Abstract
Traumatic spinal cord injury (SCI) results in some serious neurophysiological consequences that alter healthy body functions and devastate the quality of living of individuals. To find a cure for SCI, researchers around the world are working on different neurorepair and neurorehabilitation modalities. To test a new treatment for SCI as well as to understand the mechanism of recovery, animal models are being widely used. Among them, SCI rat models are arguably the most prominent. Furthermore, it is important to select a suitable behavioral test to evaluate both the motor and sensory recovery following any therapeutic intervention. In this paper, we review the rat models of spinal injury and commonly used behavioral tests to serve as a useful guideline for neuroscientists in the field of SCI research.
Keywords: Neurology, Physiology, Rehabilitation, Surgery, Anatomy, Neuroscience
1. Introduction
Spinal cord injury (SCI) is responsible for damaging the sensory, motor and autonomic functions of an individual [1]. To address this problem, over the past few decades animals have been used for developing and testing therapies for SCI [2]. Both large and small animal models are commonly used to study the injury mechanism and to assess the therapeutic outcomes after a controlled SCI in laboratory settings [3]. Large animals, especially dogs and cats were used in the early 1950s–1970s [4]. Afterward, rodent models became popular and were then standardized as a suitable animal model for SCI experiments. In rodent models, rats and mice are mostly used to evaluate the functional and morphological changes after SCI [5, 6, 7]. However, in the mouse model, following a SCI, evidence suggests that a modest degree of regeneration occurs in the injured area and no typical cyst-like structure is formed, while such type of axonal regeneration is not found in rat and non-human primates [1]. In contrast, after an injury in rats, a large fluid-filled cystic cavity is formed which is similar to the human pathology after SCI and thus useful for preclinical studies [8]. Rats are quickly adaptive to the experimental environments and to the given tasks for sensorimotor evaluations. Furthermore, high availability, low infection rate after surgery, and ease of maintenance make the rat a prime candidate for translational research. Based on the above, laboratory rats are now widely used as experimental SCI models.
In this translational research, several rat injury models were developed to examine the neurophysiological responses after a SCI. Numerous approaches are being applied to regenerate the damaged axon across the lesioned area, and confirmed immunohistochemically and by protein analysis in rats [9, 10]. Furthermore, apart from histological analysis, behavioral responses are also studied as a primary measure to assess the recovery of spinal pathways. Other reviews [11, 12, 13, 14] focused on the different animal models of SCI particularly cervical injury and some behavior tests. However, to our knowledge, no study has comprehensively described about all the injury models and their impact on the behavioral changes. In this review, we focused on different SCI rat models and their recovery assessments using different behavioral tests. We also propose some novel behavioral tests to enrich this research. Our review offers a guideline for the researchers in this field to successfully conduct SCI research using rats.
2. Main text
2.1. Rat SCI models
Experimental injury models are designed to explore the physiological changes after SCI to evaluate the treatments to repair the broken spinal cord. Laboratory rats are commonly used to evaluate neural repair after an experimental SCI. Although some SCI experiments have been conducted in non-human primates, more demanding protocols, critical manpower, health safety and a large amount of investment in the experimental setup and management make the primate model much more difficult and less used than rat injury models [15]. In rat SCI experiments, three different injury models are widely utilized: compression, contusion and transection models. Among these, contusion and compression are the most common types of injury found in humans. Hence, to evaluate the neuronal changes and behavioral outcomes, these models are essential [7].
2.1.1. Compression model
In humans, flexion and axial compression (burst fracture) are responsible for displacing bone fragments into the vertebral canal and consequently SCI occurs. However, the timing of compression can affect the outcome of pathology [16]. To address this problem, in rats the same model of injury is carried out to create persistent spinal canal occlusion that is similar to human acute SCI. This injury is varied at different levels of the spinal cord. For example, at lumbar level, compression injury impairs the ejaculation reflex in male rats [17]. There are several ways to create a compression model for rats such as calibrated forcep compression, clip compression and baloon compression [16]. However, the clip compression model at the thoracic level is much more correlated with the functional and histological outcome [18].
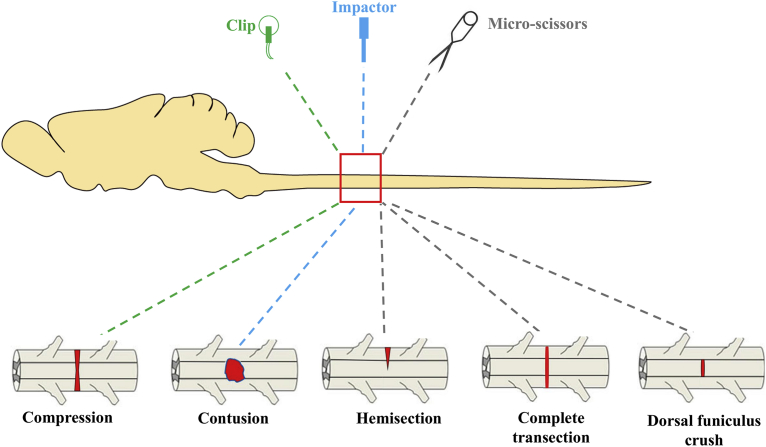
The acute clip compression model is the first non-transection model that was developed in 1978 [19] and is widely used for experimental injury in rats. It is an inexpensive method where a special clip is used to compress the spinal cord [20]. Under inhalation anesthesia, a laminectomy is performed at a desired level of the spine by retracting muscles and removing the spinous process and body of vertebrae. A modified aneurysm clip (Fig. 1) is then inserted extradurally and maintained for 60 sec and then the clip is removed to produce an acute injury [21]. Muscles and connective tissues are then sutured, and the skin is closed.
Fig. 1.
The diagram represents different injury models in rats' spinal cord. In the compression model a clip is used to initiate the injury. For the contusion model of injury an impactor is dropped from a predefined distance. In the transection model of injury, a surgical blade is used to carry out different types of transection injury. Modified from [113].
Due to the similarity to human traumatic SCI, the compression injury model is also suitable for translational research. In a chronic condition of a compression SCI model, glial scars are formed which are quite similar to those in SCI patients [22]. To examine the therapeutic effect and neuroprotective study following a traumatic SCI, the compression model of injury is a better choice for the experiment. Furthermore, this model is useful to study the minimal loss of neurons after a SCI. In addition, the compression injury model is also useful to study the secondary injury mechanism events and cell transplantation therapies [23]. An important limitation of the compression method, however, is that after the injury some neural tracts may not be properly damaged which may lead to undesired outcomes in regeneration studies. Moreover, after 4 and 6 weeks of post-injury, tissue sparing may increase, suggesting that the late subacute and early intermediate stages of the secondary injury may be associated with limited neuroanatomical recovery [24].
2.1.2. Contusion model
In humans, compression injury is caused by mainly falls from a height or by a physical impactor that crushes the spinal vertebral column which further leads the bony parts to compress the spinal cord [1]. In rats, to get the same type of injury, the contusion model is probably the most suitable method [25]. Among the several anatomical locations, in thoracic position contusion injury is commonly used to evaluate the forward and backward locomotion as well as recovery of the spinal tract [26]. Usually, the injury is induced by an impactor which is used to drop a 10-gm rod onto the spinal cord [27]. Several impactors are available worldwide to serve this purpose. In Multicenter Animal Spinal Injury Study (MASCIS), the New York university impactor, Infinite Horizon (IH) impactor and Ohio State University Electromagnetic Spinal Cord Injury Device (ESCID) are most commonly used. In MASCIS, a 10-gm computer-controlled load is used to induce a SCI in rats [28]. After laminectomy, at a desired spinal level, muscles and spinous process are removed according to the diameter (usually 2.5 mm) of the impactor (Fig. 1). Height of the impactor, drop weight and time are previously set up on a computer before initiation of the injury. After the injury, subdural hemorrhage is commonly found which is later washed with saline [27]. Finally, muscles and skin are sutured. Another reliable commercially available impactor is Infinite Horizon (IH) which is also used to produce SCI rat models. System software is implemented to apply impactor force to the spinal cord. This injury renders an ideal model of SCI by reducing the variability among other existing devices [29].
Most SCIs reported in humans are similar to contusion and compression [8, 30]. Although the contusion SCI animal model is useful to study the secondary injury mechanism and neuropathology of human injury [7], some limitations still persist [31]. In neuroregeneration research, after a thoracic contusion injury, it is difficult to study the fiber's regeneration through the contusion site [32]. Furthermore, the contusion model cannot provide information regarding axonal tract regeneration which is essential to evaluate limb function [33].
2.1.3. Transection model
The transection model of injury is the most widely utilized method that can give the information of desired neural tracts at different spinal level after injury. To investigate the neuronal and functional improvements after different transplantation therapies, transection models of SCI are commonly used [34]. This injury model is quite stable and assessment can be performed within four weeks of the injury [35]. The spinal cord transection model is also useful to evaluate axonal regeneration and behavioral responses after SCI [36]. Transection injury cannot produce any condition similar to contusion injury. However, this injury is useful to investigate the damaged tract recovery after experimental therapies. Two main transection models (complete and incomplete) are used in rats. In the incomplete transection model (often called hemisection model), different portions of spinal cord are cut such as lateral hemisection, dorsal hemisection and dorsal funiculus crush to carry out different experiments. However, incomplete surgical transections are not considered relevant to human SCI [13]. A complete injury requires a complete transection of the spinal cord including both ascending and descending tracts. To carry out a complete transection injury after laminectomy, microdissection scissors are inserted to cut the spinal cord at a desired level and depth. After an injury gelfoam is inserted into the transection site to minimize the bleeding and to confirm the separation of the spinal cord. After some complete transections ventral parts of the axons may be spared, which is responsible for some residual motor function of the hindlimb [37]. For an incomplete transection injury iridectomy scissors are used to cut both the dorsal and ventral columns of the spinal cord from lateral to the midline by closing the tip of the scissors [38]. Muscles and skin incision are then sutured, and the rat is examined for therapeutic approaches and behavioral tests after recovery. Complete and incomplete transection injuries are not very similar to the clinical SCI of humans; they are rather utilized for neuroscience and neurology research to study the neuronal circuits and pathways [13].
Transection injury can give information on both ascending and descending tract functions which is useful in neuroscience research [7]. Compared to other injury models there is no need for a special device to transect the spinal cord. Before transecting the spinal cord, it is essential to understand the neurophysiology of the different anatomical structures of the nervous system. A complete transection injury event involves the dissection of both ascending and descending tracts [22]. Special care should be taken for the pain management and urinary bladder management after a complete transection. To study specific spinal tract activity such as dorsal and dorsolateral CST, an incomplete transection (or hemisection) injury can be applied. After therapeutic interventions, this injury can also provide information regarding axonal tract regeneration. The hemisection model of injury is suitable at the level of cervical region CST which is responsible for forelimb reaching tasks. To regenerate axonal tracts for translational research after any therapeutic approach or cell transplantation, the transection model of injury can be a choice for experiments [39]. However, the transection injury model is unable to study the secondary injury mechanism.
2.1.4. Selection of an optimum injury model
Pathological and behavioral functions that alter after a SCI depend on the site and type of the injury. A correct injury model can give a proper understanding of the system and the cellular-level changes after a SCI. Furthermore, an experimental SCI model is necessary to evaluate the safety and reliability of a new therapeutic intervention before translating it to humans [40]. Every injury model has advantages and disadvantages over other models. Some selection criteria (listed in Table 1) are necessary to select the most optimum one for a specific study. For example, for translational research, contusion and compression injury models are useful as these models mimic some relevant characteristics of the lesions of traumatic injuries of the spinal cord. As they mimic real-life events, both models are also useful to study the secondary injury mechanism. Even though there are clear advantages of clinical translation of the injuries, both models fail to confirm the anatomical lesion, confidently and precisely. In contrast, transection injury model confidently cuts specific neural roots and circuits as per experimenter's demand and thus very useful for neuroscience research [13]. However, a post-experiment histology might be required to confirm the lesion. To study a specific drug for nerve regeneration in the lesioned spinal cord a specific nerve root lesion via incomplete transection model is suitable [41, 42]. However, for cell transplantation treatment, a compression model is recommended.
Table 1.
Summary of the major injury models and their selection criteria.
| Contusion | Compression | Transection |
|---|---|---|
2.2. Behavioral examinations
Laboratory rats are widely used to evaluate different types of behavioral tests that involve neurological functions. After an experimental SCI in the desired location, alteration in the spinal cord and neurological function can be confirmed with behavioral assessment. Hence, behavioral examination is one of the fundamental tests after any therapeutic intervention of SCI. Different behavioral tests are carried out to estimate the pre- and post-treatment functions of various neurological tracts of the sensorimotor system [43]. According to the data collection procedure and functional organization of the nervous system these tests are categorized as: 1) endpoint measures, in which a certain behavior receives a score according to some predefined scale, e.g. number of pellets eaten in a given time; 2) kinematic measurements, which range from a qualitative description of movements to continuous quantitative measurements, e.g. Basso-Beattie-Bresnahan (BBB) scale; 3) kinetic measurements, which quantify the force produced by the limbs, e.g. forelimb grip strength tests; and 4) electrophysiological measurements, e.g. motor and somatosensory evoked potentials [43, 44]. Based on the neurological tracts used [14], these behavioral examinations can be divided into: 1) Motor test, e.g., limb grip strength test, forelimb reaching task; 2) Locomotor test, e.g. BBB scale, swim test; 3) Sensory test, e.g. Von Frey filament test, test for hot and cold sensation; and 4) Sensorimotor test, e.g ladder walking test, grid walking test, rope climbing and walking test.
2.3. Motor test
Descending tracts of motor neurons which comprises both pyramidal and extrapyramidal tracts convey information to the lower motor centers in the spinal cord that are responsible for transferring information to the muscle fiber to initiate movements of the limbs [45]. The corticospinal tract (CST) is one of the foremost descending tracts of the motor system that plays a vital role in skilled motor tasks by conveying volitional information to the limbs. The degree of CST injury is strongly co-related to the impairment of skilled motor function after a SCI [46]. After a cervical cord injury, motor control of the forelimb is profoundly affected due to the impairment of the CST. Motor functions may be restored after skilled motor learning training that assists in the reorganization of the nerve fibers [47]. To assess the motor recovery of descending tracts, several functional tests have been developed. These tests include evaluation of the motor functions of different limbs after complete and incomplete SCI. Among these tests the Irvine, Beatties and Bresnahan (IBB) scale, skilled forelimb reaching task and limb grip strength test are the most common to evaluate the motor functions after an experimental SCI in laboratory rats. In the following sections, we discuss them in detail.
2.3.1. Skilled forelimb reaching task
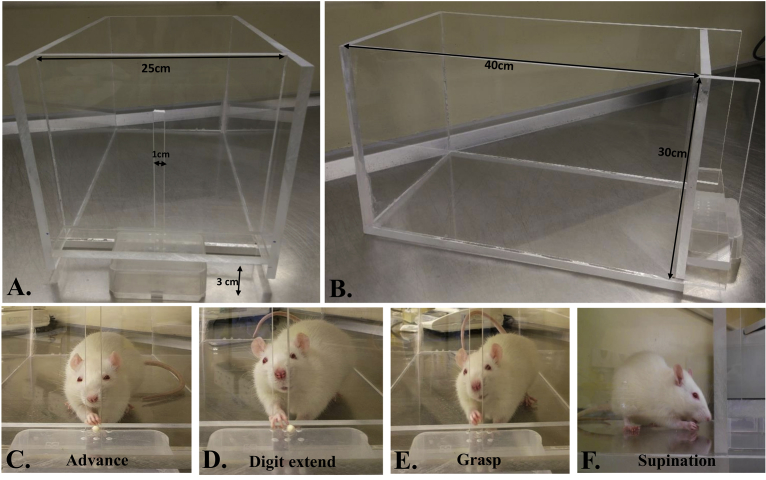
The forelimb reaching task is time-consuming and needs several weeks of training to induce motor learning for forelimb food pellet retrieval. First, rats are habituated with the apparatus and special food pellet (usually sugar pellet) for one to two weeks. Food restriction is given from 3 days prior to starting the training. Training is provided for several weeks for the rats to master the reaching and grasping of the food pellets. A pellet is placed on a pit platform in front of the box slit and it must be ensured that the rat approaches the opening in a continuous manner as shown in (Fig. 2). Ten pellets for warm up and 20 pellets per task are usually used to evaluate the reaching behavior. Qualitative and quantitative assessments of a rat's skilled reaching task are performed after 4 weeks of training (5 sessions/week) [48, 49]. For qualitative assessment, 10 components of movements (digits to the midline, digits flexed, elbow in, advance, digits extended, arpeggio, grasp, supination I and II) are rated. Animals' reaching is rated on a 3-point scale. For normal reaching a score of “0” and for abnormal movement “1” is used. If there is no movement then “2” is given [50, 51]. For quantitative assessment, the success rate of a rat's reaching is usually calculated. It includes the total number of pellets retrieved and eaten. The following equation is used for reaching success rate estimation:
Fig. 2.
Skilled forelimb reaching task for a laboratory rat. A) A special Plexiglas chamber (40 cm × 25 cm × 30 cm) with a 1–2 cm wide opening for grasping the food pellet from a pit is normally used which is 3 cm above the base. B-E) Different stages (advance-forelimb is advanced through a slot to the platform, digit extension-forepaw is extended toward the pellet, grasp-paw grasps the pellet and supination-paw is withdrawn from the slot and a pellet is successfully taken into the mouth) of reaching and grasping of a sugar pellet by a trained Sprague Dawley rat.
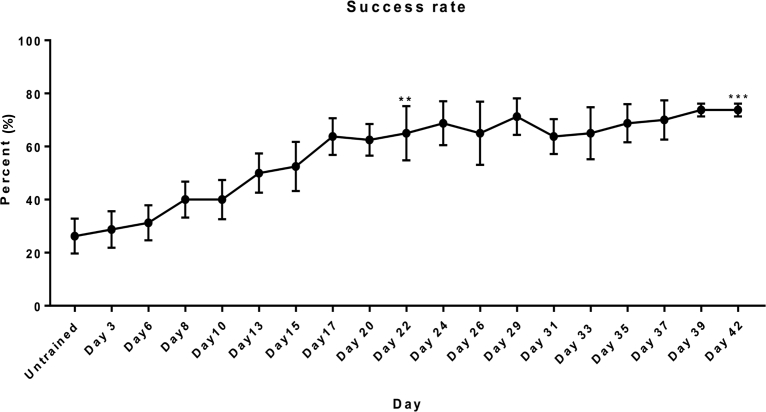
Furthermore, the amount of time required for grasping the pellets can also be measured to evaluate forelimb funtional recovery [52]. Pre-injury assessment of success rates for forelimb reaching was performed for five rats. The experimental protocol was approved by the Animals Subjects Ethics Sub-committee (ASESC) of The Hong Kong Polytechnic University. Fig. 3 demonstrates the average number of food pellets grasped and consumed in each trial (20 pellets per trial) by all the five rats. After one week of apparatus habituation, the rats were trained to grasp 45 mg of dustless sugar pellets (Bio-serv®, Flemington, NJ, USA). After 42 days of training, reaching scores for each time point were compared to the untrained day 1 score. Significant differences of motor learning were found on day 22 and day 42 of the training (P < 0.05 and P < 0.001 respectively; paired t-test).
Fig. 3.
Success rate (Mean ± SEM) of the food-pellet reaching task of uninjured rats (n = 5). Significant differences were found after 22 days (**P < 0.05) and 42 days (***P < 0.001) of reaching and grasping training from day 1 (untrained).
A major limitation of the skilled forelimb reaching task is that partial lesion may not impair the reaching success and qualitative scores including forepaw aiming, supination and food pellet release. However, it is also expected that complete restoration of the damaged corticospinal pyramidal tract will not restore the original behavioral condition [53].
2.3.2. Irvine, Beatties and Bresnahan (IBB) scale for forelimb function
The IBB scale is a 10-point scale to identify recovery of both proximal and distal forelimb functions after unilateral cervical SCI. Unlike the forelimb reaching task, acclimatization of rats and food deprivation is not necessary for this test [54]. First, a rat is placed in a cylindrical Plexiglas chamber for acclimation to the food. When the rat is well adapted to the apparatus and food, grasping and eating behavior are recorded by a video camera. Various sizes (spherical and doughnut) of cereals are generally used to assess forelimb function. From the recorded video, elbow position (extended or flexed), supporting of the paw (contact or noncontact), position of forepaw (clubbed, extended or flexed), digit movements and grasping method are evaluated [55]. This test, however, is a qualitative assessment of the forelimb function. Visual inspections by the experimenter may affect the experimental assessment and outcomes. Sometimes, the recorded video fails to provide any information for scoring as the animal often shifts position during eating the cereals [54].
2.3.3. Limb grip strength test
A grip strength test is used to evaluate both forelimb and hindlimb functions [56]. Quantitative strength of the flexor muscles can be measured by this test. Grip strength measurement is important because it can predict any motor deficit in the limbs after a SCI. Anderson and colleagues showed that after a complete unilateral hemisection, rats lose the gripping ability permanently [57]. Acclimatization of rats to the testing apparatus is necessary before starting the test [58]. At the beginning of measurement, the rat is held by the neck or back and allowed to grab the metal grid or pull bar with either forepaw or hindpaw. The rat is then gently pulled away in horizontal plane by the tail. The sensor reads the pull strength until the rat releases the bar. Maximum grip strength is then measured from the recorded force. For each limb, several testing sessions are usually conducted to evaluate the maximum grip strength. This method is a simple procedure to evaluate the recovery over time and the response is immediate. However, after daily repeated testing, rats may hesitate to grasp if they feel an unpleasant sensation when pulled by the tail [59]. Unlike to other motor tests, in grip strength test, rats are forcibly motivated to perform the task. Hence, the measured value may differ from their natural grip strength value. The test values are also experimenter-dependent, adding more variability into the measurement. Furthermore, due to multiple measurements, the muscle may become fatigue and results may differ from each measured value.
2.4. Locomotor test
In mammals, the spinal cord contains an intrinsic locomotor mechanism that is controlled by a rhythmic central pattern generator (CPG) [60]. The location of the CPG in rats is found throughout the spinal cord, but most prominently at the lower lumbar part that rhythmically activates flexor and extensive motor pools of hindlimbs during locomotion [61]. To transmit the neuronal impulse, the dorsal root of the spinal cord carries sensory information and transmits it to the CPG for integration within the spinal cord for motor output (Windhorst 2007). During locomotion, the motor output carries information for the gait control (Grillner and Jessell 2009). Hence, to evaluate the recovery after SCI, locomotion tests of rats are commonly used [62]. Common locomotion tests include open-field test, swim test, BBB scale and BBB sub-scoring scale. In the following sections, we discuss these tests in detail.
2.4.1. BBB scale and BBB sub-scoring scale
In 1954, Tarlov and Klinger designed a qualitative scale for locomotion [63]. Due to less reliability and sensitivity, it was further modified to the Basso, Beattie and Bresnahan (BBB) scale [64] and is widely used to evaluate hindlimb locomotion function. It is a 21-point qualitative measurement developed for rats where ‘0’ refers to no observable hindlimb movement and ‘21’ refers to consistent hindlimb movement. This 21-point scale represents several stages during recovery such as movement of joints, forelimb and hindlimb coordination, trunk stability, toe clearance and tail position. From 0 to 7 is related to movement of the joints and 8 to 21 are related to planter stepping and consistent coordination of gait [64, 65].
The BBB scale has some advantages over other locomotor tests. For example, there is no training needed for this assessment. Furthermore, this test does not require any special apparatus, and thus rats can incorporate a wide range of functions [66]. BBB scale is an assessment for locomotion study which is widely used for the evaluation of interlimb coordination via reticulospinal and vestibular pathways [67]. Following a SCI, electrical and pharmacological stimulation can trigger the rhythmic circuit to generate locomotion type movements [68]. For studies of reticulospinal regeneration after a thoracic cord injury, the BBB scale is a good method to evaluate the assessment. Furthermore, rhythmic activity can give information on excitatory interneurons of the spinal cord. In the case of therapeutic treatment, the scale does not follow the order of the BBB scale [69]. Furthermore, for non-contusive models of SCI, many functional state conditions cannot represent the BBB scale [70].
To further solve the sensitivity of the BBB scale and to evaluate the higher motor function, a 7-point sub-scoring system was developed by Lankhorst and colleagues. In this sub-scoring scale, all the behaviors are cumulated independently and then added together to yield a single score [71]. The major limitation of the BBB sub-score is that it is only valid for mildly injured animals [72].
2.4.2. Open field test
The open field test is also the simplest method to evaluate locomotor behavior and anxiety in rats after a SCI. In 1934, Calvin Hall developed a test where defecation was used as a measure of anxiety [73]. It is also used to evaluate the efficacy of therapeutic drugs on anxiety-like behaviors in rodents [74]. To evaluate the exploratory behavior deficit after a SCI, open field test of rat is also used [75]. Under closely controlled conditions a square open field arena (60 × 60 cm), made of black Plexiglas is required to assess behavior. The bottom of the area is subdivided into 12 × 12 cm squares. A rat is placed at the center of the area and the number of squares crossed is counted and recorded [76]. Additionally, the open field test is also used to measure the locomotor recovery of hindlimbs [77]. Another modification to this test named open field motor test (OFT) was also developed for motor evaluation [78, 79].
2.4.3. Swim test
The swim test is a standardized natural evaluation method of locomotor recovery after SCI [80, 81]. The method is also suitable for measuring the spasticity after a contusive SCI [82]. Rebecca and colleagues developed the Louisville Swimming Scale (LSS) which mainly evaluates forelimb and hindlimb movements and body position. It cannot, however, uniformly evaluate mild to moderate SCI in rats [83]. To combat this problem, in 2015, Xu and colleagues developed a swimming scale to assess the locomotor recovery after a SCI in rats [72].
For the swim test, typically a Plexiglas swim tank (150 cm × 15 cm × 30 cm) is used [72]. The tank is usually filled with tap water (28–32 °C) to a depth of 20 cm. To observe rats during swimming, a mirror is placed underneath the tank at a 45-degree angle. A total of five trials per rat are used to evaluate the swimming behavior. Forelimb and hindpaw movements, and the distance between hindpaw and tail are used to analyze the swimming behavior. All the parameters are measured on an 8-point scale as described previously [84]. An important limitation of the test is that it cannot be performed in complete SCI animals. Moreover, the rats need extensive training to obtain effective results; otherwise it may induce depression behavior [85].
2.5. Sensory test
Sensory feedback such as afferent proprioception plays a significant role in the control of motor functions. Hence, sensory tests are used to evaluate the hyperactivity of rats in the presence of heat [86], cold [87] or pain [88]. After an experimental SCI and therapeutic interventions, sensitivity tests are useful to evaluate the sensory pathways of the experimental rats [89]. By selecting a correct hindlimb sensory test for these SCI rats, a sensory assessment can be carried out. In the following sections, we further discuss some of these sensory tests in detail.
2.5.1. Tactile sensory test or neuropathic pain test
To test hindlimb sensation, the tactile sensory test is used by determining whether the rat can stand or not after a SCI [90]. A group of special filaments (Von Frey Filament) are used to evaluate the improvement of neuropathic pain after therapeutic interventions in SCI rats [91]. Two validated testing methods - up/down plantar Von Frey (VF) test, and dorsal Von Frey Hair (VFH) monofilaments - are used for the evaluation [90]. Before the evaluation, rats are habituated with the cage by providing cereal grain so that they can seek food for a reward. The up/down plantar VF test is calibrated by VF microfilaments ranging from 0.4–74 gm to elicit paw withdrawal by the stimulus. For a successful test, at least 10 sequential trials are required for each paw, and about 3–5 min of resting period must be maintained between two trials. In the dorsal VF test, first the rat is wrapped in a towel so that the hindquarters are exposed. The stimulus is then given to the dorsal surface in between the first and second metatarsal by the VFH filaments. Withdrawal of the paw indicates that the spinal reflex is active. Hair value for left and right paw are recorded and withdrawal responses are marked as positive and negative. Both of these forces produced by the Von Frey Hairs are then averaged to yield a single score [92]. The VFH test has high sensitivity to assess the tactile sensation of the hindpaws, and it can also give information on neuropathic pain in chronic conditions. A disadvantage of this test is, however, that it requires a very long time of repetitive measurements which may lead to sensitization or learnt responses [93].
2.5.2. Test for hot sensation
This is a modification of the tail flick test where a heating beam is placed under the tail for a predefined time to assess the thermal sensation [94]. Later, a preheated (50 °C) plate is used for this test to evaluate the sensory stimulation of hind paws after a thoracic injury. A rat is placed on this hot plate for a maximum of 60 seconds. If the rat shows any sign of licking its hind paws within this time, the rat is considered as positive. If the rat does not show any sign of licking, it is considered as a negative and must be removed from the hot plate after 60 seconds [95]. A major limitation of the test is that the rat may vocalize or be injured due to the heat or burn its paw skin. The problem can be overcome by wrapping the rat in a towel and placing only the plantar on the metal surface [96].
2.5.3. Test for cold sensation
Unlike the hot sensation test, the cold sensation test is also useful for sensory nociceptor testing. Ethyl chloride is mainly used for testing the cold sensation of rats. First, the area of interest of the skin of a rat is shaved. Then ethyl chloride is sprayed onto it, and the response is recorded and graded based on the following scale: 0 = no response, 1 = localized response, 2 = transient vocalization, and 3 = sustained vocalization [88]. Other chemicals such as acetone can also be used for cold sensation evaluation. Application of 100 μl of acetone onto the plantar hind paw was applied to test the cold response in rats [87]. In addition, this sensory test can evaluate only unilateral pain measurement. A complete injured animal model is not suitable due to the variability during measurements.
2.6. Sensorimotor test
Unilateral or bilateral damage to the spinal cord is responsible for chronic deficits of somatosensory functions [97] and asymmetry of limb movements and inter-limb coordination [98]. The somatosensory system also incorporates the afferent, efferent and central integration pathways of the spinal cord to maintain homeostasis during joint movements. To evaluate this sensory and motor integration after a SCI and treatment, some sensorimotor tests are commonly used in rats. In the following sections, we discuss these tests in further detail.
2.6.1. Horizontal ladder walking test
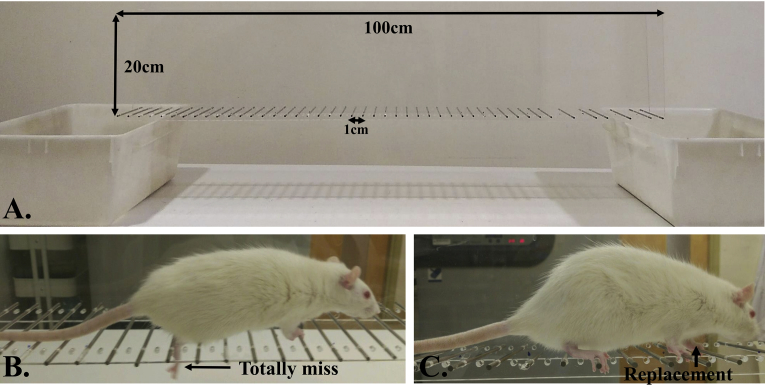
To evaluate forelimb and hindlimb stepping and coordination, a horizontal ladder walking test is frequently used [99]. Special ladder apparatus and video recording are needed for quantitative and qualitative analysis of walking. Two horizontal Plexiglas walls (100 cm × 20 cm each) with 50 small holes inserted with plane metal rungs are generally used to make the horizontal ladder (Fig. 4). The rungs are placed in a regular pattern at about 2 cm intervals. Rats are allowed to cross the horizontal ladder from a new cage to the native cage. A reward is given after each successful cross across the ladder.
Fig. 4.
Ladder rung test for rats. A) Two horizontal Plexiglas walls with 50 small inserted metal rungs; B) The rat completely misses the metal rung when walking along the ladder; C) Rat lifts its limb and replaces it on another metal rung.
After several days of training, different templates of irregular rung patterns are used to normalize the difficulty and enhance comparability during learning [70]. The recorded videos of the rat's footsteps on the rungs are used for analyses. A 7-category foot fault scoring scale (0-total miss, 1-deep slip, 2-slight slip, 3-replacement, 4-correction, 5-partial placement and 6-correct placement) is used for the quantitative measurement of foot paw placement on the rungs.
2.6.2. Grid walking test
Grid walking test is useful to evaluate and predict the efferent and afferent sensorimotor integration [100]. There are several versions of this test such as horizontal grid walking [44], ladder beam [101], inclined grid climbing [102] etc. These tests are useful for measuring sensorimotor deficit and recovery after any experimental injury and treatment.
The grid walking test is an another method to assess the sensorimotor deficits and motor incoordination in rats after a SCI [100]. The test is useful for animals with chronic neurological deficits. Because foot faults are quantified during the test, it is also termed as the foot fault test [103]. A rat is placed on a metal grid and allowed to cross a 1–1.2 m long runway with irregular gaps. A fence is used to restrict the roaming of the rat. The rat is required to put its limbs accurately on certain places. When the paw of a rat falls down, it considered as a foot fault. Errors are counted during the walking on the grid for 1 minute. A 3-point score is given to evaluate the task. In complete transected or severely contused animal models, a major limitation is that the differentiation of descending spinal influences may be difficult; however, this measure was found to be a sensitivity indicator for small residual neurological deficits [66, 78].
For inclined ladder climbing, a ladder made up of plastic frame (1280 mm × 190 mm) with 19 round rungs (6 mm in diameter) is place in an inclined position (55°). Training of animal is essential for this test. After successful training, rat is placed at the bottom of the ladder and climbing video is recorded. From the video, hindpaw placings are evaluated and correct ladder steps are noted. The test can provide an evaluation of motor behavior after severe paralysis. However, the sensory feedback cannot properly evaluated by this test. Furthermore, marginal improvements of hindlimb stepping also cannot be evaluated properly; although, for horizontal ladder walking, the hindlimb stepping can be evaluated properly.
2.6.3. Rope climbing and walking test
Pre-training of rats is needed for the rope climbing test. During this test, rats are required to climb 160 cm and 1.25-in. wide vertical rope four times. Videos are recorded using a camera to evaluate the climbing. From the video, the total number of foot slips per rope climbing are evaluated for 42 days after the injury [104]. For the horizontal rope walking test, rats are trained to cross an 83 cm horizontal rope as described previously [105]. Rats are then tested for five consecutive trials. Quantitative assessments of the rats are performed based on slips and falls from the rope during each trial. The total number of errors per 10 steps is analyzed to evaluate the locomotion on a 4-point rating (0- normal locomotion, 1- close to normal locomotion, 2- moderate or unable to achieve continuous placement, 3- frequent falls and slips).
The sensorimotor test is useful to assess gait during walking. To find out the coordination of both sensory and motor functions, ladder walking tests are performed before and after a SCI. With special apparatus, bipedal movement capacity can also be carried out during locomotion [106]. Nonetheless both quantitative and qualitative assessments are essential to validate the study after an experimental SCI.
2.6.4. Selection of an appropriate behavioral test
An appropriate behavioral test should be chosen to understand the effects of new therapeutic interventions on experimental animals. Limitations of the behavior tests should be considered before proceeding with the test. For specific tract recovery, a behavior test should be chosen very wisely. For example, to evaluate the motor recovery, especially after CST injury, skilled reaching task in rats is useful. Similarly, for drug and cell transplantation treatments for sensorimotor recovery, different grid walking tests can be considered. Behavioral assessment should also be chosen on the basis of reliability, validation and sensitivity [64, 107, 108]. The overall criteria for selecting a particular behavioral test are summarized in Table 2.
Table 2.
Summary of broad four types of behavioral tests and their selection criteria.
| Motor test | Locomotor test | Sensory test | Sensorimotor test |
|---|---|---|---|
A healthy nervous system utilizes all the functions to accomplish one particular task, it is expected that not all the functions are dominant to that task. For example, while reaching task is dominant with motor function and walking task is dominant with locomotor function; for natural reaching and walking, along with motor and locomotor functions, both sensory and sensorimotor functions are also essential. However, one or two appropriate tasks are sufficient to test the recovery of one particular function.
3. Conclusions
Laboratory rat with experimental SCI is a well-accepted animal model, frequently used worldwide for SCI-related translational research. Several surgical methods such as compression, contusion and transection models have been developed and tested on laboratory rats. Selection of a correct injury model is also essential to understand similar clinical conditions in patients. Plasticity of neural tract after SCI is very important and is a major topic in clinical research. However, there are still some questions needed to be answered. How the behavior or activity modulates the neural circuits and the synaptic strengths? What is the mechanism underlying this neuroplasticity? Each behavioral test, however, can answer only one or two specific questions. Hence, some new tests should be included into the experimental toolkits. For example, for studying bipedal locomotion, rats can be trained on a body-weight support system for locomotor training [109]. Recent studies have demonstrated successful translation of the research results to early-stage clinical interventions [110, 111]. For rat's somatosensory test, tickling test, a new method of eliciting ultrasonic vocalization by gently caressing to the trunk area, can be used to test the integrity of the neuronal tract for conveying sensory information [112]. For successful clinical tests of new interventions for SCI, an appropriate experimental plan is also needed. Nonetheless, very high ethical standards should be maintained for animal welfare and for the acceptance of the scientific outcomes.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kjell J., Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis. Model. Mech. 2016;9(10):1125–1137. doi: 10.1242/dmm.025833. Epub 2016/10/14. PubMed PMID: 27736748; PubMed Central PMCID: PMCPMC5087825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N., Fang M., Chen H., Gou F., Ding M. Evaluation of spinal cord injury animal models. Neural Regen. Res. 2014;9(22):2008–2012. doi: 10.4103/1673-5374.143436. PubMed PMID: 25598784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheriyan T., Ryan D.J., Weinreb J.H., Cheriyan J., Paul J.C., Lafage V. Spinal cord injury models: a review. Spinal Cord. 2014;52(8):588–595. doi: 10.1038/sc.2014.91. Epub 2014/06/11 PubMed PMID: 24912546. [DOI] [PubMed] [Google Scholar]
- 4.Kwon B.K., Streijger F., Hill C.E., Anderson A.J., Bacon M., Beattie M.S. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol. 2015;269:154–168. doi: 10.1016/j.expneurol.2015.04.008. Epub 2015/04/23. PubMed PMID: 25902036. [DOI] [PubMed] [Google Scholar]
- 5.Metz G.A., Curt A., van de Meent H., Klusman I., Schwab M.E., Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J. Neurotrauma. 2000;17(1):1–17. doi: 10.1089/neu.2000.17.1. Epub 2000/02/16. PubMed PMID: 10674754. [DOI] [PubMed] [Google Scholar]
- 6.van den Brand R., Heutschi J., Barraud Q., DiGiovanna J., Bartholdi K., Huerlimann M. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336(6085):1182–1185. doi: 10.1126/science.1217416. Epub 2012/06/02. PubMed PMID: 22654062. [DOI] [PubMed] [Google Scholar]
- 7.Sharif-Alhoseini M., Khormali M., Rezaei M., Safdarian M., Hajighadery A., Khalatbari M.M. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017;55(8):714–721. doi: 10.1038/sc.2016.187. Epub 2017/01/25. PubMed PMID: 28117332. [DOI] [PubMed] [Google Scholar]
- 8.Lee D.-H., Lee J.K. Animal models of axon regeneration after spinal cord injury. Neurosci. Bull. 2013;29(4):436–444. doi: 10.1007/s12264-013-1365-4. PubMed PMID: 23893429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alto L.T., Havton L.A., Conner J.M., Hollis E.R., 2nd, Blesch A., Tuszynski M.H. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat. Neurosci. 2009;12(9):1106–1113. doi: 10.1038/nn.2365. Epub 2009/08/04. PubMed PMID: 19648914; PubMed Central PMCID: PMCPMC2753201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson L. Combinatory treatments needed for spinal cord injury. Exp. Neurol. 2013;248:309–315. doi: 10.1016/j.expneurol.2013.06.024. Epub 2013/07/09. PubMed PMID: 23830948. [DOI] [PubMed] [Google Scholar]
- 11.Nardone R., Florea C., Holler Y., Brigo F., Versace V., Lochner P. Rodent, large animal and non-human primate models of spinal cord injury. Zoology (Jena) 2017;123:101–114. doi: 10.1016/j.zool.2017.06.004. Epub 2017/07/20. PubMed PMID: 28720322. [DOI] [PubMed] [Google Scholar]
- 12.Geissler S.A., Schmidt C.E., Schallert T. Rodent models and behavioral outcomes of cervical spinal cord injury. J. Spine. 2013;Suppl 4:001. doi: 10.4172/2165-7939.S4-001. PubMed PMID: 25309824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steward O., Willenberg R. Rodent spinal cord injury models for studies of axon regeneration. Exp. Neurol. 2017;287(Pt 3):374–383. doi: 10.1016/j.expneurol.2016.06.029. Epub 2016/07/05. PubMed PMID: 27374113. [DOI] [PubMed] [Google Scholar]
- 14.Sedy J., Urdzikova L., Jendelova P., Sykova E. Methods for behavioral testing of spinal cord injured rats. Neurosci. Biobehav. Rev. 2008;32(3):550–580. doi: 10.1016/j.neubiorev.2007.10.001. Epub 2007/11/27. PubMed PMID: 18036661. [DOI] [PubMed] [Google Scholar]
- 15.Sharif-Alhoseini M., Rahimi-Movaghar V. 2014. Animal Models in Traumatic Spinal Cord Injury. [PMC free article] [PubMed] [Google Scholar]
- 16.Abdullahi D., Annuar A.A., Mohamad M., Aziz I., Sanusi J. Experimental spinal cord trauma: a review of mechanically induced spinal cord injury in rat models. Rev. Neurosci. 2017;28(1):15–20. doi: 10.1515/revneuro-2016-0050. Epub 2016/11/16. PubMed PMID: 27845888. [DOI] [PubMed] [Google Scholar]
- 17.Kozyrev N., Staudt M.D., Brown A., Coolen L.M. Chronic contusion spinal cord injury impairs ejaculatory reflexes in male rats: partial recovery by systemic infusions of dopamine D3 receptor agonist 7OHDPAT. J. Neurotrauma. 2016;33(10):943–953. doi: 10.1089/neu.2015.4232. Epub 2015/10/07. PubMed PMID: 26437577. [DOI] [PubMed] [Google Scholar]
- 18.Poon P.C., Gupta D., Shoichet M.S., Tator C.H. Clip compression model is useful for thoracic spinal cord injuries: histologic and functional correlates. Spine. 2007;32(25):2853–2859. doi: 10.1097/BRS.0b013e31815b7e6b. Epub 2008/02/05. PubMed PMID: 18246008. [DOI] [PubMed] [Google Scholar]
- 19.Rivlin A.S., Tator C.H. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg. Neurol. 1978;10(1):38–43. Epub 1978/07/01. PubMed PMID: 684604. [PubMed] [Google Scholar]
- 20.Tator C.H., Poon P. Acute clip impact-compression model. In: Chen J., Xu Z.C., Xu X.-M., Zhang J.H., editors. Animal Models of Acute Neurological Injuries. Humana Press; Totowa, NJ: 2009. pp. 449–460. [Google Scholar]
- 21.Moonen G., Satkunendrarajah K., Wilcox J.T., Badner A., Mothe A., Foltz W. A new acute impact-compression lumbar spinal cord injury model in the rodent. J. Neurotrauma. 2016;33(3):278–289. doi: 10.1089/neu.2015.3937. Epub 2015/09/29. PubMed PMID: 26414192; PubMed Central PMCID: PMCPMC4744888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cregg J.M., DePaul M.A., Filous A.R., Lang B.T., Tran A., Silver J. Functional regeneration beyond the glial scar. Exp. Neurol. 2014;253:197–207. doi: 10.1016/j.expneurol.2013.12.024. Epub 2014/01/16. PubMed PMID: 24424280; PubMed Central PMCID: PMCPMC3951813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun G.D., Chen Y., Zhou Z.G., Yang S.X., Zhong C., Li Z.Z. A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord. Neural Regen. Res. 2017;12(8):1365–1374. doi: 10.4103/1673-5374.213693. Epub 2017/10/03. PubMed PMID: 28966654; PubMed Central PMCID: PMCPMC5607834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forgione N., Karadimas S.K., Foltz W.D., Satkunendrarajah K., Lip A., Fehlings M.G. Bilateral contusion-compression model of incomplete traumatic cervical spinal cord injury. J. Neurotrauma. 2014;31(21):1776–1788. doi: 10.1089/neu.2014.3388. Epub 2014/06/21. PubMed PMID: 24949719; PubMed Central PMCID: PMCPMC4186801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young W. Spinal cord contusion models. Prog. Brain Res. 2002;137:231–255. doi: 10.1016/s0079-6123(02)37019-5. Epub 2002/11/21. PubMed PMID: 12440371. [DOI] [PubMed] [Google Scholar]
- 26.van Gorp S., Leerink M., Nguyen S., Platoshyn O., Marsala M., Joosten E.A. Translation of the rat thoracic contusion model; part 2 - forward versus backward locomotion testing. Spinal Cord. 2014;52(7):529–535. doi: 10.1038/sc.2014.73. Epub 2014/05/14. PubMed PMID: 24819507. [DOI] [PubMed] [Google Scholar]
- 27.Young W. MASCIS spinal cord contusion model. In: Chen J., Xu Z.C., Xu X.-M., Zhang J.H., editors. Animal Models of Acute Neurological Injuries. Humana Press; Totowa, NJ: 2009. pp. 411–421. [Google Scholar]
- 28.Gruner J.A. A monitored contusion model of spinal cord injury in the rat. J. Neurotrauma. 1992;9(2):123–126. doi: 10.1089/neu.1992.9.123. discussion 6-8. Epub 1992/01/01. PubMed PMID: 1404425. [DOI] [PubMed] [Google Scholar]
- 29.Scheff S.W., Rabchevsky A.G., Fugaccia I., Main J.A., Lumpp J.E., Jr. Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J. Neurotrauma. 2003;20(2):179–193. doi: 10.1089/08977150360547099. Epub 2003/04/05. PubMed PMID: 12675971. [DOI] [PubMed] [Google Scholar]
- 30.Kwon B.K., Hillyer J., Tetzlaff W. Translational research in spinal cord injury: a survey of opinion from the SCI community. J. Neurotrauma. 2010;27(1):21–33. doi: 10.1089/neu.2009.1048. Epub 2009/09/16. PubMed PMID: 19751098. [DOI] [PubMed] [Google Scholar]
- 31.Onifer S.M., Rabchevsky A.G., Scheff S.W. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007;48(4):385–395. doi: 10.1093/ilar.48.4.385. Epub 2007/08/23. PubMed PMID: 17712224. [DOI] [PubMed] [Google Scholar]
- 32.Beattie M.S., Bresnahan J.C. Cell death, repair, and recovery of function after spinal cord contusion injuries in rats. In: Kalb R.G., Strittmatter S.M., editors. Neurobiology of Spinal Cord Injury. Humana Press; Totowa, NJ: 2000. pp. 1–21. [Google Scholar]
- 33.Talac R., Friedman J.A., Moore M.J., Lu L., Jabbari E., Windebank A.J. Animal models of spinal cord injury for evaluation of tissue engineering treatment strategies. Biomaterials. 2004;25(9):1505–1510. doi: 10.1016/s0142-9612(03)00497-6. Epub 2003/12/31. PubMed PMID: 14697853. [DOI] [PubMed] [Google Scholar]
- 34.Lukovic D., Moreno-Manzano V., Lopez-Mocholi E., Rodriguez-Jimenez F.J., Jendelova P., Sykova E. Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Sci. Rep. 2015;5:9640. doi: 10.1038/srep09640. Epub 2015/04/11. PubMed PMID: 25860664; PubMed Central PMCID: PMCPMC5381701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F., Huang S.-L., He X.-J., Li X.-H. Determination of the ideal rat model for spinal cord injury by diffusion tensor imaging. Neuroreport. 2014;25(17):1386–1392. doi: 10.1097/WNR.0000000000000278. Epub 2014/04/14. PubMed PMID: 25325349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores-Leal M., Morales-Guadarrama A., Salgado-Ceballos H., Sacristán-Rock E., editors. Rat Spinal Cord Transection Injury Progression: an MRI Study. Springer Singapore; Singapore: 2017. [Google Scholar]
- 37.Shah P.K., Gerasimenko Y., Shyu A., Lavrov I., Zhong H., Roy R.R. Variability in step training enhances locomotor recovery after a spinal cord injury. Eur. J. Neurosci. 2012;36(1):2054–2062. doi: 10.1111/j.1460-9568.2012.08106.x. Epub 2012/05/18. PubMed PMID: 22591277; PubMed Central PMCID: PMCPMC3389255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvanian V.L., Schnell L., Lou L., Golshani R., Hunanyan A., Ghosh A. Chronic spinal hemisection in rats induces a progressive decline in transmission in uninjured fibers to motoneurons. Exp. Neurol. 2009;216(2):471–480. doi: 10.1016/j.expneurol.2009.01.004. Epub 2009/03/26. PubMed PMID: 19320005; PubMed Central PMCID: PMCPMC2889190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bregman B.S., Coumans J.-V., Dai H.N., Kuhn P.L., Lynskey J., McAtee M. Chapter 18 Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury. In: McKerracher L., Doucet G., Rossignol S., editors. vol. 137. Elsevier; 2002. pp. 257–273. (Progress in Brain Research). [DOI] [PubMed] [Google Scholar]
- 40.Schwab M.E., Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol. Rev. 1996;76(2):319–370. doi: 10.1152/physrev.1996.76.2.319. Epub 1996/04/01. PubMed PMID: 8618960. [DOI] [PubMed] [Google Scholar]
- 41.Dai H., MacArthur L., McAtee M., Hockenbury N., Tidwell J.L., McHugh B. Activity-based therapies to promote forelimb use after a cervical spinal cord injury. J. Neurotrauma. 2009;26(10):1719–1732. doi: 10.1089/neu.2008-0592. Epub 2009/03/26. PubMed PMID: 19317604; PubMed Central PMCID: PMCPMC2788495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Girgis J., Merrett D., Kirkland S., Metz G.A., Verge V., Fouad K. Reaching training in rats with spinal cord injury promotes plasticity and task specific recovery. Brain. 2007;130(Pt 11):2993–3003. doi: 10.1093/brain/awm245. Epub 2007/10/12. PubMed PMID: 17928316. [DOI] [PubMed] [Google Scholar]
- 43.Muir G.D., Webb A.A. Mini-review: assessment of behavioural recovery following spinal cord injury in rats. Eur. J. Neurosci. 2000;12(9):3079–3086. doi: 10.1046/j.1460-9568.2000.00205.x. Epub 2000/09/21. PubMed PMID: 10998091. [DOI] [PubMed] [Google Scholar]
- 44.Kunkel-Bagden E., Dai H.N., Bregman B.S. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp. Neurol. 1993;119(2):153–164. doi: 10.1006/exnr.1993.1017. Epub 1993/02/01. PubMed PMID: 8432357. [DOI] [PubMed] [Google Scholar]
- 45.Schomburg E.D. Spinal sensorimotor systems and their supraspinal control. Neurosci. Res. 1990;7(4):265–340. doi: 10.1016/0168-0102(90)90008-3. [DOI] [PubMed] [Google Scholar]
- 46.Raineteau O., Schwab M.E. Plasticity of motor systems after incomplete spinal cord injury. Nat. Rev. Neurosci. 2001;2:263. doi: 10.1038/35067570. [DOI] [PubMed] [Google Scholar]
- 47.Starkey M.L., Bleul C., Maier I.C., Schwab M.E. Rehabilitative training following unilateral pyramidotomy in adult rats improves forelimb function in a non-task-specific way. Exp. Neurol. 2011;232(1):81–89. doi: 10.1016/j.expneurol.2011.08.006. Epub 2011/08/27. PubMed PMID: 21867701. [DOI] [PubMed] [Google Scholar]
- 48.Whishaw I.Q., Gorny B., Foroud A., Kleim J.A. Long-Evans and Sprague-Dawley rats have similar skilled reaching success and limb representations in motor cortex but different movements: some cautionary insights into the selection of rat strains for neurobiological motor research. Behav. Brain Res. 2003;145(1–2):221–232. doi: 10.1016/s0166-4328(03)00143-8. Epub 2003/10/08. PubMed PMID: 14529819. [DOI] [PubMed] [Google Scholar]
- 49.Alam M., Garcia-Alias G., Jin B., Keyes J., Zhong H., Roy R.R. Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats. Exp. Neurol. 2017;291:141–150. doi: 10.1016/j.expneurol.2017.02.006. Epub 2017/02/14. PubMed PMID: 28192079; PubMed Central PMCID: PMCPMC6219872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whishaw I.Q., Pellis S.M., Gorny B., Kolb B., Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 1993;56(1):59–76. doi: 10.1016/0166-4328(93)90022-i. Epub 1993/07/30. doi: PubMed PMID: 7691077. [DOI] [PubMed] [Google Scholar]
- 51.Whishaw I.Q. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39(5):788–805. doi: 10.1016/s0028-3908(99)00259-2. Epub 2000/03/04. PubMed PMID: 10699445. [DOI] [PubMed] [Google Scholar]
- 52.Chan C.C., Khodarahmi K., Liu J., Sutherland D., Oschipok L.W., Steeves J.D. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp. Neurol. 2005;196(2):352–364. doi: 10.1016/j.expneurol.2005.08.011. Epub 2005/09/13. PubMed PMID: 16154567. [DOI] [PubMed] [Google Scholar]
- 53.Piecharka D.M., Kleim J.A., Whishaw I.Q. Limits on recovery in the corticospinal tract of the rat: partial lesions impair skilled reaching and the topographic representation of the forelimb in motor cortex. Brain Res. Bull. 2005;66(3):203–211. doi: 10.1016/j.brainresbull.2005.04.013. Epub 2005/07/19. PubMed PMID: 16023917. [DOI] [PubMed] [Google Scholar]
- 54.Irvine K.A., Ferguson A.R., Mitchell K.D., Beattie S.B., Beattie M.S., Bresnahan J.C. A novel method for assessing proximal and distal forelimb function in the rat: the Irvine, Beatties and Bresnahan (IBB) forelimb scale. J. Vis. Exp. 2010;46 doi: 10.3791/2246. Epub 2011/01/06. PubMed PMID: 21206464; PubMed Central PMCID: PMCPMC3159659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Inoue T., Lin A., Ma X., McKenna S.L., Creasey G.H., Manley G.T. Combined SCI and TBI: recovery of forelimb function after unilateral cervical spinal cord injury (SCI) is retarded by contralateral traumatic brain injury (TBI), and ipsilateral TBI balances the effects of SCI on paw placement. Exp. Neurol. 2013;248:136–147. doi: 10.1016/j.expneurol.2013.06.006. Epub 2013/06/19. PubMed PMID: 23770071; PubMed Central PMCID: PMCPMC4617760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979;1(3):233–236. Epub 1979/01/01. PubMed PMID: 551317. [PubMed] [Google Scholar]
- 57.Anderson K.D., Gunawan A., Steward O. Quantitative assessment of forelimb motor function after cervical spinal cord injury in rats: relationship to the corticospinal tract. Exp. Neurol. 2005;194(1):161–174. doi: 10.1016/j.expneurol.2005.02.006. Epub 2005/05/19. PubMed PMID: 15899253. [DOI] [PubMed] [Google Scholar]
- 58.Alam M., Garcia-Alias G., Shah P.K., Gerasimenko Y., Zhong H., Roy R.R. Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats. J. Neurosci. Methods. 2015;247:50–57. doi: 10.1016/j.jneumeth.2015.03.012. Epub 2015/03/21. PubMed PMID: 25791014; PubMed Central PMCID: PMCPMC4465788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertelli J.A., Mira J.C. The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J. Neurosci. Methods. 1995;58(1–2):151–155. doi: 10.1016/0165-0270(94)00169-h. Epub 1995/05/01. PubMed PMID: 7475220. [DOI] [PubMed] [Google Scholar]
- 60.Brown Thomas G., Sherrington Charles S. The intrinsic factors in the act of progression in the mammal. Proc. R. Soc. Lond. Ser. B Contain. Pap. Biol. Character. 1911;84(572):308–319. [Google Scholar]
- 61.Taccola G. The locomotor central pattern generator of the rat spinal cord in vitro is optimally activated by noisy dorsal root waveforms. J. Neurophysiol. 2011;106(2):872–884. doi: 10.1152/jn.00170.2011. Epub 2011/05/27. PubMed PMID: 21613591. [DOI] [PubMed] [Google Scholar]
- 62.Singh A., Krisa L., Frederick K.L., Sandrow-Feinberg H., Balasubramanian S., Stackhouse S.K. Forelimb locomotor rating scale for behavioral assessment of recovery after unilateral cervical spinal cord injury in rats. J. Neurosci. Methods. 2014;226:124–131. doi: 10.1016/j.jneumeth.2014.01.001. Epub 2014/01/29. PubMed PMID: 24468219; PubMed Central PMCID: PMCPMC4252014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarlov I.M., Klinger H. Spinal cord compression studies. II. Time limits for recovery after acute compression in dogs. AMA Arch. Neurol. Psychiatry. 1954;71(3):271–290. Epub 1954/03/01. PubMed PMID: 13123590. [PubMed] [Google Scholar]
- 64.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. Epub 1995/02/01. PubMed PMID: 7783230. [DOI] [PubMed] [Google Scholar]
- 65.Fehlings M.G., Tator C.H. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995;132(2):220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 66.Metz G.A., Merkler D., Dietz V., Schwab M.E., Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883(2):165–177. doi: 10.1016/s0006-8993(00)02778-5. Epub 2000/11/14. PubMed PMID: 11074045. [DOI] [PubMed] [Google Scholar]
- 67.Koopmans G.C., Deumens R., Honig W.M., Hamers F.P., Steinbusch H.W., Joosten E.A. The assessment of locomotor function in spinal cord injured rats: the importance of objective analysis of coordination. J. Neurotrauma. 2005;22(2):214–225. doi: 10.1089/neu.2005.22.214. Epub 2005/02/18. PubMed PMID: 15716628. [DOI] [PubMed] [Google Scholar]
- 68.Gerasimenko Y.P., Ichiyama R.M., Lavrov I.A., Courtine G., Cai L., Zhong H. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J. Neurophysiol. 2007;98(5):2525–2536. doi: 10.1152/jn.00836.2007. Epub 2007/09/15. PubMed PMID: 17855582. [DOI] [PubMed] [Google Scholar]
- 69.Popovich P.G., Guan Z., Wei P., Huitinga I., van Rooijen N., Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158(2):351–365. doi: 10.1006/exnr.1999.7118. Epub 1999/07/23. PubMed PMID: 10415142. [DOI] [PubMed] [Google Scholar]
- 70.Metz G.A., Whishaw I.Q. The ladder rung walking task: a scoring system and its practical application. J. Vis. Exp. JoVE. 2009;(28):1204. doi: 10.3791/1204. PubMed PMID: 19525918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lankhorst A.J., Verzijl M.R., Hamers F.P.T. Experimental spinal cord contusion injury: comparison of different outcome parameters. Neurosci. Res. Commun. 1999;24(3):135–148. [Google Scholar]
- 72.Xu N., Akesson E., Holmberg L., Sundstrom E. A sensitive and reliable test instrument to assess swimming in rats with spinal cord injury. Behav. Brain Res. 2015;291:172–183. doi: 10.1016/j.bbr.2015.05.004. Epub 2015/05/20. PubMed PMID: 25986406. [DOI] [PubMed] [Google Scholar]
- 73.Hall C.S. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934;18(3):385–403. [Google Scholar]
- 74.Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 2003;463(1–3):3–33. doi: 10.1016/s0014-2999(03)01272-x. Epub 2003/02/26. PubMed PMID: 12600700. [DOI] [PubMed] [Google Scholar]
- 75.Mills C.D., Grady J.J., Hulsebosch C.E. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J. Neurotrauma. 2001;18(10):1091–1105. doi: 10.1089/08977150152693773. Epub 2001/11/01. PubMed PMID: 11686495. [DOI] [PubMed] [Google Scholar]
- 76.Laviola G., Renna G., Bignami G., Cuomo V. Ontogenetic and pharmacological dissociation of various components of locomotor activity and habituation in the rat. Int. J. Dev. Neurosci. 1988;6(5):431–438. doi: 10.1016/0736-5748(88)90049-4. Epub 1988/01/01. PubMed PMID: 3202002. [DOI] [PubMed] [Google Scholar]
- 77.Martinez M., Brezun J.M., Bonnier L., Xerri C. A new rating scale for open-field evaluation of behavioral recovery after cervical spinal cord injury in rats. J. Neurotrauma. 2009;26(7):1043–1053. doi: 10.1089/neu.2008.0717. Epub 2009/07/15. PubMed PMID: 19594382. [DOI] [PubMed] [Google Scholar]
- 78.Behrmann D.L., Bresnahan J.C., Beattie M.S., Shah B.R. Spinal cord injury produced by consistent mechanical displacement of the cord in rats: behavioral and histologic analysis. J. Neurotrauma. 1992;9(3):197–217. doi: 10.1089/neu.1992.9.197. Epub 1992/01/01. PubMed PMID: 1474608. [DOI] [PubMed] [Google Scholar]
- 79.Bohlman H.H., Bahniuk E., Field G., Raskulinecz G. Spinal cord monitoring of experimental incomplete cervical spinal cord injury: a preliminary report. Spine. 1981;6(5):428–436. doi: 10.1097/00007632-198109000-00002. Epub 1981/09/01. PubMed PMID: 7302676. [DOI] [PubMed] [Google Scholar]
- 80.de Leon R., Hodgson J.A., Roy R.R., Edgerton V.R. Extensor- and flexor-like modulation within motor pools of the rat hindlimb during treadmill locomotion and swimming. Brain Res. 1994;654(2):241–250. doi: 10.1016/0006-8993(94)90485-5. Epub 1994/08/22. PubMed PMID: 7987674. [DOI] [PubMed] [Google Scholar]
- 81.Hutchinson K.J., Gomez-Pinilla F., Crowe M.J., Ying Z., Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127(Pt 6):1403–1414. doi: 10.1093/brain/awh160. Epub 2004/04/08. PubMed PMID: 15069022. [DOI] [PubMed] [Google Scholar]
- 82.Ryu Y., Ogata T., Nagao M., Kitamura T., Morioka K., Ichihara Y. The swimming test is effective for evaluating spasticity after contusive spinal cord injury. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171937. Epub 2017/02/10. PubMed PMID: 28182676; PubMed Central PMCID: PMCPMC5300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith R.R., Burke D.A., Baldini A.D., Shum-Siu A., Baltzley R., Bunger M. The Louisville Swim Scale: a novel assessment of hindlimb function following spinal cord injury in adult rats. J. Neurotrauma. 2006;23(11):1654–1670. doi: 10.1089/neu.2006.23.1654. Epub 2006/11/23. PubMed PMID: 17115911; PubMed Central PMCID: PMCPMC2833969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liebscher T., Schnell L., Schnell D., Scholl J., Schneider R., Gullo M. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann. Neurol. 2005;58(5):706–719. doi: 10.1002/ana.20627. Epub 2005/09/21. PubMed PMID: 16173073. [DOI] [PubMed] [Google Scholar]
- 85.Bogdanova O.V., Kanekar S., D'Anci K.E., Renshaw P.F. Factors influencing behavior in the forced swim test. Physiol. Behav. 2013;118:227–239. doi: 10.1016/j.physbeh.2013.05.012. Epub 2013/05/21. PubMed PMID: 23685235; PubMed Central PMCID: PMCPMC5609482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hunskaar S., Berge O.G., Hole K. A modified hot-plate test sensitive to mild analgesics. Behav. Brain Res. 1986;21(2):101–108. doi: 10.1016/0166-4328(86)90088-4. Epub 1986/08/01. PubMed PMID: 3755945. [DOI] [PubMed] [Google Scholar]
- 87.Choi Y., Yoon Y.W., Na H.S., Kim S.H., Chung J.M. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–376. doi: 10.1016/0304-3959(94)90023-X. Epub 1994/12/01. PubMed PMID: 7708411. [DOI] [PubMed] [Google Scholar]
- 88.Yu W., Hao J.X., Xu X.J., Saydoff J., Haegerstrand A., Hokfelt T. Long-term alleviation of allodynia-like behaviors by intrathecal implantation of bovine chromaffin cells in rats with spinal cord injury. Pain. 1998;74(2–3):115–122. doi: 10.1016/s0304-3959(97)00204-2. Epub 1998/03/31. PubMed PMID: 9520225. [DOI] [PubMed] [Google Scholar]
- 89.Lavrov I., Courtine G., Dy C.J., van den Brand R., Fong A.J., Gerasimenko Y. Facilitation of stepping with epidural stimulation in spinal rats: role of sensory input. J. Neurosci. 2008;28(31):7774–7780. doi: 10.1523/JNEUROSCI.1069-08.2008. PubMed PMID: 18667609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Detloff M.R., Fisher L.C., Deibert R.J., Basso D.M. Acute and chronic tactile sensory testing after spinal cord injury in rats. JoVE. 2012;62:e3247. doi: 10.3791/3247. Epub 2012/04/18. PubMed PMID: 22508401; PubMed Central PMCID: PMCPMC3466630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gris D., Marsh D.R., Oatway M.A., Chen Y., Hamilton E.F., Dekaban G.A. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004;24(16):4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. Epub 2004/04/23. PubMed PMID: 15102919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Detloff M.R., Clark L.M., Hutchinson K.J., Kloos A.D., Fisher L.C., Basso D.M. Validity of acute and chronic tactile sensory testing after spinal cord injury in rats. Exp. Neurol. 2010;225(2):366–376. doi: 10.1016/j.expneurol.2010.07.009. Epub 2010/07/21. PubMed PMID: 20643128; PubMed Central PMCID: PMCPMC4933012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deuis J.R., Dvorakova L.S., Vetter I. Methods used to evaluate pain behaviors in rodents. Front. Mol. Neurosci. 2017;10(284) doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Merkler D., Metz G.A., Raineteau O., Dietz V., Schwab M.E., Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J. Neurosci. 2001;21(10):3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. Epub 2001/05/23. PubMed PMID: 11331396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gale K., Kerasidis H., Wrathall J.R. Spinal cord contusion in the rat: behavioral analysis of functional neurologic impairment. Exp. Neurol. 1985;88(1):123–134. doi: 10.1016/0014-4886(85)90118-9. Epub 1985/04/01. PubMed PMID: 3979506. [DOI] [PubMed] [Google Scholar]
- 96.Carter M., Shieh J. Chapter 2 - animal behavior. In: Carter M., Shieh J., editors. Guide to Research Techniques in Neuroscience. second ed. Academic Press; San Diego: 2015. pp. 39–71. [Google Scholar]
- 97.Liu Y., Kim D., Himes B.T., Chow S.Y., Schallert T., Murray M. Transplants of fibroblasts genetically modified to express BDNF promote regeneration of adult rat rubrospinal axons and recovery of forelimb function. J. Neurosci. 1999;19(11):4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. Epub 1999/05/26. PubMed PMID: 10341240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schallert T., Fleming S.M., Leasure J.L., Tillerson J.L., Bland S.T. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39(5):777–787. doi: 10.1016/s0028-3908(00)00005-8. Epub 2000/03/04. PubMed PMID: 10699444. [DOI] [PubMed] [Google Scholar]
- 99.Metz G.A., Whishaw I.Q. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J. Neurosci. Methods. 2002;115(2):169–179. doi: 10.1016/s0165-0270(02)00012-2. Epub 2002/05/07. PubMed PMID: 11992668. [DOI] [PubMed] [Google Scholar]
- 100.Chao O.Y., Pum M.E., Li J.S., Huston J.P. The grid-walking test: assessment of sensorimotor deficits after moderate or severe dopamine depletion by 6-hydroxydopamine lesions in the dorsal striatum and medial forebrain bundle. Neuroscience. 2012;202:318–325. doi: 10.1016/j.neuroscience.2011.11.016. Epub 2011/12/07. PubMed PMID: 22142899. [DOI] [PubMed] [Google Scholar]
- 101.Soblosky J.S., Colgin L.L., Chorney-Lane D., Davidson J.F., Carey M.E. Ladder beam and camera video recording system for evaluating forelimb and hindlimb deficits after sensorimotor cortex injury in rats. J. Neurosci. Methods. 1997;78(1–2):75–83. doi: 10.1016/s0165-0270(97)00131-3. Epub 1998/03/13. PubMed PMID: 9497003. [DOI] [PubMed] [Google Scholar]
- 102.Semler J., Wellmann K., Wirth F., Stein G., Angelova S., Ashrafi M. Objective measures of motor dysfunction after compression spinal cord injury in adult rats: correlations with locomotor rating scores. J. Neurotrauma. 2011;28(7):1247–1258. doi: 10.1089/neu.2010.1737. Epub 2011/03/25. PubMed PMID: 21428717. [DOI] [PubMed] [Google Scholar]
- 103.Schaar K.L., Brenneman M.M., Savitz S.I. Functional assessments in the rodent stroke model. Exp. Transl. Stroke Med. 2010;2(1):13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thallmair M., Metz G.A., Z'Graggen W.J., Raineteau O., Kartje G.L., Schwab M.E. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat. Neurosci. 1998;1(2):124–131. doi: 10.1038/373. Epub 1999/04/09. PubMed PMID: 10195127. [DOI] [PubMed] [Google Scholar]
- 105.Kim D., Schallert T., Liu Y., Browarak T., Nayeri N., Tessler A. Transplantation of genetically modified fibroblasts expressing BDNF in adult rats with a subtotal hemisection improves specific motor and sensory functions. Neurorehabil. Neural Repair. 2001;15(2):141–150. doi: 10.1177/154596830101500207. Epub 2002/01/29. PubMed PMID: 11811255. [DOI] [PubMed] [Google Scholar]
- 106.Rigosa J., Panarese A., Dominici N., Friedli L., van den Brand R., Carpaneto J. Decoding bipedal locomotion from the rat sensorimotor cortex. J. Neural Eng. 2015;12(5) doi: 10.1088/1741-2560/12/5/056014. [DOI] [PubMed] [Google Scholar]
- 107.Basso D.M. Behavioral testing after spinal cord injury: congruities, complexities, and controversies. J. Neurotrauma. 2004;21(4):395–404. doi: 10.1089/089771504323004548. Epub 2004/04/30. PubMed PMID: 15115589. [DOI] [PubMed] [Google Scholar]
- 108.Fouad K., Hurd C., Magnuson D.S.K. Functional testing in animal models of spinal cord injury: not as straight forward as one would think. Front. Integr. Neurosci. 2013;7:85. doi: 10.3389/fnint.2013.00085. PubMed PMID: 24324414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Leon R.D., Reinkensmeyer D.J., Timoszyk W.K., London N.J., Roy R.R., Edgerton V.R. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog. Brain Res. 2002;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. Epub 2002/11/21. PubMed PMID: 12440365. [DOI] [PubMed] [Google Scholar]
- 110.Courtine G., Gerasimenko Y., van den Brand R., Yew A., Musienko P., Zhong H. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009;12:1333. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harkema S., Gerasimenko Y., Hodes J., Burdick J., Angeli C., Chen Y. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–1947. doi: 10.1016/S0140-6736(11)60547-3. Epub 2011/05/24. PubMed PMID: 21601270; PubMed Central PMCID: PMCPMC3154251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishiyama S., Brecht M. Neural correlates of ticklishness in the rat somatosensory cortex. Science. 2016;354(6313):757. doi: 10.1126/science.aah5114. [DOI] [PubMed] [Google Scholar]
- 113.Walker C.L., Xu X.-M. Morphological assessments following spinal cord injury. In: Chen J., Xu X.-M., Xu Z.C., Zhang J.H., editors. Volume 2. Humana Press; Totowa, NJ: 2012. pp. 405–416. (Animal Models of Acute Neurological Injuries II: Injury and Mechanistic Assessments). [Google Scholar]
- 114.Pinzon A., Marcillo A., Quintana A., Stamler S., Bunge M.B., Bramlett H.M. A re-assessment of minocycline as a neuroprotective agent in a rat spinal cord contusion model. Brain Res. 2008;1243:146–151. doi: 10.1016/j.brainres.2008.09.047. Epub 2008/10/08. PubMed PMID: 18838063; PubMed Central PMCID: PMCPMC2643041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krishna V., Andrews H., Jin X., Yu J., Varma A., Wen X. A contusion model of severe spinal cord injury in rats. JoVE. 2013;78 doi: 10.3791/50111. Epub 2013/08/28. PubMed PMID: 23979022; PubMed Central PMCID: PMCPMC3855925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marques S.A., Garcez V.F., Del Bel E.A., Martinez A.M.B. A simple, inexpensive and easily reproducible model of spinal cord injury in mice: morphological and functional assessment. J. Neurosci. Methods. 2009;177(1):183–193. doi: 10.1016/j.jneumeth.2008.10.015. PubMed PMID: 19013194. [DOI] [PubMed] [Google Scholar]
- 117.Guertin P.A. Central pattern generator for locomotion: anatomical, physiological, and pathophysiological considerations. Front. Neurol. 2012;3:183. doi: 10.3389/fneur.2012.00183. Epub 2013/02/14. PubMed PMID: 23403923; PubMed Central PMCID: PMCPMC3567435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. Epub 2006/06/17. PubMed PMID: 16776587. [DOI] [PubMed] [Google Scholar]
- 119.Moreno-Lopez Y., Olivares-Moreno R., Cordero-Erausquin M., Rojas-Piloni G. Sensorimotor integration by corticospinal system. Front. Neuroanat. 2016;10:24. doi: 10.3389/fnana.2016.00024. Epub 2016/03/26. PubMed PMID: 27013985; PubMed Central PMCID: PMCPMC4783411. [DOI] [PMC free article] [PubMed] [Google Scholar]