Abstract
Introduction
High cut-off dialysis, increasingly used in multiple myeloma patients, is susceptible to influence anticancer drug elimination. We report about lenalidomide disposition in a patient on high cut-off dialysis for renal failure secondary to myeloma cast nephropathy.
Methods
The patient received a higher dosage of lenalidomide (5 mg b.i.d.), owing to concerns about a potential decrease in lenalidomide exposure during dialysis sessions. A set of blood samples was taken in order to develop a pharmacokinetic model accounting for lenalidomide concentrations in this setting.
Results
According to our model, the area under the curve was 3273 µg h/L, i.e., 60% higher than expected under usual dosage (25 mg q.d.) with normal renal function. Despite this, the patient did not develop major hematological toxicity.
Conclusions
Lenalidomide doses of 5 mg b.i.d. led to high exposure in a patient with renal failure undergoing high cut-off dialysis. Yet, the dosage of 5 mg q.d. recommended in conventional dialysis would probably be adequate in such patients.
Keywords: High-flux dialysis, High cut-off membrane, Lenalidomide, Pharmacokinetics
Introduction
Lenalidomide (molecular weight 259 Da) is an orally administered immunomodulatory drug with demonstrated clinical efficacy in patients with multiple myeloma. It is approximately 40% protein bound in plasma and mostly eliminated by filtration and active tubular secretion through the kidney with a small metabolized fraction. With impaired kidney function, lenalidomide elimination is delayed and dosage adaptation is required [1]. Lenalidomide disposition was studied during classical dialysis sessions, and elimination was found to be significantly increased [2]. However, lenalidomide has never been investigated during high cut-off (HCO) dialysis, nowadays increasingly used in the management of renal failure caused by multiple myeloma. Dialyzers used for HCO dialysis having a higher permeability for substances in the molecular weight range of 15–45 kDa are implemented using longer (8 h), more frequent (daily) dialysis sessions and requires albumin substitution. Higher drug elimination may therefore occur compared to classical intermittent hemodialysis. We report here the pharmacokinetic profile of lenalidomide during HCO dialysis in a patient with multiple myeloma and advanced renal failure.
Methods
Case presentation
A 69-year-old woman (54.7 kg) known for progressive IgG kappa light-chain, stage III multiple myeloma (diagnosed 7 years ago) was hospitalized for acute renal failure requiring dialysis (GFR < 5 mL/min). Renal failure was caused by cast nephropathy (light chains deposition), confirmed by biopsy. HCO dialysis (Theralite™, Gambro Lundia AB, Lund, Sweden) was started on admission, 5 days a week, 7 h per session with an albumin substitution of 7 g/h and an average blood and dialysate flow of 300–350 and 500 mL/min, respectively. Lenalidomide (Revlimid®) first cycle was started at the same time, in replacement of bortezomib, given over one year until then. We were concerned about a potential decrease in lenalidomide exposure during HCO dialysis and therefore increased lenalidomide dosage to 5 mg b.i.d. with one dose at the beginning and another one at the end of dialysis sessions (instead of 5 mg q.d. usually recommended in terminal renal failure [1]).
The patient showed a good response to combined antitumor and purification treatment, with serum kappa free light chains decreasing from 7620 to 795 mg/L (sustained reduction, 89%) after the first cycle of lenalidomide. Major toxicity was not reported, but mild and reversible thrombocytopenia occurred after 14 days of treatment. Renal function unfortunately did not recover, and the patient was eventually switched to intermittent hemodialysis 2 months later.
Samples collection
We collected a set of blood samples to determine lenalidomide plasma levels during dialysis. Blood samples were taken 3 h post-dose, at the beginning, 3 h after the beginning and at the end of three dialysis sessions. Post-filter blood samples and dialysate samples were taken 3 h after the beginning of the dialysis on two occasions. Samples were taken from day 1–7 of lenalidomide first cycle. Written patient’s consent was obtained. Plasma and dialysate concentrations were determined by validated ultra high-performance liquid chromatography with tandem mass spectrometric detection (uHPLC-MS/MS) [3].
Pharmacokinetics parameters
A one-compartment pharmacokinetic (PK) model was developed to predict plasma concentrations of lenalidomide during HCO dialysis, using published lenalidomide PK parameters, the patient’s renal status on admission and the dialysis settings [2, 4, 5]. A PK model was built up initially without taking into account our observations and then compared to the observed plasma concentrations, to check whether they were consistent with the model. Detailed information about model development is available as Online Resource 1.
The model was further refined in order to improve the model fit and to obtain a likely description of lenalidomide concentrations between and during HCO dialysis sessions (Fig. 1). The model was implemented and optimized using Microsoft Excel 2007 (Microsoft Corp., Redmond, WA, USA) with the Solver™ add-on.
Fig. 1.
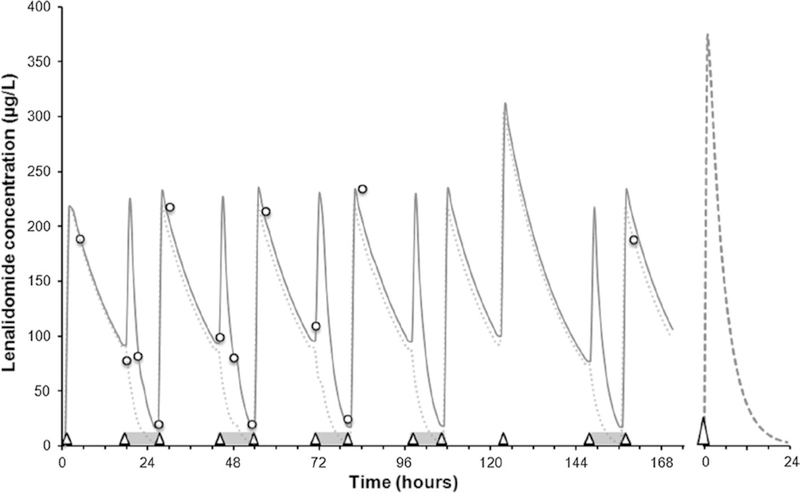
Predicted (continuous line) and measured (open circles) lenalidomide concentrations over time. High cut-off dialysis sessions are indicated by gray horizontal bars, lenalidomide doses (5 mg) by open triangles. Concentrations predicted with a dosage of 5 mg q.d. (dotted line) and at steady state with a dosage of 25 mg q.d. and normal renal function (dashed line, right panel) are represented as well
Results
The estimated AUC24h amounted to 3273 µg h/L for a dosage of 5 mg b.i.d. (Table 1). Lenalidomide extraction coefficient (E) was 53% during the first HCO dialysis and 23% during the second one (Table 1). These values are consistent with both our initial estimation based on drug’s fraction unbound (fu) and blood-to-plasma concentration ratio (rbp) of fu • rbp (43%) and data already published during intermittent hemodialysis [2]. Differences in the measured value of E are likely to derive from both variability in dialysis performance and cumulated imprecision on pre- and post-filter determinations. Patient’s lenalidomide PK parameters during HCO dialysis are detailed in Table 1.
Table 1.
Pharmacokinetic parameters of lenalidomide in a patient with HCO dialysis
| Parameters | Our patient | Reference values at steady state in a patient with normal renal function |
|---|---|---|
| Dose (mg) | 5 b.i.d.a | 25 q.d. |
| Pharmacokinetic parameters | ||
| AUC24h (µg h/L) | 3273 | 2057 |
| CLTot/F (mL/min) | 24 | 196 |
| Vd/ F(L) | 20.7 | 54 |
| t1/2 off-dialysis (h) | 10 | 3 |
| Hemodialysis elimination | ||
| E (%) | 23–53 | – |
| CLDial predicted (mL/min) | 117−128 | – |
| CLDial observed (mL/min) | 69−180 | – |
| t1/2 on-dialysis (h) | 2 | – |
At the beginning and the end of HCO dialysis. Hemodialysis clearance: CLDial predicted:Qblood · fu · rbp; CLDial observed: [(Ca–Cv) · Qblood]/Ca; Ca: concentration entering the dialyzer (µg/L); Cv: concentration leaving the dialyzer (µg/L); Qblood: blood flow (mL/min); E: extraction coefficient (%): CLDial/Qblood; t1/2 half-life: off-dialysis: ln(2) · Vd/F/CLTot, Patient; t1/2: on-dialysis: ln(2) · Vd/F/(CLTot, Patient +CLDial)
Discussion
Little is still known about drug disposition during HCO dialysis. Chen et al. [2] demonstrated that a 4-h conventional dialysis eliminates 31% of lenalidomide. HCO dialysis sessions of 8 h’ duration, 5 days a week, with a blood flow of 300–350 mL/min and high filter permeability may result in a higher lenalidomide clearance compared to classical intermittent dialysis. Furthermore, HCO dialysis leads to significant albumin loss (5–10 g/h), which might alter free drug fraction and therefore drug clearance [6]. Altered free drug fraction might, however, be somewhat mitigated by albumin substitution during HCO dialysis [7].
According to our initial evaluation, we would have expected a significant decrease in lenalidomide concentrations during the second half of the HCO dialysis. Nevertheless, measured lenalidomide concentrations showed that lenalidomide total clearance was still rather low. Dialytic clearance during HCO dialysis was similar to published data during conventional dialysis [2]. Our estimation of the AUC24h (3273 µg h/L) obtained with 5 mg b.i.d. (once before and once after dialysis) was about 60% higher than the value expected in normal conditions (2057 µg h/L for a dosage of 25 mg q.d. at steady state). Actually, we estimated that about 85% of the dose administered before dialysis was eliminated in the effluent during the session. Still, an increase in AUC24h (2476 µg h/L, +20%) was predicted for a single daily dosage of 5 mg given after dialysis in the same conditions. Predicted lenalidomide concentrations under 5 mg q.d. were similarly close to zero at the end of the dialysis, as predicted in normal conditions (Fig. 1) and supported actual guidelines for multiple myeloma which recommend 5 mg q.d. after dialysis for patients with terminal renal failure [1, 8].
With this rational PK approach, we were able to conclude a posteriori that increasing the dosage of lenalidomide before HCO dialysis was not necessary for this patient and could potentially have led to increased toxicities. However, it was well tolerated and produced a good hematological response in this patient. The immunomodulatory effect of lenalidomide was recently linked to the degradation of transcriptional suppressors, which repress interleukin-2 expression and T cell activation [9]. Time-dependent degradation of these transcriptional factors was observed after both lenalidomide and pomalidomide treatment. These data suggest that response is probably related to exposure and hence to AUC. Even though therapeutic intervals are not defined yet, lenalidomide might be a forthcoming candidate for therapeutic drug monitoring.
In conclusion, we report here about disposition of lenalidomide during HCO dialysis in a patient with advanced multiple myeloma and terminal renal failure. Our model suggests that lenalidomide exposure under a dosage of 5 mg b.i.d. before and at the end of HCO dialysis led to higher lenalidomide exposure compared to normal conditions, without toxicity in this patient. According to our model, an increased dosage does not seem necessary for HCO dialysis compared to classical intermittent dialysis. A dosage of 5 mg q.d. at the end of HCO dialysis sessions is predicted to ensure an exposure comparable to normal conditions. Lenalidomide dosage during HCO dialysis remains yet to be prospectively validated in further studies.
Supplementary Material
Footnotes
Compliance with ethical standards
Conflict of interest All the authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent Written and informed consent was obtained from the patient included in the study.
References
- 1.Celgene Corporation (2016) Product information REVLIMID® (lenalidomide) capsules, http://www.accessdata.fda.gov/drugsat-fda_docs/label/2013/021880s0341bl.pdf. Accessed 28 Oct 2016
- 2.Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, Laskin OL (2007) Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol 47(12): 1466–1475. doi: 10.1177/0091270007309563 [DOI] [PubMed] [Google Scholar]
- 3.Tohnya TM, Hwang K, Lepper ER, Fine HA, Dahut WL, Venitz J, Sparreboom A, Figg WD (2004) Determination of CC-5013, an analogue of thalidomide, in human plasma by liquid chromatography-mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 811(2): 135–141. doi: 10.1016/j.jchromb.2004.08.022 [DOI] [PubMed] [Google Scholar]
- 4.Hou J, Du X, Jin J, Cai Z, Chen F, Zhou DB, Yu L, Ke X, Li X, Wu D, Meng F, Ai H, Zhang J, Wortman-Vayn H, Chen N, Mei J, Wang J (2013) A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol 6:41. doi: 10.1186/1756-8722-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Wen L, Fau H, Surapaneni S, Kumar G (2012) Pharmacokinetics, metabolism and excretion of [(14)C]-lenalidomide following oral administration in healthy male subjects. Cancer Chemother Pharmacol 69(3):789–797. doi: 10.1007/s00280-011-1760-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchison CA, Heyne N, Airia P, Schindler R, Zickler D, Cook M, Cockwell P, Grima D (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant 27(10):3823–3828. doi: 10.1093/ndt/gfr773 [DOI] [PubMed] [Google Scholar]
- 7.Krieter DH, Devine E, Wanner C, Storr M, Krause B, Femke HD (2014) Clearance of drugs for multiple myeloma therapy during in vitro high-cutoff hemodialysis. Artif Organs 38(10):888–893. doi: 10.1111/aor.12248 [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulos M, Alegre A, Stadtmauer EA, Goldschmidt H, Zonder JA, de Castro CM, Masliak Z, Reece D, Olesnyckyj M, Yu Z, Weber DM (2010) The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer 116(16):3807–3814. doi: 10.1002/cncr.25139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, Klippel A, Handa H, Daniel TO, Schafer PH, Chopra R (2014) Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4 (CRBN). Br J Haematol 164(6):811–821. doi: 10.1111/bjh.12708 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


