Abstract
The deposition of pathologic misfolded proteins in neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia and amyotrophic lateral sclerosis is hypothesized to burden protein homeostatic (proteostatic) machinery, potentially leading to insufficient capacity to maintain the proteome. This hypothesis has been supported by previous work in our laboratory, as evidenced by the perturbation of cytosolic protein solubility in response to amyloid plaques in a mouse model of Alzheimer’s amyloidosis. In the current study, we demonstrate changes in proteome solubility are a common pathology to mouse models of neurodegenerative disease. Pathological accumulations of misfolded tau, α-synuclein and mutant superoxide dismutase 1 in CNS tissues of transgenic mice was associated with changes in the solubility of hundreds of CNS proteins in each model. We observed that changes in proteome solubility were progressive and, using the rTg4510 model of inducible tau pathology, demonstrated that these changes were dependent upon sustained expression of the primary pathologic protein. In all of the models examined, changes in proteome solubility were robust, easily detected, and provided a sensitive indicator of proteostatic disruption. Interestingly, a subset of the proteins that display a shift towards insolubility were common between these different models, suggesting that a specific subset of the proteome is vulnerable to proteostatic disruption. Overall, our data suggest that neurodegenerative proteinopathies modeled in mice impose a burden on the proteostatic network that diminishes the ability of neural cells to prevent aberrant conformational changes that alter the solubility of hundreds of abundant cellular proteins.
Keywords: Proteostasis, Protein Misfolding, Neurodegeneration, Proteinopathy, Proteomics
Introduction
Neurodegenerative disorders, including Alzheimer’s disease (AD), Frontotemporal Dementia (FTD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) are characterized pathologically by the accumulation of misfolded proteins (often referred to as “proteinopathies”) [9, 21, 22, 41, 85, 86]. Considerable evidence supports the idea that the accumulation of misfolded proteins could be deleterious to the proteostasis network [5, 8, 15, 24, 44, 56, 62, 68, 73, 90, 95]. This network includes chaperones, the ubiquitin-proteasome system, and the autophagic system working in unison to maintain protein folding or degrade misfolded/aggregated protein species. Aggregated forms of misfolded tau are degraded in the autophagy-lysosomal system, while the accumulation of misfolded tau in CNS tissues of AD, and other tauopathies, is associated with lower 26S proteasome activity [39, 55, 69, 89]. Similar reductions in proteostatic activity have been reported in the presence of α-synuclein (αSyn) pathology [16, 26, 28, 104], and in tissues from mouse models that over-express ALS-associated variants of superoxide dismutase 1 (SOD1) [6, 10, 14, 50, 51, 79]. Accumulations of misfolded protein have also been associated with overburdened chaperone function, leading to impaired folding and maturation other proteins (reviewed in [5, 44]). This idea was originally elaborated in C. elegans where muscle cells expressing aggregating mutants of huntingtin, or SOD1, failed to correctly fold co-expressed temperature-sensitive metastable proteins [33, 34]. Accordingly, it has become increasingly clear that “mixed” proteinopathies are common in neurodegenerative disease [13, 42, 46, 87, 92]. For example, humans with tauopathy, as well as transgenic mice that model tauopathy, often have pathologic deposition of TDP-43 and αSyn [12, 42, 60, 63, 103]. Thus, there is evidence that disruptions in proteostatic function could degrade proteome integrity in neurodegenerative disease. To date, however, there is limited understanding of how changes in these proteostatic functions could impact overall proteome folding in the mammalian nervous system.
We have previously shown in a mouse model of Alzheimer’s amyloidosis that, as Aβ deposition increases, the CNS proteome begins to lose solubility and a fraction of cytosolic proteins become aberrantly detectable in detergent-insoluble fractions [102]. Given that Aβ is largely deposited extracellularly, it was difficult to conclude that our observed outcomes could be explained by misfolded Aβ peptides directly over-burdening cytosolic protein chaperone functions. To assess whether the cytoplasmic, intracellular, accumulation of misfolded proteins, such as tau, might impose a burden on protein folding, we have examined proteome solubility in transgenic mouse models of mutant tau, mutant SOD1, and mutant αSyn proteinopathies. Similar to prior effort in amyloidosis models, we used bottom-up label-free proteomics to identify CNS proteins that lose solubility in detergent-containing buffers as pathology develops. The study design was to identify CNS proteins in control nontransgenic animals that are normally consistently detectable in aqueous fractions and of low abundance, or absent, from the detergent-insoluble fractions. We then assessed whether these proteins aberrantly fractionate into insoluble fractions at a greater frequency in the CNS lysates of our neurodegenerative mouse models. To study the effects of tau pathology on proteome solubility, we utilized the rTg4510 mouse (human tau-P301L), an established model of conditional tauopathy [75, 77]. We also examined changes in proteome solubility in the JNPL3 model (human tau-P301L) of spinal tau pathology [58], the M83 model (human αSyn-A53T) of spinal αSyn pathology [31], and the G93A model (human SOD1-G93A) of spinal SOD1 pathology [37]. In all of these models, we observe >100 proteins that lose solubility in buffers containing SDS as pathology develops. Although some proteins were unique to each model, many were common to all four models, with some additional overlap with Aβ amyloidosis models. Overall, our data are consistent with the hypothesis that the accumulation of misfolded proteins associated with neurodegenerative disease imposes a burden on proteostatic function such that a subpopulation of CNS cytosolic proteins fail to maintain normal soluble conformations.
Materials and Methods
Transgenic animals
The JNPL3 mouse model of tauopathy (maintained on the Swiss Webster background from Taconic) expresses mutant human tau (P301L, 4R0N) under the mouse prion promoter and develops robust tau pathology in the spinal cord and brain stem with other regions being modestly affected [59]. All of the JNPL3 mice used in this study were female due to an earlier onset of tau pathology and motor dysfunction. The rTg4510 model of tau pathology (maintained on a hybrid 129S6/FVB background) consists of bigenic mice that contain both human tau (P301L, 4R0N) behind by a minimal promoter disrupted by tetracycline responsive elements, and the tet-transactivator (tTA) driven by a calmodulin kinase II promoter (forebrain-specific); promoting high levels of mutant tau within the hippocampus and neocortex [75]. All of the rTg4510 mice used in this study were female due to the potential for differences in the severity of tau pathology between males and females [4]. The rTg21221 mice express the wild-type human tau (4R0N) gene under the same promoter systems as that of rTg4510 mice [45]. To model α-synucleinopathy, we used the M83 mouse model (maintained on the hybrid B6/C3 background). This model overexpresses mutant (A53T) human αSyn under the mouse prion promoter [31], and develops αSyn pathology primarily within the spinal cord, brain stem midbrain, hypothalamus, thalamus and periaqueductal gray regions (other brain regions less affected) resulting in a severe motor phenotype and paralysis. This pathology and phenotype occurs between 8–16 months of age in homozygous M83 mice, but later than 21 months in hemizygous M83 mice [31]. Mice expressing the G93A mutant of human SOD1 have been previously characterized [37]. The mutant gene is expressed under the human SOD1 promoter within a 12 Kb fragment of genomic human DNA. The G93A mice used for the current study were maintained on the FVB background and develop paralysis at about 150 days of age.
Sequential protein detergent extraction from mouse forebrain/spinal cord and preparation of protein samples for proteomics analysis
The protocol used here is based on changes in protein solubility in buffers containing detergents and was developed in previous studies of proteome solubility in heat-stressed cultured cells and the brains of APPswe/PS1dE9 (Line 85 or L85) mice that model Alzheimer’s amyloidosis [101, 102]. Altered protein solubility in buffers containing either nonionic (e.g. NP40) or ionic detergents (e.g. SDS) is a well-known criterion used to distinguish natively folded proteins from those that are misfolded and/or aggregated [32, 54, 64, 80, 91, 93]. The sequential extraction and sedimentation technique was used to reduce the complexity of samples that were subjected to LC-MS/MS. Mice were anesthetized with isoflurane, perfused with PBS, followed by the extraction of the forebrain or spinal cord. These tissues were then homogenized in 4 ml PBS on ice with 1% protease inhibitor cocktail in DMSO (Sigma Aldrich, St. Louis, MO) followed by sequential detergent extraction and ultracentrifugation. The sequence of detergents used was nonidet-P40 (NP40), followed by deoxycholate (DOC), and then sodium dodecyl sulfate (SDS) according to our previously utilized protocol [102]. We ultimately compared PBS-soluble (PBS-S) and SDS-insoluble fractions, the latter of which was resuspended in 300 μl of 1x Laemmli buffer, in chromatography tandem mass spectrometry (LC-MS/MS). 30 μl of each SDS-insoluble sample or 20 μl of each PBS-soluble sample was loaded into a 4–20% Tris-HCl (Bio-Rad, Hercules, CA) gel and subjected to SDS-PAGE until proteins migrated about 1.5 cm into the gel. The gel was then stained with Coomassie blue and each lane was individually excised from the gel and cut into ~1 pieces. This was followed by trypsin (Sigma Aldrich, St. Louis, MO) digestion and peptide extraction from the gel by standard protocols (Online Resource 1) executed by personnel in the University of Florida Interdisciplinary Center for Biotechnology Research Proteomics and Mass Spectrometry Core (Gainesville, FL).
Calibration, relative quantification and bioinformatics of proteomics data
The number of MS/MS spectra identified for a particular protein is presumed to be largely proportional to its abundance in a sample. To determine whether a particular protein identified in SDS-insoluble fractions from CNS tissues of transgene positive mice was over-represented relative to nontransgenic controls, we used two statistical tools to analyze the total unweighted spectrum counts for each protein from the Scaffold data on protein identity (see Online Resource 1 Table S1 for a summary of the number of animals compared by the different statistical approaches). One of the statistical tools we used is the G-test (likelihood ratio test for independence) [70, 74], which can be programmed into Microsoft Excel. In analyzing data for rTg4510 and APPswe/PS1dE9 mice across ages, we used littermate controls (Online Resource 1, Table S1). In studies of the JNPL3 tau, G93A SOD1, M83 αSyn mice, we grouped all of the data for nontransgenics together to produce a collective control group of n=6 (Online Resource 1, Table S1). For many proteins, the average number of spectral counts for a given protein was <1 in the nontransgenic littermate control samples. In such cases, the corresponding transgenic sample must have contained at least 5 spectra for the protein analyzed to achieve G-test significance (p< 0.05). In cases in which the average number of spectra for a given protein in controls was ≥1, we identified proteins in the transgenic sample that presented with 3-fold more spectra and used an unpaired t-test, as well as the G-test (at least 5 spectra in the transgenic sample), to establish whether the spectral count numbers were significantly different. GraphPad Prism (Version 7.0h, La Jolla, CA) was used when t-tests were a statistical method of comparison. In order to identify proteins that were statistically consistent by genotype and age, we used SAINT (Significance Analysis of INTeractome) analysis with a SAINT score threshold of ≥0.9 as the cut off for confidence in over-representation in SDS-insoluble fractions [11]. The SAINT scoring algorithm factors protein molecular weight in its calculation of statistical probability of over-representation. To identify proteins with the highest possible confidence level as being over-represented in SDS-insoluble fractions from samples from transgene-positive rTg4510 mice, we used the SAINT scoring system and grouped all nontransgenic littermate controls for all ages together as collective control group (n=34). Bioinformatics analysis was conducted using the PANTHER (Protein ANalysis THrough Evolutionary Relationships) database (version 12.0, Los Angeles, CA) [65, 66]. SAS JMP pro (version 13) was used to perform cluster analysis and generate heat maps (Cary, NC).
Western blot validations of LC-MS/MS data
30 μL of SDS-insoluble or 5 μL of PBS-soluble protein fractions from each animal were loaded onto a Criterion 4–20% tris-glycine gel (Bio-Rad, Hercules, CA). After overnight transfer to nitrocellulose membrane, the proteins were identified by overnight incubation in primary antibody following standard procedures. Primary antibodies included ubiquitin carboxyl-terminal hydrolase isozyme 1 (UCHL1, 1:1000 Proteintech, Rosemont, IL), alpha-enolase (ENO1, 1:200, Santacruz Biotechnology, Dallas, TX), fructose biphosphate aldolase C (ALDOC, 1:1000, Encore Biotechnology, Gainesville, FL), phosphoglycerate mutase 1 (PGAM1, 1:5000, Sigma Aldrich, St. Louis, MO), malate dehydrogenase (cytoplasmic) (MDH1, 1:1000, Abcam, Cambridge, MA) and superoxide dismutase 1 (SOD1, 1:4000, generated in house).
N2a cell culture and K18-mediated human tau seeding in vitro
Neuro 2a (N2a) cells were purchased from the American Type Culture Collection (ATCC) and cultured in media containing an equal amount of Dulbecco’s Modified Eagle Media (Thermo Fisher Scientific, Hampton, NH, USA) and Opti-MEM Reduced Serum Medium (Thermo Fisher Scientific). Media was supplemented with 5% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Life Technologies, Carlsbad, CA, USA). Cell transfections were conducted using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. The vector expressing 4R/0N human tau P301L has been previously described [88]. Twenty minutes post-transfection, wild-type human K18 tau fibrils were added to the cell media to achieve a 1 μM concentration. Cells were harvested 48 hours post-transfection followed by detergent extraction as previously described [100]. Briefly, cells were initially harvested in PBS with 1% protease inhibitor cocktail (Sigma Aldrich, St. Louis, MO, USA) followed by sequential homogenization/extraction in different detergents (NP40 and DOC), yielding the DOC-insoluble protein fraction which was resuspended in 1x Laemmli buffer prepared from a 4x Laemmli buffer stock solution. These samples were analyzed by LC-MS/MS as described above and in Online Resource 1.
Results
Neurofibrillary tangle pathology perturbs the solubility of CNS cytosolic proteins
The rTg4510 model of tau pathology produces robust neurofibrillary tangle pathology that progressively worsens with age [75, 77]. Guided by previous studies of this model, we produced cohorts of mice aged to 2.5, 4.5, 7.0 and 9.5 months before harvesting. Following methods described in Material and Methods (and Online Resource 1), we produced PBS-soluble and SDS-insoluble fractions from the homogenized forebrains of rTg4510 mice and nontransgenic littermates for proteomics analysis. The proteins we focused on were those that were readily detected in PBS-soluble fractions from the nontransgenic mice (having at least 5 peptide spectra detected in all nontransgenic PBS-soluble fractions analyzed per group). We viewed these proteins as molecules that should normally be highly soluble. We used two separate criteria for distinguishing when these proteins became over-represented in SDS-insoluble fractions of mutant mice relative to the nontransgenic controls. One criterion required at least a 3-fold difference in the number of peptide spectra in the SDS-insoluble fraction in transgenic samples relative to the average value for nontransgenic insoluble samples. There were many proteins where the average spectral counts for nontransgenic samples was less than 1 or zero. In these cases, only proteins in which there were at least five peptide spectra for that protein in the transgenic sample met statistical criteria (G-test; p <0.05) for over-representation [43, 70, 84]. In each individual animal analyzed the number of proteins that met criteria for over-representation in SDS-insoluble fractions increased progressively by age group (Fig. 1a; Online Resource 2, Table S2). Notably, the brains of rTg4510 mice at all ages exhibited significantly higher levels of insoluble tau as compared to nontransgenic mice, with the levels of insoluble tau rising steadily across the 4 age groups (Fig. 1b). The rTg4510 mouse expresses mutant human tau-P301L conditionally, using the tet-off system in which the administration of doxycycline suppresses transgene expression. In a paradigm similar to the initial characterization of this model [75, 77], two groups of rTg4510 mice received doxycycline, beginning either at 4.5 or 5.5 months with harvest at 7.0 months of age, or beginning at 5.5 months with harvest at 9.5 months of age. When we conducted proteomics analyses on rTg4510 mice that received doxycycline, number of proteins meeting criteria for over-representation in SDS-insoluble fractions was reduced in parallel to reduced levels of insoluble tau (Fig. 1a-b, shaded area). Overall, these data demonstrate that changes in protein solubility worsen as the level of misfolded tau increases and that suppression of transgene expression attenuates the shifts in proteome solubility.
Fig. 1. Quantification of changes in protein detection in SDS-insoluble fractions in brains of rTg4510 mice.
(a) Numbers of proteins that meet criteria for over-representation in SDS-insoluble fractions in rTg4510 mice at different stages of tauopathy, both with (shaded area) and without tau suppression via doxycycline. Data are presented as mean ± SD. *, p < 0.05; **, p < 0.01; ***, p<0.0001. Proteins were accepted if they achieved (i) at least a 3-fold increase in SDS-insoluble peptide spectra from nontransgenic to transgenic counterparts, (ii) when nontransgenic SDS-insoluble fractions yielded an average of <1 spectra for any given protein, the transgenic SDS-insoluble fraction contained at least 5 peptide spectra identified, and (iii) each individual protein in a given animal achieved G-test significance (p < 0.05). For doxycycline (DOX) treatment, the number shown within parentheses indicates the age (in months) at which the mice received doxycycline to suppress tau expression. Orange symbols were animals treated with DOX beginning at 4.5 months and the black symbols were animals that were treated with DOX beginning at 5.5 months of age, with all harvested at 7.0 months of age. n.s.; not significant.
(b) Numbers of SDS-insoluble tau spectra corresponding to each animal analyzed. (a-b) Each data point symbol represents an individual animal with each symbol corresponding to the same animal analyzed in panel (a). (c) Venn diagram of the affected proteins that reach SAINT score threshold (≥0.9) in each individual age group of rTg4510 mice (Online Resource 2, Table S4). (d) Venn diagram of the overlap between rTg4510 and APPswe/PS1dE9 mice based upon LC-MS/MS analysis of SDS-insoluble proteins. The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
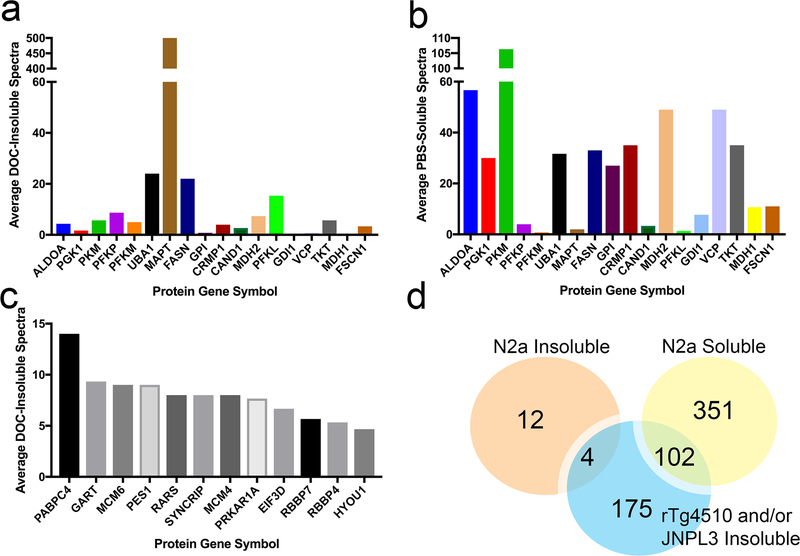
The proteins identified as losing solubility in the younger rTg4510 animals were also identified in older rTg4510 animals. Of the 148 unique proteins that were identified collectively as over-represented in the SDS-insoluble fractions for the 2.5 month old mice, 128 (86%) were identified in insoluble fractions from the forebrains of 7.0 and 9.5 month old animals; however, for many of these proteins there was considerable animal-to-animal variability in spectral count numbers (Online Resource 2, Table S2). Therefore, we used two statistical approaches to compare data between mice of different age groups to identify proteins that were consistently over-represented in the SDS-insoluble fractions. We initially used an approach in which we identified proteins that were over-represented in SDS-insoluble fractions in 7 of 10 7.0 month old rTg4510 mice and all three 9.5 month old rTg4510 mice. By this approach, we identified >200 proteins (including tau) that met statistical criteria for over-representation, relative to littermate controls (Online Resource 2, Table S3). Between the mice from the two age groups, there were 169 proteins that were common, with 255 unique proteins identified when both age groups were considered together. To further refine the data, we applied a second statistical analysis, called SAINT scoring [11], that accounts for variability in spectral data between animals and for differences in protein molecular weight in calculating the probability of over-representation in a set of samples. The SAINT system was originally developed for analyzing protein:protein interaction data, and compares spectral count numbers between samples from two different conditions. In our study, the two conditions compared were spectral counts from controls versus transgene positive animals. In this approach, we analyze all of the data collectively, comparing the transgene positive rTg4510 mice to all nontransgenic mice of any age (see Online Resource 1, Table S1). Table 1 lists the 30 proteins other than tau with the largest fold-change in spectral counts as complied by SAINT score analysis (see Online Resource 2, Table S3 for complete protein lists). Under this more stringent analysis, we identified 5 proteins that met a confidence level of 90% (0.9) probability to be over-represented in the SDS-insoluble fractions of forebrains from 2.5 month old rTg4510 mice (Fig. 1c; Online Resource 2, Table S4). All 5 of the proteins were also detected in insoluble fractions of 7.0 and 9.5 month old mice (Fig. 1c). Further, with these more stringent criteria, we observed a high level of overlap in protein identifications as over-represented in SDS-insoluble fractions among all age groups of rTg4510 mice (Fig. 1c). In the two oldest age groups (7.0 and 9.5 months), SAINT scoring identified 190 proteins that were common, with 277 unique proteins (aside from tau) identified when both age groups were considered together. Collectively, these data indicate that a nonrandom subpopulation of the murine CNS proteome is over-represented in SDS-insoluble fractions from the brains of rTg4510 mice with more severe tau pathology.
Table 1. List of the proteins that showed the highest differential of over-representation in SDS-insoluble fractions from the forebrain of 7-month-old rTg4510 mice.
Spectral counts for each protein were averaged from data from 10 individual rTg4510 animals versus 34 nontransgenic controls. All spectral count comparisons in this table exhibited a SAINT score of ≥0.9 (n=10) for over-representation in SDS-insoluble fractions. nTg = nontransgenic, Tg = transgenic, PBS-S = PBS soluble.
| Gene Symbol | Protein | Accession Number | MW kDa | Average Spectral Counts | ||
|---|---|---|---|---|---|---|
| SDS-insoluble | PBS-S | |||||
| Ctrl | rTg4510 | nTg | ||||
| Pgk1 | Phosphoglycerate kinase 1 | PGK1_MOUSE | 45 | 4.47 | 58.2 | 96.8 |
| Pkm | Pyruvate kinase | KPYM_MOUSE | 58 | 3.74 | 49.8 | 164 |
| Uba1 | Ubiquitin-activating enzyme E1 | B9EHN0_MOUSE | 118 | 3.44 | 47.7 | 103.3 |
| Fasn | Fatty acid synthase | FAS_MOUSE | 272 | 1.79 | 37.6 | 40.5 |
| Ldhb | L-lactate dehydrogenase B chain | LDHB_MOUSE | 37 | 3.91 | 36.4 | 89 |
| Gpi | Glucose-6-phosphate isomerase | G6PI_MOUSE | 63 | 3.26 | 35.7 | 107 |
| Crmp1 | Dihydropyrimidinase-related protein 1 | Q6P1J1_MOUSE | 62 | 4.41 | 33.7 | 72 |
| Rap1gds1 | RAP1 GTPase-GDP dissociation stimulator 1 | Q3TU36_MOUSE | 66 | 1.62 | 32.7 | 52.5 |
| Cand1 | Cullin-associated NEDD8-dissociated protein 1 | CAND1_MOUSE | 136 | 3.74 | 31 | 59.3 |
| Pfkl | ATP-dependent 6-phosphofructokinase, liver type | PFKAL_MOUSE | 85 | 2.03 | 27.9 | 25 |
| Gdi1 | Rab GDP dissociation inhibitor alpha | GDIA_MOUSE | 51 | 3.47 | 27.6 | 84.8 |
| Synj1 | Synaptojanin-1 | D3Z656_MOUSE | 176 | 3.62 | 26.6 | 54.3 |
| Vcp | Transitional endoplasmic reticulum ATPase | TERA_MOUSE | 89 | 0.74 | 26.6 | 56.3 |
| Tkt | Transketolase | TKT_MOUSE | 68 | 0.85 | 25.8 | 58 |
| Mdh1 | Malate dehydrogenase, cytoplasmic | MDHC_MOUSE | 37 | 3.85 | 25.4 | 56 |
| Fscn1 | Fascin | FSCN1_MOUSE | 55 | 2.38 | 24.9 | 30.8 |
| Ldha | L-lactate dehydrogenase A chain | Q3UDU4_MOUSE | 36 | 1.03 | 23.2 | 61.3 |
| Prkca | Protein kinase C | Q4VA93_MOUSE | 77 | 2.74 | 23.2 | 12.5 |
| Eef2 | Elongation factor 2 | EF2_MOUSE | 95 | 2.09 | 21.8 | 72.3 |
| Gstp1 | Glutathione S-transferase P 1 | GSTP1_MOUSE | 24 | 2.56 | 21.2 | 35.5 |
| Glul | Glutamine synthetase | GLNA_MOUSE | 42 | 3.65 | 21.1 | 50 |
| Idh3b | Isocitrate dehydrogenase [NAD] subunit, mitochondrial | Q91VA7_MOUSE | 42 | 2.94 | 21.1 | 24 |
| Pgam1 | Phosphoglycerate mutase 1 | PGAM1_MOUSE | 29 | 2.09 | 20.8 | 73.3 |
| Vps35 | Vacuolar protein sorting 35 | Q3TRJ1_MOUSE | 92 | 2.18 | 20.8 | 36 |
| Qdpr | Dihydropteridine reductase | DHPR_MOUSE | 26 | 1.00 | 20.7 | 16.8 |
| Cs | Citrate synthase, mitochondrial | CISY_MOUSE | 52 | 4.15 | 20.6 | 35.8 |
| Ppia | Peptidyl-prolyl cis-trans isomerase A | PPIA_MOUSE | 18 | 4.76 | 20.3 | 54.3 |
| Gdi2 | Rab GDP dissociation inhibitor beta | Q3UC72_MOUSE | 57 | 3.09 | 19.7 | 53.75 |
| Hspa4 | Heat shock 70 kDa protein 4 | Q571M2_MOUSE | 94 | 0.56 | 19.6 | 52.5 |
| Aldoc | Fructose-bisphosphate aldolase C | ALDOC_MOUSE | 39 | 2.53 | 19.3 | 96 |
To further compare changes in protein solubility across all rTg4510 mice that were analyzed, we performed a cluster analysis of SDS-insoluble peptide spectra from all of the mice within our rTg4510 cohort. Based upon this analysis, which groups data sets by similarity, rTg4510 mice that received Dox from 4.5/5.5 months to 7.0 months clustered together with 2.5 and 4.5-month-old rTg4510 mice (Online Resource 3). Only two out of five 9.5-month-old mice that received Dox beginning at 5.5 months clustered with aged rTg4510 mice (7.0 and 9.5 months) that did not receive Dox. This analysis indicates that changes in proteome solubility were partially mitigated by suppressing tau expression in the 4.5–5.5 month age window. Importantly, the effect of Dox on mutant tau expression was not complete as the treated mice still express the human tau-P301L transgene at levels that are 2-fold over endogenous mouse tau [77]. Thus, in mice treated with Dox at 4.5 or 5.5 months of age, the tau pathology still worsens progressively when aged to 9.5 months [77]. Overall, the data strongly indicate that the number of CNS proteins that lose solubility in rTg4510 mice is highly correlated with the severity of tau pathology.
As indicated above, the techniques used here to analyze the rTg4510 mice are very similar to what we have used in the past to examine changes in proteome solubility in the brains of the APPswe/PS1dE9 model of Alzheimer’s amyloidosis [102]. The technology in mass spectrometry has evolved rapidly in the last few years and we thus conducted a replication study of proteome solubility in the APPswe/PS1dE9 mice so that we could make better comparisons of data between the two models. Our prior study with older mass spectrometry instruments identified 28 proteins in addition to Aβ that were over-represented in SDS-insoluble fractions of 16 months old APPswe/PS1dE9 mice. Importantly, we were able to identify 21 of the same proteins, including Aβ, in the new data sets, which grew to a list of 179 proteins in 20 month old APPswe/PS1dE9 mice (Online Resource 4 and 5). Between the APPswe/PS1dE9 and rTg4510 models, there were 141 common proteins that could be identified as over-represented in SDS-insoluble fractions despite the very distinct locations of Aβ and tau pathology (Fig. 1d; Online Resource 2, Tables S4 and S5). Overall, however, the total number of proteins that show changes in solubility in the brains of rTg4510 mice was much higher than the APPswe/PS1dE9 mice.
Validation of LC-MS/MS data in tauopathy
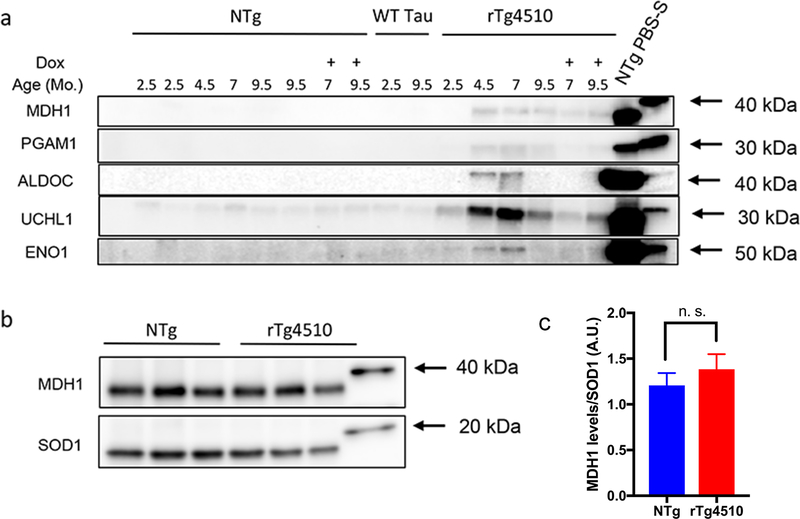
To validate the LC-MS/MS data, we conducted immunoblot confirmations for a subset of proteins identified. We obtained antibodies from commercial sources and confirmed detection of a band of the appropriate size for proteins of interest by immunoblots of PBS-soluble fractions. Importantly, the proteins that we tested spanned a wide range of relative abundance in our samples (estimated by spectral counts). We included in this validation rTg21221 mice expressing wild-type (WT) human tau, which do not exhibit NFT pathology [45]. No proteins consistently lost solubility in this model when analyzed at 9.5 months of age (see Online Resource 2; Table S2). We also included rTg4510 animals at 2.5, 4.5, 7.0, and 9.5 months of age, doxycycline-treated rTg4510 mice, and nontransgenic control mice. Because the composition and level of proteins in the insoluble fractions was expected to vary considerably by genotype and age, we normalized all fractions by volume. For each tissue sample, we initially generated a 10% weight by volume homogenate in PBS. The final volume of each fraction generated from a given sample was adjusted to be equivalent to the starting volume of the initial PBS homogenate (10% weight by volume). We then loaded an equivalent volume of sample in each lane, meaning that each lane should contain the same volumetric fraction of the original 10% homogenate. Focusing on SDS-insoluble fractions, we conducted immunoblots to ubiquitin carboxyl-terminal hydrolase isozyme 1 (UCHL1), alpha-enolase (ENO1), fructose biphosphate aldolase C (ALDOC), phosphoglycerate mutase 1 (PGAM1) and malate dehydrogenase (cytoplasmic; MDH1) (Fig. 2a). These proteins were easily detected in PBS-soluble fractions of nontransgenic control mice, indicative of their normally soluble state (Fig. 2a). In good alignment with the LC-MS/MS data, we observed an increase in the intensity of the band corresponding to these proteins in samples from 4.5 and 7.0 month old untreated rTg4510 mice. The intensity of the band for each protein was variably lower in Dox-treated mice. Overall, the intensity of the band for each protein tracked well with the LC/MS-MS data at 4.5 and 7.0 months of age (Online Resource 2, Table S6), but for unknown reasons the intensity of immunoreactivity for these proteins in 9.5 month old rTg410 mice was consistently lower than expected. By 9.5 months of age, we note significant animal-to-animal variability in the spectral count data and that the intensity of immunoreactivity for each protein in SDS-insoluble fractions does not entirely correlate to spectral count numbers. For example, the spectral count numbers for Eno1 are much higher than that of Uchl1, but the immunoblot data would suggest that the latter protein should be more abundant (Online Resource 2, Table S6). Overall, the immunoblot data provide a measure of validation of the mass spectrometry data.
Fig. 2. Immunoblot validations of LC-MS/MS data in rTg4510 mice.
(a) SDS-insoluble fractions were analyzed for rTg4510, nontransgenic littermate, and rTg21221 (wild-type) tau-expressing control mice and probed with antibodies for MDH1, PGAM1, ALDOC, UCHL1 and ENO1. 30 μL of the SDS-insoluble fractions (of the total ~300 μL volume) from the forebrains of rTg4510 or nontransgenic mice were separated on a 4–20% tris-glycine gel. DOX treated animals received the drug at 4.5 months of age (7.0 month group) or 5.5 months of age (9.5 month group). The spectral count data from the proteins analyzed by immunoblotting are provided in Online Resource 2, Table S6. (b) MDH1 immunoblot of PBS-soluble fractions of 7-month-old rTg4150 mice and nontransgenic controls. 5 μg of protein were loaded per well. Immunoblotting for SOD1 served as a loading control. All lanes in which samples were loaded are shown in each blot, with the blot cropped to include the relevant weight marker for each protein detected. (c) Quantification of MDH1 signal; data are presented as mean ± SD; n.s., not significant; A.U., arbitrary units.
Because the complexity of the PBS-soluble fractions that were analyzed by LC-MS/MS was much greater than that of the SDS-insoluble fractions, direct comparisons of protein abundance by spectral counting is difficult. Spectral count numbers for peptides from any given protein can be affected by co-elution with other abundant peptides and the number of spectra for any given peptide can reach a saturation point from sample loading. From an analysis of serially diluted PBS-soluble fractions, we observed that each protein exhibits different saturation points. In the present study, the amount of sample that was loaded for LC-MS/MS of PBS-soluble fractions was biased towards detecting enough spectra from proteins of lesser abundance to provide confidence of protein identification. In this setting, the spectral counts for very abundant proteins were at saturation. Based on the intensity of immunostaining of proteins detected in SDS-insoluble fractions relative to PBS-soluble fractions in immunoblots, we surmised that a relatively small fraction of the total amount of any given protein shows shifts in solubility in the brains of the aged rTg4510 mice. To confirm this assumption, we compared the relative intensity of the immunoreactivity for MDH1 in the PBS-soluble fraction from nontransgenic brains to that of 7.0 month old rTg4510 mice. As expected, the levels of MDH1 in the PBS-soluble fraction of brain lysate from rTg4510 mice were not significantly different from that of brain lysates from age-matched nontransgenic littermates (Fig. 2b-c). Immunoblotting for SOD1 was used as a loading control because SOD1 is only detected in PBS-soluble fractions in both nontransgenic and transgenic mice (see Online Resource 2; Table S2). We conclude that at steady-state, a relatively small fraction of these proteins exhibits altered solubility. Whether these non-natively folded proteins are cleared or whether they may be in equilibrium with a natively folded state is unknown at present.
We next asked whether the proteins detected in SDS-insoluble fractions would potentially be visible as inclusions in tissue from mice with high levels of neurofibrillary tangle pathology and if changes in the distribution of these proteins would change by Dox treatment. As with validating LC-MS/MS data using immunoblot, obtaining reliable antibodies for histology was also a challenge, particularly with less-studied proteins. One of the proteins that had a large differential in spectral counts in SDS-insoluble fractions from nontransgenics relative to older rTg4510 mice was Hspa4 (Table 1). We were able to identify an antibody that appeared to be specific for Hspa4 and demonstrated increased immunostaining for this protein in older rTg4510 mice (Online Resource 6). The increased staining for Hspa4 did not appear to overlap with tau reactivity in NFT structures, suggestive that Hspa4 was not co-depositing with tau in vivo.
Differential effects of pathological tau versus amyloid-β on proteome solubility
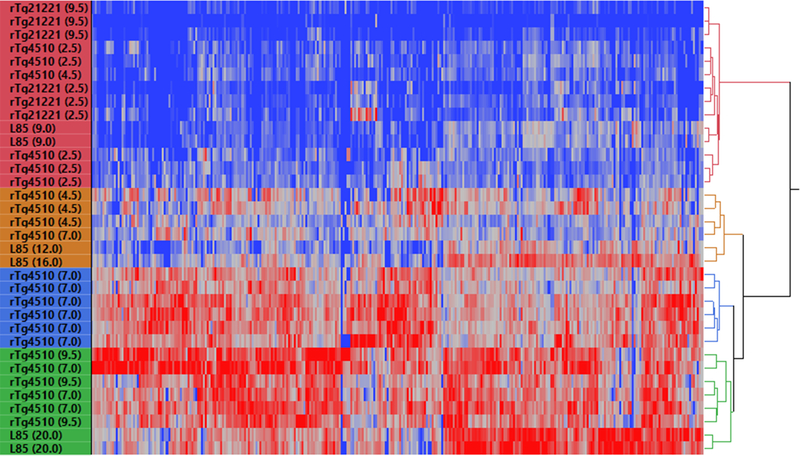
As noted above, there was a somewhat surprising degree of overlap in the identity of proteins that lost solubility in the APPswe/PS1dE9 and rTg4510 models. We used cluster analysis to compare the data from these two models to get a more in-depth assessment of similarity. For comparison, we also included data from analysis of SDS-insoluble proteins in the brains of rTg21221 mice expressing WT human tau. All rTg21221 mice clustered into a distinct subgroup, which was expected given the lack of tau pathology and misfolded tau in this model (Fig. 3). The data from rTg21221 brain analysis clustered together with younger (2.5 months) rTg4510 samples and one 4.5-month-old rTg4510 sample. The three remaining 4.5-month-old rTg4510 mice were clustered into a separate group, exemplifying the variability in spectral count data from the SDS-insoluble brain fractions of this age group (Fig. 3). Interestingly, these three mice at this stage of tauopathy were more similar to aged APPswe/PS1dE9 mice than to their older rTg4510 counterparts. As expected, all older rTg4510 mice (both 7- and 9.5-month groups) clustered together aside from one 7.0 month old anmial, reflecting their similarities in the number and identity of proteins that aberrantly fractionate in the SDS-insoluble fraction (Fig. 3). APPswe/PS1dE9 animals clustered according to age, with older mice (20 months of age) exhibiting more severe proteome insolubility compared to younger mice (9, 12 and 16 months of age) and accurately reflects our previous findings in this model [102]. Older groups of both rTg4510 and APPswe/PS1dE9 mice were more similar to each other regarding their insoluble proteomes compared their younger counterparts. Thus, it appears that as pathological burden increases with age (highest in older mice of these lines) a specific subpopulation of the proteome becomes over-represented in SDS-insoluble fractions from the brains of these mice.
Fig. 3. Two-way clustering of spectral count data from SDS-insoluble fractions from rTg4510, rTg21221 and APPswe/PS1dE9 mice.
Clustering is based upon detergent-insoluble peptide spectra for 222 total proteins identified as affected in either rTg4510, rTg21221 or APPswe/PS1dE9 Line 85 (L85) mice. Red is indicative of the highest number of peptide spectra for a given protein relative to nontransgenic control mice, while blue is indicative of absence of the peptide in SDS-insoluble fractions, or absence of a difference between transgenic and nontransgenic samples. Figure generated using JMP Pro Statistical Discovery from SAS (version 13.0, Cary, NC, USA).
Analysis of changes in proteome solubility in mouse models of spinal proteinopathy
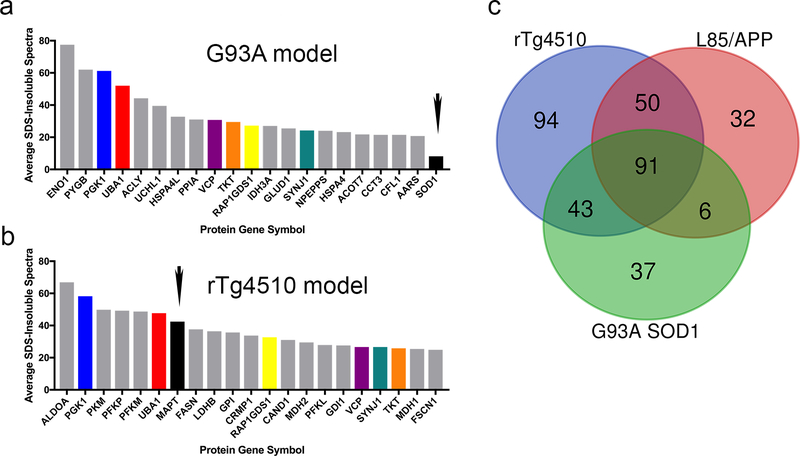
To further extend our analysis of changes in proteome solubility that occur in neurodegenerative disorders, we analyzed the SDS-insoluble proteomes of mice that model SOD1-linked ALS, spinal tauopathy and spinal α-synucleinopathy (Online Resource 5), using the same criteria and statistical analyses as described above. The G93A SOD1 model develops an ALS-like paralytic phenotype accompanied by motor neuron degeneration [37] and the accumulation of aggregated mutant SOD1 that is insoluble in non-ionic detergent [48, 53, 93, 94]. Our proteomics analysis of G93A SOD1 mice revealed many cytosolic proteins that aberrantly fractionate as SDS-insoluble in spinal cords of paralyzed G93A mice. Specifically, 177 proteins were identified that exhibited a SAINT score of ≥0.9 based on spectra from 4 independent replicates of G93A SOD1 mice compared to 6 nontransgenic controls (see Online Resource 2, Table S4; proteins with greatest differential listed in Table S7). Notably, prior studies have demonstrated that mutant SOD1 aggregates in G93A spinal cord are solubilized by SDS [53]. Neither human nor mouse SOD1 peptides were detected in SDS-insoluble fractions at levels that met statistical significance criteria (see Online Resource 2, Table S4). Thus, in the G93A model, proteins that fractionate in SDS-insoluble fractions are clearly not doing so because of some association with an SDS-insoluble SOD1. By contrast, in rTg4510 mice, tau peptide spectra were easily detected at high frequency in SDS-insoluble fractions (see Online Resource 2, Table S4). In comparing the identities of proteins with abundant peptide spectral counts in the SDS-insoluble fractions of the G93A and rTg4510 mice (based on spectral counts), there were multiple proteins in common (Fig. 4a-b). In the data for Aβ, tau and SOD1 models, there were 91 shared proteins that were over-represented in the SDS-insoluble fractions G93A spinal cords and the brains of APPswe/PS1dE9 and rTg4510 models (Fig. 4c; Online Resource 2, Table S8).
Fig. 4. Comparison of spectral count data between rTg4510 and G93A-SOD1 mice.
(a-b) Average spectral counts of the 20 most affected proteins in either paralyzed G93A mice (a) or 7-month rTg4510 (b) mice relative to either mutant SOD1 or tau, demonstrating low levels of mutant SOD1 in the SDS-insoluble fractions of spinal cord from G93A SOD1 mice. The small black arrow marks the position for tau and SOD1 in each graph.
(c) Venn diagram of the overlap between rTg4510, APPswe/PS1dE9 (L85), and G93A SOD1 mice based upon LC-MS/MS analysis of SDS-insoluble proteins. There were 91 proteins that were identified as enriched in SDS-insoluble fractions in G93A spinal cord by SAINT score analysis that were common between the three models.
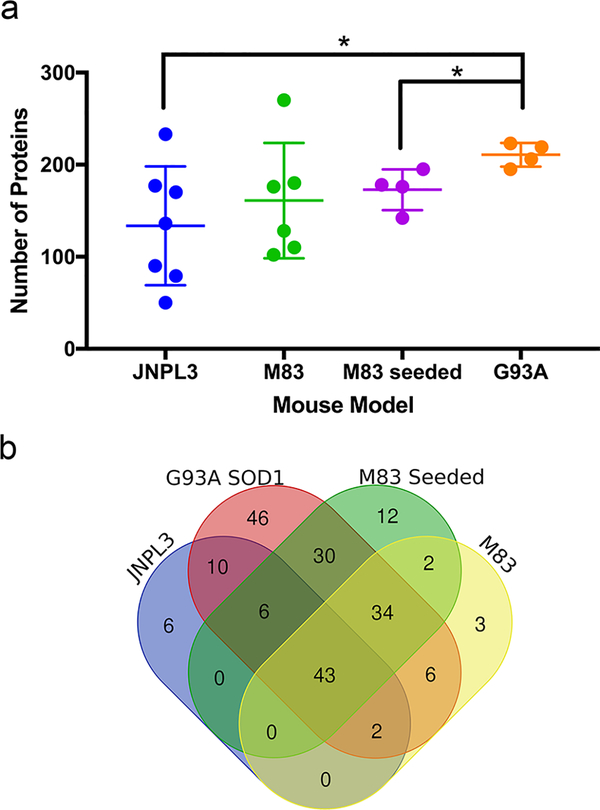
To compare the effects of tau and αSyn pathology in the spinal cord on proteome solubility, we utilized the JNPL3 (human tau-P301L [59]) and M83 (human aSyn-A53T, [31]) mouse models. Both models develop abundant spinal tau or αSyn inclusions, respectively, as well as severe motor dysfunction. We used M83 mice in two separate paradigms. These included homozygous M83 mice that intrinsically develop αSyn pathology with age [31] and hemizygous M83 mice induced to develop CNS pathology via peripheral intramuscular (IM) injection of pre-formed αSyn fibrils [76] (see Online Resource 1, Supplemental Methods). In mice from all three models that exhibited severe spinal pathology and motor deficits, we identified over 100 proteins that showed 3-fold or greater over-representation in the SDS-insoluble fraction (Fig. 5a). The harvest age for each model varied according to the age of paralytic impairment, ranging from 13 to 18 months in JNPL3 mice, 12 to 15 months in homozygous M83 mice, 5.4 to 5.8 months in the seeded M83 IM-injected mice (hereafter referred to as M83 seeded), and at 6 months in G93A SOD1 mice. The control mice for these studies, which also varied in age, were grouped as a single control population (n=6; see Online Resource 1, Table S1). Individual samples for the JNPL3 mice produced a wide variation in the number of proteins that met criteria (3-fold difference, with minimum of 5 spectral counts in the transgenic sample), ranging from 50 to 233 (134 average; Fig. 5a). SDS-insoluble fractions from individual spinal cords of aged homozygous M83 mice exhibited a range of 102 to 180 affected proteins (161 average; Fig. 5a). Similar results were achieved in heterozygous seeded M83 mice, ranging from 142 to 195 proteins identified (173 average, Fig. 5a). In comparison, in the G93A model, the range of proteins meeting criteria was 195 to 223 (210 average; Fig. 5a).
Fig. 5. Neurodegenerative models of spinal proteinopathy characterized by paralysis also induce impairments in proteome solubility.
(a) Numbers of proteins that abnormally shift to an insoluble state in JNPL3 (n = 7), homozygous M83 (n = 5), hemizygous M83 seeded (n = 4) and G93A SOD1 (n = 4). Graph displays the number of proteins in each model that were identified as over-represented in SDS-insoluble fractions of the transgenic animal relative to nontransgenic control mice. JNPL3 (all female), homozygous M83 (3 female, 2 male), hemizygous M83 seeded (2 male, 2 female) and G93A SOD1 (2 male, 2 female) mice were analyzed at end-stage phenotype (one or more limbs exhibiting paresis). The figure was generated using GraphPad Prism (version 7.0h). Significance was assessed by unpaired, two tailed T-test *, p < 0.01; **, p < 0.005. Data are presented as mean ± SD.
(b) Venn diagram of the proteins that met criteria by SAINT score (0.9 threshold) as over-represented in SDS-insoluble fractions among all spinal models.
To identify proteins in the SDS-insoluble fractions that were common between the models, we used SAINT score analysis (using a score threshold of ≥0.9), which compared data across all models and controls in statistical analyses (individual lists per transgenic model can be found in Online Resource 2, Table S4). Among these different models, we identified 43 proteins that were commonly identified as over-represented in SDS-insoluble fractions (Online Resource 2, Table S9). To further define shared features of these models, we used cluster analysis based upon peptide spectra for detergent-insoluble proteins. The proteins identified and the number of peptide spectra for SDS-insoluble fractions from tissues from each model clustered largely by genotype (Online Resource 7). Additionally, compared to the data from rTg4510 mice, the different models of spinal proteinopathy grouped together, with G93A SOD1 mice being most similar to M83 seeded mice (accelerated pathology model). Meanwhile, JNPL3 mice displayed the greatest degree of variability, having the most similarity to aged homozygous M83 mice. Overall, the proteomic data from these models clustered first according to pathological localization and second according to genotype. In summary, our data demonstrate changes in the solubility of a subpopulation of CNS proteins and identify 43 proteins that are aberrantly detectable in SDS-insoluble fractions of spinal cord from mice with mutant SOD1, tau, or αSyn pathology.
Identification of proteins in SDS-insoluble fractions from N2a cell lysates prepared with tau aggregates
To obtain additional evidence that what was observed in our proteomics analysis of transgenic mouse tissues from tau mice was not due to the binding of cellular proteins to pathologic tau aggregates, we utilized an established paradigm of exposing mouse Neuro2a (N2a) cells to human tau aggregates [30, 36]. N2a cells were expected to express many of the same proteins found in CNS tissues. Cells were transfected with vectors to express human mutant P301L tau in conjunction with seeding the cells with pre-formed aggregates of a synthetic tau fragment (K18) [36] (see Online Resource 1, Supplemental Methods). In this paradigm, a subset of cells produce full-length human tau aggregates [36]. We hypothesized that, if proteins identified in SDS-insoluble fractions from the tau mice were simply binding to misfolded tau during the fractionation process, then such proteins should exhibit similar behavior if they are present in the N2a cells. In all of the mouse studies above, the samples underwent sequential extraction starting with PBS, then in buffers containing NP40, DOC, and SDS (see Materials and Methods). In this cell culture model, we simplified the protocol to separate PBS soluble proteins from proteins insoluble in buffered DOC. We had previously determined that high quality LC-MS/MS data can be obtained from cultured cells with this simplified method [100, 101]. Proteins in which the spectra were at least 3-fold more abundant in DOC-insoluble fractions of cells seeded to induce tau aggregation compared to naïve cells in 2 out of 3 replicates were considered to meet criteria for over-representation. If a protein was absent from the DOC-insoluble protein fractions of naïve cells, then there must have been five peptide spectra present for the protein in the insoluble fractions from the tau seeded condition to meet criteria (G-test p>0.05). As previously described, the data was filtered to focus on proteins that are normally readily detectable in PBS-soluble fractions of naïve cells but aberrantly fractionate into detergent-insoluble fractions in cells exposed to aggregated tau. Of the 20 proteins with the highest number of spectral counts in SDS-insoluble fractions from the brain of rTg4510 mice (see Fig. 4b), 7 met criteria for detection in insoluble fractions generated from the seeded N2a cells (having an average spectral count ≥ 5) while 13 were detected only in PBS-soluble fractions (Fig. 6a-b). Importantly, only tau and one other protein (Fasn) met criteria for over-representation in the DOC/SDS-insoluble fractions of both N2a cells and rTg4510 mice (Fig. 6a). As expected, the number of spectral counts for tau in the insoluble fractions from the seeded N2a cells was much higher than any other protein (Fig. 6a). The vast majority of these tau spectral counts were derived from the recombinant tau seeds, which comprise the amino acids 244 to 372 of human tau. We identified 16 proteins that met criteria for over-representation in detergent-insoluble fractions in tau seeded N2a cells, 12 of which were unique to this paradigm and not present in either rTg4510 or JNPL3 mice (Fig. 6c-d). There were 3 proteins aside from Fasn (Acly, Gls and Tpi1) that were over-represented in the DOC-insoluble fraction of N2a cells that also met criterion for SDS-insoluble fractions from either rTg4510 or JNPL3 mice (Fig. 6d); however, the spectral counts for these three proteins were relatively low (Online Resource 5). Of the 453 proteins detected only in PBS-soluble fractions of tau seeded N2a cells, 102 of these proteins were among those that were over-represented in detergent-insoluble fractions of CNS lysates from the rTg4510 and JNPL3 models (Fig. 6d). Collectively, these data indicate that the proteins identified as over-represented in detergent-insoluble fractions of the tau models are not likely to be associating with misfolded tau aggregates during the fractionation process.
Fig. 6. Analysis of cellular protein co-sedimentation with tau aggregates in mouse N2a cell models.
(a) Average DOC-insoluble spectral counts in the seeded N2a cells for a subset of proteins that were identified as aberrantly fractionating to SDS-insoluble fractions from the brains of rTg4510 mice.
(b) Average PBS-soluble spectral counts for the same proteins displayed in panel (a), indicating the level of detectability of the proteins in PBS-soluble fractions.
(c) Spectral count data for the small number of proteins that meet criteria for over-representation in DOC-insoluble fractions from N2a cells seeded for tau aggregation. Proteins were accepted if they (i) achieved at least a 3-fold increase in DOC-insoluble spectra from untransfected to tau seeded cells for at least 2 out of 3 experimental replicates and (ii) during instances where untransfected cells yielded 0 peptides for any given proteins, the tau seeded condition yielded a significant G-test (p < 0.05).
(d) Venn Diagram comparing DOC-insoluble and PBS-soluble proteins from the N2a cells with the proteins identified as over-represented in SDS-insoluble fractions from either brain or spinal cord of rTg4510 and JNPL3 mice, respectively.
Bioinformatic analysis of proteins that aberrantly fractionate as SDS-insoluble reveals commonly affected protein classes
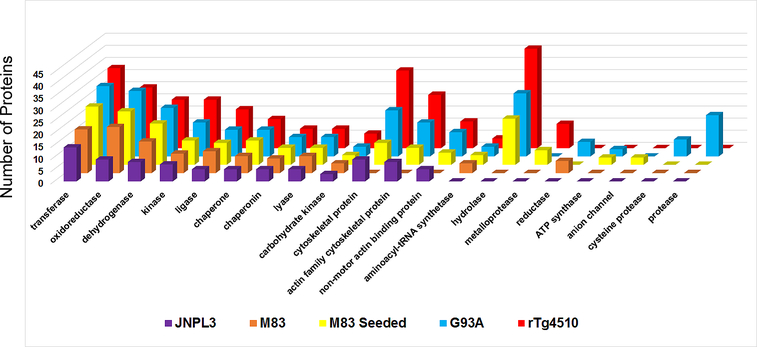
Our data to this point indicate that the brains of mice with distinct pathological features show common signatures of altered protein solubility. To determine whether particular classes of proteins might be selectively vulnerable, we conducted bioinformatics analyses on all proteins that met statistical criteria across the neurodegenerative proteinopathy models. For this gene ontology analysis, we honed in on protein classes that were significantly over-represented (statistical over-representation test, PANTHER database, see Materials and Methods) in the SDS-insoluble fraction relative to the mouse proteome. This revealed the common occurrence of transferases, oxidoreductases, dehydrogenases, kinases, ligases, chaperonins, chaperones and lyases as aberrantly fractionating into insoluble fractions in the presence of neurodegenerative pathology (Fig. 7). Many protein classes were present in three or more proteinopathy models, including cytoskeletal proteins, actin family cytoskeletal proteins, non-motor actin binding proteins, aminoacyl-tRNA synthetases and hydrolases. Other affected protein classes were unique to the presence of a particular type and/or location of pathological inclusion, such as anion channels being specific to the M83 seeded model and cysteine proteases/proteases being specific to G93A SOD1 mice. Meanwhile, several protein classes were specifically affected in the presence of cortical tauopathy (Online Resource 8). Overall, the data indicate significant commonality in the identities and classes of proteins that aberrantly fractionate into SDS-insoluble fractions of CNS tissues from neurodegenerative mouse models.
Fig. 7. Bioinformatic analysis of protein classes that are statistically over-represented in SDS-insoluble fractions of proteinopathy models analyzed via LC-MS/MS.
Statistical over-representation tests (p < 0.05) with Bonferroni correction for multiple testing were conducted using the PANTHER (Protein ANalysis THrough Evolutionary Relationships) database (version 12.0). The graph was generated using Microsoft Excel (version 16.0). The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
Discussion
In the current study, we have determined the identity of CNS proteins that exhibit changes in detergent solubility in the presence of robust neurodegenerative pathology. Building on a prior study of mice that model Alzheimer’s amyloidosis, the models examined here included mice that develop tau, αSyn, or SOD1 pathology. In mice that model cortical tau pathology, we demonstrate that the number of proteins that become aberrantly detectable in SDS-insoluble fractions increases as pathology worsens and the levels of insoluble tau increase. To control for possible associations between aggregated tau and cytosolic proteins, we used an N2a cell culture model of seeded tau aggregation. In these seeding experiments, only a fraction of the cells contain tau aggregates and, thus, this paradigm provides a control for potential interactions between tau aggregates and cytosolic proteins during cell lysis and fractionation. Our data suggest that the proteins that were over-represented in SDS-insoluble fractions of rTg4510/JNPL3 mice were not merely binding to and co-sedimenting with tau aggregates during the fractionation process. Cluster analysis of the detergent-insoluble peptide spectra across the different models demonstrated that mice with high levels of amyloidosis (aged APPswe/PS1dE9 model) clustered together with older rTg4510 mice. 141 of the 179 proteins identified as over-represented in insoluble fractions from the brains of APPswe/PS1dE9 mice by SAINT score were also found in insoluble fractions from the brains of rTg4510 mice (277 total proteins by SAINT score). Interestingly, 91 proteins identified in SDS-insoluble fractions of brains from APPswe/PS1dE9 or rTg410 mice were also found in insoluble fractions from spinal cords of G93A SOD1 models. Additionally, there were 43 proteins that were present in the insoluble fractions from spinal cords of G93A SOD1 mice that were also found in spinal fractions from αSyn and tau models. Importantly, the shifts in proteome solubility in the spinal cords of G93A mice (177 proteins total) occurred despite the fact that misfolded mutant SOD1 was not itself SDS-insoluble [53]. Collectively, these data provide strong arguments against the notion that the cytosolic proteins found in SDS-insoluble fractions of CNS tissues from these mouse models are simply binding to, or otherwise specifically co-aggregating with, a primary pathologic protein.
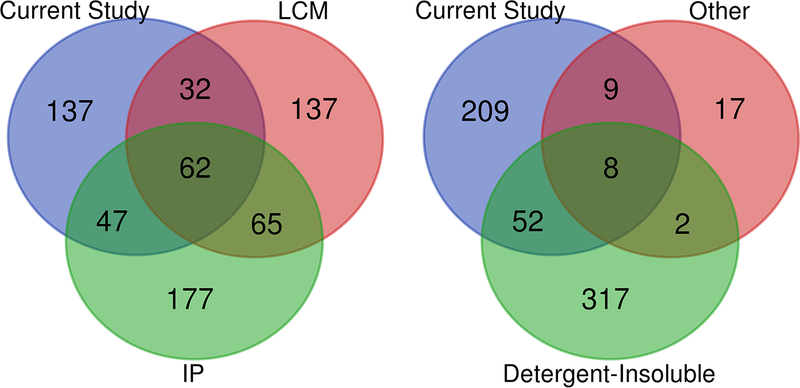
Proteomic approaches have been used extensively to investigate proteins that may associate with pathological features in neurodegenerative disorders (Online Resource 1, Table S13). One aspect of our study that must be emphasized is that the tissues we have subjected to our detergent extraction protocol were all freshly isolated. When we first began to use our protocol to analyze mice that develop Alzheimer’s amyloidosis, we noticed a remarkable difference in the LC-MS/MS data between freshly prepared and frozen tissue [102]. This aspect of our study must be taken into account when comparing our findings to previous work of human and mouse samples that had been frozen prior to tissue analysis (Online Resource 1, Table S13; one study used tissue embedded in paraffin [25]). We harvest and homogenize fresh tissue in order to avoid changes in solubility that could occur due to freeze/thaw. Nonetheless, despite methodological differences, a subset of the proteins we identified had also been identified in other studies as associated with pathological features or pathological proteins. Multiple studies have used a co-immunoprecipitation paradigm with either Aβ, or tau, on human AD and tauopathy tissues (Online Resource 1, Table S13). Of the collective 352 proteins identified in these studies, 109 overlapped with our data set (Fig. 8, left panel). One of these co-immunoprecipitation studies, identified over 100 proteins that bind to a synthetic β-structure peptide [71], but our data showed minimal overlap with this data set (only 7 proteins; Online Resource 1, Table S13). Altogether, of the 277 proteins (across all ages) we identified as over-represented in SDS-insoluble fractions of brain lysates from the rTg4510 mice, there were 115 that had been previously identified as binding Tau (Online Resource 2, Table S10). Laser capture microdissection another approach that has been used to enrich for proteins adjacent to pathological features in human tissues [25, 61, 96] (Online Resource 1, Table S13). Of the 296 proteins identified by these approaches, 94 overlap with our data set (Fig. 8, left panel). Notably, of the 36 proteins identified to be associated with Lewy bodies present in AD brains, 17 were also present in SDS-insoluble protein fractions of rTg4150 mice (Online Resource 1, Table S13) [99]. Collectively, these prior studies identified 143 proteins (in addition to tau itself) that were associated with pathologic features that we also detected as over-represented in SDS-insoluble fractions from rTg4510 mice (Online Resource 2, Table S11). Several studies have identified proteins in tissues from AD and tauopathy patients that were insoluble in detergents or otherwise exhibited altered solubility [1, 3, 35, 38, 81, 98] (Online Resource 1, Table S13), and our study identified many of the same proteins (n = 60; Fig. 8, right panel). One study of mice expressing tau-P30L transgenes, similar to our JNPL3 mice, identified proteins with shifts in solubility [17], and our study identified 10 of the 28 proteins previously characterized as altered (Online Resource 1, Table S13). Altogether, of the 277 proteins we identified as over-represented in SDS-insoluble fractions from the brains of 7.0–9.5 month old rTg4510 mice, 121 have not been identified in any previous proteomic study of neurodegenerative pathology (Online Resource 9).
Fig. 8. Overlapping protein identifications between SDS-insoluble fractions from Tg4510 mice and previous proteomic studies of disease-associated pathological features.
Venn diagram demonstrating the number of proteins identified from previous studies listed in Online Resource 1 (Table S13). These prior studies used various techniques including laser capture microdissections of amyloid plaques and tau tangles (LCM), affinity capture with antibodies to tau, Aβ−42, or synthetic β-structure proteins (IP), isolation of detergent-insoluble proteins from transgenic mouse and human disease tissue (Detergent-Insoluble), and density gradient (Other) methodologies.
Previous work in G93A SOD1 mice had looked for detergent-insoluble proteins that may specifically become sequestered into SOD1 aggregates [82]. An analysis of proteins extracted from frozen tissues that were insoluble in NP40 detergent identified mutant SOD1 as the only protein uniquely present in samples derived from the paralyzed G93A mice. Another study in G93A SOD1 mice identified 29 proteins enriched in triton-insoluble fractions of spinal cords from 26 week-old mice [7]; however, only 11 of these 29 proteins overlapped with our own data from this line of mice. Overall, the vast majority of proteins we identify in SDS-insoluble fractions from the spinal cords of G93A SOD1 mice have not been previously recognized as binding aggregated SOD1. Moreover, as noted above, aggregated SOD1 is largely soluble in SDS and, thus, the insoluble proteins we detected in spinal cords of these mice were not bound to SOD1.
The process by which a subset of cellular proteins become insoluble in SDS in these models requires further study. None of the proteins identified here have been previously described to form inclusions in CNS tissues of the mouse models we examined. While it is difficult to completely exclude interactions between misfolded pathologic proteins (tau, SOD1, αSyn, etc.) and a subset of cytosolic proteins, we also envision a scenario in which proteins form SDS-resistant conglomerates as a result of extended existence in a non-natively folded state. These non-natively folded proteins could persist longer in neural cells in neurodegenerative disease either because there is insufficient chaperone function to fold them or insufficient proteasome/autophagic function to degrade them. In non-native states of folding, hydrophobic regions within these proteins could be exposed, causing them to associate into amorphous aggregates that rapidly coalesce into larger sedimentable aggregates when the tissues are initially homogenized in PBS. Notably, in prior studies of proteostatic stress in cell culture models, we have observed that acute thermal stress causes shifts in protein solubility, but the structures generated are not resistant to SDS [101]. We hypothesize that the proteins we identify as aberrantly insoluble in mouse models of tauopathy, α-synucleinopathy, and SOD1 pathology may exhibit this property due to extended existence in a non-natively folded state. There is well-established precedence for non-natively folded proteins to organize into large aggregates in the presence of detergents from studies of misfolded prion protein (PrP). Misfolded PrPSc that accumulates in the brains of hamsters infected with prion rapidly organizes into large aggregates when membranes isolated in aqueous solutions are exposed to detergents [64]. Notably, the misfolded PrPSc in the brains of infected hamsters often accumulates without organizing into recognizable intracellular inclusions [64]. We hypothesize that a similar type of reorganization of dispersed, misfolded, cellular proteins in the brains of our models could cause these proteins to lose solubility in SDS.
There have been multiple studies that have documented changes in proteasome function in tissues from mouse models of neurodegenerative disease as well as human tissues [10, 26, 28, 49–51, 55, 69, 79, 89, 104]. Diminished proteasome or autophagic function could cause non-natively folded proteins to persist longer than normal. Similarly, there is ample evidence that insufficient chaperone function could play a role in causing proteins to lose solubility. Reports have described associations between tau many different chaperones that are engaged in multiple functions, including the facilitation of microtubule binding and its proteolytic turnover [19, 23, 27, 47, 52, 67, 72, 78]. Tau also associates with other proteostatic factors, including the E3 ligases CHIP, TRAF6, and Axotrophin/MARCH7 [2, 29, 72, 83]. Both the ubiquitin/proteasome and autophagic systems contribute to tau degradation [2, 18, 20, 27, 39, 40, 57, 72, 97] It has also recently been discovered that a deubiquinating enzyme, OTUB1, regulates the ubiquitination state of tau [95]. As previously mentioned, proteasomal impairment has been linked to tauopathy in a wide range of settings [18, 40, 55, 57, 69, 89]. Based upon the extensive degree of proteostatic elements and processes involved in tau homeostasis, it is reasonable to propose that when pathological tau accumulates to the degree that is seen in rTg4510 mice, the balance of cellular proteostasis may be compromised, leading to insufficient capacity to maintain other cellular proteins.
One of the aspects of the rTg4510 model that is particularly useful is that the expression of mutant tau is driven by promoter elements that can be regulated by treating the mice with analogs of tetracycline such as doxycycline [75]. Tau suppression was effective in improving proteome solubility when animals were harvested 2.5 or 1.5 months after initiating treatment at 4.5 or 5.5 months of age, respectively, but much less effective when the mice were aged longer (with doxycycline administered at 5.5 months of age and harvested at 9.5 months). Previous work in the rTg4510 model has characterized the effects that tau reduction has on NFT pathology, tau solubility, and cognitive performance [77]. Suppression of tau expression at 4.5 or 5.5 months of age has a limited effect on the evolution of tau pathology due to the baseline expression of the mutant tau gene (estimated to be 2-fold over endogenous in the presence of doxycycline) [77]. Our mass spectrometry data, however, show that the levels of SDS-insoluble tau are lower in mice treated with doxycycline. In prior studies of rTg4510 mice, doxycycline to suppression of mutant tau at 5.5 months of age led to statistically significant improvements (but not a full recovery) in cognitive performance when tested at either 7.0 or 9.5 months of age [77]. We observed that far fewer proteins were identified as over-represented in SDS-insoluble brain fractions from mice treated with doxycycline at 5.5 months and harvested at 7.0 months or 9.5 months. The alignment of these data suggests that changes in proteome solubility could contribute to cognitive dysfunction and neuronal death. Notably, significant losses in hippocampal neurons are first evident in rTg4510 mice at 5.5 months age, which is beyond the age that we first observe changes in proteome solubility (4.5 months; see Fig. 1a).
The present work, coupled with a prior effort using mice that model Alzheimer’s amyloidosis, demonstrates that one of the common signatures of disease in mouse models of neurodegenerative proteinopathy is a loss in proteome integrity. In each of the models we examined, we identified >100 proteins that aberrantly fractionated into detergent-insoluble fractions. This study focused on proteins that were consistently detected in PBS-soluble fractions with minimal tendency to spontaneously lose solubility with age (see Online Resource 2, Table S2). A large body of literature has described changes in protein quality control networks in neurodegenerative disease including changes in the function of chaperones, the ubiquitin-proteasome system, and the autophagy-lysosomal pathway. How these changes impact cellular function has largely been uncharacterized. Here, we identify the cellular proteins and specific protein classes that are most impacted by the disruptive features of misfolded Aβ, tau, αSyn, and SOD1. Although each proteinopathy exhibits some unique signatures of altered cellular proteome solubility dysfunction, we identified a core of vulnerable proteins that were consistently detected in SDS-insoluble fractions at much higher frequency in the transgenic animal models analyzed here. For any given cellular protein, the portion that aberrantly fractionated as SDS-insoluble was relatively modest and this limited alteration in solubility seems unlikely to be sufficient to cause loss of function of any particular protein that we identified. However, it is possible that our method does not fully account for changes in proteome folding because some, or most, of the non-natively folded proteins in the CNS of these models might not coalesce into detergent-resistant aggregates. In conclusion, our study provides the first systematic comparison of proteome solubility across different models of neurodegenerative disease and reveals the identity of proteins that consistently show aberrant fractionation into SDS-insoluble fractions and, thus, could be useful molecular biomarkers of proteostatic disruption.
Supplementary Material
Online Resource 1, Table S1. Statistical information for different proteinopathy animal groups analyzed via LC-MS/MS. Supplemental Materials and Methods.
Online Resource 1, Table S13 Overlapping protein identifications between SDS-insoluble fractions from Tg4510 mice and previous proteomic studies of disease-associated pathological features.
Online Resource 2, Table S2. Spectral counts exported from Scaffold (version Scaffold_4.7.3, Proteome Software Inc., Portland, OR) used for analysis of rTg4510 mice (with and without doxycycline treatment) and rTg21221 mice.
Online Resource 2, Table S3. Comprehensive lists of proteins that were aberrantly detected in SDS-insoluble fractions in 7.0 and 9.5 month-old based upon fold-change and G-test criteria. Analysis was conducted on rTg4510 7.0 month old (n=10) and 9.5-month-old (n=3) mice. For 7.0 month old mice, any given protein must have reached our criterion in 7 out of 10 analyzed mice.
Online Resource 2, Table S4. Comprehensive list of proteins that met SAINT score thresholds of 0.9 as over-represented in SDS-insoluble fractions in all of the proteinopathy models. Analysis was conducted on JNPL3 (n=7), rTg4510 7-month (n=10), rTg4510 9.5 month (n=3), M83 (n=6), M83 seeded (n=4), APPswe/PS1dE9 20-month (n=3) and G93A SOD1 (n=4) mice. Models of spinal proteinopathy were harvested at end-stage phenotype (paresis in one or more hind limbs). Proteins were accepted if they reached a SAINT score of ≥0.9 between control (NTg) mice and each corresponding transgenic model of neurodegenerative proteinopathy.
Online Resource 2, Table S5. Comparison of proteins in SDS-insoluble fractions across cortical models (rTg4510 & L85) and spinal models (JNPL3, M83, M83 seeded, and G93A SOD1).
Online Resource 2, Table S6. Spectral count data from proteins analyzed by immunoblotting Figure 2a
Online Resource 2, Table S7. List of proteins that are most highly over-represented in SDS-insoluble fractions from spinal cords of end-stage G93A SOD1 mice. Individual animal spectral counts are separated by a comma in the SDS-insoluble columns. All spectral count comparisons exhibited a G-test value of <0.01 (n=4). nTg = nontransgenic, Tg = transgenic, PBS-S = PBS soluble.
Online Resource 2, Table S8. List of the 91 common proteins that lose solubility in the G93A SOD1, APPswe/PS1dE9, and rTg4510 models of neurodegenerative proteinopathy. The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
Online Resource 2, Table S9 List of proteins common to SDS-insoluble fractions across all spinal proteinopathy models (JNPL3, M83, M83 seeded, and G93A SOD1). The protein list was compiled based upon the lists of proteins generated in Online Resource, Table S4.
Online Resource 2, Table S10 Compiled lists of proteins that lose solubility in rTg4510 mice that either overlap (Column A) or do not overlap (Column B) with previous studies identifying proteins that may be interacting with tau (see Online Resource 1, Table S13).
Online Resource 2, Table S11 Compiled lists of proteins that lose solubility in rTg4510 mice that either overlap (Column A) or do not overlap (Column B) with previous studies identifying proteins that may interact with pathologic features of AD (see Online Resource 1, Table S13).
Online Resource 2, Table S12 Complete lists of overlapping proteins between rTg4510 SDS-insoluble forebrains and the two first studies listed in Online Resource 1, Table S13.
Online Resource 3 Two-way clustering of spectral count data from rTg4510 mice. Clustering is based upon detergent-insoluble peptide spectra for 206 total proteins identified as affected in rTg4510 mice of any age (with and without DOX treatment). Red is indicative of the highest number of peptide spectra for a given protein relative to nontransgenic control mice, while blue is indicative of an absence of the peptide in SDS-insoluble fractions, or absence of a difference between transgenic and nontransgenic samples. Figure generated using JMP Pro Statistical Discovery from SAS (version 13.0, Cary, NC, USA).
Online Resource 4 Venn diagram of common proteins identified in APPswe/PS1dE9 (L85) across different analysis timepoints. Newly analyzed L85 mice were compared to previously analyzed animals.
Online Resource 5 Spectral counts exported from Scaffold (version Scaffold_4.7.3, Proteome Software Inc., Portland, OR) used for analysis of the APPswe/PS1E9 model of amyloidosis, the JNPL3, M83, and G93A models of spinal proteinopathy, and the seeded N2a cells.
Online Resource 6 Immunoreactivity for Hspa4 protein is not highly co-localized with neurofibrillary tangle pathology of rTg4510 mice. Nontransgenic mice (a & b) exhibit minimal positive staining of Hspa4 puncta (green). rTg4510 (c & d) exhibit dramatic increases in Hspa4 puncta (green) that do not directly co-localize with neurofibrillary tangles (stained using the MC1 antibody to misfolded human tau, red). rTg4510 mice that received doxycycline to suppress mutant tau expression from 4.5 – 7.0 months of age (e & f) exhibited reduced Hspa4 immunostaining compared to rTg4510 mice that did not receive doxycycline. The graph shows the number of spectral counts for Hspa4 in SDS-insoluble fractions from the forebrains of NTg, 7-month-old rTg4510 mice, and 7-month-old rTg4510 that began DOX treatment at 4.5 months of age (g).
Online Resource 7 Two-way clustering of SDS-insoluble spectra for rTg4510, M83, M83 seeded, JNPL3, and G93A SOD1 models. Clustering is based upon detergent-insoluble peptide spectra for 310 total proteins identified as affected in any spinal proteinopathy model. Red is indicative of the highest number of peptide spectra for a given protein relative to nontransgenic control mice, while blue is indicative of an absence of the peptide in SDS-insoluble fractions, or absence of a difference between transgenic and nontransgenic samples. Figure generated using JMP Pro Statistical Discovery from SAS (version 13.0, Cary, NC, USA).
Online Resource 8 Bioinformatic analysis of protein classes that are statistically over-represented in SDS-insoluble fractions rTg4510 mice. Pie chart of protein classes uniquely affected in rTg4510 mice. The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
Online Resource 9 Combined Venn diagram representative of both diagrams from Fig. 8, encompassing the numbers of overlapping proteins from all types of methodologies used in previous literature (IP, Detergent-Insoluble, LCM, and Other).
Acknowledgments
We thank the Interdisciplinary Center for Biotechnology Research (ICBR), specifically the Proteomics and Mass Spectrometry Core for assistance in processing LC-MS/MS samples. We also thank personnel within the University of Florida Animal Care Services for assistance with animal care for the mice used in this study. We also acknowledge the generous contribution of wild type tau K18 fibril preparations from Kevin Strang within the laboratory of Dr. Benoit Giasson.
Funding: This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (R21NS083006 to D.R.B. and J.L.; R01NS089622 to B.I.G.), the National Institute on Aging (P50AG047266; R01AG049456 to D.R.B. and J.L.), the BrightFocus Foundation (A20141085) to G.X. and by the Santa Fe HealthCare Alzheimer’s Disease Research Center.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Florida Institutional Animal Care and Use Committee (IACUC). This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Ayyadevara S, Balasubramaniam M, Parcon PA, Barger SW, Griffin WST, Alla R, Tackett AJ, Mackintosh SG, Petricoin E, Zhou W, Shmookler Reis RJ (2016) Proteins that mediate protein aggregation and cytotoxicity distinguish Alzheimer’s hippocampus from normal controls. Aging Cell 15:924–939. doi: 10.1111/acel.12501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babu JR, Geetha T, Wooten MW (2005) Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J Neurochem 94:192–203. doi: 10.1111/j.1471-4159.2005.03181.x [DOI] [PubMed] [Google Scholar]
- 3.Bai B, Hales CM, Chen P, Gozal Y, Dammer EB, Fritz JJ (2013) U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer ‘ s disease. Proc Natl Acad Sci U S A 110:16562–16567. doi: 10.1073/pnas.1310249110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1310249110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey RM, Covy JP, Melrose HL, Rousseau L, Watkinson R, Knight J, Miles S, Farrer MJ, Dickson DW, Giasson BI, Lewis J (2013) LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol 126:809–827. doi: 10.1007/s00401-013-1188-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balch WE, Morimoto RI, Dillin A, Kelly, Jeffery W (2008) Adapting Proteostasis for Disease Intervention. Science (80- ) 319:916–919. doi: 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- 6.Basso M, Massignan T, Samengo G, Cheroni C, De Biasi S, Salmona M, Bendotti C, Bonetto V (2006) Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J Biol Chem 281:33325–33335. doi: 10.1074/jbc.M603489200 [DOI] [PubMed] [Google Scholar]
- 7.Basso M, Samengo G, Nardo G, Massignan T, D’Alessandro G, Tartari S, Cantoni L, Marino M, Cheroni C, de Biasi S, Giordana MT, Strong MJ, Estevez AG, Salmona M, Bendotti C, Bonetto V (2009) Characterization of detergent-insoluble proteins in ALS indicates a causal link between nitrative stress and aggregation in pathogenesis. PLoS One 4. doi: 10.1371/journal.pone.0008130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosco DA, LaVoie MJ, Petsko GA, Ringe D (2011) Proteostasis and Movement Disorders: Parkinson’s Disease and Amyotrophic Lateral Sclerosis. Cold Spring Harb Perspect Biol 3:a007500–a007500. doi: 10.1101/cshperspect.a007500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrell RW, Lomas DA (1997) Conformational disease. Lancet 350:134–138. doi: 10.1016/S0140-6736(97)02073-4 [DOI] [PubMed] [Google Scholar]
- 10.Cheroni C, Peviani M, Cascio P, DeBiasi S, Monti C, Bendotti C (2005) Accumulation of human SOD1 and ubiquitinated deposits in the spinal cord of SOD1G93A mice during motor neuron disease progression correlates with a decrease of proteasome. Neurobiol Dis 18:509–522. doi: 10.1016/j.nbd.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 11.Choi H, Larsen B, Lin Z-Y, Breitkreutz A, Mellacheruvu D, Fermin D, Qin ZS, Tyers M, Gingras A-C, Nesvizhskii AI (2011) SAINT: probabilistic scoring of affinity purification-mass spectrometry data. Nat Methods 8:70–3. doi: 10.1038/nmeth.1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clippinger AK, D’Alton S, Lin WL, Gendron TF, Howard J, Borchelt DR, Cannon A, Carlomagno Y, Chakrabarty P, Cook C, Golde TE, Levites Y, Ranum L, Schultheis PJ, Xu G, Petrucelli L, Sahara N, Dickson DW, Giasson B, Lewis J (2013) Robust cytoplasmic accumulation of phosphorylated TDP-43 in transgenic models of tauopathy. Acta Neuropathol 126:39–50. doi: 10.1007/s00401-013-1123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, Marti MJ, Clarimon J, Lleo A (2013) Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol 72:1203–1212. doi: 10.1097/NEN.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 14.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, Galbiati M, Fontana E, Marino M, Carra S, Bendotti C, de Biasi S, Poletti A (2010) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum Mol Genet 19:3440–3456. doi: 10.1093/hmg/ddq257 [DOI] [PubMed] [Google Scholar]
- 15.Cuanalo-Contreras K, Mukherjee A, Soto C (2013) Role of protein misfolding and proteostasis deficiency in protein misfolding diseases and aging. Int J Cell Biol 2013. doi: 10.1155/2013/638083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired Degradation of Mutant -Synuclein by Chaperone-Mediated Autophagy. Science (80- ) 305:1292–1295. doi: 10.1126/science.1101738 [DOI] [PubMed] [Google Scholar]
- 17.David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, Ravid R, Dröse S, Brandt U, Müller WE, Eckert A, Götz J (2005) Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem 280:23802–23814. doi: 10.1074/jbc.M500356200 [DOI] [PubMed] [Google Scholar]
- 18.David DC, Layfield R, Serpell L, Narain Y, Goedert M, Spillantini MG (2002) Proteasomal degradation of tau protein. J Neurochem 83:176–185. doi: 10.1046/j.1471-4159.2002.01137.x [DOI] [PubMed] [Google Scholar]
- 19.Dickey CA, Koren J, Zhang Y-J, Xu Y-F, Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C, Bailey RM, Dunmore J, Soresh S, Leon C, Morgan D, Petrucelli L (2008) Akt and CHIP coregulate tau degradation through coordinated interactions. Proc Natl Acad Sci U S A 105:3622–7. doi: 10.1073/pnas.0709180105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickey CA, Yue M, Lin W-L, Dickson DW, Dunmore JH, Lee WC, Zehr C, West G, Cao S, Clark AMK, Caldwell GA, Caldwell KA, Eckman C, Patterson C, Hutton M, Petrucelli L (2006) Deletion of the Ubiquitin Ligase CHIP Leads to the Accumulation, But Not the Aggregation, of Both Endogenous Phospho- and Caspase-3-Cleaved Tau Species. J Neurosci 26:6985–6996. doi: 10.1523/JNEUROSCI.0746-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS, Tabaton M, Vonsattel JP, Wakabayashi K, Litvan I, Office of Rare Diseases of the National Institutes of Health (2002) Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–46 [DOI] [PubMed] [Google Scholar]
- 22.Dobson CM (1999) Protein misfolding, evolution and disease. Trends Biochem Sci 24:329–332. doi: 10.1016/S0968-0004(99)01445-0 [DOI] [PubMed] [Google Scholar]
- 23.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H (2003) Chaperones increase association of tau protein with microtubules. Proc Natl Acad Sci U S A 100:721–6. doi: 10.1073/pnas.242720499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Douglas PM, Dillin A (2010) Protein homeostasis and aging in neurodegeneration. J Cell Biol 190:719–729. doi: 10.1083/jcb.201005144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drummond E, Nayak S, Faustin A, Pires G, Hickman RA, Askenazi M, Cohen M, Haldiman T, Kim C, Han X, Shao Y, Safar JG, Ueberheide B, Wisniewski T (2017) Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol 133:933–954. doi: 10.1007/s00401-017-1691-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyllick-Brenzinger M, D’Souza CA, Dahlmann B, Kloetzel P-M, Tandon A (2010) Reciprocal Effects of α-Synuclein Overexpression and Proteasome Inhibition in Neuronal Cells and Tissue. Neurotox Res 17:215–227. doi: 10.1007/s12640-009-9094-1 [DOI] [PubMed] [Google Scholar]
- 27.Elliott E, Tsvetkov P, Ginzburg I (2007) BAG-1 associates with Hsc70Tau complex and regulates the proteasomal degradation of Tau protein. J Biol Chem 282:37276–37284. doi: 10.1074/jbc.M706379200 [DOI] [PubMed] [Google Scholar]
- 28.Emmanouilidou E, Stefanis L, Vekrellis K (2010) Cell-produced α-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiol Aging 31:953–968. doi: 10.1016/j.neurobiolaging.2008.07.008 [DOI] [PubMed] [Google Scholar]
- 29.Flach K, Ramminger E, Hilbrich I, Arsalan-Werner A, Albrecht F, Herrmann L, Goedert M, Arendt T, Holzer M (2014) Axotrophin/MARCH7 acts as an E3 ubiquitin ligase and ubiquitinates tau protein in vitro impairing microtubule binding. Biochim Biophys Acta - Mol Basis Dis 1842:1527–1538. doi: 10.1016/j.bbadis.2014.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frost B, Jacks RL, Diamond MI (2009) Propagation of Tau misfolding from the outside to the inside of a cell. J Biol Chem 284:12845–12852. doi: 10.1074/jbc.M808759200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VMY (2002) Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron 34:521–533. doi: 10.1016/S0896-6273(02)00682-7 [DOI] [PubMed] [Google Scholar]
- 32.Giasson BI, Uryu K, Trojanowski JQ, Lee VMY (1999) Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem 274:7619–7622. doi: 10.1074/jbc.274.12.7619 [DOI] [PubMed] [Google Scholar]
- 33.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI (2006) Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science (80- ) 311:1471–1474. doi: 10.1126/science.1124514 [DOI] [PubMed] [Google Scholar]
- 34.Gidalevitz T, Krupinski T, Garcia S, Morimoto RI (2009) Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet 5. doi: 10.1371/journal.pgen.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gozal YM, Duong DM, Gearing M, Cheng D, Hanfelt JJ, Funderburk C, Peng J, Lah JJ, Levey AI (2009) Proteomics Analysis Reveals Novel Components in the Detergent-Insoluble Subproteome in Alzheimer ‘ s Disease research articles. J Proteome Res 8:5069–5079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo JL, Lee VMY (2011) Seeding of normal tau by pathological tau conformers drives pathogenesis of Alzheimer-like tangles. J Biol Chem 286:15317–15331. doi: 10.1074/jbc.M110.209296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng H-X, Chen W, Zhai P, Sufit RL, Siddique T (1994) Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science (80- ) 264:1772–1775. doi: 10.1126/science.8209258 [DOI] [PubMed] [Google Scholar]
- 38.Hales CM, Dammer EB, Deng Q, Duong DM, Gearing M, Troncoso JC, Thambisetty M, Lah JJ, Shulman JM, Levey AI, Seyfried NT (2016) Changes in the detergent-insoluble brain proteome linked to amyloid and tau in Alzheimer’s Disease progression. Proteomics 1–27. doi: 10.1002/pmic.201600057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW (2008) Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci 27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x [DOI] [PubMed] [Google Scholar]
- 40.Han DH, Na H-K, Choi WH, Lee JH, Kim YK, Won C, Lee S-H, Kim KP, Kuret J, Min D-H, Lee MJ (2014) Direct cellular delivery of human proteasomes to delay tau aggregation. Nat Commun 5:5633. doi: 10.1038/ncomms6633 [DOI] [PubMed] [Google Scholar]
- 41.Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–9 [DOI] [PubMed] [Google Scholar]
- 42.Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H (2007) Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res 1184:284–94. doi: 10.1016/j.brainres.2007.09.048 [DOI] [PubMed] [Google Scholar]
- 43.Higgs RE, Knierman MD, Freeman AB, Gelbert LM, Patil ST, Hale JE (2007) Estimating the statistical significance of peptide identifications from shotgun proteomics experiments. J Proteome Res 6:1758–1767. doi: 10.1021/pr0605320 [DOI] [PubMed] [Google Scholar]
- 44.Hipp MS, Park SH, Hartl UU (2014) Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol 24:506–514. doi: 10.1016/j.tcb.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 45.Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, Pitstick R, Carlson GA, Lanier LM, Yuan LL, Ashe KH, Liao D (2010) Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 68:1067–1081. doi: 10.1016/j.neuron.2010.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016) TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. BRAIN 139:2983–2993. doi: 10.1093/brain/aww224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jinwal UK, O’Leary JC, Borysov SI, Jones JR, Li Q, Koren J, Abisambra JF, Vestal GD, Lawson LY, Johnson AG, Blair LJ, Jin Y, Miyata Y, Gestwicki JE, Dickey CA (2010) Hsc70 rapidly engages tau after microtubule destabilization. J Biol Chem 285:16798–16805. doi: 10.1074/jbc.M110.113753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnston JA, Dalton MJ, Gurney ME, Kopito RR (2000) Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 97:12571–6. doi: 10.1073/pnas.220417997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kabashi E, Agar JN, Hong Y, Taylor DM, Minotti S, Figlewicz DA, Durham HD (2008) Proteasomes remain intact, but show early focal alteration in their composition in a mouse model of amyotrophic lateral sclerosis. J Neurochem 105:2353–2366. doi: 10.1111/j.1471-4159.2008.05317.x [DOI] [PubMed] [Google Scholar]
- 50.Kabashi E, Agar JN, Strong MJ, Durham HD (2012) Impaired proteasome function in sporadic amyotrophic lateral sclerosis. Amyotroph Lateral Scler 13:367–371. doi: 10.3109/17482968.2012.686511 [DOI] [PubMed] [Google Scholar]
- 51.Kabashi E, Agar JN, Taylor DM, Minotti S, Durham HD (2004) Focal dysfunction of the proteasome: A pathogenic factor in a mouse model of amyotrophic lateral sclerosis. J Neurochem 89:1325–1335. doi: 10.1111/j.1471-4159.2004.02453.x [DOI] [PubMed] [Google Scholar]
- 52.Karagö GE, Duarte AMS, Akoury E, Ippel H, Biernat J, Moran Luengo T, Radli M, Didenko T, Nordhues BA, Veprintsev DB, Dickey CA, Mandelkow E, Zweckstetter M, Boelens R, Madl T, Rudiger SGD (2014) Hsp90-tau complex reveals molecular basis for specificity in chaperone action. Cell 156:963–974. doi: 10.1016/j.cell.2014.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karch CM, Prudencio M, Winkler DD, Hart PJ, Borchelt DR (2009) Role of mutant SOD1 disulfide oxidation and aggregation in the pathogenesis of familial ALS. Proc Natl Acad Sci U S A 106:7774–9. doi: 10.1073/pnas.0902505106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kawarabayashi T, Younkin L (2001) Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci 21:372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keck S, Nitsch R, Grune T, Ullrich O (2003) Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem 85:115–122. doi: 10.1046/j.1471-4159.2003.01642.x [DOI] [PubMed] [Google Scholar]
- 56.Labbadia J, Morimoto RI (2015) The Biology of Proteostasis in Aging and Disease. Annu Rev Biochem 1–30. doi: 10.1146/annurev-biochem-060614-033955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MJ, Lee JH, Rubinsztein DC (2013) Tau degradation: The ubiquitin-proteasome system versus the autophagy-lysosome system. Prog. Neurobiol 105:49–59 [DOI] [PubMed] [Google Scholar]
- 58.Lewis J, Dickson DW, Lin W-L, Chisholm L, Corral A, Jones G, Yen S, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M (2001) Enhanced Neuro brillary Degeneration in Transgenic Mice Expressing Mutant Tau and APP. Science (80- ) 293:1487–1491. doi: 10.1126/science.1058189 [DOI] [PubMed] [Google Scholar]
- 59.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M (2000) Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet 25:402–405. doi: 10.1038/79109 [DOI] [PubMed] [Google Scholar]
- 60.Liang TW, Forman MS, Duda JE, McCluskey L, Trojanowski JQ, Siderowf A (2005) Multiple pathologies in a patient with a progressive neurodegenerative syndrome. J Neurol Neurosurg Psychiatry 76:252–255. doi: DOI 10.1136/jnnp.2004.039479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liao L, Cheng D, Wang J, Duong DM, Losik TG, Gearing M, Rees HD, Lah JJ, Levey AI, Peng J (2004) Proteomic characterization of postmortem amyloid plaques isolated by laser capture microdissection. J Biol Chem 279:37061–37068. doi: 10.1074/jbc.M403672200 [DOI] [PubMed] [Google Scholar]
- 62.Lindberg I, Shorter J, Wiseman RL, Chiti F, Dickey C a, McLean PJ (2015) Chaperones in Neurodegeneration. J Neurosci 35:13853–9. doi: 10.1523/JNEUROSCI.2600-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Love S, Saitoh T, Quijada S, Cole GM, Terry RD (1988) Alz-50, ubiquitin and tau immunoreactivity of neurofibrillary tangles, Pick bodies and Lewy bodies. J Neuropathol Exp Neurol 47:393–405 [DOI] [PubMed] [Google Scholar]
- 64.McKinley MP, Meyer RK, Kenaga L, Rahbar F, Cotter R, Serban A, Prusiner SB (1991) Scrapie prion rod formation in vitro requires both detergent extraction and limited proteolysis. J Virol 65:1340–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD (2017) PANTHER version 11: Expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45:D183–D189. doi: 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mi H, Muruganujan A, Casagrande JT, Thomas PD (2013) Large-scale gene function analysis with the PANTHER classification system. Nat Protoc 8:1551–1566. doi: 10.1038/nprot.2013.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyata Y, Koren J, Kiray J, Dickey CA, Gestwicki JE (2011) Molecular chaperones and regulation of tau quality control: strategies for drug discovery in tauopathies. Future Med Chem 3:1523–1537. doi: 10.4155/fmc.11.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morimoto RI, Cuervo AM (2014) Proteostasis and the aging proteome in health and disease. Journals Gerontol - Ser A Biol Sci Med Sci 69:S33–S38. doi: 10.1093/gerona/glu049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Myeku N, Clelland CL, Emrani S, Kukushkin NV, Yu WH, Goldberg AL, Duff KE (2015) Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling. Nat Med 6:1–11. doi: 10.1038/nm.4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Old WM (2005) Comparison of Label-free Methods for Quantifying Human Proteins by Shotgun Proteomics. Mol Cell Proteomics 4:1487–1502. doi: 10.1074/mcp.M500084-MCP200 [DOI] [PubMed] [Google Scholar]
- 71.Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, Vabulas RM (2011) Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144:67–78. doi: 10.1016/j.cell.2010.11.050 [DOI] [PubMed] [Google Scholar]
- 72.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13:703–714. doi: 10.1093/hmg/ddh083 [DOI] [PubMed] [Google Scholar]
- 73.Piras A, Collin L, Grüninger F, Graff C, Rönnbäck A (2016) Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol Commun 4:22. doi: 10.1186/s40478-016-0292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prokai L, Stevens SM, Rauniyar N, Nguyen V (2009) Rapid label-free identification of estrogen-induced differential protein expression in vivo from mouse brain and uterine tissue. J Proteome Res 8:3862–3871. doi: 10.1021/pr900083v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH (2005) Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci 25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sacino AN, Brooks M, Thomas MA, McKinney AB, Lee S, Regenhardt RW, McGarvey NH, Ayers JI, Notterpek L, Borchelt DR, Golde TE, Giasson BI (2014) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc Natl Acad Sci U S A 111:1–6. doi: 10.1073/pnas.1321785111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.SantaCruz K, Lewis J, Spires-Jones TL, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH (2005) Tau Suppression in a Neurodegenerative Mouse Model Improves Memory Function. Science (80- ) 309:476–481. doi: 10.1126/science.1113694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarkar M, Kuret J, Lee G (2008) Two motifs within the tau microtubule-binding domain mediate its association with the hsc70 molecular chaperone. J Neurosci Res 86:2763–2773. doi: 10.1002/jnr.21721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sau D, De Biasi S, Vitellaro-Zuccarello L, Riso P, Guarnieri S, Porrini M, Simeoni S, Crippa V, Onesto E, Palazzolo I, Rusmini P, Bolzoni E, Bendotti C, Poletti A (2007) Mutation of SOD1 in ALS: A gain of a loss of function. Hum Mol Genet 16:1604–1618. doi: 10.1093/hmg/ddm110 [DOI] [PubMed] [Google Scholar]
- 80.Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, Bates GP, Lehrach H, Wanker EE (1999) Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci U S A 96:4604–9. doi: 10.1073/pnas.96.8.4604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seyfried NT, Gozal YM, Donovan LE, Herskowitz JH, Dammer EB, Xia Q, Ku L, Chang J, Duong DM, Rees HD, Cooper DS, Glass JD, Gearing M, Tansey MG, Lah JJ, Feng Y, Levey AI, Peng J (2012) Quantitative analysis of the detergent-insoluble brain proteome in frontotemporal lobar degeneration using SILAC internal standards. J Proteome Res 11:2721–2738. doi: 10.1021/pr2010814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw BF, Lelie HL, Durazo A, Nersissian AM, Xu G, Chan PK, Gralla EB, Tiwari A, Hayward LJ, Borchelt DR, Valentine JS, Whitelegge JP (2008) Detergent-insoluble aggregates associated with amyotrophic lateral sclerosis in transgenic mice contain primarily full-length, unmodified superoxide dismutase-1. J Biol Chem 283:8340–8350. doi: 10.1074/jbc.M707751200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimura H, Schwartz D, Gygi SP, Kosik KS (2004) CHIP-Hsc70 Complex Ubiquitinates Phosphorylated Tau and Enhances Cell Survival. J Biol Chem 279:4869–4876. doi: 10.1074/jbc.M305838200 [DOI] [PubMed] [Google Scholar]
- 84.Sokal RR, Rohlf JF (1995) Biometry, the principles and practice of statistics in biological research. W.H. Freeman [Google Scholar]
- 85.Soto C (2003) Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci 4:49–60. doi: 10.1038/nrn1007 [DOI] [PubMed] [Google Scholar]
- 86.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B (1997) Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci U S A 94:4113–8. doi: 10.1073/pnas.94.8.4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spires-Jones TL, Attems J, Thal DR (2017) Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 1–19. doi: 10.1007/s00401-017-1709-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strang KH, Croft CL, Sorrentino ZA, Chakrabarty P, Golde TE, Giasson BI (2017) Distinct differences in prion-like seeding and aggregation between tau protein variants provide mechanistic insights into tauopathies. J Biol Chem jbc.M117.815357. doi: 10.1074/jbc.M117.815357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tai HC, Serrano-Pozo A, Hashimoto T, Frosch MP, Spires-Jones TL, Hyman BT (2012) The synaptic accumulation of hyperphosphorylated tau oligomers in alzheimer disease is associated with dysfunction of the ubiquitin-proteasome system. Am J Pathol 181:1426–1435. doi: 10.1016/j.ajpath.2012.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Taylor RC, Dillin A (2011) Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol 3:1–17. doi: 10.1101/cshperspect.a004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trojanowski JQ, Wang J, Lee VM (1999) Purification of Paired Helical Filament Tau and Normal Tau from Human Brain Tissue. Methods Enzymol 309:81–89 [DOI] [PubMed] [Google Scholar]
- 92.Walker L, Kirsty ·, Mcaleese E, Thomas AJ, Johnson M, Martin-ruiz C, Parker C, Colloby SJ, Jellinger K, Attems J (2015) Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol 129:729–748. doi: 10.1007/s00401-015-1406-3 [DOI] [PubMed] [Google Scholar]
- 93.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR (2003) Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet 12:2753–64. doi: 10.1093/hmg/ddg312 [DOI] [PubMed] [Google Scholar]
- 94.Wang J, Xu G, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR (2002) Fibrillar Inclusions and Motor Neuron Degeneration in Transgenic Mice Expressing Superoxide Dismutase 1 with a Disrupted Copper-Binding Site. Neurobiol Dis 10:128–138. doi: 10.1006/nbdi.2002.0498 [DOI] [PubMed] [Google Scholar]
- 95.Wang P, Joberty G, Buist A, Vanoosthuyse A, Stancu I-C, Vasconcelos B, Pierrot N, Faelth-Savitski M, Kienlen-Campard P, Octave J-N, Bantscheff M, Drewes G, Moechars D, Dewachter I (2017) Tau interactome mapping based identification of Otub1 as Tau deubiquitinase involved in accumulation of pathological Tau forms in vitro and in vivo. Acta Neuropathol 133:731–749. doi: 10.1007/s00401-016-1663-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Q, Woltjer RL, Cimino PJ, Pan C, Montine KS, Zhang J, Montine TJ (2005) Proteomic analysis of neurofibrillary tangles in Alzheimer disease identifies GAPDH as a detergent-insoluble paired helical filament tau binding protein. FASEB J 19:869–871. doi: 10.1096/fj.04-3210fje [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow E-M, Cuervo AM, Mandelkow E (2010) Synergy and antagonism of macroautophagy and chaperone-mediated autophagy in a cell model of pathological tau aggregation. Autophagy 6:182–3 [DOI] [PubMed] [Google Scholar]
- 98.Woltjer RL, Cimino PJ, Boutte AM, Schantz AM, Montine KS, Larson EB, Bird T, Quinn JF, Zhang J, Montine TJ (2005) Proteomic determination of widespread detergent insolubility, including A but not tau, early in the pathogenesis of Alzheimer’s disease. FASEB J 22:1–23. doi: 10.1096/fj.05-4263fje [DOI] [PubMed] [Google Scholar]
- 99.Xia Q, Liao L, Cheng D, Duong DM, Gearing M, Lah JJ, Levey AI, Peng J (2008) Proteomic identification of novel proteins associated with Lewy bodies. Front Biosci 13:3850–6. doi: 10.2741/2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu G, Pattamatta A, Hildago R, Pace MC, Brown H, Borchelt DR (2016) Vulnerability of newly synthesized proteins to proteostasis stress. J Cell Sci 129:1892–901. doi: 10.1242/jcs.176479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu G, Stevens SM, Kobiessy F, Brown H, McClung S, Gold MS, Borchelt DR (2012) Identification of Proteins Sensitive to Thermal Stress in Human Neuroblastoma and Glioma Cell Lines. PLoS One 7:1–13. doi: 10.1371/journal.pone.0049021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu G, Stevens SM, Moore BD, McClung S, Borchelt DR (2013) Cytosolic proteins lose solubility as amyloid deposits in a transgenic mouse model of alzheimer-type amyloidosis. Hum Mol Genet 22:2765–2774. doi: 10.1093/hmg/ddt121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yokota O, Davidson Y, Bigio EH, Ishizu H, Terada S, Arai T, Hasegawa M, Akiyama H, Sikkink S, Pickering-Brown S, Mann DMA (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120:55–66. doi: 10.1007/s00401-010-0702-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang NY, Tang Z, Liu CW (2008) α-synuclein protofibrils inhibit 26 S proteasome-mediated protein degradation: Understanding the cytotoxicity of protein protofibrils in neurodegenerative disease pathogenesis. J Biol Chem 283:20288–20298. doi: 10.1074/jbc.M710560200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1, Table S1. Statistical information for different proteinopathy animal groups analyzed via LC-MS/MS. Supplemental Materials and Methods.
Online Resource 1, Table S13 Overlapping protein identifications between SDS-insoluble fractions from Tg4510 mice and previous proteomic studies of disease-associated pathological features.
Online Resource 2, Table S2. Spectral counts exported from Scaffold (version Scaffold_4.7.3, Proteome Software Inc., Portland, OR) used for analysis of rTg4510 mice (with and without doxycycline treatment) and rTg21221 mice.
Online Resource 2, Table S3. Comprehensive lists of proteins that were aberrantly detected in SDS-insoluble fractions in 7.0 and 9.5 month-old based upon fold-change and G-test criteria. Analysis was conducted on rTg4510 7.0 month old (n=10) and 9.5-month-old (n=3) mice. For 7.0 month old mice, any given protein must have reached our criterion in 7 out of 10 analyzed mice.
Online Resource 2, Table S4. Comprehensive list of proteins that met SAINT score thresholds of 0.9 as over-represented in SDS-insoluble fractions in all of the proteinopathy models. Analysis was conducted on JNPL3 (n=7), rTg4510 7-month (n=10), rTg4510 9.5 month (n=3), M83 (n=6), M83 seeded (n=4), APPswe/PS1dE9 20-month (n=3) and G93A SOD1 (n=4) mice. Models of spinal proteinopathy were harvested at end-stage phenotype (paresis in one or more hind limbs). Proteins were accepted if they reached a SAINT score of ≥0.9 between control (NTg) mice and each corresponding transgenic model of neurodegenerative proteinopathy.
Online Resource 2, Table S5. Comparison of proteins in SDS-insoluble fractions across cortical models (rTg4510 & L85) and spinal models (JNPL3, M83, M83 seeded, and G93A SOD1).
Online Resource 2, Table S6. Spectral count data from proteins analyzed by immunoblotting Figure 2a
Online Resource 2, Table S7. List of proteins that are most highly over-represented in SDS-insoluble fractions from spinal cords of end-stage G93A SOD1 mice. Individual animal spectral counts are separated by a comma in the SDS-insoluble columns. All spectral count comparisons exhibited a G-test value of <0.01 (n=4). nTg = nontransgenic, Tg = transgenic, PBS-S = PBS soluble.
Online Resource 2, Table S8. List of the 91 common proteins that lose solubility in the G93A SOD1, APPswe/PS1dE9, and rTg4510 models of neurodegenerative proteinopathy. The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
Online Resource 2, Table S9 List of proteins common to SDS-insoluble fractions across all spinal proteinopathy models (JNPL3, M83, M83 seeded, and G93A SOD1). The protein list was compiled based upon the lists of proteins generated in Online Resource, Table S4.
Online Resource 2, Table S10 Compiled lists of proteins that lose solubility in rTg4510 mice that either overlap (Column A) or do not overlap (Column B) with previous studies identifying proteins that may be interacting with tau (see Online Resource 1, Table S13).
Online Resource 2, Table S11 Compiled lists of proteins that lose solubility in rTg4510 mice that either overlap (Column A) or do not overlap (Column B) with previous studies identifying proteins that may interact with pathologic features of AD (see Online Resource 1, Table S13).
Online Resource 2, Table S12 Complete lists of overlapping proteins between rTg4510 SDS-insoluble forebrains and the two first studies listed in Online Resource 1, Table S13.
Online Resource 3 Two-way clustering of spectral count data from rTg4510 mice. Clustering is based upon detergent-insoluble peptide spectra for 206 total proteins identified as affected in rTg4510 mice of any age (with and without DOX treatment). Red is indicative of the highest number of peptide spectra for a given protein relative to nontransgenic control mice, while blue is indicative of an absence of the peptide in SDS-insoluble fractions, or absence of a difference between transgenic and nontransgenic samples. Figure generated using JMP Pro Statistical Discovery from SAS (version 13.0, Cary, NC, USA).
Online Resource 4 Venn diagram of common proteins identified in APPswe/PS1dE9 (L85) across different analysis timepoints. Newly analyzed L85 mice were compared to previously analyzed animals.
Online Resource 5 Spectral counts exported from Scaffold (version Scaffold_4.7.3, Proteome Software Inc., Portland, OR) used for analysis of the APPswe/PS1E9 model of amyloidosis, the JNPL3, M83, and G93A models of spinal proteinopathy, and the seeded N2a cells.
Online Resource 6 Immunoreactivity for Hspa4 protein is not highly co-localized with neurofibrillary tangle pathology of rTg4510 mice. Nontransgenic mice (a & b) exhibit minimal positive staining of Hspa4 puncta (green). rTg4510 (c & d) exhibit dramatic increases in Hspa4 puncta (green) that do not directly co-localize with neurofibrillary tangles (stained using the MC1 antibody to misfolded human tau, red). rTg4510 mice that received doxycycline to suppress mutant tau expression from 4.5 – 7.0 months of age (e & f) exhibited reduced Hspa4 immunostaining compared to rTg4510 mice that did not receive doxycycline. The graph shows the number of spectral counts for Hspa4 in SDS-insoluble fractions from the forebrains of NTg, 7-month-old rTg4510 mice, and 7-month-old rTg4510 that began DOX treatment at 4.5 months of age (g).
Online Resource 7 Two-way clustering of SDS-insoluble spectra for rTg4510, M83, M83 seeded, JNPL3, and G93A SOD1 models. Clustering is based upon detergent-insoluble peptide spectra for 310 total proteins identified as affected in any spinal proteinopathy model. Red is indicative of the highest number of peptide spectra for a given protein relative to nontransgenic control mice, while blue is indicative of an absence of the peptide in SDS-insoluble fractions, or absence of a difference between transgenic and nontransgenic samples. Figure generated using JMP Pro Statistical Discovery from SAS (version 13.0, Cary, NC, USA).
Online Resource 8 Bioinformatic analysis of protein classes that are statistically over-represented in SDS-insoluble fractions rTg4510 mice. Pie chart of protein classes uniquely affected in rTg4510 mice. The protein list was compiled based upon the lists of proteins generated in Online Resource 2, Table S4.
Online Resource 9 Combined Venn diagram representative of both diagrams from Fig. 8, encompassing the numbers of overlapping proteins from all types of methodologies used in previous literature (IP, Detergent-Insoluble, LCM, and Other).