Abstract
Study Objectives:
Recent studies show that obstructive sleep apnea (OSA) is a possible contributor to abnormal cognitive decline in older adults. These new observations create the need to identify older adults with OSA who are at risk of the developing dementia if not treated. This study's goal was to verify whether self-reported cognitive complaints could become a useful tool to screen for objective cognitive deficits in late middle-aged and older adults with OSA.
Methods:
Fifty-seven participants with OSA with an apnea-hypopnea index (AHI) ≥ 15 events/h (3% or arousal) and aged between 55 and 85 years were compared to 54 participants in a mild/non-OSA group on their ability to evaluate their objective cognitive functioning. They underwent overnight polysomnography followed by a comprehensive neuropsychological assessment. We recruited a similar proportion of participants with mild cognitive impairment (MCI) in both groups (OSA: 36.8%; mild/non-OSA: 35.2%). They filled out questionnaires assessing mood, sleep, and cognition. Group (OSA versus mild/non-OSA) × cognitive status (MCI versus non-MCI) analyses of variance were performed on cognitive complaint questionnaires.
Results:
We found that among participants without objective cognitive deficits, participants in the OSA group reported more cognitive complaints compared to those in the mild/non-OSA group. Among participants with objective cognitive deficits, those in the OSA group reported less cognitive complaints compared to those in the mild/non-OSA group.
Conclusions:
Participants with OSA and MCI were less aware of their deficits compared to those in the mild/non-OSA group, possibly reflecting a distinctive OSA-associated cognitive impairment. Our results underscore the importance of referring patients with OSA for a comprehensive neuropsychological assessment when an abnormal cognitive decline is suspected.
Citation:
Gagnon K, Baril AA, Montplaisir J, Carrier J, De Beaumont L, D'Aragon C, Chami S, Pelleieux S, Poirier J, Gauthier S, Lafond C, Gagnon JF, Gosselin N. Disconnection between self-reported and objective cognitive impairment in obstructive sleep apnea. J Clin Sleep Med. 2019;15(3):409–415.
Keywords: aging; cognitive complaint; mild cognitive impairment; neuropsychology, sleep-disordered breathing; subjective cognitive decline; subjective cognitive impairment
BRIEF SUMMARY
Current Knowledge/Study Rationale: A relationship was recently established between obstructive sleep apnea (OSA) and dementia. To help clinicians identify patients at risk of progressing to dementia, we investigated whether reporting cognitive complaints is linked to objective cognitive impairment in late middle-aged and older individuals with OSA.
Study Impact: We found that, in the presence of objectively assessed cognitive deficits using neuropsychological tests, participants with OSA were less aware of their cognitive impairment compared to those in the mild/non-OSA group. Our results are useful for clinicians, because self-reports from patients with OSA regarding their cognitive functioning might not accurately reflect their actual cognitive status. Future longitudinal studies are needed to determine whether unawareness of cognitive impairments in OSA predicts dementia.
INTRODUCTION
Obstructive sleep apnea (OSA) produces several awakenings per night and a chronic intermittent hypoxemia that may affect brain structure and function in late middle-aged and older adults.1,2 Recent large cohort studies showed that OSA is a possible contributor to abnormal cognitive decline and dementia.3,4 More specifically, hypoxemia was strongly associated with the risk of dementia,3 and OSA was linked with earlier diagnoses of mild cognitive impairment (MCI) and Alzheimer disease.4 These new observations stress the need to identify adults with OSA who are at risk of developing dementia if not treated.
An easy method to evaluate risk of cognitive decline is to measure self-reported cognitive functioning using standardized questionnaires. There is increasing evidence that having a self-reported cognitive complaint (SCC) is a risk factor for presenting future abnormal cognitive decline and dementia.5,6 Most of the time, the SCC is considered as the first manifestation of an ongoing neurodegenerative process and may appear several years before more significant objective cognitive impairments.7 For these reasons, reporting an SCC is now a core criterion for mild neurocognitive disorder, a prodromal stage of dementia.8
The prevalence of adults with OSA who report SCC is 23% to 69%.9 Of course, these SCC are not specifically associated with ongoing neurodegenerative processes as young and middle-aged patients are also among those who report SCC. Moreover, SCC reported by patients with OSA are not necessarily associated with objective cognitive impairment (ie, when tested with a comprehensive neuropsychological evaluation), and whether OSA increases the risk of reporting SCC in late middle-aged and older adults is unclear.9 Indeed, one study showed more SCC in older adults with OSA compared to healthy controls,10 but another study failed to show a significant effect of OSA.11
The objective of this study was to verify whether late middle-aged and older adults with OSA report the same level of SCC compared to individuals in a mild/non-OSA group. To achieve this goal, we tested older adults and late middle-aged adults because SCC can appear many years before the first objective neurodegenerative manifestations.7 The novelty of this study relies on the fact that we included a subgroup of participants with MCI in both the OSA and the mild/non-OSA groups. This subgroup allowed us to verify whether those in the OSA and mild/non-OSA groups were able to correctly evaluate their cognitive functioning in the presence of objective impairments. Because previous OSA studies found no correlation between objective and self-reported cognitive functioning,12,13 we hypothesized that those in the OSA group will show poorer ability than those in the mild/non-OSA group to accurately evaluate their cognitive functioning, particularly when they face objective cognitive deficits.
METHODS
Participants
Participants were recruited at the Hôpital du Sacré-Cœur de Montréal's Department of Pulmonology, Montreal, Canada, and through newspaper ads for volunteers for a study on sleep and cognitive health. Inclusion criteria were age from 55 to 85 years, a minimum of 7 years of education, no neuropsycho-logical assessment in the past year, and French or English as primary language. Exclusion criteria have been described in previous studies from our laboratory.1,14 Briefly, we excluded participants with dementia diagnosis, sleep disorders other than OSA, neurological or psychiatric disorders, morbid obesity, medication and/or drug use known to affect cognition or sleep. Because sleep deprivation may influence the cognitive abilities assessed the next morning, we excluded 18 participants whose total sleep time during the polysomnography was below their habitual self-reported sleep duration (difference of at least 2 hours on self-reported questionnaire) or whose total sleep time during the polysomnography was below 4 hours. This exclusion criteria was previously used by our group.14 All participants gave their written informed consent to participate in the study, and the hospital ethics committee approved the protocol and consent form.
Protocol Overview
Participants underwent an in-laboratory polysomnography. In the evening, they were weighed and measured for body mass index calculation and they completed the following questionnaires: Beck Depression Inventory-II,15 Beck Anxiety Inventory,16 Pittsburgh Sleep Quality Index,17 Epworth Sleepiness Scale,18 and the Vascular Burden Index.19 In the morning, participants completed a 3-hour neuropsychological assessment. Questionnaires on sleep, mood, sleepiness, and vascular burden were used to characterize our sample.
Sleep Data Acquisition and Analysis
Our group has described the polysomnography procedure in detail in previous articles.1 Briefly, we used a 17-channel electroencephalographic montage with electrooculogram and chin electromyogram. Periodic leg movements were measured with a bilateral anterior tibialis muscle electromyogram. Thoracoabdominal strain gauges, oronasal thermistors, and an oronasal cannula were used to monitor respiration, and a transcutaneous finger pulse oximeter was used to measure oxygen saturation. According to the American Academy of Sleep Medicine criteria an obstructive apnea is a decrease > 90% of the baseline airflow for 10 seconds or more, with a sustained or an increased respiratory effort. Obstructive hypopnea is a decrease > 30% of the airflow amplitude for at least 10 seconds accompanied with a desaturation > 3% or an arousal.20,21 The apnea-hypopnea index (AHI) was calculated with the sum of apneas and hypopneas divided by the total number of hours of sleep. All the data were scored by a trained sleep technologist. Participants with OSA were all referred to the sleep apnea clinic for follow-up; continuous positive airway pressure or other treatments were offered when it was appropriate.
Assessment of Self-Reported Cognitive Complaint
Standardized questionnaires were used to measure different aspects of SCC. The Cognitive Failure Questionnaire22 contains 25 questions on a 5-point Likert scale that measure cognitive slips and errors common in daily life. The Cognitive Difficulties Scale23 was first designed to assess the cognitive side effects of tricyclic antidepressants and then was validated to evaluate memory complaints among individuals between 45 and 75 years. This questionnaire contains 36 questions on a 5-point Likert scale (0 = never to 4 = very often). The Self-Evaluation Questionnaire24 was designed to assess 10 different memory problems (forgetting conversations, books and movies, persons, use of objects, general knowledge, places, actions, personal life, and distractions) encountered by patients with brain damage. It contains 64 questions on a 6-point Likert scale of which an average raw score is calculated. The raw scores of each SCC questionnaire were computed to make group comparisons.
Neuropsychological Assessment
MCI was defined as a condition where individuals demonstrate cognitive impairment with minimal impairment in activities of daily living.25 We assessed five cognitive domains: attention and processing speed, executive functions, visual and verbal episodic memory, visuospatial abilities, and language. The neuropsychological assessment included the following tests: the Digit Span, Block Design, Coding, and Vocabulary subtests from the Wechsler Adult Intelligence Scale (3rd Edition),26 Continuous Performance Test-II,27 Color-Word Interference and Verbal Fluency from the Delis-Kaplan Executive Function System,28 Trail Making Test,29 Tower of London Test,30 Rey Auditory-Verbal Learning Test,31 Brief VisuoSpatial Memory Test – Revised,32 Bells Test,33 Benton Judgment of Line Orientation Test,34 Rey-Osterrieth Complex Figure Test,35 and Boston Naming Test.36 Cognitive tasks were administered by a neuropsychologist or a psychometrician in the same order for all participants.
To determine whether participants had an objective cognitive impairment, we used the following criteria, based on MCI definition: (1) performance ≥ 1.5 standard deviation for at least two measurements in at least one cognitive domain; (2) no significant alteration in daily living activities assessed by questionnaires37; and (3) cognitive impairment not explained by a psychiatric condition or medication use.38
Genotyping
Apolipoprotein e4 (ApoE4) is a risk factor for progression from SCC to MCI.39 Therefore, genotyping was performed in this study to include ApoE4 to characterize our groups. DNA extraction and genotyping procedures have been described in a previous study from our laboratory.14
Statistical Analyses
Participants with AHI ≥ 15 events/h were considered as having OSA, whereas participants with AHI < 15 events/h were considered as the mild/non-OSA group, which included healthy volunteers and those with mild OSA.2 Demographic, sleep, and questionnaire variables were compared between groups using t tests or chi-square statistics.
To test the effect of OSA on the association between self-reported and objective cognitive impairments, we conducted group (OSA, mild/non-OSA) × cognitive status (MCI, non-MCI) analyses of variance (ANOVAs) on each SCC questionnaire total scores. We adjusted these analyses for age and education. For significant interactions, we decomposed the ANOVA with simple effect analyses.
To explore the association between OSA severity and SCC, we used Pearson correlation coefficients in all participants on each SCC questionnaire score and OSA severity measures, namely the number of awakenings, the AHI, the mean oxygen saturation, and the time with oxygen saturation < 90%. Because anxiety, depression, and sleep disturbances are known to increase the risk of SCC,5 we have also evaluated relationships between each SCC questionnaires score and the Beck Depression Inventory-II, Beck Anxiety Inventory, Pittsburgh Sleep Quality Index, and Epworth Sleepiness Scale using Pearson correlation coefficients.
All statistical analyses were performed on normally distributed data, more specifically age, Beck Anxiety Inventory, Pittsburgh Sleep Quality Index, AHI, number of awakenings, sleep efficiency, stage N1 sleep, and stage N3 sleep were log-transformed, and time with SpO2 < 90% was square-root transformed. SPSS for Mac 25.0 (SPSS Science, Chicago, Illinois, United States) was used for data analyses. The significance threshold was set at P < .05.
RESULTS
Participant and Group Characteristics
A total of 136 participants were recruited for the study. After applying exclusion criteria, 111 participants (57 in OSA group, 54 in the mild/non-OSA group) were included in the study. In the whole sample, 74.8% were men, mean age was 63.93 ± 6.12 years (range 55–82), and mean education level was 14.93 ± 3.67 years. Demographic, clinical, and sleep characteristics for OSA and mild/non-OSA groups are presented in Table 1. We recruited a similar proportion of MCI in both groups, ie, 36.8% in the OSA and 35.2% in the mild/non-OSA group. Using a group approach, we found no differences for SCC questionnaire scores between groups.
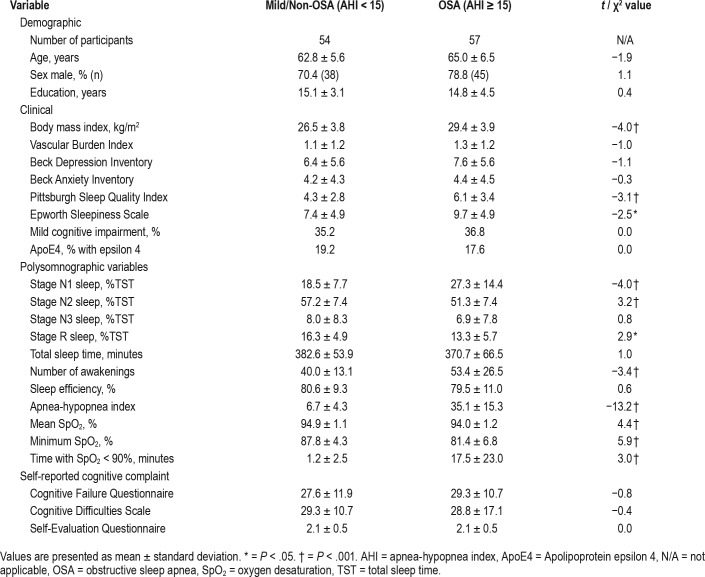
Table 1.
Demographic, clinical, and sleep variables for mild/non-OSA and OSA groups.
Association Between Self-Reported and Objective Cognitive Functioning in OSA and Mild/ Non-OSA Groups
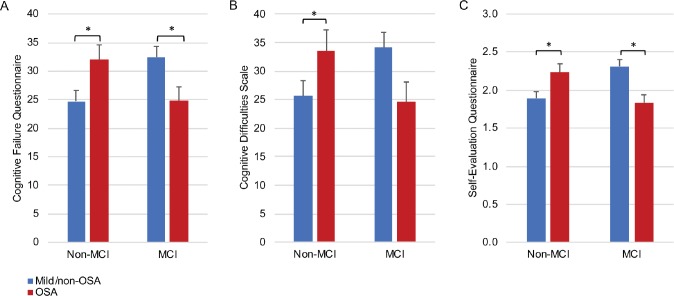
Group (OSA, mild/non-OSA) × cognitive status (MCI, non-MCI) interactions were found on Cognitive Failure Questionnaire (F1,105 = 11.84, P < .01), Cognitive Difficulties Scale (F1,104 = 7.79, P < .01), and Self-Evaluation Questionnaire (F1,83 = 17.69, P < .001) (Figure 1). Decomposition of interactions showed that, among participants without MCI, those with OSA had more SCC than those in the mild/non-OSA group (Cognitive Failure Questionnaire F1,105 = 7.61, P < .01; Cognitive Difficulties Scale F1,104 = 4.36, P < .05; Self-Evaluation Questionnaire F1,83 = 8.46, P < .01). Surprisingly, among those with MCI, the OSA group had less SCC than the mild/non-OSA group (Cognitive Failure Questionnaire F1,105 = 4.88, P < .05; Cognitive Difficulties Scale F1,104 = 3.64, P = .05; Self-Evaluation Questionnaire F1,83 = 10.02, P < .01). Although our sample size limited the number of covariables that were included in our analyses, exploratory analyses using ApoE4 and sex as covariables were performed, separately. Our group × cognitive status ANOVA remained significant for all questionnaires (see supplemental material).
Figure 1. Scores on cognitive complaint questionnaires according to group and cognitive status.
This figure represents group (mild/non-OSA, OSA) × cognitive status (MCI, non-MCI) interactions for (A) Cognitive Failure Questionnaire, (B) Cognitive Difficulties Scale and (C) Self-Evaluation Questionnaire. For all questionnaires, among participants without MCI, the OSA group had more SCC than the mild/non-OSA group. However, the reverse pattern was observed among patients with MCI where the OSA group had less SCC than the mild/non-OSA group. Error bars represent standard error. * = P < .05. MCI = mild cognitive impairment, OSA = obstructive sleep apnea.
In order to understand why those in the OSA group reported less SCC than those in the mild/non-OSA group when they have MCI, we conducted a posteriori correlation analyses between SCC questionnaire scores and neuropsychological scores in participants with OSA. These analyses could identify a particular cognitive profile associated with lower SCC scores in the presence of MCI. We found that participants reporting less SCC had lower performances on tasks involving episodic verbal learning and memory (Rey Auditory-Verbal Learning Test recognition, r = .44, P < .01) and executive functions/visuospatial abilities (Trail B-A score, r = −.39, P < .01 and Rey-Osterrieth Complex Figure, r = .48, P < .01). These positive associations between lower SCC and poorer cognitive performance were not found in participants in the mild/non-OSA group. In fact, the mild/non-OSA group showed a negative association: more SCC was associated with a lower performance on a cognitive test involving language (Boston Naming Test, r = −.39, P < .01), and no other significant association was found.
Factors Associated With More SCC
Briefly, when all participants were included, we found that OSA severity (ie, number of awakenings, AHI, mean oxygen saturation, and time with oxygen saturation < 90%) was not associated with SCC questionnaire scores (r = .003 to .14; P = .97 to .16). Correlation analyses performed between mood/sleep quality questionnaires and SCC showed significant positive correlations between all questionnaires (Beck Depression Inventory-II; Beck Anxiety Inventory; Pittsburgh Sleep Quality Index; and Epworth Sleepiness Scale) and SCC (r = .29 to .53; all P < .01), reflecting that participants who report cognitive impairments were also those reporting more depression symptoms, anxiety symptoms, worse sleep quality, and more sleepiness (Figure S1 and Figure S2 in the supplemental material).
DISCUSSION
Overview of the Main Results
In the current study, we found that among participants without MCI, those in the OSA group reported more SCC than the mild/non-OSA group. We found the reverse association among participants with MCI: in those cases, the OSA group had less SCC than the mild/non-OSA group. Moreover, in the OSA group, those with poorer performance on memory and executive function tests were those with the lowest SCC. Markers of OSA severity (AHI, sleep fragmentation, and hypoxemia) were not associated with increased SCC scores; however, symptoms of depression, anxiety, sleepiness, and poor sleep quality were all associated with increased SCC. Our results are of particular interest for clinicians, because self-reported cognitive function by patients with OSA might not accurately reflect their actual cognitive status.
Are Individuals With OSA Less Aware of Their Cognitive Impairments?
The awareness of cognitive impairment differs between the mild/non-OSA and OSA groups, with the OSA group showing a less accurate evaluation of their cognitive status. OSA could therefore play a role in the ability to properly assess cognitive functioning. Possible factors that could explain this lack of awareness include, among others, cognitive deficits specifically affecting judgment and self-criticism. In fact, lack of awareness for cognitive impairment, also referred to as anosognosia, has been reported in MCI and early-stage Alzheimer disease.40 This unawareness seems related to the severity and the nature of the cognitive impairment. Thus, individuals are more aware of cognitive changes in the very early stages of cognitive decline, but as the neurodegenerative process progresses, they lose their ability to accurately assess their cognitive performance.41 Specific brain dysfunctions have been associated with lack of awareness in MCI and early stages of Alzheimer disease, namely hypoperfusions of the right prefrontal cortex and the hippocampus.42,43 Interestingly, these brain regions have been identified as hypoper-fused in older individuals with OSA.1,44,45 Concordant with these findings, in our OSA sample, participants with less SCC had lower performances on neuropsychological tests involving the hippocampus (Rey Auditory-Verbal Learning Test recognition) and the prefrontal cortex (Trail B-A score and Rey-Osterrieth Complex Figure copy).46–48 We can therefore hypothesize that the weaker awareness of cognitive deficits in OSA is possibly related to abnormal prefrontal and hippocampal functioning.
In the mild/non-OSA group, participants with more SCC had objective cognitive impairments, or MCI. Contrary to participants in the OSA group, those in the mild/non-OSA group were able to accurately assess their cognitive functioning. Interestingly, the mild/non-OSA group participants with higher SCC had lower performances on the Boston Naming Test, a neuropsychological test sensitive to early Alzheimer disease.49 Taken together, our results suggest that the cognitive impairment of mild/non-OSA was insufficient to alter their self-assessment. The prefrontal cortex and the hippocampus are selectively affected by sleep deprivation and hypoxemia.2 Therefore, the presence of hypoxemia and sleep disturbances could contribute to the early lack of awareness in individuals with OSA by affecting brain regions associated with anosognosia.
We also cannot exclude that how cognitive impairments appear could be a contributing factor. Adults with OSA may have cognitive impairments due to daytime sleepiness that progressively evolve. This gradual occurrence of relatively mild cognitive deficits could make patients less aware of their cognitive changes.
Mood and Self-Reported Sleep Quality, But Not OSA, Are Associated With More SCC
Current results on the presence of SCC in late middle-aged and older adults with OSA are scarce and inconsistent.10,11 Our study sheds new light on the discrepancies between previous studies. We found that more severe depressive and anxiety symptoms, sleepiness, and poor sleep quality, as reported by participants, were highly correlated with higher SCC scores.
Sforza et al. found no group differences on the Cognitive Difficulties Scale between individuals with OSA and mild/ non-OSA aged 65 years and older.11 In line with our results, their participants showed low anxiety and depression scores; moreover, sleepiness scores were lower compared to what we observed in our participants with OSA. However, Vernet et al. found that individuals with OSA aged 55 years and older reported significantly more SCC than those with mild or no OSA.10 Importantly, these authors recruited participants based on high self-reported daytime sleepiness (Epworth Sleepiness Scale > 10) and these patients also had high depression and anxiety scores. Taken together, our results and those of previous studies indicate that SCC in individuals with OSA is more strongly correlated with mood and/or sleepiness complaints than with OSA severity per se.
Strengths and Limitations
This study is the first that uses different instruments to detect SCC in OSA. Furthermore, it includes a subgroup with MCI to assess the ability of participants to evaluate their cognitive functioning. The prevalence of MCI in our sample was higher than what is observed in the general population. The recruitment of participants in the sleep apnea clinic and the fact that our newspaper ads indicated that it was a study on sleep and cognitive health may have created a bias. In fact, people with concerns about their sleep or cognitive functioning were possibly more likely to participate in our study. However, we had a similar and relatively high proportion of MCI in both groups, which allowed better comparisons between groups on the nature of the self-reported cognitive complaint. Although our participants with OSA had a wide range of OSA severity (AHI from 15 to 84 events/h), they presented only mild nocturnal hypoxemia (mean minimal oxygen saturation: 84.47 ± 6.57%) and part of them had mild daytime symptoms (mean Epworth Sleepiness Scale: 9.75 ± 4.89); thus, our results might not be applicable to populations with severe OSA.
CONCLUSIONS
Self-reported alterations of mood and sleep quality were associated with an increasing risk of SCC in individuals with OSA. Interestingly, we found that OSA may lead to a disconnection between SCC and MCI. Our results underscore the importance of using objective neuropsychological assessments to obtain accurate cognitive profiles in older individuals presenting with OSA, who are probably less aware of their cognitive deficits. In the current study, all SCC questionnaires showed very similar result patterns. Our results also bring new insight to understand the low adherence to continuous positive airway pressure treatment that is particularly prevalent in older adults.50 Arguably, it would be more difficult to convince patients to adhere to an expensive and somewhat invasive treatment if they have no complaints about daytime functioning. The lack of awareness of MCI found in OSA should be considered as a specific OSA cognitive impairment. Further longitudinal studies with large cohorts are necessary to determine whether the lack of awareness of cognitive impairment in older individuals with OSA predicts dementia.
DISCLOSURE STATEMENT
The research work was conducted at the Centre for Advanced Research in Sleep Medicine of the Hôpital du Sacré-Coeur de Montréal, Montreal, Canada. All authors have seen and approved the manuscript. This research was supported by the Canadian Institutes of Health Research (MOP123294) and by the Fonds de Recherche du Québec – Santé. Ms. Gagnon, D'Aaragon, Chami and Drs. Poirier, De Beaumont and Lafond report no conflicts of interest. The Canadian Institutes of Health Research supported Ms. Baril with a doctoral scholarship and Dr. Gagnon with an Investigator Salary Award. The FRQ-S also supported Drs. Gosselin, Gagnon, and Carrier with a Salary Award. Dr. Gagnon holds a Canada Research Chair in Cognitive Decline in Pathological Aging and Dr. Montplaisir holds a Canada Research Chair in Sleep Medicine. Dr. Gauthier reports grants from Canadian Institutes of Health Research, CQDM network, Pfizer-Fond de Recherche Santé -Québec, during the conduct of the study. He reports personal fees from Lilly, Roche, TauRx, Lundbeck, AbbVie, ADvantage, Alzheon, Axovant, Boehringer-Ingelheim, Firalis, Heptares, IntelGen, Klagene, Novartis, Otsuka, Servier, Sanofi, Schwabe, and Tacked. He received nonfinancial support from Alzheimer's Disease Cooperative Study and Alzheimer's Disease Therapeutic Research Institute that is not linked to the current work.
ACKNOWLEDGMENTS
The authors thank Hélène Blais, Jean Paquet, and Marie-Josée Quinn from the Centre for Advanced Research in Sleep Medicine for their efforts and support in data acquisition and analyses.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- ApoE4
Apolipoprotein epsilon 4
- MCI
mild cognitive impairment
- OSA
obstructive sleep apnea
- SCC
self-reported cognitive complaint
- SpO2
oxygen desaturation
REFERENCES
- 1.Baril AA, Gagnon K, Arbour C, et al. Regional cerebral blood flow during wakeful rest in older subjects with mild to severe obstructive sleep apnea. Sleep. 2015;38(9):1439–1449. doi: 10.5665/sleep.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 3.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–1971. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendonca MD, Alves L, Bugalho P. From subjective cognitive complaints to dementia: who is at risk?: a systematic review. Am J Alzheimers Dis Other Demen. 2016;31(2):105–114. doi: 10.1177/1533317515592331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hohman TJ, Beason-Held LL, Lamar M, Resnick SM. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25(1):125–130. doi: 10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13:369–396. doi: 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 9.Vaessen TJ, Overeem S, Sitskoorn MM. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev. 2015;19:51–58. doi: 10.1016/j.smrv.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Vernet C, Redolfi S, Attali V, et al. Residual sleepiness in obstructive sleep apnoea: phenotype and related symptoms. Eur Respir J. 2011;38(1):98–105. doi: 10.1183/09031936.00040410. [DOI] [PubMed] [Google Scholar]
- 11.Sforza E, Roche F, Thomas-Anterion C, et al. Cognitive function and sleep related breathing disorders in a healthy elderly population: the SYNAPSE study. Sleep. 2010;33(4):515–521. doi: 10.1093/sleep/33.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CW, Yang CM, Chen NH. Objective versus subjective cognitive functioning in patients with obstructive sleep apnea. Open Sleep J. 2012;5:33–42. [Google Scholar]
- 13.Daurat A, Huet N, Tiberge M. Metamemory beliefs and episodic memory in obstructive sleep apnea syndrome. Psychol Rep. 2010;107(1):289–302. doi: 10.2466/10.13.20.22.PR0.107.4.289-302. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin N, De Beaumont L, Gagnon K, et al. BDNF Val66Met polymorphism interacts with sleep consolidation to predict ability to create new declarative memories. J Neurosci. 2016;36(32):8390–8398. doi: 10.1523/JNEUROSCI.4432-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 16.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Villeneuve S, Massoud F, Bocti C, Gauthier S, Belleville S. The nature of episodic memory deficits in MCI with and without vascular burden. Neuropsychologia. 2011;49(11):3027–3035. doi: 10.1016/j.neuropsychologia.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 21.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 23.McNair DM, Kahn RJ. Self-assessment of cognitive deficits. In: Crook T, Ferris S, Bartus R, editors. Assessment in Geriatric Psychopharmacology. New Canaan, CT: Mark Powley Associates; 1983. [Google Scholar]
- 24.Van der Linden M, Wijns C, von Frenkell R, Coyette F, Seron X. Le QAM, questionnaire d'auto-évaluation de la mémoire. Brussels, Belgium: Editions EDITEST; 1989. [Google Scholar]
- 25.Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(3):126–135. doi: 10.1212/WNL.0000000000004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 27.Conners CK. Conners' Continuous Performance Test (CPT-2) Computer Program for Windows, Technical Guide, and Software Manual. Toronto, ON: Multi Health Systems Inc.; 2000. [Google Scholar]
- 28.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 29.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 30.Culbertson WC, Zillmer EA. Tower of London-Drexel University, Second Edition. Toronto, ON: Multi-Health Systems; 2004. [Google Scholar]
- 31.Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. Arch Psychol. 1941;28(112):286–340. [Google Scholar]
- 32.Benedict RHB. Brief Visuospatial Memory Test - Revised: Professional Manual. Lutz, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 33.Gauthier L, Dehaut F, Joanette Y. The Bells Test: a quantitative and qualitative test for visual neglect. J Clin Exp Neuropsychol. 1989;11(2):49–54. [Google Scholar]
- 34.Qualls CE, Bliwise NG, Stringer AY. Short forms of the Benton Judgment of Line Orientation Test: development and psychometric properties. Arch Clin Neuropsychol. 2000;15(2):159–163. [PubMed] [Google Scholar]
- 35.Osterrieth P. Le test de copie d'une figure complexe. Arch Psychol. 1944;30:206–356. [Google Scholar]
- 36.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lee & Febiger; 1983. [Google Scholar]
- 37.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;(11 Suppl 2):S33–S39. [PubMed] [Google Scholar]
- 38.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 39.Luck T, Riedel-Heller SG, Luppa M, et al. Risk factors for incident mild cognitive impairment--results from the German Study on Ageing, Cognition and Dementia in Primary Care Patients (AgeCoDe) Acta Psychiatr Scand. 2010;121(4):260–272. doi: 10.1111/j.1600-0447.2009.01481.x. [DOI] [PubMed] [Google Scholar]
- 40.Vogel A, Stokholm J, Gade A, Andersen BB, Hejl AM, Waldemar G. Awareness of deficits in mild cognitive impairment and Alzheimer's disease: do MCI patients have impaired insight? Dement Geriatr Cogn Disord. 2004;17(3):181–187. doi: 10.1159/000076354. [DOI] [PubMed] [Google Scholar]
- 41.Starkstein SE. Anosognosia in Alzheimer's disease: diagnosis, frequency, mechanism and clinical correlates. Cortex. 2014;61:64–73. doi: 10.1016/j.cortex.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 42.Sedaghat F, Dedousi E, Baloyannis I, et al. Brain SPECT findings of anosognosia in Alzheimer's disease. J Alzheimers Dis. 2010;21(2):641–647. doi: 10.3233/JAD-2010-090631. [DOI] [PubMed] [Google Scholar]
- 43.Vogel A, Hasselbalch SG, Gade A, Ziebell M, Waldemar G. Cognitive and functional neuroimaging correlate for anosognosia in mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry. 2005;20(3):238–246. doi: 10.1002/gps.1272. [DOI] [PubMed] [Google Scholar]
- 44.Innes CR, Kelly PT, Hlavac M, Melzer TR, Jones RD. Decreased regional cerebral perfusion in moderate-severe obstructive sleep apnoea during wakefulness. Sleep. 2015;38(5):699–706. doi: 10.5665/sleep.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shiota S, Inoue Y, Takekawa H, et al. Effect of continuous positive airway pressure on regional cerebral blood flow during wakefulness in obstructive sleep apnea. Sleep Breath. 2014;18(2):289–295. doi: 10.1007/s11325-013-0881-9. [DOI] [PubMed] [Google Scholar]
- 46.Saury JM, Emanuelson I. Neuropsychological assessment of hippocampal integrity. Appl Neuropsychol Adult. 2017;24(2):140–151. doi: 10.1080/23279095.2015.1113536. [DOI] [PubMed] [Google Scholar]
- 47.Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13(2):230–239. [PubMed] [Google Scholar]
- 48.Elderkin-Thompson V, Kumar A, Mintz J, Boone K, Bahng E, Lavretsky H. Executive dysfunction and visuospatial ability among depressed elders in a community setting. Arch Clin Neuropsychol. 2004;19(5):597–611. doi: 10.1016/j.acn.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Chung SC, Choi MH, Kim HS, et al. Differences in and correlations between cognitive abilities and brain volumes in healthy control, mild cognitive impairment, and Alzheimer disease groups. Clin Anat. 2016;29(4):473–480. doi: 10.1002/ca.22684. [DOI] [PubMed] [Google Scholar]
- 50.Yang MC, Lin CY, Lan CC, et al. Factors affecting CPAP acceptance in elderly patients with obstructive sleep apnea in Taiwan. Respir Care. 2013;58(9):1504–1513. doi: 10.4187/respcare.02176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.