Significance
The liver plays crucial roles in the control of glucose homeostasis during the fast-feeding cycle. It responds to insulin and glucagon to either store or provide glucose to other organs. Insulin resistance is a hallmark of metabolic diseases such as type 2 diabetes and nonalcoholic fatty liver disease. Associations have been made between low hepatic levels of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1A) and hepatic insulin resistance, but underlying mechanisms are elusive. We show that PGC1A controls the hepatic ratio of Insulin Receptor Substrates 1 (IRS1) and IRS2 expression, crucial for defining the precise insulin signal in hepatocytes to respond to fasting and feeding. Importantly, PGC1A is key for insulin-mediated suppression of hepatic glucose production.
Keywords: liver, PGC-1, Ppargc1a, fasting, gluconeogenesis
Abstract
Precise modulation of hepatic glucose metabolism is crucial during the fasting and feeding cycle and is controlled by the actions of circulating insulin and glucagon. The insulin-signaling pathway requires insulin receptor substrate 1 (IRS1) and IRS2, which are found to be dysregulated in diabetes and obesity. The peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1A) is a fasting-induced transcriptional coactivator. In nonalcoholic fatty liver disease and in patients with type 2 diabetes, low hepatic PGC1A levels are associated with insulin resistance. However, how PGC1A activity impacts the hepatic insulin-signaling pathway is still unclear. We used gain- and loss-of-function models in mouse primary hepatocytes and measured hepatocyte insulin response by gene and protein expression and ex vivo glucose production. We found that the PGC1A level determines the relative ratio of IRS1 and IRS2 in hepatocytes, impacting insulin receptor signaling via protein kinase B/AKT (AKT). PGC1A drove the expression of IRS2 downstream of glucagon signaling while simultaneously reducing IRS1 expression. We illustrate that glucagon- or PGC1A-induced IRS2 expression was dependent on cAMP Response Element Binding Protein activity and that this was essential for suppression of hepatocyte gluconeogenesis in response to insulin in vitro. We also show that increased hepatic PGC1A improves glucose homeostasis in vivo, revealing a counterregulatory role for PGC1A in repressing uncontrolled glucose production in response to insulin signaling. These data highlight a mechanism by which PGC1A plays dual roles in the control of gluconeogenesis during the fasting-to-fed transition through regulated balance between IRS1 and IRS2 expression.
The liver plays a critical role in glucose homeostasis. It uptakes glucose, synthesizes glycogen and triglycerides following food intake, releases glucose produced by glycogenolysis or gluconeogenesis, and triggers ketogenesis during fasting. These processes are controlled by the coordinated actions of the pancreatic hormones glucagon and insulin, whose ratio in circulation determines whether the liver initiates catabolic or anabolic processes to adapt to the nutritional status. High glucagon during fasting promotes glucose release from liver to provide a continuous source of fuel for peripheral tissues (1). Conversely, following a meal, higher insulin levels stimulate hepatic glucose utilization and inhibit glucose release. The actions of these hormones are mediated by a complex series of molecular signaling events downstream of their cell-surface receptors. In healthy liver tissue, insulin binding to the insulin receptor leads to recruitment of adapter insulin receptor substrate (IRS) proteins. Phosphoinositide 3-kinase (PI3K) is then recruited and activated by the IRSs, producing phosphatidylinositol-3,4,5-trisphosphate (PIP3) from phosphatidylinositol-4,5-bisphosphate that activates the Ser/Thr kinases phosphoinositide-dependent protein kinase-1 (PDK1) and protein kinase B (also known as AKT). Activated AKT potentiates glycogenesis, stimulates fatty acid synthesis, and inhibits gluconeogenesis (2). Tight control over expression and activation of these proteins is necessary to ensure precise and rapid adaptation to feeding cycles. In metabolic disorders such as type 2 diabetes and nonalcoholic fatty liver disease (NAFLD), the liver becomes resistant to the actions of insulin, even in a hyperinsulinemic context (3). Insulin resistance is a culmination of several events and factors that hinder this signaling cascade, many of which still remain elusive.
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1A) is a transcriptional coactivator within a family that includes two other members, peroxisome proliferator-activated receptor gamma coactivator 1-beta (PGC1B) and peroxisome proliferator-activated receptor gamma coactivator-related protein 1 (PPRC1), which bind transcription factors to enhance expression of multiple genes involved in nutrient metabolism (4). PGC1A regulates the hepatic response to fasting by promoting gluconeogenesis (5), lipid catabolism (6), and detoxification of reactive oxygen species produced by mitochondria (7). Reductions in PGC1A expression are associated with hepatic insulin resistance in diabetes and NAFLD in humans (8–10). Mouse models suggest that physiological reductions of PGC1A disrupt insulin signaling in liver (6, 7), and mice with low hepatic PGC1A are more susceptible to hepatic steatosis and hypertriglyceridemia, hallmarks of insulin resistance (6, 11, 12).
Until now, it was unclear whether alterations in hepatic insulin sensitivity linked to PGC1A activity are due to effects on mitochondrial and/or fatty acid metabolism augmenting lipid accumulation and oxidative damage (6, 7, 11–14) or direct regulation of genes that control the insulin-signaling pathway (15). Using gain- and loss-of-function models in primary mouse hepatocytes, we show that PGC1A regulates expression of many upstream components of the insulin-signaling pathway in hepatocytes, most notably, levels of the IRSs. We show that PGC1A influences the balance between IRS1 and IRS2 expression in liver cells, and as a result, the coactivator plays a key role in insulin-mediated control of gluconeogenesis during the fasting-to-fed transition.
Results
PGC1A Levels Influence Insulin-Stimulated AKT Phosphorylation.
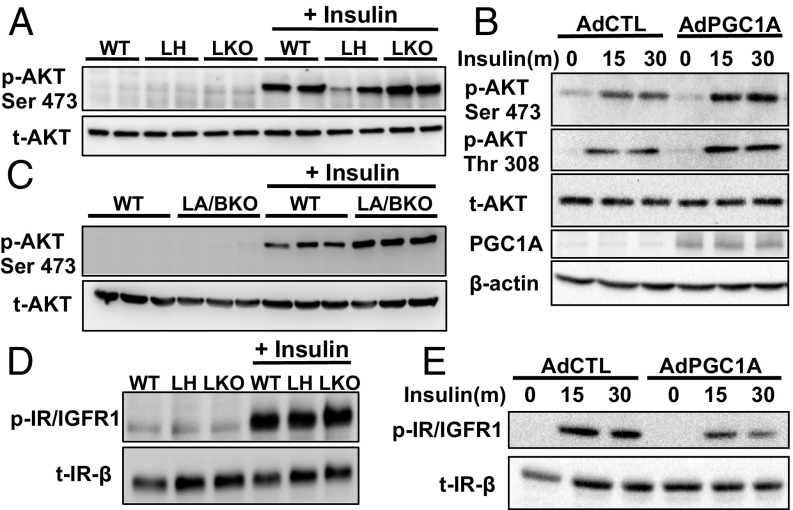
Conflicting data in various loss-of-function models has complicated understanding of whether PGC1A inhibits or activates insulin signaling in the liver (6, 7, 15). To address this question, we assessed multiple aspects of the insulin-signaling pathway using gain- and loss-of-function studies in primary mouse hepatocytes. Paradoxically, both complete knockout and overexpression of PGC1A increased phosphorylation of AKTS473 in response to insulin (Fig. 1 A and B). Increases in AKT phosphorylation were not due to compensatory increases in PGC1B (7), as insulin-induced AKT phosphorylation was similarly increased in hepatocytes of mice lacking both coactivators (LA/BKO double knockouts) (Fig. 1C and SI Appendix, Fig. S1A). Differences in AKT phosphorylation were also not due to enhanced signaling of the insulin receptor, as insulin receptor protein levels and ligand-induced phosphorylation (Y1146) were not affected by loss of PGC1A (Fig. 1D). Overexpression of PGC1A did lead to decreased phosphorylation of the insulin receptor in response to insulin, despite enhanced levels of p-AktS473 and p-AKTT308 (Fig. 1 B and E). Thus, while these data confirmed PGC1A as an important modulator of hepatic insulin signaling, the results were not easily explained by previously described links between PGC1A and insulin action (7, 15).
Fig. 1.
PGC1A levels influence insulin-stimulated AKT phosphorylation. Immunoblots of protein from primary mouse hepatocytes treated with 100 nM insulin for 15 min (or indicated time). Insulin-signaling pathway activation in hepatocytes from (A and D) male WT and LH (liver-PGC1A heterozygote) and LKO (liver-PGC1A knockout) mice. (B and E) Hepatocytes infected with adenoviruses expressing vector control (AdCTL) or PGC1A or (C) liver-specific PGC1A and PGC1B double-knockout mice (LA/BKO). All experiments were performed at least two times, and each lane represents an individual mouse.
PGC1A Levels Affect Gene Expression of Key Players of Insulin Signaling.
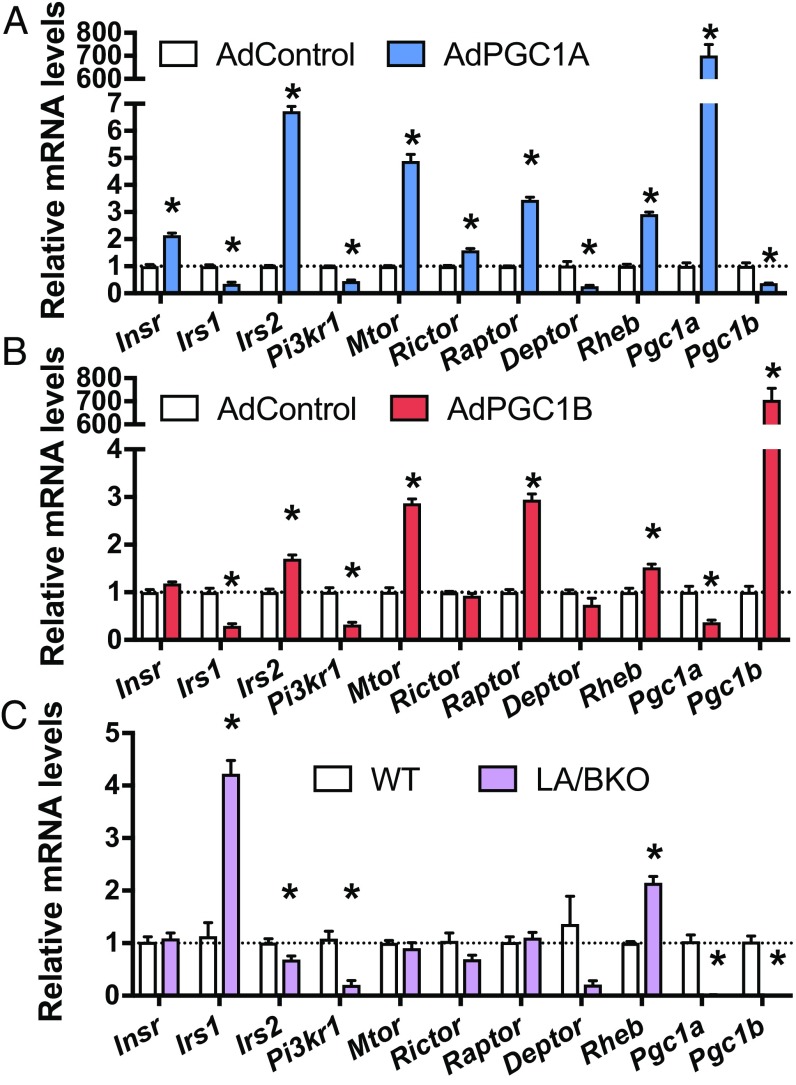
Since various proteins can directly or indirectly regulate phosphorylation of AKT in response to insulin (2), we hypothesized that, as a transcriptional coactivator, PGC1A might differentially control expression of one or many of these intermediates. Overexpression of PGC1A in primary hepatocytes significantly increased expression of Insr, Irs2, Mtor, Rictor, Raptor, and Rheb, while decreasing Irs1, Pi3kr1, and Deptor (Fig. 2A). As expected, altered PGC1A/B changed mRNA levels of genes controlling mitochondrial processes (SI Appendix, Fig. S1 B and C). Overexpression of PGC1B had similar effects on the insulin-signaling pathway, except levels of Insr, Rictor, and Deptor were unchanged (Fig. 2B). The reciprocal pattern of Irs expression was observed in PGC1A/B double KO hepatocytes, with significantly increased Irs1 and decreased Irs2 (Fig. 2C), while Pi3kr1 and Rheb were similarly decreased by either loss or gain of PGC1 expression.
Fig. 2.
PGC1A levels affect gene expression of key members of the insulin-signaling pathway. Gene expression by qPCR in primary mouse hepatocytes. WT cells infected with adenovirus overexpressing (A) PGC1A, (B) PGC1B, or (C) primary hepatocytes isolated from LA/BKO mice. Experiments were performed at least twice with n = 4 ± SEM *P < 0.05 vs. control.
To determine whether PGC1A and PGC1B are redundant regulators of this pathway and to tease out direct effects of the coactivator versus adaptive changes following chronic loss, we knocked down each family member acutely using shRNA. Knockdown of PGC1A led to significantly decreased gene expression for Irs2, Rictor, Raptor, and Rheb and increased Irs1, Pi3kr1, and Deptor mRNA (SI Appendix, Fig. S1D), a pattern opposite to that observed following PGC1A overexpression. Acute knockdown of PGC1B had no significant effect on these genes (SI Appendix, Fig. S1E), suggesting that PGC1A may have a more essential role in regulating these pathways at the gene level or that any effects of PGC1B are indirect. Despite some increase in insulin receptor mRNA with high PGC1A (Fig. 2A), total protein levels of the receptor were unchanged in all conditions (Fig. 1 D and E).
PGC1A Influences the Relative Ratio of IRS Protein in Mouse Hepatocytes.
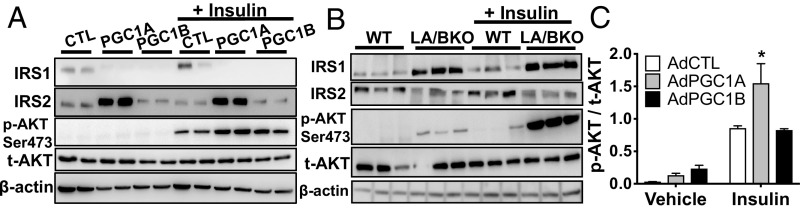
Of targets measured, concordant patterns of Irs1, Irs2, Rictor, Raptor, Deptor, and Rheb expression following gain and loss of PGC1A suggested that these genes might be proximal targets of the coactivator. Overexpression of PGC1A in primary hepatocytes increased IRS2 and decreased IRS1 proteins, with or without insulin stimulation (Fig. 3A). Conversely, IRS1 protein levels were significantly higher and IRS2 protein levels lower in LA/BKO hepatocytes compared with WT (Fig. 3B), consistent with gene expression in loss-of-function models (Fig. 2C and SI Appendix, Fig. S1 D and E). In contrast, high PGC1A increased DEPTOR and decreased RHEB proteins, and PGC1A knockdown had opposite effects (SI Appendix, Fig. S2), results inconsistent with gene expression data (Fig. 2 A and D). These data led us to conclude that PGC-1–induced changes in mRNA noted for mTOR pathway members (Fig. 2) were likely due to indirect feedback on this arm of the pathway, as seen previously (16), and not due to direct coactivation by the PGC-1s. PGC1B, while having the same ability as PGC1A to decrease IRS1 protein, did not increase IRS2 (Fig. 3A), and only PGC1A overexpression increased phosphorylation of AKT in response to insulin (Fig. 3 A and C). Thus, due to strong correlation of IRS and PGC-1A expression, we proposed that IRS proteins are direct effectors of PGC1A in liver cells and that coactivator activity promotes a shift in the relative balance of IRS1 to IRS2 to influence downstream insulin action. Thus, when PGC1A is high (e.g., during a fast), IRS2 increases and IRS1 decreases, and vice versa when PGC1A is low (e.g., during feeding). Importantly, inappropriately high levels of either IRS1 or IRS2 increase p-AKTS473 in response to insulin (17) (SI Appendix, Fig. S3), potentially explaining why overactivation of AKT was observed following both overexpression (Fig. 1B) and knockout (Figs. 1 A and C and 3B) of PGC1A.
Fig. 3.
PGC1A regulates IRS protein expression. Immunoblots of protein from primary hepatocytes treated 15 min with 100 nM insulin. (A) Cells overexpressing vector control (CTL), PGC1A, or PGC1B. (B) Cells from LA/BKO mice. (C) Ratio of p-AKT to t-AKT in cells overexpressing PGC1A or PGC1B (n = 4 ± SEM, performed at least two times).
PGC1A Potentiates Glucagon-Stimulated IRS2 Expression Through cAMP Response Element Binding Protein.
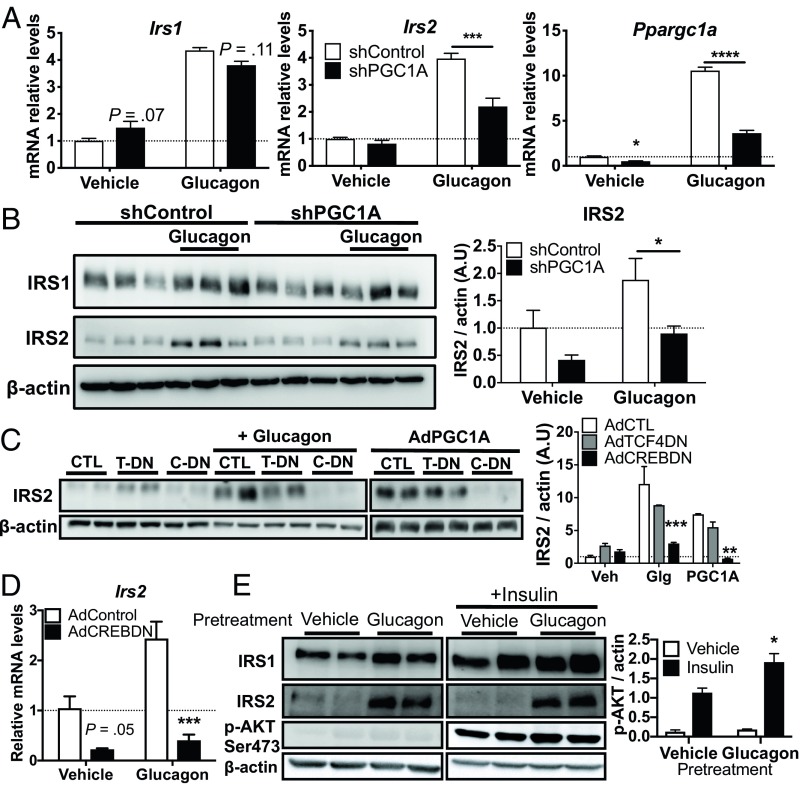
The IRS1:IRS2 ratio is known to shift in response to fasting and feeding cycles in mice, correlating closely with changing hormone levels (17). As fasting and glucagon signaling increase both hepatic PGC1A and IRS2 (18, 19), we hypothesized that PGC1A may serve as an essential mediator of glucagon’s role in modulating the insulin response. Consistent with its role as an important coactivator of gluconeogenic gene expression, PGC1A overexpression increased glucose production from primary hepatocytes in the absence of insulin (SI Appendix, Fig. S4A). Moreover, PGC1A knockdown decreased basal and glucagon-induced G6pc and Pepck mRNA, as previously described (5), as well as Hsl, another PGC1A target (20) not regulated by glucagon (SI Appendix, Fig. S4B). Following treatment of hepatocytes with glucagon, we observed significantly higher mRNA expression of Ppargc1a, Irs1, and Irs2 and protein expression of IRS2 (Fig. 4 A and B). Importantly, the ability of glucagon to induce IRS2 expression was dependent on PGC1A, as knockdown of PGC1A using shRNA blunted hormone-induced Irs2 mRNA and protein (Fig. 4 A and B). IRS1 gene and protein levels were also induced by glucagon, but this was not dependent on PGC1A (Fig. 4A). Contrary to the belief that glucagon and PGC1A actions are synonymous, our data suggest that repression of IRS1 by PGC1A counteracts this action of glucagon, further increasing the IRS2:IRS1 ratio when both PGC1A and glucagon are high. Thus, PGC1A was an essential mediator of glucagon’s role in controlling the ratio of IRS1 to IRS2 expression.
Fig. 4.
PGC1A potentiates glucagon-stimulated IRS2 expression through CREB. Primary mouse hepatocytes treated with 40 nM glucagon or vehicle. (A) Gene expression (n = 4 ± SE) and (B) immunoblots from cells expressing shControl or shPGC1A. (B, Right) Quantification of two experiments (n = 5 ± SEM). (C) Immunoblot from hepatocytes coexpressing vector control (CTL) or dominant-negative CREB (C-DN or CREBDN), dominant-negative TCF4 (T-DN or TCF4DN), or PGC1A, as indicated. (Right) Quantification of two experiments (n = 5 ± SEM). (D) mRNA in primary hepatocytes expressing CREB DN or Control vector. (E) Immunoblots from primary hepatocytes pretreated or not with glucagon (40 nM, 4 h) and treated with insulin (100 nM, 15 min). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs. control. Blots are representative of two independent experiments. A.U., arbitrary units; Glg, glucagon; veh, vehicle.
We next sought to determine how the glucagon/PGC1A axis regulates Irs gene expression. Irs expression is regulated by numerous transcription factors that are also targets of the PGC1s. cAMP Response Element Binding Protein (CREB) and its coactivator CREB-Regulated Transcription Coactivator 2 (CRTC2) increase Irs2 expression (18) and CREB is an important cofactor of PGC1A during fasting (21). T-Cell Factor 4 (TCF4/TCF7L2) regulates Irs1 expression (22, 23), binds to the Irs2 promoter (24), and is a major player in hepatic metabolic zonation, which also impacts IRS expression (22). Yin Yang 1 (YY1) and Hypoxia Inducible Factor 2A (HIF2A/Epas1) bind PGC1A (25, 26) and can also regulate IRS gene transcription (27, 28). Since expression levels of both PGC1A and its target transcription factors often correlate with target gene expression, we assessed how mRNA of Irs1/2 changed with each candidate transcription factor. Relative mRNA levels of each candidate plotted versus Irs1/2 showed that Epas1, Tcf4, and Creb1 expression positively correlated with Irs2 (R2 = 0.61, P < 0.001; R2 = 0.58, P < 0.001; R2 = 0.26, P = 0.01, respectively). Irs1 levels did not significantly correlate with the expression of any of these transcription factors (SI Appendix, Fig. S5). Although we noted a positive correlation between Epas1 and Irs2, Epas1 (HIF2A) levels in liver increase postprandially to repress glucagon action (29), a time frame when PGC1A and IRS2 are at their lowest (17, 30). Thus, we focused attention on TCF4 and CREB as potential partners of PGC1A in potentiating IRS2 expression.
To determine whether TCF4 or CREB were necessary for the glucagon/PGC1A/IRS2 axis, we assessed the ability of glucagon and PGC1A to induce IRS2 in the presence of dominant-negative TCF4 (31) or CREB (21). Glucagon- and PGC1A-induced IRS2 protein levels were unaffected by expression of the dominant-negative TCF4, but significantly decreased by inhibition of CREB activity (Fig. 4C), consistent with reductions in glucagon-induced Irs2 gene expression by dominant-negative CREB (Fig. 4D). Thus, control of IRS2 expression by glucagon and PGC1A was dependent on CREB activity. Before feeding, glucagon levels are high and insulin low. In this state, our data suggest that activation of the glucagon/PGC1A axis shifts the IRS1:IRS2 ratio, increasing the amount of IRS2 available to the insulin receptor and priming the liver for the postprandial insulin response. Accordingly, when primary hepatocytes were pretreated with glucagon, insulin-induced phosphorylation of AKT was increased (Fig. 4E).
Insulin-Induced Suppression of Hepatic Gluconeogenesis Is Regulated by the PGC1A/IRS2 Axis.
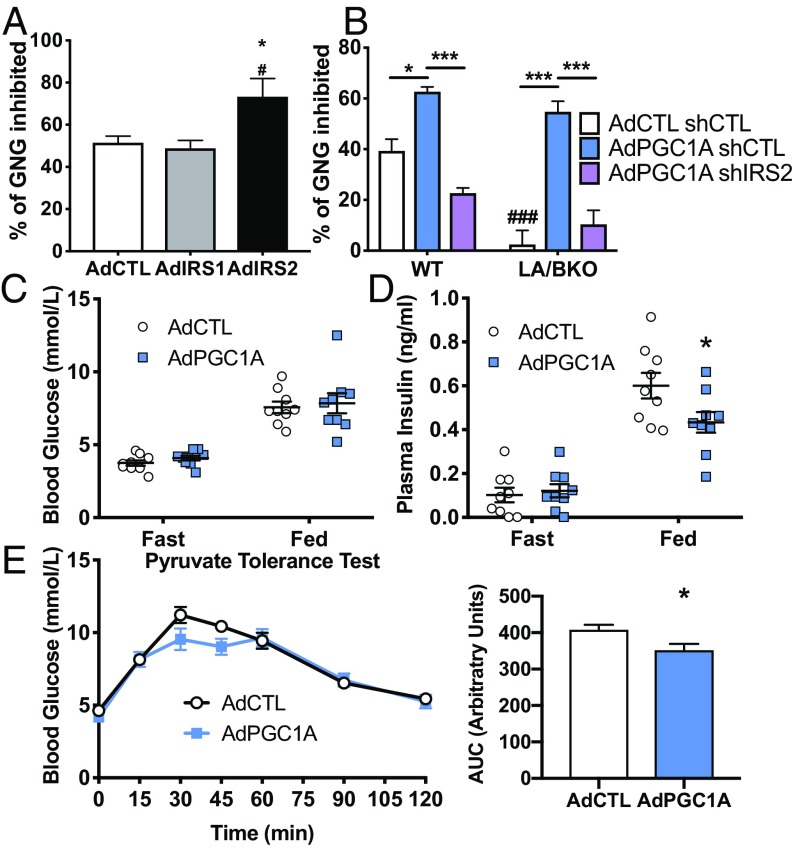
Increased signaling via IRS2 might facilitate inhibition of gluconeogenesis by insulin (17, 18), a rapid and important action of this anabolic hormone occurring shortly after its rise in circulation after feeding. In support of this, insulin treatment more efficiently suppressed glucagon-induced glucose release from primary hepatocytes following IRS2 overexpression (Fig. 5A). Consistent with this being a unique effector of IRS2, increased IRS1 expression did not impact glucose production in response to glucagon (Fig. 5A), despite activating AKT to equal levels (SI Appendix, Fig. S3). We hypothesized that high levels of PGC1A during fasting shifts the ratio of IRS1:IRS2 to favor IRS2 just before feeding, priming the insulin receptor response toward more efficient inhibition of gluconeogenesis in response to ligand binding. Consistent with this, overexpression of PGC1A in WT hepatocytes enhanced the ability of insulin to suppress gluconeogenesis in primary hepatocytes, and this inhibition was completely dependent on IRS2, as coexpression of an shRNA against IRS2 (SI Appendix, Fig. S5) reversed the effects of high PGC1A (Fig. 5B). To determine whether PGC1A played an essential role in the ability of insulin to suppress gluconeogenesis, we took advantage of our LA/BKO model, which has a conversely high ratio of IRS1:IRS2 (Figs. 2C and 3C). Consistent with PGC1A and the IRS1:IRS2 ratio being important for insulin-induced suppression of gluconeogenesis, insulin was not able to suppress glucagon-induced glucose production in primary hepatocytes from these mice (Fig. 5B). Reintroduction of PGC1A in LA/BKO cells rescued the ability of insulin to suppress gluconeogenesis, and this was dependent on IRS2. Together, these data show that PGC1A is both necessary and sufficient to enhance insulin-mediated suppression of glucogenogenesis in vitro and that this function of PGC1A is mediated via IRS2.
Fig. 5.
Insulin-induced suppression of hepatocyte gluconeogenesis is regulated by the PGC1A/IRS2 axis in vitro and in vivo. (A) Insulin-mediated (100 nM, 1 h) suppression of glucagon-induced (1 nM, 1 h) gluconeogenesis (GNG) in primary hepatocytes overexpressing vector, IRS1, or IRS2 (n = 8 ± SEM). *P < 0.05 vs. Control; #P < 0.05 IRS2 vs. IRS1. (B) Insulin suppression of GNG in WT and LA/BKO hepatocytes expressing control or PGC1A and shControl or shIRS2, as indicated (n = 4 ± SEM). ###P < 0.001 vs. WT; *P < 0.05, ***P < 0.001. Male mice tail-vein–injected with viruses encoding vector (AdCTL) or PGC1A (AdPGC1A). Blood glucose (C) and plasma insulin (D) measured after 16 h fasting or 1 h refeeding. (E) Pyruvate tolerance test and area under the curve (Right). n = 9 ± SEM; *P < 0.05 vs. control. All experiments were performed twice.
We next tested whether increased hepatic PGC1A impacts glucose homeostasis in vivo. Compared with mice expressing control adenovirus, mice overexpressing PGC1A in liver (SI Appendix, Fig. S7) had equal blood glucose levels following refeeding (Fig. 5C) within the setting of lower plasma insulin (Fig. 5D). This would suggest either more effective glucose disposal or more efficient suppression of gluconeogenesis. To assess glucose homeostasis within a setting of enhanced gluconeogenesis, we performed a pyruvate tolerance test. Mice overexpressing PGC1A had lower blood glucose levels compared with control mice following the peak of glycemia following pyruvate challenge (Fig. 5E). Taken together with lower postprandial insulin levels (Fig. 5D), these data suggest that, even when hepatic PGC1A is high and promoting gluconeogenesis (SI Appendix, Fig. S4A), circulating insulin can more effectively suppress hepatic glucose production to tightly control glucose output. Glucose disposal, more represented in later time points of the curve, was identical between the two groups. These data support the concept that PGC1A controls hepatic IRS2-dependent gluconeogenesis suppression.
Discussion
In this study, we demonstrated that PGC1A reciprocally regulates both IRS1 and IRS2 expression in hepatocytes and plays an important role in the hepatic response to insulin during the fasting-to-fed transition. We show that expression levels of PGC1A in liver directly impact the ratio of IRS1:IRS2, influencing signaling pathways downstream of insulin binding to its receptor. We also demonstrate that glucagon requires PGC1A to increase Irs2 expression and that this mechanism is dependent on the transcription factor CREB, uncovering a molecular mechanism linking fasting hormone signaling to transcriptional changes tightly controlling glucose production. Our data support previous reports illustrating a dynamic switch from IRS2 to IRS1 during the fasting-to-fed transition (17) and implicate PGC1A as an important modulator of glucagon’s role in this process. Concurrent with the well-known functions of PGC1A and CREB to induce gluconeogenesis during fasting, our data expand the role of PGC1A to also include sensitizing the liver to insulin. We propose this sensitization acts as a necessary brake to prevent uncontrolled production of glucose and prime the liver to shut down gluconeogenesis when insulin rapidly spikes after feeding. Our data illustrating the importance of the PGC1A/CREB/IRS2 axis downstream of glucagon are in line with a recent paper elegantly showing that glucagon signaling is required for the finely tuned actions of insulin in liver during fasting and feeding (32).
While increased hepatic PGC1A levels are largely believed to negatively impact blood glucose control due to induction of enzymes that drive gluconeogenesis (5, 33), we show here that increased PGC1A in fasting liver also primes the liver to respond to insulin, counteracting uncontrolled glucose production. By concurrently potentiating hepatic gluconeogenesis and sensitizing the liver to insulin, an elegant counterbalance exists to maintain blood glucose within tight parameters and ensure that increased PGC1A does not inappropriately elevate glucose. This may have implications when considering PGC1A as a target for the treatment of metabolic disease, in particular diabetes and fatty liver disease. Increased PGC1A is linked to improvements in adipose tissue function, glucose homeostasis, and liver fat catabolism (12–14). However, interest in developing therapeutics that raise PGC1A may be tempered by fears of increased PGC1A in liver promoting inappropriate glucose production. Our data show that PGC1A modulates multiple pathways in hepatocytes to control glucose production, suggesting that this potential detrimental outcome may be unwarranted.
Unexpectedly, altered PGC1A and PGC1B levels affected gene expression of numerous members of the mTOR pathway. As gene changes were not reflected at the protein level and we had identified a clear link between PGC1A coactivator activity, IRS expression, and AKT activation, we did not pursue whether modifications of mTOR gene expression had functional consequences on the pathway. Research shows that mTOR activity primarily affects the phosphorylation status of IRS1 and IRS2, not its expression level (34, 35). Interestingly, White and collaborators (34) show that, in a fed state (when PGC1A is low), IRS2 is degraded by a mTOR-dependent pathway. Therefore, changes in DEPTOR protein (an inhibitor of mTOR) driven by PGC1A may represent a convenient feedback mechanism to control IRS2 degradation during fasting. Further studies are warranted to unravel mechanistic connections between PGC1A and mTOR, which could also be complicated by the effects of PGC1A on metabolites sensed by AMPK and mTOR (36).
It is suggested that the IRS1:IRS2 ratio helps to specify or amplify specific pathways downstream of insulin signaling within different metabolic contexts. For example, immediately after fasting, higher IRS2 might favor insulin-mediated shutdown of gluconeogenesis over enhancing lipogenesis, a metabolic process more appropriately activated when glucose is high and energy storage is needed. It has also been suggested that increased IRS1 might favor the lipogenic arm of insulin signaling (22, 37); thus, low PGC1A, which we show leads to a shift toward higher IRS1, might potentiate the production of lipids in response to insulin. Livers of type 2 diabetic and NAFLD patients are shown to have decreased PGC1A (8–10), correlating with low IRS2 and high IRS1 expression (22, 38). An inability to transition from IRS1 to IRS2 due to low PGC1A might explain increased steatosis. In fact, mice with low hepatic PGC1A inappropriately accumulate lipids in their livers (6, 7, 11). Increased triglyceride in these models was attributed to decreased catabolism of lipids due to essential roles for PGC1A in fatty acid oxidation. However, our data suggest that the increased IRS1:IRS2 ratio when PGC1A is low may also aggravate lipid accumulation due to inappropriate lipogenesis, but this has yet to be tested.
Although we can speculate on the physiological impact of shifting the IRS1:IRS2 ratio, further studies are warranted to decipher the precise biological functions of these insulin receptor effectors in hepatocytes. A recent study in brown adipose tissue shows that overexpression of IRS1 or IRS2 has broad and distinctive signaling outcomes (39). Previous studies in IRS knockout mice (17, 18, 22, 40, 41) elegantly describe the requirement of the IRSs for whole-body energy metabolism during the fed-to-fasted transition, yet cell-autonomous effects on hepatic metabolic processes are still uncertain. Thus, while we show a dependence on PGC1A to regulate IRS1 and IRS2 expression in mouse liver cells, the precise consequences of this on hepatic glucose and lipid metabolism as a whole downstream of insulin are still mostly unknown.
Interestingly, we show that both PGC1A and PGC1B decrease IRS1 expression, while only PGC1A drives IRS2 expression. This highlights an important functional distinction between these structurally related coactivators, which share many downstream targets but seem to also have unique roles in liver surrounding glucose and lipid metabolism (42). Importantly, PGC1B is thought to play a more significant role in lipogenesis (43). Our data demonstrating that PGC1A and PGC1B have differential effects on key modulators of insulin receptor signaling may help explain differences and further an understanding of the impact of these coactivators on hepatic energy homeostasis. However, while we show that CREB is important for the ability of PGC1A to induce IRS2, the mechanism by which the PGC1s decrease Irs1 expression in hepatocytes has yet to be identified.
In summary, we show that PGC1A reciprocally regulates IRS protein expression and mediates the induction of IRS2 in response to glucagon, which is important for hepatic adaptation to fasting and the transition to the fed state. This sheds new light on the impact of PGC1A on the insulin-signaling pathway and potentially on pathologies stemming from dysregulation of IRS-dependent signal transduction.
Materials and Methods
Animals.
Conditional liver-specific Ppargc1a, Ppargc1b, and Ppargc1af/f:Ppargc1bf/f KO mice were generated and maintained as previously described (7) (SI Appendix, Supplemental Methods). All experiments were approved by and performed in accordance with the Institut de Recherches Cliniques de Montreal animal facility institutional animal care and use committee regulations.
Primary Hepatocyte Isolation and Culture.
Primary mouse hepatocytes from 10- to 12-wk-old male and female mice were isolated by collagenase perfusion/percoll gradient purification and infected with adenoviruses as previously described (6). For insulin or glucagon responses, cells were incubated overnight in high glucose (25 mM) media lacking dexamethasone and insulin. Insulin and glucagon were added at indicated concentrations and times.
In Vivo Metabolic Phenotyping.
Blood glucose and plasma insulin levels were measured after an overnight (16 h) fast or 1 h post refeeding. To test for pyruvate tolerance, sodium pyruvate (2 mg/kg) was injected intraperitoneally following an overnight fast, and blood glucose was measured at indicated times. For overexpression, male mice were tail-vein–injected with an adenovirus expressing vector (control) or PGC1A cDNA (2 × 109 infectious units) with recovery for 1 wk before testing.
Suppression of Gluconeogenesis and Glucose Assays.
Primary hepatocytes were switched to basic medium (DMEM with 0.2% BSA and 1 mM glutamine, with no glucose, red phenol or NaPyruvate) for 1 h to induce glycogenolysis and deplete glycogen (44). Basic media containing 10 mM lactate and 1 nM glucagon (to promote gluconeogenesis) and 100 nM insulin (to test insulin response) was exchanged and harvested every hour for 3 h. Glucose released was measured by enzymatic reaction (Hexokinase assay #GAHK20 Sigma-Millipore) and normalized to protein content per well.
Immunoblotting.
Proteins were isolated in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors (Roche). Samples were resolved by SDS/PAGE, blotted, and probed with antibodies listed in SI Appendix, Supplemental Methods.
Gene Expression.
TRIzol-isolated total RNA treated with DNase I was reverse-transcribed, and cDNA was quantified by real-time qPCR (Vii7) using SYBR Green. Data were normalized using the ΔΔCt method to hypoxanthine–guanine phosphoribosyltransferase (Hprt) mRNA and expressed relative to control. Primers sequences are presented in SI Appendix, Table S1.
Statistical Analyses.
Statistical analyses were performed using GraphPad Prism. One-way (univariate) or two-way (bivariate) ANOVA was followed by post hoc testing (Sidak) corrected for multiple comparisons.
Supplementary Material
Acknowledgments
Vectors were kindly provided by Drs. S. Guo (IRS1 and IRS2), L. Alonso (shIRS2), M. Montminy (CREB DN), B. Spiegelman (PGC-1B), and T. Jin (TCF4 DN). This work was supported by grants from the Canadian Institutes of Health Research (SVB-145591) and the Merck, Sharpe & Dohme Corporation (to J.L.E.). P.L.-D. was supported by a graduate scholarship from the Montreal Diabetes Research Centre; S.J. by the Jean-Coutu post-doc (Institut de Recherches Cliniques de Montreal); and J.L.E. by a Chercheur-boursier (Junior 2) from the Fonds de Recherche de Québec Santé.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815150116/-/DCSupplemental.
References
- 1.Moore MC, Coate KC, Winnick JJ, An Z, Cherrington AD. Regulation of hepatic glucose uptake and storage in vivo. Adv Nutr. 2012;3:286–294. doi: 10.3945/an.112.002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19:31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 6.Estall JL, et al. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-gamma coactivator-1alpha expression. Diabetes. 2009;58:1499–1508. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besse-Patin A, et al. Estrogen signals through peroxisome proliferator-activated receptor-γ coactivator 1α to reduce oxidative damage associated with diet-induced fatty liver disease. Gastroenterology. 2017;152:243–256. doi: 10.1053/j.gastro.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Ahrens M, et al. DNA methylation analysis in nonalcoholic fatty liver disease suggests distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 2013;18:296–302. doi: 10.1016/j.cmet.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Koliaki C, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab. 2015;21:739–746. doi: 10.1016/j.cmet.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Westerbacka J, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 11.Leone TC, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris EM, et al. PGC-1α overexpression results in increased hepatic fatty acid oxidation with reduced triacylglycerol accumulation and secretion. Am J Physiol Gastrointest Liver Physiol. 2012;303:G979–G992. doi: 10.1152/ajpgi.00169.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleiner S, et al. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sczelecki S, et al. Loss of Pgc-1α expression in aging mouse muscle potentiates glucose intolerance and systemic inflammation. Am J Physiol Endocrinol Metab. 2014;306:E157–E167. doi: 10.1152/ajpendo.00578.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 16.Javary J, et al. Liver Reptin/RUVBL2 controls glucose and lipid metabolism with opposite actions on mTORC1 and mTORC2 signalling. Gut. 2018;67:2192–2203. doi: 10.1136/gutjnl-2017-314208. [DOI] [PubMed] [Google Scholar]
- 17.Kubota N, et al. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8:49–64. doi: 10.1016/j.cmet.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Canettieri G, et al. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005;2:331–338. doi: 10.1016/j.cmet.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Louet JF, Hayhurst G, Gonzalez FJ, Girard J, Decaux JF. The coactivator PGC-1 is involved in the regulation of the liver carnitine palmitoyltransferase I gene expression by cAMP in combination with HNF4 alpha and cAMP-response element-binding protein (CREB) J Biol Chem. 2002;277:37991–38000. doi: 10.1074/jbc.M205087200. [DOI] [PubMed] [Google Scholar]
- 20.Oropeza D, et al. PGC-1 coactivators in β-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Mol Metab. 2015;4:811–822. doi: 10.1016/j.molmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 22.Kubota N, et al. Differential hepatic distribution of insulin receptor substrates causes selective insulin resistance in diabetes and obesity. Nat Commun. 2016;7:12977. doi: 10.1038/ncomms12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JC, et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–1518. doi: 10.1101/gad.1924910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norton L, et al. Chromatin occupancy of transcription factor 7-like 2 (TCF7L2) and its role in hepatic glucose metabolism. Diabetologia. 2011;54:3132–3142. doi: 10.1007/s00125-011-2289-z. [DOI] [PubMed] [Google Scholar]
- 25.Cunningham JT, et al. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 26.Rasbach KA, et al. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA. 2010;107:21866–21871. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blättler SM, et al. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 2012;15:505–517. doi: 10.1016/j.cmet.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei K, et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat Med. 2013;19:1331–1337. doi: 10.1038/nm.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramakrishnan SK, et al. HIF2α is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metab. 2016;23:505–516. doi: 10.1016/j.cmet.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornu M, et al. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc Natl Acad Sci USA. 2014;111:11592–11599. doi: 10.1073/pnas.1412047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip W, et al. Liver-specific expression of dominant-negative transcription factor 7-like 2 causes progressive impairment in glucose homeostasis. Diabetes. 2015;64:1923–1932. doi: 10.2337/db14-1329. [DOI] [PubMed] [Google Scholar]
- 32.Kim T, et al. Hepatic glucagon receptor signaling enhances insulin-stimulated glucose disposal in rodents. Diabetes. 2018;67:2157–2166. doi: 10.2337/db18-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharabi K, et al. Selective chemical inhibition of PGC-1α gluconeogenic activity ameliorates type 2 diabetes. Cell. 2017;169:148–160.e15. doi: 10.1016/j.cell.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rui L, Fisher TL, Thomas J, White MF. Regulation of insulin/insulin-like growth factor-1 signaling by proteasome-mediated degradation of insulin receptor substrate-2. J Biol Chem. 2001;276:40362–40367. doi: 10.1074/jbc.M105332200. [DOI] [PubMed] [Google Scholar]
- 35.Yoneyama Y, et al. Serine phosphorylation by mTORC1 promotes IRS-1 degradation through SCFβ-TRCP E3 ubiquitin ligase. iScience. 2018;5:1–18. doi: 10.1016/j.isci.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiol (Oxf) 2009;196:65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo S, et al. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29:5070–5083. doi: 10.1128/MCB.00138-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honma M, et al. Selective insulin resistance with differential expressions of IRS-1 and IRS-2 in human NAFLD livers. Int J Obes. 2018;42:1544–1555. doi: 10.1038/s41366-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabiee A, Krüger M, Ardenkjær-Larsen J, Kahn CR, Emanuelli B. Distinct signalling properties of insulin receptor substrate (IRS)-1 and IRS-2 in mediating insulin/IGF-1 action. Cell Signal. 2018;47:1–15. doi: 10.1016/j.cellsig.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong X, et al. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116:101–114. doi: 10.1172/JCI25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong XC, et al. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chambers KT, et al. PGC-1β and ChREBP partner to cooperatively regulate hepatic lipogenesis in a glucose concentration-dependent manner. Mol Metab. 2013;2:194–204. doi: 10.1016/j.molmet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chambers KT, et al. Liver-specific PGC-1beta deficiency leads to impaired mitochondrial function and lipogenic response to fasting-refeeding. PLoS One. 2012;7:e52645. doi: 10.1371/journal.pone.0052645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, et al. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS One. 2012;7:e37103. doi: 10.1371/journal.pone.0037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.