.Braud VM, Allan DS, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. (1999) 11:100–8. 10.1016/S0952-7915(99)80018-1 [DOI] [PubMed] [Google Scholar]
- 26.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, et al. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. (2002) 196:1473–81. 10.1084/jem.20020609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joosten SA, Sullivan LC, Ottenhoff TH. Characteristics of HLA-E restricted T-cell responses and their role in infectious diseases. J Immunol Res. (2016) 2016:2695396. 10.1155/2016/2695396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens J, Joly E, Trowsdale J, Butcher GW. Peptide binding characteristics of the non-classical class Ib MHC molecule HLA-E assessed by a recombinant random peptide approach. BMC Immunol. (2001) 2:5. 10.1186/1471-2172-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HL, Wiseman RW, Hughes CM, Webb GM, Abdulhaqq SA, Bimber BN, et al. The role of MHC-E in T cell immunity is conserved among humans, rhesus macaques, and cynomolgus macaques. J Immunol. (2018) 200:49–60. 10.4049/jimmunol.1700841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salerno-Gonçalves R, Fernandez-Viña M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. (2004) 173:5852–62. 10.4049/jimmunol.173.9.5852 [DOI] [PubMed] [Google Scholar]
- 31.Salerno-Goncalves R, Wahid R, Sztein MB. Ex vivo kinetics of early and long-term multifunctional human leukocyte antigen E-specific CD8+ cells in volunteers immunized with the Ty21a typhoid vaccine. Clin Vaccine Immunol. (2010) 17:1305–14. 10.1128/CVI.00234-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArthur MA, Sztein MB. Heterogeneity of multifunctional IL-17A producing S. Typhi-specific CD8+ T cells in volunteers following Ty21a typhoid immunization. PLoS ONE. (2012) 7:e38408. 10.1371/journal.pone.0038408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sztein MB, Tanner MK, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. (1995) 155:3987–93. [PubMed] [Google Scholar]
- 34.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, et al. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. (1994) 170:1508–17. 10.1093/infdis/170.6.1508 [DOI] [PubMed] [Google Scholar]
- 35.Lee N, Goodlett DR, Ishitani A, Marquardt H, Geraghty DE. HLA-E surface expression depends on binding of TAP-dependent peptides derived from certain HLA class I signal sequences. J Immunol. (1998) 160:4951–60. [PubMed] [Google Scholar]
- 36.McArthur MA, Chen WH, Magder L, Levine MM, Sztein MB. Impact of CD4+ T cell responses on clinical outcome following oral administration of wild-type enterotoxigenic Escherichia coli in humans. PLoS Negl Trop Dis. (2017) 11:e0005291. 10.1371/journal.pntd.0005291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McArthur MA, Fresnay S, Magder LS, Darton TC, Jones C, Waddington CS, et al. Activation of Salmonella Typhi-specific regulatory T cells in typhoid disease in a wild-type S. Typhi challenge model. PLoS Pathog. (2015) 11:e1004914. 10.1371/journal.ppat.1004914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Lau MC, Wong MT, Newell EW, Poidinger M, Chen J. Cytofkit: A bioconductor package for an integrated mass cytometry data analysis pipeline. PLoS Comput Biol. (2016) 12:e1005112. 10.1371/journal.pcbi.1005112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. (1999) 401:708–12. 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 40.Fulop T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. (2013) 4:271. 10.3389/fimmu.2013.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. (2013) 43:2797–809. 10.1002/eji.201343751 [DOI] [PubMed] [Google Scholar]
- 42.Nelson MH, Kundimi S, Bowers JS, Rogers CE, Huff LW, Schwartz KM, et al. The inducible costimulator augments Tc17 cell responses to self and tumor tissue. J Immunol. (2015) 194:1737–47. 10.4049/jimmunol.1401082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celik AA, Kraemer T, Huyton T, Blasczyk R, Bade-Döding C. The diversity of the HLA-E-restricted peptide repertoire explains the immunological impact of the Arg107Gly mismatch. Immunogenetics. (2016) 68:29–41. 10.1007/s00251-015-0880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. (2007) 13:843–50. 10.1038/nm1592 [DOI] [PubMed] [Google Scholar]
- 45.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. (2008) 8:247–58. 10.1038/nri2274 [DOI] [PubMed] [Google Scholar]
- 46.Van Epps P, Banks R, Aung H, Betts MR, Canaday DH. Age-related differences in polyfunctional T cell responses. Immun Ageing. (2014) 11:14 10.1186/1742-4933-11-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perdomo-Celis F, Taborda NA, Rugeles MT. Follicular CD8(+) T cells: origin, function and importance during HIV infection. Front Immunol. (2017) 8:1241. 10.3389/fimmu.2017.01241 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.