Abstract
Introduction
Anemia is a common complication of chronic kidney disease (CKD) in children; however, the role of inflammation in its pathogenesis remains incompletely understood.
Methods
To elucidate the role of interleukin (IL)-6 in renal anemia, we induced CKD by adenine diet in juvenile wild-type (WT) and IL-6 deficient (Il6KO) mice, and examined serum IL-6 and relevant parameters in children with CKD.
Results
WT-CKD mice developed anemia despite increases in serum erythropoietin and displayed low serum iron and elevated serum IL-6. IL-6 deficiency resulted in a significant improvement of red blood cell count and hemoglobin in CKD mice. This effect was associated with improvement of hypoferremia by Il6 deletion, likely mediated by hepcidin. However, correction of hypoferremia by oral iron supplementation in WT-CKD mice did not fully replicate the protective effects of Il6 deletion, suggesting an additional iron-independent role for IL-6 in CKD-anemia. Indeed, Il6 deletion mitigated the severity of renal fibrosis and alleviated relative erythropoietin insufficiency in CKD mice. Cytokine profiling in a pediatric CKD cohort demonstrated that of 10 cytokines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, tumor necrosis factor (TNF)-α, and interferon-γ), only IL-6 was significantly (inversely) associated with hemoglobin when adjusted for glomerular filtration rate (GFR). The association between IL-6 and hemoglobin in children with CKD remained significant after adjustment for CKD stage, iron therapy, and hepcidin.
Discussion
IL-6 contributes to development of anemia in juvenile CKD, through mechanisms that include induction of hypoferremia, aggravation of renal fibrosis, and alteration of the erythropoietin axis. IL-6 appears to be a promising therapeutic target in the management of CKD-anemia.
Keywords: anemia, chronic kidney disease, erythropoietin, inflammation, pediatric nephrology
CKD in children is associated with significant morbidity, mortality, and high economic burden.1, 2 Anemia complicates CKD in more than 50% of children before they reach end-stage renal disease.3 Anemia negatively affects quality of life in children with CKD4 and increases risk of hospitalization.5 The presence of anemia at initiation of dialysis is associated with higher risk of death in children.6
The pathogenesis of renal anemia (CKD-anemia) includes dysfunction of the erythropoietin (Epo) axis, decreased red blood cell (RBC) life span, impact of nutritional factors, and both occult and iatrogenic blood losses, among other factors. Recently, it was recognized that CKD-anemia shares important features with anemia of inflammation. Indeed, CKD has been characterized as a state of chronic low-grade inflammation,7 and adult patients with CKD-anemia show upregulation of proinflammatory cytokines.8
IL-6 was originally identified as a regulator of B-cell differentiation9 and subsequently shown to have pleiotropic functions regulating a multitude of physiologic processes. IL-6 is produced by numerous cell types, including monocytes, macrophages, lymphocytes, fibroblasts, and endothelial cells in response to a variety of stimuli,10 particularly in response to uremic toxins.11 Circulating levels of IL-6 inversely correlate with GFR in adult patients with CKD.12, 13 IL-6 is an independent predictor of mortality in adult patients starting peritoneal and hemodialysis.14 Peripheral blood mononuclear cells from hemodialysis patients produced more IL-6 than those from healthy subjects, when stimulated in vitro.15 IL-6 is elevated in pediatric dialysis patients.16 However, very few studies have evaluated IL-6 in children with pre-dialysis CKD.17, 18 Regulation of erythropoiesis is one of the functions of IL-6, which has been implicated in nonrenal anemias. Elevated IL-6 levels correlate with increased erythropoiesis-stimulating agent (ESA) requirements due to ESA-resistance in adult hemodialysis patients.19, 20 Use of a biocompatible dialyzer associated with enhanced IL-6 clearance improved anemia and reduced ESA response index in such patients.21 In children with CKD, inflammatory response may differ from adults.22, 23 Therefore, it is important to elucidate the role of IL-6 in the pathophysiology of CKD-anemia in children.
The effects of IL-6 on erythropoiesis likely include iron-mediated and iron-independent actions. IL-6 is the major upstream activator of hepcidin, a hepatic iron-regulatory hormone, in anemia of inflammation.24, 25, 26 Hepcidin binds to iron exporter ferroportin, expressed on enterocytes, hepatocytes, and macrophages, forming a hepcidin-ferroportin complex that is internalized to undergo lysosomal degradation, which prevents iron egress from the cells. Thus, increased hepcidin, due to higher circulating levels of IL-6, leads to iron sequestration, decreased iron absorption, and lack of iron availability for erythropoiesis. Because iron availability is a rate-limiting step in the maturation of RBCs, hepcidin overproduction leads to anemia. However, it remains to be confirmed whether IL-6 increases hepcidin in juvenile CKD.
Experimental studies have also identified iron-independent effects of IL-6 on erythropoiesis in anemia of inflammation.27, 28, 29 IL-6 directly impaired the erythroid development of human TF-1 erythroleukemic cells in vitro in a dose-dependent manner.30 Hepcidin knockout (KO) and Il6KO mice exhibit different patterns in the development and the resolution of anemia of inflammation, such that a more rapid recovery of erythropoiesis is observed in Il6KO mice.27 This suggests a direct detrimental effect of IL-6 on erythropoiesis, distinct from effects on iron metabolism.
Thus, although the effect of IL-6 in nonrenal anemias of inflammation has been established, evidence that IL-6 may be involved in the pathogenesis of CKD-anemia is preliminary.31 The possible relationship between anemia and IL-6 in pediatric CKD is largely unknown and requires investigation.18 Here we present our studies elucidating the effects of Il6KO on experimental CKD-anemia in juvenile mice, and a parallel clinical study to examine the translational significance of these observations in children with CKD.
Materials and Methods
Animals and Experimental Design
The animal protocol was approved by the Institutional Animal Care and Use Committee. B6.129S2-Il6tm1Kopf/J (Il6KO) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). At weaning (21 days of age) WT and Il6KO mice were randomly assigned to 4 groups (≥5 mice per group): WT-controls, Il6KO-controls [CKD(−)], WT CKD, and Il6KO CKD [CKD(+)]. CKD was induced by a 0.2% adenine diet (Envigo, Madison, WI). Eight weeks later, mice were euthanized via pentobarbital injection.
Blood Biochemistry and Complete Blood Count
Blood was collected via retro-orbital puncture 6 weeks through the experimental period and from the inferior vena cava at euthanasia. Blood urea nitrogen (BUN) and creatinine were measured on the Beckman Coulter (Brea, CA) AU 680 analyzer. Serum iron was measured using colorimetric assay (Pointe Scientific, Canton, MI). Enzyme-linked immunosorbent assay was used to measure hepcidin (IntrinsicLifesciences, La Jolla, CA) and Epo (R&D Systems, Minneapolis, MN). Cytokines were measured using a V-Plex kit from Meso Scale Discovery (Rockville, MD) on the SI2400 Multiplex Analyzer. Mouse blood counts were performed on the IDEXX Procyte DX analyzer (Westbrook, ME). The reticulocyte index was calculated as follows: [reticulocyte (%) × mouse hematocrit (%)] / [average hematocrit (%) in the control WT CKD(−) group of mice].
Flow Cytometry
To measure phagocytosis of RBCs by macrophages in spleen, we used fluorescein isothiocyanate–conjugated F4/80, phycoerythrin-conjugated CD115 and phycoerythrin-Cyanine7–conjugated GR-1 antibodies (Biolegend, San Diego, CA) to detect macrophage population. To identify mature RBCs, we used allophycocyanin-conjugated Ter-119 antibody (BD Biosciences, San Jose, CA). Spleen single-cell suspensions were incubated with antibody cocktails for 40 minutes at 4°C in the dark. The stained cells were washed twice, fixed with 2% paraformaldehyde (Santa Cruz Biotechnology; Dallas, TX) and captured (50,000 events per sample) using BD Accuri C6 instrument (BD Biosciences). The internalization of RBCs by macrophages was determined by gating a Ter119 histogram on F4/80 expressing multiplet population (the gating strategy is illustrated in Supplementary Figure S1).
Real-Time Quantitative Polymerase Chain Reaction
Total kidney RNA was purified using PureLink RNA Mini Kit (ThermoFisher Scientific, Waltham, MA). The reverse transcription to complementary DNA was carried out using High-Capacity cDNA Reverse Transcription Kit (ThermoFisher Scientific). Real-time quantitative polymerase chain reaction was performed using the TaqMan Gene Expression Assay and Master Mix (ThermoFisher Scientific) in an ABI 7900 HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers and probes were obtained from ThermoFisher Scientific and mouse Gapdh was used as an internal control (Fn1, Mm01256744_m1; Gapdh, Mm99999915_g1). Data were analyzed by the comparative CT method.32
Western Blotting
Kidneys were harvested in tissue protein extraction agent (ThermoFisher Scientific) lysis buffer. Protein concentrations were determined using the bicinchoninic acid assay (ThermoFisher Scientific). Protein extracts were loaded on Midi-Protein TGX gel (Bio-Rad, Hercules, CA). Proteins were electroblotted onto polyvinyl difluoride membranes (MilliporeSigma, Burlington, MA). After transfer, nonspecific binding was prevented with 5% skimmed milk prepared in 0.1% tris-buffered saline–Tween (Bio-Rad). After blocking, the membrane was incubated with anti-fibronectin antibody (Abcam, Cambridge, MA). Following secondary antibody incubation, targeted bands were visualized using enhanced chemiluminescence solution (ThermoFisher Scientific). Quantification of western blots was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Histology
Organs were fixed in 10% formalin for 24 hours and subsequently paraffin embedded. Sections were cut 5-μm thick. Kidney sections were stained with Masson trichrome. Spleens and bone marrow sections were stained with Perls Prussian blue.
Patients
Children were enrolled in pediatric nephrology clinics at Weill Cornell Medical Center after obtaining informed consent. The study was approved by the institutional review board. Eligibility criteria included the following: age <21 years, CKD stage 2 and above, no acute illnesses at the time of blood collection. Serum and plasma were separated immediately from collected blood samples and stored at −80 degrees until analyzed. Quantitative immunoassays were used to determine the serum concentrations of hepcidin (IntrinsicLifesciences, La Jolla, CA), growth hormone (ALPCO, Salem, NH), and insulin-like growth factor 1 (R&D, Minneapolis, MN).
Statistical Analyses
Experimental data were reported as means ± SD, and the differences between 2 groups were determined with Student t test. One-way analysis of variance was used for the analysis of differences among 4 groups. Human variables normality was tested using Shapiro-Wilk W test (Supplementary Table S1). Non-normally distributed variables were subjected to the common logarithmic transformation and re-tested for normality (Supplementary Table S1). The distributions of Log Il-6 and Log hepcidin passed the normality testing (Supplementary Figure S2). For cytokine profiling analysis, cytokine levels were categorized into tertiles. The χ2 test was performed to detect difference in categorical variables and analysis of variance versus Kruskal-Wallis tests to compare continuous variables. The correlations were assessed using Spearman’s rank test. Multiple linear regression was used for multivariate analyses. GraphPad-Prism (La Jolla, CA) and STATA-12.0 (Stata Corp, College Station, TX) were used for statistical analyses.
Results
Juvenile Mice With CKD-Anemia Have Elevated Serum IL-6
CKD was induced in juvenile mice by adenine diet.33 WT mice fed the adenine diet [CKD(+)] exhibited elevation of BUN and serum creatinine compared with their WT littermates fed a regular diet [CKD(−)] that served as controls (Table 1). The body growth of juvenile WT CKD(+) mice was impaired. WT CKD(+) mice developed anemia, characterized by reduced RBC counts, hemoglobin, hematocrit, and mean corpuscular volume (MCV). The percentage, but not the absolute count of reticulocytes was elevated in the peripheral blood of WT CKD(+) mice compared with WT CKD(−) mice. CKD(+) mice displayed alterations in iron metabolism, evident by elevated serum hepcidin, low serum iron, and high spleen iron content. Serum TNF-α and, importantly, serum IL-6 levels were elevated in WT CKD(+) mice.
Table 1.
Parameters of chronic kidney disease in wild-type mice
| Parameters | Control diet (n = 8) | Adenine diet (n = 8) | P |
|---|---|---|---|
| BUN (mg/dl) | 32.5 ± 1.4 | 261.3 ± 10.0 | <0.001 |
| Serum creatinine (mg/dl) | 0.18 ± 0.02 | 1.17 ± 0.06 | <0.001 |
| Creatinine / BMI (cm2/dl) | 9.58 ± 1.21 | 105.70 ± 9.76 | <0.001 |
| Body length (cm) | 17.83 ± 0.12 | 15.49 ± 0.14 | <0.001 |
| Body weight (g) | 24.70 ± 0.51 | 10.93 ± 0.54 | <0.001 |
| BMI (kg/cm2) | 19.42 ± 0.32 | 11.36 ± 0.42 | <0.001 |
| IL-6 (pg/ml) | 6.8 ± 6.5 | 86.4 ± 21.3 | <0.001 |
| TNF-α (pg/ml) | 8.0 ± 4.5 | 33.8 ± 6.2 | <0.001 |
| WBC (103/μl) | 8.91 ± 0.93 | 3.12 ± 0.53 | 0.001 |
| Neutrophil (103/μl) | 0.78 ± 0.09 | 0.89 ± 0.17 | 0.56 |
| Neutrophil % | 9.28 ± 1.32 | 28.90 ± 2.48 | <0.001 |
| Monocyte (103/μl) | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.82 |
| Monocyte % | 0.78 ± 0.14 | 2.27 ± 0.52 | 0.010 |
| Lymphocyte (103/μl) | 0.79 ± 0.83 | 2.04 ± 0.37 | <0.001 |
| Lymphocyte % | 87.12 ± 1.51 | 64.76 ± 3.27 | <0.001 |
| Platelets (103/μl) | 809.1 ± 55.5 | 1871.0 ± 190.4 | <0.001 |
| RBC (106/μl) | 10.27 ± 0.19 | 5.52 ± 0.26 | <0.001 |
| Hemoglobin (g/dl) | 14.73 ± 0.32 | 6.31 ± 0.39 | <0.001 |
| Hematocrit (%) | 52.22 ± 0.91 | 22.63 ± 1.31 | <0.001 |
| MCV (fl) | 50.86 ± 0.64 | 40.94 ± 0.51 | <0.001 |
| MCH (pg) | 14.73 ± 0.07 | 13.13 ± 0.16 | <0.001 |
| MCHC (g/dl) | 28.70 ± 0.26 | 32.30 ± 0.47 | <0.001 |
| Reticulocyte (103/μl) | 385.50 ± 32.82 | 391.70 ± 55.54 | 0.92 |
| Reticulocyte % | 3.73 ± 0.29 | 6.98 ± 1.02 | 0.003 |
| Hepcidin (ng/ml) | 669.0 ± 102.6 | 3016.0 ± 739.0 | 0.039 |
| Iron (μg/dl) | 131.6 ± 11.7 | 48.7 ± 16.2 | 0.029 |
| Spleen iron (μg/g) | 143.7 ± 40.2 | 302.9 ± 68.6 | 0.026 |
BMI, body mass index; BUN, blood urea nitrogen; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cells; WBC, white blood cells.
Deletion of Il6 Improves CKD-Anemia in Juvenile Mice
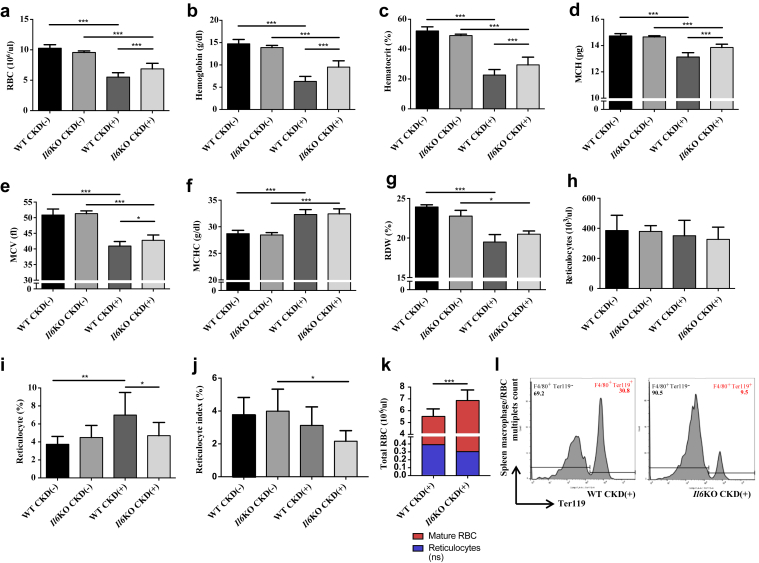
Il6 deletion led to improvement in RBC count, hemoglobin, hematocrit, and mean corpuscular hemoglobin (Figure 1a–d) in CKD(+) mice after 8 weeks of adenine diet. Hemoglobin in Il6KO CKD(+) mice was 50.9% higher than in WT CKD(+) mice. There was also an improvement of MCV in Il6KO CKD(+) group compared with WT CKD(+) (Figure 1e), although not as robust as improvement in mean corpuscular hemoglobin. Mean corpuscular hemoglobin content (Figure 1f) and RBC distribution width (Figure 1g) were not different between the WT CKD(+) and Il6KO CKD(+) groups. Absolute reticulocyte count was not different between the groups (Figure 1h). However, average reticulocyte percent was almost twice as high in WT CKD(+) mice than in WT CKD(−) mice. (Figure 1i). Because reticulocyte percentage is frequently misleading in a setting of anemia, we adjusted reticulocyte percent for the degree of anemia using reticulocyte index. No change in reticulocyte index was observed in WT mice following CKD induction. In Il6KO mice, however, induction of CKD resulted in a decrease of reticulocyte index (Figure 1j). Importantly, the differences in relative reticulocytosis between WT CKD(+) and Il6KO CKD(+) groups (Figure 1i and j) did not correspond to the differences in anemia between these groups (Figure 1a–e). This discrepancy can be explained by the differences in RBC destruction rates between the CKD(+) groups. Taken together, our reticulocyte and total RBC data suggest that Il6 deletion may have improved RBC life span in CKD(+) mice (Figure 1k). Indeed, thrice more spleen macrophage/RBC multiplets were found in WT CKD(+) group than in Il6KO CKD(+) group, indicating suppression of splenic RBC recycling (erythrophagocytosis) by Il6 deletion in CKD(+) mice (Figure 1l and Supplementary Figure S1).
Figure 1.
Interleukin (IL)-6 deletion improves anemia in juvenile mice with chronic kidney disease (CKD). Peripheral blood erythroid parameters in 4 groups of mice: wild-type controls [WT CKD(−)], IL-6–deficient controls [Il6KO CKD(−)], WT with adenine-induced CKD [WT CKD(+)], IL-6–deficient with adenine-induced CKD [Il6KO CKD(+)]. (a) Red blood cell count (RBC). (b) Hemoglobin. (c) Hematocrit. (d) Mean corpuscular hemoglobin (MCH). (e) Mean corpuscular volume (MCV). (f) Mean corpuscular hemoglobin content (MCHC). (g) Red blood cell distribution width (RDW). (h) Percentage of reticulocytes divided by the total number of RBCs × 100. (i) Reticulocyte index = [reticulocyte (%) × mouse hematocrit (%)] / [average hematocrit (%) in the control WT CKD(−) group of mice]. (j) Absolute reticulocyte count (not different between the groups). (k) Increase of mature fraction of circulating RBCs without significant changes in reticulocyte counts in Il6KO CKD(+) mice compared with WT CKD(+) mice suggest that Il6 deletion may have increased RBC life span. (l) Flow cytometry analysis of spleen cell suspensions indicates decreased hemophagocytosis in Il6KO CKD(+) mice compared with WT CKD(+) mice. The counts of Ter119-expressing RBCs (as shown in the histograms) gated from the multiplets positive for F4/80-expressing macrophages were 3 times lesser in Il6KO CKD(+) mice compared with WT CKD(+) mice (9.5% vs. 30.8%). Blood was collected at euthanasia (8 weeks after the initiation of the adenine diet). n ≥ 5 per group. No statistically significant differences in any of the peripheral blood parameters were seen between WT CKD(−) and Il6KO CKD(−) mice. Error bars are SDs. *P < 0.05; **P < 0.01; ***P < 0.001.
After 6 weeks of adenine diet, CKD(+) mice were already mildly anemic; however, the differences between RBC count, hemoglobin, and hematocrit between WT CKD(+) and Il6KO CKD(+) mice were not yet statistically significant (Supplementary Figure S3).
Kidney Function in Juvenile WT and Il6KO Mice With CKD
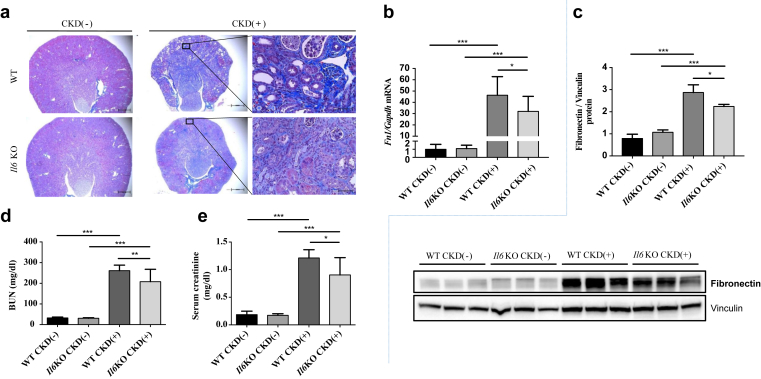
Il6KO mice were previously reported to be partially resistant to renal fibrosis.34 It is therefore plausible that improvement of renal function due to Il6 deletion may contribute to improvement of anemia in Il6KO CKD(+) mice. Indeed, Il6KO CKD(+) mice had less severe renal tubulointerstitial fibrosis as assessed by Masson trichrome staining than the WT CKD(+) mice (Figure 2a), and lower expression of kidney fibronectin mRNA and protein (Figure 2b and c). Furthermore, Il6 deletion improved BUN and serum creatinine (Figure 3d and e) in CKD(+) mice. Changes in body weight and linear body growth were similar between WT and Il6KO CKD(+) mice throughout the experimental period (Supplementary Figure S4).
Figure 2.
Kidney histology and renal function in wild-type (WT) and interleukin-6 deficient (Il6KO) juvenile mice with and without chronic kidney disease (CKD). (a) Masson trichrome staining demonstrated normal kidney histology in control WT CKD(−) and Il6KO CKD(−) mice. In WT CKD(+) mice, kidneys were affected by extensive tubulointerstitial fibrosis, tubular atrophy, and tubular dilation. These changes were attenuated in Il6KO CKD(+) mice. Representative images: original magnification ×4, scale bars = 500 μm (left and middle); original magnification ×40, scale bars = 50 μm (right). (b) Fibronectin mRNA and (c) protein expression in the kidney tissue. (d) Blood urea nitrogen (BUN). (f) Serum creatinine; n ≥ 5 per group (d,e). Error bars are SDs. *P < 0.05; **P < 0.01; ***P < 0.001.
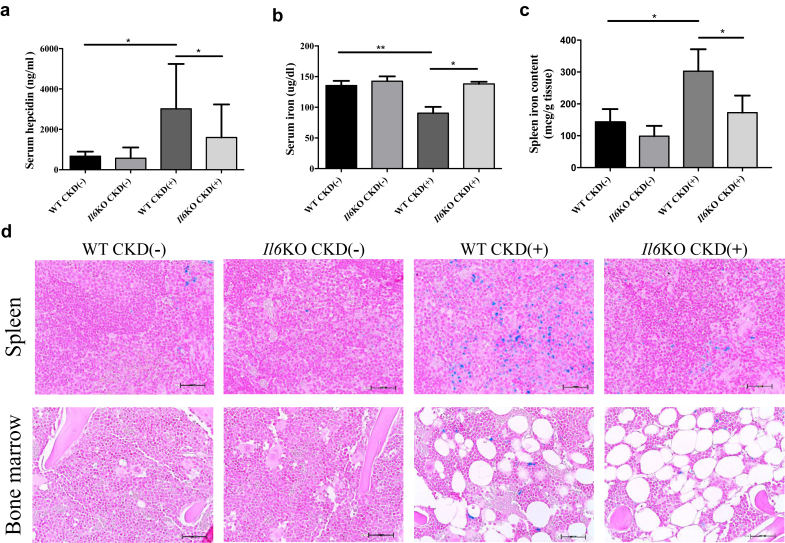
Figure 3.
Systemic iron homeostasis in wild-type (WT) and interleukin-6 deficient (Il6KO) juvenile mice with and without chronic kidney disease (CKD). (a) Serum hepcidin. (b) Serum iron. (c) Spleen tissue iron concentration. No statistically significant differences in spleen iron content were observed between Il6KO CKD(−) and Il6KO CKD(+) mice. (d) Representative images of spleen and bone marrow iron stains (Prussian blue); original magnification ×40. WT CKD(+) mice had more blue-stained ferric iron deposits in the spleen and bone marrow than their WT CKD(−) controls, whereas less iron was deposited in the spleen and bone marrow of Il6KO CKD(+) mice compared with WT CKD(+) mice. Increase in bone marrow adiposity was seen in both WT CKD(+) and Il6KO CKD(+) mice. Error bars are SDs. *P < 0.05; **P < 0.01. n ≥ 5 per group. Scale bars = 50 μm.
Deletion of Il6 Ameliorates Alteration of Iron Metabolism in Juvenile Mice With CKD
As expected, CKD led to elevation of serum hepcidin. However, Il6KO CKD(+) mice showed significantly lower serum hepcidin than WT CKD(+) mice (Figure 3a). Consequently, hypoferremia, seen in WT CKD(+) mice, was not present in Il6KO CKD(+) mice (Figure 3b). WT CKD(+) mice showed increased iron stores in the spleen (Figure 3c and d) and bone marrow (Figure 3d), compared with WT CKD(−) mice. Iron sequestration in the spleen and bone marrow was attenuated in Il6KO CKD(+) mice. Thus, Il6 deletion mitigated the overproduction of hepcidin in CKD(+) mice, reducing iron sequestration and thereby improving iron availability for erythropoiesis, which likely contributed to the improvement of anemia.
Correction of Hypoferremia With Oral Iron Does Not Recapitulate Effects of Il6 Deletion in CKD(+) Mice
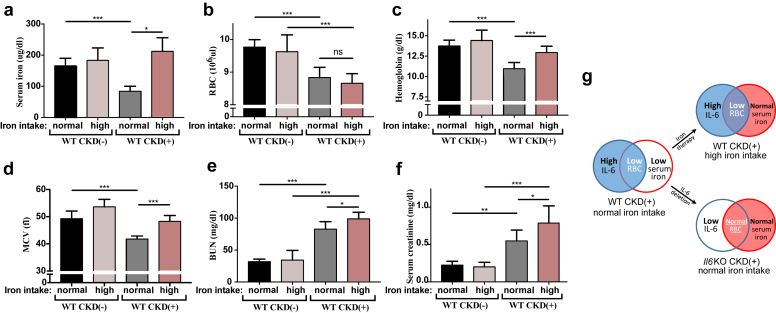
Because Il6 deletion normalized serum iron in CKD(+) mice, we sought to examine whether normalization of serum iron in the presence of intact IL-6 signaling would replicate the effects of Il6 deletion in this model of CKD. Should that be the case, one could argue that perhaps the pro-anemic action of IL-6 in CKD was solely due to the effects of IL-6 on iron metabolism. To test this, we corrected hypoferremia in WT CKD(+) mice by a therapeutic supplementation of the mouse diet with 0.5% carbonyl iron (Figure 4). This iron therapy indeed normalized serum iron in CKD(+) mice (Figure 4a). In contrast to Il6 deletion, however, iron therapy did not improve RBC count in CKD(+) mice (Figures 4b and 1a). Iron therapy improved hemoglobin and MCV (Figure 4c and d), but not as effectively as did deletion of Il6 (18% vs. 50.9% hemoglobin rise, respectively). Furthermore, iron therapy unexpectedly led to a rise in BUN and serum creatinine (Figure 4e and f). Thus, correction of hypoferremia by iron therapy did not reproduce the effects of Il6 deletion in juvenile CKD(+) mice. Normalization of serum iron in CKD(+) mice was associated with improvement of RBC production in the absence, but not in the presence of IL-6 (Figure 4g). Therefore, normalization of circulating iron per se was not sufficient to rescue RBC production in our model of CKD. Thus, the protective action of Il6 deletion against anemia was beyond its effect on circulating iron. This suggests that IL-6 may exert iron-independent effects contributing to the development of CKD-anemia.
Figure 4.
Erythroid parameters and renal function after correction of hypoferremia by oral iron in juvenile mice with chronic kidney disease (CKD). (a) Supplementation of a mouse diet with a 0.5% carbonyl iron (high iron intake) did not significantly change serum iron in control CKD(−) mice. Serum iron in CKD(+) mice fed a high iron diet was not significantly different from control CKD(−) mice. (b) Red blood cell count (RBC). (c) Hemoglobin. (d) Mean corpuscular volume (MCV). (e) Blood urea nitrogen (BUN). (f) Serum creatinine. (g) Comparative effect of 2 models (iron therapy vs. Il6 deletion) on RBC in CKD(+) mice. Normalization of serum iron improved RBC production in the absence but not in the presence of IL-6. Blood was collected at euthanasia. n ≥ 5 per group. Error bars are SDs. *P < 0.05; **P < 0.01; ***P < 0.001.
Erythropoietin in Juvenile WT and Il6KO Mice With CKD
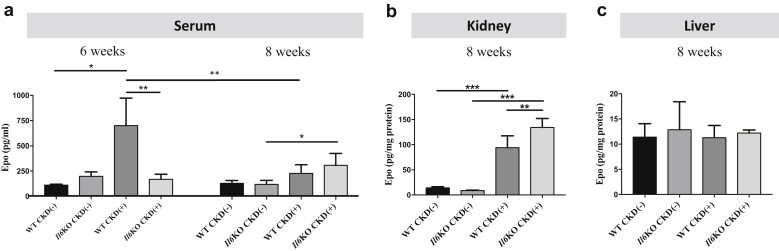
Serum Epo levels were assessed after 6 weeks and 8 weeks of adenine diet (Figure 5a). At 6 weeks, serum Epo in WT CKD(+) mice was significantly higher than in WT CKD(−) mice, but there was no difference between Il6KO CKD(+) and Il6KO CKD(−) mice. WT CKD(+) mice had higher levels of circulating Epo than Il6KO CKD(+) mice, whereas RBCs and hemoglobin at that time had the opposite trend (Supplementary Figure S4). This indicates a relative Epo resistance in WT CKD(+) mice at 6 weeks, which was mitigated by Il6 deletion. At 8 weeks, serum Epo in WT CKD(+) mice was significantly lower than at 6 weeks. Serum Epo levels were similar between WT CKD(+) and WT CKD(−) groups at 8 weeks. In contrast, Il6KO CKD(+) mice had higher serum Epo than Il6KO CKD(−) mice at that time (Figure 5a). The differences in kidney Epo protein (Figure 5b) were consistent with the differences in serum Epo levels at 8 weeks. The kidney was still able to respond to anemia as indicated by a modest increase in Epo production in both CKD(+) groups; however, the more anemic WT CKD(+) mice displayed significantly lower Epo expression in the kidney than Il6KO CKD(+) mice. Liver Epo expression was not different among the 4 groups of mice (Figure 5c). We conclude that IL-6 appears to induce Epo resistance early in the course of experimental CKD and may impair renal Epo production in advanced CKD.
Figure 5.
Erythropoietin (Epo) in wild-type (WT) and interleukin-6 deficient (Il6KO) juvenile mice with and without CKD. (a) Serum Epo at 2 different time points: 6 and 8 weeks of adenine diet exposure. (b) Kidney and (c) liver Epo protein expression. In the liver, Epo protein expression was not different among the 4 groups of mice. Serum and tissue Epo was measured by enzyme-linked immunosorbent assay. n ≥ 5 per group. Error bars are SDs. *P < 0.05; **P < 0.01; ***P < 0.001.
Characteristics of the Pediatric CKD Cohort
To elucidate the translational significance of our experimental findings, we used a cohort of children with CKD. The cohort was composed of 63 children with estimated GFR <90 ml/min per 1.73 m2 (Table 2). Most children had nonglomerular CKD etiology, mainly congenital anomalies of the kidneys and urinary tract. Stage III was the most common CKD stage (52.4%). Children overall had well-controlled CKD–mineral and bone disorder and blood pressure. Approximately one-half of the cohort (48.8%) had microalbuminuria. Although most children had a normal body mass index, many, as expected, were short (median height Z score = −1.1). Anemia, defined as hemoglobin below the fifth age- and sex-specific percentile, was present in 33.3% of children. Iron therapy was used in 38% of the cohort, whereas only 6 children were receiving ESA therapy. Serum hepcidin values were comparable with those reported by Atkinson et al.35 in a similar cohort of iron-naïve children.
Table 2.
General, renal, and hematologic characteristics of the pediatric chronic kidney disease cohort
| Cohort characteristics | Values, n (%) or median [IQR] N = 63 |
|---|---|
| Age (yr) | 12.3 [7.2–17.0] |
| Male sex | 38 (60.30) |
| Black race | 14 (22) |
| Hispanic ethnicity | 19 (30) |
| Glomerular etiology | 16 (25) |
| GFR (ml/min per 1.73 m2) | 42.0 [30.1–56.2] |
| Urine ACR (mg/g) | 54.9 [10.8–197.9] |
| Phosphorus (mg/dl) | 4.5 [3.7–5.1] |
| PTH (ng/l) | 95 [46–248] |
| 25(OH)-Vitamin D (ng/ml) | 28.1 [20.1–34.7] |
| Neutrophil/lymphocyte ratio | 1.58 [0.97–2.53] |
| Alkaline phosphatase (IU/l) | 196.5 [103.0–265.0] |
| Hemoglobin (g/dl) | 12.0 [11.1–12.9] |
| Hematocrit (%) | 35.5 [32.8–38.5] |
| RBC (106/μl) | 4.3 [3.9–4.8] |
| Serum hepcidin (ng/ml) | 42.8 [24.0–72.1] |
| Serum iron (mcg/dl) | 69.0 [47.0–90.5] |
| Transferrin saturation (%) | 23.0 [16.0–30.0] |
| Serum ferritin (ng/ml) | 58.5 [31.0–216.6] |
| Iron therapy | 24 (38) |
| ESA therapy | 6 (9.5) |
| Systolic blood pressure percentile | 64.8 [35.5–82.9] |
| Diastolic blood pressure percentile | 58.8 [41.1–86.7] |
| Serum albumin (g/dl) | 4.0 [3.7–4.3] |
| BMI percentile | 61.5 [35.7–89.6] |
| Weight Z score | −0.52 [−1.39 to 0.68] |
| Height Z score | −1.04 [−2.16 to −0.09] |
ACR, albumin-to-creatinine ratio; BMI, body mass index; ESA, erythropoiesis-stimulating agents; GFR, glomerular filtration rate (estimated using bedside Schwartz formula); PTH, parathyroid hormone.
Cytokine Profiling Identified an Association Between IL-6 and CKD-Anemia in Children
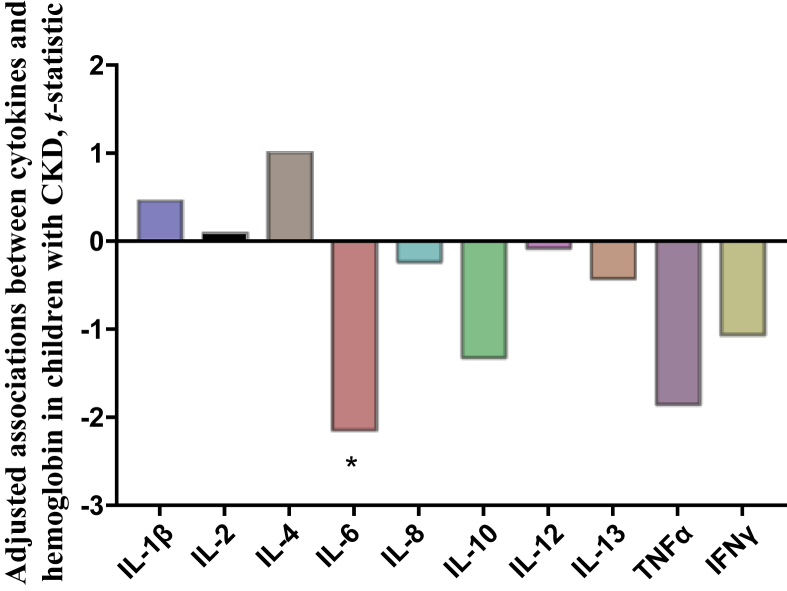
To delineate the role of inflammation in the development of anemia in children with CKD, we measured serum levels of 10 cytokines (IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-13, TNF-α, and interferon-γ). In unadjusted analysis, serum IL-6 and TNF-α were significantly associated with hemoglobin (Supplementary Table S2). Because iron supplementation was the leading treatment for anemia in our cohort, and iron can exert proinflammatory effects, we evaluated association between iron status and serum cytokines. None of the cytokine levels were associated with serum iron, transferrin saturation, or serum ferritin. Given the known inverse relationship between inflammation and GFR, we adjusted our analysis for GFR. In this adjusted analysis (Figure 6), IL-6 remained significantly associated with hemoglobin, whereas the association between TNF-α and hemoglobin was attenuated to a statistically nonsignificant trend (P = 0.068).
Figure 6.
Associations between cytokines and hemoglobin in children with chronic kidney disease (CKD). Each bar represents a separate regression analysis of hemoglobin on the respective serum cytokine, adjusted for glomerular filtration rate; t-statistic values corresponding to the significance levels (P) for the overall models were graphed. Negative values correspond to inverse correlations. n = 63. *P < 0.05. IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Clinical Correlates of IL-6 in Children With CKD
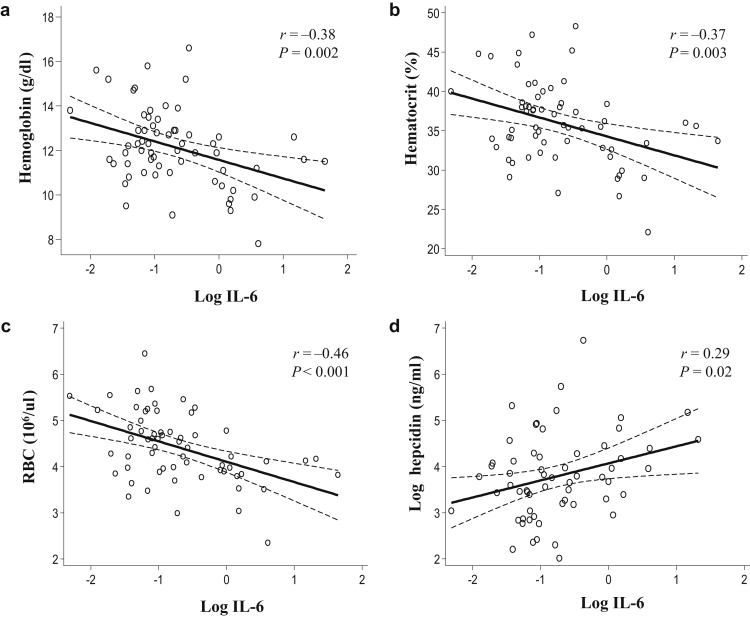
To characterize the relationship between IL-6 and clinical characteristics of CKD in children, we stratified our pediatric cohort by IL-6 tertiles. There were no differences in age, sex, race/ethnicity, CKD etiology, microalbumin-to-creatinine ratio, body mass index percentiles, body weight and height Z-scores between children from different IL-6 tertiles (Table 3). There was no association between serum IL-6 and serum insulin-like growth factor 1 or growth hormone (Supplementary Table S3). Serum IL-6 inversely correlated with GFR. Associations between IL-6, parathyroid hormone, and vitamin D were not significant when adjusted for GFR (not shown). The number of children receiving iron therapy was not significantly different between IL-6 tertiles (Table 3). IL-6 inversely correlated with hemoglobin, hematocrit, and RBC (Figure 7a–c), and positively correlated with hepcidin (Figure 7d).
Table 3.
Clinical parameters stratified by interleukin (IL)-6 tertiles in the cohort of children with chronic kidney disease
| Cohort characteristics | IL-6 tertiles |
P | ||
|---|---|---|---|---|
| I (n = 21) | II (n = 21) | III (n = 21) | ||
| Age, yr | 9.13 (6.34–13.99) | 13.64 (7.31–18.27) | 15.74 (11.89–17.78) | 0.12 |
| Male sex, n (%) | 14 (75) | 12 (57) | 12 (57) | 0.78 |
| Black race, n (%) | 4 (19) | 4 (19) | 6 (29) | 0.69 |
| Hispanic ethnicity, n (%) | 5 (24) | 8 (38) | 6 (29) | 0.59 |
| Glomerular etiology, n (%) | 4 (19) | 8 (38) | 4 (19) | 0.26 |
| GFR, ml/min per 1.73 m2 | 51.83 (37.95–73.19) | 45.50 (38.27–50.45) | 30.86 (14.61–46.20) | <0.001 |
| Urine ACR, mg/g | 30.0 (4.5–136.2) | 97.0 (29.9–160.1) | 185.5 (15.1–392.5) | 0.21 |
| Phosphorus, mg/dl | 4.60 (3.90–5.20) | 4.30 (3.70–4.80) | 4.70 (3.70–5.10) | 0.54 |
| PTH, ng/l | 38 (36–49) | 58.50 (43–95) | 255.70 (182–546.70) | <0.001 |
| Vitamin D, ng/ml | 28 (21.40–34.80) | 31.70 (28.10–35.40) | 20.15 (17.20–23.90) | 0.03 |
| Neutrophil/lymphocyte ratio | 1.21 (0.91–1.88) | 1.61 (0.73–2.21) | 2.03 (1.24–4.50) | <0.001 |
| Alkaline phosphatase, IU/l | 198.50 (124–253) | 187.50 (77.50–252) | 196 (109.50–292) | 0.73 |
| Iron therapy, n (%) | 4 (24) | 10 (48) | 9 (43) | 0.24 |
| ESA therapy, n (%) | 1 (4.80) | 0 (0) | 5 (24) | 0.02 |
| Systolic blood pressure percentile | 51.10 (37.10–70.60) | 64.90 (32.40–75.50) | 80.50 (34.40–95.20) | 0.28 |
| Diastolic blood pressure percentile | 47.30 (40.70–67.00) | 53.80 (37.10–79.60) | 86.10 (52.80–94.10) | 0.05 |
| BMI percentile | 73.30 (44.80–81.90) | 52.60 (30.20–82.10) | 65.50 (34.40–95.30) | 0.66 |
| Weight Z score | −0.67 (−1.56 to 0.49) | −0.46 (−0.86 to 0.64) | −0.18 (−1.61 to 1.60) | 0.53 |
| Height Z score | −1.32 (−2.15 to −0.55) | −0.58 (−1.69 to −0.04) | −1.01 (−2.33 to −0.22) | 0.48 |
ACR, albumin/creatinine ratio; ESA, erythropoiesis-stimulating agents; GFR, glomerular filtration rate (estimated using bedside Schwartz formula); PTH, parathyroid hormone.
Continuous variables presented as median (interquartile range).
Figure 7.
Associations of serum interleukin (IL)-6 with erythroid parameters and serum hepcidin in children with chronic kidney disease (CKD). (a) Hemoglobin, (b) hematocrit, and (c) red blood cell count (RBC) each inversely correlate with the IL-6 log. (d) The serum hepcidin log positively correlates with the IL-6 log. On the scatter plots, solid lines depict correlation trends and dashed lines show confidence intervals.
Association Between IL-6 and Erythroid Parameters in Children With CKD Remains Significant After Adjustment for Renal Function, Hepcidin, and Iron Therapy
In addition to hemoglobin, hematocrit, and RBC, parameters of iron status were included in the stepwise multivariate analyses (Table 4). In unadjusted analysis (Model 1), there was no association between serum iron, transferrin saturation, or serum ferritin with serum IL-6. After adjustment for demographic characteristics and CKD stage (Model 2), and additional adjustment for iron therapy (Model 3), hemoglobin, hematocrit, and RBC count remained significantly associated with IL-6, whereas there was still no association between iron status and IL-6. Interestingly, after adjustment for hepcidin (Model 4), the association between serum iron and IL-6 became statistically significant, which highlights the essential role of hepcidin in the relationship between IL-6 and iron status. There was still no association between transferrin saturation or ferritin with IL-6 after adjustment for hepcidin. Erythroid parameters remained significantly associated with IL-6 after adjustment for hepcidin. Thus, multivariate analyses indicate that the role of IL-6 in the development of CKD-anemia in children is distinct from hepcidin, iron therapy, and renal function.
Table 4.
Multivariate analyses of the relationship between serum interleukin (IL)-6 and erythroid parameters in children with chronic kidney disease (CKD)
| Parameters | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted: age, sex, ethnicity, CKD etiology, and CKD stage |
Adjusted: model 2 + iron therapy |
Adjusted: model 3 + hepcidin |
|||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |
| Hgb (g/dl) | −0.83 (−1.35 to −0.31) | 0.002 | −0.63 (−1.2 to −0.04) | 0.036 | −0.67 (−1.24 to −0.10) | 0.022 | −0.69 (−1.35 to −0.03) | 0.039 |
| Hct (%) | −2.41 (−3.9 to −0.85) | <0.01 | −1.85 (−3.57 to −0.13) | 0.035 | −1.96 (−3.67 to −0.26) | 0.025 | −1.97 (−3.94 to −0.01) | 0.049 |
| RBC (106/μl) | −0.44 (−0.65 to −0.22) | <0.01 | −0.38 (−0.63 to −0.13) | <0.01 | −0.38 (−0.64 to −0.13) | <0.01 | −1.97 (−3.94 to −0.01) | 0.010 |
| Iron (μg/dl) | −3.72 (−13.27 to −5.81) | 0.436 | −6.35 (−17.30 to −4.59) | 0.249 | −7.52 (−18.8 to 3.77) | 0.186 | −12.41 (−24.0 to −0.74) | 0.038 |
| TSat (%) | −0.76 (−2.69 to −4.22) | 0.657 | −1.01 (−4.92 to −2.88) | 0.602 | −1.23 (5.28 to 2.81) | 0.541 | −3.07 (−6.85 to 0.69) | 0.107 |
| Ferritin (ng/ml) | 0.11 (−0.31 to −0.52) | 0.605 | −0.26 (−0.69 to −0.15) | 0.212 | −0.33 (−0.77 to 0.09) | 0.126 | −0.21 (−0.54 to 0.12) | 0.216 |
CI, confidence interval; Hct, hematocrit; Hgb, hemoglobin; RBC, red blood cell count; TSat, transferrin saturation.
Each line represents a separate analysis for each model.
Discussion
In this study, we demonstrated the role of IL-6 in the development of juvenile CKD-anemia, using mutant Il6 deficient juvenile mice. We elucidated pleiotropic effects of IL-6 on iron metabolism, renal fibrosis, and Epo production. The translational significance of our experimental findings was confirmed by the independent inverse association between serum IL-6 and hemoglobin in children with CKD.
IL-6 is one of the major cytokines implicated in anemia of inflammation. However, it remained unknown whether IL-6 was associated with CKD-anemia independently of renal function. Consistent with a previous report,8 our unadjusted analysis demonstrated that IL-6 and TNF-α were associated with hemoglobin in children with CKD. Importantly, the association between IL-6 and hemoglobin remained significant after adjustment for GFR in our cohort, whereas the association between TNF-α and hemoglobin was no longer significant after adjustment for GFR. None of the other 8 cytokines that we screened showed an association with hemoglobin. Thus, the results of our cytokine profiling indicated a specific role for IL-6 in the development of CKD-anemia in children.
To test whether IL-6 has a causative effect on the development of CKD-anemia, we induced CKD in juvenile IL-6–deficient mice. Deletion of Il6 mitigated the severity of CKD-anemia, indicating a nonredundant pro-anemic role of IL-6 in CKD. Analysis of the peripheral blood of CKD(+) mice indicated that Il6 deletion improved the number of circulating RBCs and increased their volume (MCV), while not affecting mean corpuscular hemoglobin content. In contrast to the total RBC count, the absolute reticulocyte count was not affected by Il6 deletion. At the same time, the percentage of reticulocytes and the relative reticulocyte index was reduced in Il6KO CKD(+) mice. The seeming discrepancy between reduced relative reticulocytosis and improved anemia in Il6KO CKD(+) mice can be explained by the differences in RBC life span, a known phenomenon in CKD.36 Rescue of the relative reticulocytosis in Il6KO CKD(+) mice may therefore indicate the possible protective effect of Il6 deletion on RBC life span in CKD. Indeed, similar to Il6KO mice with anemia of inflammation,27 RBC recycling in the spleen was reduced in Il6KO CKD(+) mice.
IL-6 is one of the main described activators of hepcidin in anemia of inflammation.24 In our experiments, Il6KO CKD(+) mice had significantly lower serum hepcidin than WT CKD(+) mice. In children with CKD, we observed direct correlation between serum IL-6 and serum hepcidin. Consequently, hypoferremia, observed in WT CKD(+) mice, was not present in Il6KO CKD(+) mice, likely due to improved iron absorption and reduced iron sequestration in a setting of reduced hepcidin induction in Il6KO mice. Indeed, the iron overload that we observed in the spleen and bone marrow of WT CKD(+) mice was mitigated by Il6 deletion. In children with CKD, there was no unadjusted association between serum iron and IL-6. The seeming discrepancy between human and mouse data could be explained by the confounding effect of iron therapy that many children in our cohort were receiving. After adjustment for iron therapy, hepcidin, GFR, and demographic characteristics, serum iron was significantly associated with IL-6 in children with CKD. Thus, reduction of iron availability for erythropoiesis due to induction of hepcidin by IL-6 appears to be one of the mechanisms by which IL-6 contributes to the development of CKD-anemia. Association between iron status and RBC size (MCV) has been described in children with CKD37 and may account for improved MCV that we observed in both Il6KO and HampKO33 CKD(+) mice, as well as in WT CKD(+) mice supplemented with oral iron.
We considered the possibility that the protective action of Il6 deletion against anemia in CKD might be solely due to the effects of IL-6 on iron availability for erythropoiesis. To test this, we compared effects of Il6 deletion to the effects of oral iron supplementation in CKD(+) mice. Both deletion of Il6 and oral iron supplementation effectively normalized serum iron after CKD induction. However, no improvement in circulating RBC counts was observed after iron supplementation, in contrast to the normalization of RBC counts following Il6 deletion in CKD(+) mice. Consequently, oral iron supplementation was less effective in improving hemoglobin in CKD(+) mice than deletion of Il6. Thus, in our mouse model of juvenile CKD, normalization of serum iron per se was not sufficient to rescue dysfunctional erythropoiesis leading to anemia, contrasting the effects of Il6 deletion. Correction of hypoferremia in the absence of IL-6 was associated with essentially complete rescue of anemia, whereas correction of hypoferremia in a setting of intact IL-6 did not improve RBC production (although this slightly increased RBC size and therefore improved hemoglobin). These experiments suggested that the protective effect of Il6 deletion against anemia was only partially mediated by increased iron availability for erythropoiesis, and therefore iron-independent effects of IL-6 likely contribute to the development of CKD-anemia.
Profibrotic actions of IL-6 have been demonstrated in several models of tissue fibrosis.38 Adult Il6KO mice were protected from renal fibrosis in the angiotensin II–induced model of CKD.34 In our model, juvenile Il6KO CKD(+) mice also exhibited less severe tubulointerstitial fibrosis and tissue damage compared with WT CKD(+) mice. Improved histology corresponded to lower BUN and serum creatinine in juvenile Il6KO CKD(+) mice compared with WT CKD(+) mice. Recently, Durlacher-Betzer et al.39 reported similar BUN levels between adult WT and Il6KO mice fed adenine diet for 20 days. The differences between our results and those reported by Durlacher-Betzer et al.39 could be related to the longer duration of adenine diet in our model (56 days), and the differences in mouse age. Interestingly, we have previously found no difference in kidney function between hepcidin gene (Hamp) KO-CKD(+) and WT CKD(+) mice,33 indicating that the effects of Il6 deletion on hepcidin likely did not play a role in the improvement of kidney function by Il6KO in CKD(+) mice. Furthermore, iron supplementation to WT CKD(+) mice was associated with the advancement of kidney dysfunction, as evident by higher BUN and serum creatinine in iron supplemented WT CKD(+) mice compared with WT CKD(+) mice fed the diet with normal iron content.
In line with our animal data, serum IL-6 inversely correlated with CKD stage in children. Although progressive rise in IL-6 levels has been previously described with the decline of GFR in patients with CKD,12 the role of IL-6 in CKD progression has not been fully elucidated.13 Our data indicate that profibrotic effects exerted by IL-6 may aggravate renal fibrosis and thus accelerate disease progression in CKD. Improvement in kidney function due to Il6 deletion could have contributed to anemia improvement, in part via reduction of the toxic effects of uremia. Our human data, however, suggest that other mechanisms besides those related to kidney injury distinctly mediated worsening of anemia by IL-6, because the association between IL-6 and hemoglobin remained significant after adjustment for renal function in children with CKD.
Inflammation in CKD has been recognized as one of the mechanisms responsible for the resistance to ESAs.15 In our experiments, WT CKD(+) mice showed a 7-fold increase in serum Epo levels 2 weeks before euthanasia compared with WT CKD(−) controls, indicating that at the 6-week time point the kidney was still able to respond to anemia by significantly increasing Epo production. High serum Epo, however, was still insufficient to improve anemia in WT CKD(+) mice. At the end of experimental period (8-week time-point), we found a major drop in serum Epo levels in WT CKD(+) mice, compared with the 6-week time-point. This was probably related to the progression of renal fibrosis and loss of renal Epo-producing cells. In contrast to WT mice, in Il6KO CKD(+) mice serum levels of Epo were not different from Il6KO CKD(−) controls at 6-week time point; however, levels did rise by the 8-week time point. Given that RBCs found in peripheral blood at 8 weeks were released into circulation in response to an earlier Epo exposure, our data suggest that in the absence of IL-6, less Epo was needed to support erythropoiesis in CKD(+) mice, and that this process was more efficient in the absence of IL-6. It appears that IL-6 induces Epo resistance in juvenile CKD(+) mice and also suppresses Epo production by the kidney in advanced CKD. Thus, IL-6 alters Epo axis in juvenile CKD, likely representing one of the mechanisms whereby IL-6 promotes the development of CKD-anemia.
Iron and ESAs remain the mainstay of CKD-anemia treatment. However, ESA-resistance is increasingly common, and inflammation has been implicated as one of the factors responsible for ESA-resistance. Iron therapy may induce oxidative stress and inflammation in patients with CKD.40 Anti-inflammatory therapies were promising in preclinical models of CKD-anemia41; however, current guidelines do not recommend routine monitoring of inflammation in children with CKD, in part due to uncertainty concerning which biomarkers would provide actionable information. Clinical trials testing the efficacy of anti-inflammatory interventions in CKD are lacking. In this regard, our study highlights the specific role of IL-6 in the development of CKD-anemia, likely mediated via several pathways. Further characterization of the role of IL-6 in CKD pathophysiology is warranted using experimental models and larger patient cohorts. Based on our results, IL-6 appears to be a promising pharmacologic target in CKD. Importantly, therapeutic IL-6 blockade may have potential advantages over hepcidin blockade, because the latter may lead to iron overload, inflammation, and bone loss in CKD.33
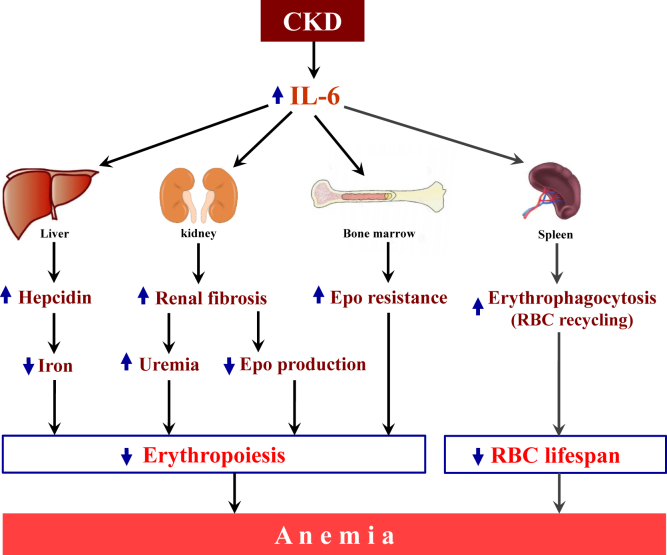
In summary, this study has elucidated the role of IL-6 in the development of CKD-anemia in both juvenile mice and children. Effects of IL-6 leading to anemia are likely mediated by several mechanisms, including activation of hepcidin, aggravation of renal fibrosis, alterations of the Epo axis, and activation of RBC destruction (Figure 8). Further investigation of the effects of existing CKD therapies on inflammation and development of novel therapeutics targeting IL-6 in CKD are warranted.
Figure 8.
Proposed model for the role of interleukin (IL)-6 in chronic kidney disease (CKD)–anemia. Increased circulating levels of IL-6 in CKD induce hepcidin overproduction, leading to decreased iron availability for erythropoiesis. In addition, IL-6 appears to aggravate resistance to high levels of circulating erythropoietin (Epo) in early CKD. Furthermore, IL-6 aggravates renal fibrosis, which worsens uremia and reduces the ability of the kidney to produce Epo in advanced CKD. RBC, red blood cell.
Disclosure
All the authors declared no competing interests.
Acknowledgements
Parts of this study were presented at the International Pediatric Nephrology Congress in Iguaçu, Brazil (September 2016), and the Pediatric Academic Societies Meeting in Toronto, Canada (May 2018). The authors appreciate the help of Angara Sureshbabu, Samantha Kimball, Richa Gautam, and Ameneh Amini.
This study was supported in part by the National Institutes of Health National Center for Advancing Translational Sciences UL1TR00457 and UL1TR002384 awarded to the Clinical and Translational Science Center, Weill Cornell Medicine (to OA and Y-SZ), National Heart, Lung, and Blood Institute R01 HL133801 (to MEC), National Institute of Diabetes and Digestive and Kidney Diseases R01 DK095112, R01 DK090554 (to SR) and K08 DK114558 (to OA), Rohr Family Clinical Scholar Award (to OA), and by the Department of Pediatrics at Weill Cornell Medicine.
Footnotes
Table S1. Assessment of study variables for normality using Shapiro-Wilk W test. Non-normally distributed variables were reassessed after common logarithmic transformation.
Table S2. Relationship among cytokines, anemia, and iron parameters in the cohort of children with CKD: univariate analysis.
Table S3. Serum IGF-1, growth hormone, and anthropometric parameters do not correlate with serum IL-6 in children with CKD.
Figure S1. Flow cytometry analysis of spleen cell suspensions indicates decreased hemophagocytosis in the spleens of Il6KO CKD(+) mice as compared with WT-CKD(+) mice. Gating steps to obtain the final plots included elimination of the Gr1+/CD115− and Gr1+/CD115+ populations (neutrophils and Gr1hi monocytes) and selection of both the Gr1−/CD115− and Gr1−/CD115+ cells, which then were gated into an F4/80hi/CD115− population. The remaining F4/80hi population of mature macrophages was analyzed by plotting SSC-H versus SSC-A to distinguish multiplets from single cells. Ter119-positive macrophage/RBC multiplets (indicating macrophages binding RBCs during the hemophagocytosis process) are shown in the histograms.
Figure S2. Distributions of log-transformed human variables.
Figure S3. Body growth in 4 groups of juvenile mice during the experimental period. (A) Body weight. Control mice were gaining weight normally throughout the experimental period. Both WT CKD(+) and Il6KO CKD(+) mice had slower rate of weight gain during the first 3 weeks and began losing weight from week 4 onward. No differences in weight changes were seen between the WT CKD(+) and Il6KO CKD(+) mice. (B) Body length, measured as a nose to tail tip length of anesthetized mice. CKD(+) mice had significant growth impairment, compared with control CKD(−) mice. No statistically significant differences were observed between the WT CKD(+) and Il6KO CKD(+) mice. Error bars are SDs. ***P < 0.001.
Figure S4. Peripheral blood erythroid parameters in 4 groups of mice after 6 weeks of adenine diet. (A) Red blood cell count (RBC), (B) hemoglobin, and (C) hematocrit were reduced after 6 weeks of adenine diet (2 weeks before the end of the experimental period); however, there was only a trend toward improvement of anemia in Il6KO CKD(+) mice, compared with the WT CKD(+) mice. n ≥5 per group. Error bars are SDs. **P < 0.01; ***P < 0.001.
Supplementary material is linked to the online version of the paper at www.kireports.org/.
Supplementary Material
Assessment of study variables for normality using Shapiro-Wilk W test. Non-normally distributed variables were reassessed after common logarithmic transformation.
Relationship among cytokines, anemia, and iron parameters in the cohort of children with CKD: univariate analysis.
Serum IGF-1, growth hormone, and anthropometric parameters do not correlate with serum IL-6 in children with CKD.
Flow cytometry analysis of spleen cell suspensions indicates decreased hemophagocytosis in the spleens of Il6KO CKD(+) mice as compared with WT-CKD(+) mice. Gating steps to obtain the final plots included elimination of the Gr1+/CD115− and Gr1+/CD115+ populations (neutrophils and Gr1hi monocytes) and selection of both the Gr1−/CD115− and Gr1−/CD115+ cells, which then were gated into an F4/80hi/CD115− population. The remaining F4/80hi population of mature macrophages was analyzed by plotting SSC-H versus SSC-A to distinguish multiplets from single cells. Ter119-positive macrophage/RBC multiplets (indicating macrophages binding RBCs during the hemophagocytosis process) are shown in the histograms.
Distributions of log-transformed human variables.
Body growth in 4 groups of juvenile mice during the experimental period. (A) Body weight. Control mice were gaining weight normally throughout the experimental period. Both WT CKD(+) and Il6KO CKD(+) mice had slower rate of weight gain during the first 3 weeks and began losing weight from week 4 onward. No differences in weight changes were seen between the WT CKD(+) and Il6KO CKD(+) mice. (B) Body length, measured as a nose to tail tip length of anesthetized mice. CKD(+) mice had significant growth impairment, compared with control CKD(−) mice. No statistically significant differences were observed between the WT CKD(+) and Il6KO CKD(+) mice. Error bars are SDs. ***P < 0.001.
Peripheral blood erythroid parameters in 4 groups of mice after 6 weeks of adenine diet. (A) Red blood cell count (RBC), (B) hemoglobin, and (C) hematocrit were reduced after 6 weeks of adenine diet (2 weeks before the end of the experimental period); however, there was only a trend toward improvement of anemia in Il6KO CKD(+) mice, compared with the WT CKD(+) mice. n ≥5 per group. Error bars are SDs. **P < 0.01; ***P < 0.001.
References
- 1.Medway M., Tong A., Craig J.C. Parental perspectives on the financial impact of caring for a child with CKD. Am J Kidney Dis. 2015;65:384–393. doi: 10.1053/j.ajkd.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System . 2018 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Chapter 6: ESRD Among Children, Adolescents, and Young Adults. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2018. [Google Scholar]
- 3.Fadrowski J.J., Pierce C.B., Cole S.R. Hemoglobin decline in children with chronic kidney disease: baseline results from the chronic kidney disease in children prospective cohort study. Clin J Am Soc Nephrol. 2008;3:457–462. doi: 10.2215/CJN.03020707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerson A., Hwang W., Fiorenza J. Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis. 2004;44:1017–1023. doi: 10.1053/j.ajkd.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Staples A.O., Wong C.S., Smith J.M. Anemia and risk of hospitalization in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:48–56. doi: 10.2215/CJN.05301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warady B.A., Ho M. Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol. 2003;18:1055–1062. doi: 10.1007/s00467-003-1214-1. [DOI] [PubMed] [Google Scholar]
- 7.Akchurin M., Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif. 2015;39:84–92. doi: 10.1159/000368940. [DOI] [PubMed] [Google Scholar]
- 8.Keithi-Reddy S.R., Addabbo F., Patel T.V. Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney Int. 2008;74:782–790. doi: 10.1038/ki.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimoto N., Kishimoto T. Interleukin 6: from bench to bedside. Nat Rev Rheumatol. 2006;2:619. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 10.Lotz M. Interleukin-6: a comprehensive review. In: Kurzrock R., Talpaz M., editors. Cytokines: Interleukins and Their Receptors. Springer; Boston, MA: 1995. pp. 209–233. [Google Scholar]
- 11.Rossi M., Campbell K.L., Johnson D.W. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res. 2014;45:309–317. doi: 10.1016/j.arcmed.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Gupta J., Mitra N., Kanetsky P.A. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amdur R.L., Feldman H.I., Gupta J. Inflammation and progression of CKD: the CRIC study. Clin J Am Soc Nephrol. 2016;11:1546–1556. doi: 10.2215/CJN.13121215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecoits-Filho R., Barany P., Lindholm B. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant. 2002;17:1684–1688. doi: 10.1093/ndt/17.9.1684. [DOI] [PubMed] [Google Scholar]
- 15.Goicoechea M., Martin J., de Sequera P. Role of cytokines in the response to erythropoietin in hemodialysis patients. Kidney Int. 1998;54:1337–1343. doi: 10.1046/j.1523-1755.1998.00084.x. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein S.L., Leung J.C., Silverstein D.M. Pro-and anti-inflammatory cytokines in chronic pediatric dialysis patients: effect of aspirin. Clin J Am Soc Nephrol. 2006;1:979–986. doi: 10.2215/CJN.02291205. [DOI] [PubMed] [Google Scholar]
- 17.Nehus E., Furth S., Warady B., Mitsnefes M. Correlates of resistin in children with chronic kidney disease: the chronic kidney disease in children cohort. J Pediatr. 2012;161:276–280. doi: 10.1016/j.jpeds.2012.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyal K.K., Saha A., Sahi P.K. Hepcidin and proinflammatory markers in children with chronic kidney disease: a case-control study. Clin Nephrol. 2018;89:363–370. doi: 10.5414/CN109132. [DOI] [PubMed] [Google Scholar]
- 19.Sitter T., Bergner A., Schiffl H. Dialysate related cytokine induction and response to recombinant human erythropoietin in haemodialysis patients. Nephrol Dial Transplant. 2000;15(8):1207–1211. doi: 10.1093/ndt/15.8.1207. [DOI] [PubMed] [Google Scholar]
- 20.Rattanasompattikul M., Molnar M.Z., Zaritsky J.J. Association of malnutrition–inflammation complex and responsiveness to erythropoiesis-stimulating agents in long-term hemodialysis patients. Nephrol Dial Transplant. 2012;28:1936–1945. doi: 10.1093/ndt/gfs368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kakuta T., Komaba H., Takagi N. A prospective multicenter randomized controlled study on interleukin-6 removal and induction by a new hemodialyzer with improved biocompatibility in hemodialysis patients: a pilot study. Ther Apher Dial. 2016;20:569–578. doi: 10.1111/1744-9987.12454. [DOI] [PubMed] [Google Scholar]
- 22.Bamgbola O.F., Kaskel F.J., Coco M. Analyses of age, gender and other risk factors of erythropoietin resistance in pediatric and adult dialysis cohorts. Pediatr Nephrol. 2009;24:571–579. doi: 10.1007/s00467-008-0954-3. [DOI] [PubMed] [Google Scholar]
- 23.Bamgbola O.F. Pattern of resistance to erythropoietin-stimulating agents in chronic kidney disease. Kidney Int. 2011;80:464–474. doi: 10.1038/ki.2011.179. [DOI] [PubMed] [Google Scholar]
- 24.Nemeth E., Rivera S., Gabayan V. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemeth E., Valore E.V., Territo M. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson M.A., Leonard M.B., Herskovitz R. Changes in hepcidin and hemoglobin after anti-TNF-alpha therapy in children and adolescents with Crohn disease. J Pediatr Gastroenterol Nutr. 2018;66:90–94. doi: 10.1097/MPG.0000000000001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardenghi S., Renaud T.M., Meloni A. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood. 2014;123:1137–1145. doi: 10.1182/blood-2013-08-521625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langdon J.M., Yates S.C., Femnou L.K. Hepcidin-dependent and hepcidin-independent regulation of erythropoiesis in a mouse model of anemia of chronic inflammation. Am J Hematol. 2014;89:470–479. doi: 10.1002/ajh.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raj D.S. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388. doi: 10.1016/j.semarthrit.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 30.McCranor B.J., Kim M.J., Cruz N.M. Interleukin-6 directly impairs the erythroid development of human TF-1 erythroleukemic cells. Blood Cells Mol Dis. 2014;52:126–133. doi: 10.1016/j.bcmd.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukaszyk E., Lukaszyk M., Koc-Zorawska E. Fibroblast growth factor 23, iron and inflammation—are they related in early stages of chronic kidney disease? Arch Med Sci. 2017;13:845–850. doi: 10.5114/aoms.2016.58647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3(6):1101. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 33.Akchurin O., Sureshbabu A., Doty S.B. Lack of hepcidin ameliorates anemia and improves growth in an adenine-induced mouse model of chronic kidney disease. Am J Physiol Renal Physiol. 2016;311(5):F877–F889. doi: 10.1152/ajprenal.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W., Wang W., Yu H. Interleukin 6 underlies angiotensin II–induced hypertension and chronic renal damage. Hypertension. 2012;59(1):136–144. doi: 10.1161/HYPERTENSIONAHA.111.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkinson M.A., Kim J.Y., Roy C.N., Warady B.A., White C.T., Furth S.L. Hepcidin and risk of anemia in CKD: a cross-sectional and longitudinal analysis in the CKiD cohort. Pediatr Nephrol. 2015;30(4):635–643. doi: 10.1007/s00467-014-2991-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ly J., Marticorena R., Donnelly S. Red blood cell survival in chronic renal failure. Am J Kidney Dis. 2004;44(4):715–719. [PubMed] [Google Scholar]
- 37.Morgan H.E., Holt R.C., Jones C.A., Judd B.A. Intravenous iron treatment in paediatric chronic kidney disease patients not on erythropoietin. Pediatr Nephrol. 2007;22(11):1963–1965. doi: 10.1007/s00467-007-0499-x. [DOI] [PubMed] [Google Scholar]
- 38.Fielding C.A., Jones G.W., McLoughlin R.M. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40(1):40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durlacher-Betzer K., Hassan A., Levi R., Axelrod J., Silver J., Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018 doi: 10.1016/j.kint.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal R., Vasavada N., Sachs N.G., Chase S. Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int. 2004;65(6):2279–2289. doi: 10.1111/j.1523-1755.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 41.Santana A.C., Degaspari S., Catanozi S. Thalidomide suppresses inflammation in adenine-induced CKD with uraemia in mice. Nephrol Dial Transplant. 2013;28(5):1140–1149. doi: 10.1093/ndt/gfs569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Assessment of study variables for normality using Shapiro-Wilk W test. Non-normally distributed variables were reassessed after common logarithmic transformation.
Relationship among cytokines, anemia, and iron parameters in the cohort of children with CKD: univariate analysis.
Serum IGF-1, growth hormone, and anthropometric parameters do not correlate with serum IL-6 in children with CKD.
Flow cytometry analysis of spleen cell suspensions indicates decreased hemophagocytosis in the spleens of Il6KO CKD(+) mice as compared with WT-CKD(+) mice. Gating steps to obtain the final plots included elimination of the Gr1+/CD115− and Gr1+/CD115+ populations (neutrophils and Gr1hi monocytes) and selection of both the Gr1−/CD115− and Gr1−/CD115+ cells, which then were gated into an F4/80hi/CD115− population. The remaining F4/80hi population of mature macrophages was analyzed by plotting SSC-H versus SSC-A to distinguish multiplets from single cells. Ter119-positive macrophage/RBC multiplets (indicating macrophages binding RBCs during the hemophagocytosis process) are shown in the histograms.
Distributions of log-transformed human variables.
Body growth in 4 groups of juvenile mice during the experimental period. (A) Body weight. Control mice were gaining weight normally throughout the experimental period. Both WT CKD(+) and Il6KO CKD(+) mice had slower rate of weight gain during the first 3 weeks and began losing weight from week 4 onward. No differences in weight changes were seen between the WT CKD(+) and Il6KO CKD(+) mice. (B) Body length, measured as a nose to tail tip length of anesthetized mice. CKD(+) mice had significant growth impairment, compared with control CKD(−) mice. No statistically significant differences were observed between the WT CKD(+) and Il6KO CKD(+) mice. Error bars are SDs. ***P < 0.001.
Peripheral blood erythroid parameters in 4 groups of mice after 6 weeks of adenine diet. (A) Red blood cell count (RBC), (B) hemoglobin, and (C) hematocrit were reduced after 6 weeks of adenine diet (2 weeks before the end of the experimental period); however, there was only a trend toward improvement of anemia in Il6KO CKD(+) mice, compared with the WT CKD(+) mice. n ≥5 per group. Error bars are SDs. **P < 0.01; ***P < 0.001.