Abstract
Among mammals, there is a positive correlation between serum uric acid (UA) levels and life span. Humans have high levels of UA because they lack a functional urate oxidase (UOX) enzyme that is present in shorter lived mammals. Here, we show that male and female mice with UOX haploinsufficiency exhibit an age-related elevation of UA levels, and that the life span of female but not male UOX+/− mice is significantly increased compared to wild-type mice. Serum UA levels are elevated in response to treadmill exercise in UOX+/− mice, but not wild-type mice, and the endurance of the UOX+/− mice is significantly greater than wild-type mice. UOX+/− mice exhibit elevated levels of brain-derived neurotrophic factor, reduced brain damage and improved functional outcome in a model of focal ischemic stroke. Levels of oxidative protein nitration and lipid peroxidation are reduced in muscle and brain tissues of UOX+/− mice under conditions of metabolic and oxidative stress (running in the case of muscle and ischemia in the case of the brain), consistent with prior evidence that UA can scavenge peroxynitrite and hydroxyl radical. Our findings reveal roles for UA in life span determination, endurance and adaptive responses to brain injury, and suggest novel approaches for protecting cells against injury and for optimizing physical performance.
Keywords: Uric acid, Endurance, Lifespan, Oxidative stress, Ischemic stroke
1. Introduction
The Uox gene encoding the urate oxidase (UOX) protein is functional in rodents, but not in humans, as the result of multiple mutations that occurred in the UOX gene during the divergence of the living genera of hominoids (gibbons, orangutans, chimpanzees, gorillas, and humans) from the Old World monkeys (Friedman et al., 1985; Varela-Echavarria et al., 1988; Wu et al., 1989). As a consequence, circulating uric acid (UA) levels in humans are 5–10 times greater than rodents. UA is one of the most abundant antioxidant molecules in humans with a potent ability to scavenge peroxynitrite, nitric oxide, and hydroxyl radicals, thereby preventing protein nitration and lipid peroxidation (Hooper et al., 1997, 1998). Studies in animal models have shown that administration of UA or soluble UA analogs that retain the antioxidant properties of UA protects the brain against ischemic injury (Aliena-Valero et al., 2018; Dhanesha et al., 2018; Haberman et al., 2007; Justicia et al., 2017; Yu et al., 1998), and a UA analog accelerates wound healing (Chigurupati et al., 2010), suggesting that UA can protect cells against injury and enhance repair of damaged tissue. The positive correlation between life span and UA levels among mammalian species suggests a potential role for UA in mitigating the aging process (Cutler, 1984). However, UA levels are elevated in gout and cardiovascular disease (Reginato et al., 2012; Zoppini et al., 2011) but are reduced in Parkinson’s disease and multiple sclerosis (Kutzing et al., 2008). The impact of endogenous UA in stroke is unclear (Dimitroula et al., 2008). Nevertheless, these findings from genetic, epidemiological, clinical, and experimental studies of UA suggest that concentrations of UA in the upper normal range are generally beneficial compared to lower concentrations, whereas higher concentrations that result in crystal formation are detrimental.
In response to physical exercise, levels of circulating and skeletal muscle UA increase in humans, as a result of ATP hydrolysis and inhibition of renal clearance of UA (Child et al., 1998; Emmerson et al., 1978; Hellsten-Westing et al., 1994; Sutton et al., 1980). It has been proposed, but not established, that UA plays a role in sustaining muscle function and reducing cellular damage during intense physical exertion (Castejon et al., 2006; Green and Fraser, 1998). It is therefore conceivable that mutation of the Uox gene during primate evolution contributed to the superior endurance runner phenotype of humans (Mattson, 2012). Targeted deletion of both Uox alleles in mice results in a more than 10-fold increase in UA levels and the development of nephrogenic diabetes insipidus and early death unless the mice are given allopurinol therapy (Kelly et al., 2001; Wu et al., 1994). However, we find that similar to humans (Gephardt et al., 1964; Kuzuya et al., 2002), circulating levels of UA increase in UOX+/− mice in an age-related manner and in response to stressful conditions, and that UOX+/− mice exhibit an extended life span, superior adaptive responses to stress that enhance physical endurance, and resistance of the brain to injury.
2. Materials and methods
2.1. Mice, UA analysis, and life span studies
Mice heterozygous for a disrupted Uox transgene (Wu et al.,1994) were obtained from Jackson Laboratories (Bar Harbor, ME) and were bred to generate the mice used in this study. The genetic background of the mice is C57BL/6J and 129Sv. Mice were maintained according to National Institutes of Health guidelines on a standard diet with free access to water. To limit the levels of hyperuricemia, the UOX−/− mice in the longevity study received water supplemented with allopurinol (0.67 mM from birth to weaning, 0.18 mM for the first year, and 0.26 mM thereafter). Separate groups of mice were used for behavior, exercise, and stroke studies. Hyperuricemic conditions were obtained by terminating the allopurinol therapy 2 weeks before the beginning of the experiment. All procedures on animals were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program. Blood was collected either by retro-orbital bleeding in live mice or by cardiac puncture at the time of euthanasia. Serum UA concentrations were measured using an assay kit (Randox, San Diego, CA) on a Roche Cobas Fara II robotic chemical analyzer according to the manufacturer’s specifications. DC-Cal and DC-Trol Level 1 and 2 Controls (Diagnostic Chemicals Limited, Oxford, CT) were used for instrument calibration and interassay accuracy, respectively.
2.2. Open field test
Open field testing was performed using the MEDOFA-MS system (Med Associated, St Albans, Vermont). Mice were placed in the center of the open field, and horizontal activity, stereotypical movements, and vertical activity were recorded for 15 minutes under dim light conditions. Spontaneous locomotor activity, stereotypical movements, and exploratory behavior were determined for each genotype.
2.3. Exercise studies
Mice were housed singly in Super Mouse Micro-Isolator cages (model 750; Lab Products Inc, Seaford, DE) with a wall-mounted 4.5 inch diameter Silent Spinner Wheel (PetSolutions, Beavercreek, OH) coupled to a bicycle odometer (Model BC506, Sigma Sport USA, Batavia, IL) with 2 neodymium magnets. Maximum speed, average speed, and total voluntary running distance were recorded twice weekly. For the training and exhaustion exercise model, mice were run on a treadmill (Model Exer-3/6 Treadmill, Columbus Instruments, Columbus, OH) at a 15° angle and 15 m/min speed for 1 hour daily or until exhaustion occurred. Exhaustion was reached when the animal fell off the treadmill 3 consecutive times within a 10-second period.
2.4. Focal ischemic stroke
The methods for focal ischemia and reperfusion brain injury were identical to those reported previously (Arumugam et al., 2010). The middle cerebral artery was occluded for 1 hour using an intraluminal thread, and only mice with more than an 85% reduction in blood flow were included in the study. The functional deficit was evaluated at 24, 48, and 72 hours after stroke using a five-point rating scale (0, no deficit; 1, failure to extend left paw; 2, circling to the left; 3, falling to the left; 4, unable to the walk spontaneously). Mice were euthanized on poststroke day 3, and their brains were removed and coronal brain slices were stained with 2% 2,3,5-triphenyltetrazolium chloride and evaluated for infarct size using standard methodology.
2.5. Western blot analysis
Muscle and brain tissues were homogenized in RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, and 1 mM sodium orthovanadate) containing protease inhibitors. The protein content of the samples was determined with Pierce BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL). Equal amounts of proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis), and transferred onto nitro-cellulose membranes (Invitrogen, Hercules, CA). Membranes were incubated with a blocking buffer (5% dry milk in 20 mM Tris–HCl, pH 7.5, 500 mM NaCl, 0.05% Tween 20) for 1 hour at room temperature, then overnight with the primary antibody at 4 °C. Immunoreactive bands were detected using horse radish peroxidase-conjugated secondary antibody and a chemiluminescence kit from Pierce (Rockford, IL). The bands were analyzed by densitometry using the ImageJ analysis software (NIH). The following antibodies were used in this study: anti-β-actin (1:5000; Sigma); anti-3-nitrotyrosine (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, and Millipore, Billerica, MA).
2.6. Lipid analysis
Lipid extraction and analysis were performed as described previously (Cutler et al., 2004). Briefly, samples were injected using a Harvard Apparatus pump at 15 μL/min into an electrospray ionization (i.e., Turbo Ion Spray module) Sciex API 3000 triple stage quadrupole tandem mass spectrometer electrospray tandem mass spectrometry (ES/MS/MS) from Sciex Inc, Thornhill, Ontario, Canada, operating in the positive mode. The ion spray voltage (V) was 5500 at a temperature of 80 °C with a nebulizer gas of 8 psi, curtain gas of 8 psi, and the collision gas set at 4 psi. The declustering potential was 80 V, focusing potential 400 V, entrance potential −10 V, collision energy 30 V, and collision cell exit potential 18 V. The ES/MS/MS scanned from 300 to 2000 atomic mass units (amu) per second at a step of 0.1 amu. Each lipid species was initially identified by a Q1 mass scan and then CAD gas breakdown products unique to each species were identified and used to make a standard curve. Samples were injected into the ES/MS/MS for 5 minutes, where the mass counts accumulated and the sum of the total counts under each peak were used to quantify each species. Positive identification and quantification of each species were achieved by precursor ion scanning or neutral loss scanning from a purified standard.
2.7. Statistical analysis
Survival curves were plotted using the Kaplan-Meier method, and log-rank Mantel-Cox and Wilcoxon tests were used to compare the curves. Results of both tests were comparable, and thus, only the results of the Mantel-Cox are reported. To assess the correlation between levels of UA and the different experiment variables, a Pearson correlation test was used. All the remaining statistical analyses were performed by either Student’s t-test or analysis of variance followed by post hoc test as appropriate. Analyses were performed using a Prism software package (GraphPad Software, San Diego, CA). Results are expressed as mean ± SEM. Values of p < 0.05 were considered statistically significant.
3. Results
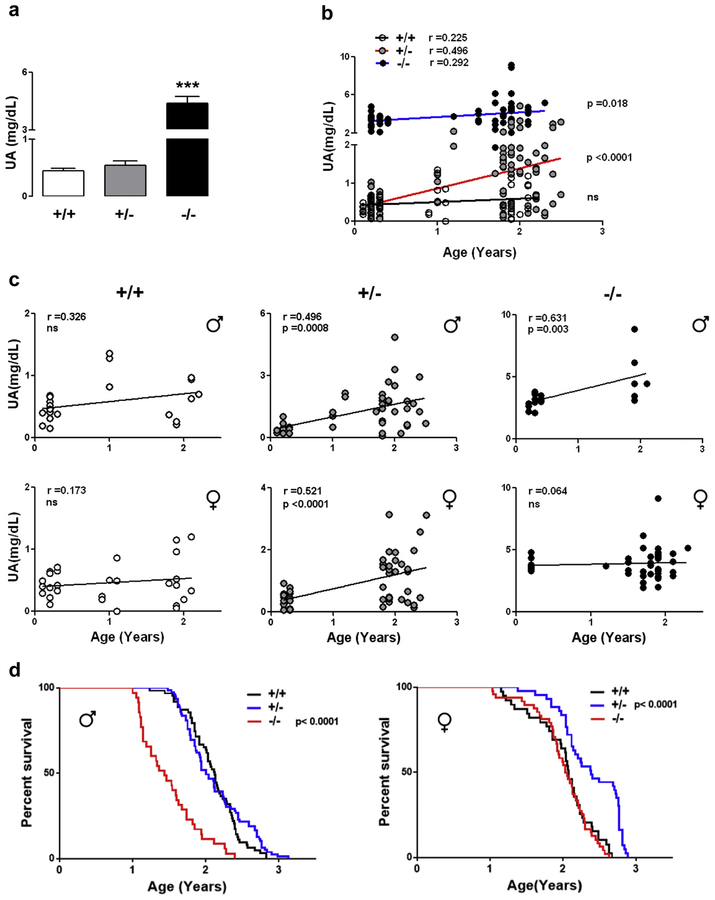
We measured circulating UA levels and life span in cohorts of UOX+/+, UOX+/−, and UOX −/− mice generated from crosses of heterozygous mice. The serum UA concentrations of young animals were (mg/dL) UOX+/+, 0.44 ± 0.05; UOX+/−, 0.54 ± 0.07; and UOX−/−, 4.4 ± 0.35 mg/dL (Fig. 1A). For the longevity study, to prevent kidney failure and early death, the UOX−/− mice were provided with allopurinol in their drinking water with the goal of maintaining UA levels within the normal range for humans. Although serum UA levels remained low during the life span of wild-type mice, UA increased by approximately 5-fold with age in UOX+/−mice (Fig. 1B). UA remained low in wild-type male and female mice throughout their life span, whereas UA levels increased with age in both male and female UOX+/− mice (Fig. 1C). Interestingly, male UOX−/− mice exhibited an age-related increase of UA levels, whereas the females did not (Fig. 1C). To determine whether UA levels might influence life span, we performed a survival study in male and female mice using each of the 3 genotypes (Fig. 1D). Female UOX+/−mice lived significantly longer than female UOX−/− mice or female wild-type mice. In males, the life spans of UOX+/− and wild-type mice were similar, whereas UOX−/− male mice exhibited a significant reduction in life span (Fig. 1D). For UOX+/− mice, the changes in UA levels with age were very similar in the both genders (male r = 0.496 females r = 0.521), yet in the survival plots, it is clear that the female UOX+/− mice live longer than wild-type females, but the male UOX+/− mice do not exhibit life span extension.
Fig. 1.
Uricase haploinsufficiency results in age-dependent elevation of circulating uric acid levels and life span extension. (A) Serum levels of UA in wild-type (+/+; n = 41), heterozygous (+/−; n = 10), and untreated (no allopurinol) homozygous (−/−; n = 40) UOX mice at 3 months of age (***p < 0.001). (B and C) The levels of UA were measured at the indicated times throughout the life span. No changes in the levels of serum UA were found in UOX+/+ mice, whereas a significant age-dependent, sex-independent increase of UA was observed in UOX+/− mice and in allopurinol-treated UOX−/− males (males +/+ n = 25, +/− n = 43, −/− n = 20; females +/+ n = 29, +/− n = 51, −/− n = 45); (D) Kaplan-Meier survival analysis of life span in mice of both genders (males +/+ n = 63, +/− n = 79, −/− n = 35; females +/+ n = 86, +/− n = 43, −/− n = 48). In males, the survival curve of UOX−/− was significantly different from the UOX +/+ mice (Mantel-Cox log-rank test χ2 = 42.6). In females, the survival curve of UOX +/− was significantly different from UOX +/+ (Mantel-Cox-log-rank test χ2 = 19.78). For these experiments, UOX−/− mice received allopurinol throughout their life. Abbreviations: UA, uric acid; UOX, urate oxidase.
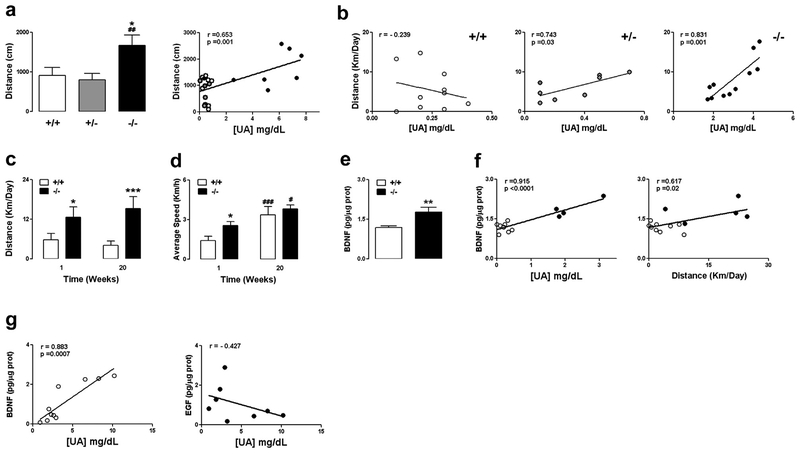
It has been proposed that UA plays an important role in animal foraging behavior (Johnson et al., 2009). Levels of UA increase during fasting conditions due to protein catabolism (Lennox, 1924), and this is associated with increased locomotor activity and decreased water excretion (Challet et al., 1995; Cherel and Le Maho, 1991). Moreover, UOX−/− mice with very high levels of UA display increased exploratory and novelty-seeking behavior compared to wild-type mice (Sutin et al., 2014). Further testing of all UOX genotypes in the open field confirmed that the overall distance covered by the UOX−/− mice in the arena was significantly greater than mice of the other 2 genotypes and was positively correlated with the levels of circulating UA (Fig. 2A). Furthermore, when mice were tested for an extended period of time in cages equipped with running wheels, we found significant positive correlations between UA levels and daily voluntary running distance in UOX+/− and UOX−/− mice but not in UOX+/+ mice (Fig. 2B). Over a period of 5 months, young UOX−/− mice consistently ran further each day on the running wheel compared to wild-type mice (Fig. 2C). By the end of the 5 month period, the UOX−/− mice ran 5 times further than the wild-type mice as a result of the UOX−/− mice spending more time running, whereas their running speed was no different than the wild-type mice (Figs. 2C and D).
Fig. 2.
Mice with elevated UA levels exhibit increased exploratory behavior, voluntary daily running distance, and brain BDNF levels. These experiments were performed using male mice. The UOX−/− mice received allopurinol for the duration of the study. (A) The total distance traveled in the open field arena was significantly greater in UOX−/− mice and positively correlated with the levels of circulating UA (7 mice per group; *p < 0.05 vs. +/+; ##p < 0.01 vs. +/−). (B) Positive correlations between levels of UA and the average daily running distance (home cage running wheel) were observed for UOX+/− mice (n = 8) and UOX−/− mice (n = 11). (C and D) Over a period of 20 weeks, UOX−/− mice consistently ran further than UOX+/+ mice (C), whereas the initial difference in average speed was lost over time; (D) (6–10 mice per group; *p < 0.05, ***p < 0.001 vs. +/+; #p < 0.05, ###p < 0.001 vs. the same genotype at week 1). (E and F) Mice were provided running wheels for a 20 week period, and were euthanized in the morning between 8:00 and 12:00. (E) BDNF levels were significantly greater in the hippocampus of running UOX−/− mice compared to UOX+/+ mice (5–9 mice per group; **p < 0.01). (F) Levels of BDNF in the hippocampus of running mice were highly significantly correlated to the levels of UA and to a lower extent to the average daily traveled distance (5–9 mice per group). (G) Sedentary UOX−/− mice were deprived of allopurinol treatment for increasing lengths of time (from 0 to 2 weeks) to allow different degrees of hyperuricemia. Levels of BDNF and EGF were measured in the hippocampus; there was a significant positive association between levels of BDNF and UA (8–10 mice per group). Abbreviations: BDNF, Brain-derived neurotrophic factor; EGF, epidermal growth factor; UA, uric acid; UOX, urate oxidase.
Brain-derived neurotrophic factor (BDNF) is produced by neurons in many brain regions where it plays critical roles in synaptic plasticity and resistance of neurons to stress (Mattson et al., 2004). In rodents, the expression of BDNF is increased in response to exercise in several brain regions including the hippocampus (Bekinschtein et al., 2011) and striatum (Marais et al., 2009), and BDNF can augment nigrostriatal dopaminergic signaling and loco-motor behavior (Horger et al., 1999; Shen et al., 1994). We found that BDNF levels in the hippocampus were significantly higher in runner UOX−/− mice compared to runner UOX+/+ mice (Fig. 2E). There was a highly significant positive association of serum UA levels and hippocampal BDNF levels and a significant positive association of serum UA levels and average daily running distance (Fig. 2F). When we extended our analysis to sedentary UOX−/− mice, we again found a significant positive correlation between the levels of hippocampal BDNF and serum UA (Fig. 2G) but not between levels of epidermal growth factor and UA (Fig. 2G), suggesting that UA may regulate the expression of BDNF in the brain.
Given the correlation between voluntary running and UA levels, we determined whether UA influences endurance. We first measured UA levels in sedentary mice and mice that had been subjected to strenuous treadmill exercise. Although treadmill running did not modify UA levels in UOX+/+ mice, it resulted in a two-fold increase in UA levels in UOX+/− mice (Fig. 3A) and a 1.5-fold increase in UOX−/− mice (data not shown). We therefore performed a study involving 3 consecutive days of intense endurance running using UOX+/−, which showed an exercise-induced increase in UA similar to what has been reported in humans. The exercise-induced elevation of UA levels in UOX+/− mice was greater 6 hours after exercise in mice trained on a treadmill 1 hour daily for 3 consecutive days compared to UOX+/− mice that were either run to exhaustion or trained for 3 days and then run to exhaustion (Fig. 3B), suggesting that UA may be partially consumed under conditions of intense muscle activity. We next evaluated the endurance of UOX+/+ and UOX+/− mice that had either been sedentary or trained for 3 days, by determining how long the mice could run until they were exhausted. Although 3 days of training did not modify the time to exhaustion in UOX+/+ mice, trained UOX+/− mice were able to run 5 times longer than nontrained UOX+/− mice (Fig. 3C). UA reacts with and neutralizes various reactive oxygen species in processes that result in degradation of UA (Kim et al., 2009). In response to vigorous exercise, protein tyrosine nitration, a modification resulting from the interaction of peroxynitrite with proteins (Souza et al., 2008), is increased in skeletal muscle cells (Vassilakopoulos et al., 2003). Because UA can scavenge peroxynitrite and can reduce protein nitration in experimental models of tissue injury (Hooper et al., 1998; Scott et al., 2005), we performed immunoblot analysis of nitrotyrosine in quadriceps muscle samples from UOX+/+ and UOX+/− mice collected at different intervals after treadmill running. Levels of nitrotyrosine were significantly increased in UOX+/+ mice after exercise but were not changed in the UOX+/− mice (Fig. 3D) although they ran significantly longer distances.
Fig. 3.
Uricase haploinsufficiency results in increased levels of UA after intense exercise and improved running endurance. These experiments were performed using male mice. (A) UA levels were measured in sedentary mice and mice subjected to treadmill exercise. Exercise induced a significant upregulation of circulating levels of UA in UOX+/− mice (5 UOX+/+ mice and 14 UOX+/− mice; **p < 0.01 vs. sedentary mice). (B) Levels of UA were measured in UOX mice that were either run to exhaustion (Ex), trained for 3 days (T), or trained for 3 days and then run to exhaustion (T+Ex). Compared to sedentary mice (−), UA levels were increased by all types of exercise and were significantly higher in mice that were not run to exhaustion (3–5 mice per group; *p < 0.05, **p < 0.01, ***p < 0.001 vs. sedentary mice; ## p < 0.01 vs. Ex and T+EX mice). (C) Endurance was measured in UOX+/+ and UOX+/− mice. (4 UOX+/+ and 5 UOX+/− mice); **p < 0.01 vs. sedentary UOX+/− mice). (D) Representative immunoblots and quantitation of 3-nitrotyrosine (3-NT) levels in quadriceps of sedentary mice (−) or mice subjected to either 1 day or 3 days of treadmill exercise. High levels of 3-NT were found in muscle of exercised UOX+/+ mice but not in exercised UOX+/− mice (3 mice per group; *p < 0.05, **p < 0.01 vs. UOX+/+ sedentary mice; #p < 0.05, ##p < 0.01 vs. corresponding exercised UOX+/− mice). Abbreviations: UA, uric acid; UOX, urate oxidase.
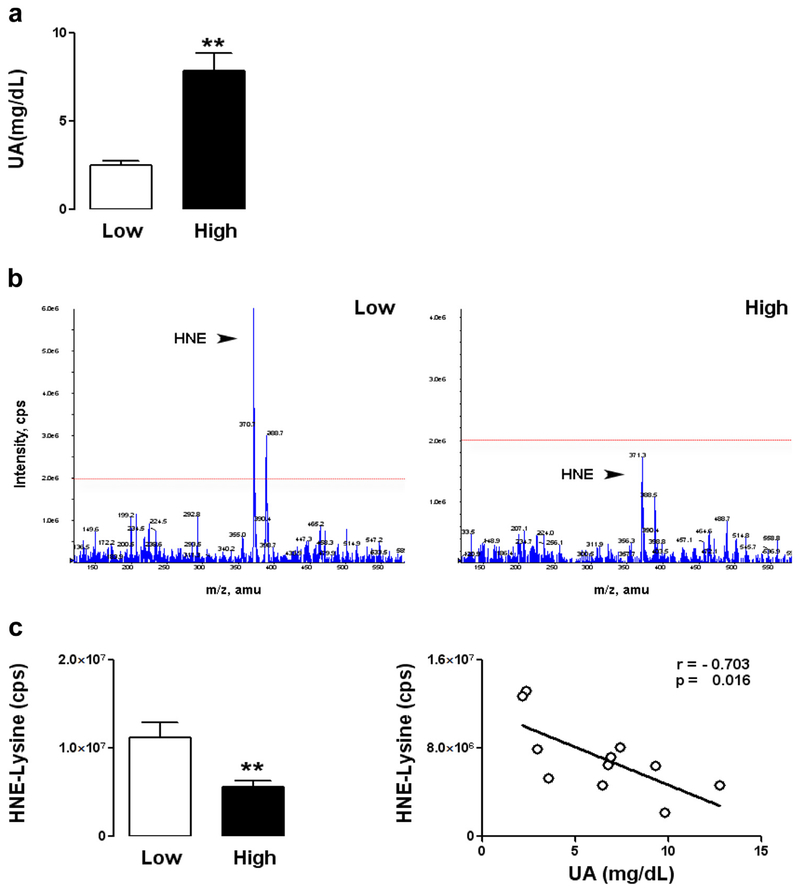
Because of their high metabolic rate and content of unsaturated fatty acids, neurons in the brain are particularly vulnerable to membrane lipid peroxidation (Scott and Hooper, 2001; Cutler et al., 2004). We chose to use the membrane lipid peroxidation product 4-hydroxynonenal (HNE) as an indicator of oxidative damage for the experiment in Figure 4 because it provides a measure complementary to 3-nitrotyrosine (3-NT). Indeed, previous studies have shown that UA can neutralize both peroxynitrite, which would be expected to reduce 3-NT levels, and hydroxyl radical, which would be expected to reduce membrane lipid peroxidation and HNE production (Becker, 1993; Cutler et al., 2015; Mattson et al., 1997). We therefore tested the hypothesis that UA levels impact the vulnerability of brain cells by using UOX−/− mice deprived of allopurinol for 2 weeks, resulting in a broad range of UA levels among individual mice (Fig. 4A). Tandem mass spectrometry analysis of cerebral cortical tissue showed significantly lower levels of lipid peroxidation product HNE adducts in mice with higher levels of UA (Fig. 4B). Levels of HNE-lysine adducts were negatively correlated with the levels of UA (Fig. 4C) indicating that UA reduces basal levels of oxidative stress in the brain.
Fig. 4.
Uric acid decreases lipid peroxidation in the brain. (A) Male UOX−/− mice (6 months old) were deprived of allopurinol for 2 weeks to allow hyperuricemia. Based on the interanimal variability in levels of UA at the time they were euthanized, the mice were divided in 2 groups, those with low and those with high UA levels (3 mice in the low-UA group and 8 mice in the high-UA group; **p < 0.01). (B) Representative spectrograms showing the relative levels of HNE adducts in the cerebral cortex of mice with low or high levels of UA. (C) Negative correlation of UA levels with levels of HNE-lysine adducts. Abbreviations: HNE, 4-hydroxynonenal; UA, uric acid; UOX, urate oxidase.
Stroke is a major cause of age-related morbidity and mortality. Treatment with exogenous UA or more soluble UA analogs can be beneficial in animal stroke models (Dhanesha et al., 2018; Haberman et al., 2007; Romanos et al., 2007; Yu et al., 1998). However, in humans, both positive and negative associations between levels of UA and the outcome of ischemic stroke have been reported. We subjected UOX+/+ and UOX+/− mice to 1 hour of middle cerebral artery occlusion (MCAO) followed by reperfusion to mimic focal ischemic stroke. Although the initial functional deficit during the first 2 days after the stroke was similar in UOX+/+ and UOX+/− mice, the deficit on day 3 was significantly less in UOX+/− mice compared to UOX+/+ mice (Fig. 5A). After behavioral evaluation on poststroke day 3, mice were euthanized, and their brains were removed and processed for quantification of ischemic infarct size. The brain infarct volume of UOX+/− mice was significantly reduced compared to that of UOX+/+ mice (Fig. 5B). A second cohort of mice was euthanized 24 hours after sham surgery or MCAO to perform biochemical analyses of cortical brain tissue. In wild-type mice, sham operation had no effect on brain UA levels, whereas MCAO caused a significant increase in cortical UA levels compared to control and sham-operated mice (Fig. 5C), possibly as a result of increased purine catabolism from the damaged areas overpowering the ability of UOX to convert UA to allantoin. Interestingly, sham-operated UOX+/−mice showed a two-fold increase in levels of cortical UA compared to control UOX+/− mice (Fig. 5C), whereas UA levels were reduced in the ischemic cortex of UOX+/− mice compared to control and sham-operated UOX+/− mice (Fig. 5C).
Fig. 5.
Endogenous UA reduces protein nitration, is neuroprotective, and improves functional outcome in a mouse model of focal ischemic stroke. These experiments were performed using male mice. (A) Neurological deficit scores at the indicated time points after middle cerebral artery occlusion (MCAO) in male UOX+/+ and UOX+/− mice (6–10 mice per group; * p < 0.05). (B) Representative images of 2,3,5-triphenyltetrazolium chloride-stained brain sections and quantitation of the infarct volume 72 hours after MCAO (6–10 mice; *p < 0.05). (C) Changes in cortical UA levels 24 hours after sham or MCAO surgery (5–6 mice; *p < 0.05; **p < 0.01 vs. UOX+/+ sham; ###p < 0.001 vs. UOX+/− sham; §§p < 0.01 vs. UOX+/+ MCAO). (D) Schematic of the UOX-mediated conversion of UA to allantoin, and nonenzymatic reactions of UA with superoxide (O2−), nitric oxide (NO), peroxynitrite (ONOO−), and other reactive oxygen species to generate the indicated products. The bottom panel shows the quantitative analysis for each of the oxidative UA derivatives in serum samples. (5–6 mice per group; *p < 0.05; **p < 0.01; ***p < 0.001 vs. UOX+/+ sham; ###p < 0.001 vs. UOX+/− sham; §p < 0.05, §§p < 0.01 §§§p < 0.001 vs. UOX+/+ MCAO). (E) Relative levels of 3-nitrotyrosine (3-NT) in the cortex of sham and MCAO mice 24 hours after surgery (5–6 mice per group; ***p < 0.001 vs. UOX+/+ sham; ###p < 0.001 vs. UOX+/+ MCAO). Abbreviations: UA, uric acid; UOX, urate oxidase.
UA is nonenzymatically converted to other compounds when it interacts with and neutralizes free radicals (Becker, 1993), which could explain the reduction in UA levels in the ischemic brain tissue of UOX+/− mice. We tested this possibility by measuring the levels of allantoin, 6-amino-uracil, triuret, and UA radical, which result from the interaction of UA with superoxide, nitric oxide, peroxynitrite, or other free radicals, respectively (Fig. 5D). Compared to sham wild-type mice, wild-type mice subjected to MCAO showed significantly decreased serum levels of allantoin and increased triuret levels (Fig. 5D). On the other hand, in UOX+/− mice subjected to MCAO, only the levels of the urate radical (Becker, 1993; Santus et al., 2001) were significantly elevated (Fig. 5D). Accordingly, a significant increase of oxidative/nitrosative damage was found in the cortex of MCAO UOX+/+ mice but not in UOX+/− mice (Fig. 5E).
4. Discussion
Our findings provide evidence that UA levels influence life span, increase rapidly in response to exercise stress, and increase the resistance of cells to oxidative damage and degeneration. UOX haploinsufficiency is one of only a few experimental genetic alterations shown to extend life span in mice, with mutations in insulin signaling-related genes also extending life span (Bartke et al., 2013). An enzyme-inactivating mutation(s) of the Uox gene was retained during primate evolution, resulting in positive correlation of UA levels with maximum life span, with humans having much higher UA levels than shorter-lived primates and lower mammals (Ames et al., 1981; Cutler, 1984). Moreover, many birds have very high UA levels and longer life spans, despite relatively high metabolic rates (Klandorf et al., 2001). The fact that UOX−/− mice did not show an extension of life span is consistent with evidence that the loss of UOX activity in hominids was gradual and spanned millions of years, which likely allowed the parallel development of adaptive measures counteracting the detrimental effects of sudden hyper-uricemic conditions (Johnson et al., 2005).
Previous studies have shown that circulating levels of UA increase during and immediately after high-intensity endurance exercise. For example, high-intensity cycling increased plasma UA by 40%, and the UA levels remained elevated for at least 24 hours (Green and Fraser, 1988), and serum UA levels increase by 25% after high-intensity swimming in adolescents (Kabasakalis et al., 2014). Moreover, the fastest horses had significantly higher UA compared to the slower horses competing in endurance races (Castejon et al., 2006). It has been proposed, but not established, that UA plays a role in sustaining muscle function and reducing cellular damage during intense physical exertion (Castejon et al., 2006; Green and Fraser, 1998). Early interventional studies that attempted to relate UA levels to endurance in humans yielded mixed results. Williams et al. (1990) and Starling et al. (1996) administered the UA precursor inosine to runners and cyclists, respectively; inosines did not improve their performance. In the latter 2 studies, exercise increased UA levels, and inosine treatment did not increase UA levels in the runners but did increase UA levels in the cyclists. A second inosine supplementation trial in cyclists reported an increase in UA levels but no benefit on performance (McNaughton et al., 1999). All of these studies involved short-term inosine administration (2–11 days). It should also be noted that the athletes in these studies were already highly trained and so may therefore not have benefited from the inosine. Indeed, administration of UA intravenously to nonathletes immediately before 20 minutes of high-intensity aerobic exercise resulted in significantly lower levels of plasma markers of oxidative stress compared to subjects who received placebo (Waring et al., 2003).
We found that UOX-deficient mice with elevated UA levels exhibit a significant increase in the distance mice ran, both voluntarily on a running wheel in their home cage and when forced to run to exhaustion on a treadmill. Our findings suggest the possibility that elevated UA levels contribute to the endurance capacity of humans compared to nonhuman primates and lower mammals (Mattson, 2012). In a longitudinal study of older people, higher serum UA levels were associated with greater muscle strength measures in both men and women; the authors suggested that UA may protect muscle cells against age-related oxidative damage (Macchi et al., 2008). However, because exercise increases UA levels, the reported association between higher UA levels and better muscle function could be a selection bias that is merely separating people who routinely exercise versus those who do not. It should also be noted that excessively high UA levels are associated with poorer physical function and greater disabilities during aging, suggesting that there may be an optimal window of UA levels conducive to disease resistance and longevity (Ruggiero et al., 2007). Our findings support this notion, albeit with apparent gender differences in the UA levels that are optimal for longevity. Thus, we found that female UOX+/− mice with serum UA levels in the range of healthy humans exhibited a significant increase in life span, whereas male UOX+/− mice did not exhibit increased life span. On the other hand, although female UOX−/− mice with high UA levels lived as long as wild-type female mice, male UOX−/− mice had a shortened life span. Based on the results of several necropsies, we believe that the main cause of shortened life span in the UOX−/−males was kidney damage caused by UA crystals. These findings in mice are of interest because in humans, women have lower blood UA than men (Anton et al., 1986).
Based on preclinical findings in rat and mouse stroke models (Haberman et al., 2007; Yu et al., 1998), clinical trials of UA in stroke patients were performed. Our results suggesting that an increase in the levels of endogenous UA in the early stages of stroke can be beneficial are supported by a phase II vehicle-controlled clinical trial of dual treatment with recombinant tissue plasminogen activator and UA within the first 3 hours of ischemic stroke onset (Amaro et al., 2007). Placebo-treated patients exhibited a 27% increase in levels of the lipid peroxidation product malondialdehyde 5 days after the infarct, whereas UA-treated patients had 30% lower levels of malondialdehyde. In a subsequent study, UA (1000 mg) was administered intravenously during a 90-minute period (in combination with thrombolytic therapy) within 5 hours of symptom onset (Chamorro et al., 2014). Patients in the UA group did not have a statistically significant improvement in outcome compared to those in the placebo group. Subsequent analysis of the data from the latter study revealed evidence that UA significantly improved outcome in women but not men (Llull et al., 2015) and that UA reduces early ischemic worsening after a stroke (Amaro et al., 2016). However, blood UA concentrations were not measured in this study and, therefore, the magnitude and duration of the UA elevation following administration is unknown. Efficacy might therefore be improved by administration of multiple doses of UA over a period of several days or more after stroke.
In addition to a potential therapeutic benefit in stroke, animal studies have shown that elevation of UA levels can ameliorate disease processes and improves functional outcome in models of multiple sclerosis and Parkinson’s disease (Chen et al., 2013; Duan et al., 2002; Hooper et al., 1998). Moreover, low UA levels are associated with an increased risk of Parkinson’s disease (Schwarzschild et al., 2011) and major depression (Bartoli et al., 2018). UA directly protects cultured neurons against degeneration induced by amyloid β peptide and iron by a mechanism involving reduction in protein nitration (Mattson et al., 1997). Oxidative stress and membrane lipid peroxidation contribute to the dysfunction and degeneration of neurons Alzheimer’s and Parkinson’s diseases and stroke (Mattson and Arumugam, 2018). The well-established anti-oxidant activity of UA is therefore a likely mechanism by which UA may retard aging and protects the brain against dysfunction and degeneration. However, we cannot rule out other potential mechanisms to explain the stress-resistant phenotype of UOX-deficient mice. In this regard, we found that allantoin levels were reduced in brain tissue of UOX+/− mice and that UA radical levels were greatly elevated in UOX+/− mice after stroke. However, reduced allantoin levels are unlikely to explain the longevity and stress resistance of UOX+/− mice because supplementing the food of Caenorhabditis elegans with allantoin significantly extends life span (Calvert et al., 2016).
With regard to its effects on the healthy brain, the positive correlation between circulating UA levels and brain BDNF levels suggests that, in addition to its intrinsic antioxidant actions, UA may also benefit neurons by enhancing neurotrophic support. Indeed, BDNF is known to protect neurons against oxidative and metabolic stress and to enhance synaptic plasticity and learning and memory (Greenberg et al., 2009; Mattson et al., 2004). In a longitudinal study of healthy adults, higher serum UA levels were associated with slower decline in visuospatial abilities and attention in men but not in women (Kueider et al., 2017). Previous studies demonstrated positive associations between blood UA levels and high energy/drive, positive affect, and achievement (Fowler, 1973; Lorenzi et al., 2010; Stevens et al., 1975). Therefore, although actions of UA on muscle cells could contribute to the enhanced endurance in UOX-deficient mice, it is also possible that UA might impact endurance by enhancing motivation and reducing psychological barriers that limit performance in endurance challenges.
Consistent with prior evidence that UA can scavenge peroxynitrite and hydroxyl radical (Haberman et al., 2007), we found that levels of oxidative protein nitration and lipid peroxidation are reduced in muscle and brain tissues of UOX+/− mice under conditions of metabolic and oxidative stress (running in the case of muscle and ischemia in the case of the brain). Although UOX+/−exhibited a complete suppression of cerebral ischemia-induced increase in brain 3-NT levels (and triuret levels) compared to UOX+/+ mice, the UOX+/− mice did not exhibit an elevation of muscle 3-NT levels under basal (no exercise) conditions. Although the antioxidant action of UA is therefore one explanation for enhanced endurance and longevity in mice with moderate elevation of UA levels, UA-independent mechanisms cannot be excluded. We were surprised that allantoin levels were reduced in brain tissue of UOX+/− mice, given that UA levels were elevated. On the other hand, an increase in UA radical levels after stroke would be expected to decrease allantoin levels, and we found that allantoin levels were indeed low after stroke (Fig. 5D). However, the possibility that reduced levels of allantoin mediate life span extension in UOX+/− mice is at odds with a recent report that supplementing the food of Caenorhabditis elegans with allantoin significantly extends life span (Calvert et al., 2016).
Our findings suggest that within the human physiological range of concentrations, UA can counteract the adverse effects of aging, strenuous exercise, and tissue damage in mice, thereby improving physical performance and resistance to metabolic and oxidative stress. If and to what extent the uricase-inactivating mutations that occurred during primate evolution contributed to the superior endurance, cognitive capabilities, and longevity of humans compared to lower primates and other mammals remains to be established. However, our findings in the present studies of UOX-deficient mice are consistent with such beneficial roles for UA on oxidative stress resistance and longevity.
Acknowledgements
This work was supported in part by the Intramural Research Program of the National Institute on Aging. SAC was supported by a Ministerio de Educacion, Cultura y Deporte of Spain postdoctoral fellowship (EX2009–0918). The authors thank the staff of the Comparative Animal Section of NIA for their outstanding service with breeding, genotyping, and handling of the mice used in this study.
Footnotes
Disclosure statement
The authors declare no actual or potential conflicts of interests.
References
- Aliena-Valero A, López-Morales MA, Burguete MC, Castelló-Ruiz M, Jover-Mengual T, Hervás D, Torregrosa G, Leira EC, Chamorro Á, Salom JB, 2018. Emergent uric acid treatment is synergistic with mechanical recanalization in improving stroke outcomes in male and female rats. Neuroscience 388, 263–273. [DOI] [PubMed] [Google Scholar]
- Amaro S, Soy D, Obach V, Cervera A, Planas AM, Chamorro A, 2007. A pilot study of dual treatment with recombinant tissue plasminogen activator and uric acid in acute ischemic stroke. Stroke 38, 2173–2175. [DOI] [PubMed] [Google Scholar]
- Amaro S, Laredo C, Renú A, Llull L, Rudilosso S, Obach V, Urra X, Planas AM, Chamorro Á, URICO-ICTUS Investigators, 2016. Uric acid therapy prevents early ischemic stroke progression: a tertiary analysis of the URICO-ICTUS trial (efficacy study of combined treatment with uric acid and r-tPA in acute ischemic stroke). Stroke 47, 2874–2876. [DOI] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P, 1981. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U. S. A 78, 6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antón FM, García Puig J, Ramos T, González P, Ordás J, 1986. Sex differences in uric acid metabolism in adults: evidence for a lack of influence of estradiol-17 beta (E2) on the renal handling of urate. Metabolism 35, 343–348. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R, 2010. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann. Neurol 67, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V, 2013. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol. Rev 93, 571–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli F, Trotta G, Crocamo C, Malerba MR, Clerici M, Carrà G, 2018. Anti-oxidant uric acid in treated and untreated subjects with major depressive disorder: a meta-analysis and meta-regression. Eur. Arch. Psychiatry Clin. Neurosci 268, 119–127. [DOI] [PubMed] [Google Scholar]
- Becker BF, 1993. Towards the physiological function of uric acid. Free Radic. Biol. Med 14, 615–631. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ, 2011. Effects of environmental enrichment and voluntary running exercise on neurogenesis, learning and memory, and patter separation: BDNF as a critical variable? Semin. Cell Dev. Biol 22, 536–542. [DOI] [PubMed] [Google Scholar]
- Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhães JP, 2016. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell 15, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castejón F, Trigo P, Muñoz A, Riber C, 2006. Uric acid responses to endurance racing and relationship with performance, plasma biochemistry and metabolic alterations. Equine Vet. J. Suppl 36, 70–73. [DOI] [PubMed] [Google Scholar]
- Challet E, le Maho Y, Robin JP, Malan A, Cherel Y, 1995. Involvement of corticosterone in the fasting-induced rise in protein utilization and locomotor activity. Pharmacol. Biochem. Behav 50, 405–412. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Castellanos M, Segura T, Arenillas J, Martí-Fábregas J, Gállego J, Krupinski J, Gomis M, Cánovas D, Carné X, Deulofeu R, Román LS, Oleaga L, Torres F, Planas AM, URICO-ICTUS Investigators, 2014. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): a randomised, double-blind phase 2b/3 trial. Lancet Neurol 13, 453–460. [DOI] [PubMed] [Google Scholar]
- Chen X, Burdett TC, Desjardins CA, Logan R, Cipriani S, Xu Y, Schwarzschild MA, 2013. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc. Natl. Acad. Sci. U. S. A 110, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherel Y, Le Maho Y, 1991. Refeeding after the late increase in nitrogen excretion during prolonged fasting in the rat. Physiol. Behav 50, 345–349. [DOI] [PubMed] [Google Scholar]
- Chigurupati S, Mughal MR, Chan SL, Arumugam TV, Baharani A, Tang SC, Yu QS, Holloway HW, Wheeler R, Poosala S, Greig NH, Mattson MP, 2010. A synthetic uric acid analog accelerates cutaneous wound healing in mice. PLoS One 5, e10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE, 1998. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half-marathon run. Med. Sci. Sports Exerc 30, 1603–1607. [DOI] [PubMed] [Google Scholar]
- Cutler RG, 1984. Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch. Gerontol. Geriatr 3, 321–348. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, Troncoso JC, Mattson MP, 2004. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. U. S. A 101, 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Camandola S, Malott KF, Edelhauser MA, Mattson MP, 2015. The role of uric acid and methyl derivatives in the prevention of age-related neurodegenerative disorders. Curr. Top Med. Chem 15, 2233–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesha N, Vázquez-Rosa E, Cintrón-Pérez CJ, Thedens D, Kort AJ, Chuong V, Rivera-Dompenciel AM, Chauhan AK, Leira EC, Pieper AA, 2018. Treatment with uric acid reduces infarct and improves neurologic function in female mice after transient cerebral ischemia. J. Stroke Cerebrovasc. Dis 27, 1412–1416. [DOI] [PubMed] [Google Scholar]
- Dimitroula HV, Hatzitolios AI, Karvounis HI, 2008. The role of uric acid in stroke: the issue remains unresolved. Neurologist 14, 238–242. [DOI] [PubMed] [Google Scholar]
- Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP, 2002. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem 80, 101–110. [DOI] [PubMed] [Google Scholar]
- Emmerson BT, 1978. Uric Acid, Handbook of Experimental Pharmacology, 51, pp. 287–324. [Google Scholar]
- Fowler MG, 1973. Relationship of serum uric acid to achievement motivation. Psychosom. Med 35, 13–22. [DOI] [PubMed] [Google Scholar]
- Friedman TB, Polanco GE, Appold JC, Mayle JE, 1985. On the loss of uricolytic activity during primate evolutione–I. Silencing of urate oxidase in a hominoid ancestor. Comp. Biochem. Physiol. B 81, 653–659. [DOI] [PubMed] [Google Scholar]
- Gephardt MC, Hanlon TJ, Matson CF, 1964. Blood uric acid values as related to sex and age. JAMA 189, 1028–1029. [DOI] [PubMed] [Google Scholar]
- Green HJ, Fraser IG, 1998. Differential effect of exercise intensity on serum uric acid concentration. Med. Sci. Sports Exerc 20, 55–59. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL, 2009. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci 29, 12764–12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberman F, Tang SC, Arumugam TV, Hyun DH, Yu QS, Cutler RG, Guo Z, Holloway HW, Greig NH, Mattson MP, 2007. Soluble neuroprotective anti-oxidant uric acid analogs ameliorate ischemic brain injury in mice. Neuromolecular. Med 9, 315–323. [DOI] [PubMed] [Google Scholar]
- Hellsten-Westing Y, Kaijser L, Ekblom B, Sjödin B, 1994. Exchange of purines in human liver and skeletal muscle with short-term exhaustive exercise. Am. J. Physiol 266, R81–R86. [DOI] [PubMed] [Google Scholar]
- Hooper DC, Bagasra O, Marini JC, Zborek A, Ohnishi ST, Kean R, Champion JM, Sarker AB, Bobroski L, Farber JL, Akaike T, Maeda H, Koprowski H, 1997. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A 94, 2528–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC, Spitsin S, Kean RB, Champion JM, Dickson GM, Chaudhry I, Koprowski H, 1998. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A 95, 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR, 1999. Enhancement of locomotor activity and conditioned reward to cocaine by brain derived neurotrophic factor. J. Neurosci 19, 4110–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Sautin YY, Oliver WJ, Roncal C, Mu W, Gabriela Sanchez-Lozada L, Rodriguez-Iturbe B, Nakagawa T, Benner SA, 2009. Lesson from comparative physiology: could uric acid represent a physiologic alarm signal gone awry in western society? J. Comp. Physiol 179, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RJ, Titte S, Cade JR, Rideout BA, Oliver WJ, 2005. Uric acid, evolution and primitive cultures. Semin. Nephrol 25, 3–8. [DOI] [PubMed] [Google Scholar]
- Justicia C, Salas-Perdomo A, Pérez-de-Puig I, Deddens LH, van Tilborg GAF, Castellví C, Dijkhuizen RM, Chamorro Á, Planas AM, 2017. Uric acid is protective after cerebral ischemia/reperfusion in hyperglycemic mice. Transl. Stroke Res 8, 294–305. [DOI] [PubMed] [Google Scholar]
- Kabasakalis A, Tsalis G, Zafrana E, Loupos D, Mougios V, 2014. Effects of endurance and high-intensity swimming exercise on the redox status of adolescent male and female swimmers. J. Sports Sci 32, 747–756. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Delnomdedieu M, Oliverio MI, Williams LD, Saifer MG, Sherman MR, Coffman TM, Johnson GA, Hershfield MS, 2001. Diabetes insipidus in uricase-deficient mice: a model for evaluating therapy with poly(ethylene glycol)-modified uricase. J. Am. Soc. Nephrol 12, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Kim KM, Henderson GN, Frye RF, Galloway CD, Brown NJ, Segal MS, Imaram W, Angerhofer A, Johnson RJ, 2009. Simultaneous determination of uric acid metabolites allantoin, 6-aminouracil, and triuret in human urine using liquid chromatography - mass spectrometry. J. Chromatogr. Analyt. Technol. Biomed. Life Sci 877, 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klandorf H, Rathore DS, Iqbal M, Shi X, Van Dyke K, 2001. Accelerated tissue aging and increased oxidative stress in broiler chickens fed allopurinol. Comp. Biochem. Physiol. C. Toxicol. Pharmacol 129, 93–104. [DOI] [PubMed] [Google Scholar]
- Kueider AM, An Y, Tanaka T, Kitner-Triolo MH, Studenski S, Ferrucci L, Thambisetty M, 2017. Sex-dependent associations of serum uric acid with brain function during aging. J. Alzheimers Dis 60, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzing MK, Firestein BL, 2008. Altered uric acid levels and disease states. J. Pharmacol. Exp. Ther 324, 1–7. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Ando F, Iguchi A, Shimokata H, 2002. Effect of aging on serum uric acid levels: longitudinal changes in a large Japanese population group. J. Gerontol. A. Biol. Sci. Med. Sci 57, M660–M664. [DOI] [PubMed] [Google Scholar]
- Lennox WG, 1924. Increase of uric acid in the blood during prolonged starvation. JAMA 82, 602. [Google Scholar]
- Llull L, Laredo C, Renú A, Pérez B, Vila E, Obach V, Urra X, Planas A, Amaro S, Chamorro Á, 2015. Uric acid therapy improves clinical outcome in women with acute ischemic stroke. Stroke 46, 2162–2167. [DOI] [PubMed] [Google Scholar]
- Lorenzi TM, Borba DL, Dutra G, Lara DR, 2010. Association of serum uric acid levels with emotional and affective temperaments. J. Affect Disord 121, 161–164. [DOI] [PubMed] [Google Scholar]
- Macchi C, Molino-Lova R, Polcaro P, Guarducci L, Lauretani F, Cecchi F, Bandinelli S, Guralnik JM, Ferrucci L, 2008. Higher Circulating Levels of Uric Acid Are Prospectively Associated with Better Muscle Function in Older persons. Mech. Ageing Dev 129, 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais L, Stein DJ, Daniels WMU, 2009. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab. Brain Dis 24, 587–597. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K, 1997. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J. Neurosci. Res 49, 681–697. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B, 2004. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci 27, 589–594. [DOI] [PubMed] [Google Scholar]
- Mattson MP, 2012. Evolutionary aspects of human exercise-born to run purposefully. Ageing Res. Rev 11, 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Arumugam TV, 2018. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab 27, 1176–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton L, Dalton B, Tarr J, 1999. Inosine supplementation has no effect on aerobic or anaerobic cycling performance. Int. J. Sport Nutr 9, 333–344. [DOI] [PubMed] [Google Scholar]
- Reginato AM, Mount DB, Yang I, Choi HK, 2012. The genetics of hyperuricemia and gout. Nat. Rev. Rheumatol 8, 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanos E, Planas AM, Amaro S, Chamorro A, 2007. Uric acid reduces brain damage and improves the benefits of rt_PA in a rat model of thromboembolic stroke. J. Cereb. Blood Flow Metab 27, 14–20. [DOI] [PubMed] [Google Scholar]
- Ruggiero C, Cherubini A, Guralnik J, Semba RD, Maggio M, Ling SM, Lauretani F, Bandinelli S, Senin U, Ferrucci L, 2007. The interplay between uric acid and antioxidants in relation to physical function in older persons. J. Am. Geriatr. Soc 55, 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santus R, Patterson LK, Filipe P, Morlière P, Hug GL, Fernandes A, Mazière JC, 2001. Redox reactions of the urate radical/urate couple with the superoxide radical anion, the tryptophan neutral radical and selected flavonoids in neutral aqueous solutions. Free Radic. Res 35, 129–136. [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Marek K, Eberly S, Oakes D, Shoulson I, Jennings D, Seibyl J, Ascherio A, Parkinson Study Group PRECEPT Investigators, 2011. Serum urate and probability of dopaminergic deficit in early “Parkinson’s disease. Mov. Disord 26, 1864–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GS, Hooper DC, 2001. The role of uric acid in protection against peroxynitrite-mediated pathology. Med. Hypotheses 56, 95–100. [DOI] [PubMed] [Google Scholar]
- Scott GS, Cuzzocrea S, Genovese T, Koprowski H, Hooper DC, 2005. Uric acid protects against secondary damage after spinal cord injury. Proc. Natl. Acad. Sci. U. S. A 102, 3483–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY, Altar CA, Chiodo LA, 1994. Brain-derived neurotropic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc. Natl. Acad. Sci. U. S. A 91, 8920–8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza JM, Peluffo G, Radi R, 2008. Protein tyrosine nitration-functional alteration or just a biomarker? Free Radic. Biol. Med 45, 357–366. [DOI] [PubMed] [Google Scholar]
- Starling RD, Trappe TA, Short KR, Sheffield-Moore M, Jozsi AC, Fink WJ, Costill DL, 1996. Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med. Sci. Sports Exerc 28, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Stevens HA, Cropley AJ, Blattler DP, 1975. Intellect and serum uric acid: an optimal concentration of serum urate for human learning? Soc. Biol 22, 229–234. [DOI] [PubMed] [Google Scholar]
- Sutton JR, Toews CJ, Ward GR, Fox IH, 1980. Purine metabolism during strenuous muscular exercise in man. Metabolism 29, 254–260. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, Terrancciano A, 2014. Impulsivity is associated with uric acid: evidence from humans and mice. Biol. Psychiatry 75, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Echavarría A, Montes de Oca-Luna R, Barrera-Saldaña HA, 1988. Uricase protein sequences:conserved during vertebrate evolution but absent in humans. FASEB J 2, 3092–3096. [DOI] [PubMed] [Google Scholar]
- Vassilakopoulos T, Deckman G, Kebbewar M, Rallis G, Harfouche R, Hussain SN, 2003. Regulation of nitric oxide production in limb and ventilatory muscle during chronic exercise training. Am. J. Physiol. Lung Cell Mol. Physiol 284, L452–L457. [DOI] [PubMed] [Google Scholar]
- Waring WS, Convery A, Mishra V, Shenkin A, Webb DJ, Maxwell SR, 2003. Uric acid reduces exercise-induced oxidative stress in healthy adults. Clin. Sci. (Lond) 105, 425–430. [DOI] [PubMed] [Google Scholar]
- Williams MH, Kreider RB, Hunter DW, Somma CT, Shall LM, Woodhouse ML, Rokitski L, 1990. Effect of inosine supplementation on 3-mile treadmill run performance and VO2 peak. Med. Sci. Sports Exerc 22, 517–522. [PubMed] [Google Scholar]
- Wu XW, Lee CC, Muzny DM, Caskey CT, 1989. Urate oxidase: primary structure and evolutionary implications. Proc. Natl. Acad. Sci. U. S. A 86, 9412–9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C Jr., Jones P, Bradley A, Caskey CT, 1994. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. U. S. A 91, 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP, 1998. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J. Neurosci. Res 53, 613–625. [DOI] [PubMed] [Google Scholar]
- Zoppini G, Targher G, Bonora E, 2011. The role of serum uric acid in cardiovascular disease in type 2 diabetic and non-diabetic subjects: a narrative review. J. Endocrinol. Invest 34, 881–886. [DOI] [PubMed] [Google Scholar]